Background: Tumor suppressors PTEN and TSC2 act upstream of mTOR kinase.
Results: An mTOR-mediated increase in Hif1α protein contributes to PTEN transcription.
Conclusion: Both TORC1 and TORC2 up-regulate PTEN levels.
Significance: An increased PTEN level in TSC2-deficient cells may contribute to reduced malignant potential of these cells.
Keywords: Akt PKB, S6 Kinase, TOR Complex (TORC), Tuberous Sclerosis (Tsc), Tumor Suppressor Gene
Abstract
Tuberous sclerosis complex 2 (TSC2) and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) function to block growth factor-induced mammalian target of rapamycin (mTOR) signaling and are mutated in autosomal dominant hamartoma syndromes. mTOR binds to a spectrum of common and different proteins to form TOR complex 1 (TORC1) and TORC2, which regulate cell growth, division, and metabolism. TSC2 deficiency induces constitutive activation of mTOR, leading to a state of insulin resistance due to a negative feedback regulation, resulting in reduced Akt phosphorylation. We have recently described an alternative mechanism showing that in TSC2 deficiency, enhanced PTEN expression contributes to reduced Akt phosphorylation. To explore the mechanism of PTEN regulation, we used rapamycin and constitutively active mTOR to show that TORC1 increases the expression of PTEN mRNA and protein. We found that in TSC2−/− mouse embryonic fibroblasts expression of a kinase-dead mutant of mTOR, which inhibits both TORC1 and TORC2, decreases the expression of PTEN via transcriptional mechanism. Furthermore, kinase-dead mTOR increased and decreased phosphorylation of Akt at catalytic loop site Thr-308 and hydrophobic motif site Ser-473, respectively. Moreover, inhibition of deregulated TORC1 in TSC2-null mouse embryonic fibroblasts or in 293 cells by down-regulation of raptor decreased the levels of the transcription factor Hif1α and blocked PTEN expression, resulting in enhanced phosphorylation of Akt at Thr-308 and Ser-473. Finally, knockdown of rictor or mSin1 attenuated the expression of Hif1α, which decreased transcription of PTEN. These results unravel a previously unrecognized cell-autonomous function of TORC1 and TORC2 in the up-regulation of PTEN, which prevents phosphorylation of Akt and may shield against the development of malignancy in TSC patients.
Introduction
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN)5 represents the most frequently deleted phosphatase and second most frequently deleted tumor suppressor gene in cancer (1). In fact, 50–70% of sporadic tumors including prostate tumors, endometrial tumors, and glioblastomas as well as 30–50% of lung, breast, and colon cancers show loss of one allele of PTEN (2). Complete loss of PTEN in endometrial tumors, glioblastomas, and BRCA1-deficient breast cancer is associated with advanced metastasis (2). Furthermore, germ line mutation of PTEN is associated with autosomal dominant developmental disorders, neurological deficiencies, and hamartoma syndromes including Cowden disease, Bannayan-Riley-Ruvalcaba syndrome, and Lhermitte-Duclos disease, which show a high frequency of cancer predisposition (3, 4).
Growth factor-stimulated class I phosphatidylinositol (PI) 3-kinase family member or its mutated constitutively active catalytic subunit produces phosphatidylinositol 3,4,5-trisphosphate (PIP3), which regulates a myriad of cellular functions such as cell migration, polarity, proliferation, and survival (5, 6). PIP3 produced in the plasma membrane recruits proteins containing pleckstrin homology domains such as Akt and PDK-1. Akt activation occurs via phosphorylation by PDK-1 and mTORC2 at two sites, Thr-308 and Ser-473, respectively (7). Structurally, PTEN shares identity with other protein phosphatases in its catalytic domain (8). Although PTEN has been infrequently reported to dephosphorylate protein substrates, its phosphatase activity toward PIP3 represents an important mechanism for its physiological tumor suppressor function (9–13). Thus, PTEN acts as a negative regulator of PI 3-kinase signal transduction, significantly attenuating the biological activity of Akt. In PTEN-negative cancer cells, Akt is constitutively activated and regulates cell growth, proliferation, angiogenesis, and metabolism via phosphorylation of a number of substrates including tuberous sclerosis complex 2 (TSC2) and PRAS40, inactivation of both of which increases rapamycin-sensitive mTORC1 activity (7, 14).
Mutation in either TSC1 or TSC2 gene contributes to the development of TSC, which manifests as disorders involving pulmonary lymphangiomyomatosis, facial angiosarcomas, and renal angiomyolipomas (15). In addition, TSC patients often display neurological disorders including mental retardation, epilepsy, and autism (16). Clinically, mutations in TSC2 locus contribute more significantly to the manifestation of TSC compared with TSC1 mutation (17). Functionally, TSC1 and TSC2 exist as a heterodimer of which TSC2 contains a GTPase-activating protein domain. The TSC1·TSC2 complex exerts its GTPase-activating protein activity on the small GTPase Ras homolog enriched in brain (Rheb) and blocks mTOR activity (18, 19). Genetic studies in Drosophila and in mammalian cells place TSC2 as a signal integration hub in the PI 3-kinase/Akt/mTOR pathway (19, 20). Activated Akt and other mitogenic kinases phosphorylate TSC2 at distinct sites, leading to its dissociation from TSC1 and inactivation (21–24). Thus, inactivated TSC2 maintains elevated levels of GTP-bound Rheb, which activates TORC1 to promote tumorigenesis. Enhanced TORC1 activity is manifested in pathologic specimens of TSC hamartomas (25, 26). Similarly, in PTEN-deficient tumors, increased Akt activity phosphorylates TSC2, resulting in its inactivation, leading to activation of mTOR (7, 27).
TOR exists in two evolutionary conserved complexes, TORC1 and TORC2; the former is more sensitive to rapamycin (28, 29). TORC1 and TORC2 contain two distinct proteins, rapamycin-sensitive adaptor protein of mTOR (raptor) and rapamycin-insensitive companion of mTOR (rictor), respectively (28, 30, 31). Both these complexes bind mLST8/GβL and deptor, whereas TORC1 contains PRAS40, and TORC2 contains protor and mSin1 (28, 32, 33). TORC1 directly phosphorylates the eukaryotic initiation factor 4E-binding proteins (4EBPs) and the ribosomal protein S6 kinase and increases ribosomal biogenesis to elicit cell growth, proliferation, and metabolism (32, 34). On the other hand, TORC2 phosphorylates Akt at the hydrophobic motif site Ser-473 to fully activate its antiapoptotic function (35, 36).
The wide spectrum of hamartomas elicited by loss of TSC2 rarely progress to malignancy. Lack of TSC2 leads to inhibition of Akt activity, which may contribute to reduced malignancy of TSC tumors (37, 38). Multiple mechanisms have been postulated for the down-regulation of Akt phosphorylation in TSC2-null cells. Disruption of TSC2 produces a state of insulin resistance by a rapamycin-sensitive TORC1-mediated negative feed back loop through insulin receptor substrate 1 (37, 38). A second level of negative regulation on PI 3-kinase/Akt signaling is achieved by down-regulation of PDGF receptor-β in TSC2-null murine embryonic fibroblasts (MEFs) via an unknown mechanism (39, 40). We have recently reported that in TSC2-null MEFs, the level of PTEN is significantly elevated, which also contributes to decreased phosphorylation of Akt (41). The signal transduction mechanism by which PTEN is up-regulated in TSC2-disrupted cells is not known.
In the present study, we found that the enhanced TORC1 activity present in the TSC2-null MEFs regulates the levels of PTEN mRNA and protein via transcriptional mechanism. Similarly, TORC1 controls expression of PTEN in normal cells. Furthermore, we show that along with TORC1 TORC2 also contributes to PTEN expression. Finally, we demonstrate that both TORC1 and TORC2 up-regulate Hif1α protein, which enhances the transcription of PTEN. Thus, up-regulation of PTEN in TSC patients may attenuate the malignant potential of the tumors despite increased mTOR activity.
EXPERIMENTAL PROCEDURES
Cell Culture
TSC2+/+ and TSC2−/− MEFs were grown as described previously in DMEM with low glucose in the presence of 10% fetal bovine serum (41). 293 cells were grown in DMEM with high glucose in 10% fetal bovine serum. The cell stocks were maintained in Plasmocin and Primocin. When necessary, the cells were incubated in serum-free medium for 24 h before the experiment.
