Background: High levels of all-trans-retinal (atRAL) are associated with photoreceptor degeneration.
Results: atRAL promotes NADPH oxidase-mediated overproduction of intracellular reactive oxygen species.
Conclusion: A cascade of signaling events is demonstrated to underlie the action of atRAL in photoreceptor degeneration in mice.
Significance: Mechanistic elucidation of atRAL-mediated photoreceptor degeneration is essential for understanding the molecular pathogenesis of Stargardt disease and other types of retinal degeneration.
Keywords: Reactive oxygen species (ROS), Retinal metabolism, Retinoid, Vision, Vitamin A
Abstract
Compromised clearance of all-trans-retinal (atRAL), a component of the retinoid cycle, increases the susceptibility of mouse retina to acute light-induced photoreceptor degeneration. Abca4−/−Rdh8−/− mice featuring defective atRAL clearance were used to examine the one or more underlying molecular mechanisms, because exposure to intense light causes severe photoreceptor degeneration in these animals. Here we report that bright light exposure of Abca4−/−Rdh8−/− mice increased atRAL levels in the retina that induced rapid NADPH oxidase-mediated overproduction of intracellular reactive oxygen species (ROS). Moreover, such ROS generation was inhibited by blocking phospholipase C and inositol 1,4,5-trisphosphate-induced Ca2+ release, indicating that activation occurs upstream of NADPH oxidase-mediated ROS generation. Because multiple upstream G protein-coupled receptors can activate phospholipase C, we then tested the effects of antagonists of serotonin 2A (5-HT2AR) and M3-muscarinic (M3R) receptors and found they both protected Abca4−/−Rdh8−/− mouse retinas from light-induced degeneration. Thus, a cascade of signaling events appears to mediate the toxicity of atRAL in light-induced photoreceptor degeneration of Abca4−/−Rdh8−/− mice. A similar mechanism may be operative in human Stargardt disease and age-related macular degeneration.
Introduction
To sustain vision, all-trans-retinal (atRAL),2 released from light-activated visual pigments, including rhodopsin, must be continuously isomerized back to its 11-cis isomer (1). This process occurs by a sequence of reactions catalyzed by membrane-bound enzymes of the retinoid cycle located in rod and cone photoreceptor cell outer segments and the retinal pigmented epithelium (RPE) (2–5). Regeneration of rhodopsin requires 11-cis-retinal (11-cis-RAL) supplied from the RPE, but cone pigments are also regenerated in cone-dominant species by a separate “cone visual cycle” (6–8). A high flux of retinoids through the retinoid cycle, as occurs during intense light exposure, can cause elevated levels of toxic retinoid intermediates, especially atRAL, that can induce photoreceptor degeneration (9). Toxic effects of atRAL include caspase activation and mitochondrial-associated cell death (10), but the precise sequence of molecular events that leads to photoreceptor degeneration remains to be clarified.
Even in the presence of a functional retinoid cycle, A2E, a retinal dimer, and other toxic atRAL condensation products (11–13) accumulate with age (14). These compounds are fluorescent biomarkers of aberrant atRAL metabolism (15). Patients affected by retinal degeneration in age-related macular degeneration, Stargardt disease, or some other retinal diseases feature abnormal accumulation of these atRAL condensation products (16). Mice carrying a double knock-out of the Rdh8 gene, which encodes one of the main enzymes that reduces atRAL in rod and cone outer segments (17), and the Abca4 gene (18, 19), which encodes the transporter of atRAL from the inside to the outside of disc membranes, rapidly accumulate atRAL condensation products and manifest RPE/photoreceptor dystrophy at an early age (20). The similarity of this retinopathy to human age-related macular degeneration makes these Abca4−/−Rdh8−/− mice invaluable for research aimed at ameliorating this devastating blinding disease (10, 21). Mutations in ABCA4 can cause Stargardt macular degeneration (22), cone-rod dystrophy (23), or recessive retinitis pigmentosa (24, 25). Heterozygous mutations in ABCA4 increase the risk of developing age-related macular degeneration as well (16).
Abca4−/−Rdh8−/− mice, which exhibits markedly delayed clearance of atRAL after photobleaching and serves as a model of cone and rod retinal degeneration (10, 21), allowed us to examine in greater detail the molecular pathways involved in the pathogenesis of this retinopathy. Oxidative stress is a major mechanism contributing to photoreceptor cell death in animal models of retinal degeneration, including light-induced retinopathy (26, 27). Tightly regulated low levels of reactive oxygen species (ROS) are needed to mediate physiological functions, including cell survival, growth, differentiation, and metabolism. But excessive production of ROS can damage macromolecules, including DNA, proteins, and lipids (28). Thus, aberrant ROS generation constitutes a major mechanism of pathological cell death.
NADPH oxidase is the main enzymatic source of superoxide and hydrogen peroxide (29), and its product ROS, which is involved in retinal degeneration (30, 31). Rac1, an essential component of the NADPH oxidase complex, is implicated in light-induced retinal degeneration, because Rac1 deficiency partially protects photoreceptor cells against photo-oxidative insult (30). Treatment with the NADPH oxidase inhibitor apocynin (1-(4-hydroxy-3-methoxyphenyl)ethanone (APO)) (32) can protect BALB/c mice from developing light-induced retinal degeneration (30). Moreover, APO is effective in preventing cone cell death in a mouse model of retinitis pigmentosa (31). These findings imply that, by causing oxidative stress, NADPH oxidase is mechanistically involved in the pathogenesis of some types of retinal degeneration.
Although atRAL stimulates the production of superoxide via NADPH oxidase (33, 34), there are observations that such stimulation is not the result of a direct interaction between atRAL and this enzyme (35). PLC activation reportedly occurs prior to NADPH oxidase-dependent ROS production in atRAL-treated neutrophils suggesting that products of PLC enzymatic activity, diacylglycerols and inositol 1,4,5-trisphosphate (IP3), could be the intermediates involved in this pathway (33). IP3 promotes release of Ca2+ from the endoplasmic reticulum into the cytosol through binding to an intracellular IP3-receptor, IP3R (36). This signaling pathway may underlie the previously unexplained observation that atRAL causes a rapid increase in intracellular Ca2+ (10). Ca2+ signaling has also been reported to increase ROS production by NADPH oxidase (37). Because PLC is typically activated by G protein-coupled receptors (GPCRs) coupled to Gq protein (38), specific GPCRs could affect overall PLC activation, thus mediating atRAL- induced toxic effects.
Results from cell culture experiments indicate that atRAL-induced generation of ROS can be mediated through NADPH oxidase. We further investigated the in vivo signaling mechanisms that mediate the action of atRAL in causing ROS production and light-induced photoreceptor degeneration. The results indicate that PLC activation and the resulting second messenger IP3 contribute to atRAL-induced NADPH oxidase activation. The toxic action of atRAL was also diminished by blocking serotonin 2A (5-HT2AR) or M3-muscarinic (M3R) receptors, implicating GPCR participation in the overall process. These observations raise the possibility that certain types of retinal degeneration could be prevented by therapies selectively targeting transient sequestration (buffering) of elevated atRAL, antagonizing a subset of GPCRs, or inhibiting PLC, IP3R, or NADPH oxidase, alone or in combination.
