Background: Abiraterone and TOK-001 are inhibitors of CYP17 activity, a crucial enzyme for the synthesis of testosterone in prostate cancer cells.
Results: These 17-heteroarylsteroids also directly down-regulate the expression and activation of the androgen receptor.
Conclusion: TOK-001 and abiraterone, down-regulate androgen receptor signaling via multiple mechanisms.
Significance: Anti-CYP17 17-heteroarylsteroids may have multiple mechanisms of action in prostate cancer cells.
Keywords: Cancer Therapy, Protein Drug Interactions, Testosterone, Translation, Translation initiation Factors, 17-Heteroarylsteroids, TOK-001, Abiraterone, Androgen Receptor, Prostate Cancer
Abstract
TOK-001 and abiraterone are potent 17-heteroarylsteroid (17-HAS) inhibitors of Cyp17, one of the rate-limiting enzymes in the biosynthesis of testosterone from cholesterol in prostate cancer cells. Nevertheless, the molecular mechanism underlying the prevention of prostate cell growth by 17-HASs still remains elusive. Here, we assess the effects of 17-HASs on androgen receptor (AR) activity in LNCaP and LAPC-4 cells. We demonstrate that both TOK-001 and abiraterone reduced AR protein and mRNA expression, and antagonized AR-dependent promoter activation induced by androgen. TOK-001, but not abiraterone, is an effective apparent competitor of the radioligand [3H]R1881 for binding to the wild type and various mutant AR (W741C, W741L) proteins. In agreement with these data, TOK-001 is a consistently superior inhibitor than abiraterone of R1881-induced transcriptional activity of both wild type and mutant AR. However, neither agent was able to trans-activate the AR in the absence of R1881. Our data demonstrate that phospho-4EBP1 levels are significantly reduced by TOK-001 and to a lesser extent by abiraterone alcohol, and suggest a mechanism by which cap-dependent translation is suppressed by blocking assembly of the eIF4F and eIF4G complex to the mRNA 5′ cap. Thus, the effects of these 17-HASs on AR signaling are complex, ranging from a decrease in testosterone production through the inhibition of Cyp17 as previously described, to directly reducing both AR protein expression and R1881-induced AR trans-activation.
Introduction
The androgen receptor (AR)2 is a ligand-dependent transcription factor responsible for the normal development of prostate tissue by regulating androgen-responsive gene expression during adolescence (1). Despite an essential role for the AR in the maturing prostate, dysregulation of AR signaling has been linked to a number of human diseases, most notably prostate cancer (2). To combat altered AR signaling in prostate cancer, anti-androgens such as bicalutamide and flutamide were developed to: (a) block the binding of testosterone and dihydrotestosterone (DHT) to AR, (b) cause the assembly of a transcriptionally inactive receptor, possibly by inducing a conformational change in the AR that either prevents receptor dimerization, diminishes double-stranded DNA binding, or (c) increase the recruitment of nuclear co-repressor complexes into holo-AR transcriptional complexes (3–5).
Although advanced prostate cancers have historically been labeled androgen independent, there is little doubt that the AR still plays a key role in their growth and viability (6, 7). Circulating levels of androgen in the blood is no longer considered an accurate marker for androgen levels in the tumor (8). Intra-tumoral levels of testosterone are approximately equivalent to those found in the normal prostate gland (9), while levels of DHT, though reduced, can still be significantly above its Kd of binding to the AR. Therefore, reduction in systemic androgen levels, though the major goal of contemporary prostate cancer therapy, is not adequate to sufficiently suppress intra-tumoral androgen levels nor to abrogate androgen receptor-mediated gene activity (8), due in part to an up-regulation of AR activity. Several mechanisms for the up-regulation of AR activity include AR gene amplification (10, 11), AR mutation (12, 13), alterations in AR-associated co-regulators (14), as well as the synthesis of intratumoral androgens (8–9, 15–16). Furthermore, the transcriptional activity of the AR may also become entirely ligand-independent (17). It has also been shown that disruption of the AR can inhibit the proliferation of ostensibly androgen-refractory cells (18, 19). Thus, a reasonable therapeutic strategy would be to drastically reduce the levels AR protein in prostate cancer cells, by targeting its stability, degradation, expression and/or activity (18, 20–21).
Many strategies, including naturally occurring compounds and gene-based oligonucleotides, have been employed to down-regulate AR expression. Molecules that have been shown to decrease the steady-state level of AR protein include: quercetin (22); the non-steroidal anti-inflammatory flufenamic acid (23); resveratrol (24); the flavone luteolin (25); docetaxel (which may be one of its major mechanisms of action clinically; (26)); phytocompounds from the oriental herbal medicine Wedelia chinensis (27); siRNAs (28); morpholino antisense oligonucleotides (oligos, (29)); antisense phosphorothioate oligos delivered by electroporation (30), and antisense locked nucleic acid (LNA (31)) and FANA (32) oligonucleotides delivered gymnotically (33). Unfortunately, all of these approaches suffer from diminished clinical utility due to the requirement for high concentrations that lead to toxicity, to high cost, and to drug delivery problems.
More recently, a novel C-17 heteroarylsteroid (3β-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene, also known as VN/124–1 and TOK-001) has been described (34). This compound shares the ability of abiraterone alcohol (the active pharmaceutical ingredient and plasma enzymatic cleavage product of abiraterone acetate (35, 36)), also a C-17 heteroarylsteroid (17-HAS), to potently inhibit the function of 17α-hydroxylase/17,20 lyase (CYP17; (37)), the rate-determining enzyme in the synthesis of testosterone from precursor steroids. However, in addition to its CYP17-inhibitory properties, TOK-001 has been shown to down-regulate AR protein levels both in vitro and in vivo in the LAPC-4 human tumor xenograft mouse model (38). TOK-001 has also been stated to inhibit cellular proliferation by induction of an endoplasmic reticulum stress response, resulting in down-regulation of cyclin D1 protein expression and arrest in the G1 phase of the cell cycle (39). Because of its multiple mechanisms of action and highly favorable pre-clinical toxicity profile, TOK-001 has recently entered a Phase 1/2 clinical trial in eight centers in the US. However, the molecular mechanism(s) underlying the inhibition of the AR by TOK-001 remain unknown.
In this study, we evaluate the effects of TOK-001 and abiraterone alcohol on AR expression and AR signaling in AR-positive LNCaP and LAPC-4 cells. Whereas both TOK-001 and abiraterone alcohol decrease steady-state expression of AR protein to a similar level, TOK-001 proved more effective at blocking androgen-induced transcriptional activation by the AR. The reduction in AR protein and AR signaling in response to 17-HASs was observed for both the WT and mutant AR proteins. Our data also demonstrate that TOK-001 and abiraterone alcohol can target the cell's own translational machinery to reduce AR protein levels. This report extends the utility of 17-HASs beyond Cyp17 inhibition and provides a novel mechanism of action for antagonism of AR activity in prostate cancer cells.
EXPERIMENTAL PROCEDURES
Cell Lines and Reagents
PC3 (CRL-1435) and LNCaP (CRL-1740) cells were maintained in RMPI media supplemented with 10% heat-inactivated fetal bovine serum, 2 mm l-glutamine, 100 units/ml penicillin G sodium/100 mg/ml streptomycin sulfate, sodium pyruvate, and non-essential amino acids at 37 °C in a humidified 5% CO2 incubator. LAPC-4 cells, a generous gift of Dr. R. Reiter (UCLA), were maintained similarly, but in IMDM media supplemented with 5% heat inactivated fetal bovine serum. Cells expressing either the wild type (WT) or AR mutant proteins were created by stable transfection of PC3 (AR-null) cells with pCIneo-hAR (WT), pCIneo-hAR-W741C, or pCIneo-hAR-W741L (generous gifts of Dr. S. P. Balk, Beth Israel Medical Center, Boston, MA). Where indicated, cells were cultured in phenol red-free, steroid-free media, consisting of basal media supplemented with 5–10% dextran-coated, charcoal-stripped FBS. TOK-001, previously known as VN/124–1, was prepared and generously provided by Tokai Pharmaceuticals (Cambridge, MA). TOK-001 was dissolved in DMSO prior to use. Bicalutamide (Casodex®) was dissolved in DMSO prior to use and was a generous gift of V. Njar (Jefferson Medical University, Philadelphia, PA). Abiraterone acetate, a prodrug that is rapidly deacetylated to abiraterone alcohol in plasma or tissue culture media, was a generous gift of V. Njar and was dissolved in 95% ethanol prior to use. R1881 was obtained from Perkin Elmer and dissolved in DMSO. Cycloheximide was obtained from Sigma and dissolved in ethanol. MG132 was dissolved in DMSO and was obtained from EMS Scientific (San Diego, CA). 7-Methyl-GTP (7mG)-Sepharose beads were obtained from Amersham Biosciences/GE Healthcare (Piscataway, NJ).
