Background: Neuronostatin peptide is a somatostatin gene derivative, whose functions are largely unknown.
Results: Neuronostatin activates p38 MAPK and JNK attenuating endothelin-1-induced cardiac contractility and compromising cardiomyocyte viability.
Conclusion: Neuronostatin has multiple biological effects in cardiomyocytes.
Significance: Receptors for neuronostatin need to be identified to further characterize the functions of the peptide.
Keywords: Cardiovascular, Jun N-terminal kinase (JNK), Necrosis (Necrotic Death), p38 MAPK, Signal Transduction, Neuronostatin
Abstract
Neuronostatin, a recently discovered peptide encoded by somatostatin gene, is involved in regulation of neuronal function, blood pressure, food intake, and drinking behavior. However, the biological effects of neuronostatin on cardiac myocytes are not known, and the intracellular signaling mechanisms induced by neuronostatin remain unidentified. We analyzed the effect of neuronostatin in isolated perfused rat hearts and in cultured primary cardiomyocytes. Neuronostatin infusion alone had no effect on left ventricular (LV) contractile function or on isoprenaline- or preload-induced increase in cardiac contractility. However, infusion of neuronostatin significantly decreased the positive inotropic response to endothelin-1 (ET-1). This was associated with an increase in phosphorylation of p38 mitogen-activated protein kinase and c-Jun N-terminal kinase (JNK). Treatment of both neonatal and adult cardiomyocytes with neuronostatin resulted in reduced cardiomyocyte viability. Inhibition of JNK further increased the neuronostatin-induced cell death. We conclude that neuronostatin regulates cardiac contractile function and cardiomyocyte survival. Receptors for neuronostatin need to be identified to further characterize the biological functions of the peptide.
Introduction
Neuronostatin (NST)2 is a recently discovered peptide, which is encoded by somatostatin gene. Neuronostatin is involved in regulation neuronal function, blood pressure, food intake, and drinking behavior (1). The amounts of immunoreactive neuronostatin are highest in spleen, pancreas, cerebrum, and hypothalamus, but it is found in diverse tissues including the heart. Unlike somatostatin, neuronostatin does not modulate basal or hormone-induced growth hormone release (1). Somatostatin (also known as somatotropin release-inhibiting factor, SRIF) is a peptide, which was initially identified in the hypotalami (2). It inhibits the secretion of growth hormone and thyroid-stimulating hormone in the anterior pituitary gland. Additionally, somatostatin is found in a number of other tissues where it generally acts as a negative regulator of a variety of biological functions. Cardiac effects of somatostatin have not been completely elucidated. Exposure of guinea pig papillary muscles to somatostatin inhibits the isoprenaline-induced positive inotropic response without altering the basal contractile tension (3) and studies with rat ventricular cardiomyocytes have also revealed a negative inotropic effect in the presence of β-adrenergic agonist isoprenaline (4). On the other hand, treatment of acromegalic patients with somatostatin analogues has suggested an improvement in myocardial contractility (5).
The diversity in cardiac effects of somatostatin may be due to expression and involvement of multiple receptor subtypes in the heart. There are five distinct somatostatin receptor proteins (somatostatin-transfected receptors, SSTRs) discovered so far, and four of those (SSTR1, SSTR2, SSTR4, and SSTR5) are expressed in the adult human atria and ventricle, whereas all subtypes (SSTR1–5) are expressed in adult rat cardiomyocytes (6–8). Somatostatin binding affinity toward different receptor subtypes varies, and therefore it is not surprising that different concentrations of somatostatin have been shown to have distinct biological effects (9). All five somatostatin receptor subtypes belong to G protein-coupled receptor subfamily, and somatostatin has been shown to participate in the regulation of adenylate cyclase, Ca2+-channels, K-channels, phosphoinositide (PI), and mitogen-activated protein kinase (MAPK) cascades (9). To date, there are no specific receptors identified for neuronostatin. Samson et al. (1) expressed the five somatostatin receptors in HEK293T cells and found that while somatostatin expectedly induced the Gi pathway, exposure of cells to neuronostatin was without effect.
Existing data of the effects of neuronostatin on the heart are derived from a study carried out in washout experiments in Langendorff-perfused hearts or in isolated cells using chemical inhibitors H89, chelerythrine, and SP600125 (10). However, the intracellular signaling mechanisms regulated by neuronostatin remain unidentified and biological effects of neuronostatin in cardiomyocytes are not known. To address these issues, we performed studies with paced perfused isolated rat hearts and isolated neonatal rat and adult mouse cardiomyocytes, and investigated the effects of neuronostatin on cardiac contractility, cardiomyocyte hypertrophy, cardiomyocyte viability, and regulation intracellular signaling mechanisms. We show that neuronostatin treatment alone has no effect on cardiac contractile function, but it significantly inhibits endothelin-1 (ET-1) induced left ventricular contractility. Neuronostatin treatment induces phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) and c-Jun N-terminal kinase (JNK) in the adult rat heart. Prolonged treatment of isolated cardiomyocytes with neuronostatin compromises cardiomyocyte viability, and inhibition of JNK results in further increase in neuronostatin-induced cell death.
EXPERIMENTAL PROCEDURES
Materials
Neuronostatin, somatostatin, insulin-like growth factor-1, and endothelin-1 were purchased from Phoenix Pharmaceuticals (Burlingame, CA). JNK inhibitor peptide (JNK inhibitor I) was from Calbiochem (Darmstadt, Germany) and SB203580 from LC Laboratories (Woburn, MA). Antibodies against phosphoprotein kinase C-α (PKCα) and GAPDH were from Millipore (Billerica, MA) and antibody against phosphorylated JNK was from Promega (Madison WI). All other antibodies were from Cell Signaling Technology (Beverly, MA) and all other reagents were from Sigma-Aldrich.
Isolated Perfused Rat Heart Preparation
All protocols were reviewed and approved by the Animal Use and Care Committee of the University of Oulu. Male 7-week-old Sprague-Dawley rats from the Center for Experimental Animals at the University of Oulu were used. Rats were decapitated, and hearts were quickly removed and arranged for retrograde perfusion by the Langendorff technique as described previously (12). The hearts were perfused with a modified Krebs-Henseleit bicarbonate buffer, pH 7.40, equilibrated with 95% O2-5% CO2 at 37 °C. Hearts were perfused at a constant flow rate of 5.5 ml/min with a peristaltic pump (Minipuls 3, model 312, Gilson, Villiers, France). Heart rate was maintained constant (305 ± 1 beats per minute) by atrial pacing using a Grass stimulator (model S88, Grass Instruments, West Warwick, RI) (11 V, 0.5 ms). Contractile force (apicobasal displacement) was obtained by connecting a force displacement transducer (FT03, Grass Instruments, West Warwick, RI) to the apex of the heart at an initial preload stretch of 2 g. Perfusion pressure reflecting coronary vascular resistance was measured by a pressure transducer (model MP-15, Micron Instruments, Los Angeles, CA) situated on a side arm of the aortic cannula. A 40-min equilibration period and a 5-min control period were followed by addition of various drugs to the perfusate at a rate of 0.5 ml/min for 10 min.
