Background: RhoA GTPase is essential for integrin αMβ2-mediated phagocytosis.
Results: Activation of Rap1 GTPase can induce phagocytosis even when RhoA is inactivated.
Conclusion: Rap1 GTPase can replace the function of RhoA GTPase in phagocytosis.
Significance: This might be the first observation that Rap1 and RhoA GTPases collectively regulate phagocytosis in macrophages.
Keywords: Actin, GTPase, Integrin, Macrophages, Phagocytosis, Reactive Oxygen Species (ROS), Rho, Rap1, Profilin
Abstract
Phagocytosis occurs primarily through two main processes in macrophages: the Fcγ receptor- and the integrin αMβ2-mediated processes. Complement C3bi-opsonized particles are known to be engulfed through integrin αMβ2-mediated process, which is regulated by RhoA GTPase. C3 toxin fused with Tat-peptide (Tat-C3 toxin), an inhibitor of the Rho GTPases, was shown to markedly inhibit the phagocytosis of serum (C3bi)-opsonized zymosans (SOZs). However, 8CPT-2Me-cAMP, an activator of exchange protein directly activated by cAMP (Epac, Rap1 guanine nucleotide exchange factor), restored the phagocytosis of the SOZs that was previously inhibited by the Tat-C3 toxin. In addition, a constitutively active form of Rap1 GTPase (CA-Rap1) also restored the phagocytosis that was previously reduced by a dominant negative form of RhoA GTPase (DN-RhoA). This suggests that Rap1 can replace the function of RhoA in the phagocytosis. Inversely, CA-RhoA rescued the phagocytosis that was suppressed by DN-Rap1. These findings suggest that both RhoA and Rap1 GTPases collectively regulate the phagocytosis of SOZs. In addition, filamentous actin was reduced by the Tat-C3 toxin, which was again restored by 8CPT-2Me-cAMP. Small interfering profilin suppressed the phagocytosis, suggesting that profilin is essential for the phagocytosis of SOZs. Furthermore, 8CPT-2Me-cAMP increased the co-immunoprecipitation of profilin with Rap1, whereas Tat-C3 toxin decreased that of profilin with RhoA. Co-immunoprecipitations of profilin with actin, Rap1, and RhoA GTPases were augmented in the presence of GTPγS rather than GDP. Therefore, we propose that both Rap1 and RhoA GTPases regulate the formation of filamentous actin through the interaction between actin and profilin, thereby collectively inducing the phagocytosis of SOZs in macrophages.
Introduction
Phagocytosis is a primary defense mechanism against infectious pathogens, and it facilitates the clearance of cells that need to be discarded, including aged red blood cells. Phagocytosis is initiated by the interaction between ligands and specific receptors on the surface of specialized phagocytes, such as macrophages and neutrophils (1). Among the variety of receptors for phagocytosis, Fcγ receptors (FcγRs),2 which recognize immunoglobulin (IgG)-opsonized particles, and complement receptor 3 (CR3; also referred to as integrin αMβ2, Mac-1, and CD11b/CD18), which recognizes complement (C3bi)-opsonized particles, have been extensively studied (2). The assembly and disassembly of actin are essential for a variety of cellular processes including phagocytosis (3). In particular, phagocytosis through CR3 is correlated with actin dynamics, including an accumulation of Arp2/3 (4). In addition, interaction of talin to CR3 is required for CR3 activation and phagocytosis (5). On the other hand, CR3 activates the small GTPase, RhoA, whereas FcγR activates Cdc42 and Rac1 GTPases (6–8).
The Rho GTPase subfamily of proteins, which belong to the Ras superfamily, regulates a variety of cellular functions, including gene expression, dynamic actin organization, and processes that govern cellular morphology, adhesion, motility, and membrane trafficking (9). Rho GTPases can be converted to an active, GTP-bound form by guanine nucleotide exchange factors (GEFs), whereas they are converted to an inactive, GDP-bound form by GTPase-activating proteins (GAPs). Active GTP-Rho is able to bind to effector proteins, which transmit signals to downstream pathways (9). Among effector proteins for RhoA, Rho-associated coiled-coil containing protein kinase (ROCK) inactivates a myosin light chain phosphatase by phosphorylation and leads to the actin filament cross-linking activity of myosin II (10). In addition, ROCK activates LIM kinase (LIMK) by phosphorylation, which, in turn inactivates cofilin by phosphorylation (11). Another effector protein for RhoA is mDiaphanous (mDia), which participates in a diverse set of actin-remodeling events, such as RhoA regulation of CR3-mediated phagocytosis (12). However, the precise mechanism by which RhoA regulates the formation of actin-rich foci during CR3-mediated phagocytosis is currently unknown (2).
Rap1 GTPase, which is also a member of the Ras GTPase family, induces integrin αLβ2 (LFA-1)-mediated adhesion to intercellular adhesion molecule (ICAM) in Jurkat cells (13). This indicates that Rap1 regulates integrin activities (14). In contrast, β2-integrin activates Rap1 and Rap2, which, in turn, promotes cell adhesion (15). In addition to the role of Rap1 in regulating integrin, Rap1 also regulates cytoskeletal dynamics (16). Furthermore, Rap1 regulates complement-mediated phagocytosis, which requires the activation of integrin αMβ2 (17), and Rap1 activation is also required for FcγR-dependent phagocytosis (18). Although Rap1 is thought to be related to cytoskeletal regulation, there is not enough evidence to understand the mechanism by which Rap1 regulates integrin αMβ2-mediated and FcγR-mediated phagocytoses.
We observed that the inhibition of RhoA GTPase suppressed the phagocytosis of serum-opsonized zymosan (SOZ) particles. Nevertheless, the activation of Rap1 GTPase attenuated this suppression, suggesting that Rap1 could assume the role of RhoA in the phagocytosis of SOZs in macrophages. In the phagocytic processes, signaling pathways that are regulated by Rap1 and RhoA GTPases may converge onto actin filament remodeling, and profilin may be a common mediator protein in these processes. Therefore, we proposed that the involvement of profilin is collectively regulated by RhoA and Rap1 GTPases in the phagocytosis of SOZs.
EXPERIMENTAL PROCEDURES
Materials
The following were purchased from Sigma: phosphate-buffered saline solution (PBS), lysophosphatidic acid (1-acyl-2-hydroxy-sn-glycero-3-phosphate; LPA), 8-(4-chloro-phenylthio)-2′-O-methyladenosine-3′-5′-cyclic monophosphate (8CPT-2Me-cAMP), zymosan A, fluorescein isothiocyanate (FITC), phenylmethylsulfonyl fluoride (PMSF), 5-amino-2,3-dihydro-1,4-phthalazinesione (Luminol), Nonidet-40, Triton X-100, and crystal violet. Tween 20 was purchased from USB Corp. Lipofectamine 2000 was purchased from Invitrogen, and protein A-Sepharose beads and glutathione (GSH)-Sepharose beads were purchased from Amersham Biosciences. Alexa Fluor 488-conjugated phalloidin was purchased from Molecular Probes. Methanol-free 16% formaldehyde was purchased from Pierce. Anti-RhoA, anti-profilin, anti-Rap1, anti-cofilin, anti-actin, anti-myosin phosphatase-1 (MYPT-1), anti-hemagglutinin (HA), and anti-Myc antibodies, and profilin-1 and profilin-2 siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-cofilin antibody was purchased from Cell Signaling (Beverly, MA). His-bound nitrilotriacetic acid-agarose (Ni2+-bead) was purchased from Novagen (Madison, WI). CN01, RhoA activator was purchased from Cytoskeleton Inc. (Denver, CO). We constructed the GST-Rho binding domain (RBD) of Rhotekin (GST-Rhotekin-RBD) plasmid. HA-RhoA, Myc-RhoA and HA-Rap1 cDNAs in pCDNA3.1 plasmid were purchased from Missouri S&T cDNA Resource Center. HA-p190 RhoGAP in Rc plasmid containing CMV promotor (Invitrogen) was obtained from Dr. Settleman at Harvard University. Plasmid of His-Rap binding domain of RalGDS (RalGDS-RapBD) was provided from Dr. Bos at the University Medical Center Utrecht. Recombinant C3 toxin fused with Tat-peptide, cell-penetrating peptide (Tat-C3 toxin) was purified from Escherichia coli (19).
