Background: Parasite fatty acid synthesis is an attractive drug target but complex and poorly understood.
Results: We delineate the molecular activity of two pathways in Toxoplasma combining metabolomic and genetic analyses.
Conclusion: The apicoplast is a significant source of fatty acids, and its products are further modified in the parasite endoplasmic reticulum.
Significance: We define the metabolic host-parasite relationship with molecular resolution in intracellular parasites.
Keywords: Chloroplast, Endoplasmic Reticulum (ER), Fatty Acid, Lipid Synthesis, Parasite, Apicomplexa, Apicoplast, Toxoplasma
Abstract
Apicomplexan parasites are responsible for high impact human diseases such as malaria, toxoplasmosis, and cryptosporidiosis. These obligate intracellular pathogens are dependent on both de novo lipid biosynthesis as well as the uptake of host lipids for biogenesis of parasite membranes. Genome annotations and biochemical studies indicate that apicomplexan parasites can synthesize fatty acids via a number of different biosynthetic pathways that are differentially compartmentalized. However, the relative contribution of each of these biosynthetic pathways to total fatty acid composition of intracellular parasite stages remains poorly defined. Here, we use a combination of genetic, biochemical, and metabolomic approaches to delineate the contribution of fatty acid biosynthetic pathways in Toxoplasma gondii. Metabolic labeling studies with [13C]glucose showed that intracellular tachyzoites synthesized a range of long and very long chain fatty acids (C14:0–26:1). Genetic disruption of the apicoplast-localized type II fatty-acid synthase resulted in greatly reduced synthesis of saturated fatty acids up to 18 carbons long. Ablation of type II fatty-acid synthase activity resulted in reduced intracellular growth that was partially restored by addition of long chain fatty acids. In contrast, synthesis of very long chain fatty acids was primarily dependent on a fatty acid elongation system comprising three elongases, two reductases, and a dehydratase that were localized to the endoplasmic reticulum. The function of these enzymes was confirmed by heterologous expression in yeast. This elongase pathway appears to have a unique role in generating very long unsaturated fatty acids (C26:1) that cannot be salvaged from the host.
Introduction
Apicomplexans are a phylum of single-celled eukaryotic parasites that includes the causative agents for important human diseases such as malaria, toxoplasmosis, and cryptosporidiosis as well as numerous veterinary pathogens. Control of these diseases is challenging, in no small part due to a rapid emergence of drug resistance that requires a continuous pipeline of new drugs with novel targets and/or modes of action. Apicomplexans are obligate intracellular pathogens and deploy complex mechanisms of synthesis and salvage to meet their essential nutritional requirements. The acquisition of lipids and fatty acids has emerged as a particularly important aspect of the metabolism of intracellular pathogens (1–3). Fatty acids are the main building blocks of membranes and also play a significant role in the post-translational modification of numerous proteins.
Apicomplexans were believed to be incapable of synthesizing their own fatty acids and to rely completely on host-derived lipids. However, the discovery of the apicoplast, a plastid-like organelle challenged this view. As found for the chloroplasts of plants and algae, the apicoplast contains a type II fatty-acid synthesis (FASII)7 pathway (4). Humans lack plastids and the associated FASII, and instead they rely on type I fatty acid synthesis for the production of bulk long chain fatty acids. A FASII-type synthase is present in human mitochondria, but there are structural differences between its components and those of bacterial or apicomplexan FASII (5). Furthermore, apicoplast FASII also differs from the mammalian FASI pathway, thereby making it a potential target for the treatment of apicomplexan infections (6). Initial pharmacological studies on Plasmodium falciparum and Toxoplasma gondii supported this idea (7, 8). Subsequent genetic studies found the essentiality of this pathway to be more complicated. We constructed a conditional mutant for the apicoplast acyl carrier protein (ACP) in T. gondii. ACP is a key molecule of the FASII pathway; fatty-acyl intermediates are covalently bound to ACP as the enzymes of the pathway act on them. Loss of ACP in this conditional mutant affects apicoplast biogenesis and results in the death of the parasite. Moreover, mice infected with a lethal dose of T. gondii can be cured by suppression of the FASII pathway, suggesting its importance in the growth and survival of the parasite (9). In contrast, this pathway is not essential for the bloodstream and mosquito stages of the malarial parasite Plasmodium, although it is essential for the liver stage that is required for establishment of infection in the mammalian host (10, 11). Other apicomplexan parasites, such as Cryptosporidium, Babesia, and Theileria appear to completely lack the FASII biosynthetic machinery suggesting that they are dependent on fatty acid salvage from the host (1).
Although both T. gondii and P. falciparum have been shown to take up radiolabeled fatty acids from the culture medium (12, 13), these parasites are predicted to contain a number of other fatty acid biosynthetic pathways that could partly compensate for the loss of FASII (1, 14). These alternative mechanisms include a FASI pathway (in T. gondii, Eimeria, and Cryptosporidium) and a putative fatty acid elongation pathway (in most apicomplexans with the exception of Theileria and Babesia). FASI in apicomplexans so far has only been studied in Cryptosporidium parvum (15, 16).
Overall, these studies suggest that apicomplexan fatty acid metabolism is complex and that multiple potentially redundant biosynthetic pathways could contribute to the total cellular fatty acid pool. Identifying the specific biochemical activities of individual components and delineating their sequence and interaction and their relative importance under different growth conditions are critical for understanding host-parasite interaction and identifying potential drug targets. Here, we develop a combined genetic and metabolomic strategy to identify the products of FASII and the elongation pathways for T. gondii. De novo fatty acid synthesis was monitored in wild type parasites and in mutant parasite lines in which specific fatty acid biosynthetic enzymes were selectively ablated by labeling parasite-infected host cells with [U-13C]glucose and analysis of parasite lipids by gas chromatography-mass spectrometry (GC-MS). The activity of specific fatty acid elongases was further examined by heterologous expression and biochemical analyses. Our analyses suggest that the FASII and elongase pathways have nonredundant functions in intracellular tachyzoite stages in maintaining the fatty acid composition of bulk lipids and that these de novo pathways cannot be by-passed by fatty acid salvage from the host cell.
EXPERIMENTAL PROCEDURES
Gene Identification and Gene Tagging
The protein sequences of the yeast fatty acid elongation machinery (ELO1–3, ketoacyl-CoA reductase, dehydratase, and enoyl-CoA reductase; GenBankTM accession numbers NP_012339, NP_009963, NP_13476, AAS56194, NP_012438, NP_010269) were used as query sequences for BLAST searches against the T. gondii genome and GenBankTM databases. To evaluate the computational predictions for these genes, we conducted RT-PCR experiments. The predictions for T. gondii ELO-A, ELO-B, ELO-C, and DEH were confirmed. However, for ECR and KCR, the beginning and ending of the coding sequences were different and were subsequently established by RT-PCR, subcloning, and sequencing. The experimentally validated T. gondii genes described in this study are ELO-A, ELO-B, ELO-C, KCR, DEH, and ECR (see supplemental Table S1 for GenBankTM accession numbers). The full-length coding sequences for all genes were amplified from T. gondii cDNA using primers introducing flanking BglII and AvrII restriction sites, subcloned into plasmid pCR2.1 (Invitrogen), and subsequently introduced into the equivalent sites of either plasmid pdt7s4H3 or pdt7s4M3 (17), placing them under the control of a tetracycline-regulatable promoter and fusing a triple HA (pDT7S4H3) or a triple c-Myc epitope tag (pDT7S4M3), respectively, to the 3′-end. All primers used are listed in supplemental Table S1.
Parasite Culture and Construction of Mutants
Parasites were cultured and genetically manipulated as described previously (18). Conditional mutants were generated using the previously described two-step strategy (9, 19). Plasmids containing a second copy of the target gene under the control of a regulatable promoter (pdt7s4H3) were stably introduced into a T. gondii tetracycline transactivator strain (20). Stable clones for ELO-A, -B, or -C were established in the presence of pyrimethamine. We next engineered a plasmid-based construct to delete the ELO-C locus and cosmid-based targeting vectors for ELO-A and -B. For ELO-C, sequences (∼2 kb) upstream and downstream of the ELO-C genomic locus were amplified by PCR and subcloned into the respective BamHI/KpnI and AatII/SpeI restriction sites of the PTCY vector flanking a chloramphenicol resistance cassette (the vector also contains a YFP cassette for negative selection (9)). This construct was linearized with SpeI and transfected into the pDT7S4-ELO-C-H3 parasite line. Transgenic parasites that were chloramphenicol-resistant and YFP-negative were isolated. Clones were tested by PCR for integration, and gene disruption was confirmed by Southern blot analyses. (Plasmids used for the probes are listed in supplemental Table S2.) Cosmids (TOXP929/ELO-B or TOXO789/ELO-A) were modified to replace the coding sequence with a chloramphenicol resistance cassette by recombineering (19). Targeting cosmids were transfected into the respective parasite, and transgenic clones were selected in the presence of chloramphenicol. Mutants were identified as described for ELO-C. We conducted plaque assays in the absence or presence of 0.5 μm ATc to evaluate the growth of mutants (18). For chemical complementation assays, myristic and palmitic acids were dissolved in equal molar amounts in ethanol, dried into a microcentrifuge tube, and complexed to fatty acid-free BSA in water (Invitrogen, 1:3 molar ratio) with sonication prior to addition to normal growth medium as indicated.
Microscopy
Immunofluorescence microscopy was performed as described previously (17). Infected coverslip cultures were fixed with 3% paraformaldehyde in PBS, followed by permeabilization in 0.25% Triton X-100 in PBS. After blocking, rat anti-HA antibodies (Roche Applied Science) were used at a dilution of 1:100, rabbit anti-ACP (a kind gift from Geoff McFadden, University of Melbourne (4)) at 1:1000, mouse anti-GFP (Torrey Pines Biolabs) at 1:400, and rabbit anti-Myc (Roche Applied Science) at 1:100. Secondary antibodies were used at a dilution of 1:200 and were anti-rat Alexa Fluor 546, anti-rabbit Alexa Fluor 546, anti-mouse Alexa Fluor 488, and anti-rat Alexa Fluor 488 (all from Invitrogen). Images were taken using an Applied Precision Delta Vision microscope, and images were deconvoluted and adjusted for contrast using Softworx. Protein localization annotations have been submitted to the ApiLoc data base of protein subcellular localization in Apicomplexa.
