Background: Adipose hypertrophy limits fat cell oxygenation, promotes scarring, and associates with increased local glucocorticoid regeneration (higher 11βHSD1 enzyme).
Results: 11βHSD1 knock-out mice have reduced scarring and better vascularization and oxygenation in their adipose tissue.
Conclusion: Elevated adipose 11βHSD1 contributes to obesity pathogenesis by suppressing adipose angiogenesis.
Significance: Enhancement of adipose oxygenation and vascularization is a novel therapeutic modality for 11βHSD1 inhibitors.
Keywords: Adipose Tissue, Collagen, Extracellular Matrix Proteins, Fibroblast, Hypoxia, Angiogenesis, Fibrosis, Glucocorticoids
Abstract
In obesity, rapidly expanding adipose tissue becomes hypoxic, precipitating inflammation, fibrosis, and insulin resistance. Compensatory angiogenesis may prevent these events. Mice lacking the intracellular glucocorticoid-amplifying enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1−/−) have “healthier” adipose tissue distribution and resist metabolic disease with diet-induced obesity. Here we show that adipose tissues of 11βHSD1−/− mice exhibit attenuated hypoxia, induction of hypoxia-inducible factor (HIF-1α) activation of the TGF-β/Smad3/α-smooth muscle actin (α-SMA) signaling pathway, and fibrogenesis despite similar fat accretion with diet-induced obesity. Moreover, augmented 11βHSD1−/− adipose tissue angiogenesis is associated with enhanced peroxisome proliferator-activated receptor γ (PPARγ)-inducible expression of the potent angiogenic factors VEGF-A, apelin, and angiopoietin-like protein 4. Improved adipose angiogenesis and reduced fibrosis provide a novel mechanism whereby suppression of intracellular glucocorticoid regeneration promotes safer fat expansion with weight gain.
Introduction
In obesity, adipose tissue expansion may exceed compensatory vascular supply. Consequently, adipose tissue hypoxia is postulated to precipitate inflammatory macrophage infiltration and impair insulin signaling (1–4). Induction of hypoxia-inducible factors (HIFs)3 is a molecular hallmark of tissue hypoxia. Both HIF-1α and HIF-2α are involved in adipocyte differentiation and responses to hypoxia in vitro (5, 6). HIF-1α associates with hypoxic and inflammatory changes in adipose tissues in vivo, and transgenic overexpression of HIF-1α in adipose tissue causes fibrosis (7).
Altered glucocorticoid action has a major impact upon adipose tissue distribution and insulin sensitivity. Elevated adipose tissue levels of the intracellular glucocorticoid-regenerating enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) are found in human obesity (8, 9). Modeling this by adipose-specific overexpression in mice drives visceral obesity and a metabolic syndrome (10). In contrast, 11βHSD1 gene knock-out (11βHSD1−/−) mice are protected from high fat diet-induced metabolic disease (11, 12) and adipose inflammation (13). In addition, 11βHSD1 deficiency is associated with enhanced angiogenesis during wound healing and in the myocardium following ischemia (14, 15). We hypothesized that the metabolically “healthy” adipose tissue found with 11βHSD1 deficiency might have its origin in improved adipose tissue angiogenesis, with consequent improvement in hypoxia-induced fibrosis. We therefore examined the impact of 11βHSD1 deficiency on adipocyte paracrine, stromal proangiogenic responses, and the consequent effects on adipose tissue vascularization and fibrosis in dietary obesity.
EXPERIMENTAL PROCEDURES
Diet-induced Obesity (DIO)
Adult male 11βHSD1−/− and congenic C57Bl/6J controls (12, 13) were single-housed (12-h light/12-h dark cycle) and given food and water ad libitum. Mice (n = 6–8 per group) were randomized to standard chow or a high fat (HF) diet, 58% calories as fat (Research Diets D12331), for 12 weeks.
Pimonidazole Staining
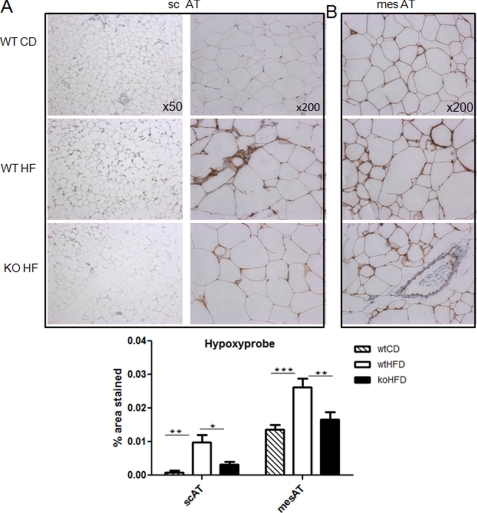
To assess adipose tissue hypoxia in vivo, mice were injected intraperitoneally with 60 mg/kg of pimonidazole (HypoxyprobeTM-1 Plus kit, Chemicon International), 30 min prior to sacrifice. Subcutaneous (scAT) and mesenteric (mesAT) adipose tissues were collected in formalin and paraffin-embedded. 4-μm sections were incubated with mAb1 conjugated with FITC at a 1:100 dilution for 30 min at room temperature. A secondary anti-FITC mAb was applied at a 1:100 dilution for 30 min (according to the manufacturer's instructions). Pimonidazole adduct staining was visualized using the diaminobenzidine chromogen-A system (DAKO). Sections were rinsed and counterstained with hematoxylin and imaged using a Zeiss microscope. 30–40 high power fields (magnification ×200) per section were randomly selected for each slide by an assessor blind to genotype. Diaminobenzidine brown staining was quantified using Photoshop PS3 and normalized to the total number of pixels.
