Background: Intracellular concentration of glutathione, an essential sulfur compound, is tightly controlled.
Results: In yeast, glutathione degradation is faster than previously published, and glutathione intracellular concentration does not affect its synthesis.
Conclusion: Glutathione degradation is a key determinant of glutathione homeostasis.
Significance: This work challenges notions on glutathione synthesis and degradation, which were considered as established.
Keywords: Glutathione, Mathematical Modeling, Metabolism, Sulfur, Yeast, Dug Complex, Glutathione
Abstract
Glutathione (GSH) has several important functions in eukaryotic cells, and its intracellular concentration is tightly controlled. Combining mathematical models and 35S labeling, we analyzed Saccharomyces cerevisiae sulfur metabolism. This led us to the observation that GSH recycling is markedly faster than previously estimated. We set up additional in vivo assays and concluded that under standard conditions, GSH half-life is around 90 min. Sulfur starvation and growth with GSH as the sole sulfur source strongly increase GSH degradation, whereas cadmium (Cd2+) treatment inhibits GSH degradation. Whatever the condition tested, GSH is degraded by the cytosolic Dug complex (composed of the three subunits Dug1, Dug2, and Dug3) but not by the γ-glutamyl-transpeptidase, raising the question of the role of this enzyme. In vivo, both DUG2/3 mRNA levels and Dug activity are quickly induced by sulfur deprivation in a Met4-dependent manner. This suggests that Dug activity is mainly regulated at the transcriptional level. Finally, analysis of dug2Δ and dug3Δ mutant cells shows that GSH degradation activity strongly impacts on GSH intracellular concentration and that GSH intracellular concentration does not affect GSH synthesis rate. Altogether, our data led us to reconsider important aspects of GSH metabolism, challenging notions on GSH synthesis and GSH degradation that were considered as established.
Introduction
Glutathione (GSH) is a very important tripeptide present in quantity ranging from 1 to 10 mm in all cellular types. GSH is involved in a multitude of cellular processes, including detoxification of xenobiotics, antioxidant defense, proliferation, and apoptosis. Deficiency in GSH contributes to oxidative stress, which plays a key role in aging and in the development of many human diseases, including neurodegenerative diseases, liver diseases, diabetes, cystic fibrosis, AIDS, heart attack, and cancers. GSH is synthesized in the cytosol by two enzymes, the γ-glutamylcysteine synthetase (γ-GCS),3 considered as the rate-limiting enzyme of the pathway, and the glutathione synthetase (see Fig. 1).
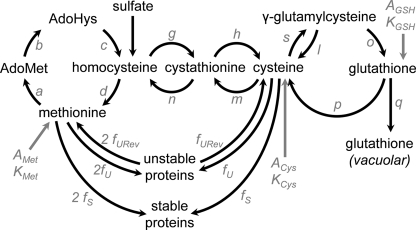
FIGURE 1.
GSH synthesis and catabolism in yeast cytosol and vacuole. 1, γ-GCS, encoded by GSH1; 2, glutathione synthetase, encoded by GSH2; 3, peptidase complex, encoded by DUG2 and DUG3; 4, Cys-Gly dipeptidase, encoded by DUG1; 5, vacuolar glutathione S-conjugate transporter, encoded by YCF1; 6, γ-GT, encoded by ECM38.
In mammalian cells GSH degradation occurs exclusively in the extracellular space. γ-Glutamyl-transpeptidase (γ-GT) cleaves GSH into cysteinylglycine (Cys-Gly) and a Glu residue that is transferred on another acceptor amino acid. Cys-Gly is further cleaved by a dipeptidase (for review see Ref. 1). GSH homeostasis is thus the result of its synthesis and utilization inside the cell, its export, and its degradation outside the cell, with a fast turnover (half-life of 2–3 h in the rat liver (2)). It is generally considered that the cellular pool of GSH is tightly controlled at the step of its synthesis through the regulation of γ-GCS activity and Cys availability (for review, see Ref. 3), but little is known concerning regulations that could occur at the level of GSH export and degradation. In yeast, GSH is transported inside the cell by Hgt1 and localized in the plasma-membrane (4) and can be transported from the cytoplasm to the vacuole by Ycf1 (5) (see Fig. 1). Preliminary experiments have suggested that GSH turnover is slow (6, 7). GSH was presumed to be degraded through the γ-GT pathway, localized in the vacuole (see Fig. 1), at least under sulfur and nitrogen starvation (8, 9), which are conditions strongly inducing γ-GT activity. However, mutants deleted for the gene coding for γ-GT, ECM38, are able to utilize GSH as an exogenous sulfur source (7). This observation led to the characterization of another degradation pathway, a cytosolic complex composed of three proteins, Dug1, Dug2 and Dug3 (see Fig. 1) (10). Dug2 and Dug3 are required to cleave the γ-glutamyl linkage between Glu and Cys-Gly, and Dug1 is a dipeptidase able to cleave Cys-Gly (11). Mutants lacking any of these proteins (dug1Δ, dug2Δ, dug3Δ) cannot grow with GSH as sole sulfur source (10). However, the respective contributions of this pathway and of the γ-GT pathway in GSH degradation under other growth conditions and under different stress conditions have not been studied.
GSH can be considered as an end-product of the sulfur metabolism. Yeast sulfur metabolism and its regulations have been extensively studied (for review see Refs. 12 and 13). One crucial actor of these regulations is the transcriptional activator Met4, which tightly controls the expression of most of the enzymes of this pathway (12, 14). Met4 transcriptional activity is repressed by addition of repressive amounts of Met in the medium (12) and highly induced under conditions leading to sulfur deprivation (e.g. sulfur starvation (14) or chromate treatment (15)) or GSH demand (e.g. cadmium (Cd2+) treatment Refs. 16 and 17).
In the course of our work on yeast sulfur metabolism, we developed quantitative techniques of 35S labeling combined to mathematical models. The data inferred from these models suggested that GSH turnover would be higher than anticipated. We set up additional experiments and models to evaluate sulfur fluxes in this pathway and, more specifically, estimate GSH half-life. We confirmed the rapid turnover of intracellular GSH and found that whatever the condition used, the only pathway involved in GSH degradation is the Dug pathway. Moreover, we showed that GSH degradation activity strongly impacts on GSH intracellular concentration.
EXPERIMENTAL PROCEDURES
Strains
Experiments were performed in W303-1A (18) and isogenic mutant strains CC950-2A (met4::TRP1 (19)), dug1Δ (dug1::KanMX4; this study), dug2Δ (dug2::KanMX4; this study), dug3Δ (dug3::KanMX4; this study), and ecm38Δ (ecm38::KanMX4; this study). We also used S288c (20), BY4742 (21), and the related isogenic mutant strain gsh1Δ (gsh1::KanMX4) (EUROSCARF).
Growth Media
The medium used in this study is yeast nitrogen base minimal medium (20 g/liter glucose), modified as in Lafaye et al. (22) to control both sulfur (as sulfate, GSH or Met) and nitrogen (as ammonium, GSH or Glu) contents, and supplemented with Lys, Leu, Trp, His, adenine, and uracil, except when specified for strain S288c. Sulfur-free medium is minimal medium with 30 mm ammonium but without any added sulfur source. Nitrogen-free medium is minimal medium with 0.1 mm sulfate but without added ammonium. Standard medium is minimal medium with 0.1 mm sulfate and 30 mm ammonium. In this medium, W303-1A doubling-time is 120 min.
