Background: Human umbilical cord blood cells are an effective experimental treatment for stroke.
Results: These cells activate Akt to increase peroxiredoxin 4 and are essential for oligodendrocyte survival during ischemia.
Conclusion: Akt and peroxiredoxin 4 are key molecules in transducing the cellular protection elicited by cord blood cells.
Significance: Identifying this signaling pathway provides new pharmaceutical targets for stroke treatment.
Keywords: Brain, Cell therapy, Gene expression, Ischemia, Signal transduction, Stroke, White matter
Abstract
Human umbilical cord blood (HUCB) cells protect the brain against ischemic injury, yet the mechanism of protection remains unclear. Using both in vitro and in vivo paradigms, this study examined the role of Akt signaling and peroxiredoxin 4 expression in human umbilical cord blood cell-mediated protection of oligodendrocytes from ischemic conditions. As previously reported, the addition of HUCB cells to oligodendrocyte cultures prior to oxygen glucose deprivation significantly enhanced oligodendrocyte survival. The presence of human umbilical cord blood cells also increased Akt phosphorylation and elevated peroxiredoxin 4 expression in oligodendrocytes. Blocking either Akt or peroxiredoxin 4 activity with Akt Inhibitor IV or a peroxiredoxin 4-neutralizing antibody, respectively, negated the protective effects of human umbilical cord blood cells. In vivo, systemic administration of human umbilical cord blood cells 48 h after middle cerebral artery occlusion increased Akt phosphorylation and peroxiredoxin 4 protein expression while reducing proteolytic cleavage of caspase 3 in oligodendrocytes residing in the ipsilateral external capsule. Moreover, human umbilical cord blood cells protected striatal white matter bundles from degeneration following middle cerebral artery occlusion. These results suggest that the soluble factors released from human umbilical cord blood cells converge on Akt to elevate peroxiredoxin 4 levels, and these effects contribute to oligodendrocyte survival.
Introduction
Human umbilical cord blood (HUCB)2 cells have shown great promise in models of experimental stroke. After systemic administration HUCB cells has been shown to migrate to the injured hemisphere of the brain to reduce infarct volume and promote behavioral recovery following permanent middle cerebral artery occlusion (MCAO) (1, 2). Besides increasing neuronal survival, HUCB cells also protected against white matter injury (3).
HUCB cells are composed of proliferative hemopoietic, colony-forming, endothelial precursor, and mesenchymal progenitor cells (4–6). The protective properties of these cells have been attributed to secreted soluble factors, many of which are known activators of the Akt signal transduction pathway (7–10). Upon phosphorylation, Akt transduces survival signals by inhibiting apoptotic cascades and promoting activation of cell survival pathways (11, 12). Indeed, previous studies have shown that HUCB cells protect cultured neurons from excitotoxic injury through activation of Akt (13).
Because converging lines of evidence demonstrate a role for Akt in the protection of neural cells, the present study examined whether the protection afforded to oligodendrocytes (OLs) by HUCB cell therapy occurs through Akt activation. For the in vitro experiments, HUCB cells were co-incubated with OLs using Transwell inserts that prevented cellular contact, thus allowing an assessment of efficacy resulting from secreted factors. Previously, we have reported that HUCB cells increased the expression of survival-associated and antioxidant genes in cultured OLs during oxygen glucose deprivation (OGD) (14). Here, we show that HUCB cell co-incubation with OLs reduced OL cell death and increased the expression of peroxiredoxin 4 (Prdx4) in an Akt-dependent manner during OGD. Prdx4 is a secreted protein, and the addition of a blocking antibody negated the protective effects of HUCB cells. Immunohistochemical analysis showed that systemic administration of HUCB cells 48 h post-MCAO increased Akt phosphorylation and Prdx4 protein expression while reducing proteolytic cleavage of caspase 3 in the external capsule. Following MCAO, HUCB cell treatment also increased intact striatal white matter bundles when evaluated 24-h post-treatment. In summary, Akt activation promotes HUCB cell-mediated oligoprotection, potentially through increased expression of Prdx4.
EXPERIMENTAL PROCEDURES
Animal Care
All animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals with a protocol approved by the Institutional Animal Care and Use Committee at the University of South Florida. Experiments were designed to minimize the number of animals required. Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN), maintained on a 12-h light/dark cycle (6 a.m. to 6 p.m.) in a climate-controlled room, and allowed access to food and water ad libitum. Neonatal rats birthed from untimed pregnant dams were used for in vitro experiments, and 300- to 350-g male rats were used for in vivo experiments.
Mixed Glial Cultures and OL Culture Purification
Mixed glial cultures were prepared, and OL cultures were purified as previously described (3, 14). Mixed glial cultures were prepared from postnatal 3-day rat pups and seeded (1.5 × 107) into flasks. After 8 days the OLs were purified from these preparations and plated onto glass poly-l-lysine-treated coverslips (15). After a 7-day proliferation period the PDGF-AA was withdrawn for 5 days to induce OL differentiation into the mature phenotype, and experiments were conducted immediately as previously described in Hall et al. (3).
Oxygen Glucose Deprivation
OLs seeded onto glass coverslips were randomly assigned to one of two conditions: OGD (DMEM without glucose) or normoxia (DMEM with glucose) as previously described (3, 14). The medium from each well was collected, and lactate dehydrogenase (LDH) analysis was performed immediately.
The following treatment conditions were tested. HUCB cells (ALLCELLS, Emeryville, CA) in DMEM with glucose were seeded onto tissue culture inserts (1 × 105 cells/insert) and placed into the wells containing OL coverslips immediately prior to OGD exposure. The inserts provided a barrier that prevented OL-HUCB cell contact but was permeable to media and soluble factors. Experimental groups not subjected to HUCB cell treatment received inserts containing an equal volume of DMEM with glucose. A negative control of media alone and wells containing 1 × 105 HUCB cells only were included as controls to quantify HUCB cell contribution to the LDH assay for each experimental condition (14). Akt Inhibitor IV (10 μm), wortmannin (200 μm), LY294002 (25 μm), and Akt Inhibitor V (20 μm, EMD Biosciences, Gibbstown, NJ) were dissolved in DMSO then placed in media. Experimental groups not subjected to Akt Inhibitor IV received equivalent quantity of DMSO. An equivalent concentration of Prdx4 and rabbit IgG was included in the media of respective groups and controls: rabbit anti-Prdx4 (0.75 μg/ml, Abcam, Cambridge, MA) and anti-rabbit IgG (0.75 μg/ml, Cell Signaling, Danvers, MA) respectively.
LDH Assay
OL cell death in culture was determined using the LDH assay (Takara Bio, Inc., Madison, WI) as previously described (3, 14). A standard curve was used to quantify OL cell death by extrapolating total numbers of dead OLs from LDH values, as previously described (14).
