Background: HCV infection results in hepatocellular carcinoma. mTORC1 and eIF4E regulate tumorigenesis through translation initiation.
Results: HCV, through NS5A, activates mTORC1 and eIF4E, resulting in enhanced eIF4F assembly. NS5A binds to eIF4F complex and associates with polysomes.
Conclusion: HCV up-regulates cap-dependent host protein translation machinery through NS5A.
Significance: Activation of host translation machinery might facilitate HCV-associated hepatocellular carcinoma.
Keywords: Cell Signaling, eIF4E, Hepatitis C Virus, mTOR, Translation Initiation Factors, Translation Regulation, MNK
Abstract
Initiation, a major rate-limiting step of host protein translation, is a critical target in many viral infections. Chronic hepatitis C virus (HCV) infection results in hepatocellular carcinoma. Translation initiation, up-regulated in many cancers, plays a critical role in tumorigenesis. mTOR is a major regulator of host protein translation. Even though activation of PI3K-AKT-mTOR by HCV non-structural protein 5A (NS5A) is known, not much is understood about the regulation of host translation initiation by this virus. Here for the first time we show that HCV up-regulates host cap-dependent translation machinery in Huh7.5 cells through simultaneous activation of mTORC1 and eukaryotic translation initiation factor 4E (eIF4E) by NS5A. NS5A, interestingly, overexpressed and subsequently hyperphosphorylated 4EBP1. NS5A phosphorylated eIF4E through the p38 MAPK-MNK pathway. Both HCV infection and NS5A expression augmented eIF4F complex assembly, an indicator of cap-dependent translation efficiency. Global translation, however, was not altered by HCV NS5A. 4EBP1 phosphorylation, but not that of S6K1, was uniquely resistant to rapamycin in NS5A-Huh7.5 cells, indicative of an alternate phosphorylation mechanism of 4EBP1. Resistance of Ser-473, but not Thr-308, phosphorylation of AKT to PI3K inhibitors suggested an activation of mTORC2 by NS5A. NS5A associated with eIF4F complex and polysomes, suggesting its active involvement in host translation. This is the first report that implicates an HCV protein in the up-regulation of host translation initiation apparatus through concomitant regulation of multiple pathways. Because both mTORC1 activation and eIF4E phosphorylation are involved in tumorigenesis, we propose that their simultaneous activation by NS5A might contribute significantly to the development of hepatocellular carcinoma.
Introduction
Hepatitis C virus (HCV),4 the lone member of the genus Hepacivirus within the family Flaviviridae (1), is a human pathogen with widespread distribution across the globe. An estimated 170 million people have been infected with HCV globally. Liver is the major site of HCV infection that leads to initial hepatitis, and chronic infections lead to liver cirrhosis and hepatocellular carcinoma (2). Non-structural protein 5A (NS5A) is a large HCV polypeptide that has been associated with a myriad of functions including virus replication, cell cycle regulation, proliferation, and modulation of antiviral effectors (3–6). NS5A migrates at 56- and 58-kDa positions in SDS-PAGE due to its differential existence in hypo or hyperphosphorylated forms (7, 8). However, studies suggested that NS5A protein from HCV genotype 2a is not hyperphosphorylated, in contrast to genotype 1b, suggesting differential requirements and regulation of NS5A functions between these genotypes (9).
Translation initiation is the major rate-limiting event in eukaryotic protein synthesis (10, 11). A majority of the eukaryotic mRNA transcripts are solely translated by cap-dependent translation. 5′Cap is a 7-methyl GTP structure at the extreme 5′ end of majority of eukaryotic mRNAs that is recognized and physically bound by eukaryotic translation initiation complex 4F (eIF4F) (12–14). The mRNA binding eIF4F complex is constituted by eukaryotic translation initiation factors 4E (eIF4E), the scaffold protein 4G (eIF4G), and RNA helicase 4A (eIF4A). eIF4E binds to the cap structure initially and is followed by the binding of eIF4G to it. Binding of eIF4A to eIF4G completes the assembly of eIF4F complex. After binding to the cap, the eIF4F complex is joined by a 43 S preinitiation complex and then searches for the first eligible AUG for translation initiation (12, 15).
Regulation of translation is an important step in various cellular events (14, 16, 17). eIF4F complex assembly and its subsequent binding to the cap structure (13, 14) are targets of regulation. A major pathway that regulates eIF4F complex assembly is the mammalian target of rapamycin (mTOR) 4E-binding protein (4EBP) pathway. mTOR is a serine/threonine kinase and functions through two distinct complexes, viz. mTORC1 and mTORC2. Host translation is regulated by mTORC1 through two of its major effectors, 4EBP1 and p70S6K1 (S6K1, p70 ribosomal S6 kinase 1) (18, 19). 4EBP1 has at least four important phosphorylation sites and exists either in hypo- or hyperphosphorylated forms. Hypophosphorylated 4EBP1 binds to eIF4E and inhibits its interaction with eIF4G, thereby inhibiting eIF4F complex formation. Phosphorylated 4EBP1 loses eIF4E binding affinity, allowing it to bind to 5′cap and form an eIF4F complex (20). mTORC2 activity is less understood, and one of its substrates is the Ser-473 residue of AKT (21).
Another molecule that regulates translation initiation is eIF4E. MAP kinase interacting serine/threonine kinase (MNK) phosphorylates eIF4E at Ser-209, and its recruitment to eIF4G is critical for eIF4E phosphorylation (22). Both p38 and ERK1/2 MAP kinases (23, 24) can phosphorylate MNK, thereby regulating its recruitment to eIF4G (22, 25). Even as a set of reports suggests that eIF4E phosphorylation at Ser-209 residue reduces its affinity for 5′cap and eIF4F complex formation (26), the incremental role of eIF4E expression and its phosphorylation in tumorigenesis is unquestionable (27–33). However, neither eIF4E overexpression nor increased eIF4F complex formation enhances global protein synthesis. Interestingly, these modifications result in preferential translation of a limited number of proteins that have unusually long and complex 5′-untranslated region (34). Many of these proteins are involved in events such as cell cycle regulation, apoptosis, and angiogenesis (35–38). Some recent reports support the implicated role of enhanced eIF4F complex assembly on cancer development and drug resistance (39, 40).
Viruses are acknowledged to interfere with and modulate host translation machinery through a variety of mechanisms (41). These interactions usually result in up-regulation of viral protein synthesis and could be inhibitory to host translation (42–52). These reports underline the intensely tangled relationship the viruses maintain with the host translation.
In this study we tried to understand the involvement of HCV NS5A protein in host protein translation initiation. HCV proteins have been shown to activate phosphatidylinositol-3 kinase (PI3K), which contributes significantly to the development of hepatocellular carcinoma (53). PI3K regulates mTOR either via AKT or independent of it, which in turn regulates translation initiation through 4EBP1 (54, 55). HCV NS5A and pseudovirus particles were reported to negatively regulate protein translation in non-hepatic cell lines (56), whereas another report demonstrated activation of mTOR-mediated anti-apoptotic activities by HCV NS5A (57). These reports present a paradox because activation of mTOR suggests a possible activation of cap-dependent translation. In addition, these studies were performed either with pseudovirus particles or HCV replicons or in systems that express viral proteins. We tried to understand the regulation of host protein translation by HCV. Our results suggest that HCV, through NS5A, enhances eIF4F complex assembly and thereby cap-dependent translation by 4EBP1 inactivation and activation of eIF4E. We also present some interesting novel findings that contribute to the up-regulation of host cap-dependent translation machinery by HCV. We propose that up-regulation of host protein translation machinery could have significant implications in the development of HCV-associated hepatocellular carcinoma.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
Human hepatoma cell lines Huh7.5 were cultured in Dulbecco's modified essential medium supplemented with 10% FBS, 1× minimum essential medium nonessential amino acids as explained previously and 1× penicillin and streptomycin (58). G418 (Invitrogen), rapamycin (Sigma), wortmannin (Sigma), LY294002 (Cell Signaling Technology, Danvers, MA), and p38 MAPK inhibitor VIII (Merck KGaA, Darmstadt, Germany) were used at 1 mg/ml, 25 nm, 1 μm, 50 μm, and 25 μm concentrations, respectively. Anti c-myc antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), anti-HA antibodies were from Sigma, anti-NS5A and core antibodies were from Abcam (Abcam Plc, Cambridge, MA), whereas all other primary antibodies were from Cell Signaling Technology. HRP-conjugated anti mouse and anti-rabbit secondary antibodies were from Santa Cruz Biotechnology.
Plasmids, Transfection, and Stable Cell Line Selection
The N terminus myc-tagged HCV genotype 2a NS5A coding sequence was amplified from pFL-J6/JFH1 plasmid (58) and cloned into pCDNA 3.1/myc-his(−)A vector. Full-length human eIF4E sequence with an HA tag and myc-tagged 4EBP1 were amplified from cDNA of hematopoietic stem cells of human origin and cloned into pCDNA 3.1/myc-his vector.
For transfection, 105 cells were seeded in 6-well plates and transfected at 70% confluency with plasmids DNA and Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. 48 h later cells stably carrying plasmids were selected in media containing G418 (1 mg/ml). Subclones were selected in 48-well plates by serial dilution and the single cell seeding method.
Proliferation Assays
Trypan blue exclusion assay was performed as per previous protocols. Cells grown in six-well plates for different time intervals were trypsinized (0.05% trypsin and 0.05% EDTA) and resuspended in growth media, and equal volumes of cell suspension and 0.4% trypan blue solution were mixed and incubated for 5 min. Cells were spotted in a hemocytometer, and viable cells were counted. Average values from at least three independent experiments were used to analyze the data.
HCV Cell Culture Production
Chimeric HCV infectious particles were produced as described previously (58). 10 μg of in vitro transcribed (Ampliscribe T7 Flash Transcription kit, Epicenter, Madison, WI) HCV RNA was electroporated into 7.5 × 106 Huh7.5 cells suspended in 400 μl of cytomix buffer at 260 V and 950 microfarads. Alternately, 105 cells were transfected with 5 μg of RNA by Lipofectamine 2000. The treated cells were resuspended in growth medium with 20% FBS and subcultured for 30 days. Supernatants were collected every 3 days, and viruses were titered and stored until further use.