RNA Extraction and Quantitative Real Time PCR
scAT and mesAT were weighed and snap-frozen in liquid nitrogen, and RNA was extracted as described (12). 1 μg of RNA was reverse-transcribed using the SuperScript III cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions. Real time PCR was performed with the LightCycler (Roche Applied Science), using TaqMan assays for all genes measured and correcting for 18 S and β-actin expression.
Immunoblotting
Frozen adipose tissues were homogenized in a urea/SDS buffer supplemented with Complete protease inhibitor (Roche Applied Science). The fat layer was removed by centrifugation (4000 rpm, 10 min). The protein concentration was determined using a Bio-Rad assay kit. Proteins were separated on 7–12% Bis-Tris gels and transferred to PVDF membranes (Millipore). The antibodies used were rabbit anti-mouse HIF-1α, HIF-2α, PHD2, PHD1, PHD3 (Novus Biologicals), anti-phospho-Smad3 (Cell Signaling), anti-Smad2/3 (Cell Signaling), anti-α-SMA (Sigma), and HRP-conjugated anti-β-actin (Abcam). The primary antibodies were detected with secondary HRP-IgGs (Dako) using ECL detection (GE Healthcare). Densitometry was performed using ImageJ, and protein levels were corrected for β-actin.
Immunohistochemistry
For the detection of collagen deposition, formalin-fixed, paraffin-embedded sections (4 μm) were stained with picrosirius red (Sigma). Myofibroblasts were detected with a mouse monoclonal antibody to α-SMA (Sigma). An anti-CD31 antibody (Abcam) was used for identifying endothelial cells/vessels. Secondary antibodies were all from Dako. Binding of primary antibody was visualized using diaminobenzidine chromogen-A (Dako) after counterstaining with hematoxylin.
SVF Cell Preparation and in Vitro Hypoxia
Adipose tissue was removed from HF-fed mice into warmed Krebs' buffer and collagenase type 1 (Worthington Biochemicals)-digested as described (30). Briefly, after 1 h of digestion and centrifugation to separate from adipocytes, stromovascular fraction (SVF) cells were seeded at 2.5 × 105 cells/well in DMEM. On day 2, culture medium was replaced with 1% O2 preconditioned medium. Cells were left at 1% O2 in a hypoxic chamber (1% O2) for 6 h and then lysed in TRIzol, and RNA was extracted as above.
Rosiglitazone Treatment of Primary Adipocytes
The ceiling-cultured adipocytes from collagenase digestion (see above) were filtered through a 200-μm size exclusion mesh and then centrifuged. Equal volumes of fractionated adipocytes were cultured in DMEM medium (Lonza, Berkshire, UK) for 16 h at 37 °C, 5% CO2 in the presence or absence of 1 μm rosiglitazone. Adipocytes were lysed in TRIzol, and total RNA was extracted as above.
Angiogenesis Bioassays
Strips of periaortic adipose tissue (immediately adjacent to the adventitia) were isolated from the thoracic aortas of C57Bl/6 or 11βHSD1−/− mice and incubated (20 mg of periaortic adipose/ml; 24 h at 37 °C) in DMEM containing 4.5 g/liter glucose, 584 mg/liter l-glutamine, 75 mg/liter l-ascorbic acid, 10 mg/liter heparin, 100 units/ml penicillin, 100 μg/ml streptomycin, 250 ng/ml amphotericin B, and 0.2% BSA. Adipose tissue was removed from the conditioned medium, which was aliquoted and stored at −80 °C. Control medium (vehicle) was obtained following the same procedure without adipose tissue. Tube formation from mouse aortic rings was performed as described (14). Briefly, aortic rings (∼1 mm in length) from C57Bl/6 mice were embedded in Matrigel and incubated (DMEM, 37 °C; 5% CO2) in 50% DMEM; 50% periaortic fat conditioned medium or 50% DMEM; 50% control medium for 7 days, with medium refreshed every 48 h. Aortic rings cultured under these conditions in Matrigel spontaneously generated endothelial tube-like structures. The impact of periaortic fat conditioned medium on angiogenesis was quantified by counting the number of tube-like structures every other day (by an operator blinded to treatment).
Statistical Analysis
Values are means ± S.E. Simple comparisons were analyzed using Student's t test. For multiple comparisons, differences between genotypes and the effect of treatment(s) were analyzed by two-way ANOVA with subsequent Tukey's post hoc test. Significance was set at p < 0.05.
RESULTS
11βHSD1−/− Adipose Tissue Is Resistant to Hypoxia with Obesity
Weight gain and fat mass were similar after 12 weeks of HF feeding in both genotypes (Table 1). This intermediate time point suggests that a convergent phase in fat accumulation follows an earlier generalized reduction in fat mass (13) and precedes a later beneficial redistribution of fat away from visceral depots and into peripheral fat depots (12).
TABLE 1.
Similar body weight and adipose mass gain in 11βHSD1−/− mice after HF feeding
11βHSD1−/− and control (C57BL/6) mice gained similar weight (n = 6). Body fat distribution and subcutaneous and mesenteric fat deposition were similar after 12 weeks on HF diet. * indicates significant differences between control chow and high fat diet in both genotypes. CD, chow-fed; HFD, high fat diet-fed; BW, body weight.
