Background: Binding of vinylogous urea-derived inhibitors to HIV-1 RT was examined by scanning mutagenesis.
Results: Mutating α-helix I of the p51 HIV-1 RT subunit induces high level resistance to RNase H inhibitors.
Conclusion: Contacts between the p51 thumb of HIV-1 RT and nucleic acid are exacerbated by inhibitor binding.
Significance: Developing allosteric RNase H inhibitors is critical for improved HIV therapies.
Keywords: Allosteric Regulation, Infectious Diseases, Protein-DNA Interaction, Reverse Transcription, Ribonuclease
Abstract
The vinylogous urea, NSC727447, was proposed to allosterically inhibit ribonuclease H (RNase H) activity of human immunodeficiency virus type 1 reverse transcriptase (HIV-1 RT) by interacting with the thumb subdomain of its non-catalytic p51 subunit. Proximity of the p51 thumb to the p66 RNase H domain implied that inhibitor binding altered active site geometry, whereas protein footprinting suggested a contribution from α-helix I residues Cys-280 and Lys-281. To more thoroughly characterize the vinylogous urea binding site, horizontal alanine scanning mutagenesis between p51 residues Lys-275 and Thr-286 (comprising α-helix I and portions of the neighboring αH/αI and αI/αJ connecting loops) was combined with a limited vertical scan of Cys-280. A contribution from Cys-280 was strengthened by our observation that all substitutions at this position rendered selectively mutated, reconstituted p66/p51 heterodimers ∼45-fold less sensitive to inhibition. An ∼19-fold reduced IC50 for p51 mutant T286A coupled with a 2–8-fold increased IC50 when intervening residues were substituted supports our original proposal of p51 α-helix I as the vinylogous urea binding site. In contrast to these allosteric inhibitors, mutant enzymes retained equivalent sensitivity to the natural product α-hydroxytropolone inhibitor manicol, which x-ray crystallography has demonstrated functions by chelating divalent metal at the p66 RNase H active site. Finally, reduced DNA strand-transfer activity together with increased vinylogous urea sensitivity of p66/p51 heterodimers containing short p51 C-terminal deletions suggests an additional role for the p51 C terminus in nucleic acid binding that is compromised by inhibitor binding.
Introduction
In the continuing effort to develop improved inhibitors of human immunodeficiency virus type 1 (HIV-1) replication, high throughput screening has identified several antagonists of reverse transcriptase (RT)-associated ribonuclease H (RNase H) activity. These include N-hydroxyimides (1, 2), N-acyl hydrazones (3), diketo acids (4, 5), naphthyridinones (6), pyrimidinol carboxylic acids (7, 8), and natural product α-hydroxytropolones (9). With the exception of N-acyl hydrazones, crystallographic studies (2, 6, 8, 10) show these inhibitors share a common property of interrupting hydrolysis by coordinating divalent metal at the RNase H active site. However, the observation that the nucleic acid substrate efficiently displaces α-hydroxytropolones from the enzyme-inhibitor complex (11) suggests that alternatives to active site RNase H inhibitors might be a more attractive strategy. This would not be without precedent, as nonnucleoside RT inhibitors currently in clinical use occupy a site at the base of the p66 thumb subdomain, interrupting catalysis by affecting the conformational dynamics of the retroviral polymerase (12–19).
High throughput screening of NCI, National Institutes of Health libraries of chemical entities identified the vinylogous ureas 2-amino-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxamide (NSC727447) and N-[3-(aminocarbonyl)-4,5-dimethyl-2-thienyl]-2-furancarboxamide (NSC727448) as moderately potent HIV-1 and HIV-2 RNase H inhibitors (11, 20). Despite their lower activity compared with α-hydroxytropolones, these compounds had the advantage of retaining activity against the enzyme-substrate complex (11). In the absence of crystallographic data, mass spectrometric protein footprinting (via biotin modification and subsequent sensitivity to proteolysis) positioned NSC727447 in the vicinity of α-helix I residues Cys-280 and Lys-281 of the p51 and p66 thumb subdomains. Proximity of the p51 thumb to the p66 RNase H domain (Fig. 1) suggested allosteric inhibition of RNase H activity, possibly by altering divalent metal coordination geometry at the active site. However, footprinting studies could not distinguish between a direct involvement of Cys-280 and Lys-281 in inhibitor binding or an inhibitor-induced alteration at an unrelated site that protected these residues from chemical modification. Moreover, a long range influence on RNase H activity by inhibitor binding to the p66 thumb could not be completely excluded.
FIGURE 1.
a, shown is the structure of the p66/p51 HIV-1 RT heterodimer. p66 subunits are color-coded as follows: blue, fingers; red, palm; green, thumb; yellow, connection (Conn.); gold, RNase H. Only the p51 thumb subdomain has been color-coded in green. b, expanded illustration of the p66 RNase H domain and p51 thumb is shown. Thumb residues substituted with alanine are denoted in white, active site carboxylates of the RNase H domain are in red, and the conserved His-539 is in magenta. Data were based the RT/manicol cocrystal of Chung et al. (24). c, procedures were as b but illustrate residues of p51 thumb (Cys-280—Thr-290, white) and the p66 RNase H domain (Pro-537—Glu-546, gold) that contribute to the buried surface at the dimer interface. The conserved His-539 of the RNase H domain is indicated in magenta.
This work comprises a detailed mutagenesis study designed to better understand vinylogous urea-induced allosteric inhibition of RNase H activity. Scanning mutagenesis was undertaken to examine the properties of selectively mutated p66/p51 HIV-1 RT heterodimers (11) containing alanine substitutions between p51 residues Lys-275 and Thr-286 together with a limited vertical scan of Cys-280. Based on proximity of the p51 C terminus to its thumb subdomain, inhibitor sensitivity of selectively deleted p66/p51 RT heterodimers was also examined. High level resistance of mutants C280A and T286A to 5,6-dimethyl-2-(4-nitrophenyl)thieno[2,3-d]pyrimidin-4(3H)-one (compound 16 (11), hereafter designated DNTP) coupled with increased sensitivity of enzymes whose intervening residues are altered strengthens our postulate that p51 αI and adjacent regions constitute the DNTP binding site. Together with observations from selectively deleted p66/p51 heterodimers, our data also suggest an indirect contribution from inhibitor binding to the p66 thumb subdomain is unlikely.