Quantitative Real-time PCR
An RNeasy Mini Kit (Qiagen, Valencia, CA) was used to collect and purify the RNA that was then subjected to qRT-PCR using an Affinity Script qRT-PCR kit and Brilliant SYBR® Green QPCR reagents (Stratagene, La Jolla, CA) as described in Rowe et al. (14). Primers specific to Prdx4 were purchased from SABiosciences (Frederick, MD; sequences are proprietary).
MCAO and HUCB Cells Treatment
The laser Doppler probe (Moor Instruments Ltd., Devon, England) placement for monitoring blood flow and MCAO surgeries was performed as previously described (1, 3, 16). Rats that did not show ≥ 60% reduction in blood flow during MCAO were excluded from the study (3).
Rats were injected (intravenously, penile vein) with either HUCB cells (1 × 106 HUCB cells in 500 μl of PBS (pH 7.4 + DNase) or vehicle (500 μl of PBS + DNase) 48 h after MCAO surgery (3, 14). Sham-operated animals also received vehicle. Animals were then euthanized 54, 72, and 96 h post-stroke and transcardially perfused, and the brains were processed as previously described (3, 14, 17).
Histochemistry
The cryopreserved brains were sectioned to include bregma 1.7 mm through bregma −3.3 mm. Staining for Fluoro-Jade and peroxidase detection for brain tissue sections and peroxidase for OL coverslips were performed as previously described (3, 14, 17). Antibodies consisted of the following: rabbit anti-Phospho-Akt (Ser-473, 1:50; Cell Signaling), rabbit anti-Caspase 3 (1:1000; Sigma-Aldrich), rabbit anti-Prdx4 (1:250; Abcam). Secondary detection was achieved using biotinylated secondary antibodies (1:300; Vector Laboratories) corresponding to the respective species of primary antibodies.
Double label immunohistochemistry was performed as previously described by Rowe et al. (14). Antibodies used for fluorescence detection consisted of the following: mouse anti-receptor interacting protein (RIP, 1:5,000; Millipore, Temecula, CA), myelin basic protein (MBP) rat monoclonal (1:10,000; Abcam), rabbit anti-Phospho-Akt (Ser-473) (1:50; Cell Signaling), and rabbit anti-Caspase 3 (1:1,000; Sigma-Aldrich). Secondary antibodies used were Alexa Fluor 488 and 594 (1:1,000; Molecular Probes, Eugene, OR). Negative controls were labeled in the absence of primary antibody but with the respective secondary.
Image Analyses
Following in vitro experiments, images were generated using a Zeiss Axioskop2 microscope controlled by OpenLab (Improvision Ltd., Lexington, MA) software. Images were captured with a Zeiss Axiocam Color camera. The ImageJ 1.41 program (National Institutes of Health) was used to measure total staining intensity and was expressed as total intensity per area. For in vivo image analyses, brain sections from ≥3 animals per group were used. Images were analyzed as previously described in Rowe et al. (14).
Statistical Analyses
Data from all experiments were quantified and analyzed using Prism 4.0 (GraphPad, La Jolla, CA) software. Main effects were determined using one-way analysis of variance, followed by Dunnett's post hoc tests to detect significant differences across treatment groups. When two variables were present, two-way analysis of variance was used followed by Bonferroni post hoc tests. A t test was completed by using the Mann-Whitney U test. A p value of <0.05 was used as the threshold for significant differences.
RESULTS
Akt Inhibition Negated HUCB Cell Protection in Vitro
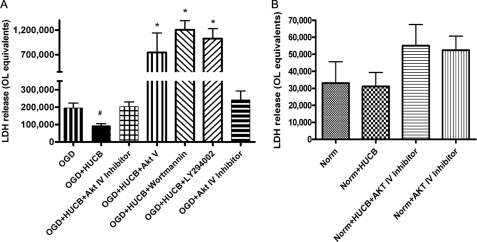
To assess the role of Akt in HUCB cell protection, four Akt inhibitors were applied to OL cultures that were co-cultured with HUCB cells and exposed to OGD (Fig. 1A). Akt Inhibitor IV specifically competes for the ATP binding site upstream of Akt to block its phosphorylation (18), and Akt Inhibitor V specifically inhibits Akt activity. Wortmannin and LY294002 block the upstream PI3K. As expected, exposure to 24-h OGD resulted in increased OL cell death, whereas the presence of HUCB cells reduced OGD-induced cell death (n ≥ 6; #, p < 0.05). The inhibition of Akt phosphorylation eliminated the protective effects of HUCB cells, because cell death was significantly increased relative to HUCB-treated groups. In the presence of Akt Inhibitor IV, cell death following HUCB cell treatment was not different from OGD groups. Treatment with Akt Inhibitor V, wortmannin, and LY294002 resulted in significantly enhanced oligodendrocyte death despite the presence of HUCB cells (n > 3; *, p < 0.0001). There was no difference in OL cell death among the treatment groups exposed to normoxic conditions (Fig. 1B). Akt Inhibitor IV was chosen to be used in subsequent experiments, because ample cells were still viable for further study of the cultures.
FIGURE 1.
Akt inhibition negates the protective effects of HUCB cells on cultured OLs. An increase in OL cell death was detected in cultures subjected to 24-h OGD compared with normoxic controls (n ≥ 6; *, p < 0.01), demonstrating OGD-induced cellular injury. OL cultures subjected to OGD were rescued by co-incubation with HUCB cells, because cell death was reduced back to levels present in normoxic controls (n ≥ 6; #, p < 0.05). Addition of Akt Inhibitor IV (10 μm, Akt Inhibitor V (20 μm), wortmannin (200 μm), or LY294002 (25 μm) eliminated HUCB cells oligoprotective effects in cultures exposed to 24-h OGD (n ≥ 6) (A). There was no effect of treatment on OLs exposed to normoxic conditions (B).
