Background: CREMα is increased in SLE T cells, and although it suppresses IL-2, it enhances IL-17A production.
Results: CREMα binds to the IL17F promoter and suppresses its expression in SLE T cells.
Conclusion: CREMα disrupts the balance between IL-17A and IL-17F in SLE T cells in favor of IL-17A.
Significance: Understanding the molecular basis of the aberrant cytokine network in SLE will help in devising approaches to correct it.
Keywords: Chromatin Modification, DNA Methylation, Epigenetics, Inflammation, Interleukin, Transcription Factors, CREMα, IL-17, IL-17F, SLE
Abstract
The proinflammatory cytokines IL-17A and IL-17F are primarily produced by Th17 lymphocytes. Both are involved in host defense mechanisms against bacterial and fungal pathogens and contribute to the development of various autoimmune diseases. T lymphocytes from patients with systemic lupus erythematosus (SLE) display increased expression of transcription factor cAMP-responsive element modulator α (CREMα), which has been documented to account for aberrant T cell function and contributes to the pathogenesis of SLE. Here, we provide evidence that IL-17F expression is reduced in SLE T cells. We demonstrate that CREMα binds to a yet unidentified CRE site within the proximal promoter. This results in reduced IL-17F expression in SLE T lymphocytes and is independent of activating epigenetic patterns (increased histone H3 Lys-18 acetylation, reduced histone H3 Lys-27 trimethylation, and CpG-DNA demethylation). Forced CREMα expression in human T lymphocytes results in reduced IL-17F expression. Our findings demonstrate extended involvement of CREMα in cytokine dysregulation in SLE by contributing to a disrupted balance between IL-17A and IL-17F. An increased IL-17A/IL-17F ratio may aggravate the proinflammatory phenotype of SLE.
Introduction
The IL-17 family of cytokines consists of six members: IL-17A though IL-17F (1). IL-17 cytokines play a key role in immune responses, and dysregulation of cytokine expression contributes to autoimmune disorders (2). The namesake cytokine IL-17A was the first member to be described in 1993 (3). The remaining cytokines, IL-17B through IL-17F, have been identified subsequently, based on their homology with IL-17A, with IL-17F being the most conserved family member (50% amino acid conservation). The IL17A and IL17F genes are located on chromosome 6p12. IL-17A and IL-17F are produced by various immune cells, including T lymphocytes, natural killer cells, invariant natural killer cells, γδT cells, and neutrophils. Both cytokines are secreted as disulfide-linked homodimers or IL-17A/F heterodimers. They share key biological properties, including involvement in host defense mechanisms against bacteria and fungi, and IL-17F exerts proinflammatory functions, including the induction of chemokines, cytokines, and the recruitment of neutrophils to the site of inflammation (2). However, IL-17A homodimers seem to promote more robust proinflammatory responses when compared with IL-17F homodimers and IL-17A/F heterodimers (2, 4–7). Over the past decade, a specialized subset of IL-17-producing T helper lymphocytes, denoted Th17 cells, has been reported and extensively studied. Th17 lymphocytes are the predominant cell type, producing IL-17A and IL-17F (4, 5, 8, 9). Th17 subsets play a central role in adaptive immune responses and are involved in the pathogenesis of autoimmune diseases, including systemic lupus erythematosus (SLE)4 (10). SLE is a chronic autoimmune disease that affects multiple organs and is characterized by severe T cell signaling abnormalities (11).
Dysregulation in IL-17A expression contributes to the pathophysiology of autoimmune disorders. Increased expression of IL-17A has been documented in SLE (11–14), rheumatoid arthritis (15), psoriasis (16), and multiple sclerosis (17, 18). Recently, we demonstrated the involvement of the transcriptional regulatory factor cAMP-responsive element modulator α (CREMα) in the induction of IL-17A expression in SLE T lymphocytes (14). The involvement of IL-17F in autoimmune disease remains to be clarified. However, its expression has been reported to be elevated in rheumatoid arthritis, inflammatory bowel disease, and psoriasis (2).
In the present report, we link overexpression of the transcription regulatory factor CREMα to reduced IL-17F production in SLE T lymphocytes. CREMα belongs to a superfamily of transcription factors that includes cAMP-responsive element (CRE)-binding protein, the inducible cAMP-response element repressor, and activating transcription factors (14). In response to activation, these transcription factors bind to CREs (consensus sequence: TGACGTCA) or CRE half-sites (5′ (TGAC) and/or 3′ (GTCA)) in cis-regulatory regions. The presence of multiple CREM isoforms is caused by the presence of various promoters, alternative initiation sites, and differential splicing (19).
We previously reported that T lymphocytes from SLE patients display increased CREMα expression that is due to increased CREM promoter activity and reflects disease activity (20–23). Several target genes have been identified that are relevant for immune cell function and undergo trans-regulation by CREMα. These include the cytokine genes IL17A and IL2 that are regulated antithetically (14, 24), the transcription factor c-Fos (20, 25), TCR/CD3ζ, (26), and the antigen-presenting cell molecule CD86 (27). We further demonstrated that CREMα is involved in epigenetic remodeling of cytokine genes through histone deacetylase 1 (HDAC1) and DNA methyltransferase 3a (DNMT3a) recruitment to regulatory gene sequences.
Here, we demonstrate reduced IL-17F expression from SLE T lymphocytes. We show that CREMα binds to a yet unidentified CRE site within the proximal IL17F promoter (−127/−123 bp upstream of the transcriptional start site). CREMα recruitment to this site is associated with reduced IL-17F expression in T lymphocytes from SLE patients. We provide evidence that CREMα suppresses IL17F promoter activity. Reduced IL-17F expression is independent of activating epigenetic patterns in SLE T lymphocytes, including increased histone H3 Lys-18 (H3K18) acetylation, decreased H3K27 trimethylation, and cytosine-phosphate-guanosine (CpG)-DNA demethylation of the human IL17 locus. Our data support the importance of CREMα in regulating the transcriptional machinery of SLE T lymphocytes and constitute the first report of reduced IL-17F expression in SLE. Because IL-17A/IL-17F heterodimers have reduced proinflammatory activities when compared with IL-17A, imbalances of the IL-17A/IL-17F ratio toward IL-17A may further contribute to the inflammatory phenotype of SLE.
EXPERIMENTAL PROCEDURES
Study Subjects and T Lymphocyte Culture
All SLE patients included in this study were diagnosed according to the American College of Rheumatology classification criteria and recruited from the Division of Rheumatology at Beth Israel Deaconess Medical Center (Boston, MA) after written informed consent under protocol 2006-P-0298. All included patients were female. Average SLE disease activity scores were 10.5, reflecting active disease. Epidemiological information including immunosuppressive medications is given in Table 1. Healthy ethnicity-, age-, and gender-matched individuals were chosen as controls. Peripheral venous blood was collected in heparin-lithium tubes, and total human T lymphocytes were purified as described previously (14). Primary human T cells and human Jurkat T cells were maintained in RPMI medium supplemented with 10% fetal bovine serum. Naive CD4+ T cells from healthy controls were purified from total T cell suspension, using the Human Naive CD4+ T Cell Isolation kit II (Miltenyi Biotec). For activation assays, T lymphocytes were incubated in the absence or presence of plate-bound anti-CD3 and anti-CD28 antibodies (BioXCell; both at 1 μg/ml) for 72 h.