For quantitation of HCV titer, a Taqman-based assay was used as described previously (58). RNA was prepared from virus supernatants using TRIzol LS (Invitrogen) according to the manufacturer's protocols. 250 ng of RNA were used in duplicates of Taqman RT-PCR assays by Taqman EZ-RTPCR kit (Invitrogen). The sequence detection reaction was as follows: 2 min at 50 °C, 30 min at 60 °C, 5 min at 95 °C, then cycled 44 times at 95 °C for 15 s, 56 °C for 30 s, and 62 °C for 30 s. For the preparation of external standards, a region spanning the Taqman RT-PCR detection sequence was amplified from pFL-J6/JFH plasmid and cloned into pCR2.1. This sequence was in vitro transcribed by Ampliscribe T7 Flash Transcription system. Serial dilutions of known copy numbers of the external standards were used to calculate absolute copy numbers of HCV genome that approximately represent the number of virus particles in the respective supernatant.
Infection of Huh7.5 Cells with HCV
Huh7.5 cells grown to about 50% confluency were infected with 1 RNA copy multiplicity of infection (m.o.i.) of HCV for 4 h, after which viruses were removed, and cells were washed and further incubated with growth medium for a total of 72 h post-infection (PI). For confirmation of successful HCV infection, the infected cells were harvested and lysed with appropriate lysis buffer, and protein lysates were analyzed for the expression of HCV core and NS5A by Western blotting as described in the following section.
Immunoblotting, Immunoprecipitation, and Analyses
Cells grown in culture were trypsinized, pelleted, washed, and resuspended either in modified radioimmune precipitation assay lysis buffer (59) or in a milder Nonidet P-40 lysis buffer (1% Nonidet P-40, 50 mm Tris HCl, 150 mm NaCl, pH 7.5) containing EGTA, 1 mm sodium orthovanadate, 10 mm sodium pyrophosphate, 100 mm NaF, and 1 mm phenylmethylsulfonyl fluoride. For inhibition studies, cells were cultured in 25-cm2 flasks until 80% confluency followed by treatment for 1 h, after which they were harvested and washed, and lysates were prepared. The lysates were vortexed and centrifuged at 13,000 rpm for 15 min, and proteins in supernatants were quantified by BCA reagent (Thermo Fisher Scientific, Rockford, IL). Proteins were resolved in SDS-PAGE, transferred to PVDF membrane (Millipore, Billerica, MA), and immunoblotted with specific primary antibodies followed by HRP-conjugated secondary antibodies. Protein bands were detected by Supersignal West Pico or Femto Chemiluminescence kit (Thermo Fisher Scientific).
For immunoprecipitation, cells were trypsinized, pelleted, washed, and resuspended in Nonidet P-40 lysis buffer and centrifuged at 13,000 rpm for 15 min. 1 mg of protein from supernatants was incubated overnight with specific primary antibodies, and the complexes were pulled down by protein G-agarose bead (Invitrogen) incubation for 4 h. The beads were washed twice with lysis buffer, resuspended in 2× SDS protein loading dye, boiled, and resolved in SDS-PAGE. Immunoprecipitated proteins were detected by immunoblotting as explained previously in this manuscript. Run-off lysates were used in Western blot to detect β-actin for normalization of samples.
Densitometric analyses of the detected bands were performed by Image J software (National Institute of Health, rsbweb.nih.gov). Average densitometric values from three different NS5A cells normalized against those of the corresponding β-actin bands were calculated from at least three independent experiments, and the -fold change for phosphorylation and expression of individual proteins between NS5A expressing and control cells were plotted in graphs.
M7 GTP Pulldown Assay
eIF4F complexes were pulled down from protein lysates by 7-methyl GTP-Sepharose 4B beads (GE Healthcare). Cells were lysed in Nonidet P-40 lysis buffer as mentioned above. Beads that were reconstituted in lysis buffer were incubated for 6 h with 1 mg of protein lysates at 4 °C, and eIF4F complexes were precipitated at 13,000 rpm for 15 min followed by 2 washes in lysis buffer. The washed beads were then boiled in 2× SDS protein loading dye, and the proteins were resolved in SDS-PAGE and detected by immunoblotting as described previously. Densitometric values of the bands were normalized against β-actin bands from the retrieved lysates.
Quantitative RT-PCR
Quantitative RT-PCR assays were performed to estimate changes in transcription of various cellular genes. Total RNA was prepared from NS5A-Huh7.5 and control cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Extracted RNAs were quantified, and 4 μg of the total RNA was converted to cDNA using Superscript TM First Strand Synthesis (Invitrogen). 100 ng of the cDNA was amplified during real time analyses using Platinum SYBR Green qPCR Supermix-UDG (Invitrogen) with gene-specific primer sets. Values were normalized using the human GAPDH endogenous control primer set. All the real time experiments and subsequent analysis were performed using ABI 7900 HT. Normalized expression values of each gene between NS5A-Huh7.5 and control cells were represented graphically in -fold changes.
Analysis of Polysomes and Associated Proteins
NS5A-Huh7.5 and control cells (2.5 × 107) grown to 80% confluency were trypsinized and washed twice in PBS, and the pellets were resuspended in 800 μl of polysome lysis buffer (20 mm Tris-Cl, pH 8.0, 140 mm KCl, 1.5 mm MgCl2, 1% Triton X-100, 0.5 mm DTT, 100 μg/ml cycloheximide, 1× protease inhibitor, 1 mg/ml heparin, 100 units of RNasin/ml). For HCV-infected lysates, mock-infected and Huh7.5 cells infected with 1 m.o.i. of HCV were harvested 72 h PI, washed, and lysed as mentioned above. For analysis of dissociated polysomes, cells treated as above were lysed in polysome lysis buffer containing 20 mm EDTA with a further incubation of 10 min at 4 °C. The lysates were clarified by centrifugation at 10,000 × g for 15 min at 4 °C. For fractionation, 175 μg of the RNA from clarified lysates were loaded on top of a 10–50% sucrose gradient and resolved by centrifugation at 36,000 rpm for 4 h at 4 °C in Beckman SW41 rotor. After centrifugation, the tubes were pierced, and gradients were separated and analyzed by absorbance at 254 nm through an auto-programmable density gradient fractionator (Teledyne Isco Inc, Lincoln, NE).
For analysis of proteins in polysomes, proteins from independent or pooled polysomal and monosomal fractions were precipitated after overnight incubation at −30 °C with two volumes of absolute ethanol. Proteins were precipitated at 14,000 rpm for 20 min at 4 °C. Precipitated proteins were solubilized by boiling in 2× SDS loading buffer and were separated on SDS-PAGE and subsequently visualized by Western blotting by specific antibodies as mentioned previously in this manuscript.
RESULTS
HCV Infection Enhances Assembly of eIF4F Complex
First we wanted to study if HCV regulates host protein translation through mTOR. Even though a few previous studies have linked HCV to activation of PI3K, AKT, and mTOR, none of these studies used HCV infectious particles. Naive Huh7.5 cells were infected with 1 m.o.i. of HCV particles for 72 h as detailed under “Experimental Procedures.” Two independent viral supernatants, one from the 18th day (HCV1) and the other from the 27th day (HCV2) of electroporation, were used in these studies. Lysates prepared after 72 h of PI were analyzed for mTOR phosphorylation. As shown in Fig. 1A, HCV infection significantly induced mTOR phosphorylation at Ser-2448 over the mock-infected cells, which is an indication of higher mTORC1 activity. In agreement with these results, mTOR substrates 4EBP1 and p70S6K1 (its substrate rpS6 was phosphorylated) showed activation during HCV infection (Fig. 1A). These results suggest that mTORC1 was activated by HCV in Huh7.5 cells. mTOR activation was detected in the serum presence, suggesting that the magnitude of this activation is very significant.
FIGURE 1.
HCV infection induces eIF4F complex assembly. A, Huh7.5 cells infected with 1 m.o.i. of HCV2a infectious viruses were harvested 72 h PI, lysates were prepared, and phosphorylation of mTOR, 4EBP1, rpS6, p38 MAPK, MNK1, eIF4E, and eIF4G was analyzed by Western blotting. Two separate batches of viruses were used in these experiments depicted as HCV1 (18th day supernatant) and HCV2 (27th day supernatant). B, eIF4F complex was precipitated from the lysates mentioned in A by m7GTP pulldown assays. The precipitates were boiled in 2× loading dye, and the component proteins were analyzed by Western blotting as shown. C, average -fold change in eIF4E and eIF4G loading into the complex, normalized to β-actin from the respective run-off lysate, is represented graphically.
Next we analyzed the phosphorylation of eIF4E and eIF4G in HCV-infected cells because of their augmentative role in cap-dependent translation. As demonstrated in Fig. 1A, eIF4E phosphorylation at Ser-209 residue was enhanced in HCV-infected cells in comparison with mock-infected cells. Its upstream regulators p38MAPK and MNK1 were both increasingly phosphorylated in HCV-infected cells. Similarly, eIF4G was also overphosphorylated during HCV infection. These results strongly suggest an up-regulation of host protein translation by HCV.