Immunoblot and Protein Analysis
Whole cell extracts were prepared by scraping the cells from the dish with a rubber spatula, washing the cell pellet with 1× cold PBS, extracting with lysis buffer at 4 °C for 1 h followed by the removal of cell debris by centrifugation at 14,000 × g for 20 min at 4 °C. Protein concentrations were determined using the Bradford protein assay system (Bio-Rad). Equal amounts of protein were resolved by SDS-PAGE, transferred to PVDF membrane and stained with SYPRO Ruby. Membranes were blocked for 1 h at room temperature or 4 °C overnight with 5% nonfat dry in TBS-T (10 mm Tris, pH 7.4 + 0.05% Tween-20). After treatment with the appropriate primary and secondary antibodies in 5% milk in TBS-T, enhanced chemiluminescence was performed (Amersham Biosciences, Piscataway, NJ). The following antibodies (clone, dilution) were used: anti-androgen receptor (clone F39.4.1, BioGenenix, San Ramon, CA, 1:400), anti-α-tubulin (clone B-5–1-2, Sigma; 1:3000), anti-β-actin (clone AC-15, Abcam, Cambridge, MA, 1:5000), anti-PSA (clone A67-B/E3) and anti-AR (Millipore, Temecula, CA, 1:500), anti-XIAP (clone 2F-1, Abcam, Cambridge, MA, 1:1000), anti-total eIF4E, eIF4G, or 4EBP1 and phospho-4EBP1 (Cell Signaling Technology, Danvers, MA). Quantitation of protein expression was determined using Image J analysis. Representative Western blots of at least three independent experiments are depicted in the figures.
Isolation of RNA and qRT-PCR
Total RNA was isolated from cells using Qiagen's RNeasy kit (Qiagen, Valencia, CA) and quantified using a Nanodrop. cDNA was primed using random hexamers and the Superscript II RT enzyme (Invitrogen) according to the manufacturers directions. The PCR step was performed using the EvaGreen-R qPCR supermix (ABM, BC, Canada) according to the manufacturer's instructions. qPCR reactions were performed using an ABI 7900 real time PCR system with the following cycling conditions: 50 °C, 2 min, 1×; 95 °C, 10 min, 1×; 94 °C, 20 s, 60 °C, 1 min, 40×. A dissociation step was also performed to confirm the amplification of a single product. The relative standard curve method was used to quantify the amount of AR and RPLPO mRNA in each sample. A cDNA standard curve of serial dilutions was prepared using cDNA from DMSO-treated cells for amplification with both AR and RPLPO primers. Relative gene expression was determined by using the relative standard curve method.
Competitive AR Binding Assay
The binding of TOK-001 to the AR was determined by a competitive binding assay as previously described (34). Briefly, 2–3 × 105 LNCaP or LAPC-4 cells were plated into poly-l-lysine coated wells in steroid-free media and allowed to attach. One day later, the media was replaced with serum-free, steroid-free RPMI media supplemented with 0.1% BSA, 10 μm triamcinolone acetonide, 15 nm [3H]R1881 (Perkin Elmer, Boston, MA) or, in some cases, excess testosterone. After 2 h at 37 °C, 5% CO2, whole cell extracts were obtained by washing cells twice with ice-cold 1× DPBS to remove unbound [3H]R1881, followed by the addition of 1× DPBS containing 0.5% SDS and 20% glycerol. Cell-associated radioactivity was counted using a liquid scintillation counter. The data were analyzed and Ki determined by non-linear regression using the One site-Fit Ki equation of Graphpad Prism software. (Note: This program determines Ki under non-equilibrium, non-steady state conditions using the Cheng-Prusoff equation (40). This Ki has often been referred to as Kc, the “competition constant,” as it henceforth will be in this work). For the analysis of WT or mutant AR proteins, PC-3 cells were first transiently transfected with pCIneo-AR-WT, pCIneo-AR-W741C, or pCIneo-AR-W741L.
AR Degradation Studies
LNCaP or LAPC-4 cells were plated onto 6-well plates in Phenol red-free media containing charcoal-stripped FBS and treated with cycloheximide (100 μm) along with vehicle (DMSO or ethanol) or TOK-001 or abiraterone alcohol. Whole cell extracts were collected at time t = 0 and then every 4 h for an additional 36 h. The amount of AR protein as a function of time was determined by Western blot analysis as described above. Differences in the rate of AR degradation between test compound and control were determined by Mann-Whitney statistical analysis. For experiments involving the proteasome inhibitor MG132, LNCaP, and LAPC-4 cells were co-treated with MG132 (5 μm) and abiraterone alcohol or TOK-001. Whole cell extracts were collected 24 h post-treatment and the level of AR protein was determined by Western blot.
Luciferase Assays and Cell Proliferation Assays- Determination of AR Transcriptional Activity
The plasmid pARE4-luciferase contains four Androgen Response Elements (AREs) cloned in tandem into pGL3 (Promega, Madison, WI). pRL-CMV-Renilla is a cytomegalovirus (CMV) promoter-driven Renilla luciferase control plasmid. PC3 cells stably expressing WT or mutant AR proteins were seeded into poly-lysine-coated plates using phenol red-free, steroid-free RMPI complete media without antibiotics and transfected 24 h later with 100 ng of pARE4-Luciferase and 100 pg pRL-CMV-Renilla using Lipofectamine 2000 (Invitrogen). 24 h post-transfection, the medium was changed to fresh phenol red-free, steroid-free RMPI complete media and hormones or drugs were added at the indicated concentrations. Firefly and Renilla luciferase activities were determined 18 h later using the Dual Luciferase Kit (Promega). Data shown represent the mean and standard deviation of three independent experiments performed in triplicate and is expressed as relative light units (RLU, firefly luciferase/Renilla luciferase). For the determination of IC50 values, dose-response data were analyzed by non-linear regression to fit the data to the log (inhibitor) versus response with variable slope using Graphpad Prism software.
Determination of AR 5′-UTR-Luciferase Activity
LNCaP cells were seeded in 24-well plates at 70% confluence a day before transfections. Cells then were transfected with plasmid DNA (0.5 μg/well) carrying pIR-AR 5′UTR-Luc (5′UTR; test) or pIRES-Luc (IRES, control) for 6 h in serum-free and antibiotic-free conditions. Transcription of both luciferase reporter genes is under the control of the CMV promoter. Constructions of the plasmid DNA were described elsewhere (see Ref. 49 for details). Lipofectamine 2000 reagent was used in all transfections according to the manufacturer's instructions (Invitrogen). Cells were then treated with doses of TOK-001 (0, 10, and 20 μm) in 5% FBS/T-Medium (Invitrogen). Luciferase reporter gene activities were measured using Luciferase Assay System from Promega (Madison, WI) at 36 h post treatment using a BMG Labtech microplate reader (Cary, NC). Relative luciferase units were normalized to total protein and then normalized to vector control (pIR-AR 5′UTR-Luc) and the result was presented as luciferase activity. For cell proliferation studies, LNCaP cells in 96-well plate were seeded 24 h prior to drug treatment and then treated with control (mock), TOK-001 (10 μm), or abiraterone alcohol (10 μm) in 5% FBS/T-medium for 72 h. Cell proliferation was determined using MTS according to manufacturer's instruction (Promega).
