Background: Molecular features governing α4β2 nAChRs efficacy have remained elusive.
Results: Binding studies, electrophysiology, and structural data from co-crystallization with Ls-AChBP are reported for a series of α4β2 agonists.
Conclusion: Direct halogen bonds and an invariant Loop-C suggest that intersubunit bridge formation governs efficacy.
Significance: The data provide a structural basis for understanding of efficacy levels at nAChRs.
Keywords: Cys-loop Receptors, Ion Channels, Nicotinic Acetylcholine Receptors, Protein Structure, Receptor Structure-Function, Acetylcholine-binding Protein, nAChR Drug Discovery
Abstract
The α4β2 subtype of the nicotinic acetylcholine receptor has been pursued as a drug target for treatment of psychiatric and neurodegenerative disorders and smoking cessation aids for decades. Still, a thorough understanding of structure-function relationships of α4β2 agonists is lacking. Using binding experiments, electrophysiology and x-ray crystallography we have investigated a consecutive series of five prototypical pyridine-containing agonists derived from 1-(pyridin-3-yl)-1,4-diazepane. A correlation between binding affinities at α4β2 and the acetylcholine-binding protein from Lymnaea stagnalis (Ls-AChBP) confirms Ls-AChBP as structural surrogate for α4β2 receptors. Crystal structures of five agonists with efficacies at α4β2 from 21–76% were determined in complex with Ls-AChBP. No variation in closure of loop C is observed despite large efficacy variations. Instead, the efficacy of a compound appears tightly coupled to its ability to form a strong intersubunit bridge linking the primary and complementary binding interfaces. For the tested agonists, a specific halogen bond was observed to play a large role in establishing such strong intersubunit anchoring.
Introduction
Through classical drug discovery efforts, the α4β2 subtype of the nicotinic acetylcholine receptor (nAChR)2 has been pursued as a drug target for its involvement in psychiatric and neurodegenerative disorders (1–7), and it is the main target for smoking cessation aids including the marketed drugs cytisine (8–10) and varenicline (10, 11). For the latter, a delicate balanced profile of potency and efficacy with respect to different nAChR subtypes has been suggested to be responsible for the beneficial clinical profile (12, 13). Likewise, success of future drug discovery projects may depend on the ability to selectively target subpopulations or individual receptor subtypes in a custom-tailored manner to produce drugs with less side effects. To reach this goal, a better understanding of the molecular determinants responsible for binding and not the least functional profiles of drugs at individual receptor subtypes is required, and comprehensive studies of high resolution co-crystal structures may significantly aid this process.
A high resolution structure of a nAChR giving detailed insight into the complete orthosteric binding site and its detailed interaction with ligands is still to come. However, x-ray structures of molluscan acetylcholine-binding proteins (AChBPs) from the water snails Aplysia californica (Ac) and Lymnaea stagnalis (Ls) have provided detailed information with regard to binding of nAChR agonists (14–18) and antagonists (15, 19–25). AChBPs are soluble pentameric proteins with a structure and folding closely resembling that of the extracellular domain of nAChRs (26, 27) and have proven valuable templates for homology modeling (22, 28, 29). Their overall sequence identity with respect to nAChRs is in the range of 21–24% (26) but is much higher in the immediate vicinity of the orthosteric agonist binding pocket. Furthermore, binding data indicate that AChBP in some cases can reproduce relative binding affinities of nAChR of series of ligands (30). Structural studies of AChBP in complex with ligands have revealed a considerable flexibility in the so-called C-loop that closes in on agonists and forms a lid on the binding site. Thus, C-loop closure has been suggested to couple to channel activation (15, 18, 20, 24), and it has even been suggested to be an important determinant for agonist efficacy (18, 25).
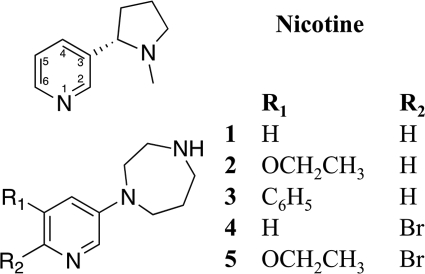
Many nicotinic α4β2 agonists arising from traditional drug discovery efforts have structural similarities to nicotine. The prototypical agonist contains a pyridine ring connected to a second nitrogen-containing ring that will typically be positively charged at physiological conditions. Based on this scaffold, a vast amount of α4β2 nAChR agonists have been prepared varying the ring containing the charged amine and the substitution pattern on the pyridine ring and replacing the pyridine itself by bioisosteric groups. The structure activity relationship knowledge accumulated from this work may be summarized very briefly as follows. Increasing the size of the ring containing the charged amine beyond a certain threshold leads to increased binding affinity but decreased functional potency (31); substitution in the pyridine ring is allowed to varying degrees where 2- and 4-positions generally seem not to be tolerated, and the 5- and 6-positions seem much more accepting. In the pyridine 5-position, introduction of many different chemical functionalities is allowed, whereas substitution in the 6-position only is allowed to a limited extent (13, 31–34). Common to the studies evaluating compounds from medicinal chemistry efforts is a thorough understanding of binding properties of pyridine ring-substituted compounds. However, much less is understood about functional profiles of the compounds due to the inherent difficulty in obtaining robust datasets. In many cases functional efficacies have been obtained using fluorescent-based assays with dyes such as Fluo-4 due to the throughput of these techniques. However, although this is a fast method, translations from a detected fluorescent signal to flux through an ion channel often suffer from a lack of linearity. Thus, for determining exact efficacy levels of agonists relative to i.e. ACh, fluorescent based assays are not ideal. Furthermore, recent data have shown that α4β2 receptors can express in two different stoichiometries with different functional properties and even different numbers of agonist binding sites (29). In light of this, previous studies clearly lack in obtaining efficacy data, which may explain why a clear understanding of functional efficacies of α4β2 compounds has been so elusive. In search of a structural explanation for the functional effects related to substituents in the pyridine 5- and 6-positions, we have investigated a series of α4β2 partial agonists (1-5, Fig. 1) belonging to a thoroughly studied compound series, the 1-(pyridin-3-yl)-1,4-diazepanes (13, 31–36). All five selected compounds bind with subnanomolar affinity to α4β2 receptors (see Table 1), and they have previously been shown to possess significantly different functional profiles using fluorescent-based assays (31). We confirmed the use of Ls-AChBP as the model system for nAChR α4β2 agonists based on a comparison of binding affinities to the two proteins and co-crystallized compounds 1-5 with Ls-AChBP. Next, we performed electrophysiological characterization on α4β2 receptors in Xenopus laevis oocytes and interpreted the functional data in a structural context after co-crystallization of 1-5 with Ls-AChBP. The results offer a detailed characterization of the interaction pattern of low partial to near-full agonists and provide new insight in the receptor activation mechanism that questions the importance of C-loop closure as the determinant for efficacy.
FIGURE 1.
Structures of nicotine and compounds 1-5.
TABLE 1.
Binding affinities, agonist potency, and efficacy (±S.E.) of compounds 1-5 and reference compounds
| Compound |
Ki |
EC50 Potency cHSa α4β2 | Efficacy cHSa α4β2 | ||
|---|---|---|---|---|---|
| Ls-AChBP ([3H]Epibatidine) | α4β2 nAChR ([3H]Cytisine) | α7 nAChR ([3H]α-Bungarotoxin) | |||
| nm | nm | nm | nm | % | |
| Acetylcholine | 890b | 33c | 1,880 ± 670 | 100 | |
| (−)-Nicotine | 83 ± 10 | 6.0 ± 3.4 | 170 ± 65 | 380 ± 45 | 22 ± 1 |
| Cytisine | 8.2 ± 2 | 0.95 ± 0.04 | 295 ± 45 | 11 ± 8 | 3.0 ± 0.5 |
| Epibatidine | 0.097 | 0.33 ± 0.21 | 18 ± 16 | 11 ± 2 | 28 ± 2 |
| 1 | 3.1 ± 0.9 | 0.72 ± 0.17 | 136 ± 20 | 21 ± 2 | 41 ± 1 |
| 2 | 2.2 ± 0.1 | 0.62 ± 0.09 | 9,970 ± 524 | 20 ± 3 | 41 ± 2 |
| 3 | 8.9 ± 0.9 | 0.80 ± 0.28 | >10,000 | 80 ± 10 | 20 ± 1 |
| 4 | 1.3 ± 0.9 | 0.32 ± 0.06 | 190 ± 18 | 56 ± 5 | 62 ± 3 |
| 5 | 0.067 ± 0.026 | 0.25 ± 0.05 | 847 ± 21 | 22 ± 2 | 76 ± 3 |
EXPERIMENTAL PROCEDURES
All chemicals were of analytical grade and were from Sigma unless otherwise specified. The preparation of 1-(pyridin-3-yl)-1,4-diazepane (1, NS3531), 1-(5-ethoxypyridin-3-yl)-1,4-diazepane (2, NS3573), 1-(5-phenylpyridin-3-yl)-1,4-diazepane (3, NS3570), 1-(6-bromopyridin-3-yl)-1,4-diazepane (4, NS3920), and 1-(6-bromo-5-ethoxypyridin-3-yl)-1,4-diazepane (5, NS3950) have been reported previously (33, 37).