| C57BL/6 CD | C57BL/6 HFD | 11βHSD1−/− HFD | p values | |
|---|---|---|---|---|
| Terminal BW (g) | 38.8 ± 3.2* | 49.2 ± 1.7 | 53 ± 1.1 | <0.0001 |
| BW gain (g) | 5.9 ± 0.3* | 16.1 ± 1.2 | 17.1 ± 1.2 | <0.0001 |
| scAT (g) | 0.4 ± 0.03* | 1.0 ± 0.06 | 1.2 ± 0.09 | <0.0001 |
| mesAT (g) | 0.7 ± 0.06* | 1.5 ± 0.1 | 1.6 ± 0.1 | <0.0001 |
| scAT/BW ratio | 0.010 ± 0.0006* | 0.020 ± 0.001 | 0.022 ± 0.001 | <0.0001 |
| mesAT/BW ratio | 0.018 ± 0.0009* | 0.030 ± 0.001 | 0.031 ± 0.001 | 0.0006 |
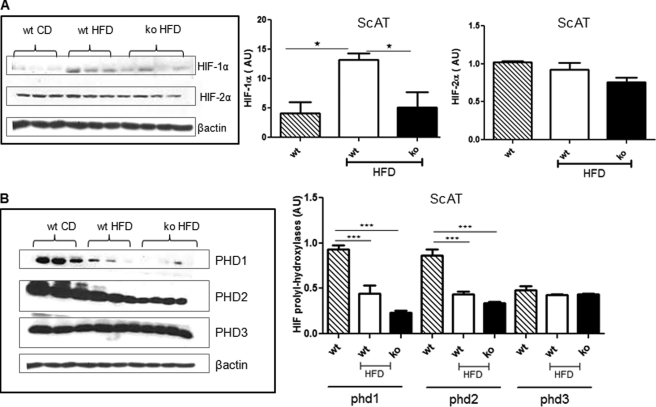
Despite similar scAT depot expansion in 12-week HF-fed 11βHSD1−/− mice, there was markedly less adipose tissue pimonidazole staining and hence hypoxia than in HF-fed control (WT) mice (Fig. 1). Importantly, mesAT from chow-fed animals exhibited 14-fold (p = 0.005) greater pimonidazole staining than scAT, suggesting that marked hypoxia occurs under basal circumstances in this depot. HF feeding significantly induced adipose HIF-1α in control mice, whereas HIF-1α remained very low in similarly obese HF-fed 11βHSD1−/− mice (Fig. 2A and supplemental Fig. S1). HIF-2α protein was unaffected by HF or 11βHSD1 genotype (Fig. 2A), suggesting that it does not play a role in adipose hypoxic responses in vivo.
FIGURE 1.
Less adipose hypoxia in 11βHSD1−/− mice. A and B, representative images of Hypoxyprobe staining of subcutaneous (A) and mesenteric (B) adipose tissue in chow-fed control (WT CD; quantification histogram: hatched bars), HF-fed control (WT HF; quantification histogram: white bars), and HF 11βHSD1−/− (KO HF; quantification histogram: black bars) mice (n = 6). 30–40 high power fields (magnification ×200) per section were quantified. Brown adducts indicate areas of low oxygen availability. Note the unstained blood vessel that acts as an internal negative control. n = 6/group, *, p < 0.05, **, p < 0.01,***, p < 0.001 by ANOVA. HFD, high fat diet.
FIGURE 2.
Reduced adipose HIF-1α in 11βHSD1−/− mice. A and B, representative immunoblots of HIF-1α and HIF-2α protein levels (A) and PHD 1–3 protein levels (B) in scAT. Right panels, quantification histograms showing the relative protein levels corrected for β-actin control mice on chow diet (hatched bars, n = 6), control HF (white bars, n = 6), and 11βHSD1−/− HF mice (black bars, n = 6). *, p < 0.05, ***, p < 0.001 by ANOVA. AU, arbitrary units; WT CD, chow-fed control; wt HFD, HF-fed control; KO HFD, HF 11βHSD1−/−.
HIF prolyl hydroxylases (PHD 1–3 (also known as EGLNs)) degrade HIFs under normoxic conditions by hydroxylation of the two proline residues in the oxygen-dependent degradation domain of HIF-α (16–18). The role of PHDs in adipose tissue and obesity is currently unknown. Adipose tissue PHD mRNAs were unaffected by HF feeding or genotype (supplemental Fig. S2). However, PHD1 and PHD2 protein levels were suppressed by HF feeding (Fig. 2B). Thus, although lower PHD1 and PHD2 (and hence reduced HIF-1α degradation) may contribute to HIF-1α elevation with DIO in controls, variation in PHDs does not explain the lower levels of HIF-1α in adipose tissue with 11βHSD1 deletion.
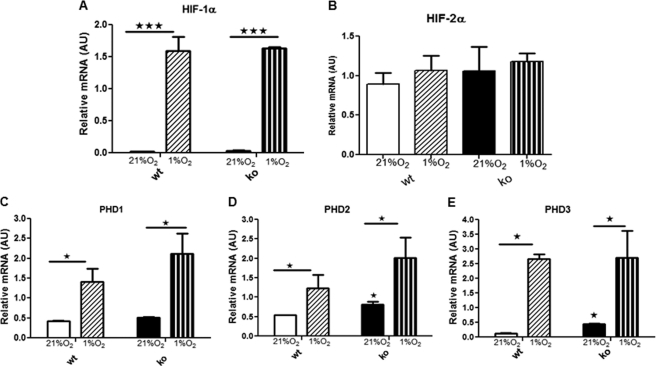
To determine whether 11βHSD1−/− mice have altered responses to hypoxia in the fibrogenic adipose SVF, HIFs and PHDs were measured in SVF cells from HF-fed mice under normoxia (21% O2) or acute hypoxia (1% O2) in vitro. In both genotypes, HIF-1α, but not HIF-2α, mRNA levels were markedly and similarly up-regulated by hypoxia (Fig. 3, A and B). All three PHDs were expressed in SVF cells and were similarly up-regulated by hypoxia (Fig. 3, C–E). Thus, the attenuation of HIF-1α responses to HF in 11βHSD1−/− adipose tissue in vivo is likely due to the reduced tissue hypoxia rather than an intrinsic difference in hypoxia responses.
FIGURE 3.