EXPERIMENTAL PROCEDURES
Animals
Abca4−/−Rdh8−/− mice, generated and genotyped as previously described (20), were used when they reached 4 to 5 weeks of age. Eight- to 12-week-old BALB/c mice were obtained from Jackson Laboratory (Bar Harbor, ME). All mice were housed in the Animal Resource Center at the School of Medicine, Case Western Reserve University, where they were routinely maintained in a 12-h light (less than 10 lux)/12-h dark cycle environment. For bright light exposure experiments, mice were dark-adapted for 24 h prior to illumination at 10,000 lux (150-watt spiral lamp, Commercial Electric) for either 30 min (Abca4−/−Rdh8−/− mice) or 2 h (BALB/c mice). Abca4−/−Rdh8−/− mouse pupils were dilated with 1% tropicamide prior to light exposure, whereas BALB/c mice did not require pupil dilation before such exposure. Analyses of retinal structural and functional changes were performed 7 days after bright light exposure. All animal-handling procedures and experiments were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University.
Chemicals
atRAL was purchased from Toronto Research Chemicals, Inc. (Toronto, Canada). All-trans-retinoic acid (atRA), apocynin (APO), diphenyliodonium (DPI), 2-aminoethoxydiphenyl borate (2-APB), ketanserin, and 8-hydroxy-N,N-dipropyl-2-aminotetralin (8-OH-DPAT) were obtained from Sigma. Pregabalin was synthesized by Ricerca Bioscience LLC (Concord, OH). A2E (39) and all-trans-retinylamine (Ret-NH2) were synthesized as previously described (40). U-73122 was purchased from Calbiochem (Gibbstown, NJ). Ritanserin and 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP) were purchased from Tocris (Ellisville, MO).
In Vitro Detection and Quantification of Intracellular ROS
ARPE19 cells were cultured in Dulbecco's modified Eagle's medium (DMEM, low glucose) supplemented with 10% fetal bovine serum. The ROS probes, 2′,7′-dichlorofluorescein diacetate (DCF-DA, Sigma) or dihydroethidium (DHE, Invitrogen) were added in DMSO at a concentration of 400 nm (final solvent concentration, 1% v/v) after indicated pretreatments and incubated at 37 °C for 10 min before cells were thoroughly washed in phosphate-buffered saline. ROS signals were subsequently observed at the same exposure setting under an inverted fluorescence microscope (Leica DMI 6000 B). Fluorescence quantification was performed with Metamorph imaging software (Molecular Devices, Downington, PA). Thresholds corresponding to fluorescent signals were set from the images, and average fluorescence intensities were recorded for statistical analyses.
In Vivo Detection of ROS
The ROS probe, DHE, at a dose of 20 mg/kg body weight in 25 μl of DMSO, was administered to Abca4−/−Rdh8−/− mice via intraperitoneal injection 30 min prior to light exposure. Eye cups obtained after removing the cornea, lens, and vitreous body from enucleated eye globes 3 h post light illumination were fixed in 4% paraformaldehyde. Cryosections were prepared from fixed eye cups and cut at 12-μm thickness for microscopic assessment of ROS fluorescence in the retina using ImageJ (National Institutes of Health).
Mouse Treatments
Ret-NH2 and pregabalin were administered by gavage to 24-h dark-adapted mice at a dose of 100 mg/kg body weight 2 h before illumination. All other experimental compounds were given to 24 h dark-adapted mice by intraperitoneal injection through a 28-gauge needle at 24 h and 1 h prior to bright light exposure. Tested compounds and their doses were as follows: APO, 50 mg/kg body weight; DPI, 1 mg/kg body weight; U-73122, 6.25 mg/kg body weight; 2-APB, 2.5 mg/kg body weight; ketanserin, 1.25 mg/kg body weight; ritanserin, 3.75 mg/kg body weight; 8-OH-DPAT, 10 mg/kg body weight; and 4-DAMP, 6.25 mg/kg body weight. The gavage volume was 100 μl per treatment. The injected volume of the injected drug did not exceeded 50 μl per animal. Ret-NH2 was prepared in soybean oil. Pregabalin and 8-OH-DPAT were dissolved in water. All other drugs were dissolved in DMSO.
OCT
Ultra-high resolution SD-Optical Coherence Tomography (OCT, Bioptigen, Research Triangle Park, NC) was used for in vivo imaging of mouse retinas. Mice were anesthetized by intraperitoneal injection of a mixture consisting of ketamine (6 mg/ml) and xylazine (0.44 mg/ml) diluted with 10 mm sodium phosphate, pH 7.2, and 100 mm NaCl given at a dose of 20 μl/g body weight. Pupils were dilated with 1% tropicamide prior to imaging. Four frames of OCT images acquired in the B-mode were averaged for presentation.
Histology and Immunohistochemistry
Retinal histology and immunohistochemistry were performed as previously described (41). Briefly, eye cups freed of cornea, lens, and vitreous body were fixed in 2% glutaraldehyde/4% paraformaldehyde and processed for Epon embedding. Sections of 1-μm thickness were cut and stained with toluidine blue for histological examination under a light microscope. Immunohistochemical analysis was performed on 12-μm thick cryosections prepared from 4% paraformaldehyde-fixed eye cups. Collected cryosections were stained with DAPI and subjected to examination for rhodopsin, and with peanut agglutinin for cone sheaths.
ERGs
All ERG procedures were performed by published methods (41). For single-flash recording, the duration of white light flash stimuli (from 20 μs to 1 ms) was adjusted to provide a range of illumination intensities (from −3.7 to 1.6 log cd·s/m2). Three to five recordings were made at sufficient intervals between flash stimuli (from 3 s to 1 min) to allow recovery from any photobleaching effects.
Retinoid Analyses
Extraction, derivatization, and separation of retinoids were performed, and 11-cis-retinal content was analyzed by HPLC by procedures previously described (41).
Statistical Analyses
Results were averaged from at least three independent experiments. Data were expressed as means ± S.E., and statistical analyses were performed using the student's t test for p value calculations.
RESULTS
atRAL Stimulates Intracellular ROS Production through NADPH Oxidase
To determine the effect of atRAL on retinal ROS production, we incubated ARPE19 cells, an immortalized human RPE-like cell line susceptible to atRAL-induced cell death, with atRAL followed by examination with a ROS probe. As shown in Fig. 1A, atRAL exposure significantly elevated intracellular ROS production prior to massive cell death in a dose-dependent manner. Because the probe used, DCF-DA, is highly selective for H2O2 and hydroxyl radicals, intracellular ROS levels were also examined by another commonly used ROS probe, DHE, which is especially sensitive to superoxide. Consistently, the intracellular ROS signal-identified DHE probe was markedly increased in ARPE19 cells treated with 30 μm atRAL (Fig. 1B), a concentration that reproducibly caused excessive ARPE19 cell death as reported previously (10). The same concentration of atRAL would be produced by a ∼1% bleach of rhodopsin under physiological conditions. Interestingly, atRAL-related metabolic products such as all-trans-retinol (atROL), all-trans-retinoic acid (atRA), and A2E did not induce overproduction of intracellular ROS (supplemental Fig. S1). The difference between atRAL and the other retinoids in triggering intracellular ROS production could explain the difference in their effect on inducing cell death, because neither atROL, atRA, nor A2E induced cell death at the concentrations examined (10).