Antibodies and Reagents
TSC2, PTEN, Myc, and Hif1α antibodies were obtained from Santa Cruz Biotechnology. Phospho-Akt antibodies recognizing either Ser-473 or Thr-308 and raptor, rictor, mTOR, phospho-S6 kinase (Thr-389), phospho-4EBP-1 (Thr-37/46), phospho-4EBP-1 (Ser-65), S6 kinase, 4EBP-1, eIF4E, eIF4G, and Akt antibodies were purchased from Cell Signaling Technology. Actin and HA antibodies were purchased from Sigma and Covance, respectively. mSin1 antibody recognizing mSin1.1 and mSin1.2 was obtained from Bethyl Laboratories. shRNA expression plasmids for human raptor (shRaptor 1 and shRaptor 2), human rictor (shRictor 1 and shRictor 2), human mSin1 (mSin1 sh1 and mSin1 sh2) targeting both mSin1.1 and mSin1.2, and scrambled vector were obtained from Addgene. Pooled siRNAs against mouse raptor and rictor and human raptor and rictor and scrambled RNAs were obtained from Santa Cruz Biotechnology. siRNA for mouse mSin1 was obtained from Sigma. Hif1α 5′ terminal oligopyrimidine tract (TOP)-Lux reporter plasmid in which the 5′-untranslated region (5′-UTR) of Hif1α flanks the luciferase gene was a kind gift from Dr. John Blenis (Harvard Medical School), who obtained it from Dr. Charles Sawyers, University of California at Los Angeles (42). The RNA isolation kit RNAzol and OneStep RT-PCR kit were purchased from Invitrogen. Primers to detect mouse and human PTEN mRNAs were obtained from SuperArray Bioscience. The luciferase assay kit was purchased from Promega. Expression plasmids for Myc-tagged raptor, rictor, mSin1.1, mSin1.2, and kinase-dead mTOR (mTOR KD) were obtained from Addgene. Constitutively active mTOR expression vector (SL1+I2017T) was kindly provided by Dr. Tatsuya Maeda, Institute of Molecular Biology, The University of Tokyo, Tokyo, Japan. In this constitutively active mutant, four amino acids of mTOR are changed (I2017T, V2198A, L2216H, and L2260P) (supplemental Fig. S2) (43). We refer to this mutant as constitutively active mutant of mTOR (CA mTOR).
Cell Lysis and Immunoblotting
MEFs and 293 cells treated with rapamycin (25 nm) or transfected cells were washed with PBS and harvested in radioimmune precipitation assay buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA, 1 mm Na3VO4, 1 mm PMSF, 0.1% protease inhibitor mixture, and 1% Nonidet P-40) (41, 44, 45). The crude cell extracts were centrifuged at 12,000 × g at 4 °C for 30 min. Cleared cell lysates were separated by SDS-polyacrylamide gel electrophoresis and transferred to a PVDF membrane. Immunoblotting was performed using the indicated primary antibodies, and the protein bands were developed with HRP-conjugated secondary antibody using ECL reagent as described (41, 44, 45).
RNA Extraction and Semiquantitative RT-PCR
Total RNAs were prepared from TSC2−/− MEFs and 293 cells using the RNAzol reagent according to the vendor's instructions. Reverse transcription-PCR was carried out using the OneStep RT-PCR kit as described previously (41).
Transfection and Luciferase Assay
The cells were transfected with the indicated plasmids or siRNAs using FuGENE HD according to the manufacturer's protocol (41, 44, 45). For determining PTEN transcription, a reporter plasmid containing the PTEN promoter driving the luciferase cDNA was transfected along with the indicated expression vector or siRNAs. The luciferase activity was determined using an assay kit according to the vendor's instructions (41, 44, 45).
Statistics
Statistical significance of the data was determined by paired t test or analysis of variance using GraphPad Prism 4 software as described previously (41, 44, 45). The significance level was considered at a p value <0.05.
RESULTS
mTOR Regulates PTEN Expression in TSC2−/− MEFs
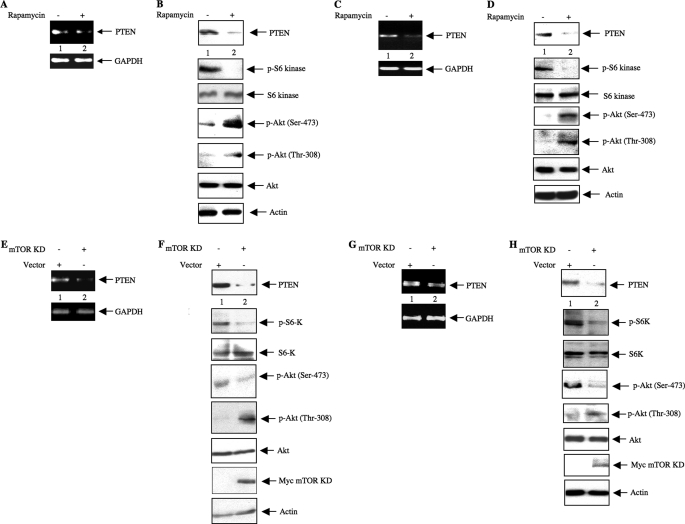
In TSC2-null cells, a negative feedback loop involving enhanced TORC1 activity inhibits phosphorylation of Akt (37, 38, 46). The tumor suppressor protein PTEN represents an important mechanism by which the PIP3 level, which regulates Akt phosphorylation, is maintained (11, 12). We have recently shown that increased expression of PTEN in the TSC2-null cells also contributes to the reduced phosphorylation of Akt (supplemental Fig. S1) (41). GTPase-activating protein activity of TSC2 blocks TORC1 activity (18, 20). Indeed, TORC1 is constitutively active in TSC2-deficient MEFs as confirmed by the increased phosphorylation of p70 S6 kinase at Thr-389 (supplemental Fig. S1). To examine the role of TORC1 in PTEN expression in TSC2−/− MEFs, we used rapamycin, a potent inhibitor of TORC1 activity (29). Rapamycin significantly reduced the expression of PTEN mRNA in TSC2-null MEFs concomitantly with a reduction in the PTEN protein level (Fig. 1, A and B). Inhibition of TORC1 activity was confirmed by the loss of phosphorylation of S6 kinase in response to rapamycin (Fig. 1B) (37, 41, 46). The reduction of the PTEN protein level resulted in the up-regulation of Akt phosphorylation in these cells (Fig. 1B). These findings suggest that TORC1 regulates the expression of PTEN in TSC2-deficient cells. To obtain more insight into this mode of regulation of PTEN expression, we extended these studies in growing human 293 cells, which express active TSC2, in the presence of serum. Incubation of these cells with rapamycin showed a reduction of PTEN mRNA and protein that was concomitant with the inhibition of TORC1 (Fig. 1, C and D). Similar to the observation obtained with TSC2-null MEFs, rapamycin increased the phosphorylation of Akt at Ser-473 and Thr-308 (Fig. 1D).
FIGURE 1.
mTOR regulates expression of PTEN and phosphorylation of Akt. A–D, rapamycin lowers PTEN expression to modulate Akt phosphorylation. A and B, TSC2−/− MEFs were grown to near confluence and serum-starved for 24 h prior to incubation with 25 nm rapamycin for 24 h. A, total RNA was used in semiquantitative RT-PCR to detect PTEN and GAPDH mRNAs as indicated. B, the cell lysates were immunoblotted with the indicated antibodies as described under “Experimental Procedures.” C and D, 293 cells were grown to confluence in the presence of 10% serum prior to incubation with 25 nm rapamycin for 24 h. C, total RNA was analyzed for the expression of PTEN and GAPDH mRNAs by semiquantitative RT-PCR. D, cleared cell lysates were immunoblotted with the indicated antibodies. E–H, dominant negative mTOR inhibits PTEN expression to affect phosphorylation of Akt. E and F, TSC2−/− MEFs in a 12-well culture dish were transfected with 500 ng of Myc-tagged mTOR KD or vector plasmid as described under “Experimental Procedures.” Twenty-four hours post-transfection, the cells were incubated with serum-free medium for 24 h. E, total RNA was analyzed for PTEN and GAPDH mRNAs by semiquantitative RT-PCR. F, the cleared cell lysates were immunoblotted with the indicated antibodies. G and H, 293 cells were transfected with Myc-mTOR KD and grown for 48 h in 10% serum. G, total RNAs were analyzed for PTEN and GAPDH mRNAs by semiquantitative RT-PCR. H, the cleared cell lysates were immunoblotted with the indicated antibodies. p-Akt, phospho-Akt; S6K, S6 kinase; p-S6K, phospho-S6 kinase.
Rapamycin has been shown to significantly inhibit TORC1 activity, although prolonged incubation (more than 12 h) with this macrolide blocked TORC2 activity in some cancer cells (29). The results described above were obtained with cells incubated with rapamycin for 24 h (Fig. 1, A–D). Under these conditions, we observed enhanced phosphorylation of Akt at Ser-473 (Fig. 1, B and D). These data suggest that rapamycin did not inhibit mTORC2 activity in TSC2−/− MEFs and in 293 cells.
To test the involvement of mTOR in more detail, we examined whether kinase-dead mTOR, which acts as a dominant negative kinase, affects expression of PTEN in TSC2-deficient MEFs. The results show that expression of kinase-dead mTOR in TSC2-null MEFs inhibited PTEN mRNA and protein expression and attenuated TORC1-mediated phosphorylation of S6 kinase (Fig. 1, E and F) similarly to the effects obtained with rapamycin above. However, unlike the results obtained with rapamycin, kinase-dead mTOR inhibited the TORC2-mediated basal phosphorylation of Akt at Ser-473 (Fig. 1F). These data are consistent with the mutation in the kinase-dead construct affecting the kinase activity of mTOR irrespective of whether it is a constituent of TORC1 or TORC2. On the other hand, we observed increased phosphorylation of Akt at Thr-308 (Fig. 1F). This is probably due to an increase in PIP3 levels associated with decreased PTEN (Fig. 1F, top panel), which would stimulate the PDK-1 activity responsible for Akt Thr-308 phosphorylation in TSC2−/− MEFs (Fig. 1F).