11βHSD1−/− show similar HIF response after acute hypoxia in vitro. A–E, SVF from HF control at 21% oxygen (white) and 1% oxygen (diagonal hatched) and HF 11βHSD1−/− at 21% oxygen (black) and 1% oxygen (vertical hatched). Cells were kept in 1% oxygen for 6 h. Data are presented as means ± S.E. of SVF from four individual mice. mRNA levels of HIF-1α (A), HIF-2α (B), PHD1 (C), PHD2 (D), and PHD3 (E) were determined. *, p < 0.05, ***, p < 0.001, by two-way ANOVA. AU, arbitrary units.
HF-fed 11βHSD1−/− Mice Have Reduced Adipose Collagen Deposition and Expression of Fibrogenic Mediators
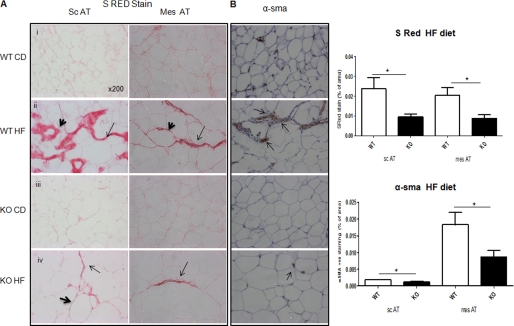
To determine whether obesity-induced hypoxia impacted adipose fibrosis, as in liver (19), we analyzed collagen deposition. Staining for picrosirius red-reactive collagens I and III was low and comparable between genotypes in chow-fed scAT and mesAT. HF diet markedly induced collagen staining in adipose tissue, observed as broad strands transversing the tissue and around individual adipocytes (Fig. 4A). 11βHSD1−/− mice exhibited significantly less collagen deposition in response to HF in both fat depots with much thinner fibrillar streaks and little staining around adipocytes (Fig. 4A).
FIGURE 4.
Attenuated adipose tissue fibrosis in HF 11βHSD1−/−. A, representative images of picrosirius red (S RED) staining in subcutaneous (sc) and mesenteric (mes) adipose. Bold arrowheads show collagen deposition surrounding the adipocytes, and arrows point to the streaks of collagen between adipocytes. WT CD, chow-fed control; wt HF, HF-fed control; KO CD, chow-fed 11βHSD1−/−; KO HF, HF 11βHSD1−/−. B, α-SMA immunohistochemistry in mesenteric adipose; brown stained myofibroblasts are indicated by arrows. 30–40 fields (magnification ×200) per section were assessed in each group. Right, quantification histograms (n = 6/genotype) showing WT HF (white bars) versus KO HF (black bars) *, p < 0.05.
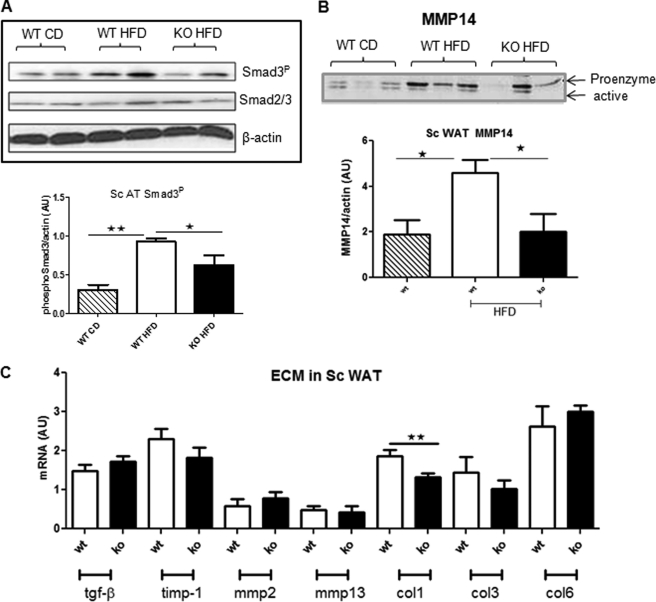
Tissue fibrosis is characterized by increased transdifferentiation of fibroblasts to activated myofibroblasts and collagen deposition (20). 11βHSD1−/− adipose tissue exhibited reduced α-SMA staining in both scAT and mesAT adipose in response to HF (Fig. 4B). Consistent with reduced fibrosis and fewer α-SMA-positive cells, glucocorticoid-regulated Col1α1 mRNA levels were lower in 11βHSD1−/− adipose tissue (Fig. 5C). Other genes involved in fibrosis were unaltered in HF-fed 11βHSD1−/− adipose tissues, including Col3α1 and Col6α1 (Fig. 5C). Activation of the TGF-β/Smad signaling pathway is critical in driving fibrogenesis in liver (21) and in adipose tissue (7). To investigate the impact of intra-adipose glucocorticoid deficiency on adipose fibrosis, we measured TGF-β/Smad signaling. TGF-β mRNA (Fig. 5C) and total Smad2/3 (Fig. 5A) levels were comparable across genotypes and diet. HF feeding significantly induced Smad3 phosphorylation in control mice (3-fold, p < 0.01, Fig. 5A and supplemental Fig. S3). However, Smad3 phosphorylation levels were lower in HF-fed 11βHSD1−/− (Fig. 5A). In vitro hypoxia rapidly up-regulated TGF-β in SVF cells from control mice, whereas TGF-β was unchanged in 11βHSD1−/− SVF cells (supplemental Fig. S4). Moreover, the fibrosis-associated 63-kDa MMP14 pro-enzyme was elevated by HF diet in adipose tissue of control but not 11βHSD1−/− mice (Fig. 5B). Other matrix remodeling enzymes were unaltered including TIMP-1, MMP2, and MMP13 mRNA levels (Fig. 5C).
FIGURE 5.