EXPERIMENTAL PROCEDURES
Site-directed Mutagenesis and Enzyme Purification
Alanine substitutions were introduced into the p51 HIV-1 RT subunit using the QuikChange protocol (Agilent Technologies Inc., Santa Clara, CA). His6p51Δ5, His6p51Δ9, and His6p51Δ13 RTs lack p51 residues Gly-536—Phe-440, Glu-432—Phe-440, and Gln-428—Phe-440, respectively (21). Selectively mutated and selectively deleted p66/His6p51 heterodimers (22) were purified from recombinant Escherichia coli by a combination of immobilized metal affinity and ion exchange chromatography (23). Purified, concentrated enzymes were stored at −20 °C in a buffer of 50 mm Tris/HCl, pH 7.0, 25 mm NaCl, 1 mm EDTA, 1 mm dithiothreitol and 50% (v/v) glycerol.
Enzyme Assays
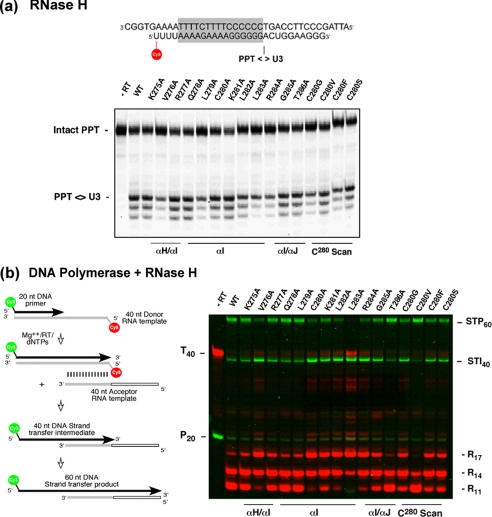
RNase H activity was initially evaluated on fluorescently labeled polypurine tract (PPT)2-containing RNA/DNA hybrid (5′-Cy5-uuu uaa aag aaa agg ggg gac ugg aag gg-3′, 5′-ATT AGC CCT TCC AGT CCC CCC TTT TCT TTT AAA AAG TGG C-3′). The reaction was initiated by adding 1 μl of 100 mm MgCl2 to 9 μl of mixture containing 10 ng of enzyme, 200 nm substrate, 50 mm Tris, pH 8.0, 80 mm KCl, and 2 mm DTT at 37 °C. Hydrolysis products were fractionated by high voltage denaturing polyacrylamide gel electrophoresis. RNA-dependent DNA polymerase and RNase H activities were simultaneously evaluated via the ability to support DNA strand transfer. This assay (Fig. 2b) comprised a Cy5 5′-end-labeled 40-nt donor RNA template annealed to a Cy3 5′-end-labeled 20-nt DNA primer (Integrated DNA Technology, Coralville, IA) in the presence of a 40-nucleotide acceptor RNA template. Donor and acceptor RNA templates shared 20 nucleotides of homology at their 5′- and 3′-termini, respectively. Initial RNA-dependent DNA synthesis produces a 40-nt strand transfer intermediate (STI) and successful, RNase H-mediated strand transfer produces a 60-nt strand transfer product (STP). DNA synthesis was initiated by adding 1 μl of RT (30 ng/μl) to 9 μl of mixture containing 50 nm donor RNA template-DNA primer, 250 nm acceptor RNA template, and 200 μm dNTPs in 10 mm Tris/HCl, pH 7.8, 9 mm MgCl2, 80 mm NaCl, 5 mm dithiothreitol. Samples were incubated at 37 °C for 20 min and quenched with an equal volume of 8 m urea in Tris borate-EDTA buffer. Polymerization and hydrolysis products were resolved by high voltage, denaturing polyacrylamide gel electrophoresis and visualized by fluorescent imaging (Typhoon Trio+, GE Healthcare).
FIGURE 2.
a, PPT cleavage activity of selectively mutated reconstituted p51 thumb mutants is shown. The hydrolysis product indicating cleavage at the PPT/U3 junction is indicated. b, shown is an evaluation of DNA polymerase and RNase H activity of p51 thumb mutants via their ability to support DNA strand transfer between 40-nt RNA templates sharing 20 nt of homology. DNA polymerase and RNase H hydrolysis products are indicated in green and red, respectively. STI40 and STP60 indicate the 40-nt strand transfer intermediate and the 60-nt strand transfer product, respectively. Lane 1, migration positions of the 20-nt Cy3-labeled DNA primer (P20) and 40-nt Cy5-lebeled donor RNA template (T40), respectively. Lane 2, wild type RT. Rn denotes the size of the RNase H hydrolysis product.
RNase H Inhibitor Analysis
IC50 values were determined as previously reported (11) using an 18-nt 3′-fluorescein-labeled RNA annealed to a complementary 18-nt 5′-dabsyl-labeled DNA. To a 96-well plate was added 1 μl of each vinylogous urea (in DMSO) followed by 10 μl of the appropriate RT (10 ng/μl) in reaction buffer. Hydrolysis was initiated by adding 10 μl of RNA/DNA hybrid (2.5 μm). Final assay conditions were 50 mm Tris·HCl, pH 8.0, 60 mm KCl, 10 mm MgCl2, 1% DMSO, 250 ng of RT, 250 nm substrate, and increasing concentrations of inhibitor. Wells containing only DMSO or lacking RT were used as negative controls and background, respectively. Plates were incubated at 37 °C in a Spectramax Gemini EM fluorescence spectrometer for 10 min, and fluorescence (λex = 485 nm; λem = 520 nm) was measured at 1-min intervals such that linear initial rates could be measured in the presence (vi) and absence (vo) of inhibitor. Percent inhibition was calculated as 100 × (vo − vi)/vo and plotted against log10[I]. IC50 values were determined using SigmaPlot software. All assays were performed in triplicate.
Viral Fitness Assays
Vesicular stomatitis virus G-pseudotyped HIV-1 vectors were produced in human embryonic kidney cell line 293T obtained from the American Type Culture Collection. These vectors were used to infect the human osteosarcoma cell line. Both cell lines were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 5% (v/v) fetal bovine serum, 5% newborn calf serum, penicillin (50 units/ml), and streptomycin (50 μg/ml) (Quality Biological, Gaithersburg, MD). Mutations were introduced to the pNLNgoMIVR+ΔEnv.LUC plasmid using the QuikChange XL system (Agilent Technologies, Inc.). One day before transfection, 9 × 105 cells were plated onto 100-mm dishes and incubated overnight at 37 °C. Using calcium phosphate precipitation, each plate was transfected with 10 μg of pNLNgoMIVR+ΔEnv.LUC (with either WT or mutant RT) and 3 μg of pHCMV-g. Plates were washed twice with 1× PBS after 8 h, at which time fresh media was added, and the plates were incubated at 37 °C for 42 h. The following day cells were diluted to 4.0 × 104 cells/ml, and 100 μl per well were added to 96-well luminescence cell culture plates, which were stored overnight at 37 °C. Transfection supernatants were clarified by low speed centrifugation, then treated with 200 units of DNase I (Roche Applied Science) at room temperature for 3 h to remove residual plasmid. Viral Gag p24 was quantified by ELISA (PerkinElmer Life Sciences). Yields are reported as the mean of two independent transfections (±S.D.) normalized to the yield of the WT transfections. Each virus stock was diluted to 25 ng/ml p24, and 100 μl of virus was added to each well of a 96-well plate. Three infections were performed for each of the two independent transfections per RT (WT, mutants, and mock), totaling six infections per RT. Luciferase activity was measured 42 h post-infection using a Steady-Lite Plus kit (PerkinElmer Life Sciences) and luminescence was detected with a microplate reader. Relative infectivity was calculated as the percent luciferase activity (±S.D.) normalized to WT.
