Abstract
Pathogenic bacteria can resist their microenvironment by changing the expression of virulence genes. In Salmonella typhimurium, some of these genes are controlled by the two-component system PhoP-PhoQ. Studies have shown that activation of the system by cationic antimicrobial peptides (AMPs) results, among other changes, in outer membrane remodeling. However, it is not fully clear what characteristics of AMPs are required to activate the PhoP-PhoQ system and whether activation can induce resistance to the various AMPs. For that purpose, we investigated the ability of a broad repertoire of AMPs to traverse the inner membrane, to activate the PhoP-PhoQ system, and to induce bacterial resistance. The AMPs differ in length, composition, and net positive charge, and the tested bacteria include two wild-type (WT) Salmonella strains and their corresponding PhoP-PhoQ knock-out mutants. A lacZ-reporting system was adapted to follow PhoP-PhoQ activation. The data revealed that: (i) a good correlation exists among the extent of the positive charge, hydrophobicity, and amphipathicity of an AMP and its potency to activate PhoP-PhoQ; (ii) a +1 charged peptide containing histidines was highly potent, suggesting the existence of an additional mechanism independent of the peptide charge; (iii) the WT bacteria are more resistant to AMPs that are potent activators of PhoP-PhoQ; (iv) only a subset of AMPs, independent of their potency to activate the system, is more toxic to the mutated bacteria compared with the WT strains; and (v) short term exposure of WT bacteria to these AMPs does not enhance resistance. Overall, this study advances our understanding of the molecular mechanism by which AMPs activate PhoP-PhoQ and induce bacterial resistance. It also reveals that some AMPs can overcome such a resistance mechanism.
Keywords: Antibiotic Resistance, Antimicrobial Peptides, Bacteria, Membrane Biophysics, Membrane Function, Peptide-Membrane Interaction
Introduction
Bacteria are unicellular organisms that can sense changing environments and respond by expressing specific sets of genes (1–3). A widespread bacterial response mechanism includes two-component signal transduction systems. They consist of a membrane sensor that senses signals in the environment and transfers this information by activating a transcriptional response regulator through its phosphorylation or dephosphorylation (2, 4–9). The PhoP-PhoQ two-component system found in several Gram-negative bacteria including Escherichia coli and Salmonella typhimurium has received much interest in recent years (9–18). In this system, the sensor PhoQ is activated by low magnesium concentrations (μm) (19), low pH (5.5) (20), or the presence of AMPs (21, 22). Recently, the biological consequences resulting from sensing multiple signals has been addressed (10, 23) and showed that the PhoP-PhoQ system regulates different Salmonella genes depending on whether the inducing signal is acidic pH or low Mg2+.
Upon activation, it promotes the transcription of many genes including LPS-modifying enzymes (1, 10, 24, 25). One of these genes, pagP, encodes an acyltransferase that catalyzes lipid A palmitoylation. This leads to an increase of both the membrane thickness and the hydrophobicity of the LPS (21, 26). These responses to environmental changes are important for Salmonella virulence, for adaptation to changing environments, and in modulating recognition by the immune system (5, 19, 27, 28). However, it has also been suggested that they are important for resistance to AMPs (14, 21, 22, 27, 29–33). For example, mutants lacking functional PhoP-PhoQ (null mutants) have been shown to be more susceptible to some AMPs2 compared with the WT bacteria (9, 14, 21, 22, 27, 34–37). Studies on the mode of PhoQ activation by AMPs suggest that they displace divalent cations (Mg2+) that bind to a highly acidic domain within the PhoQ (8, 22, 23). However, because of the limited number and types of AMPs investigated so far, it is not entirely clear what characteristics of AMPs are required to activate the PhoP-PhoQ system and whether such an activation can induce resistance to the various AMPs.
To answer these questions, we synthesized and biologically and biophysically investigated a set of native and synthetic AMPs and lipopeptides. These peptides represent different families, and they differ in their lengths, compositions, and net positive charge. The peptides and lipopeptides were tested against two WT S. typhimurium strains (ATCC 14028 and SL1344) and their isogenic mutants lacking functional PhoP-PhoQ systems. Furthermore, the four bacteria strains were constructed to express a PhoP-PhoQ reporting system to monitor the activation of the pagP gene by the various AMPs. Biophysically, all of the peptides and lipopeptides were investigated for their ability to permeate the bacterial membrane as a measure of their ability to traverse the outer membrane and interact with PhoQ. In addition, transmission electron microscopy was utilized to visualize damage to WTs and mutants caused by treatment with AMPs.
EXPERIMENTAL PROCEDURES
Materials and Bacteria
Rink amide methylbenzhydrylamine (MBHA) resin and Fmoc (N-(9-fluorenyl)methoxycarbonyl) amino acids were purchased from Calbiochem-Novabiochem. Other reagents used for peptide synthesis included TFA (Sigma-Aldrich), N,N-di-isopropylethylamine (Sigma-Aldrich), dichloromethane (peptide synthesis grade; Bio-Lab), dimethylformamide (peptide synthesis grade; Bio-Lab), and 1-hydroxybenzotriazole (HOBT) and O-(1-H-benzotriazole-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) (peptide synthesis grade; Bio-Lab). Four S. typhimurium strains were used: ATCC 14028 (WT) and the PhoP null derivative (3), a gift from Prof. Shoshi Altuvia's lab; and SL1344 (WT) and the knock-out PhoPQ::Tn10 derivative, a strain with a knock-out mutation that abolished the activity of the PhoP-PhoQ system. Polymyxin B, gentamicin, and kanamycin were purchased from Sigma (catalog numbers P1004, G1272, and K-1377, respectively).
