Background: VLDLR and apoER2 are receptors for reelin and apoE.
Results: Reelin or apoE3 induced macrophage ABCA1 expression and increased cholesterol efflux. Down-regulation of VLDLR, apoER2, or inhibition of Dab1, PI3K, PKCζ and Sp1 attenuated reelin- or apoE3-induced ABCA1 expression.
Conclusion: Activation of VLDLR- and apoER2-mediated signaling up-regulates ABCA1 expression.
Significance: Up-regulation of ABCA1 expression is a novel function of VLDLR and apoER2.
Keywords: ABC Transporter, ApoE, Cholesterol, Macrophages, Signal Transduction, ApoE Receptor2, VLDL Receptor, Reelin
Abstract
Activation of very low density lipoprotein receptor (VLDLR) and apolipoprotein E receptor 2 (apoER2) results in either pro- or anti-atherogenic effects depending on the ligand. Using reelin and apoE as ligands, we studied the impact of VLDLR- and apoER2-mediated signaling on the expression of ATP binding cassette transporter A1 (ABCA1) and cholesterol efflux using RAW264.7 cells. Treatment of these mouse macrophages with reelin or human apoE3 significantly increased ABCA1 mRNA and protein levels, and apoAI-mediated cholesterol efflux. In addition, both reelin and apoE3 significantly increased phosphorylated disabled-1 (Dab1), phosphatidylinositol 3-kinase (PI3K), protein kinase Cζ (PKCζ), and specificity protein 1 (Sp1). This reelin- or apoER2-mediated up-regulation of ABCA1 expression was suppressed by 1) knockdown of Dab1, VLDLR, and apoER2 with small interfering RNAs (siRNAs), 2) inhibition of PI3K and PKC with kinase inhibitors, 3) overexpression of kinase-dead PKCζ, and 4) inhibition of Sp1 DNA binding with mithramycin A. Activation of the Dab1-PI3K signaling pathway has been implicated in VLDLR- and apoER2-mediated cellular functions, whereas the PI3K-PKCζ-Sp1 signaling cascade has been implicated in the regulation of ABCA1 expression induced by apoE/apoB-carrying lipoproteins. Taken together, these data support a model in which activation of VLDLR and apoER2 by reelin and apoE induces ABCA1 expression and cholesterol efflux via a Dab1-PI3K-PKCζ-Sp1 signaling cascade.
Introduction
ATP binding cassette transporter A1 (ABCA1)2 is a plasma membrane protein that exports excess cholesterol derived from internalized lipoproteins (1–4). Based on findings in animal models and humans, it has been suggested that a deficiency in ABCA1 could result in cellular cholesterol accumulation, thus promoting abnormalities such as atherosclerosis (1). Our previous studies demonstrated that lipoproteins carrying apolipoprotein E (apoE) and apoB increased ABCA1 mRNA and protein levels in mouse macrophages (5), whereas apoE-free and apoB-carrying lipoproteins showed a decreased ability to induce ABCA1 expression (6). These findings suggested a regulatory role of apoE in ABCA1 expression. In addition, we demonstrated that sequential activation of phosphatidylinositol 3-kinase (PI3K), protein kinase Cζ (PKCζ), and specificity protein 1 (Sp1) is at least partially responsible for the increased ABCA1 expression induced by apoE/apoB-carrying lipoproteins (5).
It is known that apoE is able to bind several members of the low density lipoprotein receptor (LDLR) family, including LDLR, very low density lipoprotein receptor (VLDLR), LDLR-related protein-1 (LRP1), LRP2, and apoE receptor 2 (apoER2) (7). Although the role of the LDLR is limited to the regulation of cholesterol homeostasis by receptor-mediated endocytosis of lipoprotein particles, other members of this gene family have additional physiological functions (7). For example, activation of VLDLR or apoER2 has been shown to activate disabled-1 (Dab1) and PI3K (8). Thus far, this VLDLR/apoER2-Dab1-PI3K signaling pathway has been implicated in neural development and cellular uptake of lipoproteins in tissues that are active in fatty acid metabolism, such as muscle and adipose tissues (8).
Besides binding apoE, both VLDLR and apoER2 can also interact with reelin, a secreted extracellular matrix glycoprotein (9). It has been well established that interaction of reelin with VLDLR and apoER2 in the central nervous system activates a signaling transduction path that controls neuronal migration and positioning in the embryonic brain and neuronal survival and degeneration in the mature brain (9). It has been suggested that defects in this pathway impair brain development and contribute to the pathogenesis of Alzheimer disease (9). Further studies have demonstrated that some extraneuronal tissues also express reelin (10), suggesting a biological role(s) of reelin beyond neuronal development.
In this study we report that treatment of macrophages with reelin or apoE induced ABCA1 expression, enhanced Dab1, PI3K, PKCζ, and Sp1 phosphorylation, and increased cholesterol efflux. Down-regulation of VLDLR or apoER2 or inhibition of Dab1, PI3K, PKCζ, and Sp1 activities attenuated reelin- and apoE-induced ABCA1 expression. These data suggest that stimulation of VLDLR and apoER2 by reelin or apoE enhances cholesterol efflux and up-regulates ABCA1 expression via activation of the Dab1-PI3K-PKCζ-Sp1 signaling cascade. They also demonstrate a novel function of VLDLR and apoER2 beyond that of neural development and lipoprotein uptake.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), TRIzol reagent, Lipofectamine 2000, and penicillin/streptomycin as well as the primers for amplification of ABCA1, ABCG1, scavenger receptor B1 (SR-B1), β-actin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Invitrogen. Phosphorylated PI3K antibody, PI3K inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002), and PKC inhibitor bisindolylmaleimide I hydrochloride (109203X) were purchased from Cell Signaling Technology (Boston, MA). A High Capacity cDNA Reverse Transcription kit was purchased from Applied Biosystems (Carlsbad, CA). Rat PKCζ expression plasmid vector (FLAG.PKCζ) was purchased from Addgene Inc (Cambridge, MA). M-PER mammalian protein extraction reagent and a BCA protein assay kit were purchased from Pierce. Recombinant mouse reelin was purchased from R&D System (Minneapolis, MN) and a GenMute siRNA and DNA transfection reagent was purchased from SignaGen Laboratories (Ijamsville, MD). The negative control siRNA (sc-37007) and the siRNAs specific for Dab1 (sc-35166), apoER2 (sc-40098) and VLDLR (sc-36823) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The sequence information for these siRNAs is protected by the vender. Antibodies against phosphorylated PKCζ (p-PKCζ), Sp1, ABCA1 (H-220), Dab1, phosphorylated Dab1 (p-Dab1), and β-actin as well as protein A/G plus agarose, anti-rabbit serum, and horseradish peroxidase-conjugated secondary antibodies were also obtained from Santa Cruz. Antibodies against VLDLR and apoER2 were purchased from Abcam (Cambridge, MA), whereas [1,2-3H(N)]cholesterol was obtained from PerkinElmer Life Sciences. Mithramycin A was purchased from Sigma. Cell culture dishes and plates were purchased from Corning Inc. (Corning, NY). Protease inhibitor mixture was purchased from Roche Applied Science. A CellTiter-Glo assay kit for cellular ATP measurement was purchased from Promega (Madison, WI).
