Background: Activation and cytokine release by glia may be important in prion disease pathogenesis, but factors stimulating this activation are unknown.
Results: Truncated fragments of cyclophilin A in prion disease brain were found to stimulate in vitro cytokine release by glial cells.
Conclusion: Cyclophilin A appears to be important in maintenance of glial activation.
Significance: Blocking of cyclophilin A truncation might influence prion disease pathogenesis.
Keywords: Astrocytes, Chemokines, Cytokine, Glia, Microglia, Transmissible Spongiform Encephalopathy, Cyclophilin A, Gliosis, Prion Disease, Stimulating Factor
Abstract
Prion diseases or transmissible spongiform encephalopathy diseases are typically characterized by deposition of abnormally folded partially protease-resistant host-derived prion protein (PrPres), which is associated with activated glia and increased release of cytokines. This neuroinflammatory response may play a role in transmissible spongiform encephalopathy pathogenesis. We previously reported that brain homogenates from prion-infected mice induced cytokine protein release in primary astroglial and microglial cell cultures. Here we measured cytokine release by cultured glial cells to determine what factors in infected brain contributed to activation of microglia and astroglia. In assays analyzing IL-12p40 and CCL2 (MCP-1), glial cells were not stimulated in vitro by either PrPres purified from infected mouse brains or prion protein amyloid fibrils produced in vitro. However, significant glial stimulation was induced by clarified scrapie brain homogenates lacking PrPres. This stimulation was greatly reduced both by antibody to cyclophilin A (CyPA), a known mediator of inflammation in peripheral tissues, and by cyclosporine A, a CyPA inhibitor. In biochemical studies, purified truncated CyPA fragments stimulated a pattern of cytokine release by microglia and astroglia similar to that induced by scrapie-infected brain homogenates, whereas purified full-length CyPA was a poor stimulator. This requirement for CyPA truncation was not reported in previous studies of stimulation of peripheral macrophages, endothelial cell cardiomyocytes, and vascular smooth muscle cells. Therefore, truncated CyPA detected in brain following prion infection may have an important role in the activation of brain-derived primary astroglia and microglia in prion disease and perhaps other neurodegenerative or neuroinflammatory diseases.
Introduction
Prion diseases, also known as transmissible spongiform encephalopathy (TSE)3 diseases, are infectious neurodegenerative disorders that affect both humans and animals (1). These diseases include scrapie in sheep, bovine spongiform encephalopathy in cattle, chronic wasting disease in cervids, and Creutzfeldt-Jakob disease in humans. A critical event in TSE pathogenesis is the conformational transition of the cellular prion protein (PrPsen; also known as PrPC) into an abnormal, partially protease-resistant host-derived prion protein (PrPres or PrPSc). Accumulation of PrPres in brain is associated with spongiform degeneration of gray matter, neuronal cell death, and gliosis involving activation of microglia and astroglia.
In response to brain infection, injury, or neurodegeneration, microglia and astroglia often promote inflammation by releasing neurotoxic factors and proinflammatory cytokines (2–4). In various diseases, microglia and astroglia have been shown to be activated by factors such as lipopolysaccharide (LPS), interferon-γ, β-amyloid peptide, complement 1q, IL-6, IL-1β, and ATP (5–8). In prion diseases, activated microglia and astroglia (9–11) are usually found in the same regions of the brain as PrPres even prior to clinical signs of disease (12, 13), suggesting an important role for these glia during TSE disease. However, the specific roles of glia and their stimulatory molecules in TSE pathogenesis are still not clear. Microglial activation in vitro and induction of release of IL-6 and IL-1β by the prion protein peptide 106–126 have been demonstrated (14–17), but these studies did not elucidate whether a similar stimulatory process occurred in vivo.
Prion diseases are atypical in the sense that despite an extensive microgliosis and astrogliosis (2, 11, 12) the cytokine response is very restricted (18). Contrary to bacterial or viral infections, which induce high levels of many cytokines (19–21), only a few cytokines were found in vivo in scrapie-infected brains and also released in vitro by microglia or astroglia after exposure to scrapie-infected brain homogenates (22). In the present work, our goal was to identify molecules present in scrapie-infected brain that are responsible for stimulation of cytokine release by microglia and astroglia. Analysis of fractionated scrapie-infected brain homogenates identified cyclophilin A (CyPA) as an important factor in scrapie-infected brains stimulating cytokine release from microglia and astroglia in vitro. CyPA has previously been shown to be involved in inflammatory processes of peripheral tissues in diseases such as arthritis and atherosclerosis (23–26). However, this study is the first to report a possible role for CyPA in brain damage and gliosis caused by prion infection.
EXPERIMENTAL PROCEDURES
Mouse Lines
In vivo TSE infection experiments and in vitro primary glial cultures were done using the C57BL10/SnJ mouse strain. All mice experiments were conducted at Rocky Mountain Laboratories in compliance with the guidelines of their Animal Care and Use Committee.
Preparation of Brain Homogenate and Subfractions
Mice were inoculated intracerebrally at 3–4 weeks of age with scrapie brain homogenate containing the 22L TSE strain as described previously (27, 28). Wild-type mice were euthanized at the time of clinical signs (around 135–155 days postinoculation (dpi)) unless otherwise indicated. Infected and uninfected brains were homogenized at a 20% (w/v) concentration using a Mini Bead Beater (BioSpec Products) as described previously (22) in sterile PBS with 1× Complete protease inhibitor mixture (Roche Applied Science). Brain homogenates were sonicated for 1 min, vortexed aggressively for 30 s, and frozen in aliquots at −80 °C for future use. Brain homogenates were subjected to differential centrifugations to create multiple pellet and supernatant (sup) fractions from sequential processing of supernatants. The initial brain homogenate was spun at 600 × g for 5 min to create an A-sup and A-pellet. The A-sup was then spun at 3000 × g for 20 min to create a B-sup and B-pellet. Subsequent similar processing of B-sup generated fractions in a C-spin (15,000 × g for 1 h) and D-spin (100,000 × g for 4 h). The last fractions produced were D-sup and D-pellet. Each pellet was resuspended in PBS as 20% (w/v) brain homogenate. Supernatants and pellets were kept at −80 °C for future use.
SDS Gel Analysis
Protein samples were quantified using the BCA protein assay kit (Thermo Scientific). Each sample was mixed with 4× lithium dodecyl sulfate sample buffer and 10× sample reducing agent (Invitrogen), then heated for 10 min at 70 °C, and subjected to centrifugation (22,000 × g) for 5 min. Electrophoresis was done using NuPAGE 10% Bis-Tris 1.5-mm gels with MES running buffer and 120-V constant voltage. Gels used for protein analysis were stained with silver using a PlusOne silver staining kit according to the manufacturer's directions (GE Healthcare), and gels used for analysis by LC-MS/MS were stained with Coomassie Blue Imperial stain (Thermo Fisher Scientific).
Western Blots and Antibodies
For CyPA immunoblotting, proteins were transferred to PVDF using a 7-min transfer on an iBlot (Invitrogen) device. The membrane was blocked with 5% skim milk and 3% BSA in Tris-buffered saline with Tween 20 and incubated for 1 h with rabbit polyclonal α-human CyPA (immunogen was keyhole limpet hemocyanin-conjugated synthetic peptide with CyPA residues 100–165, the C-terminal third of human CyPA) at 1:1500 (Abcam). Blots were rinsed, then exposed to horseradish peroxidase-conjugated goat α-rabbit IgG (1:2500) for 1 h, and rinsed again, and bands were detected using ECL substrate as directed (GE Healthcare). To determine which bands were CyPA-specific, in some experiments prior to immunoblotting, α-CyPA was preincubated overnight at 4 °C with excess peptide corresponding to the epitope recognized by the antibody (synthetic peptide derived from residues 100–165) (Abcam). Purified recombinant human CyPA (rhCyPA) from two different lots (Abcam lot numbers 784230 and 838900) was used as a control in immunoblots and glial stimulation experiments as well as a source to purify truncated CyPA forms. LPS contamination of commercially available CyPA reagents was quantitatively assessed using the Toxin Sensor Chromogenic LAL Endotoxin Assay kit (GenScript).
