Abstract
Human embryo implantation is a critical multistep process consisting of embryo apposition/adhesion, followed by penetration and invasion. Through embryo penetration, the endometrial epithelial cell barrier is disrupted and remodeled by an unknown mechanism. We have previously developed an in vitro model for human embryo implantation employing the human choriocarcinoma cell line JAR and the human endometrial adenocarcinoma cell line Ishikawa. Using this model we have shown that stimulation with ovarian steroid hormones (17β-estradiol and progesterone, E2P4) and suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor, enhances the attachment and adhesion of JAR spheroids to Ishikawa. In the present study we showed that the attachment and adhesion of JAR spheroids and treatment with E2P4 or SAHA individually induce the epithelial-mesenchymal transition (EMT) in Ishikawa cells. This was evident by up-regulation of N-cadherin and vimentin, a mesenchymal cell marker, and concomitant down-regulation of E-cadherin in Ishikawa cells. Stimulation with E2P4 or SAHA accelerated Ishikawa cell motility, increased JAR spheroid outgrowth, and enhanced the unique redistribution of N-cadherin, which was most prominent in proximity to the adhered spheroids. Moreover, an N-cadherin functional blocking antibody attenuated all events but not JAR spheroid adhesion. These results collectively provide evidence suggesting that E2P4- and implanting embryo-induced EMT of endometrial epithelial cells may play a pivotal role in the subsequent processes of human embryo implantation with functional control of N-cadherin.
Keywords: Cadherins, Cell motility, Embryo implantation, Epithelial mesenchymal transition, Estrogen, Progesterone
Introduction
The first critical step in establishing a human pregnancy is implantation of an embryo onto the endometrial epithelial cells (EECs).2 In human, differentiation of the EECs is regulated across the menstrual cycle by changing levels of ovarian steroids. This ensures that an embryo-acceptable endometrial lining is present within 7–11 days after ovulation, the period termed the “implantation window” (1–3). Human embryonic implantation is a complex, multistep process, during which the embryo first comes into apposition with, and then later adheres onto the EEC layer. This is followed by embryo penetration through the EEC with invasion into the endometrial stromal cell (ESC) layer.
Although several regulatory mediators of implantation have been identified in mice, the one or more mechanisms governing human implantation are largely still unknown. Ethical concerns and technical limitations pose significant obstacles to elucidating these processes in humans (4). Whereas mouse implantation models have enabled detailed molecular analyses, the human implantation site has remained inaccessible in vivo. To address the limitations of existing models, we adapted an in vitro implantation assay using human EECs and simulated model embryos (5). This model has been employed by several investigators studying early events in implantation (4–7). For example, using this model, we investigated the effect of suberoylanilide hydroxamic acid (SAHA). SAHA is one of the histone deacetylase inhibitors. Reversible nucleosomal histone acetylation, a histone modification that is controlled by histone acetyltransferases and histone deacetylases, regulates gene transcription (8), therefore, histone deacetylase inhibitors are able to exchange transcription of a part of genes. We have previously demonstrated that SAHA enhanced human implantation through up-regulation of Glycodelin protein expression, which is originally induced by ovarian steroid hormones in human EECs during the implantation window (5, 9).
An early event in embryo implantation is disruption of the EEC barrier. The mechanisms underlying EEC remodeling have not been addressed. It is uncertain whether migration or proliferation is responsible for this remodeling. One process that we explored herein with this model is the epithelial-mesenchymal transition (EMT). The EMT is characteristic in migration or invasion, including early development and tumor cell metastasis (10, 11). E- and N-cadherin proteins are members of the cadherin superfamily and are transmembrane adhesion molecules that mediate homophilic cell-cell adhesion (12). During EMT, the phenomenon known as “cadherin switch,” characterized by down-regulation of E-cadherin and up-regulation of N-cadherin, is observed. In association with actin rearrangement, such as stress fiber formation and decreased cortical actin, the cadherin switch is reflected in the acceleration of cell motility during EMT (13, 14). Using our in vitro implantation assay, we provide evidence that the EEC migration through EMT plays an important role in the remodeling of the EEC barrier during implantation.
EXPERIMENTAL PROCEDURES
Materials
Phenol red-free minimum essential medium, RPMI 1640 medium, and FBS were purchased from Invitrogen. SAHA was obtained from BIOMOL (Plymouth Meeting, PA). Lipophilic dye cell tracers, DiI and DiO, were purchased from Invitrogen. Antibodies against E-cadherin, N-cadherin (BD Biosciences, Bedford, MA), N-cadherin (clone FA-5), MAPK (Upstate Biotechnology, Inc., Lake Placid, NY), Texas Red-conjugated phalloidin (Invitrogen), and Cy2-, Cy3-, and horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were purchased from commercial sources. Unless indicated otherwise, all other chemicals were obtained from Sigma-Aldrich or Wako (Osaka, Japan).
Cell Cultures
Ishikawa (clone 3-H-12) (15), a human endometrial adenocarcinoma cell line of epithelial origin, was a kind gift from Dr. M. Nishida (National Kasumigaura Hospital, Ibaragi, Japan). JAR, a human choriocarcinoma cell line, was kindly provided by Dr. N. Suzuki (St. Marianna University, Kanagawa, Japan). Ishikawa cells and JAR cells were cultured in phenol red-free minimum essential medium and RPMI 1640 medium, respectively, supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 mg/ml streptomycin. Ishikawa cells were used within 10 passages according to the provider's recommendation to avoid changes in cell characteristics, including down-regulation of estrogen receptor and progesterone receptor expression.