Isolation of Mouse ApoB-carrying Lipoproteins
All animal procedures were approved by the Institutional Animal Care and Use Committee at Meharry Medical College. Wild-type C57BL/6 mice at 3–4 months of age were obtained from The Jackson Laboratory (Bar Harbor, ME) and used for collection of blood samples. To minimize oxidation, collected blood was immediately mixed with 50 μm butylated hydroxytoluene, 2 mm EDTA, and cooled on ice. Mouse plasma was overlaid with a potassium bromide (KBr) gradient solution (d, 1.063) and centrifuged at 120,000 rpm for 2 h. ApoB-carrying lipoproteins were collected, dialyzed in phosphate-buffered saline (PBS, pH 7.4) containing 10 mm EDTA for 48 h at 4 °C, and filtered through a 0.45-μm filter (11).
Cell Culture
RAW 264.7 cells were obtained from ATCC (Manassas, VA). The cells were maintained in DMEM containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C under 5% CO2 in 100-mm2 culture dishes, and split on reaching confluence.
Cholesterol Efflux Assay
RAW264.7 cells were grown to confluence in 24-well plates and then incubated for 24 h in 400 μl of DMEM containing 5 μCi/ml [1,2-3H(N)]cholesterol (∼450,000 dpm/μCi) in the presence or absence of 20 μg/ml mouse apoB-carrying lipoproteins (12). After washing with PBS, the cells were further incubated with 2 μg/ml reelin or 3 μg/ml apoE3 in the presence or absence of 20 μg/ml human apoAI or culture medium. After a 2-h incubation, the culture medium was collected, and cells were lysed with 0.5 m NaOH. The lysate and medium were mixed with scintillation fluid to assay radioactivity using a Tri-Carb 2300TR Liquid Scintillation Analyzer (PerkinElmer Life Sciences). Cholesterol efflux was expressed as the percentage of radioactivity in the medium compared with the total radioactivity (cells plus medium).
Quantitative Real-time RT-PCR Assay
RAW264.7 cells were grown to confluence in six-well plates made quiescent by incubation in serum-free DMEM for 12 h and then treated with 2 μg/ml reelin, 3 μg/ml apoE3, or culture medium alone as a control for the time periods indicated in figure legends. In experiments involving protein kinase inhibitors and mithramycin A, macrophages were incubated with 100 nm mithramycin A, 50 μm LY294002, or 8 μm 109203X for 30 min before reelin or apoE treatment. Total RNA was extracted using TRIzol reagent and subjected to reverse transcription using a High Capacity cDNA reverse transcription kit. The resulting cDNAs were subjected to quantitative real-time PCR with an iCycler system (Bio-Rad). The following specific primers were used for amplification: ABCA1 forward (5′-GCTACCCACCCTACGAACAA-3′) and reverse (5′-GGAGTTGGATAACGGAAGCA-3′), ABCG1 forward (5′-GAAGTGGCATCAGGGGAGTA-3′) and reverse (5′-AAAGAAACGGGTTCACATCG-3′), SR-B1 forward (5′-GGGCTCGATATTGATGGAGA-3′) and reverse (5′-GGAAGCATGTCTGGGAGGTA-3′), β-actin forward (5′-GCTACAGCTTCACCACCACA-3′) and reverse (5′-TCCAGGGAGGAAGAGGATGC-3′), and APDH forward (5′-GAGCCAAAAGGGTCATCATC-3′) and reverse (5′-TAAGCAGTTGGTGGTGCAGG-3′). The expression levels of the target mRNAs were normalized to β-actin or GAPDH mRNA (13, 14).
Western Blot Analysis
Quiescent RAW264.7 cells in serum-free DMEM were treated with 2 μg/ml reelin, 3 μg/ml apoE3, or medium (control) for the time periods as indicated in the legends of Figs. 1, 2, 4, and 5 and lysed in M-PER mammalian protein extraction reagent. Samples containing 40 μg of protein were resolved on 6% (for separation of phosphorylated and non-phosphorylated Sp1) or 10% SDS-PAGE gels (for separation of the other proteins). Proteins were transferred to a polyvinyl fluoride membrane (Millipore). After blocking with 5% fat-free milk, the membranes were incubated with antibodies (13). Immunoreactive bands were visualized using ECL-plus chemiluminescence reagent (GE Healthcare) and analyzed with a GS-700 Imaging Densitometer (Bio-Rad).
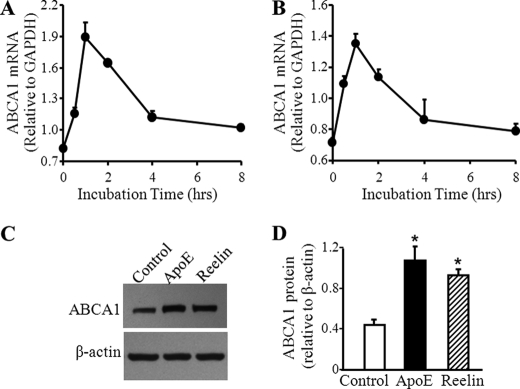
FIGURE 1.
Reelin and apoE3 induce ABCA1 expression and cholesterol efflux. Mouse macrophages were incubated with 3 μg/ml apoE3 (A) or 2 μg/ml reelin (B) for the indicated time periods. The mRNA level of ABCA1 was determined by quantitative real-time RT-PCR and normalized to GAPDH mRNA. C and D, macrophages were incubated with 3 μg/ml apoE3, 2 μg/ml reelin, or culture medium alone (control) for 1 h. The level of ABCA1 protein was determined by Western blot analysis and quantitated relative to β-actin. Values represent the mean ± S.E. of six independent experiments. *, p < 0.05 compared with controls.
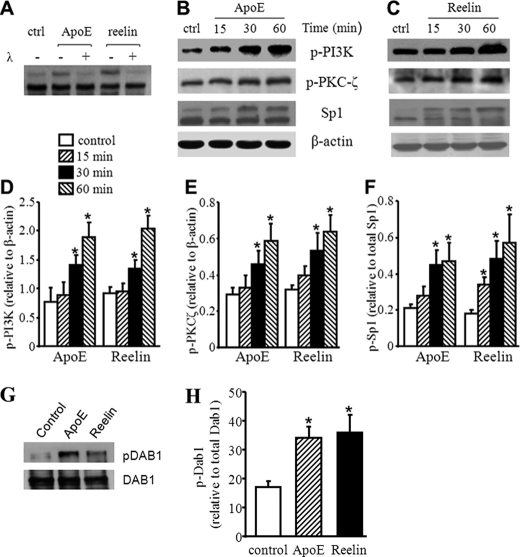
FIGURE 2.