For PrPres immunoblotting, tissue samples were analyzed as described previously (29). Briefly, 20 μl of a 20% tissue homogenate was adjusted to 100 mm Tris-HCl (pH 8.3), 1% Triton X-100, and 1% sodium deoxycholate in a total volume of 31 μl. Samples were treated with 50 μg/ml proteinase K (Roche Applied Science) for 45 min at 37 °C. The reaction was stopped by adding 2 μl of 100 mm Pefabloc (Roche Diagnostics) and placed on ice for 5 min. An equal volume of 2× Laemmli sample buffer (Bio-Rad) was added, and then tubes were heated to 100 °C for 5 min. Samples were frozen at −20 °C until electrophoresed on a 16% Tris-glycine SDS-polyacrylamide gel (Invitrogen) and blotted to PVDF using a 7-min transfer on an iBlot (Invitrogen) device. Immunoblots were probed with human α-mouse PrP antibody (D13, 1:5000; Ref. 30) followed by a peroxidase-conjugated α-human IgG secondary antibody (1:5000; Sigma).
Glial Cell Cultures
Glial cells from cerebral cortices of 1–3-day-old mice were cultured as a primary passage in T-75 flasks for 10–14 days as described previously (22). Twenty-four hours after secondary passage into 96-well plates at 5 × 104 cells/well, cells were overlaid with fresh medium containing various materials to be tested for the ability to stimulate cytokine release by the glial cells. Supernatant fluid was collected 24 h later and stored frozen until analysis for cytokine levels using a multiplex assay.
Treatment of Glial Cells with Brain Homogenate Subfractions
Different subfractions of brain homogenates from mice clinically infected with the 22L strain of scrapie or equivalent dilutions of normal uninfected brain homogenate were added to glial cell cultures at a 1% concentration (10 μl of 20% sample in 200 μl of total medium). Glial cells were also stimulated with 1% D-sup previously treated with 60 μg/ml proteinase K, 1 mg/ml trypsin, 500 μg/ml RNase, or 100 μg/ml DNase or incubated at 56 °C for 1 h. The different proteases or nucleases alone in medium were used as controls. For all of these treatments, D-sup was incubated with the different compounds for 45 min at 37 °C with gentle agitation before exposure to live cells.
Treatment of Glial Cells with PrP-containing Materials
Glial cells were exposed to 25–1000 ng/ml amyloid-like fibrils derived from PrP expressed in Escherichia coli, 25–1000 ng/ml monomeric non-fibrillar recombinant PrP(23–231) as a negative control, 1–1000 ng/ml enriched PrPres (31) from C57BL/10 mice clinically infected with the 22L strain of scrapie, or 1–1000 ng/ml normal control from uninfected brain homogenate (mock PrPres preparation) (32).
Treatment of Glial Cells with CyPA, Cyclosporine A, and Antibodies for Inhibition Assays
Glial cells were exposed to 1% D-sup preincubated for 45 min at 37 °C with 2 μg of α-CyPA, α-FK506-binding protein 12 (Santa Cruz Biotechnology), α-mammary derived growth inhibitor (also known as heart fatty acid-binding protein; Santa Cruz Biotechnology), α-brain fatty acid-binding protein (Santa Cruz Biotechnology), or control antibody. Other D-sup samples were preincubated with 0.1 μg/ml (83 nm) cyclosporine A (Sigma) and then applied to glial cultures. D-sup preincubated with medium was used as the negative control.
Glial cells were also exposed to an N-terminal polyhistidine-tagged rhCyPA (Abcam and Sigma), untagged rhCyPA (R&D Systems), or eluted rhCyPA breakdown products. As negative and positive controls, cultures were overlaid with medium alone or medium containing LPS (1 μg/ml) (Invitrogen), respectively.
Cytokine Quantification
23-cytokine multiplex kits (Bio-Rad) were used to determine the concentration of 23 cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, IFN-γ, CCL11 (Eotaxin), G-CSF, GM-CSF, CXCL1 (KC), CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), and TNF) in glial culture supernatant fluids as described previously (22). On some occasions, Single-Plex kits were also used to analyze IL-6, CCL2, and IL-12p40 in glial culture supernatants. Data were obtained using the Bio-Plex Manager software program (Bio-Rad version 4.1.1) for standardization and standard curve acquisition and exported to Microsoft Excel (Microsoft Corp., Seattle, WA) for further analysis. Statistical analysis was performed using GraphPad software with a paired t test, Wilcoxon signed rank test for pairs of individual stimulated or control glial cell cultures, or one-way analysis of variance with Dunnett's multiple comparison test for comparison of inhibition by multiple antibodies.
Size Exclusion Chromatography
D-sup samples consisting of 500 μl of 20% D-sup with ∼2 mg of protein were fractionated on a Superdex 200 10/300GL column connected to an ÄKTA Purifier 100 system (GE Healthcare). The column was pre-equilibrated at room temperature with sterile filtered PBS buffer (pH 7.2). Fractions of 1 ml were collected by isocratic elution at 0.5 ml/min and tested for stimulation of cytokine release by glial cells at a 1:4 dilution in medium.
Tryptic Digestion of Acrylamide Gel Fractions
For analysis of proteins by mass spectroscopy, ∼5 μg of total protein from each fraction was loaded on a 16% acrylamide gel for SDS-PAGE and stained with Coomassie Blue Imperial stain (Thermo Fisher Scientific). Stained bands from each lane were cut out of the gel with a razor blade for in-gel digestion as described previously (32). Each digest was then dissolved in 14 μl of LC buffer A (water, 3% acetonitrile, and 0.1% formic acid), subjected to centrifugation at 22,000 × g, and then transferred to an autosampler vial for nanospray LC-MS/MS analysis.
Nanospray LC-MS/MS
Tryptic peptides were identified by LC-MS/MS using an Agilent 1200 instrument connected to an XCT Ultra Ion Trap via a microfluidic HPLC chip interface and a nanospray source as described previously (32). Separate HPLC chips were used for infected and non-infected samples to minimize the possibility of contamination.
Data Analysis and Database Searching
Postrun data were processed into Mascot generic format peak lists using Ion Trap data analysis software, version 3.4, revision 6.1 provided by Agilent. These data were searched against the Sprot database with mouse taxonomy using Mascot Daemon (55) to search for trypsin-digested peptides, allowing for one missed cleavage site and a variable methionine oxidation mass of +16 Da. The peptide and MS/MS tolerances were set to 2.4 and 1.4 Da, respectively. The DAT output files generated by Mascot were further analyzed using Scaffold software version 2_03_01 (Proteome Software, Portland, OR). Protein identifications were only accepted at a threshold of >99% probability based upon the implementation of the Protein Prophet algorithm (33) in Scaffold and contained at least three unique peptides.
Protein Electroelution
Thirty micrograms of rhCyPA (Abcam lot number 838900) was solubilized in Laemmli buffer at 56 °C for 30 min, separated by 18% SDS-PAGE, and stained using the E-Zinc reversible stain kit (Thermo Fisher Scientific). Regions of the gel corresponding to the full-length CyPA and smaller breakdown products were excised and electroeluted using the Elutrap electroelution system (Whatman) (200 V for 3.5 h at 4 °C) in a buffer consisting of 25 mm Tris, 192 mm glycine, 0.1% SDS (pH 8.3). The elution buffer temperature in the apparatus was under constant monitoring and never reached above 35 °C. Eluted products were stored at −20 °C until concentration and precipitation. The volume of each elute (∼600 μl) was reduced to half by speed vacuum centrifugation at 30 °C for 4 h. Once the volume was reduced, proteins were precipitated by the methanol/chloroform method (34), suspended in 70 μl of sterile DMEM-F-12 supplemented with 10% FBS, and allowed to refold by incubation at 23 °C for 1 h. Eluted protein suspensions were stored at 4 °C for 24–48 h until use. Portions of the eluted products were evaluated by immunoblot with α-CyPA to determine the percent recovery relative to 1 μg of rhCyPA starting material. Immunoreactive bands were visualized and quantified using the Li-Cor Odyssey imaging system and software.
