Background: Subcellular distribution of NADPH oxidase in macrophages is unclear.
Results: In mature (activated) macrophages, NADPH oxidase is contained in a storage compartment and is internalized by clathrin-coated pits.
Conclusion: NADPH oxidase trafficking is regulated by external factors.
Significance: Subcellular localization of NADPH oxidase relates to pathogen eradication, nature, and severity of oxidative tissue stress as well as redox signaling.
Keywords: Endocytosis, Macrophages, Membrane Trafficking, NADPH Oxidase, Rab Proteins
Abstract
Here, we report that activation of different types of tissue macrophages, including microglia, by lipopolysaccharide (LPS) or GM-CSF stimulation correlates with the quantitative redistribution of NADPH oxidase (cyt b558) from the plasma membrane to an intracellular stimulus-responsive storage compartment. Cryo-immunogold labeling of gp91phox and CeCl3 cytochemistry showed the presence of gp91phox and oxidant production in numerous small (<100 nm) vesicles. Cell homogenization and sucrose gradient centrifugation in combination with transferrin-HRP/DAB ablation showed that more than half of cyt b558 is present in fractions devoid of endosomal markers, which is supported by morphological evidence to show that the cyt b558-containing compartment is distinct from endosomes or biosynthetic organelles. Streptolysin-O-mediated guanosine 5′-3-O-(thio)triphosphate loading of Ra2 microglia caused exocytosis of a major complement of cyt b558 under conditions where lysosomes or endosomes were not mobilized. We establish phagocytic particles and soluble mediators ATP, TNFα, and CD40L as physiological inducers of cyt b558 exocytosis to the cell surface, and by shRNA knockdown, we identify Rab27A/B as positive or negative regulators of vesicular mobilization to the phagosome or the cell surface, respectively. Exocytosis was followed by clathrin-dependent internalization of cyt b558, which could be blocked by a dominant negative mutant of the clathrin-coated pit-associated protein Eps15. Re-internalized cyt b558 did not reach lysosomes but associated with recycling endosomes and undefined vesicular elements. In conclusion, cyt b558 depends on clathrin for internalization, and in activated macrophages NADPH oxidase occupies a Rab27A/B-regulated secretory compartment, which allows rapid agonist-induced redistribution of superoxide production in the cell.
Introduction
Generation of superoxide by the NADPH oxidase expressed in professional phagocytes is essential for the efficient killing of invading pathogens (1, 2). The catalytic core of the NADPH oxidase is a flavocytochrome b558 complex (cyt b558)3 consisting of integral membrane proteins gp91phox (NOX-2) and p22phox. In addition, stimulus-dependent recruitment of cytosolic subunits p40phox, p47phox, p67phox, and Rac1 to cyt b558 in the membrane is required for electron abstraction from NADPH and transfer to redox centers in gp91phox (3), which channel the electrons through the membrane to react with molecular oxygen. Thus, superoxide is formed on the extracellular side of the plasma membrane (or in the phagosomal lumen).
The phagocyte NADPH oxidase is the founding member of a small family of highly homologous NADPH oxidoreductases, i.e. the NOX family (4, 5), which by redox signaling fulfill a multitude of functions also outside of the immune system. The five most conserved NOX family members NOX1–5 each have distinct subcellular distributions in different or even in the same cell types (6–10). Although superoxide or hydrogen peroxide production is presumed to be highly compartmentalized to achieve anything from pathogen killing to specific redox signaling (11), the sorting receptors and trafficking events that mediate the final subcellular distribution of NOX family members are largely unknown, and no sorting motifs in any NOX family member has been identified. Although NOX family members have been associated by morphological and biochemical techniques with different potential endocytic structures, including lipid rafts (12) and caveolae (8, 13), there is no evidence for a role of these trafficking platforms in bulk internalization of NOX family members.
Inappropriate production and release of reactive oxygen species to the extracellular milieu is known to be a major pathological factor in many inflammatory diseases (14). Therefore, phagocytes are endowed with protective mechanisms to minimize reactive oxygen species release to surrounding tissues. First and foremost, the strictly stimulus-dependent assembly of the NADPH oxidase holoenzyme ensures a low basal production of superoxide under resting conditions. Furthermore, in neutrophils and monocytes, cyt b558 is contained in intracellular secretory granules and vesicles (15, 16), which fuse with the phagosomal membrane following internalization of pathogens (17) to limit extracellular release of free radicals.
In contrast, the subcellular distribution of NADPH oxidase in macrophages has received considerably less attention despite potential implications for T-cell activation (18), redox signaling, phagocyte killing, and tissue oxidative stress in chronic inflammatory states. In immature macrophages (not presented with any activating stimulus), cyt b558 is predominantly plasma membrane-localized (19) and in mesenchymal cell types has been proposed to be distributed between the plasma membrane and transferrin (Tfn)-positive recycling endosomes (20).
We report here that activation of different types of tissue macrophages with LPS or GM-CSF correlates with an almost quantitative redistribution of cyt b558 from plasma membrane to an intracellular secretory storage compartment. This compartment can be exocytosed to the cell surface or phagosomal membrane in response to soluble or particulate stimuli, followed by clathrin-dependent re-internalization of cyt b558. Our results define the first known bulk internalization/sorting structure of any NOX family member and indicate that the subcellular distribution of NADPH oxidase in activated macrophages is not an intrinsic property of NADPH oxidase but is subject to modulation by external signals in the form of exogenous or endogenous pro-inflammatory mediators.
MATERIALS AND METHODS
ATP, fMLP PMA, luminol, HRP-II, LPS (Escherichia coli serotype O:55), nitro blue tetrazolium (NBT), NHS-SS-biotin, NHS-biotin, superoxide dismutase (SOD), and diphenyliodonium were purchased from Sigma. Antibodies used included anti-gp91phox mAb 54.1 recognizing an intracellular C-terminal epitope (21), mAb 8G11 recognizing an extracellular epitope (22), mAb 7D5 recognizing an extracellular epitope on human gp91phox (Medical and Biological Laboratories, Nagoya, 460-0002, Japan), rabbit polyclonal anti-p22phox antibody FL195 (Santa Cruz Biotechnology; Santa Cruz, CA), and anti-Rab27A mAb 4B12 (42) (generously provided by Dr. Seabra, Imperial College London, UK). See supplemental “Materials and Methods” for a discussion of anti-cyt b558 antibody specificity and for other antibodies used. All fluorophore-conjugated reagents, including zymosan, transferrin, acetylated LDL, and secondary Alexa-conjugated goat anti-mouse, anti-rat, or anti-rabbit antibodies, were from Molecular Probes (Paisley PA4 9RF, UK). TNF-α, IFN-γ, M-CSF, CD40L, and GM-CSF were all from PeproTech (Rocky Hill, NJ).
Purification and Culture of Macrophages
Purification and culture of primary microglia from newborn rats has been described previously (23). Bone marrow-derived macrophages and resident peritoneal macrophages from rat were prepared by standard techniques (see supplemental “Materials and Methods” for further information) and were cultured in RPMI 1640 medium with 10% FCS, 1% pyruvate, 2% nonessential amino acids, penicillin/streptomycin, and 10 μm β-mercaptoethanol (with 20 ng/ml M-CSF added for 8–10 days to induce bone marrow precursor differentiation to macrophages). All macrophage populations used were about 95% pure as determined by expression of CD11b and FcγR (see supplemental Fig. 1). Human monocytes were isolated from buffy coats of healthy volunteers by standard Hypaque-Ficoll and Percoll gradient purification (see supplemental “Materials and Methods”). Cells were cultured in RPMI 1640 medium as above without any stimulation for 4–7 days to allow differentiation to macrophages. The murine microglial cell line Ra2 (24) (59) was kindly provided by Dr. Makoto Sawada (Dept. of Brain Function, Research Institute of Environmental Medicine, Nagoya University, Nagoya, Japan) and was maintained in MEM with 10% FCS, 1 ng/ml GM-CSF, and 5 μg/ml bovine insulin.
Lentivirus Production and Cell Transduction
The cDNAs for mutant Eps15-EH29 (a kind gift of Drs. Dautry-Varsat and Benmerah) (25) and the CFP-gp91phox fusion protein (26) were PCR-amplified with sticky primers for cloning into lentivector pLOX TW rtTA carrying a tetracycline-responsive promoter (27). The pHR EF gp91phox lentivector, calcium phosphate transfection of HEK 293T producer cells, and lentivector production have been described (23). Vectors were used to superinfect reverse tetracycline transactivator protein (rtTA)-expressing Ra2 cells previously established, and in all cases this resulted in transgene expression in the large majority of cells (>85%). For Rab27A and -B knockdown, Ra2 microglia were infected with shRNA lentivector pLKO.1 (Sigma) with the hairpin sequences GCTTCTGTTCGACCTGACAAA and CGGGAAGACAACATTTCTCTA for Rab27A and -B, respectively, or control vector expressing noncoding shRNA (Sigma). Ra2 cells were used on day 4 or 5 after transduction for immunofluorescence or assays for free radical production.
Measurement of Superoxide Production
For the NBT test, cells were incubated in HBSS with 0.25% (w/v) NBT and 100 ng/ml PMA at 37 °C for 10–30 min before fixation in 2% paraformaldehyde in phosphate buffer and visual inspection. In one series of experiments, human macrophages were treated or not with 1 μg/ml LPS overnight, before stimulation with 100 ng/ml PMA in the presence or absence of 400 units/ml SOD in HBSS with 0.25% NBT. Luminol-enhanced chemiluminescence (luminol ECL) was used to measure fMLP or PMA-induced superoxide release as described previously (23). Briefly, Ra2 microglia kept on ice were warmed for 2 min in a 37 °C water bath in HBSS/luminol/HRP-II before being dispensed into ELISA wells at 100,000 cells/well. Subsequently, superoxide production was measured before and after stimulation with 4 μm fMLP or 100 ng/ml PMA (final concentrations) delivered through the injector module of a Synergy HT microplate reader. Superoxide generation during phagocytosis was determined by a flow cytometry-based assay. Briefly, Ra2 microglia in suspension were challenged with IgG-opsonized, Alexa 633- and 2′,7′-dichlorofluorescein (H2DCF)-conjugated zymosan or sheep red blood cells (SRBC; Fitzgerald) at a ratio of 1:100 and 1:10, respectively. Fluorescence intensity of H2DCF was followed at intervals of ∼2 min on a FACSAria (BD Biosciences) at 37 °C using Alexa 633 gating to follow Ra2 microglia containing only one or a few phagocytic particles. The entire Alexa 633 gate represented 60–70% of the whole cell population.
