Background: Structural and functional studies on G protein-coupled receptor (GPCR) benefit from reconstitution of pure GPCR into lipid bilayers.
Results: Cannabinoid CB2 receptor was successfully reconstituted and the effect of anionic lipids and cholesterol on G protein activation was studied.
Conclusion: G protein activation increased with anionic lipid content up to ∼50 mol %.
Significance: Anionic lipids regulate signal transduction by CB2 receptor and possibly other class A GPCR.
Keywords: G Protein-coupled Receptors (GPCR), Membrane Proteins, Membrane Reconstitution, Protein-Drug Interactions, Recombinant Protein Expression, Protein-Lipid Interaction
Abstract
Human cannabinoid type 2 (CB2) receptor expressed in Escherichia coli was purified and successfully reconstituted in the functional form into lipid bilayers composed of POPC, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine (POPS), and cholesteryl hemisuccinate (CHS). Reconstitution was performed by detergent removal from the protein/lipid/detergent mixed micelles either on an adsorbent column, or by rapid dilution to below the critical micelle concentration of detergent followed by removal of detergent monomers on a concentrator. Proteoliposomes prepared at a protein/phospholipid/CHS molar ratio of 1/620–650/210–220 are free of detergent as shown by 1H NMR, have a homogeneous protein/lipid ratio shown by isopycnic gradient ultracentrifugation, and are small in size with a mean diameter of 150–200 nm as measured by dynamic light scattering. Functional integrity of the reconstituted receptor was confirmed by quantitative binding of 2H-labeled agonist CP-55,940-d6 measured by 2H magic angle spinning NMR, as well as by activation of G protein. The efficiency of G protein activation by agonist-bound CB2 receptor was affected by negative electric surface potentials of proteoliposomes controlled by the content of anionic CHS or POPS. The activation was highest at an anionic lipid content of about 50 mol %. There was no correlation between the efficiency of G protein activation and an increase of hydrocarbon chain order induced by CHS or cholesterol. The results suggest the importance of anionic lipids in regulating signal transduction by CB2 receptor and other class A GPCR. The successful reconstitution of milligram quantities of pure, functional CB2 receptor enables a wide variety of structural studies.
Introduction
The human cannabinoid type 2 (CB2)3 receptor (Fig. 1) is a member of the large family of G protein-coupled receptors (GPCR) that play a vital physiological role by transducing extracellular signals to the cell interior. The receptor is primarily located in cells of immune and hematopoietic systems such as thymus, spleen, and tonsil (1), but was also detected in perivascular microglial cells in human brain (2). The discovery of the endogenous cannabinoid ligands anandamide and 2-arachidonoylglycerol (3–5) stimulated studies of the intrinsic function of cannabinoid receptors (6). CB2 receptor plays a major role in inflammatory processes (1). Migration behavior of immune cells upon activation of the receptor has also become the subject of deep interest (7). The need for novel treatments of inflammation and pain has encouraged development of specific ligands for the CB2 receptor that are free from the psychoactive side effects of conventional phytocannabinoids (8, 9).
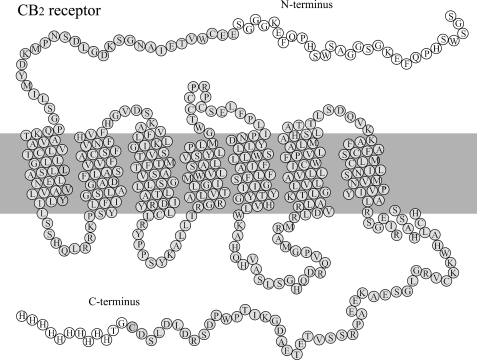
FIGURE 1.
Snake plot of the human CB2 receptor. Amino acid residues in the wild-type receptor are shown with gray background. The recombinant CB2 receptor that was cleaved from the CB2-130 fusion protein contains additional amino acids in the N- and C-terminal ends (white background). Those residues did not alter the receptor function as measured by ligand-binding and G protein activation. The α-helical domains of the receptor are shown as predicted by the CB2 receptor homology model of Xie et al. (45) derived from the crystal structure of bovine rhodopsin. The end of helix VI on the extracellular side is shown as predicted by Nebane et al. (77). The snake plot is in reasonable agreement with the reported structure of the CB2 receptor in a bilayer obtained in a molecular simulation (67).
To study structure and function of the CB2 receptor in membranes of controlled composition, the development of procedures for uniform, functional, and high-density reconstitution of the receptor into lipid bilayers is required. Despite the physiological significance of GPCR, experiments on reconstituted receptors have been performed almost exclusively on bovine rhodopsin that can be purified in bulk quantities from rod outer segment disk membranes of the retina. Reconstitution of rhodopsin is straightforward thanks in part to its relatively high stability to temperature and micellar environment in the dark adapted state (10). In difference to rhodopsin, structural instability of other GPCR in detergent micelles is a well known phenomenon that requires special attention during purification and reconstitution. Therefore, reconstitution of GPCR must be followed by functional studies to assure preservation of structural integrity.
Recently, advances in the recombinant expression of GPCR as well as in their thermostabilization by selective amino acid replacement allowed preparation of well diffracting crystals of several class A receptors (11). However, in crystals it is inevitably difficult to preserve critical features of GPCR function, e.g. the ability to interact with G proteins and arrestin. The N- and C-terminal domains as well as large loops may have to be truncated or mutated to enable crystallization. Even in the absence of such artificial modifications, the structure of the more flexible regions of GPCR may be affected by non-native interactions in the tightly packed crystals, unless they are co-crystallized with interaction partners, e.g. G protein as recently demonstrated for the β2 adrenergic receptor-Gs protein complex (12). It is obvious that studies on full-length, unaltered GPCR in their natural environment, the lipid bilayer, are critical for understanding their structure and function (11).
Expression and purification of CB2 receptor as a fusion with MBP and thioredoxin in combination with several affinity tags were reported previously (13–16). However, reconstitution of the purified receptor in a functional form into lipid bilayers, and insights into the role of phospholipids and cholesterol on function were not reported yet. The functional reconstitution of the CB2 receptor has to satisfy the following criteria: (i) efficient removal of detergents from mixed protein/lipid/detergent micelles, (ii) preservation of the structural integrity of the protein, and (iii) uniform protein to lipid ratio in the proteoliposomes. Insertion of membrane proteins into lipid bilayers was shown to occur spontaneously upon removal of detergents from the mixed micelles (17–20). We reconstituted CB2 receptor by treatment of mixed protein/lipid/detergent micelles with detergent adsorbent or by rapid dilution to below the critical micelle concentration (CMC) of detergents.
In this article, we report on the successful reconstitution of the CB2 receptor into liposomes composed of POPC, POPS, CHS, and cholesterol (Fig. 2), on the characterization of the proteoliposomes, and on first insights into the role of phospholipids and cholesterol in regulation of receptor function. Functionality of the receptor was investigated in a ligand binding study using deuterated agonist CP-55,940-d6 in combination with solid-state 2H MAS NMR (21), a method that distinguishes between specific binding of the ligand to the protein and nonspecific interactions with the lipid matrix. G protein activation by agonist-bound CB2 receptor was measured in an in vitro coupled assay. The G protein activation experiments were conducted as a function of the electric surface potential of membranes altered by the anionic CHS or POPS content, as well as of a function of lipid hydrocarbon chain order raised by addition of CHS or cholesterol. We show that G protein activation by agonist-bound CB2 receptor is most efficient at a content of anionic lipids of ∼50 mol %, whereas the lipid order has no apparent influence.
FIGURE 2.
Lipids used for reconstitution of CB2 receptor.
EXPERIMENTAL PROCEDURES
Materials
The lipids POPC, POPC-d31, POPC-d4, POPS sodium salt, and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL). CHS-Tris salt was from Anatrace (Maumee, OH). The fluorophore-labeled lipid, DilC18(5) solid (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt) was from Invitrogen. The detergents CHAPS and DM were from Anatrace. LDAO was from Sigma. The ligand CP-55,940 ((−)-cis-3[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl) cyclohexanol) was from Tocris Bioscience (Ellisville, MO) and 3H-labeled CP-55,940 was from PerkinElmer Life Sciences. 2H-Labeled CP-55,940-d6 was synthesized as reported earlier (21). The NMR solvents of deuterium-depleted H2O (2–3 ppm 2H), 2H2O (99.9% 2H), and C2H3O2H (99.8% 2H doped with 0.05% (v/v) tetramethylsilane) were from Cambridge Isotope Laboratories (Andover, MA).