RESULTS
Presence of Glial Stimulatory Activity in Brain Homogenate Fractions Lacking PrPres
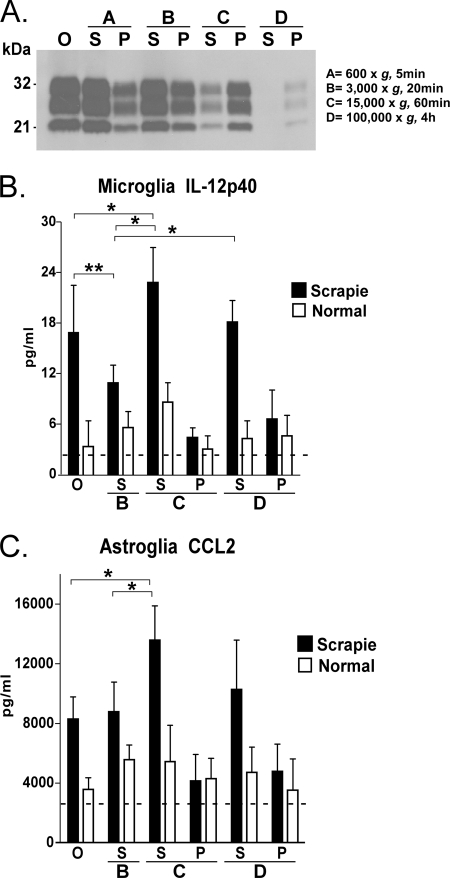
To identify the molecules from brains of prion-infected mice that might be stimulating microglia and/or astroglia to release cytokines, we fractionated scrapie-infected brain homogenates by differential centrifugation as described under “Experimental Procedures” to isolate successive pellet and supernatant fractions, which were tested for stimulatory activity in vitro. Brain homogenates were made from clinically sick C57BL/10 mice, which were sacrificed at 135–155 dpi, and age-matched uninfected mice. PrPres was detectable by Western blot in each fraction from scrapie brains except in the fraction corresponding to the supernatant after the 100,000 × g D-spin (D-sup) (Fig. 1A). The analysis of the stimulatory effect of the different fractions was performed by induction of IL-12p40 release by microglia and CCL2 release by astroglia; these were the strongest cytokine responses seen in previous experiments (22). Three points were noted as shown in Fig. 1, B and C. First, the supernatant fractions stimulated much more cytokine release than the pellet fractions. Second, the supernatants from the scrapie-infected brain homogenates stimulated much better than those from uninfected brain homogenates (Fig. 1, B and C). Third, the comparison between the concentration of PrPres in the fraction and the stimulatory effect of this fraction on glial cells showed no correlation between PrPres content and stimulatory effect (Fig. 1). The lack of detectable PrPres in the fraction D supernatant from scrapie brain (scrapie D-sup) suggested that other stimulatory factors in this sample might be responsible for glial cytokine release.
FIGURE 1.
Fractionation of scrapie and normal brain homogenates by differential centrifugation. A, Western blot detection of PrPres in fractions from mouse brain infected by scrapie strain 22L. Each lane was loaded with 2 mg of tissue equivalents treated with proteinase K, and the blot was probed using the α-PrP antibody D13. O, the original scrapie brain homogenates without centrifugation; Fractions A–D are described under “Experimental Procedures.” S, supernatant of each fraction; P, pellet of each fraction. Cultured microglia (B) or astroglia (C) were stimulated by overlay with different fractions of scrapie-infected or uninfected normal brain homogenates made in DMEM-F-12 with 10% FBS. Supernatants were analyzed for IL-12p40 and CCL2 protein level by multiplex assay 24 h after stimulation. Values shown are means of the cytokine level detected minus the level contributed by the brain-derived overlay material from seven culture wells tested in several separate experiments on different days. Dotted lines show cytokine levels induced by medium alone. *, p = 0.05; **, p = 0.005. Brackets indicate S.E.
Lack of Stimulation of Glia by PrPres or PrP Amyloid
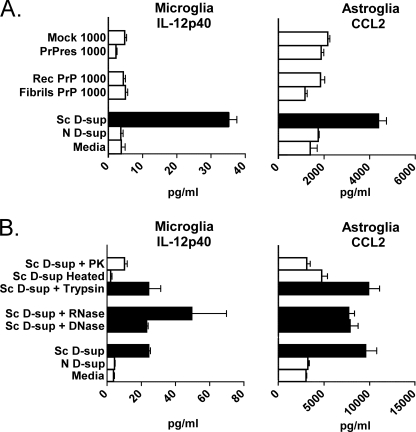
Although stimulation of microglia and astroglia by scrapie D-sup did not correlate with the presence of PrPres, we also evaluated whether purified PrPres by itself could stimulate glial cells. We incubated PrPres enriched from 22L scrapie brain homogenates or non-infectious recombinant PrP amyloid fibrils formed in vitro with microglia or astroglia cultures; however, neither of the preparations stimulated IL-12p40 release by microglia or CCL2 release by astroglia (Fig. 2A). Thus, neither purified PrPres from scrapie brain nor PrP amyloid fibrils appeared to be capable of inducing cytokine release by cultured microglia and astroglia.
FIGURE 2.
Evaluation of CCL2 and IL-12p40 release by microglia and astroglia in response to various stimuli. A, cultured microglia or astroglia were stimulated by overlay with amyloid-like fibrils derived from PrP expressed in E. coli (Fibrils PrP), monomeric non-fibrillar recombinant PrP(23–231) (Rec PrP) as a negative control, enriched PrPres from brains of C57BL/10 mice clinically infected with 22L strain of scrapie (PrPres), or mock control sample purified from normal uninfected brain homogenate (Mock). The figure shows results of tests at a stimulator concentration of 1000 ng/ml, but PrPres and PrP fibrils were also tested at several concentrations lower than 1000 ng/ml, and similar negative results were observed. Glial cells were also overlaid with medium alone or with 1% normal (N) D-sup as negative controls. White bars represent samples with significantly lower stimulation than 1% scrapie (Sc) D-sup, which was the positive control (black bars in A). B, cultured microglia or astroglia were stimulated by overlay with 1% scrapie D-sup treated either with 100 μg/ml DNase, 500 μg/ml RNase, 1 mg/ml trypsin, heat to 56 °C for 1 h, or 60 μg/ml proteinase K (PK). Medium alone or 1% normal D-sup in medium were the negative controls, whereas stimulation with 1% scrapie D-sup was the positive control. White bars in B indicate samples with significantly lower stimulation than positive control. All supernatants in A and B were analyzed by multiplex assay for IL-12p40 (microglia) or CCL2 (astroglia) 24 h after stimulation. Values shown are from 11 (A) or seven (B) culture wells tested in several separate experiments on different days. All p values in A were ≤0.005, and in B, p values were ≤0.05 when comparing scrapie D-sup treated or untreated with proteinase K or heat. Bars indicate the mean, and brackets represent the S.E.
Glial Stimulating Molecules Are Proteinaceous
To investigate the biochemical nature of the glial stimulatory materials in scrapie D-sup, the cytokine induction activity of scrapie D-sup was evaluated after various treatments (Fig. 2B). Treatment with RNase or DNase had no significant effect on the stimulation of either microglia or astroglia by scrapie D-sup, suggesting that nucleic acids were not involved. Scrapie D-sup treated with trypsin was still stimulatory for microglia and astroglia, but treatment with proteinase K significantly reduced the stimulatory activity. Moreover, heating scrapie D-sup at 56 °C for 1 h eliminated all stimulation of cytokine release by glial cells (Fig. 2B). Therefore, the stimulatory factor(s) for both microglia and astroglia appeared to be heat-labile proteins.
Glial Stimulatory Factors Fractionate with Low Molecular Weight Proteins
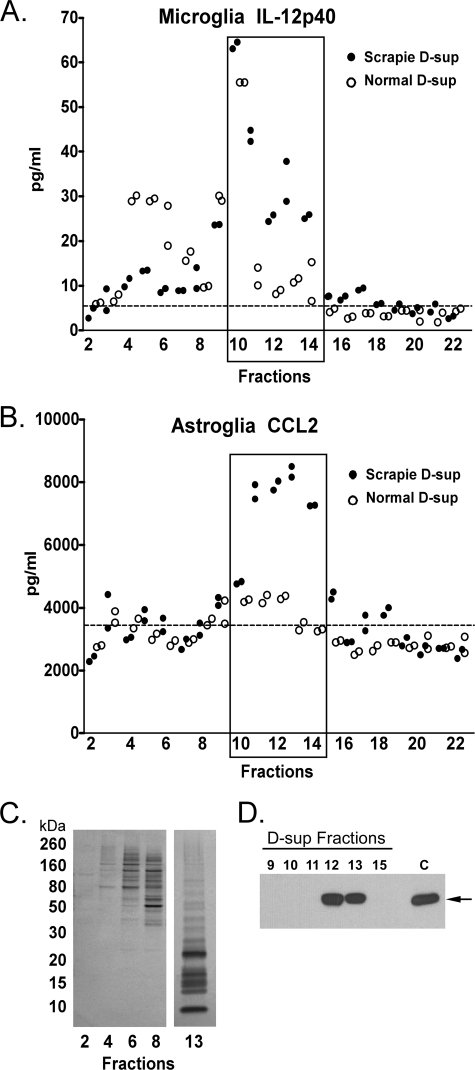
To purify molecules that might stimulate microglia or astroglia, we fractionated the scrapie D-sup by size exclusion chromatography on a Superdex 200 column (Fig. 3). The fractions were used to stimulate microglia and astroglia in vitro. Fractions 10–14 had a high scrapie-specific stimulatory effect for IL-12p40 release by microglia (Fig. 3A) and CCL2 release by astroglia (Fig. 3B). A second size exclusion chromatography experiment showed similar results (data not shown). The majority of the proteins in the stimulatory fractions were under 30 kDa in size as shown in the representative stimulating fraction 13 (Fig. 3C), and non-stimulating fractions 2–8 contained mostly larger sized proteins (Fig. 3, A and B). Thus, the glial stimulatory molecules in scrapie brain homogenates appeared to be low molecular weight proteins.