We then analyzed eIF4F complex assembly in HCV-infected cells as an indicator of translation initiation. eIF4F complexes, precipitated by an m7GTP-Sepharose pulldown assay from HCV and mock-infected cells, were analyzed by Western blotting to understand their loading of eIF4E and eIF4G to the complex. Significantly larger quantities of eIF4E and eIF4G were detected in the eIF4F complex of HCV-infected cells as compared with mock-infected cells (Fig. 1, B and C). These results are in complete agreement with the immunoblot data presented above and propose that HCV up-regulates host protein translation initiation machinery.
mTORC1 Substrates 4EBP1 and S6K1 Are Overexpressed and Phosphorylated by HCV2a NS5A
Because HCV NS5A was previously shown to activate PI3K, AKT, and mTOR molecules, we asked if this protein is responsible for the up-regulation of host protein translation that was observed in HCV-infected cells. Two independent Huh7.5 cells stably expressing myc-NS5A (NS5A-Huh7.5) and one of their subclones (NS5A2 in Figs. 2, 3, 4, 7, and 8) were used in this study. Immunoblotting of the lysates confirmed the expression of a single band of about 56 kDa size in NS5A-transfected cells but not in the empty vector control cells (supplemental Fig. 1A). Detection of a single band was in agreement with previous reports that HCV2a NS5A is not hyperphosphorylated (9). NS5A-expressing cells (NS5A-Huh7.5) proliferated 3-fold faster over the control cells at specific time intervals (supplemental Fig. S1B). Previous studies have reported activation of AKT-mTOR by HCV NS5A (53, 57). Analysis of protein lysates from NS5A-Huh7.5 cells and the control cells by Western blotting showed higher phosphorylation of AKT at both Thr-308 and Ser-473 residues by NS5A, as demonstrated in supplemental Fig. 1C. mTOR was also increasingly phosphorylated in NS5A-Huh7.5 cells, demonstrating that similar to HCV1b NS5A, HCV2a NS5A also induces PI3K-AKT and mTOR. These results also authenticate our system for further experiments.
FIGURE 2.
HCV NS5A activates mTORC1 in Huh7.5 cells. A, expression and phosphorylation of 4EBP1 in three different NS5A expressing and control Huh7.5 cells was detected by immunoblotting. B, D and E, shown are graphic representation of average -fold changes in the normalized densitometric values of 4EBP1 expression, phosphorylation, and non-phosphorylation respectively. C, shown is average -fold change in 4EBP1 transcription in NS5A-Huh7.5 cells over the control cells, as detected by qRT-PCR. F, shown are immunoblots depicting the expression and phosphorylation of S6K1 in NS5A expressing and the control Huh7.5 cells. G, average -fold change in S6K1 expression and phosphorylation are represented graphically. H, shown is a graph representation of average -fold change in S6K1 transcription in NS5A-Huh7.5 cells over the control cells, as detected by qRT-PCR. Graphic data plotted for Western blots are the averages of three different lysates. For qRT-PCR, each sample was used in duplicate, and the plotted values indicate -fold changes in transcription from a minimum of three independent experiments.
FIGURE 3.
Phosphorylation of eIF4E by HCV NS5A. A and B, immunoblot analysis of eIF4E phosphorylation by NS5A and analysis of its average -fold change in densitometric values are represented graphically, respectively. C and D, shown are phosphorylation of MNK1, as detected by Western blots and graphic representation of its average -fold change in densitometric values, respectively. E, shown is an estimation of MNK1 transcripts in NS5A-Huh7.5 and control cell by qRT-PCR. F and G, shown is are phosphorylation of p38 MAPK detected by immunoblotting and its average -fold change depicted graphically, respectively. Each set of bars for Western blot represents average -fold changes in normalized densitometric values from three independent lysates. For qRT-PCR, each sample was used in duplicate, and the plotted values indicate -fold changes in transcription from a minimum of three independent experiments. H, NS5A expression increased eIF4G phosphorylation. Protein lysates from NS5A-Huh7.5 and control cells were immunoblotted with specific antibodies as shown. I, average increase in eIF4G phosphorylation is represented graphically as -fold changes after normalization with β-actin.
FIGURE 4.
Enhanced eIF4F loading in NS5A-Huh7.5 cells. HCV NS5A enhances eIF4F complex assembly. NS5A-expressing and control Huh7.5 cells were lysed, and eIF4F complexes were pulled down by m7GTP-Sepharose beads. Precipitated proteins were separated in SDS-PAGE and immunoblotted with specific antibodies as shown. β-Actin from run-off lysates was used as normalization controls. Each set of bars for Western blot represents average -fold changes in normalized densitometric values from three independent lysates.
FIGURE 7.
Global protein synthesis is not changed in NS5A-Huh7.5 cells. NS5A-Huh7.5 and the control cells grown in culture were lysed in polysome lysis buffer and fractionated by density gradient ultracentrifugation, and fractions were separated and analyzed by absorbance at 254 nm.
FIGURE 8.
NS5A physically interacts with eIF4F complex and is detected in polysome fractions. A, pulldown of NS5A with eIF4F complex is shown. Proteins pulled down from NS5A-Huh7.5 and control cell lysates by m7GTP-Sepharose beads were detected by immunoblotting with specific antibodies. B, co-immunoprecipitation (IP) of NS5A with eIF4F complex proteins is shown. Proteins immunoprecipitated from NS5A-Huh7.5 and control cell lysates by anti-myc antibodies were immunoblotted with specific antibodies as mentioned. β-Actin from run-off lysates was used as the normalization control in both experiments. C, HCV NS5A was detected in polysome fractions. Proteins from pooled polysome and monosome fractions described in Fig. 7 were ethanol-precipitated and analyzed for NS5A and polysome-associated proteins by Western blotting. D, dissociation of NS5A from polysomes of NS5A-Huh7.5 cells by EDTA is shown. Cells grown in culture were harvested and lysed in polysome lysis buffer containing EDTA and fractionated into polysome and monosome fractions. Proteins precipitated from pooled fractions were analyzed for NS5A and polysome-associated proteins by Western blotting. PABP, poly(A) binding protein.
Because mTOR was phosphorylated by NS5A in our system, we explored the status of 4EBP1 phosphorylation. As shown in Fig. 2, A and D, 4EBP1 phosphorylation was induced in all the four residues in NS5A-Huh7.5 cells as compared with the control cells. This observation was made in cells grown under serum sufficiency (Fig. 2, A and B). Surprisingly, immunoblotting experiments revealed significant overexpression of 4EBP1 in NS5A-Huh7.5 cells as compared with the control cells (Fig. 2, A and B). The quantum of 4EBP1 overexpression was comparable with that of their hyperphosphorylation (Fig. 2, A, B, and D). This result proposes that most of the overexpressed 4EBP1 are also hyperphosphorylated, suggesting an increase in mTORC1 activity. SYBR Green quantitative real time RT-PCR studies revealed transcriptional up-regulation of 4EBP1 in NS5A-Huh7.5 cells (Fig. 2C) at levels comparable with that of protein expression (Fig. 2, A and B). Our results imply that 4EBP1 is a target of the transcriptional activities by NS5A (60, 61). These observations raised the question of whether 4EBP1 overexpression in NS5A-Huh7.5 cells activates or inhibits cap-dependent protein translation, as the majority of the studies demonstrated inhibition of cap-mediated translation during 4EBP1 overexpression (62).
We analyzed the fraction of non-phosphorylated 4EBP1 in NS5A-Huh7.5 cells by immunoblotting with an antibody that specifically detects non-phosphorylated Thr-46 residue. Slightly elevated levels of non-phospho-Thr-46 4EBP1 were detected in NS5A-Huh7.5 cells over the control cells (Fig. 2, A and E), the magnitude of which was significantly lower than that of its expression and phosphorylation (Fig. 2, A, B, and D). These results conclusively demonstrate that a significant proportion of the overexpressed 4EBP1 in NS5A-Huh7.5 cells was hyperphosphorylated, suggesting that NS5A-mediated 4EBP1 overexpression in our system might facilitate translation initiation.
S6K1, which is the other major substrate of mTORC1, was also overexpressed in NS5A-Huh7.5 cells over the control cells (Fig. 2, F and G). Overexpressed S6K1 was also phosphorylated at levels comparable with its overexpression ((Fig. 2, F and G). Quantitative real time RT-PCR studies indicated that S6K1 transcription was up-regulated in NS5A-Huh7.5 cells as compared with the control cells (Fig. 2H). Thus, NS5A transcriptionally up-regulates S6K1 expression and subsequently phosphorylates them through mTOR, similar to the case of 4EBP1. Enhancement of S6K1 phosphorylation along with hyperphosphorylation of 4EBP1 confirms the activation of mTORC1 by HCV NS5A protein. These results show significant deviation from previous report (57) in that our observations were made in serum sufficient conditions, and unlike Peng et al. (57), we observed consistent overexpression of both 4EBP1 and S6K1. Put together, these data indicate that HCV NS5A enhances host protein translation initiation by inactivating 4EBP1 and activating S6K1, both through mTORC1.
HCV2a NS5A Induces Phosphorylation of Translation Initiation Factor eIF4E
We next studied the phosphorylation of eIF4E by NS5A. Immunoblotting experiments indicated a remarkable induction of eIF4E phosphorylation at Ser-209 residue in NS5A-Huh7.5 cells as compared with the control cells (Fig. 3, A and B), suggesting the manipulation of eIF4E phosphorylation regulatory pathways by NS5A.
Because MNK1 has been shown to phosphorylate eIF4E, we explored its phosphorylation status in NS5A-expressing cells. Immunoblot analyses showed significantly enhanced MNK1 phosphorylation at Thr-197/202 in NS5A-Huh7.5 cells as opposed to the control cells (Fig. 3, C and D). Surprisingly, MNK1 was also overexpressed in NS5A-Huh7.5 cells at a magnitude that is comparable with that of its phosphorylation (Fig. 3, C and D). Quantitative RT-PCR did not detect any increase in MNK1 transcription in NS5A-Huh7.5 cells (Fig. 3E).
We next wanted to identify its upstream kinase by immunoblotting. Our analyses revealed that p38 phosphorylation at Thr-180/Tyr-182 residues was induced notably by NS5A (Fig. 3, F and G), but ERK1/2 phosphorylation was unchanged (data not shown). These results suggest that MNK1 is regulated by p38 but not by ERK1/2 in our system. These observations, along with 4EBP1 phosphorylation data substantiate our previous suggestion that NS5A possibly up-regulates cap-dependent translation in Huh7.5 cells.
