Background: Insulin actions are decreased in endothelial cells causing vascular dysfunction in diabetic and insulin-resistant states.
Results: IRS2 and p85 subunit of PI3K are targets of PKC and angiotensin activation inhibiting insulin signaling.
Conclusion: PKC and angiotensin activation inhibit selective insulin activation of Akt/eNOS in endothelial cells.
Significance: We provide a biochemical mechanism by which PKC activation inhibits insulin signaling and protective actions in endothelial cells.
Keywords: Diabetes, Endothelial Cell, Insulin, Protein Kinase C (PKC), Vascular Endothelial Growth Factor (VEGF), IRS1, PI3K
Abstract
The regulation of endothelial function by insulin is consistently abnormal in insulin-resistant states and diabetes. Protein kinase C (PKC) activation has been reported to inhibit insulin signaling selectively in endothelial cells via the insulin receptor substrate/PI3K/Akt pathway to reduce the activation of endothelial nitric-oxide synthase (eNOS). In this study, it was observed that PKC activation differentially inhibited insulin receptor substrate 1/2 (IRS1/2) signaling of insulin's activation of PI3K/eNOS by decreasing only tyrosine phosphorylation of IRS2. In addition, PKC activation, by general activator and specifically by angiotensin II, increased the phosphorylation of p85/PI3K, which decreases its association with IRS1 and activation. Thr-86 of p85/PI3K was identified to be phosphorylated by PKC activation and confirmed to affect IRS1-mediated activation of Akt/eNOS by insulin and VEGF using a deletion mutant of the Thr-86 region of p85/PI3K. Thus, PKC and angiotensin-induced phosphorylation of Thr-86 of p85/PI3K may partially inhibit the activation of PI3K/eNOS by multiple cytokines and contribute to endothelial dysfunction in metabolic disorders.
Introduction
Insulin resistance is one of the major risk factors for developing atherosclerosis and is characterized by the loss of insulin action in multiple tissues, including adipocytes, skeletal muscle, and hepatocytes (1). Besides these traditional metabolic tissues, insulin action on vascular endothelium has been shown to be physiologically significant in regulating hemodynamic functions by the activation of endothelial nitric-oxide synthase (eNOS)2 (2–4). Pathophysiologically, the loss of insulin action on the vascular endothelium can cause endothelial dysfunction, which correlates to increased risk of coronary artery disease and even insulin resistance (5). The importance of the actions of insulin on endothelial function was clearly established by the findings that mice with double knock-out of apolipoprotein E (apoE−/−) and insulin receptor (IR−/−) on the endothelial cells developed atherosclerosis at 2–3 times greater severity than apoE−/− mice (6).
The mechanism of the physiological actions of insulin on the vascular endothelium is predominantly mediated via the IRS/PI3K/Akt pathway, leading to the activation of eNOS and the expression of cytokines such as vascular endothelial growth factor (VEGF) and heme oxygenase-1 (HO-1) (4, 7–9). In insulin-resistant states most commonly associated with obesity and diabetes, selective inhibition of the IRS/PI3K/Akt pathway has been demonstrated, probably because of abnormal elevation of free fatty acid and glucose levels (10). One mechanism causing the selective inhibition of the actions of insulin is likely due to PKC activation, which has been shown to be a consistent feature in the endothelium in response to diabetes or insulin resistance (11). Inhibition of PKCβ isoforms has been reported to decrease severity of atherosclerosis in apoE−/− mice (12). We suggest that one mechanism for PKC activation to increase atherosclerosis is the inhibition of insulin's action selectivity in the endothelial cells (13). PKC activation has been reported to selectively inhibit insulin activation of Akt and eNOS in endothelial cells by decreasing the phosphorylation of Akt at Ser-473 and eNOS at Ser-1177 (1179 for bovine) (13). However, the specific step(s) of insulin signaling between insulin receptors (IR) to Akt, which are being inhibited by PKC activation, have not been identified. Previously, studies in nonendothelial cells have shown that PKC activation can decrease insulin-induced tyrosine phosphorylation of IR and IRS1 by increasing serine (Ser) or threonine (Thr) phosphorylation at several sites on these proteins (14–20). However, we have reported that PKC activation can also inhibit vascular endothelial growth factor (VEGF)-induced phosphorylation of Akt and eNOS in endothelial cells, suggesting that phosphoinositide 3-kinase (PI3K) subunits can also be targets of the inhibiting actions of PKC activation (13).
In this study, we have characterized the effects of PKC activation on multiple steps of the insulin signaling cascade, from the receptor to Akt phosphorylation, in endothelial cells. The results showed that the inhibitory effects of PKC activation in endothelial cells differ from other types of cells in the lack of changes on IR and IRS1 tyrosine phosphorylation. However, we have identified a novel phosphorylation site on the p85 subunit of PI3K, which is responsive to PKC activation and blunts insulin activation of Akt/eNOS in endothelial cells.
EXPERIMENTAL PROCEDURES
All chemicals otherwise noted were purchased from Sigma. Antibodies were purchased from Cell Signaling Technology Inc. (Danvers, MA), Upstate, Millipore (Billerica, MA), or Santa Cruz Biotechnology (Santa Cruz, CA).
Antibodies against p85, IRS1, IRS2, phosphotyrosine (Tyr(P)) (4G10®) were from Upstate. Phospho-/total eNOS, phospho-/total Akt and ERK, and HA tag are from Cell Signaling. All the phospho-IRS1 antibodies were purchased either from Cell Signaling or Upstate. Polyclonal antibody against insulin receptor (IR) β was from Santa Cruz Biotechnology, and monoclonal antibody for immunoblotting was from Cell Signaling. Anti-GST-HRP-conjugated and protein A/G PLUS-agarose were purchased from Santa Cruz Biotechnology. ECLTM and ECL Plus Western blotting detection reagents were purchased from GE Healthcare. GF109203X (bisindolylmaleimide I, GFX) and PD98059 were purchased from Calbiochem. Phosphatidylinositol (catalog no. 109853) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). VEGF was purchased from R&D Systems Inc. (Minneapolis, MN). Dulbecco's modified Eagle's medium with 100 mg/dl glucose (DMEM-L) was provided by Joslin Media Core with penicillin G and streptomycin included. Angiotensin was purchased from Sigma.
Cell Culture
Bovine aortic endothelial cells (BAEC) were isolated from bovine aorta purchased from a local vendor and propagated in DMEM-L and 10% horse serum. BAEC were used at passage 2–5. Mouse endothelial cells were isolated from lungs of IRS2KO mice or wild-type C57/B6 mice. Lungs were digested with collagenase 0.2% (w/v) in DMEM, 0.1% BSA. The dissociated cells were cultured in DMEM with 100 mg/dl glucose, 10% horse serum, 50 μg/ml endothelial cell growth supplement (ECGS), and 100 μg/ml heparin for 3 days and were then resuspended and incubated with ICAM-2 rat anti-mouse antibody (Pharmingen) with Dynabeads sheep anti-rat IgG (Invitrogen) in PBS, 0.1% BSA. The endothelial cells were magnetically isolated when passing through Dynal MPC-15 Magnetic Particle Concentrator (Invitrogen). Cells were standardized with 0.1% BSA (Sigma) for 24 h, then pretreated with 100 nm PMA for 20 min, and stimulated with 100 nm insulin for 5 min before washing and lysing the cells.
Adenoviral Infection
To overexpress IRS1 and IRS2, adenoviral vectors that encode full-length cDNA of rat IRS1 and IRS2 were used as described previously (21). Adenovirus encoding LacZ was used as a control. The multiplicity of infection used to infect the cells was 25.