FIGURE 3.
Analysis of response of cultured microglia and astroglia to D-sup fractions. D-sup was fractionated into 22 fractions of 1 ml each by chromatography on a Superdex 200 column, and fractions were overlaid on cultured microglia (A) or astroglia (B). Each fraction was tested in duplicate at a final dilution of 1:4 in medium. Supernatants were analyzed for IL-12p40 and CCL2 release by multiplex assay 24 h after stimulation. The cytokine response to scrapie D-sup or normal D-sup are as indicated. Each circle represents an independent culture. The cytokine level after overlay with medium alone is represented by the dotted line. C, approximately 5 μg of total protein from representative fractions 2, 4, 6, 8, and 13 was separated by SDS-PAGE and stained with silver. Standards in kilodaltons (kDa) are on the left. Low molecular mass proteins under 30 kDa were abundant in the stimulating fractions (fractions 10–14), but proteins in the non-stimulating fractions (fractions 2–8) were mostly of higher molecular mass (>30 kDa). D, equal amounts of column fractions and control scrapie D-sup (indicated by the C) were separated by SDS-PAGE, transferred to PVDF, and probed with α-CyPA. The arrow identifies the 18-kDa band representing CyPA, which is enriched in fractions 11, 12, and 13. Note that the CyPA-immunoreactive band in fraction 11 was faint and required longer exposure to visualize.
Inhibition of Cyclophilin A in Scrapie D-sup Blocks Glial Stimulation
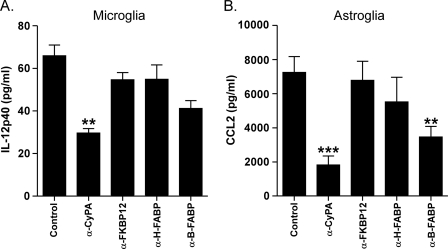
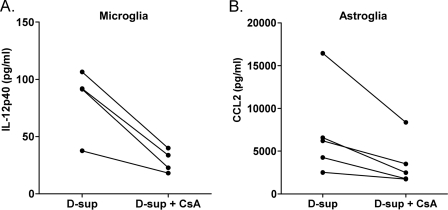
To identify possible proteins in the stimulatory fraction 13 (Fig. 3, A and B), we used tandem mass spectrometry. This analysis revealed 19 proteins with significant spectral counts (Table 1 and supplemental Table S1). Nine of the 19 proteins were known to induce cytokine release (Table 1). Therefore, to identify possible stimulating molecules, we next attempted blocking experiments using antibodies to four of these proteins. Antibody to CyPA significantly reduced the stimulatory effect of scrapie D-sup on microglia and astroglia (Fig. 4, A and B). However, antibody against brain fatty acid-binding protein also showed partial inhibition of stimulation of astroglia (Fig. 4B). Release of IL-12p40 by microglia was also reduced by a known inhibitor of CyPA, cyclosporine A (CsA) (Fig. 5A). CsA also had a significant inhibitory effect on CCL2 release by astroglia (Fig. 5B). Western blotting of size exclusion column fractions indicated that CyPA was enriched in stimulating factions 11, 12, and 13 (Fig. 3D). Furthermore, using a 23-plex kit, CsA was able to block scrapie D-sup-mediated stimulation of all cytokines usually released by astroglia and/or microglia in this system (Table 2). Together these results indicated that CyPA was likely an important molecule involved in stimulation of glial activation during prion infection and disease.
TABLE 1.
Results of analysis by tandem mass spectrometry of fraction 13
| Protein | Gene | Total spectraa | Molecular mass | Unique peptidesb |
|---|---|---|---|---|
| kDa | ||||
| Cytokine-inducing molecules | ||||
| Peptidyl-prolyl cis-trans isomerase A (cyclophilin A) | Ppia | 25 | 18 | 11 |
| Profilin-1 | Pfn1 | 21 | 15 | 7 |
| Profilin-2 | Pfn2 | 21 | 15 | 6 |
| Peptidyl-prolyl cis-trans isomerase (FKBP12)c | Fkbp1a | 15 | 12 | 3 |
| Glia maturation factor β | Gmfb | 13 | 17 | 4 |
| Heart fatty acid-binding protein (MDGI)c | Fabp3 | 13 | 15 | 4 |
| Macrophage migration inhibitory factor | Mif | 13 | 13 | 4 |
| UPF0556 protein (IL-25) | D17Wsu104e | 13 | 16 | 4 |
| Brain fatty acid-binding protein | Fabp7 | 10 | 15 | 3 |
| Non-cytokine-inducing molecules | ||||
| Cytochrome c | Cycs | 21 | 12 | 7 |
| Acyl-CoA-binding protein | Dbi | 14 | 10 | 3 |
| ADP-ribosylation factor 1 | Arf1 | 13 | 21 | 7 |
| Acylphosphatase-1 | Acyp1 | 11 | 11 | 5 |
| Polyubiquitin-B | Ubb | 8 | 34 | 3 |
| Peroxiredoxin-5 | Prdx5 | 7 | 22 | 4 |
| Ribonuclease UK114 | Hrsp12 | 7 | 14 | 4 |
| Parvalbumin α | Pvalb | 7 | 12 | 3 |
| Superoxide dismutase (Cu,Zn) | Sod1 | 6 | 16 | 3 |
| Ubiquitin-like protein ISG15 | Isg15 | 3 | 18 | 3 |
a The number of assigned spectra for a given protein.
b The number of peptides with distinct m/z values that identify a specific protein. Refer to supplemental Table S1 for individual peptides and scores.
c FKBP12, FK506-binding protein 12; MDGI, mammary derived growth inhibitor.
FIGURE 4.
Anti-cyclophilin A treatment of scrapie D-sup reduces cytokine release by glia. Cultured microglia (A) or astroglia (B) were stimulated by incubation with 1% scrapie D-sup with or without preincubation with 2 μg of α-CyPA, α-FK506-binding protein 12 (FKBP12), α-heart fatty acid-binding protein (H-FABP), α-brain fatty acid-binding protein (B-FABP), or control antibody (Control). Supernatants were analyzed by multiplex cytokine assay for IL-12p40 (microglia) and CCL2 (astroglia) 24 h after stimulation. α-CyPA significantly reduced both microglial release of IL-12p40 (**, p < 0.01) and CCL2 release by astroglia (***, p < 0.001). α-Brain fatty acid-binding protein also reduced CCL2 release by astroglia (**, p < 0.01). Results were compared by one-way analysis of variance with Dunnett's multiple comparison test. Bars indicate the means, and brackets represent the S.E.
FIGURE 5.
Cyclosporine A treatment of scrapie D-sup reduces cytokine release by glia. Cultured microglia (A) or astroglia (B) were stimulated by incubation with 1% scrapie D-sup with or without preincubation with 0.1 μg/ml CsA. Supernatants were analyzed by multiplex cytokine assay for IL-12p40 (microglia) and CCL2 (astroglia) 24 h after stimulation. CsA was able to significantly reduce both microglial release of IL-12p40 (p < 0.028) and astroglial release of CCL2 (p < 0.049) when analyzed by paired t test. In this figure, for more accurate statistical analysis, data shown are raw cytokine levels without subtraction of brain homogenate backgrounds because background was the same in paired samples but differed slightly among the various pairs where different D-sups were used. CsA did not decrease LPS stimulation of astroglia and microglia (data not shown). CsA alone did not stimulate glial cells. Values from paired experiments are linked by a line.
TABLE 2.