Determination of Intracellular Concentration of GSH
Metabolite extracts were prepared in perchloric acetic acid 0.1% as described (22). GSH concentration in the extracts was measured as described by Rahman et al. (23). GSH intracellular concentration was deduced from the average volume of a cell (4.5 × 10−14 liter) and the quantity of cells used to prepare the extract.
Metabolite Fraction (MF) and Protein Fraction (PF) Preparation
0.9 ml of a culture of 35S-labeled cells in exponential phase were mixed to 0.9 ml of 86% cold ethanol (−60 °C) and centrifuged. The cell pellet was washed in cold water, suspended in 100 μl of cold water, heated for 5 min at 95 °C, and centrifuged. The thus-obtained supernatant corresponds to the MF. The pellet, which corresponds to the PF, was suspended in 100 μl of water.
Thin-layer Chromatography (TLC) Experiments
MF was prepared as above, except that the cells were suspended in 30 μl instead of 100 μl before heating at 95 °C. 3 μl of MF were mixed to 1 μl of performic acid and incubated for 20 min at room temperature. 0.5 μl of these samples as well as controls ([35S]Cys, [35S]GSH, [35S]Met, PerkinElmer Life Sciences) were applied on cellulose plates TLC, where they migrated for ∼35 h (5 migrations of ∼7 h) using a butanol-1/acetic acid/water (90/25/15) solvent that allows the separation of Cys and GSH. The dried plates were quantified as previously described (24).
[35S]Met, [35S]Cys, and [35S]GSH Labeling
Cells were grown to early exponential phase under standard conditions. At time t = 0, [35S]Met (30 μCi/ml), [35S]Cys (30 μCi/ml), or [35S]GSH (2 μCi/ml) was added to the culture. A constant volume of culture was taken at each time point and processed to obtain the MF and PF either for radioactivity measurement (PF and MF) or TLC experiments (MF).
Stoichiometric Models of Yeast Sulfur Metabolism
The model presented in Fig. 2 is detailed in supplemental information, part 1. It is based on the assumption of steady state: each concentration and metabolite flux is constant. Therefore, for each metabolite, the difference between synthesis fluxes and catabolism fluxes is the amount of metabolite required for cellular growth (supplemental Equation 3). From this, each “forward” flux (g, h, s, o, q, d, a, b, fU) can be expressed as a combination of metabolite concentrations (considered as known; see the supplemental information), doubling-time (T, measured experimentally), and “reverse” fluxes (n, m, l, p, c, fURev). Therefore, to find flux values compatible with our experimental data, we only had to explore six reverse fluxes and two additional parameters (percentStable and percentVac; supplemental information, Part 1). The reverse fluxes were the main parameters adjusted to fit the experimental data, obtained using 35S radiolabeling (see Fig. 3 and the supplemental information). For each set of tried reverse fluxes, the kinetics of the radioactive labeling of each sulfur-containing intracellular compound were simulated when [35S]Met, [35S]Cys, or [35S]GSH was added to the growth medium and compared with the corresponding experimental data. The reverse fluxes were modified as described in the supplemental information to find the best fit of the experimental data. The selected fit is presented in Fig. 3 and discussed in the supplemental information, part 1.8, where the corresponding numerical values are also given.
FIGURE 2.
Model used for simulating 35S labeling. Entering of [35S]Met, [35S]Cys and [35S]GSH, used as tracers, are modeled with the parameters (AMet, KMet), (ACys, KCys) and (AGSH, KGSH), respectively (supplemental information, Part 1). Names of the fluxes, expressed in mm·min−1, are indicated near the corresponding arrows. AdoMet is S-adenosylmethionine, AdoHys is S-adenosylhomocysteine. Forward fluxes are the fluxes g, h, s, o, q, d, a, b, fU; reverse fluxes are the fluxes n, m, l, p, c, fURev; fixed' fluxes are the fluxes q and fS (supplemental information, Part 1).
FIGURE 3.
In vivo35S labeling and corresponding simulations. Cells grown under standard conditions were labeled at t = 0 with [35S]Met, [35S]Cys, or [35S]GSH. The same amount of culture was regularly collected and processed to extract MF and PF. A, shown is measured radioactivity in PF and MF when cells are labeled with [35S]Met, [35S]Cys, or [35S]GSH (gray dotted lines) and the corresponding simulation (black line). Measured and simulated values are relative to final total intracellular radioactivity. B, the left panel shows a representative autoradiography of 35S-labeled metabolites separated by TLC in the case of [35S]Met labeling. Time of collection is indicated below each corresponding lane. Arrows indicate [35S]Met, [35S]GSH, and [35S]cystathionine spots. The metabolite indicated with an asterisk is probably S-adenosylmethionine converted into methylthioadenosine during extraction. The right panel shows signals measured for intracellular [35S]Met, [35S]GSH, and [35S]cystathionine using TLC experiments and [35S]Met labeling (gray dotted lines) and corresponding simulations (black line). Maximal values, measured experimentally or simulated, are set to 1.
Determination of appT1/2GSH Using [35S]GSH
Cells grown to early exponential phase in standard medium were labeled for 30–40 min with [35S]GSH (1 μCi/ml). At the end of the labeling, about 90% of the intracellular 35S radioactivity was in the metabolites, the remaining 10% being associated to the proteins (supplemental Fig. S8). Culture medium was removed by centrifugation, and the cells were suspended in the same volume of a nonradioactive medium. At each time point, 0.9 ml of culture was collected, and MF and PF were prepared. Radioactivity of MF and PF was measured as previously described (24) and normalized to the total cellular radioactivity (MF + PF), which was constant along the time course (no excretion of 35S-labeled compounds). appT1/2GSH is the time for half-MF radioactivity (mostly GSH) to be transferred in PF.
Determination of ProtCys/MetaCys
Cells grown in standard medium to early exponential phase were transferred to the defined medium. After 15 min (H2O2), 30 min (sulfur starvation), 60 min (nitrogen starvation, chromate, Glu, GSH, Met, H2O2), 90 min (Cd2+), or 120 min (GSH, Met, H2O2) cells were labeled for 10 min with [35S]Cys (10–20 μCi/ml) and processed for MF and PF. In the case of exposure to H2O2, fresh H2O2 was also added at times 40 and 80 min. ProtCys/MetaCys is the ratio of radioactivity measured in PF and MF.
Determination of GSH Synthesis Rate
Cells grown to early exponential phase in standard medium were labeled with [35S]Cys for 10 min (50 μCi/ml) and processed for MF and PF. Analysis by TLC showed that MF radioactivity was mainly [35S]GSH ([35S]Cys stood for 6–10% of the signal). For each strain, the radioactivity corresponding to [35S]GSH, measured on TLC as described (24), was normalized to the radioactivity in PF.
Determination of T½ Using gsh1Δ Mutant
gsh1Δ cells were grown to early exponential phase in standard medium supplemented with 100 μm GSH. Cells were pelleted and suspended in GSH-free medium. A constant volume of culture was collected at the indicated times for extract preparation and GSH determination. Values were plotted and fitted to an exponential function (supplemental Fig. S11), from which was determined appT1/2GSH = corT1/2GSH, the time for half-GSH to be converted into something else.