Immunoprecipitation and Immunoblot Studies
Cells were lysed in modified RIPA buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% Nonidet P-40, 1 mm EDTA, 1 mm NaF) containing protease and phosphatase inhibitors (1 mm phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mm Na3VO4; Sigma). The protein amount was measured with a BCA kit (Bio-Rad). For immunoprecipitation, the lysates were immunoprecipitated with the indicated antibody and immobilized on protein A/G PLUS-agarose beads (Santa Cruz Biotechnology). The lysates (50 μg of protein) or the precipitant were fractionated by SDS-PAGE, transferred to polyvinylidene difluoride or nitrocellulose membrane, and blocked with 5% skim milk. Antigens were detected using the indicated antibodies and then with horseradish peroxidase-conjugated secondary antibodies and detected with the ECL system (GE Healthcare).
Transfection Assays
Transfection of siRNA or DNA and siRNA-DNA was performed using Lipofectamine 2000 (Invitrogen). The sequence of siRNA for IRS2 is sense 5′-GGGCUGAGGAAGCGGACUUtt-3′, and the sequence of siRNA for p85 is sense 5′-GGGAAGAGGACAUUGACUUaa-3′. One day before transfection, cells were plated in Opti-MEM reduced serum medium (Invitrogen) in 6-well dishes. On the day of transfection, siRNA or DNA or siRNA-DNA molecule LipofectamineTM 2000 complex was added to cells 48 h before harvesting.
Far Western Analysis
BAEC with overexpression of IRS1 were exposed to insulin and/or PMA (100 nm) as described above. Total cell lysates were immunoprecipitated with IRS1 (100 nm) antibody and separated on SDS-PAGE. Membranes used for transfer were incubated with 1 μg/ml synthetic p85α-GST in PBST + 5% BSA at 4 °C overnight, followed by anti-GST-HRP (sc459) in PBST + 5% skim milk. The signals were visualized with chemiluminescence and film.
PI3K Activity Assay
Method for PI3K assay was performed as described previously (21).
Isolation of p85α from BAEC for TOF-MS/MS
BAEC (20 plates of p150 culture dishes for each experiment) were infected with 1–2 × 108 infection units/dish of adenovirus encoded with human HA-tagged p85α. On the day after infection, cells were starved in 0.1% BSA/DMEM-L for 24 h and then incubated with 100 nm PMA for 20 min before washing with ice-cold PBS and harvesting with IP lysis buffer. Samples were centrifuged at 10,000 rpm for 25 min at 4 °C. Supernatant yielded ∼100 mg of protein that was incubated with 1:600 of anti-HA antibody at 4 °C for 24 h and then was centrifuged at 10,000 rpm for 25 min at 4 °C. Then supernatant was incubated with protein A/G-agarose (Santa Cruz Biotechnology) for 2 h at 4 °C. The beads were collected, washed, and boiled with sample buffer. Denatured protein was separated on SDS-PAGE (7.5%). A p85α band was identified by Coomassie staining and parallel immunoblotting.
LC-MS/MS Analysis
p85 was immunoprecipitated from bovine aortic endothelial cell lysates, and the immunoprecipitate was separated by SDS-PAGE. p85 protein was visualized by staining with Coomassie Brilliant Blue G-250 stain (Bio-Rad), and the gel slice containing p85 was digested with sequencing grade modified trypsin (Promega). The resultant tryptic digest was separated by capillary HPLC (C18, 75 μm inner diameter Picofrit column, New Objective) and analyzed using an LTQ ion trap mass spectrometer (ThermoElectron). Synthetic phosphopeptide, corresponding to tryptic sequences derived from p85, was obtained from Boston Biomolecules, Inc., and analyzed using the LC-MS/MS protocol described above. Spectra containing a neutral ion loss of −98 and 49 were analyzed using Sequest (BioWorks 3.3, Thermo Electron) with fragment ion tolerance <0.5 and amino acid modification variables, including phosphorylation (80 Da) of Ser, Thr, and Tyr.
Statistical Analysis
Data are presented as the means ± S.D. Comparisons between two groups were performed with unpaired Students' t test. Multiple comparisons were performed with one-way analysis of variance, and Student-Newman-Keuls method was used for post hoc tests. p values less than 5% were considered statistically significant.
RESULTS
Characterization of Inhibitory Effects of PKC on Insulin Signaling Cascade
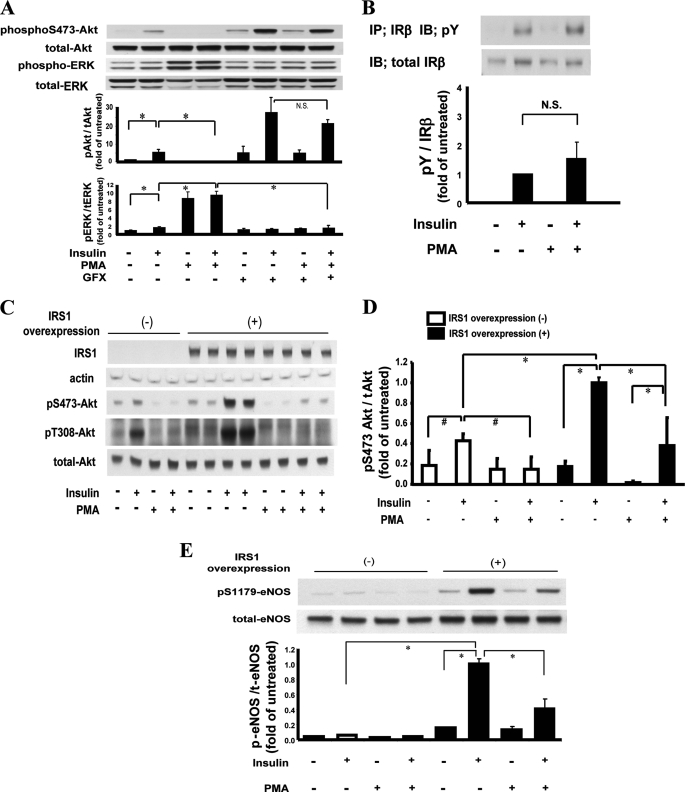
The effect of PKC activation on the induction of insulin on Akt and ERK phosphorylation was studied using PMA, which mimics diacylglycerol and can activate conventional and novel PKC isoforms (22). As reported, insulin (100 nm) increased phosphorylation of Ser-473-Akt (p-Akt Ser-473) by 6.7 ± 1.8-fold, which was inhibited completely by the addition of PMA (Fig. 1A). However, the addition of GFX, a general PKC inhibitor, prevented the actions of PMA on the induction of insulin on p-Akt Ser-473. Insulin alone increased phosphorylation of ERK1/2 (p-ERK1/2) by 1.7 ± 0.3-fold, which was increased further by 9.5 ± 1.0-fold in the presence of PMA. Addition of GFX inhibited the enhancing actions of PMA on insulin-induced p-ERK1/2 (Fig. 1A).
FIGURE 1.
A, effect of PKC activation on insulin signaling cascade. Effect of PMA on insulin induced p-Akt and p-ERK. BAEC were pretreated with PMA and stimulated with insulin with and without PKC inhibitor GFX (5 μm). The density of the bands was detected by enhanced chemiluminescence and quantitated densitometrically (n = 4; *, p < 0.01). B, effect on insulin-stimulated Tyr(P) (pY) of IRβ. BAEC were treated with PMA and/or insulin. The samples were immunoprecipitated (IP) with anti-insulin receptor β antibody and immunoblotted by anti-phosphotyrosine antibodies (IB). Bar graph shows the ratios of phosphotyrosine to IRβ signal (n = 3) (N.S. = not significant). C, immunoblot effect of insulin induced p-Akt on Ser-473 and Thr-308 in BAEC overexpressing IRS1. D, densitometrical quantitation of Akt phosphorylation on Ser-473 in BAEC overexpressing IRS1. Each bar shows the ratio of phosphoserine 473 to total Akt (n = 4; *, p < 0.01; #, p < 0.05). E, effect of insulin and PMA on p-eNOS at Ser-1179 in BAEC overexpressing IRS1 (n = 4; *, p < 0.01). IB, immunoblot analysis.
