Background: GABABR signaling blocks neuronal firing ensuring appropriate cerebellar cortex output.
Results: Loss of RGS6 results in ataxia rescued by a GABABR antagonist and enhanced GABABR-GIRK current in neurons.
Conclusion: RGS6 is an essential component of GABA signaling in cerebellum and required for motor coordination.
Significance: RGS6 dysregulation could result in cerebellar ataxia, and thus, it might represent a novel target for pharmacological intervention.
Keywords: Cerebellum, G protein-coupled receptors (GPCRs), G proteins, GABA receptors, RGS proteins, GABABR, GIRK
Abstract
γ-Aminobutyric acid (GABA) release from inhibitory interneurons located within the cerebellar cortex limits the extent of neuronal excitation in part through activation of metabotropic GABAB receptors. Stimulation of these receptors triggers a number of downstream signaling events, including activation of GIRK channels by the Gβγ dimer resulting in membrane hyperpolarization and inhibition of neurotransmitter release from presynaptic sites. Here, we identify RGS6, a member of the R7 subfamily of RGS proteins, as a key regulator of GABABR signaling in cerebellum. RGS6 is enriched in the granule cell layer of the cerebellum along with neuronal GIRK channel subunits 1 and 2 where RGS6 forms a complex with known binding partners Gβ5 and R7BP. Mice lacking RGS6 exhibit abnormal gait and ataxia characterized by impaired rotarod performance improved by treatment with a GABABR antagonist. RGS6−/− mice administered baclofen also showed exaggerated motor coordination deficits compared with their wild-type counterparts. Isolated cerebellar neurons natively expressed RGS6, GABABR, and GIRK channel subunits, and cerebellar granule neurons from RGS6−/− mice showed a significant delay in the deactivation kinetics of baclofen-induced GIRK channel currents. These results establish RGS6 as a key component of GABABR signaling and represent the first demonstration of an essential role for modulatory actions of RGS proteins in adult cerebellum. Dysregulation of RGS6 expression in human patients could potentially contribute to loss of motor coordination and, thus, pharmacological manipulation of RGS6 levels might represent a viable means to treat patients with ataxias of cerebellar origin.
Introduction
The cerebellum is an important modulatory neuronal circuit that processes diverse motor information to ensure proper coordination of movement. Two distinct afferent pathways originating from multiple regions of the central nervous system converge on the neurons of the cerebellar cortex. The mossy fibers provide excitatory input to the cerebellar granule neurons (CGNs),4 which subsequently provide excitatory input through their parallel fibers to Purkinje neurons, while the climbing fibers form direct excitatory synapses with the well differentiated dendritic arbors of Purkinje neurons in the molecular layer (1). The net effect of cerebellar cortex excitation is the release of the inhibitory neurotransmitter GABA onto the deep cerebellar nuclei, the sites of synaptic integration of motor signals from the motor cortex, and other regions of the central and peripheral nervous system. Both input elements to the cerebellar cortex also activate a number of inhibitory interneurons that limit the extent of granule and Purkinje cell excitation.
GABA release from inhibitory interneurons located within the cerebellar cortex represents an important feed-forward and feedback inhibitory circuit that modulates neuronal excitation and subsequent neurotransmitter release (2). GABA release activates two distinct channel types: ionotropic GABAA receptors and metabotropic GABAB receptors (GABABRs). Activation of GABAA receptors results in fast chloride influx and membrane hyperpolarization. GABABRs influence neuronal excitability by promoting activation of Gαi/o proteins in response to GABA binding. Proper regulation of GABA-associated signaling is critical to proper cerebellar function as loss of GABA transporter 1 results in cerebellar ataxia characterized by impaired rotarod performance and gait abnormalities due to prolongation of GABA half-life at the synapse (3).
The functional GABABR is unique in that it exists as a heterodimer of the R1 and R2 subunits responsible for agonist binding as well as membrane trafficking and coupling to G proteins, respectively (4). Gβγ released from GABABR-activated Gαi/o proteins can facilitate either opening of G protein-activated inwardly rectifying K+ (GIRK) channels leading to potassium efflux or inhibition of P/Q- and N-type voltage-gated calcium channels in both cases leading to blockade of neuronal firing and neurotransmitter release (4–8). Loss of GABABR-activated GIRK2 currents has been observed in CGNs isolated from the weaver mouse model of cerebellar ataxia, highlighting the importance of GABABR-GIRK channel activation in cerebellum (7). Indeed, mice lacking neuronal GIRK channel subunits 1 and 2 are resistant to the ataxic effects of baclofen, a GABABR agonist (9).
Regulators of G protein signaling (RGS) proteins are essential components of the G protein-coupled receptor (GPCR)-G protein-GIRK channel signaling pathway required for recapitulation of native channel gating kinetics in heterologous systems (10). By stabilizing the transition state between the GTP- and GDP-bound forms of the Gα subunit, RGS proteins accelerate GTP hydrolysis and terminate the downstream signaling activity of both the α and βγ subunits of the heterotrimeric G protein complex. In this way, RGS proteins determine the magnitude and duration of the cellular response to GPCR stimulation (11, 12). RGS proteins are known to modulate GPCR pathways involving GIRK channel activation. In particular, loss of RGS9–2 results in deficits in motor coordination and working memory (13) due to its essential role in accelerating the termination of dopamine D2 receptor-mediated activation of GIRK channels (14–16). RGS2 is known to play a similar role in dopaminergic neurons of the ventral tegmental area where it contributes to low GABAB-GIRK signaling sensitivity (17). Despite the fact that RGS proteins have been shown to modulate numerous neuronal signaling pathways in the cerebrum and peripheral nervous system, little is known about the role they play in cerebellar function and the coordinated control of motor movement. Here, we provide the first interrogation of the functional role of a specific RGS protein in adult cerebellum.
RGS6 is a member of the R7 subfamily of RGS proteins, which are characterized by a distinct three-domain structure. Their function as GTPase-accelerating proteins (GAPs) for Gαi/o is conferred by the semi-conserved RGS domain common to all RGS proteins. Two additional domains unique to R7 family members, the GGL (Gγ subunit-like) domain and DEP/DHEX domain, allow for complex formation between RGS6 and the accessory proteins Gβ5 and R7BP, respectively. Binding to Gβ5 results in stabilization of both proteins, whereas binding to R7BP is thought to primarily control the subcellular localization of R7 family members (18). R7 family RGS proteins have been implicated in controlling motor movement, because Gβ5−/− mice, which lack functional expression of all R7 family members (19), exhibit an ataxic phenotype likely due to abnormal cerebellar development (20, 21). Our recent studies have identified RGS6, which is highly expressed in the atria and sinoatrial node, as an essential modulator of parasympathetic stimulation of the heart. In cardiac tissue RGS6 acts to limit Gβγ-mediated activation of cardiac GIRK channels by acetylcholine stimulation of muscarinic M2 receptors, thus attenuating IKAch current and effectively preventing parasympathetic override and severe bradycardia (22). Here, we show enriched expression of RGS6 in the granule cell layer of mouse cerebellum along with neuronal GIRK channel subunits 1 and 2. Based on its role in regulating cardiac GIRK channels, we hypothesized that RGS6 may function to terminate GIRK channel activation by GABABRs in CGNs to limit the extent of neuronal inhibition and ensure appropriate signal output from the cerebellar cortex. In fact, hippocampal neurons isolated from Gβ5−/− mice, which lack functional expression of all R7 RGS proteins, including RGS6 (19), exhibit a loss of rapid GIRK channel deactivation in response to GABABR stimulation (23). Changes in GABABR signaling in cerebellum could exert profound effects on motor coordination and movement.
