Background: Pseudomonas aeruginosa alkaline protease is a virulence factor from the repeats in toxin (RTX) family.
Results: Calcium binding in the RTX domain induces folding and results in activation of the protease.
Conclusion: Disorder-to-order transitions in the RTX domain regulate protease structure, stability, and activity.
Significance: Understanding how calcium binding regulates the RTX family provides a basis for understanding its impact on bacterial virulence.
Keywords: Protein Folding, Protein Stability, Pseudomonas aeruginosa, Spectroscopy, Virulence Factors
Abstract
Pseudomonas aeruginosa is an opportunistic pathogen that contributes to the mortality of immunocompromised individuals and patients with cystic fibrosis. Pseudomonas infection presents clinical challenges due to its ability to form biofilms and modulate host-pathogen interactions through the secretion of virulence factors. The calcium-regulated alkaline protease (AP), a member of the repeats in toxin (RTX) family of proteins, is implicated in multiple modes of infection. A series of full-length and truncation mutants were purified for structural and functional studies to evaluate the role of Ca2+ in AP folding and activation. We find that Ca2+ binding induces RTX folding, which serves to chaperone the folding of the protease domain. Subsequent association of the RTX domain with an N-terminal α-helix stabilizes AP. These results provide a basis for the Ca2+-mediated regulation of AP and suggest mechanisms by which Ca2+ regulates the RTX family of virulence factors.
Introduction
Pseudomonas aeruginosa infection is a major complication for immunocompromised persons, contributes significantly to pathogen-induced loss of vision, and is a major contributor to the mortality of patients with cystic fibrosis (1–3). To facilitate infection and colonization, Pseudomonas utilizes multiple exoproteins to evade host defenses, facilitate adhesion, and modulate virulence (4–6). Studies of AprA, the alkaline protease (AP)2 from Pseudomonas aeruginosa, implicate this exoprotein in the virulence associated with multiple modes of infection (2, 5, 7). Alkaline protease is enriched in biofilms, and expression is correlated with increased virulence in the cystic fibrosis lung (4, 6, 8, 9).
Alkaline protease is a Zn2+-metalloprotease, a member of the serralysin family of proteases (10). Crystal structures of AP and related bacterial proteases demonstrate a two-domain architecture (11, 12). The N-terminal proteolytic domain (PD) contains the canonical HEXXHXXGXXH metal binding motif. This domain is structurally similar to human matrix metalloproteases and is active against a broad range of polypeptide sequences (13–16). The C-terminal domain contains nonapeptide Gly- and Asp-rich repeats characteristic of the repeats in toxin (RTX), Ca2+-binding domains (17, 18). Calcium binding is coordinated by the carboxylate groups of the Asp side chains and the carbonyl groups of the Gly backbones within the loops of a β-helix (12, 19).
Secretion of the RTX proteases and other RTX-containing exoproteins occurs via a Type 1 secretion system (T1SS) (20, 21). These systems are composed of at least three transport proteins and one or more polypeptide transport substrates (22). The transport complex is composed of an ABC transporter, a membrane fusion protein, and a TolC-like protein. The transport proteins putatively form a continuous channel across both bacterial membranes and the periplasm (23–25). Although the secreted proteins, or allocrites, vary by structural and functional classification, they share common physical and chemical features. The identified and predicted TISS allocrites 1) are acidic, with pI values between 3.0 and 4.8; 2) have few, if any, cysteine residues; 3) contain an aspartate/glycine-rich domain composed of multiple-nonapeptide Ca2+-binding RTX repeats; and 4) are targeted for secretion by a C-terminal signal sequence. The conservation of the RTX domain across a diverse set of secreted proteins suggests that its function may be conserved and is independent of the specific enzymatic activities associated with the particular exoprotein (17, 18, 21, 26–29).
Recent work demonstrates that an RTX domain from the Bordatella pertussis adenylyl cyclase (CyaA) protein is disordered in the absence of Ca2+ (30, 31). Calcium binding to multiple sites in the RTX domain facilitates a disorder-order transition with an apparent Ca2+ affinity higher than cytosolic [Ca2+]free (32). As such, it has been suggested that the RTX domains remain in an extended conformation within the cell. These unfolded conformations may promote secretion because the substrate protein must putatively be unfolded during secretion due to physical constraints of the T1SS pore. Alternatively, the unfolded conformations may serve to reduce unwanted intracellular enzymatic activity before the post-translational secretion of the substrate proteins. The putative dynamics associated with transport out of the cell and Ca2+-coupled folding suggest that these disorder-order transitions are critical for the regulation of T1SS exoprotein activities. However, it is not known how the Ca2+-induced RTX folding events may be coupled to the secretion, folding, and activity of their cognate enzymatic domains (33–35).
To evaluate the mechanisms by which Ca2+ regulates the folding and activity of AP, we have expressed and purified a series of AP proteins from P. aeruginosa for structural and functional studies. Calcium binding studies demonstrate that the RTX domain from AP undergoes a highly cooperative disorder-order transition upon Ca2+ binding. This Ca2+-bound, native RTX structure is critical for the folding and activation of the full-length AP and acts through two distinct mechanisms. First, the RTX domain provides a self-chaperoning activity and assists in the folding/activation of the PD through contacts across the RTX-PD interface. Second, interactions between an N-terminal helix and the RTX domain stabilize the native state. From these data, we propose a vectoral model for the Ca2+-induced secretion and folding of AP. Calcium-induced folding of the RTX domain nucleates the folding of the PD. Association of the N- and C-terminal sequences, brought into close proximity by folding of the RTX and PD, lock the protease in a stable, native conformation.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
P. aeruginosa PAO1 genomic DNA (ATCC) was used as a template for the PCR amplification of the AprA alkaline protease protein and the RTX domain. N- or C-terminal His6 tags were included for the purposes of purification and cloned into pETDuet vectors (Novagen) for T7-regulated expression in BL21(DE3) cells (Stratagene). Clones were verified by automated DNA sequencing. Domain boundaries and residue numbering were established using the Protein Data Bank entry 1KAP crystal structure (36). Full-length AP included nine N-terminal residues not visualized in the 1KAP structure and matched the NP_249940.1 protein sequence. The RTX domain included residues 253–470. ΔN-AP included residues 28–470. The PD protein included residues 28–252.
The AP proteins and domains expressed at high levels and were purified under denaturing conditions from inclusion bodies using either urea (RTX) or guanidine HCl (full-length and truncation mutants). Briefly, cells were resuspended in lysis buffer (50 mm Tris, 150 mm NaCl, 5 mm CaCl2, pH 6.8) and disrupted by sonication. Insoluble material, including inclusion bodies, was collected by centrifugation (20 min, 15,000 × g, 4 °C), solubilized in lysis buffer containing 8 m urea or 6 m guanidine HCl and clarified by centrifugation (30 min, 40,000 × g, 4 °C). The clarified supernatant was loaded onto an Ni2+-NTA column (GE Healthcare) under denaturing conditions. For RTX protein, the column was washed, and protein was eluted in the absence of urea (50 mm Tris, 150 mm NaCl, 30 mm (wash)/400 mm (elute) imidazole, pH 6.8). For the full-length and truncation mutants, the columns were washed, and protein was eluted in buffers containing 6 m guanidine HCl. The eluted proteins were concentrated and loaded onto an S-300 gel filtration column (GE Healthcare). A single major peak containing the AP or RTX proteins was identified and collected. For full-length and truncation mutations, protein purity was >98%, as assessed by SDS-PAGE and Coomassie staining.