Immunofluorescent Study
Ishikawa cells with or without JAR spheroids were fixed with 3.7% paraformaldehyde, permeabilized with 0.2% Triton X-100 in PBS, and then incubated with the indicated antibody for 1 h at room temperature, followed by incubation with the appropriate secondary antibody. Confocal images were acquired using a Leica TCS SP2 confocal microscopy system with a Leica DMIRE2 inverted microscope (Leica Microsystems). Other fluorescent or differential interference contrast images were photographed using fluorescence microscopy (BIOREVO® BZ-9000, Keyence, Osaka, Japan). To visualize actin, cells were incubated with Texas Red-conjugated phalloidin for 30 min at room temperature. Nonspecific background fluorescence was determined by staining the cells with irrelevant antibodies coupled with the appropriate dye-conjugated secondary antibodies. Signal intensities of vimentin were analyzed in the Ishikawa monolayers. A 500-μm square area of the Ishikawa monoculture was examined. In the JAR spheroid co-culture, 200-μm square areas of the Ishikawa monolayer were assessed for vimentin. Areas examined were both adjacent to the spheroid as well as 400 μm away from the spheroid. Images were analyzed using computer application ImageJ software (National Institutes of Health). N-cadherin signal intensities in the area every 80 μm from the edge of Ishikawa monolayer adjacent to the spheroid were also analyzed using ImageJ.
Immunoprecipitation and Immunoblotting
Confluent Ishikawa cells with or without 5000 JAR spheroids were cultured in the indicated medium in a 60-mm dish for 3 days and lysed on ice with RIPA buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% sodium deoxycholate, 0.1% SDS, 1 mm Na3VO4, 50 mm NaF, 1 mm Na2MoO4) containing a protease inhibitor mixture (Roche Applied Science). A separate shaking culture of JAR spheroids was also lysed with the same buffer. Protein concentrations were determined using the DC protein assay kit (Bio-Rad) with BSA as a standard. 250-μg aliquots of protein were then subjected to immunoprecipitation with anti-E-cadherin, or anti-N-cadherin antibody and protein G-Sepharose beads (GE Healthcare, Fairfield, CT) for 4 h at 4° C. Immunoprecipitates and whole cell lysates were separated by electrophoresis on an 8% or a 15% SDS-PAGE gel and transferred onto a polyvinylidene difluoride membrane. After incubation with anti-E-cadherin, anti-N-cadherin, or anti-MAPK antibodies, followed by a horseradish peroxidase-conjugated secondary antibody, the immunoreactive proteins were detected by using the ECL method (GE Healthcare). Band intensities were analyzed using ImageJ. Immunofluorescence, immunoprecipitation, and immunoblotting studies were repeated three times, and the representative images are shown.
In Vitro Implantation Assay
To generate spheroids of JAR cells for use as blastocyst models (5–7), the JAR cell concentration was adjusted to 2 × 105 cells/ml, and the cell suspension was cultured with shaking at 70 rpm in medium containing cell tracers, DiI or DiO. Spheroids of 50–200 μm in diameter, which were similar in size to an implanting blastocyst, were generated after 24-h shaking culture. Ishikawa cells were prepared in 96-well plates or 8-well chamber slides and were grown with or without various agents (ovarian steroid hormones, SAHA, N-cadherin functional blocking antibody (FA-5), and mouse IgG1 antibody (MOPC21)) for the indicated period. After the Ishikawa cells had reached confluence, co-culture of Ishikawa cells and JAR spheroids (∼100 spheroids/well) for the indicated period was performed with fresh medium containing no factors. Co-cultured cells were then fixed with 3.7% paraformaldehyde. To visualize and count the attached or adhered lipophilic-dye coated spheroids, confocal or fluorescent microscopy images were acquired. The relative implantation ratio was then calculated as the number of adhered spheroids divided by the number of implanted control cells. The relative implantation ratio of control cells was set at 1. The relative outgrowth area was calculated as the area of adhered and spread spheroids measured using ImageJ.
Wound Healing Assay
A confluent Ishikawa monolayer was scratched with a plastic micropipette tip (0 h) and then cultured in the absence or presence of 10 nm 17β-estradiol plus 1 mm progesterone (E2P4), or 2.0 μm SAHA in combination with or without 1/200 dilution of FA-5 (IgG1) or irrelevant antibody MOPC (IgG1) for 24 h. At times 0 and 24 h, the scratched monolayer cultures were photographed using fluorescence microscopy (BIOREVO® BZ-9000). Quantification was performed by measuring the number of pixels in each wound closure area using ImageJ. Each wound closure area for the experimental cells was then compared with the wound closure area of the cells cultured in the control medium, or the medium containing E2P4 alone, or SAHA alone. The wound closure area of the control cells was set at 1.
Statistical Analysis
All experimental data from the bioassays represent the results obtained from three independent experiments, each with triplicate assays, expressed as the mean ± S.E. Statistical analysis was performed utilizing the analysis of variance, with the Bonferroni correction. Immunofluorescence, immunoprecipitation, and immunoblotting studies were repeated three times, and the representative images are shown. The differences were considered significant at p < 0.05.