For analysis of the Frank-Starling response, left ventricular contractility was assessed by measuring isovolumic left ventricular pressure with a fluid-filled balloon placed in the left ventricular chamber as described (13). LVDP (left ventricular developed pressure) and LVEDP (left ventricular end diastolic pressure) were measured using a pressure transducer linked to the balloon cannula (model BP-100 Blood Pressure Transducer, iWorx Systems Inc., Washington). Data were converted to a digital form by iWorx computer interface (IX/228-S Data Acquisition System) and recorded to a computer using iWorx Labscribe 2 software. A 40-min stabilization period was followed by 10 min of pretreatment with the desired drug. Thereafter, the infusion was continued, and the volume of the intraventricular balloon was increased in 15-μl steps. Cardiac function was assessed within 1 min of each volume increment, when a new steady state was reached. The whole assessment of the Frank-Starling response was completed within 15 min. At the end of the experiments, the LVs were frozen in liquid nitrogen and stored at −80 °C until assayed.
Neonatal Cardiomyocyte Culture
Neonatal rat ventricular myocytes were prepared from 2–4-day-old Spraque-Dawley rats as described (11). Neuronostatin, somatostatin, hydroxen peroxide, insulin, and insulin-like growth factor were added to culture medium at the third day of culture. The Animal Use and Care Committee of the University of Oulu approved the experimental design.
Isolation of Adult Ventricular Cardiomyocytes
Cardiomyocytes were isolated from 8–10-week-old c57BL/6 mice as described (12). Briefly, the mouse was deeply anesthesized, and the heart was excised rapidly. The heart was cannulated through aorta and retrogradily perfused with Hepes-buffered Tyrode's solution supplemented with 0.1% collagenase type II (Worthington) and 2,3-butandione-monoxime until digested. Ventricular tissue was homogenized, and myocytes were collected with low-speed centrifugation. Myocytes were finally plated into laminin-coated multiwell plates in MEM, 5% FBS, insulin-transferrin-selenin (Invitrogen), 10 mm 2,3-butandione-monoxime, 2 mm l-glutamine, and penicillin-streptomycin (Sigma). After cell attachment, medium was changed, and FBS replaced with 0.01% BSA when starting the experiment. The protocols were reviewed and approved by the Animal Use and Care Committee of the University of Oulu.
Protein Synthesis
Cardiomyocyte protein synthesis was measured by analyzing [3H]leucine incorporation into the cells as previously described (13). Briefly, neonatal rat cardiomyocytes were cultured in 24-well plates, and on the third day in culture the medium was replaced with complete serum-free medium supplemented with [3H]leucine (5 μCi/ml) and, when appropriate, treated with neuronostatin and other compounds. After 24 h cells were lysed and processed for measurement of incorporated [3H]leucine (Amersham Biosciences-Pharmacia) by liquid scintillation counter.
Cell Viability Assays
Analysis of adenylate kinase release from ruptured cells into the cell culture medium by using a kit from Lonza (Basel, Switzerland) as described (14). Analysis for apoptotic cell death was performed by using TUNEL kit from Millipore (Billerica, MA). The effect of neuronostatin on the viability of adult cardiomyocytes was determined with resazurin assay which quantifies the reduction of resazurin to a fluorescent compound resorufin within viable cells (15). For analysis, 10 μm resazurin (Sigma) was added to the cell culture medium at the end of the experiment. 1 h later, medium samples were collected into 96-well plate and measured by excitation at 544 nm and emission at 595 nm on a multiplate reader (Victor Wallac).
Western Blot Analysis
Immunoblotting was performed as described (16). Shortly, the samples were homogenized into a buffer, which consisted of 20 mm Tris-HCl, 150 mm NaCl, 1 mm EDTA, 1 mm M EGTA, 1% (v/v) Triton-X100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, and 1 mm Na3VO4 (pH 7.5) supplemented with 1 mm dithiothreitol, protease inhibitors, and phosphatase inhibitors. Protein extracts were matched for protein concentration (20–50 μg), loaded on SDS-PAGE, and transferred to nitrocellulose filters. The membranes were blocked in blocking buffer, incubated with primary antibodies overnight at 4 °C, and amount of protein was detected by Odyssey Infrared Imaging System (LI-COR, Lincoln, Nebraska).
Statistical Analysis
Results are presented as mean ± S.E. Data were analyzed with Student's t test and for analysis of multiple experimental groups, repeated measures- or one way ANOVA followed by Bonferroni post hoc test was used. Differences were considered statistically significant at the level of p < 0.05.
RESULTS
Neuronostatin Does Not Affect Basal Cardiac Function
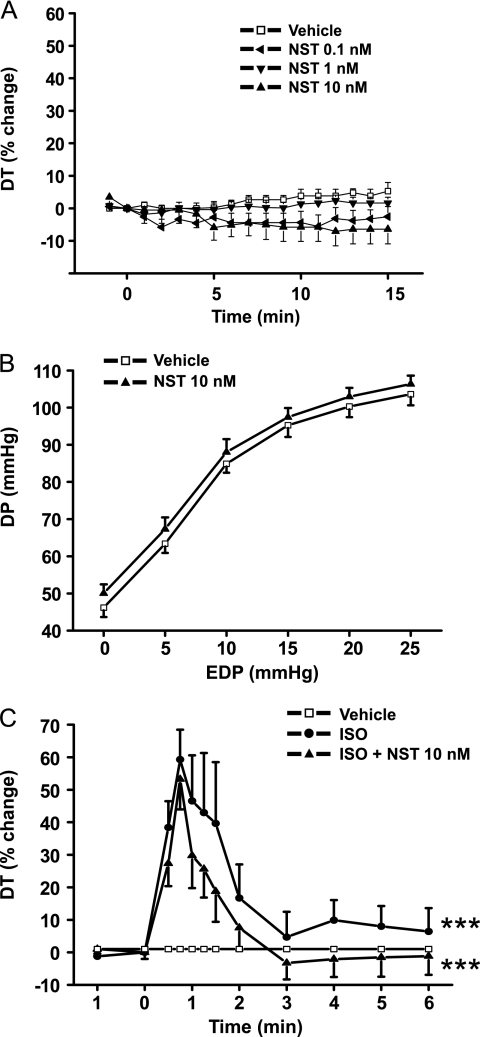
We first analyzed the effect of neuronostatin on auxotonic contractions in an isolated rat heart model (17). Previous data suggest that neuronostatin has a negative inotropic effect that is observed with a dose ranging from 0.3 to 30 nm (10). We treated the isolated paced rat hearts with 0.1 nm, 1 nm, and 10 nm doses of neuronostatin and found a modest yet nonsignificant decrease in left ventricular contractile function during the 15-min experiment (Fig. 1A). We also extended the experiment time to 30 min and treated the hearts with 10 nm of neuronostatin, but that had no significant effect on cardiac contractile function (data not shown).