Expression and Purification of CB2 Receptor
CB2 receptor was expressed in Escherichia coli as a fusion with MBP, a recognition sequence for tobacco etch virus protease and the StrepTag II sequence at the N terminus, and with a decahistidine tag at the C terminus (CB2–130) (13). The fusion protein was purified on a Ni-NTA resin, N-terminal MBP removed by treatment with specific tobacco etch virus protease, and the receptor was further purified in a second step of affinity chromatography on a StrepTactin column. Eluted CB2 receptor from the column was concentrated in a centrifugal spin concentrator (Orbital Biosciences, Topsfield, MA) with a 30-kDa molecular mass cut off, and the protein concentration was determined with a Bio-Rad DC kit (Bio-Rad). This final step of protein sample preparation raises not only the concentration of the receptor but also the concentration of detergents, almost proportionally to protein concentration. The final protein concentration was typically 1–2 mg/ml, and the concentrations of detergents were: CHAPS, 2–2.5% (w/v); DM, 0.4–0.5% (w/v); and CHS, 0.4–0.5% (w/v). To facilitate subsequent quantification of the receptor throughout the reconstitution procedure, a fraction of the purified CB2 receptor was labeled with fluorophore Alexa Fluor 488 or 532 as described under supplemental Materials S1–S6.
Reconstitution by Adsorbent Column Chromatography
Reconstitution of CB2 receptor from protein/lipid/detergent mixed micelles was performed as follows: 100 μg of purified CB2 receptor (1–2 mg/ml) was supplemented with 2 μg of the fluorophore-labeled CB2 receptor (0.45 μg/μl) dissolved in the same detergent mixture. The phospholipids POPC (0.8 mg) and POPS (0.2 mg) supplemented with 0.1 μg of fluorophore-labeled DilC18(5) were dissolved in 0.5% (w/v) LDAO in PBS buffer (10 mm sodium phosphate, 0.9% (154 mm) NaCl, pH 7.4). The protein (100 μg) in micellar solution was combined with the lipid (1.0 mg) in micellar solution and the total volume was adjusted to 315 μl by addition of 0.5% (w/v) LDAO.
A sample of 300 μl of the mixed protein/lipid/detergent micellar solution was loaded on a column (7.4 mm diameter and 35 mm length) packed with 1.5 ml of detergent removing resin (Thermo Scientific, Waltham, MA). The remaining 15 μl of solution were used to obtain a calibration curve for quantification of the receptor and lipids. PBS buffer was used as the column eluant and the flow rate was maintained at ∼25 μl/s. To monitor the elution profiles of protein and lipids, the eluate from the column was fractionated (∼150 μl each) into the wells of a 96-well plate using a Beckman SC100 fraction collector (Beckman Coulter, Fullerton, CA). The protein and lipid content in each fraction were quantified by fluorescence measurements (see supplemental Materials S1–S6). Fractions containing the initial 85% of the total fluorescence intensity were combined. This allowed for substantial recovery of CB2 receptor as proteoliposomes, whereas minimizing contamination with detergents. The CHS was not removed by the column and almost quantitatively incorporated into the lipid matrix of proteoliposomes.
Reconstitution by Rapid Dilution
The 300 μl of protein/lipid/detergent mixed micelles, prepared in the same manner as for the adsorbent column reconstitution, were diluted dropwise into 75 ml of PBS buffer at 4 °C under continuous stirring. This 250-fold dilution was sufficient to lower detergent concentrations to well below the CMC, which resulted in formation of proteoliposomes and detergent monomers. The reported CMC values of the detergents in water are: LDAO 1.5–2.1 mm (22), CHAPS 4.1–6.4 mm (23), and DM 0.17 mm (24). The CMC of detergents in mixed micelles containing the receptor and lipids are expected to be somewhat lower. After rapid dilution, the concentration of detergents was reduced ∼1300-fold by buffer exchange on a concentrator (two cycles of concentration/dilution from 75 to 5 ml, and one cycle from 30 to 5 ml using a Vivacell 70 concentrator (Vivascience, Hannover, Germany)) with a polyethersulfone filter (10-kDa molecular mass cut off). The concentration/dilution was performed at 4 °C by applying pressure of 2.8 bar of argon gas under continuous lateral shaking. For the preparation of larger samples (400 μg of CB2 receptor and higher) as required for ligand binding experiments by 2H NMR, the concentration/dilution was performed using an Amicon 8050 device (Millipore, Billerica, MA) with a polyethersulfone filter (30-kDa molecular mass cut off) and a pressure of 1 bar of argon gas.
Physical Characterization of Proteoliposomes
Solution state 1H NMR spectra of proteoliposomes were recorded to monitor (i) efficiency of detergent removal, (ii) lipid composition, and (iii) ligand concentration. Small aliquots of the dispersion of proteoliposomes were lyophilized, dissolved in deuterated methanol, and the spectra were recorded. Comparison was made with spectra of a known amount of detergent, lipid, or ligand in deuterated methanol. The density of proteoliposomes as well as their homogeneity were determined by isopycnic gradient ultracentrifugation (supplemental Materials S1–S6).
Functional Characterization of CB2 Receptor in Proteoliposomes
Functional activity of the CB2 receptor reconstituted into liposomes was measured by (i) quantitative binding of the 2H-labeled synthetic cannabinoid ligand CP-55,940-d6 in solid-state 2H MAS NMR, and (ii) G protein activation by the agonist-bound receptor.
G Protein Activation
Myristoylated Gαi1 was expressed in E. coli and purified as described earlier (25). Recombinant human β1γ2 subunits of G protein were expressed in baculovirus-infected Sf9 cells and purified as described earlier (26). The G protein activation assay was conducted as follows (final concentrations in 50 μl of reaction mixture are given in parentheses): the proteoliposome sample was diluted into ice-cold 10 mm MOPS buffer to reach a receptor concentration of 0.2–0.8 ng/μl. Ten μl of the diluted proteoliposome dispersion containing 2–8 ng of the receptor were dispensed into presiliconized glass tubes and mixed with 2 μm CP-55,940 in MOPS buffer containing 0.1% (w/v) BSA. Upon addition of a mixture of Gαi1 (100 nm) and Gβ1γ2 (500 nm), the tubes were incubated on ice for 30 min. The reaction was started by addition of MOPS buffer, pH 7.5 (50 mm), EDTA (1 mm), MgCl2 (3 mm), GDP (4 μm), BSA (0.3% w/v), NaCl (100 mm), DTT (1 mm), and [35S] GTPγS (2–6 nm, 1250 Ci/mmol), followed by rapid transfer of the tubes to a water bath at 30 °C. The incubation was continued for 20 min. The reaction was terminated by addition of 2 ml of ice-cold stop solution, TNMg (20 mm Tris-HCl, pH 8.0, 100 mm NaCl, and 25 mm MgCl2). The reaction mixture was rapidly filtered through nitrocellulose filters (Millipore, Billerica, MA). Filters were washed four times with 2 ml each of cold TNMg buffer, dried, placed in scintillation vials filled with ScintiSafe Econo F scintillation liquid (Fisher, Waltham, MA), and the radioactivity was counted.
Microelectrophoresis
Measurement of electrokinetic potentials (ζ-potentials) of CB2 proteoliposomes and protein-free liposomes was performed with a Mark II apparatus from Rank Brothers (Bottisham, UK) equipped with a cylindrical cell. The proteoliposomes or empty liposomes were centrifuged overnight at 417,200 × g at 4 °C in an Optima TLX ultracentrifuge with a TLA-100.4 rotor (Beckman Coulter). The pellet was re-dispersed in PBS buffer to yield a lipid concentration in the range of 0.02–0.1% (w/v). The voltage drop over the capillary at a constant current was measured with a high input resistance electrometer (Keithley, Cleveland, OH). Each ζ-potential was calculated from the average velocities of 10 large (∼1 μm) liposomes measured with reversal of polarity of the applied voltage. The ζ-potentials were calculated using the Helmholtz-Smoluchowski equation (27),
 |
where u is the electrophoretic mobility, η is the water viscosity, ϵ is the relative permittivity, and ϵ0 the permittivity of vacuum. All measurements were carried out at 25 °C.