To investigate further the physiological relevance of PTEN regulation by mTOR, we examined its effect in growing human 293 cells. Expression of dominant negative mTOR kinase in these cells inhibited PTEN mRNA (Fig. 1G) and protein abundance concomitantly with reduced phosphorylation of S6 kinase, which was used as a surrogate to determine TORC1 activation (Fig. 1H). Because kinase-dead mTOR would block TORC2 activity, phosphorylation of Akt at Ser-473 was inhibited in 293 cells (Fig. 1H). In contrast, phosphorylation of Akt at Thr-308 was increased by dominant negative mTOR (Fig. 1H). Together these data indicate that similarly to TSC2−/− MEFs dominant negative mTOR blocks both TORC1 and TORC2 activity to regulate phosphorylation of Akt at Thr-308 and Ser-473 in a reciprocal manner.
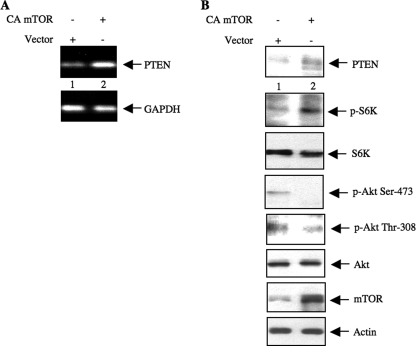
To confirm our results demonstrating a role of TORC1 in the regulation of PTEN expression, we next used a constitutively active mutant of mTOR (supplemental Fig. S2) in human 293 cells (43). Expression of CA mTOR in 293 cells increased the expression of PTEN mRNA and protein concomitantly with increased phosphorylation of S6 kinase (Fig. 2, A and B). This constitutively active mutant of mTOR has been shown to display only TORC1 activity (43). As a result, phosphorylation of Akt at Ser-473 was significantly inhibited in constitutively active mTOR-expressing cells (Fig. 2B). Furthermore, Akt phosphorylation at Thr-308 was also inhibited in the presence of constitutively active mTOR (Fig. 2B). This is probably because increased PTEN would decrease the PIP3 level, which would in turn lead to diminished PDK-1 activity. Together our results suggest that increased expression of PTEN due to active TORC1 reduces phosphorylation of Akt.
FIGURE 2.
CA mTOR increases PTEN to regulate phosphorylation of Akt in 293 cells. 293 cells were transfected with CA mTOR or vector plasmids. Twenty-four hours post-transfection, the cells were incubated in serum-free medium for 24 h prior to harvest. A, total RNA was analyzed for expression of PTEN and GAPDH mRNAs by semiquantitative RT-PCR. B, cleared cell lysates were immunoblotted with the indicated antibodies. p-Akt, phospho-Akt; S6K, S6 kinase; p-S6K, phospho-S6 kinase.
mTOR Regulates Transcription of PTEN
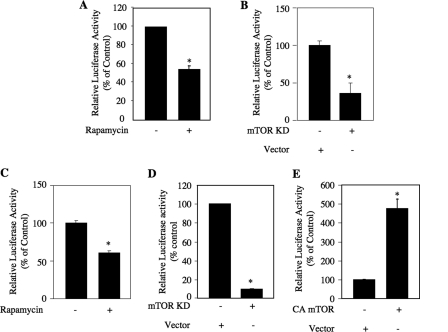
The above findings indicate that TORC1 regulates PTEN mRNA expression. To gain more insight into the mechanism, we investigated the role of TORC1 in the transcription of PTEN. We used a reporter plasmid in which the firefly luciferase gene is driven by the PTEN promoter (PTEN-Luc) (41, 47). Transient transfection of this reporter plasmid leads to significant luciferase activity in TSC2−/− MEFs (Fig. 3A) (41). Incubation of the reporter-transfected cells with rapamycin significantly inhibited the luciferase activity, indicating a reduction in transcription of PTEN (Fig. 3A). Similarly, expression of a kinase-dead mutant of mTOR prevented the activity of the reporter in TSC2−/− MEFs (Fig. 3B). To confirm these results, we transiently transfected PTEN-Luc into human 293 cells. Treatment of these cells with rapamycin attenuated the reporter activity (Fig. 3C). Furthermore, cotransfection of dominant negative mTOR with the reporter plasmid markedly inhibited the luciferase activity (Fig. 3D). Finally, we determined whether constitutively active mTOR increases this activity. Cotransfection of a constitutively active mutant of mTOR with PTEN-Luc in 293 cells resulted in a significant up-regulation of luciferase activity (Fig. 3E). These results suggest that TORC1 regulates PTEN promoter activity to increase PTEN expression.
FIGURE 3.
mTOR regulates transcription of PTEN in TSC2−/− MEFs and 293 cells. A and B, TSC2−/− MEFs were transfected with PTEN-Luc along with mTOR KD as indicated. Twenty-four hours post-transfection, the cells were serum-starved for 24 h and treated with rapamycin for 24 h before harvesting (A). Luciferase activity was determined in the cell lysates as described under “Experimental Procedures” (41, 44, 45, 65). C–E, 293 cells were transfected with PTEN-Luc along with mTOR KD or CA mTOR as indicated followed by incubation in the presence of serum for 48 h (C and D). The cells in C were incubated with rapamycin for 24 h before harvesting. For E, 24 h post-transfection, the cells were incubated in serum-free medium for 24 h. The cell lysates were used for luciferase activity assay as described (41, 44, 45, 65). For A–D, mean ± S.E. of 12 measurements is shown. For A, B, and D, * represents p = 0.001 versus control. For C, * represents p = 0.002 versus control. For E, mean ± S.E. of six measurements is shown. *, p = 0.0007 versus control.
TORC1 and TORC2 Regulate PTEN Expression via Transcription
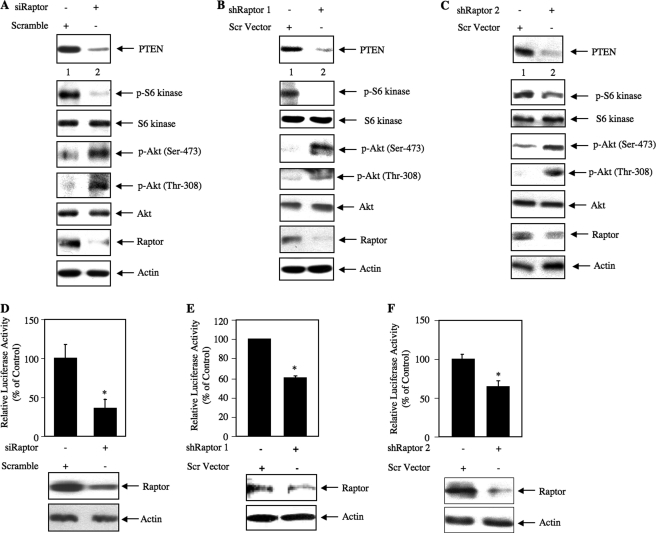
Use of rapamycin, kinase-dead mTOR, and constitutively active mTOR mutants conclusively pointed to a significant role of the kinase in the regulation of PTEN expression. To further dissect the molecular mechanisms, we first examined the contribution of TORC1 by inhibiting raptor, an exclusive component of the TORC1 kinase (31). Knockdown of raptor using a pool of siRNA in TSC2−/− MEFs decreased the level of PTEN (Fig. 4A). As expected, raptor knockdown reduced the TORC1 activity as judged by the phosphorylation of S6 kinase in these cells (Fig. 4A). Reduced levels of PTEN due to decreased TORC1 activity were associated with increased phosphorylation of Akt at the Ser-473 and Thr-308 (Fig. 4A). These findings suggest a specific requirement of TORC1 activity in the up-regulation of PTEN in TSC2 deficiency. We also examined the effect of down-regulation of raptor in growing human 293 cells. Knockdown of raptor using two independent shRNA vectors, shRaptor 1 and shRaptor 2, decreased the basal PTEN levels concomitantly with reduced phosphorylation of S6 kinase (Fig. 4, B and C). The reduction in PTEN resulted in an increase in the phosphorylation of Akt at Ser-473 and Thr-308 (Fig. 4, B and C). To confirm this observation, we also used an siRNA pool to down-regulate raptor in 293 cells. As expected, expression of PTEN was inhibited along with decreased phosphorylation of S6 kinase and increased phosphorylation of Akt (supplemental Fig. S3A).
FIGURE 4.
Down-regulation of raptor prevents expression of PTEN and increases Akt phosphorylation in TSC2−/− MEFs and 293 cells. A, TSC2−/− MEFs were transfected with siRNA pool (20 nm) targeting raptor or scrambled RNA as described under “Experimental Procedures” (41, 44, 45, 65). Twenty-four hours post-transfection, the cells were serum-starved for 24 h. The cell lysates were immunoblotted with the indicated antibodies. B and C, 293 cells were transfected with two independent shRNA expression plasmids, shRaptor 1 and shRaptor 2, targeting two different regions in the raptor gene (500 ng) or scrambled (Scr) vector as described under “Experimental Procedures” (41, 44, 45, 65). Forty-eight hours post-transfection, the cell lysates were immunoblotted with the indicated antibodies. D–F, TSC2−/− MEFs (D) or 293 cells (E and F) were transfected with PTEN-Luc along with either an siRNA pool (20 nm) against raptor (D) or plasmid expressing shRaptor 1 (E) or shRaptor 2 (F) (250 ng). Twenty-four hours post-transfection, the TSC2−/− MEFs were serum-starved for 24 h (D). In the case of 293 cells (E and F), the cells were harvested at 48 h post-transfection. The cell lysates were assayed for luciferase activity as described (41, 44, 45, 65). Mean ± S.E. of 12 measurements is shown for D and E. For F, mean ± S.E. of six measurements is shown. For D, * represents p = 0.002 versus control. For E, * represents p = 0.0008 versus control. For F, * represents p = 0.02 versus control. The expression of raptor is shown at the bottom of histograms for representative wells. p-Akt, phospho-Akt; p-S6 kinase, phospho-S6 kinase.