Reduced profibrotic signaling in 11βHSD1−/−. A and B, representative adipose Western blots of phosphorylated Smad3 (Smad3P) (A) and unphosphorylated Smad2/3 protein pro-enzyme and active MMP14 (B). Lower panels, protein quantification histograms for control mice on chow diet (WT CD, hatched bars, n = 4), control HF (WT HFD, white bars, n = 4), and 11βHSD1−/− HF mice (KO HFD, black bars, n = 4). AU, arbitrary units. C, mRNA of a panel of profibrotic genes (n = 8/genotype). White and black bars, differences between control on HF (white bars) and 11βHSD1−/− on HF (black bars) by Student's t test. *, p < 0.05, **, p < 0.01.
Increased Angiogenesis in 11βHSD1−/− Adipose Tissue
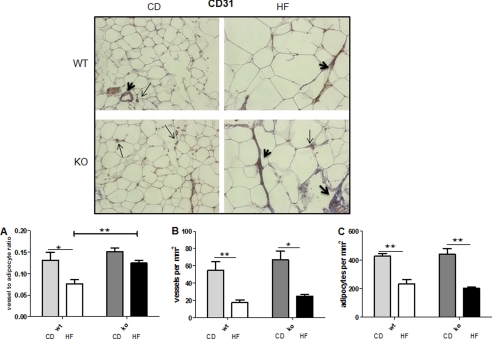
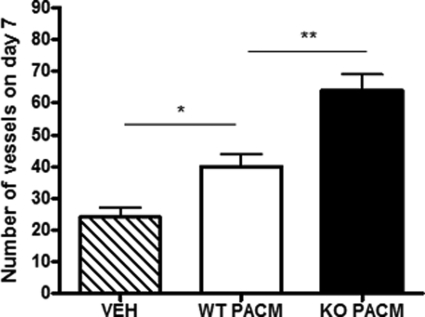
We next explored whether the attenuation of adipose tissue hypoxia and fibrosis responses in HF-fed 11βHSD1−/− adipose tissues was associated with enhanced angiogenesis. The vessel-to-adipocyte ratio (correcting for adipose hypertrophy) was higher in HF-fed 11βHSD1−/− mice than in control mice (Fig. 6A) despite comparable reductions in total vessel (Fig. 6B) and fat cell numbers (Fig. 6C). This appeared to be a direct effect of 11βHSD1−/− adipose tissue on functional induction of angiogenesis because exposure of control aortic rings to 11βHSD1−/− adipose tissue-conditioned medium induced greater angiogenesis (3-fold, p < 0.01) than control (1.5-fold, p < 0.05) adipose tissue medium (Fig. 7).
FIGURE 6.
Higher vessel-to-adipocyte ratio in 11βHSD1−/− in obesity. Top panels, representative pictures (×200 magnification) showing vessel staining using a CD31 antibody. Arrows indicate individual CD31-positive cells, and bold arrowheads indicate stained vessels. 30–40 fields per section were randomly selected, and the number of vessels positively staining for CD31 normalized to the total number of adipocytes was quantified blind to genotype. CD, chow-fed. A–C, quantification of total vessels to adipocyte ratio (A), total vessel number per area (mm2) (B), and total adipocyte number per area (mm2) (C). n = 4, *, p < 0.05, **, p < 0.01.
FIGURE 7.
Higher capacity of adipose-mediated tube-like structure formation in 11βHSD1−/− mice. The number of tube-like structures formed from mouse aortic rings after the addition of vehicle (VEH, hatched bars), wild type periaortic fat conditioned media (WT PACM, white bars), or 11βHSD1−/− periaortic fat conditioned media (KO PACM, black bars) was assessed. n = 4, *, p < 0.05, **, p < 0.01.
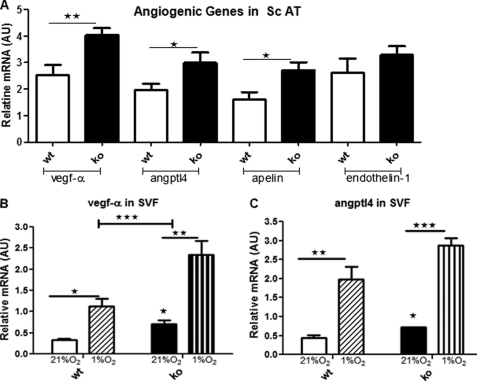
To explore the potential adipose tissue factors involved, we measured key angiogenic gene expression levels. VEGF-A, ANGPTL4, and apelin mRNA were more highly expressed in adipose tissue of 11βHSD1−/− than in control mice (Fig. 8A). SVF endothelial cells are key to adipose tissue expansion (22). Consistent with the in vivo findings, normoxic SVF cells from 11βHSD1−/− adipose tissue had higher VEGF-A and ANGPTL4 expression (Fig. 8, B and C). Hypoxia induced SVF cell VEGF-A and ANGPTL4 mRNA expression to a greater extent in 11βHSD1−/− (Fig. 8B).
FIGURE 8.
Higher expression of proangiogenic genes in 11βHSD1−/− in obesity. A, adipose mRNA levels of a panel of potent angiogenic factors, corrected for β-actin (n = 8). The bars indicate the following: SVF from HF control at 21% oxygen (white bars), 1% oxygen (diagonal hatched bars), HF 11βHSD1−/− 21% oxygen (black bars) and 1% oxygen (vertical hatched bars). Cells were kept in 1% oxygen for 6 h. Data are presented as means ± S.E. SVF was prepared from four individual mice. AU, arbitrary units. B and C, mRNA levels of VEGF-A (B) and ANGPTL4 (C). *, p < 0.05, **, p < 0.01, and ***, p < 0.001.