FIGURE 1.
Basal cardiac contractility, isoprenaline-response or Frank-Starling response are not affected by neuronostatin treatment. Effect of NST was studied in isolated rat heart preparation as described under “Experimental Procedures.” A, rat hearts were perfused in Langendorff model with either vehicle or NST (0.1, 1, or 10 nm) for 15 min. B, LV Frank-Starling response was analyzed by stepwise increasing the volume of the intraventricular balloon in Langendorff-perfused hearts treated with either vehicle or NST (10 nm). C, rat hearts were perfused in Langendorff model with either vehicle, ISO, or ISO + NST (10 nm). DT indicates developed tension, DP indicates developed pressure. A and C, results are expressed as a percent change versus baseline values. Data are mean ± S.E. (n = 6–8 for each group). ***, p < 0.001 versus vehicle.
Neuronostatin Has No Effect on Frank-Starling Response or on Isoprenaline-induced Inotropic Response
We next analyzed the effect of neuronostatin on the Frank-Starling response in isolated perfused rat hearts. In this experimental system (17–19), stepwise increases in the volume of the left ventricular balloon resulted in prominent enhancement of left ventricular systolic function. The maximal point on the Frank-Starling curve was reached at an end-diastolic pressure of 20–25 mmHg with a corresponding left ventricular systolic pressure of 90–120 mmHg. We found that treatment of isolated hearts with either 1 nm (not shown) or 10 nm neuronostatin had no effect on the Frank-Starling response (Fig. 1B). We then exposed the hearts to intracoronary perfusion with potent β1/β2-adrenergic agonist isoprenaline. Infusion of 1 μm isoprenaline induced a rapid increase in developed tension that peaked at 1 min time point (Fig. 1C). At 10 min, the left ventricular develop tension was returned near to the baseline values. Coadministration of neuronostatin (10 nm) had no effect on isoprenaline-induced developed tension.
Neuronostatin Attenuates Endothelin-1 Induced Cardiac Contractility
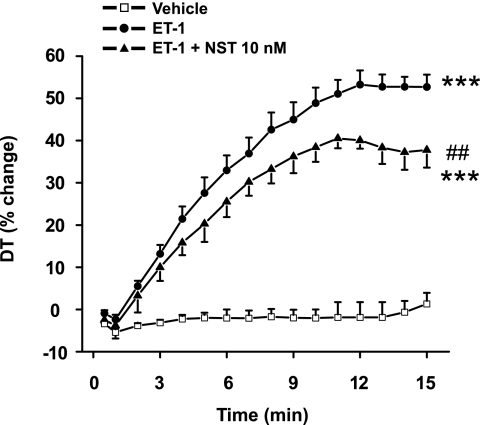
We then assessed the effect of neuronostatin on left ventricular function in paced perfused rat heart during intracoronary infusion of 1 nm ET-1 for 10 min. In agreement with the maximal response in our previous studies (20), ET-1 increased developed tension by 51 ± 4% (Fig. 2). Administration of neuronostatin (10 nm) together with ET-1 significantly attenuated the ET-1 induced increase in contractility (Fig. 2). Coadministration of neuronostatin at 1 nm dose had a modest but nonsignificant negative effect on ET-1 induced LV contractile function, whereas 0.1 nm concentration of neuronostatin was without effect (data not shown).
FIGURE 2.
Neuronostatin attenuates endothelin-1 induced cardiac contractile response. Rat hearts were perfused in Langendorff model with either vehicle, 1 nm ET-1, or ET-1 + 10 nm NST. DT indicates developed tension. Data are mean ± S.E. (n = 6–8 for each group). ***, p < 0.001 versus vehicle; ##, p < 0.01 versus ET-1.
Neuronostatin Modulates p38 and JNK Phosphorylation in Isolated Rat Heart
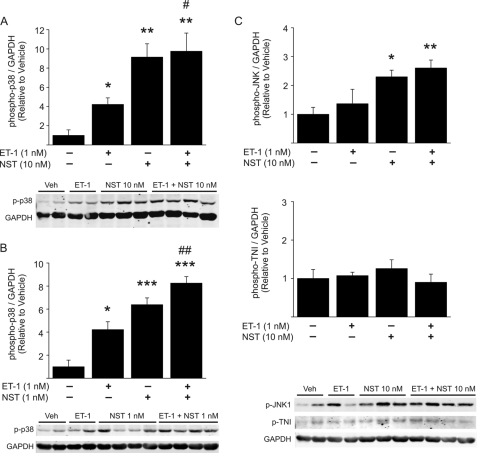
We next analyzed the effect of neuronostatin on activation of intracellular signaling pathways upon ET-1 stimulation in the perfused rat heart. We found that 10-min ET-1 infusion induced a marked increase in phosphorylation of p38 MAPK, and coadministration of 10 nm neuronostatin significantly augmented the effect (Fig. 3A). Remarkably, administration of 10 nm neuronostatin alone also resulted in comparable increase in p38 MAPK phosphorylation (Fig. 3A). As noted, 1 nm neuronostatin did not affect ET-1-induced increase in LV contractility. It was, however, sufficient to increase p38 phosphorylation in the adult heart (Fig. 3B). ET-1 treatment had no effect on JNK phosphorylation, whereas treatment with 10 nm neuronostatin alone or in combination with ET-1 resulted in a significant increase in JNK phosphorylation (Fig. 3C). 1 nm neuronostatin induced a modest but non-significant increase in JNK phosphorylation (not shown). ET-1-induced phosphorylation of extracellular signal-regulated kinase (ERK), however, was not affected by administration of neuronostatin (data not shown). We also analyzed the effect of neuronostatin on protein kinase A target troponin I. Administration of neuronostatin (10 nm) alone or in combination with ET-1 had no effect on phosphorylation of troponin I (TNI) at Ser-23/24 residue (Fig. 3C).
FIGURE 3.