FIGURE 1.
atRAL stimulates ROS production in cultured ARPE19 cells via NADPH oxidase. A, atRAL in DMSO (0.5% v/v final concentration) was applied to cultured ARPE19 cells at indicated concentrations 1 h prior to addition of the ROS probe, DCF-DA. a, images of the ROS signal (green fluorescence) obtained with the same exposure time under a fluorescence microscope. b, average fluorescence intensities recorded and compared with Metamorph imaging software for statistical analyses (means ± S.E.; *, compared with 0 μm, p < 0.01). B, atRAL-stimulated ROS production in ARPE19 cells was verified by another ROS probe, DHE, 1 h after atRAL treatment. a, images of the ROS signal detected by DHE (red fluorescence) were obtained under a fluorescence microscope. b, recorded ROS signals were then compared by using the method described above. C, atRAL and/or the NADPH oxidase inhibitor, APO was applied to cultured ARPE19 cells at concentrations indicated. ROS generation was monitored 1 h after indicated treatments via DCF-DA detection as noted above. a, fluorescence images were recorded with the same exposure times, and b, statistical analyses were performed as noted above (*, compared with control, p < 0.01; #, compared with atRAL 30 μm, p < 0.01; §, compared with control, p > 0.05).
NADPH oxidase is the primary catalyst involved in atRAL-stimulated superoxide production by neutrophils (33, 34). To examine the role of NADPH oxidase in retinal cells, we added APO, a widely used NADPH oxidase inhibitor that interrupts NADPH oxidase complex assembly, to ARPE19 cells together with atRAL. As shown in Fig. 1C, APO treatment inhibited atRAL-induced intracellular ROS generation and enhanced ARPE19 cell survival (supplemental Fig. S2). Taken together, these results indicate that NADPH oxidase is required for atRAL-induced ROS production in ARPE19 cells, a finding that implies involvement of NADPH oxidase-mediated ROS generation in atRAL-induced retinal cell death.
NADPH Oxidase Mediates Light-induced ROS Production in Abca4−/−Rdh8−/− Mouse Retina
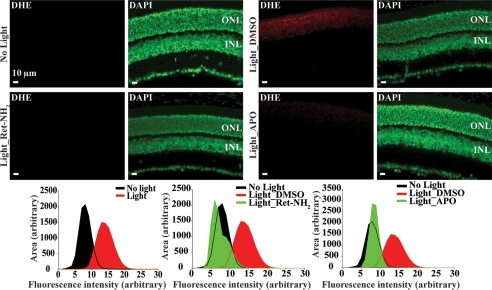
To further test the observation that atRAL induces ROS overproduction through NADPH oxidase in vivo, the ROS probe DHE was administered to Abca4−/−Rdh8−/− mice 30 min before light exposure at 10,000 lux for 30 min. This regimen was selected because this illumination intensity caused marked photoreceptor degeneration in Abca4−/−Rdh8−/− mice, whereas WT controls manifested no obvious morphological changes (supplemental Fig. S3). Compared with the ROS signal detected in the outer nuclear layer (ONL) of Abca4−/−Rdh8−/− mice unexposed to light, a strong ROS signal was recorded in the ONL of retinas from light-exposed and vehicle-treated Abca4−/−Rdh8−/− mice (Fig. 2). When APO was administered 1 h prior to illumination, these APO-treated double mutant mice displayed substantially decreased ROS production in the ONL with a signal intensity similar to that observed in retinas from non-light exposed Abca4−/−Rdh8−/− mice (Fig. 2). In addition, DPI, another commonly used NADPH oxidase inhibitor structurally different from APO (42), exhibited a similar effect on ROS production in light-challenged Abca4−/−Rdh8−/− mice (supplemental Fig. S4). The association of atRAL with ROS production in vivo was further confirmed by pretreating light-exposed Abca4−/−Rdh8−/− mice with Ret-NH2, a retinal scavenger and retinoid cycle inhibitor (40). Ret-NH2 significantly decreased ROS production as well (Fig. 2). This effect was also consistently observed in mice pretreated with pregabalin, which also reduced free atRAL (supplemental Fig. S4). Together, these results demonstrate that atRAL promotes ROS production in photoreceptors upon light exposure. This effect is mediated by NADPH oxidase, suggesting that atRAL-induced, NADPH oxidase-mediated ROS generation could be involved in the pathogenesis of acute light-induced photoreceptor degeneration.
FIGURE 2.
atRAL is associated with NADPH oxidase-mediated ROS generation in photoreceptors. Dark-adapted Abca4−/−Rdh8−/− mice at ages of 4–5 weeks were treated with the ROS probe, DHE, prior to light exposure at 10,000 lux for 30 min. DMSO vehicle control (Light_DMSO) and APO (Light_APO) were administered by intraperitoneal injection 1 h prior to light exposure. Ret-NH2 was gavaged 2 h before illumination (Light_Ret-NH2). Dark-adapted Abca4−/−Rdh8−/− mice unexposed to experimental light were included for the DHE probe treatment as well (No light). Retinas were harvested 3 h after illumination. ROS signals (in red) were obtained with the same exposure setup under a fluorescence microscope. DAPI staining (pseudo colored in green) was performed simultaneously to visualize cell nuclei and gross retinal structure. Recorded ROS fluorescence intensity averaged from various areas was plotted as a histogram for group comparisons.
Inhibition of NADPH Oxidase Protects Abca4−/−Rdh8−/− Mouse Retina against Acute Light-induced Photoreceptor Degeneration
To directly examine if atRAL-induced NADPH oxidase-mediated ROS production is mechanistically implicated in acute light-induced photoreceptor degeneration, we treated Abca4−/−Rdh8−/−mice with APO, DPI, or vehicle control (DMSO) 1 h prior to light exposure at 10,000 lux for 30 min. The effect of NADPH oxidase inhibitor treatment then was assessed 7 days after illumination. Although OCT scans revealed significantly disrupted photoreceptor structures in DMSO-treated mice, OCT of both APO-treated (Fig. 3A, panel a) and DPI-treated (data not shown) mice exhibited well preserved retinal morphology. This observation was confirmed by retinal histological examination. Retinas from DMSO-treated mice manifested prominent structural disorder with shortened lengths of photoreceptor outer/inner segments, markedly decreased cell numbers in the ONL, and increased pyknosis of photoreceptor nuclei. This morphology contrasted sharply with the nearly intact retinal morphology manifested by APO-treated (Fig. 3A, panel b) or DPI-treated mice (Fig. 3B, panel a). Immunohistochemical examination for rhodopsin in rod photoreceptor outer segments and peanut agglutinin-labeling of cone cell matrix sheaths were also performed. These images revealed abundant and organized expression of rhodopsin and peanut agglutinin in APO-treated (Fig. 3A, panel c) or DPI-treated (data not shown) mice, in sharp contrast to the residual pattern of rhodopsin and peanut agglutinin staining detected in DMSO-treated mice. Quantification of ONL thickness after DAPI staining revealed that both APO (Fig. 3A, panel d) and DPI (Fig. 3B, panel b) pretreatment greatly preserved photoreceptors compared with DMSO pretreatment. These results support the notion that NADPH oxidase-mediated ROS generation is mechanistically implicated in the action of atRAL during light-induced photoreceptor degeneration.