To identify the step(s) of the insulin signaling pathway, which is targeted by PKC activation in BAEC, we characterized in sequence from the IR to p-Akt Ser-473. Analyzing IR in BAEC showed that insulin significantly increased tyrosine phosphorylation (Tyr(P)) of the IRβ subunit, which was not reduced by the addition of PMA, suggesting that the target of PKC is post-IR in BAEC (Fig. 1B).
To clearly evaluate whether PKC activation can alter IRS1, we overexpressed IRS1 by 10-fold, which enhanced the effect of insulin on p-Akt Ser-473 by 2.3-fold (p < 0.01) (Fig. 1, C and D). PMA blocked insulin-induced p-Akt Ser-473 in wild type (WT) cells and IRS1-overexpressed BAEC by 62% (p < 0.01). Changes in phosphorylation of Thr-308-Akt were parallel to that of Ser-473-Akt in BAEC. Phosphorylation of eNOS Ser-1179, (p-eNOS Ser-1179) downstream of Akt was also enhanced with the overexpression of IRS1, which was increased with the addition of insulin by 6.6-fold and inhibited by PMA by 59% (p < 0.01), in parallel with changes in p-Akt Ser-473 (Fig. 1E).
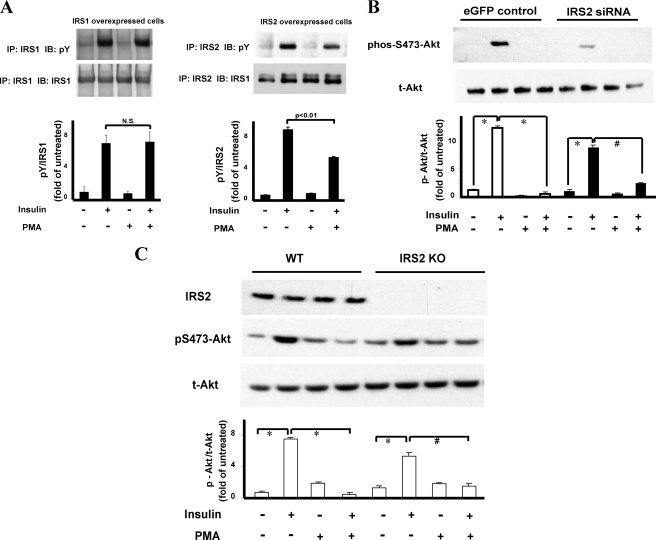
Using the BAEC overexpressing with IRS1 or IRS2, insulin significantly increased Tyr(P) of IRS1 (Tyr(P) IRS1) by 7.0-fold and IRS2 (Tyr(P) IRS2) by 8.7-fold. The addition of PMA did not inhibit insulin-induced Tyr(P) IRS1, but it significantly inhibited Tyr(P) IRS2 by 39% (Fig. 2A).
FIGURE 2.
Comparative analysis of PKC activation on IRS1 and IRS2 activation. A, effect on insulin-induced Tyr(P) (pY) on IRS1 and IRS2. BAEC were treated with PMA and/or insulin. Samples were immunoprecipitated (IP) with anti-IRS1 or IRS2 antibody and immunoblotted (IB) with antibodies to IRS1, IRS2, or Tyr(P). Bar graph shows the ratios of phosphotyrosine to IRS1 (n = 4) or IRS2 signal (n = 3) (N.S., not significant). B, effect of IRS2 knockdown on insulin activation of p-Akt. BAEC transfected with IRS2, siRNA or enhanced GFP was pretreated with and without PMA or insulin. Results show immunoblot analysis by antibodies to Akt and p-Akt as indicated. (n = 3; *, p < 0.01; #, p < 0.05). C, effect of insulin on endothelial cells from IRS2KO mouse. Lung endothelial cells from wild type mice (WT) and IRS2KO mice were pretreated with PMA and stimulated with insulin (*, p < 0.01; #, p < 0.05) and immunoblotted (IB) with antibodies to IRS2, Ser(P)-473-Akt, or total-Akt (t-Akt) (n = 4; *, p < 0.01; #, p < 0.05).
The lack of inhibitory effect of PKC activation on IRS1 tyrosine phosphorylation, although it still decreased insulin action on p-Akt, was surprising because PKC has been reported to increase Ser/Thr phosphorylation of IRS1 in nonendothelial cells and inhibit its signaling function (10). To confirm that IRS1-mediated insulin signaling can be inhibited by PKC activation in BAEC, we reduced IRS2 levels with siRNA by 59% in BAEC (supplemental Fig. 1), and we observed that insulin still strongly induced p-Akt Ser-473 by 8.5-fold, which was also inhibited in the presence of PMA by 76% (Fig. 2B). To confirm that IRS1 can clearly mediate insulin activation of Akt/eNOS, lung vascular endothelial cells were isolated from IRS2 knock-out (IRS2KO) mice and studied. As shown in Fig. 2C, insulin activation of p-Akt in IRS2KO endothelial cells was reduced compared with WT mice, but PMA still inhibited insulin activation in endothelial cells from WT (by 94%) and IRS2KO (by 77%) mice significantly. These results suggest that PKC activation can inhibit IRS1- and IRS2-mediated signaling for the activation of p-Akt/p-eNOS by different mechanisms in endothelial cells.
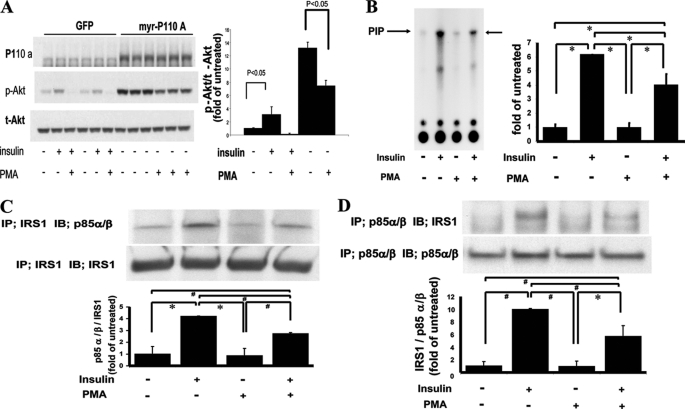
To clarify the discrepancy between the lack of effects of PKC activation on insulin-induced total Tyr(P) of IRS1 and inhibition of p-Akt, the inhibitory effects of PKC activation on several steps of the insulin signaling cascade from IRS1 to p-Akt were characterized. First, we assessed the extent of the inhibitory effects of PMA at the pre- or post-p110/PI3K step by overexpressing constitutively active p110/PI3K in BAEC (BAEC-myr-p110A) (Fig. 3A). The addition of PMA to BAEC-myr-p110A inhibited p-Akt by only 44%, indicating that ∼50% of the inhibitory effects of PKC are targeted to steps before the activation of p110/PI3K. Because IRS1 appears to mediate a majority of the activation of insulin for p-Akt in BAEC and did not exhibit a decrease in its total tyrosine phosphorylation with PKC activation, we characterized IRS1 and its association with p85/PI3K by assessing the various known phosphorylation sites on IRS1, which have been shown to affect IRS1 functions (Table 1). Surprisingly, Ser-307, the best characterized residue to be phosphorylated by PKC activation and to decrease IRS1 function, did not increase in phosphorylation level with PMA (23). Residue Ser-612 of IRS1, which is known to be phosphorylated by MAPK or PKC, increased by 1.9 ± 0.3-fold (p < 0.05) with the addition of PMA in BAEC (19). However, the phosphorylation of Tyr-608, which is adjacent to Ser-612 and needed for binding to p85/PI3K, did not show a concomitant inhibition by PKC activation when stimulated by insulin (24). Moreover, pretreatment of BAEC with PD98059, a MEK inhibitor, completely blocked the phosphorylation of Ser-612/IRS1 induced by PMA, but it did not change the inhibitory effect of PMA on insulin-induced IRS1-associated PI3K activity and Ser(P)-473 of Akt (Table 1).