Neuronostatin induces p38 MAPK and JNK phosphorylation in isolated perfused hearts. A–C, hearts were perfused in Langendorff model with either vehicle, 1 nm ET-1, 1, or 10 nm NST or ET-1 + NST (1 or 10 nm) for 15 min. At the end of the experiment, protein was extracted from left ventricular tissue samples. Shown is quantification of Western blot analysis of phosphorylation of p38 MAPK, JNK, and troponin I (TNI), and representative Western blots. GAPDH was used as a loading control. Data are mean ± S.E. (n = 4–6 for each group). *, p < 0.05 versus vehicle; **, p < 0.01 versus vehicle; ***, p < 0.001 versus vehicle; #, p < 0.05 versus ET-1; ##, p < 0.01 versus ET-1.
Neuronostatin Induces Phosphorylation of p38 MAPK in Cultured Cardiomyocytes
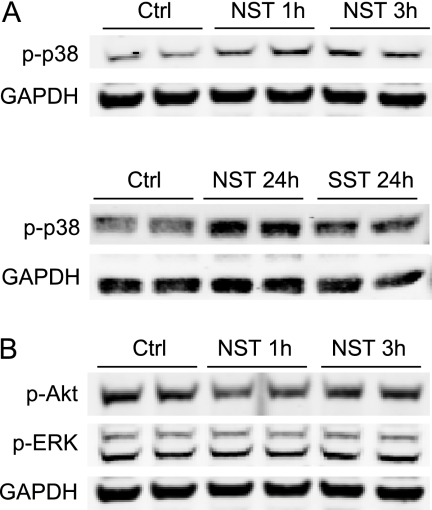
To investigate the effects of a more extended exposure of cardiomyocytes to neuronostatin, cultured neonatal rat ventricular cardiomyocytes were treated with various concentrations of neuronostatin and activation of key signaling pathways in cardiomyocytes was investigated. In agreement with our findings in the isolated heart, we found that administration of neuronostatin induced a robust increase in phosphorylation of p38 MAPK, which was most evident with 0.1 nm concentration (Fig. 4A). The neuronostatin-induced phosphorylation of p38 was seen already after 1 h of treatment, and was still strongly induced at 24 h. In contrast, exposure of cardiac myocytes to somatostatin had no effect on p38 phosphorylation (Fig. 4A). Treatment of cardiomyocytes with 0.1 nm neuronostatin had no effect on phosphorylation of Akt or ERK (Fig. 4B).
FIGURE 4.
Neuronostatin phosphorylates p38 MAPK in neonatal rat cardiomyocytes. A, cultured NRVMs were treated with vehicle (Ctrl), 0.1 nm NST, or 0.1 nm SST for times shown in the figure. Cell lysates were then immunoblotted for phosphorylated p38 MAPK. B, NRVMs were treated with 0.1 nm NST for times shown in the figure and at the end of the experiment protein samples were collected. Shown are Western blot analyses of phosphorylation of Akt and extracellular signal-regulated kinase (ERK). GAPDH was used as a loading control.
Neuronostatin Reduces Cell Viability
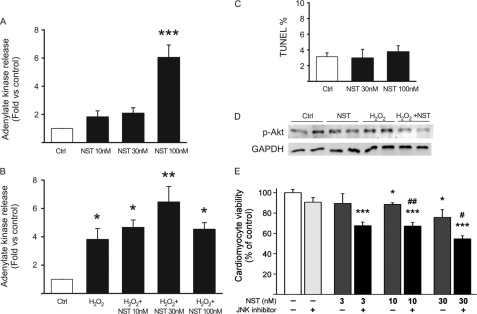
To assess the effect of neuronostatin on cardiomyocyte viability, cultured neonatal cardiac myocytes were treated with various concentrations of neuronostatin and analyzed for both necrotic and apoptotic cell death. Cardiomyocyte necrosis was analyzed by release of cellular adenylate kinase from ruptured cells into the cell culture medium. Exposure of cardiomyocytes to neuronostatin for 16 h dose-dependently increased necrotic cardiomyocyte death (significant effect only at 100 nm) (Fig. 5A). Moreover, treatment of cells with neuronostatin also resulted in augmented hydrogen peroxide (H2O2)-induced adenylate kinase release (Fig. 5B). However, 24-h treatment with 30 or 100 nm neuronostatin had no effect on cardiomyocyte apoptosis as assessed by TUNEL (Fig. 5C). To analyze the effect of neuronostatin on signaling mechanisms regulating cardiomyocyte viability, phosphorylation of key protective, and detrimental signaling pathways was investigated. We found that 90-min treatment of cultured cardiac myocytes with a high concentration of neuronostatin (100 nm) slightly decreased the amount of phosphorylated Akt, and markedly reduced the Akt phosphorylation upon H2O2 treatment (Fig. 5D). Phosphorylation of STAT3 (signal transducer and activator of transcription 3), JNK or ERK was not affected by treatment of cardiomyocytes with 10 or 100 nm neuronostatin (data not shown).
FIGURE 5.
Neuronostatin reduces cardiomyocyte viability. A, NRVMs were treated with 10, 30, or 100 nm NST for 16 h and release of adenylate kinase from ruptured cells was analyzed. B, NRVMs were treated with H2O2 (200 μm) alone or in combination with NST (10, 30, or 100 nm) for 16 h and at the end of the experiment necrotic cell death was analyzed. C, TUNEL assay. NRVMs were treated with vehicle or NST (30 or 100 nm) for 24 h before staining with TUNEL. In the graph, TUNEL-positive cells are expressed as a percentage of the number of total cells, as determined by staining post-fixation with hematoxylin. D, NRVMs were treated with vehicle versus NST (100 nm), H2O2 (200 μm) alone, or H2O2 + NST for 3 h. Shown is Western blot analysis of phosphorylation of Akt. GAPDH was used as a loading control. E, isolated adult cardiomyocytes were treated with vehicle, JNK inhibitor peptide (2 μm), or NST (3, 10, or 30 nm) with or without JNK inhibitor peptide and 24 h later cell viability was analyzed. *, p < 0.05 versus control; **, p < 0.01 versus control; ***, p < 0.001 versus control; #, p < 0.05 versus 30 nm NST; ##, p < 0.01 versus 10 nm NST.