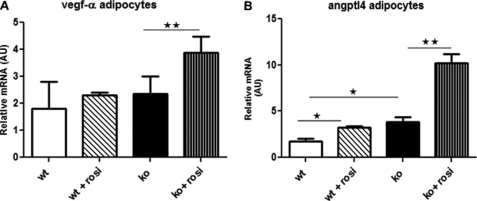
To determine whether the increased PPARγ sensitivity of 11βHSD1−/− adipocytes (13) might contribute in a paracrine manner to enhanced SVF angiogenic responses, primary mesAT adipocytes from HF-fed mice were treated with the PPARγ agonist rosiglitazone (1 μm) for 24 h. As in vivo, ANGPTL4 (although not VEGF) was more highly expressed in 11βHSD1−/− adipocytes in vitro (Fig. 9B). Rosiglitazone up-regulated adipose ANGPTL4 with a 2-fold greater effect in 11βHSD1−/− adipocytes. VEGF-A was induced by rosiglitazone in 11βHSD1−/− but not control adipocytes (Fig. 9A).
FIGURE 9.
11βHSD1−/− adipocytes are more sensitive to PPARγ-mediated angiogenic gene induction. A and B, VEGF-A (A) and ANGPTL4 (B) mRNA levels in adipocytes from HF-fed control mice (white bars, n = 4), rosiglitazone-treated mice (diagonal hatched bars, n = 6), 11βHSD1−/−-untreated mice (black bars, n = 4) and rosiglitazone-treated mice (vertical hatched bars, n = 6). Two-way ANOVA, *, p < 0.05, **, p < 0.01. AU, arbitrary units.
DISCUSSION
Here we show that relative glucocorticoid deficiency within adipose tissue is permissive for angiogenesis in response to DIO, thus preventing severe hypoxia and consequent inflammation, metabolic dysfunction, and fibrosis. We find that DIO induces more severe hypoxia in mesenteric AT than in subcutaneous AT. This is associated with HIF-1α induction through reduced PHD1- and PHD2-mediated HIF degradation. This cascade of responses associates with induction of TGF-β/Smad/α-SMA signaling, myofibroblast activation, increased collagen deposition, and MMP14 induction in HF-fed C57BL/6J but not 11βHSD1−/− mice. Increased PPARγ activation and induction of adipocyte angiogenic factors associates with the improved 11βHSD1−/− adipose tissue phenotype. This work suggests that a cascade of failed angiogenesis leads to tissue hypoxia and fibrosis in rapidly expanding adipose tissue in obesity. This is ameliorated with intra-adipose glucocorticoid deficiency.
Adipose tissue hypoxia mediates insulin resistance and inflammation in obesity (1–4). Moreover, hypoxia is profibrotic in adipose tissue, with transgenic overexpression of adipose-HIF-1α leading to exacerbated collagen deposition and fibrosis (7). Although persistent inflammation precedes fibrosis (23), it is unclear whether chronic low grade inflammation of obesity alters angiogenesis-dependent hypoxia and consequent adipose tissue fibrosis. Loss of the potent angiogenic factor VEGF in myeloid cells leads to excessive collagen deposition and fibrosis, indicating that inflammatory cell-derived VEGF is required for vascular remodeling (24). Failure of extracellular matrix remodeling and/or neovascularization during adipose expansion could feasibly lead to adipose tissue fibrosis. Indeed, genetically obese mice and obese humans show significantly reduced adipose VEGF-A accompanied by lower capillary density (4, 25), suggesting reduced adaptive angiogenesis. Although hypoxia up-regulates VEGF-A, the protective angiogenic response observed in 11βHSD1−/− mice did not follow the canonical hypoxia-HIF-dependent pathway. HIF-independent regulation of VEGF-A and angiogenesis through PPARγ coactivator PGC-1α-mediated responses occurs in skeletal muscle (26) and brain (27). Moreover, induction of HIF-1α in DIO or adipose HIF-1α overexpression does not increase VEGF-A (7). Glucocorticoids potently suppress angiogenesis through inhibition of VEGF-A (28, 29). Consistent with this, reduced hypoxia and fibrosis in adipose tissue from 11βHSD1−/− mice showed augmented PPARγ activation that is associated with induction of adipocyte VEGF-A and ANGPLT4 and sustained vessel density. Moreover, the SVF cells (fibroblast/endothelial population) are more responsive to DIO-mediated hypoxia in 11βHSD1−/− mice, driving beneficial adipose tissue remodeling. 11βHSD1 is highly expressed in the fibroblastic preadipocytes (30) and not the SVF macrophages (13), suggesting that 11βHSD1−/− preadipocytes and/or endothelial cells likely mediate the beneficial response to hypoxia/inflammation and fibrosis associated with obesity.
Distinct adipose depots exhibited different degrees of hypoxia, under basal or obesogenic conditions. Thus, mesAT is more hypoxic as compared with scAT depot. This suggests that hypoxia does not progress uniformly between depots, most probably due to differences in the initial degree of depot vascularization or in the rate/capacity of neovascularization during expansion. This is consistent with greater capillary density and angiogenic capacity in human subcutaneous versus visceral adipose tissue (25, 31). This suggests that individuals prone to visceral fat accumulation are at higher risk of hypoxia-induced metabolic dysfunction. Our finding that glucocorticoid deficiency is associated with fewer hypoxic areas, and consequently normoxic HIF-1α levels, in both depots, in contrast to what is seen in DIO or genetically obese mice (1, 32), suggests a metabolic/vascular advantage during adipose expansion. Glucocorticoids could exacerbate the induction of HIF-1α-dependent gene expression under hypoxic conditions and enhance the hypoxic metabolic insult (29, 33). Importantly, our data suggest that HIF-1α might be regulated (stabilized/activated) by other pathway(s) apart from tissue hypoxia per se during obesity such as inflammatory cytokines (34, 35) and insulin (36–38) pathways. In agreement with this, HF-fed 11βHSD1−/− mice are insulin-sensitized and have reduced adipose inflammation (12, 13).