We have demonstrated that mTOR regulates the expression of PTEN mRNA by a transcriptional mechanism (Fig. 3). To examine whether TORC1 specifically regulates the transcription of PTEN, we cotransfected PTEN-Luc reporter plasmid with an siRNA pool against raptor in TSC2−/− MEFs. Knockdown of raptor in TSC2−/− MEFs significantly inhibited the luciferase activity (Fig. 4D). Similarly, cotransfection of PTEN-Luc with shRaptor 1 or shRaptor 2 attenuated reporter activity in 293 cells (Fig. 4, E and F). Identical results were obtained when we used a pool of siRNA targeting raptor in 293 cells (supplemental Fig. S3B).
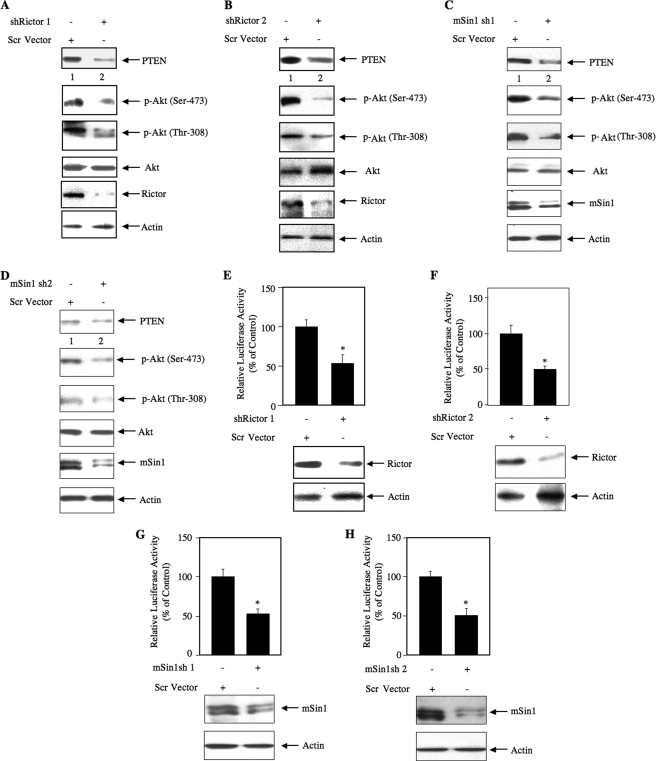
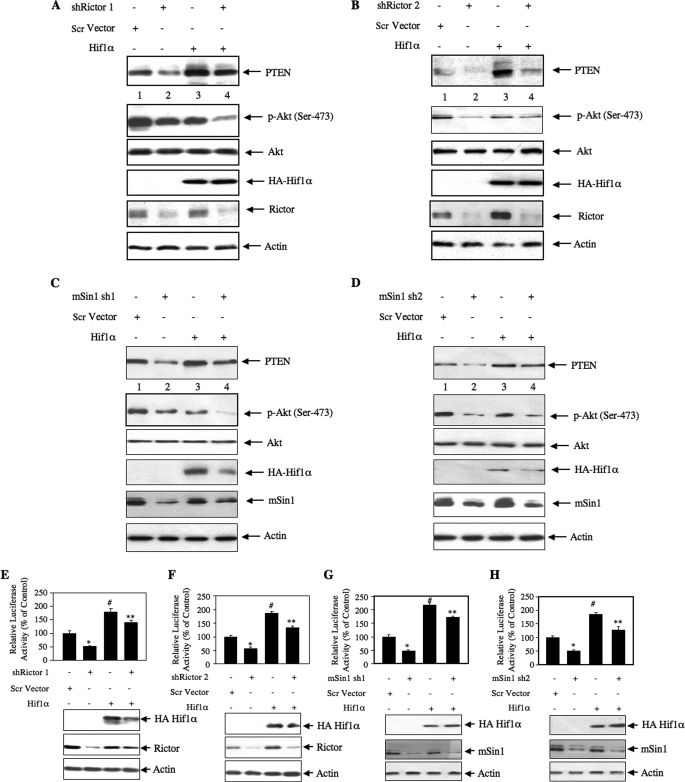
Next, we investigated whether TORC2 plays any role in PTEN expression. 293 cells grown in serum were used. We down-regulated rictor, a component of TORC2 necessary for its kinase activity (48), in growing 293 cells using two independent shRNA vectors, shRictor 1 and shRictor 2. Knockdown of rictor with both rictor shRNAs inhibited PTEN protein expression (Fig. 5, A and B). As expected, the phosphorylation of Akt at Ser-473 was decreased as it is the substrate of TORC2 (Fig. 5, A and B). Interestingly, a decrease in phosphorylation of Akt at Thr-308 was evident in response to rictor down-regulation (Fig. 5, A and B). Identical results were obtained using an siRNA pool to down-regulate rictor in 293 cells (supplemental Fig. S4A). To further confirm the role of TORC2, we used two shRNA plasmids targeting mSin1, an exclusive component of TORC2. Two isoforms of this protein, mSin1.1 and mSin1.2, are predominantly present in the TORC2 complex (33). Down-regulation of both mSin1 complexes with two independent shRNAs inhibited expression of PTEN (Fig. 5, C and D). Phosphorylation of Akt was also blocked (Fig. 5, C and D). Finally, to elucidate the role of TORC2 in PTEN transcription, 293 cells were cotransfected with PTEN-Luc reporter and two independent shRNA vectors, shRictor 1 and shRictor 2. Knockdown of rictor with both shRNAs significantly blocked the reporter activity (Fig. 5, E and F). Similar results were obtained using an siRNA pool targeting rictor in these cells to inhibit rictor expression (supplemental Fig. S4B). When shRNAs against mSin1 were used to down-regulate TORC2, identical results were obtained (Fig. 5, G and H). These results demonstrate that both TORC1 and TORC2 contribute to the expression of PTEN protein via a transcriptional mechanism.
FIGURE 5.
Down-regulation of rictor and mSin1 prevents expression of PTEN and decreases Akt phosphorylation in 293 cells. A and B, 293 cells were transfected with two independent shRNA expression plasmids, shRictor 1 and shRictor 2, targeting two different regions in the rictor gene (500 ng) or scrambled (Scr) vector. C and D, 293 cells were transfected with two independent shRNA expression plasmids, mSin1 sh1 and mSin1 sh2, targeting two different regions in the mSin1 mRNA (500 ng) or scrambled vector. Forty-eight hours post-transfection, the cell lysates were immunoblotted with the indicated antibodies. E and F, 293 cells were cotransfected with PTEN-Luc along with shRictor 1 (E), shRictor 2 (F), mSin1 sh1 (G), or mSin1 sh2 (H) expression vector. The cells were harvested at 48 h post-transfection. The cell lysates were assayed for luciferase activity as described (41, 44, 45, 65). For E, mean ± S.E. of 12 measurements is shown. *, p = 0.002 versus control. For F, mean ± S.E. of six measurements is shown. *, p = 0.03 versus control. For G, mean ± S.E. of six measurements is shown. *, p = 0.04 versus control. For H, mean ± S.E. of six measurements is shown. *, p = 0.002 versus control. The expression of rictor and mSin1 is shown at the bottom of the histogram. p-Akt, phospho-Akt.
TORC2 Contributes to Hif1α Expression by Translational Mechanism
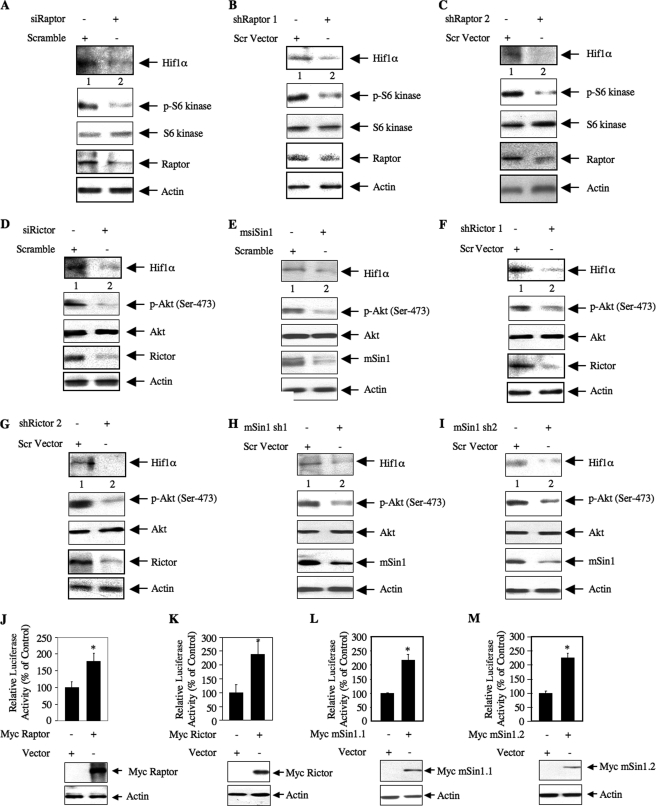
We have recently shown that a Hif1α-responsive DNA element present in the PTEN promoter increases its transcription in TSC2-null MEFs and in 293 cells (41). Previously, rapamycin was shown to inhibit mTOR activity and Hif1α-mediated transcription in TSC2-null MEFs (49). We directly tested whether TORC1 regulates Hif1α protein in TSC2-deficient MEFs. siRNA pool-mediated down-regulation of raptor in these cells decreased Hif1α protein levels (Fig. 6A). Similarly, inhibition of raptor expression using two independent shRNA vectors, shRaptor 1 and shRaptor 2, also blocked Hif1α expression in 293 cells (Fig. 6, B and C). Identical results were obtained when we used an siRNA pool to down-regulate raptor in these cells (supplemental Fig. S5). These results conclusively demonstrate that TORC1 regulates Hif1α protein expression.