Yet another factor that could influence eIF4F complex assembly and translation initiation is eIF4G phosphorylation (63, 64). Next, we analyzed eIF4G phosphorylation by Western blotting. As shown in Fig. 3, H and I, an increased phosphorylation of eIF4G at Ser-1108 residue was detected in NS5A-expressing cells in comparison with the control cells. Increased phosphorylation of eIF4G along with that of eIF4E and hyperphosphorylation of 4EBP1 strongly supported the hypothesis that HCV activates cap-dependent translation apparatus through NS5A.
eIF4F Complex Loading Is Enhanced by NS5A in Huh7.5 Cells
With the previously mentioned observations in mind, we asked how these independent events activated by NS5A influence eIF4F complex assembly in our system. eIF4F complexes were affinity-precipitated by m7GTP-Sepharose beads from equal quantities of cell lysates. Precipitated proteins were analyzed by immunoblotting. As depicted in Fig. 4, considerably higher amounts of eIF4E were detected in NS5A-Huh7.5 cells over the control cells. This was in contrast with its expression pattern, as no significant change in expression was noticed between them (Fig. 3, A and B). The majority of eIF4E loaded in the eIF4F complex was phosphorylated at Ser-209 residue (Fig. 4). β-Actin bands from retrieved lysates served as loading control for normalization. This result proposes that in our system phosphorylation of eIF4E is probably critical for its association with the 5′cap and subsequent formation of eIF4F complex in contrast to some of the previous reports (26, 65).
Next, we studied the loading pattern of eIF4G in eIF4F complex of NS5A-Huh7.5 cells. This was particularly important because of the observed overexpression of translation-inhibitory 4EBP1. Similar to eIF4E, eIF4G was more efficiently loaded to eIF4F complex in NS5A-expressing cells than in the control cells (Fig. 4). As in the case of eIF4E, the majority of the eIF4G in eIF4F complex of NS5A-Huh7.5 cells was also phosphorylated at Ser-1108 residue. These results confirm the significance of eIF4G phosphorylation in its loading in eIF4F complex. These results also substantiate our previous suggestion that overexpressed 4EBP1 is predominantly hyperphosphorylated and non-restrictive to translation initiation in NS5A-expressing cells. MNK1 was also overly loaded in NS5A-Huh7.5 cells in comparison with the control cells, confirming the presence of increased eIF4G in eIF4F complex. As an indicator of purity of the complex preparation, β-actin was not detected in eIF4F complexes.
We subsequently analyzed 4EBP1 loading in eIF4F complex. As demonstrated in Fig. 4, 4EBP1 loading to eIF4F complex was unaffected by NS5A even though its expression increased considerably in NS5A-Huh7.5 cells (Fig. 2, A and B). This result categorically demonstrates that a significantly large population of the overexpressed 4EBP1 in NS5A-Huh7.5 cells was not loaded into eIF4F complex. This along with the results described above suggests that HCV NS5A activates host translation initiation through enhanced eIF4F complex assembly.
We tried to simulate the NS5A-assisted translation regulation and enhanced proliferation by overexpressing eIF4E ectopically in Huh7.5 cells (supplemental Fig. 2A). eIF4E-Huh7.5 cells proliferated about 20–30% faster than control cells (supplemental Fig. 2B) but substantially slower than NS5A-Huh7.5 cells (supplemental Fig. 1B). Overexpression of eIF4E also resulted in increased assembly of eIF4F complex as observed from the increased loading of eIF4E and eIF4G (supplemental Fig. 2D). Consistent with this, eIF4E overexpression led to reduced expression of 4EBP1 and a proportionate decrease in its phosphorylation (supplemental Fig. 2C). This is partially in agreement with a previous report that demonstrated a similar hypophosphorylation of 4EBP1 during eIF4E overexpression (66). This part of our results implies that overexpression of eIF4E alone could not account for the enhanced proliferative properties associated with HCV NS5A.
We overexpressed 4EBP1 ectopically in Huh7.5 cells to understand the contribution of 4EBP1 overexpression to NS5A-mediated up-regulation of host translation machinery. Western blot analysis revealed that overexpressed myc-4EBP1 was also phosphorylated (supplemental Fig. 3A), rendering the system more comparable with NS5A-Huh7.5 cells. However, ectopic 4EBP1 expression resulted in decreased eIF4E expression and its phosphorylation (supplemental Fig. 2A), in contrast to the observations in NS5A-Huh7.5 cells. eIF4F complex analysis revealed reduced loading of eIF4E and eIF4G in 4EBP1-overexpressing cells, suggesting a possible inhibition of translation initiation in these cells (supplemental Fig. 3B). 4EBP1-overexpressing Huh7.5 cells also exhibited reduced proliferative properties in comparison with vector control cells (supplemental Fig. 3C). These results suggest that functionally, NS5A expression is not replicable by the overexpression of 4EBP1 alone in Huh7.5 cells.
NS5A-mediated Ser-473 Phosphorylation of AKT, but Not Thr-308, Is Uniquely Resistant to PI3K Inhibitors
A recent study suggested the activation of mTOR by HCV1b NS5A through an unidentified pathway independent of PI3K-AKT (57). We studied if the NS5A-mediated mTORC1 activation is regulated by this pathway. Phosphorylation of AKT, 4EBP1, and ribosomal protein S6 (rpS6) (as a measure of S6K1 activity) was analyzed in NS5A-Huh7.5 and control cells incubated with PI3K inhibitors wortmannin and LY294002. As demonstrated in Fig. 5, treatment with 1 μm wortmannin for 1 h remarkably reduced both Thr-308 and Ser-473 phosphorylations of AKT in control cells, confirming the inhibition of PI3K activity (Fig. 5, left panel). This was followed by inhibition of 4EBP1 and rpS6 phosphorylations, suggesting a downstream inhibition of mTORC1 activity by PI3K inhibitor. Wortmannin inhibited Thr-308 phosphorylation in NS5A-Huh7.5 cells as well. However, Ser-473 phosphorylation was intact during the drug treatment in these cells, and 4EBP1 and rpS6 remained phosphorylated as well, indicating a resistance of mTORC1 activity to PI3K inhibitor in NS5A-Huh7.5 cells. Therefore, the inability of PI3K inhibitor to inhibit mTORC1 activity in NS5A-Huh7.5 cells is more because of the constitutive activity of AKT mediated through Ser-473 phosphorylation than the use of a PI3K-independent pathway as suggested by Peng et al. (57). Treatment with 50 μm LY294002 confirmed similar results (data not shown). However, the total inability of the drugs to inhibit mTORC1 activity in NS5A-Huh7.5 cells suggests a possible involvement of multiple molecules in this process. Because mTORC2 phosphorylates the Ser-473 residue on AKT, we speculate that mTORC2 is activated by NS5A that in turn keeps AKT constitutively phosphorylated at Ser-473, thereby providing resistance against PI3K inhibitors. These results also imply sufficiency of Ser-473 phosphorylation for the activation of mTORC1 by AKT.
FIGURE 5.
AKT is constitutively phosphorylated by NS5A. NS5A-Huh7.5 and control cells were incubated with 1 μm wortmannin for 1 h after which the cells were harvested, and protein lysates were prepared and separated in SDS-PAGE and assayed by Western blotting using specific antibodies as shown. The experiment was performed from a minimum of three independent lysates.
NS5A-mediated 4EBP1 Phosphorylation and eIF4F Complex Loading Are Rapamycin-resistant
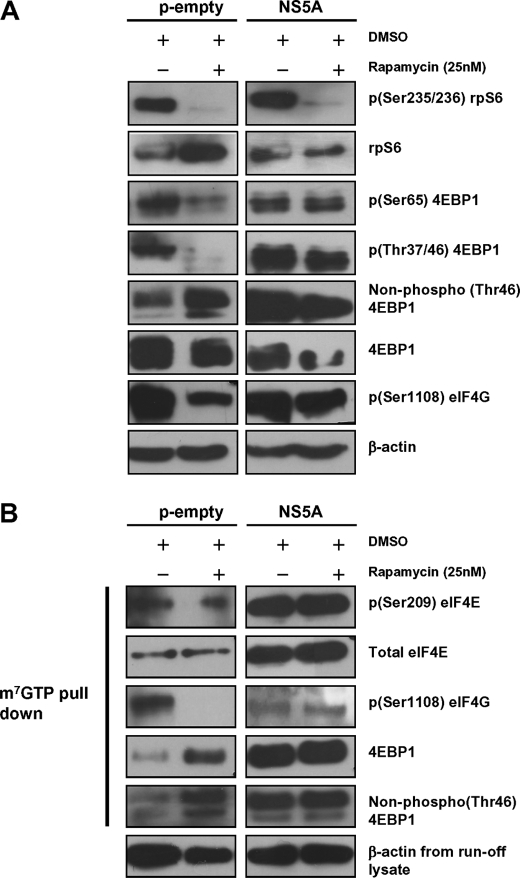
Rapamycin inhibits mTORC1 activity, and we wanted to study the effect of mTORC1 inhibition on NS5A-assisted eIF4F complex assembly. A few recent reports propose the existence of an alternate mechanism of 4EBP1 phosphorylation that is resistant to rapamycin treatment (62, 67). Of these reports, one suggests the existence of a rapamycin-resistant mechanism that discriminates between 4EBP1 and S6K1 phosphorylation (62). Peng et al. (57) demonstrated that HCV1b NS5A activates mTOR that subsequently phosphorylated 4EBP1 and S6K1 in serum-starved conditions, and both these phosphorylations were sensitive to rapamycin treatment. NS5A-Huh7.5 and the control cells were treated with rapamycin as described under “Experimental Procedures,” and the lysates were analyzed by immunoblotting. 25 nm rapamycin, not DMSO, treatment for 1 h inhibited 4EBP1 and rpS6 phosphorylation in control cells (Fig. 6A, left panel). Dephosphorylation of 4EBP1 was confirmed by the increased detection of non-phosphorylated Thr-46 residues on 4EBP1 in the rapamycin-treated cells (Fig. 6A, left panel). Rapamycin treatment also inhibited eIF4G phosphorylation at Ser-1108, in agreement with previous reports. Consistent dephosphorylation of 4EBP1, rpS6, and eIF4G by rapamycin treatment in the control cells suggested the efficacy of rapamycin-mediated inhibition of mTOR activity at 25 nm concentration.