Reelin and apoE3 increase Dab1, PI3K, PKCζ, and Sp1 phosphorylation. A, mouse macrophages were treated with 3 μg/ml apoE3, 2 μg/ml reelin, or culture medium alone as a control (ctrl) for 30 min. The lysates were then incubated with or without λ-phosphatase (λ). The Sp1 protein level was determined by Western blot analysis. B–F, mouse macrophages were treated with 3 μg/ml apoE3, 2 μg/ml reelin, or culture medium alone as a control for the indicated time periods. The levels of Sp1 and phosphorylated PI3K (p-PI3K) and PKCζ (p-PKCζ) were determined by Western blot analysis (B and C) and quantitated relative to β-actin (D and E). The level of phosphorylated Sp1 (top band for Sp1 in B and C) was quantitated relative to the level of total Sp1 (the sum of the top and bottom bands) and are shown in F. G and H, macrophages were treated with 3 μg/ml apoE3, 2 μg/ml reelin, or culture medium alone (control) for 1 h. Dab1 was immunoprecipitated. G, Western blot analysis was performed to detect the protein level of total Dab1 and phosphorylated Dab1 (pDab1) in the precipitant. H, the level of p-Dab1 was quantitated relative to the level of Dab1. *, p < 0.05 compared with controls.
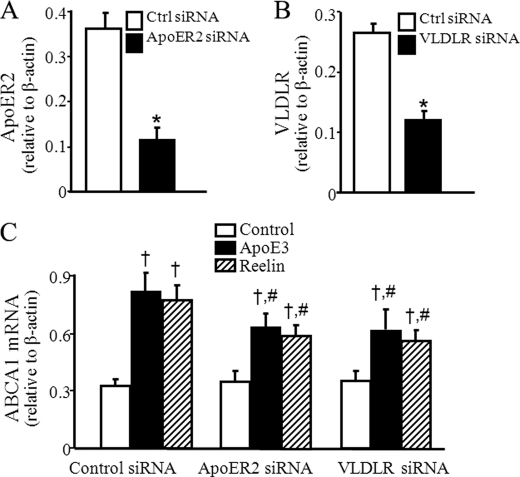
FIGURE 4.
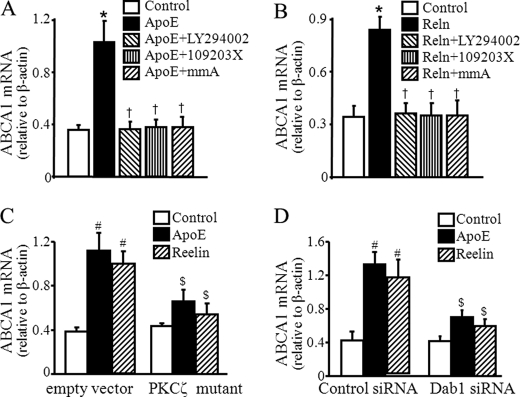
Knockdown of VLDLR and apoER2 reduces apoE3- and reelin-induced ABCA1 mRNA expression. Mouse macrophages were transfected with siRNA against VLDLR or apoER2 or control (Ctrl) siRNA. A and B, protein levels of apoER2 and VLDLR were determined by Western blot analysis and normalized to β-actin. C, transfected cells were incubated with 2 μg/ml reelin, 3 μg/ml apoE3, or culture medium alone for 1 h. ABCA1 mRNA levels were determined by quantitative real-time RT-PCR and normalized to β-actin mRNA. Values represent the mean ± S.E. of four independent experiments. *, p < 0.05 compared with cells transfected with control siRNA; †, p < 0.05 compared with cells transfected with same siRNA and untreated with apoE3 or reelin (control), and #, p < 0.05 compared with cells transfected with control siRNA and treated with apoE3 or reelin.
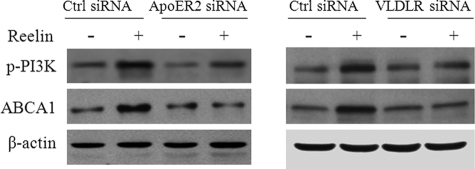
FIGURE 5.
Knockdown of VLDLR and apoER2 reduces reelin-induced PI3K phosphorylation and ABCA1 protein expression. A and B, mouse macrophages were transfected with siRNA against apoER2 or VLDLR or control (Ctrl) siRNA. The transfected cells were then incubated with 2 μg/ml reelin or culture medium alone for 1 h. The level of phosphorylated PI3K (p-PI3K) and ABCA1 protein was determined by Western blot analysis and quantitated relative to β-actin.
Immunoprecipitation of Dab1
RAW264.7 cells with the abovementioned reelin or apoE3 treatment were washed with ice-cold PBS containing 10 mm NaF, 25 mm β-glycerophosphate, and 2 mm sodium orthovanadate, centrifuged at 900 × g for 5 min at 4 °C, and resuspended in immunoprecipitation buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 1 mm MgCl2, 1% Nonidet P-40, supplemented with protease and phosphatase inhibitor cocktails). Cells were homogenized by forcing them through a 27-gauge needle. The lysates were cleaned by centrifugation at 20,000 × g and 4 °C for 30 min. One mg of protein extract was mixed with 4 μg of Dab1 antibody or 10 μl of antiserum in a total volume of 1 ml and incubated at 4 °C for 1 h. Immune complexes were precipitated with 80 μl of protein A/G plus agarose slurry. Pelleted beads were washed once with immunoprecipitation buffer, then three times with washing buffer (immunoprecipitation buffer minus Nonidet P-40) and resuspended in 50 μl of Western loading buffer. The agarose beads were discarded by centrifugation at 10,000 × g for 1 min. The immunoprecipitants were resolved on 10% SDS-PAGE gels for detection of Dab1 and p-Dab1.
Recombinant Plasmid Construction
For determination of the role of PKCζ in ABCA1 expression induced by reelin and apoE, a PKCζ kinase dead expression vector was generated by site-directed mutagenesis using the FLAG.PKCζ plasmid as a template and the oligonucleotide cagatttacgccatgtgggtggtgaagaaggag to substitute PKCζ lysine 281 with tryptophan (5, 15). The recombinant construct or an empty pEGFP vector (control) was then transfected into RAW 264.7 cells by Lipofectamine 2000 following the manufacturer's instructions. The transfected cells were treated with 2 μg/ml reelin or 3 μg/ml apoE3 for 1 h and harvested for measurement of protein and mRNA.
SiRNA Knockdown of Dab1, VLDLR, and ApoER2
RAW264.7 cells were grown in 6-well plates to ∼60–70% confluency and then transfected with control or specific siRNA against Dab1, VLDLR, or apoER2 using the GenMute siRNA and DNA transfection reagent according to the manufacturer's instructions. After 6 h, cells were replenished with fresh medium containing 10% FBS and cultured for an additional 24 h. These transfection procedures were repeated one more time. The transfected cells were then treated with 2 μg/ml reelin or 3 μg/ml apoE3 for 1 h and harvested for measurement of protein and mRNA.
Measurement of Cellular ATP Levels
The cellular ATP levels were determined using a Promega CellTiter-Glo assay kit, as previously described (16). RAW246.7 cells were grown in a white-sided, clear-bottom 96-well plate to confluence and then incubated at 37 °C with 2 μg/ml reelin, 3 μg/ml apoE3, or 100 μl/well serum-free DMEM alone as a control. At the indicated times, 100 μl of kit reagent were added to each well. After a 10-min incubation, luminescence was quantified using a BL10000 Lumicount luminometer (PerkinElmer Life Sciences). The intensity of the luminescence signal generated in this assay is proportional to the cellular ATP content (see the manufacturer's instructions).