Peptide Synthesis and Purification
The peptides were synthesized by a solid phase method on rink amide methylbenzhydrylamine (MBHA) resin by using an ABI 433A automatic peptide synthesizer (Applied Biosystems). The resin-bound peptides (0.1 meq each) were cleaved from the resins by trifluoroacetic acid (TFA), washed with dry ether, and extracted with acetonitrile/water (50% by volume). All of the peptides were amidated at their C terminus. The peptides were further purified by reverse phase HPLC on a C4 or C18 reverse phase Bio-Rad column (250 × 10 mm, 300-Å pore size, 5-μm particle size). A linear gradient of 10–70% acetonitrile in water containing 0.1% TFA (v/v) for 40 min was used at a flow rate of 1.8 ml/min. Each crude peptide contained one major peak, as revealed by reverse phase HPLC. The purified peptides were shown to be homogeneous (>98%) by analytical HPLC. Electrospray mass spectrometry confirmed their identity.
Antimicrobial Activity of Peptides
The antimicrobial activity of the peptides was examined in sterile 96-well plates (Nunc F96 microtiter plates) in a final volume of 100 μl as follows. Aliquots (50 μl) of a suspension containing bacteria with or without the reporting system plasmid (late log phase diluted 1:5000) in Luria-Bertani broth (LB, 20 g/liter LB broth, Conda) were added to 50 μl of LB broth containing the peptides in a serial 2-fold dilution. This LB is composed of 10 g/liter tryptone (pancreatic digest of casein), 5 g/liter yeast extract, and 5 g/liter sodium chloride, pH 7.0. Inhibition of growth was determined by eye (visibility of turbidity in comparison with blank with no growth) after an incubation of 18–20 h at 37 °C with agitation. Antibacterial activity was expressed as the minimal inhibitory concentration (MIC) in which no growth was seen.
Visualization of Bacteria Treated by the Peptides Using Transmission Electron Microscopy
Samples containing mid-log phase S. typhimurium SL1344 WT and its mutant were incubated with melittin, d,l-K5L7, and its fatty acid conjugated analog, C8-d,l-K5L7, at the MICs of the mutants or the WT strains. A drop of each sample was deposited onto a carbon-coated grid and negatively stained with phosphotungstic acid (2%), pH 6.8. The grids were examined using electron microscopy.
Construction of the Reporting System
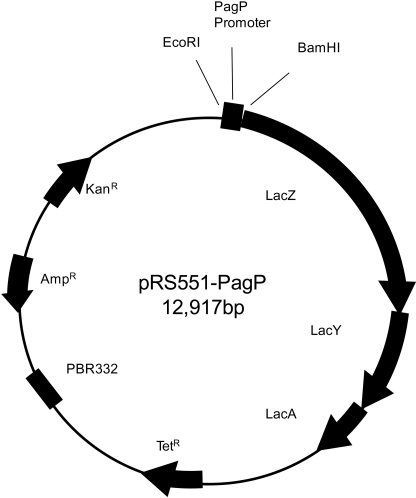
To follow the activation of the PhoP-PhoQ system in response to AMPs, we constructed a gene reporter assay based on the pagP gene, which codes for an enzyme that adds a palmitoyl chain to the lipid A part of LPS (26, 38). The pagP promoter region containing EcoRI and BamHI sites was generated using primers PagF (GCGAATTCGTCTTATCGACACCCAGAGT) and PagR (GTATGGATCCACCGTTCAAAAATTCGACTGTG). They were then amplified by polymerase chain reaction (Phusion; Promega) and used to clone into pRS551, a tightly regulated low copy number expression vector containing a promoterless lactose operon (lacZYA) (38). This vector was digested with EcoRI and BamHI, and the pagP promoter was cloned upstream of the lac genes. The pRS551-pagP plasmid was passed through a S. typhimurium LB5010 restriction mutant and then introduced by electroporation into S. typhimurium 14028 and SL1344 (WT and mutant strains).
Bacterial Growth Curve in the Presence of the Peptides
S. typhimurium ATCC 14028 WT was grown in LB broth containing peptide at various concentrations in a 96-well plate using a BioTek multiplate reader (Synergy HT). The plates were heated to 37 °C with shaking, and the absorbance (A600) was measured every 20 min. The growth curve was then plotted.
The Effect of AMPs on Induction of Reporter System
S. typhimurium ATCC 14028 WT and its null mutant were grown in LB broth containing peptide at 0.25-fold MIC of the tested strain (see under “Results” and Fig. 1) and 50 μg/ml kanamycin in a final volume of 100 μl in 96-well plates. After 6 and 20 h of incubation at 37 °C, 20 μl were sampled from each well and assayed for β-galactosidase expression (39). The experiments were performed in four wells per treatment, and their mean values were calculated. The results were expressed as the fold induction relative to the control value of normal culture in the absence of peptide. Gentamicin was used as a noninducer control antibiotic.