Cap Binding Assay
LNCaP cells were seeded in 6-well plates 24 h prior to drug exposures. Cells were then exposed to doses of TOK-001 or abiraterone alcohol (0, 10, and 20 μm) in 5%FBS/T-Medium for 36 h. Cells were washed with ice cold PBS and total cell lysates were prepared in cap binding buffer containing 150 mm NaCI, 50 mm Tris, pH 7.5, 50 mm NaF, 10 mm Na pyrophosphate, 1 mm EDTA, 2.5 mm Na orthovanadate supplemented with mixture of protease inhibitor (Calbiochem) from indicated conditions. For cap binding assay, 25 μl of pre-washed 7-methyl-GTP (7mG) Sepharose beads slurry was added to 150 μg total proteins and incubated at room temperature for 1 h. Samples were then washed with cap binding buffer and quenched with sample buffer. Samples were boiled and equal amount of elute and 10 μg of total lysates were resolved by 7.5% (for the detections of eIF4G and AR proteins) or by 12% (for the detection of eIF4E, 4EBP1, phospho-4EBP1 and AR proteins) SDS-PAGE and then transferred onto nitrocellulose membranes. Western blots were performed essentially as described in Ref. 49 using a polyclonal antibody raised against total eIF4E, eIF4G, 4EBP1, phospho-4EBP-1, or AR and the signal was detected using chemiluminescence (Thermo Scientific).
RESULTS
TOK-001 and Abiraterone Alcohol, Two 17-HASs, Reduce AR Protein Levels
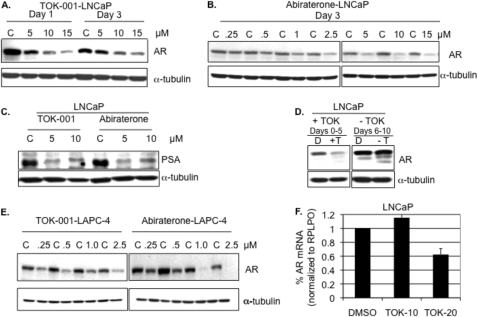
We measured the effects of treatment with these two compounds on AR expression in two prostate cancer cell lines, LNCaP and LAPC-4, that express AR and respond to androgen signaling. When LNCaP cells were cultured in medium supplemented with charcoal-stripped serum (CSS, T < 1 nm) followed by treatment with increasing concentrations of TOK-001, the steady-state levels of AR protein were markedly decreased (up to 84%, 15 μm TOK-001, Day 3, Fig. 1A). Abiraterone alcohol, another potent Cyp17 inhibitor, produced a similar dose-dependent decrease in AR protein levels in LNCaP cells (Fig. 1B). The decrease in AR protein correlated with a profound diminution of the expression of intracellular PSA protein in LNCaP cells (Fig. 1C), which in this cell line is dependent on AR signaling. For both TOK-001 and abiraterone alcohol, no significant decrease in AR protein was observed at concentrations below 2.5 μm in LNCaP cells after 3 or 5 days of treatment. When LNCaP cells initially treated for 5 days with TOK-001 were re-cultured in media that lacked TOK-001, AR protein returned to levels observed in untreated cells within 5 days, indicating that AR down-regulation is reversible (Fig. 1D). In LAPC-4 cells, which over-express the WT AR, the concentration of 17-HAS required for maximum silencing of the AR is ∼5-fold lower than in LNCaP cells (1.0 μm versus 5.0 μm) (Fig. 1E). In LAPC-4 cells, abiraterone alcohol reduced AR expression to a greater extent than TOK-001 at concentrations greater than or equal to 1 μm. When LNCaP cells were treated with 20 μm TOK-001 for 24 h, AR mRNA levels were reduced by 38%. However no reduction in AR mRNA was observed in LNCaP cells treated with 10 μm TOK-001 (Fig. 1F).
FIGURE 1.
The effect of 17-HASs on the levels of AR protein and AR mRNA. A, AR protein from LNCaP cells cultured in charcoal-stripped serum (CSS) and treated with the indicated concentrations of TOK-001 (5–15 μm). Control (C) cells were treated with an equal volume of DMSO. A representative Western blot of whole cell extracts collected on day 1 or day 3 post-treatment. The levels of α-tubulin were also determined as a loading control. B, Western blot analysis for AR protein from LNCaP cells cultured in CSS and treated for 3 days with the indicated concentrations of abiraterone alcohol (5–15 μm). Control (C) cells were treated with an equal volume of ethanol. The levels of α-tubulin were also determined as a loading control. C, Western blot analysis for intracellular PSA protein levels in LNCaP cells cultured in FBS and treated for 3 days with the indicated concentrations of TOK-001 or abiraterone alcohol. Control (C) cells were treated with an equal volume of either DMSO or ethanol. The levels of α-tubulin were also determined as a loading control. D, recovery of AR protein expression after TOK-001 treatment. Western blot analysis of AR steady-state protein levels in LNCaP cells cultured in CSS and treated with DMSO (D) or 5 μm TOK-001 (+T) for 5 days (Days 0–5, left panel). Treated cells were then subcultured into fresh media without drug (-T) and incubated for an additional 5 days (Days 6–10, right panel). The levels of α-tubulin were also determined as a loading control. E, Western blot of AR protein levels in LAPC-4 cells cultured in CSS for 3 days with the indicated concentrations (0.25–2.5 μm) of TOK-001 and abiraterone alcohol. Control (C) cells were treated with an equal volume of either DMSO or ethanol. The levels of α-tubulin were also determined as a loading control. F, qRT-PCR of AR steady-state mRNA levels in LNCaP cells cultured in CSS and treated for 24 h with the indicated concentrations of TOK-001. AR mRNA levels were normalized to RPLPO. Data represent the mean and S.D. of independent experiments.
TOK-001 and Abiraterone Alcohol Bind to the Wild Type and Mutant AR
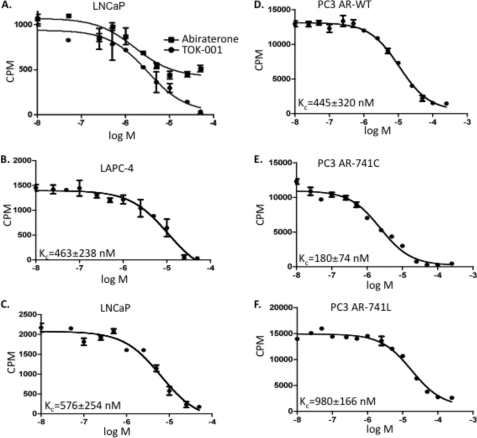
We used an established competitive binding assay with the radioligand [3H]R1881 to determine the Kc (competition constant) of TOK-001 and abiraterone alcohol for the AR-WT and various clinically-relevant mutant ARs. Under saturating concentrations (15 nm R1881), the Kc of testosterone (T) for [3H]R1881 binding to the AR was determined to be 5 nm (data not shown), similar to the previously determined value of Kd (42). We attempted to measure the competitive binding of abiraterone alcohol to the T877A mutant AR that is naturally expressed in LNCaP cells (43). Only a 2-fold reduction in [3H]R1881 binding to the T877A AR was observed in the presence of a > 6000-fold molar excess of abiraterone alcohol (Fig. 2A). Abiraterone alcohol exhibited a similar inability to compete with [3H]R1881 for binding to the AR-WT expressed in LAPC-4 cells (data not shown). Although an exact determination of the Kc for abiraterone alcohol is not possible because of (a) the aqueous insolubility of this compound at high concentrations, and (b) the probable formation of micelles, we can nevertheless estimate the Kc to be greater than 3 μm.
FIGURE 2.
Competitive binding assay of TOK-001 and abiraterone alcohol for the AR. A, competitive binding curve of TOK-001 and abiraterone alcohol for the T877A mutant AR in LNCaP cells. B–F, competitive binding curve of TOK-001 for the wild type AR (LAPC-4 (B)), mutant AR (LNCaP, T877A) (C)), (D–F) AR expressed in PC3 cells: wild type AR (PC3 AR-WT (D)), mutant W741C AR (PC3 AR-741C (E)), mutant W741L (PC3 AR-W741L (F)). In PC3 cells, the WT AR and mutant AR proteins were expressed by transient transfection, as described. Kc was determined by non-linear regression using the One-site fit Ki equation of the GraphPad Prism Software. Plotted are AR-bound counts (cpm) versus [17-HAS] (log [M]). The average kc ± S.D. (n = 3) is shown in the lower left corner of each graph.