FIGURE 6.
NS5A-induced 4EBP1 phosphorylation is rapamycin-insensitive. A, NS5A-Huh7.5 and control cells were incubated with 25 nm rapamycin for 1 h after which the cells were harvested, protein lysates were prepared, and phosphorylation of the mentioned molecules was subsequently analyzed by immunoblotting. B, eIF4F complexes from protein lysates described in A were pulled down by m7GTP-Sepharose beads, and proteins were separated in SDS-PAGE and immunoblotted with specific antibodies as described. β-Actin from run-off lysate was used as the normalization control.
However, in contrast to the control cells, rapamycin could not inhibit 4EBP1 phosphorylation at both Thr-37/46 and Ser-65 residues in NS5A-Huh7.5 cells (Fig. 6A, right panel). In contrast, rpS6 phosphorylation was inhibited substantially under the same conditions, suggesting the involvement of an alternate regulatory mechanism of 4EBP1 phosphorylation by NS5A (Fig. 6A, right panel). The effect of rapamycin on phosphorylation of these molecules was consistent across serum-supplemented and serum-starved cells (data not shown), suggesting a different mechanism in contrast to that reported previously (57). Again, rapamycin failed to dephosphorylate eIF4G at Ser-1108 residue in NS5A-Huh7.5 cells (Fig. 6A, right panel). These results propose a differential regulation of 4EBP1 and S6K1 mediated by NS5A in Huh7.5 cells.
Rapamycin inhibits eIF4F complex assembly, and we studied its effect on eIF4F complex assembly in our system. eIF4F complexes prepared from the lysates of rapamycin and DMSO-treated NS5A-Huh7.5 and empty vector control cells were analyzed by immunoblotting as done previously. In both the NS5A expressing and control cells, rapamycin treatment did not affect eIF4E affinity to the 5′cap (Fig. 6B, right panel). As expected, phosphorylation of eIF4E was also unaffected under these conditions. However, 4EBP1 loading to eIF4F complexes was enhanced in rapamycin-treated control cells, and the majority of the loaded 4EBP1 was non-phosphorylated as against the DMSO-treated cell (Fig. 6B, left panel). These results are consistent with their hypophosphorylated status as detected by immunoblotting (Fig. 6A, left panel). In contrast, 4EBP1 loading did not change in NS5A-Huh7.5 cells, confirming the existence of rapamycin-resistant 4EBP1 phosphorylating mechanism in these cells. eIF4G loading was also unchanged in rapamycin-treated NS5A-Huh7.5 cells. These results advocate that HCV2a NS5A provides resistance to rapamycin-mediated inhibition of host translation initiation that could have implications in therapeutics.
Global Protein Synthesis Is Not Changed in NS5A-Huh7.5 Cells
To understand the effect of NS5A expression on global protein synthesis in the context of enhanced eIF4F loading, we analyzed the ribosome-RNA engagement in polysome and non-polysome fractions from both NS5A-Huh7.5 and control cells. Polysomes were fractionated and analyzed as explained under “Experimental Procedures.” As depicted in Fig. 7, polysome fractions of NS5A-expressing cells consistently exhibited a similar profile as that of control Huh7.5 cells, suggesting that NS5A expression did not alter global protein synthesis pattern. Our results are in total agreement with the contemporary perception that enhanced eIF4F loading by phosphorylation or overexpression of eIF4E results in preferential translation of a specific set of transcripts but does not change the global translation pattern.
HCV NS5A Binds to eIF4F Complex
As some viral proteins have been shown to bind to eIF4F complex proteins effecting varied outcomes on translation, we were curious if HCV NS5A physically interacts with eIF4F complex proteins. The presence of NS5A was analyzed by immunoblotting in purified eIF4F complexes of NS5A-Huh7.5 and control cells. NS5A was detected in the eIF4F complex of NS5A-Huh7.5 cells but not in that of the control cells (Fig. 8A), suggesting its physical interaction with the eIF4F complex protein(s). Both eIF4E and eIF4G were detected in the complex, confirming the integrity of the complex. The absence of HCV core and β-actin in the complex showed the specificity of interaction. Similar intensities of β-actin bands were detected across the retrieved lysates, confirming comparable quantities of proteins used in this experiment.
The interaction of NS5A with the eIF4F complex protein(s) was confirmed by its reciprocal co-precipitation with eIF4F complex proteins anti-myc-tag antibodies and subsequent immunoblotting. As demonstrated in Fig. 8B, both eIF4E and eIF4G were detected in the co-precipitated complex in NS5A-Huh7.5 cells. None of these proteins was detected in the complex precipitated from empty vector control cells, indicating a specific interaction between NS5A and eIF4F complex protein(s). Similar quantities of β-actin were detected across the retrieved lysates, confirming the use of equal amounts of proteins for precipitation. However, total absence of β-actin from the complexes (data not shown) authenticated the purity of the precipitate. Unrelated antibodies could not co-precipitate eIF4E and eIF4G (data not shown). These experiments unambiguously suggest a physical interaction of HCV NS5A with eIF4F complex protein(s).
HCV NS5A Is Detected in Polysome Fractions of NS5A-Huh7.5 Cells
To learn in detail whether the eIF4F complex-associated NS5A is recruited to polysome that would confirm its involvement in host protein translation, proteins from pooled polysome fractions of NS5A-Huh7.5 and the control cells were ethanol-precipitated, and the presence of NS5A in these fractions was checked by immunoblotting. As shown in Fig. 8C, NS5A protein was detected in polysomes of NS5A-Huh7.5 cells, confirming its involvement in host cap-dependent protein synthesis. The specificity of the experiment was substantiated by the absence of this protein in the polysomes of control cells. eIF4E was detected in the polysomes confirming the integrity of the fractions. Remarkably larger amounts of eIF4E and eIF4G were detected in NS5A-Huh7.5 cells in comparison with the control cells (Fig. 8C), validating our data on the analyses of eIF4F complexes by m7GTP pulldown experiments (Fig. 4).
If NS5A interaction with the polysome is genuine, then dissociation of polysomal proteins should result in the sequestration of NS5A from the precipitate. Polysome fractions prepared from cell lysates containing 20 mm EDTA were ethanol-precipitated, and NS5A precipitation was analyzed by Western blotting. NS5A co-precipitation with polysomes was completely abolished in the presence of EDTA, whereas they were detected in its absence, suggesting that their interaction was genuine (Fig. 8D). These results confirm that HCV NS5A indeed interacts with polysomes and participates in protein translation. The consequences of this interaction are unclear. We speculate that this binding might assist in the up-regulation of host translation initiation.
NS5A Association to eIF4F and Polysome Is Detected during HCV Infection
To substantiate the association of NS5A with eIF4F complex and polysomes that we observed in NS5A-Huh7.5 cells, we analyzed these properties in HCV-infected cells. Naive Huh7.5 cells were infected as described under “Experimental Procedures” and Fig. 1. eIF4F complexes from HCV-infected cells were analyzed for NS5A association by Western blotting. As in the case of NS5A-Huh7.5 cells, NS5A was detected in the eIF4F complex of the HCV-infected Huh7.5 cells but not in mock-infected cells (Fig. 9A). Interestingly, similar to NS5A-Huh7.5 cells, eIF4E and eIF4G loading to eIF4F complex increased considerably in HCV-infected cells as well. The absence of HCV core protein in the complex demonstrated the purity of preparation and specificity of the reaction (data not shown). These results from HCV infection corroborate the data from NS5A-expressing cells.
FIGURE 9.
NS5A associates with eIF4F and is detected in polysome fractions in HCV-infected cells. A, Huh7.5 cells infected with 1 m.o.i. of HCV2a infectious viruses were harvested 72 h PI, lysates were prepared, and the eIF4F complex was precipitated by m7GTP pulldown assays. The precipitates were boiled in 2× loading dye, and the component proteins were analyzed by Western blotting for the detection of NS5A, eIF4E, and eIF4G. Two separate batches of viruses were used in these experiments depicted as HCV1 (18th day supernatant) and HCV2 (27th day supernatant). B, detection of NS5A in the polysomal fractions of HCV-infected Huh7.5 cells is shown. Polysomes from total lysates of HCV-infected (18th day supernatant) and mock-infected Huh7.5 cells were separated by sucrose density gradient centrifugation and were separated into fractions. Proteins from polysome fractions 1–5 of HCV infected cells were ethanol-precipitated and analyzed by Western blotting as shown. Individual monosome and polysome fractions from mock-infected cells were pooled before precipitation and analysis. C, dissociation of NS5A from polysomes of HCV-infected cells by EDTA is shown. HCV-infected cells were harvested and lysed in polysome lysis buffer containing EDTA and fractionated into polysome and monosome fractions. Proteins precipitated from pooled fractions were analyzed for NS5A and polysome-associated proteins by Western blotting. PABP, poly(A) binding protein.