TABLE 1.
Epidemiological data of the included SLE patients
Samples from the same patients (patients 1–6 and 7–10) were also used in Ref. 24. F, female; SLEDAI, systemic lupus erythematosus disease activity index (0, inactive; 1–5, mild activity; 6–10, moderate activity; 11–19, high activity; >20, very high activity). Aza, azathioprine; MMF, mycophenolate mofetil; HCQ, hydroxychloroquine; GC, glucocorticoids. IP, immunoprecipitation.
| Patient | Gender | Age | SLEDAI | Treatment | Application |
|---|---|---|---|---|---|
| 1 | F | 30 | 4 | MMF, HCQ, GC | CmpG-IP |
| 2 | F | 37 | 10 | HCQ, GC | CmpG-IP |
| 3 | F | 36 | 10 | MMF, GC | CmpG-IP |
| 4 | F | 39 | 8 | CmpG-IP | |
| 5 | F | 38 | 36 | GC | CmpG-IP |
| 6 | F | 54 | 14 | HCQ | CmpG-IP |
| Average | 39 | 13.67 | |||
| Range | (30–54) | (4–36) | |||
| 7 | F | 36 | 4 | MMF | ChIP |
| 8 | F | 27 | 6 | GC | ChIP |
| 9 | F | 39 | 5 | HCQ | ChIP |
| 10 | F | 32 | 8 | Aza | ChIP |
| 11 | F | 45 | 4 | HCQ, GC | ChIP |
| 12 | F | 45 | 4 | HCQ, GC | ChIP |
| 13 | F | 48 | 4 | MMF | ChIP |
| Average | 33.5 | 5.00 | |||
| Range | (27–39) | (4–8) |
mRNA Extraction and Quantitative RT-PCR
Total RNA was isolated, using the RNeasy minikit (Qiagen). Residual genomic DNA contamination was removed by DNase I (Qiagen). RNA was reverse-transcribed into cDNA, using the reverse transcription system (Promega). Sequences for primers used for qRT-PCR are given in Table 2.
TABLE 2.
qRT-PCR primers
| Primer | Forward (5′–3′) | Reverse (5′–3′) | Annealing temperature |
|---|---|---|---|
| ºC | |||
| IL-17A | ACCAATCCCAAAAGGTCCTC | CACTTTGCCTCCCAGATCAC | 60 |
| IL-17F | CACGTAACATCGAGAGCCG | AGCCCAAGTTCCTACACTGG | 60 |
| 18 S rRNA | ACTCAACACGGGAAACCTCA | AACCAGACAAATCGCTCCAC | 60 |
| IL17_CNS4 | TTGGGTCAGATGGTGAGAAG | GAAAGCAAGCAGCAAGGATG | 55 |
| IL17_CNS5 | CCTGTGTATGCCTGTGTTC | GTCAGTAACTCCGGGTCAA | 55 |
| IL17_CNS6 | CAGGAGCTGACCTTTCTA | TAGTTCTCCCAGCAGGTA | 55 |
IL-17A and IL-17F ELISAs
Primary human T lymphocytes were cultured for 72 h (in the presence or absence of plate-bound anti-CD3 and anti-CD28 antibodies as indicated). Supernatants were collected and measured for secreted IL-17A and IL-17F by ELISAs following the manufacturer's instructions (eBioscience, San Diego, CA).
Plasmids and Generation of Luciferase Reporter Constructs
An expression plasmid for human CREMα (on a pcDNA3.1/V5-His-TOPO backbone, Invitrogen) has been kindly provided by G. N. Europe-Finner (Faculty of Medical Sciences, Newcastle upon Tyne, UK) (28). Reporter constructs spanning the proximal 636 and 166 bp of the human IL17F promoter were PCR-amplified and cloned into luciferase vector pGL3-Basic (Promega), using primers with attached restriction sites for MluI and BglII. All plasmid DNA preparations were carried out with DNA purification kits (Qiagen) and sequence-verified (Genewiz, Cambridge, MA). Site-directed mutagenesis at the −127/−123 CRE site within reporter construct IL17A_p(−166)_luc was performed using a DNA oligonucleotide harboring a mutated CRE (5′-gacccggagttacctgcgaatgcaggggtttttttttttattcc-3′; MWG Operon) and PfuTurbo® DNA polymerase (Stratagene) following the manufacturer's instructions.
Forced Expression of CREMα in Primary Human T Lymphocytes and Jurkat T Cells
Primary human T lymphocytes or Jurkat T cells (as indicated) were transfected with a total amount of 3.0 μg of plasmid DNA, using the Amaxa transfection system (Lonza). Five hours after transfection, cells were collected and lysed, and mRNA was harvested and processed as indicated previously (14). All experiments were repeated at least three times, and values in the bar diagrams are given as mean ± S.D.
Transfection of Jurkat T Cells with Control and CREM siRNA
Jurkat T cells were transfected with 5 nm irrelevant control siRNA and CREM-specific siRNA (OriGene) using Lipofectamine (Invitrogen). Prior to these experiments, experimental conditions were optimized, applying Cy-3-labeled control siRNA (OriGene) through transfection with Lipofectamine (Invitrogen). Transfection efficiency was >90%. Cells were collected after culture for 5 h in the absence and presence (as indicated) of soluble anti-CD3/CD28 antibodies (3 μg/ml) and processed for mRNA analysis as indicated previously (14). All experiments were repeated three times, and values in the bar diagrams are given as mean ± S.D.
Luciferase Assays in Jurkat T Cells
One million Jurkat T cells were transfected with the indicated amounts of plasmid DNA, using the Amaxa transfection system (Lonza). Effector/reporter transfection experiments were performed at a molar ratio of 3:1. Each reporter experiment included 10 ng of Renilla luciferase construct as an internal control. Five hours after transfection, cells were collected and lysed, and luciferase activity was quantified, using the Promega Dual-Luciferase assay system (Promega) following the manufacturer's instructions. Luciferase experiments were repeated at least three times, and values in the bar diagrams are given as mean ± S.D.