Quantitative Real-time-PCR Analysis
Around 5 × 108 cells grown in the indicated condition were collected, washed in cold water, pelleted, and stored at −80 °C. Total RNA was extracted as described (25). Expression of the target genes was determined using the ΔΔCt method with ACT1 as the reference gene. The primers used are given in supplemental information, Part 11.
RESULTS
Stoichiometric Models of Yeast Sulfur Metabolism Suggest Significant Recycling of GSH into Cys
In a precedent article, we built mathematical models of the yeast sulfur metabolism to optimize a new method of proteomic quantifications using radiolabeling (24). We pursued this work to develop a comprehensive representation of the yeast sulfur metabolism and evaluate the sulfur fluxes in its different parts. The model of the sulfur pathway that we used to simulate [35S]Met, [35S]Cys, or [35S]GSH labeling is presented in Fig. 2. It corresponds to growth on sulfate as unique sulfur source (standard condition). Thanks to their high specific radioactivity, 35S-labeled compounds can be used in very small amounts as tracers without the risk of affecting yeast metabolism. In this model GSH is divided into two stocks; one that can be recycled, with the flux p, and one (called “vacuolar” for simplicity) that is not recycled. Similarly, proteins are divided into two stocks, one that can be recycled and one that is stable (Fig. 2). Under the assumption of steady state, each forward or fixed flux can be expressed as a combination of reverse fluxes, concentrations, and doubling-time (see “Experimental Procedures” and supplemental information, Part 1.4). Therefore, to find flux values compatible with our experimental data, we only had to explore eight parameters (“Experimental Procedures” and supplemental information, Part 1). We fitted two kinds of experimental data: (i) kinetics of radioactivity distribution between the MF and the PF (for [35S]Met, [35S]Cys, and [35S]GSH labeling, Fig. 3A) and (ii), in the case of [35S]Met labeling, kinetics of radioactivity distribution between intracellular Met, GSH, and cystathionine, based on thin layer experiments (TLC) (Fig. 3B). Experimental data and the simulations corresponding to the chosen values of fluxes are presented in Fig. 3. The best fit we found required the flux p, from GSH to Cys (Fig. 2), to be equal to the minimal flux required for synthesis of GSH from γ-glutamylcysteine. We were surprised that the best fit was only obtained with a high recycling of GSH into Cys. As this observation was at odds with previous results (6, 7), we refined this work. We labeled the cells with [35S]Cys and [35S]GSH for, respectively, 30 and 45 min, put them at time t = 0 in nonradioactive medium, and measured the radioactivity in MF and PF (see below for details). Again, the flux values found above were in good accordance with these experiments (supplemental Fig. S5). Thus, our model strongly suggests that the time required for converting half the concentration of total GSH into Cys (corT1/2GSH) is around 90 min (supplemental information, Part1).
Determination of GSH Half-lives
The half-life of a molecule is usually defined as the time required to degrade 50% of the initial amount of this molecule (e.g. corT1/2GSH for GSH; see above). For practical reasons, we also use in this work the notion of apparent half-life of GSH (appT1/2GSH), which we define as the time required to incorporate into proteins 50% of the sulfur atoms of the initial pool of GSH. This conversion requires GSH to be first degraded into Cys, from which it can either be incorporated into proteins, into cystathionine, or converted back into GSH (Fig. 2). To measure appT1/2GSH, cells were labeled with [35S]GSH, washed, and suspended in nonradioactive medium. A constant volume of culture, corresponding to a constant amount of radioactivity, was harvested at each time point, and the amounts of intracellular 35S in MF and PF were quantified (Fig. 4). We found appT1/2GSH to be around 174 min (Table 1), confirming that GSH degradation is higher than previously anticipated. Of note, the decrease of 35S in metabolites correlates with the increase of 35S incorporated in the proteins (Fig. 4B), indicating that [35S]GSH was not exported outside the cells but was degraded to Cys and finally incorporated into proteins as [35S]Cys and possibly [35S]Met. We checked that the incorporation of 35S into proteins was not the result of protein S-thiolation (supplemental information, Part 3).
FIGURE 4.
Determination of GSH apparent half-life (appT1/2GSH). Cells grown under standard conditions were labeled with [35S]GSH for 30–40 min, washed, and suspended either in nonradioactive standard medium or sulfur-free medium. The same amount of culture was regularly collected and processed to extract MF and PF. A, shown is autoradiography (left panel) and the corresponding quantifications (right panel) of the 35S-labeled metabolites separated by TLC. Time of collection (min) is indicated below each lane. The arrow indicates [35S]GSH spots. GSH is the sole radioactive metabolite that is detectable under these conditions. [35S]GSH radioactivity at time 0 is set to 100%. B, shown is evolution of the radioactivity in MF (black diamonds) and PF (asterisks) of representative experiments. Values are relative to total radioactivity.
TABLE 1.
GSH half-lives in cells grown under different conditions
Apparent (appT1/2GSH) and corrected (corT1/2GSH) half-lives of GSH under different growth conditions are shown. fGSH, given as 100 ×fGSH for better readability, is the fraction of intracellular GSH converted into Cys in 1 min. Values between parentheses are the S.D. of experiments repeated at least three times (see supplemental information, Part 4). Except for the standard conditions, fGSH and corT1/2GSH are only given as rough guides, as their calculation is based on assumptions that may not be true under the corresponding conditions (see supplemental information, Part 4 for details). Measurement of ProtCys/MetaCys is described under “Experimental Procedures.” NA, not applicable.
| Condition | appT1/2GSH | ProtCys/MetaCys | 100 × fGSH | corT1/2GSH |
|---|---|---|---|---|
| min | min−1 | min | ||
| Standard (NH4+, SO42−) | 174 (17) | 1.0 | 0.80 (0.10) | 63 (7) |
| Sulfur starvation (NH4+, −) | 26 (6) | 3.5 | 4.47 (0.93) or 3.89 (0.88)a | 12 (3.5) or 14 (4)a |
| GSH 0.1 mm (NH4+, GSH) | 57 (19) | 1.5 | NA | NA |
| Chromate 10 μm (NH4+, SO42−) | 71 (27) | 2.4 | 1.96 (0.63) or 1.67 (0.51)a | 29 (10) or 34 (13)a |
| Met 0.5 mm (NH4+, Met) | > 500 | 0.2 | NA | NA |
| Cadmium 5 μm (NH4+, SO42−) | > 500 | 0.2 | NA | NA |
| H2O2b (NH4+, SO42−) | > 500 | 0.1 | NA | NA |
| Glu 10 mm (Glu, SO42−) | 214 (5) | 1 | 0.62 (0.05) | 82 (7) |
| Nitrogen starvation (−, SO42−) | 201 (29) | 0.9 | 1.96 (0.63) | 66 (23) |
a In this case the model is different (see supplemental information, Part 4.2).
b 0.2 μm (final concentration) of H2O2 was added 3 times, at t = 0, 40 min, and 80 min.
