Background: Giardia lamblia parasitizes the human small intestine to cause diseases. It differentiates into infectious cysts by intestinal stimulation.
Results: Giardial Cdk2 is associated with up-regulation of cyst wall protein genes.
Conclusion: Cdk2 may phosphorylate a Myb transcription factor, leading to activation of cyst wall protein genes during encystation.
Significance: The results may help us to develop new drugs to inhibit cyst formation by disrupting the Cdk2 pathway.
Keywords: CDK (Cyclin-dependent Kinase), Cell Cycle, Cell Differentiation, Gene Regulation, Signal Transduction, Giardia, Myb
Abstract
The protozoan Giardia lamblia parasitizes the human small intestine to cause diseases. It undergoes differentiation into infectious cysts by responding to intestinal stimulation. How the activated signal transduction pathways relate to encystation stimulation remain largely unknown. During encystation, genes encoding cyst wall proteins (CWPs) are coordinately up-regulated by a Myb2 transcription factor. Because cell differentiation is linked to cell cycle regulation, we tried to understand the role of cell cycle regulators, cyclin-dependent kinases (Cdks), in encystation. We found that the recombinant Myb2 was phosphorylated by Cdk-associated complexes and the levels of phosphorylation increased significantly during encystation. We have identified a putative cdk gene (cdk2) by searching the Giardia genome database. Cdk2 was found to localize in the cytoplasm with higher expression during encystation. Interestingly, overexpression of Cdk2 resulted in a significant increase of the levels of cwp gene expression and cyst formation. In addition, the Cdk2-associated complexes can phosphorylate Myb2 and the levels of phosphorylation increased significantly during encystation. Mutations of important catalytic residues of Cdk2 resulted in a significant decrease of kinase activity and ability of inducing cyst formation. Addition of a Cdk inhibitor, purvalanol A, significantly decreased the Cdk2 kinase activity and the levels of cwp gene expression and cyst formation. Our results suggest that the Cdk2 pathway may be involved in phosphorylation of Myb2, leading to activation of the Myb2 function and up-regulation of cwp genes during encystation. The results provide insights into the use of Cdk inhibitory drugs in disruption of Giardia differentiation into cysts.
Introduction
Giardia lamblia is one of the most common human intestinal parasites (1–3). Its infection is prevalent in developing countries and contributes greatly to malnutrition and malabsorption leading to delayed child development (4). After infection of G. lamblia, some people may suffer from post-infectious irritable bowel syndrome (5–9). Like Entamoeba histolytica and other intestinal protozoan parasites, G. lamblia undergoes differentiation from a trophozoite form into a cyst form that is essential for disease transmission in the life cycle (10–12). Cysts can survive in the hostile environment and infect a new host because they have a resistant extracellular wall (1, 2). The life cycle of G. lamblia may provide perspectives on cell differentiation in response to different environments.
Because of the importance of the cyst stage, many researchers are working on identifying the key components of the cyst wall (13–15). Three important cyst wall proteins (CWP)2 (CWP1, -2, and -3) have been found to be highly up-regulated during encystation (13–15). However, there is little understanding of the molecular mechanisms governing their transcriptional or post-transcriptional regulation. A microRNA-mediated post-transcriptional regulation was found to regulate the expression of variant surface proteins (16), but little is known of this kind of regulation in the CWP expression. The lack of clear giardial homologs to many basal transcription factors suggests that Giardia may have diverged early and represents a transition during the evolution of eukaryotic transcription systems (17, 18). Only four of the 12 general transcription initiation factors have giardial homologs (17, 18). Many giardial transcription factors diverge at a higher rate than those of crown group eukaryotes (17, 19). In addition, unusually short 5′-flanking regions (<65 bp) are sufficient for the expression of many giardial protein-coding genes (20–22). Within the short promoter regions, no consensus TATA boxes or other cis-acting elements characteristic of higher eukaryotic promoters have been observed (13, 14, 20–25). Instead, AT-rich sequences that are functionally similar to the initiator element in late-branching eukaryotes have been found around the transcription start sites of many genes (13–15, 20–27). Few transcription factors that have been characterized to date are involved in cwp gene regulation. The GARP, ARID, Pax, WRKY, and E2F family transcription factors may be involved in transcriptional regulation of many different genes including the encystation-induced cwp genes (27, 28, 31). In addition, we have identified an encystation-induced Myb2 protein (open reading frame 8722, Myb1-like protein in the Giardia genome database), which can bind to the promoters of four key encystation-induced genes, cwp1-3, and myb2 itself, suggesting that Myb2 may be involved in coordinating their differential expression (26, 32). Interestingly, overexpression of Myb2 resulted in an increase of expression of CWP1 at both protein and mRNA levels and the Myb2-overexpressing trophozoites had increased capability to differentiate into cysts (32). However, little is known about encystation-induced signal transduction pathways that are involved in the regulation of Myb2 function and synthesis of CWPs. Many encystation-induced genes have been identified to contain putative Myb2-binding sequences in their promoters (33).
Myb family transcription factors are DNA-binding transcription factors important in regulating developmental processes in organisms as diverse as fungi, plants, and mammals (34–36). In higher eukaryotes, Myb proteins can function as a transcriptional activator or repressor to regulate specific gene expression and differentiation of different cell types (35, 37, 38). In mammals and plants, Myb proteins also play important roles in cell cycle regulation, acting at G1/S or G2/M transitions (39, 40). Some of the Myb target genes are required for the S or M phase, such as cyclin-dependent kinase 1 (cdk1), cyclin a, cyclin b, and pcna (39–42). Mammalian B-Myb is a transcription factor whose expression is up-regulated by an E2F-dependent transcriptional mechanism at the G1/S border of the cell cycle (43). B-Myb is phosphorylated by the G1/S phase-specific Cdk2-cyclin A or Cdk2-cyclin E complexes and cyclin A or cyclin E can enhance its transactivation activity (39, 44–47). The Myb proteins (NtMybA1–3) in plants control the G2/M phase by regulating transcription of the G2/M phase-specific genes, such as the cyclin b gene (48). The levels of Myb (NtMybA1–2) transcripts are peaked before the cyclin b transcripts reach a peak level (48). The Myb (NtMybA2) protein is phosphorylated by Cdk in a G2/M-specific manner and cyclin A and B can enhance its transactivation activity (49).
Cdks are a family of Ser/Thr protein kinases that play a central role in coordinating cell cycle progression, cell proliferation, and cell differentiation in eukaryotes (50–52). Some Cdks need to be active by association with different types of cyclins and phosphorylation by Cdk-activating kinases (50–52). Cyclin D forms complexes with Cdk4 or Cdk6 to phosphorylate and inactivate the Cdk inhibitors, Cip/Kip (50–52). The above complexes also phosphorylate and inactivate pRB to release E2F. E2F activates transcription of cyclin E and A genes and S phase genes. Cdk2-cyclin E or Cdk2-cyclin A complexes have S phase promoting activity by phosphorylation of proteins required for DNA synthesis, including histone biosynthesis, transcription factors, and DNA replication factors (50–52). At the onset of mitosis, Cdk1-cyclin B complexes phosphorylate proteins that function in mitosis events, including nuclear envelope breakdown and spindle assembly (50–52). The levels of active Cdks remain low during G1 phase, increase during progression into S phase, and then reduce during mitosis (53).
Cdks regulate transcription by direct association and phosphorylation of transcription factors (50, 54, 55). For example, Cdk2-cyclin A and Cdk1-cyclin B complexes phosphorylate p53 and enhance its sequence-specific DNA binding ability (56). Cdk7, Cdk8, or Cdk9 and their associated cyclin complexes phosphorylate RNA polymerase II to enhance transcription initiation or elongation (50, 56). Cdks may regulate transcription by indirect association and phosphorylation of activators or suppressors of transcription factors (50). For example, Cdk2 can phosphorylate linker protein histone H1 to relax the chromatin structure and this may result in activation of the DNA replication and gene transcription (50). Cdk4- or Cdk6-cyclin D complexes phosphorylate and inactivate pRB to release E2F and this may result in transcriptional activation of S phase genes (50).
A normal Giardia vegetative trophozoite in G1 phase contains a ploidy of 4N (57–60). A vegetative trophozoite in stationary phase may be arrested in G2 phase and contain a ploidy of 8N (57–60). During encystation, a trophozoite may differentiate into a cyst by dividing 2 nuclei into 4 nuclei and by replicating DNA, generating a cyst with a ploidy of 16N (57–60). Encystation in Giardia has been proposed to initiate in the G2 phase of the cell cycle, suggesting that encystation is coupled with cell cycle regulation (57–60). Because Cdks can coordinate cell cycle progression, cell proliferation, and cell differentiation, we tested the hypothesis that Cdk may regulate Giardia encystation. We found that the encystation-inducing transcription factor Myb2 was phosphorylated by Cdks and the levels of phosphorylation increased significantly during encystation. To identify a specific Cdk-cyclin complex that may phosphorylate the Myb2 protein, we further dissected the function of a giardial homologue of the Cdk family (Cdk2). We found that expression levels of the giardial Cdk2 increased during encystation. In addition, Cdk2 can phosphorylate the Myb2 and the levels of phosphorylation increased significantly during encystation. We also found that the cwp1, cwp2, and myb2 mRNA levels and cyst formation increased by Cdk2 overexpression. In addition, a cdk inhibitor, purvalanol A (61, 62), can inhibit Cdk2-dependent kinase activity. We also found an interaction between Myb2 and Cdk2-associated complexes. Our results suggest that Cdk2 may phosphorylate Myb2, resulting in activation of the specific activity of the Myb2 protein during encystation.
EXPERIMENTAL PROCEDURES
G. lamblia Culture
Trophozoites of G. lamblia WB (ATCC 50803), clone C6, were cultured in modified TYI-S33 medium (63). Encystation was performed as previously described (15). Briefly, trophozoites that were grown to late log phase in growth medium were harvested and encysted for 24 h in TYI-S-33 medium containing 12.5 mg/ml of bovine bile at pH 7.8 at a beginning density of 5 × 105 cells/ml.
Cyst Count
Cyst count was performed on the stationary phase cultures (∼2 × 106 cells/ml) during vegetative growth as previously described (64) and the data shown in Fig. 3C. Cells were subcultured in growth medium with suitable selection drugs at an initial density of 1 × 106 cells/ml. Cells seeded at this density became confluent within 24 h. Confluent cultures were maintained for an additional 8 h to ensure that the cultures were in stationary phase (at a density of∼2 × 106 cells/ml). Cyst count was performed on these stationary phase cultures. Cultures were chilled and cells were washed twice in double-distilled water at 4 ºC and trophozoites were lysed by incubation in double-distilled water overnight at 4 ºC. Cysts were washed three times in double-distilled water at 4 ºC. Water-resistant cysts were counted in a hemacytometer chamber. Cyst count was also performed on 24-h encysting cultures.
FIGURE 3.
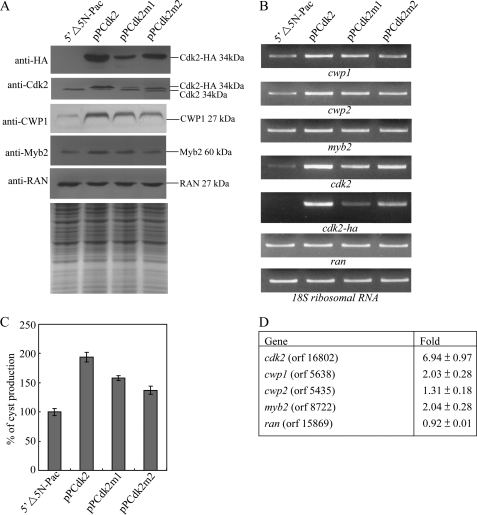
Induction of cwp1, cwp2, and myb2 gene expression in the Cdk2 overexpressing cell line. A, overexpression of Cdk2 increased the levels of CWP1 and Myb2 proteins. The 5′Δ5N-Pac, pPCdk2, pPCdk2m1, and pPCdk2m2 stable transfectants were cultured in growth medium and then subjected to SDS-PAGE and Western blot. The blot was probed by anti-HA, anti-Cdk2, anti-CWP1, anti-Myb2, and anti-RAN antibodies. Equal amounts of protein loading were confirmed by SDS-PAGE and Coomassie Blue staining. Representative results are shown. B, RT-PCR analysis of gene expression in the Cdk2, Cdk2m1, and Cdk2m2 overexpressing cell lines. The 5′Δ5N-Pac, pPCdk2, pPCdk2m1, and pPCdk2m2 stable transfectants were cultured in growth medium and then subjected to RT-PCR analysis. PCR was performed using primers specific for cwp1, cwp2, myb2, cdk2, cdk2-ha, ran, and 18S ribosomal RNA genes. C, overexpression of Cdk2 increased cyst formation. The 5′Δ5N-Pac, pPCdk2, pPCdk2m1, and pPCdk2m2 stable transfectants were cultured in growth medium and then subjected to cyst count as described under “Experimental Procedures.” The sum of total cysts is expressed as relative expression level over control. Values are shown as mean ± S.E. D, microarray analysis. Microarray data were obtained from the 5′Δ5N-Pac and pPCdk2 cell lines during vegetative growth. Fold-changes are shown as the ratio of transcript levels in the pPCdk2 cell line relative to the 5′Δ5N-Pac cell line. Results are expressed as the mean ± S.E. of at least three separate experiments.