Western Blotting
Western blot analyses were performed as described previously (17) and used rat anti-HA antibodies (Roche Applied Science) at 1:100 and anti-GRA8 antibodies at 1:2000 (a kind gift from Gary Ward, University of Vermont (21)).
[14C]Acetate Radiolabeling and Thin Layer Chromatography
Parasites were grown in the absence and presence ATc for 48 h, and free tachyzoites were metabolically labeled as described previously (9). Briefly, 108 tachyzoites were incubated with 10 μCi of sodium [14C]acetate (Movarek) in 1 ml of DMEM for 4 h at 37 °C and 5% CO2. Total lipids were extracted with chloroform/methanol (2:1). The extract was dried, and fatty acid methyl esters were subjected to acidic methanolysis. The resulting fatty acid methyl esters were extracted with hexane and analyzed on RP-18 HPTLC plates developed in methanol/chloroform/water (75:25:5) and exposed to film for 1 week.
Stable Isotope Labeling and Metabolomic Analyses
T. gondii-infected fibroblasts were grown in DMEM in a T175 flask in the absence or presence of ATc for 48 h. The medium was supplemented with 8 mm [U-13C]glucose (final concentration of [13C/12C]glucose was 16 mm) 24 h prior to egress. Free parasites were separated from host cells by filtration through a membrane with a 3-μm pore size. Parasites were quenched by rapid chilling of the cell suspension in a dry ice/ethanol bath, and parasites were recovered by centrifugation (4000 × g, 25 min, 0 °C). Cell pellets were washed three times with ice-cold PBS, and cell aliquots (2 × 108 cells) were transferred to microcentrifuge tubes and centrifuged (10,000 × g, 30 s, 0 °C). Pellets were suspended in chloroform (50 μl) and vortex-mixed thoroughly prior to the addition of 200 μl of methanol/water (3:1). Samples were sonicated for 1 min at RT and then incubated at 60 °C for 20 min. Extracts were centrifuged at 10,000 × g for 5 min, and the supernatant was subjected to biphasic partitioning by addition of 100 μl of water. The organic phase was transferred to a partially sealed capillary for methanolysis, dried with an internal standard of 1 nmol of scyllo-inositol, and subsequently washed twice with methanol. Dried residues were dissolved in 0.5 n methanolic HCl (50 μl, Sigma), and the capillaries were sealed under vacuum and incubated at 95 °C overnight. Samples were neutralized with 10 μl of pyridine (20 min) before being transferred to GC-MS vials, dried, and derivatized by addition of 25 μl of N-methyl-N-(trimethylsilyl)-trifluoroacetamide/trimethylchlorisane. Samples were analyzed on an Agilent 7890A-5975C GC-MS system. Splitless injection (injection temperature 280 °C) onto a 30 m + 10 m × 0.25 mm DB-5MS + DG column (J&W, Agilent Technologies) was used, using helium as the carrier gas. The initial oven temperature was 80 °C (2 min), followed by temperature gradients to 140 °C at 30 °C/min, from 140 to 250 °C at 5 °C/min, and from 250 to 265 °C at 15 °C/min. The final temperature was held for 10 min. Data analysis was performed using Chemstation software (MSD Chemstation D.01.02.16, Agilent Technologies). Abundance and label incorporation was calculated as described previously (22). Data shown are the average of three technical replicates and their standard deviation. They are representative of two biological experiments. Statistical significance was evaluated by Wilcoxon Rank Sum Testing with continuity corrections, using the R data analysis package (version 2.14.0), where p values of <0.05 were considered significant.
Yeast Media
Yeast strains were grown on YP (1% yeast extract, 2% peptone) or yeast synthetic complete (SC) media (yeast synthetic dropout medium supplement (Sigma)), supplemented with 0.67% Difco yeast nitrogen base (BD Biosciences), containing either 2% (w/v) d-galactose and 0.05% (w/v) d-glucose (GalGlu), 4% (w/v) d-glucose or 2% (w/v) raffinose as the carbon source(s). For solid media, 2% agar was added. Selections were carried out on plates lacking leucine (SCGalGlu−Leu). Counter-selections against plasmids containing a URA3 marker were done on 5′-fluoroorotic acid plates (23) supplemented with GalGlu (5′FOAGalGlu). Liquid culture with 4% (w/v) d-glucose was used to repress expression from the GAL1 promoter. Strains subjected to spotting assay were grown overnight in YPGalGlu, kept for 24 h below an A600 <1, and then grown for 8 h in YPRaf. The cell density was normalized to an A600 = 0.5 before being spotted on YPGalGlu, SCGalUra GalGlu, and SC 5′FOAGalGlu.
Yeast Strain Construction
The yeast strain Saccharomyces cerevisiae W1536 Δelo2Δelo3/YCp33 GAL1 ScELO3 (URA3) (Δade2, Δade3, his3-11, his3-12, trp1-1, ura3-1, elo2::kanMX4, elo3::kanMX4) has been described before (24–26). The yeast strain was transformed with either one of the following plasmids (all LEU2): YCp111 GAL1-TgELO-B, YCp111 GAL1-TgELO-C, or YCpP111 GAL1-TgELO-A on SCGalGlu-Leu. (Primers used in the construction of these plasmids are listed in supplemental Table S3.) Candidate transformants on SCGalGlu-Leu plates were picked and streaked on 5′-FOAGalGlu plates to select against the presence of the YCp33 GAL1 ScELO3 plasmid. Complementation of the Δelo2Δelo3 mutations was indicated by the production of a large amount of FOA-resistant colonies. Presence of the plasmids carrying T. gondii constructs was verified by the ability of the FOA-resistant colonies to grow on SCGalGlu-Leu. These candidates were used in further experiments. Strain W1536-5B Δtsc13/YCplac33 GAL1 ScTSC13 (MATa; Δade2, Δade3, his3-11, his3-12, trp1-1, ura3-1, tsc13::kanMX4) was transformed with YCp111 GAL1-TgTSC13 on SCGalGlu-Leu, and colonies complemented by T. gondii TSC1 were identified and isolated in an analogous fashion as described above. Finally, yeast mutants complemented by T. gondii PHS1 were generated in a similar manner, using strain W1536-5B Δphs1/pYES2 PHS1 (MATa; Δade2, Δade3, his3-11, his3-12, trp1-1, ura3-1, phs1::kanMX4) transformed with YCp111 GAL1-TgPHS1.
Preparation of ER Extracts from Yeast Cells
Yeast cells were grown to exponential phase (2·106 cells·ml−1) and then harvested by centrifuging for 15 min at 1300 × g, 4 °C. The pellet was washed with sterile water and suspended in ice-cold lysis buffer. The cells were disrupted with glass beads and centrifuged for 15 min at 15,000 × g at 4 °C to remove the cell debris. The supernatants were centrifuged for 90 min at 85,000 × g in a Sorvall Ti70 rotor at 4 °C to generate pellet (P85) and supernatant (S85) fractions. The P85 was suspended in lysis buffer and used for the elongase assay. The protein concentrations were determined by the method of Lowry using bovine serum albumin as the standard. Equivalent amounts of total lysate, P85, and S85 were precipitated by adding trichloroacetic acid (TCA) to a 10% final concentration and processed for immunoblotting.
Yeast Fatty Acid Analysis
Yeast cultures were inoculated from an overnight starter culture to an A600 of 0.05 in 100 ml of YPGalGlu, allowed to grow to an A600 of 0.5, and harvested by 5 min centrifugation at 4300 × g. Cells were washed in 50 ml of water and harvested by centrifugation. The cell pellet was flash-frozen in liquid nitrogen and stored at −70 °C. Fatty acid analysis was performed following methods published earlier (27) with minor modifications. Briefly, the cell pellets were extracted with 1 ml of chloroform/methanol/formic acid (10:10:1), followed by a second extraction with 1 ml of chloroform/methanol/water (5:5:1 v/v). The combined organic phases were washed with an equal volume of 1 m KCl in 0.2 m phosphoric acid and dried. Lipids were dissolved in 1 ml of toluene and treated with 1 ml of methanolic HCl (prepared by adding 0.5 ml of acetyl chloride to 5 ml of cold dry methanol) overnight at 50 °C. The solution was washed with 1 ml of 4 m KCl; the organic phase was separated, and the aqueous phase was extracted with 1 ml of hexane. The organic phases were pooled, washed with 0.5 ml of 0.5 m ammonium bicarbonate, dried, redissolved in 100 μl of hexane, and subjected to gas chromatography on an HP6890 gas chromatograph with an HP5973 mass analyzer and equipped with a J&W Scientific DB624 (30 m × 0.32 mm, stationary phase 1.8 μm) column. 1 μl was injected splitless (detector block 250 °C) and subjected to a temperature program from 60 to 250 °C (20 °C/min) and a hold period of 35 min, flow 2 ml/min. Masses were scanned from m/z 35 to 800, and a Wiley 275 spectral library was used for identification.