ChIP Assays
Anti-HDAC1, anti-H3K18ac, and anti-H3K27me3 antibodies, and nonspecific normal rabbit mouse IgG were obtained from Upstate (Millipore). Polyclonal anti-CREMα antibody detecting human CREMα has been described before (23). ChIP grade Protein A/G Plus-agarose has been purchased from Pierce (ThermoScientific). ChIP assay was carried out according to the manufacturer's instructions (Upstate Biotechnology/Millipore). Briefly, cells were cross-linked with 1% formaldehyde, washed with cold phosphate-buffered saline, and lysed in buffer containing protease inhibitors (Roche Applied Science). Cell lysates were sonicated to shear DNA and sedimented, and diluted supernatants were immunoprecipitated with the indicated antibodies. A proportion (20%) of the diluted supernatants was kept as input control. Protein-DNA complexes were eluted in 1% SDS, 0.1 m NaHCO3 and reverse-cross-linked at 65 °C. DNA was recovered, using the QIAamp DNA minikit (Qiagen) and subjected to PCR analysis on an ABI OneStepPlus real-time PCR system. Real-time qPCR primer sequences are given in Table 2. The amount of immunoprecipitated DNA was subtracted by the amplified DNA that was bound by the nonspecific normal IgG and subsequently calculated relative to the respective input DNA.
IL-17A Secretion Assay
Human naive CD4+ T cells were activated, using anti-CD3/anti-CD28 antibodies for 72 h and PMA (50 ng/ml)/ionomycin (500 ng/ml) for another 5 h. Because IL-17A and IL-17F are largely co-expressed in human CD4+ T lymphocytes, the commercially available IL-17A secretion assay was performed according to the manufacturer's instructions (Miltenyi Biotec).
Methyl-CpG-DNA Immunoprecipitation
The methyl-CpG-DNA immunoprecipitation assay was carried out following the manufacturer's instructions (Zymo Research). Briefly, genomic DNA from T lymphocytes obtained from SLE patients and healthy control individuals was purified, applying the AllPrep RNA/DNA/protein minikit (Qiagen), and sheared to fragments of ∼200-bp length, using DNA shearase (Zymo Research). Subsequently, 100 ng of sheared genomic DNA were used as input for methyl-CpG-DNA immunoprecipitation. Methylated CpG-DNA was recovered and subjected to PCR analysis on an ABI OneStepPlus real-time PCR system, using primers as listed in Table 2. Equal amounts (100 ng) of completely methylated (100%) human CpG-DNA and demethylated human CpG-DNA (Zymo Research) were included as “input” and negative control.
Statistical Analysis
Paired two-tailed Student's t test was used for statistical analysis.
RESULTS
T Lymphocytes from SLE Patients Fail to Express IL-17F
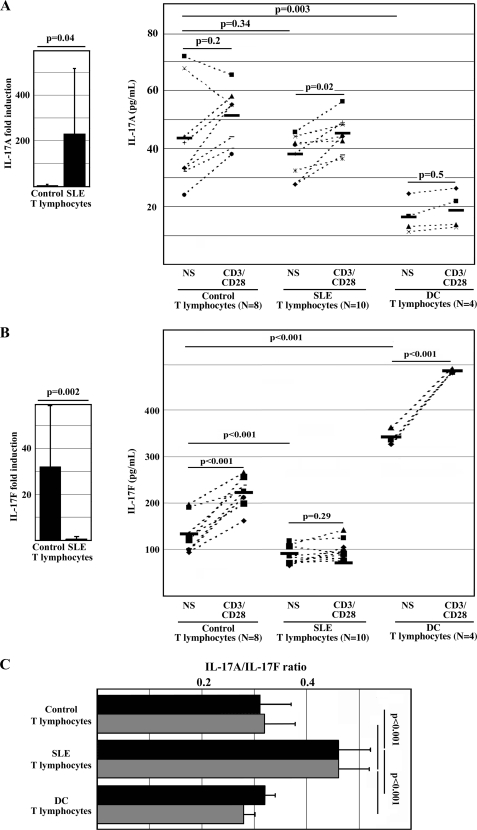
Because IL-17A is overexpressed by T cells from SLE patients (11–13), and IL-17A and IL-17F are usually co-expressed in IL-17 expressing T lymphocytes (9), we asked whether this is also the case in SLE T cells. Thus, we cultured total T lymphocytes from healthy controls, SLE patients, and autoimmune disease control patients (psoriatic arthritis and rheumatoid arthritis) for 72 h in the presence or absence of anti-CD3/CD28 antibodies and determined IL-17A and IL-17F expression at the mRNA and protein level. In response to stimulation, T lymphocytes from SLE patients produced significantly more IL-17A mRNA when compared with healthy controls (Fig. 1A, left, p = 0.04). Interestingly, resting T lymphocytes from healthy controls and SLE patients expressed comparable amounts of IL-17A protein in the cell culture supernatant. IL-17A expression was inducible in T lymphocytes from SLE patients (p = 0.02), whereas the changes in IL-17A expression in control T cells were variable and did not reach statistical significance (Fig. 1A, right, p = 0.2). Interestingly, T lymphocytes from autoimmune disease control patients produced significantly less IL-17A protein when compared with control and SLE T lymphocytes (p = 0.003). In contrast to these observations, T lymphocytes from SLE patients failed to produce IL-17F mRNA when compared with control T cells (Fig. 1B, left, p = 0.002). Resting T lymphocytes from healthy donors and autoimmune disease control patients expressed significantly more IL-17F protein when compared with SLE T lymphocytes (Fig. 1B, left, p < 0.001). Stimulation with anti-CD3/CD28 resulted in increased IL-17F expression in T lymphocytes from all control individuals (p < 0.001), whereas the IL-17F expression from SLE T lymphocytes did not increase further (p = 0.29).
FIGURE 1.
Activated T lymphocytes from SLE patients fail to produce IL-17F. Primary T lymphocytes from healthy control individuals, SLE patients, and control individuals with other autoimmune diseases (DC; rheumatoid arthritis and psoriatic arthritis) were cultured in the presence or absence (not significant; NS) of anti-CD3 and anti-CD28 antibodies. After 72 h, cells were harvested and subjected to qRT-PCR; supernatants were subjected to IL-17A and IL-17F ELISA. A, T lymphocytes from SLE patients express significantly more IL-17A mRNA in response to stimulation with anti-CD3/CD28 antibodies when compared with T lymphocytes from healthy controls (left). T lymphocytes from healthy controls and SLE patients produce comparable amounts of IL-17A protein in response to T cell activation (72 h), whereas resting and activated T lymphocytes from DC patients produce lower amounts of IL-17A (right). B, T lymphocytes from SLE patients express significantly lower amounts of IL-17F mRNA in response to stimulation with anti-CD3/CD28 antibodies when compared with T lymphocytes from healthy controls (left). T lymphocytes from healthy controls and DC patients produce significantly more IL-17F in response to T cell activation, whereas resting and activated T lymphocytes from SLE patients fail to produce IL-17F (right). C, failure to express IL-17F protein results in an increased IL-17A/IL-17F (protein) ratio of SLE T lymphocytes when compared with healthy control and autoimmune disease control T cells. Black bars, IL-17A/IL-17F ratio in unstimulated cells; gray bars, cells after stimulation with anti-CD3/CD28 antibodies for 72 h. Error bars, S.D.