Cell Cultures
Mouse macrophage Raw264.7 cell lines were purchased from the Korean Cell Line Bank (Seoul, Korea) and cultured with Dulbecco's modified Eagle's/F-12 medium (DMEM-F12) (Invitrogen) supplemented with 5% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin (Lonza; Wakersville, MD).
FlTC-conjugated Zymosan Preparation and Serum Opsonization
FITC-conjugated SOZs (F-SOZs) were prepared as follows. First, 10 mg of FITC was dissolved in 0.1 ml of dimethyl sulfoxide, which was then added to 0.9 ml of conjugation buffer (0.25 m sodium carbonate and 0.1 m sodium chloride, pH 9.0) and filtered using a filter with a 0.2-μm diameter (Sartorius Stedim Biotech). Zymosan (40 mg) was washed three times with 1 ml of PBS and then resuspended in 1 ml of PBS. The FITC solution (0.2 ml) was combined with 20 mg of zymosan in 0.7 ml of PBS in a microcentrifuge tube, which was wrapped in foil to protect the solution from light and incubated at 4 °C overnight with rotation. The F-SOZs were washed with PBS at least 10 times to remove unconjugated FITC completely. Finally, FITC-conjugated and unconjugated zymosan particles (20 mg) were opsonized with non-heat-inactivated serum for 60 min at 37 °C to coat the zymosan particles with C3bi, which were referred as SOZ particles; these SOZ particles were regarded as C3bi-opsonized zymosans. SOZ particles were washed twice with PBS, resuspended in PBS, divided into aliquots, and stored at −20 °C (19).
Phagocytosis Assay
Raw264.7 cells were cultured to 60% confluence and then incubated in antibiotics-containing DMEM-F12 media without serum for at least 10 h to abolish any signals from serum stimuli, such as growth factors. F-SOZ particles (4 × 105) were added to macrophages (2 × 104) that were cultured in 6-well culture plates, and the plates were incubated at 37 °C for 30 min for the phagocytosis of SOZs. Unbound particles were removed by washing with 1× PBS, and macrophages were detached from the 6-well plates and resuspended in 2 ml of PBS. The phagocytosis was stopped by incubating the tubes on ice. The total fluorescence of the FITC engulfed by the cells was measured by a fluorescence spectrophotometer (Kontron SFM25; München, Germany) by excitation at 490 nm and emission at 520 nm. Phagocytosis was evaluated based on the fluorescence in the presence of 10 μm crystal violet because the fluorescence of F-SOZs bound to the surface of cells, but not that of internalized F-SOZs, is quenched by the addition of crystal violet (19).
Binding of SOZ Particles to Cells
F-SOZ particles (2 × 106) were applied for 30 min on ice for particles to bind to Raw264.7 cells (2 × 105), and then unbound FITC-SOZ particles were washed out three times with cold PBS. Total fluorescence of SOZ particles bound to cells after washing was measured by a fluorescence spectrophotometer (Kontron, SFM25). At this step, crystal violet (10 μm) could completely quench the fluorescence of FITC, demonstrating that phagocytosis was rarely undergone because of low temperature.
Transient Transfections
For the DNA transfection experiment, 2–5 × 106 cells at 60–90% confluence in a 60-mm plate that contained 2 ml of DMEM-F12 or a 6-well plate that contained 1 ml of DMEM-F12 were combined with 1 ml of transfecting DNA solution, which consisted of 0.5 ml of DMEM-F12 containing 8–10 μl of Lipofectamine 2000 and 0.5 ml of DMEM-F12 containing 4 μg of DNA and incubated for 4–6 h. The cells were washed and incubated for an additional 16–18 h in DMEM-F12 containing 5% FBS to allow maximal expression of DNA. For siRNA transfection, 2 × 104 cells in 500 μl of DMEM-F12 media were mixed with 200 μl of DMEM-F12 media containing 10 nmol of siRNA and 5 μl of Hiperfect transfection reagent, incubated for 20 min, and then incubated in DMEM-F12 media containing 5% FBS for 72 h.
Western Blotting
Cells were lysed in ice-cold lysis buffer containing 0.5% Nonidet P-40 (v/v) in 20 mm Tris-HCl, pH 8.0, 50 mm NaCl, and protease inhibitors (1 μg/ml each aprotinin, pepstatin, and leupeptin, 1 mm PMSF, 1 mm Na4VO3, and 10 mm NaF). Lysates were incubated for 30 min on ice prior to centrifugation at 13,000 × g for 15 min at 4 °C. Proteins in the supernatant were denatured by boiling for 15 min in Laemmli sample buffer and separated on SDS-PAGE. The proteins in the gel were transferred to PVDF membranes (Millipore), and the membranes were blocked overnight at 4 °C in a 10% solution of nonfat dried milk in Tris-buffered saline solution (TBS: 10 mm Tris-HCl, pH 7.4, and 150 mm NaCl) containing 0.5% Tween 20 (TBST). The membranes were incubated for 60 min at room temperature with primary antibodies that were diluted 1:500, and they were then washed three times with TBST. The membranes were incubated with horseradish peroxidase-conjugated anti-IgG antibody (1:1000) for 60 min and washed three times with TBST. The specific proteins were detected using enhanced chemiluminescence (ECL) reagents (Amersham Biosciences).
Immunoprecipitation
For immunoprecipitation experiments, 1 μg of anti-profilin antibody or control IgG was added to 350 μg of cell lysate protein for 1 h at 4 °C, and then 30–40 μl of protein A-Sepharose (50% slurry solution) was added for an additional 2–4 h under constant rocking. Protein concentrations of the samples were quantified using the BCA assay (Pierce). Precipitated proteins were dissolved in 20 μl of Laemmli sample buffer and subjected to SDS-PAGE. Proteins were detected by Western blotting using antibodies.
Pulldown Assay of GTP-RhoA and GTP-Rap1
To measure GTP-RhoA and GTP-Rap1 levels, cells (2 × 106) were lysed in a lysis buffer (25 mm Tris-HCl, pH 7.5, 1% Nonidet P-40, 150 mm NaCl, 10 mm MgCl2, 1 mm DTT, 5% glycerol, 1 mm PMSF, and protease inhibitors) and centrifuged at 13,000 × g for 15 min at 4 °C. The supernatants of the lysates were incubated for 2 h at 4 °C with GST-Rhotekin-RBD immobilized with GSH-Sepharose beads to detect GTP-RhoA or His-RalGDS-RapBD immobilized Ni2+-beads (His-bind purification kit) to detect GTP-Rap1. The beads were collected and washed three times with a lysis buffer, and the bound proteins on the beads were subjected to SDS-PAGE. GTP-RhoA and GTP-Rap1 were detected by Western blotting using anti-RhoA and anti-Rap1 antibodies, respectively (20, 21).
Fluorescence Microscopy
For fluorescence microscopy, cells were fixed and immunolabeled according to the methods described in a previous study (22). Briefly, Raw264.7 macrophages (1.5 × 106 cell) were grown on 12-mm glass coverslips (BD Biosciences) and incubated with SOZs or FITC-SOZs. Stimulated cells were fixed using 4% formaldehyde in 1× PBS with 1% BSA for 15 min at room temperature. Samples were washed in 1× PBS containing 1% BSA and were permeabilized using 0.2% Triton X-100 in 1× PBS for 10 min at room temperature.
Filamentous (F)-actin in cells was stained by incubating the cells with 1 μm Alexa Fluor 488-phalloidin for 30 min at room temperature and washing the cells with 1× PBS. Samples were observed using an Axiovert 200 microscope (Zeiss) under ×40 magnification without oil immersion. Images were recorded with a camera (Cool SNAP ES; Roper Scientific Photometrics, Tucson, AZ) driven by IPLab Spectrum software (Scanalytics Inc., Fairfax, VA). Image processing was performed with Adobe Photoshop 7.0 software (Adobe Systems). Total fluorescence intensity of F-actin visualized by Alexa Fluor 488-phalloidin within a cell was quantified from the images of equal exposure using the ImageJ program (National Institutes of Health). The ImageJ program can assign the contour and quantify the fluorescence intensity of a cell.