FIGURE 6.
TORC1 and TORC2 regulate Hif1α expression in TSC2−/− MEFs and 293 cells. A, D, and E, TSC2−/− MEFs were transfected with siRNAs (20 nm) targeting raptor, rictor, mSin1, or scrambled RNA as described under “Experimental Procedures.” Twenty-four hours post-transfection, the cells were serum-starved for 24 h. The cell lysates were immunoblotted with the indicated antibodies. B, C, F, G, H, and I, 293 cells were transfected with 500 ng of two independent shRNA expression plasmids, shRaptor 1 (B) and shRaptor 2 (C), shRictor 1 (F) and shRictor 2 (G), or mSin1 sh1 (H) and mSin1 sh2 (I), or scrambled (Scr) vector as described under “Experimental Procedures.” Forty-eight hours post-transfection, the cell lysates were immunoblotted with the indicated antibodies. J–M, Hif1α 5′ TOP-Lux reporter plasmid (42) was cotransfected with either Myc-tagged raptor (J), Myc-tagged rictor (K), Myc-tagged mSin1.1 (L), or Myc-tagged mSin1.2 (M) in 293 cells. Luciferase activity was determined in the cell lysates. Mean ± S.E. of six measurements is shown. For J, * represents p = 0.005 versus control. For K, * represents p = 0.03 versus control. For L, * represents p = 0.005 versus control. For M, * represents p = 0.001 versus control. p-Akt, phospho-Akt; p-S6 kinase, phospho-S6 kinase.
We have shown above that along with TORC1 TORC2 also contributes to the expression of PTEN (Figs. 4 and 5). Because PTEN is a Hif1α target gene (41), we tested the involvement of TORC2 in Hif1α expression using rictor siRNA. A pooled siRNA-directed down-regulation of rictor in TSC2-null MEFs decreased Hif1α protein levels (Fig. 6D). siRNA-directed down-regulation of mSin1 in these cells also suppressed Hif1α expression (Fig. 6E). Analogous to these observations, down-regulation of either rictor or mSin1 using two independent shRNA vectors for each protein reduced Hif1α protein abundance in 293 cells (Figs. 6, F–I). Expression of Hif1α protein has been ascribed to increased translation of its mRNA due to the presence of a TOP in the 5′-UTR (50, 51). In fact, in TSC2-null MEFs, Hif1α protein expression is sensitive to rapamycin, indicating a role for TORC1 (supplemental Fig. S6) (49, 50). To test directly the translational mechanism, we used a Hif1α 5′-UTR-driven reporter plasmid along with a raptor expression vector (42). Overexpression of raptor increased Hif1α 5′-UTR-mediated reporter activity (Fig. 6J). Interestingly, expression of rictor and both mSin1.1 and mSin1.2 also increased the Hif1α 5′-UTR-driven luciferase activity (Fig. 6, K–M).
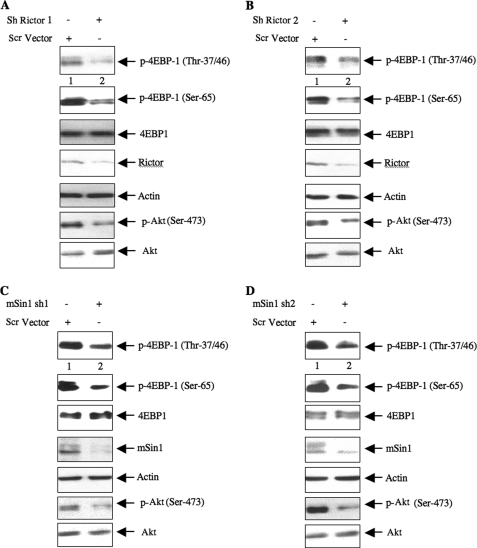
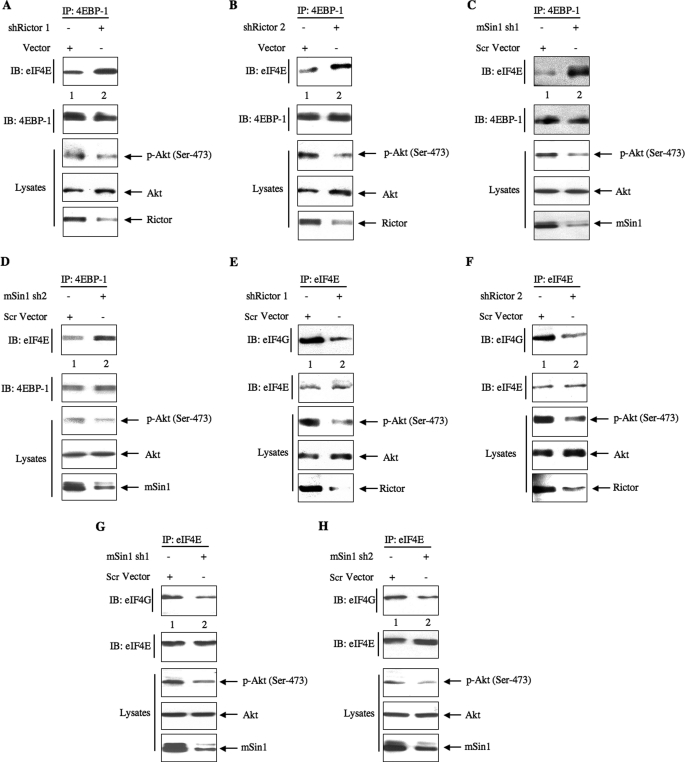
The above results suggest a role of TORC2 in increasing translation from the Hif1α 5′-UTR. The rate-limiting step in protein synthesis involves phosphorylation of the translation repressor 4EBP-1 by TORC1 (52). We tested the role of TORC2 in phosphorylation of 4EBP-1 using rictor and mSin1 shRNAs. Down-regulation of either rictor or mSin1 inhibited the phosphorylation of 4EBP-1 at Thr-37/46 and Ser-65 (Fig. 7, A–D). Phosphorylation induces dissociation of 4EBP-1 from the eIF4E·4EBP-1 complex to release eIF4E, allowing the eIF4E to associate with eIF4G to form the initiation complex to commence mRNA translation (52). We tested the role of TORC2 in this process using shRNAs targeting rictor and mSin1 in 293 cells. Coimmunoprecipitation experiments revealed that down-regulation of rictor as well as mSin1 using two independent shRNAs for each protein increased the association of eIF4E with 4EBP-1 (Fig. 8, A–D). Reciprocally, immunoprecipitation of eIF4E from rictor shRNA- or mSin1 shRNA-down-regulated samples followed by immunoblotting with 4EBP-1 showed increased an association of 4EBP-1 with eIF4E (supplemental Fig. S7, A–D).
FIGURE 7.
Down-regulation of rictor or mSin1 decreases phosphorylation of 4EBP-1. 293 cells were transfected with two independent shRNA plasmids targeting either rictor (A and B) or mSin1 (C and D) or with scrambled (Scr) vector as indicated. The cell lysates were immunoblotted with phospho-4EBP-1 (p-4EBP-1) (Thr-37/46), phospho-4EBP-1 (Ser-65), and 4EBP-1 antibodies. Immunoblotting of the same samples with antibodies against rictor, mSin1, phospho-Akt (p-Akt) (Ser-473), Akt, and actin is also shown.
FIGURE 8.
TORC2 regulates dissociation of eIF4E·4EBP-1 complex and formation of eIF4E·eIF4G complex. 293 cells were transfected with either shRictor 1 (A and E), shRictor 2 (B and F), mSin1 sh1 (C and G), or mSin1 sh2 (D and H) expression vectors. Forty-eight hours post-transfection, the cells were lysed, and the extracts were immunoprecipitated with 4EBP-1 followed by immunoblotting (IB) with eIF4E and 4EBP-1 antibodies (A–D). E–H, the same cell lysates were immunoprecipitated (IP) with eIF4E followed by immunoblotting with eIF4G and eIF4E antibodies. Phosphorylation of Akt at Ser-473 to demonstrate TORC2 activity in rictor-down-regulated cells and expression of rictor and mSin1 in the cell lysates are shown in the bottom panels. Scr, scrambled; p-Akt, phospho-Akt.
Initiation of translation occurs due to binding of the eIF4F complex composed of eIF4E, eIF4G, and eIF4A to the cap of the mRNA. Therefore, we tested the involvement of TORC2 in formation of the eIF4E·eIF4G complex. eIF4E immunoprecipitates from shRictor- or shmSin1-transfected cells using two independent shRNAs for each protein were immunoblotted with eIF4G. Down-regulation of rictor or mSin1 suppressed the formation of the eIF4E·eIF4G complex (Fig. 8, E–H). Reciprocal immunoprecipitation-immunoblotting of the same samples showed similar results (supplemental Fig. S7, E and H). Together these results provide evidence that TORC2 regulates the initiation phase of mRNA translation and support our observation of the increased Hif1α translation described above.