Neuronostatin Reduces Viability of Adult Cardiomyocytes
We next analyzed the effect of neuronostatin on viability of adult cardiomyocytes. Isolated mouse cardiomyocytes were treated with neuronostatin for 24 h and cell death was analyzed by resazurin assay. This method is based on the reduction of resazurin to resorufin, a quantifiable fluorescent dye, within viable cells. We found that adult cardiomyocyte viability was already compromised by treatment of cells with 10 nm neuronostatin and further reduced by treatment with 30 nm neuronostatin (Fig. 5E). As shown in Fig. 3, neuronostatin induced a significant increase in p38 and JNK phosphorylation in the adult heart. To analyze if p38 or JNK played a role in neuronostatin induced cardiomyocyte death, we then cotreated the cardiomyocytes with p38 inhibitor SB203580 (5 μm) or with a cell-permeable JNK inhibitor peptide (2 μm). We found that p38 inhibition did not affect neuronostatin-induced cardiomyocyte death (not shown), while JNK inhibition further reduced the cardiomyocyte viability (Fig. 5E). Treatment of cells with JNK inhibitor alone did not affect cell viability (Fig. 5E).
Neuronostatin Regulates Eukaryotic Elongation Factor 2
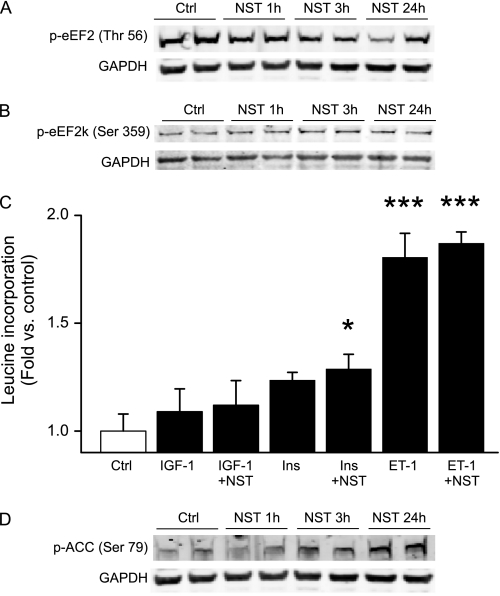
Postnatal cardiomyocytes rarely enter the cell cycle and, in fact, many of the signaling mechanisms promoting cell proliferation in cycling cells actually induce cell growth (i.e. hypertrophy) in cardiomyocytes. To assess the effect of neuronostatin on cell growth, we first analyzed the effects of neuronostatin on regulation of protein synthesis machinery in cardiomyocytes. Mammalian target of rapamycin, mTOR, is a central regulator of protein synthesis and cell growth. mTor complex 1 (mTORC1) regulates the activity of the eukaryotic elongation factor 2 kinase (eEF2k) and its target, the elongation factor eEF2, which is a central regulator of protein synthesis (21). We found that 24-hour treatment of cultured cardiomyocytes with 0.1 nm neuronostatin resulted in a significant decrease in phosphorylation of eEF2 (Fig. 6A). However, 1 nm and 10 nm doses of neuronostatin had little or no effect (data not shown). Prior studies have suggested that eEF2 kinase (eEF2k) is regulated by p38 MAPK, which negatively regulates eEF2k activity (22). We therefore analyzed the phosphorylation of eEF2k and found that neuronostatin treatment resulted in increased phosphorylation of eEF2k at serine 359, a putative p38 MAPK target site (Fig. 6B). To then analyze the effect of neuronostatin on protein synthesis in cultured cardiomyocytes, cells were treated with neuronostatin alone or in combination with insulin-like growth factor-1 (IGF-1), insulin, or ET-1, and analyzed for incorporation of radioactive leucine. We found that treatment of cultured neonatal cardiac myocytes with neuronostatin alone (0.1 nm, 1 nm or 10 nm) had no effect on protein synthesis (data not shown). ET-1, IGF-1, and insulin all had a positive effect on leucine incorporation and co-treatment of cells with 0.1 nm concentration of neuronostatin only modestly enhanced the protein synthesis induced by all three agonists (Fig. 6C). Considering the underlying reason for a weak biological response to signaling events facilitating the ribosome function, we finally analyzed the effect of neuronostatin on phosphorylation of AMP-activated protein kinase (AMPK), a key regulator and sensor of cellular energy status. Treatment of cardiomyocytes with neuronostatin (0.1 nm) induced a marked increase in phosphorylation of acetyl-co-carboxylase (ACC), a downstream target of AMPK, suggesting activation of AMPK (Fig. 6D). This phosphorylation was detectable after 3 h of treatment with neuronostatin and was further increased at 24 h.
FIGURE 6.
Neuronostatin regulates protein synthesis machinery. A, NRVMs were treated with 0.1 nm NST or vehicle (Ctrl) for times indicated in the figure. Cell lysates were then immunoblotted for phosphorylated eukaryotic translation elongation factor 2 (eEF2). B, cells were treated as in A, and cell lysates were then immunoblotted for phosphorylated eEF2 kinase (eEF2k). C, NRVMs were treated with insulin-like growth factor-1 (IGF-1), insulin (Ins), or ET-1 alone, or in combination with 0.1 nm NST. Shown is analysis of incorporation of radioactive leucine after 24 h of treatment. D, NRVMs were treated with 0.1 nm NST versus vehicle for times indicated in the figure, and cell lysates were then immunoblotted for phosphorylated ACC. *, p < 0.05 versus control; ***, p < 0.001 versus control.
DISCUSSION
Cardiac contractile performance is mainly regulated by circulating catecholamines, which bind to β-adrenergic receptors on cardiomyocyte cell membrane. In addition, a number of neurohumoral agonists regulate cardiac function and play a role in cardiac physiology especially in the stressed heart. These include vasoactive peptides ET-1, urotensin II, apelin, and adrenomedullin (17, 20, 23, 24). However, the possible effects of neuronostatin on cardiac function have only just begun to be studied. Our studies performed in tightly controlled experimental settings (17, 20, 24) show that neuronostatin alone has no significant effect on LV contractile performance. Under similar conditions, ET-1 infusion induced a robust increase in LV contractility as we have also previously reported (20). Interestingly, we found that coadministration of neuronostatin markedly attenuated the ET-1 induced LV contractile response. Analysis of possible signaling mechanisms involved revealed that neuronostatin treatment induced a robust increase in phosphorylation of p38 MAPK, and, importantly, coadministration of neuronostatin with ET-1 significantly augmented the ET-1-induced phosphorylation of p38 MAPK.
p38 MAPK belongs to the group of stress-activated MAPKs (25). It is activated by extracellular stimuli such as osmotic stress, radiation, G protein-coupled receptor agonists and cardiomyocyte stretch (26). p38 regulates the activity of numerous downstream kinases and transcription factors via phosphorylation (27). In the heart, p38 is an important regulator of cardiac hypertrophy, cardiac fibrosis and cardiomyocyte survival (28–32). In addition, a number of studies have indicated that activation or overexpression of members of p38 MAPKs or upstream kinases leads to deterioration of cardiac function (33, 34). While the mechanism is not totally clear, the central factor appears to be desensitization of myofilaments to elevation of intracellular calcium (35). In addition, transgenic mice overexpressing either MKK3 or MKK6 show reduced expression of sarcoplasmic reticulum calcium ATPase 2A (SERCA2A) and phospholamban (PLN), which may contribute to worsening of the LV contractile function (29). In a recent study, we found that pharmacological inhibition of p38 enhances ET-1 induced LV contractility in isolated heart preparation, and this was associated with an increase in phosphorylation of PLN at serine 16 residue (20) providing an additional mechanism for p38-dependent regulation of LV contractile function. Thus, our observation that neuronostatin is a potent p38 activator, may indeed be critical for observed attenuation of ET-1 induced LV contractility.