F-actin Extraction and Determination
F-actin was isolated from cells using Triton X-100 (23, 24). Briefly, cells (5 × 106) were precipitated at 15,000 × g for 10 min at 4 °C, and then resuspended in 200 μl of cytoskeleton buffer (10 mm Tris-HCl, pH 7.4, 100 mm NaCl, 1 mm EDTA, 1% Triton X-100, 10% glycerol, 2 μg/ml each leupeptin, aprotinin, and pepstatin, and 1 mm PMSF) on ice for 15 min. The cell lysates were centrifuged at 400 × g for 6 min at 4 °C, and the supernatants were loaded on 300 μl of cytoskeleton buffer containing 6% sucrose and then centrifuged at 230,000 × g for 45 min at 4 °C a using table-top ultracentrifuge (Optima TLX, TLS55 rotor; BD Biosciences). The pellets were rinsed once in cytoskeleton buffer, dissolved in Laemmli sample buffer, and run on SDS-PAGE. F-actin was identified by Western blotting using anti-actin antibody.
Statistical Analysis
All experiments were replicated independently at least three times. Means of triplicate experiments were compared using one-tailed unpaired t tests or with one- or two-way analyses of variance (GraphPad Prism version 4.03 program).
RESULTS
Rap1 Replaces RhoA during Phagocytosis of SOZs
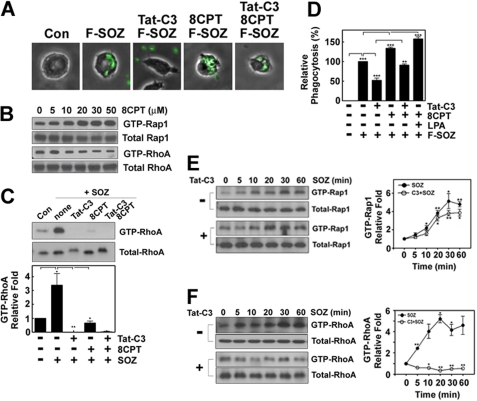
It was reported previously that RhoA regulates phagocytosis (19); and using fluorescence microscopy we confirmed again that Tat-C3 toxin, which is an inhibitor of Rho GTPase, markedly suppressed the phagocytosis of SOZ particles (Fig. 1A). However, we found that 8CPT-2Me-cAMP attenuated the Tat-C3 toxin-mediated suppression of the phagocytosis of SOZs (Fig. 1A). Recently, it was documented that a novel cAMP analog, 8CPT-2Me-cAMP, can activate exchange protein directly activated by cAMP (Epac, GEF for Rap1), thereby activating Rap1 (25). As expected, 8CPT-2Me-cAMP enhanced GTP-Rap1 levels in macrophages in a concentration-dependent manner (Fig. 1B). Actually, Tat-C3 toxin suppressed GTP-RhoA levels, which were otherwise increased in response to the SOZs, and shifted the migration mobility of RhoA on SDS-PAGE, which most likely represents an ADP-ribosylation of RhoA (Fig. 1C). We quantified the phagocytosis of FITC-SOZs by measuring the fluorescence intensity of engulfed FITC-SOZs using a fluorescence spectrophotometer. Similar to the results shown in Fig. 1A, Tat-C3 toxin suppressed the levels of the phagocytosis of FITC-SOZs. In contrast, LPA, which is a known activator of RhoA, augmented the phagocytosis of SOZs (Fig. 1D). Furthermore, the activation of both Rap1 and RhoA by 8CPT-2Me-cAMP and LPA, respectively, induced more phagocytosis than either activation (Fig. 1D), suggesting that Rap1 and RhoA collectively regulate the phagocytosis. In particular, 8CPT-2Me-cAMP increased the levels of the phagocytosis of FITC-SOZ particles and significantly restored the levels that were suppressed by Tat-C3 toxin (Fig. 1D). These findings suggest that Rho and Rap1 GTPase are independently and collectively essential for the regulation of the phagocytosis of SOZs and that Rap1 could rescue or replace the role of RhoA in these processes. Therefore, we also examined whether 8CPT-2Me-cAMP rescues the Tat-C3 toxin-suppressed GTP-RhoA levels. Contrary to our expectations, 8CPT-2Me-cAMP markedly suppressed GTP-RhoA levels (Fig. 1, B and C). This suggests that 8CPT-2Me-cAMP could not restore the Tat-C3 toxin-suppressed GTP-RhoA levels. Next, we examined whether SOZs activate Rap1 in macrophages by determining the GTP-Rap1 levels. SOZs elevated the GTP-Rap1 levels in a time-dependent manner (Fig. 1E), which suggests that SOZs activates Rap1 during the phagocytosis of SOZs. In particular, Tat-C3 toxin did not affect the GTP-Rap1 levels in response to SOZs (Fig. 1E). These results indicate that Rap1 activation is independent of RhoA activity and that RhoA is not upstream of Rap1. Instead, Rap1 antagonistically down-regulates RhoA activity, most likely through ARAP3 (26).
FIGURE 1.
8CPT-2Me-cAMP restores Tat-C3 toxin-suppressed phagocytosis. A, Raw264.7 macrophages were pretreated for 1 h with Tat-C3 (10 μg/ml) and/or 8CPT-2Me-cAMP (50 μm). The cells were incubated at 37 °C with F-SOZ particles (4 × 105) for 15 min. F-SOZ particles were observed by fluorescence microscopy and visualized in green. B, Raw264.7 macrophages were incubated with various concentrations of 8CPT-2Me-cAMP for 60 min at 37 °C. GTP-Rap1 and GTP-RhoA were pulled down using His-RalGDS-RapBD beads and GST-Rhotekin-RBD beads, respectively, and Rap1 and RhoA were measured by Western blotting using anti (α)-Rap1 and anti (α)-RhoA antibodies, respectively. C, cells were treated with Tat-C3 and/or 8CPT-2Me-cAMP as shown in A. SOZ particles were applied to the cells for 30 min, and the pulldown assay to measure GTP-RhoA was performed using GST-Rhotekin-RBD beads in cytosol. RhoA was measured by Western blotting using anti (α)-RhoA antibody. D, cells were pretreated with Tat-C3 (10 μg/ml), 8CPT-2Me-cAMP (50 μm), and LPA (10 μm) for 1 h. F-SOZ particles (100 μl, 2 × 106) were applied to the cells for 30 min. Phagocytosis of F-SOZ particles was measured as described under “Experimental Procedures.” E and F, macrophage cells in 6-well plates (2 × 105 cells/well) were pretreated with Tat-C3 toxin (10 μg/ml) and then incubated with 100 μl of SOZ (2 × 106 particles) for defined time periods. Cell lysates (500 μg of protein) were used for pulldown GTP-Rap1 and GTP-RhoA assays. His-RalGDS-RapBD beads and GST-Rhotekin-RBD beads were mixed with cell lysates for 2 h at 4 °C, and bound Rap1 (E) and RhoA (F) were determined by Western blotting using anti (α)-Rap1 and anti (α)-RhoA antibodies, respectively. They were quantified with densitometry and are shown in on the right. Data are presented as representative (A and B) and means ± S.E. (error bars) (C–F) of three independent experiments (*, p < 0.05; **, p < 0.01; ***, p < 0.001). The spots between with and without Tat-C3 toxin do not differ significantly (E).
As a control, we showed again that SOZs also increased the GTP-RhoA levels in a time-dependent manner. Consistently, Tat-C3 toxin completely suppressed SOZ-stimulated RhoA activity (Fig. 1F).