RTX and AP Refolding
The purified proteins were refolded by rapid dilution into buffer (50 mm Tris, 150 mm NaCl, 0–50 mm Ca2+, pH 6.8) on ice for 15 min. Prior to activity and/or spectroscopic measurements, the refolding reactions were clarified by centrifugation and/or filtration through a Microcon YM-100 filter. Data presented were collected from at least two independent protein purifications with n > 4 for all experiments.
CD Spectroscopy
The purified protein samples were analyzed by far UV CD spectroscopy to evaluate changes in secondary structure as a function of Ca2+ binding. Protein samples between 4 and 10 μm were analyzed using a 1-mm path length cuvette. Spectra were collected from 280 to 195 nm on a Jasco 810 CD spectrophotometer at 25 °C. Buffer and blank absorbance measurements were collected and subtracted from experimental samples. For Ca2+ titrations, the ratio of absorbance at 204 nm (apo) and 217 nm (folded) were calculated from buffer-corrected spectra. These data were subsequently normalized for comparison with fluorescence data.
Intrinsic Protein Fluorescence
Fluorescence emission spectra of the RTX domain were collected on a BioTek Synergy 4 multimode plate reader and a Jobin-Yvon Fluorolog 3 fluorometer. Protein samples were excited at 280 nm, and emission spectra were collected between 300 and 400 nm. Buffers were analyzed for background and subtracted from total protein fluorescence. Changes in the ratio of fluorescence intensity at 328 nm (folded) and 338 nm (apo) were used to derive the fraction folded in Ca2+ binding experiments. The ratios were normalized for comparison with other spectrophotometric measurements.
Changes in the ratio of fluorescence between 328 nm (folded) and 348 nm (urea-denatured) were used to evaluate urea denaturation. Linear regression was used to fit the urea-induced transition to determine thermodynamic parameters, as described previously (37).
Gel Filtration Chromatography
Gel filtration chromatography of the purified RTX domain was accomplished using an AKTA FPLC and a Superdex 75 300GL size exclusion column (GE Healthcare) at 4 °C. The sizing column was equilibrated in buffer (50 mm Tris, 150 mm NaCl, 0–10 mm CaCl2, pH 6.8), and protein elution was monitored by absorbance at 280 nm. Calcium binding was assessed by injecting apoprotein onto a column equilibrated with increasing concentrations of calcium. Calcium release was measured by injection of calcium-bound protein onto the column equilibrated with decreasing concentrations of calcium. The apo and fully bound protein elution volumes were verified by prebinding protein and pre-equilibrating the column in solutions containing saturating calcium and solutions buffered with EGTA to <1 nm free calcium. Columns were calibrated using Bio-Rad GFC standards.
Data Analysis
Non-linear regression was performed using SigmaPlot. For Ca2+ binding experiments, data were fit using a four-parameter Hill equation.
Western Blotting
Western blots were performed using a rabbit polyclonal antibody raised against the purified, Ca2+-bound RTX domain (Genscript).
Protease Assay
Activity of the AP protein was assessed using a BODIPY-conjugated casein substrate (EnzChek, Invitrogen). Hydrolysis of the casein polypeptide results in a dequenching of the BODIPY fluorophore. Protease assays were measured as a function of emission intensity. Reactions were evaluated either kinetically or as end point measurements in a BioTek Synergy 4 plate reader with excitation at 485 nm and emission at 525 nm.
RESULTS
Calcium-induced Folding of RTX
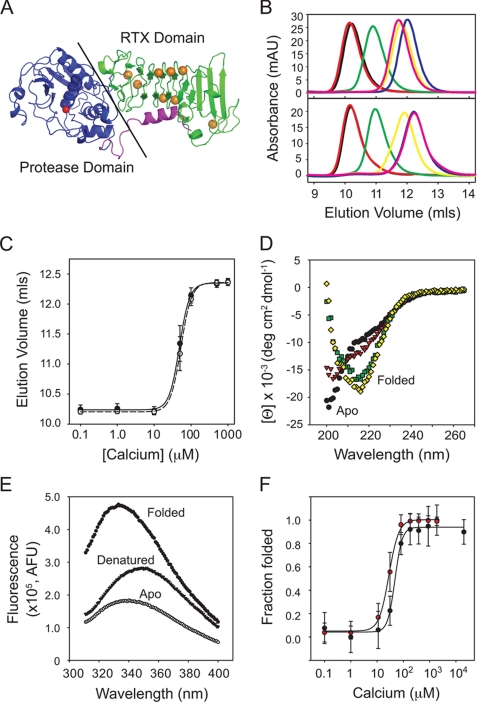
Alkaline protease is a multidomain protein composed of two globular domains: an N-terminal proteolytic domain and a C-terminal Ca2+-binding RTX domain (Fig. 1A) (12, 36). The domains pack together, forming a ∼1000 Å2 interface, although neither domain contributes to the core structure of the other. The structures of the Ca2+-bound AP contain eight ions, coordinated by side chain and backbone contacts in a β-helix formed by the RTX sequences (Fig. 1A). To assess the role of the RTX domain in the folding and stabilization of the native AP structure, RTX domain was purified, and its Ca2+-dependent folding was characterized.
FIGURE 1.
Calcium-induced folding of the alkaline protease RTX domain. The isolated RTX domain from alkaline protease was purified for in vitro Ca2+ binding and structural studies. A, a schematic representation of the 1KAP crystal structure is shown (36). The Ca2+-binding RTX domain is shown in green, the protease domain is shown in blue, and the N-terminal helix is shown in magenta. The active site Zn2+ is shown as a red sphere, and the RTX-bound Ca2+ ions are shown in orange. B, representative analytical gel filtration chromatographs show the changes in Ca2+-induced hydrodynamic radii. Calcium-induced protein folding was evaluated by injecting Ca2+-free protein onto a column pre-equilibrated with increasing [Ca2+] (top). Bottom, unfolding was monitored by injecting Ca2+-bound protein onto the column with decreasing [Ca2+]. Calcium concentrations were 1 μm (black), 10 μm (red), 50 μm (green), 100 μm (yellow), 500 μm (magenta), and 1 mm (blue). C, a summary of the Ca2+-induced hydrodynamic changes is shown and fit using non-linear regression. The calcium-induced folding transition is shown by the solid circles and line. The unfolding transition is shown in open circles and dashed lines. D, representative circular dichroism spectra of the Ca2+-induced changes in RTX secondary structure are shown. Calcium concentrations were 0 μm (black circles), 20 μm (red triangles), 50 μm (green squares), and 200 μm (yellow diamonds). E, representative fluorescence spectra are shown for the apo state (filled circles), Ca2+-bound folded state (open circles), and urea-induced denatured state (solid triangles). F, summary of the folding transitions monitored by CD (black circles) and fluorescence (red circles). Data are shown as mean ± S.D. (error bars).
The hydrodynamic radius of the purified RTX protein was first assessed using analytical gel filtration chromatography. Concentration of the Ca2+-free RTX domain (>20 μm) resulted in the formation of amyloid-like aggregates that bound thioflavin-T and appeared ordered by negative stain EM (data not shown). In dilute solution (<10 μm), the Ca2+-free and Ca2+-bound RTX protein migrated as single symmetrical peaks. Protein in either the apo state (unfolded) or prebound with 2 mm Ca2+ (folded) was injected onto a column pre-equilibrated in the presence and absence of Ca2+. Protein elution was monitored by absorbance at 280 nm. Injection of the apo RTX protein onto a column equilibrated with increasing concentrations of Ca2+ resulted in an increase in retention volume (Fig. 1, B (top) and C). No peak splitting, significant changes in peak tailing, or broadening were observed.