FIGURE 3.
Regulation of insulin-induced PI3K activity and association with IRS1 by PKC activation. A, effect of constitutively active p110/PI3K (myr-P110A) on p-Akt. BAEC, transfected with GFP or constitutively active p110/PI3K plasmid, were treated with insulin or PMA. p110α, p-Akt, and t-Akt were assessed by immunoblot analysis in three separate experiments (n = 3). B, effect of insulin and PMA on PI3K activity associated with IRS1. Left, representative thin layer chromatography with arrows point to 32P incorporation into phosphatidylinositol (PIP). Right, bar graph shows densitometrical quantitation of 32P-phosphorylated phosphatidylinositol (n = 3; *, p < 0.001). C, effect of PKC activation on IRS1-associated p85α/β in IRS1-overexpressed cells. Top, immunoprecipitation (IP) with anti-IRS1 antibodies followed by immunoblotting (IB) with indicated antibodies. Bottom, densitometry quantitation of p85α/β to IRS1 levels (n = 3; *, p < 0.001; #, p < 0.005). D, effect of PKC activation on the amount of p85-associated IRS1 in IRS1-overexpressed cells. Top, immunoprecipitation with anti-p85α/β antibodies followed by immunoblot with indicated antibodies are shown. Bottom, densitometry quantitation of IRS1 to p85α/β signal ratio are shown (n = 3; *, p = 0.001; #, p < 0.001).
TABLE 1.
Effect of insulin and PMA on various phosphorylation sites on IRS1
| Residue | Treatment with insulin | Treatment with PMA |
|---|---|---|
| Ser-307 | No change | No change |
| Ser-612 | No change | 1.9 ± 0.3-fold increasea (p < 0.05) |
| Tyr-608 | 2.6 ± 0.3-fold increase (p < 0.05) | No effect on insulin-stimulated increase |
| Tyr-891 | No change | No change |
a Reversal of phosphorylation with PD98059 did not affect the inhibitory effect of PMA as assessed by PI3K activity and Akt phosphorylation.
Assessing PI3K Activities and Interactions with IRS1
Because PMA did not alter insulin-induced tyrosine phosphorylation of the IRβ subunit and IRS1, we assessed PI3K activities induced by insulin with and without the addition of PMA. As shown in Fig. 3B, insulin increased IRS1-associated PI3K activity by 6.3-fold, which was significantly inhibited by 35 ± 12.8% with the addition of PMA (p < 0.001). In addition, we characterized the association between IRS1 and p85α/β/PI3K isoforms and found that insulin increased the association between IRS1 and p85α/β subunit of PI3K by 4.2-fold, which was deceased by 35% (p < 0.005) in the presence of PMA when the complex was immunoprecipitated with antibodies to IRS1 (Fig. 3C). Similarly, when immunoprecipitation was performed with antibodies to p85α/β, insulin increased IRS1 association with p85α/β/PI3K by 9.9-fold, which was reduced by 43% in the presence of PMA(p < 0.001) (Fig. 3D).
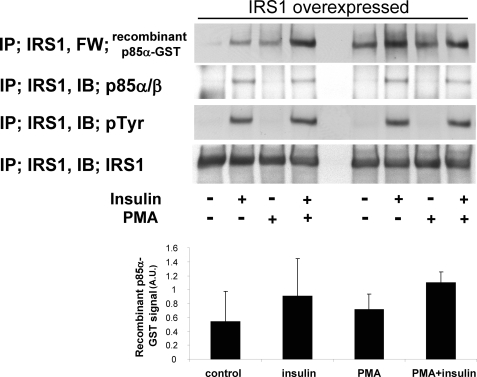
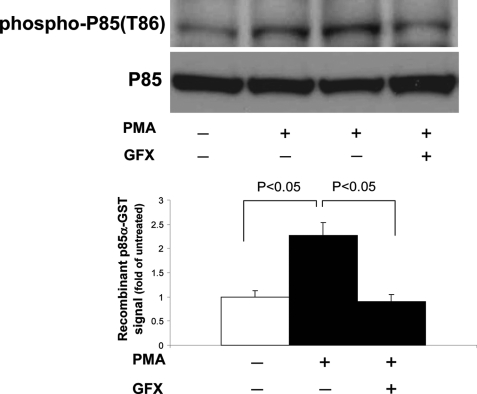
To confirm that the change in p85/PI3K is partially responsible for PKC-induced inhibition of interactions between IRS1 and p85, we performed far Western analysis using recombinant p85α-GST fusion protein. As shown in Fig. 4, insulin increased Tyr(P) of IRS1 in BAEC-overexpressing IRS1, which was not reduced by PMA, but the association between IRS1 and endogenous p85/PI3K was decreased by 35% (Fig. 3C). In contrast, the association between recombinant p85α-GST protein and endogenous IRS1 was not inhibited by PMA (Fig. 4). No association was observed between IRS1 and the carboxyl-terminal peptide of p85α as a control (supplemental Fig. 3). These data suggest that PKC affects endogenous p85α/β subunit of PI3K in BAEC and decreases its binding to IRS1.
FIGURE 4.
Far Western (FW) analysis of IRS1 association with recombinant p85α-GST and endogenous p85α/β. Upper panels, representative blots of immunoprecipitation (IP) with indicated probe or antibodies. Lower panels, bar graph of densitometry quantitations of p85α-GST signals. PMA, phorbol myristate acetate; PY, antibody to phosphotyrosine. AU, arbitrary units.
Analysis of p85α/β/P13K Phosphorylation Sites with PKC Activation
To determine the potential phosphorylation sites on p85/P13K that are induced by PMA, p85α was overexpressed in BAEC by adenoviral vector infection containing p85α and exposed to PMA for 30 min, and p85α was immunoprecipitated by anti-p85α antibodies and separated by gel electrophoresis. We identified MS2 spectra corresponding to the phosphopeptide ISPPT*PK using LC-MS/MS analysis of the tryptic digest of p85 isolated from PMA-stimulated BAEC were identified (Fig. 5). MS2 spectra from the p85-derived tryptic peptide and the corresponding synthetic peptide (ISPPT*PK) displayed similar distributions of y and b fragment ions for 1+ and 2+ precursor ions. In addition, MS2 spectra for 1+ precursors from the tryptic and synthetic peptides contained a prominent fragment, a 721 m/z, which is consistent with neutral ion loss. To document further that PKC activation by PMA increased the phosphorylation of Thr-86 of p85α in BAEC, antibodies were raised against phosphorylated Thr-86/p85α (Thr(P)-86/p85α) peptides. Τhe specificity of the antibody was validated against Thr(P)-86 peptide in vitro. As shown in Fig. 6, PMA increased the level of Thr(P)-86/p85α in BAEC by 2.3-fold (p < 0.05). The increase of Thr(P)-86/p85α was inhibited completely by the addition of PKC inhibitor GFX.