Radioligand Competition Assay
Binding affinities of 1–5 for α4β2 nAChR were determined in tissue suspension of rat cortical membranes using 1 nm [3H]cytisine as previously reported (38). Nonspecific binding was determined in the presence of 100 μm (−)-nicotine. Binding affinities for α7 nAChR were determined as reported (39) in tissue preparation of rat cortical membranes using 1 nm [3H]α-bungarotoxin. Nonspecific binding was determined in the presence of 1 mm (−)-nicotine. Binding affinities for AChBP were determined by displacement of [3H]epibatidine (PerkinElmer Life Sciences) to Ls-AChBP linked to the transmembrane domain of the 5HT3 receptor and expressed in HEK293 cells as described by Bouzat et al. (40). AChBP-expressing cells were harvested and washed once with 50 mm Tris-HCl, pH 7.4, and stored at −80 °C until the day of the experiment. The thawed membrane pellets were resuspended in 15 ml of ice-cold Tris-HCl buffer using an Ultra-Turrax homogenizer (Rose Scientific Ltd.) and centrifuged for 10 min (27,000 × g) at 4 °C. The final pellet was resuspended in Tris-HCl buffer and used for binding experiments. Binding to AChBP was performed using 0.3 nm [H]epibatidine at 8–12 μg of protein/assay. The samples were incubated in a final volume of 1 ml for 1 h at room temperature, and nonspecific binding was determined in the presence of 30 μm (−)-nicotine. Binding was terminated by rapid filtration through Whatman GF/C glass fiber filters presoaked in 0.1% polyethyleneimine for at least 30 min on either a Millipore filtration devise or a Brandel Harvester. Filters were washed with ice-cold buffer, and radioactivity was determined by conventional liquid scintillation counting using a Tri-CarbTM counter (PerkinElmer Life Sciences). All compounds were tested in triplicate and were repeated to at least n = 3. Estimates of IC50 values in binding experiments were calculated with the nonlinear curve-fitting program GraphPad Prism (Version 4.03; Graph-Pad Software Inc., San Diego, CA). Ki values were calculated from IC50 values using the Cheng and Prusoff equation: Ki = IC50/(1+(L/Kd)). The Kd values were as follows: 0.95 nm for [3H]cytisine, 0.90 nm for [α-3H]bungarotoxin, and 0.097 nm for [3H]epibatidine. All results are given as the means ± S.E.
Electrophysiological Studies of High Sensitivity α4β2 nAChR
The electrophysiological measurements were carried out as concentration-response measurements on α4β2 nAChR expressed in oocytes from X. laevis. To ensure a near-homogeneous receptor population, a concatenated α4β2 construct was expressed with native β2 subunits by injection of cRNA in a ratio of 4:1 to yield high sensitivity α4β2 receptors corresponding to the (α4)2(β2)3 stoichiometry. The construct was prepared as previously described (41). Approximately 25 ng of cRNA mixture was injected into each oocyte using a Pico Pump (World Precision Instruments). After injection, oocytes were incubated at 17 °C for 2–3 days to allow expression of α4β2 nAChR to occur in modified Barth's solution containing 10 mm Hepes, 90 mm NaCl, 1 mm KCl, 0.82 mm MgCl2, 0.74 mm CaCl2, and 0.66 mm NaNO3 to which was added 100 mg/liter gentamycin, and the pH was adjusted to 7.5. Voltage-clamp recordings were performed using a two-electrode system. Oocytes placed individually in a custom-designed recording chamber were continuously perfused with a flow of 2 ml/min OR2 containing in 5 ml of Hepes, 90 ml of NaCl, 2.5 ml of CaCl2, 2.5 ml of KCl, 1 ml of MgCl2, and the pH adjusted to 7.5. Recording electrodes were fabricated from borosilicate glass with filament (Sutter BF150–110-10) using a DMZ-Universal puller (Zeitz Instrumente) and backfilled with 2 m KCl. Electrodes were placed in the oocytes using manual micro manipulators and had an open bath serial resistance of 0.5–2.0 megaohms. Oocytes were voltage-clamped at a holding potential of −50 to −80 mV using a Geneclamp 500B amplifier (Axon), and signals were low-pass filtered at 20 Hz, digitized at 200 Hz by a Digidata 1322A (Axon). All drug solutions were prepared on the same day of the experiment in OR2 and applied through a capillary tube with an inner diameter of 1.5 mm (Modulohm 214813) placed ∼2 mm from the oocyte and connected through Teflon tubing to a Gilson 233XL auto sampler. A flow rate of 2.5 ml/min through the capillary tube during applications ensured a rapid exchange of liquid surrounding the oocyte in the order of few seconds. Recordings were controlled by Gilson 735 software controlling all the Gilson equipment (233XL autosampler, 402 diluter, and Minipuls 3 pumps) and triggering recording by pCLAMP9. Data were evaluated using Clampfit (Axon Instruments), and peak current amplitudes were base-line-subtracted and normalized to a 100% response evoked by 100 μm ACh. Data were fitted by nonlinear regression to the Hill equation with a fixed Hill slope of 1 and bottom of 0 using GraphPad Prism (GraphPad Software Inc.) as previously described (29).
Expression and Crystallization of Ls-AChBP
A pFastbac I vector containing the L. stagnalis AChBP gene including its signal sequence as previously described (14) was generously provided by Prof. Titia Sixma (Netherlands Cancer Institute). Ls-AChBP was expressed in Sf9 cells and purified as described (14). Ls-AChBP was crystallized at 20 °C using the hanging-drop vapor diffusion technique. Drops contained protein solution and reservoir solution in a ratio 1:1 in drops with a total volume of either 2 μl or 4 μl. The protein solution contained 2.5 mg/ml protein in 20 mm Tris, 20 mm NaCl, pH 8.0, and a ratio of compound to Ls-AChBP of 1:10 for 1 and 1:50 for 2-5. The reservoir solutions (0.5 ml) consisted of 1.8–2.1 m (NH4)2SO4, 0–3% PEG 400, 0.1 m Tris, pH 8.0. Crystals were collected after 8–41 days and soaked in a cryoprotection buffer of 2.0 m (NH4)2SO4, 1% PEG 400, 0.1 m Tris, pH 8.0, and 15–30% glycerol before flash-freezing in liquid nitrogen. X-ray diffraction data of Ls-AChBP in complex with 2, 4, and 5 were collected on beamline 911-2 and 3 on beamline 911-3 at MAX-lab, Lund, Sweden, whereas the data of Ls-AChBP in complex with 1 were collected on beamline ID14-EH1 at ESRF, Grenoble, France. Ls-AChBP crystallized in orthorhombic or monoclinic space groups (supplemental Table 2). Data were indexed, integrated, scaled, and merged using Mosflm and Scala within CCP4 (42), except from the data set of 4, which was indexed and integrated using XDS (43). Data collection statistics are reported in supplemental Table 2.
Structure Refinement
All structures were solved by molecular replacement using Phaser (44) within CCP4 (1, 2, and 4) or auto build within PHENIX (45) (3 and 5) using the structure of Ls-AChBP with nicotine as template (PDB code 1UW6; one pentamer; protein atoms only). All five agonists were built in Maestro (Maestro, version 9.2, Schrödinger, LLC, New York, NY, 2011) with a positive charge on the secondary amine. To obtain low energy conformations, especially of the 1,4-diazepane ring, the agonists were subjected to a conformational search in MacroModel (MacroModel, Version 9.9, Schrödinger, LLC) using the MCMM sampling method, a 13-kJ energy cutoff and otherwise default settings. The 10 conformations with the lowest energy were docked into each binding site of the proteins after the first refinement step using Glide Version 5.7, Schrödinger, LLC (46) with default setting and Trp-143 to define the center of the grid used to represent the binding site. Based on the confirmed importance for binding of nicotine-like ligands of the water molecule that interacts with Leu-102 and Met-114, it was included in the structure to facilitate the docking. The 10 output conformations were then evaluated based on docking score and visual comparison to the density map. The best conformation was selected for further structure refinement. The structure of AChBP in complex with 3 had, in addition to the nicotine binding site, three alternative binding sites. The structures were refined in program PHENIX (45) using TLS and NCS. The structures of Ls-AChBP in complex with 1, 3, and 5 were refined with individual B-factors, whereas grouped B-factors were used for Ls-AChBP in complex with 2 and 4. The structures were inspected and manually corrected in program COOT (47). In the refinement procedure, the disordered regions including some N-acetylglucosamine residues at position Asp-66 were excluded due to weak electron densities. The program PROCHECK (48) was applied for the final validation of the models. The atomic coordinates and structure factor amplitudes of the five complexes have been deposited with the RCSB Protein Data Bank codes 3U8J, 3U8K, 3U8L, 3U8M, 3U8N).