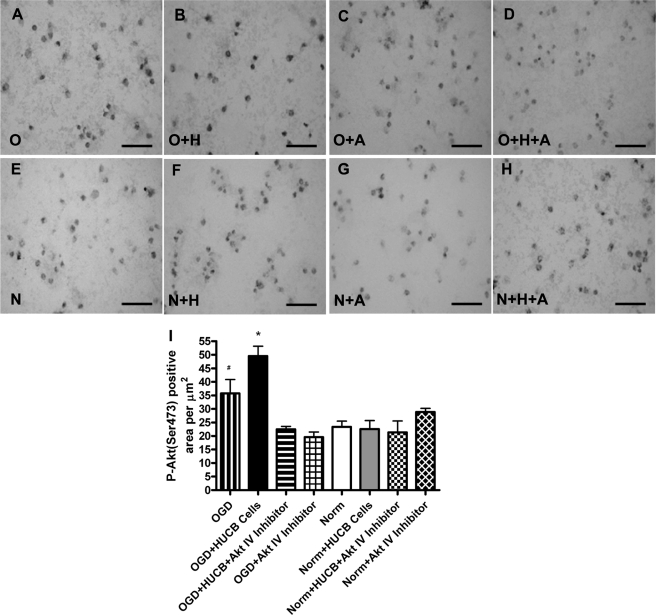
HUCB Cells Increased Akt Phosphorylation in Vitro
Serine 473 is one of two essential phosphorylation sites for activation of Akt to promote cell survival (19). An antibody specific for Akt phosphorylated at serine 473 was used to evaluate HUCB cell-induced phosphorylation of Akt in cultured OLs (Fig. 2). Exposure to OGD increased Akt phosphorylation compared with normoxic controls (n = 3; *, p < 0.05). HUCB cell treatment significantly increased Akt phosphorylation relative to OGD alone (n = 3; #, p < 0.05), whereas co-incubation of HUCB cell-treated OLs with Akt Inhibitor IV reduced phosphorylation to levels that were not different from normoxic conditions (n = 3). Akt phosphorylation was not different among any of the treatment groups exposed to normoxia (Fig. 2, E–H).
FIGURE 2.
HUCB cells increase Akt(Ser-473) phosphorylation in OLs subjected to 24-h OGD. Immunohistochemistry was performed using an antibody generated against Akt phosphorylated at serine 473. Micrographs show immunoreactivity in OLs exposed to OGD (A–D) or normoxia (E–H). Treatment groups were vehicle (A and E), HUCB cells (B and F), Akt Inhibitor IV (C and G), or HUCB cells + Akt Inhibitor IV (D and H). Quantification (I) revealed increased Akt phosphorylation in OLs exposed to OGD relative to normoxic controls (n = 3; *, p < 0.05). Addition of HUCB cells resulted in an additional increase in phosphorylation during OGD relative to OGD alone (n = 3; #, p < 0.05). Akt Inhibitor IV reduced phosphorylation during OGD when added in the presence or absence of HUCB cells, such that immunoreactivity was not different from normoxic controls. Scale bars = 50 μm.
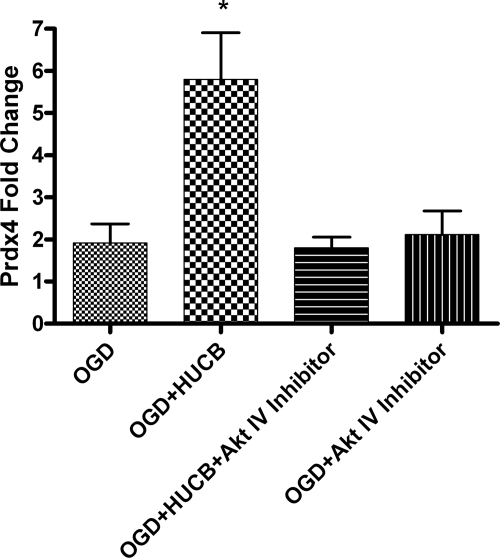
HUCB Cell-induced Expression of Prdx4 Is Suppressed by AKT Inhibition in Vitro
Prdx4 is a secreted antioxidant that reduces extracellular oxidative stress (20). We have previously reported that HUCB cells increase Prdx4 expression in OLs exposed to OGD (14). To determine if Prdx4 expression is dependent on Akt activity, qRT-PCR was performed to quantify expression of the Prdx4 gene transcript in OLs subjected to OGD and treated with HUCB cells and/or Akt Inhibitor IV (Fig. 3). As expected, HUCB cell treatment increased Prdx4 mRNA when compared with OLs subjected to OGD alone (n ≥ 7; *, p < 0.05). Akt inhibition blocked the HUCB cell-induced elevation in Prdx4 mRNA (*, p < 0.05), and these levels were not different from those observed in OLs subjected to OGD alone.
FIGURE 3.
Akt Inhibitor IV suppresses HUCB cell-induced Prdx4 expression during OGD. qRT-PCR was performed to quantify mRNA expression of the antioxidant enzyme Prdx4. OLs were subjected to OGD and treated with HUCB cells in the presence or absence of Akt Inhibitor IV. HUCB cells increased Prdx4 gene transcript compared with OGD alone (n ≥ 7; *, p < 0.05). The addition of the Akt inhibitor with HUCB cells reduced the HUCB cell-induced elevation in Prdx4 mRNA (*, p < 0.05) back to levels detected in OLs treated with OGD or Akt inhibitor alone.
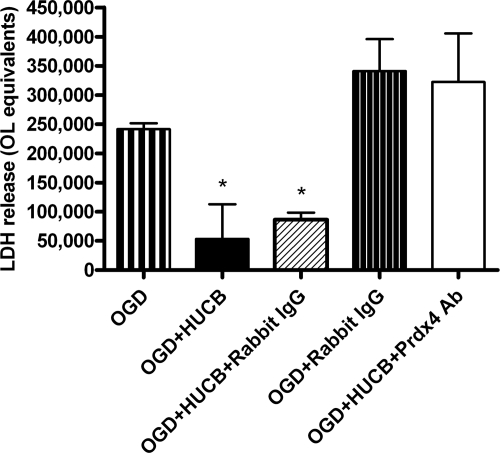
Prdx4-neutralizing Antibodies Blocked HUCB Cell Activity
Corroborating our previous reports, co-cultured HUCB cells significantly reduced OL cell death following 24-h OGD (*, p < 0.05), whereas the addition of 0.75 μg/ml Prdx4 antibody blocked this protection (Fig. 4). No differences in OL survival were observed when comparing OGD alone to OLs co-incubated with HUCB in the presence of Prdx4 antibodies. The anti-rabbit control antibody (negative control) did not interfere with HUCB cell protection of OLs from OGD and is significantly different from the other three groups (*, p < 0.05).
FIGURE 4.
Prdx4-neutralizing antibody blocked HUCB cell activity. HUCB cells blocked OGD-induced OL cell death in cultured OLs (*, p < 0.05). With the addition of 0.75 μg/ml Prdx4 antibody, HUCB cell-induced oligoprotection following 24-h OGD was blocked. The anti-rabbit control antibody (negative control) did not interfere with HUCB cell protection of OLs from OGD and is significantly different from the other three groups (*, p < 0.05).