Polysomes from HCV-infected cells were analyzed, and proteins from each fraction were precipitated and examined by Western blotting. As shown in Fig. 9B, NS5A was detected in both the monosomal and the polysomal fractions, whereas it was absent in the fractions of mock-infected cells. HCV core protein was not detected in these fractions (data not shown). EDTA inclusion in lysis buffer effectively abolished NS5A interaction with polysomes (Fig. 9C). More amounts of eIF4E and eIF4G were detected in the polysomes of HCV-infected cells as compared with the mock-infected cells, which is consonant with the data from eIF4F complex analysis (Fig. 9A). These studies confirm that HCV NS5A physically associates with eIF4F complex and propose that this protein is actively involved in cap-dependent translation in HCV-infected cells by associating with polysomes.
eIF4E Phosphorylation Does Not Contribute to NS5A-mediated eIF4F Complex Assembly
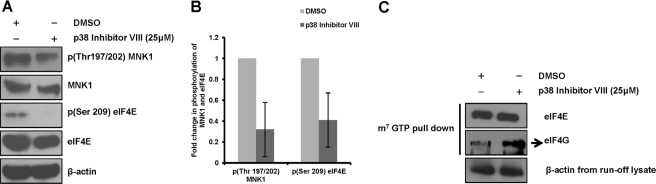
We explored the requirement of eIF4E phosphorylation in the assembly of eIF4F complex in NS5A-Huh7.5 cells through MNK1 inhibition by p38MAPK inhibitor VIII. Treatment of NS5A-Huh7.5 cells with the inhibitor for 1 h at 25 μm concentration resulted in the inhibition of MNK1 and eIF4E as shown in Fig. 10, A and B. However, p38 inhibition did not have any effect on the assembly of the eIF4F complex, as no change in the loading of eIF4E and eIF4G was observed between inhibitor-treated and DMSO-treated NS5A-Huh7.5 cells (Fig. 10C). These results indicate that eIF4E phosphorylation is dispensable for the NS5A-assisted, enhanced eIF4F complex loading. Although these results are in agreement with previous reports that question the significance of eIF4E phosphorylation in its cap binding affinity (26, 65), they also pose a serious challenge to understanding the significance of eIF4E phosphorylation in our system. We speculate eIF4E phosphorylation could contribute to the development of HCV-associated hepatocellular carcinoma as reported for other tumors.
FIGURE 10.
eIF4F complex loading is not affected by eIF4E dephosphorylation. A, NS5A-Huh7.5 cells were incubated with 25 μm p38 inhibitor VIII for 1 h, the cells were lysed, and protein lysates were subjected to Western blotting by specific antibodies mentioned. B, shown is a graphic representation of normalized, average -fold inhibition of MNK1,and eIF4E phosphorylations by p38 inhibitor VIII. C, eIF4F complexes were precipitated from protein lysates described in A by m7GTP-Sepharose pulldown assay, and the eIF4F component proteins were assayed by Western blotting.
DISCUSSION
Interaction of viral proteins to members of the eIF4F complex is not unique (41–43, 50). Although a large number of viral infections exerts a repressive effect on eIF4F complex, a few studies have revealed a facilitator role of viral proteins on eIF4F loading (48, 51), and our results are in agreement with the latter set. We propose from our studies that HCV2a NS5A enhances cap-dependent translation machinery by synchronously orchestrating the up-regulation of two independent pathways. We have been able to convincingly demonstrate that NS5A physically interacts with eIF4F complex in our system. At this point, the identity of its binding partner in eIF4F complex is not obvious. The implications of this binding on viral biology and pathogenesis are being studied. We hypothesize that binding of NS5A to the complex is facilitates translation initiation based on the increased proliferative properties associated with its expression. Because HCV is an oncovirus, it makes a lot of sense for the virus to induce host translation initiation. Because global protein translation did not increase in NS5A-Huh7.5 cells, it is speculated that the increased eIF4F assembly in our system might result in enhanced translation of a select group of proteins that might play significant roles in HCV infection. NS5A has been shown previously to be able to bind to RNA (68), and the proposition of this protein binding to a specific set of mRNA population on eIF4F complex is appealing. Identification of specific domains on NS5A that interact with eIF4F complex and the use of mutants that lack this interaction would provide important inputs on the regulation of translation by NS5A in HCV infection. Our studies assign one more critical function to the already burgeoning list of NS5A activities besides adding one more viral protein to the list of those capable of directly regulating host translational machinery. Our findings are specific to NS5A as two other HCV proteins, E1 and p7, could not up-regulate host translation machinery in our hands.
The increased loading of eIF4E and eIF4G into eIF4F complex in NS5A-Huh7.5 cells could be attributed to their phosphorylation status and to the decreased 4EBP1 affinity for eIF4F complex. It could be extrapolated that, due to increased 4EBP1 exclusion, more eIF4E was available for cap binding, similar to a previous report (50). However, this reasoning stands repudiated in our system because neither the 4EBP1 loading changed nor the eIF4E expression levels increased during NS5A expression. This strengthens the case for the role of eIF4E and eIF4G phosphorylation in this process. Ironically, the inability of eIF4E phosphorylation inhibition by p38 inhibitor to inhibit eIF4F complex assembly poses a serious question on the effect of eIF4E phosphorylation on eIF4F complex assembly in our system. Whether NS5A-initiated signaling cascades are critical in these events is worth exploring. Detailed studies on understanding the mechanism of increased eIF4F complex assembly by NS5A are in progress.
Overexpression of 4EBP1 has been demonstrated to be inhibitory to translation initiation (62), which seems to be counterintuitive to our hypothesis and observation of enhanced translation initiation. In contrast, other studies have detected overexpression and hyperphosphorylation of 4EBP1 in high grade tumors (70), indicating a facilitator role of hyperphosphorylated 4EBP1 on translation initiation. It is not apparent why NS5A induced 4EBP1 overexpression and then hyperphosphorylated the majority of them, thus inactivating its binding to eIF4E. We believe that enhanced 4EBP1 expression might work outside its established role as a translation inhibitor to facilitate viral infection through inactivating processes such as autophagy (71) and apoptosis.
Similar to a previous report that identified a unique rapamycin-resistant 4EBP1 phosphorylation mechanism that was persistent and specific to 4EBP1, but not to S6K1 (62), we detected a phenomenon induced by NS5A. This unique mechanism was indicative of an upstream regulator of 4EBP1 in addition to mTORC1. A second molecule implicated in 4EBP1 phosphorylation is PIM2, which is a proto-oncogene that has been implicated in various leukemia, lymphoma, and hepatocellular carcinoma (72, 73). We analyzed PIM2 expression in NS5A-Huh7.5 cells and did not find any change in their expression over that in the control cells (data not shown). At this stage, the identity of these regulatory molecules is not evident. It is likely that this molecule is at least partially regulated by mTORC1.
Recent reports suggest that c-myc overexpression induces 4EBP1 expression that resulted in inhibition of autophagy (71). In a separate study c-myc was shown to augment its own expression through enhanced eIF4F complex assembly and translational up-regulation, suggesting the existence of a forward loop regulation (74). These authors also reported overexpression of eIF4E and eIF4G that resulted in enhanced eIF4F complex assembly consequential to c-myc overexpression (71, 74). These studies, when juxtaposed, seemed to be very similar to our observations. However, cellular c-myc overexpression in NS5A-Huh7.5 cells could not be detected (data not shown).
Similar to a previous study (57), we also failed to inhibit mTORC1 activity by PI3K inhibitors in NS5A-Huh7.5 cells. Contrary to that report, our detailed studies revealed that it is in fact not probably by employing a PI3K-independent pathway but by a differential regulation of AKT itself. Because Ser-473 of AKT is a substrate for mTORC2, we propose that NS5A up-regulates mTORC2 activity. mTORC2 activation by NS5A could be either through AKT itself or independent of it. mTORC2 is activated by S6K1 through phosphorylation of rictor, and this could be a possible mechanism in our system as well. It seems that the activation loop of AKT-mTORC1-mTORC2-AKT is perpetuated by NS5A, and this “chicken or egg first” question needs to be addressed in detail. Because differential phosphorylation of mTOR and its partners has been implicated in their associations into mTORC1 and C2 complexes, detailed examination of these phosphorylations is needed. The possibilities of NS5A functioning similar to Us3 of HSV-1 as suggested by Chuluunbaatar et al. cannot be ruled out (48).
Although we have identified changes in cap-dependent host protein translation by HCV in this study, we have not attempted to understand its implications on IRES 5′TOP translation. Although we observed activation of cap-dependent translation machinery by NS5A, we cannot rule out the effects on IRES or TOP mRNA translation. This is particularly true in the context of activation of p70 S6K1 that is a positive regulator of 5′TOP translation and also because of increased phosphorylation of eIF4G by HCV NS5A. Although eIF4G is required for efficient IRES translation of viruses such as Picorna and Rota, its requirement in HCV IRES translation has been ruled out (75, 76), suggesting no direct effect of enhanced eIF4F complex assembly on HCV IRES translation. However, more studies such as profiling of the polysome-associated RNA population by microarray analyses and those employing bicistronic reporter vectors carrying HCV IRES would expand our understanding on these questions in detail.
The expression of NS5A in our stable cell lines was comparable with that during HCV infection as demonstrated in supplemental Fig. 4. We also compared NS5A expression in Huh7.5 cells that were electroporated with HCV mRNA for infectious virus preparation. These studies showed comparable levels of NS5A among some of the HCV RNA-transfected, HCV-infected, and NS5A-Huh7.5 cells, suggesting that our system is genuine and our results with NS5A-expressing cells are acceptable.
Overall, our study provides insights into the ways by which HCV manipulates host signaling pathways regulating protein translation through its protein NS5A (Fig. 11). The implications of this regulation on HCV infection and subsequent pathogenesis could be enormous. We hypothesize that NS5A-induced up-regulation of host protein translation could be a major contributor to enhanced cell proliferation and tumorigenesis. Because enhanced eIF4F complex loading and translation initiation are trademarks of some tumors, it would not be surprising if it is a significant contributor to HCV-associated hepatocellular carcinoma. The concomitant up-regulation of both mTORC1 and eIF4E is particularly important for tumor aggression, which is more evident with recent reports on the increased efficacy of apoptosis induction in cutaneous T-cell lymphoma cells by simultaneously inhibiting these molecules than inhibiting them alone (77). Profiling of transcripts that are preferentially translated in these cells could provide important information in this direction. It would not be surprising if some of these proteins are used by the virus for the establishment of its infection. The implication of enhanced host translation by NS5A on HCV infection biology is currently being investigated.
FIGURE 11.