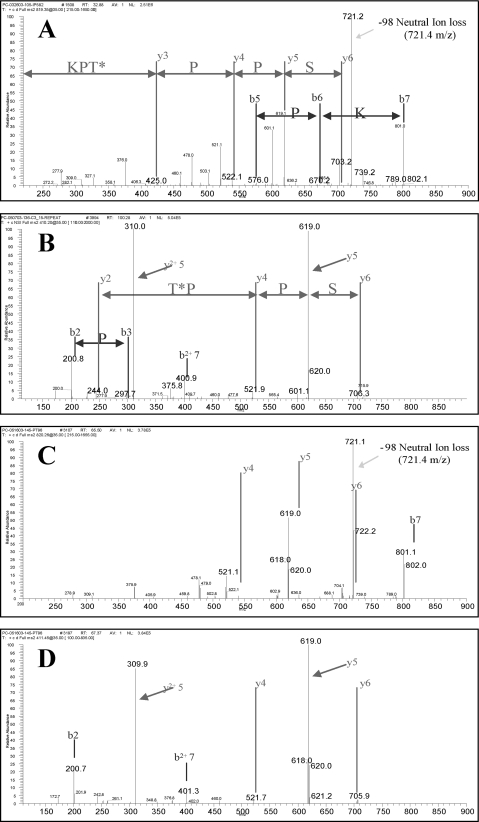
FIGURE 5.
MS2 spectra corresponding to p85α phosphorylation at Thr-86. Panel A shows the MS2 spectra of a tryptic peptide (identified as ISPPT*PK) from gel-purified p85 isolated from BAEC. Spectra shown is from a 1+ precursor with 819.2 m/z. This precursor displayed a prominent neutral ion loss at 721.2 m/z. (* indicates the phosphorylated residue Thr-86). The phosphopeptide sequence was assigned using Sequest. Panel B shows the MS2 spectra of 2+ ion at 410.2 m/z, which co-elutes with 819.2. The MS2 of this 2+ precursor provided 1+ and 2+ ions associated with ISPPT*PK fragmentation. These spectra and sequence assignments were confirmed in three separate experiments. Panels C and D show MS2 spectra of synthetic peptide ISPPT*PK as a 1+ and 2+ precursor, respectively.
FIGURE 6.
Effect of PKC activation on phosphorylation Thr-86/p85α levels in BAEC. Cells were treated with PMA or GFX (PKC inhibitor). Total cell lysates were separated on SDS-PAGE and immunoblotted with antibodies as directed at Thr(P)-86/p85α. Densitometry of immunoblot analysis from three different experiments (n = 3).
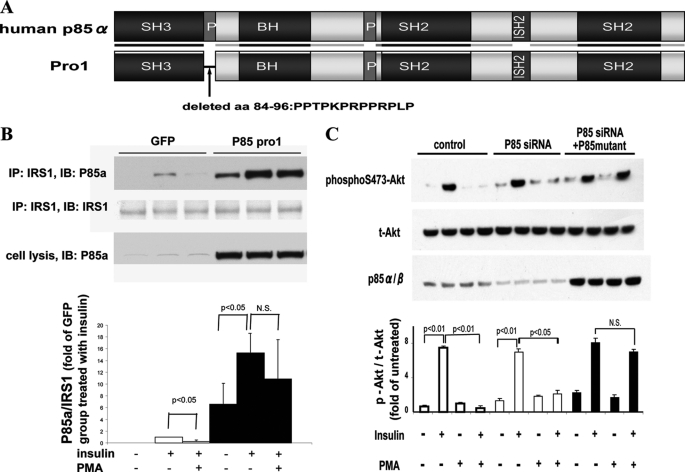
The functional role of Thr(P)-86/p85a was evaluated by overexpressing a plasmid containing human p85α deletion mutant, lacking 13 amino acids of p85α (84–96) (Fig. 7A). In BAEC overexpressing deletion mutant of human p85α (84–96), named pro-1, insulin increased its association with IRS1 by 1.5-fold similar to control BAEC. The addition of PMA decreased insulin-induced association between IRS1/p85α in the control cells by 72%. In the BAEC overexpressing deletion mutant p85α (84–96) pro-1, the inhibiting effects of PMA on the association between IRS1 and p85α was significantly reduced (Fig. 7B). The effect of overexpressing pro-1 was characterized further on activation of insulin on Akt (p-Akt). Because overexpression of p85α subunit inhibits p110/PI3K activities, we reduced the amount of endogenous p85α subunit using siRNA of p85α in BAEC with simultaneous transfection of pro-1. In these conditions, the levels of endogenous p85α in BAEC were reduced by 67%, and the amount of pro-1 mutant was significantly increased (Fig. 7C). p-Akt levels were increased by 7.5- and 7.0-fold with insulin in control BAEC and in cells transfected with siRNA of p85α, respectively. The addition of PMA inhibited insulin-induced p-Akt levels completely in wild type BAEC. In BAEC transfected with siRNA of p85α and pro-1 (p85 mutant), insulin again increased p-Akt levels by 8.0-fold (p < 0.05). However, the inhibitory effect of insulin-induced PMA p-Akt levels was significantly reduced (Fig. 7C).
FIGURE 7.
Effect of p85α deletion mutant on actions of insulin and PMA. A, schema of wild type p85α and p85 deletion mutant p85 pro-1 deletion mutant; human p85α with deletion of 13 amino acids (aa) (84–96). B, effect of p85 deletion mutant (p85 pro-1) on association to IRS1 with and without insulin and PMA. Top, immunoprecipitation (IP) with anti-IRS1 antibodies followed by immunoblotting (IB) with indicated antibodies are shown. Bottom, densitometry analysis of p85α/β to IRS1 ratio in bar graph (n = 3). N.S., not significant. C, BAEC transfected with p85 siRNA and/or p85 pro-1 deletion mutant, pretreated with PMA, and stimulated with insulin. Total cell lysates were separated on SDS-PAGE and immunoblotted with antibodies as indicated (n = 3). N.S., not significant.
PKC Activation by PMA and Angiotensin on p-Akt
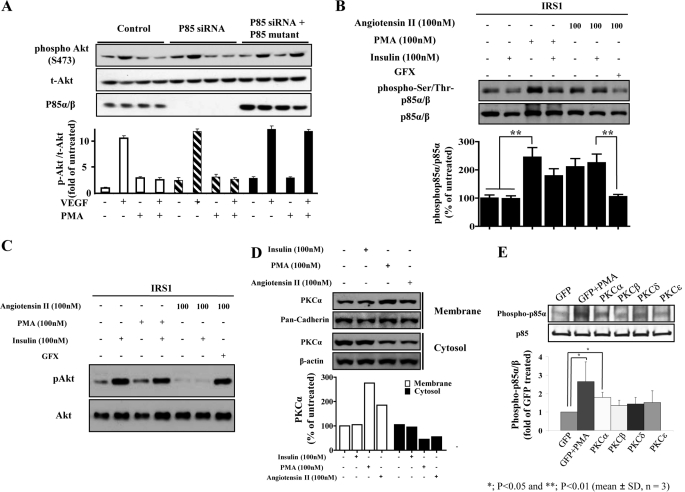
VEGF can also increase the levels of p-Akt after binding to vascular endothelial growth factor receptor 2 (VEGFR-2/KDR) and activating PI3K (25). However, unlike insulin, KDR can bind and activate p85/PI3K without IRS1/2. Thus, PKC activation by PMA with increased phosphorylation of p85/PI3K should also inhibit the actions of VEGF. As shown in Fig. 8, VEGF increased p-Akt by 10.6-fold (p < 0.001), which was inhibited completely by the addition of PMA. Furthermore, the adding of PKC inhibitor GFX alone or with VEGF did not alter p-Akt levels. However, GFX prevented the inhibitory actions of PMA on activation of VEGF by p-Akt. To support Thr-86/p85 as the site for the inhibiting effects of PMA on activation of VEGF by p-Akt, the effect of overexpressing p85α pro-1 in BAEC was studied. In control BAEC, VEGF increased Ser(P)-473Akt by 10-fold, which was significantly inhibited by PMA. In BAEC overexpressing p85α siRNA, the endogenous levels of p85α/β were significantly decreased. However, VEGF was still able to increase p-Akt levels by 10-fold, which again was inhibited by PMA by 90% even though the levels of p85α/β were significantly decreased. In BAEC, transfected with p85 siRNA pro-1, the amounts of p85α/β were elevated by 11.7-fold above their endogenous levels. In contrast to wild type BAEC, VEGF was able to increase p-Akt/Akt levels equally with and without PMA in BAEC transfected with pro-1 (Fig. 8A).