EXPERIMENTAL PROCEDURES
Materials
GIRK1 and GIRK2 antibodies were from Alomone Laboratories (Israel), GABABR2 and Kv4.2 antibodies were from the University of California Davis/National Institutes of Health NeuroMab Facility, Davis, CA, and R7BP (W-16) and Gαi3 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Gβ5 and RGS7 antibodies were provided by Dr. Jason Chen (Virginia Commonwealth). Baclofen and actin antibodies were from Sigma, SCH-50911 was from Tocris Biosciences, Supersignal® West Pico chemiluminescent substrate was from Thermo Scientific, and nitrocellulose membrane was from Bio-Rad.
Mice
RGS6−/− mice were generated as described recently (22). Experiments were performed using age-matched (3–4 months old) wild-type (WT) and RGS6−/− mice. Experiments were performed in agreement with the Guide for the Use and Care of Laboratory Animals.
CGN Isolation
CGNs were isolated from cerebella of 4- to 6-day-old WT and RGS6−/− pups using the Papain Dissociation System (Worthington Biochemical Corp.) according to the manufacturer's protocol. Greater than 90% of the isolated CGNs stained positive for Kv4.2, which is expressed only in neurons in the granule cell layer of the cerebellum (see Fig. 1C).
FIGURE 1.
Expression of RGS6 in mouse cerebellum. A, immunohistochemical staining of RGS6 in cerebellar sections (5 μm) from WT and RGS6−/− mice (scale bar = 100 μm; white boxes, regions shown in enlarged images). B, RGS6 and Gβ5 protein expression in cerebellum of WT, RGS6+/−, and RGS6−/− mice. C, co-immunostaining of RGS6 and Kv4.2 in WT and RGS6−/− mice confirmed CGN-specific localization of RGS6 in cerebellum (scale bar = 100 μm; white boxes, regions shown in enlarged merged images).
Immunohistochemistry
Formaldehyde (4%)-perfused frozen brain sections and CGNs (grown on coverslips) from WT and RGS6−/− mice were processed to examine protein expression and localization. Briefly, cryosections were washed in PBS, blocked with 5% BSA, and incubated overnight at 4 °C with and without (control) anti-RGS6 rabbit polyclonal antibody (developed in our laboratory) or other antibodies. Following washing four times in PBS (10 min each), sections were incubated for 1 h at room temperature with Alexa Fluor® secondary antibodies (Invitrogen). Sections were visualized using epifluorescence microscopy as we described previously (22).
Electrophysiological Measurements of GIRK Currents in CGNs
Whole cell, patch clamp recordings were performed in CGNs from WT and RGS6−/− mice. CGNs were bathed in 500 nm tetrodotoxin-containing extracellular buffer composed of (mm) 120 NaCl, 20 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH 7.4. The intracellular/pipette buffer contained (mm) 120 KCl, 20 NaCl, 1 CaCl2, 1 MgCl2, 10 EGTA, 10 HEPES, 3 Mg-ATP, and 0.3 Na-GTP, pH 7.4. GIRK currents were recorded with the application of 200 nm baclofen for 30–35 s at a holding potential of −90 mV using an Axopatch 200B amplifier, connected to a Digidata 1440A digitizer, with pClamp10 acquisition software (Molecular Devices). Current recordings (n = 8–10) were analyzed for current density, activation/deactivation time constants, and desensitization, as described earlier (22), using Clampfit and Origin 7 software.
Co-immunoprecipitation
Mouse cerebras and cerebella were harvested and lysed separately in RIPA buffer. Lysates containing 1 mg of protein were pre-cleared at 4 °C for 1.5 h, by incubating with 10 μl of Protein A/G-agarose beads (Santa Cruz Biotechnology) and 0.4 μg of rabbit anti-FLAG IgG (Sigma). Cleared lysates were then incubated at 4 °C for 1.5 h with 2 μg of antibody against RGS6, followed by an additional overnight incubation with 20 μl of Protein A/G-agarose beads at 4 °C. At the end of the incubation, beads were collected by centrifugation at 1000 × g for 5 min at 4 °C, and washed three times with lysis buffer. After the final wash, immunoprecipitates were eluted from the beads with 30 μl of 1.5× SDS-PAGE sample buffer by heating the tube at 95 °C for 10 min. Resultant proteins were subjected to SDS-PAGE followed by transfer to nitrocellulose membranes and visualized using appropriate primary antibodies and Protein A-HRP conjugates (Abcam, Cambridge, MA).
GIRK1 was immunoprecipitated from the membrane fraction of lysed hippocampi, cerebella, and cerebra isolated from WT and RGS6−/− mice according to a previously published protocol (23). Resultant protein complexes were subjected to SDS-PAGE followed by transfer to nitrocellulose membranes and visualized using appropriate primary antibodies and Protein A-HRP conjugates.
Immunoblotting
Immunoblotting was performed either utilizing HRP-conjugated anti-rabbit protein A (Abcam) or anti-mouse secondary antibody (Millipore, Bedford, MA) and chemiluminescent substrate as previously described (24) or labeled secondary antibodies and the Odyssey Infrared Imaging System (LI-COR Biotechnology, Lincoln, NE).
Rotarod Performance Tests
WT and RGS6−/− mice (14 weeks old, n = 9 each) were tested on a motorized rotarod apparatus (Columbus Instruments). Mice were placed on the roller, and the time they remained on it during rotation was measured. Tests were performed at fixed speeds of 5, 10, or 15 rpm with acceleration (3 rpm/s) from 5 rpm. A maximum of 120 s was allowed per mouse for fixed speed tests. In subsequent experiments, WT and RGS6−/− mice (15 weeks old, n = 9 each) were injected with baclofen (10 mg/kg, subcutaneously) or SCH-50911 (30 mg/kg, intraperitoneally) prior to rotarod testing.
Gait Analysis
WT and RGS6−/− mice (14 weeks old, n = 8 each) were tested using the DigiGaitTM Imaging System (Mouse Specifics Inc., Boston, MA). This system uses video capture of the paws of the mice during treadmill locomotion (belt speed = 35 cm/s). The DigiGaitTM software is used to determine when individual paws are in contact with the treadmill and to calculate standard gait parameters.