Morphological Methods
Immunofluorescence was essentially performed on cells fixed in 2% (w/v) paraformaldehyde in phosphate buffer, pH 7.2, as described previously (28) using secondary Alexa 488-, 568-, or 633-conjugated goat anti-rabbit, -mouse, or -rat antibodies. In some cases cells were allowed to internalize Alexa 633-conjugated Tfn or DiI- or Alexa 594-conjugated AcLDL before or concurrent with experiments. For mAb tracer experiments (internalization of anti-gp91phox, TfnR, and CD3ϵ antibodies), Ra2 microglia were starved of GM-CSF 24–48 h before experiments and then incubated with 2 μg/ml antibody and 50 μm EIPA to avoid macropinocytosis during the 6-h incubation. Leupeptin (50 μg/ml) and pepstatin (67 μg/ml) were included as specified to block lysosomal degradation. Medial images of cells, mostly encompassing the nucleus, were acquired with a Zeiss LSM510 confocal laser scanning microscope with a C-Apochromat ×63, 1.2 oil immersion objective, using the argon 488-nm and helium-neon 543- and 594-nm laser lines for excitation of Alexa 488, 568, and 633, respectively. Optical sections (1.0–1.5 μm) were collected and saved as 512 × 512-pixel images at 8-bit resolution before import into Adobe Photoshop for compilation. For cryo-immunogold labeling, Ra2 cell pellets were fixed in 2% paraformaldehyde and 0.1% (w/v) glutaraldehyde in phosphate buffer, pH 7.2, and subsequent gelatin-embedding, ultra-cryosectioning, and cryo-immunogold labeling performed as described previously (28) with anti-gp91phox mAb 54.1 followed by secondary 10-nm gold-conjugated goat anti-mouse antibodies. Oxidant production was visualized by NBT assay and by a cerium chloride (CeCl3)-based detection method for EM (16). Briefly, Ra2 cells incubated with 100 ng/ml LPS overnight were stimulated with 100 ng/ml PMA in Hepes buffer (135 mm NaCl, 5 mm KCl, 1 mm CaCl2, 5 mm glucose, 20 mm Hepes, pH 7.4) containing 20 mm Tricine, 1 mm CeCl3, and 1 mm NaN3. After incubation for 1 h in a 37 °C water bath, cells were washed twice in cold buffer containing 20 mm Tricine and then fixed in 2% (w/v) glutaraldehyde in Hepes buffer for 20 min on ice. After post-fixation in 0.1 m cacodylate buffer, pH 7.4, containing 1% (w/v) OsO4 and 1.5% (w/v) potassium ferrocyanide for 30 min on ice, cells were finally processed for Epon embedding. Sections were examined in a Phillips CM 100 electron microscope.
Streptolysin-O (SLO) Permeabilization
Ra2 CFP-gp91phox cells contained in a thin glass-bottomed 8-chamber culture vessel were incubated in advance with DiI-conjugated AcLDL for 1 h and chased 2–3 h for lysosomal loading. Alternatively, cells were incubated with Alexa 633-conjugated Tfn for 1 h to load early endosomes. Then cells were washed and incubated at 37 °C with permeabilization buffer (30 mm NaCl, 110 mm KCl, 0.4 mm NaH2PO4, and 15 mm Hepes, pH 7.3) with 100 μm ATP, and 50 μg/ml Mr 10,000 Alexa 633-conjugated dextran further containing 250–400 ng/ml SLO (Sigma) and 400 ng/ml GTPγS. The 8-chamber culture vessel was mounted on a thermostated microscope stage in atmospheric air, and fluorescence of CFP, DiI, and Alexa 633 was followed by time lapse using the LSM510 laser lines as described above. Alternatively, a more gentle protocol allowing SLO-mediated loading and plasma membrane resealing was followed (29). Briefly, Ra2 cells were incubated for 12 min at 37 °C with 400 ng/ml SLO in permeabilization buffer with 1 mm EGTA and 100 μm ATP. The concentration of SLO was titrated on each experimental day to obtain permeabilization of ∼60–80% of the cells as judged by trypan blue exclusion (29). Subsequently, permeabilization buffer was exchanged for cold HBSS, and cells were allowed to reseal at 4 °C for 1 h before incubation at 37 °C in HBSS for up to 10 min before fixation and immunofluorescence.
Subcellular Fractionation, DAB/HRP Ablation, and Gradient Centrifugation
Coupling of apotransferrin to HRP was done with the EZ-Link Plus activated peroxidase kit (Pierce) according to the manufacturer's instructions. For DAB ablation, Ra2 cells expressing human gp91phox and TfnR were incubated in HBSS with Tfn-HRP at a concentration of 20 μg/ml for 30 min at 37 °C and then washed three times for 5 min in ice-cold HBSS and in PBS without Ca2+ and Mg2+ for a further 10 min. Cells were then resuspended in ice-cold hypotonic buffer (75 mm NaCl, 10 mm Hepes, 170 mm sucrose, 1 mm MgCl2, and 1 mm EGTA, pH 7.4) and sonicated. The post-nuclear homogenate was divided in two and subjected to DAB reaction (100 μg/ml) with or without 0.02% (w/v) H2O2 for 1 h at 4 °C. Subsequently, supernatants were centrifuged at 16,000 × g for 10 min at 4 °C, to remove the mitochondrial fraction, and the resulting supernatant was separated on a 15–45% (w/v) sucrose density gradient by velocity centrifugation in a Beckman SW40Ti rotor for 18–20 h at 155,000 × g. Standard cell homogenization and centrifugation were performed in ice-cold hypotonic buffer as above, but cells were disrupted by repeated rounds of aspiration with a 27-gauge needle, and the resulting post-mitochondrial homogenate was applied to a 10–45% sucrose gradient for 18–20 h at 155,000 × g. In both instances, the fractions were collected with a peristaltic pump from the bottom of the tube and analyzed by Western blotting with antibodies directed against cyt b558 or different organelle markers as indicated. Cell surface biotinylation with 3-sulfo-N-hydroxysuccinimide ester-biotin or the reducible variant, NHS-SS-biotin, to measure levels of cell surface-exposed or internalized cyt b558, respectively, was performed essentially as described previously (28).
RESULTS
LPS and GM-CSF Cause Redistribution of cyt b558 from the Cell Surface to an Intracellular Compartment in Macrophages
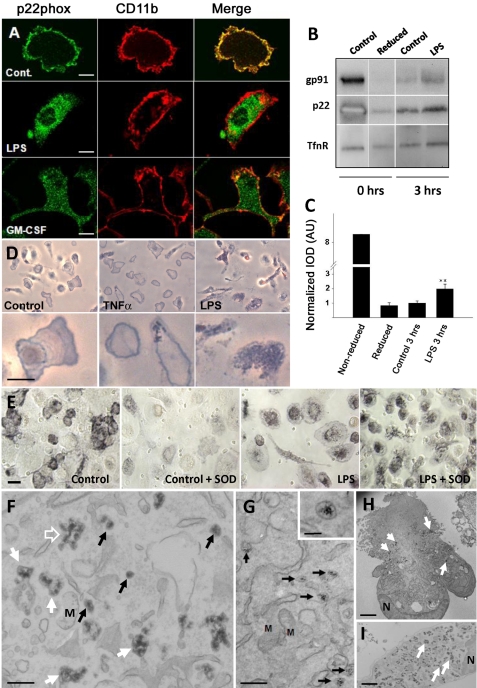
BMDM differentiated in vitro with M-CSF, resident peritoneal macrophages, and purified microglia, all from rat, were isolated and cultured as described under “Materials and Methods.” Populations were about 95% pure as determined by CD11b labeling and flow cytometry analysis (see supplemental Fig. 1). If macrophages are purified and cultured with care, cyt b558 is present mainly on the cell surface similar to the findings of others (19). However, as shown in Fig. 1A for primary microglia (see supplemental Fig. 2 for other macrophage types), activation obtained by overnight stimulation with 100 ng/ml LPS or 10–20 ng/ml GM-CSF caused a dramatic redistribution of cyt b558 from the plasma membrane to numerous small cytosolic vesicles in all macrophage populations examined. In contrast, CD11b (integrin αM and FcγRIIA/III; data not shown) remained at the cell surface. Generally, prolonged times of incubation (minimum 10 h) with LPS or GM-CSF were required to obtain a complete intracellular localization of cyt b558 as observed by immunofluorescence. However, an increased rate of cyt b558 internalization could be detected 3 h after LPS stimulation by a cell surface biotinylation technique with the reduction-sensitive NHS-SS-biotin analog (Fig. 1, B and C). Bone marrow-derived macrophages were used for this purpose because high cell numbers could be obtained with few animals.
FIGURE 1.
LPS and GM-CSF induce redistribution of cyt b558 from the cell surface to an intracellular membrane compartment in primary macrophages. A, rat primary microglia were treated with 100 ng/ml LPS or 10–20 ng/ml GM-CSF overnight and then processed for immunofluorescence with polyclonal rabbit anti-p22phox antibodies (green, left column) and anti-CD11b mAb (red, middle column). The merged channels are shown in the right column. Bars, 10 μm. B, internalization of cyt b558 subunits in rat BMDMs after stimulation with 100 ng/ml LPS for 3 h was detected by a cell surface biotinylation protocol (NHS-SS-biotin). The bar graph in C represents optical density mean ± S.E. of Western blot bands for p22phox derived from three independently performed experiments. Note that the reduced and “treated” lanes, which appear as separate gel strips in B, were derived from nonadjacent lanes from the same gel. AU, arbitrary units. D, primary microglia were stimulated overnight with 20 ng/ml GM-CSF or 100 ng/ml LPS before stimulation with 100 ng/ml PMA in the presence of NBT. Note the redistribution of the blue reaction product from the cell surface of untreated control cells (delineating the cell periphery) to an intracellular punctate pattern following stimulation. Bars, 10 μm. E, human monocyte-derived macrophages treated or not with 2 μg/ml LPS overnight were stimulated with 100 ng/ml PMA with or without 400 units/ml SOD in the presence of NBT salt. Bars, 10 μm. F–I, CeCl3 cytochemistry on LPS-treated BMDM or Ra2 microglia, which were stimulated with PMA in the presence of CeCl3 for 1 h before processing for EM. F, in BMDM, the electron-dense cerium hydroxide reaction product is deposited in small 100 nm vesicles (black arrows), tubulovesicular elements (open arrow), and large vacuoles (white arrows). G, cerium hydroxide deposition in Ra2 cells was less intense, but the same compartments as above could be identified. Shown here are examples of 100 nm vesicles with clear internal precipitates (see inset for detail). Note that other membranes, including mitochondria (M), are devoid of reaction product. Bars, F and G, 500 nm (inset in G, 100 nm). H, Ra2 microglia; I, BMDM at low magnification. The reaction product is deposited almost exclusively inside both cell types (arrows) with little or no product on the plasma membrane. N, nucleus. Bars, 1 μm.
It should be mentioned here that redistribution of cyt b558 took place without any exogenous stimulation simply by culturing the cells for prolonged times (more than a week to 10 days). This implies that the redistribution of cyt b558 correlates with general activation of macrophages rather than LPS or GM-CSF signaling specifically, as it is well known that macrophages in mono-culture will eventually assume a matured phenotype. However, here we used LPS and GM-CSF activation because this was easier to control and resulted in healthier cells. LPS concentrations required to achieve redistribution of cyt b558 varied according to cell type ranging from 100 ng/ml for microglia to over 500 ng/ml for bone marrow-derived macrophages to 1–2 μg/ml for peritoneal macrophages and human monocyte-derived macrophages.
To verify our observations by nonimmunological techniques, we analyzed oxidant production by cytochemistry. By light microscopy, we analyzed superoxide production of control, TNFα, or LPS-stimulated primary macrophages by the use of NBT, which specifically reacts with superoxide to form an insoluble formazan reaction product. In nontreated microglia stimulated with PMA, a strong agonist of NADPH oxidase activity, the bluish formazan precipitate was confined mainly to the plasma membrane delineating the cell shape (Fig. 1D). Pretreatment with the pro-inflammatory agent TNF-α did not change this distribution; however, in LPS-stimulated microglia, most of the reaction product was observed as intracellular punctate foci within the great majority of cells. We also performed the NBT test with human monocyte-derived macrophages this time, including SOD, to distinguish between extracellular and intracellular superoxide. As shown in Fig. 1E, the reaction product was redistributed from the cell surface of control macrophages to an intracellular compartment in macrophages treated with 1–2 μg/ml LPS overnight, which is corroborated by the inability of SOD to block superoxide generation only in LPS-treated cells. We extended these observations to the ultrastructural level by making use of a protocol introduced by Kobayashi et al. (16), which relies on the ability of oxidants to reduce CeCl3 to an insoluble, electron-dense cerium hydroxide precipitate. For this purpose, we made use of BMDM and the murine microglial cell line Ra2 (24), which expresses all phagocyte NADPH oxidase subunits (23). Ra2 microglia are cultured in medium with GM-CSF for growth and thus under standard culture conditions contain cyt b558 in intracellular stores. Deprivation of GM-CSF causes a slow return of cyt b558 to the plasma membrane (see supplemental Fig. 3). BMDM and Ra2 microglia treated with 100 ng/ml LPS overnight were stimulated with PMA in the presence of CeCl3 for 1 h (time required for sufficient build up of the cerium hydroxide precipitate) before fixation and processing for EM. As seen in Fig. 1, F and G, in both BMDMs and Ra2 microglia many vesicular profiles (<100 nm) with cerium hydroxide precipitate were identifiable, likely corresponding to the vesicles observed by light microscopy. But additionally larger vacuolar or tubulovesicular elements were observed, presumably generated by PMA-induced fusion of the 100-nm vesicles with themselves or with endosomes. Cell surface deposition of cerium hydroxide could be observed, but the great majority of cerium precipitate was deposited in intracellular locations in both cell types (Fig. 1, H and I). Control cells not receiving PMA stimulation contained no or little visible cerium precipitate, and diphenyliodonium, an inhibitor of NADPH oxidase, significantly reduced the precipitate (data not shown).