RESULTS
Toxoplasma Is Capable of de Novo Fatty Acid Synthesis
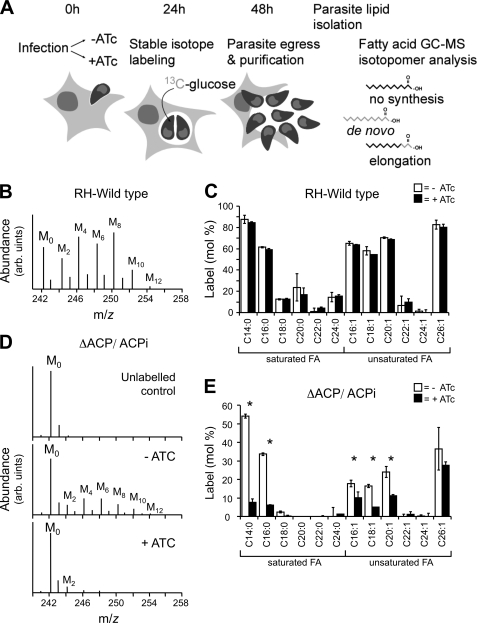
In apicomplexans, the enzymes of the FASII pathway are localized to the apicoplast (4). In plants, the homologous chloroplast pathway is the sole source of de novo-synthesized fatty acids. We have previously constructed a T. gondii mutant with a conditional defect in this pathway. Although the growth of this mutant was severely reduced upon inhibition of FASII, no difference was observed in the rate of fatty acid biosynthesis as measured using [14C]acetate (9). We considered that FASII may not use acetyl-CoA generated from exogenous [14C]acetate. To test this, we have established a new metabolic labeling procedure, in which the incorporation of 13C-derived from [U-13C]glucose into the entire cellular pool of fatty acids is detected by GC-MS analysis (Fig. 1, A–C). When infected fibroblasts were labeled with [U-13C]glucose, label was rapidly incorporated into tachyzoite lipids that were recovered from extracellular stages after host cell lysis and parasite egress. [U-13C]Glucose labeling of extracellular tachyzoites resulted in negligible labeling of parasite lipids indicating that de novo fatty acid synthesis occurs predominantly in intracellular stages and decreases dramatically following egress (data not shown). Host fibroblast cells are also capable of synthesizing fatty acids via a FASI system. In principal, 13C derived from exogenous [13C]glucose could be incorporated into host fatty acids and subsequently scavenged by intracellular tachyzoites. To investigate the extent to which fibroblasts synthesize fatty acids, uninfected fibroblasts were metabolically labeled with [U-13C]glucose, and incorporation of 13C into fatty acids was assessed by GC-MS. In contrast to the intracellularly labeled tachyzoites, negligible label was detected in the uninfected fibroblast lipids (Table 1). These experiments, together with the data presented below, indicate that 13C labeling of tachyzoite fatty acids with [13C]glucose represents de novo fatty acid biosynthesis within the parasite rather than uptake of labeled host lipids. In this context, it is notable that significant differences occur in the extent to which different fatty acids are labeled. Although the long and very long chain fatty acids, C14:0, C16:0, C16:1, C18:1, C20:1, and C26:1, were strongly labeled (>60%) others, including C18:0, C22:0, and C24:1, were labeled to less than 20%. These differences could reflect differences in the turnover and achievement of isotopic equilibrium in different fatty acids pools or, more likely, differences in the rate of salvage of “unlabeled” host fatty acids. Regardless, the results indicate that a number of major fatty acids in intracellular stages are primarily synthesized de novo rather than taken up from the host.
FIGURE 1.
Metabolic labeling of intracellular tachyzoites with [U-13C]glucose and analysis of de novo fatty acid biosynthesis. A, T. gondii-infected fibroblasts were precultured in the presence or absence of ATc for 24 h and then labeled with [U-13C]glucose 24 h prior to parasite egress. After host cell lysis, extracellular tachyzoites were metabolically quenched in a dry ice/ethanol bath and separated from host cell debris by filtration prior to metabolite extraction. Examples of different labeling patterns (13C indicated in gray) and their interpretation are schematically shown for myristate. B, lipids from [U-13C]glucose-labeled tachyzoites were subjected to methanolysis and trimethylsilyl derivatization and analyzed by GC-MS. A portion of the mass spectrum containing the molecular ions of the methyl ester of myristate is shown. Unlabeled myristoyl methyl ester (M0) has an m/z of 242 atomic mass units. Higher mass isotopomers (M1–12) contain between 1 and 12 13C atoms, corresponding to incorporation of 13C into the fatty acid (FA) biosynthetic pathways via [13C]acetyl-CoA. arb., arbitrary units. C, incorporation of 13C into the major fatty acids of intracellular wild type T. gondii tachyzoites (labeling is given as mol % of all labeled mass isotopomers (M1–12) relative to M0, after correction for natural abundance). The fatty acid notation Cn:m indicates the length of the fatty acid (n, carbon number) and degree of unsaturation (m, number of double bonds). Note that treatment of wild type parasites with ATc (black) does not result in any significant changes of the fatty acid labeling pattern when compared with untreated controls (white). D, intracellular tachyzoites of the T. gondii ΔACP/ACPi mutant carrying an inducible copy of FASII ACP were labeled with [U-13C]glucose in the presence or absence of ATc. Total lipids were extracted from the purified tachyzoites and released fatty acid methyl esters analyzed by GC-MS. The mass spectrum (molecular ion region from m/z 240 to 250) of the myristate fatty acid methyl esters from unlabeled parasites, and from [13C]glucose-labeled parasites cultured in the absence or presence of ATc is shown. Incorporation of multiple 13C2H4 units into myristate is observed in the absence of ATc (ACP active) and largely inhibited in the presence of ATc (ACP repressed). The spectrum is representative of three individual experiments. E, total 13C incorporation into fatty acids in ΔACP/ACPi tachyzoites labeled in the presence (black) or absence (white) of ATc. Error bars represent standard deviation where n = 3. Fatty acids for which significant changes in labeling were observed in the presence and absence of ATc using the Wilcoxon rank sum test (p values less than 0.05) are indicated with an asterisk. The absolute amount of incorporation can vary from experiment to experiment depending on the stage of the culture (no ATc in C and E). We therefore always directly compare ATc-treated samples with an untreated control from the same culture batch.
TABLE 1.
Percent labeling of fatty acids derived from [U-13C]glucose-fed tachyzoites and HFF
Wild type RH parasites were used to infect HFF and labeled in situ with [13C]glucose. Uninfected HFF were labeled with lsqb]13C]glucose under identical conditions. Percent labeling was determined by GC-MS analysis.
| Cell type | RH | RH | HFF |
|---|---|---|---|
| +/− ATc | − | + | − |
| C14:0 | 87.73 (± 3.86) | 84.68 (± 0.50) | ND |
| C16:0 | 61.56 (± 0.58) | 59.01 (± 1.09) | 0.00 (± 0.00) |
| C18:0 | 12.60 (± 0.55) | 12.64 (± 0.73) | 0.00 (± 0.00) |
| C20:0 | 23.64 (± 12.81) | 16.84 (± 6.41) | 0.00 (± 0.00) |
| C22:0 | 1.14 (± 2.95) | 4.23 (± 1.05) | 0.00 (± 0.00) |
| C24:0 | 14.57 (± 4.41) | 15.38 (± 1.53) | 0.00 (± 0.00) |
| C16:1 | 65.15 (± 1.62) | 63.76 (± 0.47) | 0.00 (± 0.00) |
| C18:1 | 57.97 (± 3.88) | 54.59 (± 0.15) | 1.76 (± 1.77) |
| C20:1 | 70.41 (± 0.82) | 68.62 (± 0.71) | 0.00 (± 0.00) |
| C22:1 | 6.81 (± 8.79) | 9.76 (± 3.44) | 0.00 (± 0.00) |
| C24:1 | 1.31 (± 1.87) | 0.00 (± 2.58) | 0.00 (± 0.00) |
| C26:1 | 82.83 (± 4.14) | 80.37 (± 2.75) | ND |
Apicoplast FASII Is Required for Synthesis of Long Chain Fatty Acids
The detection of multiple mass isotopomers of the long chain fatty acids, differing by 2 atomic mass units in [U-13C]glucose fed-parasites (Fig. 1B) indicated multiple rounds of incorporation of [13C]acetyl-CoA and [12C]acetyl-CoA into these chains, consistent with the operation of a FAS system. To investigate the contribution of the apicoplast-located FASII complex to cellular fatty acid synthesis, fibroblasts were infected with the T. gondii ΔACP/ACPi mutant that expresses components of the apicoplast FASII complex under the control of a tetracycline-regulated promoter (9). When T. gondii ΔACP/ACPi-infected fibroblasts were incubated with [U-13C]glucose in the absence of ATc, high levels of 13C incorporation were observed in tachyzoite lipids (Fig. 1, D and E). In contrast, when labeling was done in the presence of ATc, resulting in selective down-regulation of parasite FASII activity, the labeling of tachyzoite fatty acids was strongly inhibited. In particular, labeling of the long chain saturated and unsaturated fatty acid C14:0 and C16:0 was reduced by >80% in the presence of ATc (Fig. 1E). In a control experiment, ATc was found to have no effect on the labeling of wild type tachyzoite fatty acids, indicating that ATc by itself does not inhibit [U-13C]glucose uptake and catabolism to acetyl-CoA or fatty acid synthesis (Fig. 1C and Table 1). These experiments provide further direct support for the conclusion that labeling of parasite fatty acids with [U-13C]glucose reflects parasite de novo synthesis rather than salvage. They also suggest that the FASII pathway is directly responsible for the de novo synthesis of saturated long chain fatty acids (C14:0 and C16:0) that are utilized, at least in part, for the synthesis of unsaturated and very long chain fatty acids.
Growth Defects Due to Loss of FASII Pathway Are Only Partially Restored by Chemical Complementation with Myristic and Palmitic Acid
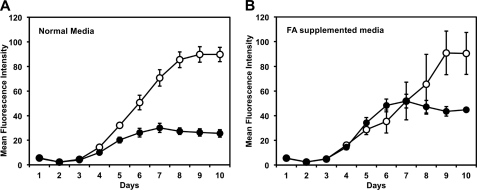
We have previously shown that loss of FASII activity leads to inhibition of parasite growth (9). As loss of FASII results in a deficiency in myristic and palmitic acid synthesis, we investigated whether nutritional supplementation of FASII-deficient parasites with these fatty acids would restore growth. Parasite lines expressing red fluorescent protein were cultured in 96-well plates in medium supplemented with fatty acids complexed to BSA at concentrations ranging from 20 nm to 2 μm. These fatty acid concentrations have no effect on the growth of the wild type parasites (data not shown). As expected, intracellular growth of the ΔACP/ACPi mutant was markedly reduced after 6 days in the presence of ATc (Fig. 2A) (9). Growth inhibition in the presence of ATc was delayed by 2 days in the presence of a combination of 1 μm myristic and 1 μm palmitic acid. However, fatty acid supplementation did not restore wild type growth or prevent death over longer periods, possibly reflecting inefficient transport to the parasite vacuole, increased fatty acid demand at later time points, and/or the involvement of FASII in other lipid biosynthetic pathways, such as the synthesis of lipoic acid.
FIGURE 2.
Growth of ΔACP/ACPi parasites in media supplemented with myristic and palmitic acid. Growth of ΔACP/ACPi parasites stably expressing a dTomatoRFP transgene was evaluated by measuring parasite fluorescence daily in a 96-well culture format (35). Parasites were grown in normal growth media supplemented with fatty acid-free BSA (A) or media supplemented with 1 μm of each myristic and palmitic acid coupled to BSA (3:1 molar ration) (B). Parasites were grown in the presence (black) or absence (white) of ATc. The average of fluorescence intensity (arbitrary units) for three independent replicates is shown, and error bars represent the standard deviation for each data point. Continuous culture in BSA-conjugated fatty acids (or higher serum supplementation) did not lead to continuous growth of the ACP mutant in the presence of Atc. FA, fatty acids.