In contrast to the very low competitive ability of abiraterone alcohol, TOK-001 was an effective competitor of [3H]R1881 binding to the WT and mutant ARs (Fig. 2A). No significant difference in the TOK-001 Kc was found between the endogenously expressed WT AR in LAPC-4 cells (Kc = 460 nm, Fig. 2B) and the transiently expressed WT AR in PC3 cells (Kc = 445 nm, Fig. 2D). The Kc of TOK-001 inhibition of binding of [3H]R1881 to the T877A mutant AR expressed in LNCaP cells (Kc = 575 nm, Fig. 2C) was only slightly greater than that measured for the WT AR (Kc = 460 nm). These data are in agreement with previous studies demonstrating that TOK-001 was an effective competitor for radioligand binding to the AR in LAPC-4 and LNCaP cells (34, 38).
In clinical practice, the non-steroid anti-androgen bicalutamide (Casodex-®) is now employed more frequently than flutamide. However, its use has been associated with the emergence of two additional AR ligand binding domain (LBD) mutations, W741C and W741L (44). PC3 (AR-null) cells were capable of supporting high levels of WT, W741C, or W741 mutant AR expression (supplemental Fig. S1). The W741C and W741L mutant AR proteins allow bicalutamide to act as an agonist for AR transcriptional activation (see Fig. 3C). TOK-001 was an effective competitor of [3H]R1881 binding to AR containing either the W741C or W741L mutations (KcW741C = 180 nm; KcW741L = 980 nm), albeit with different magnitudes (Fig. 2, D--F).
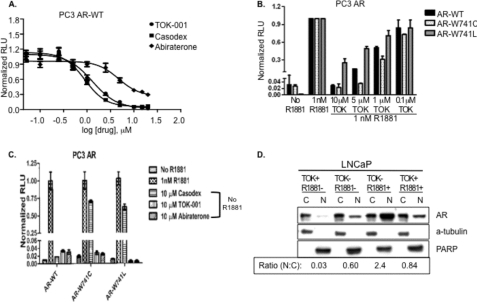
FIGURE 3.
Suppression of androgen receptor trans-activation by 17-HASs. A, PC3 clones that stably express the WT AR protein were transiently transfected with the reporter vector pARE4-Luciferase and pCMV-Renilla. 24 h later, cells were stimulated with R1881 (1 nm) and treated with serial dilutions of TOK-001, Casodex, or abiraterone alcohol (n = 6 ± S.E.). The amount of transcriptional activity was normalized to Renilla luciferase and is expressed as normalized relative light units (RLU). Solid lines represent the best-fit sigmoidal dose response (variable slope). IC50[TOK-001] = 1.3 μm; IC50[abiraterone alcohol] = 4.7 μm; IC50[Casodex] = 0.89 μm. B, PC3 clones that stably express the wild type (black), W741C (white), or W741L (gray) AR proteins were transiently transfected with the reporter vector pARE-4X-Luciferase and then stimulated with R1881 (1 nm). Cells were co-treated with the indicated concentrations of TOK-001. The amount of transcriptional activity was normalized to Renilla luciferase and is expressed as normalized relative light units (RLU). C, PC3 clones that stably express the WT, W741C, or W741L AR proteins were transiently transfected with the reporter vector pARE-4-Luciferase. The cells were untreated (mock or control), treated with 1 nm R1881, or treated with 10 μm Casodex, 10 μm TOK-001, or 10 μm abiraterone in the absence of R1881. The amount of transcriptional activity was normalized to Renilla luciferase and is expressed as normalized relative light units (RLU). D, LNCaP cells were either pretreated with DMSO (TOK-) or pretreated with 20 μm TOK-001 (TOK+) for 1 h. Cells were then stimulated with either 1 nm R1881 (R1881+) or DMSO (R1881-) for an additional 2 h before fractionation of the cytoplasmic (C) and nuclear (N) proteins by differential lysis and centrifugation. A representative Western blot is depicted showing the levels of AR in the C and N fractions. The levels of PARP and α-tubulin indicate the quality of the fractionation procedure.
TOK-001 and Abiraterone Alcohol Antagonize AR Transcriptional Activity
The disparate binding of these 17-HASs to the WT and mutant AR proteins suggests that abiraterone alcohol and TOK-001 might also exhibit different abilities to antagonize AR activity. To examine this possibility, we measured AR-dependent promoter reporter gene activation (pARE4-Luc) following transient transfection of PC3 cells that were co-transfected with WT AR. These cells were then treated by R1881 and either TOK-001, abiraterone alcohol or bicalutamide. TOK-001 exhibited a significantly greater ability to block R1881-induced AR transcriptional activation compared with abiraterone alcohol (IC50[TOK-001] = 1.3 μm versus IC50[abiraterone alcohol] = 4.7 μm) (Fig. 3A). We also compared the ability of the 17-HASs to block ligand-dependent AR transactivation against that of bicalutamide. Bicalutamide (IC50 = 0.89 μm) was only slightly more effective than TOK-001 (IC50 = 1.3 μm), but greater than 5-fold more effective than abiraterone alcohol, at blocking AR transactivation (Fig. 3A).
We then examine the inhibitor effects of TOK-001 on the ability of mutant ARs (versus WT AR) to mediate promoter reporter activation in PC3 cells. In cells expressing the W741C mutant AR, R1881-induced transcriptional activation was reduced by more than 49-fold in the presence in 10 μm TOK-001. Below 10 μm, TOK-001 exhibited a superior ability to block transcriptional activation of the mutant AR-W741C mutant compared with the decrease in transactivation observed for the AR-WT (Fig. 3B). In cells treated with 5 μm TOK-001, ligand-induced AR transcriptional activation was reduced by more than 28-fold in PC3 cells expressing the AR-W741C mutant, compared with a ∼6.2-fold reduction observed in AR-WT cells (WT, 6.2 ± 0.6 versus W741C, 28 ± 0.4; p < 0.01). In cells treated with 1 μm TOK-001, a significant difference in the reduction of AR trans-activation was also observed for the WT versus W741C mutant AR (fold reduction: WT, 1.8 ± 0.3 versus W741C, 3.2 ± 0.5; p < 0.05) (Fig. 3B).
The reduction in AR transcriptional activation at 10 μm TOK-001 was significantly less for the W741L AR compared with the AR-WT (fold reduction: WT, 37 ± 1.0 versus W741L, 4.0 ± 1.1; p < 0.01). The more limited ability of TOK-001 to block W741L AR transcriptional activation compared with the AR-WT was also seen at a 5 μm concentration (fold reduction: WT, 6.2 ± 0.6 versus W741L, 2.0 ± 0.1; p < 0.05) (Fig. 3B). Thus, TOK-001 exhibited the most effective trans-activational block in cells expressing the AR-W741C protein, for which the drug also exhibits the highest [3H]R1881 competitive ability (KcW741C = 180 nm). In contrast, TOK-001 did not as potently compete with radio-ligand binding to the AR-W741L mutant protein (KcW741L = 980 nm) (Fig. 3B).
Next, we determined whether TOK-001 possessed agonist or partial agonist activity for the WT or mutant AR. This was done because mutations in the AR LBD can create AR proteins with broadened specificity for non-steroidal antiandrogens such as flutamide and bicalutamide (12, 44). Therefore, we measured reporter gene activity in cells treated with TOK-001 or bicalutamide in the absence of R1881. Both TOK-001 and bicalutamide failed to induce transcriptional activity of the WT AR in the absence of R1881 (Fig. 3C). However, under similar conditions, bicalutamide promoted transcriptional activation in the W741C and W741L mutant ARs (Fig. 3C). This demonstrates that TOK-001 does not possess agonist activity against the WT AR or mutant W741C/L mutant (Fig. 3C). Similarly, abiraterone alcohol also failed to stimulate the AR activation of the WT AR or AR-W741 mutants in the absence of R1881 (data not shown). Our data not only confirm the agonist activity of bicalutamide for the W741C/L LBD mutations (Fig. 3C) but also provide evidence that the 17-HASs evaluated here do not possess the ability to trans-activate either wild type or mutant AR proteins in the absence of androgen.