Rap1 and RhoA Collectively Regulate Phagocytosis of SOZs
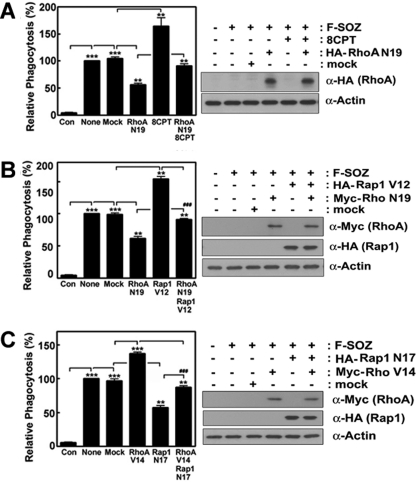
By using transfection of RhoA and Rap1 cDNAs into macrophages, we again verified that Rap1 rescues RhoA defects. DN-RhoA (N19) reduced the phagocytosis of SOZs, and 8CPT-2Me-cAMP also attenuated the DN-RhoA (N19)-mediated suppression of the phagocytosis of SOZs (Fig. 2A). Consistently, CA-Rap1 (V12) transfection restored the phagocytosis of SOZs, which was significantly suppressed by transfection of DN-RhoA (N19) (Fig. 2B). In addition, CA-Rap1 (V12) significantly enhanced the phagocytosis of SOZs, whereas DN-Rap1 (N17) markedly reduced it, suggesting that Rap1 is involved in the regulation of the phagocytosis of SOZs. Herein, we also determined whether RhoA could rescue the suppression of the phagocytosis of SOZs when Rap1 was blocked. To demonstrate this, DN-Rap1 (N17) and CA-RhoA (V14) DNA constructs were co-transfected into the cells, and the phagocytosis of SOZs were assessed. The CA-RhoA (V14) construct rescued the DN-Rap1 (N17)-mediated suppression of the phagocytosis of SOZs (Fig. 2C). These findings suggest that Rap1 and RhoA independently and collectively regulate the phagocytosis of SOZs.
FIGURE 2.
Rap1 and RhoA induce the phagocytosis of SOZ-particles. HA-RhoA or Myc-RhoA N19 and V14 cDNA constructs, and Rap1 V12 and N17 (each 5 μg/ml) were transiently transfected into macrophages (2 × 105) in 6-well plates, which were incubated for 48 h in serum-free DMEM-F12 media. F-SOZ particles (2 × 106) were applied for 30 min, and phagocytosis was determined using a fluorescence spectrophotometer. Effects of 8CPT-2Me-cAMP (50 μm) and DN-RhoA (N19) (A), effects of CA-Rap1 (V12) and DN-RhoA (N19) (B), and effects of CA-RhoA (V14) and DN-Rap1 (N17) (C) were assessed. Transfection with each construct was identified by Western blotting using anti (α)-HA antibody for RhoA (A), for Rap1 (B and C), and anti (α)-Myc antibody for RhoA (B and C). The amounts of actin were also determined as controls (right). Data are presented as means ± S.E. (error bars) of three independent experiments (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Effects of RhoA and Rap1 on Binding of SOZ to Cells
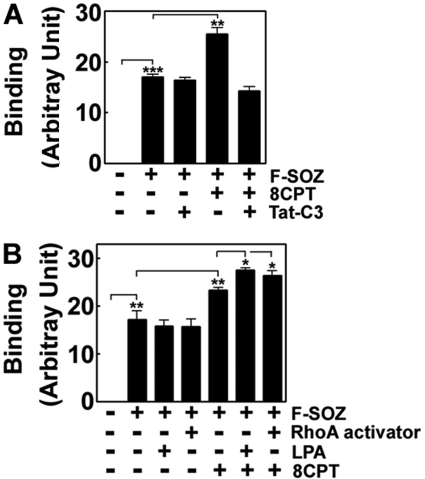
It has been well established that Rap1 induces integrin αLβ2-mediated adhesion to ICAM (13) and controls inside-out signaling to integrin αMβ2 (27). Therefore, we investigated whether the binding of SOZs is dependent on 8CPT-2Me-cAMP and Tat-C3 toxin; 8CPT-2Me-cAMP enhanced the binding of SOZs (Fig. 3A), suggesting that Rap1 regulates the binding of SOZ. However, neither Rho inhibitor-like Tat-C3 toxin (Fig. 3A) nor RhoA activators including LPA and CN01 alter the binding of SOZs (Fig. 3B), suggesting that RhoA activity is not directly relevant to the binding of SOZs. Nevertheless, 8CPT-2Me-cAMP in the presence of Tat-C3 toxin could not enhance the binding of SOZ (Fig, 3A), suggesting that RhoA is involved in Rap1-mediated SOZ binding.
FIGURE 3.
Effects of reagents to regulate RhoA and Rap1 activities on the binding of SOZ particle. Raw264.7 cells (2 × 105) were pretreated with 50 μm 8CPT-2Me-cAMP, 10 μg/ml Tat-C3 toxin, 10 μm LPA, 5 μm RhoA activator I (CN01) for 60 min at 37 °C. FITC-conjugated zymosan particle (2 × 106) were applied for 30 min on ice for particles to bind to cells, and then unbound F-SOZ particles were washed out three times with cold PBS. Total fluorescence of SOZ particles bound to cells after washing was measured by a fluorescence spectrophotometer. At this step, crystal violet could completely quench the fluorescence of FITC, demonstrating that phagocytosis is almost blocked, and all F-SOZ particles were bound to the surface of cells. Data are presented as mean ± S.E. (error bars) of five (A) or three (B) independent experiments.
Involvement of Actin in Phagocytosis
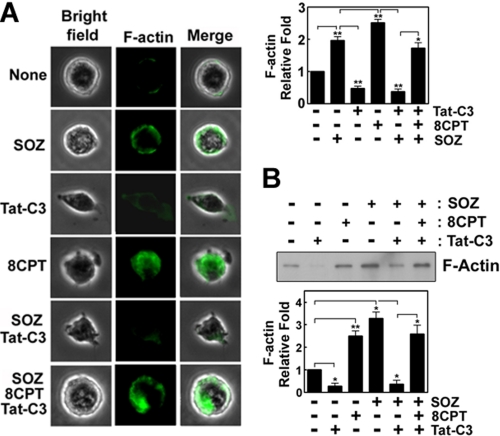
To assess the mechanism of the 8CPT-2Me-cAMP-mediated rescue of the Tat-C3 toxin-mediated suppression of the phagocytosis of SOZs, we examined whether 8CPT-2Me-cAMP regulates the formation of F-actin by using Alexa Fluor 488-phalloidin in macrophages during the phagocytosis of SOZs. The SOZs and 8CPT-2Me-cAMP enhanced F-actin content, whereas Tat-C3 toxin reduced it. As expected, 8CPT-2Me-cAMP rescued the F-actin content, which was reduced by Tat-C3 toxin (Fig. 4A). In another way, F-actin was extracted by Triton X-100, precipitated with ultracentrifugation, and identified by Western blotting. By this procedure, we again confirmed that SOZs and 8CPT-2Me-cAMP enhanced F-actin content and that Tat-C3 toxin reduced F-actin formation of SOZ-treated cells, which was rescued after adding 8CPT-2Me-cAMP (Fig. 4B).
FIGURE 4.
Rap1 activation rescues actin filament formation impaired by RhoA inactivation. A, Raw264.7 macrophages (2 × 105) were pretreated for 60 min with Tat-C3 (10 μg/ml) or/and 8CPT-2Me-cAMP (50 μm). SOZ particles (2 × 106) were applied for 10 min at 37 °C, fixed with 4% methanol-free formaldehyde, and permeabilized with 0.1% Triton X-100; F-actin was then stained with Alexa Fluor 488-phalloidin (1 μm) for 60 min at room temperature. Green fluorescence in the cells was observed under fluorescence microscopy. Total fluorescence intensity of F-actin within a cell was quantified using the ImageJ program. The fluorescence of the image was quantified (right). B, F-actin was isolated from cells using sucrose cushion ultracentrifugation (see “Experimental Procedures”). F-actin was identified by Western blotting using anti (α)-actin antibody and quantified using densitometry (lower). Data are presented as means ± S.E. (error bars) of three independent experiments (*, p < 0.05; **, p < 0.01).