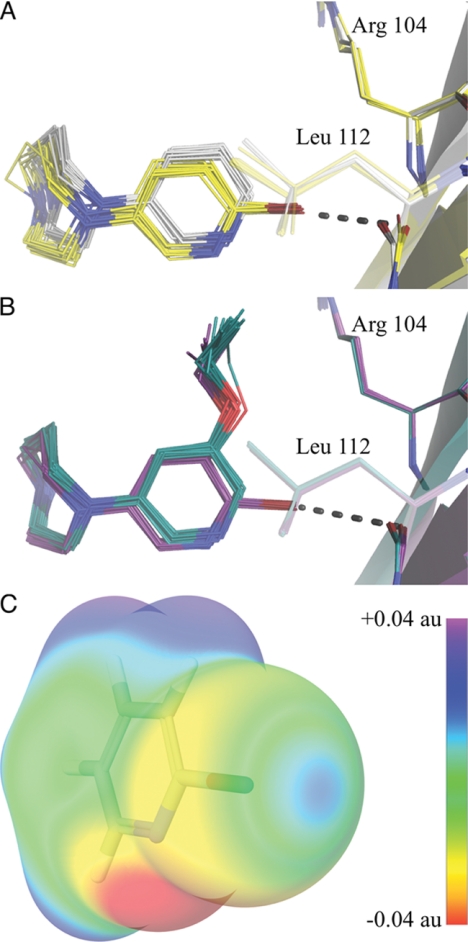
Comparison of relative binding modes of 1 versus 4 and 2 versus 5 (see Fig. 5, A and B) were made in PyMOL (The PyMOL Molecular Graphics System, Version 1.3, Schrodinger, LLC, DeLano, W. L., 2002) by creating an entry for each dimer comprising a binding site and superimposing the entries by using the backbone atoms of residues 102, 106, 110, and 114 in the protein chain making up the complementary side of the binding site. The mentioned residues were used for the superimposition to ensure that compound-induced changes in the backbone of nearby residues, Arg-104 and Leu-112, did not affect the result.
FIGURE 5.
Halogen bonding and binding mode of halogen-substituted compounds 4 and 5. All binding sites are superimposed as described under “Experimental Procedures.” A, shown is a comparison of the binding modes of compounds 1 (gray carbons) and 4 (yellow carbons). B, shown is a comparison of the binding modes of compounds 2 (green carbons) and 5 (purple carbons). The broken line indicates halogen bond. C, shown is the electrostatic potential of 2-bromopyridine mapped on the surface of the molecular electron density calculated using the B3LYP hybrid potential and a cc-pvdzs basis set. au, atomic units.
RESULTS AND DISCUSSION
Evaluation of Ls-AChBP as Model System for nAChR α4β2 Agonists
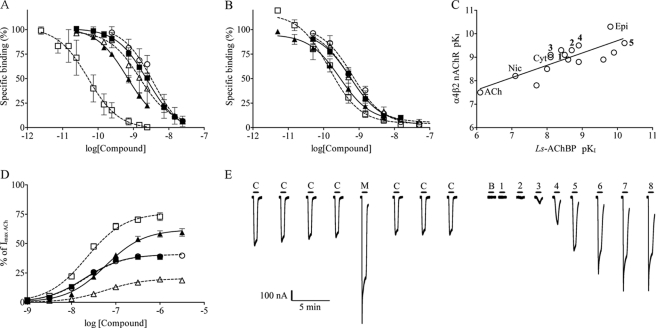
We selected Ls-AChBP as the model protein for our structural studies because Ls-AChBP, unlike Ac-AChBP, contains a, with respect to nAChRs, conserved tryptophan, Trp-53 (Ls-AChBP numbering), which is known to be functionally important in nAChRs (25, 49–51) (52) and to influence ligand binding affinities (15, 53) and even ligand binding modes as evident from comparing Ls- and Ac-AChBP crystal structures with the same ligand (16, 17). To facilitate a comparison of binding affinities between α4β2 nAChRs and Ls-AChBP, we established a new [3H]epibatidine binding assay by linking Ls-AChBP to a 5-hydroxytryptamine receptor ion channel domain and subsequently expressing the construct in HEK293 cells as previously reported (40). In the following, this construct is referred to as Ls-AChBP. This allowed use of the same techniques as for α4β2 brain homogenate and recombinantly expressed membrane protein assays, and the assay was scaled up to run in parallel as part of a platform for structure based ion channel drug discovery. To validate this assay, nicotine, epibatidine, and cytisine were initially tested as reference compounds resulting in inhibition constants of 83, 0.097, and 8.2 nm, respectively (Table 1), which is in agreement with previously reported data (14, 15, 54). The binding affinities at Ls-AChBP and α4β2 and α7 nAChRs for compounds 1–5 and reference compounds are summarized in Table 1, and displacement curves for 1-5 at Ls-AchBP and α4β2 nAChRs are shown in Figs. 2, A and B. Compounds 1–3 all bound with subnanomolar affinity to α4β2 receptors, whereas 2–3-fold higher affinities were observed for the halogenated compounds 4 and 5. A similar rank order was observed at Ls-AChBP, whereas binding affinities at α7 nAChRs stand out being significantly lower (Table 1). It is worth noticing that compounds 1 and 4 bind with equal affinities at α7 nAChRs, whereas the addition of an R1 position substituent as in 2 and 3 is highly detrimental for binding. This detrimental effect can be partly overcome by additionally having bromine in the R2 position as in 5. To further substantiate the correlation between binding affinities on α4β2 and Ls-AChBP, we tested seven additional α4β2 agonists and partial agonists in both assays (supplemental Table S1). Plotting α4β2 binding data as a function of Ls-AChBP binding data for all tested compounds resulted in a linear correlation with a correlation coefficient of 0.7 and slope of 0.5 (Fig. 2C). Thus, the Ls-AChBP assay generally results in slightly lower binding affinity than α4β2 nAChRs, but it clearly captures the rank order of agonist binding affinities across a range of 3 orders of magnitude. This confirms that Ls-AChBP is a valid model system suitable as a structural surrogate for investigating details of binding of typical nicotine-like agonists at the α4β2 nAChR.
FIGURE 2.
Binding and functional profile of compounds 1–5: 1, ○; 2, ■; 3, △; 4, ▴; 5, □. A, binding affinity (±S.E.) at Ls-AChBP ([3H]epibatidine) is shown. B, binding affinity (±S.E.) at α4β2 nAChR ([3H]cytisine) is shown. C, correlation between binding affinities of agonists at Ls-AChBP and α4β2 nAChR is shown. Slope = 0.5, R2 = 0.7. Nic, nicotine; epi, epibatidine; Cyt, cytisine; unnumbered compounds correspond to compounds listed in supplemental Table S1. D, functional concentration-response profiles for 1–5 from HS α4β2 nAChRs are expressed from the dimeric concatenated β2–6-α4 construct and β2 in a 4:1 ratio (±S.E.). Responses are relative to a maximum response of ACh. E, representative current traces for compound 5 in two-electrode voltage-clamp electrophysiological experiments in X. laevis oocytes. Oocyte were injected with β2–6-α4 and β2 nAChR subunits in a 4:1 ratio. Application of the compound is indicated by a bar above each trace, and C denotes an ACh control concentration of 1 μm, M denotes a 100 μm AChmax concentration, B denotes a buffer application; numbers 1–8 denote increasing concentrations of compound 5 in half-log-unit increments with a minimal concentration of 316 pm in application, 1, and maximal concentration of 1 μm in application 8.
Functional Properties of Compounds
The functional properties of compounds 1–5 were characterized along with reference compounds by electrophysiological studies in Xenopus oocytes. Co-expression studies have shown that α4 and β2 subunits can assemble with different stoichiometries to form two distinct functional receptor subtypes, (α4)2(β2)3 and (α4)3(β2)2 (55, 56). Furthermore, we have recently shown that the (α4)3(β2)2 subtype in addition to two high sensitivity agonist sites in α4β2 interfaces contains a low ACh sensitivity orthosteric agonist site in the α4α4 interface (29). The presence of two distinctly different agonist sites in the (α4)3(β2)2 receptor complicates interpretation of structure-activity data, and we have, therefore, chosen (α4)2(β2)3 receptors for these studies. This way, we ensure that the functional data only reflect agonist binding in the orthosteric α4β2 interfaces. In a structural context, this makes further sense because residues in the complementary side of the binding interfaces in α4β2 and Ls-AChBP sites are hydrophobic in nature, whereas the corresponding residues in an α4α4 site are hydrophilic (29). The receptor is expressed as concatenated β2–6-α4 subunits (where “6” means an AGS sequence repeated six times to link the β2 N terminus to the α4 C terminus) and single β2 subunits in oocytes as previously reported (41).