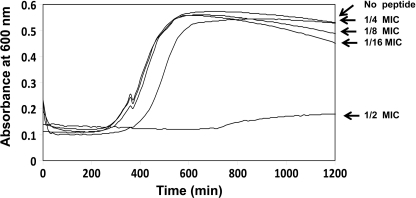
FIGURE 1.
Growth curves of bacteria in the presence of LL-37. S. typhimurium ATCC 14028 WT were grown in LB containing LL-37 peptide at different concentrations in a 96-well plate. The assay was done at 37 °C with agitation for 1200 min. Automatic measurements at A600 (Biotek Synergy HT, multiplate reader) were done every 10 min. Concentrations used are expressed as folds of the MIC.
Sytox Green Uptake Assay
S. typhimurium ATCC 14028 WT and its mutant were grown to mid-log phase, centrifuged, and suspended in sodium phosphate buffer (8 mm Na2HPO4, 2 mm NaH2PO4, pH 7.4). Their absorbance was measured, and sodium phosphate buffer was added to reach A600 = 0.1 in 10 ml. Two μl of Sytox Green (Invitrogen; S7020) were added to the bacterial stock, and they were incubated with agitation for 15 min at room temperature. A standard MIC assay was then done in a black 96-wells plate (Nunc) in sodium phosphate buffer, and the kinetics were measured over 20 min using a BioTek multiplate reader (Synergy HT) fluorescent detector (excitation = 485 nm, emission = 528 nm). At the end point, the fluorescence had reached equilibrium.
RESULTS
Peptide Design and Synthesis
We synthesized and investigated a broad repertoire of AMPs differing in length, composition, and net positive charge. To reduce the contribution of drastic structural changes to their function, we selected amphipatic peptides that are either fully α-helical or preserved partial helical structures (40). The list of peptides and lipopeptides is presented in Table 1 and includes: (i) the human AMP LL-37; (ii) the bee venom melittin; (iii) a de novo designed 15-mer AMP-designated K6L9; (iv) its d,l-amino acid-containing analog d,l-K6L9; (v) its d,l-amino acid histidine derivative d,l-H6L9; (vi) a 12-mer d,l-K5L7; and (vii) its fatty-acid conjugated analog C8-d,l-K5L7. The antibiotic polymyxin B, which specifically targets LPS, and the antibiotic gentamicin, which targets the ribosome, were used as controls. Table 1 also lists the net positive charge of the peptides under the experimental conditions. In addition, the reverse phase HPLC retention times are shown and used to measure the experimental hydrophobicities. A longer retention time would indicate higher hydrophobicity.
TABLE 1.
Peptide designations, sequences, and RP-HPLC retention times
| Peptide number | Peptide designation | Sequencea | Net charge (at pH 7.0) | HPLC retention time |
|---|---|---|---|---|
| min | ||||
| 1 | LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-NH2 | +7 | 34.13 |
| 2 | Melittin | GIGAVLKVLTTGLPALISWIKRKRQQG-NH2 | +6 | 32.38 |
| 3 | l-K6L9 | LKLLKKLLKKLLKLL-NH2 | +7 | 33.85 |
| 4 | d,l-K6L9 | LKLLKKLLKKLLKLL-NH2 | +7 | 27.71 |
| 5 | d,l-H6L9 | LHLLHHLLHHLLHLL-NH2 | +1 | 29.31 |
| 6 | d,l-K5L7 | KKLLKLLLKLLK-NH2 | +6 | 23.57 |
| 7 | C8-d,l-K5L7 | CH3(CH2)6CO-KKLLKLLLKLLK-NH2 | +5 | 29.15 |
| 8 | Polymyxin B | C56H100N16O17S | +5 | 22.56 |
a Underlined and bold amino acids are d-enantiomers. All of the peptides are amidated in their C termini.
Antibacterial Activity of the Peptides
The antibacterial activity of the eight peptides and lipopeptides was tested against the two strains of the WT and mutants of S. typhimurium. Our results show that polymyxin B and d,l-K6L9 were the most potent molecules against the WT strains, whereas LL-37, melittin, d,l-H6L9, and d,l-K5L7 were significantly less active. Note that the addition of a C8 hydrophobic tail to d,l-K5L7 enhanced its activity (Table 2). Our data also revealed that, as compared with the WT strains, the two isogenic PhoP-PhoQ mutants were 4-fold more susceptible to melittin and C8-d,l-K5L7 (Table 2). However, these mutants did not display increased susceptibility to LL-37, l-K6L9, and d,l-H6L9 and were only 2-fold more susceptible to d,l-K6L9, d,l-K5L7, and polymyxin B.
TABLE 2.
MICs of various peptides against S. typhimurium strains
MICs are in μm. The experiments were done in LB medium.
| Peptide designation | 14028 WT | 14028 PhoP null | SL1344 WT | SL1344 PhoPQ::Tn10 |
|---|---|---|---|---|
| LL-37 | 50 | 50 | >50 | 50 |
| Melittin | 50 | 12.5 | 50 | 12.5 |
| l-K6L9 | 50 | 25 | 50 | 50 |
| d,l-K6L9 | 3.12 | 1.56 | 3.12 | 1.56 |
| d,l-H6L9 | 50 | 50 | >50 | >50 |
| d,l-K5L7 | 50 | 25 | 50 | 25 |
| C8-d,l-K5L7 | 12.5 | 3.12 | 12.5 | 3.12 |
| Polymyxin B | 1.56 | 0.78 | 1.56 | 0.39 |
| Gentamicina | 1.5 | 1.5 | 1.5 | 1.5 |
a The aminoglycoside gentamicin was used as a reference molecule.