Molecular Modeling
Molecular modeling of the HIV-1 RT p51 thumb and adjacent p66 RNase H domain was performed with PyMol (Delano Scientific, LLC, San Francisco, CA) using our recently reported 2.7 Å structure of p66/p51 HIV-1 RT containing the nonnucleoside RT inhibitor rilpirivine (TMC278) in the DNA polymerase domain and the α-hydroxytropolone manicol in the RNase H active site (24).
RESULTS
Construction and Analysis of Selectively Mutated p66/p51 RT Heterodimers
To more precisely define p51 thumb residues contributing to DNTP binding, we performed alanine scanning mutagenesis between Lys-275 and Thr-286, which constitutes α-helix I and a portion of the adjacent αH/αI and αI/αJ connecting loops (Fig. 1b). Based on mass spectrometric protein footprinting data (20), Cys-280 was also substituted with Gly, Val, Ser, Phe, and Asp. Selectively mutated, reconstituted p66/His6p51 heterodimers (22) were purified by a combination of metal chelate and ion exchange chromatography. Because the p66 RNase H domain and p51 thumb comprise a major contact surface of the HIV-1 RT heterodimer (25), metal chelate affinity chromatography also assessed whether mutagenesis inhibited dimerization. As illustrated in supplementary Fig. S1, all mutants eluted from Ni2+-NTA-Sepharose as p66/p51 heterodimers with a 1:1 subunit stoichiometry, confirming that the integrity of their dimer interface was not compromised. Our previous communication illustrated that unlike active site inhibitors, which can be displaced from the RNase H active site by nucleic acid, vinylogous ureas are still effective in the presence of substrate (11). The order-of-addition experiment of supplementary Fig. S2 confirmed that DNTP inhibition is likewise not affected by substrate.
Activity of Reconstituted Enzymes
Based on reports that substituting Cys-280 of the HIV-1 p51 thumb compromises activity (26), we first examined RNase H and RNA-dependent DNA polymerase activity of selectively mutated p66/p51 heterodimers. As a measure of specificity, we monitored cleavage of a PPT-containing RNA/DNA hybrid. Fig. 2a indicates that although a 40–60% reduction in overall activity is evident for mutants Val-276, Leu-279, Leu-283, and Arg-284 (supplemental Fig. S3a), all enzymes catalyzed specific cleavage at the PPT/U3 junction and to a lesser extent at positions −1 and −2. Next, RNase H- and RNA-dependent DNA polymerase activities of mutant enzymes were simultaneously determined via their ability to support DNA strand transfer between donor and acceptor RNA templates sharing limited sequence homology. The approach is similar to our previously reported assay (21), differing inasmuch as the DNA primer and donor RNA template were conjugated at their 5′ termini to Cy3 and Cy5, respectively. After electrophoretic fractionation, the emission maxima of these fluorophores allowed differentiation between DNA synthesis (green) and RNA degradation products (red).
Fig. 2b indicates mutant enzymes retained sufficient RNA-dependent DNA polymerase activity to extend the primer to the 5′ terminus of the donor template without significant pausing. As shown in supplemental Fig. S3b, equivalent levels of DNA synthesis were evident for all enzymes, differing only in the ratio of the strand transfer intermediate (STI40) to strand transfer product (STP60). Thereafter, RNase H activity generates a series of hydrolysis products resulting from cleavage 17, 14, and 11 nt (R17, R147 and R11, respectively) from the 5′ terminus of the Cy5-labeled donor RNA template. R17 is the product of polymerization-dependent hydrolysis, defined by the spatial separation of the active sites of the N-terminal DNA polymerase and C-terminal RNase H domains of HIV-1 RT (27, 28). In contrast, R14 and R11 are derived by polymerization-independent hydrolysis, requiring repositioning of RT 3 and 6 nt, respectively, beyond the 5′ terminus of the donor template. Although quantification indicates the ratio of polymerization-dependent and -independent products differs (supplemental Fig. S3c), they cumulatively demonstrate that all mutants retain significant levels of RNase H activity, confirming PPT primer selection data of Fig. 2a. As we (21, 29) and others (28) previously demonstrated, the efficiency of DNA strand transfer is paralleled by accumulation of the shortest R11 polymerization-independent product. After −11 cleavage, crystallographic data for HIV-1 RT predicts all contacts with p66 α-helix H, and the majority of contacts with p66 α-helix I would be relinquished, inducing dissociation of the donor template and hybridization of nascent DNA to the acceptor RNA. Data of Fig. 2b indicate that Ala substitutions of p51 α-helix I residues Val-276 and Cys-280–Arg-284 reduce accumulation of the R11 polymerization-independent hydrolysis product, resulting in accumulation of STI40 and reduced levels of STP60. In the case of mutant L283A, accumulation of intermediate-sized hydrolysis products reflects RT stalling shortly after initiation of DNA synthesis and concomitant cleavage of the RNA/DNA hybrid. Fig. 2b thus suggests a stabilizing contribution from p51 α-helix I to DNA strand transfer via contacts with nucleic acid after −14 cleavage and relocation of RT on the 14-nt RNA/40-nt nascent DNA hybrid before −11 cleavage. Although speculative, this notion is in line with modeling studies proposing that the amphiphilic p51 α-helix I contacts the phosphate backbone between primer nucleotides −23 and −25 (30). This postulate will be addressed later.
DNTP Sensitivity of p51 RT Thumb Mutants
We next determined DNTP sensitivity of reconstituted heterodimers. Based on proximity of the proposed vinylogous urea binding site to the p66 RNase H domain, sensitivity to the active site α-hydroxytropolone inhibitor, manicol (9, 24), was also determined to confirm that RNase H active site architecture was preserved. Table 1 illustrates that within experimental error, manicol sensitivity of all p51 thumb mutants was equivalent to that of wild type RT.
TABLE 1.