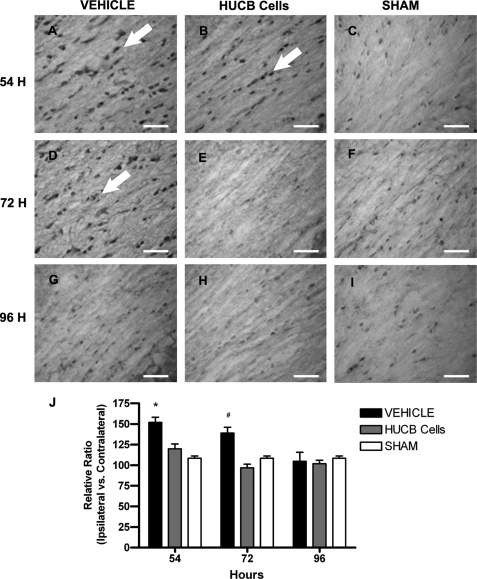
Delayed HUCB Cell Treatment Increased Akt Phosphorylation in Cerebral White Matter after MCAO
Immunoreactivity of Akt phosphorylation was assessed in the white matter-rich region of the external capsule following MCAO. Rats received HUCB cells or vehicle 48 h after MCAO and were euthanized at 54, 72, or 96 h post-stroke (Fig. 5). Constitutive phosphorylation was observed in sham-operated animals, and these levels were not different from vehicle-treated rats at any of the time points examined. HUCB cell treatment increased Akt phosphorylation in the ipsilateral hemisphere at 72 h (Fig. 5E) and 96 h (Fig. 5H) relative to both vehicle controls (Fig. 5, A, D, and G; * and ^, p < 0.05 compared with 72 and 96 h vehicle, respectively) and 54-h HUCB cell-treated rats (Fig. 5B) (n = 3; #, p < 0.05).
FIGURE 5.
HUCB cells increase Akt (Ser-473) phosphorylation in vivo. Immunohistochemistry was performed using an antibody generated against Akt phosphorylated at serine 473. Micrographs show immunoreactive cells (arrows) in the ipsilateral external capsule of vehicle-treated (A, D, and G), HUCB cell-treated (B, E, and H), and sham-operated (C, F, and I) rats. These rats were euthanized at 54 (A–C), 72 (D–F), or 96 h (G–I) after MCAO. Quantification (J) revealed increased phosphorylation of Akt in tissues from HUCB cell-treated rats euthanized at 72 (^, p < 0.05) and 96 h (*, p < 0.05) compared with vehicle-treated rats at the respective time points. Additionally, phosphorylated Akt was increased after HUCB cell treatment at 72 and 96 h relative to 54 h (n = 3; #, p < 0.05), whereas no differences were detected across groups at the 54-h time point. Scale bars = 50 μm.
HUCB Cells Reduce Infarct Volume and Damage to Striatal White Matter
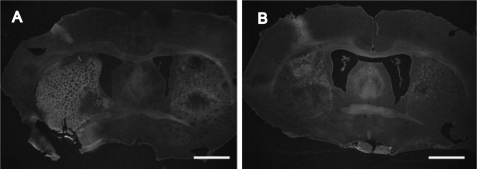
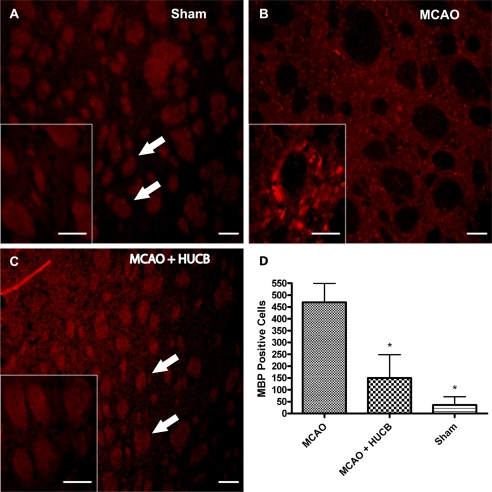
Infarct volumes were assessed with the histochemical stain, Fluoro-Jade, and representative images are shown in Fig. 6. The quantification of these infarct volumes at 72 h was previously reported (14). At 72 h post-MCAO, extensive damage was displayed in the striatal-cortical regions of the rat brain (Fig. 6A), whereas systemic treatment with HUCB cells at 48 h reduced the infarct (Fig. 6B). An antibody generated against MBP was used to evaluate striatal white matter damage in rats subjected to MCAO (21). Following MCAO, increased MBP immunoreactivity was detected throughout the infarct (Fig. 7B). Systemic administration of HUCB cells 48 h post-MCAO inhibited this rise in MBP immunoreactivity (Fig. 7C). Quantification (Fig. 7D) showed that MCAO increased MBP immunoreactivity in the striatum compared with HUCB cell treatment groups and sham controls (n = 3; *, p < 0.05). In these experiments, sham-operated controls were not statistically different from HUCB cell treatment groups (p > 0.05). Only brain regions adversely affected by MCAO exhibited alterations in MBP immunostaining.
FIGURE 6.
HUCB cells reduce MCAO-induced injury. Photomicrographs depict ipsilateral striatal area of animals subjected to MCAO (A) and MCAO + HUCB cells (B). Fluoro-Jade staining increased in the striatum 72 h after MCAO (A) while systemic administration of HUCB cells 48 h following MCAO reduced this increase (B). Scale bars = 100 μm.
FIGURE 7.
HUCB cells reduce damage to striatal white matter. Photomicrographs depict ipsilateral striatal area of animals subjected to Sham (A), MCAO (B), and MCAO + HUCB cells (C). Antibodies generated against MBP were used to evaluate striatal white matter damage in rats subjected to MCAO and euthanized 72 h following surgery. Immunoreactivity for MBP (D) increased in the striatum after MCAO (B), whereas systemic administration of HUCB cells 48 h following MCAO blocked this increase in MBP immunoreactivity (C, n = 3; *, p < 0.05). Sham-operated controls were not statistically different from groups treated with HUCB cells (p > 0.05). Arrows point to white matter in the striatum. Scale bars = 100 μm; inset images, 50 μm.