Involvement of Profilin in Phagocytosis of SOZs
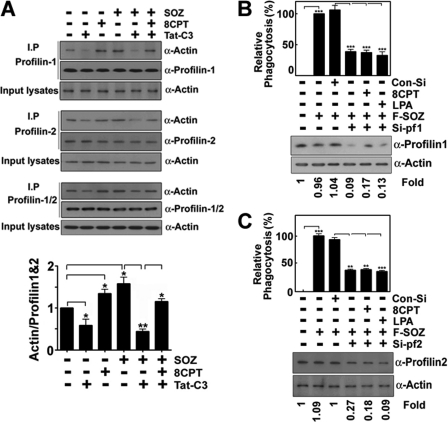
To elucidate further the mechanisms of actin behavior, the interaction between profilin and actin was studied because profilin is an essential regulator of F-actin assembly and is linked to RhoA and Rap1 through a variety of mediator molecules (28–33). First, we examined the interaction of profilin-1, profilin-2, and mixture of profilin-1 and profilin-2 (profilin-1/2) with actin; profilin-1, profilin-2, and profilin-1/2 were pulled down with an anti-profilin-1, anti-profilin-2, and anti-profilin-1/2 antibodies, and co-immunoprecipitated actins were detected by Western blotting using an anti-actin antibody. The interactions occurred to a considerable extent in the resting state; Tat-C3 toxin disrupted the interactions of actin with profilin-1, profilin-2, and profilin-1/2, but 8CPT-2Me-cAMP restored them during the phagocytosis of SOZs (Fig. 5). The amount of actin interacting with profilin-1/2 was also quantified (Fig. 5A, bottom panel). The results suggest that both Rap1 and RhoA may stimulate the interaction of profilin-1/2 with actin, and each molecule can supplement defects in the other. Next, we examined whether profilin is essential for the phagocytosis of SOZs in macrophages. Therefore, we addressed the effect of profilin small interfering (si)-RNA on the phagocytosis of SOZs; siRNAs for profilin-1 and profilin-2 markedly suppressed the phagocytosis of SOZs (Fig. 5, B and C). Indeed, transfection with siRNAs for profilin-1 and profilin-2 reduced profilin expression (Fig. 5, B and C, bottom panels), and neither 8CPT-2Me-cAMP, which is an activator of Epac that leads to Rap1 activation, nor LPA, which is an activator of RhoA, restored the suppression of the phagocytosis that was induced by siRNAs for profilin-1 and profilin-2 (Fig. 5, B and C), suggesting that profilin-1 and profilin-2 are downstream of RhoA and Rap1 in the phagocytotic process.
FIGURE 5.
Rap1 and RhoA regulate the interaction between actin and profilin. A, Raw264.7 macrophages (2 × 105) were pretreated for 60 min with Tat-C3 (10 μg/ml) and/or 8CPT-2Me-cAMP (50 μm). F-SOZ particles (2 × 106) were applied for 30 min at 37 °C. Raw264.7 macrophages were lysed, and profilin was pulled down with anti-profilin-1, anti-profilin-2, and anti-profilin-1/2 antibodies overnight in precleared cell lysates. The samples were loaded on 10% SDS-PAGE, and actin was detected by Western blotting using an anti (α)-actin antibody. The actin was quantified with densitometry, and the ratio of actin to profilin-1/2 is presented (lower panel). B and C, profilin-1 and profilin-2 siRNAs (10 nm) were transfected into cells using Hiperfect transfection reagent for 20 min and incubated for 72 h; cells were pretreated with 8CPT-2Me-cAMP (50 μm) or LPA (10 μm) for 60 min, and F-SOZ particles (2 × 106) were applied for 30 min. Phagocytosis of the SOZ particles was measured. Actual loss of profilin-1 and profilin-2 by profilin-1 and profilin-2 siRNAs was identified by Western blotting using anti (α)-profilin-1 and anti (α)-profilin-2 antibodies (lower panels). Data are presented as means ± S.E. (error bars) of three independent experiments (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
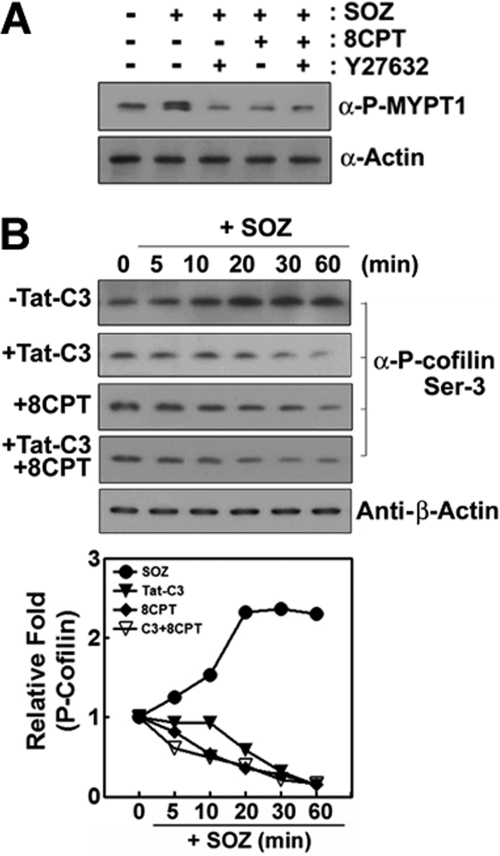
Because RhoA can also activate ROCK, it was determined whether ROCK is regulated by Rap1 during the phagocytosis of SOZs. To demonstrate the effect of 8CPT-2Me-cAMP on ROCK activity, we examined the phosphorylation of MYPT-1, a substrate of ROCK. The stimulation of cells by SOZ induced the phosphorylation of MYPT-1 which was abolished by Y27632, a specific ROCK inhibitor. As expected, 8CPT-2Me-cAMP could not recover its phosphorylation (Fig. 6A). Instead, 8CPT-2Me-cAMP inhibited the phosphorylation of MYPT-1 (Fig. 6A) because 8CPT-2Me-cAMP inactivates RhoA (Fig. 1C) likely through ARAP3.
FIGURE 6.
Epac activator, 8CPT-2Me-cAMP does not rescue ROCK and LIMK activities. A, cells were pretreated with Y27632 (30 μm) and 8CPT-2Me-cAMP (50 μm) for 60 min, and then phospho-MYPT-1 was determined by Western blotting using anti (α)-phospho-MYPT-1 antibody. Actin was determined as a control by Western blotting using anti (α)-actin antibody. B, Raw264.7 cells were pretreated with Tat-C3 (10 μg/ml) and/or 8CPT-2Me-cAMP (50 μm) for 60 min. F-SOZ particles were applied for the specified times at 37 °C. Phospho-cofilin was detected by Western blotting using anti (α)-phospho-cofilin (at Ser(P)-3 residue) antibody. As a control, actin was determined by Western blotting using anti-β-actin antibody. The bands of Western blotting were quantified by a densitometer. Data are expressed as representative of three (A) or two (B) independent experiments.
It was known that RhoA-ROCK-LIMK can phosphorylate cofilin to produce its inactive form and lead to the formation of actin filaments; the dephosphorylated form of cofilin can disassemble actin filaments. Indeed, we found that SOZs induced the phosphorylation of cofilin. However, as expected, Tat-C3 toxin suppressed cofilin phosphorylation (Fig. 6B). To identify whether Rap1 affects the phosphorylation of cofilin, the phospho-cofilin was assessed upon treatment with 8CPT-2Me-cAMP; 8CPT-2Me-cAMP suppressed the SOZ-stimulated phosphorylation levels of cofilin (Fig. 6B). Furthermore, 8CPT-2-Me-cAMP could not restore the Tat-C3 toxin-mediated suppression of the phosphorylation of cofilin (Fig. 6B). These findings suggest that the cofilin phosphorylation by LIMK is not involved in the restoration of the phagocytosis of SOZs that is mediated by Rap1 activation.