Statistical Analysis
Data were analyzed by using Student's t test and one-way analysis of variance. Results were considered significantly different at p < 0.05. Values are expressed as means ± S.E.
RESULTS
Cerebellar Expression of RGS6
Our laboratory first cloned RGS6 in 1998 (GenBankTM accession no. AF073920) and later described the existence of complex alternative splicing of RGS6 in brain (25). Immunohistochemistry of whole mouse brain sections using an antibody that recognizes all splice forms of RGS6 showed robust expression of RGS6 in cerebellum that is absent in RGS6−/− mice (Fig. 1A). RGS6 expression is most prominent in the cerebellar granule layer, which was confirmed by co-immunostaining with a specific antibody against the Kv4.2 channel protein, which is strictly localized to CGNs (Fig. 1C). Western blotting of tissue lysates from cerebella of WT mice revealed the existence of multiple RGS6 isoforms whose expression was decreased in heterozygous mice and completely lost in the RGS6−/− animals indicating a gene dosage effect in these tissues (Fig. 1B). The requisite RGS6 binding partner Gβ5 was also expressed in cerebellum with no significant decrease in expression seen in mice lacking RGS6 (Fig. 1B).
Loss of RGS6 Results in Cerebellar Ataxia and Gait Abnormalities
Due to its high expression level in cerebellum, we hypothesized that loss of RGS6-mediated inhibition of cerebellar GPCRs might perturb cerebellar neurotransmission. Cerebellar dysfunction manifests phenotypically as impaired performance on the rotating rod (rotarod) (26), a classic test for motor coordination in rodent models, with occasional gait abnormalities (27–30). Gross examination of the carriage of RGS6−/− mice on the rotarod revealed both a lowered stance and hip position compared with their WT counterparts (Fig. 2A). RGS6−/− mice also showed a reduced ability to remain on the rotarod at both fixed speed (15 rpm) and when the rod was accelerating (Fig. 2, B and C). These data indicate that loss of RGS6 results in ataxia.
FIGURE 2.
Altered motor function phenotype of RGS6−/− mice. Mice lacking RGS6 exhibit (A) lower hip position, flattened stance, and impaired ability to stay on the rotarod at (B) fixed speed and (C) during acceleration from 5 rpm compared with their WT counterparts (n = 9). Data are presented as mean ± S.E.*, p < 0.05; **, p < 0.01 versus WT.
We next utilized a digital gait analysis system (DigiGaitTM) that is capable of quantitatively measuring numerous parameters in ambulatory animals to detect subtle changes in gait resulting from pharmacological or genetic manipulation without the confounding effects of inconsistencies in mouse walking speed (31). Gait analysis of mice of both genotypes revealed that RGS6−/− mice exhibited a number of differences in forelimb gait parameters. An example data curve with parameter descriptions can be found in Fig. 3A. Although mice of the two genotypes showed no change in total stride time or length (supplemental Table S1), the percent time spent in the swing phase (paw in air) decreased in RGS6−/− mice with a corresponding increase in the stance phase (time of paw contact with surface) (Fig. 3B). There was also a significant increase in the ratio of stance time to swing time in RGS6−/− animals (Fig. 3D) likely due, in part, to a decrease in total swing time (supplemental Table S1). The stance interval is composed of both the brake (time when animal is decelerating) and propulsion (time required for force generation) phases, which decreased and increased in RGS6−/− animals, respectively (Fig. 3C). The propulsion, but not brake phase of total stride time (Fig. 3B) as well as the total propulsion time (supplemental Table S1) also increased in mice lacking RGS6. RGS6−/− animals also exhibited decreases in stance width variability (supplemental Table S1). None of these parameters differed for hind limb tracings (Fig. 3, E and F), but RGS6−/− animals did exhibit increased hind limb stance width (Fig. 3G). Other measured gait parameters did not differ significantly between WT and RGS6−/− mice (supplemental Table S1). These data indicate subtle alterations in the gait of mice lacking RGS6, which could presumably contribute to their altered performance on the rotarod.
FIGURE 3.
Gait abnormalities of RGS6−/− mice. A, example trace from DigiGaitTM system with mouse gait indices indicated. Mice lacking RGS6 exhibit alterations in forelimb gait parameters including: B, an increase in the percentage of total stride time spent in the propel and stance phases with a corresponding decrease in swing time; C, an increase in percentage of total stance time spent in the propel phase with a corresponding decrease in braking; and D, an increase in the ratio of stance time to swing time (p values indicated on graph). E, percent stride time spent in each phase did not differ for hind limb nor did (F) percent stance time spent in braking and propulsion phases. G, RGS6−/− mice exhibit an increase in hind limb stance width. Data are presented as mean ± S.E. (n = 8 each WT and RGS6−/− mice). p values are indicated on the graph.
RGS6 Modulates GABAB Receptor Signaling
Based on the robust expression of RGS6 in cerebellum and the critical role of GABAB receptors in controlling cerebellar function, we hypothesized that RGS6 might function to terminate activation of neuronal GABABRs to limit the extent of neuronal inhibition and ensure appropriate signal output from the cerebellar cortex. If so, mice lacking RGS6 would be expected to exhibit a phenotype of enhanced GABABR signaling.
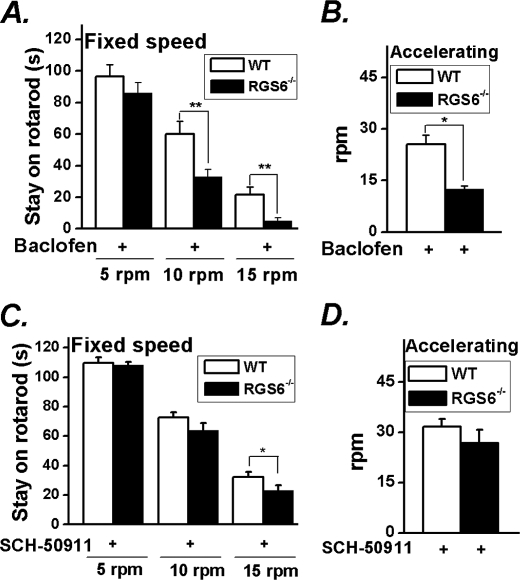
In rodents, baclofen induces deficits in motor coordination due to activation of neuronal GABABRs, which manifests as impaired performance on the rotarod instrument (32). We found that administration of baclofen resulted in an exaggerated ataxic phenotype in mice lacking RGS6. More specifically, RGS6−/− animals showed a reduced capacity to remain on the rotarod both at a fixed speed and when the rod was accelerating upon baclofen administration (Fig. 4, A and B) compared with their WT counterparts, although drug administration to mice of both genotypes caused a loss of performance when compared with untreated animals (fixed speeds of 10 or 15 rpm, p < 0.001; accelerating p < 0.05, p < 0.001 for WT and KO, respectively). These results indicate that RGS6 is an essential modulator of GABABR-signaling pathways controlling motor coordination.