Intracellular Stores of cyt b558 Are Mobilized by Phagocytosis
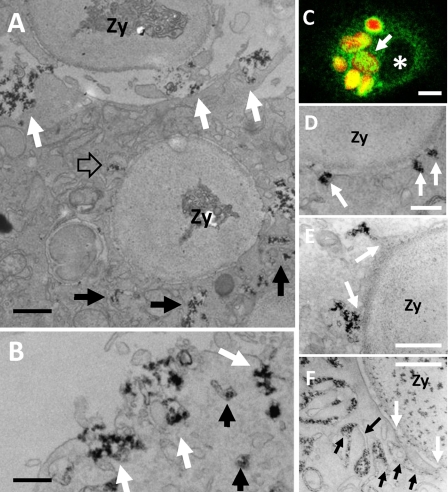
We also analyzed oxidant production following phagocytosis of IgG-opsonized zymosan particles in LPS-stimulated Ra2 microglia. When Ra2 microglia were challenged with phagocytic prey, the cytosolic vesicles containing cyt b558 were efficiently recruited to nascent or internalized phagosomes as determined by both immunofluorescence (Fig. 2C and supplemental Fig. 4) and CeCl3 cytochemistry (Fig. 2, A, B, and D–F). In fact, by immunofluorescence, there was no discernable difference in the complement of cyt b558 acquired by phagosomes in control or LPS-stimulated cells, despite the fact that cyt b558 is recruited to phagosomes from the cell surface or intracellular storage vesicles in these cells, respectively (see supplemental Fig. 4). Typically, large vacuolar structures (hundreds of nanometers up to a few micrometers) with cerium hydroxide were formed in the cortical area beneath nascent phagosomes or around already internalized phagosomes (Fig. 2A) before fusion with the phagosome membrane and expulsion of their content of cerium hydroxide precipitate into the phagosome lumen (Fig. 2, A and E). In addition, individual small vesicles (<100 nm) with precipitate could be seen to fuse directly with phagosomes (Fig. 2D), consistent with the “patchy” distribution of cyt b558 immunoreactivity on phagosomes observed by confocal microscopy (Fig. 2C). Finally, in a minor fraction of Ra2 microglia, we observed the development of complex networks of tubuli and/or lamella, which fused with the limiting membrane of the phagosomes to deliver the cerium precipitate to the lumen (Fig. 2F).
FIGURE 2.
CeCl3 cytochemistry in Ra2 microglia phagocytosing IgG-opsonized zymosan. A–F, LPS-stimulated Ra2 microglia were challenged with IgG-opsonized zymosan particles and either used for immunofluorescence (C) or for CeCl3 cytochemical detection of superoxide production (A, B, and D–F). A shows a cell that has completed uptake of one IgG-opsonized zymosan particle (Zy) and is in the process of phagocytosing another (at top). Note the build up of membrane-bound compartments containing cerium hydroxide reaction product around the phagosome (black arrows), one of which is seen to fuse with the limiting membrane of the phagosome (open arrow). Reaction product is also released at the base of the phagocytic cup by exocytosis of larger vacuolar elements (white arrows). Bar, 1 μm. B, exocytosis of vacuolar elements with cerium hydroxide deposit could also be seen on the general plasma membrane (white arrows). Bar, 500 nm. C shows by immunofluorescence one Ra2 microglial cell that has phagocytosed several IgG-opsonized, Texas Red-conjugated zymosan particles (red), which are all surrounded by an intense patchy immunoreactivity for gp91phox (green). Asterisk denotes nucleus. Bar, 10 μm. D–F, examples of delivery of reaction product to phagosomes containing IgG-opsonized zymosan. D shows direct fusion of 100 nm vesicles with the limiting membrane of the phagosome (arrows), but more commonly cerium hydroxide reaction product was released into nascent phagosomes by fusion with larger vesicles and vacuoles (arrows in E). Some phagosomes were surrounded by lamellar and/or tubular membranes containing the reaction product (black arrows), some of which fused with the limiting membrane of the phagosome (white arrows). Bars, 250 nm in D; 500 nm in E and F.
cyt b558 Localizes to Clathrin-coated Pits and Vesicles
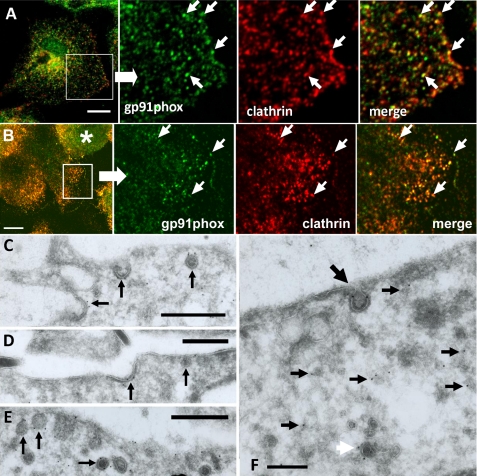
In our initial morphological trials, we observed that immunoreactivity of cyt b558 on the cell surface sometimes resembled clathrin-coated pit (CCP) distribution. We therefore performed immunofluorescence co-localization studies with anti-clathrin heavy chain and anti-gp91phox antibodies in LPS-treated primary microglia or GM-CSF-treated Ra2 microglia. As seen in Fig. 3, A and B, a considerable fraction of cyt b558-immunoreactive punctae was positive for clathrin heavy chain. These observations were extended to the ultrastructural level by cryo-immunogold labeling. Immunolocalization of gp91phox in GM-CSF-stimulated Ra2 microglia with mAb 54.1, which recognizes an intracellular epitope on the C-terminal domain of gp91phox (21), showed the presence of gp91phox in CCPs and clathrin-coated vesicular profiles close to the plasma membrane (Fig. 3, C–F). Within deeper regions of the cells, gp91phox immunoreactivity was confined to small (<100 nm) vesicular or tubulovesicular structures, most with no apparent coat (small black arrows in Fig. 3F) but some with a coat (white arrow).
FIGURE 3.
Immunofluorescence and ultracryo-immunogold EM shows localization of gp91phox in clathrin-coated pits and vesicles. Immunofluorescence was performed on LPS-stimulated primary microglia (A) and Ra2 microglia (a rounded cell (asterisk) and more well spread cells are seen) (B) with antibodies to gp91phox (green) or clathrin heavy chain (red). Boxed areas are enlarged in the next three panels and demonstrate significant co-localization of clathrin and gp91phox (arrows). Bars, 10 μm. C–F, anti-gp91phox mAb 54.1 cryo-immunogold labeling of GM-CSF-stimulated Ra2 microglia. C–E, three panels show immunoreactivity for gp91phox in CCPs and clathrin-coated vesicular profiles (arrows) close to the cell surface. F, in deeper areas of Ra2 cells gp91phox could be seen to localize to small vesicles and/or tubulovesicular structures devoid of apparent coating (small black arrows) and some with coating (white arrow). Also seen is an invaginated CCP with gp91phox immunolabeling (large black arrow). Bars, 500 nm in C–E; 250 nm in F.
GM-CSF-induced Internalization of cyt b558 in Ra2 Microglia Is Clathrin-dependent
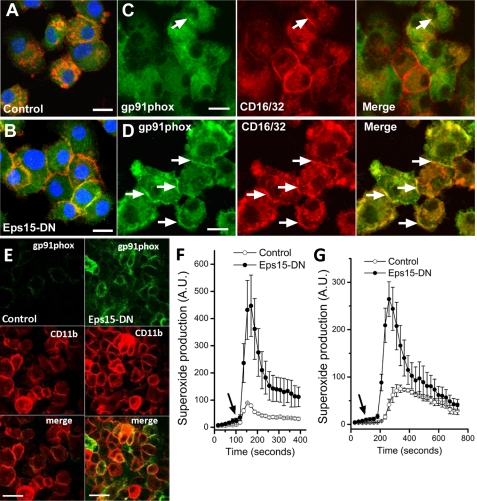
To test the significance of these findings, we created by lentiviral transduction a Ra2 microglial cell line conditionally (Tet-On) expressing a dominant negative Eps15 mutant (Eps15-EH29), which inhibits clathrin-mediated internalization by preventing CCPs from budding off the membrane (25). Ra2 microglia were induced to express Eps15-EH29 by 24 h of doxycycline treatment in the absence of GM-CSF (to allow cyt b558 to reach the cell surface; see supplemental Fig. 3). As shown in Fig. 4, A and B, Eps15-EH29 expression yielded a near complete inhibition of internalization of monomeric rabbit IgG, which is bound by cell surface FcγRs and endocytosed through CCPs, confirming an effective block of clathrin-dependent uptake by Eps15-EH29. Furthermore, Eps15-EH29 expression caused a shift in both gp91phox and CD16/32 (FcγR-IIA/III) distributions from intracellular localizations to the cell surface (Fig. 4, C and D). An increased plasma membrane expression of gp91phox in EPS15-EH29-expressing cells was confirmed by use of mAb 7D5, which recognizes an external epitope on human gp91phox (Fig. 4E). To evaluate the consequences of Eps15-EH29 expression on superoxide release, we next measured by luminol-enhanced chemiluminescence the respiratory burst of Ra2 microglia stimulated with fMLP or PMA. In Ra2 microglia, intracellular peroxidase content is low, and the luminol ECL signal is inhibited by ∼95% in the presence of SOD in the incubation medium and therefore represents almost exclusively extracellular superoxide (23). As seen in Fig. 4, F and G, Eps15-EH29 expression increased the release of superoxide to the medium severalfold following fMLP or PMA stimulation.
FIGURE 4.
Expression of dominant negative Eps15-EH29 mutant inhibits clathrin-coated pit endocytosis and retains NADPH oxidase at the cell surface. A and B, control and Eps15-EH29-expressing Ra2 microglia were incubated for several hours with 10 μg/ml monomeric rabbit IgG. Subsequently, immunofluorescence was performed with anti-gp91phox mAb (green) followed by Alexa 488 goat anti-mouse antibodies, Alexa 568-conjugated goat anti-rabbit antibodies to detect rabbit IgG, and ToPro-3 to visualize the nucleus (blue). Note that control cells efficiently internalize and concentrate rabbit IgG in endosomes and lysosomes (A), although expression of Eps15-EH29 traps bound rabbit IgG on the cell surface (B). Control (C) or Ra2 Eps15-EH29-expressing (D) cells were GM-CSF-starved for 20 h and processed for immunofluorescence with anti-gp91phox mAb 54.1 (green) and rat anti-CD16/32 mAb (red). Note increased co-localization (yellow) of CD16/32 and gp91phox on the cell surface of Eps15-EH29 cells (arrows in C and D). Bars, A–D, 10 μm. E, Ra2 cells expressing CFP-(h)gp91phox with or without expression of Eps15-EH29 were cell surface-labeled on ice with FITC-conjugated anti-(h)gp91phox mAb 7D5. Immunofluorescence was then performed with rat anti-CD11b mAb (red), and 7D5 mAb was visualized (further) by secondary Alexa 488-conjugated goat anti-mouse antibodies. F and G, control (open dots) or Eps15-EH29-expressing (filled dots) Ra2 microglia were stimulated with fMLP (F) or PMA (G), and superoxide release was measured by luminol ECL. Injection of fMLP or PMA is marked by arrows. The ordinate in arbitrary units (A.U.) represents ECL counts/s normalized to control cells. Mean ECL and S.E. of five independent experiments are shown in F and G.