FIGURE 3.
NADPH oxidase inhibitor protects Abca4−/−Rdh8−/− mouse photoreceptors from light-induced degeneration. A, 4- to 5-week-old Abca4−/−Rdh8−/− mice were exposed to white light at 10,000 lux for 30 min after pretreatment with either vehicle control DMSO or APO. B, Abca4−/−Rdh8−/− mice were exposed to white light at 10,000 lux for 30 min after pretreatment with either vehicle control (DMSO) or another NADPH oxidase inhibitor, DPI. For both APO and DPI pretreatment, evaluations performed 7 days after illumination included OCT imaging (a, * indicates disrupted photoreceptors in the retinal structure), retinal histological examination (b, * indicates disrupted and decreased length of outer and inner photoreceptor segments; 63×), photoreceptor immunohistochemistry (c, rhodopsin in red, peanut agglutinin in green, and DAPI in blue; 20×) and measurements of outer nuclear layer thickness after DAPI staining (d, RPE, retinal pigmented epithelium; OS, outer segment; IS, inner segment; ONH, optic nerve head).
Involvement of PLC/IP3/Ca2+ Signaling in Light-induced atRAL-mediated Photoreceptor Degeneration
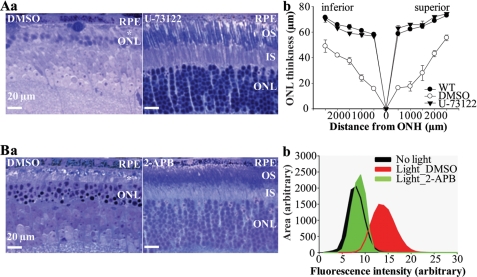
To test the hypothesis that PLC/IP3/Ca2+ signaling is involved in the cascade of events related to atRAL toxicity in light-induced photoreceptor degeneration, we pretreated Abca4−/−Rdh8−/− mice with the selective PLC inhibitor, U-73122 (43), prior to light exposure. In contrast to Abca4−/−Rdh8−/− mice pretreated with DMSO that reproducibly manifested severe histological photoreceptor degeneration, Abca4−/−Rdh8−/−mice pretreated with U-73122 exhibited markedly less light-induced photoreceptor damage (Fig. 4A, panel a) and ONL thickness measurements provided further evidence of a protective effect (Fig. 4A, panel b). These results strongly support the involvement of PLC activation in light-induced atRAL-mediated photoreceptor degeneration.
FIGURE 4.
Inhibition of PLC/IP3/Ca2+ signaling preserves retinal morphology in light-challenged Abca4−/−Rdh8−/− mice. A, 4- to 5-week-old Abca4−/−Rdh8−/− mice were exposed to white light at 10,000 lux for 30 min after pretreatment with either vehicle control (DMSO) or the PLC inhibitor, U-73122. In A: a, retinal histology (63×), with * indicating disorganized and reduced length of outer/inner segments, and b, analysis of ONL thickness were performed 7 days after illumination. B, light-challenged Abca4−/−Rdh8−/− mice were pretreated with either vehicle control (DMSO) or 2-APB, an antagonist against IP3-mediated intracellular Ca2+ release. In B: a, retinal histology was analyzed 7 days after illumination; b, in situ ROS production after 2-APB treatment was assessed as described in Fig. 2.
To further validate the involvement of PLC/IP3/Ca2+ signaling in atRAL-mediated photoreceptor degeneration in vivo, 2-APB, an antagonist of IP3/IP3R-mediated Ca2+ release (44), was administered to Abca4−/−Rdh8−/− mice prior to light exposure. Retinal morphological examination revealed that 2-APB pretreatment significantly preserved retinal morphology after illumination compared with DMSO pretreatment (Fig. 4B, panel a). Further, 2-APB pretreatment reduced ROS production in light-exposed Abca4−/−Rdh8−/− mouse photoreceptors to a level comparable to that observed in photoreceptors of mice without light exposure (Fig. 4B, panel b). Thus, IP3-mediated Ca2+ elevation is mechanistically associated with atRAL-induced ROS production during light-induced photoreceptor degeneration. Taken together, our results demonstrate that the PLC/IP3/Ca2+ pathway acts upstream of light-induced atRAL-mediated ROS generation and subsequent photoreceptor degeneration.
Involvement of Gq-coupled Receptors in Light-induced atRAL-mediated Retinal Degeneration
5-HT2AR has been suggested to be involved in NADPH oxidase activation (45). Additionally, chronic or acute 5-HT2AR activation causes considerable reduction in 5-HT1AR activity (46–49). The 5-HT1AR is involved in light-induced photoreceptor degeneration, because selective 5-HT1AR agonists protect the rat retina against photo-oxidative stress (50). Moreover, 5-HT2AR activates PLC (54), and PLC activation is involved in the in vivo action of atRAL (Fig. 4). Therefore we hypothesized that increased functionality of the 5-HT2AR could contribute to the pathogenesis of light-induced photoreceptor degeneration in Abca4−/−Rdh8−/−mice. To test this hypothesis, Abca4−/−Rdh8−/− mice were treated with selective 5-HT2AR antagonist ketanserin (51) prior to light exposure. A substantial protective effect of ketanserin against light-induced photoreceptor degeneration was observed compared with DMSO pretreatment (Fig. 5A, panels a and b). A similar observation was made when Abca4−/−Rdh8−/− mice were treated with another selective 5-HT2AR antagonist, ritanserin (52) (supplemental Fig. S5). A role for 5-HTRs in light-induced atRAL-mediated retinal degeneration in Abca4−/−Rdh8−/− mice is additionally supported by the protective effect of the 5-HT1AR agonist, 8-OH-DPAT (53) (supplemental Fig. S6).
FIGURE 5.
Inhibition of either 5-HT2A or M3R protects against light-induced atRAL-mediated photoreceptor degeneration in Abca4−/−Rdh8−/− mice. A, 4- to 5-week-old Abca4−/−Rdh8−/− mice were exposed to white light at 10,000 lux for 30 min after pretreatment with either vehicle control (DMSO) or the 5-HT2A receptor antagonist, ketanserin. B, the M3R antagonist, 4-DAMP, was independently tested and compared with a vehicle control (DMSO) in light-exposed Abca4−/−Rdh8−/− mice. Seven days later, retinal histological examination (63×) (a, * indicates disrupted and reduced length of outer/inner segments) and measurements of ONL thickness (b) were performed.
Considering that PLC can be activated by multiple Gq-coupled receptors, we tested whether 5-HT2AR is the only GPCR involved in atRAL-induced PLC activation. Interestingly, the M3R antagonist, 4-DAMP (54), also preserved retinal morphology in Abca4−/−Rdh8−/− mice challenged by acute light exposure (Fig. 5B, panels a and b), supporting the idea that multiple Gq-coupled receptors could be activated to mediate the effect of atRAL on PLC activation in light-induced photoreceptor degeneration.