In good agreement with the relatively close binding affinities at α4β2 receptors, the functional potencies of compounds 1–5 ranged from 20 to 80 nm and thus differed by only 4-fold (Table 1 and Fig. 2D). However, the efficacies ranged from low partial agonism (20%) to near-full agonism (76%) (Table 1, Fig. 2D) showing that pyridine R1- and R2-position substitution patterns play an important role in fine-tuning the functional profiles of these compounds. Compared with compound 1, the introduction of an ethoxy substituent (2) in the R1 position had no influence on functional potency or efficacy, whereas introduction of a more bulky phenyl substituent (3) led to a decrease in efficacy from 41 to 20% (Table 1). The most pronounced substituent effect is a gain of efficacy associated with the presence of a bromine substituent in the pyridine R2 position, which is evident by comparing compound 1 to the brominated analog 4 where efficacy was increased from 41 to 62% (Table 1). A similar increase in efficacy from 41 to 76% was observed for the ethoxy derivative 2 and the corresponding brominated analog 5. Interestingly, the effects of R1 and R2 substituents are not entirely additive in nature. As mentioned above, adding an ethoxy substituent to the naked pyridine scaffold did not affect efficacy (compare 1 and 2), whereas a gain was observed when bromine was already present in the R2 position (compare 4 and 5). To ensure a consistent dataset facilitating interpretation of the functional data in a structural context, we have additionally evaluated three reference compounds, epibatidine, nicotine, and cytisine, in the same oocyte assay as 1-5. Despite being often referred to as full agonists, epibatidine and nicotine were observed to be low efficacy partial agonists at (α4)2(β2)3 receptors on a par with 3. Cytisine displayed only marginal agonism close to the detection limit.
Structural Studies of AChBP Co-crystallized with Partial α4β2 Agonists
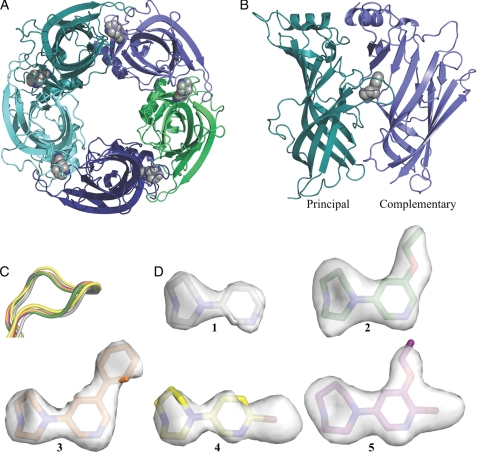
To study the details of agonist binding, compounds 1–5 were crystallized in complex with Ls-AChBP (Fig. 3). Structures were solved at resolutions of 2.3–2.7 Å from crystals belonging to four different space groups with two or four pentamers in the corresponding asymmetric units (crystallization conditions are shown under “Experimental Procedures,” and statistics of data collection and refinement are shown in Table 2 and supplemental Table S2). In general, compound occupancy is high and uniform in all structures, and electron densities are well defined (Fig. 3D) in particular in and around compound binding sites. Disorder is observed in some of the F-loop regions (Thr-156 to Ser-162). Despite different unit cells, all pentamers show a high degree of similarity with compounds in all binding sites and an overall similar orientation of the ligands. An exception from this is the structure co-crystallized with 3, which contains additional molecules bound in one interface in each pentamer. These results are discussed separately below. The cysteines in the C-loops (Cys-187 and Cys-188) are observed in different oxidation states: reduced, oxidized, and a mixed state of reduced and oxidized. As complexes were crystallized under nonreducing conditions, reduction of disulfides could potentially be caused by radiation damage (57–59). Superimposition and comparison of the individual monomers did not reveal oxidation state-dependent variations in the position of the C-loop backbone. However, for clarity reasons, the analysis of the structures presented below focuses on dimer interfaces containing oxidized C-loops.
FIGURE 3.
Structure of Ls-AChBP co-crystallized with partial agonists. A, shown is a bottom view of the pentameric Ls-AChBP with compound 1 (gray) in the orthosteric agonist binding pockets. Subunits are colored individually. B, shown is the Ls-AChBP interface with compound 1 (gray) bound under the C-loop. The principal and complementary sides are in teal and blue, respectively. C, the C-loop of AChBP in complex with compound 1 (gray), 2 (green), 3 (orange), 4 (yellow) and 5 (purple) is shown. The structures were superimposed on the principal subunit. D, the conformations of compounds 1–5 when bound in the binding pocket of Ls-AChBP. Fo − Fc omit maps are shown and contoured at 3.0δ.
TABLE 2.
Refinement statistics of Ls-AChBP complexes
For further details on crystal data, data collection and refinement statistics and validation of Ls-AChBP complexes, see supplemental Table S2. GlcNac, 2-(acetylamino)-2-deoxy-d-glucose.
| Data set | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| PDB code | 3U8J | 3U8K | 3U8L | 3U8M | 3U8N |
| Space group | P212121 | P21212 | P21 | P21212 | C2 |
| Resolution range (Å) | 44.89-2.35 (2.42-2.35)b | 52.51-2.47 (2.50-2.47) | 42.35-2.32 (2.35-2.32) | 30.01-2.70 (2.75-2.70) | 33.19-2.35 (2.43-2.35) |
| Pentamers | 2 | 4 | 2 | 4 | 4 |
| Residues | 2050 | 4044 | 2022 | 4010 | 4043 |
| Compound | 10 | 20 | 14 | 20 | 20 |
| Water | 525 | 1382 | 599 | 330 | 1832 |
| GlcNac | 1 | 3 | 0 | 0 | 1 |
| Sulfate | 6 | 10 | 3 | 17 | 0 |
| Rworkb (%) | 20.5 (26.6) | 19.8 (24.7) | 19.1 (23.7) | 21.9 (24.2) | 19.7 (26.4) |
| Rfreec (%) | 25.8 (33.6) | 23.7 (30.3) | 24.4 (30.4) | 27.5 (29.7) | 23.0 (29.6) |
a Values in parentheses correspond to the outermost resolution shell.
b Rwork = Σhkl(‖Fo,hkl| − |Fc,hkl‖)/|Fo,hkl|, where |Fo,hkl| and | Fc,hkl| are the observed and calculated structure factor amplitudes.
c Rfree is equivalent to Rwork but calculated with reflections omitted from the refinement process (5% of reflections omitted in data set 1, 1% in data set 5, and 2% in the other data sets).
Binding Mode of Compound 1
Compound 1 defines the binding mode of the unsubstituted 1-(pyridin-3-yl)-1,4-diazepane scaffold, which is in general agreement with the classical nicotinic pharmacophore (60). As shown in Fig. 4A, the 1,4-diazepane ring points toward the principal subunit and is capped by the C-loop, whereas the pyridine ring faces the complementary subunit. The 1,4-diazepane ring, which is likely protonated at the distal nitrogen N4, is within hydrogen-bonding distance (2.8 Å) of the backbone carbonyl of Trp-143 and the hydroxyl group of Tyr-89 in loop B (2.9 Å). Both hydrogen bonds have been observed in other AChBP co-crystal structures, e.g. with epibatidine (15), and are known to be important determinants for high affinity agonist binding (31). In addition to these two direct hydrogen bonds, contacts to the principal subunit are supported by cation-π interactions mediated through hydrogens on electron-deficient alkyl groups adjacent to the cationic center (61). The interactions are evident from short contact distances from C3 and C5 in the 1,4-diazepane ring to the aromatic centers of Trp-143 (3.3 Å), Tyr-185 (3.8 Å), and Tyr-192 (3.4 Å). Apart from these interactions, compound 1 is stabilized by van der Waals interactions (3.6–4.0 Å) to the disulfide bridge (Cys-187—Cys-188) and to Thr-144 (3.6–4.0 Å) (Fig. 4A). On the complementary side, the major contact between compound 1 and the receptor is water-mediated. A water molecule bound between the carbonyl oxygen of Leu-102, the backbone nitrogen of Met-114, and a second water molecule donates a hydrogen bond to the pyridine nitrogen (2.9 Å). Similar water-mediated contacts have previously been reported for agonists (14, 15, 17, 18) and represent a now well established interaction point for the hydrogen bond acceptor in the nicotinic pharmacophore that was recently experimentally verified by Blum et al. (62) using incorporation of unnatural amino acids in the nAChR α4β2 receptor. The interaction to the complementary subunit is further supported by van der Waals contacts to Trp-53, Arg-104, Leu-112, and Met-114. It is worth noting the interactions to Trp-53, as the corresponding residue in α4β2 and α7 nAChRs, is known to play an important role in channel activation (52) As mentioned above, this residue constitutes the reason for selecting the Ls-AChBP over the Ac-AChBP, and from our structure it is predicted that the 1,4-diazepane ring would not be able to interact favorably if Trp-53 was a Tyr as it is in Ac-AChBP. Arg-104, Leu-112, and Met-114 correspond to Leu-111, Phe-119, and Leu-121 in the β2 subunit. With the guanidine moiety of Arg-104 pointing away from the ligand binding site, the three residues in the β2 subunit expose the ligand to a hydrophobic environment similar in nature to the one seen in Ls-AChBP, which is also evident from the correlation of binding affinities.