Parasites Express an Endoplasmic Reticulum-associated Fatty Acid Elongation System
Several very long chain fatty acids were labeled with [U-13C]glucose in the absence of FASII suggesting that additional fatty acid biosynthetic pathways are active during intracellular tachyzoite development. Bioinformatic analysis of the T. gondii genome indicates that these parasites may have a functional fatty acid elongase system. Using the sequences of the components of the yeast elongation pathway as query in similarity searches, we found three candidate genes coding for fatty acid elongases and a single candidate for each of the downstream reductases and the dehydratase in T. gondii (see supplemental Table S1).
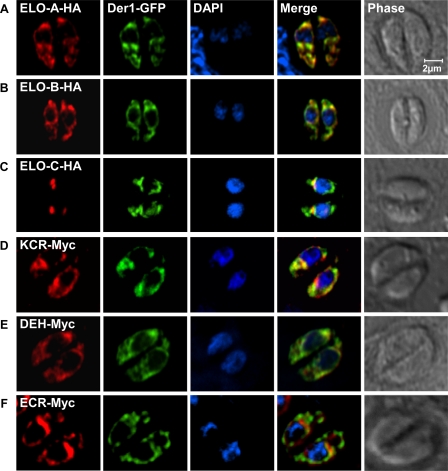
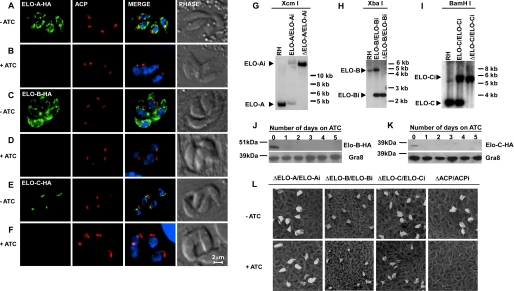
We evaluated the coding sequence for all six genes by cDNA and rapid amplification of cDNA ends-PCR followed by subcloning and sequencing. This analysis validated the computationally predicted gene models for four genes: ELO-A–C and the dehydratase, which is annotated as a hypothetical protein tyrosine phosphatase-like domain-containing protein in the T. gondii genome database. We established a diverging coding sequence for the presumptive enoyl-reductase and keto-acyl-CoA reductase (see “Experimental Procedures” and supplemental Table S1 for details). The coding sequences were amplified and introduced into a parasite expression vector that appended a C-terminal epitope tag. These constructs were transfected into parasites, and stable transgenic lines were established through drug selection and cloning (see “Experimental Procedures” for details). The six epitope-tagged proteins localized to the same perinuclear structure in the parasite, as determined by immunofluorescence assays. For comparison, we transfected these lines with an expression plasmid for Der1-GFP (17), a marker for the T. gondii endoplasmic reticulum. This marker co-localized with the epitope-tagged proteins indicating that these enzymes localize to the endoplasmic reticulum (Fig. 3) (note that ELO-C had a more restricted pattern at the apical face of the nuclear envelope reminiscent of the previously characterized ER exit site (28)). These observations are consistent with reports from other organisms where the components of the fatty acid elongation pathway have been found to form a multienzyme complex on the membrane of the endoplasmic reticulum (29, 30).
FIGURE 3.
T. gondii fatty acid elongation pathway is localized to the parasite endoplasmic reticulum. Transgenic parasite lines were allowed to infect coverslip cultures and were fixed and processed for immunofluorescence assay 24 h later. Transgenic proteins were marked with an HA (A–C) or a Myc (D–F) epitope tag detected with suitable antibodies (red channel). All strains carried a Der1GFP marker (green) previously shown to localize to the endoplasmic reticulum (17). DAPI and phase contrast images are shown for comparison. See supplemental Table S1 for reference to T. gondii gene models and “Experimental Procedures” for details on gene identification and cloning. Note that all six candidate fatty acid elongation enzymes are found to be associated with the endoplasmic reticulum. Tagged ELO-C produces a more restricted pattern at the apical side of the nuclear envelope.
Candidate T. gondii Genes Rescue Lethal Yeast Fatty Acid Elongation Mutants
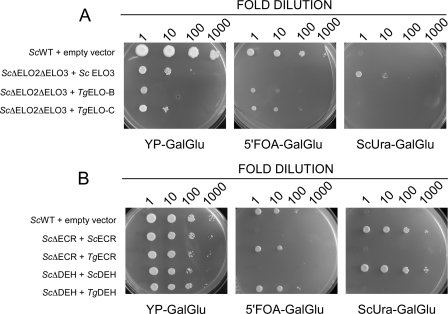
We next tested the molecular function of each of the six enzymes by heterologous expression in well defined yeast mutants lacking equivalent enzymes. For these phenotypic complementation experiments, we constructed yeast expression vectors under the control of the galactose-activated/glucose-repressed GAL1 promoter. Constructs encoding the putative T. gondii fatty acid elongases were transformed into S. cerevisiae strain W1536 Δelo2Δelo3/YCp33 GAL1-ScELO3 (26). This mutant lacks the genes for both fatty acid elongase 2 and 3. This is a lethal double mutation (27), and the strain is only viable due to episomal expression of elongase 3 from a GAL1 promoter. Following transformation with parasite test plasmids, loss of the URA3-containing yeast plasmid was achieved by counter-selection on media containing the URA3 activated toxic substrate 5′-fluoroorotic acid. T. gondii ELO-B and ELO-C but not ELO-A rescued the growth deficiency of the Δelo2Δelo3 mutant strain (Fig. 4A). Functional complementation was further validated by incubation of microsomal fractions prepared from the two respective complemented strains with radiolabeled malonyl-CoA and palmitoyl-CoA (31). As expected, this resulted in chain elongation of the substrate (data not shown). Similarly, when expressed in yeast cells, the T. gondii candidates for dehydratase and enoyl reductase were found to complement the growth of mutants in their respective yeast homologs (S. cerevisiae Phs1p and Tsc13, see Fig. 4B and “Experimental Procedures” for detail). Overall, these experiments suggest that the T. gondii candidate genes indeed encode the enzymes of the fatty acid elongation system. We cannot make firm conclusions for TgELO-A and TgKCR that failed to complement. This may be due to lack of expression or poor interaction of the heterologous enzymes with the native yeast machinery.
FIGURE 4.
T. gondii genes complement yeast fatty acid elongation mutants. Yeast mutants with deletions in specific genes encoding fatty acid elongation enzymes were transformed with plasmids carrying T. gondii candidate genes as detailed under “Experimental Procedures.” The resulting strains were used to conduct spotting assays on YPGalGlu media and demonstrate complementation (panels on the left). Control experiments using the same yeast cultures were done on media that select against the yeast rescue plasmid and thus indicate that phenotypic complementation is due to presence of the T. gondii constructs only (5′FOA-GalGlu), or media that indicate presence of the yeast rescue plasmids only (ScUra-GalGlu) (center and right panels, respectively). Yeast cultures were progressively diluted prior to plating from left to right as indicated. A, complementation analyses for the yeast elongase ScΔelo2Δelo3 double mutant and the yeast (B) enoyl-CoA reductase (ScΔECR) and dehydratase (ScΔDEH) mutants, respectively. Note that under restrictive conditions (5′FOA-GalGlu) growth depends on the presence of the T. gondii genes. Only experiments that resulted in phenotypic complementation are shown here. See Table 2 for full detail on the genotype of all yeast strains.
Genetic Ablation of Fatty Acid Elongases in T. gondii
To further investigate the functional significance of the fatty acid elongase system in Toxoplasma, we generated conditional parasite mutants for the condensing enzymes of the pathway. A parasite strain expressing epitope-tagged ELO-B under a tetracycline-regulated promoter from an ectopic locus was constructed. In this background, we replaced the chromosomal copy of this gene with a chloramphenicol resistance cassette using homologous recombination. Chloramphenicol-resistant parasites were screened by PCR to identify the clones with genetic disruption for ELO-B. Using a similar strategy, we also engineered conditional mutants for ELO-C and ELO-A (see “Experimental Procedures” for details and note that some deletions were constructed using plasmids (9) and others with cosmids (19)). Deletion of the respective locus was confirmed for each mutant by Southern blot (Fig. 5, G–I). We evaluated the ATc dependence of the expression of each of the genes by immunofluorescence assay and Western blot assays. For immunofluorescence assay, mutant parasites were grown in the absence and presence of ATc for 24 h prior to fixation and processing. For Western blots, parasites were grown in ATc for 0–4 days. Both assays show rapid loss of the tagged proteins upon ATc addition. The proteins are essentially undetectable after 24 h of treatment (Fig. 5). To determine the effect of the loss of these proteins on parasite growth, we next performed plaque assays. Confluent host cell monolayers were infected with the mutant parasites in the absence or presence of ATc. Plaques in the host monolayer were visualized by staining after 10 days. We detected no significant difference in plaque number or size for each of the three elongase mutants (Fig. 5L). The ACP mutant was used as a positive control, and reduced plaque formation was readily observed as described previously (9). We conclude that individual loss of ELO-A, ELO-B, or ELO-C does not impede parasite growth in tissue culture.
FIGURE 5.
Conditional mutants lacking individual fatty acid elongases exhibit normal growth. We constructed conditional mutants for each of the three T. gondii ELO genes. A–F, immunofluorescence assay detecting the expression of the HA epitope-tagged ectopic allele for the indicated ELO genes when grown in the absence (−ATc) or presence (+ATc) for 24 h. Merge images also show DAPI staining of DNA. G–I, Southern blot analysis probing genomic DNA from RH wild type, a strain carrying both the native and the conditional allele, and the mutant carrying only the conditional allele (from left to right for each of the respective mutants as indicated). Probes detect the respective coding region of each gene and are detailed in supplemental Table S2. In each blot the expected size of the restriction fragment for the native locus (e.g. ELO-B) and the conditional locus (e.g. ELO-Bi) is indicated with an arrowhead. Note loss of native loci. J and K, Western blot analysis detecting ELO-Bi and ELO-Ci expression using an antibody against the HA tag. GRA8 is shown as a loading control. L, growth of the conditional ELO mutants was measured by plaque assay in absence and presence of ATc as indicated. ΔACP/ACPi serves as positive control.