TORC2 Regulates Hif1α-dependent PTEN Expression
We have shown previously that expression of PTEN is regulated by Hif1α (41). Also, we have demonstrated that TORC2 regulates the expression of Hif1α protein (Fig. 6, F–I). We examined the hypothesis that TORC2-regulated Hif1α contributes to the expression of PTEN. 293 cells were transfected with either shRictor or shmSin1 plasmids along with the Hif1α expression vector. As expected, down-regulation of rictor or mSin1 and expression of Hif1α decreased and increased the levels of PTEN protein, respectively (Fig. 9, A–D). Phosphorylation of Akt at Ser-473 was inhibited due to inhibition of TORC2 and increased PTEN expression. Interestingly, expression of Hif1α prevented the shRictor- or shmSin1-induced inhibition of PTEN expression, which resulted in decreased Akt (Ser-473) phosphorylation (Fig. 9, A–D, compare lanes 4 with lanes 2). These results indicate that TORC2-mediated expression of Hif1α regulates PTEN levels.
FIGURE 9.
TORC2 regulates Hif1α-driven PTEN expression. A–D, 293 cells were transfected with HA-tagged Hif1α along with either shRictor 1 (A), shRictor 2 (B), mSin1 sh1 (C), or mSin1 sh2 (D). Forty-eighty hours post-transfection, the cells were lysed, and the lysates were immunoblotted with the indicated antibodies. E and F, TORC2 regulates Hif1α-dependent PTEN transcription. 293 cells were cotransfected with PTEN-Luc and HA-Hif1α along with shRictor 1 (E), shRictor 2 (F), mSin1 sh1 (G), or mSin1 sh2 (H). Forty-eight hours post-transfection, luciferase activity was determined in the cell lysates. Mean ± S.E. of six determinations is shown. In E, * represents p < 0.01 versus control, # represents p < 0.001 versus control, and ** represents p < 0.001 versus shRictor 1 alone. In F, * represents p < 0.001 versus control, # represents p < 0.001 versus control, and ** represents p < 0.001 versus shRictor 2 alone. In G, * represents p < 0.001 versus control, # represents p < 0.001 versus control, and ** represents p < 0.001 versus mSin1 sh1 alone. In H, * represents p < 0.01 versus control, # represents p < 0.001 versus control, and ** represents p < 0.001 versus mSin1 sh2 alone. Scr, scrambled; p-Akt, phospho-Akt.
Next, we tested the involvement of the TORC2-Hif1α axis in the transcription of PTEN. 293 cells were transiently transfected with PTEN-Luc reporter and shRictor or shmSin1 in the presence of the Hif1α expression vector. As expected, expression of shRictor or shmSin1 inhibited luciferase activity (Fig. 9, E–H). Overexpression of Hif1α prevented the shRictor- or shmSin1-induced inhibition of reporter activity (Fig. 9, E–H). These results conclusively demonstrate that TORC2 contributes to the increased expression of Hif1α to enhance PTEN expression.
DISCUSSION
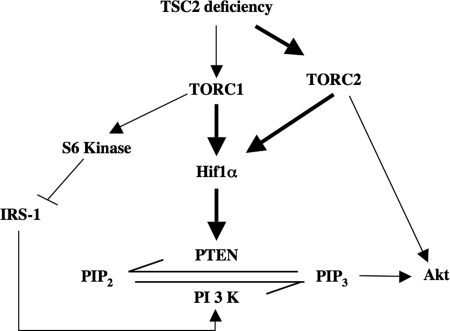
This study establishes an important contribution of mTOR activity to the up-regulation of PTEN in TSC2 deficiency (Fig. 10). Our results provide the first evidence that both TORC1 and TORC2 regulate the expression of PTEN to control Akt activation in a reciprocal manner. Finally, we demonstrate the involvement of TORC2 in regulating the Hif1α transcription factor, which results in the increased transcription of PTEN (Fig. 10).
FIGURE 10.
Schema summarizing our results. The solid arrows indicate the results described in our study. PIP2, phosphatidylinositol 4,5-bisphosphate.
Tumors from TSC patients display increased phosphorylation of S6 kinase and 4EBP-1, which is mimicked by cultured cells lacking TSC2, indicating a constitutive activation of TORC1 (25, 49, 53). Elevated TORC1 activity has been recognized as the principal driver of cancer cell proliferation in culture and in animal models (34, 54). Enhanced inactivating TSC2 phosphorylation has been detected in human cancers with elevated mTOR signaling (55). However, recent clinical trials using different mTOR inhibitors showed rather modest antitumor activity only in a minority of patients (56, 57). These disappointing results may be partially explained by the release of the feedback inhibition of the PI 3-kinase pathway similar to that observed in TSC2-null cells involving IRS-1. The increased TORC1-mediated S6 kinase activity reduces the levels of IRS-1, leading to inhibition of PI 3-kinase-dependent Akt phosphorylation (37, 53). Indeed, in TSC2-deficient cells, rapamycin blocks S6 kinase activity and relieves the negative regulation of IRS-1, leading to the restoration of Akt phosphorylation in response to insulin/IGF-1. A recent report demonstrated increased expression and hence tyrosine phosphorylation of multiple receptor tyrosine kinases including members of the human epidermal growth factor receptor family and insulin and IGF-1 receptors in response to chronic Akt inhibition (58). However, reduced Akt activity in TSC2 deficiency is not sufficient to increase insulin/IGF-1 signaling (37). TSC2 deficiency causes a loss of Akt phosphorylation in response to multiple growth factors such as EGF and PDGF, which do not use IRS-1 to signal (19, 39, 40, 59). These results indicate that multiple feedback mechanisms exist to regulate Akt phosphorylation.
Recently, we have reported that in TSC2 deficiency the level of PTEN is significantly elevated, and this prevents Akt phosphorylation (41). In the present study, we show that treatment of TSC2-null MEFs with rapamycin diminishes PTEN protein with a concomitant increase in Akt phosphorylation at Thr-308 (Fig. 1B). Phosphorylation at this site is mediated by PDK-1 downstream of PIP3 (12). This observation was also confirmed in growing human 293 cells (Fig. 1D). Together our results show that PTEN gene expression is downstream of TORC1. Note that the incubation of cells with rapamycin was for 24 h, which has been shown to down-regulate TORC2 activity in many cancer cells (29). However, under this incubation condition, rapamycin acted as a classical TORC1 inhibitor, blocking the phosphorylation of S6 kinase in TSC2−/− MEFs and in 293 cells (Fig. 1, B and D). Moreover, rapamycin increased the hydrophobic site phosphorylation of Akt at Ser-473 concomitantly with PTEN down-regulation (Fig. 1, B and D). It has been shown recently that PIP3 regulates the activation of TORC2 (60). Our data indicate that due to down-regulation of PTEN the resulting increase in PIP3 enhances the TORC2 activity to augment the phosphorylation of Akt at Ser-473. Furthermore, rapamycin does not inhibit TORC2 activity.
The results obtained with rapamycin were further strengthened with the use of a kinase-dead mutant of mTOR that mimicked the action of rapamycin on the expression of PTEN and on TORC1 activity in TSC2-deficient MEFs and in 293 cells (Fig. 1, E–H). Because mTOR is the common kinase subunit for both TORC1 and TORC2, kinase-dead mTOR is expected to block the activity of both these kinase complexes. Indeed, we observed inhibition of TORC1 and TORC2 activity in both TSC2−/− MEFs and in 293 cells expressing dominant negative mTOR (Fig. 1, F and H). The phosphorylation of Akt at Thr-308 by PDK-1 is under the direct regulation of PTEN (12). However, this phosphorylation was reported to be regulated by phosphorylation of Ser-473, and the loss of components of TORC2 also diminished the phosphorylation of both these sites in Akt (35, 61, 62). In contrast to these results, we demonstrate increased phosphorylation of Thr-308 in the presence of reduced phosphorylation of Akt at Ser-473 (Fig. 1, F and H). These results support the idea that down-regulation of PTEN by dominant negative mTOR increases the phosphorylation of Akt at Thr-308. Furthermore, inhibition of TORC1 also relieves the negative feed back loop, resulting in increased phosphorylation of Akt at Thr-308.
In contrast to our data obtained with rapamycin and kinase-dead mTOR, as expected, we found increased expression of PTEN when we used a constitutively active mutant of mTOR (Fig. 2, A and B). This mutant increased phosphorylation of S6 kinase due to enhanced TORC1 activity, resulting in the inhibition of Akt phosphorylation (Fig. 2B). Unlike the kinase-dead mutant, constitutively active mTOR did not affect TORC2 activity as we observed no increase in Akt phosphorylation at Ser-473. Rather, the phosphorylation was inhibited in the presence of constitutively active mTOR (Fig. 2B), indicating that this mutant affects only TORC1 activity in cells. These results are consistent with the studies in which Ohne et al. (43) established that this constitutively active mutant exclusively up-regulated TORC1 activity in the absence of any effect on TORC2.
Thus, our data point to a specific involvement of TORC1 in the up-regulation of PTEN, which contributes to the decreased phosphorylation of Akt in TSC2 deficiency. This conclusion is further supported by our results with raptor down-regulation in TSC2-null MEFs and in 293 cells, which show reduced PTEN protein levels in association with increased phosphorylation of Akt at both Thr-308 and Ser-473 (Fig. 4, A–C). The increase in phosphorylation of both these sites may result from attenuation of PTEN, which increases PIP3 levels to increase PDK-1 and TORC2 activity (12, 60). Another possibility is that due to raptor down-regulation more mTOR kinase may be available for assembly into TORC2 in the absence of TORC1 formation. This will result in increased phosphorylation of Akt at Ser-473 by TORC2.