Elution volume was determined using the maximum absorbance intensity for each Ca2+ concentration and plotted against [Ca2+]. The apparent Kd, starting from the apo state, was 48 ± 7 μm with a Hill coefficient of 3.1 ± 0.3 based on differential retention volume. Elution volumes of the calcium-bound protein injected onto the gel filtration column matched those of the apoprotein for each Ca2+ concentration, indicating reversibility (Fig. 1, B (bottom) and C). The apparent Kd, as calculated from the Ca2+-bound protein state, was 53 ± 8 μm with a Hill coefficient of 3.1 ± 0.2.
Structural changes associated with Ca2+ binding to RTX were then measured using far UV CD spectroscopy. In low Ca2+, the RTX domain showed an absorbance minimum near 200 nm (Fig. 1D). In addition to the predominant absorbance minimum, a shoulder of absorbance was seen near 220 nm, consistent with some residual secondary structure in the extended apo conformation. Calcium addition shifted the absorbance minimum to 217 nm, consistent with a β-rich structure. Calcium titrations between 1 μm and 2 mm were performed to further characterize the influence of calcium on the secondary structure of the purified RTX domain. At and above 50 μm, the absorbance shifted to a single minimum at 217 nm. No significant changes in CD signal were seen as [Ca2+] increased above ∼200 μm.
Calcium binding affinity and cooperativity were assessed using the ratio of absorbance at 204 nm (random coil) and 217 nm (β-structure). This ratio of absorbance was normalized based on minimum and maximum values for comparison with other spectroscopic data (Fig. 1F). The CD data showed a single cooperative transition between the unfolded and folded states with an apparent Kd of 30 ± 11 μm and a Hill coefficient of 3.0 ± 0.2. These structural transitions were reversible upon the addition of EGTA or dilution into low Ca2+ solutions. In addition, the transitions were specific for Ca2+ because other divalent cations failed to induce the spectral shift (data not shown).
Calcium binding to the RTX domain was further assessed using intrinsic protein fluorescence. Two tryptophan residues are found in the sequence of the RTX domain. In the absence of calcium, the apo RTX fluoresced with a peak wavelength of 338–340 nm (Fig. 1E). At 2 mm Ca2+, the RTX protein fluorescence increased, and peak wavelength shifted to 328–330 nm. These changes in fluorescence were not seen when the purified RTX domain was incubated with other divalent cations, consistent with the GFC and CD measurements. Denaturation of the Ca2+-bound, folded RTX protein by incubation in 7 m urea decreased intrinsic fluorescence and shifted the peak wavelength to 348 nm (Fig. 1E).
Calcium titrations between 100 nm and 2 mm were performed to assess the Ca2+ dependence of the fluorescence spectra. Fluorescence was assessed using the ratio of intensities at 328 nm (Ca2+-bound maximum) and 338 nm (apo maximum). The resulting ratio data were normalized for comparison with the CD data (Fig. 1F). Peak fluorescence increased with Ca2+ concentrations above 10 μm and appeared to saturate at 100–200 μm Ca2+. The apparent Kd for calcium binding was 49 ± 23 μm with a Hill coefficient of 3.2 ± 0.3.
Calcium-induced Folding of AP
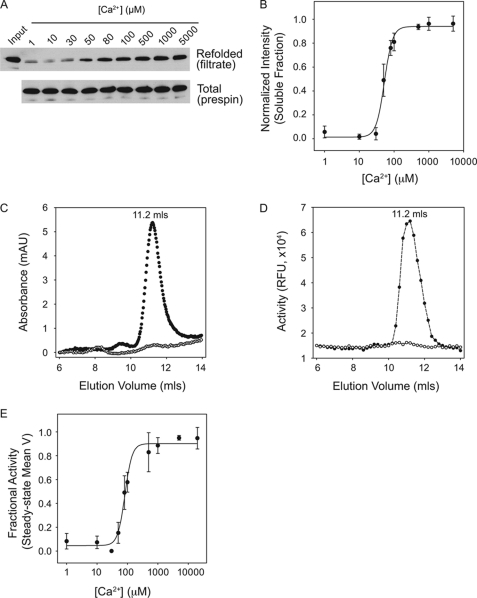
To evaluate the calcium-dependent folding of full-length AP, recombinant AP was purified for spectroscopic, functional, and hydrodynamic studies. Overexpression of AP in Escherichia coli resulted in large quantities of protease produced insolubly in inclusion bodies. The insoluble AP was purified under denaturing conditions and refolded by rapid dilution. Refolding was initially assessed as the fraction of protein in the filtrate of YM-100 centrifugal filters or after centrifugation (Fig. 2, A and B). Below ∼50 μm Ca2+, a large fraction of the full-length AP protein aggregated and could be removed from solution by filtration or centrifugation (Fig. 2, A and B). Recovery increased between 50 and 100 μm Ca2+ and saturated between 200 and 500 μm Ca2+. Refolding efficiency was >85% in saturating Ca2+. Non-linear regression was used to fit the Hill equation, using densitometric intensities plotted against [Ca2+]. The apparent [Ca2+]½ was 58 ± 13 μm with a Hill coefficient of 2.8 ± 0.3. These data are presented as [Ca2+]½ because the folding appeared irreversible under the conditions tested, in contrast to the reversible folding seen with the isolated RTX.
FIGURE 2.
Calcium-induced AP folding. Full-length alkaline protease was purified for binding and activation studies to evaluate the role of Ca2+ binding on AP folding. A, AP refolding was assessed after rapid dilution into Ca2+-containing buffers and filtration through a Microcon YM-100 filter. A representative Western blot of the filtrate (top) and total protein (bottom) is shown for Ca2+ concentrations between 1 μm and 5 mm. B, the refolded fraction of AP after filtration was assessed by densitometry and is shown as a function of Ca2+ in the refolding buffer. C, analytical gel filtration chromatography was used to evaluate the hydrodynamic radius of purified AP. Protein was refolded in the absence of Ca2+ (open circles) or in 20 mm Ca2+ (filled circles) and filtered prior to column injection. D, protease activity was assessed from fractions collected after gel filtration. Proteins refolded in the absence of Ca2+ (open circles) and in 20 mm Ca2+ (filled circles) were evaluated utilizing the BODIPY-casein substrate. E, mean velocities of the steady-state activities of AP refolded are shown plotted against Ca2+. Non-linear, least squares regression fit to a four-parameter Hill equation is shown as a solid line in B and E. Data are shown as mean ± S.D. (error bars).
Analytical GFC was used to assess the hydrodynamic properties of refolded AP. Dilution from guanidine HCl in the absence of Ca2+ resulted in protein that precipitated in solution and was removed upon filtration by YM-100 centrifugal filters (Fig. 2C, open circles). Analysis of the unfiltered apo AP resulted in protein that was retained on the column or eluted in the void (supplemental Fig. 1A). Analysis of the AP refolded into Ca2+-containing buffer and filtered using a YM-100 filter resulted in a predominant elution peak at 11.2 ml, consistent with a protein of ∼50 kDa (Fig. 2C, filled circles). In the absence of filtration and/or centrifugation of the Ca2+-bound AP, a small void peak was seen in GFC analysis. The monomeric fraction appeared unaffected by Ca2+ dilution or chelation after folding in high Ca2+ (data not shown).
To evaluate the enzymatic activities of the refolded AP, eluates from the GFC were collected by fractionation. Activity was evaluated using a BODIPY-conjugated casein digestion assay. The intact BODIPY-casein is quenched as a result of intramolecular interactions. Casein digestion leads to dequenching of the BODIPY fluorophores and can be followed as an increase in fluorescence intensity. Proteolytic activity was seen in the AP monomeric fractions after refolding into Ca2+ with and without filtration (Fig. 2D and supplemental Fig. 1B). No significant protease activity was seen in the void fractions or in fractions outside of the monomeric peak.