T. gondii ELO Proteins Are Required for the Synthesis of Very Long Chain Fatty Acids
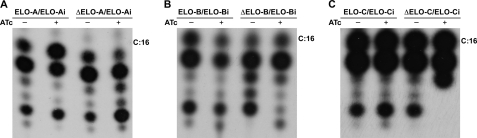
We do not detect a growth phenotype in single elongase-deficient parasites. This could reflect the possibility that fatty acids synthesized by the elongase system are not essential, the ELO enzymes are functionally redundant (27), and/or the intracellular parasites can compensate for the loss of the pathways by salvaging necessary fatty acids from the host. To determine the specific biochemical role of individual elongases, the fatty acid biosynthesis capabilities of each mutant were initially analyzed using [14C]acetate labeling. Parasites were grown in the absence or presence of ATc, liberated from their host cells, and incubated in fatty acid-free medium supplemented with 10 μCi of [14C]acetate. Lipids were extracted after 4 h, and the corresponding fatty acid methyl esters were analyzed by thin layer chromatography (Fig. 6). The loss of ELO-B resulted in a moderate yet reproducible decrease in the labeling of all long chain fatty acids. Loss of ELO-C produced the complete loss of labeling in a single very long chain fatty acid. In addition, we noted a marked increase in the abundance of a shorter fatty acid, suggesting that this may be a precursor that accumulates in the absence of the downstream enzyme (Fig. 6C). Ablation of ELO-A did not produce recognizable changes in the labeling pattern. Although these analyses indicate clear changes in the biosynthetic capabilities of two of our mutants, they are limited in their sensitivity and resolution. In particular, they do not provide accurate measurement for the length and saturation of the affected fatty acids.
FIGURE 6.
Radiolabeling analysis shows a reduction in long chain fatty acid synthesis in the T. gondii ELO-B and ELO-C mutant. T. gondii ELO mutants (A, ΔELO-A; B, ΔELO-B; and C, ΔELO-C) were metabolically labeled with [14C]acetate; lipids were extracted, and fatty acid methyl esters were analyzed by reverse phase thin layer chromatography, and representative autoradiographs for each mutant are shown. Each panel shows the parental strain carrying native and conditional locus (e.g. ELO-B/ELO-Bi) to the left and the mutant (ΔELO-B/ELO-Bi) to the right grown in the absence (−) or presence (+) of ATc.
ELO-B and ELO-C Produce Monounsaturated Long Chain Fatty Acids in a Two-step Mechanism
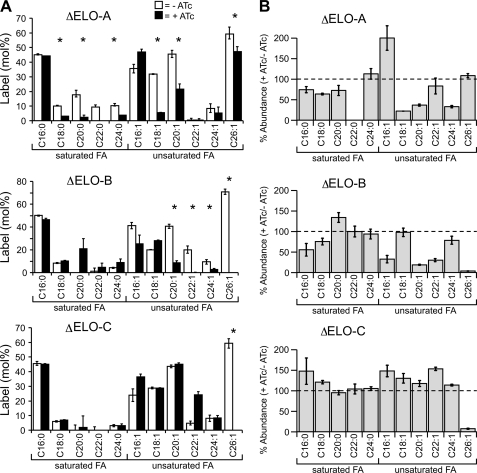
To further investigate the substrate specificity of the different T. gondii fatty acid elongases, each of the ELO mutant parasites was metabolically labeled with [13C]glucose in absence or presence of ATc, and fatty acid mass isotopomers were analyzed by GC-MS. The labeling of saturated long chain fatty acids up to C16:0 was unaffected by the individual loss of activity of ELO-A, ELO-B, and ELO-C (Fig. 7), consistent with their synthesis being regulated by FASII. Loss of ELO-A activity was associated with a marked reduction in labeling of saturated and unsaturated fatty acids with chain lengths of 18–24 carbons. Ablation of ELO-A also resulted in a reduction in the cellular levels of these fatty acids. Significantly, both the abundance and level of labeling of the unsaturated fatty acid C16:1 increased in the absence of ELO-A. These results suggest that ELO-A is required for efficient elongation of C16:0 or C16:1 to longer species. In the absence of ELO-A, de novo synthesized C16:0 is apparently channeled into increased synthesis of C16:1.
FIGURE 7.
Loss of elongases results in selective reductions in the rate of synthesis and cellular levels of unsaturated fatty acids. Intracellular tachyzoites of the three ΔELO mutants were labeled with [U-13C]glucose in the presence (black) or absence (white) of ATc as detailed in Fig. 1. A, level of 13C incorporation into the major fatty acids (FA) of the isolated tachyzoites. Error bars represent standard deviation where n = 3–4. B, changes in the relative abundance of individual fatty acids in ΔELO tachyzoites in the presence of ATc as measured by GC-MS (values are given relative to the abundance of this fatty acid species in the no ATc control). Results are indicative of >3 individual replicates. Individual values for all experiments are listed in Table 4. Fatty acids for which significant changes in labeling were observed in the presence and absence of ATc using the Wilcoxon Rank Sum Test (p values less than 0.05) are indicated with an asterisk.
In contrast, loss of expression of ELO-B or ELO-C resulted in enhanced labeling of long chain fatty acids but reduced labeling of several very long chain fatty acids. Specifically, down-regulation of ELO-B activity severely ablated labeling of the monounsaturated very long chain fatty acids C20:1, C22:1, C24:1, and C26:1 (Fig. 7A and Table 2). However, down-regulation of ELO-C activity led to a highly selective loss of labeling of C26:1 and the accumulation of C22:1. These results suggest that ELO-B is largely responsible for the elongation of C18:1/C20:1 to C22:1, whereas the primary role of ELO-C is to elongate fatty acids from C22:1 to C26:1. This model is also supported by our measurements of overall fatty acid abundance (Fig. 7B). Loss of ELO-C results in a severe drop in the abundance of only C26:1, in contrast, loss of ELO-B affected several shorter monounsaturated fatty acids in addition to C26:1. This is reminiscent of the situation in S. cerevisiae, where ScElo2p (equivalent to TgELO-B) is capable of elongating fatty acids up to 24 carbons, preferring a substrate with <22 carbon length. Likewise, ScElo3p and TgELO-C are alike in preferential production of very long chain fatty acids. Note that both T. gondii enzymes complement the yeast double mutant indicating that they can have overlapping and slightly broader specificity.
TABLE 2.
Percent labeling of fatty acids derived from [U-13C]glucose-fed tachyzoites
Mutant parasites were used to infect HFF and labeled in situ with [13C]glucose in the presence or absence of ATc. Percent labeling was determined by GC-MS analysis. Fatty acids for which significant changes in labeling were observed in the presence and absence of ATc using the Wilcoxon rank sum test (p values less than 0.05) are indicated by asterisk.
| Cell type | ACP | ELOA | ELOA | ELOB | ELOB | ELOC | ELOC | |
|---|---|---|---|---|---|---|---|---|
| +/−ATc | + | − | + | − | + | − | + | |
| C14:0 | 54.08* (± 0.99) | 7.66* (± 1.78) | ND | ND | ND | ND | ND | ND |
| C16:0 | 33.58* (± 0.68) | 6.06* (± 0.22) | 45.17 (± 0.64) | 44.24 (±0.19) | 50.01 (± 0.45) | 46.34 (± 1.47) | 45.65 (± 1.28) | 45.14 (± 0.35) |
| C18:0 | 2.38* (± 0.51) | 0.26* (± 0.28) | 10.02* (± 0.50) | 3.08* (± 0.03) | 8.40 (± 0.40) | 10.18 (± 0.54) | 5.95 (± 0.53) | 7.02 (± 0.18) |
| C20:0 | 0.00 (±0.00) | 0.00 (± 0.00) | 17.79* (± 3.14) | 2.28* (± 1.60) | 0.00 (± 0.00) | 20.99 (± 8.83) | 0.00 (± 3.65) | 1.97 (± 7.81) |
| C22:0 | 0.00 (± 0.00) | 0.00 (± 0.39) | 9.27 (± 1.61) | ND | 1.26 (± 2.64) | 4.62 (± 3.55) | 0.00 (± 2.47) | 0.00 (± 0.00) |
| C24:0 | 0.00 (± 4.82) | 1.23 (± 0.08) | 10.21* (± 1.47) | 3.75*(± 0.12) | 4.18 (± 0.42) | 8.87 (± 3.04) | 3.12 (± 0.81) | 3.17 (± 1.14) |
| C16:1 | 17.76* (± 1.76) | 10.01* (± 3.17) | 35.63 (± 3.90) | 47.04 (± 1.82) | 41.27 (± 2.78) | 25.38 (± 7.47) | 23.94 (± 4.33) | 36.37 (± 2.07) |
| C18:1 | 16.37* (± 0.86) | 4.96* (± 0.15) | 31.95* (± 0.25) | 5.42* (± 0.25) | 20.15 (± 0.08) | 27.88 (± 0.64) | 28.86 (± 0.53) | 28.83 (± 0.19) |
| C20:1 | 24.05* (± 2.99) | 11.21* (± 0.61) | 45.56* (± 1.48) | 21.54* (± 3.51) | 40.60* (± 1.72) | 8.46* (± 1.93) | 43.64 (± 0.96) | 45.09 (± 0.83) |
| C22:1 | 0.00 (± 1.82) | 1.05 (± 1.50) | 0.51 (± 1.26) | 0.55 (± 0.82) | 20.12* (± 3.21) | 0.00* (± 0.19) | 4.91 (± 1.38) | 24.13 (2.22) |
| C24:1 | 0.31 (± 0.52) | 0.00 (± 1.58) | 8.36 (± 1.75) | 5.29 (± 4.18) | 9.42* (± 2.07) | 2.63* (± 1.14) | 8.10 (± 2.10) | 8.39 (± 1.70) |
| C26:1 | 36.45 (± 11.48) | 27.56 (± 1.86) | 59.32* (± 4.71) | 47.30* (± 3.37) | 70.80* (± 2.30) | 0.00* (± 0.00) | 59.41* (± 3.28) | 0.00* (± 0.00) |
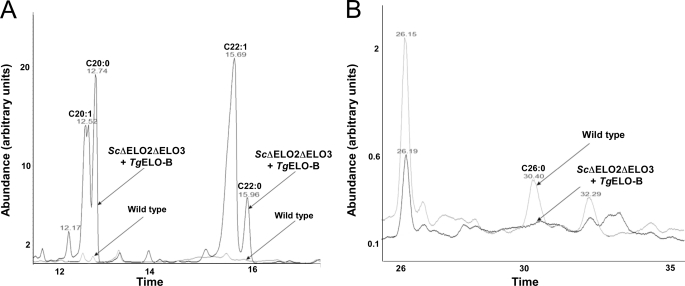
To distinguish between the potential activities of ELO-B, we returned to the complemented yeast strains and analyzed their fatty acid contents. The wild type yeast, mutant Δelo2Δelo3 complemented S. cerevisiae ELO3, as well as the mutant complemented by expression of TgELO-B were grown on YPGalGlu medium. Total lipids were extracted and analyzed by GC-MS (see under “Experimental Procedures”). As shown previously, when compared with wild type, the Δelo2Δelo3/ScELO3 mutant shows a much decreased level of fatty acids C20 and higher. The double deletion strain carrying the TgELO-B construct, displayed some restoration of its longer chain fatty acids (C20–24) but not C26 or C26:1. Interestingly, there seemed to be an overabundance of unsaturated fatty acids with no increase or only a modest increase in saturated fatty acids (see GC-MS tracks in Fig. 8, A and B). In particular, there was a striking accumulation of C22:1 compared with all other strains (see Tables 3 and 4 and Fig. 8A).