The real degradation rate of GSH is faster than the rate measured in our assay, as some of the [35S]Cys is converted back into [35S]GSH (Fig. 1). We evaluated the importance of Cys back-conversion into GSH using [35S]Cys labeling (Table 1). The ProtCys/MetaCys ratio thus measured gives an estimate of the relative importance of the fluxes coming from Cys to proteins and to other sulfur-containing metabolites. Using these additional data in small mathematical models, we calculated fGSH, defined as the fraction of GSH converted into Cys in 1 min (Table 1, supplemental information, Part 4). If we consider that the intracellular concentration of GSH is constant, which is the case under standard conditions, we can calculate the corrected half-life of GSH (corT1/2GSH) as corT1/2GSH = 0.5/fGSH. As expected, corT1/2GSH(63 min) is markedly shorter than appT1/2GSH (174 min). As previous studies concluded that GSH is highly stable (6, 7), we tried to confirm our results by a different method (7). It consists of using a gsh1Δ mutant unable to synthesize GSH in monitoring the pool of intracellular GSH when cells are transferred to a GSH-free medium. In the case of stability, the amount of intracellular GSH for a constant volume of culture should be constant. As shown in supplemental Fig. S11, the amount of GSH decreases exponentially with a half-life (corresponding to both appT1/2GSH and corT1/2GSH in this case) around 92 min. This result, obtained with a mutant strain, is in good accordance with the corT1/2GSH calculated for the wild type (WT) strain using radioactivity and confirms that GSH degradation is higher than previously thought.
GSH Turnover Depends on Physiological State of Cell
We measured appT1/2GSH in S288c and BY4742, two strains with a genetic background different from W303-1A, and found that appT1/2GSH was slightly shorter in S288c and BY4742 compared with W303-1A (around 135 min, supplemental information, Part 8). Sulfate concentration of the culture medium seems to have no effect on appT1/2GSH (supplemental information, Part 8). We pursued the study with the W303-1A strain and tested different conditions that were expected to increase GSH degradation: sulfur starvation (8), nitrogen starvation (9, 26), growth with GSH as sole sulfur source (7), growth with Glu as nitrogen source (6), and chromate treatment (15). We also tested Cd2+ treatment (22), the effect of adding repressive amounts of Met to the culture, and exposure to H2O2. appT1/2GSH measured under these conditions are reported in Table 1. We also measured ProtCys/MetaCys to extrapolate fGSH and corT1/2GSH (Table 1, details are in supplemental information, Part 4). A notable result is that GSH degradation is strongly stimulated by sulfur starvation (appT1/2GSH around 32 min, Table 1 and Fig. 4). This is consistent with the notion that GSH is an endogenous sulfur supply during sulfur starvation (8). The appT1/2GSH of 57 min when GSH is used as the sole sulfur source reflects an important activity of GSH degradation. When cells are treated with chromate, appT1/2GSH is also short (around 71 min), in accordance with the observation that chromate causes a depletion of sulfur metabolites (15). Supplementation of repressive amounts of Met, exposure to Cd2+, or exposure to H2O2, each, block 35S incorporation into proteins (appT1/2GSH > 500 min, Table 1). This could be due to the important conversion of [35S]Cys into [35S]GSH, as under these conditions only 11% (H2O2), 14% (Cd2+), and 18% (Met) of the sulfur flux coming from Cys is converted into proteins or to a real inhibition of GSH degradation. To directly detect such an inhibition, we used a gsh1Δ strain and confirmed that exposure to Cd2+ or Met markedly reduces GSH degradation (supplemental information, Part 6), whereas H2O2 has no measurable effect on GSH recycling (supplemental information, Part 7).
When cells are starved for nitrogen or when Glu is used as a unique source of nitrogen, appT1/2GSH is longer than under standard conditions (Table 1). The high appT1/2GSH observed under nitrogen starvation contrasts with previous results, suggesting that under this condition, intracellular GSH could be used as a nitrogen source (9, 26). We decided to carefully re-examine this question in the WT strains used in this study, W303-1A, BY4742, and S288c. We found that neither GSH (10 mm) nor glutathione disulfide (GSSG, 5 mm) was able to sustain growth of these strains, whereas Glu (5 to 10 mm) allowed it (supplemental information, Part 10). Therefore, in the three tested genetic backgrounds, GSH cannot be used as a unique nitrogen source. As Glu is the first amino acid to be released when GSH is degraded (Fig. 1), this result shows that GSH is not degraded at a sufficient rate to provide enough nitrogen for growth. This is not surprising as, in yeast, nitrogen requirement is about 30-fold higher than sulfur requirement. To complete this study, we measured GSH intracellular concentration in W303-1A cells grown in nitrogen-free medium and observed that this concentration is significantly increased (Table 2). This result is consistent with a recent study in Schizosaccharomyces pombe (27) but contrasts with other data in Saccharomyces cerevisiae (9).
TABLE 2.
GSH intracellular concentration in different conditions and genetic backgrounds
S.D. among 13 (WT in standard conditions) or 4 (other conditions/strains) independent experiments is indicated between parentheses.
| Strain | Condition | Intracellular concentration |
|---|---|---|
| mm | ||
| WT | Standard | 3.0 (0.5) |
| WT | Sulfur starvation (1 h) | 0.1 (1.10−3) |
| WT | Nitrogen starvation (2 h) | 4.2 (0.5) |
| WT | Nitrogen starvation (6 h) | 4.8 (0.1) |
| dug2Δ | Standard | 6.2 (0.6) |
| dug3Δ | Standard | 6.6 (0.2) |
| ecm38Δ | Standard | 2.5 (0.2) |
GSH Is Degraded via Dug Pathway
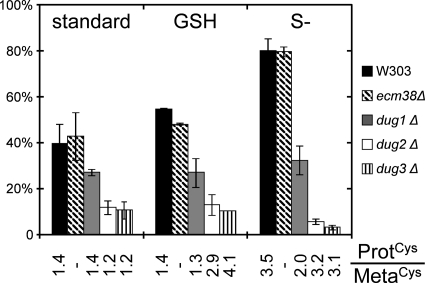
Two pathways of GSH degradation have been described in yeast, the γ-GT pathway and the Dug pathway (Fig. 1). To evaluate the relative importance of these pathways, we built deletion mutants ecm38Δ, dug1Δ, dug2Δ, and dug3Δ. In standard medium, growth of the mutant strains was indistinguishable from growth of W303-1A, the isogenic WT strain. Consistent with previous results (7), dug1Δ, dug2Δ, and dug3Δ (but not ecm38Δ) were unable to grow using GSH or GSSG as unique sulfur source. We compared their ability to incorporate the sulfur atoms of 35S-labeled GSH in proteins. The test was performed under three conditions; (i) growth in standard medium, (ii) growth with GSH as unique sulfur source, and (iii) sulfur starvation (Fig. 5). Notably, synthesis of 35S-labeled proteins was not affected in the ecm38Δ strain, suggesting that the γ-GT pathway is not involved in GSH turnover in any of the three tested conditions. In contrast, incorporation of GSH sulfur atoms into proteins was markedly reduced in the dug2Δ and dug3Δ mutants in all tested conditions. We checked that this decrease was not due to a reduced protein synthesis by measuring the ProtCys/MetaCys ratio under the same conditions (Fig. 5). The Dug pathway is thus of major importance for GSH degradation under these three conditions. The dug1Δ mutant presented a less marked defect than dug2Δ and dug3Δ. This intermediate pattern suggests that other peptidases can complement Dug1 absence.