The AR is rapidly translocated to the nucleus upon androgen binding. We measured AR levels in the cytoplasmic and nuclear fractions following pretreatment of LNCaP cells with TOK-001 prior to exposure to the synthetic androgen R1881 (Fig. 3D). In cells pretreated with vehicle and then exposed to androgen (TOK-/R1881+), AR levels in the nucleus were ∼5-fold higher than in untreated cells (TOK-001-/R1881-). While pretreatment of LNCaP cells with TOK-001 had little effect on AR levels in the cytoplasm, AR levels in the nucleus were reduced ∼3-fold in cells pretreated with TOK-001 (TOK-001+/R1881-) relative to untreated cells. Even after stimulation with R1881, cells that were pretreated with TOK-001 (TOK-001+/R1881+) showed reduced nuclear AR levels compared with cells exposed to R1881 alone (TOK-001-/R1881+). These findings suggest that TOK-001 reduces androgen-stimulated AR nuclear translocation, which is an important mechanism of AR-dependent gene expression.
TOK-001 and Abiraterone Alcohol Do Not Affect the Rate of AR Degradation
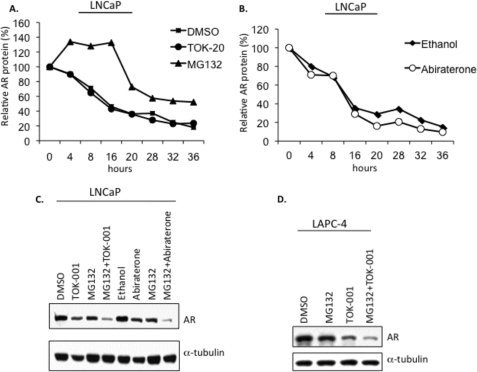
To determine whether these compounds promote AR is degradation, we measured AR protein levels by Western blot following treatment of LNCaP cells cultured in charcoal-stripped serum with the protein synthesis inhibitor cycloheximide (CHX) and either TOK-001 or abiraterone. No difference (Mann-Whitney, p = NS) in the rate of AR protein loss was observed when cells were treated with either DMSO (vehicle) or TOK-001 in the presence of CHX. In LNCaP cells treated with CHX plus the reversible 26 S proteasome inhibitor MG132 (5 μm), AR protein levels remained stable for at least 16 h before declining, confirming that under these culture conditions normal turnover of the AR is controlled by the proteasome (Fig. 4A). Abiraterone alcohol increased the rate of AR degradation compared with ethanol (vehicle)-treated cells; however this small change was only observed at later time points (Fig. 4B). Over the entire time course of the experiment, however, there was no difference in the rate of protein loss when cells were treated either with ethanol (vehicle) or abiraterone alcohol (Mann-Whitney, p = NS). The decrease in steady-state AR protein expression after treatment of LNCaP cells with either abiraterone alcohol or TOK-001 was not reversed by co-treatment with MG132; co-treatment of LNCaP cells with MG132 plus 17-HASs resulted in an additive decrease in AR protein levels (Fig. 4C). Similarly, MG132 failed to reverse the loss of AR-WT protein in LAPC-4 cells following treatment with TOK-001 (Fig. 4D). In fact, co-treatment of MG132 plus 17-HASs caused a more profound decrease in AR protein compared with the treatment using MG132 or 17-HAS alone. However, it is known that proteasome inhibition is associated with down-regulation of AR mRNA expression (14, 45, 47, 48). These findings suggests that TOK-001 does not contribute to an increase in the proteasome-mediated degradation of the AR.
FIGURE 4.
Neither TOK-001 nor abiraterone increase the rate of AR degradation. A and B, representative AR degradation time-course experiment in LNCaP cells is shown. AR protein was measured at 4-h intervals from LNCaP cells cultured in androgen-free media and co-treated with cycloheximide (CHX, 100 μm) and 20 μm TOK-001 (TOK-20) or 20 μm abiraterone alcohol (abiraterone). AR protein expression was also measured from vehicle control cells treated with CHX+DMSO (for TOK-001 (A)) or CHX+ethanol (for abiraterone alcohol (B)). AR levels are expressed relative to the time at t = 0. For both compounds, compared with vehicle, p = NS (Mann-Whitney). In a separate experiment, LNCaP cells were co-treated with cycloheximide (100 μm) and MG132 (5 μm). C, Western blot analysis of AR steady-state protein levels in LNCaP cells treated for 24 h with 17-HASs (TOK, 20 μm TOK-001; ABIR, 20 μm abiraterone alcohol) in the absence or presence of MG132 (5 μm). The levels of α-tubulin were determined as a loading control. D, Western blot analysis of AR steady-state protein levels in LAPC-4 cells treated for 24 h with TOK-001 (20 μm) in the absence or presence of MG132 (5 μm). The levels of α-tubulin were determined as a loading control.
TOK-001 and Abiraterone Alcohol Target Translational Machinery to Reduce AR Protein Levels
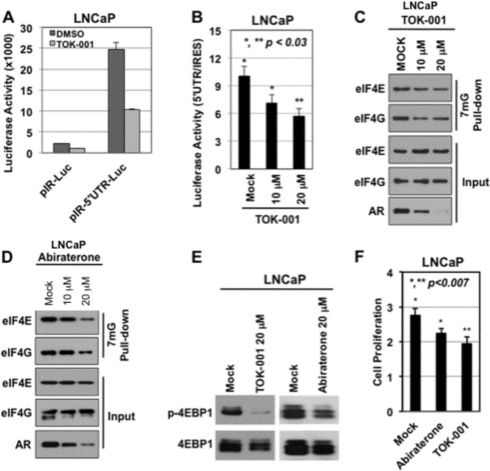
Published studies indicate that AR expression can also be regulated at the translational level through alterations in the cap-dependent translational machinery (49). To investigate whether TOK-001 or abiraterone alcohol could reduce AR expression by regulating the translation machinery, we first measured AR 5′-UTR-directed luciferase (pIR-AR 5′-UTR-Luc) gene expression at the translational level, along with control pIRES-Luc (49, 50). The inhibition of pIRES-Luc and pIR-AR 5′-UTR-Luc by TOK-001 are individually shown in Fig. 5A ([TOK-001] = 20 μm) in LNCaP cells grown in serum-fed conditions. Treatment of LNCaP cells under these conditions with TOK-001 reduced normalized luciferase reporter activity in a dose-dependent manner (Fig. 5B). In contrast, abiraterone alcohol did not significantly alter luciferase reporter activity under the control of either the IRES or AR 5′-UTR promoters. Next, we conducted a cap binding experiment after treatment of serum-fed LNCaP cells with TOK-001 or abiraterone alcohol. This was done because the binding of eIF4E along with eIF4G to cap-mRNA plays an important role in the modulation of mRNA translation, including that of the AR (49). The data in Fig. 5C demonstrated that TOK-001, in a dose-dependent manner, reduced the binding of eIF4E and eIF4G to 7mG-Sepharose by 50% compared with untreated control cells (DMSO); this closely correlated with reduced levels of AR protein as demonstrated by Western blot (Fig. 5C, input). Similar results were obtained with abiraterone alcohol (Fig. 5D). Furthermore, the reduction in AR protein by TOK-001, and to a lesser extent by abiraterone alcohol, also correlated with a significant reduction in the levels of phosphorylated 4EBP1 protein (Fig. 5E) By laser scanning densitometry, the p-4EBP1/4EBP1 ratio was 13% (i.e. an 87% decrease in 4EBP1 phosphorylation) for the TOK-001 treated LNCaP cells versus mock under serum-fed conditions. In contrast, the ratio for cells treated with abiraterone alcohol was 74% (i.e. a 26% decrease in 4EBP1 phosphorylation)) This protein, when unphosphorylated, functions as a suppressor of cap-dependent mRNA translation (51). These findings indicate that TOK-001 alters the cap-dependent machinery and reduces AR protein expression. This also occurs, but to a lesser extent, with abiraterone alcohol. Given that androgenic signaling is important for cell growth in LNCaP prostate cancer cells (52), we then measured the growth of LNCaP cells under similar experimental conditions to assess the biological significance of these data. The results demonstrate that TOK-001 and abiraterone cause a significant reduction of LNCaP cell growth after 72 h in serum-fed conditions (Fig. 5F, p < 0.01), which is consistent with the reduced AR protein levels.
