Background: PARC sequesters p53 in the cytoplasm; dysregulation of p53 localization/function contributes to chemoresistance in OVCA.
Results: Cisplatin promotes calpain-mediated PARC down-regulation, mitochondrial and nuclear p53 accumulation, and apoptosis in chemosensitive but not resistant OVCA cells.
Conclusion: Dysregulation of calpain-mediated PARC processing promotes chemoresistance in OVCA cells.
Significance: PARC down-regulation represents a novel strategy to prevent/reverse resistance to cisplatin-based chemotherapy.
Keywords: Akt, Apoptosis, Calpain, Intracellular Trafficking, Ovarian Cancer, p53, PARC, Chemoresistance, Cisplatin, Mitochondria
Abstract
Resistance to cisplatin (CDDP)-based therapy is a major hurdle to the successful treatment of human ovarian cancer (OVCA), and the chemoresistant phenotype in OVCA cells is associated with Akt-attenuated p53-mediated apoptosis. Pro-apoptotic functions of p53 involve both transcription-dependent and -independent signaling pathways, and dysfunctional localization and/or inactivation of p53 contribute to the development of chemoresistance. PARC is a cytoplasmic protein regulating p53 subcellular localization and subsequent function. Little is known about the molecular mechanisms regulating PARC. Although PARC contains putative caspase-3 cleavage sites, and CDDP is known to induce the activation of caspases and calpains and induce proteasomal degradation of anti-apoptotic proteins, if and how PARC is regulated by CDDP in OVCA are unknown. Here, we present evidence that CDDP promotes calpain-mediated PARC down-regulation, mitochondrial and nuclear p53 accumulation, and apoptosis in chemosensitive but not resistant OVCA cells. Inhibition of Akt is required to sensitize chemoresistant cells to CDDP in a p53-dependent manner, an effect enhanced by PARC down-regulation. CDDP-induced PARC down-regulation is reversible by inhibition of calpain but not of caspases or the 26 S proteasome. Furthermore, in vitro experiments confirm the ability of calpain in mediating Ca2+-dependent PARC down-regulation. The role of Ca2+ in PARC down-regulation was further confirmed as ionomycin-induced PARC down-regulation in both chemosensitive and chemoresistant ovarian cancer cells. The data presented here implicate the regulation of p53 subcellular localization and apoptosis by PARC as a contributing factor in CDDP resistance in OVCA cells and Ca2+/calpain in PARC post-translational processing and chemosensitivity.
Introduction
Ovarian cancer (OVCA)3 is the most lethal of the gynecological cancers. Although cisplatin (CDDP) and its derivatives are effective chemotherapeutic agents for OVCA, CDDP resistance remains a major hurdle for long term treatment success. The PI3K/Akt signaling pathway is frequently activated/overexpressed in human OVCA and is a determinant of chemoresistance. Down-regulation of Akt activity can sensitize resistant OVCA cells to CDDP in a p53-dependent manner. Conversely, Akt activation inhibits mitochondrial p53 accumulation and attenuates p53 phosphorylation and nuclear function in OVCA cells, conferring resistance to CDDP (1–4). Phosphorylation of p53 (Ser-15) is necessary for CDDP-induced apoptosis, although the mechanism(s) by which Akt regulates p53 phosphorylation is unclear. OVCA cells expressing activated Akt are characterized by increased p53 content, suggesting that Akt attenuates p53 function rather than its content (5).
p53 mediates CDDP-induced apoptosis in both a transcription-dependent (6) and -independent manner (7–9). Although the loss of p53 function by inactivating mutations has been widely demonstrated (10), evidence suggest that the control of intracellular localization of p53 is also important in the regulation of apoptosis and chemosensitivity (5, 9).
The p53-associated, Parkin-like cytoplasmic protein (PARC, Cul9) is a cytoplasmic protein that regulates p53 subcellular localization and function. Described as an atypical cullins protein and a putative E3 ubiquitin ligase, the endogenous substrates of PARC and mediators of PARC regulation are unknown (11, 12). PARC forms a multiprotein complex of ∼1 million kDa in size, with its N terminus binding to the C terminus of p53 (11). The anti-apoptotic role of PARC is demonstrated in cellular models where cytoplasmic p53 content is high and is characterized by poor response to drug therapy (11, 13). The majority of cytoplasmic p53 is bound to PARC in these models as determined by immunodepletion assays (11). PARC down-regulation in these cells promotes nuclear p53 accumulation, transcriptional activation, and apoptosis. In contrast, overexpression of PARC prevents nuclear p53 accumulation and apoptosis (11, 13). The role of PARC in the regulation of mitochondrial p53 accumulation and transcription-independent apoptosis has not been examined.