HUCB Cells Induce Prdx4 Expression in the External Capsule
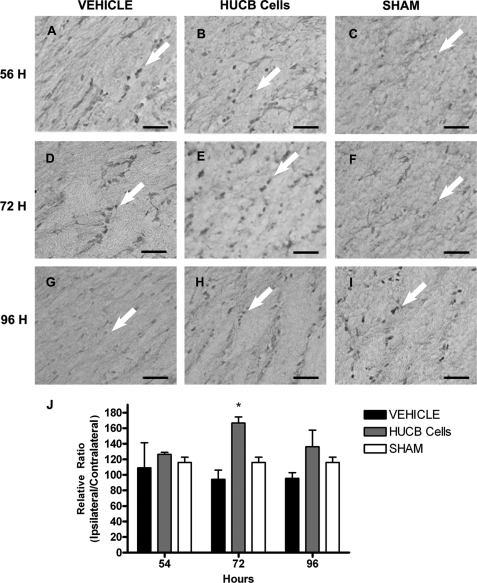
Levels of Prdx4 protein were assessed to determine whether increased mRNA in vitro would translate into gene product expression in vivo (Fig. 8). Rats received HUCB cells or vehicle 48 h after MCAO or sham-MCAO and were euthanized at 54, 72, or 96 h post-stroke for immunohistochemical evaluation of the external capsule. Prdx4 immunoreactivity was restricted to cell bodies. In general, sham-operated controls (Fig. 8, C, F, and I) showed nearly identical expression patterns as vehicle-treated controls (Fig. 8, A, D, and G) with no apparent differences in Prdx4 expression over the time course examined. Quantification (Fig. 8J) showed that levels of Prdx4 were significantly increased in the ipsilateral external capsule of rats treated with HUCB cells compared with sham-operated and vehicle-treated controls at 72-h post-MCAO (*, p < 0.01). No differences in Prdx4 levels were detected 54 or 96 h following MCAO.
FIGURE 8.
HUCB cells induce Prdx4 expression in vivo. Immunohistochemistry was performed to determine the effects of HUCB cell therapy on Prdx4 protein expression. Micrographs show immunoreactive cells (arrows) in the ipsilateral external capsule of vehicle-treated (A, D, and G), HUCB cell-treated (B, E, and H), and sham-operated (C, F, and I) rats. These rats were euthanized at 54 (A–C), 72 (D–F), or 96 h (G–I) after MCAO. Quantification (J) revealed increased expression of Prdx4 in tissues from rats treated with HUCB cells and euthanized at 72 h post-MCAO relative to vehicle-treated and sham-operated animals at the same time point (n = 3; *, p < 0.01). No differences were detected across treatment groups at any other time points examined. Scale bars = 50 μm.
HUCB Cell Treatment Reduces Caspase 3 Activation in the External Capsule following MCAO
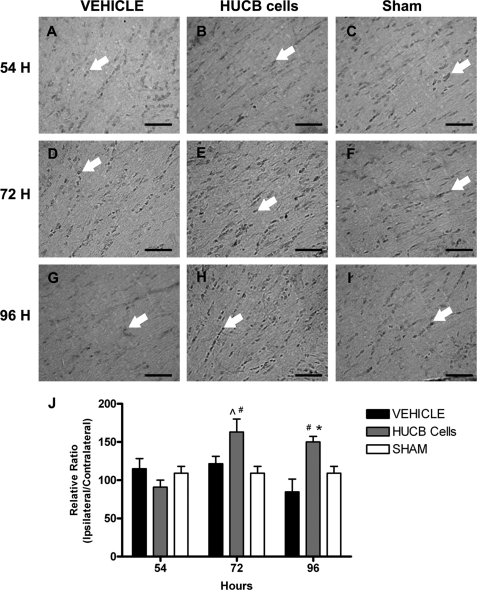
An antibody generated against activated caspase 3 was used to identify apoptotic signaling in the external capsule of rats following MCAO (Fig. 9). As expected, low levels of activated caspase 3 were detected in sham-operated controls. In vehicle-treated animals, levels of activated caspase 3 were elevated at 54 h (Fig. 9A) and 72 h (Fig. 9D) following MCAO compared with HUCB cell-treated and sham-operated groups (n = 3; *, p < 0.05; #, p < 0.01, respectively). Caspase 3 activation returned to basal levels at 96 h (Fig. 9G) following MCAO in vehicle-treated animals (n = 3). Additionally, HUCB cell-treated rats showed no differences in caspase 3 levels compared with sham-operated rats.
FIGURE 9.
Reduced caspase 3 activation after HUCB cell administration in vivo. Immunohistochemistry was performed to determine the effects of HUCB cell therapy on caspase 3 activation. Micrographs show immunoreactive cells (arrows) in the ipsilateral external capsule of vehicle-treated (A, D, and G), HUCB cell-treated (B, E, and H), and sham-operated (C, F, and I) rats. These rats were euthanized at 54 (A–C), 72 (D–F), or 96 h (G–I) after MCAO. MCAO-induced caspase 3 activation was detected 54 (A) and 72 h (D) post MCAO. Quantification (J) revealed HUCB cell treatment blocked the stroke-induced activation of caspase 3 detected in vehicle-treated rats at 54 and 72 h (n = 3, * and #, p < 0.05) such that levels were not different from sham-operated controls. No differences were detected across groups at the 96-h time point. Scale bars = 50 μm.
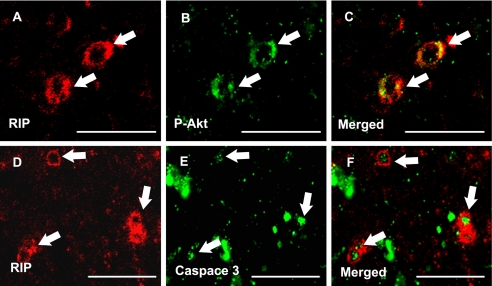
Phosphorylated Akt and Caspase 3 Co-localized with OL Marker RIP
Immunofluorescent staining was performed to determine localization of activated caspase 3 and phosphorylated Akt in OLs within the cerebral white matter. The OL-specific marker RIP was used to double label OLs in conjunction with antibodies raised against activated caspase 3 or phosphorylated Akt. Both activated caspase 3 and phosphorylated Akt were expressed by OLs residing within the cerebral white matter. Phosphorylated Akt was evident in OL cytoplasm (Fig. 10C), whereas nuclear staining was observed in micrographs depicting caspase 3/RIP co-localization (Fig. 10F).
FIGURE 10.
Akt and caspase 3 are activated in OLs in vivo. Confocal micrographs show immunofluorescent co-localization using the OL-specific antibody anti-RIP (A and D) in combination with antibodies generated against phosphorylated Akt (Ser-473) (B, MCAO sham-operated vehicle treated rat) or activated caspase 3 (E, MCAO vehicle-treated rat) in the external capsule. Merged images demonstrate expression of phosphorylated Akt (C) and activated caspase 3 (F) in RIP-positive OLs. Arrows denote immunoreactivity. Scale bars = 50 μm.