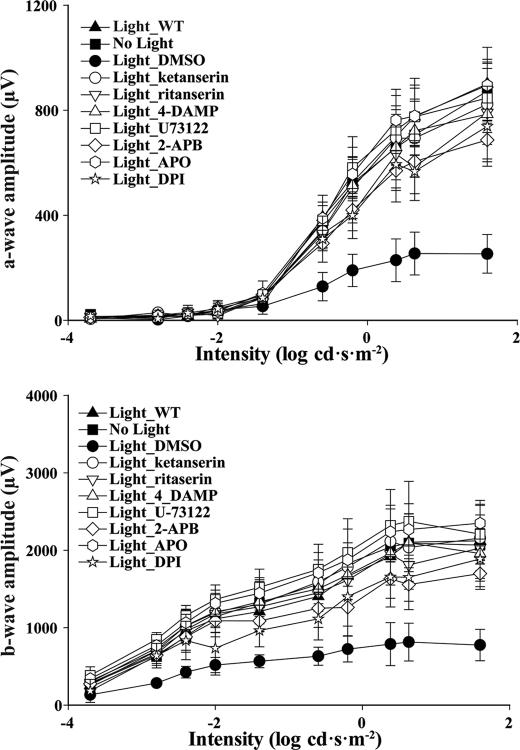
Involvement of these mechanisms in light-induced atRAL-mediated photoreceptor degeneration was also shown by improved retinal function of light-challenged Abca4−/−Rdh8−/− mice after pretreatment with several pharmacological agents that protected against histological damage. As indicated in Fig. 6, compared with light-challenged WT control and Abca4−/−Rdh8−/− mice without light exposure, light-challenged Abca4−/−Rdh8−/− mice pretreated with DMSO exhibited decreased amplitudes of both a-waves and b-waves, indicating marked impairment of their retinal function. The protective effect of these treatments on retinal function was observed by increased a-wave and b-wave amplitudes compared with those observed in DMSO-treated Abca4−/−Rdh8−/− mice.
FIGURE 6.
Retinal function in Abca4−/−Rdh8−/− mice is substantially preserved by several different treatments. Scotopic ERGs were recorded and both a-waves (top) and b-waves (bottom) were plotted to evaluate retinal function in Abca4−/−Rdh8−/− mice 7 days after they were pretreated with the indicated compounds. Compared with WT mice exposed to bright light (Light_WT) and Abca4−/−Rdh8−/− mice without light exposure (No light), light exposure at 10,000 lux for 30 min significantly impaired retinal function as indicated by decreased a-wave and b-wave amplitudes in mice treated with DMSO vehicle control (Light_DMSO). Compounds showing a protective effect against this light-induced retinopathy included ketanserin, ritanserin, 4-DAMP, U-73122, 2-APB, APO, and DPI.
Data presented above were derived from studies with Abca4−/−Rdh8−/− mice, a genetically modified animal model with deficiencies in atRAL transport and clearance owing to targeted deletion of the Rdh8 and Abca4 genes. To determine if the mechanisms proposed were merely secondary to genetic modification or arose from some unidentified off-target effects in Abca4−/−Rdh8−/− mice, we further tested our hypotheses in light-challenged BALB/c mice, a classic model of light-induced photoreceptor degeneration. Compared with unexposed control mice, BALB/c mice exposed to intense light exhibited severe photoreceptor degeneration indicated by disrupted retinal histology (Fig. 7A), decreased ocular 11-cis-RAL content (supplemental Fig. S7), and impaired retinal function (Fig. 7B). In contrast, pharmacological pretreatment targeting each proposed mechanism displayed significant protection of photoreceptors against acute light-induced degeneration as assessed by morphological (Fig. 7A), biochemical (supplemental Fig. S7), and functional tests (Fig. 7B).
FIGURE 7.
Light-induced retinal degeneration in BALB/c mice. Twelve-week-old BALB/c mice were dark-adapted followed by indicated pharmacological treatments via intraperitoneal injection 1 h prior to their exposure to white light at 10,000 lux for 2 h. All experimental evaluations were carried out 7 days later. Controls either without light exposure (No light) or with DMSO vehicle treatment followed by light exposure (Light_DMSO) were included for all analyses. A, retinal thin sections examined under light microscopy (63×) after toluidine blue staining. Scale bar, 20 μm on all panels. B, retinal function assessed by scotopic ERG in BALB/c mice 7 days after the indicated pretreatments.
DISCUSSION
Although atRAL is cytotoxic in cultured cells and associated with light-induced photoreceptor cell death in vivo (21), the involved mechanisms remain to be clarified. atRAL induces high levels of superoxide in neutrophils via NADPH oxidase, the primary enzymatic source of generated superoxide (29). Experimental results described here identify a series of intrinsically linked events, including the participation of GPCRs, PLC/IP3/Ca2+ signaling, and NADPH oxidase-mediated ROS production, which are responsible for the pathogenesis of atRAL-mediated light-induced retinal degeneration in Abca4−/−Rdh8−/− mice, a model for rod/cone degeneration. We further show that these mechanisms could play a role in the pathogenesis of photo-oxidative retinal degeneration in BALB/c mice as well.
atRAL has recently emerged as a critical player in the pathogenesis of retinal degeneration through its association with photoreceptor cell death (10, 55). However, how this retinoid exerts its toxic effects during retinal degeneration has not been previously investigated in vivo. The present study revealed that atRAL rapidly induced ROS overproduction in cultured RPE-like cells prior to cell death. This effect was also observed in the retinas of Abca4−/−Rdh8−/− mice after bright light exposure sufficient to cause prominent photoreceptor cell death in vivo, suggesting that atRAL release upon rhodopsin photobleaching is involved in ROS production. Consistent with this hypothesis, treatment of mice with Ret-NH2, a retinal scavenger and retinoid cycle inhibitor, and the primary amine-containing pregabalin that buffers atRAL significantly reduced light-induced ROS production in the ONL.
Oxidative stress is a major mechanism contributing to photoreceptor cell death in various animal models of retinal degeneration, including acute light-induced retinopathy. This is supported primarily by the protective effect of antioxidants in animal models of retinal degeneration and by the observation that photoreceptor cell death induced by light exposure involves overproduction of superoxide. NADPH oxidase has only recently been implicated as the enzymatic source of ROS generated in retinas exposed to bright light (30). Studies performed in neutrophils have demonstrated that atRAL acts as a potent stimulator of superoxide production through NADPH oxidase (33, 34). In the present study, two structurally different NADPH oxidase inhibitors independently reduced ROS generation to levels similar to those in non-light exposed control mice and provided substantial protection against light-induced retinal degeneration in Abca4−/−Rdh8−/− mice, supporting a direct role for NADPH oxidase in atRAL-mediated light-induced ROS production.