FIGURE 5.
The AR translational machinery is a target of 17-HASs. A and B, luciferase reporter assay, in which translation of the luciferase mRNA is under the control of AR 5′-UTR (test) or IRES (control). Cells were treated with 20 μm TOK-001 or an equal volume of vehicle (DMSO) in A or treated with increasing doses of TOK-001 in B. C and D, cap-binding assay with 7mG-pull down in total lysates from LNCaP cells treated with 10 μm or 20 μm TOK-001 in C or 10 μm or 20 μm abiraterone alcohol (ABI) in D for 36 h. Western blot analysis with the indicated antibodies is depicted. E, Western blot analysis of 4EPB1 and phospho-4EBP1 (p-4EBP1) in total lysates from LNCaP cells treated with vehicle (mock), 20 μm TOK-001, or 20 μm abiraterone alcohol (ABI). F, cell proliferation with MTS; *,** p < 0.007. LNCaP cells were incubated with mock (control), TOK-001 (10 μm), or abiraterone (10 μm) in serum-fed media for 72 h. Statistical analyses were performed using unpaired Student's t test. The data represent at least three independent experiments.
DISCUSSION
The progression of prostate cancer to castration resistance (CRPC) is driven by the selection of tumor cells that can proliferate in a low androgen environment. These cells often have alterations in expression and/or mutations in the AR that impart a selective growth advantage, allowing them to utilize the relatively low concentrations of systemic androgens, or available intra-tumoral androgens, to drive cellular proliferation. Therefore, therapeutic strategies seeking to increase the number of durable remissions in patients with CRPC should focus on the elimination of AR signaling entirely (18–21). Here we now provide evidence that abiraterone alcohol and TOK-001, two C-17 heteroarylsteroid (17-HAS) drugs that were originally designed to block testosterone synthesis by inhibiting CYP17 activity, also possess anti-androgen properties that decrease AR protein expression and AR signaling in human prostate cancer cell lines through multiple mechanisms. (We could not, however, demonstrate a stress response in cells after treatment with TOK-001).
The steady-state levels of AR protein depend on two balanced processes: The rate of AR protein synthesis versus the rate of its proteasomal degradation. Many natural and synthetic products reduce AR steady-state protein levels by enhancing proteasome-mediated degradation of AR. For example, the flavonoid luteolin increases the rate of AR degradation by disrupting the function of HSP90 (25), a protein chaperone essential for AR stability that is the target of ansamycin antibiotic-based drugs such as geldanamycin. We did observe a small increase in the rate of AR degradation when LNCaP cells were treated with abiraterone alcohol for greater than 20 h. In contrast, TOK-001 had no significant impact on the rate of AR protein degradation in LNCaP cells (Fig. 4, A and C), suggesting that the mechanism of AR protein loss by these two compounds may be different. This difference is also supported by our observation that TOK-001 reduced AR protein levels in LNCaP cells to a greater degree than abiraterone alcohol at time points less than or equal to 36 h post-treatment.
Post-transcriptional control of AR expression is regulated in part by specific cis-acting sequences in the long 5′-untranslated region (5′-UTR) that are important for translation initiation (49, 50). A ∼0.5 kb region of the AR 5′-UTR containing binding sites for the nucleic acid-binding protein HnRNPK has been implicated in the attenuation of AR translation initiation by PI 3-kinase/Akt/mTOR signaling (53). A mechanism by which TOK-001 (and to a lesser extent abiraterone alcohol) alters AR translation initiation is supported by the reduced expression of a reporter gene fused to the AR 5′-UTR. In addition, inefficient translation initiation could also be due to poor recruitment of eIF4E and eIF4G to the 7mG cap of the AR mRNA in response to 17-HASs as a class. Our observation that phospho-4EBP1 levels are significantly reduced after treatment with these both TOK-001 and, to a lesser extent, abiraterone alcohol suggests a mechanism by which cap-dependent translation is suppressed by blocking assembly of the eIF4F complex to the mRNA 5′ cap (54). Although the mechanism by which 17-HASs suppress the phosphorylation of 4EBP1 is unclear at this time, previous data indicates that TOK-001 can suppress mTORC1 signaling (55), which is a key regulator of protein synthesis in mammalian cells. TOK-001, but not abiraterone alcohol (which is a less potent inducer of AR protein down-regulation than TOK-001) also had a negative impact on a luciferase reporter gene whose translation relies on the encephalomyocarditis virus (ECMV) internal ribosomal entry site (IRES; Fig. 5A), suggesting that TOK-001 affects the global translational machinery. A role for TOK-001 in suppressing IRES-dependent translation could explain the observed decrease in XIAP expression in treated cells (supplemental Fig. S2), whose translation is mediated by an IRES element within its 5′-UTR (56). The down-regulation of XIAP protein may well be a beneficial effect of TOK-001 treatment, given that overexpression of this protein appears to contribute to treatment failure in clinical prostate cancer (57).
The defects in cap- and IRES-dependent translation are not the result of apoptotic signaling in response to TOK-001 or abiraterone alcohol, as there was no evidence of an increase in the percentage of apoptotic cells in response to TOK-001 alone, nor did we observe an increase in the cleavage of PARP-1, eIF4G (Fig. 5, C and D) or pro-caspase-3 to caspase-3 (supplemental Fig. S3A). Similarly, there were no consistent changes as a function of time in the levels of expression of Bcl-xL or Bax proteins (supplemental Fig. S3A). In addition, TOK-001 did not interfere with the pro-apoptotic effects of docetaxel, a drug used extensively in CRPC patients (supplemental Fig. S3B). It is important to note that LNCaP proliferation was not suppressed by concentrations of TOK-001 below 20 μm, even though the reduction in cap-dependent translation correlated with a 50% decrease in AR protein expression. Thus, these observations suggest the possibility that significant changes in the proliferation rate of prostate cancer cells requires near complete loss of AR protein expression, which is likely to require the inhibition of multiple pathways involved in the transcriptional and post-transcriptional regulation of AR expression. Further, inhibition of cap-dependent translation by 17-HASs is undoubtedly not specific for the AR. This process may also effect the translation of other genes, but any potential toxicity associated with nonspecific effects in normal cells can best be addressed during on-going clinical studies.
Both abiraterone alcohol and TOK-001 proved effective at blocking AR-mediated trans-activation in response to androgen, albeit with different potencies. The 3.6-fold greater antagonism of the WT AR by TOK-001 compared with abiraterone alcohol is likely due to the higher affinity of TOK-001 for the AR. The ability of 17-HASs to inhibit androgen-mediated trans-activation of WT AR and mutant-W741 AR proteins is an important property when considering additional anti-androgen treatments for patients who have failed bicalutamide. Our observation that abiraterone alcohol exhibits a significant ability to block AR trans-activation despite its weak binding to the AR suggests that apparent competitive blocking of androgen binding to the AR cannot by itself account for the inhibitory effect on AR gene activation by abiraterone alcohol. Moreover, pretreatment of LNCaP cells with TOK-001 prevents the increase in nuclear AR that occurs in response to ligand-mediated AR translocation. Thus, 17-HASs possess two different mechanisms to decrease AR signaling in prostate cancer cells: (i) inhibition of AR trans-activation, and (ii) reduction in AR steady-state protein expression.
In summary, the properties of 17-HASs extend beyond a single mechanism of action (i.e. CYP17 inhibition). The inhibition of AR signaling by 17-HASs likely occurs through multiple mechanisms, including inhibition of AR trans-activation, reduction in AR steady-state protein expression and reduced AR-nuclear translocation. Combined with their inhibitory effects on CYP17 activity and intratumoral testosterone production, the ability to target multiple signaling axes critical for prostate cancer progression makes 17-HASs optimal candidates for development as clinical therapeutic drugs for prostate cancer (46, 58–59).