Isolation and Analysis of the cdk2 Gene
The G. lamblia genome database (18, 65) was searched with the keyword “cdk” for annotated genes. This search detected six putative homologues for Cdk. Only two of them contain PSTAIRE-like cyclin binding motifs, which were named Cdk1 and Cdk2 (GenBankTM accession numbers XP_001704058.1 and XP_001709931.1, open reading frames 8037 and 16802, respectively, in the G. lamblia genome database). The Cdk2 coding region with 350 nucleotides of the 5′-flanking regions was cloned and the nucleotide sequence was determined. The cdk2 gene sequence in the database was correct. To isolate the cDNA of the cdk2 gene, we performed RT-PCR with cdk2-specific primers using total RNA from G. lamblia. For RT-PCR, 5 μg of DNase-treated total RNA from vegetative and 24-h encysting cells was mixed with oligo(dT)12–18 and random hexamers and SuperScript II RNase H-reverse transcriptase (Invitrogen). Synthesized cDNA was used as a template in subsequent PCR with primers cdk2F (CACCATGACTGACCCCTTGAAC) and cdk2R (CTTTGCAAAGTACGGATGCTTG). Genomic and RT-PCR products were cloned into pGEM-T easy vector (Promega) and sequenced (Applied Biosystems, ABI).
RNA Extraction and RT-PCR Analysis and Quantitative Real-time PCR Analysis
Total RNA was extracted from G. lamblia cell lines at the differentiation stages indicated in the legends to Figs. 1, 3, and 6 using TRIzol reagent (Invitrogen). For RT-PCR, 5 μg of DNase-treated total RNA was mixed with oligo(dT)12–18 and random hexamers and SuperScript II RNase H− reverse transcriptase (Invitrogen). Synthesized cDNA was used as a template in subsequent PCR. Semiquantitative RT-PCR analysis of cdk2 (XP_001709931.1, open reading frame 16802), cdk2-ha, cwp1 (U09330, open reading frame 5638), cwp2 (U28965, open reading frame 5435), myb2 (AY082882, open reading frame 8722), ran (U02589, open reading frame 15869), and 18S ribosomal RNA (M54878, open reading frame r0019) gene expression was performed using primers cdk2F and cdk2R, cdk2F and HAR (AGCGTAATCTGGAACATCGTATGGGTA); cwp1F (ATGATGCTCGCTCTCCTT) and cwp1R (TCAAGGCGGGGTGAGGCA); cwp2F (ATGATCGCAGCCCTTGTTCTA) and cwp2R (CCTTCTGCGGACAATAGGCTT); myb2F (ATGTTACCGGTACCTTCTCAGC) and myb2R (GGGTAGCTTCTCACGGGGAAG); ranF (ATGTCTGACCCAATCAGC) and ranR (TCAATCATCGTCGGGAAG); and 18S realF (AAGACCGCCTCTGTCAATCAA) and 18S realR (GTTTACGGCCGGGAATACG), respectively. For quantitative real-time PCR, SYBR Green PCR master mixture was used (Kapa Biosystems). PCR was performed using an Applied Biosystems PRISMTM 7900 Sequence Detection System (Applied Biosystems). Specific primers were designed for detection of the cdk2, cdk2-ha, cwp1, cwp2, cwp3, myb2, ran, and 18S ribosomal RNA genes: cdk2realF (GCGTCTTGCACCGTGATCTA) and cdk2realR (TTAAGGAGCCCAGAGCTGGTAA); cdk2HAF (AAGATGATGGCTCTTGACC) and HAR; cwp1realF (AACGCTCTCACAGGCTCCAT) and cwp1realR (AGGTGGAGCTCCTTGAGAAATTG); cwp2realF (TAGGCTGCTTCCCACTTTTGAG) and cwp2realR (CGGGCCCGCAAGGT); myb2realF (TCCCTAATGACGCCAAACG) and myb2realR (AGCACGCAGAGGCCAAGT); ranrealF (TCGTCCTCGTCGGAAACAA) and ranrealR (AACTGTCTGGGTGCGGATCT); 18SrealF and 18SrealR. Two independently generated stably transfected lines were made from each construct and each of these cell lines was assayed three separate times. The results are expressed as relative expression levels over control. Student's t tests were used to determine statistical significance of differences between samples.
FIGURE 1.
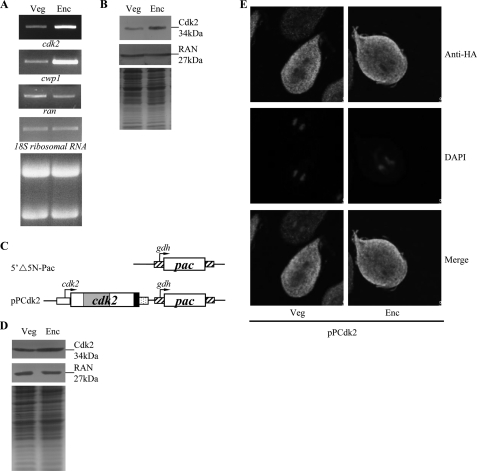
Analysis of cdk2 gene expression. A, RT-PCR analysis of cdk2 gene expression. RNA samples were prepared from G. lamblia wild-type nontransfected WB cells cultured in growth (Veg, vegetative growth) or encystation (Enc, encystation) medium and harvested at 24 h. RT-PCR was performed using primers specific for cdk2, cwp1, ran, and 18S ribosomal RNA genes. Ribosomal RNA quality and loading controls are shown in the bottom panel. Representative results are shown. B, Cdk2 protein levels in different stages. The wild-type nontransfected WB cells were cultured in growth (Veg) or encystation medium for 24 h (Enc) and then subjected to SDS-PAGE and Western blot. The blot was probed by anti-Cdk2 and anti-RAN antibody. Representative results are shown. Equal amounts of protein loading were confirmed by SDS-PAGE and Coomassie Blue staining. C, diagrams of the 5′Δ5N-Pac and pPCdk2 plasmids. The pac gene (open box) is under the control of the 5′- and 3′-flanking regions of the gdh gene (striated box). The cdk2 gene is under the control of its own 5′-flanking region (open box) and the 3′-flanking region of the ran gene (dotted box). The filled black box indicates the coding sequence of the HA epitope tag. D, Cdk2 protein levels in pPCdk2 stable transfectants. The pPCdk2 stable transfectants were cultured in growth (Veg) or encystation medium for 24 h (Enc) and then subjected to SDS-PAGE and Western blot. HA-tagged Cdk2 protein was detected using an anti-HA antibody by Western blot analysis. Equal amounts of protein loading were confirmed by SDS-PAGE and Coomassie Blue staining. E, cytoplasmic localization of the Cdk2 protein. The pPCdk2 stable transfectants were cultured in growth (Veg, left panels) or encystation media for 24 h (Enc, right panels), and then subjected to immunofluorescence analysis using anti-HA antibody for detection. The product of pPCdk2 localizes to the cytoplasm in both vegetative and encysting trophozoites (upper panels). The middle panels show the DAPI staining of cell nuclei. The bottom panels are the merged images of the DAPI staining and images of Cdk2-HA.
FIGURE 6.
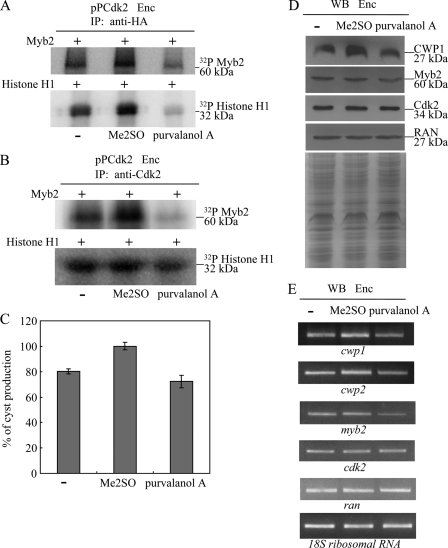
Inhibition of cyst formation by a Cdk inhibitor. A and B, inhibition of phosphorylation of Myb2 by a Cdk inhibitor. The pPCdk2 stable transfectants were cultured in encystation (Enc) medium for 24 h and then subjected to IP kinase assay using anti-HA (A) or anti-Cdk2 (B) antibody. A Cdk inhibitor, purvalanol A, was dissolved in Me2SO and added in a kinase assay reaction to a final concentration of 10 μm. The control samples were treated with the same volume of water (−) or Me2SO. Kinase activity was measured using purified recombinant Myb2 or bovine histone H1 protein as a substrate. C, addition of a Cdk inhibitor decreased the levels of cyst formation. The wild-type nontransfected WB cells were cultured in encystation medium containing 10 μm purvalanol A, or the same volume of water (−) or Me2SO for 24 h (Enc) and then subjected to cyst count. The sum of total cysts is expressed as the relative expression level over control. Values are shown as mean ± S.E. of three independent experiments. D, addition of a Cdk inhibitor decreased the levels of CWP1 and Myb2 proteins. The wild-type nontransfected WB cells were cultured in encystation medium containing 10 μm purvalanol A, or the same volume of water (−) or Me2SO for 24 h (Enc) and then subjected to SDS-PAGE and Western blot. The blot was probed by anti-CWP1, anti-Myb2, anti-Cdk2, and anti-RAN antibodies. Representative results are shown. Equal amounts of protein loading were confirmed by SDS-PAGE and Coomassie Blue staining. E, addition of a Cdk inhibitor decreased the mRNA levels of the cwp1, cwp2, and myb2 genes. The wild-type nontransfected WB cells were cultured in encystation medium containing 10 μm purvalanol A, or the same volume of water (−) or Me2SO for 24 h (Enc) and then subjected to RT-PCR analysis. PCR was performed using primers specific for the cwp1, cwp2, myb2, cdk2, ran, and 18S ribosomal RNA genes.
Plasmid Construction
All constructs were verified by DNA sequencing with a BigDye Terminator 3.1 DNA Sequencing kit and an Applied Biosystems 3100 DNA Analyzer (Applied Biosystems). Plasmid 5′Δ5N-Pac was a gift from Dr. Steven Singer and Dr. Theodore Nash (67). To make the pPCdk2, the cdk2 gene and its 300-bp 5′-flanking region were amplified with oligonucleotides cdk2XF (GGCGTCTACCTCGTCGTTGTAAGAGCGTA) and cdk2MR (GGCGACGCGTCTTTGCAAAGTACGGATGCT), digested with XbaI/MluI, and cloned into NheI/MluI-digested pPop2NHA (66). To make the pPCdk2m1, the cdk2 gene was amplified using two primer pairs cdk2m1F (GAATGCGGACGAGGGTATCgctgctgctaataatgctgctaatGCTATACTCAAAGAGATGA, mutated nucleotides are shown in lowercase type) and cdk2MR, and cdk2m1R (TCATCTCTTTGAGTATAGCattagcagcattattagcagcagcGATACCCTCGTCCGCATTC) and cdk2XF. The two PCR products were purified and used as templates for a second PCR. The second PCR also included primers cdk2XF and cdk2MR, and the product was digested with XbaI and MluI and cloned into the NheI/MluI-digested pPop2NHA vector to generate plasmid pPCdk2m1. To make the pPCdk2m2, the cdk2 gene was amplified using two primer pairs cdk2m2F (CTCCTTAAGCTCGCAaatTTTGGCCTGGCGCGT) and cdk2MR, and cdk2m2R (ACGCGCCAGGCCAAAattTGCGAGCTTAAGGAG) and cdk2XF. The two PCR products were purified and used as templates for a second PCR. The second PCR also included primers cdk2XF and cdk2MR, and the product was digested with XbaI and MluI and cloned into the NheI/MluI-digested pPop2NHA vector to generate plasmid pPCdk2m2. To make the pPcyclinA, the cyclin a gene (open reading frame 14488) and its 300-bp 5′-flanking region was amplified with oligonucleotides cycAKF (GGCGACGCGTTACGGTTGGAGATAATAAGG) and cycAMR (GGCGACGCGTCTTTGCAAAGTACGGATGCT), digested with KpnI/MluI, and cloned into KpnI/MluI-digested pPop2NHA (66).
Transfection and Western Blot Analysis
Cells transfected with the pP series plasmid containing the pac gene were selected and maintained with 54 μg/ml of puromycin. Western blots were probed with anti-V5-horseradish peroxidase (HRP) (Invitrogen), anti-HA monoclonal antibody (1/5,000 in blocking buffer; Sigma), anti-RAN antibody (1/10,000 in blocking buffer) (91), anti-CWP1 antibody (1/10,000 in blocking buffer) (32), anti-Myb2 (1/10,000 in blocking buffer) (31), or anti-Cdk2 (1/10,000 in blocking buffer), and detected with HRP-conjugated goat anti-mouse IgG (Pierce, 1/5,000 in blocking buffer) or HRP-conjugated goat anti-rabbit IgG (Pierce, 1/5,000) and enhanced chemiluminescence (GE Healthcare).
Generation of Anti-Cdk2 Antibody
The genomic cdk2 gene was amplified using oligonucleotides cdk2F and cdk2R. The product was cloned into the expression vector pET101/D-TOPO (Invitrogen) in-frame with the C-terminal His and V5 tag to generate plasmid pCdk2. The pCdk2 plasmid was freshly transformed into Escherichia coli BL21 StarTM(DE3) (Invitrogen). An overnight pre-culture was used to start a 250-ml culture. E. coli cells were grown to an A600 of 0.5, and then induced with 1 mm isopropyl d-thiogalactopyranoside (Promega) for 4 h. Bacteria were harvested by centrifugation and sonicated in 10 ml of buffer A (100 mm sodium phosphate, 10 mm Tris-Cl, 6 m guanidine hydrochloride, pH 8.0) containing 10 mm imidazole and complete protease inhibitor mixture (Roche Applied Science). The samples were centrifuged and the supernatant was mixed with 1 ml of a 50% slurry of nickel-nitrilotriacetic acid Superflow (Qiagen). The resin was washed with buffer B (100 mm sodium phosphate, 10 mm Tris-Cl, 8 m urea, pH 8.0) and buffer C (100 mm sodium phosphate, 10 mm Tris-Cl, 8 m urea, pH 6.3) and eluted with buffer E (100 mm sodium phosphate, 10 mm Tris-Cl, 8 m urea, pH 4.5). Fractions containing Cdk2 were pooled, dialyzed in phosphate-buffered saline (PBS), and stored at −70 °C. Protein purity and concentration were estimated by Coomassie Blue and silver staining compared with bovine serum albumin. Cdk2 was purified to apparent homogeneity (>95%). Purified Cdk2 protein was used to generate rabbit polyclonal antibodies through a commercial vendor (Angene, Taipei, Taiwan).