Calpains comprise a family of calcium-dependent cysteine proteases that are activated in apoptosis and are also prominent in necrosis. The two major isoforms, named μ-calpain (calpain I) and m-calpain (calpain II), are activated by micromolar and millimolar amounts of Ca2+, respectively. Their importance in apoptosis is reflected in the growing list of calpain substrates, including p53 (14), Bax, Bid (15), apoptosis-inducing factor, and several cytoskeletal proteins (16). Although CDDP can induce calpain activation, whether calpain plays a role in the regulation of PARC in OVCA cells is not known.
In this study, we have demonstrated for the first time the control of p53 subcellular localization and CDDP sensitivity by PARC in OVCA cells, and the possible involvement of Akt in this regulation. We have also presented evidence for the Ca2+-activated, calpain-mediated PARC processing in CDDP-induced apoptosis and that CDDP resistance is associated with PARC stabilization and up-regulation.
EXPERIMENTAL PROCEDURES
Reagents
CDDP, DMSO, Hoechst 33258, phenylmethylsulfonyl fluoride (PMSF), sodium orthovanadate (Na3VO4), aprotinin, EGTA, 5′-aza-2′-deoxycytidine, calcium chloride dehydrate (CaCl2-2H2O), lactacystin and calpain 1, and ionomycin calcium salt were purchased from Sigma. Rabbit polyclonal α-fodrin, p-p53 (Ser-15), p-Akt (Ser-473), p-GSK-3β (Ser-9), and PARP antibodies were from Cell Signaling Technology, Inc. (Beverly, MA). Mouse monoclonal GAPDH and active and pro-caspase-3 were from Abcam (Cambridge, MA). Mouse monoclonal p53 (Western blot, DO-1; immunofluorescence, pAb1801), goat polyclonal calpain 1 antibody, and peroxidase-conjugated donkey anti-goat immunoglobulin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal PARC was obtained from Bethyl Laboratories Inc. (Montgomery, TX). Peroxidase-conjugated goat anti-mouse and goat anti-rabbit immunoglobulin were purchased from Bio-Rad. Calpeptin and epoxomicin were obtained from EMD Chemicals (Gibbstown, NJ). API-2, Z-DEVD-FMK and Z-VAD-FMK were from Tocris Bioscience (Ellisville, MO).
Cell Lines and Cell Culture
CDDP-sensitive OV2008 (17) and A2780s (18, 19) and CDDP-resistant C13* (20), OVCA420 (21), OVCA433 (22, 23), and HEY (24) human ovarian cancer cell lines were derived from serous cystadenocarcinomas of the ovary. The C13* cell line is the isogenic resistant counterpart to OV2008, selected by chronic exposure of increasing concentrations of CDDP. IOSE397 (25) is a human ovarian surface epithelial cell line immortalized by transfection of the SV40 large T antigen. All cells lines are p53 wild-type, as reported previously (9, 21, 26–28), and were gifts from Drs. Rakesh Goel and Barbara Vanderhyden (Ottawa Regional Cancer Centre, Ottawa, Ontario, Canada).
Cells were maintained in RPMI 1640 medium (chemosensitive OV2008 and chemoresistant C13* and HEY) and DMEM/F-12 medium (chemosensitive A2780s and chemoresistant OVCA420 and OVCA433), and the immortalized ovarian surface epithelial cell line IOSE397 was cultured in MCDB/M199. RPMI 1640 and DMEM/F-12 media contained heat-inactivated FBS (10%), streptomycin (50,000 μg/liter), penicillin (50,000 units/liter), and Fungizone (625 μg/liter; Invitrogen) (29). MCDB/M199 media (1:1) contained heat-inactivated FBS (15%). Cells were plated in 60-mm culture dishes at 40% confluency unless stated otherwise and cultured at 37 °C with 5% CO2, 95% O2.