In order to further assess differences in the regulation of IL-17A and IL-17F expression in T lymphocytes from controls, SLE patients, and autoimmune disease control individuals, we calculated IL-17A/IL-17F protein expression ratios of cell culture supernatants in resting total T lymphocytes and in response to T cell activation with anti-CD3/CD28 antibodies (Fig. 1C). Interestingly, the IL-17A/IL-17F ratio of both control and autoimmune disease control T lymphocytes was significantly lower when compared with T lymphocytes from SLE patients (p < 0.001). This reflects “symmetrically” increased IL-17A and IL-17F protein expression from autoimmune disease control T lymphocytes. However, T lymphocytes from SLE patients produce significantly increased amounts of IL-17A but fail to produce IL-17F.
Chromatin Modifications in Conserved Regions of Human IL17F Gene
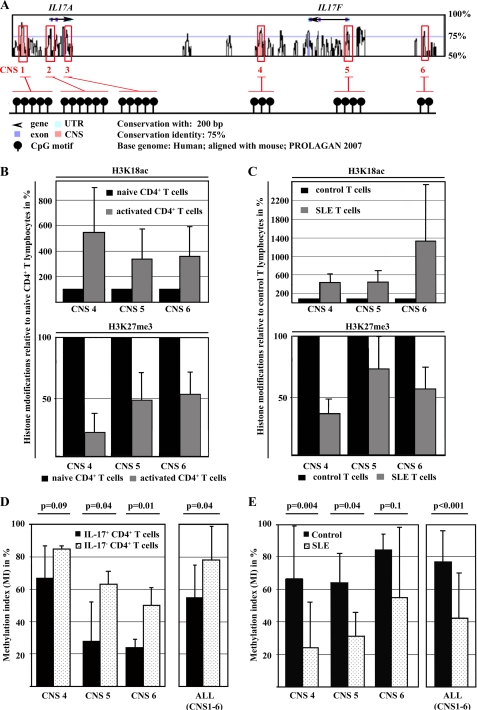
We previously reported that epigenetic modifications of IL2 and IL17 contribute to the dysregulation of these genes in SLE T lymphocytes. Next, we aimed to investigate epigenetic patterns across the IL17F gene in naive and activated CD4+ T lymphocytes from healthy control individuals and total T lymphocytes from SLE patients and controls. Therefore, we defined regions of interest within and around the IL17F gene, based on bioinformatic approaches. We aligned the mouse and human IL17F genes (VISTA Genome Browser, available on the World Wide Web) and determined conserved non-coding sequences (CNS; Fig. 2A). CNS regions were defined as regions with sequence homology of >75% between the human and mouse genes over at least 200 bp. Based on the degree of conservation, we identified three regions of interest, one of which was ∼15 kbp upstream of the IL17F gene (CNS6), one mapping to the proximal promoter (CNS5) that contains a yet unidentified CRE site (−127/−123), and one 3′ of the IL17F gene (CNS4). We performed ChIP analyses in naive and activated CD4+ T cells, using antibodies against activating histone H3 acetylation (H3K18ac) or repressive H3 methylation (H3K27me3). Throughout the analyzed regions, we detected decreased H3K27 trimethylation and enriched H3K18 acetylation following T cell activation (Fig. 2B). Subsequently, we compared these histone modifications in total T cells obtained from SLE patients and matched healthy individuals. T lymphocytes from SLE patients display reduced H3K27 trimethylation and increased H3K18 acetylation when compared with control T lymphocytes (Fig. 2C). Thus, our data suggest a similar pattern in SLE T cells as observed in activated naive CD4+ T cells.
FIGURE 2.
Epigenetic remodeling of the human IL17F gene in response to T cell activation and in SLE T lymphocytes. A, alignment of the human and mouse IL17 gene locus. Conserved CNS (pink) were defined as regions with sequence homology of >75% between humans and mice over a length of at least 200 bp. Exons of the human IL17A and IL17F genes are displayed in purple, conserved non-coding sequences are shown in pink, and untranslated regions (UTR) are shown in turquoise. B, H3K18 acetylation (top) and H3K27 trimethylation (bottom) were analyzed in resting (black bars) and activated (gray bars) naive CD4+ T cells from four healthy individuals by ChIP assays. The ratio of relative histone acetylation/methylation was determined between unstimulated and the activated naive T cells (from the same individual). Black bars represent histone methylation/acetylation in unstimulated cells (set to 100%). Changes in the methylation or acetylation status following T cell activation are given in the bar diagram (mean ± S.D. (error bars)). C, H3K18 acetylation (top) and H3K27 trimethylation (bottom) were analyzed in T lymphocytes from four matched healthy controls (black bars) and four SLE patients (gray bars) by ChIP assays. The ratio of relative histone acetylation/methylation was determined between control and SLE T cells. Black bars represent histone methylation/acetylation in healthy control T cells (set to 100%). D, naive CD4+ T cells from healthy controls that had been sequentially stimulated with anti-CD3/anti-CD28 antibodies for 72 h and with PMA/ionomycin for another 5 h were subjected to an IL-17A secretion assay. ChIP was performed in both IL-17A-enriched T cells (black bars) and non-IL-17A-secreting T cells (dotted bars), using antibodies that specifically bind methyl-CpG-DNA. Methylated DNA was recovered, and CNS regions were amplified by real-time qPCR. Completely methylated (input; 100%) and unmethylated human DNA samples (negative control; 0%) were included. Values are given as mean ± S.D. from six independent experiments. E, total T cells from six matched SLEs (gray bars) and healthy control individuals (CON; black bars) were subjected to CpG-DNA immunoprecipitation. Percentages of methylated DNA are given as mean ± S.D.
CpG-DNA Methylation in Activated T Lymphocytes from SLE Patients and Healthy Controls
Histone hypermethylation is frequently accompanied by concordant CpG-DNA methylation (29–31). Because the investigated CNS regions and the proximal promoter of the human IL17F gene contain CpG-rich regions (Fig. 2A), we investigated CpG-DNA methylation patterns across these regions in resting and activated naive CD4+ T lymphocytes from healthy donors and in total T lymphocytes from SLE patients and matched control individuals.