Immunofluorescence Assay
The pPCdk2, pPCdk2m1, and pPCdk2m2 stable transfectants were cultured in growth medium under puromycin selection. Cells cultured in growth medium or encystation medium for 24 h were harvested, washed in PBS, and attached to glass coverslips (2 × 106 cells/coverslip) and then fixed and stained (22). Cells were reacted with anti-HA monoclonal antibody (1/300 in blocking buffer; Molecular Probes) and anti-mouse Alexa 488 (1/500 in blocking buffer, Molecular Probes) as the detector. ProLong antifade kit with 4′,6-diamidino-2-phenylindole (Invitrogen) was used for mounting. Cdk2, Cdk2m1, and Cdk2m2 were visualized using a Leica TCS SP5 spectral confocal system.
Expression and Purification of Recombinant Myb2 Proteins
Plasmid pMyb2 has been previously described (26). The genomic myb2 gene encoding the N-terminal or C-terminal regions of Myb2 was amplified using oligonucleotides Myb2NF (CACCATGTTACCGGTACCTTCTCAG) and Myb2NR (CGGCTTCCTACGTATTACGTA) or Myb2CF (CACCATGTGCACGAACTGGGCACCA) and Myb2CR (GGGTAGCTTCTCACGGGGAAG), and the products were cloned into the expression vector pCRT7/CT-TOPO (Invitrogen) to generate plasmids pMyb2N or pMyb2C. To make the pMyb2Nm1 expression vector, the myb2 gene encoding the N-terminal region of Myb2 was amplified using oligonucleotides Myb2Nm1F (CACCATGTTACCGGTACCTTCTCAGCCATCAgcaCCGAAACGCGTACCCCAGCCAgcaCCGCCACAGTGCGTCCACgcaCCGTTTGCTC)and Myb2NR, and the products were cloned into expression vector pCRT7/CT-TOPO (Invitrogen). To make the pMyb2Nm2 (or pMyb2Nm3–6) expression vector, the myb2 gene encoding the N-terminal region of Myb2 was amplified using two primer pairs, Myb2Nm2F (AGTAGTATACgcaCCACCTGCCTTGgcaCCGATGCCTT)(or Myb2Nm3F, TGCCGTTTCCgcaCCCTTCTCTA; Myb2Nm4F, GGCTCGTATCgcaCCGTGCTACGTGGCACCAGCTGGCCCTTTCCGCgcaCCTATGGTAG; Myb2Nm5F, ACAGCTTTCCgcaCCTTCCCTAT; Myb2Nm6F, CCAATTAAATgcaCCTATTAAAA) and Myb2NR, and Myb2NF and Myb2Nm2R (or Myb2Nm3–6R). The two PCR products were purified and used as templates for a second PCR. The second PCR also included primers Myb2NF and Myb2NR, and the product was cloned into the expression vector to generate plasmids pMyb2Nm2–6. The pMyb2, pMyb2N, pMyb2C, or pMyb2Nm1–6 plasmids were freshly transformed into E. coli BL21(DE3)pLysS (QIAexpressionist, Qiagen). An overnight preculture was used to start a 250-ml culture. E. coli cells were grown to an A600 of 0.6, and then induced with 1 mm isopropyl d-thiogalactopyranoside (Promega) for 6 h. Bacteria were harvested by centrifugation and sonicated in 10 ml of buffer A (50 mm sodium phosphate, pH 8.0, 300 mm NaCl) containing 10 mm imidazole and complete protease inhibitor mixture (Roche Applied Science). The samples were centrifuged and the supernatant was mixed with 1 ml of a 50% slurry of nickel-nitrilotriacetic acid Superflow (Qiagen). The resin was washed with buffer A containing 20 mm imidazole and eluted with buffer A containing 250 mm imidazole. Fractions containing Myb2, Myb2N, Myb2C, or Myb2Nm1–6 were pooled, dialyzed in 25 mm HEPES, pH 7.9, 40 mm KCl, 0.1 mm EDTA, and 15% glycerol, and stored at −70 °C. Protein purity and concentration were estimated by Coomassie Blue and silver staining compared with bovine serum albumin. Myb2, Myb2N, Myb2C, or Myb2Nm1–6 were purified to apparent homogeneity (>95%).
Kinase Assay
Kinase assays were performed as described with modification (49). Cdk kinase activity in the p13SUC1-associated complexes were assayed from the wild-type nontransfected WB cells cultured in growth or encystation medium for 24 h. IP kinase assays were performed using the 5′Δ5N-Pac, pPCdk2, pPCdk2m1, or pPCdk2m2 stable transfectants cultured in growth or encystation medium for 24 h and anti-HA antibody for immunoprecipitation. Giardia trophozoites (∼108 cells) were harvested by centrifugation and lysed in buffer X (25 mm Tris-HCl, pH 7.5, 10 mm EDTA, 10 mm EGTA, 20 mm NaCl, 10% glycerol, 1 mm dithiothreitol, 20 mm β-glycerophosphate, 1 mm sodium o-vanadate, 1 mm NaF, 1% Triton X-100, and 1% Nonidet P-40) containing glass beads and complete protease inhibitor mixture (Roche Applied Science). The samples were centrifuged and the concentration of the supernatant was estimated by SDS-PAGE. The supernatant was mixed and rotated with p13SUC1-agarose beads (Upstate Biotechnology) or anti-HA beads (Covance) at 4 °C for 2 h. Beads were washed four times with buffer X and twice with kinase buffer (50 mm HEPES-KOH, pH 7.5, 20 mm MgCl2, 5 mm EGTA, 1 mm dithiothreitol, 20 mm β-glycerophosphate, and 1 mm sodium o-vanadate). The beads were mixed in a reaction mixture containing 50 μm ATP, and 10 μCi of [γ-32P]ATP in kinase buffer. Recombinant Myb2 protein (4 μg) or histone H1 (2 μg) (Upstate) was added to the reaction mixture and incubated for 1 h. A Cdk inhibitor, purvalanol A, may be added to the reaction at a final concentration of 10 μm. Addition of 2× sample buffer for SDS-PAGE was used to terminate the reaction. Proteins were separated on SDS-PAGE and the gels were dried. Signals were imaged using a Typhoon system (GE healthcare). The Cdk2-HA protein was detected by anti-HA antibody in Western blot.
Co-immunoprecipitation Assay
The 5′Δ5N-Pac and pPCdk2 stable transfectants were inoculated into encystation medium (5 × 107 cells in 45 ml of medium) and harvested after 24 h in encystation medium under drug selection and washed in phosphate-buffered saline. Cells were lysed in luciferase lysis buffer (Promega) and protease inhibitor (Sigma) and then vortexed with glass beads. The cell lysates were collected by centrifugation and then incubated with anti-HA antibody conjugated to beads (Bethyl Laboratories Inc.). The beads were washed three times with luciferase lysis buffer (Promega). Finally the beads were then resuspended in sample buffer and analyzed by Western blot and probed with anti-HA monoclonal antibody (1/5,000 in blocking buffer; Sigma) and anti-Myb2 (1/10,000 in blocking buffer), and detected with HRP-conjugated goat anti-mouse IgG (Pierce, 1/5,000) or HRP-conjugated goat anti-rabbit IgG (Pierce, 1/5,000) and enhanced chemiluminescence (GE Healthcare).
Flow Cytometry Analysis
The 5′Δ5N-Pac, pPCdk2, pPCdk2m1, and pPCdk2m2 stable transfectants were cultured in growth medium. Flow cytometry analysis was performed as described (68), using Sytox green (Sigma) stain. Flow cytometry was performed on a FACScalibur system (BD Biosciences). Data were analyzed by CellQuest software.
Microarray Analysis
RNA was quantified by A260 nm by an ND-1000 spectrophotometer (Nanodrop Technology) and qualified by a Bioanalyzer 2100 (Agilent Technology) with an RNA 6000 Nano LabChip kit. RNA from the pPCdk2 cell line was labeled with Cy5 and RNA from the 5′Δ5N-Pac cell line was labeled with Cy3. RNA from the wild-type nontransfected WB cells cultured in growth medium was labeled with Cy5 and RNA from the wild-type nontransfected WB cells cultured in encystation medium for 24 h were labeled with Cy3. 0.5 μg of total RNA was amplified by a Low RNA Input Fluor Linear Amp kit (Agilent Technologies) and labeled with Cy3 or Cy5 (CyDye, PerkinElmer Life Sciences) during the in vitro transcription process. 0.825 μg of Cy-labeled cRNA was fragmented to an average size of about 50–100 nucleotides by incubation with fragmentation buffer at 60 °C for 30 min. Correspondingly fragmented labeled cRNA was then pooled and hybridized to a G. lamblia oligonucleotide microarray (Agilent Technologies) at 60 °C for 17 h. After washing and drying by nitrogen gun blowing, microarrays were scanned with an Agilent microarray scanner (Agilent Technologies) at 535 nm for Cy3 and 625 nm for Cy5. Scanned images were analyzed by Feature Extraction version 9.1 software (Agilent Technologies), and image analysis and normalization software were used to quantify signal and background intensity for each feature; data were substantially normalized by the rank consistency filtering LOWESS method.
RESULTS
Phosphorylation of Myb2 by Cdks
We tested the hypothesis that the Myb2 transcription factor, which is responsible for activation of encystation-induced cwp genes, may be regulated by Cdk-mediated phosphorylation. We used p13SUC1-agarose beads to prepare Cdk-associated complexes from Giardia. p13SUC1 protein was originally identified as a suppressor in yeast cdc2 mutants (69). It has a strong affinity for Cdks that can be used to isolate Cdks (70). We used purified Myb2 and histone H1 as substrates for a kinase assay and found that the p13SUC1 fraction can phosphorylate both the Myb2 and histone H1 proteins (supplemental Fig. S1). Interestingly, the kinase activity for both substrates increased during encystation (supplemental Fig. S1). The results indicate that Myb2 may be a target of the Cdk pathway and the function of Myb2 may coincide with the increased Cdks activity during encystation.
Identification and Characterization of cdk2 Gene
To identify genes encoding novel Cdk proteins from G. lamblia, we searched the G. lamblia genome database (18, 65) with the genes annotated as “Cdk.” This search detected six putative homologues for Cdk. Structural study of human Cdk2 suggests that the PSTAIRE helix directly interacts with the cyclin subunit (52, 53). Only two of the putative giardial Cdks contain PSTAIRE-like cyclin binding motifs, which were annotated as Cdk1 and Cdk2 (GenBank accession numbers XP_001704058.1 and XP_001709931.1, open reading frames 8037 and 16802, respectively, in the G. lamblia genome database) (71). We first focused on understanding the role of Cdk2 in Giardia. Comparison of genomic and cDNA sequences showed that the cdk2 gene contained no introns. The deduced giardial Cdk2 protein contains 291 amino acids with a predicted molecular mass of ∼32.87 kDa and a pI of 9.24. It has a putative protein kinase domain as predicted by Pfam (72). Like the human Cdk family (52), the kinase domains of the giardial Cdk2 spans an entire polypeptide chain (residues 8–289) (supplemental Fig. S2A). The giardial Cdk2 also has a PATAIRE motif, which is similar to the conserved PSTAIRE motif (supplemental Fig. S2B) (52, 53). A conserved Asp (residue 145) important for ATP binding in human cdk2 is also conserved in the giardial Cdk2 (arrow in supplemental Fig. S2B) (73). We constructed a phylogram of human Cdk1–20 and giardial Cdk2 (supplemental Fig. S3). The giardial Cdk2 is more similar to Cdk1, -2, -3, and -5 (supplemental Fig. S3). Sequence alignment shows that the giardial Cdk2 is highly similar to the human Cdk1, -2, -3, and -5 (supplemental Fig. S2B). The full-length of giardial Cdk2 has 49.83, 51.32, 49.84, and 50.51% identity and 66.78, 67.88, 66.99, and 66.33% similarity to that of human Cdk1, -2, -3, and -5, respectively. This indicates that the giardial Cdk2 is very similar to human Cdk2.
Encystation-induced Expression of cdk2 Gene
RT-PCR analysis of total RNA showed that the cdk2 transcript was present in vegetative cells and increased significantly in 24-h encysting cells (Fig. 1A). As controls, we found that the mRNA levels of the cwp1 and ran genes increased and decreased significantly during encystation, respectively (Fig. 1A). The products of the cwp1 and ran genes are components of the cyst wall and the ras-related nuclear protein (13, 20, 74). We also found that the cdk2 gene is up-regulated by ∼2.69-fold during encystation in quantitative real-time PCR analysis (data not shown). To determine expression of the Cdk2 protein, we generated an antibody specific to the full-length Cdk2. Western blot analysis confirmed that this antibody recognized Cdk2 at a size of ∼34 kDa (Fig. 1B), which was almost matched to the predicted molecular mass of Cdk2 (∼32.9 kDa). Cdk2 was expressed in vegetative cells and its levels increased significantly during encystation (Fig. 1B). As a control, the levels of the giardial RAN protein (∼27 kDa) decreased significantly during encystation (Fig. 1B).