FIGURE 4.
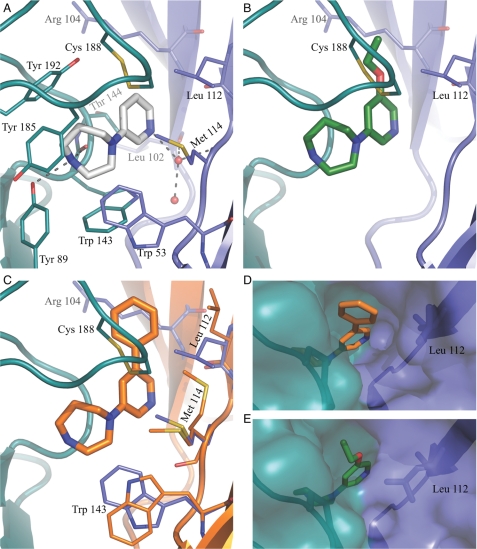
Detailed binding modes for compounds 1–3. A, shown is the binding mode of compounds 1 (gray) in Ls-AChBP. Residues interacting with 1 are colored teal at the principal side and blue at the complementary side. Broken lines indicate ionic interactions with the protein or water molecule (red sphere) within 3.5 Å. B, the binding mode of compounds 2 (green) is shown. Side chains interacting with the ethoxy substituent are shown. C, the binding mode of compound 3 (orange) is shown. Orange residues (complementary side) are shown. Blue residues from the 1-AChBP complex are included to illustrate the conformational changes in the protein associated with binding of 3. D and E, shown is the surface representation of the 3-AChBP and 2-AChBP complexes illustrating the broad (D) and narrow (E) channel to the surface of the protein.
Effect of Pyridine R1 Substituents
Compounds 2 and 3 were co-crystallized with Ls-AChBP to investigate the effect of increasing amounts of steric bulk in the pyridine R1 position of the 1-(pyridin-3-yl)-1,4-diazepane scaffold. Compound 2 contains an ethoxy substituent, and 3 contains a more bulky phenyl substituent. In both co-crystal structures, the core 1-(pyridin-3-yl)-1,4-diazepane scaffold binds and interacts essentially as observed in the structure with 1 (Fig. 4, A–C). In the 2-AChBP structure, the ethoxy substituent captures additional van der Waals contacts to Cys-188 in the C-loop (3.5 Å) and to Arg-104 (3.5 Å) as well as to Leu-112 (3.5 Å) on the complementary subunit (Fig. 4B). Overall, these relatively minor changes do not appear to affect binding or efficacy levels to any significant degree. The co-crystal structure with the more bulky compound 3 (Fig. 4C) is generally more disordered compared with the other co-crystal structures. Compound 3 is bound in all five orthosteric agonist binding sites in each of the two pentamers in the asymmetric unit of the crystal. Binding of the more bulky phenyl substituent forces Leu-112 to adopt an alternative rotameric state (Fig. 4C), which is clearly indicated in the electron density and is necessary to avoid a clash between the phenyl substituent and the side chain. As a consequence, the van der Waals contacts from Leu-112 to the tip of the C-loop observed with the original rotameric state in the 1 and 2 AChBP structures are abolished, which may contribute to the decreased efficacy of 3 compared with 1 and 2. Further rotameric states of Trp-53 and Met-114 will be discussed below. Interestingly, the conformational change of Leu-112 significantly increases the size of an otherwise narrow channel (Fig. 4, D and E), connecting the binding site to the solvent, indicating that the protein can accommodate ligands with pyridine R1 substituents of sizes considerably longer than those co-crystallized in this work. A similar effect is expected in the α4β2 receptor where the residue corresponding to Leu-112 is Phe-119, which, based on rotameric preferences, may adopt similar distinct states. Apart from the contacts described for 1, the binding mode of 3 is characterized by a short interaction distance of 2.9 Å from the edge of the phenyl ring to the hydroxy group of Tyr-192. This contact likely represents an electrostatic interaction from the positive edge of the aromatic ring to the electronegative oxygen of the tyrosine. Furthermore, the phenyl substituent is stabilized by van der Waals interactions with Arg-104 (3.5 Å) and Leu-112 (3.6 Å) on the complementary side and by the C-loop (3.7 Å), and as a consequence, the substituent is very well defined and buried in all directions.
Effect of Pyridine R2 Substituents
The overall binding mode of the pyridine ring of compounds substituted with bromine in the R2 position (4 and 5) was consistently shifted away from the complementary subunit by ∼0.5 Å (Fig. 5, A and B) compared with the corresponding analogs not containing bromine (1 and 2). This shift is necessary to allow space for the bulky bromine substituent. R2-position bromines play an important functional role as evident by an efficacy increase from 41 to 62% for compounds 1 and 4 and from 41 to 76% for compounds 2 and 5 (Table 1). In the 4-AChBP complex the bromine atom is located 3.2 Å from the backbone carbonyl oxygen of Leu-112 on the complementary subunit, and a similar situation was observed in the 5-AChBP complex. This distance is shorter than a favorable van der Waals contact distance between such atoms and indicates the presence of a specific and directional halogen bond interaction, i.e. an electrostatic interaction between the electrons in the π-system of the carbonyl oxygen and a positive electrostatic potential on the bromine atom tip (61, 63). To illustrate this polarized charge distribution on the bromine substituent, the electrostatic potential mapped onto the electron density surface of 2-bromopyridine is shown in Fig. 5C. In addition, bromine is in close van der Waals contact to the complementary subunit, and together with the directional halogen bond, the bromine substituent significantly contributes to anchoring the compound at the complementary subunit, which in turn could qualitatively explain the efficacy increases of brominated analogs 4 and 5. As seen from compounds 1 and 5, the combination of the R1 and R2 substituents results in a larger efficacy increase than predicted from an additive effect from the individual substituents. The interaction pattern observed for compound 5 bound to Ls-AChBP is essentially the combination of the interactions described for compounds 2 and 4 with the exception that the bromine results in a slight displacement of the pyridine ring and with it the attached ethoxy substituent. The structures indicate that the shift is associated with alleviation of a mild steric clash between the oxygen in the ethoxy substituent and the β carbon in Arg-104 as this distance increased from ∼3.5 to ∼3.9 Å between 2 and 5. Although this is a relatively minor change, the altered distance is consistent within all binding sites in the two/four pentamers in the asymmetric unit cells, and the slight shift in binding mode of the pyridine scaffold could potentially explain the non-additive behavior of the R1 and R2 substituents. This could also explain the binding behavior of 1, 2, and 5 at α7 nAChRs, where an ∼70-fold loss of binding was observed by introducing an R1 position ethoxy substituent, which was then counteracted by an ∼10-fold rescue of binding by further introducing a R2-position bromine. Whereas the above offer a plausible explanation to the non-additive behavior of R1- and R2-position substituents, a further confirmation probably requires a move from an AChBP model system to co-crystallization of the compounds with an α4β2 or α7 receptor.
Additional Observations from Binding of Compound 3
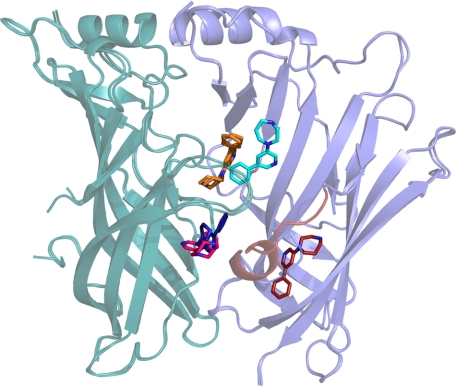
Focusing on Trp-53 and Met-114 in the complementary subunit, it was observed that the binding of 3 generally introduces some distortion of the density, showing that these two residues can adopt alternative conformations (Fig. 4C). In monomers A, F, and G in the complex with 3, Trp-53 adopts an alternative rotameric state, whereas Met-114 clearly indicates two distinct rotameric states that were built into these monomers with partial occupancy. Interestingly, the alternative rotameric states seem to be coupled to the alternative rotameric state adopted by Leu-112, which allows alternative conformations of Met-114 and Trp-53. The result is a rearrangement of the entire complementary part of the binding site. The alternative conformation of Trp-53 opens up the binding site toward the lower part of the dimer interface and offers a new hydrogen bonding possibility as the indole nitrogen is now accessible. This presents new possibilities in structure-based drug design of ligands for nAChRs using Ls-AChBP as a model system. Compound 3 was observed to bind at additional sites in one interface per pentamer between monomers A and B and between monomers H and I (monomers named according to definition in pdb file 3U8L). The conformation of the A/B and H/I interfaces is influenced by binding of two additional molecules of 3 outside the core of the orthosteric agonist binding site, and in these sites the C-loop is more open compared with the other C-loops in the same pentamer. The alternative sites are located pair-wise close to the orthosteric binding site in one of the interfaces of each pentamer (Fig. 6). As the ligand molecules are not involved in any crystal packing contacts, these additional binding sites do not seem to be a crystallization artifact (for further information see supplemental Fig. S1). The extra sites could trigger speculations about allosteric modulation as follows. (i) The extra binding sites only appear in one interface in each pentamer; (ii) the cyan copy of compound 3 in Fig. 6, which forms interaction with the protein and the molecule of 3 in the orthosteric binding site, binds with high resemblance to a recently suggested binding mode of a negative allosteric α4β2 modulator (64); (iii) the area below the orthosteric site has been suggested as a potential modulatory site (65) as well as the binding site for galantamine in the α7 nAChR (66); finally (iv) the red copy of 3 forms primarily hydrophobic contacts to the residues in the β-sandwich core, which is conserved in nature across the α4β2 nAChR. At this point the significance of these observations are uncertain, but it would seem an interesting option to synthesize new compounds particularly suited to match these sites, as such molecules could potentially be allosteric modulators.