FIGURE 4.
RGS6 modulates GABABR signaling. WT and RGS6−/− mice (n = 9 each) were tested at fixed speed (A) and during acceleration from 5 rpm (B) on the rotarod after administration of baclofen (10 mg/kg), a GABABR agonist. Administration of blood-brain barrier permeant GABABR antagonist SCH-50911 (30 mg/kg) partially rescued ataxic phenotype of RGS6−/− mice on rotarod at fixed speed (C) and completely rescued the phenotype during acceleration from 5 rpm (D). Data are presented as mean ± S.E. *, p < 0.05; **, p < 0.01 versus WT.
We also hypothesized that potentiation of GABABR signaling due to loss of RGS6-mediated inactivation of Gα might underlie the ataxic phenotype observed in our RGS6−/− animals. To test this, we administered the brain permeant GABABR antagonist SCH-50911 to mice of both genotypes at a concentration known to prevent absence seizures in mouse models (33). Blockade of GABAB receptors was able to completely reverse the deficits of RGS6−/− mice on the accelerating rotarod (Fig. 4D) and partially rescue the phenotype at fixed speed (Fig. 4C) as measured by improved mouse performance in treated (SCH-50911) versus untreated KO animals (fixed speed, p < 0.01; accelerating p < 0.01). This dose of drug did not affect the performance of wild-type animals. These results suggest that the ataxia observed in mice lacking RGS6 occurs, at least in part, through excessive GABABR signaling.
RGS6 Is Co-enriched with GIRK Channel Subunits in the Granule Cell Layer
Simulation of GABABRs in CGNs, where RGS6 expression is enriched, is known to activate robust GIRK current in isolated cells (7). Therefore, we hypothesized that RGS6 might modulate GABAB-GIRK signaling in CGNs. Native GIRK channels in cerebellum appear to be composed primarily of GIRK1 and GIRK2 complexes (34), a finding consistent with the observed loss of GABABR·GIRK current in the GIRK2 mutant weaver mouse and GIRK2−/− mouse (7) and the reduced sensitivity of mice to the behavioral effects of GABAB agonists in GIRK 1/2 but not GIRK3-null animals (9). We first confirmed that RGS6 loss in cerebellum did not alter the expression levels of key components of the GABABR-GIRK signaling axis. We observed equivalent levels of GIRK1, GIRK2, GABABR2, and Gαi3 in cerebellar lysates from WT and RGS6−/− mice (Figs. 5A and 6A), and similar results were observed in cerebrum (Figs. 5A and 6A). Genetic ablation of RGS6 did not cause loss of expression of its binding partners Gβ5 or R7BP (Figs. 1B and 6A). The distribution of Gβ5, an obligate RGS6 binding partner responsible for RGS6 protein stability, completely mirrored the distribution of RGS6 expression in cerebellum with enrichment of both proteins in CGNs (Fig. 5B). Interestingly, GIRK1/2 are co-enriched with RGS6 and Gβ5 in this cell population (Fig. 5B), suggesting RGS6 is positioned to regulate GABA/GABABR-mediated activation of GIRK channels in these cells. Although less robust compared with its expression in the molecular layer, expression of the R2 subunit of the GABABR was also detectable in the cerebellar granule cell layer (Fig. 5B).
FIGURE 5.
Localization of RGS6 and different components of the GABABR-GIRK signaling axis in mouse cerebellum. A, genetic ablation of RGS6 does not change expression of GABABR2 or Gαi3 in cerebellum or cerebrum by Western blot. Actin serves as a loading control. B, co-immunostaining of RGS6 with Gβ5, GABABR2, GIRK1, GIRK2, and in cerebellar sections (5 μm) from WT mice reveals expression of all proteins in cerebellar granule layer (scale bar = 100 μm; white boxes, regions shown in enlarged merged images; white arrows, regions of granule cell specific protein enrichment).
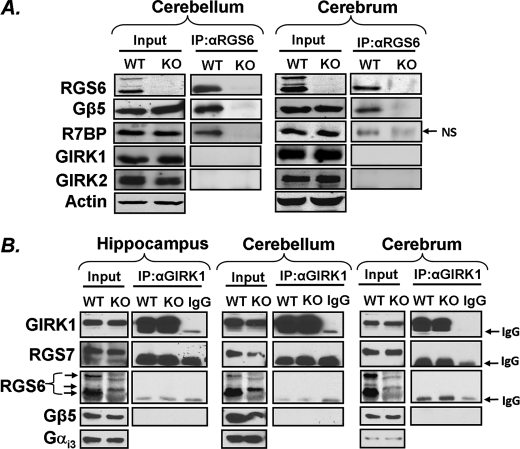
FIGURE 6.
RGS6 complex formation in cerebellum and cerebrum. A, RGS6 co-immunoprecipitates with Gβ5 and R7BP but not GIRK1 and GIRK2 in cerebellar and cerebral lysates. Actin serves as a loading control for Western blots. Input represents total tissue lysates used for subsequent immunoprecipitation. B, immunoprecipitation of GIRK1 from hippocampus, cerebrum, and cerebellum fails to reveal complex formation between GIRK1 and RGS7, Gβ5, or RGS6. Input represents blots from isolated membrane fractions used for subsequent immunoprecipitation and Gαi3 served as a loading control for Western blots. NS = nonspecific immunoreactive band, IgG = immunoglobulin heavy chain.
RGS6 Forms a Complex with R7BP and Gβ5 but Not GIRK Channels
Recently, it was discovered that RGS7, recruited by Gβ5, forms a direct complex with GIRK1 in the hippocampus to mediate fast channel inactivation and coupling to the GABAB receptor (23). This suggests that close association between GIRK channel subunits and RGS proteins might be necessary for proper regulation of channel activity. However, immunoprecipitation of RGS6 from cerebellar and cerebral lysates confirmed complex formation between RGS6 and its known binding partners Gβ5 and R7BP (35, 36), but not with GIRK1 or GIRK2 (Fig. 6A), a finding consistent with what we had previously shown in heart (22). We were also unable to recapitulate the previously reported direct interaction between GIRK1, RGS7, and Gβ5 in immunoprecipitation experiments from hippocampus, cerebellum, or cerebrum (Fig. 6B). RGS6 was also not detectable in complex with GIRK1 (Fig. 6B) in these tissues.
CGNs Isolated from RGS6−/− Mice Exhibit Delayed GABABR-mediated GIRK Channel Deactivation
Because we have shown excessive GABAB receptor signaling underlies the ataxic phenotype of our RGS6−/− animals, we sought to identify possible signaling cascades downstream of the GABABR regulated by RGS6 in cerebellum. CGNs are known to exhibit a robust GIRK channel current coupled to GABABRs through pertussis toxin-sensitive G proteins, which could be susceptible to negative regulation by RGS6 (7). To test this hypothesis, we isolated CGNs from WT and RGS6−/− mice to perform electrophysiological recordings of baclofen (a selective GABABR agonist)-induced GIRK channel current.