FIGURE 5.
GSH degradation is impaired in dugΔ mutants. Cells grown under standard conditions were labeled with [35S]GSH for 30–40 min and transferred at t = 0 to nonradioactive standard medium, 0.5 mm GSH medium (GSH), or sulfur-free medium (S−). Cells were collected at t = 0 and t = 1 h (S−) or 2 h (standard, GSH) and processed to extract MF and PF. The percent of the radioactivity initially present in MF then found in PF after 1 or 2 h is represented by histograms. The ratio of radioactivity found in PF and MF (ProtCys/MetaCys) after 10 min labeling with [35S]Cys in the different mutants and conditions is indicated. Error bars represent S.D. of independent experiments (supplemental information, Part 8).
Regulation of GSH Degradation
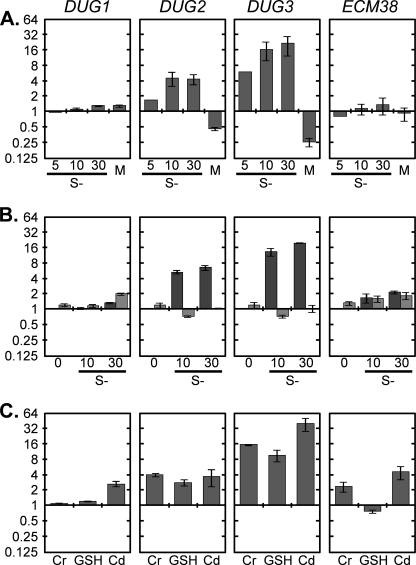
As above, transferring cells to sulfur-free medium leads to a strong and rapid activation of GSH degradation. This suggests a tight control of the activity and/or amounts of the Dug enzymes. As this may involve a very fast transcriptional induction, we measured the transcript levels of DUG1, DUG2, and DUG3 by quantitative real-time-PCR analyses. ECM38 was also analyzed, and ACT1 was used as an internal standard. Cells were collected 5, 10, and 30 min after transfer to the sulfur-free medium. Whereas DUG1 and ECM38 mRNA levels did not change significantly, DUG2 and DUG3 transcript levels increased rapidly after the onset of sulfur deprivation (Fig. 6A). On the contrary, exposure to repressive amounts of Met (0.5 mm) repressed DUG2 and DUG3 expression. Notably, induction and repression were more pronounced for DUG3 than for DUG2. To test whether these inductions were controlled by Met4, the transcriptional activator of the sulfur pathway, we analyzed DUG2 and DUG3 mRNA levels in a met4Δ mutant. W303-1A and met4Δ cells were grown using 50 μm Met as a unique sulfur source (for growth of the mutant) and transferred to sulfur-free medium, and cells were collected 10 and 30 min after the transfer. The results presented in Fig. 6B show that DUG2 and DUG3 inductions are dependent of the presence of Met4.
FIGURE 6.
DUG1, DUG2, DUG3, and ECM38 mRNA relative levels in WT and met4Δ strains. In all the quantitative real-time-PCRs experiments, ACT1 was used as the internal standard. Error bars represent the S.D. of two independent experiments. A, shown is -fold induction after 5, 10, or 30 min in sulfur-free medium (S−) or after supplementation with 0.5 mm Met (M) for 1 h relative to cells grown under standard conditions. B, -fold induction 0, 10, or 30 min after transfer from 50 μm Met-containing medium to sulfur-free medium in WT (dark gray) or met4Δ (light gray) strains relative to the WT strain grown with 50 μm Met as unique sulfur source. C, shown is -fold induction after 30 min of treatment with chromate (Cr, 10 μm), 1 h of growth in 0.5 mm GSH as unique sulfur source, or 1 h of treatment with Cd2+ (5 μm) relative to cells grown under standard conditions.
We also observed marked inductions of DUG2 and very high inductions of DUG3 when cells were treated with chromate for 30 min or grown with GSH as unique sulfur source. Under these conditions, DUG1 and ECM38 were not significantly induced (Fig. 6C). When cells were treated with Cd2+ for 1 h, DUG1, DUG2, DUG3, and ECM38 all appear to be induced. However, these apparent inductions may be a consequence of using ACT1 as the internal control. In fact, in the presence of Cd2+, ACT1 transcription is slightly repressed. Therefore, only DUG2 and DUG3 induction may be relevant under this condition. Finally, we measured GSH turnover in a met4Δ mutant; the mutant and its isogenic WT strain W303-1A were labeled with [35S]GSH and transferred to sulfur-free medium to induce sulfur starvation, and MF and PF radioactivity was measured. As shown in Fig. 7, 35S incorporation in proteins is almost totally blocked in the mutant strain, although the basal level of ECM38, DUG1, DUG2, and DUG3 mRNAs were similar in both strains before the shift (Fig. 6B, 0 min). Altogether, these data suggest that the increased degradation of GSH during sulfur starvation is mainly controlled by the transcriptional activation of DUG2 and DUG3.
FIGURE 7.
GSH degradation is impaired in met4Δ mutants during sulfur starvation. WT and met4Δ cells grown with 50 μm Met as unique sulfur source were labeled with [35S]GSH for 40 min and transferred to sulfur-free medium, and the proportion of 35S incorporated in proteins was measured after different incubation times. Error bars represent the S.D. of two independent experiments.
Dug Complex Contributes to GSH Homeostasis
The above results strongly suggest that GSH turnover is an important feature of GSH metabolism. In particular, GSH degradation could affect the intracellular content of GSH. We measured GSH concentration in WT, dug2Δ, and dug3Δ strains and found that under standard conditions, the amount of intracellular GSH in the degradation mutants is approximately twice that in the WT strain (Table 2). Interestingly, this is the concentration we find in our mathematical model by simply suppressing GSH degradation (flux p = 0, Fig. 2) and adapting GSH concentration to satisfy the steady state hypothesis. Indeed, the best fit of WT experimental data with our mathematical model was obtained with a flux from γ-glutamylcysteine to GSH (Fig. 2) that was twice the minimal flux required for growth of the GSH pool (above and supplemental information, Part 1). Transposing the metabolic model to a mutant strain without changing any flux in the GSH cycle (except the one put to 0) postulates that changes in GSH concentration do not affect the GSH synthesis rate. This is not trivial because in vitro experiments have shown that GSH is an inhibitor of γ-GCS in organisms as diverse as Escherichia coli (28), rats (28, 29), Arabidopsis thaliana (30), and yeasts (31, 32), and it is generally admitted that S. cerevisiae γ-GCS is also subjected to such a feedback inhibition in vivo (33).
We evaluated GSH synthesis rate in WT, dug2Δ, and dug3Δ strains under standard conditions. For this, cells were labeled with [35S]Cys for 10 min, and the amount of radioactivity in GSH was measured on TLC plates and, to allow comparison between different strains, normalized to the 35S incorporated into proteins during the same time. The values obtained were 0.67, 0.72, and 0.72 for the WT, dug2Δ, and dug3Δ strains, respectively, with relative S.D. among two independent experiments of 12, 7, and 7% respectively. They show that GSH synthesis was not significantly changed among the three strains. In particular, doubling the cellular content of GSH had no significant effect on GSH synthesis rate in vivo.