Involvement of Rap1 and RhoA in Regulation of Interaction between Actin and Profilin
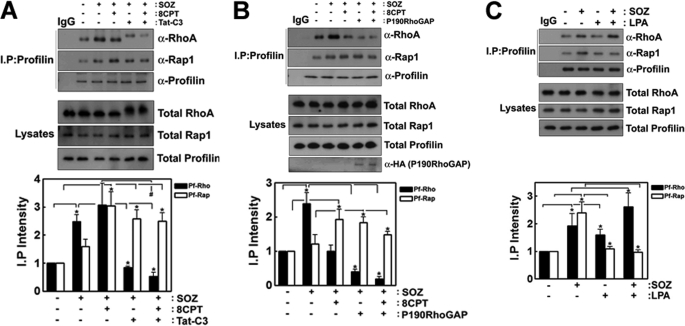
Next, we tried to determine the interactions between profilin and RhoA and between profilin and Rap1, which may be regulated by the activities of RhoA and Rap1, respectively. Profilin was pulled down with anti-profilin antibody, and the co-immunoprecipitated RhoA and Rap1 were detected by Western blotting using anti-RhoA and anti-Rap1 antibodies, respectively. SOZs promoted the co-immunoprecipitation of profilin with RhoA, and profilin with Rap1, and 8CPT-2Me-cAMP increased the co-immunoprecipitation of profilin with Rap1 but decreased the co-immunoprecipitation of profilin with RhoA (Fig. 7A). The RhoA inhibitors, Tat-C3 toxin (Fig. 7A) and p190RhoGAP (Fig. 7B), markedly reduced the levels of the co-immunoprecipitation of profilin with RhoA but significantly enhanced the levels of the co-immunoprecipitation of profilin with Rap1. The levels of the co-immunoprecipitation of profilin with RhoA were still reduced in the cells that were treated with 8CPT-2Me-cAMP plus Tat-C3 toxin (Fig. 7A) or with 8CPT-2Me-cAMP plus p190RhoGAP transfection (Fig. 7B), whereas the levels of the co-immunoprecipitation of profilin with Rap1 were still relatively high. However, LPA enhanced the co-immunoprecipitation of profilin with RhoA, which reduced the co-immunoprecipitation of profilin with Rap1 (Fig. 7C). Because there are many intermediate mediator proteins between RhoA and profilin, and between Rap1 and profilin, RhoA and Rap1 seem to be indirectly bound to profilin (28–33).
FIGURE 7.
Interactions of profilin with RhoA, and Rap1 regulated by SOZ phagocytosis. A, Raw264.7 (2 × 105) cells were pretreated for 60 min with 8CPT-2Me-cAMP (50 μm). F-SOZ particles (100 μl, 2 × 106) were applied for 30 min at 37 °C. Profilin was pulled down using an anti (α)-profilin antibody, and profilin, co-immunoprecipitated Rap1 and RhoA were determined by Western blotting using anti (α)-profilin, anti (α)-Rap1, and anti (α)-RhoA antibodies, respectively. B, p190RhoGAP (2 μg DNA) was transfected into Raw264.7 cells, and the cells were treated with 8CPT-2Me-cAMP (50 μm) for 60 min. After F-SOZ treatment for 30 min, profilin was immunoprecipitated from the cell lysate using anti-profilin antibody, and co-immunoprecipitated RhoA and Rap1 were detected by Western blotting using anti (α)-RhoA and anti (α)-Rap1 antibodies. Transfected HA-p190RhoGAP was detected by Western blotting using anti (α)-HA antibody. C, cells were treated with 10 μm LPA for 30 min. After F-SOZ treatment for 30 min, profilin was immunoprecipitated from the cell lysate using an anti-profilin antibody, and co-immunoprecipitated RhoA and Rap1 were detected by Western blotting using anti (α)-RhoA and anti (α)-Rap1 antibodies, respectively. Quantitative data are presented in the lower panels (A–C). Data are presented means ± S.E. (error bars) of three independent experiments (*, p < 0.05).
GDP/GTP Regulates Interaction of Profilin with RhoA and Rap1
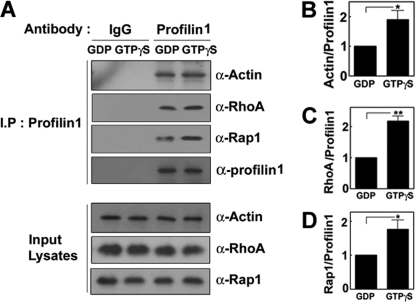
The interactions between the small GTPase and effector proteins are generally regulated by GDP or GTP. Indeed, SOZs simultaneously stimulated both GTP-Rap1 and GTP-RhoA in a time-dependent manner (Fig. 1, E and F). Therefore, we tried to determine whether GTP/GDP-bound proteins variably interact with profilin. Co-immunoprecipitations of profilin with actin, Rap1, and RhoA were all enhanced in the presence of GTPγS compared with GDP (Fig. 8, A–D), suggesting that active GTP-RhoA and GTP-Rap1 stimulate the interaction of profilin with actin and probably lead to actin filament formation or influence the dynamics of actin filaments during phagocytosis.
FIGURE 8.
GTP enhances the interactions of profilin with actin, RhoA, and Rap1. A, cell lysates were preincubated with 1 mm GDP or 0.1 mm GTPγS in the presence of 10 mm EDTA for 30 min in a lysis buffer (25 mm Tris-HCl, pH 7.5, 1% Nonidet P-40, 150 mm NaCl, 10 mm MgCl2, 1 mm DTT, 5% glycerol, 1 mm PMSF, and protease inhibitors), and then more MgCl2 was added to a lysis buffer to make a final 20 mm. Profilin was immunoprecipitated, and co-immunoprecipitated actin, RhoA, and Rap1 levels were determined by Western blotting using anti (α)-RhoA and anti (α)-Rap1 antibodies. Actin (B), RhoA (C), and Rap1 (D) were quantified by densitometry. Data are presented as means ± S.E. (error bars) of three independent experiments (*, p < 0.05).
DISCUSSION
Rap1 and RhoA Regulate Phagocytosis of SOZ Particles
Among Ras-related GTPases, RhoA is involved in the regulation of phagocytosis of SOZ, which is mediated through integrin αMβ2. Here, we showed that Rap1 also participates in the regulation of the phagocytosis of SOZs (Fig. 2). Although Rap1 activation by C3G is required for FcγR-dependent phagocytosis in rat alveolar macrophages (18), a controversial finding was also reported; C3G, the Rap1 GEF, induced actin cytoskeletal reorganization and promoted filopodia formation, but these effects were independent of its catalytic activity (34), which suggests that Rap1 activity is not required for actin reorganization in this case. However, in our study, active Rap1 rescued the CR3-mediated phagocytosis that was suppressed by RhoA inactivation (Figs. 1 and 2). In fact, CR3 activates the small GTPase, RhoA (6–8). Particularly, cytoplasmic regions of integrin β2 are required for the recruitment and activation of RhoA and for phagocytic uptake in phagosomes (34). In our study, we found that Rap1 activation by 8CPT-2Me-cAMP, which activates the Rap1 GEF, Epac, significantly recovers the phagocytosis of SOZs that is suppressed by Tat-C3 toxin-mediated inactivation of RhoA (Fig. 1D). Nevertheless, 8-CPT-2Me-cAMP markedly reduced GTP-RhoA rather than restoring RhoA activity (Fig. 1C). This is likely to be caused by the Rap-dependent RhoGAP, ARAP3, which can be activated by Rap1 leading to down-regulation of RhoA (26). From this result, it can be inferred that RhoA is not essentially required for the phagocytosis of SOZs when Rap1 is fully activated. SOZs actually induce the activation of Rap1 in parallel with the activation of RhoA in macrophages (Fig. 1, E and F), which suggests that the endogenous activation of Rap1 by SOZs does not inhibit RhoA and that the endogenous activation of Rap1 and RhoA is controlled separately during the phagocytosis of SOZs. Similarly, constitutive activation of RhoA rescues the phagocytotic process in the cells that are transfected with the DN-Rap1 plasmid. This suggests that Rap1 and RhoA collectively regulate the phagocytosis of SOZs.
It was known that superoxide is consequently generated by phagocytosis. Tat-C3 toxin also suppressed superoxide production by SOZs. Similarly, 8CPT-2Me-cAMP recovered the Tat-C3 toxin-mediated inhibition of superoxide production (supplemental Fig. 1A). As expected, LPA, which is an activator of RhoA, stimulated superoxide generation in response to SOZs (supplemental Fig. 1B). DN-RhoA (N19) reduced superoxide generation, which was restored by treatment with 8CPT-2Me-cAMP (supplemental Fig. 1C). These results suggest that Rap1 activation rescues superoxide generation, which is blocked by the inhibition of RhoA during the phagocytosis of SOZs. Herein, it is likely that superoxide production depends on the phagocytosis of SOZs.