Cytokine release by astroglia and microglia after blocking CyPA activity in scrapie D-sup with CsA
| Cytokinesa | Medium | Sc D-supb | Sc D-sup + CsAb |
|---|---|---|---|
| Astroglia | |||
| IL-12p40 | 12.4 ± 0.3c | 31.7 ± 6.7 | 3.1 ± 3.0 |
| CCL2 | 1,567 ± 312 | 16,328 ± 1613 | 8,228 ± 912 |
| IL-6 | 11.6 ± 0.2 | 88.8 ± 9.7 | 30.1 ± 5.2 |
| G-CSF | 6.7 ± 0.4 | 34.7 ± 1.5 | 13.6 ± 2.5 |
| CXCL1 | 83.4 ± 20.5 | 2,453 ± 155 | 955 ± 70.6 |
| CCL3 | 47 ± 7.7 | 120 ± 11.5 | 49 ± 12.5 |
| CCL5 | 835 ± 33 | 1,770 ± 169 | 761 ± 46 |
| IL-13 | 20.3 ± 7.2 | 36.1 ± 5.1 | 13.3 ± 5.0 |
| Microglia | |||
| IL-12p40 | 4.6 ± 2.1 | 50 ± 1.0 | 1.4 ± 0.5 |
| CCL2 | 553 ± 200 | 1,289 ± 347 | 261 ± 107 |
a Of the 23 cytokines assayed, those shown were significantly increased after scrapie D-sup stimulation of glial cells relative to medium alone.
b 1% scrapie (Sc) D-sup in medium was added to glial cells with or without the addition of 0.1 μg/ml CsA.
c All values (in pg/ml) represent the mean and S.D. of three replicate cultures from one experiment. Differences between scrapie D-sup treatments in the presence and absence of CsA were significant (p < 0.05) for all cytokines shown in this table.
Glial Stimulatory Activity in D-sup from Normal Brain
In our previous studies, normal brain homogenates were able to induce release of low levels of several cytokines by astroglia (22). Similarly, in the present study, normal brain D-sup induced a low level release of eight cytokines released at higher levels by astroglia or microglia after scrapie D-sup stimulation (Fig. 6A). This result might possibly be due to the presence of CyPA in both normal and scrapie D-sups. Most cell types express CyPA in cytoplasm, and normal and scrapie D-sups, which are both derived from brain tissue, should both have CyPA. When we compared CyPA levels in these D-sups using two-dimensional difference in-gel analysis, CyPA was present in both, but the level was 35% higher in scrapie versus normal D-sup (data not shown). However, in blocking experiments, neither α-CyPA nor CsA was capable of blocking the release of several cytokines from our glial cell cultures after stimulation by normal D-sup (Fig. 6B). Thus, the CyPA present in normal D-sup lacked the same stimulating activity that was seen in scrapie D-sup.
FIGURE 6.
Profile of cytokine expression in mouse glial cells exposed to normal D-sup versus scrapie D-sup. A, cultured microglia or astroglia were stimulated by incubation with 1% scrapie (Sc) D-sup, 1% normal (N) D-sup, or medium. Sups from cultures were tested in a 23-cytokine multiplex assay. The 23 cytokines tested were IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, IFN-γ, CCL11 (Eotaxin), G-CSF, GM-CSF, CXCL1 (KC), CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), and TNF. Four independent culture wells were tested. p values comparing scrapie versus normal D-sup for each panel shown were ≤0.05. Cytokines showing a response significantly above medium with at least one of the sample variables analyzed are included in the figure. B, cultured astroglia were stimulated by incubation with 1% normal D-sup or 1% normal D-sup preincubated either with 2 μg of α-CyPA antibody or 0.1 μg/ml CsA. p values comparing normal D-sup versus D-sup treated with α-CyPA or CsA were >0.05. Bars indicate the mean, and brackets represent the S.E.
Presence of Truncated CyPA in Scrapie D-sup
To study possible qualitative differences of CyPA expressed in scrapie D-sup and normal D-sup, we tested these samples by immunoblot with α-CyPA antibody, which was directed to an epitope in the C-terminal third of the CyPA molecule (residues 100–165). An 18-kDa CyPA band was detected in D-sup from scrapie and normal mice, but some scrapie D-sup samples contained four additional immunoreactive bands with apparent molecular masses from 8 to 15 kDa (Fig. 7, left panel, lane 1). Reactivity with both the 18-kDa band and these lower molecular mass bands was shown to be specific for CyPA by blocking the antibody by preincubation with CyPA peptide p100–165 (Fig. 7, right panel, lane 3). Therefore, these lower molecular weight bands appeared to be truncated CyPA peptides containing C-terminal sequences. These truncations were present in relatively small amounts because long exposure times were required to visualize them. Furthermore, they were not seen in all scrapie D-sups, suggesting that they were below the level of detection by immunoblot in some mice. Because these truncated forms of CyPA were observed only in scrapie D-sups and never in normal D-sups, they might contribute to the stimulation of glial cells observed in our system.
FIGURE 7.
Truncated forms of CyPA are present in scrapie brain D-sup. In the left panel, 5–40 μg of protein from scrapie D-sup or normal D-sup was separated by SDS-PAGE and analyzed by immunoblotting using α-CyPA. Truncated CyPA bands with molecular masses from 8 to 15 kDa were seen only in scrapie D-sup. In the right panel, an immunoblot with 20 μg of scrapie D-sup (Sc) or normal D-sup (N) was probed with α-CyPA preincubated with (+) or without (−) an antibody-blocking peptide CyPA p100–165 (pep) (Abcam). α-Actin reactivity, used as a loading control for both panels, is shown in the lower panels. Lane numbers for the individual immunoblots are indicated at the bottom of each panel.
Cytokine Release by Astroglia in Response to Clinical and Preclinical Scrapie D-sups
To determine whether the glial stimulatory activity found in clinical scrapie mice could also be detected earlier during the course of scrapie infection, we tested D-sups prepared from mice at five preclinical time points from 61 to 125 dpi. Significant stimulation of astroglial CCL2 release was found using D-sups from 99 to 135 dpi (Fig. 8). At 61 and 83 dpi, CCL2 release was also elevated compared with normal brain D-sups, but this elevation was not statistically significant. Some of these samples were also tested by immunoblot, but truncated CyPA bands were not observed. The glial stimulating activity detected in our in vitro cultures first appeared in brain around the midpoint of the disease course and therefore might be important in contributing to progressive gliosis during scrapie disease in vivo.
FIGURE 8.
Evaluation of CCL2 release by astroglia in response to scrapie-infected mouse brain homogenates from different times postinfection. Cultured astroglia were overlaid with a final concentration of 1.0% scrapie D-supernatants from mice harvested at 61, 83, 99, 115, 125, and 135 dpi (three mice per time point in duplicate). Astroglia were also overlaid with a final concentration of 1.0% mock-challenged normal brain homogenate D-supernatants (N D-sup) from mice sacrificed at 61 and 135 dpi (two mice per time point in duplicate), medium alone, or 1.0 μg/ml crude LPS as controls. CCL2 released from astroglia cultures was quantified by Bio-Plex assay after 24-h incubation. Astroglial release of CCL2 in response to scrapie (Sc) D-sups significantly exceeding controls was observed from 99 to 135 dpi and increased with time relative to normal brain homogenate D-supernatants or medium alone. Results were compared by one-way analysis of variance with Dunnett's multiple comparison test (* denotes p < 0.05, ** denotes p < 0.01, and *** denotes p < 0.001). Bars indicate the mean, and brackets represent the S.E.
Stimulation of Cultured Glial Cells with Recombinant Human CyPA
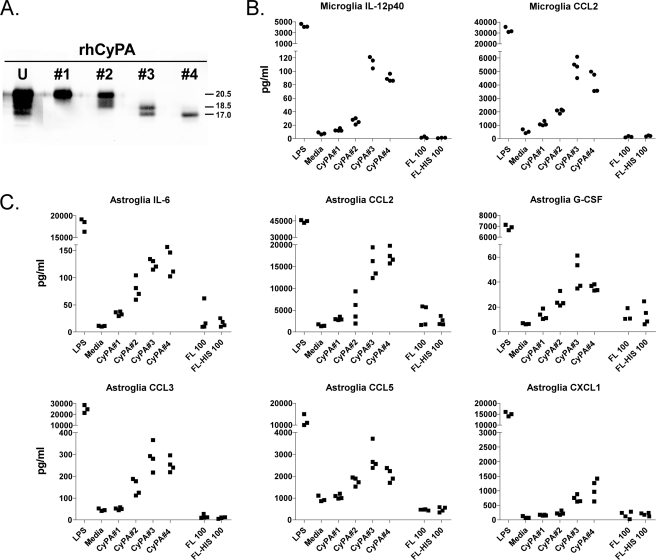
Results in Fig. 6, A and B, suggested that full-length CyPA found in both normal and scrapie D-sup was not responsible for the cytokine release observed. To determine the level of cytokine release by glial cells in response to exposure to full-length CyPA, we tested two commercially available rhCyPA proteins. Untagged rhCyPA (R&D Systems) and N-terminal polyhistidine-tagged rhCyPA (Sigma) were both unable to stimulate cytokine release by microglia or astroglia at 100 (Fig. 9, B and C) or 500 nm (data not shown).