DISCUSSION
Oligodendroglia are highly susceptible to ischemic injury (22, 23) due to the energy necessary to synthesize and maintain myelin, the high iron content necessary to produce myelin and low intracellular antioxidant content (24). In previous studies, soluble factors from HUCB cells protected and increased the expression of proliferative, myelin-associated, and antioxidant genes in cultured OLs exposed to OGD (3, 14). In vivo experiments have demonstrated that HUCB cell administration reduced infarct volume and preserved cerebral white matter integrity. These effects occurred concomitantly with increased expression of the antioxidant enzymes Prdx4 and metallothionein 3 (14). Here, we show that HUCB cell signaling is transduced via phosphorylation of Akt kinase leading to the increase in Prdx4 expression and that this activity of Akt and Prdx4 is critical for HUCB oligoprotection from ischemic conditions.
Following MCAO, we previously demonstrated that MBP detection in striatal white matter bundles was significantly reduced 96 h post-surgery (3, 17). Here, a significant increase in MBP immunoreactivity was evident within the infarcted striatum at 72 h in vehicle-treated rats, whereas striatal bundles remained intact following HUCB treatment. Similar results were shown in Hall et al. (3), where these white matter bundles remained intact following HUCB cell administration at 48 h post-MCAO. One possible explanation for the increased MBP immunoreactivity in 72-h vehicle-treated rats is that this time point is prior to maximal infarction when microglia and macrophages have phagocytosed cellular debris. We have reported that microglia/macrophages become activated after 48 h post-MCAO, and the administration of HUCB cells at this time point abolishes this activation (17). These activated microglia/macrophages produce neurotoxic substances as well as proteases to degrade proteins in injured cells. Therefore, it is likely that this increase represents MBP fragments generated from tissue injury and/or synthesis of de novo protein that is subsequently degraded due to lack of cellular support. This result is consistent with the fact that MBP has been suggested as a biomarker for stroke in the clinic due to documented increases in injured tissues (21).
Numerous survival-related factors secreted by HUCB cells have been shown to induce Akt activation, including glial cell-derived neurotrophic factor, brain derived neurotrophic factor, leukemia inhibitory factor, IL-1β, IL-6, insulin like growth factor 1, VEGF, and tissue inhibitor of metalloproteinases metallopeptidase inhibitor 1 (7, 8, 25–28). The pro-survival actions of Akt are well known. For example, cellular proliferation and survival have been shown to result from Akt-induced phosphorylation of bcl2-associated death promoter, the forkhead-related family of mammalian transcription factors and glycogen synthase kinase 3β (19). In the present study, HUCB cells rescued cultured OLs subjected to 24-h OGD, whereas the addition of Akt Inhibitor IV completely blocked this effect. Similar results were demonstrated by Dasari et al. (13), where the ability of HUCB cells to rescue neurons from glutamate excitotoxicity was blocked by Akt inhibition. Thus, the mechanism of oligoprotection observed after HUCB cell treatment is consistent with previous findings and further supports the contention that soluble factors activate Akt signal transduction to increase the viability of cells exposed to ischemic conditions.
Systemic treatment with HUCB cells at 48 h post-MCAO increased Akt phosphorylation at serine 473 in OLs of the ipsilateral external capsule. Previous reports have shown that Akt was rapidly phosphorylated at serine 473 following ischemia in vitro and in vivo, specifically 1 h following MCAO. These studies also emphasized the link between reductions of Akt phosphorylation over time and increased cell death (19, 29, 30). In disease states such as stroke, unphosphorylated Akt initiates the activation of apoptotic cascades resulting in the release of cytochrome c, caspase 3, and poly(ADP-ribose) polymerase cleavage (31–33). In the present study, the HUCB-induced increase in Akt phosphorylation was associated with decreased caspase cleavage, which is consistent with a cytoprotective role.
Peroxiredoxins are a group of six proteins most noted for their antioxidant activity (34) but are also involved in signal transduction (35, 36). Their actions have not only been implicated in neurodegenerative diseases but also as a target for cancer treatment and immune functioning (37–40). Administration of peroxiredoxin 3 protected neurons from ischemic insult resulting in enhanced behavioral recovery (41). The excitotoxic effects of ibotenate in the brain are blocked with the systemic treatment of peroxiredoxin 5 (42).
Prdx4 is a secreted protein that has been reported to reduce extracellular oxidative stress (20) through peroxidase activity (43) and also serves as a chaperone that functions to prevent intracellular protein aggregation and subsequent apoptosis (44, 45). Addition of this protein to human myeloid cells results in the activation of NF-κB and a subsequent increase in the expression of its known target genes, Icam-1 and iNOS (36). Expression of Prdx4 has been previously reported to be localized to OLs in the brain (46). Blocking secreted Prdx4 with a neutralizing antibody suppressed the protective effects of HUCB cells on OLs exposed to OGD. Thus, Prdx4 could be enhancing cell survival through its antioxidant activity and/or activation of NF-κB. In fact, NF-κB activation has been shown to provide cellular protection in models of stroke and other neurodegenerative pathologies (47, 48). Additionally, transcription of the peroxiredoxin 2 gene is increased by NF-κB, which would result in a further reduction in oxidative stress (49). Our results show that secreted Prdx4 mediates the protective effects of HUCB cells, either through its antioxidant properties, activation of survival signaling, or both. These data indicate that Prdx4 is a key downstream component of this cellular therapy.
Administration of HUCB cells reduced infarct volume and related behavioral deficits after stroke and spinal cord injury (1, 3, 50–52). In fact, HUCB treatment has consistently improved motor function using several different testing methods (1, 50). Yet the precise mechanisms underlying these protective effects remain unknown. The present study has identified a distinct pathway utilized by HUCB cells to mediate cellular protection under ischemic conditions. The phosphorylation of both Akt and Prdx4 are required for oligoprotection in vitro. Furthermore, systemic administration of HUCB cells increases Akt phosphorylation and Prdx4 protein expression in ischemic white matter. Taken together, these data provide strong evidence that the soluble factors secreted by HUCB cells converge on Akt to increase Prdx4 expression, and these effects result in enhanced cellular survival. Future studies investigating specific factors secreted by HUCB cells and the function of Prdx4 will be instrumental in defining novel therapeutic approaches to ischemic injury.
This work was supported, in whole or in part, by National Institutes of Health Grant 1-R01-NS052839.
- HUCB
- human umbilical cord blood
- MCAO
- middle cerebral artery occlusion
- OL
- oligodendrocyte
- OGD
- oxygen glucose deprivation
- Prdx4
- peroxiredoxin 4
- LDH
- lactate dehydrogenase
- RIP
- receptor interacting protein
- MBP
- myelin basic protein.