Because IL-17A and IL-17F are usually co-expressed in T lymphocytes from healthy donors (9), we sorted between IL-17A-secreting and -non-secreting cells, using cytokine capture assays. Therefore, naive CD4+ T cells from healthy blood donors were stimulated with anti-CD3/anti-CD28 antibodies for 72 h, followed by PMA/ionomycin treatment for another 5 h. CD4+ T lymphocytes were enriched for IL-17A-secreting cells, applying antibodies that yielded a purity of ∼30% within the “IL-17A secretor” group (as reported previously (14) and measured by flow cytometry (data not shown)). Cells in the non-IL-17A secretor group did not stain for intracellular IL-17A. CpG-DNA methylation within and around the IL7F gene was significantly decreased among the IL-17A-secreting T cells, suggesting an “open” chromatin conformation that is accessible for transcription regulatory factors (Fig. 2D). Subsequently, we analyzed CpG-DNA methylation of the human IL17F gene in total T lymphocytes from six age-, gender-, and ethnicity-matched SLE and control individuals (Fig. 2E). Total T cells were isolated and activated by subsequent CD3/CD28 and PMA/ionomycin stimulation as aforementioned. Throughout the analyzed regions, SLE T cells displayed lower degrees of CpG-DNA methylation in response to T cell activation as compared with control T lymphocytes. These findings suggest that IL-17F production in human T lymphocytes can be regulated by CpG-DNA methylation and that T cell activation in SLE T lymphocytes results in remodeling of the IL17 locus and increases the accessibility for trans-regulatory factors. However, this does not explain why IL-17F expression is reduced in SLE T lymphocytes.
Forced CREMα Expression Suppresses IL-17F Expression in Primary Human T Lymphocytes
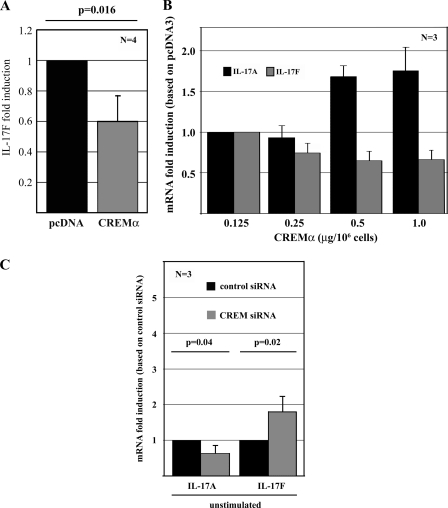
SLE T cells display reduced IL-17F production and elevated cellular CREMα protein levels (Fig. 1A) (21). Increased CREMα expression has been demonstrated to contribute to decreased IL-2 and increased IL-17A production in T lymphocytes from SLE patients. Thus, we considered that CREMα may also be involved in IL17F gene regulation. Primary human T cells from healthy individuals display low levels of CREMα. In order to mimic the conditions in SLE T lymphocytes, we transiently transfected primary human T cells (from healthy blood donors) with a His6-tagged CREMα expression plasmid (as previously reported in Ref. 14). mRNA obtained from these cells was tested for relative IL-17F expression and proved to express significantly reduced IL-17F transcript numbers after forced CREMα expression as assessed by real-time quantitative RT-PCR (Fig. 3B; relative change of 0.6; p = 0.016).
FIGURE 3.
CREMα down-regulates IL-17F expression. A, pcDNA3 or CREMα expression plasmid were transfected into primary human T cells and 5 h after transfection, RNA was analyzed for IL-17A and 18 S rRNA expression, using qRT-PCR. Experiments were performed in T cells from four different healthy blood donors. The bar diagram displays the mean relative IL-17F expression (after CREMα overexpression) ± S.D. (error bars) from four experiments. B, different concentrations (as indicated) of pcDNA3 or CREMα expression plasmid were transfected into Jurkat T cells. Five hours after transfection, mRNA was analyzed for IL-17A and 18 S rRNA expression, using qRT-PCR. The bar diagrams display the mean relative IL-17A (black bars) and IL-17F (gray bars) expression as induced by CREMα overexpression ± S.D. from three experiments. C, Jurkat T cells were transfected with 5 nm control and CREMα siRNA. Five hours after transfection, mRNA was analyzed for IL-17A and 18 S rRNA expression, using qRT-PCR. Cytokine expression levels in response to transfection with control siRNA were determined as a relative value of 1. Bar diagrams display the mean relative IL-17A and IL-17F expression as induced by CREMα knockdown ± S.D. from three unrelated experiments.
In order to assess dose-dependent effects of CREMα on IL-17A and IL-17F expression, we transfected Jurkat T cells with increasing amounts of CREMα expression plasmids. We detected a dose-dependent increase in IL-17A mRNA expression, whereas IL-17F mRNA expression was subject to transcriptional repression (Fig. 3C). This reflects the characteristics of T lymphocytes from SLE patients that show elevated cellular levels of CREMα, resulting in overexpression of IL-17A and failure to express IL-17F.
CREMα Knockdown with CREM siRNA Suppresses IL-17A and Enhances IL-17F mRNA Expression
To further establish the involvement of CREMα in the regulation of IL-17A and IL-17F, we transfected Jurkat T cells with unrelated control siRNA and siRNA directed against CREMα. mRNA obtained from transfected cells was tested for relative IL-17A and IL-17F expression and proved to express significantly reduced IL-17A (p = 0.04) and increased IL-17F (p = 0.02) transcript numbers (Fig. 3C). This provides further prove of the involvement of CREMα in the regulation of IL-17A and IL-17F.
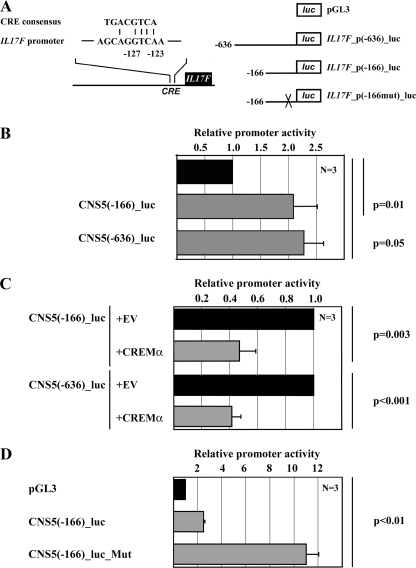
CREMα Negatively Regulates IL17F Promoter Activity
A bioinformatic analysis of the human IL17F promoter revealed a putative CRE site located between 127 and 123 bp upstream of the transcription initiation site, thus denoted the −127/−123 CRE site (Fig. 4A). This site defines a 4-bp sequence that matches the perfect CRE 3′-half-site (GTCA), which has been shown to serve as a minimum binding motif for CREM proteins in other cis-regulatory target sequences (14, 21, 27). To investigate whether CREMα regulates IL-17F expression at the transcriptional level, we performed reporter studies, using promoter constructs spanning the proximal 636 or 166 bp of the human IL17F promoter (Fig. 4B). Indeed, CREMα overexpression resulted in significantly down-regulation of IL17F promoter activities in both constructs (-fold change of 0.47 in IL17F_p(−166)_luc, p = 0.003, and 0.41 in IL17F_p(−636)_luc, p < 0.001) (Fig. 4C). Next, we mutated the −127/−122 CRE site within the 166 bp-spanning IL17F reporter construct and noted a significantly increased activity of the IL17F promoter construct (-fold change of 10.99, p < 0.001) (Fig. 4D). These results suggest that this site is crucial for CREMα-mediated down-regulation of IL17F transcription in human T lymphocytes.