Preparation of Proteoliposomes for Solid-state NMR
Proteoliposomes for measurement of ligand binding were prepared as follows: 400 μg of CB2 receptor supplemented with 1 mol % of fluorophore-labeled CB2 receptor were loaded onto 500 μl of Ni-NTA resin suspended at 50% (v/v) in Tris buffer (50 mm Tris-HCl, pH 7.5, 200 mm NaCl, 30%(v/v) glycerol, 0.5% CHAPS, 0.1% DM, 0.1% CHS, and 10.0 μm CP-55,940). The protein-loaded resin was transferred to a small column and washed with 2× 800 μl of the same buffer as above. Ligand was exchanged and lipids for reconstitution were added by passing buffer (10× 800 μl) supplemented with ligand and lipids through the column. The buffer contained 9.08 μm CP-55,940-d6 and the lipids: 3.2 mg/ml of POPC, 0.8 mg/ml of POPS, 1 mol % of POPC-d4 of total lipids, and 0.4 μg/ml of DilC18(5). Samples for competition binding experiments were prepared by adding both 9.08 μm CP-55,940-d6 and 90.8 μm protonated CP-55,940 to the buffer above. The protein was then eluted from the resin with 5× 200 μl of the same buffer that was used for ligand exchange but supplemented with 250 mm imidazole, pH adjusted to 7.5. The wash, exchange, elution, and reconstitution of the receptor by rapid dilution were conducted at 4 °C. The receptor was quantified by fluorescence intensity from the fluorophore-labeled receptor. The concentration of CP-55,940-d6 was measured by the intensity of the 2H NMR signal at 1.2 ppm. The intensity of the ligand signal was normalized by comparison to the internal standard, the head group Cα,Cβ-deuterated POPC-d4 with resonances at 3.6 and 4.3 ppm. The yields of CB2 receptor and phospholipids in the reconstituted samples were 230 μg and 6.5 mg, respectively.
Proteoliposomes for lipid order parameter studies were prepared using POPC with a perdeuterated palmitoyl chain (POPC-d31), i.e. 80 weight % POPC-d31 and 20 weight % POPS.
Proteoliposomes were precipitated by overnight centrifugation at 417,200 × g at 4 °C in the Optima TLX ultracentrifuge. The supernatant was discarded and the proteoliposome pellet re-dispersed in 2 ml of deuterium-depleted H2O for lipid order parameter measurements, or of de-ionized water for CP-55,940-d6 binding measurements and pelleted again. Each pellet was then transferred to a 4-mm outer diameter zirconia MAS rotor with a Kel-F insert for liquid samples (Bruker Biospin, Inc., Billerica, MA).
Solid-state NMR Measurements
1H MAS NMR experiments on proteoliposomes were conducted at 20 °C and a MAS frequency of 14.5 kHz on a AV800 spectrometer equipped with a 1H/13C/2H HR-MAS probe operating at the resonance frequency of 800.18 MHz (Bruker Biospin, Inc., Billerica, MA). Spectra were acquired with a relaxation delay time of 10 s. Static 31P NMR spectra of proteoliposomes were recorded at ambient temperature, nonspinning, on a DMX500 spectrometer with a 1H/X-PFG-MAS probe (Doty Scientific, Columbia, SC) at a resonance frequency of 202.46 MHz, using a Hahn echo sequence (d1-π/2-d2-π-d2-acquire)n with d1 = 1s, π/2 = 3 μs, d2 = 25 μs, n = 20,000, and 1H-continuous wave-decoupling at γHB1/2π = 20 kHz beginning with the π/2-pulse. The 2H MAS NMR measurements on CP-55,940-d6 binding were conducted at 20 °C and 14.5 kHz MAS on the Bruker AV800 spectrometer at 122.83 MHz. Typically 1,000,000 scans were acquired at a delay time between scans of 250 ms. The integral intensities of the 2H resonances of CP-55,940-d6 and POPC-d4 added as intensity standards were determined by spectral fitting (TopSpin 2.1, Bruker Biospin, Inc.).
Order parameters of lipid acyl chains in proteoliposomes were determined by recording static 2H NMR powder spectra of proteoliposomes at 25 °C using the AV800 spectrometer equipped with a MAS probe but without sample spinning. A quadrupolar echo sequence (d1-π/2-d2-π/290-d2-acquire)n (28) was used with d1 = 0.2 s, π/2 = 3.5 μs, d2 = 25 μs, n = 53,000–380,000, and the carrier frequency placed exactly at the center of the spectrum. Order parameters were determined from the 2H NMR quadrupolar splittings as described in supplemental Materials S1–S6.
RESULTS AND DISCUSSION
Reconstitution of CB2 Receptor into Liposomes
Formation of proteoliposomes harboring the human CB2 receptor was initiated by detergent removal from protein/lipid/detergent mixed micelles. In general, detergent removal can be performed by (i) treatment with detergent adsorbent, or (ii) rapid dilution below the CMC of detergent, followed by removal of detergent monomers in a filtration device, or (iii) dialysis. Those methods were applied to reconstitution of bovine rhodopsin (10, 29–31) and more recently to other recombinant GPCR (32–36). The methods differ in their efficiency of detergent removal and the yield of proteoliposomes. Here we conducted reconstitution of the receptor by (i) use of detergent adsorbent or (ii) rapid dilution. The dialysis method was not applied because it was reported that it may yield proteoliposomes of non-uniform protein/lipid ratio (37).
We tested several commercially available detergent adsorbents. Reconstitution using adsorbent took from minutes to several hours, depending on the type of adsorbent. Particular attention was devoted to finding a proper balance between efficiency of detergent removal and reduction of nonspecific adsorption of lipids and protein to the adsorbent. For each adsorbent, the effect of adsorbent/sample volume ratio, the time course of detergent removal, and the extent of unwanted adsorption of lipid and protein were examined. Careful optimization of the reconstitution conditions was performed, especially because we used mixtures of detergents to stabilize the CB2 receptor (13, 15, 16). An important advantage of using adsorbent is that detergents with a relatively low CMC can be removed efficiently. Detergent removing resin from Thermo Scientific (previously known as Extracti-Gel D from Pierce) performed satisfactorily with respect to yield of functional CB2 receptor in proteoliposomes and efficient removal of the detergents CHAPS, DM, and LDAO.
Protein/lipid/detergent mixed micelles containing the protein and phospholipids at a molar ratio of 1/580 were applied to a column packed with the adsorbent. The time course of protein and lipid elution was monitored by fluorescence of Alexa Fluor-532 labeled CB2 receptor (2 weight % added to unlabeled protein) and the fluorophore-labeled lipid DilC18(5) (0.01 weight % added to unlabeled phospholipids), respectively. The elution profiles of the receptor and lipids were identical indicating homogeneous incorporation of protein into liposomes upon detergent removal. Combined fractions (∼0.8 ml) corresponding to the initial 85% of total fluorescence in the eluate yielded proteoliposomes containing 62% of the receptor and 66% of phospholipids applied to the column. The residual detergent content in the combined fractions was below 1 mol % of total lipid as determined by high-resolution 1H NMR. Subsequent fractions contained higher amounts of residual detergent and were discarded.
The high resolution 1H NMR spectra also showed that CHS, an anionic analog of cholesterol (Fig. 2) that is added for stabilization of CB2 receptor in micelles (13, 15, 16), was also incorporated into the proteoliposomes upon reconstitution. The CHS content of proteoliposomes in a typical preparation was 25 mol % of total lipids, yielding a protein/phospholipid/CHS molar ratio of 1/620/210. In analogy to bovine rhodopsin, we expect the number of boundary lipids at the CB2 receptor-lipid interface to be 25–30 (38). Thus a few percent of all lipids are interacting directly with the protein at any given time, which is sufficient to study modulation of membrane structure and dynamics by CB2 receptor while maintaining a significant pool of unperturbed lipid molecules outside of the boundary shell. The mean diameter of proteoliposomes was ∼150 nm as measured by dynamic light scattering (Beckman Coulter model N4 Plus) suggesting that proteoliposomes in this preparation are predominantly unilamellar. The adsorbent column method has thus advantages for protein reconstitution in the submilligram range because preparation of detergent-free proteoliposomes is completed within minutes at optimal conditions. The detergent-free proteoliposomes enabled the influence of the lipid matrix on CB2 receptor function to be studied.
Alternatively, the receptor was reconstituted by rapid dilution of protein/lipid/detergent mixed micelles to below the CMC of detergents. Proteoliposome formation by this method can be completed within seconds to minutes. The method requires that the dominant detergent in the mixed micelles has a CMC near 0.1 mm or higher. Use of detergents with much lower CMC is not practical because it yields very dilute proteoliposomes. Despite the fast initial dilution step, subsequent removal of residual detergent may take from minutes to several hours or even days, depending on the method. Detergent removal by buffer exchange on membranes that are immobilized on a solid surface could be complete within minutes (39). Buffer exchange on a concentrator, or removal of detergents by dialysis takes from hours to days. Recovery of proteoliposomes is typically higher than for the detergent-adsorbent method.