FIGURE 6.
Collective image of additional binding orientations observed for compound 3. Superimposition of A/B and H/I interfaces. The principal side is colored teal, and the complementary side is blue. Compound 3 in the orthosteric binding site as it is found in all interfaces is colored orange, and 3 in the H/I and A/B interfaces are bluish (dark blue and cyan) and reddish (pink and red), respectively. The molecule of 3 binding to the β-sheets sandwich on the complementary side induces an ordered α-helical turn in loop-F (also colored red).
Mechanistic Considerations
The functional studies at the (α4)2(β2)3 receptors indicate that substituents in the R1 position play a role in fine-tuning functional properties of the compounds. The data for compounds 1, 2, and 3 are in agreement with general consensus in the literature describing a tendency toward decreased efficacy as a result of bulky substituents in the R1 position (13, 35, 36). Partial agonism has previously been suggested to be due to prevention of C-loop closure and incomplete ligand binding (18). Furthermore, a recent comparison of all available AChBP crystal structures indicates that full agonists result in a closure distance below 8 Å, as measured from the backbone carbonyl oxygen of the conserved Trp-143 and the sulfur atom of Cys-187 (Ls-AChBP numbering), whereas partial agonists display a distance between 8 and 10 Å (25). Our structural studies and the corresponding functional data, which stem from the same and, thus, a comparable functional assay, show only marginal variation in the C-loop closure (Fig. 3C) and, using the same measurement as Brams et al. (25), result in distances ranging from 7.4 to 7.9 Å for the five compounds studied. The observed difference in efficacy for the studied compounds and for nicotine and epibatidine, which also bind under fully closed C-loops despite being partial agonists at (α4)2(β2)3 receptors, does not indicate that the degree of C-loop closure is directly correlated with the degree of efficacy for agonists. Rather, the decrease in efficacy may be coupled to an energetically less favorable stabilization of a fully closed C-loop in native channels, thus lowering the probability of prolonged loop closure without preventing it from closing. The shift in conformation of Leu-112 and loss of interactions from the C-loop to the complementary subunit as observed in the 3-AChBP structure could point toward this as a general mechanism. The functional consequence would be less stabilization of the C-loop in its closed conformation and thus decreased ion channel activation probability.
These results suggest that compound-mediated contacts from the complementary to the principal side play an important role with respect to agonist efficacies. Intuitively, this makes sense, as a contact across the dimer interface could trigger or alternatively stabilize spontaneous subunit rotation, eventually leading to channel opening, as has been suggested from electron microscopy studies of the torpedo nAChR (27, 67). For the compounds in this study, contacts from the positively charged nitrogen-containing ring to Trp-143, Tyr-89, and to other aromatic residues of the aromatic box form an anchor point on the principal subunit. On the complementary side, compounds are anchored to Trp-53 and a water molecule by acceptance of a hydrogen bond, and C-loop closure is then further stabilized through van der Waals contacts to Leu-112. To reach high levels of efficacy, a strong interaction to the complementary side appear necessary. When bromine is present in the R2 position of a compound, it forms an additional tight contact to the complementary subunit through a halogen bond to the carbonyl oxygen of Leu-112, and this is accompanied with increases in primarily efficacy but also binding. The importance of halogen in relation to efficacy is consistent, also for other 1-(pyridin-3-yl)-1,4-diazepanes (31). A hypothesis based on these observations could be that interaction with the principal interface and C-loop closure is important for agonist binding affinity, whereas agonist efficacy is dependent on a strong interaction with both the principal and the complementary part of the interface. This hypothesis is consistent with a significant role of the complementary subunit in regulating the efficacy of cytisine (68). It seems very likely, however, that this may be close to as detailed a description of an agonist binding and gating process one can expect using an AChBP model system. The snail proteins are soluble proteins that are not “wound up” on an ion channel pore domain, and hence some of the features that make up the intricate gating process in a native channel, such as subunit rotations, could be impossible to observe. In this respect, AChBPs probably best resemble a desensitized state of the receptor, a feature in common with binding assays.
The establishment of a clear correlation between binding of agonists shows that Ls-AChBP can be used as a model system for the α4β2 nAChRs and adds credibility to the structural interpretation of functional properties. The structural analysis suggests that two parameters are essential for agonism. This and several other studies have emphasized the importance of C-loop closure in triggering channel activation, but in contrast to others, we observe full C-loop closure for several agonists across a range of efficacy levels; to obtain high efficacy, a strong agonist-mediated interaction between the principal and complementary side of the agonist binding site is an important determinant. These studies also indicate that such a strong inter-interface interaction can be greatly aided through halogen bonding. The compound-mediated contacts between the two subunits in the binding interface has also been suggested to be important for the efficacy on GABAA receptors (69), and it is, therefore, likely to be a general feature for the Cys-loop receptors.
The present investigation of a series of structurally related α4β2 nAChRs agonists interpreting binding and functional data in light of their detailed interactions with Ls-AChBP, as determined by x-ray crystallography, offers a deep understanding of the determinants for efficacy in nAChRs. Given how difficult it appears to have been for the pharmaceutical industry to design agonists that have high potency selectivity for α4β2 versus α3β4, such an understanding may be used to rationally design new selective agonists that have combinations of potency and efficacy selectivity. The concept of efficacy selectivity has successfully been pursued in the development of benzodiazepine binding site ligands where sufficient affinity selectivity could not be obtained (70).
Supplementary Material

This article contains supplemental Tables S1 and S2 and Fig. S1.
- nAChR
- nicotinic acetylcholine (ACh) receptor
- Ac
- Aplysia californica
- Ls
- L. stagnalis
- AChBP
- acetylcholine-binding protein.