Hua et al. (10) recently suggested that neuronostatin regulates cardiac contractile function via protein kinase A (PKA) and JNK, but not PKC, mediated mechanism. These data were obtained using pharmacological inhibitors and actual activation of intracellular signaling pathways was not investigated. Our data suggest that neuronostatin has no effect on activation of PKA, since exposure of cardiomyocytes to neuronostatin had no effect on the phosphorylation of PKA target troponin I and, importantly, neuronostatin had no effect on left ventricular contractile performance when administered either alone or in combination with isoprenaline. Analysis of signaling pathways in isolated hearts following administration of neuronostatin revealed increased JNK phosphorylation. JNK is another member of stress-activated protein kinases and data from numerous studies suggest that activation of JNK signaling in cardiomyocytes can have both prosurvival and apoptotic effects depending on the stimulus and experimental model (36–38) for review, see Ref. 39. However, in contrast to p38 MAPK, c-Jun N-terminal kinase (JNK) has not been reported to play a major role in the regulation of LV contractile function (19, 40).
Among the numerous signaling pathways involved in regulation of cell survival, the serine-threonine kinase Akt/protein kinase B and its upstream regulator PI-3 kinase play a crucial role, and strategies activating Akt in cardiomyocytes offers protection from ischemia-induced cell death (41–43). We found that treatment of neonatal cardiomyocytes with a high concentration of neuronostatin led to increased necrotic cardiomyocyte death, and neuronostatin further augmented the H2O2-induced cell death. The latter was associated with a marked decrease in the amount of phosphorylated Akt. We then isolated cardiomyocytes from adult mouse hearts, and found that neuronostatin treatment also reduced the viability of adult cardiomyocytes. The neuronostatin-induced cell death was further augmented by JNK inhibition, whereas p38 MAPK inhibition had no effect. While JNK is known to play a central role in regulating cell viability, the exact intracellular mechanisms are not fully elucidated. In fact, in the context of tumor necrosis factor stimulation, early phase of JNK activation promotes cell survival, whereas sustained JNK activation is proapoptotic (44). Interestingly, exposure of neonatal cardiomyocytes to even high concentrations of neuronostatin had no effect on cardiomyocyte apoptosis as assessed by TUNEL. This may actually signal for shortage in cellular energy levels upon neuronostatin treatment, since as opposed to necrosis, apoptosis is dependent on cellular energy supplies as caspases require ATP for function.
MAPKs as well as numerous other signaling molecules also signal to mTor. Binding of mTor to Raptor and other cofactors results in formation of mTORC1, which is sensitive to rapamycin and positively regulates translational machinery (21). Peptide chain elongation in mammalian cells is regulated by two downstream targets of mTORC1, the inhibitory 4E binding protein 1 and eEF2 (45). Phosphoprotein eEF2 is canonically phosphorylated by a specific kinase, eEF2k, at Thr-56 residue resulting in inactivation of the kinase and shutdown of protein synthesis (21). eEF2k is calcium/calmodulin-dependent kinase, which is under the control of mTORC1 at several sites. Stimuli that activate protein synthesis, such as insulin, result in inactivation of eEF2k and dephosphorylation of eEF2 (21). p70 S6 kinase 1, a central regulator of mTORC1, induces phosphorylation of eEF2k at Ser-366 and Thr-78 leading to inhibition of the eEF2k activity (21). Recent studies have revealed an additional phosphorylation site of eEF2k, Ser-359, that is regulated by cyclin-dependent kinase 2, p38 MAPKδ and is also sensitive to p38 inhibitor SB203580 (22, 46, 47). In the current study, we found that eEF2k was consistently phosphorylated at Ser-359 residue with a low concentration of neuronostatin. While this can be mediated by a number of kinases, a putative factor is p38 MAPK, which was substantially activated by neuronostatin. As a consequence of reduced eEF2k activity, we also observed grossly reduced eEF2 phosphorylation upon neuronostatin treatment.
However, to our surprise, the neuronostatin-induced ribosomal regulation was associated with only a modest increase in actual protein synthesis. Growth stimuli such as insulin and other growth factors signal to mTor via a number of signaling pathways including Akt and ERK. On the other hand, activation of AMPK signaling has been shown to result in shutdown of mTor signaling involving phosphorylation of tuberous sclerosis complex 2 (48). AMPK is activated in situations of energy and amino acid depletion and functions to reduce energy consumption and to increase the cellular ATP levels (45). In our studies exposure of cardiomyocytes to low concentration of neuronostatin induced a marked increase in AMPK phosphorylation, but had no effect on Akt or ERK. It is therefore possible, that despite neuronostatin evokes stimuli to facilitate ribosome function by inducing activation of eEF2, cellular energy shortage, as witnessed by increased AMPK activation, is sufficient to block increased protein synthesis in an attempt to conserve cellular ATP supplies.
The present results suggest that exposure of cardiac myocytes to neuronostatin induces signaling effects that are distinct from those observed with somatostatin. The biological effects of neuronostatin on cardiomyocytes are concentration-dependent and may result from expression of multiple receptor subtypes. In summary, we found that in isolated rat heart neuronostatin administration attenuates ET-1 -induced left ventricular contractility and induces p38 MAPK and JNK phosphorylation. Prolonged treatment of isolated cardiomyocytes with neuronostatin reduces cardiomyocyte survival, which is further compromised by inhibition of JNK. We conclude that neuronostatin activates p38 MAPK and JNK in the heart regulating cardiac contractile function and cardiomyocyte survival. Receptors for neuronostatin need to be identified to further characterize the biological functions of the peptide.
Acknowledgments
We thank the expert technical assistance of Marja Arbelius, Tuulikki Kärnä, Kaisa Penttilä, and Kirsi Salo. We thank Dr. Willis Samson for support and ideas.