However, atRAL does not directly activate NADPH oxidase (35). In neutrophils, PLC activation occurs prior to production of superoxide, and therefore, products generated from PLC activation, IP3, and diacylglycerol were initially suggested as required intermediates for atRAL-induced and NADPH oxidase-mediated superoxide generation in neutrophils (33, 35). Diacylglycerol functions as a physiological activator of protein kinase C, which has been shown to be unaffected by atRAL stimulation (56). IP3 causes a rapid and substantial Ca2+ release from intracellular storage sites such as the endoplasmic reticulum by activating the IP3R, resulting in increased cytosolic Ca2+ levels. Elevated cytosolic Ca2+ concentration is a key event closely associated with cell death by multiple mechanisms, including excessive NADPH oxidase-mediated ROS production (37). NADPH oxidase is activated by rising Ca2+ in cortical and hippocampal astrocytes as manifested by increased ROS production in response to Ca2+ ionophore application, an effect blocked by incubating the cells with the NADPH oxidase inhibitor, DPI. Interestingly, a previous study demonstrated that atRAL caused a rapid increase in intracellular Ca2+ levels in cultured cells, although the underlying mechanisms were not defined (10). In the current study, exposure to U-73122, a pharmacological agent that inhibits PLC activity and therefore effectively blocks IP3/IP3R-mediated intracellular Ca2+ mobilization, significantly protected the Abca4−/−Rdh8−/− mouse retina from light-induced degeneration. Similarly 2-APB, which primarily acts by antagonizing IP3/IP3R-mediated Ca2+ release from intracellular Ca2+ storage sites, significantly inhibited ROS overproduction in light-exposed Abca4−/−Rdh8−/− retinas and protected photoreceptors against light-induced damage. Together, these data support the notion that PLC/IP3-mediated intracellular Ca2+ elevation precedes superoxide production in this experimental model. This explanation agrees with previous findings in neutrophils suggesting that PLC activation may be required for atRAL-stimulated superoxide production (33, 35, 56). It is also consistent with the observation that atRAL application increases intracellular Ca2+ levels in cultured cells (7).
It is worth noting that, once overproduced, ROS and Ca2+ may also engage in crosstalk during retinal degeneration. Intracellular ROS are important second messengers in cell signaling, including elevation of intracellular Ca2+ levels by damaging intracellular Ca2+ regulatory mechanisms. NADPH oxidase activation may also enhance intracellular Ca2+ levels by increasing the sensitivity of the endoplasmic reticulum to IP3, thereby promoting Ca2+ release from these intracellular stores. The rise in Ca2+ levels could be abolished by treatment with the NADPH oxidase inhibitor, DPI, or by a deficiency of Rac1 in these cells (37). NADPH inhibitors and antagonists of PLC/IP3/Ca2+ signaling had similar effects in protecting retinas from atRAL-mediated degeneration, implying that these mechanisms are involved in the same signaling pathway.
The PLC pathway is activated by multiple GPCRs coupled to Gq protein, suggesting that GPCRs could mediate the effect of atRAL on PLC activation. Among known pharmacologically distinct GPCRs associated with PLC activation, 5-HT2AR is an excellent candidate for activating PLC, although little previous data exists regarding its involvement in light-induced retinal degeneration. 5-HT2AR expression is readily detectible in the retina and 5-HT2AR activation mainly leads to elevations in cytosolic Ca2+ through PLC activation (57). Our results further demonstrate that atRAL-mediated PLC activation during light-induced retinal degeneration could result from upstream activation of multiple GPCRs, such as 5-HT2AR and M3R, that employ PLC/IP3/Ca2+ as their primary intracellular signaling pathway (58). However, further studies are required to elucidate the mechanism of Gq-coupled GPCR activation in the context of atRAL-mediated, light-induced retinal degeneration.
Collectively, these findings demonstrate that atRAL toxicity in light-induced retinal degeneration could be mediated through a signaling cascade implicating GPCRs, PLC/IP3/Ca2+ signaling, and NADPH oxidase (Fig. 8). Pharmacological interventions targeting these mechanisms can provide novel therapeutic strategies for treating blinding retinal disorders such as Stargardt disease and age-related macular degeneration.
FIGURE 8.
Cytotoxic effect of atRAL in light-induced photoreceptor degeneration occurs through a signaling cascade implicating GPCRs, PLC/IP3/Ca2+ signaling, and NADPH oxidase. Increased functionality of Gq-coupled GPCRs is involved in mediating atRAL toxicity during light-induced photoreceptor degeneration; however, the mechanism remains to be clarified (black arrow with dotted line). Activation of Gq-coupled GPCRs causes activation of PLC/IP3/Ca2+ signaling, which in turn leads to NADPH oxidase-mediated ROS production and photoreceptor degeneration (black arrows). Pharmacological interventions targeting Gq-coupled GPCRs, PLC/IP3/Ca2+, and NADPH oxidase protect the photoreceptor from light-induced, atRAL-mediated degeneration (red bars).
Supplementary Material
Acknowledgments
We thank Dr. Zhiqian Dong for expert handling of mice, Dr. Satomi Shiose and Dr. Kaede Ishikawa for help with the treatment regimes, Satsumi Roos for block preparation and plastic sectioning, and Hiroko Matsuyama for retinoid analyses. We also thank Dr. L. T. Webster, Jr., Dr. Jack Saari, Dr. Michael E. Maguire, and members of the Palczewski laboratory for critical comments on the manuscript.
Note Added in Proof
During the review of our manuscript, we came upon a recently published paper (59), which indicates that unsaturated fatty acids activate PLC/IP3/Ca2+ signaling through GPCR activation and induce ROS overproduction in TM4t mouse mammary tumor cells. This complex mechanism highlights the effect of unsaturated fatty acids on apoptosis. Given that all-trans-retinal shares common properties with unsaturated fatty acids with respect to stimulating superoxide production and activating PLC signaling, this paper corroborates our findings of the effect of all-trans-retinal on GPCR, PLC signaling, and ROS generation.
This work was supported, in whole or in part, by National Institutes of Health Grants EY009339, EY021126, EY019031, EY019880, and P30 EY11373. This work was also supported by the Research to Prevent Blindness Foundation and the Ohio Lions Eye Research Foundation.

This article contains supplemental Figs. S1–S7.
- atRAL
- all-trans-retinal
- atROL
- all-trans-retinol
- atRA
- all-trans-retinoic acid
- A2E
- diretinoid-pyridinium-ethanolamine
- 2-APB
- 2-aminoethoxydiphenyl borate
- 4-DAMP
- 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide
- 5-HT2AR
- serotonin 2A receptor
- 11-cis-RAL
- 11-cis-retinal
- 8-OH-DPAT
- 8-hydroxy-N,N-dipropyl-2-aminotetralin
- ABCA4/ABCR
- photoreceptor specific ATP-binding cassette transporter
- APO
- apocynin
- DCF-DA
- 2′,7′-dichlorofluorescein diacetate
- DHE
- dihydroethidium
- DPI
- diphenyleneiodonium
- GPCR
- G protein-coupled receptor
- IP3
- inositol 1,4,5-trisphosphate
- IP3R
- IP3 receptor
- M3R
- M3-muscarinic receptor
- OCT
- optical coherence tomography
- ONH
- optic nerve head
- ONL
- outer nuclear layer
- PLC
- phospholipase C
- Ret-NH2
- retinylamine
- ROS
- reactive oxygen species
- RPE
- retinal pigmented epithelium
- ERG
- electroretinogram.