We propose that increased local glucocorticoid amplification exacerbates the metabolic insult caused by hypoxia and consequent fibrosis in adipose tissue. The detrimental impact on adipose tissue architecture may then lead to further metabolic compromise of adipocytes. Thus, selective 11βHSD1 inhibition might prove to be beneficial both by increasing adipocyte insulin sensitivity and function and by favoring enhanced angiogenesis signals, allowing escape from adipose hypoxia and fibrosis in obesity.
Supplementary Material
Acknowledgments
We acknowledge the support of the British Heart Foundation Centre of Research Excellence. We are grateful to the Centre for Inflammation Research Histology staff for excellent technical support and Sheila Macpherson in the MRC-Centre for Reproductive Biology (CRB) for expertise and generous help on CD31 immunohistochemistry.
This work was supported by a Henry Wellcome Postdoctoral Fellowship (to Z. M.), a Wellcome Trust Programme Grant (to J. R. S.), and a grant from the European Community 7th Framework Programme (FP7/2007-2013) under Grant Agreement 201608, entitled Targeting Obesity-driven Inflammation (TOBI). B. R. W., J. R. S., and N. M. M. are inventors on relevant patents owned by the University of Edinburgh. B. R. W. and J. R. S. have consulted on 11βHSD1 inhibitors for a number of pharmaceutical companies.

This article contains supplemental Figs. S1–S4.
- HIF
- hypoxia-inducible factor
- SVF
- stromovascular fraction
- ANGPTL4
- angiopoietin protein-like 4
- scAT
- subcutaneous adipose tissue
- mesAT
- mesenteric adipose tissue
- PHD
- HIF prolyl hydroxylase
- KO
- 11βHSD1 knock-out
- 11βHSD1
- 11β-hydroxysteroid dehydrogenase type 1
- DIO
- diet-induced obesity
- PPAR
- peroxisome proliferator-activated receptor
- HF
- high fat
- α-SMA
- α-smooth muscle actin
- ANOVA
- analysis of variance
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Hosogai N., Fukuhara A., Oshima K., Miyata Y., Tanaka S., Segawa K., Furukawa S., Tochino Y., Komuro R., Matsuda M., Shimomura I. (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56, 901–911 [DOI] [PubMed] [Google Scholar]
- 2. Rausch M. E., Weisberg S., Vardhana P., Tortoriello D. V. (2008) Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. (Lon.) 32, 451–463 [DOI] [PubMed] [Google Scholar]
- 3. Yin J., Gao Z., He Q., Zhou D., Guo Z., Ye J. (2009) Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 296, E333–E342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pasarica M., Sereda O. R., Redman L. M., Albarado D. C., Hymel D. T., Roan L. E., Rood J. C., Burk D. H., Smith S. R. (2009) Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yun Z., Maecker H. L., Johnson R. S., Giaccia A. J. (2002) Inhibition of PPARγ2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev. Cell 2, 331–341 [DOI] [PubMed] [Google Scholar]
- 6. Shimba S., Wada T., Hara S., Tezuka M. (2004) EPAS1 promotes adipose differentiation in 3T3-L1 cells. J. Biol. Chem. 279, 40946–40953 [DOI] [PubMed] [Google Scholar]
- 7. Halberg N., Khan T., Trujillo M. E., Wernstedt-Asterholm I., Attie A. D., Sherwani S., Wang Z. V., Landskroner-Eiger S., Dineen S., Magalang U. J., Brekken R. A., Scherer P. E. (2009) Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Molecular and cellular biology 29, 4467–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rask E., Olsson T., Söderberg S., Andrew R., Livingstone D. E., Johnson O., Walker B. R. (2001) Tissue-specific dysregulation of cortisol metabolism in human obesity. J. Clin. Endocrinol. Metab. 86, 1418–1421 [DOI] [PubMed] [Google Scholar]
- 9. Michailidou Z., Jensen M. D., Dumesic D. A., Chapman K. E., Seckl J. R., Walker B. R., Morton N. M. (2007) Omental 11β-hydroxysteroid dehydrogenase 1 correlates with fat cell size independently of obesity. Obesity 15, 1155–1163 [DOI] [PubMed] [Google Scholar]
- 10. Masuzaki H., Paterson J., Shinyama H., Morton N. M., Mullins J. J., Seckl J. R., Flier J. S. (2001) A transgenic model of visceral obesity and the metabolic syndrome. Science 294, 2166–2170 [DOI] [PubMed] [Google Scholar]
- 11. Kotelevtsev Y., Holmes M. C., Burchell A., Houston P. M., Schmoll D., Jamieson P., Best R., Brown R., Edwards C. R., Seckl J. R., Mullins J. J. (1997) 11β-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc. Natl. Acad. Sci. U.S.A. 94, 14924–14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morton N. M., Paterson J. M., Masuzaki H., Holmes M. C., Staels B., Fievet C., Walker B. R., Flier J. S., Mullins J. J., Seckl J. R. (2004) Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11β-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes 53, 931–938 [DOI] [PubMed] [Google Scholar]
- 13. Wamil M., Battle J. H., Turban S., Kipari T., Seguret D., de Sousa Peixoto R., Nelson Y. B., Nowakowska D., Ferenbach D., Ramage L., Chapman K. E., Hughes J., Dunbar D. R., Seckl J. R., Morton N. M. (2011) Novel fat depot-specific mechanisms underlie resistance to visceral obesity and inflammation in 11β-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes 60, 1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Small G. R., Hadoke P. W., Sharif I., Dover A. R., Armour D., Kenyon C. J., Gray G. A., Walker B. R. (2005) Preventing local regeneration of glucocorticoids by 11β-hydroxysteroid dehydrogenase type 1 enhances angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 102, 12165–12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McSweeney S. J., Hadoke P. W., Kozak A. M, Small G. R., Khaled H., Walker B. R., Gray G. A. (2010) Improved heart function follows enhanced inflammatory cell recruitment and angiogenesis in 11βHSD1-deficient mice post-MI. Cardiovasc Res. 88, 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. (1999) The tumor suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 [DOI] [PubMed] [Google Scholar]