Supplementary Material
This work was generously funded by Tokai Pharmaceuticals, Cambridge, MA, the David and Rose Himelberg Foundation (to C. A. S.), the Edwin Beer Fellowship Grant R24D00 from the New York Academy of Medicine, and the Garber Foundation from Cedars-Sinai Medical Center (to B. C.).

This article contains supplemental Figs. S1–S3.
- AR
- androgen receptor
- ARE
- androgen response element
- WT
- wild-type
- CMV
- cytomegalovirus
- XIAP
- X-linked inhibitor of apoptosis
- DHT
- dihydrotestosterone
- CRPC
- castrate-resistant prostate cancer
- PSA
- prostate specific antigen
- LNA
- locked nucleic acid
- FANA
- 2′-fluoro, 2′-deoxyarabinose nucleic acid
- Cyp
- cytochrome P450
- T
- testosterone
- RLU
- relative light units.
REFERENCES
- 1. Thomson A. A. (2001) Role of androgens and fibroblast growth factors in prostatic development. Reproduction 121, 187–195 [DOI] [PubMed] [Google Scholar]
- 2. Yong E. L., Lim J., Qi W., Ong V., Mifsud A. (2000) Molecular basis of androgen receptor diseases. Ann. Med. 32, 15–22 [DOI] [PubMed] [Google Scholar]
- 3. Masiello D., Cheng S., Bubley G. J., Lu M. L., Balk S. (2002) Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J. Biol. Chem. 277, 26321–26326 [DOI] [PubMed] [Google Scholar]
- 4. Farla P., Hersmus R., Trapman J., Houtsmuller A. (2005) Antiandrogens prevent stable DNA binding of the androgen receptor. J. Cell Sci. 118, 4187–4198 [DOI] [PubMed] [Google Scholar]
- 5. Singh P., Uzgare A., Litvinov I., Denmeade S., Isaacs J. (2006) Combinatorial androgen receptor targeted therapy for prostate cancer. Endocr. Related Cancer 13, 653–666 [DOI] [PubMed] [Google Scholar]
- 6. Chen C. D., Welsbie D. S., Tran C., Baek S. H., Chen R., Vessella R., Rosenfeld M. G., Sawyers C. L. (2004) Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10, 33–39 [DOI] [PubMed] [Google Scholar]
- 7. Snoek R., Cheng H., Margiotti K., Wafa L. A., Wong C. A., Wong E. C., Fazli L., Nelson C. C., Gleave M. E., Rennie P. S. (2009) In vivo knockdown of the androgen receptor results in growth inhibition and regression of well-established, castration-resistant prostate tumors. Clin. Cancer Res. 15, 39–47 [DOI] [PubMed] [Google Scholar]
- 8. Mostaghel E., Montgomery R., Lin D. (2007) The Basic Biochemistry and Molecular Events of Hormone Therapy. Curr. Urol. Rep. 8, 224–232 [DOI] [PubMed] [Google Scholar]
- 9. Mohler J. L., Gregory C. W., Ford O. H., 3rd, Kim D., Weaver C. M., Petrusz P., Wilson E. M., French F. S. (2004) The androgen axis in recurrent prostate cancer. Clin. Cancer Res. 10, 440–448 [DOI] [PubMed] [Google Scholar]
- 10. Visakorpi T., Hyytinen E., Koivisto P., Tanner M., Keinänen R., Palmberg C., Palotìe A., Tammela T., Isola J., Kallioniemi O. P. (1995) In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 9, 401–406 [DOI] [PubMed] [Google Scholar]
- 11. Koivisto P., Kononen J., Palmberg C., Tammela T., Hyytinen E., Isola J., Trapman J., Cleutjens K., Noordzij A., Visakorpi T., Kallioniemi O. P. (1997) Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 57, 314–319 [PubMed] [Google Scholar]
- 12. Taplin M. E., Bubley G. J., Shuster T. D., Frantz M. E., Spooner A. E., Ogata G. K., Keer H. N., Balk S. P. (1995) Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N. Engl. J. Med. 332, 1393–1398 [DOI] [PubMed] [Google Scholar]
- 13. Tilley W. D., Buchanan G., Hickey T. E., Bentel J. M. (1996) Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin. Cancer Res. 2, 277–285 [PubMed] [Google Scholar]
- 14. Lin H. K., Altuwaijri S., Lin W. J., Kan P. Y., Collins L. L., Chang C. (2002) Proteasome activity is required for androgen receptor transcriptional activity via regulation of androgen receptor nuclear translocation and interaction with coregulators in prostate cancer cells. J. Biol. Chem. 277, 36570–36576 [DOI] [PubMed] [Google Scholar]
- 15. Geller J., Albert J., Loza D., Geller S., Stoeltzing W., de la Vega D. (1978) DHT concentrations in human prostate cancer tissue. J. Clin. Endocrinol. Metab. 46, 440–444 [DOI] [PubMed] [Google Scholar]
- 16. Geller J., Albert J., Loza D. (1979) Steroid levels in cancer of the prostate–markers of tumour differentiation and adequacy of anti-androgen therapy. J. Steroid Biochem. 11, 631–636 [DOI] [PubMed] [Google Scholar]
- 17. Huang Z. Q., Li J., Wong J. (2002) AR possesses an intrinsic hormone-independent transcriptional activity. Mol. Endocrinol. 16, 924–937 [DOI] [PubMed] [Google Scholar]
- 18. Zegarra-Moro O. L., Schmidt L. J., Huang H., Tindall D. J. (2002) Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 62, 1008–1013 [PubMed] [Google Scholar]
- 19. Li T. H., Zhao H., Peng Y., Beliakoff J., Brooks J. D., Sun Z. (2007) A promoting role of androgen receptor in androgen-sensitive and -insensitive prostate cancer cells. Nucleic Acids Res. 35, 2767–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balk S. (2002) Androgen receptor as a target in androgen-independent prostate cancer. Urology 60, 132–138 [DOI] [PubMed] [Google Scholar]
- 21. Balk S., Knudsen K. (2008) AR, the cell cycle, and prostate cancer. Nucl. Recep. Signal 6, e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xing N., Chen Y., Mitchell S., Young C. (2001) Quercetin inhibits the expression and function of the androgen receptor In LNCaP prostate cancer cells. Carcinogenesis 22, 401–414 [DOI] [PubMed] [Google Scholar]
- 23. Zhu W., Smith A., Young C. Y. (1999) A nonsteroidal anti-inflammatory drug, flufenamic acid, inhibits the expression of the androgen receptor in LNCaP cells. Endocrinology 140, 5451–5454 [DOI] [PubMed] [Google Scholar]
- 24. Mitchell S. H., Zhu W., Young C. Y. (1999) Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Cancer Res. 59, 5892–5895 [PubMed] [Google Scholar]
- 25. Chiu F. L., Lin J. K. (2008) Down-regulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate 68, 61–71 [DOI] [PubMed] [Google Scholar]
- 26. Kuroda K., Liu H., Kim S., Guo M., Navarro V., Bander N. (2009) Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: Implications for PSA surrogacy. Prostate 69, 1579–1585 [DOI] [PubMed] [Google Scholar]
- 27. Lin F. M., Chen L. R., Lin E. H., Ke F. C., Chen H. Y., Tsai M. J., Hsiao P. W. (2007) Compounds from Wedelia chinensis synergistically suppress androgen activity and growth in prostate cancer cells. Carcinogenesis 28, 2521–2529 [DOI] [PubMed] [Google Scholar]
- 28. Liao X., Tang S., Thrasher J. B., Gribling T., Li B. (2005) Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol. Cancer Therap. 4, 505–515 [DOI] [PubMed] [Google Scholar]
- 29. Ko Y. J., Devi G. R., London C. A., Kayas A., Reddy M. T., Iversen P. L., Bubley G. J., Balk S. P. (2004) Androgen receptor down-regulation in prostate cancer with phosphorodiamidate morpholino antisense oligomers. J. Urol. 172, 1140–1144 [DOI] [PubMed] [Google Scholar]
- 30. Eder I. E., Culig Z., Ramoner R., Thurnher M., Putz T., Nessler-Menardi C., Tiefenthaler M., Bartsch G., Klocker H. (2000) Inhibition of LncaP prostate cancer cells by means of androgen receptor antisense oligonucleotides. Cancer Gene Therap. 7, 997–1007 [DOI] [PubMed] [Google Scholar]