REFERENCES
- 1. Palczewski K. (2006) G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. von Lintig J., Kiser P. D., Golczak M., Palczewski K. (2010) The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travis G. H., Golczak M., Moise A. R., Palczewski K. (2007) Diseases caused by defects in the visual cycle. Retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 47, 469–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiser P. D., Golczak M., Maeda A., Palczewski K. (2011) Biochim. Biophys. Acta [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiser P. D., Palczewski K. (2010) Membrane-binding and enzymatic properties of RPE65. Prog. Retin. Eye Res. 29, 428–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones G. J., Crouch R. K., Wiggert B., Cornwall M. C., Chader G. J. (1989) Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc. Natl. Acad. Sci. U.S.A. 86, 9606–9610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mata N. L., Radu R. A., Clemmons R. C., Travis G. H. (2002) Isomerization and oxidation of vitamin a in cone-dominant retinas. A novel pathway for visual-pigment regeneration in daylight. Neuron 36, 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fleisch V. C., Schonthaler H. B., von Lintig J., Neuhauss S. C. (2008) Subfunctionalization of a retinoid-binding protein provides evidence for two parallel visual cycles in the cone-dominant zebrafish retina. J. Neurosci. 28, 8208–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rózanowska M., Sarna T. (2005) Light-induced damage to the retina. Role of rhodopsin chromophore revisited. Photochem. Photobiol. 81, 1305–1330 [DOI] [PubMed] [Google Scholar]
- 10. Maeda A., Maeda T., Golczak M., Chou S., Desai A., Hoppel C. L., Matsuyama S., Palczewski K. (2009) Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 284, 15173–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mata N. L., Weng J., Travis G. H. (2000) Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 97, 7154–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim Y. K., Wassef L., Hamberger L., Piantedosi R., Palczewski K., Blaner W. S., Quadro L. (2008) Retinyl ester formation by lecithin. Retinol acyltransferase is a key regulator of retinoid homeostasis in mouse embryogenesis. J. Biol. Chem. 283, 5611–5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fishkin N., Yefidoff R., Gollipalli D. R., Rando R. R. (2005) On the mechanism of isomerization of all-trans-retinol esters to 11-cis-retinol in retinal pigment epithelial cells. 11-Fluoro-all-trans-retinol as substrate/inhibitor in the visual cycle. Bioorg. Med. Chem. 13, 5189–5194 [DOI] [PubMed] [Google Scholar]
- 14. Yannuzzi L. A., Ober M. D., Slakter J. S., Spaide R. F., Fisher Y. L., Flower R. W., Rosen R. (2004) Ophthalmic fundus imaging. Today and beyond. Am. J. Ophthalmol. 137, 511–524 [DOI] [PubMed] [Google Scholar]
- 15. Palczewska G., Maeda T., Imanishi Y., Sun W., Chen Y., Williams D. R., Piston D. W., Maeda A., Palczewski K. (2010) Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat. Med. 16, 1444–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allikmets R. (2000) Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. Am. J. Hum. Genet. 67, 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rattner A., Smallwood P. M., Nathans J. (2000) Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J. Biol. Chem. 275, 11034–11043 [DOI] [PubMed] [Google Scholar]
- 18. Molday R. S., Beharry S., Ahn J., Zhong M. (2006) Binding of N-retinylidene-PE to ABCA4 and a model for its transport across membranes. Adv. Exp. Med. Biol. 572, 465–470 [DOI] [PubMed] [Google Scholar]
- 19. Tsybovsky Y., Wang B., Quazi F., Molday R. S., Palczewski K. (2011) Posttranslational modifications of the photoreceptor-specific ABC transporter ABCA4. Biochemistry 50, 6855–6866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maeda A., Maeda T., Golczak M., Palczewski K. (2008) Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 283, 26684–26693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maeda A., Golczak M., Chen Y., Okano K., Kohno H., Shiose S., Ishikawa K., Harte W., Palczewska G., Maeda T., Palczewski K. (2011) Nature Chem. Biol. 8, 170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allikmets R., Singh N., Sun H., Shroyer N. F., Hutchinson A., Chidambaram A., Gerrard B., Baird L., Stauffer D., Peiffer A., Rattner A., Smallwood P., Li Y., Anderson K. L., Lewis R. A., Nathans J., Leppert M., Dean M., Lupski J. R. (1997) A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 15, 236–246 [DOI] [PubMed] [Google Scholar]
- 23. Cremers F. P., van de Pol D. J., van Driel M., den Hollander A. I., van Haren F. J., Knoers N. V., Tijmes N., Bergen A. A., Rohrschneider K., Blankenagel A., Pinckers A. J., Deutman A. F., Hoyng C. B. (1998) Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Genet 7, 355–362 [DOI] [PubMed] [Google Scholar]
- 24. Martínez-Mir A., Paloma E., Allikmets R., Ayuso C., del Rio T., Dean M., Vilageliu L., Gonzàlez-Duarte R., Balcells S. (1998) Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat. Genet. 18, 11–12 [DOI] [PubMed] [Google Scholar]
- 25. Zhang Q., Zulfiqar F., Xiao X., Riazuddin S. A., Ayyagari R., Sabar F., Caruso R., Sieving P. A., Riazuddin S., Hejtmancik J. F. (2005) Severe autosomal recessive retinitis pigmentosa maps to chromosome 1p13.3-p21.2 between D1S2896 and D1S457 but outside ABCA4. Hum Genet 118, 356–365 [DOI] [PubMed] [Google Scholar]
- 26. Donovan M., Carmody R. J., Cotter T. G. (2001) Light-induced photoreceptor apoptosis in vivo requires neuronal nitric-oxide synthase and guanylate cyclase activity and is caspase-3-independent. J. Biol. Chem. 276, 23000–23008 [DOI] [PubMed] [Google Scholar]
- 27. Organisciak D. T., Darrow R. M., Jiang Y. I., Marak G. E., Blanks J. C. (1992) Protection by dimethylthiourea against retinal light damage in rats. Invest. Ophthalmol. Vis. Sci. 33, 1599–1609 [PubMed] [Google Scholar]
- 28. Finkel T. (2003) Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15, 247–254 [DOI] [PubMed] [Google Scholar]
- 29. Lambeth J. D. (2004) NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 [DOI] [PubMed] [Google Scholar]
- 30. Haruta M., Bush R. A., Kjellstrom S., Vijayasarathy C., Zeng Y., Le Y. Z., Sieving P. A. (2009) Depleting Rac1 in mouse rod photoreceptors protects them from photo-oxidative stress without affecting their structure or function. Proc. Natl. Acad. Sci. U.S.A. 106, 9397–9402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Usui S., Oveson B. C., Lee S. Y., Jo Y. J., Yoshida T., Miki A., Miki K., Iwase T., Lu L., Campochiaro P. A. (2009) NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J. Neurochem. 110, 1028–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stolk J., Hiltermann T. J., Dijkman J. H., Verhoeven A. J. (1994) Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 11, 95–102 [DOI] [PubMed] [Google Scholar]