FIGURE 8.
GC-MS analysis of fatty acid methyl esters from S. cerevisiae complemented with T. gondii ELO-B. Fatty acids from yeast Δelo2/Δelo3 double deletion strain complemented by TgELO-B (see Fig. 5) were extracted and subjected to GC-MS analysis as described under “Experimental Procedures.” An overlay of the chromatogram of fatty acid methyl esters from this strain and the wild type is shown. A, enlargement of the region of the trace showing C22 and C24 methyl esters. B, region showing C26 methyl esters.
TABLE 3.
Yeast strains constructed for complementation analysis
| Name | Genotype of the yeast strain |
|---|---|
| ScΔELO2ΔELO3 + ScELO3 | W1536 elo2Δelo3Δ/YCp33 GAL1-ScELO3 |
| ScΔELO2ΔELO3 + TgELO-B | W1536 Δelo2Δelo3/YCP111 GAL TgELO-B |
| ScΔELO2ΔELO3 + TgELO-C | W1536 Δelo2Δelo3/YCP111 GAL TgELO-C |
| ScΔECR + ScECR | W1536 Δtsc13/YCP33 GAL ScTsc13 |
| ScΔECR + TgECR | W1536 Δtsc13/YCP111 GAL TgECR |
| ScΔDEH + ScDEH | W1536 Δphs1/YCP33 GAL ScPhs1 |
| ScΔDEH + TgDEH | W1536 Δphs1/YCP111 GAL TgDEH |
TABLE 4.
Fatty acid profiles of yeast wild type and complement elo2/elo3 mutant strain
Values indicated are % of relative response units of total fatty acid methyl esters. p values were calculated using a two-tailed Student's t test, setting not detected fatty acid species to 0. The results of fatty acid extractions from two independent sets of cultures are shown. Fatty acyl species were identified by GC/MALDI mass spectroscopy as described under “Experimental Procedures.” ND means not detected.
| C14 | C15 | C16:1/16 | C18:1/18 | C20:1/20 | C22:1 | C22 | C24:1 | C24 | C26 | C26-OH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TgELO-B | 2.5 ± 1.2 | 4.4 ± 1.9 | 26.1a ± 3.8 | 36.3 ± 5.0 | 12.4b ± 3.2 | 14.8b ± 1.3 | 2.2 ± 1 | 1.6b ± 0.2 | ND/0.1 | ND | NDa |
| TgELO-C | 2.1 ± 0.6 | 3.6 ± 2.3 | 47.2 ± 3.9 | 45.0a ± 0.4 | 2.2 ± 1.6 | 0.2 / ND | ND | ND | ND | ND | NDa |
| ScELO3 | 2.5 ± 0.1 | 2.4 ± 1.5 | 40.6 ± 0.8 | 51.9 ± 1.8 | ND | ND | ND | ND | ND | 1.1 ± 0.6 | 1 ± 0.1 |
| WT | 3.1 ± 1.4 | 1.6 ± 1.5 | 47.2 ± 6.5 | 46.1 ± 2.2 | 0.5 ± 0.1 | 0.3 ± 0.2 | 0.5 ± 0.5 | ND | 0.1/ND | 0.6 ± 0,4 | 0.7/ND |
a p < 0.05 is only in comparison with the S. cerevisiae elo2/elo3 double mutant complemented with S. cerevisiae ELO3.
b p < 0.05 is in comparison with both S. cerevisiae WT and elo2/elo3 double mutant complemented with S. cerevisiae ELO3.
DISCUSSION
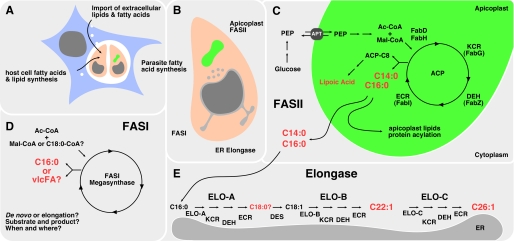
Intracellular pathogens need to either scavenge or synthesize lipids to maintain the integrity of their own membranes and/or the membrane of specialized vacuoles within which they survive and proliferate. Lipid and fatty acid salvage mechanisms have been shown to be important for the intracellular growth of several intracellular bacteria. Similarly, apicomplexan parasites have been shown to salvage lipids from the host endocytic pathway or cytoplasmic lipid bodies (12, 32–34, 36). Nonetheless, recent bioinformatic, genetic, and biochemical studies have suggested that intracellular T. gondii tachyzoite stages express multiple pathways of fatty acid biosynthesis and may be dependent on de novo synthesis of fatty acids for the synthesis of bulk and specialized lipid classes (1). To dissect this complex network of uptake and synthesis, we have combined reverse genetics with metabolomic analyses. We define the function of individual fatty acid synthesis pathways and have developed a model that incorporates these findings (Fig. 9). The FASII pathway is localized to the apicoplast (Fig. 9, shown in green) and is thought to utilize acetyl-CoA derived from glycolytic triose phosphates that are imported into the apicoplast via a phosphate translocator (19, 37). Our labeling studies provide strong support for this model. Specifically, although [14C]acetate was not effectively incorporated into FASII products, 13C derived from [U-13C]glucose was strongly incorporated into this pathway. Ablation of FASII activity resulted in a global reduction in fatty acid labeling. This was most apparent for the saturated long chain fatty acids C14:0 and C16:0, indicating that FASII is largely responsible for maintaining the cellular levels of these fatty acids. The pattern of 13C labeling of these fatty acids in FASII-competent parasites was also consistent with de novo synthesis from short chain (∼C4) fatty acid precursors. Synthesis of this precursor is most likely dependent on apicoplast FabD (38), but apicoplast ACP has also been demonstrated to be capable of self-malonylation (39). Note that T. gondii also expresses two differentially localized acetyl-CoA carboxylases, one in the apicoplast the other in the cytoplasm (40), that could provide malonyl-CoA for FASII and the elongation pathway, respectively. These findings demonstrate that the apicoplast, like the plant chloroplast, is the main cellular site of fatty acid de novo synthesis. There are a number of important functions for fatty acid synthesis directly in the plastid, including lipoylation, acylation of plastid proteins, and the synthesis of plastid lipids. Indeed, the loss of FASII in the apicoplast and the plant chloroplast perturbs organelle biogenesis and division leading to organelle loss (9, 41).
FIGURE 9.
Toxoplasma acquires fatty acids through a complex network of synthesis and uptake. A, T. gondii (pink) is an intracellular pathogen capable of fatty acid and lipid salvage from the host cell (blue). This process can intersect host cell import as well as synthesis routes. B, in addition, the parasite harbors three fatty acid synthesis pathways that are localized to different cellular compartments. C, apicoplast (green)-localized FASII pathway produces significant amounts of myristic and palmitic acid in addition to lipoic acid relying on cytoplasmic glycolysis for precursors. E, Toxoplasma also maintains an ER-associated elongase system that synthesizes very long chain monounsaturated fatty acids, subsequently using the activity of ELO-B and ELO-C. D, T. gondii FASI remains largely uncharacterized. Its stage-specific expression pattern and localization are not established. It is also unclear whether this megasynthase synthesizes fatty acids de novo like the FASI of humans or acts as an elongase for saturated fatty acids, as demonstrated for the FASI of C. parvum. Major products are highlighted in red. Des, desaturase; PEP, phosphoenolpyruvate; Mal, malonate; Ac, acetate; vlcFA, very long chain fatty acids.
Does this suggest the significance for FASII to be entirely local? Following this idea, the main function of FASII could be to maintain the apicoplast to support the organelle as the site of isoprenoid precursor synthesis (42, 43). In plant chloroplasts, FASII-derived fatty acids are rapidly exported from the plastid into the cytoplasm (44). Our experiments do not formally test this and do not establish the localization of FASII-derived lipids. We note, however, that our measurements indicate that most (60–80%) of the parasite C14:0 and C16:0 are 13C-labeled, a finding that is inconsistent with FASII only supplying a small organellar pool of lipids. Moreover, our data suggest that C16:0 and C16:1 fatty acids synthesized by the apicoplast FASII complex are further elongated by the ER elongases, indicating that a significant fraction of the apicoplast-synthesized fatty acids are exported to the ER. We propose that the apicoplast is the main source of C14:0 and C16:0 for intracellular stages of T. gondii and the liver stage of Plasmodium, but in the Plasmodium erythrocyte and mosquito stages, this need can be satisfied by salvage of fatty acids from the host. A greater dependence of T. gondii tachyzoites on de novo fatty acid synthesis is further supported by our finding that loss of FASII was only partially rescued by providing excess C14:0 and C16:0 in the culture medium. Overall, these observations are consistent with important functions of FASII-derived products within and beyond the apicoplast in T. gondii.