We described the effect of dominant negative mTOR kinase on the expression of PTEN in 293 cells (Fig. 1H). However, this mutant affects activity of both TORC1 and TORC2 as evident by the reduced phosphorylation of S6 kinase and Akt Ser-473, respectively (Fig. 1, F and H). Consequently, TORC2 along with TORC1 may be involved in the regulation of PTEN expression. Down-regulation of rictor or mSin1 to specifically block TORC2 activity indeed decreased the level of PTEN in 293 cells (Fig. 5, A–D). Interestingly, a decreased level of PTEN was not sufficient to increase the phosphorylation of Akt at Thr-308. This could be due to the fact that rictor or mSin1 down-regulation, which results in the loss of TORC2 formation, leaves more mTOR kinase available to form TORC1. We tested this hypothesis. mTOR immunoprecipitates from rictor- or mSin1-down-regulated 293 cells were immunoblotted with raptor antibody. Independent down-regulation of each of these TORC2 components increased the formation of mTOR·raptor complex, indicating enhanced TORC1 formation (supplemental Fig. S8, A–D). Consequently, down-regulation of rictor or mSin1 increased TORC1 activity as judged by phosphorylation of S6 kinase (supplemental Fig. S9, A–D). Increased TORC1 activity is known to diminish phosphorylation of Akt at Thr-308 due to the presence of a negative feed back loop involving IRS-1 (37, 38, 53). Therefore, this phenomenon may explain our observation of diminished phosphorylation of Akt at Thr-308 under the condition of reduced rictor or mSin1 expression (Fig. 5, A–D).
Our study shows that the effect of TORC1 and TORC2 on up-regulation of PTEN appears to be due to an increased level of its mRNA (Fig. 1, E and G). Multiple transcription factors have been reported to regulate the transcription of PTEN including NFκB, p53, and Egr1 (47, 63, 64). We identified a Hif1α recognition element in the PTEN promoter and showed that this transcription factor regulates the transcription of PTEN in TSC2-null MEFs (41). Previously, it was shown that an increased Hif1α protein level in TSC2-null MEFs is due to the presence of constitutively active TORC1 and enhanced translation of 5′ TOP-containing Hif1α mRNA (49, 50). Now we show that both rapamycin and dominant negative mTOR as well as down-regulation of raptor block the PTEN promoter activity in both TSC2−/− MEFs and in 293 cells (Figs. 3 and 4). These results raise the possibility that TORC1-sensitive Hif1α regulates the expression of PTEN. In fact, treatment of the TSC2-null MEFs with rapamycin and siRNA-mediated down-regulation of raptor decreased Hif1α protein levels (Fig. 6A and supplemental Fig. S6), suggesting a role of TORC1 in PTEN expression (41). Furthermore, in the present study, we demonstrate a contribution of TORC2 to the expression of Hif1α in TSC2-deficient MEFs and in 293 cells (Fig. 6, D–I). We provide evidence that TORC2 regulates the TOP-mediated translation of Hif1α due to its effect on mRNA translation initiation (Fig. 6, K–M). Indeed, TORC2 increased the phosphorylation of 4EBP-1 and the formation of the eIF4E·eIF4G complex (Figs. 7 and 8). Moreover, we present direct evidence that TORC2-mediated up-regulation of Hif1α increases the protein levels of PTEN due to enhanced transcription (Fig. 9). These data also suggest that TORC1 and TORC2 share a common regulatory pathway to control PTEN expression.
Our results demonstrate a reciprocal relationship between TORC1 and TORC2 in regulating the phosphorylation of Akt as evident by the down-regulation of raptor and rictor (Figs. 4, A–C, and 5, A–D). Furthermore, our data show that increased expression of PTEN in TSC2-deficient cells may contribute to a reduced malignant potential of TSC tumors in a cell-autonomous manner. TSC2 phosphorylation and inactivation are evident due to increased growth factor signaling in many cancers. Our results showing rapamycin-mediated down-regulation of PTEN leading to an increase in Akt phosphorylation represent a novel mechanism that may explain why different TORC1 inhibitors are not very effective in cancer clinical trials (56, 57). Finally, in patients with TSC, the efficacy of TORC1 inhibitors may depend upon the PTEN status of their tumors.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 DK50190 (to G. G. C.), RO1 AR 52425 (to N. G. C.). This work was also supported by Veterans Affairs Merit Review grant (to G. G. C.).

This article contains supplemental Figs. S1–S9.
- PTEN
- phosphatase and tensin homolog deleted on chromosome 10
- TSC
- tuberous sclerosis complex
- mTOR
- mammalian target of rapamycin
- TORC
- TOR complex
- MEF
- murine embryonic fibroblast
- PI
- phosphatidylinositol
- PIP3
- phosphatidylinositol 3,4,5-trisphosphate
- Rheb
- Ras homolog enriched in brain
- raptor
- rapamycin-sensitive adaptor protein of mTOR
- rictor
- rapamycin-insensitive companion of mTOR
- 4EBP
- eukaryotic initiation factor 4E-binding protein
- KD
- kinase-dead
- CA
- constitutively active
- TOP
- terminal oligopyrimidine tract.
REFERENCES
- 1. Stiles B. L. (2009) Phosphatase and tensin homologue deleted on chromosome 10: extending its PTENtacles. Int. J. Biochem. Cell Biol. 41, 757–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salmena L., Carracedo A., Pandolfi P. P. (2008) Tenets of PTEN tumor suppression. Cell 133, 403–414 [DOI] [PubMed] [Google Scholar]
- 3. Eng C. (2003) PTEN: one gene, many syndromes. Hum. Mutat. 22, 183–198 [DOI] [PubMed] [Google Scholar]
- 4. Saal L. H., Gruvberger-Saal S. K., Persson C., Lövgren K., Jumppanen M., Staaf J., Jönsson G., Pires M. M., Maurer M., Holm K., Koujak S., Subramaniyam S., Vallon-Christersson J., Olsson H., Su T., Memeo L., Ludwig T., Ethier S. P., Krogh M., Szabolcs M., Murty V. V., Isola J., Hibshoosh H., Parsons R., Borg A. (2008) Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat. Genet. 40, 102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wymann M. P., Marone R. (2005) Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr. Opin. Cell Biol. 17, 141–149 [DOI] [PubMed] [Google Scholar]
- 6. Samuels Y., Wang Z., Bardelli A., Silliman N., Ptak J., Szabo S., Yan H., Gazdar A., Powell S. M., Riggins G. J., Willson J. K., Markowitz S., Kinzler K. W., Vogelstein B., Velculescu V. E. (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554. [DOI] [PubMed] [Google Scholar]
- 7. Manning B. D., Cantley L. C. (2007) AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R., Bigner S. H., Giovanella B. C., Ittmann M., Tycko B., Hibshoosh H., Wigler M. H., Parsons R. (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 [DOI] [PubMed] [Google Scholar]
- 9. Tamura M., Gu J., Matsumoto K., Aota S., Parsons R., Yamada K. M. (1998) Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614–1617 [DOI] [PubMed] [Google Scholar]
- 10. Mahimainathan L., Choudhury G. G. (2004) Inactivation of platelet-derived growth factor receptor by the tumor suppressor PTEN provides a novel mechanism of action of the phosphatase. J. Biol. Chem. 279, 15258–15268 [DOI] [PubMed] [Google Scholar]
- 11. Maehama T., Dixon J. E. (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 [DOI] [PubMed] [Google Scholar]
- 12. Cully M., You H., Levine A. J., Mak T. W. (2006) Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer 6, 184–192 [DOI] [PubMed] [Google Scholar]
- 13. Ji S. P., Zhang Y., Van Cleemput J., Jiang W., Liao M., Li L., Wan Q., Backstrom J. R., Zhang X. (2006) Disruption of PTEN coupling with 5-HT2C receptors suppresses behavioral responses induced by drugs of abuse. Nat. Med. 12, 324–329 [DOI] [PubMed] [Google Scholar]
- 14. Laplante M., Sabatini D. M. (2009) mTOR signaling at a glance. J. Cell Sci. 122, 3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheadle J. P., Reeve M. P., Sampson J. R., Kwiatkowski D. J. (2000) Molecular genetic advances in tuberous sclerosis. Hum. Genet. 107, 97–114 [DOI] [PubMed] [Google Scholar]
- 16. Crino P. B., Nathanson K. L., Henske E. P. (2006) The tuberous sclerosis complex. N. Engl. J. Med. 355, 1345–1356 [DOI] [PubMed] [Google Scholar]
- 17. Dabora S. L., Jozwiak S., Franz D. N., Roberts P. S., Nieto A., Chung J., Choy Y. S., Reeve M. P., Thiele E., Egelhoff J. C., Kasprzyk-Obara J., Domanska-Pakiela D., Kwiatkowski D. J. (2001) Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am. J. Hum. Genet. 68, 64–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kwiatkowski D. J., Manning B. D. (2005) Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum. Mol. Genet. 14, R251–R258 [DOI] [PubMed] [Google Scholar]
- 19. Huang J., Dibble C. C., Matsuzaki M., Manning B. D. (2008) The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol. Cell. Biol. 28, 4104–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Y., Corradetti M. N., Inoki K., Guan K. L. (2004) TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem. Sci. 29, 32–38 [DOI] [PubMed] [Google Scholar]
- 21. Inoki K., Li Y., Zhu T., Wu J., Guan K. L. (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 [DOI] [PubMed] [Google Scholar]