Assessment of Apoptosis
Apoptotic cells were identified morphologically using Hoechst 33258 nuclear stain (1, 30, 31). At least 300 cells per treatment group were assessed in randomly selected fields with the investigator blinded to the sample group to avoid experimental bias. Apoptosis was further confirmed by detecting PARP cleavage from a full-length 116-kDa peptide into its cleaved forms (89- and 24-kDa polypeptides) by Western blot (32).
Protein Extraction and Western Blot Detection
Protein extraction and Western blot analysis was performed as reported previously (29). Membranes were incubated overnight at 4 °C in primary antibody (PARC (1:10,000), p53 (1:10,000), p-p53 (Ser-15) (1:2,000), p-Akt (Ser-473) (1:1,000), α-fodrin (1:2,500), PARP (1:2,000), active- and pro-caspase-3 (1:1,000), calpain 1 (1:1,100), V5 (1:5,000), and GAPDH (1:20,000)), followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:1,000 RT, 1 h; 1:10,000 for GAPDH and p53). Signal intensity (enhanced chemiluminescence kit (Amersham Biosciences)) was assessed densitometrically (Scion Image software, version 4.02; Scion Corp., Frederick, MD).
Reverse Transcriptase-Polymerase Chain Reaction
Reverse transcriptase-polymerase chain reaction was carried out as described previously (5). PCR primers (Invitrogen) were as follows: PARC (sense 5′-TGTACCCTTTGCCGTACCTC-3′ and antisense 5′-AGACGAGCTGCTTGGTTCAT-3′); GAPDH (sense 5′-ACAGTCAGCCGCATCTTCTT-3′ and antisense 5′-GACAAGCTTCCCGTTCTCAG-3′). PCRs were performed using HotStarTaq polymerase (Qiagen) after activation (15 min; 95 °C) as follows: denaturation, 30 s at 94 °C; annealing, (30 at 60 °C); extension, 1 min at 72 °C; 34 and 21 cycles for PARC and GAPDH, respectively.
Fluorescence Microscopy
Cells were plated on poly-d-lysine-coated (0.05% w/v; Sigma) 8-well glass culture slides (BD Biosciences) and cultured in growth media (48 h) prior to CDDP treatment. For immunostaining, cells were fixed in paraformaldehyde (4%, 1 h, RT), washed in PBS, and blocked with 1% BSA and 1% goat serum. p53 was detected using an anti-p53 mouse monoclonal antibody (1:50; Santa Cruz Biotechnology) and Alexa Fluor 488 goat anti-mouse secondary antibody (1:500; Invitrogen). TOM20 was detected with an anti-TOM20 rabbit polyclonal antibody (1:200; Santa Cruz Biotechnology) and Texas Red goat anti-rabbit secondary antibody (1:500; Invitrogen). Confocal images were obtained (×100 objective NA1.4) on an Olympus IX81 inverted microscope with appropriate argon lasers (DAPI, 405 nm; Alexa Fluor 488; 488 nm; Texas Red, 543 nm).
Determination of p53 Subcellular Localization
Cells on culture slides were incubated with antibodies against p53, TOM20 (mitochondria marker), and DAPI (nuclear stain). At least 400 cells were analyzed per treatment group for co-localization of p53 signal with TOM20 and/or DAPI.
Immunoprecipitation
The cell lysate was incubated (4 °C, 1 h) in Pierce immunoprecipitation cell lysis buffer (Thermo Fisher Scientific Inc., Rockford, IL) supplemented with 1× Protease Inhibitor Mixture I (Sigma) and 1× PhoSTOP phosphatase inhibitor (Roche Applied Science) and centrifuged (14,000 × g, 10 min, 4 °C). One mg of protein of the supernatant (200 μl) was incubated with protein A Dynabeads (Invitrogen) coated with rabbit polyclonal anti-PARC antibody (2 μg/200 μl; 1 h, RT) and immunoprecipitated (4 °C, 2 h). The beads were pelleted, resuspended in Laemmli sample buffer (2×; 40 μl; Bio-Rad), boiled (10 min), and loaded onto 9% SDS-PAGE. Following protein transfer to nitrocellulose, PARC and p53 were detected by Western blotting.
RNA Interference
According to manufacturer's instructions, cells were transfected (48 h) with PARC (Santa Cruz Biotechnology) and p53 siRNA (Dharmacon) and scrambled sequence control (Dharmacon), using Lipofectamine 2000 (Invitrogen).