Internalized cyt b558 Resides in a Compartment Distinct from Biosynthetic or Endocytic Organelles
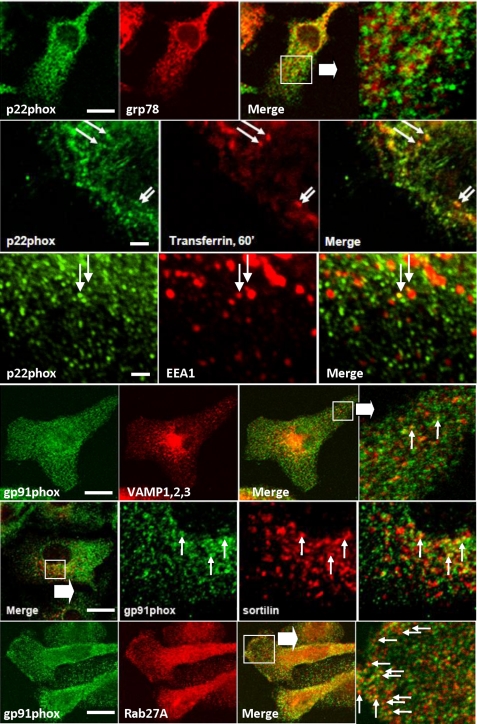
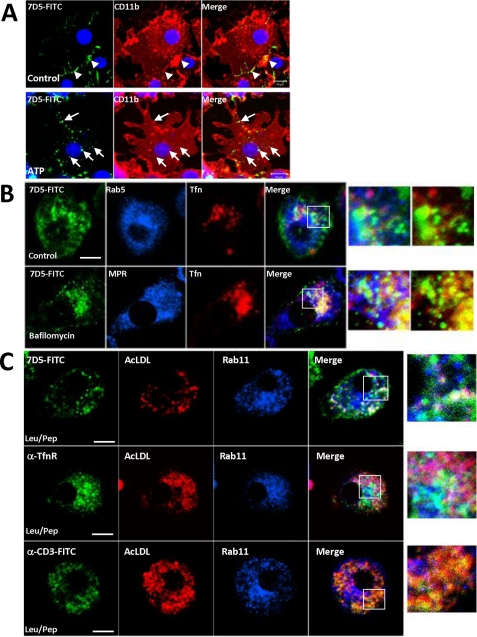
We next sought to establish the identity of the compartment harboring internalized cyt b558. When LPS-treated primary microglial cells were examined by immunofluorescence, cyt b558 subunits did not co-localize with markers of endoplasmic reticulum (ER) (Fig. 5). However, with a low frequency small cyt b558-containing vesicles did contain internalized Tfn or were co-localized with EEA1 or VAMP-3 indicating an endocytic origin. The cyt b558 complex was not present in lysosomes, but low co-localization with endosomal/late endosomal marker sortilin could be observed.
FIGURE 5.
cyt b558-containing vesicles are largely devoid of endosomal markers and distinct from biosynthetic organelles. Indirect immunofluorescence on primary microglial cells was performed with anti-p22phox or anti-gp91phox antibodies and either anti-grp94 (ER membranes), anti-EEA1 (early endosomes), anti-VAMP-1–3 (Golgi vesicles and early endosomes), anti-sortilin (early and late endosomes), or anti-Rab27A (secretory granules/lysosomes) antibodies. Alternatively, cells were allowed to internalize Alexa 568-conjugated transferrin before fixation to label recycling/early endosomes. Note the absence of p22phox co-localization with the ER and the small fraction of early endosomes (marked by EEA1, transferrin, and VAMP-1,2,3) or late endosomal elements (marked by sortilin) containing cyt b558 (arrows). Bars, 10 μm, except for 2nd and 3rd panels where it is 1 μm.
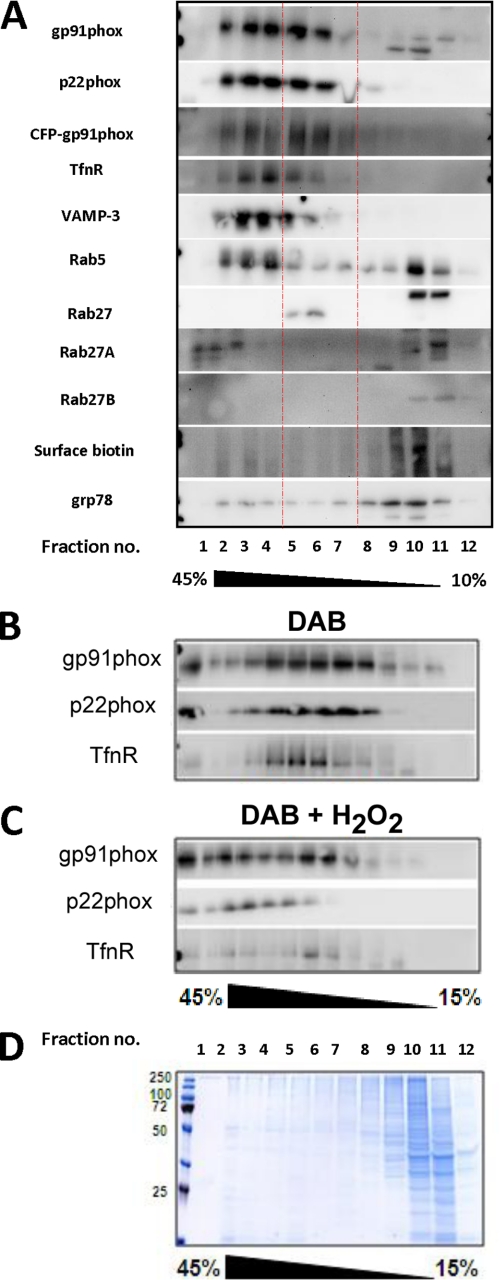
In neutrophils and dendritic cells, cyt b558-containing compartments co-localize partially with Rab27A, which is also involved with their trafficking (30–32). Of the many antigens that we tested, Rab27A gave the most convincing level of co-localization with cyt b558, typically at the periphery of cells (Fig. 5, bottom panels). Because the cyt b558-containing vesicles were numerous and their size falls below the resolution of light microscopy, it was difficult to obtain a reliable measure of co-localization with endosomal markers by counting. Therefore, to gain a perspective on the distribution of cyt b558 in endosomes, we also performed subcellular fractionation of Ra2 microglia. As shown in Fig. 6A, the great majority of cyt b558 was contained in fractions well separated from light membranes, including the plasma membrane, identified by cell surface biotinylation, and ER, identified by grp78, at the top of the gradient (fraction 12). A CFP-gp91phox fusion protein expressed in Ra microglia (see below) fractionated identically to endogenous mouse gp91phox and p22phox. Early endosomal markers TfnR, VAMP-3, and Rab5 co-localized with the denser cyt b558-containing fractions (roughly fractions 2–4), but notably these endosomal antigens were not present in fractions 5–7 harboring half of cyt b558 immunoreactivity. Rab27A and Rab27B were either soluble or associated with light membranes in fractions 10 and 11, presumably plasma membrane, but Rab27A also was contained within the densest fractions 1–3, partially overlapping the distribution of cyt b558. Use of a polyclonal rabbit antibody that does not distinguish between the two isoforms of Rab27 also indicated the presence of Rab27 with cyt b558 contained in fractions 5–7.
FIGURE 6.
cyt b558-containing vesicles and endosomes have similar sedimentation profile but are distinct. A, GM-CSF-treated Ra2 microglia (expressing CFP-gp91phox) were homogenized, and the post-mitochondrial supernatant overlaid a continuous 10–45% sucrose gradient for ultracentrifugation. Collected fractions were Western-blotted to detect cyt b558 or other antigens as indicated. Note that ∼50% of cyt b558 immunoreactivity resides in fractions 5–7 (indicated by red lines) that only slightly overlap the early endosomal markers TfnR, Rab5, and VAMP-3. Also note than cyt b558 is virtually excluded from light fractions 9–11 containing ER (Grp78) and plasma membrane (identified by cell surface biotinylation). A CFP-gp91phox fusion protein distributed as endogenous gp91phox. B and C, Ra2 microglia endocytosed Tfn-HRP for 60 min and then a post-nuclear homogenate was prepared, which was subjected to DAB reaction (with or without activator H2O2) before ultracentrifugation of the homogenate on a 15–45% sucrose gradient. Collected fractions were analyzed by Western blotting using anti-gp91phox, -p22phox, and -TfnR antibodies. Note that DAB and H2O2 to a much larger extent ablates immunoreactivity of TfnR than cyt b558 subunits. The blots are representative of two independent experiments. D, gel from an experiment as performed above was Coomassie Blue-stained to reveal protein abundance in the fractions.
To obtain a quantitative estimate of the degree of physical co-localization of cyt b558 and TfnR, a marker of early endosomes, we made use of the so-called HRP/DAB ablation technique (33). DAB permeates membranes and in the presence of H2O2 and peroxidase activity reacts to form a dense polymer, causing a density-dependent loss of vesicles/organelles in the ensuing sucrose gradient centrifugation step by pelleting. Ra2 microglia were incubated with HRP-conjugated Tfn for a total of 60 min at 37 °C to load early endosomes. A post-nuclear homogenate was then prepared by sonication, and this was DAB-treated (with or without activator H2O2 added) and fractionated by velocity sucrose gradient ultracentrifugation. Collected fractions were analyzed by Western blotting for the presence of cyt b558 subunits or TfnR (Fig. 6, B and C). In control homogenates (not exposed to H2O2), cyt b558 subunits and TfnR were distributed in overlapping fractions roughly in the middle of the gradient separated from soluble proteins and plasma membrane, which was contained within fractions 9–12. However, although H2O2/DAB ablation caused a 63 ± 6% reduction of the cumulated TfnR Western blot band intensity of all fractions, gp91phox experienced only a 16 ± 1% decrease (n = 2, mean ± S.E.). The slightly skewed distribution of cyt b558 subunits in DAB control versus DAB/H2O2-treated homogenates was consistently observed and is not due to variation in sucrose gradients, but rather it is the result of background DAB reaction causing a slight density increase of all organelles. In conclusion, although cyt b558 is internalized through the CCP pathway, it is not accessible to internalized Tfn, which occupies early endosomes (sorting and recycling endosomes).