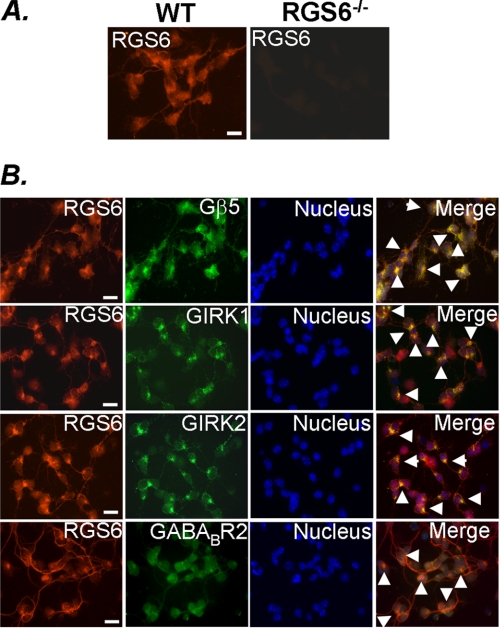
Similar to what we observed in tissue sections, isolated CGNs showed expression of RGS6 that was absent in cells isolated from RGS6−/− animals (Fig. 7A). Furthermore, these cells also expressed Gβ5, GIRK1, GIRK2, and GABABR2 (Fig. 7B). Expression of RGS6 and Gβ5 overlapped indicating, as evidenced by our co-immunoprecipitation experiments, that these two proteins co-localize and likely form a complex within CGNs. In addition, RGS6 exhibited co-localization with GIRK1, GIRK2, and GABABR2 in these cells (Fig. 7B), a finding consistent with previous reports of coupling between GABABRs and functional GIRK channels in neurons (37) and indicative that RGS6 is positioned within these cells to influence Gβγ-induced GIRK channel activity.
FIGURE 7.
Native expression of RGS6, Gβ5, GABABR2, GIRK1, and GIRK2 in isolated CGNs. A, RGS6 immunostaining is localized to the soma and neurites of CGNs from WT mice and absent in RGS6−/− mice. B, co-immunostaining of RGS6 with Gβ5, GABABR2, GIRK1, and GIRK2 in CGNs from WT mice (scale bar = 100 μm; white arrows, regions of overlapping expression of RGS6 with indicated protein).
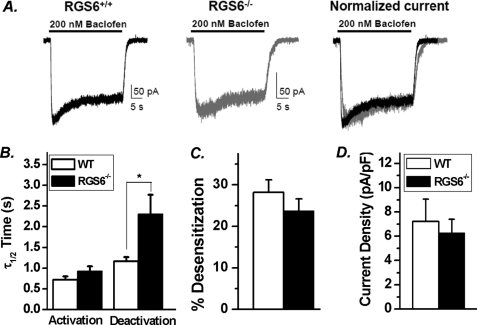
To investigate the functional consequences of RGS6 loss on GABABR-mediated GIRK channel activity, baclofen-induced inward K+ currents were recorded under whole cell, voltage clamp configuration in CGNs from mice of both genotypes. In CGNs isolated from WT mice, baclofen application induced rapid GIRK currents that exhibit slow desensitization over time followed by a rapid deactivation upon removal of baclofen (Fig. 8A). CGNs from RGS6−/− mice, conversely, exhibited a significant reduction in the rate of deactivation of baclofen-induced GIRK currents (Fig. 8, A and B) with no significant alteration in the current density, extent of desensitization, and the time course of activation (Fig. 8, A–D). These results indicate that RGS6 is required for the normal gating kinetics of GABABR-induced GIRK current in CGNs. It is well established that the GAP activity of RGS proteins accelerates the rate of activation and deactivation of GPCR activated GIRK current (38). Because GIRK channel deactivation is rate-limiting for termination of inhibitory postsynaptic currents, RGS proteins function to prevent excessive membrane hyperpolarization and blockade of neuronal firing. Thus, our results reiterate that RGS6, acting as a GAP for Gαi/o, effectively terminates Gβγ-mediated GIRK current downstream of GABABR activation in isolated CGNs.
FIGURE 8.
Loss of RGS6 potentiates GABABR-GIRK signaling in isolated CGNs. A, representative baclofen (200 nm)-induced whole cell GIRK current recordings in isolated CGNs from WT and RGS6−/− mice. GIRK currents from RGS6−/− mice exhibit (B) a significant delay in the half-maximal deactivation but not activation time constant (τ1/2) without any significant changes in the extent of desensitization (C) and current density (D) (WT, n = 8; RGS6−/−, n = 10). Data are presented as mean ±S.E.; *, p < 0.05 versus WT.
DISCUSSION
This work establishes RGS6 as a crucial modulator of neuronal signaling necessary for coordinated motor movement. RGS6−/− mice exhibited gait and stance abnormalities and impaired performance on the rotarod, the latter indicative of ataxia due to cerebellar dysfunction. Mice lacking RGS6 exhibit enhanced sensitization to the ataxic effects of baclofen, a selective agonist for GABABR. Indeed, inhibition of GABABR signaling through administration of a selective GABAB antagonist can rescue the ataxic phenotype of our RGS6−/− mice without impacting the performance of wild-type animals. These results suggest that RGS6 plays a critical role in suppressing GABABR signaling. Unsurprisingly, RGS6 is highly expressed in the cerebellum, particularly in the granule cell layer. In isolated CGNs, RGS6 functions to inactivate Gαi/o released from GABABRs and induce re-sequestration of Gβγ and termination of downstream GIRK channel activation. This is the first evidence of a functional role for an RGS protein in cerebellum.
GABA released from inhibitory interneurons located in the cerebellar cortex controls the extent of neuronal excitation and is essential for motor coordination (2). We show here that RGS6 is required for rapid deactivation of GABABR-mediated GIRK current in isolated CGNs. Loss of rapid GIRK channel deactivation would be expected to potentiate membrane hyperpolarization resulting in prolonged inhibition of CGN excitation. The net result is a loss of mossy fiber/CGN/parallel fiber-mediated Purkinje cell excitation and reduced GABAergic inhibition of the deep cerebellar nuclei, an imbalance known to manifest phenotypically as an impaired coordination of motor movement (39). Indeed, the 2-fold delay in GIRK channel deactivation seen here is consistent with that observed in cardiac myocytes and hippocampal neurons lacking RGS protein-mediated regulation of M2 receptor and GABABR-induced GIRK currents, respectively (22, 23, 45). In each case, loss of RGS protein expression leads to increased agonist sensitivity and exaggeration of agonist-induced phenotypic changes. Thus, the gait abnormalities and ataxic phenotype observed in RGS6−/− mice could result from delayed deactivation of GABABR-GIRK current in CGNs.