RESULTS
Ovarian Steroid Hormones and JAR Spheroid Attachment/Adhesion Triggered Up-regulation of Vimentin Expression in Ishikawa Cells
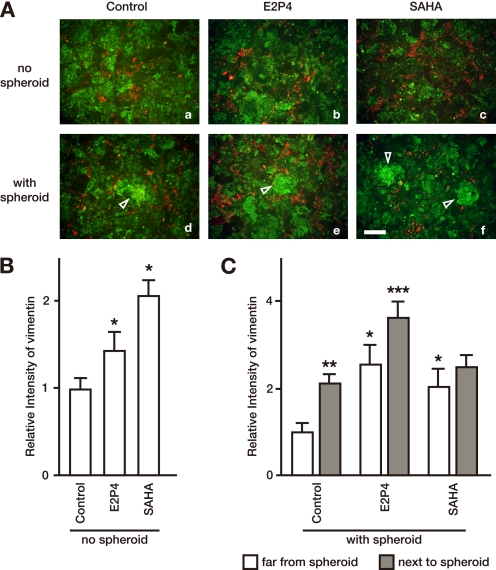
To investigate whether the EMT occurred in EECs during human implantation, we employed an in vitro implantation assay (4–7) using the human EEC line, Ishikawa (4, 15), and spheroids of the human choriocarcinoma cell line JAR as an embryo model. Prior to the addition of JAR spheroids, we pretreated Ishikawa cells for 3 days with or without E2P4 or SAHA, a histone deacetylase inhibitor, capable of enhancing implantation (5). Ishikawa cells were immunostained with anti-cytokeratin (green) and anti-vimentin (red) antibodies (Fig. 1). Prior to any stimulation, broad positive staining of the epithelial marker cytokeratin was observed in Ishikawa cells, whereas only a subset of Ishikawa cells was positive for vimentin, a mesenchymal cell marker (Fig. 1A). Pretreatment with both E2P4 and SAHA significantly up-regulated vimentin expression in Ishikawa cells in the absence of JAR spheroids (Fig. 1, A (panels b and c) and B). In the in vitro implantation assay, co-culture of Ishikawa cells and JAR spheroids resulted in spheroid adhesion onto the Ishikawa cells (5). In co-culture, vimentin expression by E2P4 or SAHA was similar to the baseline (monoculture) increase in areas away from the JAR spheroid (baseline) (Fig. 1, A–C). Vimentin positivity was more pronounced proximal to where the model embryos adhered in comparison to the baseline (2.14 ± 0.22-fold (control), 1.42 ± 0.37-fold (E2P4), and 1.22 ± 0.26-fold (SAHA)). Vimentin expression was also significantly increased in untreated Ishikawa cells adjacent to the spheroids in comparison to cells away from the spheroids. When the treatment groups were compared with the control, significant up-regulation of vimentin in the treated cells adjacent to the spheroid only occurred in Ishikawa cells pretreated with E2P4, but not with SAHA (Fig. 1, A (panels d–f) and C). These results suggest that up-regulation of the mesenchymal cell marker protein, vimentin, is triggered by both hormonal stimulation prior to implantation and subsequent embryo attachment. The magnitude of the effect varied with the stimulatory agent used.
FIGURE 1.
Vimentin staining is up-regulated following treatment with either ovarian steroid hormones or SAHA, and following JAR spheroid implantation in Ishikawa cells. A, a confluent monolayer of Ishikawa cells was cultured without (a–c) or with DiO-coated JAR spheroids (d–f) and stained with anti-cytokeratin or anti-vimentin antibodies, followed by Cy2-conjugated or Cy3-conjugated secondary antibodies, respectively. Prior to the addition of JAR spheroids, Ishikawa cells were pretreated with E2P4 (b and e) or 2.0 μm SAHA (c and f) for 3 days. Bright green fluorescent JAR spheroids are indicated by white triangles. Bar, 200 μm. B, total area vimentin signal intensities in the Ishikawa monolayer, cultured alone for 3 days in the indicated medium, were measured in 500-μm square areas. Each bar represents the mean ± S.E. of relative intensity obtained from three independent experiments. Asterisks show significant differences compared with the intensity in Ishikawa cells cultured in the control medium (p < 0.05). C, total area vimentin signal intensities in Ishikawa monolayers pre-cultured with the indicated medium and co-cultured with JAR spheroids in refreshed medium containing no stimulants were measured in 200-μm square areas 400 μm away from a JAR spheroid (white bar) or adjacent to a spheroid (gray bar). Each bar represents the mean ± S.E. of relative intensity obtained from three independent experiments. Asterisks show significant differences compared with the intensity of the Ishikawa monolayer far from the spheroid in the control medium (single asterisks), compared between far from spheroids and next to spheroids in each medium (double asterisks), or compared with next to the spheroid in the control medium (triple asterisks) (p < 0.05).
Cadherin Switch Occurs in the Peri-implantation Period
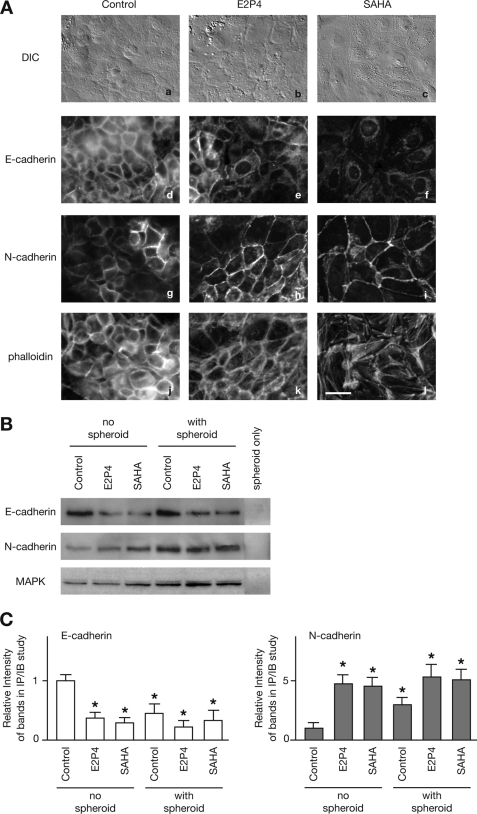
We then assessed for alterations in the expression levels of E-cadherin and N-cadherin in Ishikawa cells. Pretreatment with E2P4 or SAHA altered the morphology of the Ishikawa cells, rendering them wider and flatter (9) (Fig. 2A, panels a–c). In an immunofluorescence study, the expression of E-cadherin was down-regulated, whereas the expression of N-cadherin was up-regulated (Fig. 2A, panels d–i). The cadherin switch, characteristic of the EMT (10, 16), was confirmed by significant difference in the expression levels of both cadherins by immunoprecipitation and immunoblotting (Fig. 2, B and C). Moreover, both suppression of E-cadherin expression and enhancement of N-cadherin expression were induced by the simple addition of JAR spheroid in the absence of any stimulatory agents (Fig. 2, B and C). This suggests that implantation alone is sufficient to trigger the cadherin switch without additional stimuli. No staining of either cadherin protein was detected in cell lysates of the JAR spheroids (Fig. 2B); therefore, the changes in cadherin proteins levels in the immunoblotting experiments reflected alterations in the Ishikawa cells. Treatment with E2P4 or SAHA induced actin rearrangement, which reflected an increase in actin stress fiber formation and a decrease in cortical actin observed in cell periphery (Fig. 2A, panels j–l). This indicates that implantation-induced treatments induce EEC motility via the EMT.