GTPγS Stimulation of SLO-permeabilized Ra2 Microglia Cause Exocytosis of cyt b558 to the Cell Membrane
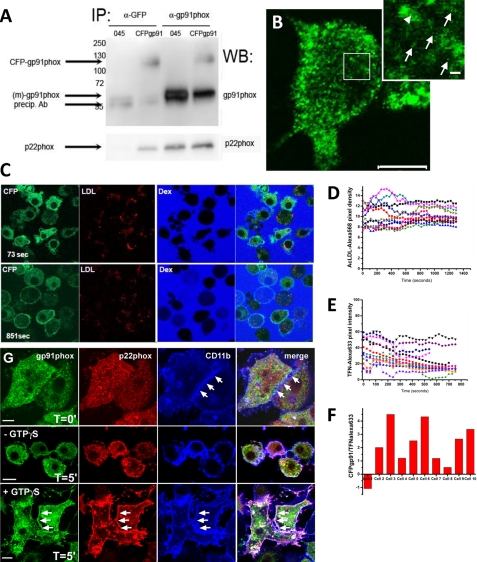
To explore exocytosis of the NADPH oxidase storage vesicles, we transduced Ra2 cells to conditionally express a CFP-gp91phox fusion protein with CFP fused to the N terminus of human gp91phox (26). CFP-gp91phox was expressed as a protein with the correctly deduced molecular mass in Ra2 microglia (∼120 kDa); it co-precipitated with anti-p22phox antibodies indicating heterodimerization (Fig. 7A), and it distributed as endogenous mouse gp91phox by cell fractionation (Fig. 6A). For the morphological analysis, CFP-gp91phox was induced with tetracycline overnight followed by 24 h of chase in tetracycline-free medium to avoid a sizeable pool of newly synthesized CFP-gp91phox in biosynthetic compartments. By confocal microscopy, CFP-gp91phox showed a predominantly intracellular, punctate distribution in resting Ra2 microglia similar to endogenous gp91phox, but weak cell surface staining could also be seen in a proportion of the cells (Fig. 7B). We initially performed time lapse imaging of CFP-gp91phox in SLO-permeabilized Ra2 microglia stimulated with GTPγS. To be able to correlate trafficking of CFP-gp91phox with endosomal mobilization and exocytosis, we pre-loaded the cells with either fluorophore-conjugated Ac-LDL to load lysosomes or Tfn to load early endosomes. We performed the experiment in a KCl-based permeabilization buffer without calcium and with 1 mm EGTA to avoid rapid clearance of SLO from the plasma membrane (34), which caused the internalization of cyt b558 into large vacuolar structures (data not shown). Fig. 7, C and D, shows that under conditions of marked mobilization of CFP-gp91phox to the plasma membrane there was no equivalent loss of lysosomal Ac-LDL. Similarly, although Alexa 633-conjugated Tfn was partially lost during the ∼10 min of time lapse, this corresponds to normal recycling of Tfn, and there was no correlation between Tfn exocytosis and trafficking of CFP-gp91phox to the plasma membrane (Fig. 7, E and F). SLO permeabilization as performed here causes the gradual washing out of the cytosol. We therefore also tried a more gentle and reversible SLO permeabilization protocol (29). In this protocol, GTPγS is loaded concurrently with SLO treatment in calcium-free buffer (as above), but after brief permeabilization and loading, cells were shifted to a calcium-containing buffer at 4 °C, and resealing of SLO pores in the plasma membrane was allowed to take place. The now resealed and intact cells were then chased at 37 °C in HBSS with calcium. As shown in Fig. 7G, 5 min of chase at 37 °C caused the GTPγS-dependent exocytosis of a major fraction of cyt b558 to the cell surface. In conclusion, cyt b558-containing vesicles can be induced to undergo exocytosis under conditions where neither lysosomes or early endosomes are exocytosed.
FIGURE 7.
cyt b558-containing vesicles are exocytosed by GTPγS in SLO-permeabilized Ra2 microglia. A, immunoprecipitates (IP) prepared from control or CFP-gp91phox-expressing Ra2 microglia using either anti-GFP or anti-gp91phox antibodies were analyzed by either anti-gp91phox (top panel) or anti-p22phox (lower panel) antibodies. Molecular weight markers are indicated on the left. Arrows indicate endogenous mouse gp91phox around 58 kDa (unglycosylated) and CFP-(human) gp91phox migrating at ∼120 kDa. WB, Western blot. B, distribution of CFP-gp91phox in Ra2 microglia visualized by immunofluorescence using an anti-GFP antibody. Shown is a medial confocal plane showing predominantly intracellular localization of CFP-gp91phox in larger endosome-like structures and in a grainy punctate appearance. In the enlarged inset, corresponding to the outlined box, individual puncta are marked by arrows, although a potential budding event from endosomes is marked by arrowheads. Bar, 10 μm; bar inset, 500 nm. C, Ra2 cells expressing CFP-gp91phox were allowed to internalize DiI-conjugated acetylated LDL for an hour followed by a chase to lysosomes for 2–3 h. The cells were then SLO-permeabilized at time point 0 in the presence of 400 nm GTPγS and 100 μm ATP, as well as Alexa 633-conjugated dextran (Mr 10,000) to mark permeabilized cells. After stimulation, the fluorescence signal of CFP-gp91phox was followed by time lapse (time shown in lower left corner of each row) for up to 15 min. Dex, dextran. D, fluorescence intensity of DiI-conjugated acetylated LDL during an experiment as performed in C. Individual traces of 10 cells are shown and are representative of two individual experiments. E, fluorescence intensity of Alexa 633-conjugated Tfn during an experiment as performed in C, except cells were loaded with Alexa 633-conjugated Tfn (instead of Ac-LDL) for 1 h and used immediately for imaging. Individual traces of 10 cells are shown and are representative of two individual experiments. F, translocation of CFP-gp91phox to the plasma membrane was assessed at the end of the time-lapse experiment as the fraction of total CFP-gp91phox fluorescence intensity residing in the plasma membrane as determined by defining regions of interest of individual cells using the LSM510 software. For individual cells, this value was then divided by the fractional loss of Alexa 633-conjugated Tfn during the time period of observation to obtain a ratio of CFP-gp91phox (plasma membrane) to exocytosed Tfn, which is depicted on the y axis in arbitrary values for 10 individual cells. G, Ra2 microglia were SLO-permeabilized with or without GTPγS present at 37 °C as above for pore formation before change to HBSS for 60 min at 4 °C to allow resealing. Cells were then incubated at 37 °C cells for 5 min before fixation and immunofluorescence with mouse anti-gp91phox mAb (green), rabbit anti-p22phox antibodies (red), or rat anti-CD11b antibodies (blue). Note robust GTPγS-mediated redistribution of cyt b558 to the cell surface marked by CD11b (arrows). Bars, 10 μm.
ATP, TNFα, and CD40L Induce Exocytosis of cyt b558-containing Vesicles Followed by Re-internalization
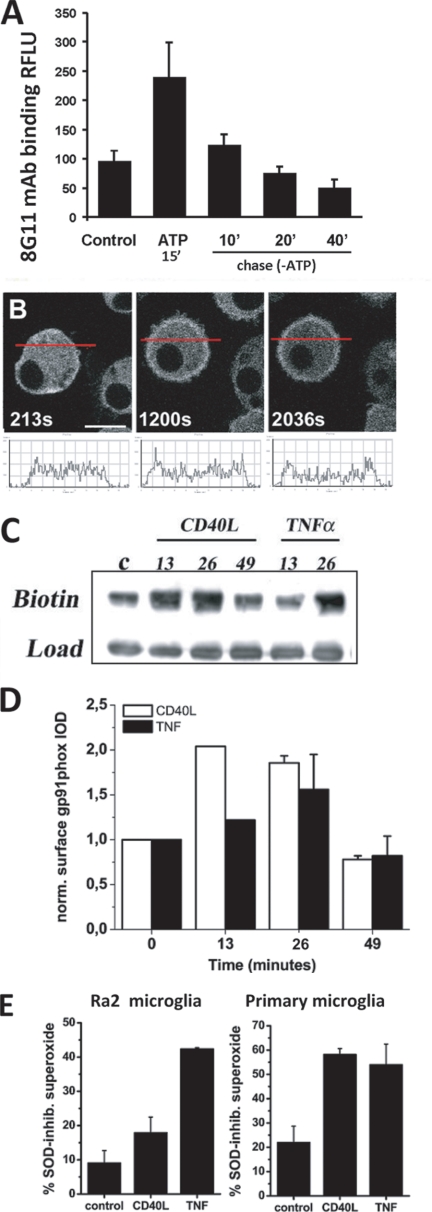
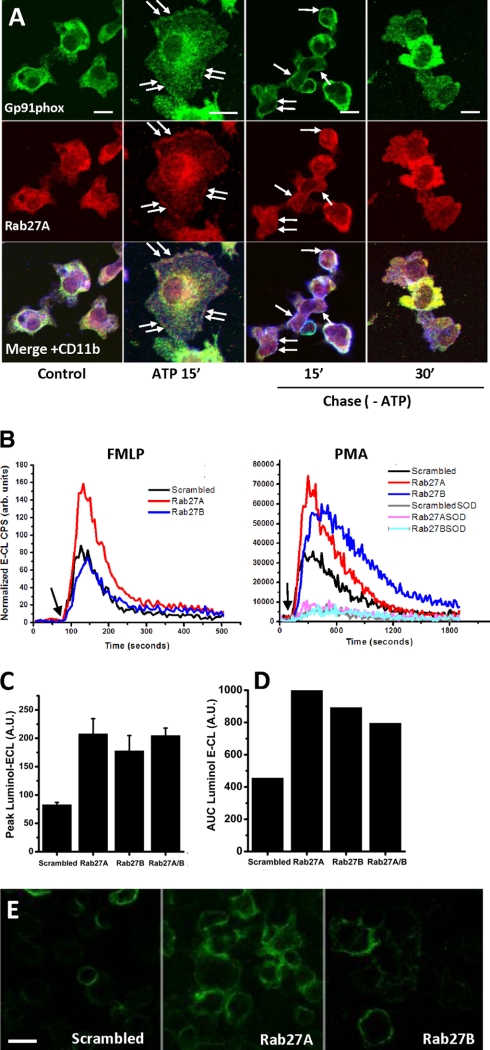
Having established the potential for exocytosis of the cyt b558-containing compartment, we next sought to identify physiological agonists of cyt b558 mobilization. During our SLO trials, we observed that some cells seemingly not permeabilized (no dextran) nevertheless showed redistribution of CFP-gp91phox to the surface (see Fig. 7C). The KCl buffer in addition to GTPγS contains ATP at 100 μm. ATP is known to induce exocytosis of small “enlargeosome” vesicles in different cell types (35) and is known to affect microglia chemotaxis through purinergic receptors (36, 37). As shown in Fig. 8A, 1 mm ATP stimulation for 15 min resulted in a 2–3-fold increased expression of endogenous gp91phox on the cell surface of Ra2 microglia as measured by flow cytometry-based detection of mAb 8G11 binding to an external epitope of gp91phox (22). Also by time-lapse confocal microscopy of Ra2 cells expressing the CFP-gp91phox fusion protein, we could observe a translocation of gp91phox to the cell surface 10–20 min after ATP stimulation (Fig. 8B; see also supplemental video 1). Subsequent chase in ATP-free medium caused the surface level of gp91phox to drop back to slightly below control levels in the course of 40 min suggesting re-internalization of cyt b558 (Fig. 8A).
FIGURE 8.
ATP, TNF-α, and CD40L induce exocytosis of cyt b558-containing vesicles to the cell surface, which is followed by re-internalization of cyt b558. A, Ra2 microglia were stimulated with 1 mm ATP for 15 min and then chased in the absence of ATP for a further 10, 20, or 40 min. Then cell surface levels of gp91phox were determined by a FACS protocol on fixed unpermeabilized cells using 8G11, an anti-gp91phox mAb with an extracellular epitope on gp91phox. The graph depicts background-subtracted (isotype-matched control) mean fluorescence intensity of 8G11 staining normalized to unstimulated control Ra2 cells and represents mean ± S.E. (n = 3). RFLU, relative fluorescent light units. B, Ra2 cells expressing CFP-gp91phox were stimulated with 1 mm ATP, and live cell imaging was performed. Shown are 1.0-μm confocal medial sections of a single cell over time (indicated in seconds) before and after ATP stimulation (at 250 s). Note that CFP-gp91phox fluorescence is redistributed in part from cytosol to the cell surface. A histogram of CFP fluorescence intensity as a function of linear distance across the cell (red line in images) is depicted below each confocal image. Bar, 10 μm. C, cell surface expression of gp91phox in Ra2 microglia after stimulation with 100 ng/ml CD40L or 20 ng/ml TNFα was followed over time (indicated in minutes) by cell surface biotinylation with NHS-biotin. The anti-gp91phox Western blot shown is representative of three independent experiments and shows streptavidin-precipitate (upper lanes) and cell lysate load control (C, lower lanes). D, mean ± S.E. (n = 3) densitometric values for gp91phox Western blots as shown above. The ordinate in arbitrary units represents gp91phox band optical density of streptavidin-precipitates normalized to load control. E, PMA-evoked production of superoxide by Ra2 microglia or primary microglia stimulated with CD40L or TNFα was estimated by NBT reduction assay with or without inclusion of 400 units/ml SOD. The ordinate represents the fraction of SOD-sensitive (extracellular) to total NBT (no SOD) absorbance at 570 nm after 15 or 30 min of PMA stimulation for CD40L and TNFα, respectively. Mean ± S.E. (n = 3) is shown.