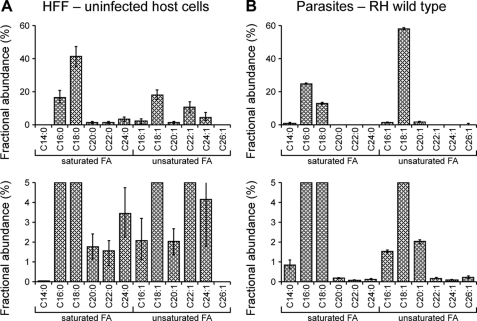
In addition to the FASII complex, we provide direct evidence for the activity of an elaborate fatty acid elongation pathway localized to the parasite endoplasmic reticulum. This fatty acid elongation pathway consists of four enzymatic steps. The extension of the carbon chain is initiated by a condensation reaction between acyl-CoA and malonyl-CoA catalyzed by a fatty acid elongase. This is followed by reduction through keto-acyl-CoA reductase, dehydration to enoyl-CoA, and final reduction to the elongated fatty acyl-CoA. We identified a full complement of six enzymes and support the sequence similarity-based assignments experimentally by yeast complementation analysis for several candidates. Our [U-13C]glucose-labeling experiments in parasite mutants and heterologous expression in yeast mutants suggest that each of the elongases may act sequentially as outlined schematically in Fig. 9E. The T. gondii elongation system generates very long chain monounsaturated fatty acids. ELO-B elongates C18:1 to C22:1 in two carbon increments. Based on the yeast data, its activity from C20:1 to C22:1 appears to be particularly robust. C22:1 is elongated in two final rounds by ELO-C to C26:1 (note that we do not fully resolve whether the synthesis of C24 is an overlapping function between the two elongases). The T. gondii ELO-B and ELO-C pathway is the sole source of C26:1 for the parasite and does not appear to elongate saturated fatty acids. The significance of this striking preference for unsaturated fatty acids is unclear at this point. Unsaturated fatty acids have distinct physical properties resulting in differences in e.g. membrane fluidity, and those may be more suited to the needs of the parasite. It is notable that the levels of C26:1 in fibroblasts are very low (Table 1 and Fig. 10), possibly accounting for the inability to generate multiple ELO genes.
FIGURE 10.
Total fatty acid composition of uninfected HFF host cells and RH tachyzoites. The total lipid extract of human foreskin fibroblasts (HFF) (A) and isolated purified tachyzoites (labeled in their host cells as detailed in the “Experimental Procedures”) (B) was subjected to solvolysis in methanolic-HCl and derivatized in trimethylsilyl reagent, and fatty acid methyl esters were detected by GC-MS. The mol % of the major fatty acids are shown (mean ± S.D.). The lower panels show same data set scaled to a maximum of 5% fractional abundance to display low abundance species.
What is the source of the ELO-B substrates? Monounsaturated fatty acids can be synthesized by the desaturation of a corresponding saturated fatty acid. A route used by many organisms is the conversion of C18:0 to C18:1 oleic acid by stearoyl-CoA desaturase (45). This enzyme is present in T. gondii (TGGT1_053030) and has been characterized in detail in P. falciparum (46). In Plasmodium, it was found to be localized to the membrane of the endoplasmic reticulum and thus could work hand in hand with the ELO system. This system could potentially tap into the FASII-generated fatty acid pool through the activity of ELO-A, which appears to be required for elongation of C16:0 and C16:1 to C18:0 and C18:1, respectively. Specifically, ablation of ELO-A resulted in a marked decrease in synthesis of C18:0, which was associated with the concomitant increase in both the labeling and steady state concentration of C16:0/C16:1 (Fig. 7). It is notable that only a minor pool of C18:0 is labeled with [U-13C]glucose in wild type parasites (<10%) indicating that this fatty acid is primarily salvaged from the host. In contrast, C18:1 is labeled to a similar extent as C16:0 and C16:1 (>40%). These findings suggest that ELO-A primarily utilizes C16:0 synthesized in the apicoplast and C16:1 synthesized by the putative tachyzoite ER desaturase. The reduced cellular levels and rates of labeling of longer chain saturated/unsaturated fatty acids in ELO-A ablated parasites indicate that the C18:0 and C18:1 synthesized by ELO-A is selectively utilized by ELO-B and ELO-C, whereas the larger cellular pool of salvaged C18:0 appears to be poorly used. Collectively, these observations indicate that synthesis of very long chain fatty acids in intracellular tachyzoites involves extensive intraorganellar transport as well as some degree of substrate channeling. It should be noted that the T. gondii genome encodes a FASI pathway (Fig. 9D), but its role in the T. gondii fatty acid metabolism is not yet established.
Although we demonstrate clear biochemical effects of suppressing individual ELO genes, we do not measure parasite growth impairment under tissue culture conditions. It is possible that the parasite can satisfy its needs through uptake. A recently described lipid uptake system in T. gondii may facilitate this process (47). Alternatively, the lack of growth defects may be due to redundancy within the ELO system itself. In yeast, the loss of a fatty acid elongase 2 or fatty acid elongase 3 can be tolerated. However, loss of both elongases results in synthetic lethality. It has been suggested that the enzymes partially complement each other and that the cells can tolerate the resulting slight differences in their fatty acid pools (27). Our yeast complementation results indicate that the T. gondii enzymes may also have overlapping substrate specificity. To establish whether a similar redundancy exists in T. gondii, we sought to engineer a more complete ablation of the parasite ELO system. Our first approach was to attempt to generate a conditional double mutant in ELO-B and -C. However, our attempts to generate such a mutant failed recurrently despite the fact that gene targeting was efficient for individual loci in previous experiments (data not shown). An alternative approach would be to delete a nonredundant downstream enzyme of the pathway, thereby affecting the activity of all three elongases. We attempted to generate conditional mutants for dehydratase and enoyl-CoA reductase. Again, we were unsuccessful in generating a mutant (data not shown). These negative results do not formally establish the essential nature of the T. gondii ELO pathway, but they are consistent with such a role.
Which aspect of parasite biology generates the heavy need for elongated fatty acids served by these elaborate and potentially redundant synthesis and uptake pathways? Very long chain fatty acids are required for the synthesis of highly specialized lipids such as waxes in plants, but they are also important components of phospholipids (27, 48). Loss of the elongation pathway affects the overall phospholipid pool in these organisms. T. gondii harbors a variety of phospholipids that not only differ in their headgroup but also in the length of the acyl chains (49). The parasites can take up phospholipids but appear to depend on the synthesis of some species (47, 50, 51). Overall, our results demonstrate that despite its substantial complexity the parasite fatty acid metabolism can be unraveled in molecular detail. Stable isotope labeling followed by GC-MS can track and quantify the synthesis of a large number of metabolites in vivo and was particularly useful for resolving the activities of different fatty acid biosynthetic enzymes. Furthermore, the fact that 13C-labeled precursors can be added at normal physiological concentrations may increase the efficiency with which they can access intracellular compartments such as the T. gondii parasitophorous vacuole rather than being diverted into host cell metabolism. Conditional gene ablation provides focus and specificity to this broader metabolomic analysis. We provide functional annotation for two important parasite pathways here and suggest that this strategy could be used to great effect to understand the metabolic interaction between host and parasite in T. gondii and many other intracellular pathogens.
Supplementary Material
Acknowledgments
We thank Dr. Ulrich Bergmann (Department of Biochemistry, University of Oulu) and Dr. Päivi Joensuu (Department of Chemistry, University of Oulu) for assistance with the yeast fatty acid analyses and Benjamin Woodcroft (Department of Biochemistry and Molecular Biology, University of Melbourne) for assistance with the statistical analyses and ApiLoc annotations.
This work was supported, in whole or in part, by National Institutes of Health Grants AI084414 and AI064671 (to B. S.). This work was also supported by National Health and Medical Research Council Grant 1006024 (to M. J. M.).

This article contains supplemental Tables S1–S3.
- FASII
- type II fatty-acid synthase
- ER
- endoplasmic reticulum
- ACP
- acyl carrier protein
- 5′FOA
- 5′-fluoroorotic acid
- ATc
- anhydrotetracycline.
REFERENCES
- 1. Mazumdar J., Striepen B. (2007) Make it or take it. Fatty acid metabolism of apicomplexan parasites. Eukaryot. Cell 6, 1727–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valdivia R. H. (2008) Chlamydia effector proteins and new insights into chlamydial cellular microbiology. Curr. Opin. Microbiol. 11, 53–59 [DOI] [PubMed] [Google Scholar]
- 3. Bhatt A., Molle V., Besra G. S., Jacobs W. R., Jr., Kremer L. (2007) The Mycobacterium tuberculosis FAS-II condensing enzymes. Their role in mycolic acid biosynthesis, acid-fastness, pathogenesis, and in future drug development. Mol. Microbiol. 64, 1442–1454 [DOI] [PubMed] [Google Scholar]
- 4. Waller R. F., Keeling P. J., Donald R. G., Striepen B., Handman E., Lang-Unnasch N., Cowman A. F., Besra G. S., Roos D. S., McFadden G. I. (1998) Nuclear encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 95, 12352–12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hiltunen J. K., Chen Z., Haapalainen A. M., Wierenga R. K., Kastaniotis A. J. (2010) Mitochondrial fatty acid synthesis. An adopted set of enzymes making a pathway of major importance for the cellular metabolism. Prog. Lipid Res. 49, 27–45 [DOI] [PubMed] [Google Scholar]
- 6. Goodman C. D., McFadden G. I. (2007) Fatty acid biosynthesis as a drug target in apicomplexan parasites. Curr. Drug Targets 8, 15–30 [DOI] [PubMed] [Google Scholar]
- 7. Surolia N., Surolia A. (2001) Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 7, 167–173 [DOI] [PubMed] [Google Scholar]
- 8. McLeod R., Muench S. P., Rafferty J. B., Kyle D. E., Mui E. J., Kirisits M. J., Mack D. G., Roberts C. W., Samuel B. U., Lyons R. E., Dorris M., Milhous W. K., Rice D. W. (2001) Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of apicomplexan Fab I. Int. J. Parasitol. 31, 109–113 [DOI] [PubMed] [Google Scholar]