Visualization of Cell Damage by Transmission Electron Microscopy
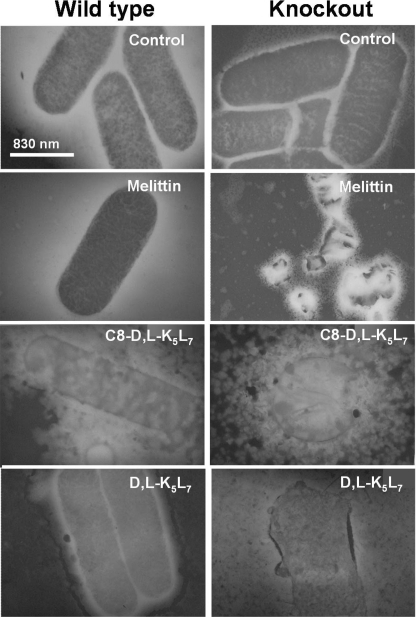
Transmission electron microscopy was used to visualize the effect of the peptides against mid-log phase cells of S. typhimurium SL1344 WT and its mutant. Three peptides were tested: two displaying significantly higher antibacterial activity on the mutant strains compared with the WT strains (melittin and C8-d,l-K5L7) and one that is only slightly more active on the mutant strains (d,l-K5L7; Table 2). Melittin and d,l-K5L7 were used at concentrations corresponding to the MIC of the mutant strain. Therefore, they induced major physical damage to the mutant that was not observed in WT bacteria (Fig. 2). In contrast, when the more potent C8-d,l-K5L7 was used at the MIC of the WT strain, massive damage to both WT and mutant bacteria occurred. The results are in agreement with the MIC values of these peptides against these strains (Table 2).
FIGURE 2.
Electron microscopy images of S. typhimurium SL1344 WT and phoPQ::Tn10 knock-out mutant incubated with peptides. Bacteria were incubated with either melittin (mutant MIC value), d,l-K5L7 (mutant MIC value), or C8-d,l-K5L7 (WT MIC value). The reason for selecting these MICs is detailed under “Experimental Procedures.” A drop of each sample was deposited onto a carbon-coated grid and negatively stained with phosphotungstic acid (2%, pH 6.8), and the grids were examined by electron microscopy.
Minimal Peptide Concentration Required to Activate PhoP-PhoQ System
To determine whether a short term induction of PhoP-PhoQ would increase the resistance of the bacteria toward the peptides, we first determined the peptide concentration that activates PhoP-PhoQ but does not affect bacterial growth. For that purpose, we grew the bacteria in the presence of the peptides at declining concentrations and measured absorbance (A600) for cell growth. The data summarized in Fig. 1 reveal that the highest peptide concentration of LL-37 that did not affect growth was 25% of the MIC concentration. Therefore, we used this concentration in the following experiments.
The Ability of Peptides to Activate PhoP-PhoQ Reporter System
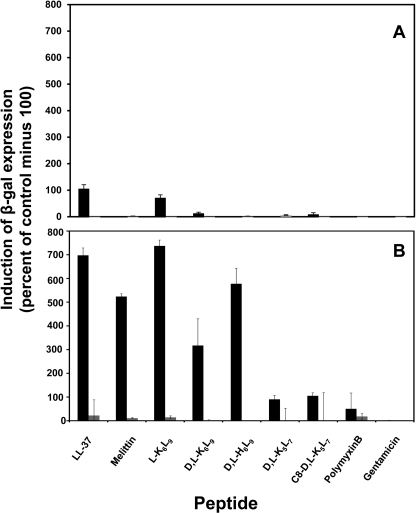
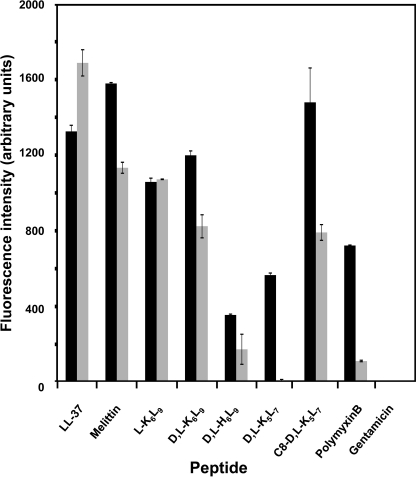
The pagP reporter system constructed in this study (Fig. 3) was used to investigate the potency of the AMPs to activate PhoP-PhoQ. This system uses the promotor of pagP, a known PhoP activated gene, to express β-galactosidase. Because both WT strains and their mutants had similar MICs (Table 2), we decided to continue with S. typhimurium ATCC 14028 WT and its null mutant. The bacteria were grown in the absence or presence of subinhibitory concentrations of a peptide (0.25 MIC), and the fold induction was measured at 6 and 20 h. Our results show that a slight induction of the reporter system in the WT strain at 6 h occurred only with LL-37 and l-K6L9 (Fig. 4A). After 20 h, however, a stronger induction was observed with LL-37, melittin, l-K6L9, d,l-K6L9, and d,l-H6L9 (Fig. 4B). In contrast, polymyxin B and the shorter peptides d,l-K5L7 and C8-d,l-K5L7 were less potent inducers. The data also reveal that as expected, the PhoP null strain could not activate the reporter system. Interestingly, the His-containing peptide d,l-H6L9 (net charge +1 under the experimental conditions) was as potent as melittin (net charge +6) in activating pagP (see “Discussion”).