DISCUSSION
The stoichiometric model of S. cerevisiae sulfur metabolism presented in this work led us to reappraise the values of yeast sulfur fluxes, especially GSH recycling into Cys. In this model, fed with in vivo 35S labeling data, half of the synthesized GSH is used for cellular growth, and the other half is degraded. This result is corroborated by the observation that a mutant strain impaired for GSH recycling has twice as much GSH as a WT strain and also by the estimation of GSH half-life by two independent methods (giving, respectively, 63 and 92 min), which is in good accordance with the value inferred from our model (87 min). A major result of this work is thus that under standard conditions, GSH is degraded at a high rate. Considering that two ATP molecules are consumed at each synthesis of one GSH molecule from Cys, this “waste” of cellular energy may be surprising. It is actually in accordance with studies showing that under optimal growth conditions, microbial cells produce energy in excess, leading to the concept of “energy spilling” (34). Our results are at odds with the general belief, based on [14C]GSH labeling experiments, that GSH is very stable in yeast (6). Because detailed experimental data of this previous study were not provided, it is difficult to comment on this discrepancy.
A major interest of the [35S]GSH-based assay developed in this work is that it allows the determination of GSH apparent half-life in any physiological condition and mutant as long as GSH can enter the cell. Without any additional measurements, this assay shows that GSH recycling is faster than previously published. Combined with [35S]Cys-labeling experiments and small mathematical models, it allows the estimate of GSH degradation rate. However, in some cases (H2O2 or Cd2+ treatment, Met supplementation) our assay can be ineffective due to a strong recycling of Cys into GSH under the considered conditions. Despite this shortcoming, our [35S]GSH assay represents an important improvement for the study of GSH turnover. An alternative method consists of monitoring GSH intracellular concentration in gsh1Δ mutant cells transferred to a GSH-free medium (7). Although this method has the drawback of requiring a specific genetic background, we tested it and confirmed a good consistency with our 35S tracer method. This shows that GSH degradation is not significantly altered in a strain unable to synthesize GSH and when GSH intracellular concentration drops. Therefore, the intracellular concentration of GSH does not seem to play a major role in GSH degradation.
We used our 35S tracer assay to analyze degradation mutants under different growth conditions. We established that, whatever the physiological condition tested, GSH is degraded through the Dug pathway. Degradation of GSH is almost completely blocked in dug2Δ and dug3Δ mutants, indicating that both Dug2 and Dug3 are essential for this process. However, GSH turnover is only partly impaired in the dug1Δ mutant. This result suggests that another peptidase(s) can partially complement the absence of Dug1. Consistent with this hypothesis, the defect observed in the dug1Δ mutant is more marked under sulfur starvation and GSH growth conditions, which are conditions with higher rates of GSH degradation, than under standard conditions. Although the ability of the dug1Δ strain to degrade GSH is not completely impaired, the mutant cannot grow using GSH as unique sulfur source (10). This apparent discrepancy could be due to the accumulation of Cys-Gly, a putative toxic compound (35, 36), in the dug1Δ mutant strain (11).
GSH intracellular concentration results from a balance between its synthesis, its degradation, and cellular growth. Previous studies have shown that increasing GSH synthesis through overexpression of GSH1 augments the GSH pool by 50–66% (33, 37). This work shows that blocking GSH degradation by deletion of DUG2 or DUG3 augments GSH cellular content by 100%. Both genetic data thus confirm that synthesis and degradation have a major impact on GSH intracellular concentration. While studies generally focus on GSH synthesis, regulation of GSH homeostasis through degradation has never been explored in yeast. We show here that sulfur starvation and growth with GSH as a unique sulfur source highly activate GSH degradation. These regulations are physiologically relevant, as they provide the cell with sulfur. Conversely, Cd2+ treatment or Met supplementation slow GSH turnover. In response to Cd2+, GSH intracellular content increases, probably to optimize Cd2+ detoxification (22). This increase was considered to be the consequence of an enhanced synthesis of GSH (22, 38). Our present study shows that it is also the consequence of a decreased degradation of GSH.
Sulfur starvation stimulates GSH degradation by the Dug2/3 enzyme. Because yeast sulfur metabolism is mainly regulated at the mRNA level (12, 14), we examined the hypothesis of a transcriptional regulation of this activation. DUG2 and DUG3 mRNAs are highly and quickly induced by sulfur starvation, and both the induction of DUG2 and DUG3 transcription and the activation of GSH degradation by sulfur starvation are dependent on the presence of the transcriptional activator Met4. The data thus suggest that GSH-increased degradation in response to sulfur starvation is mainly controlled by the level of Dug2/3 complex. Similarly, we show that Met supplementation, which is known to repress Met4 activity (12), strongly decreases DUG2 and DUG3 mRNA levels and reduces GSH degradation. Therefore, the effect of Met supplementation on GSH degradation may be merely a consequence of the Met4-dependent regulation of DUG2 and DUG3 expression.
Of note, the transcriptional control observed under sulfur starvation or upon Met supplementation is not physiologically relevant in the case of Cd2+ exposure. Indeed, Cd2+ strongly induces DUG2 and DUG3 transcription, consistent with Met4 activation by exposure to Cd2+ (16, 17), but decreases GSH degradation. Different hypotheses may account for this discrepancy; (i) the Dug complex may be inhibited by the metal, (ii) when chelated to Cd2+, GSH (in the form of Cd(GS)2) may not be a substrate of the Dug complex, and (iii) Cd(GS)2 complexes may be rapidly translocated into the vacuole where they cannot be processed by the cytosolic enzyme Dug2/3. The development of an in vitro test of the Dug complex activity would help to identify mechanisms involved in the response to Cd2+ exposure.
γ-GT (coded by ECM38) is highly expressed under different conditions, including nitrogen starvation (9), sulfur starvation (8), and growth with Glu as nitrogen source (6). Therefore, it was thought to be implicated in the utilization of GSH under these conditions. This work shows that whatever the condition (standard condition, sulfur starvation, and nitrogen starvation, growth with GSH as the sole sulfur source, or growth with Glu as nitrogen source), γ-GT is never required for GSH degradation. In addition, we show that GSH cannot be used as a nitrogen source, and GSH degradation is low under nitrogen starvation or growth with Glu as unique nitrogen source despite the strong γ-GT activity of protein extracts prepared under these conditions (6, 9). We thus have no evidence that γ-GT is involved in GSH degradation in vivo. Although GSH is a substrate of γ-GT in vitro (39), our data suggest that free GSH is not a substrate of γ-GT in vivo, raising the question of the real function of γ-GT in yeast.