In this work, the detergent monomers produced by rapid dilution were removed by buffer exchange on a concentrator. The process was complete within hours. The concentrator had a filter that is impermeable for proteoliposomes. Material recoveries for a sample containing 100 μg of the receptor and 1 mg of phospholipid were 74% for the receptor and 84% for phospholipid as measured by fluorescence. Efficiency of reconstitution was somewhat higher than in the adsorbent column method. The material loss in the rapid dilution method is most likely caused by trapping of proteoliposomes on the filter of the concentration device. Losses were minimized by optimizing the stirring and the flow rate of the buffer through the filter that was regulated by argon gas pressure. Reconstitution by rapid dilution is particularly well suited for preparation of larger samples as required for NMR structural studies (40). This is because relative losses of protein and lipid tend to get smaller with increasing sample size. For example, protein losses for a sample containing 1 mg of the receptor and 10 mg of phospholipids were just a few percent. This is in contrast to the adsorbent column method that requires use of larger columns with increasing sample size, whereas fractional losses remain constant or even increase with sample size. The properties of proteoliposomes obtained by rapid dilution were similar to those from the adsorbent column preparation. The protein/phospholipid/CHS molar ratio was 1/650/220, and the content of residual detergent was below 1 mol % of total lipids. The mean diameter of proteoliposomes was ∼200 nm, slightly larger than for liposomes from the adsorbent column preparation.
The proteoliposomes were pelleted by ultracentrifugation and the pellet was transferred to a MAS rotor for NMR experiments. The pellet contained about 30 weight % of proteoliposomes (dry weight) and 70 weight % of buffer. Fig. 3a shows a 1H MAS NMR spectrum (20 °C) of the proteoliposomes prepared by rapid dilution. The well resolved resonances of POPC/POPS/CHS (4.0/1.0/1.67, mol/mol/mol) indicate that the lipid matrix of proteoliposomes is in a liquid-crystalline phase. The removal of detergents to below detection limits is confirmed by the absence of detergent resonances. The static 31P NMR spectrum of the lipid phosphate in proteoliposomes (Fig. 3b) shows a well resolved chemical shift anisotropy of −44 ppm that is characteristic of a uniform, liquid crystalline, lamellar phase. The isotropic peak in the spectrum is from the PBS buffer.
FIGURE 3.
a, 1H MAS NMR spectrum of CB2 receptor proteoliposomes recorded at 20 °C and a MAS frequency of 14.5 kHz. Signal assignments were aided by a previous report from our laboratory (79); b, static 31P NMR spectrum of the proteoliposomes recorded at ambient temperature; c, density distribution of proteoliposomes measured by isopycnic centrifugation using a sucrose gradient of 1.010–1.081 mg/cm3.
Homogeneity of Proteoliposomes
The homogeneity of the protein/lipid ratio of proteoliposome preparations was tested by isopycnic gradient ultracentrifugation. This method probes density differences between proteoliposomes due to heterogeneity of protein content, by taking advantage of the significant density differences between the lipid matrix and proteins. Centrifugation was conducted using a linear sucrose gradient from 1.010 to 1.081 mg/cm3. Fig. 3c shows photographs taken after completion of centrifugation. The proteoliposomes prepared both by a detergent-adsorbent column or rapid dilution yielded a single, narrow band (indicated by an arrow) confirming high homogeneity of the protein/lipid ratio. For a quantitative analysis, the sucrose solution was carefully aliquoted into 100-μl fractions without disturbing the sucrose gradient, and protein and lipid content in each fraction were quantified by fluorescence (Fig. 3c). The sucrose concentration at the center of the band was determined by measurement of the refractive index. The sucrose densities at the band of proteoliposomes were 1.056 and 1.058 g/cm3 for proteoliposomes prepared by the detergent adsorbent column and by rapid dilution, respectively. Assuming a protein density of 1.35 g/cm3 (41) and taking the measured protein/phospholipid/CHS molar ratio into account, the density of the lipid matrix of proteoliposomes is estimated to be ∼1.04 g/cm3, which is in reasonable agreement with expectations from density measurements on protein-free liposomes (42–44).
Functional Activity of Reconstituted CB2 Receptor. CB2 receptor is activated upon specific binding of cannabinoid agonists to a putative binding site formed by transmembrane helices III, V, VI, and VII near the extracellular membrane surface (21, 45). Current models propose that ligand binding induces a conformational change in CB2 receptor, resulting in formation of a catalytic surface on the cytoplasmic side of the receptor where binding and activation of cognate G proteins takes place. We studied functional activity of the reconstituted CB2 receptor both by ligand binding and G protein activation.
Ligand binding studies with cannabinoids are technically challenging because of their amphiphilicity that favors nonspecific partitioning into the lipid matrix. The octanol-water partition coefficient of agonist CP-55,940 used in this study is 1.35 × 106 (46). We investigated ligand binding by 2H MAS NMR using 2H-labeled CP-55,940-d6 (Fig. 4a). The affinity of CP-55,940-d6 for CB2 receptor is indistinguishable from the affinity of protonated CP-55,940 (Kd = 1–2 nm) as determined in a radioligand binding assay using membrane preparations of E. coli cells expressing CB2 receptor. The advantage of NMR is that it can distinguish between nonspecific binding of ligand to the lipid matrix and specific binding to CB2 receptor because of drastic differences between the spectra of ligand from both locations. Nonspecifically bound CP-55,940-d6 in the lipid matrix yields a well resolved 2H NMR resonance at 1.2 ppm (21), whereas specific binding to the CB2 receptor broadens the resonance beyond detection due to motional restrictions of the ligand in the binding pocket.
FIGURE 4.
a, 2H-labeled cannabinoid agonist CP-55,940-d6. b, 2H MAS NMR spectra of CP-55,940-d6 in CB2 receptor proteoliposomes containing 1 mol % of POPC-d4 as an internal intensity standard. Spectra were recorded at 20 °C and a MAS frequency of 14.5 kHz. Spectra without (black) and with (blue) a 10-fold excess of unlabeled CP-55,940 were recorded. The inset shows the same spectra with intensity adjustment to match signal intensities of the ligand. The asterisk denotes a weak natural abundance 2H signal from the terminal methyl groups of lipid hydrocarbon chains. c, activation of G protein by agonist-bound CB2 receptor reconstituted into liposomes. A plasma membrane preparation of E. coli cells expressing CB2 receptor-130 fusion protein was used as control. Its CB2 receptor content was determined to be 2.5 μg/mg of total protein. An average of 7,560 counts per minute (cpm) for the [35S] GTPγS·Gαi1 complex per ng of CB2 receptor, after subtraction of background reactivity without CB2 receptor, was measured as control and is reported as 100%. The data shown in c are the average of four G protein activation assays conducted on one set of samples (n = 4).
In a separate study we have determined that the CB2 receptor must be purified and reconstituted in the presence of a strongly binding ligand to ensure its stability in the micellar state.4 Therefore, the protein sample was extracted and purified in the presence of protonated CP-55,940. For the 2H NMR measurement of ligand binding, exchange to the 2H-labeled CP-55,940-d6 was conducted in the micellar state upon immobilization of the receptor on a Ni-NTA resin (see ”Experimental Procedures“ for details). Two CB2-receptor samples in micelles were prepared: one containing only CP-55,940-d6, and a second one containing CP-55,940-d6 plus a 10-fold molar excess of unlabeled CP-55,940. Both samples were then reconstituted into liposomes (POPC/POPS/CHS = 4/1/1.67, mol/mol/mol). The POPC was doped with 1 mol % of POPC-d4, an internal intensity standard for the 2H NMR experiments. The samples were transferred to MAS rotors and the 2H MAS NMR spectra were recorded. The intensity of the resonance at 1.2 ppm reports the amount of nonspecifically bound CP-55,940-d6. The intensity difference of the 2H NMR resonance between the spectra yields the molar concentration of ligand binding-competent CB2 receptor; note that the ligand occupies ∼100% of binding sites on functional CB2 receptor at the ligand concentrations used in this experiment.