FIGURE 3.
Construction of a reporting system using pRS551-pagP. S. typhimurium 14028, SL1344, and mutants were transformed with the pRS551-Lactose operon plasmid having the Salmonella pagP promoter. The pagP promoter region containing the EcoRI and BamHI sites was generated with primers pagF and pagR. It was then amplified by PCR and inserted into pRS551, a tightly regulated expression vector with low copy number that was digested with EcoRI and BamHI. The lacZ gene is controlled by the pagP promoter. The activity of β-galactosidase was determined by using a standard assay.
FIGURE 4.
The ability of AMPs to activate PhoP-PhoQ as determined by the reporter gene assay. S. typhimurium ATCC 14028 WT (black bars) and null mutant (gray bars) were grown in LB-containing peptide at 0.25-fold MIC of the tested strain. After 6 (A) and 20 h (B) of incubation at 37 °C, 20 μl were sampled from each well and assayed for β-galactosidase (β-gal) expression. The experiments were performed in quintuplet, and their mean values were calculated. The results are expressed as the fold inductions relative to the control value of normal culture in the absence of peptide. The noninducer gentamicin was used as a control antibiotic. The error bars represent the standard error.
Overnight Induction of PhoP-PhoQ and Its Influence on Resistance
To test whether induction of the system would increase resistance toward the peptides, we precultivated the WT bacteria overnight (20 h) in the presence of subinhibitory concentrations (0.25 MIC) of AMPs. The MIC values for the peptides did not change. This indicates that none of the peptides induced PhoP-PhoQ-mediated self-resistance under these conditions. As expected, the MIC of the control, the aminoglycoside gentamicin, an antibiotic that binds the ribosome to inhibit protein synthesis, was identical for all the strains (Table 2).
Permeation of Bacterial Membrane Induced by Peptides
A Sytox Green assay was used to rule out the possibility that the inability of some of the AMPs to activate the PhoP-PhoQ system is due to their inability to traverse the outer membrane to interact with PhoQ. Sytox is a dye with high affinity for nucleic acids that penetrate only into membrane-compromised cells. Upon binding to nucleic acids, the fluorescence of the dye is enhanced dramatically. We measured the activity of all the peptides in promoting Sytox entrance into both WT and the mutant strain incubated at a concentration of peptides corresponding to 0.25-fold of their MICs. The data revealed that all the peptides generated a significant emission of fluorescence (Fig. 5). In buffer without a peptide or with gentamicin used as a control, no entrance of dye into the bacteria was observed (data not shown). The data indicate that these AMPs, when used at concentrations below their MICs, reach the inner membrane and perturb it. This supports the assumption that all of the peptides can reach PhoP-PhoQ.
FIGURE 5.
Sytox entrance in S. typhimurium ATCC 14028 WT and null mutant. Bacteria (WT or null) were grown in LB until they reached A600 = 1 and then were centrifuged and transferred to sodium phosphate buffer and diluted to A600 = 0.1. The bacteria were incubated at room temperature with Sytox Green dye and then transferred to a 96-well plate containing LB supplemented with peptides at 0.25 MIC of each peptide. The black and gray bars represent WT and null, respectively. After 20 min of incubation, the fluorescence was measured. The error bars represent the standard error.
DISCUSSION
We explored a select series of native and synthetic AMPs (Table 1), which allowed us to study how several properties of antimicrobial peptides contributed to their ability to activate the bacterial PhoP-PhoQ two-component system and as a result to induce bacterial resistance toward them. The selection of two WT strains and their corresponding nonfunctional PhoP-PhoQ mutants, all bearing the reporting system plasmid, allowed us to monitor PhoP-PhoQ activation in a WT genetic background. All of the assays were carried out in LB medium with low concentrations of magnesium to enable efficient activation of PhoP-PhoQ (19). The antibiotic gentamicin was used as a control because it does not activate the PhoP-PhoQ system (Fig. 4). Our data, which are summarized in Table 3 and Fig. 6, revealed several important findings.
TABLE 3.
Summary of the various properties of the peptides
| Peptide designation | Amphipathic helixa | Antimicrobial activity | Increased susceptibility of PhoP-PhoQ mutants | Sytox activityb |
Charge (pH 7.0) | HPLC retention time | PagP activationb | |
|---|---|---|---|---|---|---|---|---|
| WT | mutant | |||||||
| min | ||||||||
| LL-37 | Yes | Weak | No | High | High | +7 | 34.13 | High |
| Melittin | Yes | Weak | Yes | High | High | +6 | 32.38 | High |
| l-K6L9 | Yes | Weak | No | Low | Med | +7 | 33.85 | High |
| d,l-H6L9 | Distorted | Weak | No | Low | High | +1 | 29.31 | High |
| d,l-K6L9 | Distorted | Potent | No | Low | Low | +7 | 27.71 | Low |
| d,l-K5L7 | Distorted | Weak | No | Low | Low | +6 | 23.57 | Low |
| C8-d,l-K5L7 | Distorted | Potent | Yes | Med | High | +5 | 29.15 | Low |
| Polymyxin B | Potent | Yes | High | Med | +5 | 22.56 | Low | |
| Gentamicin | Potent | No | Low | Low | +3 | Void volume | Low | |
a Taken from previous studies.
b Sytox activity and pagP activation were determined in the presence of subinhibitory concentrations of AMPs (0.25 MIC).