FIGURE 4.
CREMα binding reduces IL17F promoter activity. A, alignment of the CRE consensus sequence with the CRE site (−127/−123) of the proximal human IL17F promoter. The schematic on the right displays IL17F reporter plasmids used for luciferase assays (636 and 166 bp). IL17F_p(−166mut)_luc indicates a reporter plasmid containing a site-directed mutation at the CRE site (−127/−123). B, human Jurkat T cells were transfected with the empty pGL3-Basic reporter (black bar) or the IL17F promoter-driven reporter plasmids (gray bars). Cells were collected 5 h after transfection, and firefly luciferase activity was measured and normalized by Renilla luciferase activity. n = 3 independent experiments. C, human Jurkat T cells were co-transfected with the indicated reporter constructs and either pcDNA3 empty vector (EV; black bars) or CREMα expression plasmid (gray bars). For each reporter, pcDNA3 empty vector (EV) co-transfection was set to 1.0, and the relative effect mediated by CREMα was calculated. Each experiment was performed three times, and values are given as mean ± S.D. D, mutation of the CRE site (−127/−123) results in a significantly increased spontaneous promoter activity. n = 3 independent experiments.
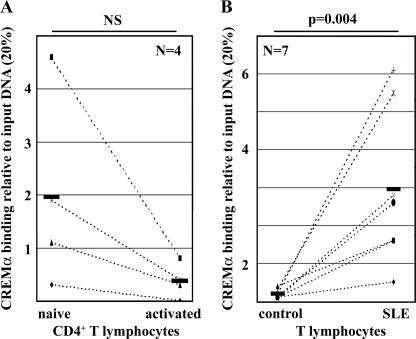
CREMα Binding to the −127/−123 CRE Site Is Increased in Activated Naive CD4+ T Cells and T Lymphocytes from SLE Patients
We addressed the in vivo relevance of CREMα binding to the −127/−123 CRE site in the human IL17F promoter by ChIP assays, using a CREMα-specific polyclonal antibody. We used naive CD4+ T cells that were freshly isolated from total human T lymphocytes and compared them with naive CD4+ T cells that were stimulated with anti-CD3 and anti-CD28 antibodies for 72 h (Fig. 5, A and B). We observed a trend toward reduced CREMα binding to this site in activated naive T lymphocytes after individual comparison with CREMα binding in unstimulated naive CD4+ T cells (-fold increase of 0.05, not significant (NS); Fig. 5A). Because T lymphocytes from SLE patients display signaling abnormalities resembling an activated phenotype, we aimed to determine CREMα recruitment to the IL17F promoter in total T lymphocytes from seven SLE patients and seven healthy control individuals that were individually matched by age, gender, and ethnicity (Fig. 5B). In agreement with the reduced expression of IL-17F in SLE T lymphocytes and reduced IL-17F expression in response to forced CREMα overexpression, we determined significantly increased CREMα binding to this site in SLE T lymphocytes (-fold increase of 9.28, p = 0.004). Our data suggest that increased CREMα binding to the IL17F promoter in SLE T cells results in potent transcriptional repression of IL17F gene expression.
FIGURE 5.
CREMα binds to the −127/−123 CRE site within the proximal IL17F promoter. A, naive human CD4+ T lymphocytes were isolated from four healthy individuals and cultured in the absence or presence of anti-CD3/anti-CD28 antibodies for 72 h. ChIP assays were performed in order to detect CREMα recruitment to the −127/−123 CRE site. Immunoprecipitated DNA was analyzed by real-time qPCR, amplifying a region that covers the CRE site within the proximal IL17F promoter. Ratios between anti-CREMα-immunoprecipitated and input DNA are shown. Dotted lines associate data between unstimulated and corresponding activated cells obtained from the same individual. Black bars represent the mean of the four experiments. B, CREMα ChIP was performed with total T lymphocytes from seven individually matched pairs of SLE patients and healthy controls. Immunoprecipitated DNA was analyzed by real-time qPCR. Ratios between anti-CREMα-immunoprecipitated and input DNA are shown. Dotted lines associate data between matched control/SLE pairs. Black bars, mean values. NS, not significant.
DISCUSSION
Here we present evidence that the transcription regulatory factor CREMα mediates reduced IL-17F expression in T lymphocytes from SLE patients, regardless of an “open” chromatin conformation of the IL17 locus. CREMα levels were documented to be increased in T lymphocytes from SLE patients (21, 32). This is mediated by increased mRNA expression of the CREM gene in a disease activity-dependent manner (20, 21, 32). We previously documented that overexpression of CREMα in human T lymphocytes results in increased IL-17A expression, which is mediated by direct transcriptional effects (14).
IL-17 cytokines have been documented to be key players in adaptive immune responses (2). The namesake cytokine IL-17A was first discovered as “cytotoxic T lymphocyte-associated antigen 8” but has been documented to be expressed by multiple cells, including immune cells, fibroblasts, and synoviocytes (2, 3, 15). Over the past decade, the IL-17 cytokine family has been expanded by five new members, IL-17B through IL-17F, with IL-17A and IL-17F being the most closely related and well studied (2). Both cytokines, IL-17A and IL-17F, are co-expressed by specialized T helper subsets, which are referred to as Th17 cells, and exhibit similar expression patterns (9). To this point, it remained unclear whether IL-17A and IL-17F underlie similar, overlapping, or divergent expression profiles in autoimmune disorders. IL-17A and IL-17F have been reported to induce proinflammatory responses through chemokine and cytokine induction as well as the recruitment of proinflammatory immune cells to the site of inflammation (2, 33). Both cytokines are involved in the pathogenesis of several autoimmune diseases, including rheumatoid arthritis (15), psoriasis (16), and multiple sclerosis (17, 18). In rheumatoid arthritis, IL-17A and IL-17F exert proinflammatory functions through Stat3, NF-κB, MAPK pathways, and CCAAT/enhancer-binding protein transcription factors. Thereby, IL-17A and IL-17F enhance innate immune responses that mediate inflammation.
Imbalances between pro- and anti-inflammatory cytokines that result from a disrupted transcriptional network are hallmarks of the pathogenesis of various autoimmune diseases, including SLE (34). We previously documented that SLE patients display increased IL-17A levels and that IL-17A-producing T lymphocytes infiltrate target organs, including the kidneys (12, 13). Recent reports from our group suggest antithetic effects of the transcription factor CREMα on IL2 and IL17A gene expression at both the transcriptional and epigenetic level (14, 20). Interestingly, CREMα recruitment is increased to a comparable extent at CRE sites of the IL2, IL17A, and IL17F promoter (∼4–6-fold). However, the functional downstream effects of increased CREMα binding on gene expression of these cytokines are diametric. CREMα contributes to increased IL-17A expression through 1) direct trans-activating effects on the IL17A promoter and 2) associated activating epigenetic modifications, including increased H3K18 acetylation, reduced H3K27 trimethylation, and reduced CpG-DNA methylation of the IL17A gene in SLE T cells (14). IL2 undergoes 1) direct transcriptional repression at the −180 CRE element of the IL2 promoter, 2) HDAC1 recruitment to the same site, and 3) CpG-DNA methylation because CREMα recruits DNMT3a to the IL2 −180 CRE element (24, 35–37). Furthermore, reduced expression of the transcription factor AP-1 (c-Fos) results in impaired IL-2 expression (25).