It has been shown, however, that granule cell inhibition in the adult cerebellum is dominated by tonic GABAA-mediated responses without a reported GABAB-dependent component (40). Thus, it remains to be determined whether the ataxic phenotype of our RGS6−/− mice is uniquely determined by loss of GABAB-mediated GIRK current deactivation in CGNs. GIRK channels, which appear to be exclusively expressed in the granule cell layer of the cerebellum, have been implicated in cerebellar function as their mutation or loss results in the weaver mouse ataxic phenotype (7) or loss of baclofen-induced rotarod performance deficits (9), respectively. Activation of GABAB receptors does block neurotransmitter release from inhibitory Golgi cells in the granule cell layer (41) and inhibits Purkinje cell firing even in the absence of a detectable change in membrane potential (42). Therefore, we cannot exclude the possibility that RGS6 regulates GPCR-signaling cascades not involving GIRK channel activation (Gβγ-mediated inhibition of voltage-gated calcium channels for example), functions in a different cerebellar cell type, or acts at extracerebellar sites to control motor behavior. Nevertheless, we have identified RGS6 as a key modulator of GABAB receptor signaling whose loss results in cerebellar ataxia.
In cerebellum, RGS6 forms a complex with known binding partners Gβ5 and R7BP. R7BP is reversibly palmitoylated, targeting itself and the associated R7 family member to the membrane (43). Thus, it could function to localize RGS6 to the plasma membrane to mediate fast deactivation of GABABR signaling. Similarly, Gβ5 is known to stabilize and enhance the activity of R7 family RGS proteins (19, 44) and would be expected to enhance RGS6-dependent modulation of GABABRs. Indeed, loss of Gβ5 is associated with enhanced inhibition of locomotor activity in mice administered baclofen (23), presumably due to loss of all R7 family members. Gβ5 knock-out mice also exhibit rotarod performance deficits, although this likely results from the abnormal cerebellar development identified in these animals (20, 21).
Neuronal GIRK channels 1 and 2 are enriched in CGNs where they are known to mediate membrane hyperpolarization in response to activation of GABABRs in isolated CGNs (7, 8). Despite the demonstration of Xie et al. that Gβ5 mediates recruitment of RGS7 to form a complex with neuronal GIRK channels, we did not observe direct coupling of RGS6 with GIRK channels 1 or 2 despite co-precipitation of RGS6 and Gβ5 in both cerebral and cerebellar homogenates. Immunoprecipitation of GIRK1 from brain lysates using this previously published protocol also failed to pull down RGS6, RGS7, and Gβ5. In heart, RGS6 also fails to directly bind GIRK1, although it does interact with GIRK4, a subunit with which RGS7 fails to associate, in a heterologous expression system (22, 23, 45). These results indicate that, despite a previous report, we cannot detect complex formation between neuronal GIRK channels and R7 family RGS proteins in hippocampus, cerebellum, or cerebrum, suggesting that this interaction is either transient or of relatively low affinity if it occurs in vivo. The fact that RGS6 does not form a direct complex with neuronal GIRK channels does not imply, however, that it is not involved in GIRK channel regulation. In fact, it is the GAP activity of RGS6 toward Gα that we believe is required for RGS6-mediated inhibition of GIRK channel activation. We have shown that RGS6 along with GIRK1, GIRK2, and GABABR2 are uniformly localized throughout the soma and neurites, suggesting that RGS6 is positioned within CGNs to modulate the GABABR-GIRK channel-signaling axis despite the lack of its direct binding to GIRK channels.
Maintenance of Gβ5 expression in RGS6−/− animals suggests that other R7 family members are also expressed in cerebellum and, thus, able to stabilize Gβ5 in the absence of RGS6. The fact that loss of RGS6 alone results in deficits in motor coordination and movement suggests that other R7 RGS family members, although expressed in cerebellum, are unable to compensate for loss of RGS6. In fact, R7 family members have been implicated both in regulation of GABAB receptor signaling as well as motor coordination. Loss of Gβ5, which results in destabilization of all R7 family members, is associated with increased sensitivity to baclofen-mediated inhibition of locomotor activity. Based on the critical role of RGS6 in inhibiting baclofen-induced loss of motor coordination reported here, loss of RGS6 expression may underlie this phenotype. R7BP−/− mice also exhibit impaired performance on the rotarod, although this phenotype is primarily due to loss of striatal-specific expression of RGS9–2 (46). Thus, it appears R7 family members differentially control GPCR signaling to influence motor behavior. Investigation into the motor phenotypes of other R7 family-specific knockouts might provide additional insight into how this group of G protein regulators contributes to cerebellar function.
Numerous ataxias of cerebellar origin are caused by genetic mutations, viral infections, toxins, autoimmune diseases or traumatic injuries and often result from aberrations in neuronal communication between the cerebellar cortex and its efferent targets that are consequences of either neuronal degeneration or alterations in neurotransmitter signaling and electrical conductivity (for review see Ref. 47). Modulation of RGS6 levels would be expected to affect the extent of GABA-mediated neuronal inhibition and, thus, the extent of CGN and Purkinje cell excitation, as well as the magnitude of inhibitory output to the deep cerebellar nuclei, the motor cortex, and the periphery. Our work underscores the critical role for GPCR signaling and the regulatory actions of RGS proteins in modulation of neurotransmitter signaling in cerebellum. RGS6 is likely not the only RGS protein to function in this neuronal circuitry, however. In fact, RGS8 has been shown to be expressed in developing Purkinje cells of the cerebellum, although its physiological significance remains unclear (48, 49). The fact that loss of RGS6 has a significant consequence on motor coordination underscores the need to investigate the roles of RGS proteins in cerebellum and to examine their possible participation in cerebellar pathologies. Indeed, alterations in RGS6 expression or function could potentially contribute to human cerebellar ataxias, and thus it might represent a novel target for pharmacological intervention.
Supplementary Material
Acknowledgments
We thank Dr. John Koland for his careful reading of and useful suggestions for the manuscript and Dr. Ching-Kang Jason Chen for his generous gift of antibodies to RGS7 and Gβ5.
This work was supported, in whole or in part, by National Institutes of Health Grants GM075033-02 and American Recovery & Reinvestment Act (ARRA) grant GM075033-03S1 (to R. A. F.) and NS069898 (to D. P. M.). This work was also supported by a University of Iowa Carver Collaborative Pilot Grant (to R. A. F. and D. P. M.).

This article contains supplemental Table S1.