According to recent publications (40, 41), γ-GT plays a critical role in the detoxification of monochlorobimane. This electrophilic xenobiotic is first conjugated to GSH, giving glutathione S-bimane. Glutathione S-bimane is then catabolized to Cys-bimane through two pathways, both requiring γ-GT for the cleavage of a γ-bond (cleavage of glutathione S-bimane into Cys-Gly-bimane and cleavage of γ-Glu-Cys-bimane) (41). This suggests that the physiological substrates of the γ-GT are the GSH conjugates. The vacuolar localization of both γ-GT and GSH conjugates (transported with high affinity by Ycf1) is consistent with such a function. Therefore, in yeast, GSH would be degraded in the cytosol mainly by the Dug pathway, and the GSH conjugates would be metabolized in the vacuole by γ-GT. In mammalian cells, GSH and GSH conjugated are described as being exclusively catabolized outside the cell by γ-GT (1). Mammals do not seem to possess true orthologs of Dug2 and Dug3 components (10). However, a Dug1 ortholog, CNDP2, can complement a dug1Δ yeast mutant in regard to the Cys-Gly peptidase function (11). Such a function in mammals remains to be confirmed.
An important result of this work is that in vivo, a 2-fold increase of GSH does not seem to affect GSH synthesis, although based on in vitro experiments, GSH is considered as an inhibitor of γ-GCS in many organisms (28–30), including S. cerevisiae (32). In yeast, the Ki value for GSH, 2.1 mm (32), is close to the intracellular concentration of GSH (Refs. 15 and 22) and this work). However, our data suggest that either this regulation does not occur in S. cerevisiae or this inhibition does not impact on GSH synthesis rate. Such a possibility is well explained by the metabolic control theory (42, 43). In particular, in a metabolic pathway, decreasing the activity of one enzyme may lead to an increase of the precursor metabolites, thus restoring a close-to-normal metabolic flux (44). Therefore, inhibition of γ-GCS may increase Cys concentration and restore the normal rate of GSH synthesis. Another explanation could simply be the fact that GSH is a competitive inhibitor of γ-GCS with respect to Glu (32). Considering that the Km for Glu is 1.2 mm (32) and that in yeast Glu intracellular concentration is more than 20 mm (45, 46), doubling GSH concentration from 3 to 6 mm may not significantly decrease γ-GCS activity. We thus suggest that the notion that γ-GCS would be the “rate-limiting enzyme” of the GSH synthesis pathway, a notion mainly based on in vitro data (3), should be reconsidered, at least in yeast, in the light of in vivo data. Although γ-GCS activity is undeniably a factor influencing GSH synthesis, there are certainly other major determinants in this control, one of them being the Cys availability (47).
Supplementary Material
Acknowledgments
We thank D. Thomas for providing the strain CC950-2A and C. Junot for preliminary experiments.
This work was supported by the Agence Nationale de la Recherche.

This article contains supplemental Parts 1–12, Equations 1–18, and Figs. S1–S14.
- γ-GCS
- γ-glutamylcysteine synthetase
- γ-GT
- γ- glutamyl-transpeptidase
- Cys-Gly
- cysteinylglycine
- fGSH
- fraction of total intracellular GSH converted into Cys in 1 min
- MF
- metabolite fraction
- PF
- protein fraction
- appT1/2GSH
- apparent half-life
- corT1/2GSH
- corrected half-life
- TLC
- thin layer chromatography.
REFERENCES
- 1. Ballatori N., Krance S. M., Notenboom S., Shi S., Tieu K., Hammond C. L. (2009) Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 390, 191–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu S. C. (1999) Regulation of hepatic glutathione synthesis. Current concepts and controversies. FASEB J. 13, 1169–1183 [PubMed] [Google Scholar]
- 3. Lu S. C. (2009) Regulation of glutathione synthesis. Mol. Aspects Med. 30, 42–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bourbouloux A., Shahi P., Chakladar A., Delrot S., Bachhawat A. K. (2000) Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 275, 13259–13265 [DOI] [PubMed] [Google Scholar]
- 5. Rebbeor J. F., Connolly G. C., Dumont M. E., Ballatori N. (1998) ATP-dependent transport of reduced glutathione on YCF1, the yeast orthologue of mammalian multidrug resistance- associated proteins. J. Biol. Chem. 273, 33449–33454 [DOI] [PubMed] [Google Scholar]
- 6. Jaspers C. J., Gigot D., Penninckx M. J. (1985) Pathways of glutathione degradation in the yeast Saccharomyces cerevisiae. Phytochemistry 24, 703–707 [Google Scholar]
- 7. Kumar C., Sharma R., Bachhawat A. K. (2003) Utilization of glutathione as an exogenous sulfur source is independent of γ-glutamyl-transpeptidase in the yeast Saccharomyces cerevisiae. Evidence for an alternative gluathione degradation pathway. FEMS Microbiol. Lett. 219, 187–194 [DOI] [PubMed] [Google Scholar]
- 8. Elskens M. T., Jaspers C. J., Penninckx M. J. (1991) Glutathione as an endogenous sulfur source in the yeast Saccharomyces cerevisiae. J. Gen. Microbiol. 137, 637–644 [DOI] [PubMed] [Google Scholar]
- 9. Mehdi K., Penninckx M. J. (1997) An important role for glutathione and γ-glutamyl-transpeptidase in the supply of growth requirements during nitrogen starvation of the yeast Saccharomyces cerevisiae. Microbiology 143, 1885–1889 [DOI] [PubMed] [Google Scholar]
- 10. Ganguli D., Kumar C., Bachhawat A. K. (2007) The alternative pathway of glutathione degradation is mediated by a novel protein complex involving three new genes in Saccharomyces cerevisiae. Genetics 175, 1137–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaur H., Kumar C., Junot C., Toledano M. B., Bachhawat A. K. (2009) Dug1p Is a Cys-Gly peptidase of the γ-glutamyl cycle of Saccharomyces cerevisiae and represents a novel family of Cys-Gly peptidases. J. Biol. Chem. 284, 14493–14502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas D., Surdin-Kerjan Y. (1997) Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 61, 503–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baudouin-Cornu P., Labarre J. (2006) Regulation of the cadmium stress response through SCF-like ubiquitin ligases. Comparison between Saccharomyces cerevisiae, Schizosaccharomyces pombe, and mammalian cells. Biochimie 88, 1673–1685 [DOI] [PubMed] [Google Scholar]
- 14. Lee T. A., Jorgensen P., Bognar A. L., Peyraud C., Thomas D., Tyers M. (2010) Dissection of combinatorial control by the Met4 transcriptional complex. Mol. Biol. Cell 21, 456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pereira Y., Lagniel G., Godat E., Baudouin-Cornu P., Junot C., Labarre J. (2008) Chromate causes sulfur starvation in yeast. Toxicol. Sci. 106, 400–412 [DOI] [PubMed] [Google Scholar]
- 16. Fauchon M., Lagniel G., Aude J. C., Lombardia L., Soularue P., Petat C., Marguerie G., Sentenac A., Werner M., Labarre J. (2002) Sulfur sparing in the yeast proteome in response to sulfur demand. Mol. Cell 9, 713–723 [DOI] [PubMed] [Google Scholar]