Proposed model for the host translation regulation by HCV NS5A. NS5A enhances host translation through up-regulation of regulatory pathways and direct interaction with mRNA-binding eIF4F complex. NS5A activates mTOR through PI3K-AKT pathway. Activated mTOR enhances host translation through phosphorylation of 4EBP1 and S6K1, both the events acting positively on translation initiation. NS5A enhances eIF4E phosphorylation that also up-regulates translation initiation machinery. NS5A also associates with eIF4F complex and was detected in polysomal fractions. All these events augmented eIF4F complex assembly that is an indicator of enhanced translation initiation.
Supplementary Material
Acknowledgments
We sincerely thank Dr. Charles Rice (Rockefeller University) for pFL/J6-JFH plasmid and Apath, Inc. for Huh7.5 cells. Special thanks go to Dr. Jomon Joseph and Dr. Vasudevan Seshadri (National Centre for Cell Science, Pune, India) for valuable suggestions. Sangeeta Gopalan helped tremendously in generating the myc-4EBP1 clone. Special thanks go to Dr. Puran Singh Sijwali (Centre for Cellular and Molecular Biology) and Dr. Rukhsana Chowdhury (Indian Institute of Chemical Biology, Kolkata, India) for critically reading the manuscript.
This work was supported by the Department of Biotechnology, Government of India (BT/PR11339/MED/29/103/2008 (to H. H. K.)) and the Council of Scientific and Industrial Research, Government of India.

This article contains supplemental Experimental Procedures and Figs. 1–4.
- HCV
- hepatitis C virus
- NS5A
- nonstructural protein 5A of hepatitis C virus
- eIF4E
- eukaryotic translation initiation factor 4E
- eIF4G
- eukaryotic translation initiation factor 4G
- eIF4A
- eukaryotic translation initiation factor 4A
- 4EBP1
- eukaryotic translation initiation factor 4E-binding protein 1
- eIF4F
- eukaryotic translation initiation complex 4F
- mTOR
- mammalian target of rapamycin
- S6K1
- ribosomal S6 protein kinase
- MNK1
- MAPK interacting kinase
- m7GTP
- 7-methyl GTP
- m.o.i.
- multiplicity of infection
- PI
- post-infection
- rpS6
- ribosomal protein S6
- qRT
- quantitative real-time
- IRES
- internal ribosome entry site.
REFERENCES
- 1. Maniloff J. (1995) Identification and classification of viruses that have not been propagated. Arch. Virol. 140, 1515–1520 [DOI] [PubMed] [Google Scholar]
- 2. Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. (1989) Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244, 359–362 [DOI] [PubMed] [Google Scholar]
- 3. Arima N., Kao C. Y., Licht T., Padmanabhan R., Sasaguri Y., Padmanabhan R. (2001) Modulation of cell growth by the hepatitis C virus nonstructural protein NS5A. J. Boil. Chem. 276, 12675–12684 [DOI] [PubMed] [Google Scholar]
- 4. Giménez-Barcons M., Wang C., Chen M., Sánchez-Tapias J. M., Sáiz J. C., Gale M., Jr. (2005) The oncogenic potential of hepatitis C virus NS5A sequence variants is associated with PKR regulation. J. Interferon Cytokine Res. 25, 152–164 [DOI] [PubMed] [Google Scholar]
- 5. Tan S. L., Katze M. G. (2001) How hepatitis C virus counteracts the interferon response. The jury is still out on NS5A. Virology 284, 1–12 [DOI] [PubMed] [Google Scholar]
- 6. Macdonald A., Harris M. (2004) Hepatitis C virus NS5A. Tales of a promiscuous protein. J. Gen. Virol. 85, 2485–2502 [DOI] [PubMed] [Google Scholar]
- 7. Kaneko T., Tanji Y., Satoh S., Hijikata M., Asabe S., Kimura K., Shimotohno K. (1994) Production of two phosphoproteins from the NS5A region of the hepatitis C viral genome. Biochem. Biophys. Res. Commun. 205, 320–326 [DOI] [PubMed] [Google Scholar]
- 8. Tanji Y., Kaneko T., Satoh S., Shimotohno K. (1995) Phosphorylation of hepatitis C virus-encoded nonstructural protein NS5A. J. Virol. 69, 3980–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirota M., Satoh S., Asabe S., Kohara M., Tsukiyama-Kohara K., Kato N., Hijikata M., Shimotohno K. (1999) Phosphorylation of nonstructural 5A protein of hepatitis C virus. HCV group-specific hyperphosphorylation. Virology 257, 130–137 [DOI] [PubMed] [Google Scholar]
- 10. Pestova T. V., Kolupaeva V. G., Lomakin I. B., Pilipenko E. V., Shatsky I. N., Agol V. I., Hellen C. U. (2001) Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. U. S. A. 98, 7029–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Preiss T., Hentze M. W. (2003) Starting the protein synthesis machine. Eukaryotic translation initiation. Bioessays 25, 1201–1211 [DOI] [PubMed] [Google Scholar]
- 12. Jackson R. J., Hellen C. U., Pestova T. V. (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sonenberg N., Hinnebusch A. G. (2009) Regulation of translation initiation in eukaryotes. Mechanisms and biological targets. Cell 136, 731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hershey J. W. B., Merrick W. C. (2000) in Translational Regulation of Gene Expression (Sonenberg N., Hershey J. W. B., Mathews M. B., eds) pp. 87–128, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 15. Gebauer F., Hentze M. W. (2004) Molecular mechanisms of translational control. Nat. Rev. Mol. Cell Biol. 5, 827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dever T. E. (2002) Gene-specific regulation by general translation factors. Cell 108, 545–556 [DOI] [PubMed] [Google Scholar]
- 17. Lodish H. F. (1976) Translational control of protein synthesis. Annu. Rev. Biochem. 45, 39–72 [DOI] [PubMed] [Google Scholar]
- 18. Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 19. Sarbassov D. D., Ali S. M., Sabatini D. M. (2005) Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17, 596–603 [DOI] [PubMed] [Google Scholar]
- 20. Haghighat A., Mader S., Pause A., Sonenberg N. (1995) Repression of cap-dependent translation by 4E-binding protein 1. Competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 14, 5701–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guertin D. A., Sabatini D. M. (2007) Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 [DOI] [PubMed] [Google Scholar]
- 22. Pyronnet S., Imataka H., Gingras A. C., Fukunaga R., Hunter T., Sonenberg N. (1999) Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 18, 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morley S. J., Pain V. M. (1995) Hormone-induced meiotic maturation in Xenopus oocytes occurs independently of p70s6k activation and is associated with enhanced initiation factor (eIF)-4F phosphorylation and complex formation. J. Cell Sci. 108, 1751–1760 [DOI] [PubMed] [Google Scholar]
- 24. Morley S. J., Pain V. M. (1995) Translational regulation during activation of porcine peripheral blood lymphocytes. Association and phosphorylation of the α and γ subunits of the initiation factor complex eIF-4F. Biochem. J. 312, 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shveygert M., Kaiser C., Bradrick S. S., Gromeier M. (2010) Regulation of eukaryotic initiation factor 4E (eIF4E) phosphorylation by mitogen-activated protein kinase occurs through modulation of Mnk1-eIF4G interaction. Mol. Cell. Biol. 30, 5160–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scheper G. C., Proud C. G. (2002) Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur. J. Biochem. 269, 5350–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerekatte V., Smiley K., Hu B., Smith A., Gelder F., De Benedetti A. (1995) The proto-oncogene/translation factor eIF4E. A survey of its expression in breast carcinomas. Int. J. Cancer 64, 27–31 [DOI] [PubMed] [Google Scholar]
- 28. Li B. D., Liu L., Dawson M., De Benedetti A. (1997) Overexpression of eukaryotic initiation factor 4E (eIF4E) in breast carcinoma. Cancer 79, 2385–2390 [PubMed] [Google Scholar]
- 29. Nathan C. O., Franklin S., Abreo F. W., Nassar R., de Benedetti A., Williams J., Stucker F. J. (1999) Expression of eIF4E during head and neck tumorigenesis. Possible role in angiogenesis. Laryngoscope 109, 1253–1258 [DOI] [PubMed] [Google Scholar]
- 30. Rosenwald I. B., Hutzler M. J., Wang S., Savas L., Fraire A. E. (2001) Expression of eukaryotic translation initiation factors 4E and 2α is increased frequently in bronchioloalveolar but not in squamous cell carcinomas of the lung. Cancer 92, 2164–2171 [DOI] [PubMed] [Google Scholar]
- 31. Seki N., Takasu T., Mandai K., Nakata M., Saeki H., Heike Y., Takata I., Segawa Y., Hanafusa T., Eguchi K. (2002) Expression of eukaryotic initiation factor 4E in atypical adenomatous hyperplasia and adenocarcinoma of the human peripheral lung. Clin. Cancer Res. 8, 3046–3053 [PubMed] [Google Scholar]
- 32. Wendel H. G., Silva R. L., Malina A., Mills J. R., Zhu H., Ueda T., Watanabe-Fukunaga R., Fukunaga R., Teruya-Feldstein J., Pelletier J., Lowe S. W. (2007) Dissecting eIF4E action in tumorigenesis. Genes Dev. 21, 3232–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bianchini A., Loiarro M., Bielli P., Busà R., Paronetto M. P., Loreni F., Geremia R., Sette C. (2008) Phosphorylation of eIF4E by MNKs supports protein synthesis, cell cycle progression, and proliferation in prostate cancer cells. Carcinogenesis 29, 2279–2288 [DOI] [PubMed] [Google Scholar]
- 34. Clemens M. J., Bommer U. A. (1999) Translational control. The cancer connection. Int. J. Biochem. Cell Biol. 31, 1–23 [DOI] [PubMed] [Google Scholar]
- 35. Kevil C. G., De Benedetti A., Payne D. K., Coe L. L., Laroux F. S., Alexander J. S. (1996) Translational regulation of vascular permeability factor by eukaryotic initiation factor 4E. Implications for tumor angiogenesis. Int. J. Cancer 65, 785–790 [DOI] [PubMed] [Google Scholar]
- 36. Pardo O. E., Arcaro A., Salerno G., Raguz S., Downward J., Seckl M. J. (2002) Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway. Correlation with resistance to etoposide-induced apoptosis. J. Boil. Chem. 277, 12040–12046 [DOI] [PubMed] [Google Scholar]