Localization of Cdk2 Protein
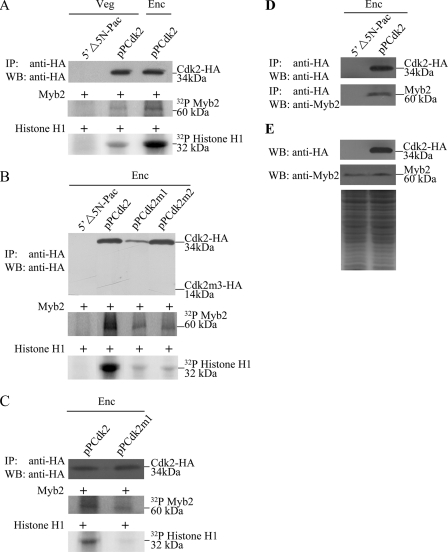
To determine the role of Cdk2 protein, we prepared construct pPCdk2, in which the cdk2 gene is controlled by its own promoter and contains an HA epitope tag at its C terminus (Fig. 1C) and stably transfected it into Giardia. Similar to the expression pattern of the endogenous Cdk2 protein, the levels of the Cdk2-HA protein increased significantly during encystation (data not shown). The HA-tagged Cdk2 was detected in the cytoplasm during vegetative growth and encystation (Fig. 1D). As a negative control, there was no staining for anti-HA antibody detection in the 5′Δ5N-Pac cell line, which expressed only the puromycin selection marker (Fig. 1C and data not shown).
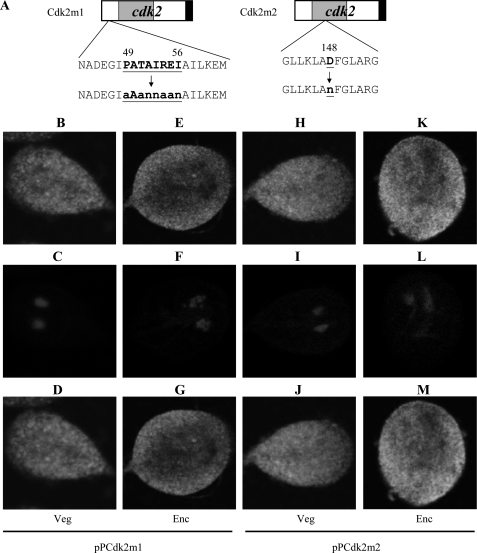
We also performed mutation analysis to understand the role of Cdk2. We found that mutation of the PATAIRE residues that may be important for cyclin binding (residues 49–56) did not affect cytoplasmic localization (pPCdk2m1, Fig. 2, A–G). Mutation of the conserved Asp that may be important for ATP binding (residue 148) did not affect cytoplasmic localization (pPCdk2m2, Fig. 2, A and H–M).
FIGURE 2.
Localization of Cdk2 mutants. A, diagrams of the pPCdk2m1 and pPCdk2m2 plasmids. The PATAIREI (residues 49–56) region, which is important for cyclin binding, is mutated in Cdk2m1. The residue Asp-148, which is important for ATP binding, is mutated in Cdk2m2. The cdk2 gene was mutated and subcloned to replace the wild-type cdk2 gene in the backbone of pPCdk2 (Fig. 1C), and the resulting plasmids pPCdk2m1 and pPCdk2m2 were transfected into Giardia. B, immunofluorescence analysis of pPCdk2m1 and pPCdk2m2 distribution. The pPCdk2m1 and pPCdk2m2 stable transfectants were cultured in growth (Veg, vegetative growth) or encystation medium (Enc, encystation) for 24 h and then subjected to immunofluorescence analysis using anti-HA antibody for detection. The products of pPCdk2m1 and pPCdk2m2 localize to the cytoplasm in both vegetative and encysting trophozoites (panels B and E and panels H and K, respectively). Panels C, F, I, and L shows the DAPI staining of cell nuclei. Panels D, G, J, and M is the merged images of B and C, E and F, H and I, K and L, respectively.
Overexpression of Cdk2 Induced Expression of cwp1, cwp2, and myb2 Genes
To study the role of Cdk2 in G. lamblia, we expressed the cdk2 gene by its own promoter (pPCdk2; Fig. 1C) and observed its expression. The Cdk2-HA protein (∼34 kDa) was expressed in the pPCdk2 stable cell line but not in the control cell line (5′Δ5N-Pac) (Fig. 1C) as detected by anti-HA antibody in Western blots (Fig. 3A). Overexpression of Cdk2 in the pPCdk2 cell line also can be confirmed by the anti-Cdk2 antibody (Fig. 3A). The size of the overexpressed Cdk2-HA is slightly larger than that of the endogenous Cdk2 in the pPCdk2 cell line (Fig. 3A). We found that Cdk2 overexpression resulted in a significant increase of the CWP1 and Myb2 protein levels during vegetative growth (Fig. 3A). As a control, similar levels of intensity of the giardial RAN protein (∼27 kDa) were detected by anti-RAN antibody (Fig. 3A). RT-PCR and quantitative real-time PCR analysis showed that the mRNA levels of the endogenous cdk2 plus vector-expressed cdk2 in the Cdk2-overexpressing cell line increased by ∼8.2-fold (p < 0.05) relative to the 5′Δ5N-Pac control cell line (Fig. 3B and data not shown). The mRNA levels of the endogenous cwp1, cwp2, and myb2 genes in the Cdk2 overexpressing cell line increased by ∼1.9-, 1.6-, and 1.5-fold (p < 0.05) relative to the 5′Δ5N-Pac control cell line (Fig. 3B and data not shown). Similar mRNA levels of the ran and 18S ribosomal RNA genes were detected (Fig. 3B and data not shown). The results suggest that overexpression of Cdk2 can increase expression of the cwp1, cwp2, and myb2 genes.
We further investigated the effect of giardial Cdk2 on cyst formation. In previous studies, we have found that some G. lamblia trophozoites may undergo spontaneous differentiation (64). We obtained consistent cyst count data for vegetative G. lamblia cultures during growth to stationary phase (∼4800 cysts/ml for 5′Δ5N-Pac cell line) (64). In this study, we found that the cyst number in the Cdk2 overexpressing cell line increased by ∼2.0-fold (p < 0.05) relative to the control cell line, which expresses only the puromycin selection marker (5′Δ5N-Pac) (Fig. 1C), indicating that the overexpressed Cdk2 can increase cyst formation (Fig. 3C). Similar results were obtained during encystation (data not shown). The results suggest that overexpression of Cdk2 can increase cyst formation.
To further understand the function of giardial Cdk2, we analyzed the effect of mutation of Cdk2. The cytoplasmic localization of Cdk2m1 and Cdk2m2 is similar to wild-type Cdk2 (Fig. 2). We found that the levels of Cdk2m1 and Cdk2m2 proteins decreased significantly compared with that of wild-type Cdk2 during vegetative growth in both anti-HA and anti-Cdk2 Western blots (Fig. 3A). The endogenous Cdk2 and HA-tagged Cdk2m1 and Cdk2m2 can be detected by anti-Cdk2 antibody (Fig. 3A). We also found that levels of the CWP1 and Myb2 proteins decreased significantly in the Cdk2m1 and Cdk2m2 overexpressing cell lines relative to the wild-type Cdk2 overexpressing cell line (Fig. 3A). As a control, similar levels of intensity of the giardial RAN protein (∼27 kDa) were detected by anti-RAN antibody (Fig. 3A). We further analyzed whether the transcript levels of the Cdk2m1 and Cdk2m2 were changed. As shown by RT-PCR analysis, the levels of HA-tagged cdk2m1 and cdk2m2 mRNA decreased significantly compared with that of wild-type HA-tagged cdk2 during vegetative growth (Fig. 3B). We did not detect any HA-tagged cdk2 transcripts in the 5′Δ5N-Pac control cell line (Fig. 3B). We also found that the levels of cwp1, cwp2, and myb2 mRNA decreased significantly in the Cdk2m1 and Cdk2m2 overexpressing cell lines relative to the wild-type Cdk2 overexpressing cell line (Fig. 3B). Similar mRNA levels of the ran and 18S ribosomal RNA genes were detected (Fig. 3B). The levels of cyst formation decreased significantly in the Cdk2m1 and Cdk2m2 overexpressing cell lines relative to the wild-type Cdk2 overexpressing cell line (Fig. 3C). Similar results were obtained during encystation (data not shown). The results suggest a decrease of encystation-induced activity of Cdk2m1 and Cdk2m2.
Oligonucleotide microarray assays confirmed up-regulation of cwp1, cwp2, and myb2 gene expression in the Cdk2 overexpressing cell line ∼1.31- to ∼2.04-fold of the levels in the control cell line (Fig. 3D). Similar mRNA levels of the ran gene were detected (Fig. 3D). We found that 26 and 53 genes were significantly up-regulated (>2-fold) and down-regulated (<1/2) (p < 0.05) in cdk2 overexpression cells relative to the vector control, respectively (Fig. 3D and Table 1). Expression levels of the cdk2 gene in the Cdk2 overexpressing cell line increased by ∼6.94-fold (p < 0.05) (Fig. 3D and Table 1).
TABLE 1.
Genes up- or down-regulated by Cdk2 overexpression
| Number | Annotation | ORF number | Fold-change (pPCdk2/5′Δ5N-Pac)a | Fold-change (Enc/Veg)b |
|---|---|---|---|---|
| 1 | High cysteine membrane protein, group 1 | 25,816 | 7.11 (*p < 0.05) | 30.43 (p < 0.05)c |
| 2 | Cdk2 | 16,802 | 6.94 (p < 0.05) | 2.69 (p < 0.05) |
| 3 | High cysteine membrane protein, group 1 | 11,309 | 3.69 (p < 0.05) | 1.96 |
| 4 | Hypothetical protein | 10,552 | 3.22 (p < 0.05) | 18.84 (p < 0.05) |
| 5 | VSP with INR | 113,450 | 2.72 (p < 0.05) | 1.04 |
| 6 | VSP | 103,992 | 2.57 (p < 0.05) | 1.49 |
| 7 | Hypothetical protein | 15,125 | 2.55 (p < 0.05) | 5.22 (p < 0.05) |
| 8 | Hypothetical protein | 10,238 | 2.52 (p < 0.05) | 0.16 |
| 9 | High cysteine protein | 17,380 | 2.51 (p < 0.05) | 0.56 |
| 10 | Hypothetical protein | 22,502 | 2.49 (p < 0.05) | 3.77 (p < 0.05) |
| 11 | VSP with INR | 101,074 | 2.42 (p < 0.05) | 1.03 |
| 12 | Spindle pole protein, putative | 13,372 | 2.22 (p < 0.05) | 1.00 |
| 13 | Variant-specific surface protein | 9,276 | 2.21 (p < 0.05) | 1.74 |
| 14 | Hypothetical protein | 16,424 | 2.20 (p < 0.05) | 5.31 (p < 0.05) |
| 15 | Hypothetical protein | 41,212 | 2.20 (p < 0.05) | 0.68 |
| 16 | β-Giardin | 4,812 | 2.19 (p < 0.05) | 0.37 (p < 0.05) |
| 17 | Heat-shock protein, putative | 16,412 | 2.14 (p < 0.05) | 3.24 (p < 0.05) |
| 18 | Hypothetical protein | 22,439 | 2.05 (p < 0.05) | 4.38 (p < 0.05) |
| 19 | Myb2 | 8,722 | 2.04 (p < 0.05) | 2.87 (p < 0.05) |
| 20 | Hypothetical protein | 6,010 | 2.04 (p < 0.05) | 1.00 |
| 21 | High cysteine membrane protein, group 4 | 114,930 | 2.04 (p < 0.05) | 0.72 |
| 22 | Protein 21.1 | 17,585 | 2.04 (p < 0.05) | 0.35 |
| 23 | Protein 21.1 | 15,965 | 2.03 (p < 0.05) | 0.90 |
| 24 | Cyst wall protein 1 | 5,638 | 2.03 (p < 0.05) | 47.88 (p < 0.05) |
| 25 | Hypothetical protein | 13,239 | 2.01 (p < 0.05) | 0.85 |
| 26 | Hypothetical protein | 10,425 | 2.00 (p < 0.05) | 16.87 (p < 0.05) |
| 27 | VSP | 115,796 | 0.09 (p < 0.05) | 1.25 |
| 28 | VSP | 13,390 | 0.10 (p < 0.05) | 1.07 |
| 29 | VSP | 90,215 | 0.23 (p < 0.05) | 0.97 |
| 30 | VSP with INR | 119,707 | 0.23 (p < 0.05) | 1.02 |
| 31 | VSP | 34,357 | 0.24 (p < 0.05) | 0.96 |
| 32 | VSP | 40,571 | 0.24 (p < 0.05) | 0.97 |
| 33 | VSP | 101,765 | 0.30 (p < 0.05) | 1.03 |
| 34 | VSP | 99,743 | 0.31 (p < 0.05) | 0.97 |
| 35 | VSP | 111,903 | 0.31 (p < 0.05) | 0.96 |
| 36 | VSP | 115,047 | 0.33 (p < 0.05) | 1.33 |
| 37 | VSP | 122,566 | 0.35 (p < 0.05) | 1.25 |
| 38 | VSP | 113,093 | 0.37 (p < 0.05) | 1.01 |
| 39 | Hypothetical protein | 26,727 | 0.37 (p < 0.05) | 0.38 (p < 0.05) |
| 40 | VSP with INR | 113,439 | 0.38 (p < 0.05) | 0.83 |
| 41 | Hypothetical protein | 125,106 | 0.38 (p < 0.05) | 1.05 |
| 42 | High cysteine protein | 32,701 | 0.38 (p < 0.05) | 1.00 |
| 43 | VSP | 40,591 | 0.39 (p < 0.05) | 1.04 |
| 44 | Hypothetical protein | 114,044 | 0.40 (p < 0.05) | 0.47 (p < 0.05) |
| 45 | VSP | 137,606 | 0.40 (p < 0.05) | 1.08 |
| 46 | Hypothetical protein | 112,017 | 0.40 (p < 0.05) | 0.44 (p < 0.05) |
| 47 | VSP | 111,874 | 0.41 (p < 0.05) | 0.82 |
| 48 | VSP | 114,672 | 0.41 (p < 0.05) | 1.08 |
| 49 | Pyruvate-flavodoxin oxidoreductase | 114,609 | 0.41 (p < 0.05) | 1.78 |
| 50 | VSP AS8 | 13,194 | 0.42 (p < 0.05) | 1.04 |
| 51 | Dynein heavy chain | 101,138 | 0.43 (p < 0.05) | 0.87 |
| 52 | High cysteine protein | 6,372 | 0.43 (p < 0.05) | 1.31 |
| 53 | VSP | 116,477 | 0.43 (p < 0.05) | 0.83 |
| 54 | Hypothetical protein | 3,269 | 0.44 (p < 0.05) | 0.83 |
| 55 | Pyruvate-flavodoxin oxidoreductase | 17,063 | 0.44 (p < 0.05) | 2.67 (p < 0.05) |
| 56 | VSP S8 | 137,604 | 0.45 (p < 0.05) | 1.17 |
| 57 | High cysteine membrane protein, group 1 | 15,317 | 0.45 (p < 0.05) | 2.23 (p < 0.05) |
| 58 | Hypothetical protein | 31,427 | 0.46 (p < 0.05) | 0.87 |
| 59 | Hypothetical protein | 114,043 | 0.47 (p < 0.05) | 0.74 |
| 60 | Hypothetical protein | 112,018 | 0.47 (p < 0.05) | 0.75 |
| 61 | VSP, putative | 92,835 | 0.47 (p < 0.05) | 0.99 |
| 62 | High cysteine membrane protein, group 3 | 114,891 | 0.47 (p < 0.05) | 0.71 |
| 63 | VSP | 137,723 | 0.47 (p < 0.05) | 0.91 |
| 64 | Hypothetical protein | 6,391 | 0.47 (p < 0.05) | 1.00 |
| 65 | VSP | 98,058 | 0.47 (p < 0.05) | 1.00 |
| 66 | VSP | 40,621 | 0.48 (p < 0.05) | 1.14 |
| 67 | High cysteine membrane protein, group 1 | 32,607 | 0.48 (p < 0.05) | 1.05 |
| 68 | Hypothetical protein | 6,493 | 0.49 (p < 0.05) | 0.91 |
| 69 | Hypothetical protein | 99,071 | 0.49 (p < 0.05) | 0.80 |
| 70 | VSP | 113,357 | 0.49 (p < 0.05) | 0.20 (p < 0.05) |
| 71 | Hypothetical protein | 17,332 | 0.49 (p < 0.05) | 0.82 |
| 72 | VSP with INR | 16,501 | 0.49 (p < 0.05) | 1.04 |
| 73 | Hypothetical protein | 20,020 | 0.49 (p < 0.05) | 1.64 |
| 74 | VSP | 111,936 | 0.49 (p < 0.05) | 1.21 |
| 75 | Zinc finger protein | 106,320 | 0.49 (p < 0.05) | 0.73 |
| 76 | VSP | 111,933 | 0.50 (p < 0.05) | 1.25 |
| 77 | VSP | 113,242 | 0.50 (p < 0.05) | 0.76 |
| 78 | EGFCP3d | 114,815 | 0.50 (p < 0.05) | 0.98 |
| 79 | EGFCP2 | 113,038 | 0.50 (p < 0.05) | 1.31 |
a The 5′Δ5N-Pac and pPCdk2 stable transfectants were cultured in growth medium for 24 h and then subjected to microarray assays.