Sensitivity of p51 thumb mutants to inhibition of RNase H activity by vinylogous ureas (DNTP, left) and α-hydroxytropolones (manicol, right)
The structures of the two inhibitors are indicated above each panel. IC50 values are the average of triplicate assays.
Ala substitutions between Lys-275 and Gln278 induced a small but reproducible increase in DNTP sensitivity, the effect being most pronounced with mutant V276A (IC50 = 0.13 ± 0.1 μm versus 1.10 ± 0.1 μm for wild type RT). Although mutant L279A exhibited similar DNTP sensitivity as wild type RT, high level resistance of C280A RT (>50 μm) strengthened previous protein footprinting data, suggesting this residue contributes to vinylogous urea binding (20). Surprisingly, although protein footprinting also implicated Lys-281 in inhibitor binding, RT containing a K281A substitution was DNTP-sensitive (IC50 0.63 ± 0.01 μm). Increased DNTP sensitivity was again observed with enzymes containing Ala substitutions between residues Leu-281 and Arg-284, whereas mutant G285A exhibited wild type sensitivity. Finally, analogous to mutant C280A, T286A RT was significantly DNTP-resistant (28.2 ± 3.8 μm). Although the structural consequences of these mutations remains to be established, the results of horizontal alanine-scanning mutagenesis strengthens our earlier hypothesis (20) that vinylogous urea inhibition of HIV-1 RNase H is mediated by an interaction with the p51 RT subunit.
Cys-280 of the p51 Thumb Is a Critical Determinant of DNTP Resistance
Our original protein footprinting studies (20) and alanine scanning data of Fig. 3 together implicate p51 residue Cys-280 in DNTP binding, The role of Cys-280 was further examined via a limited vertical scan by introducing Gly, Ser, Val, Phe, and Asp substitutions. Although mutant C280D successfully reconstituted into heterodimer, it displayed negligible RNase H activity and was not further investigated (data not shown). Data of supplementary Fig. S1 indicate that the additional p51 Cys-280 substitutions did not affect the ability to reconstitute into heterodimer, whereas Fig. 2b shows that reconstituted mutants retained RNA-dependent polymerase, RNase H, and strand transfer activity. However, Table 2 indicates that all p51 Cys-280 substitutions resulted in high level DNTP resistance, supporting a contribution from this residue to inhibitor binding.
FIGURE 3.
DNA strand transfer activity of selectively deleted p66/p51 HIV-1 RT heterodimers. The extent of the p51 C-terminal deletion is indicated above the figure. Nucleic acid substrate, DNA synthesis, and RNase H hydrolysis product notations are described in the legend of Fig. 2.
TABLE 2.
Vertical mutagenesis of p51 thumb residue Cys-280 consistently results in DNTP resistance
IC50 values represent the average of triplicate assays.
p51 Thumb Mutants Are Less Sensitive to Inhibition by Vinylogous Urea NSC727447
The most active compound identified in our original screen, NSC727447 (20), contains a cycloheptane-substituted thiophene ring that structure/activity studies showed to be the optimally sized cycloalkane substitution (11). In contrast, DNTP contains a dimethyl-substituted thiophene ring, yet is 2∼3-fold more potent. To determine whether amino acid substitutions of p51 α-helix I had equivalents effect on structurally distinct vinylogous ureas, IC50 values were determined for NSC727447. In the experiment of Table 3, an IC50 of 1.20 ± 0.10 μm was determined for wild type RT, which agrees with the value of 2.0 μm reported earlier (20). Overall, the response of all mutant enzymes to NSC727447 was lower than to DNTP. However, the overall trend with respect to the horizontal scan was in fact similar. As an example, maximum resistance to both inhibitors was noted for p66/His6p51C280A RT, whereas mutants p66/His6p51V276A, p66/His6p51L283A, and p66/His6p51R284A exhibited enhanced sensitivity. In the absence of crystallographic data for HIV-1 RT containing either of these vinylogous ureas, data of Table 2 suggest interactions of the NSC727447 cycloalkane substituent with hydrophobic residues of the p51 thumb may compensate for alterations to amino acids that directly contact DNTP and whose substitution may be more deleterious. We cannot, however, exclude the possibility that NSC727447 and DNTP occupy partially overlapping regions of the p51 thumb.
TABLE 3.
Sensitivity of p51 thumb mutants to inhibition of RNase H activity by the cycloalkane- containing vinylogous urea NSC727447, the structure of which is indicated above the panel
IC50 values are the average of triplicate assays.
Selectively Deleted p66/p51 Heterodimers Are Sensitized to DNTP
An indirect contribution of the p51 C terminus of HIV-1 RT to RNase H function was previously evidenced by loss of polymerization-independent RNase H activity and an inability to support DNA strand transfer when residues Gln-428—Phe-440 were deleted (21, 31). Although the extreme p51 C terminus is not evident in current RT structures, proximity to its thumb subdomain suggests it could provide an architectural role in accommodating the nucleic acid substrate. We, therefore, asked whether reduced strand transfer function affected inhibitor sensitivity using p66/p51 heterodimers whose p51 subunit contained short C-terminal deletions. Accumulation of STI40 in Fig. 3 indicates that all mutants retained RNA-dependent DNA polymerase activity. However, although wild type RT catalyzes polymerization-dependent (R17 product) and -independent RNase H activities (R14 and R11 products), p66/p51Δ13 RT, as previously reported (21), lacks polymerization-independent activity and, as a consequence, failed to support DNA strand transfer. Surprisingly, although polymerization-independent RNase H activity of p66/p51Δ5 and p66/p51Δ9 RT generated significant levels of R14 hydrolysis product, STI40 accumulates at the expense of STP60. At the same time, Table 4 indicates that these subtle alterations to polymerization-independent RNase H activity induce ∼3-fold increased sensitivity of p66/p51Δ5 RT to DNTP (IC50 0.35 ± 0.06 μm), and ∼10-fold increased sensitivity of mutant p66/p51Δ9 (IC50 0.11 ± 0.01 μm). Rather than invoking direct involvement of p51 C-terminal residues in inhibitor binding, we believe these short deletions compromise interactions with the RNA/DNA hybrid in the vicinity of the RNase H active site that are further exacerbated after DNTP binding to the p51 thumb. Our postulate implies a role for p51 in nucleic acid binding that hitherto was not evident from crystal structures of HIV-1 RT (32–34) but would be in line with the “helix clamp” model proposed by Heumann and co-workers (30, 35). This will be discussed in more detail later. Finally, we were unable to determine an IC50 for p66/p51Δ13 RT for DNTP, as this mutant was inactive on the 18-bp RNA/DNA duplex employed in our high throughput screening efforts. Because p66/p51Δ13 RT has significantly reduced polymerization-dependent RNase H activity (Fig. 3), the 13-residue p51 truncation either prevented binding to the 18-bp RNA/DNA hybrid or positioned the scissile phosphodiester bond within the active site in a manner inconsistent with hydrolysis.