FIGURE 9.
Truncated forms of CyPA stimulate cytokine release by glial cells, but full-length CyPA does not. Two microliters of the eluted CyPA products from each rhCyPA fraction (fractions 1–4) was separated by SDS-PAGE and probed with α-CyPA (A) to determine the recovery relative to 1 μg of unfractionated rhCyPA (U) starting material (Abcam). Immunoreactive bands were visualized and quantified using the Li-Cor Odyssey imaging system and software. Relative molecular masses in kDa are marked to the right of the panel. Cultured microglia (B) or astroglia (C) were treated by overlaying with the different eluted rhCyPA fractions (fractions 1–4) at a concentration of 50 nm or with 100 nm untagged full-length (FL) CyPA (R&D Systems) or polyhistidine-tagged (N terminus) full-length (FL-HIS) CyPA (Sigma). Glial cells incubated with medium alone or 1 μg/ml LPS constituted negative and positive controls, respectively. Twenty-four hours after stimulation, supernatants from microglia and astroglia cultures were tested in a 9- or 23-cytokine multiplex assay. Cytokines showing a response significantly above medium with at least one of the CyPA samples analyzed are included in the figure. Each data point represents an individual culture well. In repeated assays using D-sup or rhCyPA products as stimulators of microglia and astroglia, cytokines CCL3, CCL5, and IL-13 were variable in statistical significance. This accounts for their variable presence in Figs. 6 and 9 and Table 2.
Purified Truncated Forms of rhCyPA Are Strong Glial Stimulators
Because truncated murine CyPA proteins were present in scrapie D-sup at low concentrations in a complex mixture of brain-derived proteins, obtaining pure proteins from these D-sups would be difficult. However, we tested two lots of commercially prepared N-terminal polyhistidine-tagged rhCyPA from Abcam that were both found to contain truncated forms of CyPA similar to those seen in scrapie D-sup (Fig. 9A). Both lots stimulated cytokine release from glial cultures. From one of these lots, we isolated truncated forms of rhCyPA into four fractions by SDS-PAGE and electroelution (Fig. 9A, fractions 1–4). These fragments reacted with antibodies directed to the C-terminal third of CyPA but were slightly larger than fragments identified in scrapie D-sup (Fig. 7A). These four different fractions of rhCyPA bands were tested for their stimulatory effect on cytokine release by microglia and astroglia. Fraction 1, which contained non-truncated CyPA, did not stimulate cytokine release by microglia or astroglia (Fig. 9, B and C). This result was similar to what we observed with both tagged and untagged full-length rhCyPA. Fraction 2, which contained both the non-truncated form and two slightly smaller molecules, stimulated both microglia and astroglia at low levels. However, fractions 3 and 4 with the smallest CyPA peptides (17–18 kDa) induced a pattern of cytokine release from microglia and astroglia similar to that seen previously after scrapie D-sup stimulation (Fig. 9, B and C and Table 3).
TABLE 3.
Similar cytokine profiles are released by glial cells stimulated with scrapie d-sup and truncated recombinant human CyPA molecules
| Cytokinesa,b | Scrapie D-sup | rhCyPA |
|
|---|---|---|---|
| Fraction 3 | Fraction 4 | ||
| IL-12p40c | + | + | + |
| CCL2c | + | + | + |
| IL-6d | + | + | + |
| G-CSFd | + | + | + |
| CXCL1d | + | + | + |
| CCL3e | +/− | + | + |
| CCL5e | +/− | + | + |
| IL-13e | +/− | + | + |
| IL-1βe | +/− | − | − |
a Data summarized from results shown in Figs. 5, 7, 8, and 9 as well as previously published data (22).
b The following 14 cytokines (IL-1α, IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, IL-12p70, IL-17, IFN-γ, CCL11, GM-CSF, CCL4, and TNF) were not found in microglial or astroglial supernatant fluids after stimulation with scrapie D-sup or rhCyPA p100–165, fraction 3, or fraction 4 (see Fig. 9). However, these were detected after stimulation with LPS (not shown).
c These cytokines were released by both microglia and astroglia.
d These cytokines were released only by astroglia.
e These cytokines were released only by astroglia, but release was variable (+/−) in different sets of astroglial cultures.
A summary comparison of cytokines released by primary glia cultures when stimulated with scrapie D-sup (Fig. 6) and rhCyPA electroelution fractions 3 and 4 (Fig. 9) can be found in Table 3. The collective results indicated that these smallest truncated forms of CyPA were better stimulators of glia than the intact full-length CyPA, indicating that fragments containing the C-terminal third of CyPA are important for glial stimulation in our system.
DISCUSSION
CypA is a widely expressed multifunctional protein that is involved in a variety of inflammatory situations (23–26). Extracellular CyPA has been reported to stimulate release of cytokines and adhesion molecules from vascular smooth muscle cells, cardiomyocytes, endothelial cells, and macrophages (23–26, 35–39), and CyPA is chemotactic for both neutrophils and monocytes (38, 40). However, this is the first report describing a potential role for CyPA in the activation of brain microglia and astroglia. We now demonstrate that CyPA from scrapie-infected brains is a potent stimulator of cytokine release by both microglia and astroglia in vitro. Inhibiting CyPA activity in scrapie D-sups by treatment with α-CyPA or with CsA, a CyPA inhibitor, decreased cytokine release by both microglia and astroglia in vitro. Furthermore, we identified truncated forms of CyPA in glial stimulating preparations such as scrapie brains and rhCyPA. The purified full-length form of rhCyPA did not induce a glial cytokine release, but purified lower molecular weight forms of rhCyPA induced a pattern of cytokine release in cultured microglia or astroglia similar to that induced by scrapie D-sup (summarized in Table 3). This pattern of glial cytokines differed from that released by astroglia after stimulation by a brain stab wound (41) or by exposure to HIV Tat protein (42) but was similar to that described earlier in brains of scrapie-infected animals (22). The correlation in the cytokine patterns induced by scrapie brain extracts and truncated rhCyPA strengthened the conclusion that CyPA contributes to the stimulation of astroglia and microglia observed during prion disease pathogenesis. Moreover, preclinical scrapie D-sups from 61 to 135 days postinfection demonstrated a progressively increasing ability to stimulate CCL2 release by astroglia (Fig. 8). The presence of this stimulating activity correlated with the timing of the progressive gliosis following scrapie infection and suggested a role for CyPA in the gliosis observed at preclinical as well as clinical stages of prion disease. We also analyzed these preclinical samples by immunoblot for the presence of truncated CyPA products, but the truncations were likely below the level of detection by Western blotting (data not shown).
In our experiments, neither purified PrPres nor amyloid PrP fibrils were capable of stimulating microglia or astroglia to release cytokines. One might expect that the disease-associated PrPres would be capable of stimulating glial cells as deposition of PrPres is usually colocalized with gliosis (10, 43–45). Furthermore, various in vitro studies have demonstrated microglial activation by the neurotoxic fibrillar form of PrP peptide 106–126 (14–17). However, our findings using PrPres were contrary to those using peptide 106–126 and suggested that the role of PrPres in glial cytokine release and induction of brain inflammation during prion disease may be due to indirect neurotoxic effects rather than direct effects of PrPres on glial cells.
Possibly, the damage caused by PrPres during the disease process may induce active secretion and truncation of CyPA by either neurons or microglia (23, 38, 46). CyPA is not secreted in normal tissues and is considered a cytoplasmic molecular chaperone protein (47–49). Therefore, in tissues, CyPA may need to be secreted into the extracellular space to be able to stimulate glial cells by its interaction with its extracellular receptor, CD147 (23, 35, 38, 46, 50, 51). During the process of prion infection, truncated and/or full-length CyPA may be released by dying neuronal or glial cells (23, 38) into the extracellular space where further truncation of CyPA might occur. In our experiments, these truncated forms of CyPA appeared to be the most potent activators of glial cytokine release. In contrast, such truncated CyPA forms were not present in the normal D-sup. This might explain why CyPA in normal D-sup was not a stimulant of glial cytokine release.