- CGN
- cerebellar granule neuron
- DEP/DHEX
- Dishevelled, Egl-10 and Pleckstrin homology domain
- GABA
- γ-aminobutyric acid
- GABABR
- metabotropic GABA receptor
- GAP
- GTPase-accelerating protein
- GGL
- Gγ subunit-like domain
- GIRK
- G protein-activated inwardly rectifying potassium channel
- GPCR
- G protein-coupled receptor
- KO
- knockout
- RGS
- regulator of G protein signaling
- RGS6
- regulator of G protein signaling 6
- R7BP
- R7 family RGS binding protein.
REFERENCES
- 1. De Zeeuw C. I., Hoebeek F. E., Bosman L. W., Schonewille M., Witter L., Koekkoek S. K. (2011) Spatiotemporal firing patterns in the cerebellum. Nat. Rev. Neurosci. 12, 327–344 [DOI] [PubMed] [Google Scholar]
- 2. Watanabe D., Inokawa H., Hashimoto K., Suzuki N., Kano M., Shigemoto R., Hirano T., Toyama K., Kaneko S., Yokoi M., Moriyoshi K., Suzuki M., Kobayashi K., Nagatsu T., Kreitman R. J., Pastan I., Nakanishi S. (1998) Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell 95, 17–27 [DOI] [PubMed] [Google Scholar]
- 3. Chiu C. S., Brickley S., Jensen K., Southwell A., Mckinney S., Cull-Candy S., Mody I., Lester H. A. (2005) GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J. Neurosci. 25, 3234–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pinard A., Seddik R., Bettler B. (2010) GABAB receptors. Physiological functions and mechanisms of diversity. Adv. Pharmacol. 58, 231–255 [DOI] [PubMed] [Google Scholar]
- 5. Doupnik C. A., Dessauer C. W., Slepak V. Z., Gilman A. G., Davidson N., Lester H. A. (1996) Time resolved kinetics of direct Gβ1γ2 interactions with the carboxyl terminus of Kir3.4 inward rectifier K+ channel subunits. Neuropharmacology 35, 923–931 [DOI] [PubMed] [Google Scholar]
- 6. Sodickson D. L., Bean B. P. (1996) GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J. Neurosci. 16, 6374–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slesinger P. A., Stoffel M., Jan Y. N., Jan L. Y. (1997) Defective γ-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc. Natl. Acad. Sci. U.S.A. 94, 12210–12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rossi P., Mapelli L., Roggeri L., Gall D., de Kerchove d'Exaerde A., Schiffmann S. N., Taglietti V., D'Angelo E. (2006) Inhibition of constitutive inward rectifier currents in cerebellar granule cells by pharmacological and synaptic activation of GABA receptors. Eur. J. Neurosci. 24, 419–432 [DOI] [PubMed] [Google Scholar]
- 9. Pravetoni M., Wickman K. (2008) Behavioral characterization of mice lacking GIRK/Kir3 channel subunits. Genes Brain Behav. 7, 523–531 [DOI] [PubMed] [Google Scholar]
- 10. Doupnik C. A., Davidson N., Lester H. A., Kofuji P. (1997) RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. U.S.A. 94, 10461–10466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berman D. M., Wilkie T. M., Gilman A. G. (1996) GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell 86, 445–452 [DOI] [PubMed] [Google Scholar]
- 12. Watson N., Linder M. E., Druey K. M., Kehrl J. H., Blumer K. J. (1996) RGS family members. GTPase-activating proteins for heterotrimeric G protein a-subunits. Nature 383, 172–175 [DOI] [PubMed] [Google Scholar]
- 13. Blundell J., Hoang C. V., Potts B., Gold S. J., Powell C. M. (2008) Motor coordination deficits in mice lacking RGS9. Brain Res. 1190, 78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kovoor A., Seyffarth P., Ebert J., Barghshoon S., Chen C. K., Schwarz S., Axelrod J. D., Cheyette B. N., Simon M. I., Lester H. A., Schwarz J. (2005) D2 dopamine receptors colocalize regulator of G protein signaling 9–2 (RGS9–2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways. J. Neurosci. 25, 2157–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gold S. J., Hoang C. V., Potts B. W., Porras G., Pioli E., Kim K. W., Nadjar A., Qin C., LaHoste G. J., Li Q., Bioulac B. H., Waugh J. L., Gurevich E., Neve R. L., Bezard E. (2007) RGS9–2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson's disease. J. Neurosci. 27, 14338–14348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahman Z., Schwarz J., Gold S. J., Zachariou V., Wein M. N., Choi K. H., Kovoor A., Chen C. K., DiLeone R. J., Schwarz S. C., Selley D. E., Sim-Selley L. J., Barrot M., Luedtke R. R., Self D., Neve R. L., Lester H. A., Simon M. I., Nestler E. J. (2003) RGS9 modulates dopamine signaling in the basal ganglia. Neuron 38, 941–952 [DOI] [PubMed] [Google Scholar]
- 17. Labouèbe G., Lomazzi M., Cruz H. G., Creton C., Luján R., Li M., Yanagawa Y., Obata K., Watanabe M., Wickman K., Boyer S. B., Slesinger P. A., Lüscher C. (2007) RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat. Neurosci. 10, 1559–1568 [DOI] [PubMed] [Google Scholar]
- 18. Anderson G. R., Posokhova E., Martemyanov K. A. (2009) The R7 RGS protein family. Multi-subunit regulators of neuronal G protein signaling. Cell Biochem. Biophys. 54, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen C. K., Eversole-Cire P., Zhang H., Mancino V., Chen Y. J., He W., Wensel T. G., Simon M. I. (2003) Instability of GGL domain-containing RGS proteins in mice lacking the G protein β-subunit Gβ5. Proc. Natl. Acad. Sci. U.S.A. 100, 6604–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J. H., Pandey M., Seigneur E. M., Panicker L. M., Koo L., Schwartz O. M., Chen W., Chen C. K., Simonds W. F. (2011) Knockout of G protein β5 impairs brain development and causes multiple neurologic abnormalities in mice. J. Neurochem. 119, 544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xie K., Ge S., Collins V. E., Haynes C. L., Renner K. J., Meisel R. L., Lujan R., Martemyanov K. A. (2011) Psychopharmacology doi: 10.1007/S00213-011-2409-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang J., Huang J., Maity B., Gao Z., Lorca R. A., Gudmundsson H., Li J., Stewart A., Swaminathan P. D., Ibeawuchi S. R., Shepherd A., Chen C. K., Kutschke W., Mohler P. J., Mohapatra D. P., Anderson M. E., Fisher R. A. (2010) RGS6, a modulator of parasympathetic activation in heart. Circ. Res. 107, 1345–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie K., Allen K. L., Kourrich S., Colón-Saez J., Thomas M. J., Wickman K., Martemyanov K. A. (2010) Gb5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat. Neurosci. 13, 661–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maity B., Yang J., Huang J., Askeland R. W., Bera S., Fisher R. A. (2011) Regulator of G protein signaling 6 (RGS6) induces apoptosis via a mitochondrial-dependent pathway not involving its GTPase-activating protein activity. J. Biol. Chem. 286, 1409–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chatterjee T. K., Liu Z., Fisher R. A. (2003) Human RGS6 gene structure, complex alternative splicing, and role of N terminus and G protein γ-subunit-like (GGL) domain in subcellular localization of RGS6 splice variants. J. Biol. Chem. 278, 30261–30271 [DOI] [PubMed] [Google Scholar]