TABLE 4.
Effect of p51 C-terminal deletions on DNTP sensitivity
ND, not determined, due to inactivity of this mutant on the 18-bp RNA/DNA hybrid.
| Mutation | DNTP IC50 |
|---|---|
| μm | |
| WT | 1.1 ± 0.1 |
| p66/p51Δ5 | 0.35 ± 0.06 |
| p66/p51Δ9 | 0.11 ± 0.01 |
| p66/p51Δ13 | ND |
Cys-280 Mutations Reduce HIV-1 Replicative Capacity
The limited activity of vinylogous ureas in reducing HIV-1 infectivity3 prevented direct selection of drug-resistant variants. However, based on our in vitro data for the p51C280A mutant, we elected to examine the relative viral fitness of HIV-1 vectors whose RT contained Ala, Ser, Gly, or Val substitutions for Cys-280 and where the mutation would be introduced into both subunits. As indicated in Table 5, we observed a substantial reduction in p24 yield for all mutants. These replication-defective vectors can spread in cell cultures co-transfected with a vesicular stomatitis virus G-expressing plasmid (36), and if the vector has a replication deficit, p24 production from the transduced cells can be significantly reduced relative to cells transfected with a fully WT vector. Reduced p24 production shown in Table 5 suggests that for the Cys-280 mutants, viral spread in the transfected cell culture is restricted. However, virions produced from the mutant plasmids showed modest (∼2-fold or less) changes in relative infectivity compared with WT virus. Luciferase expression depends on synthesis and subsequent integration of a complete viral DNA. This suggests that RTs carrying Cys-280 mutations retain sufficient enzymatic activity to synthesize a full-length DNA whose LTR termini, which depend on accurate RNase H-mediated hydrolysis, are also normal or nearly normal. Reduced yield observed after transfection may, therefore, reflect another defect caused by the mutations. Thus, although the exact nature of additional consequences of Cys-280 mutations is presently unclear, its substitution clearly has important implications for HIV replication.
TABLE 5.
p66/p51 Cys-280 mutations in HIV-1 RT reduce viral Gag p24 yield but have a smaller effect on specific infectivity
| Mutation | p24 yield | Relative infectivity |
|---|---|---|
| % WT | % WT | |
| WT | 100.0 ± 6.2 | 100.0 ± 5.7 |
| Mock | 0.1 ± 0.04 | 0.3 ± 0.7 |
| C280A | 6.7 ± 1.8 | 142.3 ± 3.8 |
| C280 G | 21.3 ± 3.6 | 56.4 ± 5.5 |
| C280S | 9.3 ± 1.2 | 62.4 ± 4.9 |
| C280V | 7.3 ± 0.1 | 152.5 ± 7.5 |
DISCUSSION
In addition to chain-terminating nucleoside inhibitors, DNA polymerase activity of HIV-1 RT can be interrupted by several distinct classes of small molecules, the most significant being nonnucleosides, which create and occupy a hydrophobic pocket at the base of the p66 thumb (37). Indolopyridones represent another example proposed to occupy the p66 dNTP binding pocket, “freeze” the ternary complex in a post-translocational state, and restrict access of the subsequent, complementary dNTP (38). Destabilization of the p66-p51 dimer interface with [1-[2′,5′-bis-O-(tert-butyldimethylsilyl)-beta-dribofuranosyl]thymine]-3′-spiro-5″-(4″-amino-1″,2″-oxathiole-2″,2″-dioxide) (TSAO-T) (39) and related derivatives has also been reported by Sluis-Cremer et al. (40). Last, dihydroxybenzoylnaphthyl hydrazone and its derivatives bind close to the nonnucleoside RT inhibitor binding site, affecting both DNA polymerase and RNase H activity (3). Together, these studies demonstrate the importance of combining active site and allosteric inhibitors of DNA polymerase activity. It is, therefore, not unreasonable that the same principle might be applied to RNase H inhibitors. Indeed, observations that divalent metal-sequestering inhibitors are displaced from the RNase H active site by nucleic acid (41) suggests small molecules that bind within or adjacent to the RNase H domain to interrupt catalysis by an allosteric mechanism could be a more productive strategy. Although vinylogous ureas represent one such inhibitor class (11, 20), the precise nature of the pharmacophore and detailed structural information on their binding site remain to be established. Based on protein footprinting data (20), we, therefore, elected to examine the consequences of mutating p51 α-helix I and immediately adjacent thumb residues for vinylogous urea sensitivity. We also examined p51 C-terminal deletion derivatives whose subtle alterations in RNase H activity have severe consequences for DNA strand transfer function (31).
An important issue in interpreting our mutagenesis data was demonstrating that DNA polymerase and polymerization-independent RNase H activities of mutant enzymes are not severely compromised (Figs. 2, a and b). Moreover, despite differential sensitivity to vinylogous ureas, all mutants were equally responsive to an active site inhibitor (Table 1), indicating that mutagenesis preserved RNase H active site geometry. Our data may, therefore, be in part reconciled by observations that the interaction of p51 thumb residues Cys-280-Thr-290 and p66 RNase H residues Pro-537—Glu-546 constitute ∼33% of the buried surface at the p51-p66 dimer interface (Ref. 42 and Fig. 1c). Because the p66 thumb does not participate in an equivalent intersubunit interaction, this provides a structural basis for vinylogous urea inhibition of RNase H activity mediated by the p51 thumb. Data of Fig. 2b) indicate that several p51 thumb mutants have diminished polymerization-independent RNase H activity and, consequently, reduced DNA strand transfer function. Thus, in the absence of inhibitor, relatively benign substitutions (e.g. L283A) may perturb the p66-p51 interface sufficiently to affect catalysis, precedent for which is the p66 primer grip mutation L234A, which inhibits dimerization (43). Assuming that p51 residues Lys-275–Thr-286 comprise the DNTP binding pocket, Ala substitutions may, in the context of neighboring residues, locally increase hydrophobicity and enhance inhibitor binding. As a consequence, intersubunit contacts would be further exacerbated, thereby compromising RNase H activity. Conceivably, these DNTP-induced alterations to p51-p66 dimer contacts could also re-align His-539 and Gln-500 of the RNase H domain, which are in the immediate vicinity of the scissile phosphodiester bond of the RNA strand (32, 34).