GTP-Rap1 activates integrin αMβ2 in the phagocytotic process, and the direct binding of the talin head domain to the cytoplasmic tail of many integrin β subunits was reported to be a powerful way of activating integrins, including integrin β2 (5, 27). In particular, regulated binding of talin to integrin β tails leads to integrin affinity (35). Conversely, RhoA regulates outside-in signaling downstream of integrin αMβ2 (36); integrin αMβ2 requires RhoA activity to induce actin polymerization and facilitate particle uptake (6). Consistently, in this study Rap1 seems to regulate SOZ binding to cells whereas RhoA is not likely to be relevant to SOZ binding (Fig. 3). Therefore, the possibility cannot be excluded that the increase of phagocytosis by 8CPT-2Me-cAMP is at least in part due to the increase of the binding of SOZs. Here, it is worth noting that Rap1 activation along with the inactivation of RhoA could not enhance the binding of SOZs whereas RhoA activator slightly promoted the effect of 8CPT-2Me-cAMP on the binding of SOZs (Fig. 3). This suggests that RhoA is likely to control Rap1-mediated binding of SOZs, although RhoA does not affect Rap1 activity (Fig. 1E). However, the detailed mechanism by which RhoA regulates Rap1-mediated binding of SOZ to cells remains to be elucidated.
F-actin Formation by RhoA and Rap1 Is Involved in Regulation of Phagocytosis
To elucidate the mechanism by which Rap1 and RhoA could supplement the phagocytosis that was previously abolished by defects on RhoA and Rap1, respectively, we examined the actin behavior related to the phagocytosis process because actin filaments are required for the phagocytosis of SOZs. F-actin formation is disrupted by RhoA inhibition, which is restored when Rap1 is activated (Fig. 4). Therefore, it can be inferred that to induce the phagocytosis the actin cytoskeleton is regulated by both RhoA and Rap1. It is well known that RhoA is involved in actin remodeling. However, the underlying molecular mechanisms by which Rap1 regulates actin remodeling remain unclear, although it is likely that Rap1 regulates the dynamics of actin filaments. When activated by the Rap1 GEF, Epac, Rap1 stimulates Rac-specific GEFs, including Tiam1 and Vav2; this, in turn, activates Rac1 and leads to cytoskeletal remodeling (37, 38).
It is well known that RhoA transmits signals to the following molecules: ROCK, which regulates actin-myosin interactions, and activates LIMK that controls phosphorylation of cofilin; and mDia, which modulates profilin (9). Indeed, the Rho-ROCK-myosin II pathway is required for CR3-mediated phagocytosis (39). Cofilin antisense enhances the phagocytosis of SOZs in J774.1 cells, which indicates that cofilin is inactivated during phagocytosis (40) and is consistent with our results. However, Rap1 activation by 8CPT-2Me-cAMP did not recover the phosphorylation of cofilin that is suppressed by the Tat-C3 toxin-mediated inactivation of RhoA (Fig. 6). The ROCK inhibitor, Y27632, inhibits the phosphorylation of MYPT-1, but 8CPT-2Me-cAMP cannot restore the phosphorylation in the presence of Y27632. These findings suggest that Rap1 controls the formation of actin filaments not through ROCK, LIMK, or phospho-cofilin, but probably through other mediators, such as mDia and profilin.
Interestingly, we observed that RhoA inhibition disrupted the actin-profilin interaction (Fig. 7), which was recovered by an activator of Epac (8CPT-2Me-cAMP), a Rap1 GEF. Profilins are actin-binding proteins and promote nucleotide exchange activity that accelerates the ADP-ATP exchange on G-actin; they thus replenish the pool of ATP-actin in cells (28). ATP-actin filaments are protected from depolymerizing proteins, such as gelsolin and cofilin, whereas ADP-actin filaments are rapidly disassembled (28). However, it is still not evident whether actin interacts directly with profilin during phagocytosis of SOZ particles at the moment because we showed only the result of co-immunoprecipitation of profilin with actin (Fig. 5).
Rap1 has a variety of downstream effector-binding proteins. Profilin-interacting proteins that are linked to Rap1 include AF-6, RIAM, Ena/VASP, and Rgl3 (28–31). On the other hand, profilin-interacting factors, which are affected by RhoA, include Citron-N, mDia, and ROCK (28, 32). In particular, mDia binds directly to both profilin and RhoA, which are recruited around phagocyticcups that are induced by fibronectin-coated beads, which suggests that RhoA regulates actin polymerization by targeting profilin through p140mDia beneath the specific region of plasma membranes (32). In addition, IQGAP interaction with Dia1 is required for phagocytosis and phagocytic cup formation, and IQGAP mediates the localization of the actin filament nucleator Dia1 (41). IQGAP also binds to Rap1 and modulates its activity (16). Based on the literature, it could be speculated that IQGAP may be a common converging molecule that interacts with effector proteins for both Rap1 and RhoA, although the interaction between IQGAP and profilin remains to be elucidated. As a whole, the indirect interaction between RhoA and profilin is likely to be reciprocally and complementarily correlated with the indirect interaction between Rap1 and profilin (Fig. 7), suggesting that profilin is an essential component for both RhoA and Rap1-mediated phagocytoses. Therefore, we assumed that profilin activity can be regulated by Rap1 and RhoA activities through the specific mediator protein(s) by a process that remains poorly understood (Fig. 9). Herein, it also remains to be determined whether Rap1 and RhoA regulate profilin-mediated exchange of ATP/ADP bound to actin, which is an attractive hypothesis.
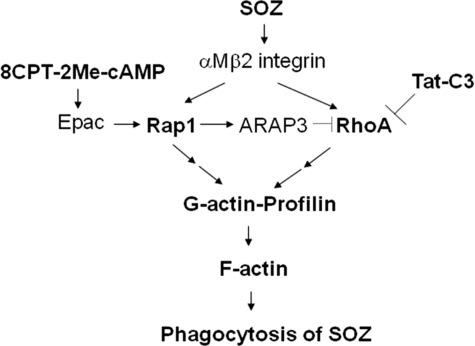
FIGURE 9.
Scheme of phagocytosis collectively regulated by RhoA and Rap1. SOZs activate both RhoA and Rap1 and induce interactions of profilin with RhoA and Rap1 via intermediate proteins, which lead to F-actin formation in Raw264.7 cells, resulting in the phagocytosis of SOZs. The molecules denoted as bold words indicate experimental components, the others mean putative molecules.
Supplementary Material
Acknowledgments
We thank Dr. Settleman at Harvard University for providing p190RhoGAP plasmid and Dr. Bos at University Medical Center Utrecht for providing RalGDS-RapBD plasmid.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by Ministry of Education, Science, and Technology Grants MRC-2011-0006211 and 2010-0007865.

This article contains supplemental Fig. 1.
- FcγR
- Fcγ receptor
- CA
- constitutively active
- 8CPT-2Me-cAMP
- 8-(4-chloro-phenylthio)-2′-O-methyladenosine-3′,5′-cAMP
- CR3
- complement receptor 3
- DN
- dominant negative
- Epac
- exchange protein directly activated by cAMP
- F-actin
- filamentous actin
- F-SOZ
- FITC-conjugated SOZ
- GAP
- GTPase-activating protein
- GEF
- guanosine nucleotide exchange factor
- GST-RalGDS-RapBD
- GST-RalGDS Rap binding domain
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- ICAM
- intercellular adhesion molecule
- LIMK
- LIM kinase
- LPA
- lysophosphatidic acid
- mDia
- mDiaphanous
- MYPT-1
- myosin phosphatase-1
- ROCK
- Rho-associated coiled-coil containing protein kinase
- Rhotekin-RBD
- Rhotekin Rho binding domain
- SOZ
- serum-opsonized zymosan.