The 2H MAS NMR spectra of the CP-55,940-d6 in CB2 proteoliposomes with and without the 10-fold excess of protonated CP-55,940 are presented in Fig. 4b. The molar ratio of CB2 receptor to CP-55,940-d6 in the proteoliposomes was 1.00/3.45. The intensity of the CP-55,940-d6 resonance in the sample, to which excess protonated CP-55,940 was added, is higher as indicated by an arrow. The observation indicates release of CP-55,940-d6 from the receptor as a result of competition with protonated CP-55,940.
Intensities of the 2H NMR resonances of CP-55,940-d6 in the spectra were compared as follows: after baseline correction of the spectra, the intensity of the signal of deuterated standard was adjusted to match and the intensities of the 2H resonance of the deuterated ligand were compared (Fig. 4b). As a cross-check, we also matched the signals of deuterated ligand and compared intensities of the lipid standard (see inset in Fig. 4b). Addition of a 10-fold molar excess of protonated CP-55,940 resulted in a 35 ± 1% increase in the intensity of the CP-55,940-d6 2H resonance. An increase by 37% was expected if 100% of the CB2 receptor would have been ligand binding competent (see supplemental Materials S1–S6). Therefore, 95 ± 3% of the reconstituted CB2 receptor binds ligand at a 1:1 stoichiometry. Considering that the error margin for determination of protein content by fluorescence in the samples is ±5%, we conclude that ≥90% of the reconstituted receptor is ligand-binding competent.
The functional activity of the reconstituted receptor was further studied in a G protein activation assay that measures nucleotide exchange on the Gα subunit of heterotrimeric G protein catalyzed by the agonist-activated receptor. We report as activation the quantity of the GTPγS·Gαi1 complex produced per ng of CB2 receptor and unit of time, in the presence of a large excess of CP-55,940. Fig. 4c shows the G protein activation measured on the CB2 receptor in liposomes (POPC/CHS = 3/1, mol/mol). The G protein activation by reconstituted CB2 receptor was comparable with the activation observed for MBP fusion CB2 receptor in the plasma membranes of E. coli. We reported previously that the MBP fusion CB2 receptor in E. coli membranes exhibited a dose-dependent activation of G protein in response to binding of CP-55,940 characterized by an EC50 in the nanomolar range, indicating full receptor functionality (13). The current results indicate that cleavage of the MBP fusion, purification, and reconstitution of the CB2 receptor do not interfere with its ability to activate G protein.
Function of the CB2 receptor in immune cells is intricately linked to the type of agonist that activates the receptor (7, 47). Agonists are characterized by their efficacy and potency in triggering signaling events. On our reconstituted CB2 receptor, it is technically challenging to accurately assess efficacy and potency of ligands in regulating G protein activation due to the need to stabilize the receptor with an excess of a strongly binding ligand during sample preparation.4 The very high lipophilicity of cannabinoids makes it difficult to remove them from reconstituted samples. Therefore, we are unable to report efficacy and potency of activation of our recombinant CB2 receptor by CP-55,940 at this time. In the following, we analyzed G protein activation by ∼90% ligand-bound CB2 receptor in different lipid environments, measured in the presence of a large excess of CP-55,940.
As we reported previously (13), without a stabilizing ligand, only about one-third of the reconstituted CB2 receptor maintains ligand-binding competence. Importantly, in these experiments the affinity of the reconstituted CB2 to the radiolabeled agonist CP-55,940 was in the low nanomolar range (13), similar to values for the native receptor in mammalian cells, suggesting that the structure of this ligand-binding competent fraction of the reconstituted receptor remained intact.
Influence of Lipids on CB2 Receptor Function
The successful reconstitution of CB2 receptor enables studies on the influence of the lipid matrix on structure and function of the receptor. Here we investigated the effect of the electric membrane-surface potential, which is closely related to the ζ-potential, and the lipid acyl-chain order on CB2-receptor function. G protein activation by CP-55,940-bound CB2 receptor was measured as a function of the anionic lipid content in the membrane that was varied systematically (see scheme in Fig. 5a), either by adding more CHS (pKa = 5.8 (48)) (Fig. 5b) or by varying the POPC/POPS ratio (Fig. 5c).
FIGURE 5.
Effect of negative electric surface potentials of proteoliposomes on G protein activation by CB2 receptor. a, scheme showing sample preparation with variations of anionic lipid content (CHS or POPS) in proteoliposomes. All proteoliposomes contain a basal level of 25 mol % of CHS (marked with asterisk) to stabilize CB2 receptor in micelles. Either an extra 16 mol % of CHS was added at a constant POPC/POPS molar ratio of 4/1, or an equivalent amount of Chol was added instead of CHS, or the POPC/POPS ratio was varied from 5/0 to 1/4. b, influence of an extra 16 mol % of CHS or Chol on G protein activation by CB2 receptor. The control sample I (G protein activation = 1) had a composition of CB2/POPC/POPS/CHS, 1/520/130/220 (mol/mol/mol/mol). Results are the average of two independently prepared samples for each composition assayed two times each (n = 4). c, G protein activation by CB2 receptor as a function of the POPC/POPS molar ratio. The G protein activation of the sample with a composition of CB2/POPC/CHS, 1/650/220 (mol/mol/mol), labeled as 5/0 (control II), was set to 1. Results are the average of four G protein activation assays conducted on one set of five samples (n = 4).
Fig. 5b shows the effect of an increase of the CHS concentration from 25 to 41 mol % of total lipid on the degree of G protein activation. At 41 mol % of CHS, the activation is 2-fold higher than at 25 mol % CHS. The concentration of CHS had no influence on the basal rate of nucleotide exchange on Gαi1 as confirmed by experiments using empty liposomes without the receptor (not shown). The increase of CHS content from 25 to 41 mol % increased the negative ζ-potential of the proteoliposomes measured by their electrophoretic mobility from −32 to −38 mV (Table 1). Empty liposomes (containing no protein) with identical lipid composition had a similar ζ-potential difference of 6 mV, but the potentials were more negative, indicating that the receptor contributes a net positive charge to the liposome-water interface.
TABLE 1.
ξ-Potentials (in mV) of CB2 proteoliposomes, and liposomes, containing 25 mol % CHS (control I), 41 mol % CHS (+CHS), and 41 mol % CHS + cholesterol (+Chol)
The POPC/POPS molar ratio was 4/1.
| 25 mol % CHS (control I) | 41 mol % CHS (+ CHS) | 41 mol % CHS + cholesterol (+ Chol) | |
|---|---|---|---|
| CB2 proteoliposomes | −32.0 ± 3.5 | −38.1 ± 3.9 | −34.3 ± 1.9 |
| Liposomes | −41.1 ± 3.2 | −46.7 ± 5.2 | −42.6 ± 2.9 |
Because CHS is an analog of cholesterol, it not only increases negative surface potentials but also raises lipid acyl-chain order. Lipid acyl-chain ordering due to the presence of CHS was measured using POPC with a perdeuterated palmitoyl chain (POPC-d31). Order parameters Sn of the palmitoyl chain segments were determined by measurement of the 2H quadrupolar splittings, ΔνQ, by static 2H NMR (Fig. 6a). Larger ΔνQ were observed for proteoliposomes with higher CHS contents, indicating that order parameters of the palmitoyl chains increased. The corresponding order parameter profiles are shown in Fig. 6b. The order increase caused by addition of more CHS was comparable with the order increase by addition of an equivalent amount of cholesterol (Fig. 6c). However, whereas the addition of CHS enhanced G protein activation by the CB2 receptor, addition of cholesterol did not (Fig. 5b). The result strongly suggests that the observed increase of G protein activation with higher CHS content stems from the higher negative electric surface potential of the membrane rather than an effect from hydrocarbon chain order.
FIGURE 6.
a, static 2H NMR spectra of POPC-d31 in CB2 proteoliposomes (protein/phospholipid molar ratio 1/650) containing 25 and 41 mol % of CHS, respectively, recorded at 25 °C. The POPC-d31/POPS molar ratio was 4/1. b, order parameter profiles Sn calculated from the 2H quadrupolar splittings (78). c, effect of addition of Chol instead of CHS on the order parameters of proteoliposomes.
From experiments on rhodopsin, another GPCR of class A, formation of meta-rhodopsin-II, the photoisomer that activates G protein, involves an outward radial movement of helix VI away from the core composed of transmembrane helices I-IV (49). This results in a change of shape of the receptor that may trigger an elastic deformation of the surrounding lipid matrix and may link GPCR activation to membrane elastic properties. For rhodopsin the degree of receptor activation is known to decrease with increasing concentrations of cholesterol (50). There was no decline in G protein activation by the CB2 receptor with increasing cholesterol content, suggesting that the CB2 receptor remained fully activated, despite the increase in membrane stiffness as demonstrated by the significant increase in lipid hydrocarbon chain order parameters (see Fig. 6).