Activated CD4+ T lymphocytes from healthy donors exhibit chromatin modifications that reflect an “open” chromatin conformation, because it is accessible for transcription regulatory factors, and express IL-17F. In T lymphocytes from SLE patients that fail to produce IL-17F, CREMα binds to the IL17F promoter and induces direct transcriptional repression at the −127/−123 CRE element. This results in reduced IL-17F expression that is independent of the aforementioned “open” chromatin conformation of the entire IL17 locus. Whether our observations have the potential to be used as biomarkers for disease activity or disease outcome remains to be elucidated in a larger cohort. All patients included in our studies had moderate to high disease activity, and the pharmacological treatment of the single patients was rather heterogeneous. Because environmental factors, including medication, have been reported to impact epigenetic patterns, this could be a limitation of this study. However, epigenetic patterns and CREMα recruitment to the IL17F promoter was comparable in all investigated individuals. Further studies in larger cohorts are warranted in order to investigate the influence of 1) disease activity, 2) disease progression, and 3) pharmacological treatment on epigenetic marks and cytokine expression in T lymphocytes from SLE patients.
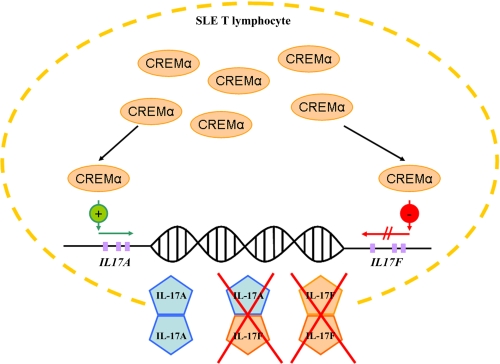
Our findings may well be significant in the pathophysiology of SLE because reduced IL-17F expression in SLE T lymphocytes may result in reduced assembly of IL-17A/F heterodimers. It has been documented that IL-17F homodimers and IL-17A/F heterodimers exert reduced proinflammatory capacities when compared with IL-17A homodimers (2, 4–7). In concert with increased IL-17A expression in SLE T lymphocytes, reduced IL-17F expression contributes to a disrupted IL-17A/IL-17F ratio toward IL-17A (Fig. 6). It appears likely that this imbalance orchestrates the inflammatory phenotype of SLE and is responsible for at least some of the differences in organ-specific symptoms between SLE and other (less severe) autoimmune disorders. Our observation that forced expression of CREMα mediates diametric effects on the expression of IL-17A and IL-17F, mimicking the phenotype of SLE T lymphocytes, and that this can be reversed by the application of CREM-specific siRNA stresses the involvement of CREMα in the pathophysiology of SLE and its role as a putative therapeutic target.
FIGURE 6.
CREMα effects on IL-17A/IL-17F expression in SLE T lymphocytes. Overexpression of CREMα in T lymphocytes from SLE patients results in increased CREMα recruitment to both IL17A and IL17F promoter. As we reported previously (14), CREMα binding results in increased expression of IL-17A in SLE T cells. CREMα recruitment to the IL17F promoter mediates reduced IL-17F expression. These diametric mechanisms result in an “SLE-characteristic” imbalance between IL-17A and IL-17F levels and probably impaired assembly of IL-17A/IL-17F heterodimers, which have reduced proinflammatory activity as compared with IL-17A homodimers. This mechanism may have severe implications for the inflammatory cascades in SLE disease expression.
Acknowledgment
We thank Melissa Zajdel for skilled technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants K23 AR055672 (to V. C. K.) and R01 AI42269, R01 AI49954, and R01 AI85567 (to G. C. T.). This work was also supported by Deutsche Forschungsgemeinschaft Grant RA1927-1/1 (to T. R.).
- SLE
- systemic lupus erythematosus; cAMP-responsive element modulator α
- CRE
- cAMP-responsive element
- H3K18
- histone H3 Lys-18
- H3K27
- histone H3 Lys-27
- qPCR
- quantitative PCR
- qRT-PCR
- quantitative RT-PCR
- CNS
- conserved non-coding sequence(s).
REFERENCES
- 1. Zhang X., Angkasekwinai P., Dong C., Tang H. (2011) Structure and function of interleukin-17 family cytokines. Protein Cell 2, 26–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pappu R., Ramirez-Carrozzi V., Sambandam A. (2011) The interleukin-17 cytokine family. Critical players in host defense and inflammatory diseases. Immunology 134, 8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rouvier E., Luciani M. F., Mattéi M. G., Denizot F., Golstein P. (1993) CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences and homologous to a herpesvirus saimiri gene. J. Immunol. 150, 5445–5456 [PubMed] [Google Scholar]
- 4. Liang S. C., Long A. J., Bennett F., Whitters M. J., Karim R., Collins M., Goldman S. J., Dunussi-Joannopoulos K., Williams C. M., Wright J. F., Fouser L. A. (2007) An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 179, 7791–7799 [DOI] [PubMed] [Google Scholar]
- 5. Wright J. F., Guo Y., Quazi A., Luxenberg D. P., Bennett F., Ross J. F., Qiu Y., Whitters M. J., Tomkinson K. N., Dunussi-Joannopoulos K., Carreno B. M., Collins M., Wolfman N. M. (2007) Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 282, 13447–13455 [DOI] [PubMed] [Google Scholar]
- 6. Wright J. F., Bennett F., Li B., Brooks J., Luxenberg D. P., Whitters M. J., Tomkinson K. N., Fitz L. J., Wolfman N. M., Collins M., Dunussi-Joannopoulos K., Chatterjee-Kishore M., Carreno B. M. (2008) The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J. Immunol. 181, 2799–2805 [DOI] [PubMed] [Google Scholar]
- 7. Chang S. H., Dong C. (2007) A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 17, 435–440 [DOI] [PubMed] [Google Scholar]
- 8. Hu Y., Shen F., Crellin N. K., Ouyang W. (2011) The IL-17 pathway as a major therapeutic target in autoimmune diseases. Ann. N.Y. Acad. Sci. 1217, 60–76 [DOI] [PubMed] [Google Scholar]