FIGURE 2.
Cadherin switch in stimulated Ishikawa cells. A, differential interference contrast images (a–c) and immunofluorescence images (d–l) of Ishikawa cells, immunoreacted with anti-E-cadherin mAb (d–f), anti-N-cadherin mAb (g–i), or Texas Red-conjugated phalloidin (j–l) are shown. All layers with the exception of E-cadherin staining were photographed in the same location. Bar, 20 μm. B, 200 μg of Ishikawa cell lysates, pretreated with E2P4 or SAHA and cultured with or without JAR spheroids, or the cell lysate of the same number of JAR spheroids, which is applied in other lanes, were immunoprecipitated and immunoblotted with anti-E-cadherin or anti-N-cadherin antibodies. Total cell lysates corresponding to each treatment condition were immunoblotted with anti-MAPK mAb. C, intensities of each of the cadherins immunoblotting bands, shown in B, were measured, normalized to the intensities of the MAPK bands, and graphed. Each bar represents the mean ± S.E. of relative intensity obtained from three independent experiments. Asterisks show significant differences compared with the each control (no spheroid) (p < 0.05).
E2P4 or SAHA-accelerated Ishikawa Cell Motility through Involvement of N-cadherin
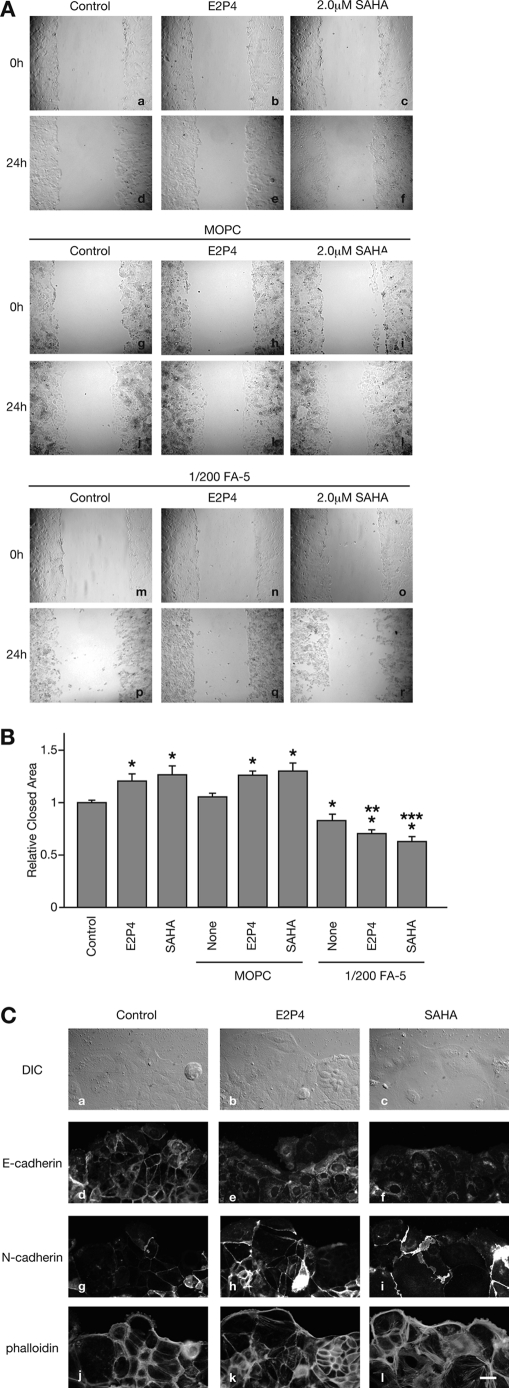
To elucidate whether E2P4- or SAHA-induced EMT accelerates collective cell movement, we next measured Ishikawa cell motility using wound healing assays. In comparison to untreated cells, cells treated with E2P4 or SAHA (120.6 ± 5.4% and 126.6 ± 7.2%, respectively (versus control)) quickly closed the monolayer defect (Fig. 3, A and B), as reported previously (17). Co-treatment with an N-cadherin functional blocking antibody FA-5 (IgG1) (none, 82.9 ± 4.9%; E2P4, 70.5 ± 3.8%; and SAHA, 62.9 ± 4.2% (versus control)) completely abrogated the effects of E2P4 and SAHA (Fig. 3, A and B). Irrelevant IgG1 MOPC (none, 105.1 ± 3.1%; E2P4, 125.8 ± 3.9%; and SAHA, 129.7 ± 6.2% (versus control)) showed no suppressive effects (Fig. 3, A and B; data not shown in Fig. 3A). These results suggest that N-cadherin is required for the E2P4- or SAHA-induced acceleration of cell motility. At the periphery of the collectively moving cells, up-regulation of N-cadherin expression, down-regulation of E-cadherin, and actin rearrangement were more pronounced in comparison to areas of the intact monolayer (Figs. 2A and 3C). This suggests that the EMT demonstrated by cadherin switch and cytoskeletal alterations occur at the motile cell front.
FIGURE 3.