In a related observation, alteration of the cholesterol content in the plasma membranes of human DAUDI leukemia cells by treatment with methyl-β-cyclodextrin did not affect binding affinity of CP-55,940 to the CB2 receptor (51). Furthermore, the [35S]GTPγS binding on G proteins, activities of adenylate cyclase, and mitogen-activated protein kinase have not been affected by changes in the cholesterol content in these cells, either. This is in marked contrast to the central-type CB1 receptor studied in rat C6 glioma cells that showed inhibited CP-55,940 binding upon an increase in cholesterol concentration and enhanced ligand binding upon cholesterol depletion as well as corresponding changes in downstream signaling (52, 53). The results obtained on mammalian cell cultures were interpreted as localization of CB1 in cholesterol-rich microdomains and exclusion of the CB2 receptor from such domains (54, 55). Although our results, obtained on recombinant CB2 receptor, show an absence of cholesterol effects on CB2 receptor activation as well, they may offer an alternative interpretation for the difference between CB1 and CB2 receptors. It could be that the CB2 receptor reacts intrinsically different to changes in membrane stiffness resulting from an increase in cholesterol concentrations.
The influence of electric surface potentials on G protein activation by the CB2 receptor was studied further by changing the POPS content of proteoliposomes (Fig. 5c). The POPC/POPS molar ratio was varied from 5/0 to 1/4, whereas the content of CHS remained constant at 25 mol %. Because POPS is fully ionized at pH of 7.5, regardless of the PC/PS ratio in the lipid matrix, we were able to systematically change the negative electric surface potentials (56, 57). POPS has a gel-fluid phase transition temperature of 13 °C (58) and is in the fluid lamellar phase at our experimental conditions of G protein activation measurements. The efficiency of activation increased with increasing POPS content up to the ratio of 3/2 and decreased again at higher POPS concentrations. This observation was confirmed to be highly reproducible for several preparations of proteoliposomes.
The results highlight the importance of anionic lipid content of membranes for signal transduction by the CB2 receptor and possibly by other class A GPCR in general. The increase in negative electric surface potentials may affect interactions between the membrane interface and amphipathic helix VIII of class A GPCR, which typically carries a net positive charge (Fig. 1) (59). Using model peptides of rhodopsin and angiotensin II, it was shown that anionic lipids have a large influence on the α-helical content of helix VIII that reaches a maximum at ∼50 mol % of anionic lipids in membranes (60, 61). The crystal structure of the complex of bovine rhodopsin with the C-terminal fragment of Gα suggests that helix VIII constitutes an important element for binding G proteins to the receptor (62, 63). Therefore, it is possible that structural stabilization of helix VIII in the CB2 receptor, regulated by anionic lipids, results in an increase in G protein activation. Structural investigations of helix VIII of CB1 and CB2 receptors were attempted using model peptides in micellar solution (64–66). Negative surface potentials attract hydronium ions to the membrane surface and lower the surface pH, which favors the opening of the proposed ionic lock between transmembrane helices III and VI in the CB2 receptor (67), a putatively critical step in activation of the receptor.
According to literature, the partitioning of Gαi to the membrane interface is not affected by the anionic-lipid content (68); Gβ1γ2 was provided in 10-fold excess to Gαi in our experiments that makes it less likely that the surface potential limits recruitment of G protein to the membrane. Furthermore, according to molecular simulations, recruitment of the homologous G protein transducin is almost independent on concentration of the negatively charged PS in bilayers at concentrations of PS higher than 20 mol % (69). Therefore, our measured dependence of G protein activation on PS and CHS content may be explained by modulation of the structure of the CB2 receptor rather than by a change of the binding behavior of G protein subunits at the membrane surface. However, more work is required to lend solid support to this statement.
It is intriguing that G protein activation efficiency is highest at a content of anionic lipids that exceeds typical concentrations in the mammalian plasma membranes, even when considering that anionic phospholipids are predominantly located in the inner leaflet of the cytoplasmic membrane where G protein activation occurs (70, 71). The highest degree of G protein activation by the receptor may not necessarily be optimal for signaling at physiological conditions. The content of anionic lipids in the inner leaflet of various types of immune cells expressing CB2 receptor has yet to be determined (72). It is possible that activities of immune cells are finely regulated by the electric surface potential of the plasma membrane via differences in activation of the CB2 receptor. In plasma membranes of viable (nonapoptotic) immune cells, PS was even observed in the outer monolayer (73–75), further suggesting that the PS content of membranes in general may play an important role in regulating cell function.
Structural studies on G protein binding CB2 receptor by solid-state NMR as well as G protein binding studies to the reconstituted receptor at a solid interface by surface plasmon resonance are in progress at our lab to further unveil molecular mechanisms of the receptor activation and of the receptor-G protein interaction (40, 76).
Supplementary Material
Acknowledgments
We thank Lioudmila Zoubak for assistance with preparation of the recombinant CB2 receptor and G protein activation analysis, Kirk Hines and Walter Teague for assistance with the isopycnic centrifugation experiments, and Elena Mekhedov and Joshua Zimmerberg for assistance with the dynamic light scattering measurements.
This work was supported, in whole or in part, by a National Institutes of Health grant from the Intramural Research Programs of the NIAAA and NIDA.

This article contains supplemental Materials S1–S6.
K. Vukoti, T. Kimura, L. B. Macke, K. Gawrisch, and A. A. Yeliseev, manuscript in preparation.
- CB2
- cannabinoid type 2 receptor
- GPCR
- G protein-coupled receptor
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPC-d31
- 1-palmitoyl(D31)-2-oleoyl-sn-glycero-3-phosphocholine
- POPC-d4
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine-1,1,2,2-d4
- POPS
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine
- CHS
- cholesteryl hemisuccinate
- Chol
- cholesterol
- MAS
- magic angle spinning
- DM
- n-dodecyl-β-d-maltoside
- LDAO
- N,N-dimethyldodecylamine N-oxide
- CMC
- critical micelle concentration
- MBP
- maltose-binding protein
- Ni-NTA
- nickel-nitrilotriacetic acid
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate.