- 37. Rosenwald I. B., Lazaris-Karatzas A., Sonenberg N., Schmidt E. V. (1993) Elevated levels of cyclin D1 protein in response to increased expression of eukaryotic initiation factor 4E. Mol. Cell. Biol. 13, 7358–7363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saito H., Hayday A. C., Wiman K., Hayward W. S., Tonegawa S. (1983) Activation of the c-myc gene by translocation. A model for translational control. Proc. Natl. Acad. Sci. U. S. A. 80, 7476–7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zindy P., Bergé Y., Allal B., Filleron T., Pierredon S., Cammas A., Beck S., Mhamdi L., Fan L., Favre G., Delord J. P., Roché H., Dalenc F., Lacroix-Triki M., Vagner S. (2011) Formation of the eIF4F translation-initiation complex determines sensitivity to anticancer drugs targeting the EGFR and HER2 receptors. Cancer Res. 71, 4068–4073 [DOI] [PubMed] [Google Scholar]
- 40. Avdulov S., Li S., Michalek V., Burrichter D., Peterson M., Perlman D. M., Manivel J. C., Sonenberg N., Yee D., Bitterman P. B., Polunovsky V. A. (2004) Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer cell 5, 553–563 [DOI] [PubMed] [Google Scholar]
- 41. Roberts L. O., Jopling C. L., Jackson R. J., Willis A. E. (2009) Viral strategies to subvert the mammalian translation machinery. Prog. Mol. Biol. Transl. Sci. 90, 313–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Etchison D., Milburn S. C., Edery I., Sonenberg N., Hershey J. W. (1982) Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap-binding protein complex. J. Boil. Chem. 257, 14806–14810 [PubMed] [Google Scholar]
- 43. Mader S., Lee H., Pause A., Sonenberg N. (1995) The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4γ and the translational repressors 4E-binding proteins. Mol. Cell. Biol. 15, 4990–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ohlmann T., Rau M., Pain V. M., Morley S. J. (1996) The C-terminal domain of eukaryotic protein synthesis initiation factor (eIF) 4G is sufficient to support cap-independent translation in the absence of eIF4E. EMBO J. 15, 1371–1382 [PMC free article] [PubMed] [Google Scholar]
- 45. Gingras A. C., Svitkin Y., Belsham G. J., Pause A., Sonenberg N. (1996) Activation of the translational suppressor 4E-BP1 following infection with encephalomyocarditis virus and poliovirus. Proc. Natl. Acad. Sci. U. S. A. 93, 5578–5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Edgil D., Polacek C., Harris E. (2006) Dengue virus utilizes a novel strategy for translation initiation when cap-dependent translation is inhibited. J. Virol. 80, 2976–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cuesta R., Xi Q., Schneider R. J. (2000) Adenovirus-specific translation by displacement of kinase Mnk1 from cap-initiation complex eIF4F. EMBO J. 19, 3465–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chuluunbaatar U., Roller R., Feldman M. E., Brown S., Shokat K. M., Mohr I. (2010) Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev. 24, 2627–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Everly D. N., Jr., Feng P., Mian I. S., Read G. S. (2002) mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus. Genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76, 8560–8571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aoyagi M., Gaspar M., Shenk T. E. (2010) Human cytomegalovirus UL69 protein facilitates translation by associating with the mRNA cap-binding complex and excluding 4EBP1. Proc. Natl. Acad. Sci. U. S. A. 107, 2640–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walsh D., Mohr I. (2004) Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 18, 660–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Walsh D., Perez C., Notary J., Mohr I. (2005) Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J. Virol. 79, 8057–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Street A., Macdonald A., Crowder K., Harris M. (2004) The Hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J. Boil. Chem. 279, 12232–12241 [DOI] [PubMed] [Google Scholar]
- 54. Mamane Y., Petroulakis E., LeBacquer O., Sonenberg N. (2006) mTOR, translation initiation, and cancer. Oncogene 25, 6416–6422 [DOI] [PubMed] [Google Scholar]
- 55. Mannová P., Beretta L. (2005) Activation of the N-Ras-PI3K-Akt-mTOR pathway by hepatitis C virus. Control of cell survival and viral replication. J. Virol. 79, 8742–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. He Y., Tan S. L., Tareen S. U., Vijaysri S., Langland J. O., Jacobs B. L., Katze M. G. (2001) Regulation of mRNA translation and cellular signaling by hepatitis C virus nonstructural protein NS5A. J. Virol. 75, 5090–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peng L., Liang D., Tong W., Li J., Yuan Z. (2010) Hepatitis C virus NS5A activates the mammalian target of rapamycin (mTOR) pathway, contributing to cell survival by disrupting the interaction between FK506-binding protein 38 (FKBP38) and mTOR. J. Biol. Chem. 285, 20870–20881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lindenbach B. D., Evans M. J., Syder A. J., Wölk B., Tellinghuisen T. L., Liu C. C., Maruyama T., Hynes R. O., Burton D. R., McKeating J. A., Rice C. M. (2005) Complete replication of hepatitis C virus in cell culture. Science 309, 623–626 [DOI] [PubMed] [Google Scholar]
- 59. Schlaepfer D. D., Jones K. C., Hunter T. (1998) Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase. Summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol. Cell. Biol. 18, 2571–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Majumder M., Ghosh A. K., Steele R., Ray R., Ray R. B. (2001) Hepatitis C virus NS5A physically associates with p53 and regulates p21/waf1 gene expression in a p53-dependent manner. J. Virol. 75, 1401–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Majumder M., Ghosh A. K., Steele R., Zhou X. Y., Phillips N. J., Ray R., Ray R. B. (2002) Hepatitis C virus NS5A protein impairs TNF-mediated hepatic apoptosis, but not by an anti-FAS antibody, in transgenic mice. Virology 294, 94–105 [DOI] [PubMed] [Google Scholar]
- 62. Choo A. Y., Yoon S. O., Kim S. G., Roux P. P., Blenis J. (2008) Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. U. S. A. 105, 17414–17419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Raught B., Gingras A. C., Gygi S. P., Imataka H., Morino S., Gradi A., Aebersold R., Sonenberg N. (2000) EMBO J. 19, 434–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vary T. C., Anthony J. C., Jefferson L. S., Kimball S. R., Lynch C. J. (2007) Rapamycin blunts nutrient stimulation of eIF4G, but not PKCepsilon phosphorylation, in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 293, E188–E196 [DOI] [PubMed] [Google Scholar]
- 65. Scheper G. C., van Kollenburg B., Hu J., Luo Y., Goss D. J., Proud C. G. (2002) Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J. Boil. Chem. 277, 3303–3309 [DOI] [PubMed] [Google Scholar]
- 66. Khaleghpour K., Pyronnet S., Gingras A. C., Sonenberg N. (1999) Translational homeostasis. Eukaryotic translation initiation factor 4E control of 4E-binding protein 1 and p70 S6 kinase activities. Mol. Cell. Biol. 19, 4302–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moorman N. J., Shenk T. (2010) Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J. Virol. 84, 5260–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huang L., Hwang J., Sharma S. D., Hargittai M. R., Chen Y., Arnold J. J., Raney K. D., Cameron C. E. (2005) Hepatitis C virus nonstructural protein 5A (NS5A) is an RNA-binding protein. J. Boil. Chem. 280, 36417–36428 [DOI] [PubMed] [Google Scholar]
- 69. Deleted in proof.
- 70. Armengol G., Rojo F., Castellví J., Iglesias C., Cuatrecasas M., Pons B., Baselga J., Ramón y Cajal S. (2007) 4E-binding protein 1. A key molecular “funnel factor” in human cancer with clinical implications. Cancer Res. 67, 7551–7555 [DOI] [PubMed] [Google Scholar]
- 71. Balakumaran B. S., Porrello A., Hsu D. S., Glover W., Foye A., Leung J. Y., Sullivan B. A., Hahn W. C., Loda M., Febbo P. G. (2009) MYC activity mitigates response to rapamycin in prostate cancer through eukaryotic initiation factor 4E-binding protein 1-mediated inhibition of autophagy. Cancer Res. 69, 7803–7810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gong J., Wang J., Ren K., Liu C., Li B., Shi Y. (2009) Serine/threonine kinase Pim-2 promotes liver tumorigenesis induction through mediating survival and preventing apoptosis of liver cell. J. Surg. Res. 153, 17–22 [DOI] [PubMed] [Google Scholar]
- 73. Cohen A. M., Grinblat B., Bessler H., Kristt D., Kremer A., Schwartz A., Halperin M., Shalom S., Merkel D., Don J. (2004) Increased expression of the hPim-2 gene in human chronic lymphocytic leukemia and non-Hodgkin lymphoma. Leukemia lymphoma 45, 951–955 [DOI] [PubMed] [Google Scholar]
- 74. Lin C. J., Malina A., Pelletier J. (2009) c-Myc and eIF4F constitute a feed-forward loop that regulates cell growth. Implications for anticancer therapy. Cancer Res. 69, 7491–7494 [DOI] [PubMed] [Google Scholar]
- 75. Bradrick S. S., Walters R. W., Gromeier M. (2006) The hepatitis C virus 3′-untranslated region or a poly(A) tract promote efficient translation subsequent to the initiation phase. Nucleic acids Res. 34, 1293–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Otto G. A., Puglisi J. D. (2004) The pathway of HCV IRES-mediated translation initiation. Cell 119, 369–380 [DOI] [PubMed] [Google Scholar]
- 77. Marzec M., Liu X., Wysocka M., Rook A. H., Odum N., Wasik M. A. (2011) Simultaneous inhibition of mTOR-containing complex 1 (mTORC1) and MNK induces apoptosis of cutaneous T-cell lymphoma (CTCL) cells. PloS One 6, e24849. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.