The Ca2+ dependence of AP refolding was then assessed using the BODIPY-casein assay after refolding in increasing [Ca2+]. Enzymatic activity was evaluated kinetically. Steady-state mean velocities were determined using linear regression. Mean velocities are plotted against [Ca2+] in Fig. 2E. Protease activity showed a Ca2+ dependence similar to that of the solubility experiments (Fig. 2B), with a [Ca2+]½ of 65 ± 19 μm and a Hill coefficient of 2.6 ± 0.2.
Ca2+-induced Chaperone Activity of RTX
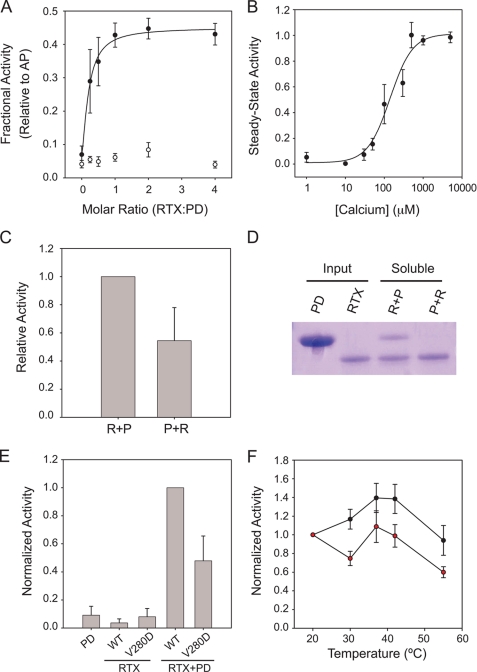
The close agreement between the observed Ca2+-induced folding of RTX and folding and activation of AP suggested that the Ca2+-induced folding of the AP RTX domain is tightly coupled to the folding and activation of the PD. To further evaluate the coupling of RTX folding to AP activity, the individual RTX and PD domains were expressed and purified for trans-complementation studies. The PD and RTX domain boundaries were established using available crystal structures and purified under denaturing conditions (Fig. 1A) (11, 12). Folding and trans-activation of the protease domain were evaluated by casein digestion in the presence and absence of Ca2+ and the RTX domain. The PD showed minimal protease activity when evaluated in isolation in the presence or absence of 2 mm Ca2+ (Fig. 3A). Protease activity increased when the PD was refolded in buffers containing the Ca2+-bound, native RTX protein. This trans-activation saturated with RTX/PD molar ratios between 1.5 and 2.0 (Fig. 3A). In 2 mm Ca2+, maximal protease activity in the trans-activation experiment was ∼45% that of the full-length AP. Activation of the PD by the RTX domain was not observed in the absence of Ca2+ or in the presence of Mg2+, suggesting that folded RTX was critical for the activation of PD. This RTX-regulated trans-activation showed a cooperative Ca2+ dependence, with an apparent [Ca2+]½ of 146 ± 27 μm and a Hill coefficient of 1.8 ± 0.4, when evaluated with saturating RTX (RTX/PD molar ratio of 2) (Fig. 3B).
FIGURE 3.
Calcium-regulated interactions of the RTX and PD domains. To assess the coupling of RTX folding and PD folding and activation, the interaction between these two domains was evaluated by trans-complementation studies and mutagenesis. A, trans-activation of the PD by refolded RTX is shown as a function of the molar ratio between the RTX and PD domains. PD was diluted into buffers containing refolded RTX in 2 mm Ca2+ (filled circles) or in the absence of Ca2+ (open circles). Data are normalized to the activity of the full-length AP protein refolded under identical conditions. The non-linear regression fit is shown. B, a summary of Ca2+ titration with a 2:1 ratio of RTX to PD is shown in filled circles and fit using non-linear regression. As in A, the RTX domain was refolded prior to dilution of the PD. C, order of addition studies were used to assess the potential role of RTX in the folding or activation of PD. Refolding the RTX prior to the PD (R + P) resulted in a nearly 2-fold increase in protease activity when compared with the activity seen when the PD was refolded and then activated by the addition of RTX (P + R). D, a representative Coomassie Blue-stained gel of refolded RTX and PD proteins from the trans-activation studies is shown. The input protein is shown for both the PD and RTX proteins. Refolding the PD in the presence of the folded RTX (R + P) results in solubilization of the PD after separation by centrifugation. The addition of the RTX domain to PD refolded alone resulted in significantly reduced recovery of the PD after centrifugation, demonstrating that folding of PD was dependent on the presence of the folded RTX. E, disruption of the RTX-PD interaction results in decreased trans-activation. A summary of protease activities of the PD, the WT and V280D RTX domains, and mixtures containing the PD with the RTX domains is shown after refolding in 2 mm Ca2+. The V280D substitution, in the core of the RTX-PD interface, decreases trans-activation of the PD by ∼50% as compared with WT, consistent with a disruption of the appropriate RTX-PD association. F, full-length AP is sensitive to the introduction of the V280D mutation. Protease activity as a function of reaction temperature is shown for the WT (black circles) and V280D (red circles) proteins using the casein digestion assay. The V280D mutant refolds with an efficiency similar to that of wild type but shows an increased thermal sensitivity at all elevated reaction temperatures. Data shown are mean ± S.D. (error bars).
The trans-activation of the PD could potentially be explained by multiple mechanisms. The PD may be allosterically regulated by the Ca2+-bound, folded RTX domain. The association of the two folded domains would lead to increased protease activity. Alternatively, the PD activation seen in the trans-complementation experiments could be the result of RTX-induced folding of the PD. In such a model, the RTX domain would serve to chaperone the proper folding of the PD. Acquisition of the PD native state would lead to the increase in protease activity. To evaluate what role the RTX domain had in the folding and activation of the PD, order of addition experiments were performed in the presence of 2 mm Ca2+. For these experiments, the RTX and PD domains were refolded and centrifuged to clear misfolded species. Subsequently, the complementary domain was then refolded by rapid dilution into the cleared reactions. When the RTX domain was refolded prior to the dilution of the PD, the resulting activity was similar to that presented in Fig. 3, A and B. When the PD was refolded prior to the addition of the RTX domain, protease activity decreased by ∼45% (Fig. 3C). Similarly, when evaluated by SDS-PAGE and Coomassie Blue staining, the refolding of PD showed a strong dependence on the presence of refolded RTX domain (Fig. 3D). Refolding of the PD, as measured by its solubility and function, required the presence of the folded RTX domain.
We hypothesized that disruption of the RTX-PD interface would result in altered activity in the trans-complementation studies. Sites within the interface were chosen for substitution based on their electrostatic and steric contributions to the RTX-PD interface. A single site mutation, located centrally in the RTX-PD interface, V280D, decreased PD activation by 50% when compared with the wild type RTX in saturating Ca2+ and equimolar RTX/PD ratios (Fig. 3E). Calcium-induced folding of the V280D was not significantly altered when compared with wild type in hydrodynamic and spectroscopic measurements (data not shown), suggesting that RTX folding was not altered by this mutation. The Asp substitution mutant was also evaluated in the context of the full-length AP protein (Fig. 3F). The apparent [Ca2+]½ and Hill coefficients were indistinguishable from those of the wild type protein (data not shown), as assessed by the Ca2+ dependence of protease activity. Similarly, the specific activity of V280D was similar to that of wild type at 20 °C, suggesting that folding and activation were not dramatically affected by the V280D substitution in the full-length protein.
To evaluate AP stability, protease assays were performed at increasing reaction temperatures. Under these conditions, the wild type protein showed a biphasic response to increasing temperature (Fig. 3F). Between 20 and 37 °C, protease activity increased by ∼40%. Protease activity was maximal between 37 and 42 °C and then decreased between 42 and 55 °C. At 55 °C, wild type AP activity was similar to that measured at 37 °C (Fig. 3F). In contrast, the V280D mutant showed decreased activity at all elevated temperatures. Mutant protease activity was reduced by 30–40% at all elevated temperatures when compared with wild type (Fig. 3F). The data were consistent with a key role for regulating AP activity via interactions across the RTX-PD interface.