TNFα is known to evoke increased expression of cyt b558 on the surface of neutrophils (38), and we therefore also tested the efficacy of TNF-α or the family member CD40L as secretagogues of cyt b558 in Ra2 microglia. Using a cell surface biotinylation protocol to quantitate cell surface expression of gp91phox after stimulation, we found that both proinflammatory agents, albeit with different kinetics, caused an up-regulation of cyt b558 on the plasma membrane, followed by down-regulation to a level slightly below base line (Fig. 8, C and D). Finally, we performed a simple microplate-based NBT test to evaluate the consequences of TNF-α or CD40L-induced cyt b558 exocytosis for the release of superoxide to the surroundings. Fig. 8E shows that CD40L (15 min) and TNF-α (30 min) stimulation caused the SOD-sensitive fraction of the total NBT absorbance to increase 2–3-fold both in Ra2 microglia and primary microglia, demonstrating a significantly increased release of superoxide to the external environment.
Internalized cyt b558 Is Not Transported to Lysosomes
To obtain a morphological correlate of re-internalization, we incubated GM-CSF-stimulated Ra2 microglia expressing human CFP-gp91phox with FITC-conjugated anti-gp91phox mAb 7D5 in the absence or presence of 100 μm ATP for 1 h. As shown in Fig. 9A, mAb 7D5 labeled the cell surface of a small fraction of control cells but showed no or limited internalization. In contrast, ATP-stimulated Ra2 microglia showed a punctate pattern of mAb 7D5 distribution inside a large fraction of the cells indicative of cyt b558 endocytosis. To determine the fate of endocytosed cyt b558, we established an assay where GM-CSF-starved Ra2 microglia (allowing a fraction of cyt b558 to return to the cell surface; see supplemental Fig. 3) were allowed to internalize mAb 7D5 together with either Alexa-conjugated Tfn or AcLDL for 6 h. As shown in Fig. 9B, internalized cyt b558 was partially contained in recycling endosomes containing Tfn, and this co-localization could be increased by 20–50 nm bafilomycin A1, which retards exit from the recycling compartment about 2-fold (39). However, most of cyt b558 was present in organelles or vesicles negative for either Rab5 (sorting endosomes) or Tfn (recycling endosomes). In the presence of bafilomycin, which also blocks transport from late endosomes to lysosomes, we saw little co-localization of cyt b558 with late endosomal marker mannose phosphate receptor, indicating that cyt b558 exits the endosomal pathway before late endosomes. To verify this, we incubated Ra2 cells with either mAb 7D5, anti-TfnR (also recognizing an external epitope), or isotype-matched control anti-CD3e antibodies together with Alexa 594-conjugated AcLDL in the presence of leupeptin and pepstatin to block lysosomal degradation. Fig. 9C shows that anti-gp91phox and -TfnR antibodies only to a limited extent co-localized with AcLDL in lysosomes, although anti-CD3ϵ mAb (likely taken up by FcγRs) was present mainly in lysosomes.
FIGURE 9.
Internalized cyt b558 passes through recycling endosomes and is not routed for lysosomal degradation. A, Ra microglia expressing CFP-gp91phox were left untreated or stimulated with 100 μm ATP in the presence of anti-gp91phox mAb 7D5 for 1 h and then processed for immunofluorescence to visualize CD11b and nucleus (ToPro-3; blue). In control cells, scant surface label may be present (arrowheads), but mainly in ATP-stimulated cells there is internalized 7D5 mAb (arrows). Bars, 10 μm. B, GM-CSF-starved Ra2 microglia expressing (h)gp91phox and (h)TfnR were incubated for 6 h in culture medium containing 2 μg/ml 7D5 mAb and Alexa 633-conjugated Tfn with or without 50 nm bafilomycin A1. Cells were processed for immunofluorescence to visualize internalized 7D5 mAb and Rab5 or mannose phosphate receptor (MPR). Note that the limited overlap of internalized 7D5 mAb with the Tfn-positive recycling compartment in the control situation is enhanced by bafilomycin A1. Boxed rectangles in merged pictures are shown at higher magnification on right. Bars, 10 μm. C, GM-CSF-starved Ra2 microglia expressing (h)gp91phox were incubated for 6 h in culture medium containing either 2 μg/ml 7D5, anti-TfnR, or anti-CD3ϵ mAb together with 1 μg/ml AcLDL and leupeptin/pepstatin. Note that internalized 7D5 and anti-TfnR mAbs are segregated away from AcLDL-positive lysosomes but that control antibody anti-CD3ϵ co-localizes with AcLDL. Boxed rectangles in merged pictures are shown at higher magnification on right. Bars, 10 μm.
Rab27A and -B Regulate cyt b558 Vesicle Mobilization during Phagocytosis of IgG-opsonized Targets
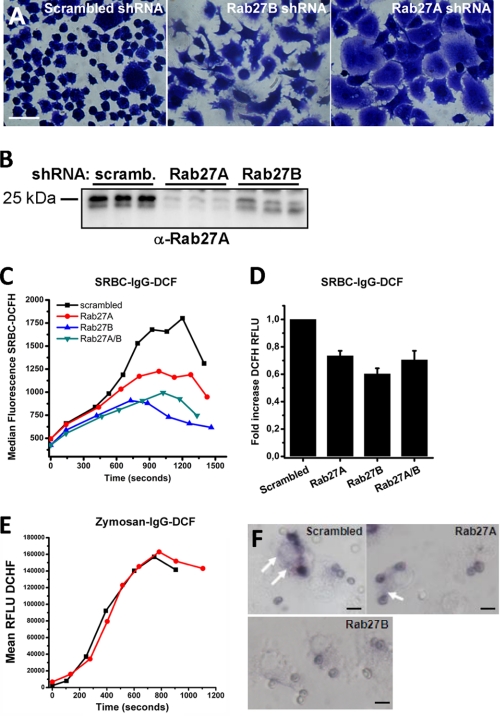
In neutrophils and dendritic cells, Rab27A regulates cyt b558 trafficking to phagosomes (30, 32) in an opsonin-dependent fashion (40). By shRNA silencing, we therefore addressed the functional significance of Rab27 with regard to cyt b558 recruitment and oxidant production in phagosomes. This was analyzed by a flow cytometry-based assay in Ra2 microglia (41) following phagocytosis of Alexa 633- and H2DCF-conjugated IgG-opsonized sheep erythrocytes (SRBC) or zymosan particles. Knockdown of Rab27A or Rab27B resulted in marked morphological phenotypes (Fig. 10A), distinct from Ra2 control cells transduced with noncoding shRNA or untreated Ra2 microglia. Efficient knockdown of Rab27A could be verified with specific anti-Rab27A mAb 4B12 (42), but we were unable to identify a specific anti-Rab27B antibody suitable for Western blotting (Fig. 10B). As seen in Fig. 10, C and D, knockdown of either Rab27A or -B inhibited phagosomal superoxide generation following uptake of IgG-SRBC with up to 40%, but no additive effects were obtained by co-transduction with both shRNA vectors. In contrast, the oxidative burst during phagocytosis of IgG-opsonized zymosan, which in addition to FcγRs also binds and/or signals through mannose receptor and CR3, was unaffected by Rab27A knockdown (Fig. 10E). As a morphological correlate, we exposed Ra2 microglia to IgG-SRBC in the presence of NBT. Fig. 10F shows a reduced deposition of formazan in phagosomes in Rab27A and, in particular, Rab27B knocked down cells, and in addition the extra-phagosomal formazan precipitation was strongly reduced in Rab27B knocked down cells.
FIGURE 10.
Rab27A and -B co-translocate with cyt b558 in stimulated cells and regulate phagosomal superoxide production in response to IgG-opsonized targets. A, Ra2 microglia transduced with scrambled, Rab27B, or Rab27A shRNA lentivectors were fixed and Coomassie Blue-stained to show morphological phenotype. B, cell lysates of Ra2 microglia transduced with shRNA vectors as above were Western-blotted with anti-Rab27A mAb 4B12. The Western blot shows results from three independent knockdown experiments. C, Ra2 microglia transduced as specified were challenged in suspension with IgG-opsonized Alexa 633- and DCF-conjugated SRBC, and development of H2DCF fluorescence was followed for the population of cells with bound SRBC. The graph shows median H2DCF fluorescence over time from a single representative experiment out of four. D, fold increase in H2DCF fluorescence (from start to maximum) of SRBC in experiments carried out as in B. mean ± S.E. of four independent experiments is shown. RFLU, relative fluorescent light units. E, Ra2 microglia transduced with control or Rab27A shRNA were challenged with IgG-opsonized Alexa 633- and H2DCF-conjugated zymosan particles, and fluorescence was followed over time. The graph shows median DCF fluorescence over time from a single representative experiment out of three. F, NBT assay of IgG-opsonized SRBC-challenged Ra2 cells expressing scrambled, Rab27A, or Rab27B shRNA. Note that there is superoxide production at locations some distance from phagosomes in control and Rab27A shRNA cells (arrows), although this phenomenon is suppressed in Rab27B shRNA-expressing cells, which also show a diminished formazan deposition in phagosomes. Bars, 10 μm.
Rab27A and -B Regulate Cell Surface Expression of cyt b558
In neutrophils Rab27A has been reported to regulate exocytosis of cyt b558-containing compartments (32). Therefore, we performed additional studies to address the role of Rab27 in response to soluble stimuli. Fig. 11A shows that Rab27A in ATP-stimulated Ra2 microglia co-translocated to the plasma membrane with gp91phox and subsequently, following withdrawal of ATP, disappeared from the cell surface (as did cyt b558). We then measured both fMLP and PMA-stimulated superoxide generation in Ra2 microglia transduced with Rab27A or -B shRNA vectors. As evidenced in Fig. 11B, the fMLP response, which is a measure of the acute expression of NADPH oxidase on the cell surface (because the response is so fast and short lived), was approximately doubled in Rab27A knocked down cells (compared with control cells transduced with noncoding shRNA), although Rab27B knockdown had no effect. In contrast, PMA worked on a post-receptor level to induce intracellular signaling events, many of them relating to endosome dynamics, and gives rise to a slower and long lasting oxidative burst. Both Rab27A and -B knocked down cells showed a more than 2-fold increased superoxide generation following PMA stimulation, with the peak of superoxide production in Rab27B knocked down cells always lagging slightly behind cells expressing control or Rab27B shRNA (Fig. 11, B–D). As expected the relative levels of superoxide production following fMLP stimulation corresponded well to the expression levels of (h)gp91phox on the cell surface detected with mAb 7D5 (Fig. 11E).
FIGURE 11.