FIGURE 6.
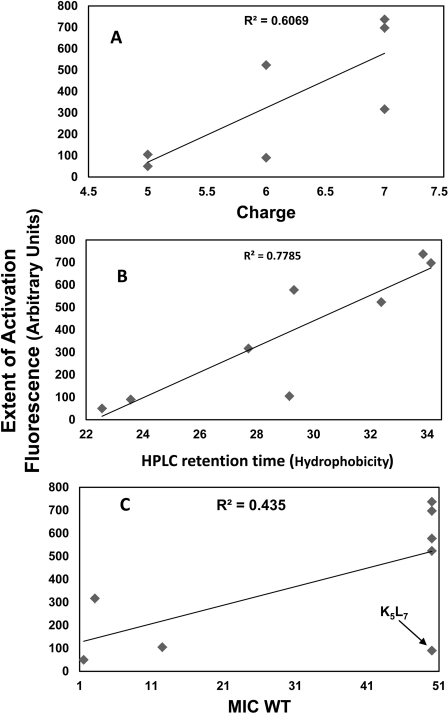
Analysis of the contribution of various parameters on the ability of the peptides to activate PhoP-PhoQ. We plotted graphs of hydrophobicity (A), charge (B), and antibacterial activity (C) versus the ability of the AMP to activate PhoP-PhoQ. Each dot represents the characteristics of one of the peptides.
Role of Positive Charge, Hydrophobicity, and Amphipaticity of the Peptides in PhoP-PhoQ Activation
In addition to d,l-H6L9, which has a +1 net charge under the experimental conditions, all of the other AMPs have a net charge ranging from +5 to +7 (Table 3). Taking into consideration the unique properties of the histidine peptide, we plotted the extent of activation as a function of charge for all other peptides (Fig. 6A). The figure reveals a general trend in which peptides with high positive charge are more potent in activating the system. Bader et al. (22) suggested a model in which cationic AMPs displace divalent cations from PhoQ metal-binding sites to initiate signal transduction. This model can explain the higher potency of the more charged AMPs to activate the system. However, it cannot explain why the histidine peptide d,l-H6L9, carrying only a +1 net charge, activated PhoP-PhoQ significantly more than its analog d,l-K6L9 carrying a +7 net charge. A plausible explanation is that whereas the highly charged AMPs activate PhoQ by replacing Mg2+in the binding site of the protein (22), d,l-H6L9 does not replace Mg2+ ions but rather removes them from the protein and LPS because of the ability of histidine to complex with divalent cations.
Fig. 6B shows a plot of the extent of activation versus the experimental hydrophobicity (HPLC retention times) of the peptides. This figure demonstrates a reasonable linear dependence between the two properties, i.e. peptides with high hydrophobicity are more potent in activating the system compared with peptides with low hydrophobicity. The lipopeptide C8-d,l-K5L7 is an exception, possibly because its hydrophobicity is due to the fatty acid part and not to the peptidic part, d,l-K5L7, which is the least hydrophobic among the peptides.
The peptide's amphipaticity also seems to contribute to its activating ability because the three all-l-amino acid peptides (LL-37, melittin, and l-K6L9) with the highest experimental hydrophobicity also have an α-helical structure in membranes (41, 42). Note that all of the peptides can reach the inner membrane to interact with PhoQ (Fig. 5).
Relationship between Extent of PhoP-PhoQ Activation and Antimicrobial Activity of Peptides against WTs and Mutants
Fig. 6C shows a plot of the extent of PhoP-PhoQ activation versus the antimicrobial activity of the peptides against the WT bacteria. The figure shows that in addition to d,l-K5L7, there is a general trend in which peptides that only slightly activate the system (C8-d,l-K5L7, d,l-K6L9, and polymyxin B) have low MICs (are more potent) compared with those that are strong activators (LL-37, melittin, l-K6L9, and d,l-H6L9). These results agree with the concept that AMPs that can highly activate PhoP-PhoQ can also induce bacterial resistance to some extent (6, 9, 11, 12, 14, 28).
Interestingly, however, comparing the antimicrobial activity of the peptides against the WT versus the mutant bacteria (Table 2) revealed that only some of the peptides are more active on the mutants compared with the WT bacteria. Furthermore, this activity was independent of whether the peptides are weak or potent activators (i.e. melittin is a potent activator, and C8-d,l-K5L7 and polymyxin B are both weak activators).