This work was supported, in whole or in part, by the Finnish Foundation for Cardiovascular Research, Sigrid Juselius Foundation, Academy of Finland (Center of Excellence) and Emil Aaltonen Foundation (to R. K.) and by Hungarian Scientific Research Fund (K69118) (to I. S.).
- NST
- neuronostatin
- SSTR
- somatostatin-transfected receptor
- PI
- phosphoinositide
- AMPK
- AMP-activated protein kinase
- LV
- left ventricular
- ET-1
- endothelin-1
- NRVM
- neonatal rat ventricular cardiomyocytes
- ACC
- acetyl-co-carboxylase
- LVEDP
- LV end-diastolic pressure.
REFERENCES
- 1. Samson W. K., Zhang J. V., Avsian-Kretchmer O., Cui K., Yosten G. L., Klein C., Lyu R. M., Wang Y. X., Chen X. Q., Yang J., Price C. J., Hoyda T. D., Ferguson A. V., Yuan X. B., Chang J. K., Hsueh A. J. (2008) Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J. Biol. Chem. 283, 31949–31959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. (1973) Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179, 77–79 [DOI] [PubMed] [Google Scholar]
- 3. Endou M., Hattori Y., Nakaya H., Kanno M. (1989) Differential effects of somatostatin on atrial and ventricular contractile responses in guinea pig heart: influence of pretreatment with islet-activating protein. J. Pharmacol. Exp. Ther. 250, 726–733 [PubMed] [Google Scholar]
- 4. Murray F., Bell D., Kelso E. J., Millar B. C., McDermott B. J. (2001) Positive and negative contractile effects of somatostatin-14 on rat ventricular cardiomyocytes. J. Cardiovasc. Pharmacol. 37, 324–332 [DOI] [PubMed] [Google Scholar]
- 5. Bogazzi F., Di B., V, Palagi C., Donne M. G., Di C. A., Gavioli S., Talini E., Cosci C., Sardella C., Brogioni S., Mariani M., Martino E. (2005) Improvement of intrinsic myocardial contractility and cardiac fibrosis degree in acromegalic patients treated with somatostatin analogues: a prospective study. Clin. Endocrinol. 62, 590–596 [DOI] [PubMed] [Google Scholar]
- 6. Bruno J. F., Xu Y., Song J., Berelowitz M. (1993) Tissue distribution of somatostatin receptor subtype messenger ribonucleic acid in the rat. Endocrinology 133, 2561–2567 [DOI] [PubMed] [Google Scholar]
- 7. Schwabe W., Brennan M. B., Hochgeschwender U. (1996) Isolation and characterization of the mouse (Mus musculus) somatostatin receptor type-4-encoding gene (mSSTR4). Gene 168, 233–235 [DOI] [PubMed] [Google Scholar]
- 8. Bell D., Zhao Y., McMaster B., McHenry E. M., Wang X., Kelso E. J., McDermott B. J. (2008) SRIF receptor subtype expression and involvement in positive and negative contractile effects of somatostatin-14 (SRIF-14) in ventricular cardiomyocytes. Cell Physiol. Biochem. 22, 653–664 [DOI] [PubMed] [Google Scholar]
- 9. Møller L. N., Stidsen C. E., Hartmann B., Holst J. J. (2003) Somatostatin receptors. Biochim. Biophys. Acta 1616, 1–84 [DOI] [PubMed] [Google Scholar]
- 10. Hua Y., Ma H., Samson W. K., Ren J. (2009) Neuronostatin inhibits cardiac contractile function via a protein kinase A- and JNK-dependent mechanism in murine hearts. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R682-R689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kerkelä R., Pikkarainen S., Majalahti-Palviainen T., Tokola H., Ruskoaho H. (2002) Distinct roles of mitogen-activated protein kinase pathways in GATA-4 transcription factor-mediated regulation of B-type natriuretic peptide gene. J. Biol. Chem. 277, 13752–13760 [DOI] [PubMed] [Google Scholar]
- 12. Martini J. S., Raake P., Vinge L. E., DeGeorge B., Jr., Chuprun J. K., Harris D. M., Gao E., Eckhart A. D., Pitcher J. A., Koch W. J. (2008) Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc. Natl. Acad. Sci. U.S.A. 105, 12457–12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pikkarainen S., Kerkelä R., Pöntinen J., Majalahti-Palviainen T., Tokola H., Eskelinen S., Vuolteenaho O., Ruskoaho H. (2002) Decoy oligonucleotide characterization of GATA-4 transcription factor in hypertrophic agonist induced responses of cardiac myocytes. J. Mol. Med. 80, 51–60 [DOI] [PubMed] [Google Scholar]
- 14. Kerkelä R., Grazette L., Yacobi R., Iliescu C., Patten R., Beahm C., Walters B., Shevtsov S., Pesant S., Clubb F. J., Rosenzweig A., Salomon R. N., Van Etten R. A., Alroy J., Durand J. B., Force T. (2006) Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med. 12, 908–916 [DOI] [PubMed] [Google Scholar]
- 15. Pollari E., Savchenko E., Jaronen M., Kanninen K., Malm T., Wojciechowski S., Ahtoniemi T., Goldsteins G., Giniatullina R., Giniatullin R., Koistinaho J., Magga J. (2011) Granulocyte colony stimulating factor attenuates inflammation in a mouse model of amyotrophic lateral sclerosis. J. Neuroinflammation. 8, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kerkela R., Woulfe K. C., Durand J. B., Vagnozzi R., Kramer D., Chu T. F., Beahm C., Chen M. H., Force T. (2009) Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin. Transl. Sci. 2, 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szokodi I., Tavi P., Földes G., Voutilainen-Myllylä S., Ilves M., Tokola H., Pikkarainen S., Piuhola J., Rysä J., Tóth M., Ruskoaho H. (2002) Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 91, 434–440 [DOI] [PubMed] [Google Scholar]
- 18. Piuhola J., Szokodi I., Kinnunen P., Ilves M., deChâtel R., Vuolteenaho O., Ruskoaho H. (2003) Endothelin-1 contributes to the Frank-Starling response in hypertrophic rat hearts. Hypertension 41, 93–98 [DOI] [PubMed] [Google Scholar]
- 19. Tenhunen O., Sármán B., Kerkelä R., Szokodi I., Papp L., Tóth M., Ruskoaho H. (2004) Mitogen-activated protein kinases p38 and ERK 1/2 mediate the wall stress-induced activation of GATA-4 binding in adult heart. J. Biol. Chem. 279, 24852–24860 [DOI] [PubMed] [Google Scholar]
- 20. Szokodi I., Kerkelä R., Kubin A. M., Sármán B., Pikkarainen S., Kónyi A., Horváth I. G., Papp L., Tóth M., Skoumal R., Ruskoaho H. (2008) Functionally opposing roles of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in the regulation of cardiac contractility. Circulation 118, 1651–1658 [DOI] [PubMed] [Google Scholar]