An alternative hypothesis invokes a role of p51 α-helix I in contacting nucleic acid downstream of the RNase H active site. In silico modeling (30, 35) suggested HIV-1 RT could contact nucleic acid as far as position −25 (defining −1 as the first base pair in the DNA polymerase active site). In particular, Hermann and Heumann (30) proposed that residues of the amphiphilic p51 α-helix I could contact primer nucleotides, whereas we demonstrated that template and primer nucleotides as far as position −25 could be cross-linked to the p66 C terminus (44). Fig. 2b shows that substituting residues Lys-281 and Arg-284 slightly diminishes polymerization-independent RNase H activity, whereas Table 1 indicates that mutating each of these positions confers increased DNTP sensitivity. Although crystallographic data for HIV-1 RT containing and extended RNA/DNA hybrid is presently unavailable, we propose that replacing basic residues of α-helix I disrupts contacts with the phosphate backbone in the vicinity of the RNase H active site. Conceivably, DNTP binding disrupts these interactions in wild type RT such the scissile phosphodiester bond is incorrectly positioned at the RNase H active site, and this effect is exacerbated when combined with p51 α-I mutations. A contribution from the p51 C terminus to extended interactions with the nucleic acid duplex is also indirectly suggested by data of Table 4, where progressive loss of polymerization-independent RNase H activity is paralleled by increased DNTP sensitivity. Electrophoretic mobility shift analysis (supplementary Fig. S4 and Table S1) indicates that this is a simple consequence of reduced affinity of mutant enzymes for the RNA/DNA hybrid, as the Kd is unaffected by the extent of C-terminal deletion. However, p51 C-terminal residues 420–427 form an α-helix that is buried between its connection and thumb subdomains (8). The N terminus of this helix forms extensive interactions with the p66 subunit and aligns the nucleic acid binding surface. Deleting the C terminus of p51 as far as residue 427 would be predicted to perturb the stability of this helix, formation of the p66/p51 heterodimer, and binding of nucleic acid substrate.
High level DNTP resistance of several p51 Cys-280 mutants re-enforces previous protein footprinting data, implicating this residue in inhibitor binding (20). However, a surprising finding was that the relatively conservative C280S substitution also conferred resistance. If we assume that the Cys-280 side chain is within hydrogen bonding distance of the inhibitor, this result may be attributed to steric effects, as the atomic radius of sulfur is 0.4 Å larger than oxygen, and their bond lengths and angles are significantly different (45). Loss of hydrogen bonding interactions mediated by the Thr-286 side chain may underlie DNTP resistance of p51 mutant T286A. An alternative, although less likely explanation is that replacing Cys-280 and Thr-286 with hydrophobic residues may strengthen intersubunit contacts and restrict access of the inhibitor to the binding pocket.
The magnitude of the response of p51 thumb mutants to DNTP (Table 1) compared with the originally identified vinylogous urea NSC727447 (Table 3) was unexpected. This is particularly striking with mutant C280A, which is highly resistant to DNTP but only 3-fold less sensitive to NSC727447. Despite this, the pattern of NSC727447 sensitivity as a function of the position of alanine substitution is retained. This may indicate that the NSC727447 cycloheptane substituent makes a significant hydrophobic contribution toward binding, which is less sensitive to amino acid substitutions that increase hydrophobicity at the p51-p66 interface. An alternative interpretation is that NSC727447 binds to an adjacent and partially overlapping regions of the p51 thumb, with the consequence that it is less responsive to the mutations we have introduced. Finally, in addition to providing important mechanistic insights into vinylogous urea binding, HIV RT mutants such as those described here will find use in future screening efforts to identify different classes of allosteric inhibitors of HIV-1 RNase H function.
Supplementary Material
This work was supported, in whole or in part, by the National Institutes of Health Intramural Research Program, NCI, Center for Cancer Research.

This article contains supplemental Figs. S1–S4 and Table S1.
J. Beutler, unpublished data.
- PPT
- polypurine tract
- STI
- strand transfer intermediate
- nt
- nucleotide(s)
- STP
- strand transfer product
- DNTP
- 5,6-dimethyl-2-(4-nitrophenyl)thieno[2,3-d]pyrimidin-4(3H)-one.
REFERENCES
- 1. Klumpp K., Hang J. Q., Rajendran S., Yang Y., Derosier A., Wong Kai In P., Overton H., Parkes K. E., Cammack N., Martin J. A. (2003) Two-metal ion mechanism of RNA cleavage by HIV RNase H and mechanism-based design of selective HIV RNase H inhibitors. Nucleic Acids Res. 31, 6852–6859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klumpp K., Mirzadegan T. (2006) Recent progress in the design of small molecule inhibitors of HIV RNase H. Curr. Pharm. Des. 12, 1909–1922 [DOI] [PubMed] [Google Scholar]
- 3. Himmel D. M., Sarafianos S. G., Dharmasena S., Hossain M. M., McCoy-Simandle K., Ilina T., Clark A. D., Jr., Knight J. L., Julias J. G., Clark P. K., Krogh-Jespersen K., Levy R. M., Hughes S. H., Parniak M. A., Arnold E. (2006) HIV-1 reverse transcriptase structure with RNase H inhibitor dihydroxy benzoyl naphthyl hydrazone bound at a novel site. ACS Chem. Biol. 1, 702–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shaw-Reid C. A., Feuston B., Munshi V., Getty K., Krueger J., Hazuda D. J., Parniak M. A., Miller M. D., Lewis D. (2005) Dissecting the effects of DNA polymerase and ribonuclease H inhibitor combinations on HIV-1 reverse transcriptase activities. Biochemistry 44, 1595–1606 [DOI] [PubMed] [Google Scholar]
- 5. Shaw-Reid C. A., Munshi V., Graham P., Wolfe A., Witmer M., Danzeisen R., Olsen D. B., Carroll S. S., Embrey M., Wai J. S., Miller M. D., Cole J. L., Hazuda D. J. (2003) Inhibition of HIV-1 ribonuclease H by a novel diketo acid, 4-[5-(benzoylamino)thien-2-yl]-2,4-dioxobutanoic acid. J. Biol. Chem. 278, 2777–2780 [DOI] [PubMed] [Google Scholar]