- 17. Epstein A. C., Gleadle J. M., McNeill L. A., Hewitson K. S., O'Rourke J., Mole D. R., Mukherji M., Metzen E., Wilson M. I., Dhanda A., Tian Y. M., Masson N., Hamilton D. L., Jaakkola P., Barstead R., Hodgkin J., Maxwell P. H., Pugh C. W., Schofield C. J., Ratcliffe P. J. (2001) Caenorhabditis elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 [DOI] [PubMed] [Google Scholar]
- 18. Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr. (2001) HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 [DOI] [PubMed] [Google Scholar]
- 19. Copple B. L., Bai S., Moon J. O. (2010) Hypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic Kupffer cells. Hepatol. Res. 40, 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iredale J. P. (2007) Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. invest. 117, 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eickelberg O. (2001) Endless healing: TGF-β, SMADs, and fibrosis. FEBS Lett. 506, 11–14 [DOI] [PubMed] [Google Scholar]
- 22. Keophiphath M., Achard V., Henegar C., Rouault C., Clément K., Lacasa D. (2009) Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol. Endocrinol. 23, 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friedman S. L. (2010) Evolving challenges in hepatic fibrosis. Nat. Rev. Gastroenterol. Hepatol. 7, 425–436 [DOI] [PubMed] [Google Scholar]
- 24. Stockmann C., Kerdiles Y., Nomaksteinsky M., Weidemann A., Takeda N., Doedens A., Torres-Collado A. X., Iruela-Arispe L., Nizet V., Johnson R. S. (2010) Loss of myeloid cell-derived vascular endothelial growth factor accelerates fibrosis. Proc. Natl. Acad. Sci. U.S.A. 107, 4329–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gealekman O., Burkart A., Chouinard M., Nicoloro S. M., Straubhaar J., Corvera S. (2008) Enhanced angiogenesis in obesity and in response to PPARγ activators through adipocyte VEGF and ANGPTL4 production. Am. J. Physiol. Endocrinol. Metab. 295, E1056–E1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arany Z., Foo S. Y., Ma Y., Ruas J. L., Bommi-Reddy A., Girnun G., Cooper M., Laznik D., Chinsomboon J., Rangwala S. M., Baek K. H., Rosenzweig A., Spiegelman B. M. (2008) HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 451, 1008–1012 [DOI] [PubMed] [Google Scholar]
- 27. Ndubuizu O. I., Tsipis C. P., Li A., LaManna J. C. (2010) Hypoxia-inducible factor-1 (HIF-1)-independent microvascular angiogenesis in the aged rat brain. Brain Res. 1366, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nauck M., Karakiulakis G., Perruchoud A. P., Papakonstantinou E., Roth M. (1998) Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur. J. Pharmacol. 341, 309–315 [DOI] [PubMed] [Google Scholar]
- 29. Leonard M. O., Godson C., Brady H. R., Taylor C. T. (2005) Potentiation of glucocorticoid activity in hypoxia through induction of the glucocorticoid receptor. J. Immunol. 174, 2250–2257 [DOI] [PubMed] [Google Scholar]
- 30. De Sousa Peixoto R. A., Turban S., Battle J. H., Chapman K. E., Seckl J. R., Morton N. M. (2008) Preadipocyte 11β-hydroxysteroid dehydrogenase type 1 is a keto-reductase and contributes to diet-induced visceral obesity in vivo. Endocrinology 149, 1861–1868 [DOI] [PubMed] [Google Scholar]
- 31. Villaret A., Galitzky J., Decaunes P., Estève D., Marques M. A., Sengenès C., Chiotasso P., Tchkonia T., Lafontan M., Kirkland J. L., Bouloumié A. (2010) Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes 59, 2755–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ye J., Gao Z., Yin J., He Q. (2007) Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 293, E1118–E1128 [DOI] [PubMed] [Google Scholar]
- 33. Kodama T., Shimizu N., Yoshikawa N., Makino Y., Ouchida R., Okamoto K., Hisada T., Nakamura H., Morimoto C., Tanaka H. (2003) Role of the glucocorticoid receptor for regulation of hypoxia-dependent gene expression. J. Biol. Chem. 278, 33384–33391 [DOI] [PubMed] [Google Scholar]
- 34. Haddad J. J., Land S. C. (2001) A non-hypoxic, ROS-sensitive pathway mediates TNF-α-dependent regulation of HIF-1α. FEBS Lett. 505, 269–274 [DOI] [PubMed] [Google Scholar]
- 35. Jung Y. J., Isaacs J. S., Lee S., Trepel J., Neckers L. (2003) IL-1β-mediated up-regulation of HIF-1α via an NFκB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 17, 2115–2117 [DOI] [PubMed] [Google Scholar]
- 36. Feldser D., Agani F., Iyer N. V., Pak B., Ferreira G., Semenza G. L. (1999) Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res. 59, 3915–3918 [PubMed] [Google Scholar]
- 37. Treins C., Giorgetti-Peraldi S., Murdaca J., Semenza G. L., Van Obberghen E. (2002) Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J. Biol. Chem. 277, 27975–27981 [DOI] [PubMed] [Google Scholar]
- 38. He Q., Gao Z., Yin J., Zhang J., Yun Z., Ye J. (2011) Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am. J. Physiol. Endocrinol. Metab. 300, E877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.