REFERENCES
- 1. Vendrame M., Cassady J., Newcomb J., Butler T., Pennypacker K. R., Zigova T., Sanberg C. D., Sanberg P. R., Willing A. E. (2004) Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke 35, 2390–2395 [DOI] [PubMed] [Google Scholar]
- 2. Newman M. B., Willing A. E., Manresa J. J., Davis-Sanberg C., Sanberg P. R. (2005) Stroke-induced migration of human umbilical cord blood cells. Time course and cytokines. Stem Cells Dev. 14, 576–586 [DOI] [PubMed] [Google Scholar]
- 3. Hall A. A., Guyer A. G., Leonardo C. C., Ajmo C. T., Jr., Collier L. A., Willing A. E., Pennypacker K. R. (2009) Human umbilical cord blood cells directly suppress ischemic oligodendrocyte cell death. J. Neurosci. Res. 87, 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nieda M., Nicol A., Denning-Kendall P., Sweetenham J., Bradley B., Hows J. (1997) Endothelial cell precursors are normal components of human umbilical cord blood. Br. J. Haematol. 98, 775–777 [DOI] [PubMed] [Google Scholar]
- 5. Erices A., Conget P., Minguell J. J. (2000) Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 109, 235–242 [DOI] [PubMed] [Google Scholar]
- 6. Nakahata T., Ogawa M. (1982) Hemopoietic colony-forming cells in umbilical cord blood with extensive capability to generate mono- and multipotential hemopoietic progenitors. J. Clin. Invest. 70, 1324–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kulik G., Klippel A., Weber M. J. (1997) Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol. Cell. Biol. 17, 1595–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin K. L., Mao X. O., Greenberg D. A. (2000) Vascular endothelial growth factor. Direct neuroprotective effect in in vitro ischemia. Proc. Natl. Acad. Sci. U.S.A. 97, 10242–10247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neuhoff S., Moers J., Rieks M., Grunwald T., Jensen A., Dermietzel R., Meier C. (2007) Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro. Exp. Hematol. 35, 1119–1131 [DOI] [PubMed] [Google Scholar]
- 10. Newman M. B., Willing A. E., Manresa J. J., Sanberg C. D., Sanberg P. R. (2006) Cytokines produced by cultured human umbilical cord blood (HUCB) cells. Implications for brain repair. Exp. Neurol. 199, 201–208 [DOI] [PubMed] [Google Scholar]
- 11. Mullonkal C. J., Toledo-Pereyra L. H. (2007) Akt in ischemia and reperfusion. J. Invest. Surg. 20, 195–203 [DOI] [PubMed] [Google Scholar]
- 12. Zhao Y., Patzer A., Herdegen T., Gohlke P., Culman J. (2006) Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 20, 1162–1175 [DOI] [PubMed] [Google Scholar]
- 13. Dasari V. R., Veeravalli K. K., Saving K. L., Gujrati M., Fassett D., Klopfenstein J. D., Dinh D. H., Rao J. S. (2008) Neuroprotection by cord blood stem cells against glutamate-induced apoptosis is mediated by Akt pathway. Neurobiol. Dis. 32, 486–498 [DOI] [PubMed] [Google Scholar]
- 14. Rowe D. D., Leonardo C. C., Hall A. A., Shahaduzzaman M. D., Collier L. A., Willing A. E., Pennypacker K. R. (2010) Cord blood administration induces oligodendrocyte survival through alterations in gene expression. Brain Res. 1366, 172–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCarthy K. D., de Vellis J. (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85, 890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butler T. L., Kassed C. A., Sanberg P. R., Willing A. E., Pennypacker K. R. (2002) Neurodegeneration in the rat hippocampus and striatum after middle cerebral artery occlusion. Brain Res. 929, 252–260 [DOI] [PubMed] [Google Scholar]
- 17. Leonardo C. C., Hall A. A., Collier L. A., Ajmo C. T., Jr., Willing A. E., Pennypacker K. R. (2010) Human umbilical cord blood cell therapy blocks the morphological change and recruitment of CD11b-expressing, isolectin-binding proinflammatory cells after middle cerebral artery occlusion. J. Neurosci. Res. 88, 1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kau T. R., Schroeder F., Ramaswamy S., Wojciechowski C. L., Zhao J. J., Roberts T. M., Clardy J., Sellers W. R., Silver P. A. (2003) A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell 4, 463–476 [DOI] [PubMed] [Google Scholar]
- 19. Zhao H., Sapolsky R. M., Steinberg G. K. (2006) Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol. Neurobiol. 34, 249–270 [DOI] [PubMed] [Google Scholar]
- 20. Okado-Matsumoto A., Matsumoto A., Fujii J., Taniguchi N. (2000) Peroxiredoxin IV is a secretable protein with heparin-binding properties under reduced conditions. J. Biochem. 127, 493–501 [DOI] [PubMed] [Google Scholar]
- 21. Brouns R., De Vil B., Cras P., De Surgeloose D., Mariën P., De Deyn P. P. (2010) Neurobiochemical markers of brain damage in cerebrospinal fluid of acute ischemic stroke patients. Clin. Chem. 56, 451–458 [DOI] [PubMed] [Google Scholar]
- 22. Lyons S. A., Kettenmann H. (1998) Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J. Cereb. Blood Flow Metab. 18, 521–530 [DOI] [PubMed] [Google Scholar]
- 23. Pantoni L., Garcia J. H., Gutierrez J. A. (1996) Stroke 27, 1641–1646; discussion, 1647 [DOI] [PubMed] [Google Scholar]
- 24. McTigue D. M., Tripathi R. B. (2008) The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 107, 1–19 [DOI] [PubMed] [Google Scholar]
- 25. Lee S. J., Yoo H. J., Bae Y. S., Kim H. J., Lee S. T. (2003) TIMP-1 inhibits apoptosis in breast carcinoma cells via a pathway involving pertussis toxin-sensitive G protein and c-Src. Biochem. Biophys. Res. Commun. 312, 1196–1201 [DOI] [PubMed] [Google Scholar]
- 26. Lentzsch S., Chatterjee M., Gries M., Bommert K., Gollasch H., Dörken B., Bargou R. C. (2004) PI3-K/AKT/FKHR and MAPK signaling cascades are redundantly stimulated by a variety of cytokines and contribute independently to proliferation and survival of multiple myeloma cells. Leukemia 18, 1883–1890 [DOI] [PubMed] [Google Scholar]
- 27. Wegiel B., Bjartell A., Culig Z., Persson J. L. (2008) Interleukin-6 activates PI3K/Akt pathway and regulates cyclin A1 to promote prostate cancer cell survival. Int. J. Cancer 122, 1521–1529 [DOI] [PubMed] [Google Scholar]