Ishikawa cell motility was accelerated by the EMT. A, a confluent monolayer of Ishikawa cells was cultured without (a, d, g, j, m, and p), or with E2P4 (b, e, h, k, n, and q), 2.0 μm SAHA (c, f, i, l, o, and r), or additional 1/200 dilution of irrelevant MOPC21 antibody (g–l), or the same concentration of FA-5 (m–r). Ishikawa cells were photographed at 0 h (a–c, g–i, and m–o) and 24 h (d–f, j–l, and p–r) after scratching of the monolayer. Bar, 200 μm. B, each wound area was measured. Each gray bar represents the mean ± S.E. relative to the closed area at 24 h obtained from three independent experiments. Asterisks, double asterisks, and triple asterisks show significant differences compared with the area of Ishikawa cells, which are unstimulated (Control), stimulated with only E2P4, or only SAHA, respectively (p < 0.05). C, differential interference contrast images (a–c) and immunofluorescent images of E-cadherin (d–f), N-cadherin (g–i), and actin (j–l) of motile Ishikawa cells in the wound edge are shown. All layers with the exception of E-cadherin staining were photographed at the same location. Bar, 20 μm.
N-cadherin-mediated Ishikawa Cell Motility Provided an Area for JAR Spheroid Outgrowth
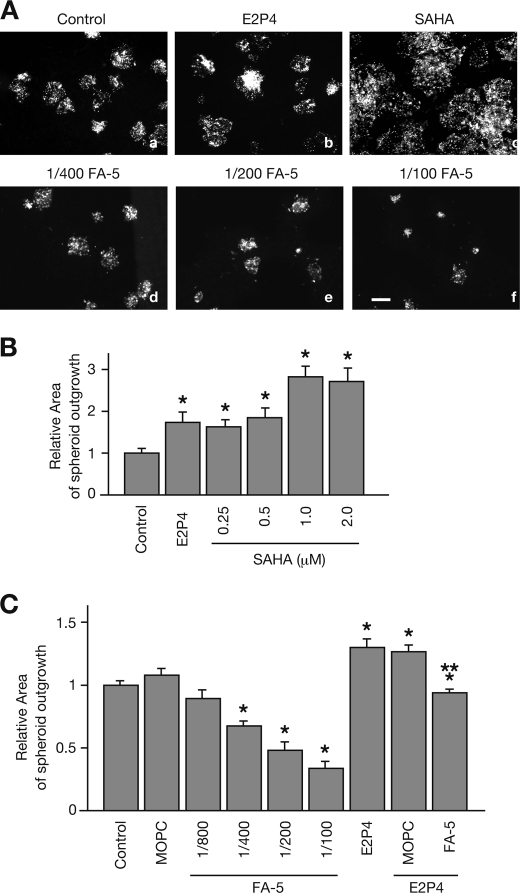
Given that implantation-enhancing reagents such as E2P4 or SAHA accelerate collective cell movement, we hypothesized that Ishikawa cell migration away from the implantation site may provide the embryo with space for expansion. In an in vitro implantation assay, we compared the outgrowth area of JAR spheroids between unstimulated or E2P4- or SAHA-stimulated Ishikawa cells. The JAR spheroid outgrowth area was significantly larger in E2P4- or SAHA-stimulated cells than in unstimulated cells in a dose-dependent manner (Fig. 4, A and B). In unstimulated cells, the outgrowth area was decreased following treatment with the N-cadherin-blocking antibody FA-5 in a dose-dependent fashion (Fig. 4, A and C). Likewise, the addition of FA-5, but not MOPC-21 significantly inhibited the outgrowth area of JAR spheroids in the E2P4-pretreated Ishikawa cells (Fig. 4C).
FIGURE 4.
JAR spheroids outgrowth in the in vitro implantation assay. A, prior to the addition of JAR spheroids, Ishikawa cells were pretreated without (a) or with E2P4 (b), SAHA (c), or the indicated concentrations of FA-5 (d–f). JAR spheroids were co-cultured with confluent monolayered Ishikawa cells for 24 h. B and C, JAR spheroid outgrowth areas were measured and graphed. Each gray bar represents the mean ± S.E. of relative area obtained from three independent experiments. Asterisks and double asterisks show significant differences compared with the area of JAR spheroids in unstimulated Ishikawa cells (Control) or E2P4-treated Ishikawa cells with no antibodies, respectively (p < 0.05).
N-cadherin-mediated Ishikawa Cell Motility Was More Evident in the Vicinity of Implanted JAR Spheroids
We expected that the N-cadherin-mediated up-regulation of Ishikawa cell motility would be focused near the JAR spheroids. Following attachment of the JAR spheroids, N-cadherin in the Ishikawa cells and the JAR spheroids coated with fluorescent lipophilic dye was visualized with either N-cadherin antibody followed by a fluorescent secondary antibody (Fig. 5A, panels a–c). N-cadherin expression was not detected in the JAR spheroids (Fig. 5A, panel c). Because motile cells were strongly positive for N-cadherin (Fig. 3C), and non-motile cells were not (Fig. 2A), we compared the signal intensity of N-cadherin staining in Ishikawa cells at constant distances relative to an adhered JAR spheroid (Fig. 5A, panel d). N-cadherin signal intensity increased in proximity to the JAR spheroids, representing the highly motile state in untreated Ishikawa cells. In the first several hours following the addition of JAR spheroids onto Ishikawa cells, the distribution of N-cadherin was uniform across the entire Ishikawa monolayer. The characteristic gradient redistribution of N-cadherin, however, gradually formed in a time-dependent fashion (Fig. 5B) and was more pronounced in cells stimulated by E2P4 or SAHA in a concentration-dependent manner (Fig. 5C). The N-cadherin gradient distribution pattern was preserved, but the magnitude of the gradient was attenuated by the N-cadherin-blocking antibody in a dose-dependent fashion. This suggests that functional homophilic dimerization of N-cadherin is essential for its graded redistribution (Fig. 5D).