b The wild-type nontransfected WB cells were cultured in growth (Veg, vegetative growth) or encystation medium for 24 h (Enc, encystation) and then subjected to microarray assays.
c p values were determined for groups in which the average means were changed by a factor of ≤2.0 or ≤0.5.
d Epidermal growth factor-like cyst protein 3 (30).
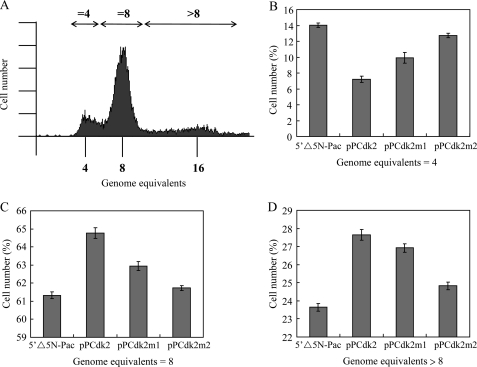
We further analyzed whether overexpression of Cdk2 influenced cell cycle progression. A Giardia vegetative trophozoite in the G1 and G2 phase may contain a ploidy of 4N and 8N, respectively (57–60). Encystation of Giardia has been proposed to initiate in the G2 phase of the cell cycle (57–60). A cyst with 4 nuclei may contain a ploidy of 16N (57–60). We used flow cytometry to analyze the genome ploidy of the Cdk2 overexpressing cell line. A one-parameter histogram showed the population of cells containing 4N, 8N, or more than 8N in the vegetative wild-type nontransfected WB cell line (Fig. 4A). We found that overexpression of Cdk2 resulted in a decreased number of cells containing 4N DNA content and an increase of the number of cells containing 8N or more than 8N DNA content compared with the control cell line (Fig. 4). The results suggest that overexpression of Cdk2 can increase populations of cells in the G2 phase and/or differentiation into cysts. In addition, mutation of Cdk2 resulted in an increased number of cells containing 4N DNA content and a decrease of the number of cells containing 8N or more than 8N DNA content compared with the Cdk2 overexpressing cell line, suggesting that the ability of inducing encystation decreased in Cdk2m1 and Cdk2m2 overexpressing cell lines compared with the Cdk2 overexpressing cell line (Fig. 4).
FIGURE 4.
Change in cell cycle progression in the Cdk2 overexpressing cell line. A, an example of cell cycle analysis by flow cytometry. The wild-type nontransfected WB cells were cultured in growth medium to late log/early stationary phase (1.5 × 106 cells/ml), then subjected to flow cytometry analysis. Cells in the G1 phase of the cell cycle may contain a 4N genome equivalent (58, 60, 61). Positions of the peaks in flow cytometry indicate cells with genome equivalent = 4, = 8, or >8 (double-headed arrows). B–D, cell cycle analysis of different cell lines. The 5′Δ5N-Pac, pPCdk2, pPCdk2m1, and pPCdk2m2 stable transfectants were cultured in growth medium to late log/early stationary phase (1.5 × 106 cells/ml), then subjected to flow cytometry analysis. The number of cells with genome equivalents = 4 (B), = 8 (C), and >8 (D) were quantified. The results are expressed as the mean ± S.E. of three independent experiments.
Phosphorylation of Myb2 by Cdk2-associated Complexes
Because Cdk2 can induce encystation, we further tested whether Cdk2 is responsible for phosphorylation of Myb2. The kinase activity of Cdk2-HA in the pPCdk2 cell line was determined by an IP kinase assay using anti-HA antibody. We used purified Myb2 and histone H1 as substrates for kinase assays. We found that the Cdk2-HA-associated complexes can phosphorylate both Myb2 and histone H1 proteins (Fig. 5A). Interestingly, the kinase activity for both substrates increased during encystation (Fig. 5A). Note that Cdk2 protein levels increased during encystation (Fig. 1B), but similar levels of the immunoprecipitated Cdk2-HA from the vegetative and encysting pPCdk2 cultures were used in the IP kinase assays as confirmed by Western blot using anti-HA antibody (Fig. 5A). As a negative control, Cdk2-HA-associated kinase activity was not detected in the 5′Δ5N-Pac cell line, which did not express the Cdk2-HA protein (Figs. 1C and 5A). The results indicate that Myb2 may be a target of the Cdk2 pathway and the function of Myb2 coincided with the increased Cdk2 activity during encystation. We also found that the Cdk2 carrying mutations at the PATAIRE motif (Cdk2m1) or residue Asp-148 (Cdk2m2) had lower kinase activity for Myb2 and histone H1 substrates as compared with the wild-type Cdk2 (Fig. 5B). As Cdk2m1 was expressed in a lower level (Fig. 3A), lower levels of Cdk2m1 were immunoprecipitated (Fig. 5B). We further diluted the immunoprecipitated wild-type Cdk2 and used similar levels of the immunoprecipitated wild-type Cdk2 and Cdk2m1 in the IP kinase assays as confirmed by Western blot using anti-HA antibody (Fig. 5C). We still found that Cdk2m1 had lower kinase activity for Myb2 and histone H1 substrates as compared with the wild-type Cdk2 (Fig. 5C). The results suggest that the Cdk2 pathway may regulate Giardia differentiation into cysts through the regulation of Myb2 phosphorylation.
FIGURE 5.
Phosphorylation of Myb2 protein by Cdk2-associated complexes. A, encystation-induced kinase activity of Cdk2. The pPCdk2 stable transfectants were cultured in growth (Veg, vegetative growth) or encystation (Enc, encystation) medium for 24 h and then subjected to IP kinase assay using anti-HA antibody. Kinase activity was measured using purified recombinant Myb2 as a substrate (middle panel). The bovine histone H1 protein was used as a positive control substrate (bottom panel). As a negative control, an IP kinase assay was performed with the vegetative 5′Δ5N-Pac cultures, which did not express the HA-tagged Cdk2 protein. Similar levels of immunoprecipitated Cdk2 protein from the vegetative and encysting pPCdk2 cultures were used for the kinase assay. The addition of similar levels of Cdk2 protein from the vegetative and encysting pPCdk2 cultures in each kinase reaction was confirmed by Western blot using an anti-HA antibody (upper panel). B, kinase activity of Cdk2m1 and pPCdk2m2 mutants. The 5′Δ5N-Pac, pPCdk2, pPCdk2m1, and pPCdk2m2 stable transfectants were cultured in encystation medium for 24 h, and then subjected to an IP kinase assay using anti-HA antibody. Kinase activity was measured as described above. Similar levels of immunoprecipitated Cdk2 and Cdk2m2 proteins were used for the kinase assay. Cdk2m1 was an exception because of its lower expression levels. The addition of similar levels of Cdk2 and Cdk2m2 proteins in each kinase reaction was confirmed by Western blot using an anti-HA antibody (upper panel). C, kinase activity of the Cdk2m1 mutant. The pPCdk2 and pPCdk2m1 stable transfectants were cultured in encystation medium for 24 h, and then subjected to IP kinase assay using anti-HA antibody. Kinase activity was measured as described above. Similar levels of immunoprecipitated Cdk2 and Cdk2m1 proteins were used for the kinase assay. The addition of similar levels of Cdk2 and Cdk2m1 proteins in each kinase reaction was confirmed by Western blot (WB) using an anti-HA antibody (upper panel). D, interaction between Cdk2 and Myb2 detected by co-immunoprecipitation assays. The 5′Δ5N-Pac and pPCdk2 stable transfectants were cultured in encystation medium for 24 h. Proteins from cell lysates were immunoprecipitated using anti-HA antibody conjugated to beads. The precipitates were analyzed by Western blot with anti-HA or anti-Myb2 antibody as indicated. E, expression of HA-tagged Cdk2 and Myb2 proteins in whole cell extracts. The 5′Δ5N-Pac and pPCdk2 stable transfectants were cultured in encystation for 24 h (Enc) and then subjected to Western blot analysis. The blot was probed by anti-HA and anti-Myb2 antibody. Equal amounts of protein loading were confirmed by SDS-PAGE and Coomassie Blue staining.
Interaction between Myb2- and Cdk2-associated Complexes
We further tried to understand whether Myb2 can interact with Cdk2. We performed co-immunoprecipitation experiments using the Cdk2 overexpressing cell line. We found that Cdk2 overexpression resulted in an increase of the Myb2 protein levels (Figs. 3A and 5E). We lysed the cells and immunoprecipitated HA-tagged Cdk2 with anti-HA antibody. Western blots of immunoprecipitates probed with anti-HA and anti-Myb2 indicate that Myb2 co-precipitates with Cdk2 (Fig. 5, D and E). As a control, the anti-HA antibody did not immunoprecipitate Cdk2 and Myb2 in the control cell line, which expressed only the puromycin selection marker (5′Δ5N-Pac) (Fig. 1C), suggesting that Myb2 co-immunoprecipitated with anti-HA requires the HA-tagged Cdk2 protein (Fig. 5, D and E). The results suggest an interaction between Myb2- and Cdk2-associated complexes.
A Cdk Inhibitor Can Decrease the Expression of cwp1, cwp2, and myb2 Genes
It has been shown that purvalanol A is a permeable inhibitor of the human Cdk2-cyclin A and Cdk1-cyclin B kinase complex (61, 62). Purvalanol A can compete for ATP binding sites of Cdks and has been used to inhibit the eukaryotic Cdk activity (62). We also found that addition of purvalanol A significantly decreased the Cdk2-associated kinase activity (Fig. 6, A and B). Addition of purvalanol A also significantly decreased cyst formation but did not affect cell growth (Fig. 6C and data not shown). Interestingly, addition of purvalanol A also significantly decreased the levels of CWP1 and Myb2 proteins (Fig. 6D). As a control, similar levels of intensity of the giardial RAN protein (∼27 kDa) were detected by anti-RAN antibody (Fig. 6D). However, addition of purvalanol A did not change the Cdk2 levels and localization (Fig. 6D). We also found that addition of purvalanol A also significantly decreased the mRNA levels of cwp1, cwp2, and myb2 genes (Fig. 6E). Similar mRNA levels of the cdk2, ran, and 18S ribosomal RNA genes were detected (Fig. 6E). The results suggest that the Cdk2 pathway may regulate Giardia differentiation into cysts through the regulation of Myb2 phosphorylation.