Statistical Analysis
Data are reported as the mean ± S.E. Differences between treatment and control groups were analyzed by analysis of variance followed by Tukey's post hoc test or Student's unpaired t test. Statistical significance was considered when p was less than 0.05. Statistix software (Statistix, Tallahassee, FL) was used for statistical analyses.
RESULTS
Reelin and ApoE3 Induce Macrophage ABCA1 Expression and Cholesterol Efflux
Reelin is able to bind VLDLR and apoER2, whereas apoE binds these two receptors as well as several other members of the LDL receptor family. Here we studied the impact of reelin and apoE on ABCA1 expression. Incubation of mouse macrophages with human apoE3 or reelin resulted in a time-related increase in ABCA1 mRNA expression that reached a peak value at 1 h and returned to basal levels within 4 h (Fig. 1, A and B). The increase in apoE3- and reelin-induced ABCA1 mRNA expression was dose-dependent when doses used were lower than 3 and 2 μg/ml, respectively. Higher doses (than 3 and 2 μg/ml, respectively) did not further increase ABCA1 mRNA expression (data not shown). ApoE3 and reelin also significantly increased the protein level of ABCA1 in mouse macrophages (Fig. 1, C and D).
ABCA1 reportedly transports cholesterol from the cell membrane to lipid-free apoAI (17). We, therefore, studied the effect of apoE3 and reelin on apoAI-mediated cholesterol efflux. As shown in Table 1, reelin did not significantly affect cholesterol efflux in the absence of apoAI. In contrast, apoE3 induced about a 30% increase in macrophage cholesterol efflux in cells without apoAI treatment (Table 1). This finding agrees with the notion that apoE is able to induce apoAI-independent cholesterol efflux, in which apoE functions as a cholesterol acceptor (18). The data in Table 1 also show that treatment of macrophages with 3 μg/ml apoE3 and 2 μg/ml reelin induced approximately a 5.2- and 3.7-fold increase in apoAI-mediated cholesterol efflux. Namely, apoAI-mediated cholesterol efflux was increased from about 0.39% to about 2.0% and 1.4% in cells treated with apoE3 and reelin, respectively. Taken together, these results suggest that both reelin and apoE can activate an ABCA1-mediated cholesterol efflux system that exports excess macrophage cholesterol.
TABLE 1.
Reelin and apoE3 increase apoAI-mediated cholesterol efflux
Mouse macrophages were preincubated for 24 h with [3H]cholesterol in the presence or absence of mouse apoB-carrying lipoproteins. After washing with PBS, 3 μg/ml apoE3 and 2 μg/ml reelin or culture medium alone (control) were added and incubated for an additional 2 h. The radioactivity (dpm) in the medium and the cell lysates was then measured.
| Treatments | Medium | Cell lysate | Cholesterol effluxa | ApoAI-mediated effluxb |
|---|---|---|---|---|
| dpm | dpm | % | % | |
| Without apoAI | ||||
| Control | 104 ± 9 | 38362 ± 4072 | 0.27 ± 0.04 | |
| ApoE3 | 138 ± 6 | 39427 ± 3610 | 0.35 ± 0.05 | |
| Reelin | 114 ± 16 | 39310 ± 4258 | 0.29 ± 0.04 | |
| With apoAI | ||||
| Control | 261 ± 17 | 39574 ± 5038 | 0.66 ± 0.06 | 0.39 ± 0.05 |
| ApoE3 | 937 ± 71c | 39886 ± 3077 | 2.35 ± 0.29c | 2.00 ± 0.24c |
| Reelin | 640 ± 103c | 37854 ± 4908 | 1.69 ± 0.22c | 1.40 ± 0.21c |
a Cholesterol efflux was expressed as the percentage of radioactivity in the medium compared to the total radioactivity (cell lysate plus medium).
b ApoAI-mediated cholesterol efflux was calculated as the difference between the values obtained in the presence or absence of ApoAI in the medium. Values represent the mean ± S.E. of four independent experiments.
c p < 0.05 compared to controls.
Reelin and ApoE3 Activate Dab1, PI3K, PKCζ, and Sp1
Sp1 is one of the transcription factors that controls ABCA1 expression (5). An increase in the phosphorylation of Sp1 enhances its capacity to bind to the ABCA1 promoter and to increase ABCA1 expression (5). To explore the mechanism(s) underlying the increased ABCA1 expression induced by reelin and apoE3, we studied not only the expression of Sp1 but its phosphorylation. Two Sp1 immunoreactive bands were detectable on Western blots (Fig. 2, A–C). Both apoE3 and reelin produced a time-related increase in the intensity of the top band (Fig. 2, B and C). To determine whether the slow migrating form of Sp1 was generated by phosphorylation, we incubated the cell lysates with λ-phosphatase before Western blot analysis. As shown in Fig. 2A, λ-phosphatase abolished the top band induced by reelin and apoE3. This observation thus provides direct evidence that the top immunoblot band, i.e. the lower mobility form of Sp1, is a result of Sp1 phosphorylation. The data in Fig. 2, B, C, and F indicate that both reelin and apoE3 induced a time-related increase in Sp1 phosphorylation with a peak value at about 1 h of treatment.
PKCζ is one of the kinases that can phosphorylate Sp1 (19), whereas activation of PI3K is a step in the signaling pathway that induces PKCζ phosphorylation (20). We previously demonstrated that activation of PKCζ is responsible for both Sp1 phosphorylation and ABCA1 expression induced by apoE/apoB-carrying lipoproteins. Activation of PI3K, on the other hand, is a step in the process by which apoE/apoB lipoproteins induce PKCζ phosphorylation (5). Fig. 2, B and C, also demonstrate that reelin and apoE3 elevated PI3K phosphorylation in a time-dependent manner (reached a peak level at 1 h). Similarly, reelin and apoE3 treatment increased phosphorylation of PKCζ in mouse macrophages.
Dab1 is an adaptor protein that links extracellular reelin with intracellular signaling pathways (8). Phosphorylated Dab1 recruits a variety of signaling molecules, including PI3K; it also induces PI3K phosphorylation (8). Although it has been suggested that mouse macrophages, including primary peritoneal macrophages and RAW264.7 cells, do not express Dab1 (19), we found that RAW264.7 cells expressed low but demonstrable levels of phosphorylated Dab1 (Fig. 2, G and H). Both reelin and apoE3 increased the level of phosphorylated Dab1 protein (Fig. 2, G and H). Taken together, these data suggest that reelin and apoE3 activate a signaling pathway involving Dab1, PI3K, PKCζ and Sp1 in macrophages.