In this study, we found apparent similarities between one commercially available rhCyPA (Abcam) and the scrapie D-sup. Both have intact full-length and truncated CyPA. These truncated forms of CyPA in both scrapie D-sup and rhCyPA were recognized by our α-CyPA antibody, which is directed against residues 100–165. Thus, the truncated CyPA forms contain an epitope within the C-terminal third of CyPA. Based on our fractionation studies of the mixture of intact full-length and truncated rhCyPA, the intact form is at best a weak glial stimulator, whereas the fractions with truncated forms are strong glial stimulators. By immunoblot in fractions 3 and 4, we could detect fragments containing an epitope in the C-terminal third (Fig. 9A), but by mass spectroscopy analysis, forms lacking C-terminal sequences could not be excluded (data not shown). The fact that this C-terminal CyPA region was present in the truncated fragments in the stimulatory fractions of both purified rhCyPA and scrapie D-sup strongly supported the conclusion that these fragments in scrapie brain D-sup are important stimulatory molecules for both astroglia and microglia.
This last finding raises the question of why truncated forms of CyPA are better glial stimulators than the full-length CyPA. Possibly, the folding of truncated forms of CyPA might be more favorable for the binding to the CD147 cellular receptor. Residue 126 present in the C-terminal region of CyPA, which is necessary for the binding to CD147 (50), might be more accessible in the truncated forms. Alternatively, some sequences present in full-length CyPA might interfere with the binding of the C-terminal region to the receptor CD147. The extra sequences might be involved in interactions with additional cellular molecules that might alter the availability of CyPA for binding to CD147. It is also plausible that truncated CyPA might interact with an as yet unidentified alternate receptor other than CD147 on glial cells. Further studies of glial stimulation and inhibition with other CyPA fragments will be required to distinguish among these possibilities.
Although extracellular CyPA has been reported to stimulate the activation and release of various molecules from several cell types derived from peripheral tissues, including vascular smooth muscle cells, cardiomyocytes, endothelial cells, and macrophages (23–26, 35–39), until our present data, there have been no previous reports noting a requirement for truncated forms of CyPA for stimulation of these cells. Possibly, the spontaneous breakdown found in some of our commercial CyPA preparations might also have occurred in the experiments of others. Alternatively, full-length CyPA might be a strong stimulator in these other cell types, suggesting a distinct difference in the ligand requirements for stimuli of astroglia and microglia.
The cytokine response by glial cells after scrapie infection is restricted to a few cytokines at low levels (22). It is still not understood whether glial activation is part of the pathogenic process or part of the host response to brain damage or both. Controversy exists over the neurodegenerative versus neuroregenerative roles of microglia in many brain diseases (52–54). Possibly, CyPA might also be released and contribute to gliosis during CNS inflammatory or degenerative diseases unrelated to prion infections. Our present results showing a role for CyPA in glial activation during prion diseases may provide new approaches such as using CyPA knock-out mice or inhibiting CyPA in vivo with cyclosporine A for studying the effects of gliosis and CyPA on disease pathogenesis in vivo.
Supplementary Material
Acknowledgments
We thank Brent Race and Kimberly Meade-White for technical assistance with the mouse experiments and Karin E. Peterson, Suzette A. Priola, Byron Caughey, and Gerry S. Baron for critical reading of the manuscript. Cells producing antibody D13 were kindly provided by Dr. Anthony Williamson.
This work was supported, in whole or in part, by the National Institutes of Health through the Division of Intramural Research, NIAID.

This article contains supplemental Table S1.
- TSE
- transmissible spongiform encephalopathy
- PrP
- prion protein
- PrPres
- partially protease-resistant host-derived prion protein
- CyPA
- cyclophilin A
- CsA
- cyclosporine A
- dpi
- days postinoculation
- sup
- supernatant
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- rhCyPA
- recombinant human CyPA
- RANTES
- regulated on activation normal T cell expressed and secreted
- G-CSF
- granulocyte colony-stimulating factor.
REFERENCES
- 1. Prusiner S. B. (1991) Molecular biology of prion diseases. Science 252, 1515–1522 [DOI] [PubMed] [Google Scholar]
- 2. Rock R. B., Gekker G., Hu S., Sheng W. S., Cheeran M., Lokensgard J. R., Peterson P. K. (2004) Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 17, 942–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burwinkel M., Riemer C., Schwarz A., Schultz J., Neidhold S., Bamme T., Baier M. (2004) Role of cytokines and chemokines in prion infections of the central nervous system. Int. J. Dev. Neurosci. 22, 497–505 [DOI] [PubMed] [Google Scholar]
- 4. Ransohoff R. M., Perry V. H. (2009) Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 [DOI] [PubMed] [Google Scholar]
- 5. Brown G. C., Neher J. J. (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 41, 242–247 [DOI] [PubMed] [Google Scholar]
- 6. Dheen S. T., Kaur C., Ling E. A. (2007) Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 14, 1189–1197 [DOI] [PubMed] [Google Scholar]
- 7. Nakamura Y. (2002) Regulating factors for microglial activation. Biol. Pharm. Bull. 25, 945–953 [DOI] [PubMed] [Google Scholar]
- 8. Na Y. J., Jin J. K., Kim J. I., Choi E. K., Carp R. I., Kim Y. S. (2007) JAK-STAT signaling pathway mediates astrogliosis in brains of scrapie-infected mice. J. Neurochem. 103, 637–649 [DOI] [PubMed] [Google Scholar]
- 9. Perry V. H., Cunningham C., Boche D. (2002) Atypical inflammation in the central nervous system in prion disease. Curr. Opin. Neurol. 15, 349–354 [DOI] [PubMed] [Google Scholar]
- 10. Van Everbroeck B., Dobbeleir I., De Waele M., De Leenheir E., Lübke U., Martin J. J., Cras P. (2004) Extracellular protein deposition correlates with glial activation and oxidative stress in Creutzfeldt-Jakob and Alzheimer's disease. Acta Neuropathol. 108, 194–200 [DOI] [PubMed] [Google Scholar]
- 11. Rezaie P., Lantos P. L. (2001) Microglia and the pathogenesis of spongiform encephalopathies. Brain Res. Brain. Res. Rev. 35, 55–72 [DOI] [PubMed] [Google Scholar]
- 12. Williams A., Lucassen P. J., Ritchie D., Bruce M. (1997) PrP deposition, microglial activation, and neuronal apoptosis in murine scrapie. Exp. Neurol. 144, 433–438 [DOI] [PubMed] [Google Scholar]
- 13. Giese A., Brown D. R., Groschup M. H., Feldmann C., Haist I., Kretzschmar H. A. (1998) Role of microglia in neuronal cell death in prion disease. Brain Pathol. 8, 449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peyrin J. M., Lasmézas C. I., Haïk S., Tagliavini F., Salmona M., Williams A., Richie D., Deslys J. P., Dormont D. (1999) Microglial cells respond to amyloidogenic PrP peptide by the production of inflammatory cytokines. Neuroreport 10, 723–729 [DOI] [PubMed] [Google Scholar]
- 15. Brown D. R., Schmidt B., Kretzschmar H. A. (1996) Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380, 345–347 [DOI] [PubMed] [Google Scholar]
- 16. Veerhuis R., Hoozemans J. J., Janssen I., Boshuizen R. S., Langeveld J. P., Eikelenboom P. (2002) Adult human microglia secrete cytokines when exposed to neurotoxic prion protein peptide: no intermediary role for prostaglandin E2. Brain Res. 925, 195–203 [DOI] [PubMed] [Google Scholar]
- 17. Thellung S., Corsaro A., Villa V., Venezia V., Nizzari M., Bisaglia M., Russo C., Schettini G., Aceto A., Florio T. (2007) Amino-terminally truncated prion protein PrP90–231 induces microglial activation in vitro. Ann. N.Y. Acad. Sci. 1096, 258–270 [DOI] [PubMed] [Google Scholar]
- 18. Brown A. R., Webb J., Rebus S., Walker R., Williams A., Fazakerley J. K. (2003) Inducible cytokine gene expression in the brain in the ME7/CV mouse model of scrapie is highly restricted, is at a strikingly low level relative to the degree of gliosis and occurs only late in disease. J. Gen. Virol. 84, 2605–2611 [DOI] [PubMed] [Google Scholar]