Ca2+-regulated Stabilization of AP
The trans-complementation experiments demonstrate that the RTX-PD interface is important for the folding and subsequent activation of AP. Disruption of the RTX-PD interface in the trans-complementation studies resulted in a decrease in protease activation. In contrast, the full-length protein showed no significant changes in Ca2+-dependent folding and activity at low temperature, instead showing changes in activity at elevated temperatures. Despite yielding subtly different results, both experiments suggest that RTX-PD association is important for the folding and activity of the AP. We hypothesized that these differences could be the result of at least two different parameters. First, the dynamics of an intramolecular domain-domain interaction in the full-length AP are probably different from those of the intermolecular RTX-PD complex. Second, an α-helix N-terminal to the PD was not present in the complementation studies. For these experiments, the RTX and PD proteins were produced as minimal globular domains and lacked the N-terminal helix present in this family of proteins (Figs. 1A and 4A). This conserved N-terminal helix packs against the Ca2+-bound, folded RTX domain, burying ∼600 Å2.
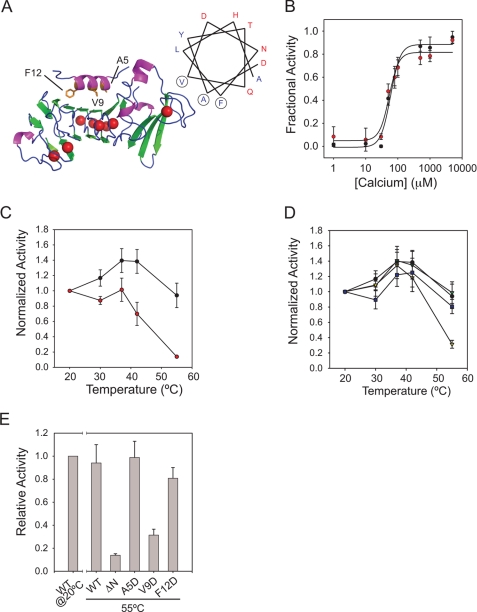
FIGURE 4.
N-terminal helix stabilization of AP. The role of the N-terminal helix in AP folding and stabilization was assessed using functional assays. A, schematic diagram of the RTX and N-terminal helix sequences of AP is shown with a helical wheel projection of the N-terminal helix. The locations of the A5D, V9D, and F12D missense mutations are indicated in the structure and circled in the helical wheel. B, calcium-induced refolding and activation were assessed for the wild type (black circles) and ΔN-AP proteins (red circles). Activities of the wild type and ΔN-AP proteins are normalized to their respective maxima in saturating Ca2+. C, the temperature sensitivities of AP (black circles) and ΔN-AP (red circles) activities are shown. D, the temperature sensitivity of the N-terminal helix missense mutations is shown as follows: wild type (black circles), A5D (purple squares), V9D (yellow triangles), and F12D (green triangles). E, summary data for the wild type, ΔN-AP, and N-terminal helix missense mutants are shown at 55 °C. Activity of the wild type protein at 20 °C is shown for reference. Data are presented as mean ± S.D. (error bars).
To assess the potential impact of the N-terminal helix on AP folding, activity, and stability, N-terminal truncations (ΔN-AP) of the amphipathic helix were expressed and purified. As with the full-length AP, ΔN-AP showed cooperative Ca2+-dependent refolding (Fig. 4B). The apparent [Ca2+]½ of the ΔN-AP protein was 63 ± 13 μm with a Hill coefficient of 2.6 ± 0.2. Gel filtration, fluorescence, and CD measurements also showed similar shifts in hydrodynamic and spectroscopic properties between the Ca2+-free and Ca2+-bound full-length and ΔN-AP proteins, consistent with the acquisition of similar native structures (data not shown). Specific activity of the ΔN-AP was similar to or higher than that of the wild type when measured at 20 °C using the casein digest assay. When protease activity was evaluated at elevated temperatures, ΔN-AP showed increased thermal sensitivity compared with wild type (Fig. 4C). At 42 °C, protease activity decreased by ∼50%, and at 55 °C, protease activity decreased by ∼90% when compared with the full-length AP in 2 mm Ca2+. The decreased thermal stability of the ΔN-AP protein suggested that the N-terminal helix-RTX interactions were critical for maintaining native state structural stability.
The N-terminal helix is a conserved amphipathic helix that binds across one face of the folded RTX domain (Fig. 4A and supplemental Fig. 2). To further probe the N-terminal helix-RTX interaction, aspartate substitutions within the interface were introduced at Ala-5, Val-9, and Phe-12. These proteins showed Ca2+-dependent folding similar to that of the wild type and ΔN-AP proteins. Specific activity and GFC profiles were similar for the wild type and N-terminal helix substitution mutants (data not shown). This was consistent with the structure and folding of the ΔN-AP protein at low temperature. The A5D mutant, located at the N-terminal end of the amphipathic helix, showed activities similar to those of wild type across a range of reaction temperatures (Fig. 4D). Similarly, the F12D mutant, located at the C-terminal end of the helix, showed a minimal decrease in activity at temperatures above 30 °C when compared with wild type. In contrast, the V9D mutant, located centrally in the N-terminal helix, showed a 70% decrease in protease activity at 55 °C, similar to the changes in activity seen with the ΔN-AP protein at high temperature (Fig. 4E). These data suggested that interactions between the N-terminal helix and the RTX domain were critical for the stability of AP and that disruption of that interaction decreased protease stability.
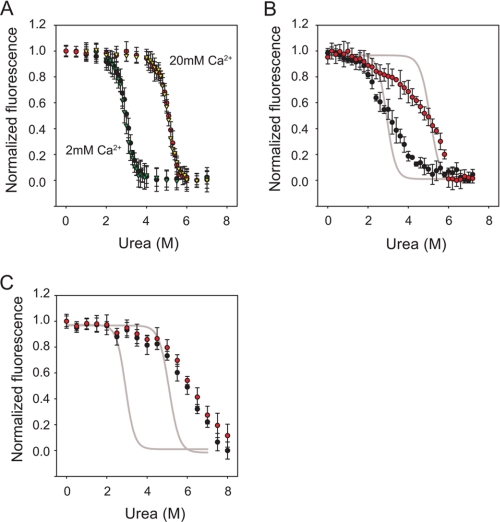
To further assess protease stability and the domain-domain interactions in AP, urea titrations were performed on the RTX, ΔN-AP, and AP proteins. Calcium concentrations were chosen to saturate the Ca2+-binding sites in the RTX domain and to be in range with environmental Ca2+. At both 2 and 20 mm Ca2+, the RTX proteins showed cooperative, urea-dependent unfolding transitions that were reversible (Fig. 5A). Analysis of the transition region using linear regression was performed to ascertain ΔGunfolding, Cm, and m values (Table 1) (37). The ΔGunfolding was measured as 6.7 ± 0.2 kcal mol−1 when protein was evaluated in 2 mm Ca2+ and 11.3 ± 0.3 kcal mol−1 in 20 mm Ca2+. Changes in ΔGunfolding were associated with an increase in Cm (2.9 versus 5.0 m). No significant changes in m value at 2 and 20 mm Ca2+ were observed (2.3 kcal mol−1 m−1), suggesting similar buried surface areas in both Ca2+ conditions.
FIGURE 5.