FIGURE 5.
N-cadherin redistribution in the in vitro implantation assay. A, Ishikawa cells were cultured with E2P4 (e), indicated concentrations of SAHA (f–h), or FA-5 (i–l). The media were replaced, and the green fluorescent JAR spheroids were then co-cultured for 24 h without stimulants. Co-cultured cells were fixed and stained with anti-N-cadherin mAb. Bar, 200 μm. B–D, total area intensities of N-cadherin signals were measured every 80 μm from the edge of the Ishikawa cell sheet adjacent to the spheroid. The in vitro implantation assay was performed for the indicated periods (B) or for 24 h (C and D), with or without 3 days of pretreatment with E2P4 or SAHA (C), or FA-5 (D). Each gray bar represents the mean ± S.E. of relative intensity obtained from three independent experiments. Asterisks show significant differences compared with the intensity in the area 400–480 μm from the edge of Ishikawa cell sheet adjacent to the spheroid (p < 0.05).
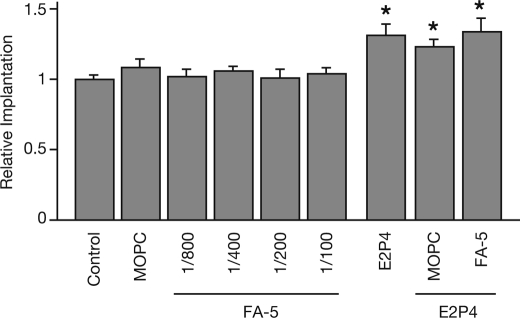
N-cadherin Was Not Required for Attachment/Adhesion of the JAR Spheroids
Finally, we tested whether the EMT-mediated N-cadherin function affected Ishikawa cell receptivity to the JAR spheroids. Although the JAR spheroids were significantly more adherent to Ishikawa cells pretreated by E2P4, as reported previously (5), the adhesion between the JAR spheroids and Ishikawa cells was not completely prevented in the presence of antibodies blocking N-cadherin function (Fig. 6).
FIGURE 6.
The implantation rate was not affected by EMT-induced stimulation, but not N-cadherin function. Ishikawa cells were pretreated without (Control) or with MOPC, various concentrations of FA-5, E2P4 alone or in combination with MOPC, or 1/200 of FA-5, or the indicated concentrations of SAHA for 24 h, prior to the addition of JAR spheroids. Implanted spheroids were counted and graphed. Each gray bar represents the mean ± S.E. of relative implantation obtained from three independent experiments. Asterisks show significant differences compared with the number of implanted spheroids in untreated Ishikawa cells (Control) (p < 0.05).
DISCUSSION
We demonstrate in the present study that the EMT, with remodeling of the EEC monolayer, is required for the adhesion and invasion of simulated embryos in our model. To study the interactions on a cellular level, we stimulated Ishikawa cells with E2P4 or one of the histone deacetylase inhibitors, SAHA. SAHA induces differentiation (9), cell motility (17), and implantation ability (5) to a greater degree than E2P4. Human EECs, like other epithelial cells, are polarized and normally do not permit the adhesion of other EECs, positioned face-to-face, or embryonic cells. During the mid-secretory phase in the menstrual cycle, which is also termed the “implantation window,” EECs require alteration of their polarity to present an adherent area to the early embryo. This is presumably achieved through reprogramming via the EMT. Ishikawa cells were nearly uniformly positive for cytokeratin, whereas only a subset was positive for vimentin, a mesenchymal cell marker protein. This suggests that a percentage of cells was transiently undergoing the EMT and likely to produce rearrangement of the EEC sheet alignment disrupted by proliferation (Fig. 1). In association with vimentin up-regulation, the cadherin switch, consisting of down-regulation of E-cadherin and up-regulation of N-cadherin, was observed in Ishikawa cells treated with E2P4 or SAHA in both immunofluorescence and immunoprecipitation studies (Fig. 2). Similar alterations in cadherin patterns were also present following addition of the model embryos. These results suggest that stimulation by E2P4 or SAHA locally induces the EMT in preparation for implantation. Implantation itself then goes on to up-regulate vimentin expression and accelerate the EMT further (Figs. 1 and 2). E2P4 or SAHA increased the number of model embryos adhering (Fig. 6) (5), probably due to the alteration of cell polarity via the EMT.
In Ishikawa cells, alteration in structural polarity with rearrangement of the actin cytoskeleton is observed during implantation in vitro (17). Treatment of Ishikawa cells with E2P4 or SAHA altered cell morphology, rendering them wider and flatter. These cells had increased actin stress fiber formation and decreased peripheral actin cortical fibers (Fig. 2A), suggesting that alteration of both EEC polarity and actin cytoskeletal structure occurs prior to implantation rather than after adhesion of the embryo. The trigger for these morphological changes is likely the change in ovarian steroid hormone levels across the menstrual cycle. The actin rearrangements are associated with increased motility in the Ishikawa cells, which is required both for reorienting cell polarity during adhesion, as well as facilitating the movement of cells out of the area in which the implanting embryo is expanding.
The acquisition of cell motility via the EMT is well described in the setting of tumor metastasis (10, 11). Down-regulation of E-cadherin expression has long been thought to be critical in malignant cell metastasis, because it results in disruption of the tight epithelial cell-cell contacts and release of invasive tumor cells from the primary tumor (18). Recently, however, N-cadherin up-regulation has been shown to play a more critical role than E-cadherin down-regulation in metastasis progression (10). Collective cell migration of Ishikawa cells was accelerated by treatment with E2P4 or SAHA and inhibited by the N-cadherin functional blocking antibody, FA-5 (Fig. 3). Furthermore, near the leading edge of motile cells, the cadherin switch and actin rearrangement were significantly enhanced in comparison to the EEC monolayer (Figs. 2 and 3). These results, taken together, suggest that the EMT-derived up-regulation of N-cadherin, with subsequent actin rearrangement, is necessary for EEC mobility during implantation.