- 19. Griffin D. E. (2010) Recovery from viral encephalomyelitis: immune-mediated noncytolytic virus clearance from neurons. Immunol. Res. 47, 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kielian T. (2004) Microglia and chemokines in infectious diseases of the nervous system: views and reviews. Front. Biosci. 9, 732–750 [DOI] [PubMed] [Google Scholar]
- 21. Lim J. K., Murphy P. M. (2011) Chemokine control of West Nile virus infection. Exp. Cell Res. 317, 569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tribouillard-Tanvier D., Striebel J. F., Peterson K. E., Chesebro B. (2009) Analysis of protein levels of 24 cytokines in scrapie agent-infected brain and glial cell cultures from mice differing in prion protein expression levels. J. Virol. 83, 11244–11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin Z. G., Melaragno M. G., Liao D. F., Yan C., Haendeler J., Suh Y. A., Lambeth J. D., Berk B. C. (2000) Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ. Res. 87, 789–796 [DOI] [PubMed] [Google Scholar]
- 24. Schmidt R., Bültmann A., Fischel S., Gillitzer A., Cullen P., Walch A., Jost P., Ungerer M., Tolley N. D., Lindemann S., Gawaz M., Schömig A., May A. E. (2008) Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor κB-dependent inflammation in monocytes. Circ. Res. 102, 302–309 [DOI] [PubMed] [Google Scholar]
- 25. Yang Y., Lu N., Zhou J., Chen Z. N., Zhu P. (2008) Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology 47, 1299–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan W., Ge H., He B. (2010) Pro-inflammatory activities induced by CyPA-EMMPRIN interaction in monocytes. Atherosclerosis 213, 415–421 [DOI] [PubMed] [Google Scholar]
- 27. Race B., Meade-White K., Oldstone M. B., Race R., Chesebro B. (2008) Detection of prion infectivity in fat tissues of scrapie-infected mice. PLoS Pathog. 4, e1000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trifilo M. J., Sanchez-Alavez M., Solforosi L., Bernard-Trifilo J., Kunz S., McGavern D., Oldstone M. B. (2008) Scrapie-induced defects in learning and memory of transgenic mice expressing anchorless prion protein are associated with alterations in the γ aminobutyric acid-ergic pathway. J. Virol. 82, 9890–9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meade-White K., Race B., Trifilo M., Bossers A., Favara C., Lacasse R., Miller M., Williams E., Oldstone M., Race R., Chesebro B. (2007) Resistance to chronic wasting disease in transgenic mice expressing a naturally occurring allelic variant of deer prion protein. J. Virol. 81, 4533–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsunaga Y., Peretz D., Williamson A., Burton D., Mehlhorn I., Groth D., Cohen F. E., Prusiner S. B., Baldwin M. A. (2001) Cryptic epitopes in N-terminally truncated prion protein are exposed in the full-length molecule: dependence of conformation on pH. Proteins 44, 110–118 [DOI] [PubMed] [Google Scholar]
- 31. Bolton D. C., Bendheim P. E., Marmorstein A. D., Potempska A. (1987) Isolation and structural studies of the intact scrapie agent protein. Arch. Biochem. Biophys. 258, 579–590 [DOI] [PubMed] [Google Scholar]
- 32. Moore R. A., Timmes A., Wilmarth P. A., Priola S. A. (2010) Comparative profiling of highly enriched 22L and Chandler mouse scrapie prion protein preparations. Proteomics 10, 2858–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 [DOI] [PubMed] [Google Scholar]
- 34. Wessel D., Flügge U. I. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141–143 [DOI] [PubMed] [Google Scholar]
- 35. Seko Y., Fujimura T., Taka H., Mineki R., Murayama K., Nagai R. (2004) Hypoxia followed by reoxygenation induces secretion of cyclophilin A from cultured rat cardiac myocytes. Biochem. Biophys. Res. Commun. 317, 162–168 [DOI] [PubMed] [Google Scholar]
- 36. Kim S. H., Lessner S. M., Sakurai Y., Galis Z. S. (2004) Cyclophilin A as a novel biphasic mediator of endothelial activation and dysfunction. Am. J. Pathol. 164, 1567–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jin Z. G., Lungu A. O., Xie L., Wang M., Wong C., Berk B. C. (2004) Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24, 1186–1191 [DOI] [PubMed] [Google Scholar]
- 38. Sherry B., Yarlett N., Strupp A., Cerami A. (1992) Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc. Natl. Acad. Sci. U.S.A. 89, 3511–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim J. Y., Kim H., Suk K., Lee W. H. (2010) Mediators Inflamm. 2010,821940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arora K., Gwinn W. M., Bower M. A., Watson A., Okwumabua I., MacDonald H. R., Bukrinsky M. I., Constant S. L. (2005) Extracellular cyclophilins contribute to the regulation of inflammatory responses. J. Immunol. 175, 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takamiya M., Fujita S., Saigusa K., Aoki Y. (2007) Simultaneous detections of 27 cytokines during cerebral wound healing by multiplexed bead-based immunoassay for wound age estimation. J. Neurotrauma 24, 1833–1844 [DOI] [PubMed] [Google Scholar]
- 42. Fitting S., Zou S., Chen W., Vo P., Hauser K. F., Knapp P. E. (2010) Regional heterogeneity and diversity in cytokine and chemokine production by astroglia: differential responses to HIV-1 Tat, gp120, and morphine revealed by multiplex analysis. J. Proteome Res. 9, 1795–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kovács G. G., Preusser M., Strohschneider M., Budka H. (2005) Subcellular localization of disease-associated prion protein in the human brain. Am. J. Pathol. 166, 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Budka H., Aguzzi A., Brown P., Brucher J. M., Bugiani O., Gullotta F., Haltia M., Hauw J. J., Ironside J. W., Jellinger K. (1995) Neuropathological diagnostic criteria for Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol. 5, 459–466 [DOI] [PubMed] [Google Scholar]
- 45. Wells G. A. (1993) Pathology of nonhuman spongiform encephalopathies: variations and their implications for pathogenesis. Dev. Biol. Stand. 80, 61–69 [PubMed] [Google Scholar]
- 46. Boulos S., Meloni B. P., Arthur P. G., Majda B., Bojarski C., Knuckey N. W. (2007) Evidence that intracellular cyclophilin A and cyclophilin A/CD147 receptor-mediated ERK1/2 signalling can protect neurons against in vitro oxidative and ischemic injury. Neurobiol. Dis. 25, 54–64 [DOI] [PubMed] [Google Scholar]
- 47. Fischer G., Aumüller T. (2003) Regulation of peptide bond cis/trans isomerization by enzyme catalysis and its implication in physiological processes. Rev. Physiol. Biochem. Pharmacol. 148, 105–150 [DOI] [PubMed] [Google Scholar]
- 48. Kruse M., Brunke M., Escher A., Szalay A. A., Tropschug M., Zimmermann R. (1995) Enzyme assembly after de novo synthesis in rabbit reticulocyte lysate involves molecular chaperones and immunophilins. J. Biol. Chem. 270, 2588–2594 [DOI] [PubMed] [Google Scholar]
- 49. Cohen E., Taraboulos A. (2003) Scrapie-like prion protein accumulates in aggresomes of cyclosporin A-treated cells. EMBO J. 22, 404–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yurchenko V., Zybarth G., O'Connor M., Dai W. W., Franchin G., Hao T., Guo H., Hung H. C., Toole B., Gallay P., Sherry B., Bukrinsky M. (2002) Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J. Biol. Chem. 277, 22959–22965 [DOI] [PubMed] [Google Scholar]
- 51. Payeli S. K., Schiene-Fischer C., Steffel J., Camici G. G., Rozenberg I., Lüscher T. F., Tanner F. C. (2008) Cyclophilin A differentially activates monocytes and endothelial cells: role of purity, activity, and endotoxin contamination in commercial preparations. Atherosclerosis 197, 564–571 [DOI] [PubMed] [Google Scholar]
- 52. Mallat M., Chamak B. (1994) Brain macrophages: neurotoxic or neurotrophic effector cells? J. Leukoc. Biol. 56, 416–422 [DOI] [PubMed] [Google Scholar]
- 53. Minton K. (2001) Immune mechanisms in neurological disorders: protective or destructive? Trends Immunol. 22, 655–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Streit W. J. (2002) Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40, 133–139 [DOI] [PubMed] [Google Scholar]
- 55. Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.