REFERENCES
- 1. Cabral G. A., Griffin-Thomas L. (2009) Emerging role of the cannabinoid receptor CB2 in immune regulation. Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 11, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Núñez E., Benito C., Pazos M. R., Barbachano A., Fajardo O., González S., Tolón R. M., Romero J. (2004) Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain. An immunohistochemical study. Synapse 53, 208–213 [DOI] [PubMed] [Google Scholar]
- 3. Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N. E., Schatz A. R., Gopher A., Almog S., Martin B. R., Compton D. R., Pertwee R. G., Griffin G., Bayewitch M., Barg J., Vogel Z. (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50, 83–90 [DOI] [PubMed] [Google Scholar]
- 4. Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 [DOI] [PubMed] [Google Scholar]
- 5. Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. (1995) 2-Arachidonoylglycerol. A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215, 89–97 [DOI] [PubMed] [Google Scholar]
- 6. Pacher P., Bátkai S., Kunos G. (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 58, 389–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller A. M., Stella N. (2008) CB2 receptor-mediated migration of immune cells. It can go either way. Br. J. Pharmacol. 153, 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ibrahim M. M., Deng H., Zvonok A., Cockayne D. A., Kwan J., Mata H. P., Vanderah T. W., Lai J., Porreca F., Makriyannis A., Malan T. P., Jr. (2003) Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. U.S.A. 100, 10529–10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibrahim M. M., Porreca F., Lai J., Albrecht P. J., Rice F. L., Khodorova A., Davar G., Makriyannis A., Vanderah T. W., Mata H. P., Malan T. P., Jr. (2005) CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. U.S.A. 102, 3093–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jackson M. L., Litman B. J. (1985) Rhodopsin-egg phosphatidylcholine reconstitution by an octyl glucoside dilution procedure. Biochim. Biophys. Acta 812, 369–376 [DOI] [PubMed] [Google Scholar]
- 11. Baker M. (2010) Structural biology. The gatekeepers revealed. Nature 465, 823–826 [DOI] [PubMed] [Google Scholar]
- 12. Rasmussen S. G., DeVree B. T., Zou Y., Kruse A. C., Chung K. Y., Kobilka T. S., Thian F. S., Chae P. S., Pardon E., Calinski D., Mathiesen J. M., Shah S. T. A., Lyons J. A., Caffrey M., Gellman S. H., Steyaert J., Skiniotis G., Weis W. I., Sunahara R. K., Kobilka B. K. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeliseev A., Zoubak L., Gawrisch K. (2007) Use of dual affinity tags for expression and purification of functional peripheral cannabinoid receptor. Protein Expr. Purif. 53, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calandra B., Tucker J., Shire D., Grisshammer R. (1997) Expression in Escherichia coli and characterization of the human central CB1 and peripheral CB2 cannabinoid receptors. Biotechnol. Lett. 19, 425–428 [Google Scholar]
- 15. Yeliseev A. A., Wong K. K., Soubias O., Gawrisch K. (2005) Expression of human peripheral cannabinoid receptor for structural studies. Protein Sci. 14, 2638–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krepkiy D., Gawrisch K., Yeliseev A. (2007) Expression and purification of CB2 for NMR studies in micellar solution. Protein Pept. Lett. 14, 1031–1037 [DOI] [PubMed] [Google Scholar]
- 17. Schubert R. (2003) Liposome preparation by detergent removal. Methods Enzymol. 367, 46–70 [DOI] [PubMed] [Google Scholar]
- 18. Rigaud J. L., Lévy D. (2003) Reconstitution of membrane proteins into liposomes. Methods Enzymol. 372, 65–86 [DOI] [PubMed] [Google Scholar]
- 19. Silvius J. R. (1992) Solubilization and functional reconstitution of biomembrane components. Annu. Rev. Biophys. Biomol. Struct. 21, 323–348 [DOI] [PubMed] [Google Scholar]
- 20. Eytan G. D. (1982) Use of liposomes for reconstitution of biological functions. Biochim. Biophys. Acta 694, 185–202 [DOI] [PubMed] [Google Scholar]
- 21. Kimura T., Cheng K., Rice K. C., Gawrisch K. (2009) Location, structure, and dynamics of the synthetic cannabinoid ligand CP-55,940 in lipid bilayers. Biophys. J. 96, 4916–4924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herrmann K. W. (1962) Non-ionic-cationic micellar properties of dimethyldodecylamine oxide. J. Phys. Chem. 66, 295–300 [Google Scholar]
- 23. Chattopadhyay A., Harikumar K. G. (1996) Dependence of critical micelle concentration of a zwitterionic detergent on ionic strength. Implications in receptor solubilization. FEBS Lett. 391, 199–202 [DOI] [PubMed] [Google Scholar]
- 24. VanAken T., Foxall-VanAken S., Castleman S., Ferguson-Miller S. (1986) Alkyl glycoside detergents. Synthesis and applications to the study of membrane proteins. Methods Enzymol. 125, 27–35 [DOI] [PubMed] [Google Scholar]
- 25. Mumby S. M., Linder M. E. (1994) Heterotrimeric 6 Proteins (Iyengar R., ed) pp. 254–268, Academic Press, New York [Google Scholar]
- 26. Wildman D. E., Tamir H., Leberer E., Northup J. K., Dennis M. (1993) Prenyl modification of guanine nucleotide regulatory protein γ2 subunits is not required for interaction with the transducin α subunit or rhodopsin. Proc. Natl. Acad. Sci. U.S.A. 90, 794–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aveyard R., Haydon D. A. (1973) An Introduction to the Principles of Surface Chemistry, Cambridge University Press, London [Google Scholar]
- 28. Davis J. H., Jeffrey K. R., Bloom M., Valic M. I., Higgs T. P. (1976) Quadrupolar echo deuteron magnetic-resonance spectroscopy in ordered hydrocarbon chains. Chem. Phys. Lett. 42, 390–394 [Google Scholar]
- 29. Jackson M. L., Litman B. J. (1982) Rhodopsin-phospholipid reconstitution by dialysis removal of octyl glucoside. Biochemistry 21, 5601–5608 [DOI] [PubMed] [Google Scholar]
- 30. De Grip W. J., Olive J., Bovee-Geurts P. H. M. (1983) Reversible modulation of rhodopsin photolysis in pure phosphatidylserine membranes. Biochim. Biophys. Acta 734, 168–179 [Google Scholar]
- 31. De Grip W. J., Vanoostrum J., Bovee-Geurts P. H. (1998) Selective detergent-extraction from mixed detergent/lipid/protein micelles, using cyclodextrin inclusion compounds. A novel generic approach for the preparation of proteoliposomes. Biochem. J. 330, 667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leitz A. J., Bayburt T. H., Barnakov A. N., Springer B. A., Sligar S. G. (2006) Functional reconstitution of β2-adrenergic receptors utilizing self-assembling Nanodisc technology. BioTechniques 40, 601–602, 604,, 606 [DOI] [PubMed] [Google Scholar]
- 33. Sarramegn V., Muller I., Milon A., Talmont F. (2006) Recombinant G protein-coupled receptors from expression to renaturation. A challenge towards structure. Cell. Mol. Life Sci. 63, 1149–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Swaminath G., Deupi X., Lee T. W., Zhu W., Thian F. S., Kobilka T. S., Kobilka B. (2005) Probing the β2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J. Biol. Chem. 280, 22165–22171 [DOI] [PubMed] [Google Scholar]
- 35. Whorton M. R., Bokoch M. P., Rasmussen S. G., Huang B., Zare R. N., Kobilka B., Sunahara R. K. (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. U.S.A. 104, 7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Granier S., Kim S., Shafer A. M., Ratnala V. R., Fung J. J., Zare R. N., Kobilka B. (2007) Structure and conformational changes in the C-terminal domain of the β2-adrenoceptor. Insights from fluorescence resonance energy transfer studies. J. Biol. Chem. 282, 13895–13905 [DOI] [PubMed] [Google Scholar]
- 37. Niu S. L., Doctrow B., Mitchell D. C. (2009) Rhodopsin activity varies in proteoliposomes prepared by different techniques. Biochemistry 48, 156–163 [DOI] [PubMed] [Google Scholar]
- 38. Watts A., Volotovski I. D., Marsh D. (1979) Rhodopsin-lipid associations in bovine rod outer segment membranes. Identification of immobilized lipid by spin labels. Biochemistry 18, 5006–5013 [DOI] [PubMed] [Google Scholar]
- 39. Soubias O., Polozov I. V., Teague W. E., Yeliseev A. A., Gawrisch K. (2006) Functional reconstitution of rhodopsin into tubular lipid bilayers supported by nanoporous media. Biochemistry 45, 15583–15590 [DOI] [PubMed] [Google Scholar]
- 40. Kimura T., Vukoti K., Lynch D. L., Hurst D. P., Grossfield A., Pitman M. C., Reggio P. H., Yeliseev A. A., Gawrisch K. (2010) Secondary-structure analysis of human peripheral cannabinoid receptor CB2 based on solid-state 13C− 15N-MAS NMR and molecular dynamics simulations. Biophys. J. 98, 625a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Messerschmidt A., Huber R. (2007) X-Ray Diffraction of Biological Macromolecules, Wiley-VCH, Weinheim, Germany [Google Scholar]