FIGURE 8.
Inhibitory effect of angiotensin II on insulin signaling via increased Thr(P)-86/p85α in BAECs. A, BAECs were treated with or without PMA and followed by stimulation of VEGF after transfection with p85 siRNA and/or p85 pro-1 deletion mutant. B and C, BAEC cells were treated with angiotensin II at 100 nm for 2 h or PMA for 20 min followed by 100 nm insulin stimulation. B and C, same amount of total proteins (60 μg) was separately applied for immunoblot analysis using the antibodies specific for Thr(P)-85/p85α (B) and pAkt (C). The Thr(P)-85/p85α level was quantified by densitometry, normalized by p85α level, and expressed as % of untreated (mean ± S.D., n = 3). **, p < 0.01. D, under same conditions as B and C. The cells were then harvested and fractionated. The membrane and cytosolic protein were applied for immunoblot analysis using the specific antibody against PKCα. The membranes were stripped and re-blotted with antibodies for pan-cadherin and β-actin. E, different PKC isoforms were overexpressed in BAECs by using adenovirus or GFP adenovirus as a control. The cells were treated with 100 nm PMA and lysed. Total cell lysates were blotted with indicated antibodies. Densitometry of immunoblot analysis was from four different experiments (n = 4; *, p < 0.05).
We also studied the effect of angiotensin II (AngII, 100 nm) to inhibit the actions of insulin and to induce phosphorylation of phospho-Ser/Thr-p85α/β. As shown in Fig. 8B, AngII increased phospho-Ser/Thr-p85α/β by 2.1-fold similar to PMA, which was prevented completely by GFX, a general PKC inhibitor. Insulin did not have any effect on phospho-Ser/Thr-p85α/β in BAEC overexpressing IRS1. In BAEC overexpressing IRS1, AngII also inhibited the activation of insulin of pAkt similarly to PMA. The inhibition of AngII of insulin-induced p-Akt was also prevented by GFX (Fig. 8C). The effect of AngII to activate PKC isoforms was characterized and showed that PKCα isoform was the predominant isoform to be translocated from cytosol to the membrane by at least 2-fold similarly to PMA (Fig. 8D). AngII also caused translocation of PKCβ and -δ (data not shown). PMA also can activate PKCβI, -βII, -δ, and -ϵ isoforms at 20 min without causing down-regulation (supplemental Fig. 4). To confirm further, the effect of the PKCα isoform in mediating the phosphorylation of phospho-Ser/Thr p85α/β, we overexpressed the adenoviral vector containing PKCα, -βI, -δ, and -ϵ isoforms. The results, as shown in Fig. 8E, suggested that the overexpression of the PKCα isoform induced the phosphor-Ser/Thr p85α to a greater extent than PKCβI, -δ, and -ϵ isoforms.
DISCUSSION
We have reported that metabolic abnormalities due to insulin resistance and diabetes can cause selective inhibition of insulin signaling via the IRS/PI3K/Akt pathway and a decrease of insulin activation or expression of eNOS (10). In contrast, these metabolic abnormalities enhanced insulin signaling via the MAPK pathway, which are known to increase the expression of PAI-1, endothelin 1, and extracellular matrix in the arterial wall (26–29). This hypothesis of selective diminution of the actions of insulin via the IRS/Akt pathway may lead to accelerated atherosclerosis as observed in insulin resistance, and diabetes has been supported recently by the report that the deletion of insulin receptors specific to the endothelial cells increased the severity of atherosclerotic lesions in apoE null mice by 2–3-fold (6).
PKC activation, especially the β isoform, has been associated with endothelial dysfunction and atherosclerosis (12, 13). Mice with double knock-out of the PKC-β isoform and apoE exhibited significantly less atherosclerosis than apoE null alone (12). Treatment with the PKCβ-selective inhibitor improved forearm blood flow in people with hyperglycemia and also ameliorated endothelial dysfunction in insulin-resistant rodents (30). Multiple reports have shown that PKC activation can increase serine/threonine phosphorylation of IR and IRS1/2 and decrease their levels of tyrosine phosphorylation and functions induced by insulin (15–20, 31). Conventional and novel PKCα/β/δ/ϵ and -τ isoforms, activated by diacylglycerol, due to hyperlipidemia or hyperglycemia (19), have been reported to cause insulin resistance (15–20, 31) mainly in liver, muscle, or adipose tissue where there is an increase in threonine/serine phosphorylation of IR or IRS.
In endothelial cells, these results indicate PKC activation can inhibit several steps of the signaling pathways of insulin such as IRS2, p85/PI3K, and unknown post-PI3K steps. Our results also support the idea that IRS1 and IRS2 participates significantly in mediating activation of insulin of Akt and eNOS. For this study, we have focused on the role of IRS1 because elucidating all the PKC targets are beyond the scope of a single report.
Our data clearly established that IRS1 is functionally important in endothelial cells because endothelial cells from IRS2KO mice still responded to insulin. In addition, the global or endothelial cell-targeted IRS1KO mouse exhibited vascular endothelial dysfunction (32). Interestingly, PKC activation inhibited the insulin signaling cascade differently between IRS1 and IRS2 in endothelial cells. Further studies are in progress to provide an explanation for the differential effects of PKC activation on Tyr(P) of IRS1 and IRS2. Although we did not observe any decrease of insulin-induced tyrosine phosphorylation of IRS1 by PKC, we did find decreased binding of IRS1 to p85/PI3K and PI3K activity. Activation of PKC and MAPK has been reported to phosphorylate serine 612 on IRS1 (15, which in mouse liver can lead to the attenuation of downstream insulin signaling by decreasing the adjacent 608/IRS1 tyrosine phosphorylation that is essential for docking with p85/PI3K (24). Our study in endothelial cells also showed that PKC activation increased phosphorylation of serine 612/IRS1, but this did not inhibit insulin-induced phosphorylation of tyrosine 608/IRS1. Moreover, the MEK inhibitor, which completely blocked the phosphorylation of serine 612 induced by PMA, did not inhibit the effect of PMA on the activation of insulin by PI3K activity and p-Akt levels. These results indicate that phosphorylation of serine 612/IRS1 induced by PMA is not responsible for the inhibitory effect of PKC on the PI3K/Akt pathway in vascular endothelial cells. Another well documented effect of PKC activation is the phosphorylation at serine 307 of IRS1. PKCτ has been reported to phosphorylate serine 307/IRS1, followed by a decrease in its tyrosine phosphorylation in skeletal muscle and muscular insulin resistance (23). Furthermore, Ser-24, -318, -357, and -1101 of IRS1 have all been reported to be phosphorylated by PKC (15–20, 31). However, PMA did not increase the phosphorylation of these sites on IRS1 in endothelial cells.