REFERENCES
- 1. Underhill D. M., Ozinsky A. (2002) Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20, 825–852 [DOI] [PubMed] [Google Scholar]
- 2. Chimini G., Chavrier P. (2000) Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2, E191–196 [DOI] [PubMed] [Google Scholar]
- 3. Pollard T. D., Borisy G. G. (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 [DOI] [PubMed] [Google Scholar]
- 4. May R. C., Caron E., Hall A., Machesky L. M. (2000) Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat. Cell Biol. 2, 246–248 [DOI] [PubMed] [Google Scholar]
- 5. Lim J., Wiedemann A., Tzircotis G., Monkley S. J., Critchley D. R., Caron E. (2007) An essential role for talin during αMβ2-mediated phagocytosis. Mol. Biol. Cell 18, 976–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caron E., Hall A. (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717–1721 [DOI] [PubMed] [Google Scholar]
- 7. Massol P., Montcourrier P., Guillemot J. C., Chavrier P. (1998) Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J. 17, 6219–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cox D., Chang P., Zhang Q., Reddy P. G., Bokoch G. M., Greenberg S. (1997) Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 186, 1487–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall A. (2005) Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 33, 892–895 [DOI] [PubMed] [Google Scholar]
- 10. Riento K., Ridley A. J. (2003) Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Biol. 6, 446–456 [DOI] [PubMed] [Google Scholar]
- 11. Ohashi K., Nagata K., Maekawa M., Ishizaki T., Narumiya S., Mizuno K. (2000) Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J. Biol. Chem. 275, 3577–3582 [DOI] [PubMed] [Google Scholar]
- 12. Colucci-Guyon E., Niedergang F., Wallar B. J., Peng J., Alberts A. S., Chavrier P. (2005) A role for mammalian diaphanous-related formins in complement receptor (CR3)-mediated phagocytosis in macrophages. Curr. Biol. 15, 2007–2012 [DOI] [PubMed] [Google Scholar]
- 13. Katagiri K., Hattori M., Minato N., Irie S., Takatsu K., Kinashi T. (2000) Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol. Cell. Biol. 20, 1956–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bos J. L., de Bruyn K., Enserink J., Kulperij B., Rangarajan S., Rehmann H., Riedl J., de Rooij J. (2003) The role of Rap1 in integrin–mediated cell adhesion. Biochem. Soc. 31, 81–86 [DOI] [PubMed] [Google Scholar]
- 15. Jenei V., Deevi R. K., Adams C. A., Axelsson L., Hirst D. G., Andersson T., Dib K. (2006) Nitric oxide produced in response to engagement of β2 integrins on human neutrophils activates the monomeric GTPases Rap1 and Rap2 and promotes adhesion. J. Biol. Chem. 281, 35008–35020 [DOI] [PubMed] [Google Scholar]
- 16. Jeong H. W., Li Z., Brown M. D., Sacks D. B. (2007) IQGAP1 binds Rap1 and modulates its activity. J. Biol. Chem. 282, 20752–20762 [DOI] [PubMed] [Google Scholar]
- 17. Caron E., Self A. J., Hall A. (2000) The GTPase Rap1 controls functional activation of macrophage integrin αMβ2 by LPS and other inflammatory mediators. Curr. Biol. 10, 974–978 [DOI] [PubMed] [Google Scholar]
- 18. Chung J., Serezani C. H., Huang S. K., Stern J. N., Keskin D. B., Jagirdar R., Brock T. G., Aronoff D. M., Peters-Golden M. (2008) Rap1 activation is required for Fcγ receptor-dependent phagocytosis. J. Immunol. 181, 5501–5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim J. S., Diebold B. A., Kim J. I., Kim J., Lee J. Y., Park J. B. (2004) Rho is involved in superoxide formation during phagocytosis of opsonized zymosans. J. Biol. Chem. 279, 21589–21597 [DOI] [PubMed] [Google Scholar]
- 20. Ren X. D., Schwartz M. A. (2000) Determination of GTP loading on Rho. Methods Enzymol. 325, 264–272 [DOI] [PubMed] [Google Scholar]
- 21. Herrmann C., Horn G., Spaargaren M., Wittinghofer A. (1996) Differential interaction of the Ras family GTP-binding proteins H-Ras, Rap1A, and R-Ras with the putative effector molecules Raf kinase and Ral-guanine nucleotide exchange factor. J. Biol. Chem. 271, 6794–6800 [DOI] [PubMed] [Google Scholar]
- 22. Geese M., Schlüter K., Rothkegel M., Jockusch B. M., Wehland J., Sechi A. S. (2000) Accumulation of profilin II at the surface of Listeria is concomitant with the onset of motility and correlates with bacterial speed. J. Cell Sci. 113, 1415–1426 [DOI] [PubMed] [Google Scholar]
- 23. Curnutte J. T., Erickson R. W., Ding J., Badwey J. A. (1994) Reciprocal interactions between protein kinase C and components of the NADPH oxidase complex may regulate superoxide production by neutrophils stimulated with a phorbol ester. J. Biol. Chem. 269, 10813–10819 [PubMed] [Google Scholar]
- 24. El Benna J., Dang P. M., Andrieu V., Vergnaud S., Dewas C., Cachia O., Fay M., Morel F., Chollet-Martin S., Hakim J., Gougerot-Pocidalo M. A. (1999) p40phox associates with the neutrophil Triton X-100-insoluble cytoskeletal fraction and PMA-activated membrane skeleton: a comparative study with p67phox and p47phox. J. Leukocyte Biol. 66, 1014–1020 [DOI] [PubMed] [Google Scholar]
- 25. Enserink J. M., Christensen A. E., de Rooij J., van Triest M., Schwede F., Genieser H. G., Døskeland S. O., Blank J. L., Bos J. L. (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4, 901–906 [DOI] [PubMed] [Google Scholar]
- 26. Krugmann S., Williams R., Stephens L., Hawkins P. T. (2004) ARAP3 is a PI3K- and Rap-regulated GAP for RhoA. Curr. Biol. 14, 1380–1384 [DOI] [PubMed] [Google Scholar]
- 27. Dupuy A. G., Caron E. (2008) Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J. Cell Sci. 121, 1773–1783 [DOI] [PubMed] [Google Scholar]
- 28. Witke W. (2004) The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 14, 461–469 [DOI] [PubMed] [Google Scholar]
- 29. Lafuente E. M., van Puijenbroek A. A., Krause M., Carman C. V., Freeman G. J., Berezovskaya A., Constantine E., Springer T. A., Gertler F. B., Boussiotis V. A. (2004) RIAM, an Ena/VASP and profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev. Cell 7, 585–595 [DOI] [PubMed] [Google Scholar]
- 30. Boettner B., Govek E. E., Cross J., Van Aelst L. (2000) The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc. Natl. Acad. Sci. U.S.A. 97, 9064–9069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu J., Shi S., Matsumoto N., Noda M., Kitayama H. (2007) Identification of Rgl3 as a potential binding partner for Rap-family small G-proteins and profilin II. Cell. Signal. 19, 1575–1582 [DOI] [PubMed] [Google Scholar]
- 32. Watanabe N., Madaule P., Reid T., Ishizaki T., Watanabe G., Kakizuka A., Saito Y., Nakao K., Jockusch B. M., Narumiya S. (1997) p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 16, 3044–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Da Silva J. S., Medina M., Zuliani C., Di Nardo A., Witke W., Dotti C. G. (2003) RhoA/ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. J. Cell Biol. 162, 1267–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Radha V., Rajanna A., Mitra A., Rangaraj N., Swarup G. (2007) C3G is required for c-Abl-induced filopodia and its overexpression promotes filopodia formation. Exp. Cell Res. 313, 2476–2492 [DOI] [PubMed] [Google Scholar]
- 35. Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H., Calderwood D. A. (2003) Talin binding to integrin β tails: a final common step in integrin activation. Science 302, 103–106 [DOI] [PubMed] [Google Scholar]
- 36. Wiedemann A., Patel J. C., Lim J., Tsun A., van Kooyk Y., Caron E. (2006) Two distinct cytoplasmic regions of the β2 integrin chain regulate RhoA function during phagocytosis. J. Cell Biol. 172, 1069–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arthur W. T., Quilliam L. A., Cooper J. A. (2004) Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J. Cell Biol. 167, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Birukova A. A., Zagranichnaya T., Alekseeva E., Bokoch G. M., Birukov K. G. (2008) Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J. Cell. Physiol. 215, 715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olazabal I. M., Caron E., May R. C., Schilling K., Knecht D. A., Machesky L. M. (2002) Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcγR, phagocytosis. Curr. Biol. 12, 1413–1418 [DOI] [PubMed] [Google Scholar]
- 40. Adachi R., Takeuchi K., Suzuki K. (2002) Antisense oligonucleotide to cofilin enhances respiratory burst and phagocytosis in opsonized zymosan-stimulated mouse macrophage J774.1 cells. J. Biol. Chem. 277, 45566–45571 [DOI] [PubMed] [Google Scholar]
- 41. Brandt D. T., Marion S., Griffiths G., Watanabe T., Kaibuchi K., Grosse R. (2007) Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 178, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.