Urea-induced unfolding of RTX, ΔN-AP, and AP proteins. Urea denaturation titrations were performed to assess the relative stabilities of the RTX and AP proteins. A, RTX proteins in 2 and 20 mm Ca2+ were assessed by monitoring changes in tryptophan fluorescence. Folding/unfolding transitions were reversible in both 2 and 20 mm Ca2+ and are shown as follows: unfolding, 2 mm Ca2+ (black circles); refolding, 2 mm Ca2+ (green triangles); unfolding, 20 mm Ca2+ (red circles); refolding, 20 mm Ca2+ (yellow triangles). B, unfolding of the ΔN-AP proteins at 2 mm Ca2+ (black circles) and 20 mm Ca2+ (red circles) is shown. Sigmoidal fits of the RTX unfolding transitions at 2 and 20 mm Ca2+ are shown in gray lines for reference. The increase in apparent stability as a function of increasing [Ca2+] from 2 to 20 mm was similar for both the RTX and ΔN-AP proteins. C, unfolding of the full-length AP is shown at 2 mm Ca2+ (black circles) and 20 mm Ca2+ (red circles). The RTX unfolding transition is shown as a gray line, as in B. Data are presented as mean ± S.D. (error bars).
TABLE 1.
Summary of urea titration data
| 2 mm Ca2+ |
20 mm Ca2+ |
|||
|---|---|---|---|---|
| ΔG | Cm or [urea]½ | ΔG | Cm or [urea]½ | |
| kcal/mol | m | kcal/mol | m | |
| RTX | 6.7 ± 0.2 | 2.9 ± 0.2 | 11.3 ± 0.3 | 5.0 ± 0.2 |
| ΔN-AP | NDa | 3.1 ± 0.3 | ND | 4.9 ± 0.1 |
| AP | ND | 5.7 ± 0.2 | ND | 5.8 ± 0.2 |
a ND, not determined.
Urea titrations were then performed using the purified ΔN-AP and full-length proteins to evaluate their stabilities. As with the RTX protein, the ΔN-AP proteins showed a cooperative, Ca2+-sensitive unfolding transition (Fig. 5B). The transition midpoints at both 2 and 20 mm Ca2+ were similar to those of the RTX domain (Table 1). In contrast to the relatively stable pre- and post-transition base lines for the RTX domains, the ΔN-AP protein showed a decrease in pretransition fluorescence and a broadening of the unfolding/refolding transition. The broadened transition may reflect the dynamics of multiple domains in the ΔN-AP protein. As such, we did not attempt to fit thermodynamic parameters to the folding and unfolding transitions of the ΔN-AP protein. However, the apparent urea dependence (transition slope) of the ΔN-AP proteins at 2 and 20 mm Ca2+ were similar, suggesting similar desolvation and buried surface areas in both conditions.
Compared with both the RTX and ΔN-AP proteins, the full-length AP protein showed an increase in relative stability at 2 and 20 mm Ca2+ (Fig. 5C). The full-length protein in both 2 and 20 mm Ca2+ showed a urea-induced unfolding transition that was slightly higher than that of the RTX and ΔN-AP proteins in 20 mm Ca2+. Unlike the RTX and ΔN-AP proteins, the increase in Ca2+ from 2 to 20 mm had only marginal effects on the apparent unfolding transition midpoint in the full-length protein. As with the ΔN-AP, the transition appeared to broaden slightly from that of the RTX denaturation curves, although the pretransition fluorescence appeared more stable in the full-length protease. Thermodynamic parameters were not fit due to the multidomain nature of the full-length AP protein. The apparent slope of the transition was comparable in the ΔN-AP and full-length proteins, suggesting that similar buried surface areas were desolvated in both proteins.
DISCUSSION
Because alkaline protease and other RTX toxins have been implicated in a variety of disease models, understanding the mechanisms of their secretion, folding, and regulation provides insight into pathogen virulence (20). Characterization of the wild type RTX domain indicates that Ca2+-induced folding occurs cooperatively and promotes the stabilization of a compact native state. Intrinsic fluorescence, CD spectroscopy, and GFC revealed a mean apparent affinity of 42 μm and a Hill coefficient of 3.1 (Fig. 1). The general agreement of the apparent affinity and cooperativity suggests that these independent measures are reporting on similar structural transitions.
Solution-based characterization of the Ca2+-bound RTX protein is consistent with the previously solved crystal structures of AP and related proteases (11, 12). Characterization of the apo state suggests that the RTX domain is extended and largely disordered. Although the extended conformation is predominantly unfolded, both CD and fluorescence suggest that some residual secondary structure may be present in the apo state (Fig. 1, D and E).
The folding and activity of the full-length protein showed a Ca2+ dependence that was similar to that of the RTX domain in isolation. Spectroscopic, hydrodynamic, and functional measurements indicated the apparent [Ca2+]½ to be 60 μm with a Hill coefficient of 2.7. These data suggest that folding and activation of the PD are coupled to the Ca2+-induced disorder-order transitions associated with RTX domain folding (Figs. 1–3).
Two intramolecular domain-domain interactions can be facilitated by RTX domain folding: the RTX-PD association and the RTX-N-terminal helix association. Although the global fold of the AP would putatively be disrupted by alteration across either of these interfaces, analysis of these two interfaces suggested that they contribute to AP folding and stability in different manners. The RTX-PD interface is critical for PD folding and activation based on the trans-complementation experiments (Fig. 3). Specifically, order of addition experiments suggest that PD folding, as measured by solubility and activity, was coupled to its association with the folded RTX. Consistent with this, protease activity was not fully rescued when the PD was refolded in the absence of the folded RTX and subsequently mixed with folded RTX. These data suggest that PD folding is impacted by the presence of the folded RTX domain. The tendency for the PD to aggregate in solution precluded many of the structural studies performed on the RTX domain and AP protein. As a result, the specific natures of the PD structural changes are not known.
Truncation or disruption of the N-terminal helix resulted in a marked reduction in protease stability, measured by activity assays and urea titrations (Figs. 4 and 5). These mutations had no discernible effects on protease folding under the conditions tested. This suggested that the RTX-N-terminal helix interface is critical for native state stability but not for AP folding. This observation was further supported by the similarity of urea-induced unfolding between the RTX and ΔN-AP proteins. The similarity in urea-induced unfolding at both 2 and 20 mm Ca2+ suggested that the native state stabilities of ΔN-AP and RTX were similarly dependent on Ca2+ binding (Table 1). The increased stability of the full-length AP suggested that the native conformation was significantly stabilized by interactions between the RTX domain and the N-terminal helix.
In the RTX and ΔN-AP proteins, unfolding appeared arrested by Ca2+ occupancy in the RTX repeats, as seen by the increase in Cm values for 2 and 20 mm Ca2+. In the presence of the N-terminal helix, the global fold was stabilized and unfolding appeared less sensitive to supersaturating concentrations of Ca2+. These data suggest that N-terminal helix binding may stabilize the global fold of AP by locking the RTX structure in a Ca2+-bound conformation. This is consistent with previous reports suggesting that minimal RTX structures are stabilized by self-association (oligomerization) and association with their flanking protein domains (31, 38). These native domain-domain associations also suggest a physical basis for the relatively slow chelation of Ca2+ from a homologous protease despite a relatively low apparent affinity for Ca2+. The N-terminal helix binding may stabilize the Ca2+-bound RTX structures, prevent their unfolding, and slow the kinetics of Ca2+ exchange (35).