- 6. Su H. P., Yan Y., Prasad G. S., Smith R. F., Daniels C. L., Abeywickrema P. D., Reid J. C., Loughran H. M., Kornienko M., Sharma S., Grobler J. A., Xu B., Sardana V., Allison T. J., Williams P. D., Darke P. L., Hazuda D. J., Munshi S. (2010) Structural basis for the inhibition of RNase H activity of HIV-1 reverse transcriptase by RNase H active site-directed inhibitors. J. Virol. 84, 7625–7633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirschberg T. A., Balakrishnan M., Squires N. H., Barnes T., Brendza K. M., Chen X., Eisenberg E. J., Jin W., Kutty N., Leavitt S., Liclican A., Liu Q., Liu X., Mak J., Perry J. K., Wang M., Watkins W. J., Lansdon E. B. (2009) RNase H active site inhibitors of human immunodeficiency virus type 1 reverse transcriptase: design, biochemical activity, and structural information. J. Med. Chem. 52, 5781–5784 [DOI] [PubMed] [Google Scholar]
- 8. Lansdon E. B., Liu Q., Leavitt S. A., Balakrishnan M., Perry J. K., Lancaster-Moyer C., Kutty N., Liu X., Squires N. H., Watkins W. J., Kirschberg T. A. (2011) Structural and binding analysis of pyrimidinol carboxylic acid and N-hydroxy quinazolinedione HIV-1 RNase H inhibitors. Antimicrob. Agents Chemother. 55, 2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Budihas S. R., Gorshkova I., Gaidamakov S., Wamiru A., Bona M. K., Parniak M. A., Crouch R. J., McMahon J. B., Beutler J. A., Le Grice S. F. (2005) Selective inhibition of HIV-1 reverse transcriptase-associated ribonuclease H activity by hydroxylated tropolones. Nucleic Acids Res. 33, 1249–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Himmel D. M., Maegley K. A., Pauly T. A., Bauman J. D., Das K., Dharia C., Clark A. D., Jr., Ryan K., Hickey M. J., Love R. A., Hughes S. H., Bergqvist S., Arnold E. (2009) Structure of HIV-1 reverse transcriptase with the inhibitor β-Thujaplicinol bound at the RNase H active site. Structure 17, 1625–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung S., Wendeler M., Rausch J. W., Beilhartz G., Gotte M., O'Keefe B. R., Bermingham A., Beutler J. A., Liu S., Zhuang X., Le Grice S. F. (2010) Structure-activity analysis of vinylogous urea inhibitors of human immunodeficiency virus-encoded ribonuclease H. Antimicrob. Agents Chemother. 54, 3913–3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abbondanzieri E. A., Bokinsky G., Rausch J. W., Zhang J. X., Le Grice S. F., Zhuang X. (2008) Dynamic binding orientations direct activity of HIV reverse transcriptase. Nature 453, 184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Das K., Ding J., Hsiou Y., Clark A. D., Jr., Moereels H., Koymans L., Andries K., Pauwels R., Janssen P. A., Boyer P. L., Clark P., Smith R. H., Jr., Kroeger Smith M. B., Michejda C. J., Hughes S. H., Arnold E. (1996) Crystal structures of 8-Cl and 9-Cl TIBO complexed with wild-type HIV-1 RT and 8-Cl TIBO complexed with the Y181C HIV-1 RT drug-resistant mutant. J. Mol. Biol. 264, 1085–1100 [DOI] [PubMed] [Google Scholar]
- 14. Hsiou Y., Das K., Ding J., Clark A. D., Jr., Kleim J. P., Rösner M., Winkler I., Riess G., Hughes S. H., Arnold E. (1998) Structures of Y188L mutant and wild-type HIV-1 reverse transcriptase complexed with the non-nucleoside inhibitor HBY 097: inhibitor flexibility is a useful design feature for reducing drug resistance. J. Mol. Biol. 284, 313–323 [DOI] [PubMed] [Google Scholar]
- 15. Das K., Clark A. D., Jr., Lewi P. J., Heeres J., De Jonge M. R., Koymans L. M., Vinkers H. M., Daeyaert F., Ludovici D. W., Kukla M. J., De Corte B., Kavash R. W., Ho C. Y., Ye H., Lichtenstein M. A., Andries K., Pauwels R., De Béthune M. P., Boyer P. L., Clark P., Hughes S. H., Janssen P. A., Arnold E. (2004) Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J. Med. Chem. 47, 2550–2560 [DOI] [PubMed] [Google Scholar]
- 16. Das K., Lewi P. J., Hughes S. H., Arnold E. (2005) Crystallography and the design of anti-AIDS drugs: conformational flexibility and positional adaptability are important in the design of non-nucleoside HIV-1 reverse transcriptase inhibitors. Prog. Biophys. Mol. Biol. 88, 209–231 [DOI] [PubMed] [Google Scholar]
- 17. Janssen P. A., Lewi P. J., Arnold E., Daeyaert F., de Jonge M., Heeres J., Koymans L., Vinkers M., Guillemont J., Pasquier E., Kukla M., Ludovici D., Andries K., de Béthune M. P., Pauwels R., Das K., Clark A. D., Jr., Frenkel Y. V., Hughes S. H., Medaer B., De Knaep F., Bohets H., De Clerck F., Lampo A., Williams P., Stoffels P. (2005) In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2- pyrimidinyl]amino]benzonitrile (R278474, rilpivirine). J. Med. Chem. 48, 1901–1909 [DOI] [PubMed] [Google Scholar]
- 18. Das K., Bauman J. D., Clark A. D., Jr., Frenkel Y. V., Lewi P. J., Shatkin A. J., Hughes S. H., Arnold E. (2008) High resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. Proc. Natl. Acad. Sci. U.S.A. 105, 1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cullen M. D., Ho W. C., Bauman J. D., Das K., Arnold E., Hartman T. L., Watson K. M., Buckheit R. W., Pannecouque C., De Clercq E., Cushman M. (2009) Crystallographic study of a novel subnanomolar inhibitor provides insight on the binding interactions of alkenyldiarylmethanes with human immunodeficiency virus-1 reverse transcriptase. J. Med. Chem. 52, 6467–6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wendeler M., Lee H. F., Bermingham A., Miller J. T., Chertov O., Bona M. K., Baichoo N. S., Ehteshami M., Beutler J., O'Keefe B. R., Götte M., Kvaratskhelia M., Le Grice S. (2008) Vinylogous ureas as a novel class of inhibitors of reverse transcriptase-associated ribonuclease H activity. ACS Chem. Biol. 3, 635–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cameron C. E., Ghosh M., Le Grice S. F., Benkovic S. J. (1997) Mutations in HIV reverse transcriptase which alter RNase H activity and decrease strand transfer efficiency are suppressed by HIV nucleocapsid protein. Proc. Natl. Acad. Sci. U.S.A. 94, 6700–6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le Grice S. F., Naas T., Wohlgensinger B., Schatz O. (1991) Subunit-selective mutagenesis indicates minimal polymerase activity in heterodimer-associated p51 HIV-1 reverse transcriptase. EMBO J. 10, 3905–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Grice S. F., Grüninger-Leitch F. (1990) Rapid purification of homodimer and heterodimer HIV-1 reverse transcriptase by metal chelate affinity chromatography. Eur. J. Biochem. 187, 307–314 [DOI] [PubMed] [Google Scholar]