REFERENCES
- 1. Schneider J. S., Van Velson M., Menzaghi F., Lloyd G. K. (1998) Effects of the nicotinic acetylcholine receptor agonist SIB-1508Y on object retrieval performance in MPTP-treated monkeys. Comparison with levodopa treatment. Ann. Neurol. 43, 311–317 [DOI] [PubMed] [Google Scholar]
- 2. Levin E. D., Christopher N. C., Weaver T., Moore J., Brucato F. (1999) Ventral hippocampal ibotenic acid lesions block chronic nicotine-induced spatial working memory improvement in rats. Brain Res. Cogn. Brain Res. 7, 405–410 [DOI] [PubMed] [Google Scholar]
- 3. Steinlein O. K., Mulley J. C., Propping P., Wallace R. H., Phillips H. A., Sutherland G. R., Scheffer I. E., Berkovic S. F. (1995) A missense mutation in the neuronal nicotinic acetylcholine receptor α4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 11, 201–203 [DOI] [PubMed] [Google Scholar]
- 4. Grottick A. J., Higgins G. A. (2000) Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 117, 197–208 [DOI] [PubMed] [Google Scholar]
- 5. Jensen A. A., Frølund B., Liljefors T., Krogsgaard-Larsen P. (2005) Neuronal nicotinic acetylcholine receptors. Structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 48, 4705–4745 [DOI] [PubMed] [Google Scholar]
- 6. Lamb P. W., Melton M. A., Yakel J. L. (2005) Inhibition of neuronal nicotinic acetylcholine receptor channels expressed in Xenopus oocytes by β-amyloid 1–42 peptide. J. Mol. Neurosci. 27, 13–21 [DOI] [PubMed] [Google Scholar]
- 7. Gotti C., Zoli M., Clementi F. (2006) Brain nicotinic acetylcholine receptors. Native subtypes and their relevance. Trends Pharmacol. Sci. 27, 482–491 [DOI] [PubMed] [Google Scholar]
- 8. Etter J. F., Stapleton J. A. (2006) Nicotine replacement therapy for long term smoking cessation. A meta-analysis. Tob. Control 15, 280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cunningham C. S., McMahon L. R. (2011) The effects of nicotine, varenicline, and cytisine on schedule-controlled responding in mice. Differences in α4β2 nicotinic receptor activation. Eur. J. Pharmacol. 654, 47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tonstad S., Rollema H. (2010) Varenicline in smoking cessation. Expert Rev. Respir. Med. 4, 291–299 [DOI] [PubMed] [Google Scholar]
- 11. Rollema H., Chambers L. K., Coe J. W., Glowa J., Hurst R. S., Lebel L. A., Lu Y., Mansbach R. S., Mather R. J., Rovetti C. C., Sands S. B., Schaeffer E., Schulz D. W., Tingley F. D., 3rd, Williams K. E. (2007) Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52, 985–994 [DOI] [PubMed] [Google Scholar]
- 12. Rucktooa P., Smit A. B., Sixma T. K. (2009) Insight in nAChR subtype selectivity from AChBP crystal structures. Biochem. Pharmacol. 78, 777–787 [DOI] [PubMed] [Google Scholar]
- 13. Bunnelle W. H., Tietje K. R., Frost J. M., Peters D., Ji J., Li T., Scanio M. J., Shi L., Anderson D. J., Dyhring T., Grønlien J. H., Ween H., Thorin-Hagene K., Meyer M. D. (2009) Octahydropyrrolo[3,4-c]pyrrole. A diamine scaffold for construction of either α4β2 or α7-selective nicotinic acetylcholine receptor (nAChR) ligands. Substitutions that switch subtype selectivity. J. Med. Chem. 52, 4126–4141 [DOI] [PubMed] [Google Scholar]
- 14. Celie P. H., van Rossum-Fikkert S. E., van Dijk W. J., Brejc K., Smit A. B., Sixma T. K. (2004) Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41, 907–914 [DOI] [PubMed] [Google Scholar]
- 15. Hansen S. B., Sulzenbacher G., Huxford T., Marchot P., Taylor P., Bourne Y. (2005) Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 24, 3635–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ihara M., Okajima T., Yamashita A., Oda T., Hirata K., Nishiwaki H., Morimoto T., Akamatsu M., Ashikawa Y., Kuroda S., Mega R., Kuramitsu S., Sattelle D. B., Matsuda K. (2008) Crystal structures of Lymnaea stagnalis AChBP in complex with neonicotinoid insecticides imidacloprid and clothianidin. Invert Neurosci. 8, 71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Talley T. T., Harel M., Hibbs R. E., Radic Z., Tomizawa M., Casida J. E., Taylor P. (2008) Atomic interactions of neonicotinoid agonists with AChBP. Molecular recognition of the distinctive electronegative pharmacophore. Proc. Natl. Acad. Sci. U.S.A. 105, 7606–7611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hibbs R. E., Sulzenbacher G., Shi J., Talley T. T., Conrod S., Kem W. R., Taylor P., Marchot P., Bourne Y. (2009) Structural determinants for interaction of partial agonists with acetylcholine-binding protein and neuronal α7 nicotinic acetylcholine receptor. EMBO J. 28, 3040–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bourne Y., Talley T. T., Hansen S. B., Taylor P., Marchot P. (2005) Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake α-neurotoxins and nicotinic receptors. EMBO J. 24, 1512–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Celie P. H., Kasheverov I. E., Mordvintsev D. Y., Hogg R. C., van Nierop P., van Elk R., van Rossum-Fikkert S. E., Zhmak M. N., Bertrand D., Tsetlin V., Sixma T. K., Smit A. B. (2005) Crystal structure of nicotinic acetylcholine receptor homolog AChBP in complex with an α-conotoxin PnIA variant. Nat. Struct. Mol. Biol. 12, 582–588 [DOI] [PubMed] [Google Scholar]
- 21. Ulens C., Hogg R. C., Celie P. H., Bertrand D., Tsetlin V., Smit A. B., Sixma T. K. (2006) Structural determinants of selective α-conotoxin binding to a nicotinic acetylcholine receptor homolog AChBP. Proc. Natl. Acad. Sci. U.S.A. 103, 3615–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dutertre S., Ulens C., Büttner R., Fish A., van Elk R., Kendel Y., Hopping G., Alewood P. F., Schroeder C., Nicke A., Smit A. B., Sixma T. K., Lewis R. J. (2007) AChBP-targeted α-conotoxin correlates distinct binding orientations with nAChR subtype selectivity. EMBO J. 26, 3858–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulens C., Akdemir A., Jongejan A., van Elk R., Bertrand S., Perrakis A., Leurs R., Smit A. B., Sixma T. K., Bertrand D., de Esch I. J. (2009) Use of acetylcholine-binding protein in the search for novel α7 nicotinic receptor ligands. In silico docking, pharmacological screening, and X-ray analysis. J. Med. Chem. 52, 2372–2383 [DOI] [PubMed] [Google Scholar]
- 24. Bourne Y., Radic Z., Aráoz R., Talley T. T., Benoit E., Servent D., Taylor P., Molgó J., Marchot P. (2010) Structural determinants in phycotoxins and AChBP conferring high affinity binding and nicotinic AChR antagonism. Proc. Natl. Acad. Sci. U.S.A. 107, 6076–6081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brams M., Pandya A., Kuzmin D., van Elk R., Krijnen L., Yakel J. L., Tsetlin V., Smit A. B., Ulens C. (2011) A structural and mutagenic blueprint for molecular recognition of strychnine and d-tubocurarine by different cys-loop receptors. PLoS Biol. 9, e1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brejc K., van Dijk W. J., Klaassen R. V., Schuurmans M., van Der Oost J., Smit A. B., Sixma T. K. (2001) Crystal structure of an ACh-binding protein reveals the ligand binding domain of nicotinic receptors. Nature 411, 269–276 [DOI] [PubMed] [Google Scholar]
- 27. Unwin N. (2005) Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 346, 967–989 [DOI] [PubMed] [Google Scholar]
- 28. Bisson W. H., Westera G., Schubiger P. A., Scapozza L. (2008) Homology modeling and dynamics of the extracellular domain of rat and human neuronal nicotinic acetylcholine receptor subtypes α4β2 and α7. J. Mol. Model 14, 891–899 [DOI] [PubMed] [Google Scholar]
- 29. Harpsøe K., Ahring P. K., Christensen J. K., Jensen M. L., Peters D., Balle T. (2011) Unraveling the high and low sensitivity agonist responses of nicotinic acetylcholine receptors. J. Neurosci. 31, 10759–10766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smit A. B., Syed N. I., Schaap D., van Minnen J., Klumperman J., Kits K. S., Lodder H., van der Schors R. C., van Elk R., Sorgedrager B., Brejc K., Sixma T. K., Geraerts W. P. (2001) A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature 411, 261–268 [DOI] [PubMed] [Google Scholar]
- 31. Tosco P., Ahring P. K., Dyhring T., Peters D., Harpsøe K., Liljefors T., Balle T. (2009) Complementary three-dimensional quantitative structure-activity relationship modeling of binding affinity and functional potency. A study on α4β2 nicotinic ligands. J. Med. Chem. 52, 2311–2316 [DOI] [PubMed] [Google Scholar]
- 32. Carroll F. I. (2004) Epibatidine structure-activity relationships. Bioorg. Med. Chem. Lett. 14, 1889–1896 [DOI] [PubMed] [Google Scholar]
- 33. Audouze K., Nielsen E. Ø., Olsen G. M., Ahring P., Jørgensen T. D., Peters D., Liljefors T., Balle T. (2006) New ligands with affinity for the α4β2 subtype of nicotinic acetylcholine receptors. Synthesis, receptor binding, and three-dimensional QSAR modeling. J. Med. Chem. 49, 3159–3171 [DOI] [PubMed] [Google Scholar]
- 34. Bunnelle W. H., Daanen J. F., Ryther K. B., Schrimpf M. R., Dart M. J., Gelain A., Meyer M. D., Frost J. M., Anderson D. J., Buckley M., Curzon P., Cao Y. J., Puttfarcken P., Searle X., Ji J., Putman C. B., Surowy C., Toma L., Barlocco D. (2007) Structure-activity studies and analgesic efficacy of N-(3-pyridinyl)-bridged bicyclic diamines, exceptionally potent agonists at nicotinic acetylcholine receptors. J. Med. Chem. 50, 3627–3644 [DOI] [PubMed] [Google Scholar]