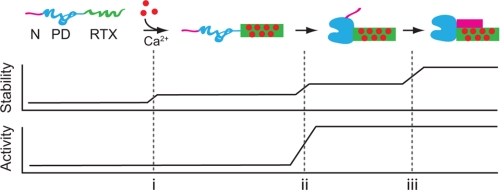
Together, these data suggest a vectoral folding pathway for the AP protein (Fig. 6). Calcium-induced folding of the RTX domain results in acquisition of the native, β-rich, compact structure and occurs without the need for additional AP domains. Subsequently, the PD associates and folds via interactions with the folded, Ca2+-bound RTX domain. The RTX-PD interaction is necessary for PD folding and activation, providing a structural scaffold through the RTX-PD interface. Finally, the N-terminal helix binds the folded RTX domain along a grove composed of the side of the folded RTX β-helix and the C-terminal sequences. This interaction stabilizes the local Ca2+-bound RTX structure and the global fold of AP.
FIGURE 6.
Alkaline protease folding pathway. A putative pathway for the vectoral folding and stabilization of AP is presented. Ca2+ binding to the RTX domain initiates its folding (i). The native RTX domain facilitates the folding and activation of the PD via the 1000-Å2 RTX-PD interface (ii). The folded PD, in turn, facilitates the N-terminal helix-RTX interaction by placing the N-terminal helix in position to bind the folded RTX domain (iii). Stability of AP increases with each domain-domain association. Stabilization of the RTX domain structure occurs upon its interaction with Ca2+, its association with the PD, and its association with the N-terminal helix. The activity of AP is regulated by the association of the PD with the folded, Ca2+-bound RTX domain. The AP domains are colored as follows: N-terminal helix (magenta), PD (blue), and RTX (green) as in Fig. 1A. Folded protein is shown as a solid shape; unfolded protein domains are shown as extended lines.
Conceptually, the N-terminal helix serves as a “key” that engages a “tumbler” formed by the folded RTX domain. This association does not facilitate folding per se but instead “locks” the native state. In an extended conformation, the unfolded RTX domain does not provide a native surface for either the PD or the N-terminal helix to bind. When folded, the RTX domain facilitates PD folding through the RTX-PD interface, which subsequently is stabilized by the RTX-N-terminal helix interaction.
Such a pathway might facilitate secretion in multiple ways. First, based on the apparent affinities and activities of the RTX and AP proteins, AP would remain unfolded or destabilized within the cell. In an extended conformation, transport could occur without the need for a cellular unfoldase activity because secretion is thought to occur post-translationally and require an unfolded polypeptide substrate (21, 27). Maintaining the cytosolic AP in an unfolded conformation would also limit unwanted enzymatic activity prior to secretion. Second, the vectoral folding of AP could be coupled to Ca2+-stimulated secretion seen in P. aeruginosa clinical isolates (6). If Ca2+-induced RTX folding occurs co-translocationally, it is possible that the free energy of folding could be actively coupled to the extraction of AP, similar to the coupled folding and translocation seen in the autotransporter systems (39). If protein secretion occurs by diffusion through the T1SS pore, co-translocational folding might physically inhibit retrotranslocation, shifting a stochastic process to a vectoral one. A similar model for protein import into mitochondria has been proposed wherein chaperone binding and protein folding prevent retrotranslocation through channels in the mitochondrial membrane (40). Both of these models would be consistent with previous reports that ATPase activity of the T1SS ABC transporters is inhibited by interactions with their protein substrates (41, 42).
It is intriguing to think that multiple structural states associated with Ca2+-induced folding may contribute to AP secretion and activity. These Ca2+-induced structural changes in RTX proteins may serve to regulate protein function prior to and after secretion into the extracellular environment. Understanding the folding and structural dynamics of AP provides insight into the virulence of Pseudomonas and other Gram-negative pathogens that utilize T1SSs and RTX-containing virulence factors to regulate host-pathogen interactions.
Supplementary Material
Acknowledgments
We thank members of the Butterworth, Devor, Frizzell, and Thibodeau laboratories for constructive comments and criticism.
This work was supported, in whole or in part, by National Institutes of Health, NIDDK, Grants DK083284 and DK072506 (to P. H. T.). This work was also supported by faculty development funding from the University of Pittsburgh School of Medicine, Department of Cell Biology and Physiology.

This article contains supplemental Figs. 1 and 2.
- AP
- alkaline protease
- PD
- proteolytic domain
- RTX
- repeats in toxin
- T1SS
- Type 1 secretion system
- ΔN-AP
- N-terminal truncation of AP.
REFERENCES
- 1. Lyczak J. B., Cannon C. L., Pier G. B. (2000) Establishment of Pseudomonas aeruginosa infection. Lessons from a versatile opportunist. Microbes Infect. 2, 1051–1060 [DOI] [PubMed] [Google Scholar]
- 2. Hobden J. A. (2002) Pseudomonas aeruginosa proteases and corneal virulence. DNA Cell Biol. 21, 391–396 [DOI] [PubMed] [Google Scholar]
- 3. Lyczak J. B., Cannon C. L., Pier G. B. (2002) Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15, 194–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leidal K. G., Munson K. L., Johnson M. C., Denning G. M. (2003) Metalloproteases from Pseudomonas aeruginosa degrade human RANTES, MCP-1, and ENA-78. J. Interferon Cytokine Res. 23, 307–318 [DOI] [PubMed] [Google Scholar]
- 5. Kharazmi A., Høiby N., Döring G., Valerius N. H. (1984) Pseudomonas aeruginosa exoproteases inhibit human neutrophil chemiluminescence. Infect. Immun. 44, 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarkisova S., Patrauchan M. A., Berglund D., Nivens D. E., Franklin M. J. (2005) Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J. Bacteriol. 187, 4327–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaffar-Bandjee M. C., Lazdunski A., Bally M., Carrère J., Chazalette J. P., Galabert C. (1995) Production of elastase, exotoxin A, and alkaline protease in sputa during pulmonary exacerbation of cystic fibrosis in patients chronically infected by Pseudomonas aeruginosa. J. Clin. Microbiol. 33, 924–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kharazmi A., Döring G., Høiby N., Valerius N. H. (1984) Interaction of Pseudomonas aeruginosa alkaline protease and elastase with human polymorphonuclear leukocytes in vitro. Infect. Immun. 43, 161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guyot N., Bergsson G., Butler M. W., Greene C. M., Weldon S., Kessler E., Levine R. L., O'Neill S. J., Taggart C. C., McElvaney N. G. (2010) Functional study of elafin cleaved by Pseudomonas aeruginosa metalloproteinases. Biol. Chem. 391, 705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guzzo J., Murgier M., Filloux A., Lazdunski A. (1990) Cloning of the Pseudomonas aeruginosa alkaline protease gene and secretion of the protease into the medium by Escherichia coli. J. Bacteriol. 172, 942–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baumann U. (1994) Crystal structure of the 50-kDa metalloprotease from Serratia marcescens. J. Mol. Biol. 242, 244–251 [DOI] [PubMed] [Google Scholar]