Short Term Preincubation of WT Bacteria with AMPs Does Not Induce Resistance
The likelihood that bacteria will develop resistance to AMPs by activation of PhoP-PhoQ suggests that these bacteria should be more resistant to AMPs upon preincubation with the peptides (22, 33, 37). We therefore determined the MICs of the peptides against WT bacteria cultivated for 20 h with subinhibitory concentrations of AMPs (0.25 MIC) (Fig. 1). No differences were observed between the MICs of the naïve and the AMP-pretreated WT bacteria. These data suggest that short term activation of PhoP-PhoQ does not promote in vitro resistance to any of these selected WT peptides.
Overall, these data support two possible mechanisms for removing Mg2+ and activation of PhoP-PhoQ by the AMPs studied herein. The first mechanism is in line with the suggestion that cationic AMPs displace metal ions in the acidic sensory domain of PhoQ (8, 22, 23). Therefore, this requires a high positive charge. The second mechanism that can explain our data with the histidine-containing peptide (only a +1 net charge) also suggests the removal of cations from the acidic sensory domain. However, in that case the ions are probably removed by chelating with histidine. Importantly, whereas a correlation exists between the extent of PhoP-PhoQ activation and the antimicrobial activity of the WT bacteria, short time incubation of bacteria with the AMPs investigated did not induce resistance. Furthermore, the removal of PhoP-PhoQ had an effect only on a few AMPs, which suggests that some AMPs can overcome LPS palmitoylation.
Acknowledgment
We thank Yehuda Marikovsky for help with electron microscopy.
This work was supported in part by the German-Israel Foundation for Scientific Research and Development (GIF) and by the Weizmann-Pasteur Joint Research Program.
- AMP
- antimicrobial peptide
- MIC
- minimal inhibitory concentration.
REFERENCES
- 1. Groisman E. A., Chiao E., Lipps C. J., Heffron F. (1989) Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl. Acad. Sci. U.S.A. 86, 7077–7081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller S. I., Kukral A. M., Mekalanos J. J. (1989) A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. U.S.A. 86, 5054–5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller S. I., Mekalanos J. J. (1990) Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J. Bacteriol. 172, 2485–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roland K. L., Martin L. E., Esther C. R., Spitznagel J. K. (1993) Spontaneous pmrA mutants of Salmonella typhimurium LT2 define a new two-component regulatory system with a possible role in virulence. J. Bacteriol. 175, 4154–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia Véscovi E., Soncini F. C., Groisman E. A. (1994) The role of the PhoP/PhoQ regulon in Salmonella virulence. Res. Microbiol. 145, 473–480 [DOI] [PubMed] [Google Scholar]
- 6. Gunn J. S., Miller S. I. (1996) PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178, 6857–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Groisman E. A., Kayser J., Soncini F. C. (1997) Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J. Bacteriol. 179, 7040–7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Véscovi E. G., Ayala Y. M., Di Cera E., Groisman E. A. (1997) Characterization of the bacterial sensor protein PhoQ. Evidence for distinct binding sites for Mg2+ and Ca2+. J. Biol. Chem. 272, 1440–1443 [DOI] [PubMed] [Google Scholar]
- 9. Macfarlane E. L., Kwasnicka A., Hancock R. E. (2000) Role of Pseudomonas aeruginosa PhoP-PhoQ in resistance to antimicrobial cationic peptides and aminoglycosides. Microbiology 146, 2543–2554 [DOI] [PubMed] [Google Scholar]
- 10. Groisman E. A. (2001) The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183, 1835–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trent M. S., Pabich W., Raetz C. R., Miller S. I. (2001) A PhoP/PhoQ-induced Lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J. Biol. Chem. 276, 9083–9092 [DOI] [PubMed] [Google Scholar]
- 12. McPhee J. B., Lewenza S., Hancock R. E. (2003) Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol. Microbiol. 50, 205–217 [DOI] [PubMed] [Google Scholar]
- 13. Mouslim C., Groisman E. A. (2003) Control of the Salmonella ugd gene by three two-component regulatory systems. Mol. Microbiol. 47, 335–344 [DOI] [PubMed] [Google Scholar]
- 14. Shi Y., Cromie M. J., Hsu F. F., Turk J., Groisman E. A. (2004) PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 53, 229–241 [DOI] [PubMed] [Google Scholar]
- 15. Kato A., Mitrophanov A. Y., Groisman E. A. (2007) A connector of two-component regulatory systems promotes signal amplification and persistence of expression. Proc. Natl. Acad. Sci. U.S.A. 104, 12063–12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murata T., Tseng W., Guina T., Miller S. I., Nikaido H. (2007) PhoPQ-mediated regulation produces a more robust permeability barrier in the outer membrane of Salmonella enterica serovar typhimurium. J. Bacteriol. 189, 7213–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prost L. R., Miller S. I. (2008) The Salmonellae PhoQ sensor. Mechanisms of detection of phagosome signals. Cell Microbiol. 10, 576–582 [DOI] [PubMed] [Google Scholar]