Analysis of Myb2 Phosphorylation Sites by Cdk2-associated Complexes
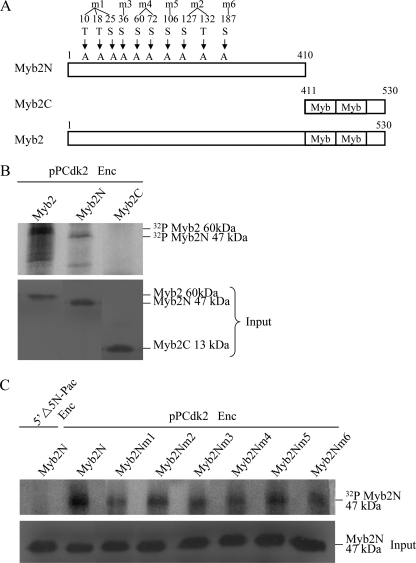
The Myb repeats are known to be important for DNA binding of Myb proteins in higher eukaryotes (75–77). We found that deletion of the N-terminal domain or the C-terminal domain containing the Myb repeats resulted in a decrease of transactivation and autoregulation function of giardial Myb2 (32). We tried to understand whether the N- or C-terminal regions of giardial Myb2 contains important phosphorylation sites. The N- (residues 2–410) or C-terminal regions (residues 411–530) were deleted and the resulting Myb2N or Myb2C was expressed in E. coli and purified (Fig. 7A). We found that Cdk2-HA-associated complexes can phosphorylate the Myb2N but not the Myb2C protein (Fig. 7B), indicating that the N-terminal region may contain important phosphorylation sites. Similar levels of wild-type Myb2, Myb2N, and Myb2C were added to the kinase reaction mixtures (Fig. 7B).
FIGURE 7.
Identification of phosphorylation sites of Myb2. A, diagrams of Myb2, Myb2N, and Myb2C proteins. The Myb repeats are indicated as open boxes labeled Myb. Myb2N (residues 1–410) does not contain the C-terminal Myb repeats and C-terminal 10 amino acids. Myb2C (residues 411–530) does not contain the N-terminal 409 amino acids. The putative phosphorylated serines and threonines of Myb2N in consensus ((S/T)PX(K/R)) and nonconsensus ((S/T)PXX) sequences of Cdk phosphorylation sites were mutated to alanines. Myb2Nm1 contains mutations on Thr-10, Thr-18, and Ser-25. Myb2Nm2 contains mutations on Ser-127 and Thr-132. Myb2Nm4 contains mutations on Ser-60 and Ser-72. Myb2Nm3, Myb2Nm5, and Myb2Nm6 contain mutations on Ser-36, Ser-106, and Ser-187, respectively. B, phosphorylation of Myb2N by Cdk2. The pPCdk2 stable transfectants were cultured in encystation (Enc, encystation) medium for 24 h and then subjected to IP kinase assay using anti-HA antibody. Kinase activity was measured using purified recombinant Myb2, Myb2N, or Myb2C as a substrate (upper panel). The addition of similar levels of Myb2, Myb2N, or Myb2C protein (input) in each kinase reaction was confirmed by Western blot using an anti-V5-HRP antibody (bottom panel). C, mutation of putative phosphorylation sites decreased phosphorylation of Myb2N by Cdk2. The Myb2N or Myb2Nm1–6 with a V5 and His tag at its C terminus was expressed in E. coli and purified by affinity chromatography. The pPCdk2 stable transfectants were cultured in encystation medium for 24 h (Enc) and then subjected to IP kinase assay using anti-HA antibody. Kinase activity was measured using purified recombinant Myb2N or Myb2Nm1–6 as a substrate (upper panel). The addition of similar levels of Myb2N or Myb2Nm1–6 protein (input) in each kinase reaction was confirmed by Western blot using an anti-V5-HRP antibody (bottom panel).
Cdk may phosphorylate serines and threonines on consensus ((S/T)PX(K/R)) and nonconsensus ((S/T)PXX) sequences (52, 53). We further tried to identify the putative phosphorylation sites of Myb2N. The serines and threonines of consensus and nonconsensus sequences of Myb2N were mutated to alanines. The Myb2Nm1 contains mutations on Thr-10, Thr-18, and Ser-25. The Myb2Nm2 contains mutations on Ser-127 and Thr-132. The Myb2Nm4 contains mutations on Ser-60 and Ser-72. The Myb2Nm3, Myb2Nm5, and Myb2Nm6 contain mutations on Ser-36, Ser-106, and Ser-187, respectively. Mutation of the putative phosphorylation sites of Myb2N (Myb2Nm1–6) significantly decreased phosphorylation by Cdk2-associated complexes (Fig. 7C). The results suggest that Myb2N may contain typical phosphorylation sites for Cdk2 pathway.
Interaction between Cdk2 and Cyclin A-associated Complexes
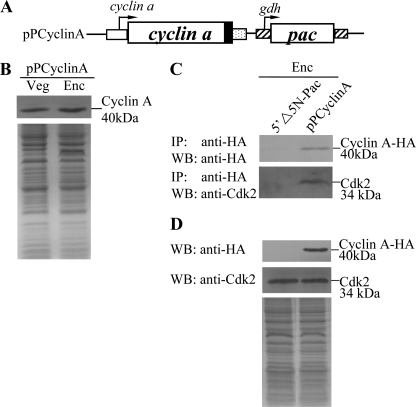
We further tried to understand whether Cyclin A can interact with Cdk2. To identify genes encoding Cyclin A proteins from G. lamblia, we searched the G. lamblia genome database (18, 65) with the genes annotated as “cyclin a.” The searches detected one putative homologue for Cyclin A (open reading frame 14488 in the G. lamblia genome database). The deduced giardial Cyclin A protein contains 354 amino acids with a predicted molecular mass of ∼40.09 kDa and a pI of 7.42. Like human Cyclin A1, the giardial Cyclin A has two putative cyclin domains as predicted by Pfam (residues 101–190 and 230–354) (72). We prepared the construct pPCyclinA in which the cyclin a gene is controlled by its own promoter and contains an HA epitope tag at its C terminus (Fig. 8A) and stably transfected it into Giardia. The levels of the Cyclin A-HA protein increased significantly during encystation (Fig. 8B). We further performed co-immunoprecipitation experiments in the Cyclin A overexpressing cell line. We lysed the cells and immunoprecipitated HA-tagged Cyclin A with anti-HA antibody. Western blots of immunoprecipitates probed with anti-HA and anti-Cdk2 indicate that Cyclin A co-precipitates with Cdk2 (Fig. 8, C and D). As a control, the anti-HA antibody did not immunoprecipitate Cyclin A and Cdk2 in the control cell line, which expressed only the puromycin selection marker (5′Δ5N-Pac) (Fig. 1C), suggesting that Cdk2 co-immunoprecipitated with anti-HA requires the HA-tagged Cyclin A protein (Fig. 8, C and D). The results suggest an interaction between Cdk2- and Cyclin A-associated complexes.
FIGURE 8.
Interaction between Cyclin A and Cdk2. A, diagrams of the 5′Δ5N-Pac and pPCyclinA plasmids. The pac gene (open box) is under control of the 5′- and 3′-flanking regions of the gdh gene (striated box). The cyclin a gene is under control of its own 5′-flanking region (open box) and the 3′-flanking region of the ran gene (dotted box). The filled black box indicates the coding sequence of the HA epitope tag. B, cyclin A protein levels in pPCyclinA stable transfectants. The pPCyclinA stable transfectants were cultured in growth (Veg, vegetative growth) or encystation (Enc, encystation) medium for 24 h and then subjected to SDS-PAGE and Western blot (WB). HA-tagged Cyclin A proteins were detected using an anti-HA antibody by Western blot analysis. Equal amounts of protein loading were confirmed by SDS-PAGE and Coomassie Blue staining. C, interaction between Cyclin A and Cdk2 detected by co-immunoprecipitation assays. The 5′Δ5N-Pac and pPCyclinA stable transfectants were cultured in encystation medium for 24 h. Proteins from cell lysates were immunoprecipitated using anti-HA antibody conjugated to beads. The precipitates were analyzed by Western blot with anti-HA or anti-Cdk2 antibody as indicated. D, expression of HA-tagged Cyclin A and Cdk2 proteins in whole cell extracts. The 5′Δ5N-Pac and pPCyclinA stable transfectants were cultured in encystation for 24 h (Enc) and then subjected to Western blot analysis. The blot was probed by anti-HA and anti-Cdk2 antibody. Equal amounts of protein loading were confirmed by SDS-PAGE and Coomassie Blue staining.
DISCUSSION
The Cdk protein family is a group of Ser/Thr protein kinases that regulate cell cycle progression, cell proliferation, and cell differentiation in yeast, animals, and plants (50–52, 76). It has also been identified in protozoan parasites, including Plasmodium falciparum, Trypanosoma brucei, Trypanosoma cruzi, Leishmania mexicana, Leishmania donovani, Leishmania major, and E. histolytica (79–84). Four Giardia Cdk homologues have been reported (84). This suggests that the Cdk family may have evolved before divergence of G. lamblia from the main eukaryotic line of descent. In this study, we carried out the first characterization of a Cdk homologue, Cdk2, and found that it is involved in Giardia differentiation into cysts.
G. lamblia colonizes the intestinal tract, and it must encyst to survive outside and infect new hosts (1). The key components of the giardial cyst wall, cyst wall proteins, are synthesized during encystation (1). The Myb2 transcription factor is up-regulated and can activate cwp gene expression during encystation (26, 32). The myb2 gene may be positively autoregulated, and this may help attain a higher level of Myb2 for induction of cwp genes during encystation (26, 32). Because encystation has been proposed to be coupled with cell cycle regulation (57–60) and Cdk can coordinate cell cycle progression and cell differentiation, we tested the hypothesis that Cdks can regulate Giardia encystation. The giardial Cdk2 is most similar to the human Cdk2 from sequence comparison. We found that the giardial Cdk2 localizes to the cell cytoplasm and the expression levels of the Cdk2 mRNA and protein increased significantly during encystation (Fig. 1), suggesting that Cdk2 may also play a role in encystation. Similarly, it has been found that the expression of plant Cdks was induced in specific tissues with a high proliferative or mitotic competence (78). Interestingly, the giardial Cdk2 was detected in the basal body-enriched fraction, suggesting its role in giardial cell division (85). We also found that Cdk2-associated complexes can phosphorylate Myb2 and the levels of phosphorylation increased during encystation (Fig. 5A). Interestingly, overexpression of Cdk2 increased levels of the cwp1, cwp2, and myb2 mRNA (Fig. 3). The levels of CWP1 protein and cyst formation also increased in the Cdk2 overexpressing cell line (Fig. 3). We also found an interaction between Myb2- and Cdk2-associated complexes using in vivo co-immunoprecipitation assays (Fig. 5). Addition of a Cdk inhibitor, purvalanol A, decreased levels of the CWP1 protein, cyst formation, and kinase activity (Fig. 6). The results suggest that Cdk2 signaling cascades may modulate the amount and transactivation function of Myb2 during encystation, leading to up-regulation of encystation-induced cwp and myb2 genes. We also found an interaction between Cdk2- and Cyclin A-associated complexes using in vivo co-immunoprecipitation assays (Fig. 8). Similarly, it has been found that mammalian Cdk2-cyclin E or Cdk2-cyclin A complexes can phosphorylate B-Myb to enhance its transactivation activity (39, 44–47).
The Myb domain of giardial Myb2 is near the C terminus (25). Deletion of the N-terminal region or C-terminal Myb domain resulted in a decrease of transactivation function and autoregulation ability of Myb2 (32). The N-terminal region containing a stretch of acidic amino acids (residues 225–232) may function as a transactivation domain (25). In this study, we found that Cdk2-associated complexes may phosphorylate the N-terminal region of Myb2, which contains the serines and threonines on consensus ((S/T)PX(K/R)) and nonconsensus ((S/T)PXX) sequences (Fig. 7) (52, 53). Mutation of putative phosphorylation sites of Myb2 decreased phosphorylation by Cdk2-associated complexes (Fig. 7). However, the Cdk2-HA-associated complexes cannot phosphorylate the C-terminal region of Myb2 (Fig. 7). This suggests that phosphorylation of the N-terminal region of Myb2 by Cdk2-associated complexes may be involved in regulation of the transactivation ability of Myb2.
Cdk activation required Cyclin binding to form activated Cdk-cyclin complexes (50–52). It is possible that Cdk2 may need to cooperate with encystation-induced cyclins to function as a kinase and to induce encystation. It has been found that mammalian Cdk2-cyclin A complexes can phosphorylate B-Myb to enhance its transactivation activity (39, 44–47). We searched the G. lamblia genome database and identified a putative Cyclin A. Like human Cyclin A1, the giardial Cyclin A has two putative cyclin domains that may form α-fold to provide interaction surfaces for Cdks or regulatory molecules (86, 87). We found that Cyclin A is also highly expressed during encystation and there is an interaction between Cdk2 and Cyclin A-associated complexes (Fig. 8). We also found that mutation of the PATAIRE residues of Cdk2 might impair the binding of cyclins (Fig. 3), leading to a significant decrease of levels of the CWP1 protein, cyst formation, and kinase activity. Three residues of human Cdk2 are involved in ATP phosphate orientation and magnesium coordination and important for catalytic activity, including Lys-33, Glu-51, and Asp-145 (88). These residues are conserved in Giardia Cdk2. We tried to understand whether Asp-148 of the giardial Cdk2, which corresponds to Asp-145 of the human Cdk2, is also important for kinase catalytic activity. We found that mutation of this conserved Asp residue might impair the kinase function (Fig. 3), leading to a significant decrease of levels of the CWP1 protein, cyst formation, and kinase activity. The results suggest that Cdk2 may induce the expression of encystation-induced cwp and myb2 genes in vivo through its kinase activity and an interaction of Cdk2 and cyclins may be important for encystation.