Inhibition of Dab1, PI3K, PKCζ, and Sp1 Reduces ABCA1 Expression Induced by Reelin and ApoE
Having established the regulatory role of reelin and apoE on ABCA1 expression and Dab1, PI3K, PKCζ, and Sp1 phosphorylation, we next studied the impact of these signaling proteins on ABCA1 expression by using previously reported inhibitors (5). Mithramycin A is a chemotherapeutic drug that binds to GC-rich DNA sequences, thereby blocking the binding of transcription factors such as Sp1 to GC-specific regions of DNA (21). LY294002 (22) and 109203X (23) are PI3K and PKC inhibitors, respectively. We previously observed that treatment of macrophages with LY294002, 109203X, or mithramycin A diminished ABCA1 expression induced by apoE/apoB-carrying lipoproteins. Thus in this study, we incubated macrophages with these inhibitors for 30 min before adding reelin or apoE3. Each inhibitor abolished the up-regulation of ABCA1 mRNA expression by apoE3 and reelin, i.e. apoE3 and reelin did not significantly elevate ABCA1 mRNA level in cells pretreated with any of these inhibitors (Fig. 3, A and B).
FIGURE 3.
Inhibition of Dab-1, PI3K, PKCζ, and Sp1 reduces ABCA1 mRNA expression induced by reelin and apoE3. A and B, mouse macrophages were treated with 50 μm LY294002, 8 μm 109203X, 100 nm mithramycin A (mmA) or culture medium alone for 30 min and then treated with 3 μg/ml apoE3, 2 μg/ml reelin, or culture medium (Control) for 1 h. C, macrophages were transfected with a kinase-dead PKCζ mutant or an empty pEGFP vector. D, macrophages were transfected with Dab1 siRNA or control siRNA. The transfected cells were treated with 2 μg/ml reelin, 3 μg/ml apoE3, or culture medium alone (Control) for 1 h. ABCA1 mRNA levels were determined by quantitative real-time RT-PCR and normalized to β-actin mRNA. Values represent the mean ± S.E. of three separate experiments. *, p < 0.05 compared with cells without reelin or apoE3 treatment; †, p < 0.05 compared with cells treated with reelin or apoE3 alone; #, p < 0.05 compared with cells transfected with empty vector or control siRNA alone; $, p < 0.05 compared with cells treated with apoE3/reelin and transfected with empty vector or control siRNA.
To confirm the regulatory role of PKCζ in ABCA1 expression, we next transfected macrophages with a kinase-dead PKCζ mutant that significantly reduced apoE or reelin-induced ABCA1 mRNA expression compared with cells transfected with the empty vector (Fig. 3C). Specifically, 3 μg/ml apoE3 and 2 μg/ml reelin elevated ABCA1 mRNA by about 192 and 161%, respectively, in macrophages transfected with the control empty vector, whereas the same dose of apoE3 and reelin elevated ABCA1 mRNA only about 51 and 25%, respectively, in cells transfected with the PKCζ mutant.
The causal role of Dab1 in apoE3-and reelin-induced ABCA1 expression was investigated by knockdown of Dab1 with siRNA. We observed that transfection of macrophages with Dab1 siRNA reduced Dab1 mRNA by about 68% (data not shown). Dab1 siRNA did not significantly alter the ABCA1 mRNA level in cells without apoE3 or reelin treatment but significantly diminished apoE3- and reelin-induced ABCA1 mRNA expression. Although 3 μg/ml apoE3 and 2 μg/ml reelin elevated ABCA1 mRNA by about 201 and 172%, respectively, in macrophages transfected with control siRNA, the same doses of apoE3 and reelin elevated ABCA1 mRNA only about 69 and 44%, respectively, in cells transfected with Dab1 siRNA (Fig. 3D). Collectively, these results suggest that activation of Dab1, PI3K, PKCζ, and Sp1 is critical to the enhanced ABCA1 expression induced by reelin or apoE3.
Knockdown of ApoER2 and VLDLR Diminishes ApoE3- and Reelin-induced ABCA1 Expression
Both apoER2 and VLDLR are cell surface receptors for reelin. To address the involvement of apoER2 and VLDLR in apoE3- and reelin-induced ABCA1 expression, apoER2 and VLDLR expression was down-regulated by siRNA. The knockdown efficiency induced by siRNA was confirmed by detection of VLDLR and apoER2 protein. The protein levels of both apoER2 and VLDLR were relatively low in RAW264.7 cells. However, transfection of these cells with siRNA against apoER2 and VLDLR reduced the protein level of apoER2 and VLDLR about 69 and 55%, respectively (Fig. 4, A and B). The basal ABCA1 mRNA levels were comparable in macrophages transfected with VLDLR siRNA, apoER2 siRNA, or control siRNA, suggesting that knockdown of VLDLR or apoER2 did not affect basal ABCA1 expression. In contrast, transfection of VLDLR or apoER2 siRNA significantly reduced apoE3- and reelin-induced ABCA1 mRNA levels (Fig. 4C). Thus, 3 μg/ml apoE3 elevated the ABCA1 mRNA level about 147% in cells transfected with the control siRNA, whereas the same concentration of apoE3 elevated the ABCA1 mRNA level only about 77 and 74%, respectively, in cells transfected with the apoER2 siRNA and VLDLR siRNA. The data in Fig. 4C also showed that 2 μg/ml reelin elevated the ABCA1 mRNA level about 138% in cells transfected with the control siRNA but only 69 and 60%, respectively, in cells transfected with the apoER2 siRNA or VLDLR siRNA. Therefore, the level of reelin-induced ABCA1 mRNA was significantly lower in macrophages transfected with apoER2 siRNA or VLDLR siRNA as compared with those transfected with the control siRNA (Fig. 4C). Transfection of macrophages with apoER2 or VLDLR siRNA also reduced reelin-induced ABCA1 protein expression (Fig. 5). These findings suggest that apoE and reelin up-regulate ABCA1 expression via a mechanism involving VLDLR and apoER2.
Knockdown of ApoER2 and VLDLR Diminishes Reelin-induced PI3K Phosphorylation
Induction of PI3K phosphorylation is a critical step for the signal transduction initiated by VLDLR or apoER2 (8). Having established the causal role of PI3K phosphorylation in reelin-induced ABCA1 expression (Figs. 2 and 3), we next studied the impact of VLDLR or apoER2 knockdown on reelin-induced PI3K phosphorylation. Knockdown of VLDLR or apoER2 by siRNA clearly reduced reelin-induced PI3K phosphorylation (Fig. 5). This observation together with the data in Figs. 2–4 suggests that VLDLR or apoER2 activates a signaling pathway involving Dab1, PI3K, PKCζ, and Sp1, resulting in ABCA1 expression.
The data in Fig. 5 also show that knockdown of apoER2 and VLDLR by siRNAs reduced the level of p-PI3K but not the level of ABCA1 in cells without apoE3 or reelin treatment. These observations suggest that apoER2 and VLDLR under control conditions regulate the phosphorylation of PI3K via an unknown mechanism; however, this mechanism does not appear to regulate ABCA1 expression.