- 9. Yang X. O., Nurieva R., Martinez G. J., Kang H. S., Chung Y., Pappu B. P., Shah B., Chang S. H., Schluns K. S., Watowich S. S., Feng X. H., Jetten A. M., Dong C. (2008) Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29, 44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ouyang W., Kolls J. K., Zheng Y. (2008) The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crispín J. C., Tsokos G. C. (2010) IL-17 in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 943254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crispín J. C., Tsokos G. C. (2009) Human TCR-α β+ CD4− CD8− T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. J. Immunol. 183, 4675–4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crispín J. C., Oukka M., Bayliss G., Cohen R. A., Van Beek C. A., Stillman I. E., Kyttaris V. C., Juang Y. T., Tsokos G. C. (2008) Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 181, 8761–8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rauen T., Hedrich C. M., Juang Y. T., Tenbrock K., Tsokos G. C. (2011) cAMP-responsive element modulator (CREM)α protein induces interleukin 17A expression and mediates epigenetic alterations at the interleukin-17A gene locus in patients with systemic lupus erythematosus. J. Biol. Chem. 286, 43437–43446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hot A., Miossec P. (2011) Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann. Rheum. Dis. 70, 727–732 [DOI] [PubMed] [Google Scholar]
- 16. Tokura Y., Mori T., Hino R. (2010) Psoriasis and other Th17-mediated skin diseases. J. UOEH 32, 317–328 [DOI] [PubMed] [Google Scholar]
- 17. Zepp J., Wu L., Li X. (2011) IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol. 32, 232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fletcher J. M., Lalor S. J., Sweeney C. M., Tubridy N., Mills K. H. (2010) T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 162, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foulkes N. S., Borrelli E., Sassone-Corsi P. (1991) CREM gene. Use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell 64, 739–749 [DOI] [PubMed] [Google Scholar]
- 20. Rauen T., Benedyk K., Juang Y. T., Kerkhoff C., Kyttaris V. C., Roth J., Tsokos G. C., Tenbrock K. (2011) A novel intronic cAMP response element modulator (CREM) promoter is regulated by activator protein-1 (AP-1) and accounts for altered activation-induced CREM expression in T cells from patients with systemic lupus erythematosus. J. Biol. Chem. 286, 32366–32372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solomou E. E., Juang Y. T., Gourley M. F., Kammer G. M., Tsokos G. C. (2001) Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J. Immunol. 166, 4216–4222 [DOI] [PubMed] [Google Scholar]
- 22. Juang Y. T., Rauen T., Wang Y., Ichinose K., Benedyk K., Tenbrock K., Tsokos G. C. (2011) Transcriptional activation of the cAMP-responsive modulator promoter in human T cells is regulated by protein phosphatase 2A-mediated dephosphorylation of SP-1 and reflects disease activity in patients with systemic lupus erythematosus. J. Biol. Chem. 286, 1795–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Juang Y. T., Wang Y., Solomou E. E., Li Y., Mawrin C., Tenbrock K., Kyttaris V. C., Tsokos G. C. (2005) Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J. Clin. Invest. 115, 996–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hedrich C. M., Rauen T., Tsokos G. C. (2011) cAMP-responsive Element Modulator (CREM)α Protein Signaling Mediates Epigenetic Remodeling of the Human Interleukin-2 Gene. Implications in systemic lupus erythematosus. J. Biol. Chem. 286, 43429–43436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kyttaris V. C., Juang Y. T., Tenbrock K., Weinstein A., Tsokos G. C. (2004) Cyclic adenosine 5′-monophosphate response element modulator is responsible for the decreased expression of c-fos and activator protein-1 binding in T cells from patients with systemic lupus erythematosus. J. Immunol. 173, 3557–3563 [DOI] [PubMed] [Google Scholar]
- 26. Tenbrock K., Kyttaris V. C., Ahlmann M., Ehrchen J. M., Tolnay M., Melkonyan H., Mawrin C., Roth J., Sorg C., Juang Y. T., Tsokos G. C. (2005) The cyclic AMP response element modulator regulates transcription of the TCR ζ-chain. J. Immunol. 175, 5975–5980 [DOI] [PubMed] [Google Scholar]
- 27. Ahlmann M., Varga G., Sturm K., Lippe R., Benedyk K., Viemann D., Scholzen T., Ehrchen J., Müller F. U., Seidl M., Matus M., Tsokos G. C., Roth J., Tenbrock K. (2009) The cyclic AMP response element modulator α suppresses CD86 expression and APC function. J. Immunol. 182, 4167–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bailey J., Tyson-Capper A. J., Gilmore K., Robson S. C., Europe-Finner G. N. (2005) Identification of human myometrial target genes of the cAMP pathway. The role of cAMP-response element binding (CREB) and modulator (CREMα and CREMτ2α) proteins. J. Mol. Endocrinol. 34, 1–17 [DOI] [PubMed] [Google Scholar]
- 29. Hedrich C. M., Tsokos G. C. (2011) Epigenetic mechanisms in systemic lupus erythematosus and other autoimmune diseases. Trends Mol. Med. 17, 714–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brenner C., Fuks F. (2007) A methylation rendezvous. Reader meets writers. Dev. Cell 12, 843–844 [DOI] [PubMed] [Google Scholar]
- 31. Brenner C., Fuks F. (2006) DNA methyltransferases. Facts, clues, mysteries. Curr. Top. Microbiol. Immunol. 301, 45–66 [DOI] [PubMed] [Google Scholar]
- 32. Kyttaris V. C., Wang Y., Juang Y. T., Weinstein A., Tsokos G. C. (2006) CAMP-response element modulator a expression in patients with systemic lupus erythematosus. Lupus 15, 840–844 [DOI] [PubMed] [Google Scholar]
- 33. Iwakura Y., Nakae S., Saijo S., Ishigame H. (2008) The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol. Rev. 226, 57–79 [DOI] [PubMed] [Google Scholar]
- 34. Crispín J. C., Kyttaris V. C., Juang Y. T., Tsokos G. C. (2008) How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype. Trends Immunol. 29, 110–115 [DOI] [PubMed] [Google Scholar]
- 35. Tenbrock K., Juang Y. T., Gourley M. F., Nambiar M. P., Tsokos G. C. (2002) Antisense cyclic adenosine 5′-monophosphate response element modulator up-regulates IL-2 in T cells from patients with systemic lupus erythematosus. J. Immunol. 169, 4147–4152 [DOI] [PubMed] [Google Scholar]
- 36. Tenbrock K., Juang Y. T., Tolnay M., Tsokos G. C. (2003) The cyclic adenosine 5′-monophosphate response element modulator suppresses IL-2 production in stimulated T cells by a chromatin-dependent mechanism. J. Immunol. 170, 2971–2976 [DOI] [PubMed] [Google Scholar]
- 37. Tenbrock K., Juang Y. T., Leukert N., Roth J., Tsokos G. C. (2006) The transcriptional repressor cAMP response element modulator α interacts with histone deacetylase 1 to repress promoter activity. J. Immunol. 177, 6159–6164 [DOI] [PubMed] [Google Scholar]