Rab27A and -B regulate expression of cyt b558 on the cell surface in response to soluble stimuli. A, Ra2 microglia were left untreated or stimulated with 1 mm ATP for 15 min and then chased in the absence of ATP for 15 or 30 min at 37 °C. Immunofluorescence to localize Rab27A, gp91phox, and CD11b was then carried out. Arrows indicate co-localization of Rab27A and gp91phox on the cell surface delineated by CD11b staining. Bars, 10 μm. B, superoxide production was measured by luminol-ECL in Ra2 microglia transduced with noncoding, Rab27A, or Rab27B shRNA lentivectors and stimulated in suspension with 4 μm fMLP or 100 ng/ml PMA (arrows). The graphs show in arbitrary units chemiluminescent cps normalized to control (scrambled shRNA) cells over time and are representative of four individual experiments. For PMA-stimulated cells parallel wells additionally containing 200 units/ml superoxide dismutase (SOD) were included to confirm extracellular release of superoxide. C, normalized mean ± S.E. (n = 4) peak superoxide production for PMA-stimulated cells as carried out in B. A.U., arbitrary units. D, normalized mean cumulative superoxide production (area under curve (AUC)) for PMA-stimulated cells as in B. E, Ra2 microglia expressing (h)gp91phox were transduced with scrambled, Rab27A, or Rab27B shRNA vectors and then surface-labeled on ice with anti-(h)gp91phox FITC-conjugated mAb 7D5. Bars, 20 μm.
DISCUSSION
We show here in different tissue macrophages that activation, here induced with LPS and GM-CSF, causes redistribution of cyt b558 from the cell surface to a Rab27A and -B-regulated exocytic storage compartment.
Regulated Clathrin-coated Pit Endocytosis of NADPH Oxidase
Based on our current evidence, we cannot formally rule out that agonist-induced redistribution of NADPH oxidase in primary macrophages is a consequence of internalization of surface-resident cyt b558 followed by lysosomal degradation concurrent with de novo synthesis and altered biosynthetic sorting of cyt b558 to secretory vesicles. However, we never observed any appreciable rough ER localization of cyt b558, and the pronounced co-localization of cyt b558 with clathrin-coated pits and vesicles during this time period indicates that the 100 nm storage vesicles containing cyt b558 constitute a post-endocytic compartment. Such a phenomenon has been described previously in neutrophils, which contain internalized cyt558, CD11b, and serum albumin in so-called secretory vesicles, which constitute the most labile secretory storage compartment of these cells (15, 43). LPS and GM-CSF-induced effects specifically affected cyt b558 inasmuch as the cell surface expression of other phagocyte cell surface antigens CD11b (integrin αM) and CD16/32 (FcγRIIA/III) was left unaltered. As mentioned, the long term culture of immature macrophages without addition of exogenous stimuli also results in cyt b558 redistribution, indicating that redistribution likely is a consequence of macrophage activation rather than an effect specifically related to LPS or GM-CSF. Blockade of CCP internalization by expression of dominant negative EPS15-EH29 increased the cell surface levels of cyt b558 in Ra2 microglia. A CCP internalization route of cyt b558 may seem surprising as cyt b558 on the plasma membrane resides in cholesterol-rich membrane microdomains (lipid rafts) (12), which is sometimes taken to indicate internalization by routes other than the CCP pathway. However, we and others have shown that distribution of membrane proteins in lipid rafts does not preclude endocytosis through coated pits (28, 44, 45). As estimated from the endocytic uptake of mAbs aimed at external epitopes of gp91phox or TfnR, it is clear that the rate of internalization of cyt b558 is severalfold lower than that of a typical recycling surface receptor such as TfnR, perhaps because CCP internalization of cyt b558 requires diffusion out of lipid rafts. Subunit gp91phox contains potential internalization consensus sequences for CCP endocytosis within its cytosolic domains (201YFEV and 456DLLQLL), but preliminary analysis by site-directed mutagenesis and expression in COS cells containing p22phox failed to show any significance of these sites (see supplemental Fig. 5).
Therefore, we speculate that LPS and GM-CSF induce de novo synthesis of internalization or sorting co-receptors that regulate the association of cyt b558 subunits with CCPs. Alternatively, cell activation may induce post-translational modification of cyt b558 subunits by phosphorylation, SUMOylation, or ubiquitination, for example, to allow conditional interaction with CCP-associated protein adaptors.
Stimulated Exocytosis of cyt b558-containing Vesicles
As indicated by the very modest internalization of mAb 7D5 in GM-CSF-stimulated microglia, cyt b558 does not cycle between internal stores and the plasma membrane under basal conditions. However, GM-CSF deprival or stimulation with soluble agonists caused a fraction of cyt b558 to escape from internal stores to the cell surface where it could be re-internalized (tagged by 7D5 mAb). In the brain ATP is an important mediator of paracrine signaling after release from injured or dying neurons, and ATP is a principal chemoattractant of microglia (36, 37). Interestingly, ATP-stimulated microglia modulate neuropathic pain perception through NADPH oxidase activity, which is required in part for cytokine expression (46, 47). Here, we found that 100 μm ATP caused exocytosis of cyt b558-containing vesicles to the plasma membrane of microglia followed by rapid re-internalization. Endosomal localization of NADPH oxidase may be highly relevant to redox signaling (9, 13, 48) and cytokine expression. Higher concentrations of ATP (1 mm) caused a more sustained up-regulation of cyt b558 on the cell surface, and also pro-inflammatory mediators TNF-α and CD40L induced cyt b558 expression on the surface in accord with their documented role in neutrophils (38). As a consequence, the fraction of total superoxide production released to the external environment after NADPH oxidase activation was expectedly increased, which in vivo should be relevant for the magnitude of oxidative tissue damage incurred during chronic inflammatory reactions.
The cyt b558-containing storage compartment was also readily redistributed to phagosomes, and in accord with data for neutrophils (40), we implicate both Rab27A and B in this process for IgG-opsonized targets specifically; the knockdown of Rab27A had no effect on phagosomal oxidant production following internalization of zymosan. In line with pioneering observations by Kobayashi et al. (16) and more recent results (40), we also observed superoxide production in vacuolar membrane compartments some distance from phagosomes, presumably because of fusion activity of cyt b558-containing vesicles before final fusion with the phagosomal membrane or cell surface. In particular, Rab27B activity seemed to be required for extra-phagosomal oxidant production. This pre-exocytosis fusion activity is reminiscent of the role of Rab27A in cytotoxic T-lymphocytes, where it regulates the fusion of lysosome-related cytotoxic granules with endosomal elements before final fusion with the plasma membrane (49).
What Is the Nature of cyt b558-containing Vesicular Compartment?
On the basis of Tfn-HRP accessibility and morphological co-localization studies, our results indicate that the intracellular pool of cyt b558 in macrophages is divided in two as follows: a major fraction of cyt b558 is contained in a compartment inaccessible to transferrin and negative for markers of endosomal and biosynthetic organelles, and a minor fraction of cyt b558 (<20%) is contained in early endosomes also containing internalized Tfn and positive for Rab5, Rab11, and/or VAMP-3. This distribution is similar to that of glucose transporter-4 (GLUT-4), which in muscle and adipose cells traffics in an insulin-regulated manner between the cell surface and exocytic storage vesicles, but it is also partially contained in early (recycling) endosomes (50, 51).
Rab27A regulates mobility and exocytosis of lysosome-related organelles in different cell types, and specifically Rab27A/B has been shown to promote trafficking of NADPH oxidase to the cell surface in neutrophils (32) or phagosomes in dendritic cells and neutrophils (30, 40). Rab27 is thought to mediate SNAP/SNARE assembly for vesicle fusion in neutrophils (31, 52), but in other cell types Rab27 regulates vesicle mobility through association with the microtubuli (53) or actin cytoskeleton (54, 55). In PMA or fMLP-stimulated Ra2 microglia, our results are clearly in accord with the latter role of Rab27, as removal of either Rab27A or -B by shRNA knockdown resulted in enhanced exocytosis of cyt b558 to the cell surface (with different kinetics). Thus, Rab27A negatively regulated cyt b558 surface expression and fMLP-stimulated superoxide production (only the surface pool of NADPH oxidase is activated), likely by tethering cyt b558-containing vesicles to the cortical actin cytoskeleton (55), which acts as a barrier to fusion of vesicles with the plasma membrane (56). This corroborates with the ability of latrunculin, which depolymerizes cortical F-actin, to greatly increase fMLP- and PMA-induced release of superoxide to the surroundings (57). In contrast, Rab27B may be involved with the transition from microtubule to actin-based translocation as proposed in mast cells (54) as an effect was only seen after PMA stimulation, which is known to cause mobilization also of intracellular stores of NADPH oxidase.
Exocytosis of cyt b558 to the cell surface by GM-CSF deprival or ATP, TNFα, or CD40L stimulation was followed by re-internalization of cyt b558. Tagging of surface-exposed gp91phox with mAb 7D5 demonstrated that internalized cyt b558 was not delivered to lysosomes for degradation but rather localized to Tfn-positive recycling endosomes and compartments of currently unknown identity. More sophisticated methods will have to be implemented to determine whether cyt b558 traffics through recycling endosomes before being returned to the vesicular storage compartment for repeated rounds of cyt b558 trafficking between cell surface and storage compartments. If so, the storage compartment described here would be a likely candidate member to be included in the family of “nonsecretory”-type of exocytic vesicles (58), whose primary function is modification of the cell surface rather than secretion of soluble compounds. Examples include the GLUT-4-containing vesicles of muscle and fat cells, and the water channel (aquaporin)-containing vesicles of kidney tubule epithelium.
Supplementary Material
Acknowledgments
We greatly appreciate the expert help of Izabela Rasmussen with cell culture and electrophoresis and Mette Ohlsen with electron microscopy.
This work was supported by NOVO Nordisk Foundation Grant 50-65074, The Aase and Ejnar Danielsens Foundation Grant 105560, and The Desiree and Niels Yde Foundation (to F. V.).

This article contains supplemental Figs. 1–5, “Materials and Methods,” and additional references.
- cyt b558
- cytochrome b558
- AcLDL
- acetylated low density lipoprotein
- fMLP
- formyl-methionyl-leucine-phenylalanine
- H2DCF
- 2′7′-dichlorofluorescein
- HBSS
- Hanks' buffered saline solution
- PMA
- phorbol 12-myristate 13-acetate
- SLO
- streptolysin-O
- SRBC
- sheep red blood cells
- Tfn
- transferrin
- TfnR
- transferrin receptor
- SOD
- superoxide dismutase
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- NBT
- nitro blue tetrazolium
- DAB
- diaminobenzidine
- NHS
- N-hydroxysuccinimide
- ER
- endoplasmic reticulum
- BMDM
- bone marrow-derived macrophage
- CFP
- cyan fluorescent protein
- CCP
- clathrin-coated pit
- DiI
- 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- h
- human.