ApoE3 and Reelin Do Not Affect Cellular ATP Content or ABCG1 and SR-B1 mRNA Levels
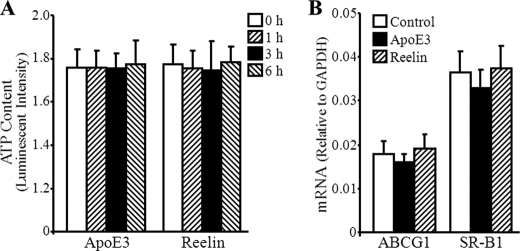
To determine whether the elevated ABCA1 expression is due to a general effect of apoE3 and reelin on cells, we measured the cellular ATP content and the mRNA levels of two other genes, i.e., ABCG1 and SR-B1. The proteins encoded by both ABCG1 and SR-B1 have been reported to mediate cholesterol efflux (17). The data in Fig. 6A show that incubation of macrophages with 2 μg/ml reelin or 3 μg/ml apoE3 for up to 6 h did not significantly affect cellular ATP levels. This observation suggests that the up-regulatory effect of reelin- and apoE3 on ABCA1 expression is not due to an increased viability in cells treated with these reagents. The data in Fig. 6B indicate that apoE3 and reelin treatment did not significantly induce ABCG1 and SR-B1 expression. These observations suggest that the reelin- and apoE3-dependent increase in ABCA1 expression is a gene-specific response rather than a general effect on the genome.
FIGURE 6.
ApoE3 and reelin do not affect cellular ATP content and ABCG1 and SR-B1 mRNA levels. A, mouse macrophages were treated with 2 μg/ml reelin or 3 μg/ml apoE3 for time periods as indicated. Cellular ATP levels were determined by luminescence assay. B, mouse macrophages were incubated with 3 μg/ml apoE3, 2 μg/ml reelin, or culture medium alone (Control) for 1 h. The mRNA levels of ABCG1 and SR-B1 were determined by quantitative real-time RT-PCR and normalized to GAPDH mRNA. Values represent the mean ± S.E. of three independent experiments.
DISCUSSION
ABCA1 expression is thought to be controlled by cellular cholesterol content (24), i.e. an elevation in cellular cholesterol or oxysterol levels up-regulates ABCA1 expression via activation of the liver X receptor (LXR) and its partner retinoid X receptor (RXR) (25, 26). For the first time this report demonstrates that treatment of macrophages with reelin and lipid-poor apoE3 induces macrophage ABCA1 expression, and knockdown of VLDLR and apoER2 attenuates apoE3- and reelin-induced ABCA1 expression. These observations suggest that the expression of ABCA1 can be regulated by something other than an elevation in cellular cholesterol content, namely by activation of VLDLR or apoER2 via extracellular signaling molecules.
Binding of reelin to VLDLR and apoER2 has been shown to induce tyrosine phosphorylation of the cytoplasmic adaptor protein Dab1 (8). which in turn activates a common set of signaling molecules, including PI3K. In agreement with these findings, apoE3 and reelin increased Dab1 and PI3K phosphorylation in these studies. In addition, knockdown of Dab1 attenuated PI3K phosphorylation and ABCA1 expression induced by these ligands. These findings provide direct evidence for the involvement of Dab1-mediated signaling transduction in reelin- and apoE-induced ABCA1 expression.
We previously reported that activation of the PI3K-PKCζ-Sp1 pathway is a mechanism for induction of ABCA1 expression by apoB-carrying lipoproteins (5). Transcription factor Sp1 is a C2H2 zinc-finger DNA-binding protein (27). The mouse ABCA1 promoter contains one Sp1 binding motif. We previously observed the increase in ABCA1 mRNA expression induced by apoB-carrying lipoproteins is associated with an increased amount of Sp1 bound to the ABCA1 promoter region (5). Furthermore, we observed that mutation of the Sp1 binding motif diminished the inducibility of ABCA1 promoter by apoB-carrying lipoproteins and that chemical inhibition of Sp1-DNA binding suppressed lipoprotein-induced ABCA1 promoter activity and mRNA expression (5). These findings suggest a causal role of Sp1 in induction of ABCA1 mRNA expression by apoB-carrying lipoproteins. Similarly, data from the present report showed that reelin and lipid-poor apoE3 augmented Sp1 phosphorylation and that inhibition of Sp1-DNA binding by mithramycin A reduced reelin- and apoE3-induced ABCA1 mRNA expression.
It is known that phosphorylation of Sp1 enhances its DNA binding and transcriptional activity (19). Several protein kinases, including DNA-dependent protein kinase, PKCζ, casein kinase II, extracellular signal-regulated kinase (ERK), and cyclin-dependent kinase 2 (Cdk2), have been shown to mediate Sp1 phosphorylation (19). Our previous studies demonstrated that PKCζ is responsible for the increased Sp1 phosphorylation by apoE/apoB-carrying lipoproteins (5). Specifically, we observed that apoB-carrying lipoproteins increased PKCζ phosphorylation and induced physical interaction of PKCζ and Sp1. Inhibition of PKCζ activity attenuated lipoprotein-induced Sp1 phosphorylation and ABCA1 expression. Moreover, we observed that lipoprotein-induced PKCζ phosphorylation was reduced by inhibition of PI3K (5), a protein kinase that mediates PKCζ phosphorylation (20). In this report we observed that reelin and lipid-poor apoE increased PI3K and PKCζ phosphorylation and that transfection of macrophages with a kinase dead PKCζ or treatment of the cells with a PKC or PI3K inhibitor diminished ABCA1 mRNA expression induced by reelin and apoE. Taken together, it is highly likely that binding of reelin or apoE to VLDLR and apoER2 results in sequential phosphorylation of Dab1, PI3K, PKCζ, and Sp1, enhancing the transcription factor activity of Sp1 and up-regulating ABCA1 mRNA expression.
Up-regulation of ABCA1 expression by activation of VLDLR and apoER2 could be physiologically important. ABCA1 is one of the plasma membrane proteins that exports excess cholesterol derived from internalized lipoproteins (1–4). Increasing evidence clearly indicates an anti-atherogenic role for ABCA1-mediated cholesterol efflux in macrophages (1–4, 28). Data from the present report showed that reelin and apoE increased ABCA1 expression and cholesterol efflux. However, these findings do not allow any conclusion about the role of VLDLR and apoER2 in atherosclerosis. Previous studies have shown both pro- and anti-atherogenic effects of VLDLR and apoER2. For example, transplantation of VLDLR-expressing macrophages into VLDLR-deficient mice accelerated the development of atherosclerotic lesions (29). Overexpression of VLDLR increased β-VLDL-induced lipid accumulation (30). In contrast, it has been shown that treatment of VLDLR- and apoER2-overexpressing macrophages with apoE down-regulated proinflammatory gene and up-regulated anti-inflammatory gene expression in these macrophages (31). Furthermore, a VLDLR deficiency increased intimal thickening after vascular injury and increased necrosis in atherosclerotic lesions (32). In addition, an apoER2 variant has been shown to increase the risk of atherosclerotic coronary artery disease (33). These observations imply that the pro-atherogenic effect of VLDLR (possibly apoER2) arising from the lipoprotein uptake function of macrophages may be counterbalanced by their anti-atherogenic effects, such as conversion of macrophages from a proinflammatory to an anti-inflammatory phenotype (31) and the inhibition of macrophage death in atherosclerotic lesions (32). Up-regulation of ABCA1 expression and augmentation of cholesterol efflux in macrophages could be a mechanism to offset the pro-atherogenic effect of VLDLR and apoER2 related to uptake of cholesterol-rich lipoproteins. In addition to macrophages, cells in vascular tissues, including endothelial cells and vascular smooth muscle cells, also express ABCA1 (34, 35), VLDLR, and apoER2 (36, 37). The ABCA1 protein expressed in these cells has been suggested to play a protective role against atherosclerosis (34, 35). Based on the findings from this report, it is highly possible that activation of apoER2 and VLDLR in endothelial cells and vascular smooth muscle cells increases ABCA1 expression, which in turn plays an anti-atherogenic role. Thus, lipid-free apoE and other ligands that activate these receptors (without increasing lipid uptake) in these cells might provide a therapeutic strategy for atherosclerosis.