A vegetative Giardia trophozoite in the G1 phase may have 2 eq nuclei with a ploidy of 4N (57–60). A trophozoite in stationary phase may be arrested in the G2 phase and contain a ploidy of 8N. During encystation, a trophozoite may differentiate into a cyst by dividing nuclei and replicating DNA. A thick walled cyst with 4 eq nuclei and a ploidy of 16N is finally formed (57–60). Encystation in Giardia has been proposed to initiate in the G2 phase of the cell cycle (57–60). In humans, Cdk2-cyclin E complexes function in G1/S progression, and Cdk2-cyclin E or Cdk2-cyclin A complexes have S phase promoting activity (50). Because Giardia encystation may need a process of DNA replication and nuclear division, we suggest that the giardial Cdk2 may be involved in differentiation of G. lamblia trophozoites into cysts. To understand the function of Cdk2, we used flow cytometry to analyze the genome ploidy of the Cdk2 overexpressing cell line. We found that overexpression of Cdk2 resulted in an increase of the number of cells containing 8N or more than 8N DNA content and a decreased number of cells containing 4N DNA content compared with the control cell line (Fig. 4). The results suggest that overexpression of Cdk2 can increase populations of cells in the G2 phase and differentiation into cysts. Our mutation analysis provides further evidence of the function of Cdk2 in encystation. Mutation of the PATAIRE residues or a conserved Asp residue might impair the activity of Cdk2, resulting in a decrease of the number of cells containing 8N or more than 8N DNA content (Fig. 4). The results suggest that the Cdk2 mutants have a reduced ability of inducing encystation.
Oligonucleotide microarray assays confirmed the up-regulation of cwp1, cwp2, and myb2 gene expression in the Cdk2 overexpressing cell line to ∼1.31- to ∼2.04-fold (Fig. 3D). Interestingly, of the 26 genes up-regulated in Cdk2 overexpression cells, 11 genes were also up-regulated during encystation and 1 gene was down-regulated during encystation (Table 1). Of the 53 genes down-regulated in the Cdk2 overexpression cells, 4 genes were also down-regulated during encystation and 2 genes were up-regulated during encystation (Table 1). The results suggest a significant overlap with the genes that were up-regulated by Cdk2 overexpression and encystation.
It has been shown that purvalanol A is a potent inhibitor of the human Cdk2-cyclin A and Cdk1-cyclin B kinase complex (61, 62). Purvalanol A can compete for ATP binding sites of Cdks and it has a high preference to inhibit Cdks but not the mitogen-activated protein kinase (61, 62). We also found that addition of purvalanol A significantly decreased the Cdk2-associated kinase activity, and the levels of CWP1 and Myb2 proteins (Fig. 6, A–C). Addition of purvalanol A significantly decreased cyst formation but did not affect cell growth (Fig. 6C and data not shown). Addition of purvalanol A did not change Cdk2 protein levels and localization (Fig. 6B and data not shown), indicating that change of Cdk2 activity but not change of Cdk2 protein levels and localization is responsible for the inhibitory effect of purvalanol A. The concentration of purvalanol A used in the assays is 10 μm, and this concentration may kill many established human cell lines (89). Further studies are required to find more suitable Cdk inhibitors to inhibit the Giardia cyst formation but not to harm human cells.
Our results indicate that the Cdk2 pathway may be involved in phosphorylation of Myb2, leading to activation of the Myb2 function and activation of cwp gene expression during Giardia differentiation into cysts. The Myb2 transcription factor may act downstream of the Cdk2 signaling pathway to induce cwp gene expression. The results may help us to develop new drugs to inhibit cyst formation by disrupting the Cdk2 pathway.
Supplementary Material
Acknowledgments
We thank Dr. A. J. Smith for helpful comments, Yi-Li Liu and I-Ching Huang for technical support in DNA sequencing, and Dr. Show-Li Chen and Tsung-Wei Ma for technical support in flow cytometry. We thank the staff of the cell imaging core at the First Core Labs, National Taiwan University College of Medicine, for technical assistance. We are also very grateful to the researchers and administrators of the G. lamblia genome database for providing genome information.
This work was supported by National Science Council Grants NSC 98-2320-B-002-018-MY2, NSC 99-2320-B-002-017-MY3, and NSC 100-2325-B-002-039, National Health Research Institutes Grants NHRI-EX98-9510NC and NHRI-EX99-9510NC in Taiwan, the Department of Medical Research in the National Taiwan University Hospital, and the Aim for the Top University Program of the National Taiwan University.

This article contains supplemental Figs. S1–S3.
- CWP
- cyst wall protein
- IP
- immunoprecipitation.
REFERENCES
- 1. Adam R. D. (2001) Biology of Giardia lamblia. Clin. Microbiol. Rev. 14, 447–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ankarklev J., Jerlström-Hultqvist J., Ringqvist E., Troell K., Svärd S. G. (2010) Behind the smile. Cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 8, 413–422 [DOI] [PubMed] [Google Scholar]
- 3. Robertson L. J., Hanevik K., Escobedo A. A., Mørch K., Langeland N. (2010) Giardiasis–why do the symptoms sometimes never stop? Trends Parasitol. 26, 75–82 [DOI] [PubMed] [Google Scholar]
- 4. Celiksöz A., Aciöz M., Deǧerli S., Cinar Z., Elaldi N., Erandaç M. (2005) Effects of giardiasis on school success, weight, and height indices of primary school children in Turkey. Pediatr. Int. 47, 567–571 [DOI] [PubMed] [Google Scholar]
- 5. Stark D., van Hal S., Marriott D., Ellis J., Harkness J. (2007) Irritable bowel syndrome. A review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int. J. Parasitol. 37, 11–20 [DOI] [PubMed] [Google Scholar]
- 6. Hanevik K., Dizdar V., Langeland N., Hausken T. (2009) Development of functional gastrointestinal disorders after Giardia lamblia infection. BMC Gastroenterol. 21, 9–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morken M. H., Lind R. A., Valeur J., Wilhelmsen I., Berstad A. (2009) Subjective health complaints and quality of life in patients with irritable bowel syndrome following Giardia lamblia infection. A case control study. Scand. J. Gastroenterol. 44, 308–313 [DOI] [PubMed] [Google Scholar]
- 8. Mørch K., Hanevik K., Rortveit G., Wensaas K. A., Eide G. E., Hausken T., Langeland N. (2009) Severity of Giardia infection associated with post-infectious fatigue and abdominal symptoms two years after. BMC Infect. Dis. 15, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cotton J. A., Beatty J. K., Buret A. G. (2011) Host parasite interactions and pathophysiology in Giardia infections. Int. J. Parasitol. 41, 925–933 [DOI] [PubMed] [Google Scholar]
- 10. Lauwaet T., Davids B. J., Reiner D. S., Gillin F. D. (2007) Encystation of Giardia lamblia. A model for other parasites. Curr. Opin. Microbiol. 10, 554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ondarza R. N. (2007) Drug targets from human pathogenic amoebas, Entamoeba histolytica, Acanthamoeba polyphaga, and Naegleria fowleri. Infect. Disord. Drug Targets 7, 266–280 [DOI] [PubMed] [Google Scholar]
- 12. Carranza P. G., Lujan H. D. (2010) New insights regarding the biology of Giardia lamblia. Microbes Infect. 12, 71–80 [DOI] [PubMed] [Google Scholar]
- 13. Luján H. D., Mowatt M. R., Conrad J. T., Bowers B., Nash T. E. (1995) Identification of a novel Giardia lamblia cyst wall protein with leucine-rich repeats. Implications for secretory granule formation and protein assembly into the cyst wall. J. Biol. Chem. 270, 29307–29313 [DOI] [PubMed] [Google Scholar]
- 14. Mowatt M. R., Luján H. D., Cotten D. B., Bowers B., Yee J., Nash T. E., Stibbs H. H. (1995) Developmentally regulated expression of a Giardia lamblia cyst wall protein gene. Mol. Microbiol. 15, 955–963 [DOI] [PubMed] [Google Scholar]
- 15. Sun C. H., McCaffery J. M., Reiner D. S., Gillin F. D. (2003) Mining the Giardia lamblia genome for new cyst wall proteins. J. Biol. Chem. 278, 21701–21708 [DOI] [PubMed] [Google Scholar]
- 16. Saraiya A. A., Li W., Wang C. C. (2011) A microRNA derived from an apparent canonical biogenesis pathway regulates variant surface protein gene expression in Giardia lamblia. RNA 17, 2152–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Best A. A., Morrison H. G., McArthur A. G., Sogin M. L., Olsen G. J. (2004) Evolution of eukaryotic transcription. Insights from the genome of Giardia lamblia. Genome Res. 14, 1537–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morrison H. G., McArthur A. G., Gillin F. D., Aley S. B., Adam R. D., Olsen G. J., Best A. A., Cande W. Z., Chen F., Cipriano M. J., Davids B. J., Dawson S. C., Elmendorf H. G., Hehl A. B., Holder M. E., Huse S. M., Kim U. U., Lasek-Nesselquist E., Manning G., Nigam A., Nixon J. E., Palm D., Passamaneck N. E., Prabhu A., Reich C. I., Reiner D. S., Samuelson J., Svard S. G., Sogin M. L. (2007) Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science 317, 1921–1926 [DOI] [PubMed] [Google Scholar]
- 19. Seshadri V., McArthur A. G., Sogin M. L., Adam R. D. (2003) Giardia lamblia RNA polymerase II. Amanitin-resistant transcription. J. Biol. Chem. 278, 27804–27810 [DOI] [PubMed] [Google Scholar]
- 20. Sun C. H., Tai J. H. (1999) Identification and characterization of a ran gene promoter in the protozoan pathogen Giardia lamblia. J. Biol. Chem. 274, 19699–19706 [DOI] [PubMed] [Google Scholar]
- 21. Yee J., Mowatt M. R., Dennis P. P., Nash T. E. (2000) Transcriptional analysis of the glutamate dehydrogenase gene in the primitive eukaryote, Giardia lamblia. Identification of a primordial gene promoter. J. Biol. Chem. 275, 11432–11439 [DOI] [PubMed] [Google Scholar]
- 22. Knodler L. A., Svärd S. G., Silberman J. D., Davids B. J., Gillin F. D. (1999) Developmental gene regulation in Giardia lamblia. First evidence for an encystation-specific promoter and differential 5′ mRNA processing. Mol. Microbiol. 34, 327–340 [DOI] [PubMed] [Google Scholar]
- 23. Elmendorf H. G., Singer S. M., Pierce J., Cowan J., Nash T. E. (2001) Initiator and upstream elements in the α2-tubulin promoter of Giardia lamblia. Mol. Biochem. Parasitol. 113, 157–169 [DOI] [PubMed] [Google Scholar]
- 24. Holberton D. V., Marshall J. (1995) Analysis of consensus sequence patterns in Giardia cytoskeleton gene promoters. Nucleic Acids Res. 23, 2945–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davis-Hayman S. R., Hayman J. R., Nash T. E. (2003) Encystation-specific regulation of the cyst wall protein 2 gene in Giardia lamblia by multiple cis-acting elements. Int. J. Parasitol. 33, 1005–1012 [DOI] [PubMed] [Google Scholar]
- 26. Sun C. H., Palm D., McArthur A. G., Svärd S. G., Gillin F. D. (2002) A novel Myb-related protein involved in transcriptional activation of encystation genes in Giardia lamblia. Mol. Microbiol. 46, 971–984 [DOI] [PubMed] [Google Scholar]
- 27. Wang C. H., Su L. H., Sun C. H. (2007) A novel ARID/Bright-like protein involved in transcriptional activation of cyst wall protein 1 gene in Giardia lamblia. J. Biol. Chem. 282, 8905–8914 [DOI] [PubMed] [Google Scholar]
- 28. Sun C. H., Su L. H., Gillin F. D. (2006) Novel plant-GARP-like transcription factors in Giardia lamblia. Mol. Biochem. Parasitol. 146, 45–57 [DOI] [PubMed] [Google Scholar]
- 29. Pan Y. J., Cho C. C., Kao Y. Y., Sun C. H. (2009) A novel WRKY-like protein involved in transcriptional activation of cyst wall protein genes in Giardia lamblia. J. Biol. Chem. 284, 17975–17988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chiu P. W., Huang Y. C., Pan Y. J., Wang C. H., Sun C. H. (2010) A novel family of cyst proteins with epidermal growth factor repeats in Giardia lamblia. PLoS Negl. Trop. Dis. 4, e677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Su L. H., Pan Y. J., Huang Y. C., Cho C. C., Chen C. W., Huang S. W., Chuang S. F., Sun C. H. (2011) A novel E2F-like protein involved in transcriptional activation of cyst wall protein genes in Giardia lamblia. J. Biol. Chem. 286, 34101–34120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang Y. C., Su L. H., Lee G. A., Chiu P. W., Cho C. C., Wu J. Y., Sun C. H. (2008) Regulation of cyst wall protein promoters by Myb2 in Giardia lamblia. J. Biol. Chem. 283, 31021–31029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morf L., Spycher C., Rehrauer H., Fournier C. A., Morrison H. G., Hehl A. B. (2010) The transcriptional response to encystation stimuli in Giardia lamblia is restricted to a small set of genes. Eukaryot. Cell 9, 1566–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wieser J., Adams T. H. (1995) flbD encodes a Myb-like DNA-binding protein that coordinates initiation of Aspergillus nidulans conidiophore development. Genes Dev. 9, 491–502 [DOI] [PubMed] [Google Scholar]