- 24. Chung S., Himmel D. M., Jiang J. K., Wojtak K., Bauman J. D., Rausch J. W., Wilson J. A., Beutler J. A., Thomas C. J., Arnold E., Le Grice S. F. (2011) Synthesis, activity, and structural analysis of novel α-hydroxytropolone inhibitors of human immunodeficiency virus reverse transcriptase-associated ribonuclease H. J. Med. Chem. 54, 4462–4473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Srivastava S., Sluis-Cremer N., Tachedjian G. (2006) Dimerization of human immunodeficiency virus type 1 reverse transcriptase as an antiviral target. Curr. Pharm. Des. 12, 1879–1894 [DOI] [PubMed] [Google Scholar]
- 26. Sevilya Z., Loya S., Duvshani A., Adir N., Hizi A. (2003) Mutagenesis of cysteine 280 of the reverse transcriptase of human immunodeficiency virus type-1: the effects on the ribonuclease H activity. J. Mol. Biol. 327, 19–30 [DOI] [PubMed] [Google Scholar]
- 27. Gopalakrishnan V., Peliska J. A., Benkovic S. J. (1992) Human immunodeficiency virus type 1 reverse transcriptase: spatial and temporal relationship between the polymerase and RNase H activities. Proc. Natl. Acad. Sci. U.S.A. 89, 10763–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peliska J. A., Benkovic S. J. (1992) Mechanism of DNA strand transfer reactions catalyzed by HIV-1 reverse transcriptase. Science 258, 1112–1118 [DOI] [PubMed] [Google Scholar]
- 29. Rausch J. W., Lener D., Miller J. T., Julias J. G., Hughes S. H., Le Grice S. F. (2002) Altering the RNase H primer grip of human immunodeficiency virus reverse transcriptase modifies cleavage specificity. Biochemistry 41, 4856–4865 [DOI] [PubMed] [Google Scholar]
- 30. Hermann T., Heumann H. (1996) Strained template under the thumbs. How reverse transcriptase of human immunodeficiency virus type 1 moves along its template. Eur. J. Biochem. 242, 98–103 [DOI] [PubMed] [Google Scholar]
- 31. Jacques P. S., Wöhrl B. M., Howard K. J., Le Grice S. F. (1994) Modulation of HIV-1 reverse transcriptase function in “selectively deleted” p66/p51 heterodimers. J. Biol. Chem. 269, 1388–1393 [PubMed] [Google Scholar]
- 32. Huang H., Chopra R., Verdine G. L., Harrison S. C. (1998) Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282, 1669–1675 [DOI] [PubMed] [Google Scholar]
- 33. Jacobo-Molina A., Ding J., Nanni R. G., Clark A. D., Jr., Lu X., Tantillo C., Williams R. L., Kamer G., Ferris A. L., Clark P. (1993) Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc. Natl. Acad. Sci. U.S.A. 90, 6320–6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarafianos S. G., Das K., Tantillo C., Clark A. D., Jr., Ding J., Whitcomb J. M., Boyer P. L., Hughes S. H., Arnold E. (2001) Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA. EMBO J. 20, 1449–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hermann T., Meier T., Götte M., Heumann H. (1994) The “helix clamp” in HIV-1 reverse transcriptase: a new nucleic acid binding motif common in nucleic acid polymerases. Nucleic Acids Res. 22, 4625–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohishi M., Shioda T., Sakuragi J. (2007) Retro-transduction by virus pseudotyped with glycoprotein of vesicular stomatitis virus. Virology 362, 131–138 [DOI] [PubMed] [Google Scholar]
- 37. Sahlberg C., Zhou X.-X. (2008) Development of non-nucleoside reverse transcriptase inhibitors for anti-HIV therapy. Antiinfect. Agents Med. Chem. 7, 101–117 [Google Scholar]
- 38. Jochmans D., Deval J., Kesteleyn B., Van Marck H., Bettens E., De Baere I., Dehertogh P., Ivens T., Van Ginderen M., Van Schoubroeck B., Ehteshami M., Wigerinck P., Götte M., Hertogs K. (2006) Indolopyridones inhibit human immunodeficiency virus reverse transcriptase with a novel mechanism of action. J. Virol. 80, 12283–12292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sluis-Cremer N., Dmitrienko G. I., Balzarini J., Camarasa M. J., Parniak M. A. (2000) Human immunodeficiency virus type 1 reverse transcriptase dimer destabilization by 1-[spiro[4″-amino-2″,2″-dioxo-1″,2″-oxathiole-5″,3′-[2′,5′-bis-O-(tert-butyldimethylsilyl)-β-d-ribofuranosyl]]]-3-ethylthy mine. Biochemistry 39, 1427–1433 [DOI] [PubMed] [Google Scholar]
- 40. Sluis-Cremer N., Hamamouch N., San Félix A., Velazquez S., Balzarini J., Camarasa M. J. (2006) Structure-activity relationships of [2′,5′-bis-O-(tert-butyldimethylsilyl)-β-d-ribofuranosyl]-3′-spiro-5″-(4″-amino-1″,2″-oxathiole-2″,2″-dioxide)thymine derivatives as inhibitors of HIV-1 reverse transcriptase dimerization. J. Med. Chem. 49, 4834–4841 [DOI] [PubMed] [Google Scholar]
- 41. Beilhartz G. L., Wendeler M., Baichoo N., Rausch J., Le Grice S., Götte M. (2009) HIV-1 reverse transcriptase can simultaneously engage its DNA/RNA substrate at both DNA polymerase and RNase H active sites: implications for RNase H inhibition. J. Mol. Biol. 388, 462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang J., Smerdon S. J., Jäger J., Kohlstaedt L. A., Rice P. A., Friedman J. M., Steitz T. A. (1994) Structural basis of asymmetry in the human immunodeficiency virus type 1 reverse transcriptase heterodimer. Proc. Natl. Acad. Sci. U.S.A. 91, 7242–7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ghosh M., Williams J., Powell M. D., Levin J. G., Le Grice S. F. (1997) Mutating a conserved motif of the HIV-1 reverse transcriptase palm subdomain alters primer utilization. Biochemistry 36, 5758–5768 [DOI] [PubMed] [Google Scholar]
- 44. Rausch J. W., Sathyanarayana B. K., Bona M. K., Le Grice S. F. (2000) Probing contacts between the ribonuclease H domain of HIV-1 reverse transcriptase and nucleic acid by site-specific photocross-linking. J. Biol. Chem. 275, 16015–16022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.