- 21. Wang X., Proud C. G. (2006) The mTOR pathway in the control of protein synthesis. Physiology 21, 362–369 [DOI] [PubMed] [Google Scholar]
- 22. Knebel A., Haydon C. E., Morrice N., Cohen P. (2002) Stress-induced regulation of eukaryotic elongation factor 2 kinase by SB 203580-sensitive and -insensitive pathways. Biochem. J. 367, 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Russell F. D., Molenaar P., O'Brien D. M. (2001) Cardiostimulant effects of urotensin-II in human heart in vitro. Br. J. Pharmacol. 132, 5–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szokodi I., Kinnunen P., Ruskoaho H. (1996) Inotropic effect of adrenomedullin in the isolated perfused rat heart. Acta Physiol. Scand. 156, 151–152 [DOI] [PubMed] [Google Scholar]
- 25. Kyriakis J. M., Avruch J. (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869 [DOI] [PubMed] [Google Scholar]
- 26. Chadee D. N., Kyriakis J. M. (2004) MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat. Cell Biol. 6, 770–776 [DOI] [PubMed] [Google Scholar]
- 27. Zarubin T., Han J. (2005) Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15, 11–18 [DOI] [PubMed] [Google Scholar]
- 28. Zechner D., Thuerauf D. J., Hanford D. S., McDonough P. M., Glembotski C. C. (1997) A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J. Cell Biol. 139, 115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liao P., Georgakopoulos D., Kovacs A., Zheng M., Lerner D., Pu H., Saffitz J., Chien K., Xiao R. P., Kass D. A., Wang Y. (2001) The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 98, 12283–12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braz J. C., Bueno O. F., Liang Q., Wilkins B. J., Dai Y. S., Parsons S., Braunwart J., Glascock B. J., Klevitsky R., Kimball T. F., Hewett T. E., Molkentin J. D. (2003) Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through up-regulation of calcineurin-NFAT signaling. J. Clin. Invest. 111, 1475–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nishida K., Yamaguchi O., Hirotani S., Hikoso S., Higuchi Y., Watanabe T., Takeda T., Osuka S., Morita T., Kondoh G., Uno Y., Kashiwase K., Taniike M., Nakai A., Matsumura Y., Miyazaki J., Sudo T., Hongo K., Kusakari Y., Kurihara S., Chien K. R., Takeda J., Hori M., Otsu K. (2004) p38α mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol. Cell. Biol. 24, 10611–10620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rose B. A., Force T., Wang Y. (2010) Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol. Rev. 90, 1507–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellahcene M., Jacquet S., Cao X. B., Tanno M., Haworth R. S., Layland J., Kabir A. M., Gaestel M., Davis R. J., Flavell R. A., Shah A. M., Avkiran M., Marber M. S. (2006) Activation of p38 mitogen-activated protein kinase contributes to the early cardiodepressant action of tumor necrosis factor. J. Am. Coll. Cardiol. 48, 545–555 [DOI] [PubMed] [Google Scholar]
- 34. Kerkela R., Force T. (2006) p38 mitogen-activated protein kinase: a future target for heart failure therapy? J. Am. Coll. Cardiol. 48, 556–558 [DOI] [PubMed] [Google Scholar]
- 35. Liao P., Wang S. Q., Wang S., Zheng M., Zheng M., Zhang S. J., Cheng H., Wang Y., Xiao R. P. (2002) p38 Mitogen-activated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circ. Res. 90, 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shao Z., Bhattacharya K., Hsich E., Park L., Walters B., Germann U., Wang Y. M., Kyriakis J., Mohanlal R., Kuida K., Namchuk M., Salituro F., Yao Y. M., Hou W. M., Chen X., Aronovitz M., Tsichlis P. N., Bhattacharya S., Force T., Kilter H. (2006) c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ. Res. 98, 111–118 [DOI] [PubMed] [Google Scholar]
- 37. Andreka P., Zang J., Dougherty C., Slepak T. I., Webster K. A., Bishopric N. H. (2001) Cytoprotection by Jun kinase during nitric oxide-induced cardiac myocyte apoptosis. Circ. Res. 88, 305–312 [DOI] [PubMed] [Google Scholar]
- 38. Ferrandi C., Ballerio R., Gaillard P., Giachetti C., Carboni S., Vitte P. A., Gotteland J. P., Cirillo R. (2004) Inhibition of c-Jun N-terminal kinase decreases cardiomyocyte apoptosis and infarct size after myocardial ischemia and reperfusion in anaesthetized rats. Br. J. Pharmacol. 142, 953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baines C. P., Molkentin J. D. (2005) STRESS signaling pathways that modulate cardiac myocyte apoptosis. J. Mol. Cell Cardiol. 38, 47–62 [DOI] [PubMed] [Google Scholar]
- 40. Minamino T., Yujiri T., Terada N., Taffet G. E., Michael L. H., Johnson G. L., Schneider M. D. (2002) MEKK1 is essential for cardiac hypertrophy and dysfunction induced by Gq. Proc. Natl. Acad. Sci. U.S.A. 99, 3866–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Datta S. R., Brunet A., Greenberg M. E. (1999) Cellular survival: a play in three Akts. Genes Dev. 13, 2905–2927 [DOI] [PubMed] [Google Scholar]
- 42. Matsui T., Nagoshi T., Rosenzweig A. (2003) Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle 2, 220–223 [PubMed] [Google Scholar]
- 43. Matsui T., Tao J., del Monte F., Lee K. H., Li L., Picard M., Force T. L., Franke T. F., Hajjar R. J., Rosenzweig A. (2001) Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104, 330–335 [DOI] [PubMed] [Google Scholar]
- 44. Ventura J. J., Hübner A., Zhang C., Flavell R. A., Shokat K. M., Davis R. J. (2006) Chemical genetic analysis of the time course of signal transduction by JNK. Mol. Cell 21, 701–710 [DOI] [PubMed] [Google Scholar]
- 45. Ma X. M., Blenis J. (2009) Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 [DOI] [PubMed] [Google Scholar]
- 46. Knebel A., Morrice N., Cohen P. (2001) A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38δ. EMBO J. 20, 4360–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith E. M., Proud C. G. (2008) cdc2-cyclin B regulates eEF2 kinase activity in a cell cycle- and amino acid-dependent manner. EMBO J. 27, 1005–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Inoki K., Zhu T., Guan K. L. (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 [DOI] [PubMed] [Google Scholar]