REFERENCES
- 1. Nauseef W. M. (2004) Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 122, 277–291 [DOI] [PubMed] [Google Scholar]
- 2. Vignais P. V. (2002) The superoxide-generating NADPH oxidase. Structural aspects and activation mechanism. Cell. Mol. Life Sci. 59, 1428–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diebold B. A., Bokoch G. M. (2001) Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat. Immunol. 2, 211–215 [DOI] [PubMed] [Google Scholar]
- 4. Lambeth J. D. (2004) NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 [DOI] [PubMed] [Google Scholar]
- 5. Bedard K., Krause K. H. (2007) The NOX family of ROS-generating NADPH oxidases. Physiology and pathophysiology. Physiol. Rev. 87, 245–313 [DOI] [PubMed] [Google Scholar]
- 6. Chen K., Kirber M. T., Xiao H., Yang Y., Keaney J. F., Jr. (2008) Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 181, 1129–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu R. F., Ma Z., Liu Z., Terada L. S. (2010) Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell. Biol. 30, 3553–3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hilenski L. L., Clempus R. E., Quinn M. T., Lambeth J. D., Griendling K. K. (2004) Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 24, 677–683 [DOI] [PubMed] [Google Scholar]
- 9. Li Q., Zhang Y., Marden J. J., Banfi B., Engelhardt J. F. (2008) Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem. J. 411, 531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diaz B., Shani G., Pass I., Anderson D., Quintavalle M., Courtneidge S. A. (2009) Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci. Signal. 2, ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ushio-Fukai M. (2006) Localizing NADPH oxidase-derived ROS. Sci. STKE 2006, re8. [DOI] [PubMed] [Google Scholar]
- 12. Vilhardt F., van Deurs B. (2004) The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J. 23, 739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oakley F. D., Smith R. L., Engelhardt J. F. (2009) Lipid rafts and caveolin-1 coordinate interleukin-1β (IL-1β)-dependent activation of NFκB by controlling endocytosis of Nox2 and IL-1β receptor 1 from the plasma membrane. J. Biol. Chem. 284, 33255–33264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Babior B. M. (2000) Phagocytes and oxidative stress. Am. J. Med. 109, 33–44 [DOI] [PubMed] [Google Scholar]
- 15. Calafat J., Kuijpers T. W., Janssen H., Borregaard N., Verhoeven A. J., Roos D. (1993) Evidence for small intracellular vesicles in human blood phagocytes containing cytochrome b558 and the adhesion molecule CD11b/CD18. Blood 81, 3122–3129 [PubMed] [Google Scholar]
- 16. Kobayashi T., Garcia del Saz E., Hendry J., Seguchi H. (1999) Detection of oxidant producing sites in glutaraldehyde-fixed human neutrophils and eosinophils stimulated with phorbol myristate acetate. Histochem. J. 31, 181–194 [DOI] [PubMed] [Google Scholar]
- 17. DeLeo F. R., Allen L. A., Apicella M., Nauseef W. M. (1999) NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 163, 6732–6740 [PubMed] [Google Scholar]
- 18. Gelderman K. A., Hultqvist M., Pizzolla A., Zhao M., Nandakumar K. S., Mattsson R., Holmdahl R. (2007) Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J. Clin. Invest. 117, 3020–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johansson A., Jesaitis A. J., Lundqvist H., Magnusson K. E., Sjölin C., Karlsson A., Dahlgren C. (1995) Different subcellular localization of cytochrome b and the dormant NADPH oxidase in neutrophils and macrophages. Effect on the production of reactive oxygen species during phagocytosis. Cell. Immunol. 161, 61–71 [DOI] [PubMed] [Google Scholar]
- 20. Casbon A. J., Allen L. A., Dunn K. W., Dinauer M. C. (2009) Macrophage NADPH oxidase flavocytochrome b localizes to the plasma membrane and Rab11-positive recycling endosomes. J. Immunol. 182, 2325–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burritt J. B., Quinn M. T., Jutila M. A., Bond C. W., Jesaitis A. J. (1995) Topological mapping of neutrophil cytochrome b epitopes with phage-display libraries. J. Biol. Chem. 270, 16974–16980 [DOI] [PubMed] [Google Scholar]
- 22. Campion Y., Paclet M. H., Jesaitis A. J., Marques B., Grichine A., Berthier S., Lenormand J. L., Lardy B., Stasia M. J., Morel F. (2007) New insights into the membrane topology of the phagocyte NADPH oxidase. Characterization of an anti-gp91-phox conformational monoclonal antibody. Biochimie 89, 1145–1158 [DOI] [PubMed] [Google Scholar]
- 23. Vilhardt F., Plastre O., Sawada M., Suzuki K., Wiznerowicz M., Kiyokawa E., Trono D., Krause K. H. (2002) The HIV-1 Nef protein and phagocyte NADPH oxidase activation. J. Biol. Chem. 277, 42136–42143 [DOI] [PubMed] [Google Scholar]
- 24. Inoue H., Sawada M., Ryo A., Tanahashi H., Wakatsuki T., Hada A., Kondoh N., Nakagaki K., Takahashi K., Suzumura A., Yamamoto M., Tabira T. (1999) Serial analysis of gene expression in a microglial cell line. Glia 28, 265–271 [DOI] [PubMed] [Google Scholar]
- 25. Benmerah A., Poupon V., Cerf-Bensussan N., Dautry-Varsat A. (2000) Mapping of Eps15 domains involved in its targeting to clathrin-coated pits. J. Biol. Chem. 275, 3288–3295 [DOI] [PubMed] [Google Scholar]
- 26. Petry A., Djordjevic T., Weitnauer M., Kietzmann T., Hess J., Görlach A. (2006) NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid. Redox. Signal. 8, 1473–1484 [DOI] [PubMed] [Google Scholar]
- 27. Vutskits G. V., Salmon P., Mayor L., Vutskits L., Cudré-Mauroux C., Soriano J., Montesano R., Maillet P., Sappino A. P. (2006) A role for atm in E-cadherin-mediated contact inhibition in epithelial cells. Breast Cancer Res. Treat. 99, 143–153 [DOI] [PubMed] [Google Scholar]
- 28. Vilhardt F., Nielsen M., Sandvig K., van Deurs B. (1999) Urokinase-type plasminogen activator receptor is internalized by different mechanisms in polarized and nonpolarized Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 10, 179–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walev I., Bhakdi S. C., Hofmann F., Djonder N., Valeva A., Aktories K., Bhakdi S. (2001) Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc. Natl. Acad. Sci. U.S.A. 98, 3185–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jancic C., Savina A., Wasmeier C., Tolmachova T., El-Benna J., Dang P. M., Pascolo S., Gougerot-Pocidalo M. A., Raposo G., Seabra M. C., Amigorena S. (2007) Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat. Cell Biol. 9, 367–378 [DOI] [PubMed] [Google Scholar]
- 31. Brzezinska A. A., Johnson J. L., Munafo D. B., Crozat K., Beutler B., Kiosses W. B., Ellis B. A., Catz S. D. (2008) The Rab27a effectors JFC1/Slp1 and Munc13-4 regulate exocytosis of neutrophil granules. Traffic 9, 2151–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson J. L., Brzezinska A. A., Tolmachova T., Munafo D. B., Ellis B. A., Seabra M. C., Hong H., Catz S. D. (2010) Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic 11, 533–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Livingstone C., James D. E., Rice J. E., Hanpeter D., Gould G. W. (1996) Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3-L1 adipocytes. Biochem. J. 315, 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Idone V., Tam C., Goss J. W., Toomre D., Pypaert M., Andrews N. W. (2008) Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 180, 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cocucci E., Racchetti G., Podini P., Meldolesi J. (2007) Enlargeosome traffic. Exocytosis triggered by various signals is followed by endocytosis, membrane shedding, or both. Traffic 8, 742–757 [DOI] [PubMed] [Google Scholar]
- 36. Haynes S. E., Hollopeter G., Yang G., Kurpius D., Dailey M. E., Gan W. B., Julius D. (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9, 1512–1519 [DOI] [PubMed] [Google Scholar]
- 37. Davalos D., Grutzendler J., Yang G., Kim J. V., Zuo Y., Jung S., Littman D. R., Dustin M. L., Gan W. B. (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 [DOI] [PubMed] [Google Scholar]
- 38. Ward R. A., Nakamura M., McLeish K. R. (2000) Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J. Biol. Chem. 275, 36713–36719 [DOI] [PubMed] [Google Scholar]
- 39. Presley J. F., Mayor S., McGraw T. E., Dunn K. W., Maxfield F. R. (1997) Bafilomycin A1 treatment retards transferrin receptor recycling more than bulk membrane recycling. J. Biol. Chem. 272, 13929–13936 [DOI] [PubMed] [Google Scholar]
- 40. Anderson K. E., Chessa T. A., Davidson K., Henderson R. B., Walker S., Tolmachova T., Grys K., Rausch O., Seabra M. C., Tybulewicz V. L., Stephens L. R., Hawkins P. T. (2010) PtdIns3P and Rac direct the assembly of the NADPH oxidase on a novel, pre-phagosomal compartment during FcR-mediated phagocytosis in primary mouse neutrophils. Blood 116, 4978–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roepstorff K., Rasmussen I., Sawada M., Cudre-Maroux C., Salmon P., Bokoch G., van Deurs B., Vilhardt F. (2008) Stimulus-dependent regulation of the phagocyte NADPH oxidase by a VAV1, Rac1, and PAK1 signaling axis. J. Biol. Chem. 283, 7983–7993 [DOI] [PubMed] [Google Scholar]
- 42. Hume A. N., Collinson L. M., Rapak A., Gomes A. Q., Hopkins C. R., Seabra M. C. (2001) Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 152, 795–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sengeløv H., Nielsen M. H., Borregaard N. (1992) Separation of human neutrophil plasma membrane from intracellular vesicles containing alkaline phosphatase and NADPH oxidase activity by free flow electrophoresis. J. Biol. Chem. 267, 14912–14917 [PubMed] [Google Scholar]
- 44. Kirkham M., Parton R. G. (2005) Clathrin-independent endocytosis: new insights into caveolae and noncaveolar lipid raft carriers. Biochim. Biophys. Acta 1746, 349–363 [DOI] [PubMed] [Google Scholar]
- 45. Hommelgaard A. M., Roepstorff K., Vilhardt F., Torgersen M. L., Sandvig K., van Deurs B. (2005) Caveolae. Stable membrane domains with a potential for internalization. Traffic 6, 720–724 [DOI] [PubMed] [Google Scholar]
- 46. Kim D., You B., Jo E. K., Han S. K., Simon M. I., Lee S. J. (2010) NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc. Natl. Acad. Sci. U.S.A. 107, 14851–14856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coull J. A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., Gravel C., Salter M. W., De Koninck Y. (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 [DOI] [PubMed] [Google Scholar]
- 48. Mumbengegwi D. R., Li Q., Li C., Bear C. E., Engelhardt J. F. (2008) Evidence for a superoxide permeability pathway in endosomal membranes. Mol. Cell. Biol. 28, 3700–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ménager M. M., Ménasché G., Romao M., Knapnougel P., Ho C. H., Garfa M., Raposo G., Feldmann J., Fischer A., de Saint Basile G. (2007) Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat. Immunol. 8, 257–267 [DOI] [PubMed] [Google Scholar]
- 50. Martin S., Tellam J., Livingstone C., Slot J. W., Gould G. W., James D. E. (1996) The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J. Cell Biol. 134, 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hashiramoto M., James D. E. (2000) Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes. Mol. Cell. Biol. 20, 416–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Neeft M., Wieffer M., de Jong A. S., Negroiu G., Metz C. H., van Loon A., Griffith J., Krijgsveld J., Wulffraat N., Koch H., Heck A. J., Brose N., Kleijmeer M., van der Sluijs P. (2005) Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol. Biol. Cell 16, 731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arimura N., Kimura T., Nakamuta S., Taya S., Funahashi Y., Hattori A., Shimada A., Ménager C., Kawabata S., Fujii K., Iwamatsu A., Segal R. A., Fukuda M., Kaibuchi K. (2009) Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Dev. Cell 16, 675–686 [DOI] [PubMed] [Google Scholar]
- 54. Mizuno K., Tolmachova T., Ushakov D. S., Romao M., Abrink M., Ferenczi M. A., Raposo G., Seabra M. C. (2007) Rab27b regulates mast cell granule dynamics and secretion. Traffic 8, 883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Desnos C., Schonn J. S., Huet S., Tran V. S., El-Amraoui A., Raposo G., Fanget I., Chapuis C., Ménasché G., de Saint Basile G., Petit C., Cribier S., Henry J. P., Darchen F. (2003) Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites. J. Cell Biol. 163, 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Muallem S., Kwiatkowska K., Xu X., Yin H. L. (1995) Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J. Cell Biol. 128, 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rasmussen I., Pedersen L. H., Byg L., Suzuki K., Sumimoto H., Vilhardt F. (2010) Effects of F/G-actin ratio and actin turnover rate on NADPH oxidase activity in microglia. BMC Immunol. 11, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chieregatti E., Meldolesi J. (2005) Regulated exocytosis. New organelles for nonsecretory purposes. Nat. Rev. Mol. Cell Biol. 6, 181–187 [DOI] [PubMed] [Google Scholar]
- 59. Sawada M. (January 10, 2007) U. S. Patent 6.673,6,5; JP3410738; EP10/602,234
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.