Successful implantation requires the embryo to penetrate through the EECs and invade the ESCs. Following adhesion of the embryo onto the EEC, apoptosis of the EEC in the vicinity of the embryo is observed in mice (19) and human (20, 21), to provide an area in which the embryo can expand. In support of this observation, treatment with E2P4 or SAHA induces cell apoptosis in Ishikawa cells3; however, it is plausible that mechanisms other than apoptosis may aid in embryo penetration. Human ESCs migrate away from the implantation site to increase the surface area available for embryo invasion (22). Embryo outgrowth, one indicator reflecting two-dimensional embryo penetration, was increased by treatment with E2P4 or SAHA, and decreased by N-cadherin-blocking antibody in a dose-dependent manner (Fig. 4). This suggests that N-cadherin-induced EMT produces EEC migration away from the implantation site.
Although the EEC barrier is physically and immunologically important, it is disrupted during normal embryo penetration. Therefore, rapid remodeling of the disrupted EEC barrier is required to restore its function as an immunological barrier to protect the developing embryo. The mechanism of EEC remodeling is still, however, unclear. When disruption by the penetrating embryo releases cells from contact inhibition, EEC in the vicinity of the implantation site will begin to proliferate. In the remodeling of the EEC disrupted by embryo penetration, we hypothesized that the monolayer defect would first become occupied by EECs migrating into the area and subsequently be closed with proliferating epithelial cells.
In our in vitro implantation assay, the Ishikawa cells co-cultured with model embryos partially seemed to cover the model embryos (Fig. 5). The intensity of N-cadherin staining was most prominent around the adhered spheroids in a co-culture time-dependent fashion (Fig. 5). The gradient staining pattern of N-cadherin was enhanced by treatment with E2P4 and dose-dependent SAHA and was attenuated by the N-cadherin blocking antibody (Fig. 5). These results suggest that the unique redistribution of N-cadherin in epithelial cells is triggered by pre-implantation stimulatory factors, such as E2P4, and embryo adhesion-induced reprogramming. This eventually may produce EEC migration to cover the implanted embryo. The mechanism by which the direction of migration is switched from away to toward the embryo is an area for future investigation. Finally, we did determine that N-cadherin function, whereas essential for cell migration, is not required for embryo adhesion itself (Fig. 6).
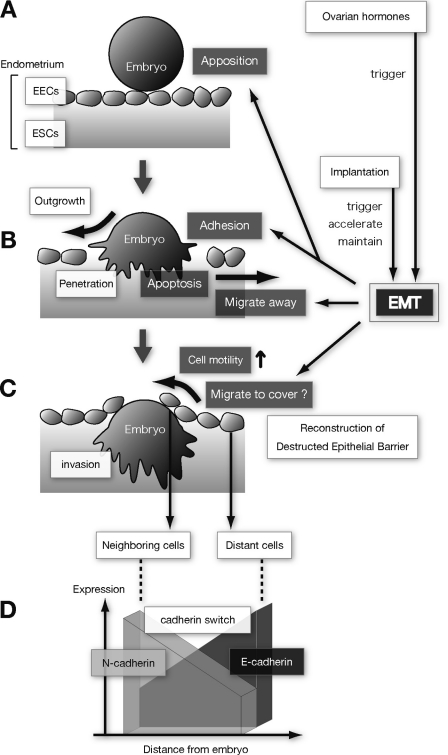
Based on the observations in our in vitro implantation model, we propose the following mechanism. Prior to embryo apposition, menstrual cycle-dependent secretion of ovarian steroid hormones (E2P4) regulates the initiation of the EMT in EEC (Fig. 7A). The local EMT reprograms the EEC, altering cell polarity to receive the embryo. The cadherin switch and actin rearrangement are initiated to prepare the site for the apposed/adhered embryo but are not helpful for adhesion itself. Studies on endometrial cadherins suggest that E2P4 plays only a minor role in their regulation (23). Embryo apposition/adhesion mainly involves N-cadherin up-regulation. The EMT induced by E2P4 and N-cadherin up-regulation produces EEC migration away from the implantation site to assist embryo outgrowth and penetration, in association with EEC apoptosis (Fig. 7B). During embryo penetration through the EEC and embryo invasion into the ESC, the implantation-accelerated and -maintained EMT redistributes N-cadherin and further up-regulates EEC motility and induces them to cover the exposed embryo (Fig. 7, C and D).
FIGURE 7.
Proposed mechanisms by which EMT and ovarian steroid hormones participate in human embryo implantation. Human implantation is a multistep process that begins with embryo apposition (A), followed by adhesion (B) onto endometrial epithelial cells (EECs), penetration, and invasion (C) into endometrial stromal cells (ESCs). During embryonal outgrowth (B), EECs likely undergo apoptosis as well as migrate away from the implantation site to create space for embryonic penetration. In the embryonic invasion step (C), the disrupted endometrial epithelial sheet is reconstructed partially by EEC proliferation as well as EEC motility. The embryo is then covered with the epithelial cells and the immunologic barrier restored. Up-regulation of EEC motility is induced by ovarian steroid hormones and implantation-mediated EMT. In these processes, implantation itself not only triggers, but also maintains and accelerates the EMT. The cadherin switch occurs in implantation and is characterized by N-cadherin up-regulation and redistribution at the implantation site.
Acknowledgments
We thank Drs. Masato Nishida (National Kasumigaura Hospital, Ibaragi, Japan) and Nao Suzuki (St. Marianna University, Kanagawa, Japan) for gifts of Ishikawa cells and JAR cells, respectively. We are also grateful to Rika Shibata for her secretarial work.