It has been reported that ABCA1 has other functions besides regulation of cholesterol efflux. One of them is to induce the expression of apoE (38). Data from this report clearly demonstrated that apoE increases ABCA1 expression via a mechanism involving VLDLR/apoER2. Thus, apoE and ABCA1 are able to up-regulate the expression of each other, i.e. binding of apoE to VLDLR and apoER2 up-regulates the expression of ABCA1, which in turn increases apoE expression. Such a positive feedback path might be important to enable cells to remove excess cholesterol. However, it has been reported that Raw 264.7 cells lack endogenous apoE (39). Thus, the above-mentioned positive feedback regulation would not occur in these particular cells. Further experiments are required to test the above-mentioned hypothesis in cells that express both ABCA1 and apoE.
In summary, data in this report clearly indicate that treatment of mouse macrophages with reelin and apoE up-regulated ABCA1 expression, accelerated apoAI-mediated cholesterol efflux, and augmented the phosphorylation of Dab1, PI3K, PKCζ, and Sp1. Knockdown of VLDLR or apoER2 or inhibition of Dab1, PI3K, PKCζ, or Sp1 attenuated reelin- and apoE-induced ABCA1 expression. These findings demonstrate that ABCA1 expression can be regulated by extracellular signaling molecules reelin and apoE via activation of the VLDLR/apoER2-Dab1-PI3K-PKCζ-Sp1 signaling cascade.
Acknowledgment
We thank Dr. Diana Marver for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants SC1HL101431 and U54RR026140 (to H. Y.) and R01ES014471 and R01HL089382 (to Z. G.).
- ABCA1
- ATP binding cassette transporter A1
- Sp1
- specificity protein 1
- LDLR
- LDL receptor
- VLDLR
- very low density lipoprotein receptor
- apoER2
- apoE receptor 2
- Dab1
- disabled-1
- SR-B1
- scavenger receptor B1.
REFERENCES
- 1. van Eck M., Bos I. S., Kaminski W. E., Orsó E., Rothe G., Twisk J., Böttcher A., Van Amersfoort E. S., Christiansen-Weber T. A., Fung-Leung W. P., Van Berkel T. J., Schmitz G. (2002) Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc. Natl. Acad. Sci. U.S.A. 99, 6298–6303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orsó E., Broccardo C., Kaminski W. E., Böttcher A., Liebisch G., Drobnik W., Götz A., Chambenoit O., Diederich W., Langmann T., Spruss T., Luciani M. F., Rothe G., Lackner K. J., Chimini G., Schmitz G. (2000) Transport of lipids from Golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat. Genet. 24, 192–196 [DOI] [PubMed] [Google Scholar]
- 3. Van Eck M., Singaraja R. R., Ye D., Hildebrand R. B., James E. R., Hayden M. R., Van Berkel T. J. (2006) Macrophage ATP binding cassette transporter A1 overexpression inhibits atherosclerotic lesion progression in low density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 26, 929–934 [DOI] [PubMed] [Google Scholar]
- 4. Singaraja R. R., Fievet C., Castro G., James E. R., Hennuyer N., Clee S. M., Bissada N., Choy J. C., Fruchart J. C., McManus B. M., Staels B., Hayden M. R. (2002) Increased ABCA1 activity protects against atherosclerosis. J. Clin. Invest. 110, 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen X., Zhao Y., Guo Z., Zhou L., Okoro E. U., Yang H. (2011) Transcriptional regulation of ATP binding cassette transporter A1 expression by a novel signaling pathway. J. Biol. Chem. 286, 8917–8923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao Y., Chen X., Yang H., Zhou L., Okoro E. U., Guo Z. (2011) A novel function of apolipoprotein E. Up-regulation of ATP binding cassette transporter A1 expression. PLoS One 6, e21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blacklow S. C. (2007) Versatility in ligand recognition by LDL receptor family proteins. Advances and frontiers. Curr. Opin. Struct. Biol. 17, 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herz J., Chen Y., Masiulis I., Zhou L. (2009) Expanding functions of lipoprotein receptors. J. Lipid Res. 50, S287–S292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Myant N. B. (2010) Reelin and apolipoprotein E receptor 2 in the embryonic and mature brain. Effects of an evolutionary change in the apoER2 gene. Proc. Biol. Sci. 277, 345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Samama B., Boehm N. (2005) Reelin immunoreactivity in lymphatics and liver during development and adult life. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 285, 595–599 [DOI] [PubMed] [Google Scholar]
- 11. Wu D., Sharan C., Yang H., Goodwin J. S., Zhou L., Grabowski G. A., Du H., Guo Z. (2007) Apolipoprotein E-deficient lipoproteins induce foam cell formation by down-regulation of lysosomal hydrolases in macrophages. J. Lipid Res. 48, 2571–2578 [DOI] [PubMed] [Google Scholar]
- 12. Dove D. E., Su Y. R., Zhang W., Jerome W. G., Swift L. L., Linton M. F., Fazio S. (2005) ACAT1 deficiency disrupts cholesterol efflux and alters cellular morphology in macrophages. Arterioscler. Thromb. Vasc. Biol. 25, 128–134 [DOI] [PubMed] [Google Scholar]
- 13. Wang Z., Yang H., Ramesh A., Roberts L. J., 2nd, Zhou L., Lin X., Zhao Y., Guo Z. (2009) Overexpression of Cu2+/Zn2+ superoxide dismutase and/or catalase accelerates benzo(a)pyrene detoxification by up-regulation of the aryl hydrocarbon receptor in mouse endothelial cells. Free Radic. Biol. Med. 47, 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin X., Yang H., Zhou L., Guo Z. (2011) Nrf2-dependent induction of NQO1 in mouse aortic endothelial cells overexpressing catalase. Free Radic. Biol. Med. 51, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chou M. M., Hou W., Johnson J., Graham L. K., Lee M. H., Chen C. S., Newton A. C., Schaffhausen B. S., Toker A. (1998) Regulation of protein kinase Cζ by PI 3-kinase and PDK-1. Curr. Biol. 8, 1069–1077 [DOI] [PubMed] [Google Scholar]
- 16. Onumah O. E., Jules G. E., Zhao Y., Zhou L., Yang H., Guo Z. (2009) Overexpression of catalase delays G0/G1- to S-phase transition during cell cycle progression in mouse aortic endothelial cells. Free Radic. Biol. Med. 46, 1658–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen W., Sun Y., Welch C., Gorelik A., Leventhal A. R., Tabas I., Tall A. R. (2001) Preferential ATP binding cassette transporter A1-mediated cholesterol efflux from late endosomes/lysosomes. J. Biol. Chem. 276, 43564–43569 [DOI] [PubMed] [Google Scholar]