- 22. Ma L., Teruya-Feldstein J., Bonner P., Bernardi R., Franz D. N., Witte D., Cordon-Cardo C., Pandolfi P. P. (2007) Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res. 67, 7106–7112 [DOI] [PubMed] [Google Scholar]
- 23. Roux P. P., Ballif B. A., Anjum R., Gygi S. P., Blenis J. (2004) Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. U.S.A. 101, 13489–13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma L., Chen Z., Erdjument-Bromage H., Tempst P., Pandolfi P. P. (2005) Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121, 179–193 [DOI] [PubMed] [Google Scholar]
- 25. El-Hashemite N., Zhang H., Henske E. P., Kwiatkowski D. J. (2003) Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet 361, 1348–1349 [DOI] [PubMed] [Google Scholar]
- 26. Goncharova E. A., Goncharov D. A., Eszterhas A., Hunter D. S., Glassberg M. K., Yeung R. S., Walker C. L., Noonan D., Kwiatkowski D. J., Chou M. M., Panettieri R. A., Jr., Krymskaya V. P. (2002) Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). J. Biol. Chem. 277, 30958–30967 [DOI] [PubMed] [Google Scholar]
- 27. Manning B. D. (2004) Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J. Cell Biol. 167, 399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L., Bonenfant D., Oppliger W., Jenoe P., Hall M. N. (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457–468 [DOI] [PubMed] [Google Scholar]
- 29. Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 [DOI] [PubMed] [Google Scholar]
- 30. Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 [DOI] [PubMed] [Google Scholar]
- 31. Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 [DOI] [PubMed] [Google Scholar]
- 32. Zoncu R., Efeyan A., Sabatini D. M. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frias M. A., Thoreen C. C., Jaffe J. D., Schroder W., Sculley T., Carr S. A., Sabatini D. M. (2006) mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 16, 1865–1870 [DOI] [PubMed] [Google Scholar]
- 34. Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 35. Guertin D. A., Stevens D. M., Thoreen C. C., Burds A. A., Kalaany N. Y., Moffat J., Brown M., Fitzgerald K. J., Sabatini D. M. (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev. Cell 11, 859–871 [DOI] [PubMed] [Google Scholar]
- 36. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 37. Harrington L. S., Findlay G. M., Gray A., Tolkacheva T., Wigfield S., Rebholz H., Barnett J., Leslie N. R., Cheng S., Shepherd P. R., Gout I., Downes C. P., Lamb R. F. (2004) The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J. Cell Biol. 166, 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shah O. J., Wang Z., Hunter T. (2004) Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 14, 1650–1656 [DOI] [PubMed] [Google Scholar]
- 39. Zhang H., Bajraszewski N., Wu E., Wang H., Moseman A. P., Dabora S. L., Griffin J. D., Kwiatkowski D. J. (2007) PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J. Clin. Investig. 117, 730–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang H., Cicchetti G., Onda H., Koon H. B., Asrican K., Bajraszewski N., Vazquez F., Carpenter C. L., Kwiatkowski D. J. (2003) Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J. Clin. Investig. 112, 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahimainathan L., Ghosh-Choudhury N., Venkatesan B., Das F., Mandal C. C., Dey N., Habib S. L., Kasinath B. S., Abboud H. E., Ghosh Choudhury G. (2009) TSC2 deficiency increases PTEN via HIF1α. J. Biol. Chem. 284, 27790–27798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thomas G. V., Tran C., Mellinghoff I. K., Welsbie D. S., Chan E., Fueger B., Czernin J., Sawyers C. L. (2006) Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat. Med. 12, 122–127 [DOI] [PubMed] [Google Scholar]
- 43. Ohne Y., Takahara T., Hatakeyama R., Matsuzaki T., Noda M., Mizushima N., Maeda T. (2008) Isolation of hyperactive mutants of mammalian target of rapamycin. J. Biol. Chem. 283, 31861–31870 [DOI] [PubMed] [Google Scholar]
- 44. Choudhury G. G. (2004) A linear signal transduction pathway involving phosphatidylinositol 3-kinase, protein kinase Cϵ, and MAPK in mesangial cells regulates interferon-γ-induced STAT1α transcriptional activation. J. Biol. Chem. 279, 27399–27409 [DOI] [PubMed] [Google Scholar]
- 45. Das F., Ghosh-Choudhury N., Mahimainathan L., Venkatesan B., Feliers D., Riley D. J., Kasinath B. S., Choudhury G. G. (2008) Raptor-rictor axis in TGFβ-induced protein synthesis. Cell. Signal. 20, 409–423 [DOI] [PubMed] [Google Scholar]
- 46. Shah O. J., Hunter T. (2006) Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol. Cell. Biol. 26, 6425–6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Virolle T., Adamson E. D., Baron V., Birle D., Mercola D., Mustelin T., de Belle I. (2001) The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat. Cell Biol. 3, 1124–1128 [DOI] [PubMed] [Google Scholar]
- 48. Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 [DOI] [PubMed] [Google Scholar]
- 49. Brugarolas J. B., Vazquez F., Reddy A., Sellers W. R., Kaelin W. G., Jr. (2003) TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4, 147–158 [DOI] [PubMed] [Google Scholar]
- 50. Düvel K., Yecies J. L., Menon S., Raman P., Lipovsky A. I., Souza A. L., Triantafellow E., Ma Q., Gorski R., Cleaver S., Vander Heiden M. G., MacKeigan J. P., Finan P. M., Clish C. B., Murphy L. O., Manning B. D. (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Laughner E., Taghavi P., Chiles K., Mahon P. C., Semenza G. L. (2001) HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol. 21, 3995–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kasinath B. S., Feliers D., Sataranatarajan K., Ghosh Choudhury G., Lee M. J., Mariappan M. M. (2009) Regulation of mRNA translation in renal physiology and disease. Am. J. Physiol. Renal Physiol. 297, F1153–F1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shah O. J., Hunter T. (2005) Tuberous sclerosis and insulin resistance. Unlikely bedfellows reveal a TORrid affair. Cell Cycle 4, 46–51 [DOI] [PubMed] [Google Scholar]
- 54. Bhaskar P. T., Hay N. (2007) The two TORCs and Akt. Dev. Cell 12, 487–502 [DOI] [PubMed] [Google Scholar]
- 55. Riemenschneider M. J., Betensky R. A., Pasedag S. M., Louis D. N. (2006) AKT activation in human glioblastomas enhances proliferation via TSC2 and S6 kinase signaling. Cancer Res. 66, 5618–5623 [DOI] [PubMed] [Google Scholar]
- 56. Cloughesy T. F., Yoshimoto K., Nghiemphu P., Brown K., Dang J., Zhu S., Hsueh T., Chen Y., Wang W., Youngkin D., Liau L., Martin N., Becker D., Bergsneider M., Lai A., Green R., Oglesby T., Koleto M., Trent J., Horvath S., Mischel P. S., Mellinghoff I. K., Sawyers C. L. (2008) Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 5, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lamborn K. R., Yung W. K., Chang S. M., Wen P. Y., Cloughesy T. F., DeAngelis L. M., Robins H. I., Lieberman F. S., Fine H. A., Fink K. L., Junck L., Abrey L., Gilbert M. R., Mehta M., Kuhn J. G., Aldape K. D., Hibberts J., Peterson P. M., Prados M. D. (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro. Oncol. 10, 162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chandarlapaty S., Sawai A., Scaltriti M., Rodrik-Outmezguine V., Grbovic-Huezo O., Serra V., Majumder P. K., Baselga J., Rosen N. (2011) AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19, 58–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Manning B. D., Logsdon M. N., Lipovsky A. I., Abbott D., Kwiatkowski D. J., Cantley L. C. (2005) Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 19, 1773–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zinzalla V., Stracka D., Oppliger W., Hall M. N. (2011) Activation of mTORC2 by association with the ribosome. Cell 144, 757–768 [DOI] [PubMed] [Google Scholar]
- 61. Scheid M. P., Woodgett J. R. (2003) Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 546, 108–112 [DOI] [PubMed] [Google Scholar]
- 62. Yang J., Cron P., Thompson V., Good V. M., Hess D., Hemmings B. A., Barford D. (2002) Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol. Cell 9, 1227–1240 [DOI] [PubMed] [Google Scholar]
- 63. Stambolic V., MacPherson D., Sas D., Lin Y., Snow B., Jang Y., Benchimol S., Mak T. W. (2001) Regulation of PTEN transcription by p53. Mol. Cell 8, 317–325 [DOI] [PubMed] [Google Scholar]
- 64. Chandrasekar B., Valente A. J., Freeman G. L., Mahimainathan L., Mummidi S. (2006) Interleukin-18 induces human cardiac endothelial cell death via a novel signaling pathway involving NF-κB-dependent PTEN activation. Biochem. Biophys. Res. Commun. 339, 956–963 [DOI] [PubMed] [Google Scholar]
- 65. Ghosh-Choudhury T., Mandal C. C., Woodruff K., St Clair P., Fernandes G., Choudhury G. G., Ghosh-Choudhury N. (2009) Fish oil targets PTEN to regulate NFκB for downregulation of anti-apoptotic genes in breast tumor growth. Breast Cancer Res. Treat. 118, 213–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.