- 26. Lalonde R., Strazielle C. (2007) Spontaneous and induced mouse mutations with cerebellar dysfunctions. Behavior and neurochemistry. Brain Res. 1140, 51–74 [DOI] [PubMed] [Google Scholar]
- 27. Oliver P. L., Keays D. A., Davies K. E. (2007) Behavioural characterisation of the robotic mouse mutant. Behav. Brain Res. 181, 239–247 [DOI] [PubMed] [Google Scholar]
- 28. Boy J., Schmidt T., Wolburg H., Mack A., Nuber S., Böttcher M., Schmitt I., Holzmann C., Zimmermann F., Servadio A., Riess O. (2009) Reversibility of symptoms in a conditional mouse model of spinocerebellar ataxia type 3. Hum. Mol. Genet 18, 4282–4295 [DOI] [PubMed] [Google Scholar]
- 29. Xie G., Harrison J., Clapcote S. J., Huang Y., Zhang J. Y., Wang L. Y., Roder J. C. (2010) A new Kv1.2 channelopathy underlying cerebellar ataxia. J. Biol. Chem. 285, 32160–32173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perkins E. M., Clarkson Y. L., Sabatier N., Longhurst D. M., Millward C. P., Jack J., Toraiwa J., Watanabe M., Rothstein J. D., Lyndon A. R., Wyllie D. J., Dutia M. B., Jackson M. (2010) Loss of b-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans. J. Neurosci. 30, 4857–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cendelín J., Voller J., Vozeh F. (2010) Ataxic gait analysis in a mouse model of the olivocerebellar degeneration. Behav. Brain Res. 210, 8–15 [DOI] [PubMed] [Google Scholar]
- 32. Jacobson L. H., Cryan J. F. (2005) Differential sensitivity to the motor and hypothermic effects of the GABA B receptor agonist baclofen in various mouse strains. Psychopharmacology 179, 688–699 [DOI] [PubMed] [Google Scholar]
- 33. Hosford D. A., Wang Y., Liu C. C., Snead O. C., 3rd (1995) Characterization of the antiabsence effects of SCH 50911, a GABA-B receptor antagonist, in the lethargic mouse, γ-hydroxybutyrate, and pentylenetetrazole models. J. Pharmacol. Exp. Ther. 274, 1399–1403 [PubMed] [Google Scholar]
- 34. Liao Y. J., Jan Y. N., Jan L. Y. (1996) Heteromultimerization of G protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J. Neurosci. 16, 7137–7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Snow B. E., Betts L., Mangion J., Sondek J., Siderovski D. P. (1999) Fidelity of G protein b-subunit association by the G protein γ-subunit-like domains of RGS6, RGS7, and RGS11. Proc. Natl. Acad. Sci. U.S.A. 96, 6489–6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martemyanov K. A., Yoo P. J., Skiba N. P., Arshavsky V. Y. (2005) R7BP, a novel neuronal protein interacting with RGS proteins of the R7 family. J. Biol. Chem. 280, 5133–5136 [DOI] [PubMed] [Google Scholar]
- 37. Ciruela F., Fernández-Dueñas V., Sahlholm K., Fernández-Alacid L., Nicolau J. C., Watanabe M., Luján R. (2010) Evidence for oligomerization between GABAB receptors and GIRK channels containing the GIRK1 and GIRK3 subunits. Eur. J. Neurosci. 32, 1265–1277 [DOI] [PubMed] [Google Scholar]
- 38. Lambert N. A., Johnston C. A., Cappell S. D., Kuravi S., Kimple A. J., Willard F. S., Siderovski D. P. (2010) Regulators of G protein signaling accelerate GPCR signaling kinetics and govern sensitivity solely by accelerating GTPase activity. Proc. Natl. Acad. Sci. U.S.A. 107, 7066–7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamamoto M., Wada N., Kitabatake Y., Watanabe D., Anzai M., Yokoyama M., Teranishi Y., Nakanishi S. (2003) Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J. Neurosci. 23, 6759–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossi D. J., Hamann M., Attwell D. (2003) Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J. Physiol. 548, 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mapelli L., Rossi P., Nieus T., D'Angelo E. (2009) Tonic activation of GABAB receptors reduces release probability at inhibitory connections in the cerebellar glomerulus. J. Neurophysiol. 101, 3089–3099 [DOI] [PubMed] [Google Scholar]
- 42. Vigot R., Batini C. (1997) GABA(B) receptor activation of Purkinje cells in cerebellar slices. Neurosci. Res. 29, 151–160 [DOI] [PubMed] [Google Scholar]
- 43. Drenan R. M., Doupnik C. A., Boyle M. P., Muglia L. J., Huettner J. E., Linder M. E., Blumer K. J. (2005) Palmitoylation regulates plasma membrane-nuclear shuttling of R7BP, a novel membrane anchor for the RGS7 family. J. Cell Biol. 169, 623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kovoor A., Chen C. K., He W., Wensel T. G., Simon M. I., Lester H. A. (2000) Co-expression of Gb5 enhances the function of two Gg subunit-like domain-containing regulators of G protein signaling proteins. J. Biol. Chem. 275, 3397–3402 [DOI] [PubMed] [Google Scholar]
- 45. Posokhova E., Wydeven N., Allen K. L., Wickman K., Martemyanov K. A. (2010) RGS6/Gß5 complex accelerates IKACh gating kinetics in atrial myocytes and modulates parasympathetic regulation of heart rate. Circ. Res. 107, 1350–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anderson G. R., Cao Y., Davidson S., Truong H. V., Pravetoni M., Thomas M. J., Wickman K., Giesler G. J., Jr., Martemyanov K. A. (2010) R7BP complexes with RGS9–2 and RGS7 in the striatum differentially control motor learning and locomotor responses to cocaine. Neuropsychopharmacology 35, 1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manto M., Marmolino D. (2009) Cerebellar ataxias. Curr. Opin. Neurol. 22, 419–429 [DOI] [PubMed] [Google Scholar]
- 48. Saitoh O., Masuho I., Itoh M., Abe H., Komori K., Odagiri M. (2003) Distribution of regulator of G protein signaling 8 (RGS8) protein in the cerebellum. Cerebellum 2, 154–160 [DOI] [PubMed] [Google Scholar]
- 49. Saitoh O., Odagiri M. (2003) RGS8 expression in developing cerebellar Purkinje cells. Biochem. Biophys. Res. Commun. 309, 836–842 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.