- 35. Oh I. H., Reddy E. P. (1999) The myb gene family in cell growth, differentiation, and apoptosis. Oncogene 18, 3017–3033 [DOI] [PubMed] [Google Scholar]
- 36. Stracke R., Werber M., Weisshaar B. (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 4, 447–456 [DOI] [PubMed] [Google Scholar]
- 37. Weston K. (1998) Myb proteins in life, death, and differentiation. Curr. Opin. Genet. Dev. 8, 76–81 [DOI] [PubMed] [Google Scholar]
- 38. Allen R. D., 3rd, Bender T. P., Siu G. (1999) c-Myb is essential for early T cell development. Genes Dev. 13, 1073–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Müller-Tidow C., Wang W., Idos G. E., Diederichs S., Yang R., Readhead C., Berdel W. E., Serve H., Saville M., Watson R., Koeffler H. P. (2001) Cyclin A1 directly interacts with B-myb and cyclin A1/cdk2 phosphorylate B-myb at functionally important serine and threonine residues. Tissue-specific regulation of B-myb function. Blood 97, 2091–2097 [DOI] [PubMed] [Google Scholar]
- 40. Nakata Y., Shetzline S., Sakashita C., Kalota A., Rallapalli R., Rudnick S. I., Zhang Y., Emerson S. G., Gewirtz A. M. (2007) c-Myb contributes to G2/M cell cycle transition in human hematopoietic cells by direct regulation of cyclin B1 expression. Mol. Cell. Biol. 27, 2048–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ku D. H., Wen S. C., Engelhard A., Nicolaides N. C., Lipson K. E., Marino T. A., Calabretta B. (1993) c-myb transactivates cdc2 expression via Myb binding sites in the 5′-flanking region of the human cdc2 gene. J. Biol. Chem. 268, 2255–2259 [PubMed] [Google Scholar]
- 42. Travali S., Ferber A., Reiss K., Sell C., Koniecki J., Calabretta B., Baserga R. (1991) Effect of the myb gene product on expression of the PCNA gene in fibroblasts. Oncogene 6, 887–894 [PubMed] [Google Scholar]
- 43. Bennett J. D., Farlie P. G., Watson R. J. (1996) E2F binding is required but not sufficient for repression of B-myb transcription in quiescent fibroblasts. Oncogene 13, 1073–1082 [PubMed] [Google Scholar]
- 44. Ziebold U., Bartsch O., Marais R., Ferrari S., Klempnauer K. H. (1997) Phosphorylation and activation of B-Myb by cyclin A-Cdk2. Curr. Biol. 7, 253–260 [DOI] [PubMed] [Google Scholar]
- 45. Ansieau S., Kowenz-Leutz E., Dechend R., Leutz A. (1997) B-Myb, a repressed trans-activating protein. J. Mol. Med. 75, 815–819 [DOI] [PubMed] [Google Scholar]
- 46. Sala A., Kundu M., Casella I., Engelhard A., Calabretta B., Grasso L., Paggi M. G., Giordano A., Watson R. J., Khalili K., Peschle C. (1997) Activation of human B-MYB by cyclins. Proc. Natl. Acad. Sci. U.S.A. 94, 532–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bessa M., Saville M. K., Watson R. J. (2001) Inhibition of cyclin A/Cdk2 phosphorylation impairs B-Myb transactivation function without affecting interactions with DNA or the CBP coactivator. Oncogene 20, 3376–3386 [DOI] [PubMed] [Google Scholar]
- 48. Ito M., Araki S., Matsunaga S., Itoh T., Nishihama R., Machida Y., Doonan J. H., Watanabe A. (2001) G2/M-phase-specific transcription during the plant cell cycle is mediated by c-Myb-like transcription factors. Plant Cell 13, 1891–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Araki S., Ito M., Soyano T., Nishihama R., Machida Y. (2004) Mitotic cyclins stimulate the activity of c-Myb-like factors for transactivation of G2/M phase-specific genes in tobacco. J. Biol. Chem. 279, 32979–32988 [DOI] [PubMed] [Google Scholar]
- 50. Doonan J. H., Kitsios G. (2009) Functional evolution of cyclin-dependent kinases. Mol. Biotechnol. 42, 14–29 [DOI] [PubMed] [Google Scholar]
- 51. Sherr C. J., Roberts J. M. (2004) Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18, 2699–2711 [DOI] [PubMed] [Google Scholar]
- 52. Pavletich N. P. (1999) Mechanisms of cyclin-dependent kinase regulation. Structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 287, 821–828 [DOI] [PubMed] [Google Scholar]
- 53. Morgan D. O. (1997) Cyclin-dependent kinases, engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13, 261–291 [DOI] [PubMed] [Google Scholar]
- 54. Malumbres M., Barbacid M. (2005) Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 30, 630–641 [DOI] [PubMed] [Google Scholar]
- 55. Lalioti V., Pulido D., Sandoval I. V. (2010) Cdk5, the multifunctional surveyor. Cell Cycle 9, 284–311 [DOI] [PubMed] [Google Scholar]
- 56. Dynlacht B. D., Moberg K., Lees J. A., Harlow E., Zhu L. (1997) Specific regulation of E2F family members by cyclin-dependent kinases. Mol. Cell. Biol. 17, 3867–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bernander R., Palm J. E., Svard S. G. (2001) Genome ploidy in different stages of the Giardia lamblia life cycle. Cell Microbiol. 1, 55–62 [DOI] [PubMed] [Google Scholar]
- 58. Svärd S. G., Hagblom P., Palm J. E. (2003) Giardia lamblia, a model organism for eukaryotic cell differentiation. FEMS Microbiol. Lett. 218, 3–7 [DOI] [PubMed] [Google Scholar]
- 59. Reiner D. S., Ankarklev J., Troell K., Palm D., Bernander R., Gillin F. D., Andersson J. O., Svärd S. G. (2008) Synchronization of Giardia lamblia. Identification of cell cycle stage-specific genes and a differentiation restriction point. Int. J. Parasitol. 38, 935–944 [DOI] [PubMed] [Google Scholar]
- 60. Ghosh E., Ghosh A., Ghosh A. N., Nozaki T., Ganguly S. (2009) Oxidative stress-induced cell cycle blockage and a protease-independent programmed cell death in microaerophilic Giardia lamblia. Drug Des. Devel. Ther. 3, 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gray N. S., Wodicka L., Thunnissen A. M., Norman T. C., Kwon S., Espinoza F. H., Morgan D. O., Barnes G., LeClerc S., Meijer L., Kim S. H., Lockhart D. J., Schultz P. G. (1998) Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281, 533–538 [DOI] [PubMed] [Google Scholar]
- 62. Bain J., McLauchlan H., Elliott M., Cohen P. (2003) The specificities of protein kinase inhibitors. An update. Biochem. J. 371, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Keister D. B. (1983) Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 77, 487–488 [DOI] [PubMed] [Google Scholar]
- 64. Su L. H., Lee G. A., Huang Y. C., Chen Y. H., Sun C. H. (2007) Neomycin and puromycin affect gene expression in Giardia lamblia stable transfection. Mol. Biochem. Parasitol. 156, 124–135 [DOI] [PubMed] [Google Scholar]
- 65. McArthur A. G., Morrison H. G., Nixon J. E., Passamaneck N. Q., Kim U., Hinkle G., Crocker M. K., Holder M. E., Farr R., Reich C. I., Olsen G. E., Aley S. B., Adam R. D., Gillin F. D., Sogin M. L. (2000) The Giardia genome project database. FEMS Microbiol. Lett. 189, 271–273 [DOI] [PubMed] [Google Scholar]
- 66. Chen Y. H., Su L. H., Sun C. H. (2008) Incomplete nonsense-mediated mRNA decay in Giardia lamblia. Int. J. Parasitol. 38, 1305–1317 [DOI] [PubMed] [Google Scholar]
- 67. Singer S. M., Yee J., Nash T. E. (1998) Episomal and integrated maintenance of foreign DNA in Giardia lamblia. Mol. Biochem. Parasitol. 92, 59–69 [DOI] [PubMed] [Google Scholar]
- 68. Poxleitner M. K., Dawson S. C., Cande W. Z. (2008) Cell cycle synchrony in Giardia intestinalis cultures achieved by using nocodazole and aphidicolin. Eukaryot. Cell 7, 569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hayles J., Aves S., Nurse P. (1986) suc1 is an essential gene involved in both the cell cycle and growth in fission yeast. EMBO J. 5, 3373–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang H., Huang X., Tang L., Zhang Q. J., Frankel J., Berger J. D. (2002) A cyclin-dependent protein kinase homologue associated with the basal body domains in the ciliate Tetrahymena thermophila. Biochim. Biophys. Acta 1591, 119–128 [DOI] [PubMed] [Google Scholar]
- 71. Gourguechon S., Cande W. Z. (2011) Rapid tagging and integration of genes in Giardia intestinalis. Eukaryot. Cell 10, 142–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Finn R. D., Mistry J., Schuster-Böckler B., Griffiths-Jones S., Hollich V., Lassmann T., Moxon S., Marshall M., Khanna A., Durbin R., Eddy S. R., Sonnhammer E. L., Bateman A. (2006) Pfam, clans, web tools, and services. Nucleic Acids Res. 34, D247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van den Heuvel S., Harlow E. (1993) Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262, 2050–2054 [DOI] [PubMed] [Google Scholar]
- 74. Sun C. H., Su L. H., Gillin F. D. (2005) Influence of 5′ sequences on expression of the Tet repressor in Giardia lamblia. Mol. Biochem. Parasitol. 142, 1–11 [DOI] [PubMed] [Google Scholar]
- 75. Klempnauer K. H., Sippel A. E. (1987) The highly conserved amino-terminal region of the protein encoded by the v-myb oncogene functions as a DNA-binding domain. EMBO J. 6, 2719–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sakura H., Kanei-Ishii C., Nagase T., Nakagoshi H., Gonda T. J., Ishii S. (1989) Delineation of three functional domains of the transcriptional activator encoded by the c-myb protooncogene. Proc. Natl. Acad. Sci. U.S.A. 86, 5758–5762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jin H., Martin C. (1999) Multifunctionality and diversity within the plant MYB gene family. Plant Mol. Biol. 41, 577–585 [DOI] [PubMed] [Google Scholar]
- 78. Chiappetta A., Bruno L., Salimonti A., Muto A., Jones J., Rogers H. J., Francis D., Bitonti M. B. (2011) Differential spatial expression of A- and B-type CDKs, and distribution of auxins and cytokinins in the open transverse root apical meristem of Cucurbita maxima. Ann. Bot. 107, 1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jirage D., Chen Y., Caridha D., O'Neil M. T., Eyase F., Witola W. H., Mamoun C. B., Waters N. C. (2010) The malarial CDK Pfmrk and its effector PfMAT1 phosphorylate DNA replication proteins and co-localize in the nucleus. Mol. Biochem. Parasitol. 172, 9–18 [DOI] [PubMed] [Google Scholar]
- 80. Ellis J., Sarkar M., Hendriks E., Matthews K. (2004) A novel ERK-like, CRK-like protein kinase that modulates growth in Trypanosoma brucei via an autoregulatory C-terminal extension. Mol. Microbiol. 53, 1487–1499 [DOI] [PubMed] [Google Scholar]
- 81. Santori M. I., Laría S., Gómez E. B., Espinosa I., Galanti N., Téllez-Iñón M. T. (2002) Evidence for CRK3 participation in the cell division cycle of Trypanosoma cruzi. Mol. Biochem. Parasitol. 121, 225–232 [DOI] [PubMed] [Google Scholar]
- 82. Banerjee S., Sen A., Das P., Saha P. (2006) Leishmania donovani cyclin 1 (LdCyc1) forms a complex with cell cycle kinase subunit CRK3 (LdCRK3) and is possibly involved in S-phase-related activities. FEMS Microbiol. Lett. 256, 75–82 [DOI] [PubMed] [Google Scholar]
- 83. Gomes F. C., Ali N. O., Brown E., Walker R. G., Grant K. M., Mottram J. C. (2010) Recombinant Leishmania mexicana CRK3:CYCA has protein kinase activity in the absence of phosphorylation on the T-loop residue Thr-178. Mol. Biochem. Parasitol. 171, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guo Z., Stiller J. W. (2004) Comparative genomics of cyclin-dependent kinases suggest co-evolution of the RNAP II C-terminal domain and CTD-directed CDKs. BMC Genomics 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lauwaet T., Smith A. J., Reiner D. S., Romijn E. P., Wong C. C., Davids B. J., Shah S. A., Yates J. R., 3rd, Gillin F. D. (2011) Mining the Giardia genome and proteome for conserved and unique basal body proteins. Int. J. Parasitol. 41, 1079–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Martin-Castellanos C., Moreno S. (1997) Recent advances on cyclins, CDKs, and CDK inhibitors. Trends Cell Biol. 7, 95–98 [DOI] [PubMed] [Google Scholar]
- 87. Noble M. E., Endicott J. A., Brown N. R., Johnson L. N. (1997) The cyclin box fold. Protein recognition in cell cycle and transcription control. Trends Biochem. Sci. 22, 482–487 [DOI] [PubMed] [Google Scholar]
- 88. Jeffrey P. D., Russo A. A., Polyak K., Gibbs E., Hurwitz J., Massagué J., Pavletich N. P. (1995) Mechanism of CDK activation revealed by the structure of a cyclin A-CDK2 complex. Nature 376, 313–320 [DOI] [PubMed] [Google Scholar]
- 89. Villerbu N., Gaben A. M., Redeuilh G., Mester J. (2002) Cellular effects of purvalanol A. A specific inhibitor of cyclin-dependent kinase activities. Int. J. Cancer 97, 761–769 [DOI] [PubMed] [Google Scholar]
- 90. Chenna R., Sugawara H., Koike T., Lopez R., Gibson T. J., Higgins D. G., Thompson J. D. (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31, 3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chuang S. F., Su L. H., Cho C. C., Pan Y. J., Sun C. H. (2012) Functional redundancy of two pax-like proteins in transcriptional activation of cyst wall protein genes in Giardia lamblia. PLoS ONE, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.