- 18. Yu J. L., Guo L. (2011) Quantitative proteomic analysis of Salmonella enterica serovar typhimurium under PhoP/PhoQ activation conditions. J. Proteome Res 10, 2992–3002 [DOI] [PubMed] [Google Scholar]
- 19. García Véscovi E., Soncini F. C., Groisman E. A. (1996) Mg2+ as an extracellular signal. Environmental regulation of Salmonella virulence. Cell 84, 165–174 [DOI] [PubMed] [Google Scholar]
- 20. Prost L. R., Daley M. E., Le Sage V., Bader M. W., Le Moual H., Klevit R. E., Miller S. I. (2007) Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol. Cell 26, 165–174 [DOI] [PubMed] [Google Scholar]
- 21. Guo L., Lim K. B., Poduje C. M., Daniel M., Gunn J. S., Hackett M., Miller S. I. (1998) Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95, 189–198 [DOI] [PubMed] [Google Scholar]
- 22. Bader M. W., Sanowar S., Daley M. E., Schneider A. R., Cho U., Xu W., Klevit R. E., Le Moual H., Miller S. I. (2005) Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122, 461–472 [DOI] [PubMed] [Google Scholar]
- 23. Choi E., Groisman E. A., Shin D. (2009) Activated by different signals, the PhoP/PhoQ two-component system differentially regulates metal uptake. J. Bacteriol. 191, 7174–7181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soncini F. C., García Véscovi E., Solomon F., Groisman E. A. (1996) Molecular basis of the magnesium deprivation response in Salmonella typhimurium. Identification of PhoP-regulated genes. J. Bacteriol. 178, 5092–5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gunn J. S. (2008) The Salmonella PmrAB regulon. Lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol. 16, 284–290 [DOI] [PubMed] [Google Scholar]
- 26. Bishop R. E., Gibbons H. S., Guina T., Trent M. S., Miller S. I., Raetz C. R. (2000) Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria. EMBO J. 19, 5071–5080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bader M. W., Navarre W. W., Shiau W., Nikaido H., Frye J. G., McClelland M., Fang F. C., Miller S. I. (2003) Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50, 219–230 [DOI] [PubMed] [Google Scholar]
- 28. Bishop J. L., Finlay B. B. (2006) Friend or foe? Antimicrobial peptides trigger pathogen virulence. Trends Mol. Med 12, 3–6 [DOI] [PubMed] [Google Scholar]
- 29. Guina T., Yi E. C., Wang H., Hackett M., Miller S. I. (2000) A PhoP-regulated outer membrane protease of Salmonella enterica serovar typhimurium promotes resistance to α-helical antimicrobial peptides. J. Bacteriol. 182, 4077–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ernst R. K., Guina T., Miller S. I. (2001) Salmonella typhimurium outer membrane remodeling. Role in resistance to host innate immunity. Microbes Infect. 3, 1327–1334 [DOI] [PubMed] [Google Scholar]
- 31. Bijlsma J. J., Groisman E. A. (2005) The PhoP/PhoQ system controls the intramacrophage type three secretion system of Salmonella enterica. Mol. Microbiol. 57, 85–96 [DOI] [PubMed] [Google Scholar]
- 32. Matson J. S., Yoo H. J., Hakansson K., Dirita V. J. (2010) Polymyxin B resistance in El Tor Vibrio cholerae requires lipid acylation catalyzed by MsbB. J. Bacteriol. 192, 2044–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kindrachuk J., Paur N., Reiman C., Scruten E., Napper S. (2007) The PhoQ-activating potential of antimicrobial peptides contributes to antimicrobial efficacy and is predictive of the induction of bacterial resistance. Antimicrob. Agents Chemother. 51, 4374–4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baker S. J., Daniels C., Morona R. (1997) PhoP/Q regulated genes in Salmonella typhi identification of melittin-sensitive mutants. Microb. Pathog. 22, 165–179 [DOI] [PubMed] [Google Scholar]
- 35. Kox L. F., Wösten M. M., Groisman E. A. (2000) A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 19, 1861–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Detweiler C. S., Monack D. M., Brodsky I. E., Mathew H., Falkow S. (2003) virK, somA and rcsC are important for systemic Salmonella enterica serovar typhimurium infection and cationic peptide resistance. Mol. Microbiol. 48, 385–400 [DOI] [PubMed] [Google Scholar]
- 37. Schurek K. N., Sampaio J. L., Kiffer C. R., Sinto S., Mendes C. M., Hancock R. E. (2009) Involvement of pmrAB and phoPQ in polymyxin B adaptation and inducible resistance in non-cystic fibrosis clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53, 534345–534351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simons R. W., Houman F., Kleckner N. (1987) Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53, 85–96 [DOI] [PubMed] [Google Scholar]
- 39. Miller J. H. (1972) Experiments in Molecular Genetics, pp. 352–355, Cold Spring Harbor, NY [Google Scholar]
- 40. Papo N., Oren Z., Pag U., Sahl H. G., Shai Y. (2002) The consequence of sequence alteration of an amphipathic α-helical antimicrobial peptide and its diastereomers. J. Biol. Chem. 277, 33913–33921 [DOI] [PubMed] [Google Scholar]
- 41. Makovitzki A., Fink A., Shai Y. (2009) Suppression of human solid tumor growth in mice by intratumor and systemic inoculation of histidine-rich and pH-dependent host defense-like lytic peptides. Cancer Res. 69, 3458–3463 [DOI] [PubMed] [Google Scholar]
- 42. Papo N., Shai Y. (2004) Effect of drastic sequence alteration and d-amino acid incorporation on the membrane binding behavior of lytic peptides. Biochemistry 43, 6393–6403 [DOI] [PubMed] [Google Scholar]