This work was supported in part by Grant-in-aid for Scientific Research C 21592108 (to H. U.) from the Japan Society for the Promotion of Science and by grants from the Kanzawa Medical Research Foundation (to H. U.).
H. Uchida, unpublished data.
- EEC
- endometrial epithelial cell
- E2P4
- 17β-estradiol plus progesterone
- EMT
- epithelial-mesenchymal transition
- ESC
- endometrial stromal cell
- SAHA
- suberoylanilide hydroxamic acid.
REFERENCES
- 1. Kao L. C., Tulac S., Lobo S., Imani B., Yang J. P., Germeyer A., Osteen K., Taylor R. N., Lessey B. A., Giudice L. C. (2002) Global gene profiling in human endometrium during the window of implantation. Endocrinology 143, 2119–2138 [DOI] [PubMed] [Google Scholar]
- 2. Norwitz E. R., Schust D. J., Fisher S. J. (2001) Implantation and the survival of early pregnancy. N. Engl. J. Med. 345, 1400–1408 [DOI] [PubMed] [Google Scholar]
- 3. Achache H., Revel A. (2006) Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod Update 12, 731–746 [DOI] [PubMed] [Google Scholar]
- 4. Hannan N. J., Paiva P., Dimitriadis E., Salamonsen L. A. (2010) Models for study of human embryo implantation. Choice of cell lines? Biol. Reprod. 82, 235–245 [DOI] [PubMed] [Google Scholar]
- 5. Uchida H., Maruyama T., Ohta K., Ono M., Arase T., Kagami M., Oda H., Kajitani T., Asada H., Yoshimura Y. (2007) Histone deacetylase inhibitor-induced glycodelin enhances the initial step of implantation. Hum. Reprod. 22, 2615–2622 [DOI] [PubMed] [Google Scholar]
- 6. Li H. Y., Chang S. P., Yuan C. C., Chao H. T., Ng H. T., Sung Y. J. (2003) Induction of p38 mitogen-activated protein kinase-mediated apoptosis is involved in outgrowth of trophoblast cells on endometrial epithelial cells in a model of human trophoblast-endometrial interactions. Biol. Reprod. 69, 1515–1524 [DOI] [PubMed] [Google Scholar]
- 7. Hohn H. P., Linke M., Denker H. W. (2000) Adhesion of trophoblast to uterine epithelium as related to the state of trophoblast differentiation. In vitro studies using cell lines. Mol. Reprod. Dev. 57, 135–145 [DOI] [PubMed] [Google Scholar]
- 8. Berger S. L. (2002) Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12, 142–148 [DOI] [PubMed] [Google Scholar]
- 9. Uchida H., Maruyama T., Nagashima T., Asada H., Yoshimura Y. (2005) Histone deacetylase inhibitors induce differentiation of human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology 146, 5365–5373 [DOI] [PubMed] [Google Scholar]
- 10. Hazan R. B., Qiao R., Keren R., Badano I., Suyama K. (2004) Cadherin switch in tumor progression. Ann. N.Y. Acad. Sci. 1014, 155–163 [DOI] [PubMed] [Google Scholar]
- 11. Thiery J. P. (2003) Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15, 740–746 [DOI] [PubMed] [Google Scholar]
- 12. Takeichi M. (1990) Cadherins. A molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 59, 237–252 [DOI] [PubMed] [Google Scholar]
- 13. Hay E. D. (1995) An overview of epithelio-mesenchymal transformation. Acta Anat. 154, 8–20 [DOI] [PubMed] [Google Scholar]
- 14. Anastasiadis P. Z., Reynolds A. B. (2001) Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13, 604–610 [DOI] [PubMed] [Google Scholar]
- 15. Nishida M. (2002) The Ishikawa cells from birth to the present. Hum. Cell 15, 104–117 [DOI] [PubMed] [Google Scholar]
- 16. Wheelock M. J., Johnson K. R. (2003) Cadherin-mediated cellular signaling. Curr. Opin. Cell Biol. 15, 509–514 [DOI] [PubMed] [Google Scholar]
- 17. Uchida H., Maruyama T., Ono M., Ohta K., Kajitani T., Masuda H., Nagashima T., Arase T., Asada H., Yoshimura Y. (2007) Histone deacetylase inhibitors stimulate cell migration in human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology 148, 896–902 [DOI] [PubMed] [Google Scholar]
- 18. Vleminckx K., Vakaet L., Jr., Mareel M., Fiers W., van Roy F. (1991) Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66, 107–119 [DOI] [PubMed] [Google Scholar]
- 19. Kamijo T., Rajabi M. R., Mizunuma H., Ibuki Y. (1998) Biochemical evidence for autocrine/paracrine regulation of apoptosis in cultured uterine epithelial cells during mouse embryo implantation in vitro. Mol. Hum. Reprod. 4, 990–998 [DOI] [PubMed] [Google Scholar]
- 20. Galán A., O'Connor J. E., Valbuena D., Herrer R., Remohí J., Pampfer S., Pellicer A., Simón C. (2000) The human blastocyst regulates endometrial epithelial apoptosis in embryonic adhesion. Biol. Reprod. 63, 430–439 [DOI] [PubMed] [Google Scholar]
- 21. Simón C., Dominguez F., Remohí J., Pellicer A. (2001) Embryo effects in human implantation. Embryonic regulation of endometrial molecules in human implantation. Ann. N.Y. Acad. Sci. 943, 1–16 [DOI] [PubMed] [Google Scholar]
- 22. Grewal S., Carver J. G., Ridley A. J., Mardon H. J. (2008) Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc. Natl. Acad. Sci. U.S.A. 105, 16189–16194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Linden P. J., de Goeij A. F., Dunselman G. A., Erkens H. W., Evers J. L. (1995) Expression of cadherins and integrins in human endometrium throughout the menstrual cycle. Fertil. Steril. 63, 1210–1216 [DOI] [PubMed] [Google Scholar]