PI3K is a key node for insulin signaling and is a critical member of various growth factors and cytokine signaling such as VEGF/Akt signaling. PI3K consists of heterodimer of regulatory subunits and catalytic subunits, and it is fully activated when the regulatory subunit binds to a phosphotyrosine residue of adapter proteins like IRS1 at the submembrane area of a cell (33). A p85 subunit of PI3K is not only an abundant regulatory subunit, but it has a unique regulatory function for this enzyme (34). Excessive amounts of p85 monomer can inhibit the activity of PI3K by competing with a PI3K heterodimer for binding to phosphotyrosines (34). The presence of excessive amounts of p85 monomer and its inhibitory function could explain the findings that its reduction by siRNA did not reduce the actions of insulin and VEGF (34). Although a great deal is known about the regulation of PI3K, only a few of the Ser/Thr phosphorylation sites on PI3K have been reported especially those due to PKC activation. One is Ser-608 on p85α, which is autophosphorylated by the catalytic subunit of p110/PI3K and serves as a negative regulator of the enzyme. The other residue is Ser-83 on p85α that is phosphorylated by protein kinase A, and kinase activity is enhanced (35). In this study, we have identified a novel phosphorylation site, Thr-86, on p85α that was phosphorylated with PKC activation induced by PMA and angiotensin. Our data suggest that it is phosphorylated by PKC and serves as a negative regulator because deletion of this region causes the loss of the effect of PKC on insulin-induced activation on PI3K. Our results suggest that the phosphorylation of Thr-86 reduces the binding of p85α to IRS1 and possibly IRS2 (supplemental Fig. 2). However, Thr-86/p85α is not within or adjacent to the SH2 domain, which is known as a binding site to phosphotyrosine. However, it is within one of the two proline-rich domains, which is reported to bind with the SH3 domain (36). It is not well understood what proteins are associated with p85α through proline-rich domains. Because various molecules, including p85α itself, have SH3 domains, it is possible that changes in interaction with a protein with an SH3 domain will reduce the affinity with IRS1. Thus, it is possible that a conformational change, induced by the phosphorylation of Thr-86/p85α, can lead to a change in subcellular sites and a decrease of the kinase activity with IRS1. Because the proline-rich domain is positively charged and phosphorylation may change this site to negative, it is possible for Thr-86 to modify its interactions with IRS1 and result in decreases in PI3K activation.
Because p85/PI3K activation is critical for the intracellular signaling of many cytokines and hormones, it was not surprising that the inhibitory action of PKC activation also reduced VEGF signaling via PI3K/Akt. Previously, we have identified PKCβ isoforms as most likely to inhibit the actions of insulin in endothelial cells via inhibition of the actions of insulin on Akt and eNOS. This is supported by recent reports that PKCβ isoform-selective inhibitors or the PKCβKO/apoE−/− mouse have decreased severity of atherosclerosis. It is also likely that activation of other PKC isoforms may even enhance VEGF action and angiogenesis (37). AngII appears to activate multiple PKC isoforms with the PKCα isoform being the most effective in phosphorylating Thr-86 of p85α/PI3K. Because AngII has been reported to inhibit insulin activation of p-AKT in the myocardium, it is likely that one of the mechanisms by which AngII is selectively inhibiting the action of insulin to cause endothelial dysfunction is by activating PKC, predominantly the α isoform and phosphorylating Thr-86/p85α/PI3K.
In summary, this report has characterized several potential sites by which PKC activation can inhibit the actions of insulin via the p-IRS/P13K/Akt pathway in endothelial cells. One such novel specific site was identified to be Thr-86 on p85/PI3K, which interfered with its association with IRS1 to decrease insulin and the action of VEGF on Akt and eNOS. Because elevation of fatty acid levels and enhancement of AngII actions can activate PKC, we suggest that increased phosphorylation on p85/PI3K by PKC activation could be partially responsible for endothelial dysfunction observed in these pathological conditions.
Acknowledgments
We acknowledge the assistance of Jonathon Winnay for the protocol of the PI3K assay, Dr. C. Ronald Kahn for kindly providing the IRS1 and IRS2 adenoviruses and suggestions, and Dr. Jonathan Becker for kindly providing the p85 mutant (pro-1) plasmid.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-DK53105 from NIDDK and P30-DK36836-21 from NIDDK (DERC). This work was also supported by American Diabetes Association Grant 1-08-RA-93.

This article contains supplemental Figs. 1–4.
- eNOS
- endothelial NOS
- BAEC
- bovine aortic endothelial cell
- PMA
- phorbol 12-myristate 13-acetate
- AngII
- angiotensin II
- IR
- insulin receptor
- IRS
- insulin-resistant state
- SH
- Src homology
- GFX
- GF109203X.
REFERENCES
- 1. Moller D. E., Flier J. S. (1991) Insulin resistance. Mechanisms, syndromes, and implications. N. Engl. J. Med. 325, 938–948 [DOI] [PubMed] [Google Scholar]
- 2. Steinberg H. O., Brechtel G., Johnson A., Fineberg N., Baron A. D. (1994) Insulin-mediated skeletal muscle vasodilation is nitric oxide-dependent. A novel action of insulin to increase nitric oxide release. J. Clin. Invest. 94, 1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuboki K., Jiang Z. Y., Takahara N., Ha S. W., Igarashi M., Yamauchi T., Feener E. P., Herbert T. P., Rhodes C. J., King G. L. (2000) Regulation of endothelial constitutive nitric-oxide synthase gene expression in endothelial cells and in vivo. A specific vascular action of insulin. Circulation 101, 676–681 [DOI] [PubMed] [Google Scholar]
- 4. Zeng G., Quon M. J. (1996) Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J. Clin. Invest. 98, 894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rask-Madsen C., King G. L. (2007) Mechanisms of disease. Endothelial dysfunction in insulin resistance and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 3, 46–56 [DOI] [PubMed] [Google Scholar]
- 6. Rask-Madsen C., Li Q., Freund B., Feather D., Abramov R., Wu I. H., Chen K., Yamamoto-Hiraoka J., Goldenbogen J., Sotiropoulos K. B., Clermont A., Geraldes P., Dall'Osso C., Wagers A. J., Huang P. L., Rekhter M., Scalia R., Kahn C. R., King G. L. (2010) Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 11, 379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng G., Nystrom F. H., Ravichandran L. V., Cong L. N., Kirby M., Mostowski H., Quon M. J. (2000) Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101, 1539–1545 [DOI] [PubMed] [Google Scholar]
- 8. Jiang Z. Y., He Z., King B. L., Kuroki T., Opland D. M., Suzuma K., Suzuma I., Ueki K., Kulkarni R. N., Kahn C. R., King G. L. (2003) Characterization of multiple signaling pathways of insulin in the regulation of vascular endothelial growth factor expression in vascular cells and angiogenesis. J. Biol. Chem. 278, 31964–31971 [DOI] [PubMed] [Google Scholar]