- 12. Baumann U., Wu S., Flaherty K. M., McKay D. B. (1993) Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa. A two-domain protein with a calcium binding parallel β roll motif. EMBO J. 12, 3357–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bertini I., Calderone V., Fragai M., Jaiswal R., Luchinat C., Melikian M., Mylonas E., Svergun D. I. (2008) Evidence of reciprocal reorientation of the catalytic and hemopexin-like domains of full-length MMP-12. J. Am. Chem. Soc. 130, 7011–7021 [DOI] [PubMed] [Google Scholar]
- 14. Lang R., Kocourek A., Braun M., Tschesche H., Huber R., Bode W., Maskos K. (2001) Substrate specificity determinants of human macrophage elastase (MMP-12) based on the 1.1 A crystal structure. J. Mol. Biol. 312, 731–742 [DOI] [PubMed] [Google Scholar]
- 15. Louis D., Bernillon J., Wallach J. M. (1998) Specificity of Pseudomonas aeruginosa serralysin revisited, using biologically active peptides as substrates. Biochim. Biophys. Acta 1387, 378–386 [DOI] [PubMed] [Google Scholar]
- 16. Louis D., Bernillon J., Wallach J. M. (1999) Use of a 49-peptide library for a qualitative and quantitative determination of pseudomonal serralysin specificity. Int. J. Biochem. Cell Biol. 31, 1435–1441 [DOI] [PubMed] [Google Scholar]
- 17. Linhartová I., Bumba L., Mašín J., Basler M., Osička R., Kamanová J., Procházková K., Adkins I., Hejnová-Holubová J., Sadílková L., Morová J., Sebo P. (2010) RTX proteins. A highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 34, 1076–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Omori K., Idei A. (2003) Gram-negative bacterial ATP-binding cassette protein exporter family and diverse secretory proteins. J. Biosci. Bioeng. 95, 1–12 [DOI] [PubMed] [Google Scholar]
- 19. Baumann U., Bauer M., Létoffé S., Delepelaire P., Wandersman C. (1995) Crystal structure of a complex between Serratia marcescens metalloprotease and an inhibitor from Erwinia chrysanthemi. J. Mol. Biol. 248, 653–661 [DOI] [PubMed] [Google Scholar]
- 20. Delepelaire P. (2004) Type I secretion in gram-negative bacteria. Biochim. Biophys. Acta 1694, 149–161 [DOI] [PubMed] [Google Scholar]
- 21. Delepelaire P., Wandersman C. (1990) Protein secretion in gram-negative bacteria. The extracellular metalloprotease B from Erwinia chrysanthemi contains a C-terminal secretion signal analogous to that of Escherichia coli α-hemolysin. J. Biol. Chem. 265, 17118–17125 [PubMed] [Google Scholar]
- 22. Duong F., Lazdunski A., Cami B., Murgier M. (1992) Sequence of a cluster of genes controlling synthesis and secretion of alkaline protease in Pseudomonas aeruginosa. Relationships to other secretory pathways. Gene 121, 47–54 [DOI] [PubMed] [Google Scholar]
- 23. Binet R., Létoffé S., Ghigo J. M., Delepelaire P., Wandersman C. (1997) Protein secretion by Gram-negative bacterial ABC exporters. A review. Gene 192, 7–11 [DOI] [PubMed] [Google Scholar]
- 24. Blight M. A., Pimenta A. L., Lazzaroni J. C., Dando C., Kotelevets L., Séror S. J., Holland I. B. (1994) Identification and preliminary characterization of temperature-sensitive mutations affecting HlyB, the translocator required for the secretion of haemolysin (HlyA) from Escherichia coli. Mol. Gen. Genet. 245, 431–440 [DOI] [PubMed] [Google Scholar]
- 25. Delepelaire P., Wandersman C. (1991) Characterization, localization, and transmembrane organization of the three proteins PrtD, PrtE, and PrtF necessary for protease secretion by the gram-negative bacterium Erwinia chrysanthemi. Mol. Microbiol. 5, 2427–2434 [DOI] [PubMed] [Google Scholar]
- 26. Létoffé S., Wandersman C. (1992) Secretion of CyaA-PrtB and HlyA-PrtB fusion proteins in Escherichia coli. Involvement of the glycine-rich repeat domain of Erwinia chrysanthemi protease B. J. Bacteriol. 174, 4920–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palacios J. L., Zaror I., Martínez P., Uribe F., Opazo P., Socías T., Gidekel M., Venegas A. (2001) Subset of hybrid eukaryotic proteins is exported by the type I secretion system of Erwinia chrysanthemi. J. Bacteriol. 183, 1346–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma Q., Zhai Y., Schneider J. C., Ramseier T. M., Saier M. H., Jr. (2003) Protein secretion systems of Pseudomonas aeruginosa and P. fluorescens. Biochim. Biophys. Acta 1611, 223–233 [DOI] [PubMed] [Google Scholar]
- 29. Duong F., Bonnet E., Géli V., Lazdunski A., Murgier M., Filloux A. (2001) The AprX protein of Pseudomonas aeruginosa. A new substrate for the Apr type I secretion system. Gene 262, 147–153 [DOI] [PubMed] [Google Scholar]
- 30. Chenal A., Guijarro J. I., Raynal B., Delepierre M., Ladant D. (2009) RTX calcium binding motifs are intrinsically disordered in the absence of calcium. Implication for protein secretion. J. Biol. Chem. 284, 1781–1789 [DOI] [PubMed] [Google Scholar]
- 31. Sotomayor Pérez A. C., Karst J. C., Davi M., Guijarro J. I., Ladant D., Chenal A. (2010) Characterization of the regions involved in the calcium-induced folding of the intrinsically disordered RTX motifs from the Bordetella pertussis adenylate cyclase toxin. J. Mol. Biol. 397, 534–549 [DOI] [PubMed] [Google Scholar]
- 32. Gangola P., Rosen B. P. (1987) Maintenance of intracellular calcium in Escherichia coli. J. Biol. Chem. 262, 12570–12574 [PubMed] [Google Scholar]
- 33. Bakás L., Veiga M. P., Soloaga A., Ostolaza H., Goñi F. M. (1998) Calcium-dependent conformation of E. coli α-hemolysin. Implications for the mechanism of membrane insertion and lysis. Biochim. Biophys. Acta 1368, 225–234 [DOI] [PubMed] [Google Scholar]
- 34. Soloaga A., Veiga M. P., García-Segura L. M., Ostolaza H., Brasseur R., Goñi F. M. (1999) Insertion of Escherichia coli α-hemolysin in lipid bilayers as a non-transmembrane integral protein. Prediction and experiment. Mol. Microbiol. 31, 1013–1024 [DOI] [PubMed] [Google Scholar]
- 35. Ravaud S., Gouet P., Haser R., Aghajari N. (2003) Probing the role of divalent metal ions in a bacterial psychrophilic metalloprotease. Binding studies of an enzyme in the crystalline state by x-ray crystallography. J. Bacteriol. 185, 4195–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hege T., Feltzer R. E., Gray R. D., Baumann U. (2001) Crystal structure of a complex between Pseudomonas aeruginosa alkaline protease and its cognate inhibitor. Inhibition by a zinc-NH2 coordinative bond. J. Biol. Chem. 276, 35087–35092 [DOI] [PubMed] [Google Scholar]
- 37. Pace C. N., Shaw K. L. (2000) Linear extrapolation method of analyzing solvent denaturation curves. Proteins 4, 1–7 [DOI] [PubMed] [Google Scholar]
- 38. Lilie H., Haehnel W., Rudolph R., Baumann U. (2000) Folding of a synthetic parallel β-roll protein. FEBS Lett. 470, 173–177 [DOI] [PubMed] [Google Scholar]
- 39. Junker M., Besingi R. N., Clark P. L. (2009) Vectorial transport and folding of an autotransporter virulence protein during outer membrane secretion. Mol. Microbiol. 71, 1323–1332 [DOI] [PubMed] [Google Scholar]
- 40. Huang S., Ratliff K. S., Schwartz M. P., Spenner J. M., Matouschek A. (1999) Mitochondria unfold precursor proteins by unraveling them from their N-termini. Nat. Struct. Biol. 6, 1132–1138 [DOI] [PubMed] [Google Scholar]
- 41. Benabdelhak H., Kiontke S., Horn C., Ernst R., Blight M. A., Holland I. B., Schmitt L. (2003) A specific interaction between the NBD of the ABC transporter HlyB and a C-terminal fragment of its transport substrate haemolysin A. J. Mol. Biol. 327, 1169–1179 [DOI] [PubMed] [Google Scholar]
- 42. Létoffé S., Ghigo J. M., Wandersman C. (1994) Secretion of the Serratia marcescens HasA protein by an ABC transporter. J. Bacteriol. 176, 5372–5377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.