- 9. Mazumdar J. H., Wilson E., Masek K. A., Hunter C., Striepen B. (2006) Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc. Natl. Acad. Sci 103, 13192–13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaughan A. M., O'Neill M. T., Tarun A. S., Camargo N., Phuong T. M., Aly A. S., Cowman A. F., Kappe S. H. (2009) Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell. Microbiol. 11, 506–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu M., Kumar T. R., Nkrumah L. J., Coppi A., Retzlaff S., Li C. D., Kelly B. J., Moura P. A., Lakshmanan V., Freundlich J. S., Valderramos J. C., Vilcheze C., Siedner M., Tsai J. H., Falkard B., Sidhu A. B., Purcell L. A., Gratraud P., Kremer L., Waters A. P., Schiehser G., Jacobus D. P., Janse C. J., Ager A., Jacobs W. R., Jr., Sacchettini J. C., Heussler V., Sinnis P., Fidock D. A. (2008) The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe 4, 567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charron A. J., Sibley L. D. (2002) Host cells. Mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 115, 3049–3059 [DOI] [PubMed] [Google Scholar]
- 13. Gerold P., Dieckmann-Schuppert A., Schwarz R. T. (1994) Glycosylphosphatidylinositols synthesized by asexual erythrocytic stages of the malarial parasite, Plasmodium falciparum. Candidates for plasmodial glycosylphosphatidylinositol membrane anchor precursors and pathogenicity factors. J. Biol. Chem. 269, 2597–2606 [PubMed] [Google Scholar]
- 14. Zhu G. (2004) Current progress in the fatty acid metabolism in Cryptosporidium parvum. J. Eukaryot. Microbiol. 51, 381–388 [DOI] [PubMed] [Google Scholar]
- 15. Zhu G., Li Y., Cai X., Millership J. J., Marchewka M. J., Keithly J. S. (2004) Expression and functional characterization of a giant Type I fatty acid synthase (CpFAS1) gene from Cryptosporidium parvum. Mol. Biochem. Parasitol. 134, 127–135 [DOI] [PubMed] [Google Scholar]
- 16. Zhu G., Shi X., Cai X. (2010) The reductase domain in a Type I fatty acid synthase from the apicomplexan Cryptosporidium parvum. Restricted substrate preference towards very long chain fatty acyl thioesters. BMC Biochem. 11, 46–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agrawal S., van Dooren G. G., Beatty W. L., Striepen B. (2009) Genetic evidence that an endosymbiont-derived endoplasmic reticulum-associated protein degradation (ERAD) system functions in import of apicoplast proteins. J. Biol. Chem. 284, 33683–33691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Striepen B., Soldati D. (2007) in Toxoplasma gondii, the Model Apicomplexan- Perspective and Methods (Weiss L. D., Kim K., eds) pp. 391–415, Elsevier, London [Google Scholar]
- 19. Brooks C. F., Johnsen H., van Dooren G. G., Muthalagi M., Lin S. S., Bohne W., Fischer K., Striepen B. (2010) The Toxoplasma apicoplast phosphate translocator links cytosolic and apicoplast metabolism and is essential for parasite survival. Cell Host Microbe 7, 62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meissner M., Schlüter D., Soldati D. (2002) Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298, 837–840 [DOI] [PubMed] [Google Scholar]
- 21. Carey K. L., Donahue C. G., Ward G. E. (2000) Identification and molecular characterization of GRA8, a novel, proline-rich, dense granule protein of Toxoplasma gondii. Mol. Biochem. Parasitol. 105, 25–37 [DOI] [PubMed] [Google Scholar]
- 22. Saunders E. C., Ng W. W., Chambers J. M., Ng M., Naderer T., Krömer J. O., Likic V. A., McConville M. J. (2011) Isotopomer profiling of Leishmania mexicana promastigotes reveals important roles for succinate fermentation and aspartate uptake in tricarboxylic acid cycle (TCA) anaplerosis, glutamate synthesis, and growth. J. Biol. Chem. 286, 27706–27717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amberg D. C., Burke D., Strathern J. N., and Cold Spring Harbor, L. (2005) Methods in Yeast Genetics, p. 207, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 24. Kastaniotis A. J., Autio K. J., Sormunen R. T., Hiltunen J. K. (2004) Htd2p/Yhr067p is a yeast 3-hydroxyacyl-ACP dehydratase essential for mitochondrial function and morphology. Mol. Microbiol. 53, 1407–1421 [DOI] [PubMed] [Google Scholar]
- 25. Song W. Q., Qin Y. M., Saito M., Shirai T., Pujol F. M., Kastaniotis A. J., Hiltunen J. K., Zhu Y. X. (2009) Characterization of two cotton cDNAs encoding trans-2-enoyl-CoA reductase reveals a putative novel NADPH-binding motif. J. Exp. Bot. 60, 1839–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qin Y. M., Pujol F. M., Hu C. Y., Feng J. X., Kastaniotis A. J., Hiltunen J. K., Zhu Y. X. (2007) Genetic and biochemical studies in yeast reveal that the cotton fiber-specific GhCER6 gene functions in fatty acid elongation. J. Exp. Bot. 58, 473–481 [DOI] [PubMed] [Google Scholar]
- 27. Oh C. S., Toke D. A., Mandala S., Martin C. E. (1997) ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J. Biol. Chem. 272, 17376–17384 [DOI] [PubMed] [Google Scholar]
- 28. Hager K. M., Striepen B., Tilney L. G., Roos D. S. (1999) The nuclear envelope serves as an intermediary between the ER and Golgi complex in the intracellular parasite Toxoplasma gondii. J. Cell Sci. 112, 2631–2638 [DOI] [PubMed] [Google Scholar]
- 29. Cinti D. L., Cook L., Nagi M. N., Suneja S. K. (1992) The fatty acid chain elongation system of mammalian endoplasmic reticulum. Prog. Lipid Res. 31, 1–51 [DOI] [PubMed] [Google Scholar]
- 30. Denic V., Weissman J. S. (2007) A molecular caliper mechanism for determining very long-chain fatty acid length. Cell 130, 663–677 [DOI] [PubMed] [Google Scholar]
- 31. Qin Y. M., Pujol F. M., Shi Y. H., Feng J. X., Liu Y. M., Kastaniotis A. J., Hiltunen J. K., Zhu Y. X. (2005) Cloning and functional characterization of two cDNAs encoding NADPH-dependent 3-ketoacyl-CoA reductased from developing cotton fibers. Cell Res. 15, 465–473 [DOI] [PubMed] [Google Scholar]
- 32. Coppens I., Dunn J. D., Romano J. D., Pypaert M., Zhang H., Boothroyd J. C., Joiner K. A. (2006) Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125, 261–274 [DOI] [PubMed] [Google Scholar]
- 33. Mikolajczak S. A., Jacobs-Lorena V., MacKellar D. C., Camargo N., Kappe S. H. (2007) L-FABP is a critical host factor for successful malaria liver stage development. Int. J. Parasitol. 37, 483–489 [DOI] [PubMed] [Google Scholar]
- 34. Sharma A., Yogavel M., Akhouri R. R., Gill J., Sharma A. (2008) Crystal structure of soluble domain of malaria sporozoite protein UIS3 in complex with lipid. J. Biol. Chem. 283, 24077–24088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gubbels M. J., Li C., Striepen B. (2003) High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob. Agents Chemother. 47, 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coppens I. (2006) Contribution of host lipids to Toxoplasma pathogenesis. Cell. Microbiol. 8, 1–9 [DOI] [PubMed] [Google Scholar]
- 37. Lim L., Linka M., Mullin K. A., Weber A. P., McFadden G. I. (2010) The carbon and energy sources of the nonphotosynthetic plastid in the malaria parasite. FEBS Lett. 584, 549–554 [DOI] [PubMed] [Google Scholar]
- 38. Prigge S. T., He X., Gerena L., Waters N. C., Reynolds K. A. (2003) The initiating steps of a type II fatty-acid synthase in Plasmodium falciparum are catalyzed by pfACP, pfMCAT, and pfKASIII. Biochemistry 42, 1160–1169 [DOI] [PubMed] [Google Scholar]
- 39. Misra A., Surolia N., Surolia A. (2009) Catalysis and mechanism of malonyl transferase activity in type II fatty acid biosynthesis acyl carrier proteins. Mol. Biosyst. 5, 651–659 [DOI] [PubMed] [Google Scholar]
- 40. Jelenska J., Crawford M. J., Harb O. S., Zuther E., Haselkorn R., Roos D. S., Gornicki P. (2001) Subcellular localization of acetyl-CoA carboxylase in the apicomplexan parasite Toxoplasma gondii. Proc. Natl. Acad. Sci. U.S.A. 98, 2723–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu G. Z., Xue H. W. (2010) Arabidopsis β-ketoacyl-[acyl carrier protein] synthase I is crucial for fatty acid synthesis and plays a role in chloroplast division and embryo development. Plant Cell 22, 3726–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nair S. C., Brooks C. F., Goodman C. D., Strurm A., McFadden G. I., Sundriyal S., Anglin J. L., Song Y., Moreno S. N., Striepen B. (2011) Apicoplast isoprenoid precursor synthesis and the molecular basis of fosmidomycin resistance in Toxoplasma gondii. J. Exp. Med. 208, 1547–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeh E., DeRisi J. L. (2011) Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 9, e1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koo A. J., Ohlrogge J. B., Pollard M. (2004) On the export of fatty acids from the chloroplast. J. Biol. Chem. 279, 16101–16110 [DOI] [PubMed] [Google Scholar]
- 45. Stukey J. E., McDonough V. M., Martin C. E. (1990) The OLE1 gene of Saccharomyces cerevisiae encodes the Δ9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J. Biol. Chem. 265, 20144–20149 [PubMed] [Google Scholar]
- 46. Gratraud P., Huws E., Falkard B., Adjalley S., Fidock D. A., Berry L., Jacobs W. R., Jr., Baird M. S., Vial H., Kremer L. (2009) Oleic acid biosynthesis in Plasmodium falciparum: characterization of the stearoyl-coA desaturase and investigation as a potential therapeutic target. PLoS ONE 4, e6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lige B., Jayabalasingham B., Zhang H., Pypaert M., Coppens I. (2009) Role of an ancestral d-bifunctional protein containing two sterol-carrier protein-2 domains in lipid uptake and trafficking in Toxoplasma. Mol. Biol. Cell 20, 658–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bach L., Michaelson L. V., Haslam R., Bellec Y., Gissot L., Marion J., Da Costa M., Boutin J. P., Miquel M., Tellier F., Domergue F., Markham J. E., Beaudoin F., Napier J. A., Faure J. D. (2008) The very long-chain hydroxy fatty acyl-CoA dehydratase PASTICCINO2 is essential and limiting for plant development. Proc. Natl. Acad. Sci. U.S.A. 105, 14727–14731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Welti R., Mui E., Sparks A., Wernimont S., Isaac G., Kirisits M., Roth M., Roberts C. W., Botté C., Maréchal E., McLeod R. (2007) Lipidomic analysis of Toxoplasma gondii reveals unusual polar lipids. Biochemistry 46, 13882–13890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coppens I., Vielemeyer O. (2005) Insights into unique physiological features of neutral lipids in Apicomplexa. From storage to potential mediation in parasite metabolic activities. Int. J. Parasitol. 35, 597–615 [DOI] [PubMed] [Google Scholar]
- 51. Gupta N., Zahn M. M., Coppens I., Joiner K. A., Voelker D. R. (2005) Selective disruption of phosphatidylcholine metabolism of the intracellular parasite Toxoplasma gondii arrests its growth. J. Biol. Chem. 280, 16345–16353 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.