- 35. Ji J., Schrimpf M. R., Sippy K. B., Bunnelle W. H., Li T., Anderson D. J., Faltynek C., Surowy C. S., Dyhring T., Ahring P. K., Meyer M. D. (2007) Synthesis and structure-activity relationship studies of 3,6-diazabicyclo[3.2.0]heptanes as novel α4β2 nicotinic acetylcholine receptor-selective agonists. J. Med. Chem. 50, 5493–5508 [DOI] [PubMed] [Google Scholar]
- 36. Frost J. M., Bunnelle W. H., Tietje K. R., Anderson D. J., Rueter L. E., Curzon P., Surowy C. S., Ji J., Daanen J. F., Kohlhaas K. L., Buckley M. J., Henry R. F., Dyhring T., Ahring P. K., Meyer M. D. (2006) Synthesis and structure-activity relationships of 3,8-diazabicyclo[4.2.0]octane ligands, potent nicotinic acetylcholine receptor agonists. J. Med. Chem. 49, 7843–7853 [DOI] [PubMed] [Google Scholar]
- 37. Peters D., Olsen G., Nielsen E. Ø., Nielsen S. F. (1999) Heteroaryl diazabicycloalkanes as cholinergic ligands at nicotinic acetylcholine receptors, WO9921834, NeuroSearch A/S, Ballerup, Denmark [Google Scholar]
- 38. Nielsen S. F., Nielsen E. O., Olsen G. M., Liljefors T., Peters D. (2000) Novel potent ligands for the central nicotinic acetylcholine receptor. Synthesis, receptor binding, and three-dimensional-QSAR analysis. J. Med. Chem. 43, 2217–2226 [DOI] [PubMed] [Google Scholar]
- 39. Andreasen J. T., Nielsen E. Ø., Christensen J. K., Olsen G. M., Peters D., Mirza N. R., Redrobe J. P. (2011) Subtype-selective nicotinic acetylcholine receptor agonists enhance the responsiveness to citalopram and reboxetine in the mouse forced swim test. J. Psychopharmacol. 25, 1347–1356 [DOI] [PubMed] [Google Scholar]
- 40. Bouzat C., Gumilar F., Spitzmaul G., Wang H. L., Rayes D., Hansen S. B., Taylor P., Sine S. M. (2004) Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature 430, 896–900 [DOI] [PubMed] [Google Scholar]
- 41. Zhou Y., Nelson M. E., Kuryatov A., Choi C., Cooper J., Lindstrom J. (2003) Human α4β2 acetylcholine receptors formed from linked subunits. J. Neurosci. 23, 9004–9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Winn M. D., Ballard C. C., Cowtan K. D., Dodson E. J., Emsley P., Evans P. R., Keegan R. M., Krissinel E. B., Leslie A. G. W., McCoy A., McNicholas S. J., Murshudov G. N., Pannu N. S., Potterton E. A., Powell H. R., Read R. J., Vagin A., Wilson K. S. (2011) Overview of the CCP4 suite and current developments. Acta. Cryst. D67, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kabsch W. (2010) XDS. Acta Crystallogr. D. Biol. Crystallogr. 66, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX, A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Friesner R. A., Banks J. L., Murphy R. B., Halgren T. A., Klicic J. J., Mainz D. T., Repasky M. P., Knoll E. H., Shelley M., Perry J. K., Shaw D. E., Francis P., Shenkin P. S. (2004) Glide, a new approach for rapid, accurate docking, and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749 [DOI] [PubMed] [Google Scholar]
- 47. Emsley P., Cowtan K. (2004) Coot, model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 48. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) PROCHECK - a program to check the stereochemical quality of protein structures. J. App. Cryst. 26, 283–291 [Google Scholar]
- 49. Gay E. A., Bienstock R. J., Lamb P. W., Yakel J. L. (2007) Structural determinates for apolipoprotein E-derived peptide interaction with the α7 nicotinic acetylcholine receptor. Mol. Pharmacol. 72, 838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gay E. A., Giniatullin R., Skorinkin A., Yakel J. L. (2008) Aromatic residues at position 55 of rat α7 nicotinic acetylcholine receptors are critical for maintaining rapid desensitization. J. Physiol. 586, 1105–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yakel J. L. (2010) Gating of nicotinic ACh receptors. Latest insights into ligand binding and function. J. Physiol. 588, 597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williams D. K., Stokes C., Horenstein N. A., Papke R. L. (2009) Differential regulation of receptor activation and agonist selectivity by highly conserved tryptophans in the nicotinic acetylcholine receptor binding site. J. Pharmacol. Exp. Ther. 330, 40–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sander T., Bruun A. T., Balle T. (2010) Docking to flexible nicotinic acetylcholine receptors. A validation study using the acetylcholine-binding protein. J. Mol. Graph. Model 29, 415–424 [DOI] [PubMed] [Google Scholar]
- 54. Geitmann M., Retra K., de Kloe G. E., Homan E., Smit A. B., de Esch I. J., Danielson U. H. (2010) Interaction kinetic and structural dynamic analysis of ligand binding to acetylcholine-binding protein. Biochemistry 49, 8143–8154 [DOI] [PubMed] [Google Scholar]
- 55. Nelson M. E., Kuryatov A., Choi C. H., Zhou Y., Lindstrom J. (2003) Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol. Pharmacol. 63, 332–341 [DOI] [PubMed] [Google Scholar]
- 56. Moroni M., Zwart R., Sher E., Cassels B. K., Bermudez I. (2006) α4β2 nicotinic receptors with high and low acetylcholine sensitivity. Pharmacology, stoichiometry, and sensitivity to long term exposure to nicotine. Mol. Pharmacol. 70, 755–768 [DOI] [PubMed] [Google Scholar]
- 57. Burmeister W. P. (2000) Structural changes in a cryo-cooled protein crystal owing to radiation damage. Acta Crystallogr. D. Biol. Crystallogr. 56, 328–341 [DOI] [PubMed] [Google Scholar]
- 58. Ravelli R. B., McSweeney S. M. (2000) The “fingerprint” that X-rays can leave on structures. Structure 8, 315–328 [DOI] [PubMed] [Google Scholar]
- 59. Weik M., Ravelli R. B., Kryger G., McSweeney S., Raves M. L., Harel M., Gros P., Silman I., Kroon J., Sussman J. L. (2000) Specific chemical and structural damage to proteins produced by synchrotron radiation. Proc. Natl. Acad. Sci. U.S.A. 97, 623–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Glennon R. A., Dukat M. (2004) α4β2 nACh receptor pharmacophore models. Bioorg. Med. Chem. Lett. 14, 1841–1844 [DOI] [PubMed] [Google Scholar]
- 61. Bissantz C., Kuhn B., Stahl M. (2010) A medicinal chemist's guide to molecular interactions. J. Med. Chem. 53, 5061–5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Blum A. P., Lester H. A., Dougherty D. A. (2010) Nicotinic pharmacophores. The pyridine N of nicotine and carbonyl of acetylcholine hydrogen bond across a subunit interface to a backbone NH. Proc. Natl. Acad. Sci. U.S.A. 107, 13206–13211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lu Y., Shi T., Wang Y., Yang H., Yan X., Luo X., Jiang H., Zhu W. (2009) Halogen bonding. A novel interaction for rational drug design? J. Med. Chem. 52, 2854–2862 [DOI] [PubMed] [Google Scholar]
- 64. Henderson B. J., Pavlovicz R. E., Allen J. D., González-Cestari T. F., Orac C. M., Bonnell A. B., Zhu M. X., Boyd R. T., Li C., Bergmeier S. C., McKay D. B. (2010) Negative allosteric modulators that target human α4β2 neuronal nicotinic receptors. J. Pharmacol. Exp. Ther. 334, 761–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Luttmann E., Ludwig J., Höffle-Maas A., Samochocki M., Maelicke A., Fels G. (2009) Structural model for the binding sites of allosterically potentiating ligands on nicotinic acetylcholine receptors. ChemMedChem 4, 1874–1882 [DOI] [PubMed] [Google Scholar]
- 66. Ludwig J., Höffle-Maas A., Samochocki M., Luttmann E., Albuquerque E. X., Fels G., Maelicke A. (2010) Localization by site-directed mutagenesis of a galantamine binding site on α7 nicotinic acetylcholine receptor extracellular domain. J. Recept. Signal Transduct. Res. 30, 469–483 [DOI] [PubMed] [Google Scholar]
- 67. Unwin N., Miyazawa A., Li J., Fujiyoshi Y. (2002) Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the α subunits. J. Mol. Biol. 319, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 68. Houlihan L. M., Slater Y., Guerra D. L., Peng J. H., Kuo Y. P., Lukas R. J., Cassels B. K., Bermudez I. (2001) Activity of cytisine and its brominated isosteres on recombinant human α7, α4β2, and α4β4 nicotinic acetylcholine receptors. J. Neurochem. 78, 1029–1043 [DOI] [PubMed] [Google Scholar]
- 69. Sander T., Frølund B., Bruun A. T., Ivanov I., McCammon J. A., Balle T. (2011) New insights into the GABA(A) receptor structure and orthosteric ligand binding. Receptor modeling guided by experimental data. Proteins 79, 1458–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Atack J. R. (2008) GABA(A) receptor subtype-selective efficacy. TPA023, an α2/α3 selective non-sedating anxiolytic and α5IA, an α5 selective cognition enhancer. CNS Neurosci. Ther. 14, 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hansen S. B., Radic' Z., Talley T. T., Molles B. E., Deerinck T., Tsigelny I., Taylor P. (2002) Tryptophan fluorescence reveals conformational changes in the acetylcholine binding protein. J. Biol. Chem. 277, 41299–41302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jensen A. A., Mikkelsen I., Frølund B., Bräuner-Osborne H., Falch E., Krogsgaard-Larsen P. (2003) Carbamoylcholine homologs. Novel and potent agonists at neuronal nicotinic acetylcholine receptors. Mol. Pharmacol. 64, 865–875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.