- 31. Koch T., Oerum. H. (2008) in Antisense Drug Technology, 2 Ed (Crooke S., ed), pp. 519–562, CRC Press, Boca Raton, FL [Google Scholar]
- 32. Ferrari N., Bergeron D., Tedeschi A. L., Mangos M. M., Paquet L., Renzi P. M., Damha M. J. (2006) Characterization of antisense oligonucleotides comprising 2'-deoxy-2'-fluoro-beta-D-arabinonucleic acid (FANA): specificity, potency, and duration of activity. Ann. N.Y. Acad. Sci. 1082, 91–102 [DOI] [PubMed] [Google Scholar]
- 33. Stein C. A., Hansen J. B., Lai J., Wu S., Voskresenskiy A., Høg A., Worm J., Hedtjärn M., Souleimanian N., Miller P., Soifer H. S., Castanotto D., Benimetskaya L., Ørum H., Koch T. (2010) Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 38, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Handratta V. D., Vasaitis T. S., Njar V. C., Gediya L. K., Kataria R., Chopra P., Newman D., Jr., Farquhar R., Guo Z., Qiu Y., Brodie A. M. (2005) Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J. Med. Chem. 48, 2972–2984 [DOI] [PubMed] [Google Scholar]
- 35. Attard G., Reid A. H., Yap T. A., Raynaud F., Dowsett M., Settatree S., Barrett M., Parker C., Martins V., Folkerd E., Clark J., Cooper C. S., Kaye S. B., Dearnaley D., Lee G., de Bono J. S. (2008) Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 [DOI] [PubMed] [Google Scholar]
- 36. Attard G., Reid A. H., A'Hern R., Parker C., Oommen N. B., Folkerd E., Messiou C., Molife L. R., Maier G., Thompson E., Olmos D., Sinha R., Lee G., Dowsett M., Kaye S. B., Dearnaley D., Kheoh T., Molina A., de Bono J. S. (2009) Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 27, 3742–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hall P. F. (1991) Cytochrome P-450 C21scc: one enzyme with two actions: hydroxylase and lyase. J. Steroid Biochem. Mol. Biol. 40, 527–532 [DOI] [PubMed] [Google Scholar]
- 38. Vasaitis T., Belosay A., Schayowitz A., Khandelwal A., Chopra P., Gediya L. K., Guo Z., Fang H. B., Njar V. C., Brodie A. M. (2008) Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol. Cancer Therap. 7, 2348–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bruno R., Gover T., Burger A., Brodie A., Njar V. (2008) 17 alpha-Hydroxylase/17,20 lyase inhibitor VN/124-1 inhibits growth of androgen-independent prostate cancer cells via induction of the endoplasmic reticulum stress response. Mol. Cancer Therap. 7, 2828–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng Y., Prusoff W. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 [DOI] [PubMed] [Google Scholar]
- 41.Deleted in proof
- 42. Kampa M., Papakonstanti E. A., Hatzoglou A., Stathopoulos E. N., Stournaras C., Castanas E. (2002) The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. FASEB J. 16, 1429–1431 [DOI] [PubMed] [Google Scholar]
- 43. Veldscholte J., Berrevoets C. A., Ris-Stalpers C., Kuiper G. G., Jenster G., Trapman J., Brinkmann A. O., Mulder E. J. (1992) The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. Steroid Biochem. Mol. Biol. 41, 665–669 [DOI] [PubMed] [Google Scholar]
- 44. Hara T., Miyazaki J., Araki H., Yamaoka M., Kanzaki N., Kusaka M., Miyamoto M. (2003) Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 63, 149–153 [PubMed] [Google Scholar]
- 45. Reddy G. P., Barrack E. R., Dou Q. P., Menon M., Pelley R., Sarkar F. H., Sheng S. (2006) Regulatory processes affecting androgen receptor expression, stability, and function: potential targets to treat hormone-refractory prostate cancer. J. Cell. Biochem. 98, 1408–1423 [DOI] [PubMed] [Google Scholar]
- 46. Danila D. C., Morris M. J., de Bono J. S., Ryan C. J., Denmeade S. R., Smith M. R., Taplin M. E., Bubley G. J., Kheoh T., Haqq C., Molina A., Anand A., Koscuiszka M., Larson S. M., Schwartz L. H., Fleisher M., Scher H. I. (2010) Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J. Clin. Oncol. 28, 1496–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ikezoe T., Yang Y., Saito T., Koeffler H. P., Taguchi H. (2004) Proteasome inhibitor PS-341 down-regulates prostate-specific antigen (PSA) and induces growth arrest and apoptosis of androgen-dependent human prostate cancer LNCaP cells. Cancer Sci. 95, 271–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pajonk F., van Ophoven A., McBride W.H. (2005) Hyperthermia-induced proteasome inhibition and loss of androgen receptor expression in human prostate cancer cells. Cancer Res. 65, 4836–4843 [DOI] [PubMed] [Google Scholar]
- 49. Cinar B., De Benedetti A., Freeman M.R. (2005) Post-transcriptional regulation of the androgen receptor by Mammalian target of rapamycin. Cancer Res. 65, 2547–2553 [DOI] [PubMed] [Google Scholar]
- 50. Mizokami A., Chang C. (1994) Induction of translation by the 5'-untranslated region of human androgen receptor mRNA. J. Biol. Chem. 269, 25655–25659 [PubMed] [Google Scholar]
- 51. Shamji A. F., Nghiem P., Schreiber S. L. (2003) Integration of growth factor and nutrient signaling: implications for cancer biology. Mol. Cell 12, 271–280 [DOI] [PubMed] [Google Scholar]
- 52. Horoszewicz J. S., Leong S. S., Kawinski E., Karr J. P., Rosenthal H., Chu T. M., Mirand E. A., Murphy G. P. (1983) LNCaP model of human prostatic carcinoma. Cancer Res. 43, 1809–1818 [PubMed] [Google Scholar]
- 53. Mukhopadhyay N. K., Kim J., Cinar B., Ramachandran A., Hager M. H., DiVizio D., Adam R. M., Rubin M. A., Raychaudhuri P., DeBenedettì A., Freeman M. R. (2009) Heterogeneous nuclear ribonucleoprotein K is a novel regulator of androgen receptor translation. Cancer Res. 69, 2210–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. She Q. B., Halilovic E., Ye Q., Zhen W., Shirasawa S., Sasazuki T., Solit D. B., Rosen N. (2010) 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell 18, 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schayowitz A., Sabnis G., Njar V. C., Brodie A. M. (2008) Synergistic effect of a novel antiandrogen, VN/124–1, and signal transduction inhibitors in prostate cancer progression to hormone independence in vitro. Mol. Cancer Ther. 7, 121–132 [DOI] [PubMed] [Google Scholar]
- 56. Holcik M., Lefebvre C., Yeh C., Chow T., Korneluk R. G. (1999) A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat. Cell Biol. 1, 190–192 [DOI] [PubMed] [Google Scholar]
- 57. Seligson D. B., Hongo F., Huerta-Yepez S., Mizutani Y., Miki T., Yu H., Horvath S., Chia D., Goodglick L., Bonavida B. (2007) Expression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrence. Clin. Cancer Res. 15, 6056–6063 [DOI] [PubMed] [Google Scholar]
- 58. Ryan C. J., Smith M. R., Fong L, Rosenberg J. E., Kantoff P., Raynaud F., Martins V., Lee G., Kheoh T., Kim J., Molina A., Small E. J. (2010) Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin. Oncol. 28, 1481–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reid A. H., Attard G., Danila D. C., Oommen N. B., Olmos D., Fong P. C., Molife L. R., Hunt J., Messiou C., Parker C., Dearnaley D., Swennenhuis J. F., Terstappen L. W., Lee G., Kheoh T., Molina A., Ryan C. J., Small E., Scher H. I., de Bono J. S. (2010) Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J. Clin. Oncol. 28, 1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.