- 18. Krimbou L., Denis M., Haidar B., Carrier M., Marcil M., Genest J., Jr. (2004) Molecular interactions between apoE and ABCA1. Impact on apoE lipidation. J. Lipid Res. 45, 839–848 [DOI] [PubMed] [Google Scholar]
- 19. Thymiakou E., Zannis V. I., Kardassis D. (2007) Physical and functional interactions between liver X receptor/retinoid X receptor and Sp1 modulate the transcriptional induction of the human ATP binding cassette transporter A1 gene by oxysterols and retinoids. Biochemistry 46, 11473–11483 [DOI] [PubMed] [Google Scholar]
- 20. Shenoy N. G., Gleich G. J., Thomas L. L. (2003) Eosinophil major basic protein stimulates neutrophil superoxide production by a class IA phosphoinositide 3-kinase and protein kinase Cζ-dependent pathway. J. Immunol. 171, 3734–3741 [DOI] [PubMed] [Google Scholar]
- 21. Blume S. W., Snyder R. C., Ray R., Thomas S., Koller C. A., Miller D. M. (1991) Mithramycin inhibits SP1 binding and selectively inhibits transcriptional activity of the dihydrofolate reductase gene in vitro and in vivo. J. Clin. Invest. 88, 1613–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo M., Joiakim A., Reiners J. J., Jr. (2000) Suppression of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-mediated aryl hydrocarbon receptor transformation and CYP1A1 induction by the phosphatidylinositol 3-kinase inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1- benzopyran-4-one (LY294002). Biochem. Pharmacol. 60, 635–642 [DOI] [PubMed] [Google Scholar]
- 23. Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. (1991) The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 266, 15771–15781 [PubMed] [Google Scholar]
- 24. Wang N., Tall A. R. (2003) Regulation and mechanisms of ATP binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23, 1178–1184 [DOI] [PubMed] [Google Scholar]
- 25. Costet P., Luo Y., Wang N., Tall A. R. (2000) Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275, 28240–28245 [DOI] [PubMed] [Google Scholar]
- 26. Janowski B. A., Grogan M. J., Jones S. A., Wisely G. B., Kliewer S. A., Corey E. J., Mangelsdorf D. J. (1999) Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc. Natl. Acad. Sci. U.S.A. 96, 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu J. H., Schwartzbauer G., Kazlman A., Menon R. K. (1999) Role of the Sp family of transcription factors in the ontogeny of growth hormone receptor gene expression. J. Biol. Chem. 274, 34327–34336 [DOI] [PubMed] [Google Scholar]
- 28. Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., Loubser O., Ouelette B. F., Fichter K., Ashbourne-Excoffon K. J., Sensen C. W., Scherer S., Mott S., Denis M., Martindale D., Frohlich J., Morgan K., Koop B., Pimstone S., Kastelein J. J., Genest J., Jr., Hayden M. R. (1999) Mutations in ABC1 in Tangier disease and familial high density lipoprotein deficiency. Nat. Genet. 22, 336–345 [DOI] [PubMed] [Google Scholar]
- 29. Eck M. V., Oost J., Goudriaan J. R., Hoekstra M., Hildebrand R. B., Bos I. S., van Dijk K. W., Van Berkel T. J. (2005) Role of the macrophage very low density lipoprotein receptor in atherosclerotic lesion development. Atherosclerosis 183, 230–237 [DOI] [PubMed] [Google Scholar]
- 30. Suzuki J., Takahashi S., Oida K., Shimada A., Kohno M., Tamai T., Miyabo S., Yamamoto T., Nakai T. (1995) Lipid accumulation and foam cell formation in Chinese hamster ovary cells overexpressing very low density lipoprotein receptor. Biochem. Biophys. Res. Commun. 206, 835–842 [DOI] [PubMed] [Google Scholar]
- 31. Baitsch D., Bock H. H., Engel T., Telgmann R., Müller-Tidow C., Varga G., Bot M., Herz J., Robenek H., von Eckardstein A., Nofer J. R. (2011) Apolipoprotein E induces anti-inflammatory phenotype in macrophages. Arterioscler. Thromb. Vasc. Biol. 31, 1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tacken P. J., Delsing D. J., Gijbels M. J., Quax P. H., Havekes L. M., Hofker M. H., van Dijk K. W. (2002) VLDL receptor deficiency enhances intimal thickening after vascular injury but does not affect atherosclerotic lesion area. Atherosclerosis 162, 103–110 [DOI] [PubMed] [Google Scholar]
- 33. Shen G. Q., Li L., Girelli D., Seidelmann S. B., Rao S., Fan C., Park J. E., Xi Q., Li J., Hu Y., Olivieri O., Marchant K., Barnard J., Corrocher R., Elston R., Cassano J., Henderson S., Hazen S. L., Plow E. F., Topol E. J., Wang Q. K. (2007) An LRP8 variant is associated with familial and premature coronary artery disease and myocardial infarction. Am. J. Hum. Genet. 81, 780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaisman B. L., Demosky S. J., Stonik J. A., Ghias M., Knapper C. L., Sampson M. L., Dai C., Levine S. J., Remaley A. T. (2012) Endothelial expression of human ABCA1 in mice increases plasma HDL cholesterol and reduces diet-induced atherosclerosis. J. Lipid Res. 53, 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choi H. Y., Rahmani M., Wong B. W., Allahverdian S., McManus B. M., Pickering J. G., Chan T., Francis G. A. (2009) ATP binding cassette transporter A1 expression and apolipoprotein A-I binding are impaired in intima-type arterial smooth muscle cells. Circulation 119, 3223–3231 [DOI] [PubMed] [Google Scholar]
- 36. Hiltunen T. P., Luoma J. S., Nikkari T., Ylä-Herttuala S. (1998) Expression of LDL receptor, VLDL receptor, LDL receptor-related protein, and scavenger receptor in rabbit atherosclerotic lesions. Marked induction of scavenger receptor and VLDL receptor expression during lesion development. Circulation 97, 1079–1086 [DOI] [PubMed] [Google Scholar]
- 37. Yang X. V., Banerjee Y., Fernández J. A., Deguchi H., Xu X., Mosnier L. O., Urbanus R. T., de Groot P. G., White-Adams T. C., McCarty O. J., Griffin J. H. (2009) Activated protein C ligation of apoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc. Natl. Acad. Sci. U.S.A. 106, 274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Von Eckardstein A., Langer C., Engel T., Schaukal I., Cignarella A., Reinhardt J., Lorkowski S., Li Z., Zhou X., Cullen P., Assmann G. (2001) ATP binding cassette transporter ABCA1 modulates the secretion of apolipoprotein E from human monocyte-derived macrophages. FASEB J. 15, 1555–1561 [DOI] [PubMed] [Google Scholar]
- 39. Hara M., Matsushima T., Satoh H., Iso-o N, Noto H., Togo M., Kimura S., Hashimoto Y., Tsukamoto K. (2003) Isoform-dependent cholesterol efflux from macrophages by apolipoprotein E is modulated by cell surface proteoglycans. Arterioscler. Thromb. Vasc. Biol. 23, 269–274 [DOI] [PubMed] [Google Scholar]