- 9. Geraldes P., Yagi K., Ohshiro Y., He Z., Maeno Y., Yamamoto-Hiraoka J., Rask-Madsen C., Chung S. W., Perrella M. A., King G. L. (2008) Selective regulation of heme oxygenase-1 expression and function by insulin through IRS1/phosphoinositide 3-kinase/Akt-2 pathway. J. Biol. Chem. 283, 34327–34336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang Z. Y., Lin Y. W., Clemont A., Feener E. P., Hein K. D., Igarashi M., Yamauchi T., White M. F., King G. L. (1999) Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J. Clin. Invest. 104, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geraldes P., King G. L. (2010) Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 106, 1319–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harja E., Chang J. S., Lu Y., Leitges M., Zou Y. S., Schmidt A. M., Yan S. F. (2009) Mice deficient in PKCβ and apolipoprotein E display decreased atherosclerosis. FASEB J. 23, 1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naruse K., Rask-Madsen C., Takahara N., Ha S. W., Suzuma K., Way K. J., Jacobs J. R., Clermont A. C., Ueki K., Ohshiro Y., Zhang J., Goldfine A. B., King G. L. (2006) Activation of vascular protein kinase C-β inhibits Akt-dependent endothelial nitric-oxide synthase function in obesity-associated insulin resistance. Diabetes 55, 691–698 [DOI] [PubMed] [Google Scholar]
- 14. Taniguchi C. M., Emanuelli B., Kahn C. R. (2006) Critical nodes in signaling pathways. Insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 [DOI] [PubMed] [Google Scholar]
- 15. Nawaratne R., Gray A., Jørgensen C. H., Downes C. P., Siddle K., Sethi J. K. (2006) Regulation of insulin receptor substrate 1 pleckstrin homology domain by protein kinase C. Role of serine 24 phosphorylation. Mol. Endocrinol. 20, 1838–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waraich R. S., Weigert C., Kalbacher H., Hennige A. M., Lutz S. Z., Häring H. U., Schleicher E. D., Voelter W., Lehmann R. (2008) Phosphorylation of Ser-357 of rat insulin receptor substrate-1 mediates adverse effects of protein kinase Cδ on insulin action in skeletal muscle cells. J. Biol. Chem. 283, 11226–11233 [DOI] [PubMed] [Google Scholar]
- 17. Li Y., Soos T. J., Li X., Wu J., Degennaro M., Sun X., Littman D. R., Birnbaum M. J., Polakiewicz R. D. (2004) Protein kinase Cθ inhibits insulin signaling by phosphorylating IRS1 at Ser-1101. J. Biol. Chem. 279, 45304–45307 [DOI] [PubMed] [Google Scholar]
- 18. Pillay T. S., Xiao S., Olefsky J. M. (1996) Glucose-induced phosphorylation of the insulin receptor. Functional effects and characterization of phosphorylation sites. J. Clin. Invest. 97, 613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Fea K., Roth R. A. (1997) Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry 36, 12939–12947 [DOI] [PubMed] [Google Scholar]
- 20. Greene M. W., Morrice N., Garofalo R. S., Roth R. A. (2004) Modulation of human insulin receptor substrate-1 tyrosine phosphorylation by protein kinase Cδ. Biochem. J. 378, 105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ueki K., Yamauchi T., Tamemoto H., Tobe K., Yamamoto-Honda R., Kaburagi Y., Akanuma Y., Yazaki Y., Aizawa S., Nagai R., Kadowaki T. (2000) Restored insulin sensitivity in IRS-1-deficient mice treated by adenovirus-mediated gene therapy. J. Clin. Invest. 105, 1437–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koya D., King G. L. (1998) Protein kinase C activation and the development of diabetic complications. Diabetes 47, 859–866 [DOI] [PubMed] [Google Scholar]
- 23. Rui L., Aguirre V., Kim J. K., Shulman G. I., Lee A., Corbould A., Dunaif A., White M. F. (2001) Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser-307 via distinct pathways. J. Clin. Invest. 107, 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Esposito D. L., Li Y., Cama A., Quon M. J. (2001) Tyr(612) and Tyr(632) in human insulin receptor substrate-1 are important for full activation of insulin-stimulated phosphatidylinositol 3-kinase activity and translocation of GLUT4 in adipose cells. Endocrinology 142, 2833–2840 [DOI] [PubMed] [Google Scholar]
- 25. Guo D., Jia Q., Song H. Y., Warren R. S., Donner D. B. (1995) Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J. Biol. Chem. 270, 6729–6733 [DOI] [PubMed] [Google Scholar]
- 26. Oliver F. J., de la Rubia G., Feener E. P., Lee M. E., Loeken M. R., Shiba T., Quertermous T., King G. L. (1991) Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J. Biol. Chem. 266, 23251–23256 [PubMed] [Google Scholar]
- 27. Grenett H. E., Benza R. L., Fless G. M., Li X. N., Davis G. C., Booyse F. M. (1998) Genotype-specific transcriptional regulation of PAI-1 gene by insulin, hypertriglyceridemic VLDL, and Lp(a) in transfected, cultured human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 18, 1803–1809 [DOI] [PubMed] [Google Scholar]
- 28. Nordt T. K., Sawa H., Fujii S., Bode C., Sobel B. E. (1998) Augmentation of arterial endothelial cell expression of the plasminogen activator inhibitor type-1 (PAI-1) gene by proinsulin and insulin in vivo. J. Mol. Cell. Cardiol. 30, 1535–1543 [DOI] [PubMed] [Google Scholar]
- 29. Goldstein R. H., Poliks C. F., Pilch P. F., Smith B. D., Fine A. (1989) Stimulation of collagen formation by insulin and insulin-like growth factor I in cultures of human lung fibroblasts. Endocrinology 124, 964–970 [DOI] [PubMed] [Google Scholar]
- 30. Beckman J. A., Goldfine A. B., Gordon M. B., Garrett L. A., Creager M. A. (2002) Inhibition of protein kinase Cβ prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ. Res. 90, 107–111 [DOI] [PubMed] [Google Scholar]
- 31. Schmitz-Peiffer C., Biden T. J. (2008) Protein kinase C function in muscle, liver, and beta-cells and its therapeutic implications for type 2 diabetes. Diabetes 57, 1774–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abe H., Yamada N., Kamata K., Kuwaki T., Shimada M., Osuga J., Shionoiri F., Yahagi N., Kadowaki T., Tamemoto H., Ishibashi S., Yazaki Y., Makuuchi M. (1998) Hypertension, hypertriglyceridemia, and impaired endothelium-dependent vascular relaxation in mice lacking insulin receptor substrate-1. J. Clin. Invest. 101, 1784–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Backer J. M., Myers M. G., Jr., Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. (1992) Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 11, 3469–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ueki K., Fruman D. A., Brachmann S. M., Tseng Y. H., Cantley L. C., Kahn C. R. (2002) Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol. Cell. Biol. 22, 965–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cosentino C., Di Domenico M., Porcellini A., Cuozzo C., De Gregorio G., Santillo M. R., Agnese S., Di Stasio R., Feliciello A., Migliaccio A., Avvedimento E. V. (2007) p85 regulatory subunit of PI3K mediates cAMP-PKA and estrogen's biological effects on growth and survival. Oncogene 26, 2095–2103 [DOI] [PubMed] [Google Scholar]
- 36. Yu H., Chen J. K., Feng S., Dalgarno D. C., Brauer A. W., Schreiber S. L. (1994) Structural basis for the binding of proline-rich peptides to SH3 domains. Cell 76, 933–945 [DOI] [PubMed] [Google Scholar]
- 37. Xia P., Aiello L. P., Ishii H., Jiang Z. Y., Park D. J., Robinson G. S., Takagi H., Newsome W. P., Jirousek M. R., King G. L. (1996) Characterization of vascular endothelial growth factor's effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J. Clin. Invest. 98, 2018–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]