- 42. Koenig B. W., Gawrisch K. (2005) Specific volumes of unsaturated phosphatidylcholines in the liquid crystalline lamellar phase. Biochim. Biophys. Acta 1715, 65–70 [DOI] [PubMed] [Google Scholar]
- 43. Heerklotz H., Tsamaloukas A. (2006) Gradual change or phase transition. Characterizing fluid lipid-cholesterol membranes on the basis of thermal volume changes. Biophys. J. 91, 600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Petrache H. I., Tristram-Nagle S., Gawrisch K., Harries D., Parsegian V. A., Nagle J. F. (2004) Structure and fluctuations of charged phosphatidylserine bilayers in the absence of salt. Biophys. J. 86, 1574–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xie X. Q., Chen J. Z., Billings E. M. (2003) Three-dimensional structural model of the G-protein-coupled cannabinoid CB2 receptor. Proteins 53, 307–319 [DOI] [PubMed] [Google Scholar]
- 46. Thomas B. F., Compton D. R., Martin B. R. (1990) Characterization of the lipophilicity of natural and synthetic analogs of δ9-tetrahydrocannabinol and its relationship to pharmacological potency. J. Pharmacol. Exp. Ther. 255, 624–630 [PubMed] [Google Scholar]
- 47. Shoemaker J. L., Ruckle M. B., Mayeux P. R., Prather P. L. (2005) Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J. Pharmacol. Exp. Ther. 315, 828–838 [DOI] [PubMed] [Google Scholar]
- 48. Hafez I. M., Cullis P. R. (2000) Cholesteryl hemisuccinate exhibits pH-sensitive polymorphic phase behavior. Biochim. Biophys. Acta 1463, 107–114 [DOI] [PubMed] [Google Scholar]
- 49. Farrens D. L., Altenbach C., Yang K., Hubbell W. L., Khorana H. G. (1996) Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274, 768–770 [DOI] [PubMed] [Google Scholar]
- 50. Mitchell D. C., Straume M., Miller J. L., Litman B. J. (1990) Modulation of metarhodopsin formation by cholesterol-induced ordering of bilayer lipids. Biochemistry 29, 9143–9149 [DOI] [PubMed] [Google Scholar]
- 51. Bari M., Spagnuolo P., Fezza F., Oddi S., Pasquariello N., Finazzi-Agrò A., Maccarrone M. (2006) Effect of lipid rafts on CB2 receptor signaling and 2-arachidonoyl-glycerol metabolism in human immune cells. J. Immunol. 177, 4971–4980 [DOI] [PubMed] [Google Scholar]
- 52. Bari M., Battista N., Fezza F., Finazzi-Agrò A., Maccarrone M. (2005) Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J. Biol. Chem. 280, 12212–12220 [DOI] [PubMed] [Google Scholar]
- 53. Bari M., Paradisi A., Pasquariello N., Maccarrone M. (2005) Cholesterol-dependent modulation of type 1 cannabinoid receptors in nerve cells. J. Neurosci. Res. 81, 275–283 [DOI] [PubMed] [Google Scholar]
- 54. Sarnataro D., Grimaldi C., Pisanti S., Gazzerro P., Laezza C., Zurzolo C., Bifulco M. (2005) Plasma membrane and lysosomal localization of CB1 cannabinoid receptor are dependent on lipid rafts and regulated by anandamide in human breast cancer cells. FEBS Lett. 579, 6343–6349 [DOI] [PubMed] [Google Scholar]
- 55. Bari M., Oddi S., De Simone C., Spagnolo P., Gasperi V., Battista N., Centonze D., Maccarrone M. (2008) Type-1 cannabinoid receptors colocalize with caveolin-1 in neuronal cells. Neuropharmacology 54, 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cevc G., Watts A., Marsh D. (1981) Titration of the phase transition of phosphatidylserine bilayer membranes. Effects of pH, surface electrostatics, ion binding, and head group hydration. Biochemistry 20, 4955–4965 [DOI] [PubMed] [Google Scholar]
- 57. Roy M. T., Gallardo M., Estelrich J. (1998) Influence of size on electrokinetic behavior of phosphatidylserine and phosphatidylethanolamine lipid vesicles. J. Colloid Interface Sci. 206, 512–517 [DOI] [PubMed] [Google Scholar]
- 58. Chen N. R., Ho J. T. (1985) Raman study of effect of phospholipid chain unsaturation on bilayer phase transitions. Biochem. Biophys. Res. Commun. 127, 220–225 [DOI] [PubMed] [Google Scholar]
- 59. Kimura T. (2006) Human opioid peptide Met-enkephalin binds to anionic phosphatidylserine in high preference to zwitterionic phosphatidylcholine. Natural abundance 13C NMR study on the binding state in large unilamellar vesicles. Biochemistry 45, 15601–15609 [DOI] [PubMed] [Google Scholar]
- 60. Krishna A. G., Menon S. T., Terry T. J., Sakmar T. P. (2002) Evidence that helix 8 of rhodopsin acts as a membrane-dependent conformational switch. Biochemistry 41, 8298–8309 [DOI] [PubMed] [Google Scholar]
- 61. Mozsolits H., Unabia S., Ahmad A., Morton C. J., Thomas W. G., Aguilar M. I. (2002) Electrostatic and hydrophobic forces tether the proximal region of the angiotensin II receptor (AT1A) carboxyl terminus to anionic lipids. Biochemistry 41, 7830–7840 [DOI] [PubMed] [Google Scholar]
- 62. Scheerer P., Park J. H., Hildebrand P. W., Kim Y. J., Krauss N., Choe H. W., Hofmann K. P., Ernst O. P. (2008) Crystal structure of opsin in its G-protein-interacting conformation. Nature 455, 497–502 [DOI] [PubMed] [Google Scholar]
- 63. Choe H. W., Kim Y. J., Park J. H., Morizumi T., Pai E. F., Krauss N., Hofmann K. P., Scheerer P., Ernst O. P. (2011) Crystal structure of metarhodopsin II. Nature 471, 651–655 [DOI] [PubMed] [Google Scholar]
- 64. Grace C. R., Cowsik S. M., Shim J. Y., Welsh W. J., Howlett A. C. (2007) Unique helical conformation of the fourth cytoplasmic loop of the CB1 cannabinoid receptor in a negatively charged environment. J. Struct. Biol. 159, 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Choi G., Guo J., Makriyannis A. (2005) The conformation of the cytoplasmic helix 8 of the CB1 cannabinoid receptor using NMR and circular dichroism. Biochim. Biophys. Acta 1668, 1–9 [DOI] [PubMed] [Google Scholar]
- 66. Xie X. Q., Chen J. Z. (2005) NMR structural comparison of the cytoplasmic juxtamembrane domains of G-protein-coupled CB1 and CB2 receptors in membrane mimetic dodecylphosphocholine micelles. J. Biol. Chem. 280, 3605–3612 [DOI] [PubMed] [Google Scholar]
- 67. Hurst D. P., Grossfield A., Lynch D. L., Feller S., Romo T. D., Gawrisch K., Pitman M. C., Reggio P. H. (2010) A lipid pathway for ligand binding is necessary for a cannabinoid G protein-coupled receptor. J. Biol. Chem. 285, 17954–17964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Escribá P. V., Ozaita A., Ribas C., Miralles A., Fodor E., Farkas T., García-Sevilla J. A. (1997) Role of lipid polymorphism in G protein-membrane interactions. Nonlamellar-prone phospholipids and peripheral protein binding to membranes. Proc. Natl. Acad. Sci. U.S.A. 94, 11375–11380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kosloff M., Alexov E., Arshavsky V. Y., Honig B. (2008) Electrostatic and lipid anchor contributions to the interaction of transducin with membranes. Mechanistic implications for activation and translocation. J. Biol. Chem. 283, 31197–31207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van Meer G., Voelker D. R., Feigenson G. W. (2008) Membrane lipids. Where they are and how they behave. Nat. Rev. Mol. Cell. Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zachowski A. (1993) Phospholipids in animal eukaryotic membranes. Transverse asymmetry and movement. Biochem. J. 294, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Galiègue S., Mary S., Marchand J., Dussossoy D., Carrière D., Carayon P., Bouaboula M., Shire D., Le Fur G., Casellas P. (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 232, 54–61 [DOI] [PubMed] [Google Scholar]
- 73. Callahan M. K., Williamson P., Schlegel R. A. (2000) Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ. 7, 645–653 [DOI] [PubMed] [Google Scholar]
- 74. Dillon S. R., Mancini M., Rosen A., Schlissel M. S. (2000) Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J. Immunol. 164, 1322–1332 [DOI] [PubMed] [Google Scholar]
- 75. Appelt U., Sheriff A., Gaipl U. S., Kalden J. R., Voll R. E., Herrmann M. (2005) Viable, apoptotic, and necrotic monocytes expose phosphatidylserine. Cooperative binding of the ligand annexin V to dying but not viable cells and implications for PS-dependent clearance. Cell Death Differ. 12, 194–196 [DOI] [PubMed] [Google Scholar]
- 76. Berger C., Ho J. T., Kimura T., Hess S., Gawrisch K., Yeliseev A. (2010) Protein Exp. Purif. 70, 236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nebane N. M., Hurst D. P., Carrasquer C. A., Qiao Z., Reggio P. H., Song Z. H. (2008) Residues accessible in the binding-site crevice of transmembrane helix 6 of the CB2 cannabinoid receptor. Biochemistry 47, 13811–13821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holte L. L., Peter S. A., Sinnwell T. M., Gawrisch K. (1995) 2H nuclear magnetic resonance order parameter profiles suggest a change of molecular shape for phosphatidylcholines containing a polyunsaturated acyl chain. Biophys. J. 68, 2396–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Polozov I. V., Gawrisch K. (2006) Characterization of the liquid-ordered state by proton MAS NMR. Biophys. J. 90, 2051–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.