- 28. Dolcet X., Egea J., Soler R. M., Martin-Zanca D., Comella J. X. (1999) Activation of phosphatidylinositol 3-kinase, but not extracellular-regulated kinases, is necessary to mediate brain-derived neurotrophic factor-induced motoneuron survival. J. Neurochem. 73, 521–531 [DOI] [PubMed] [Google Scholar]
- 29. Shibata M., Yamawaki T., Sasaki T., Hattori H., Hamada J., Fukuuchi Y., Okano H., Miura M. (2002) Upregulation of Akt phosphorylation at the early stage of middle cerebral artery occlusion in mice. Brain Res. 942, 1–10 [DOI] [PubMed] [Google Scholar]
- 30. Noshita N., Lewén A., Sugawara T., Chan P. H. (2001) Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 21, 1442–1450 [DOI] [PubMed] [Google Scholar]
- 31. Datta S. R., Brunet A., Greenberg M. E. (1999) Cellular survival. A play in three Akts. Genes Dev. 13, 2905–2927 [DOI] [PubMed] [Google Scholar]
- 32. Le Rhun Y., Kirkland J. B., Shah G. M. (1998) Cellular responses to DNA damage in the absence of poly(ADP-ribose) polymerase. Biochem. Biophys. Res. Commun. 245, 1–10 [DOI] [PubMed] [Google Scholar]
- 33. Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A. (1995) Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376, 37–43 [DOI] [PubMed] [Google Scholar]
- 34. Hall A., Sankaran B., Poole L. B., Karplus P. A. (2009) Structural changes common to catalysis in the Tpx peroxiredoxin subfamily. J. Mol. Biol. 393, 867–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ho J. N., Lee S. B., Lee S. S., Yoon S. H., Kang G. Y., Hwang S. G., Um H. D. (2010) Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Mol. Cancer Ther. 9, 825–832 [DOI] [PubMed] [Google Scholar]
- 36. Haridas V., Ni J., Meager A., Su J., Yu G. L., Zhai Y., Kyaw H., Akama K. T., Hu J., Van Eldik L. J., Aggarwal B. B. (1998) TRANK, a novel cytokine that activates NF-kappa B and c-Jun N-terminal kinase. J. Immunol. 161, 1–6 [PubMed] [Google Scholar]
- 37. Carnero A., Blanco-Aparicio C., Renner O., Link W., Leal J. F. (2008) The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 8, 187–198 [DOI] [PubMed] [Google Scholar]
- 38. Kwon J., Lee S. R., Yang K. S., Ahn Y., Kim Y. J., Stadtman E. R., Rhee S. G. (2004) Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. U.S.A. 101, 16419–16424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Radyuk S. N., Michalak K., Klichko V. I., Benes J., Orr W. C. (2010) Peroxiredoxin 5 modulates immune response in Drosophila. Biochim. Biophys. Acta 1800, 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jung H., Kim T., Chae H. Z., Kim K. T., Ha H. (2001) Regulation of macrophage migration inhibitory factor and thiol-specific antioxidant protein PAG by direct interaction. J. Biol. Chem. 276, 15504–15510 [DOI] [PubMed] [Google Scholar]
- 41. Hwang I. K., Yoo K. Y., Kim D. W., Lee C. H., Choi J. H., Kwon Y. G., Kim Y. M., Choi S. Y., Won M. H. (2010) Changes in the expression of mitochondrial peroxiredoxin and thioredoxin in neurons and glia and their protective effects in experimental cerebral ischemic damage. Free Radic. Biol. Med. 48, 1242–1251 [DOI] [PubMed] [Google Scholar]
- 42. Plaisant F., Clippe A., Vander Stricht D., Knoops B., Gressens P. (2003) Recombinant peroxiredoxin 5 protects against excitotoxic brain lesions in newborn mice. Free Radic. Biol. Med. 34, 862–872 [DOI] [PubMed] [Google Scholar]
- 43. Hofmann B., Hecht H. J., Flohé L. (2002) Peroxiredoxins. Biol. Chem. 383, 347–364 [DOI] [PubMed] [Google Scholar]
- 44. Jang H. H., Lee K. O., Chi Y. H., Jung B. G., Park S. K., Park J. H., Lee J. R., Lee S. S., Moon J. C., Yun J. W., Choi Y. O., Kim W. Y., Kang J. S., Cheong G. W., Yun D. J., Rhee S. G., Cho M. J., Lee S. Y. (2004) Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117, 625–635 [DOI] [PubMed] [Google Scholar]
- 45. Kang S. W., Rhee S. G., Chang T. S., Jeong W., Choi M. H. (2005) 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol. Med. 11, 571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jin M. H., Lee Y. H., Kim J. M., Sun H. N., Moon E. Y., Shong M. H., Kim S. U., Lee S. H., Lee T. H., Yu D. Y., Lee D. S. (2005) Characterization of neural cell types expressing peroxiredoxins in mouse brain. Neurosci. Lett. 381, 252–257 [DOI] [PubMed] [Google Scholar]
- 47. Kassed C. A., Willing A. E., Garbuzova-Davis S., Sanberg P. R., Pennypacker K. R. (2002) Lack of NF-kappaB p50 exacerbates degeneration of hippocampal neurons after chemical exposure and impairs learning. Exp. Neurol. 176, 277–288 [DOI] [PubMed] [Google Scholar]
- 48. Fridmacher V., Kaltschmidt B., Goudeau B., Ndiaye D., Rossi F. M., Pfeiffer J., Kaltschmidt C., Israël A., Mémet S. (2003) Forebrain-specific neuronal inhibition of nuclear factor-κB activity leads to loss of neuroprotection. J. Neurosci. 23, 9403–9408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Won H., Lim S., Jang M., Kim Y., Rashid M. A., Jyothi K. R., Dashdorj A., Kang I., Ha J., Kim S. S. (2012) Peroxiredoxin-2 up-regulated by NF-κB attenuates oxidative stress during the differentiation of muscle-derived C2C12 cells. Antioxid. Redox Signal. 16, 245–261 [DOI] [PubMed] [Google Scholar]
- 50. Newcomb J. D., Ajmo C. T., Jr., Sanberg C. D., Sanberg P. R., Pennypacker K. R., Willing A. E. (2006) Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant 15, 213–223 [DOI] [PubMed] [Google Scholar]
- 51. Chua S. J., Bielecki R., Yamanaka N., Fehlings M. G., Rogers I. M., Casper R. F. (2010) The effect of umbilical cord blood cells on outcomes after experimental traumatic spinal cord injury. Spine 35, 1520–1526 [DOI] [PubMed] [Google Scholar]
- 52. Garbuzova-Davis S., Willing A. E., Zigova T., Saporta S., Justen E. B., Lane J. C., Hudson J. E., Chen N., Davis C. D., Sanberg P. R. (2003) Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J. Hematother. Stem Cell Res. 12, 255–270 [DOI] [PubMed] [Google Scholar]