- 17. Barbey R., Baudouin-Cornu P., Lee T. A., Rouillon A., Zarzov P., Tyers M., Thomas D. (2005) Inducible dissociation of SCF(Met30) ubiquitin ligase mediates a rapid transcriptional response to cadmium. EMBO J. 24, 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wallis J. W., Chrebet G., Brodsky G., Rolfe M., Rothstein R. (1989) A hyper-recombination mutation in Saccharomyces cerevisiae identifies a novel eukaryotic topoisomerase. Cell 58, 409–419 [DOI] [PubMed] [Google Scholar]
- 19. Thomas D., Becker A., Surdin-Kerjan Y. (2000) Reverse methionine biosynthesis from S-adenosylmethionine in eukaryotic cells. J. Biol. Chem. 275, 40718–40724 [DOI] [PubMed] [Google Scholar]
- 20. Mortimer R. K., Johnston J. R. (1986) Genealogy of principal strains of the yeast genetic stock center. Genetics 113, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., Hieter P., Boeke J. D. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C. A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132 [DOI] [PubMed] [Google Scholar]
- 22. Lafaye A., Junot C., Pereira Y., Lagniel G., Tabet J. C., Ezan E., Labarre J. (2005) Combined proteome and metabolite-profiling analyses reveal surprising insights into yeast sulfur metabolism. J. Biol. Chem. 280, 24723–24730 [DOI] [PubMed] [Google Scholar]
- 23. Rahman I., Kode A., Biswas S. K. (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 1, 3159–3165 [DOI] [PubMed] [Google Scholar]
- 24. Baudouin-Cornu P., Lagniel G., Chédin S., Labarre J. (2009) Development of a new method for absolute protein quantification on two-dimensional gels. Proteomics 9, 4606–4615 [DOI] [PubMed] [Google Scholar]
- 25. Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman I. G., Smith J. A., Struhl K. (2002) Short Protocols in Molecular Biology (5th Ed.), pp. 13-45–13-46, John Wiley & Sons, Inc., New York [Google Scholar]
- 26. Perrone G. G., Grant C. M., Dawes I. W. (2005) Genetic and environmental factors influencing glutathione homeostasis in Saccharomyces cerevisiae. Mol. Biol. Cell 16, 218–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song S. H., Lim C. J. (2008) Nitrogen depletion causes up-regulation of glutathione content and γ-glutamyl-transpeptidase in Schizosaccharomyces pombe. J. Microbiol. 46, 70–74 [DOI] [PubMed] [Google Scholar]
- 28. Huang C. S., Moore W. R., Meister A. (1988) On the active site thiol of γ-glutamylcysteine synthetase. Relationships to catalysis, inhibition, and regulation. Proc. Natl. Acad. Sci. U.S.A. 85, 2464–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richman P. G., Meister A. (1975) Regulation of γ-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J. Biol. Chem. 250, 1422–1426 [PubMed] [Google Scholar]
- 30. Jez J. M., Cahoon R. E., Chen S. (2004) Arabidopsis thaliana glutamate-cysteine ligase. Functional properties, kinetic mechanism, and regulation of activity. J. Biol. Chem. 279, 33463–33470 [DOI] [PubMed] [Google Scholar]
- 31. Dennda G., Kula M. R. (1986) Assay of the glutathione-synthesizing enzymes by high performance liquid chromatography. Biotechnol. Appl. Biochem. 8, 459–464 [PubMed] [Google Scholar]
- 32. Biterova E. I., Barycki J. J. (2010) Structural basis for feedback and pharmacological inhibition of Saccharomyces cerevisiae glutamate cysteine ligase. J. Biol. Chem. 285, 14459–14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grant C. M., MacIver F. H., Dawes I. W. (1997) Glutathione synthetase is dispensable for growth under both normal and oxidative stress conditions in the yeast Saccharomyces cerevisiae due to an accumulation of the dipeptide γ-glutamylcysteine. Mol. Biol. Cell 8, 1699–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Russell J. B. (2007) The energy spilling reactions of bacteria and other organisms. J. Mol. Microbiol. Biotechnol. 13, 1–11 [DOI] [PubMed] [Google Scholar]
- 35. Paolicchi A., Tongiani R., Tonarelli P., Comporti M., Pompella A. (1997) γ-Glutamyl-transpeptidase-dependent lipid peroxidation in isolated hepatocytes and HepG2 hepatoma cells. Free Radic. Biol. Med. 22, 853–860 [DOI] [PubMed] [Google Scholar]
- 36. Paolicchi A., Dominici S., Pieri L., Maellaro E., Pompella A. (2002) Glutathione catabolism as a signaling mechanism. Biochem. Pharmacol. 64, 1027–1035 [DOI] [PubMed] [Google Scholar]
- 37. Cuozzo J. W., Kaiser C. A. (1999) Competition between glutathione and protein thiols for disufide bond formation. Nat. Cell Biol. 1, 130–135 [DOI] [PubMed] [Google Scholar]
- 38. Vido K., Spector D., Lagniel G., Lopez S., Toledano M. B., Labarre J. (2001) A proteome analysis of the cadmium response in Saccharomyces cerevisiae. J. Biol. Chem. 276, 8469–8474 [DOI] [PubMed] [Google Scholar]
- 39. Penninckx M. J., Jaspers C. J. (1985) Molecular and kinetic properties of purified γ-glutamyl transpeptidase from yeast (Saccharomyces cerevisiae). Phytochemistry 24, 1913–1918 [Google Scholar]
- 40. Ubiyvovk V. M., Blazhenko O. V., Gigot D., Penninckx M., Sibirny A. A. (2006) Role of γ-glutamyl-transpeptidase in detoxification of xenobiotics in the yeasts Hansenula polymorpha and Saccharomyces cerevisiae. Cell Biol. Int. 30, 665–671 [DOI] [PubMed] [Google Scholar]
- 41. Wünschmann J., Krajewski M., Letzel T., Huber E. M., Ehrmann A., Grill E., Lendzian K. J. (2010) Dissection of glutathione conjugate turnover in yeast. Phytochemistry 71, 54–61 [DOI] [PubMed] [Google Scholar]
- 42. Kacser H., Burns J. A. (1995) The control of flux. Biochem. Soc. Trans. 23, 341–366 [DOI] [PubMed] [Google Scholar]
- 43. Fell D. A. (2005) Enzymes, metabolites, and fluxes. J. Exp. Bot. 56, 267–272 [DOI] [PubMed] [Google Scholar]
- 44. Fendt S. M., Buescher J. M., Rudroff F., Picotti P., Zamboni N., Sauer U. (2010) Tradeoff between enzyme and metabolite efficiency maintains metabolic homeostasis upon perturbations in enzyme capacity. Mol. Syst. Biol. 6, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Messenguy F., Colin D., ten Have J. P. (1980) Regulation of compartmentation of amino acid pools in Saccharomyces cerevisiae and its effects on metabolic control. Eur. J. Biochem. 108, 439–447 [DOI] [PubMed] [Google Scholar]
- 46. Godat E., Madalinski G., Muller L., Heilier J. F., Labarre J., Junot C. (2010) Mass spectrometry-based methods for the determination of sulfur and related metabolite concentrations in cell extracts. Methods Enzymol. 473, 41–76 [DOI] [PubMed] [Google Scholar]
- 47. Spector D., Labarre J., Toledano M. B. (2001) A genetic investigation of the essential role of glutathione. Mutations in the proline biosynthesis pathway are the only suppressors of glutathione auxotrophy in yeast. J. Biol. Chem. 276, 7011–7016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.