- 33. Lochner J. E., Badwey J. A., Horn W., Karnovsky M. L. (1986) All-trans-retinal stimulates superoxide release and phospholipase C activity in neutrophils without significantly blocking protein kinase C. Proc. Natl. Acad. Sci. U.S.A. 83, 7673–7677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Badwey J. A., Robinson J. M., Curnutte J. T., Karnovsky M. J., Karnovsky M. L. (1986) Retinoids stimulate the release of superoxide by neutrophils and change their morphology. J. Cell. Physiol. 127, 223–228 [DOI] [PubMed] [Google Scholar]
- 35. Steinbeck M. J., Hegg G. G., Karnovsky M. J. (1991) Arachidonate activation of the neutrophil NADPH-oxidase. Synergistic effects of protein phosphatase inhibitors compared with protein kinase activators. J. Biol. Chem. 266, 16336–16342 [PubMed] [Google Scholar]
- 36. Bootman M. D., Collins T. J., Peppiatt C. M., Prothero L. S., MacKenzie L., De Smet P., Travers M., Tovey S. C., Seo J. T., Berridge M. J., Ciccolini F., Lipp P. (2001) Calcium signaling. An overview. Semin. Cell Dev. Biol. 12, 3–10 [DOI] [PubMed] [Google Scholar]
- 37. Movitz C., Sjölin C., Dahlgren C. (1997) A rise in ionized calcium activates the neutrophil NADPH-oxidase but is not sufficient to directly translocate cytosolic p47phox or p67phox to b cytochrome containing membranes. Inflammation 21, 531–540 [DOI] [PubMed] [Google Scholar]
- 38. Rhee S. G. (2001) Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70, 281–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parish C. A., Hashimoto M., Nakanishi K., Dillon J., Sparrow J. (1998) Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. U.S.A. 95, 14609–14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Golczak M., Kuksa V., Maeda T., Moise A. R., Palczewski K. (2005) Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc. Natl. Acad. Sci. U.S.A. 102, 8162–8167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maeda A., Maeda T., Imanishi Y., Kuksa V., Alekseev A., Bronson J. D., Zhang H., Zhu L., Sun W., Saperstein D. A., Rieke F., Baehr W., Palczewski K. (2005) Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J. Biol. Chem. 280, 18822–18832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doussière J., Vignais P. V. (1992) Diphenylene iodonium as an inhibitor of the NADPH oxidase complex of bovine neutrophils. Factors controlling the inhibitory potency of diphenylene iodonium in a cell-free system of oxidase activation. Eur. J. Biochem. 208, 61–71 [DOI] [PubMed] [Google Scholar]
- 43. Bleasdale J. E., Thakur N. R., Gremban R. S., Bundy G. L., Fitzpatrick F. A., Smith R. J., Bunting S. (1990) Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 255, 756–768 [PubMed] [Google Scholar]
- 44. Maruyama T., Kanaji T., Nakade S., Kanno T., Mikoshiba K. (1997) 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 122, 498–505 [DOI] [PubMed] [Google Scholar]
- 45. Greene E. L., Houghton O., Collinsworth G., Garnovskaya M. N., Nagai T., Sajjad T., Bheemanathini V., Grewal J. S., Paul R. V., Raymond J. R. (2000) 5-HT(2A) receptors stimulate mitogen-activated protein kinase via H2O2 generation in rat renal mesangial cells. Am. J. Physiol. Renal Physiol. 278, F650–F658 [DOI] [PubMed] [Google Scholar]
- 46. Hensler J. G., Truett K. A. (1998) Effect of chronic serotonin-2 receptor agonist or antagonist administration on serotonin-1A receptor sensitivity. Neuropsychopharmacology 19, 354–364 [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y., D'Souza D., Raap D. K., Garcia F., Battaglia G., Muma N. A., Van de Kar L. D. (2001) Characterization of the functional heterologous desensitization of hypothalamic 5-HT(1A) receptors after 5-HT(2A) receptor activation. J. Neurosci. 21, 7919–7927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang Y., Gray T. S., D'Souza D. N., Carrasco G. A., Damjanoska K. J., Dudas B., Garcia F., Zainelli G. M., Sullivan Hanley N. R., Battaglia G., Muma N. A., Van de Kar L. D. (2004) Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J. Pharmacol. Exp. Ther. 310, 59–66 [DOI] [PubMed] [Google Scholar]
- 49. Naumenko V. S., Bazovkina D. V., Kondaurova E. M., Zubkov E. A., Kulikov A. V. (2010) The role of 5-HT2A receptor and 5-HT2A/5-HT1A receptor interaction in the suppression of catalepsy. Genes Brain Behav 9, 519–524 [DOI] [PubMed] [Google Scholar]
- 50. Collier R. J., Patel Y., Martin E. A., Dembinska O., Hellberg M., Krueger D. S., Kapin M. A., Romano C. (2011) Agonists at the serotonin receptor (5-HT(1A)) protect the retina from severe photo-oxidative stress. Invest. Ophthalmol. Vis. Sci. 52, 2118–2126 [DOI] [PubMed] [Google Scholar]
- 51. Leysen J. E., Niemegeers C. J., Van Nueten J. M., Laduron P. M. (1982) [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol. Pharmacol. 21, 301–314 [PubMed] [Google Scholar]
- 52. Leysen J. E., Gommeren W., Van Gompel P., Wynants J., Janssen P. F., Laduron P. M. (1985) Receptor-binding properties in vitro and in vivo of ritanserin. A very potent and long acting serotonin-S2 antagonist. Mol. Pharmacol. 27, 600–611 [PubMed] [Google Scholar]
- 53. Arvidsson L. E., Hacksell U., Nilsson J. L., Hjorth S., Carlsson A., Lindberg P., Sanchez D., Wikstrom H. (1981) 8-Hydroxy-2-(di-n-propylamino)tetralin, a new centrally acting 5-hydroxytryptamine receptor agonist. J. Med. Chem. 24, 921–923 [DOI] [PubMed] [Google Scholar]
- 54. Michel A. D., Stefanich E., Whiting R. L. (1989) Direct labeling of rat M3-muscarinic receptors by [3H]4-DAMP. Eur. J. Pharmacol. 166, 459–466 [DOI] [PubMed] [Google Scholar]
- 55. Shiose S., Chen Y., Okano K., Roy S., Kohno H., Tang J., Pearlman E., Maeda T., Palczewski K., Maeda A. (2011) Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J. Biol. Chem. 286, 15543–15555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Badwey J. A., Horn W., Heyworth P. G., Robinson J. M., Karnovsky M. L. (1989) Paradoxical effects of retinal in neutrophil stimulation. J. Biol. Chem. 264, 14947–14953 [PubMed] [Google Scholar]
- 57. Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., Humphrey P. P. (1994) International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol. Rev. 46, 157–203 [PubMed] [Google Scholar]
- 58. Fisher S. K., Heacock A. M., Agranoff B. W. (1992) Inositol lipids and signal transduction in the nervous system. An update. J. Neurochem. 58, 18–38 [DOI] [PubMed] [Google Scholar]
- 59. Hsu Y. C., Ip M. M. (2011) Conjugated linoleic acid-induced apoptosis in mouse mammary tumor cells is mediated by both G protein coupled receptor-dependent activation of the AMP-activated protein kinase pathway and by oxidative stress. Cell Signal. 23, 2013–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.