Adenovirus Infection
Cells were infected with an HA-tagged “triple-A” (K179A, T308A, and S473A) dominant-negative Akt (DN-Akt) or LacZ cDNA control (9). Infection efficiency at multiplicities of infection of 40, as determined by X-gal staining, was >90%. DN-Akt expression was confirmed by Western blot against the HA epitope.
Construction of PARC Overexpression Plasmid
The plasmid pcDNA3-HA2-PARC and primers for PCR were from Addgene (Cambridge, MA) and Invitrogen, respectively. Primers for cloning were as follows: PARC forward 5′-AAGGTACCATGGTGGGGGAAC-3′ and PARC-reverse 5′-GGTCTAGACCGTCATAGGCCTCA-3′. The human coding sequence of PARC was amplified from the pcDNA3-HA2-PARC plasmid using the primers above. The PARC-V5-His fusion was constructed by cloning the human coding sequence of PARC into a pEF6/V5-His-TOPO/LacZ expression vector (Invitrogen) through KpnΙ and XbaΙ sites.
Transient Transfection
Transfections with pEF6-derived vectors (0–1 μg; 1 ml serum-free medium) used Lipofectamine and PLUS reagent (Invitrogen). Media were removed (24 h post-transfection), and cells were harvested or treated as required.
In Vitro Degradation of PARC Protein by Purified Calpain
Purified calpain (μ-calpain; human plasma, 1 unit/6.25 μg of protein) was incubated with whole cell lysate (30 μg protein; 1 h, 30 °C) as described previously (33). Calpain was inactivated by boiling (10 min) and/or addition of EGTA (10 mm) and CaCl2 (0–1 mm). The samples were boiled (10 min) and centrifuged, and supernatant proteins were resolved on 9% SDS-PAGE.
In Vitro Caspase-3 Treatment
Whole cell lysates (50 μg) were incubated (30 °C, 2 h) in Pipes assay buffer (Pipes (20 mm), NaCl (100 mm), dithiothreitol (DDT, 10 mm), EDTA (1 mm), Chaps (0.1%, w/v), sucrose (10%, w/v), pH 7.2) containing recombinant active caspase-3 (0–15 μg/ml). The samples were boiled in Laemmli sample buffer (10 min) and centrifuged, and supernatant proteins were resolved on 9% SDS-PAGE.
Statistical Analysis
Results are presented as mean ± S.E. of at least three independent experiments. Data were analyzed by one-, two-, and three-way ANOVA and subsequently by post hoc test (PRISM software version 3.0, GraphPad, San Diego). Statistical significance was inferred at p < 0.05.
RESULTS
CDDP Induces p53 Mitochondrial and Nuclear Accumulation in Chemosensitive but Not Chemoresistant OVCA Cells, Regulation by PARC
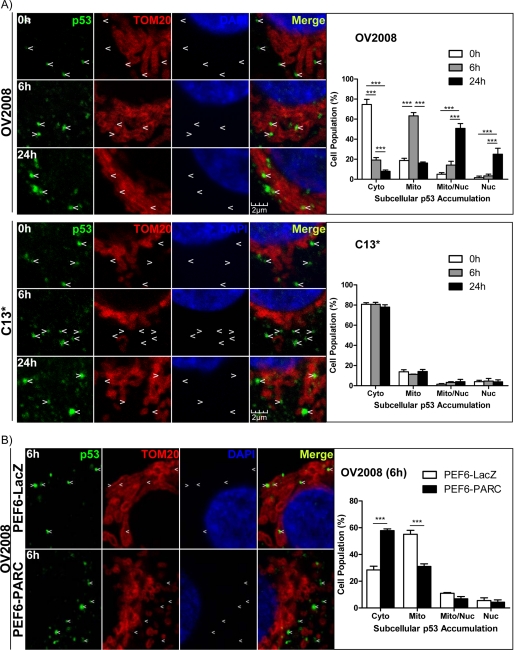
CDDP-induced apoptosis in OVCA cells is p53-dependent, and p53-loss-of-function at the nucleus and mitochondria is a determinant of chemoresistance (5, 9). To determine the influence of CDDP on p53 subcellular localization in chemosensitive (OV2008) and chemoresistant (C13*) OVCA cells, cells were treated with CDDP, and p53 subcellular localization was assessed by immunofluorescence/confocal microscopy (Fig. 1A). TOM20 and DAPI served as mitochondrial and nuclear markers, respectively. Differential subcellular p53 accumulation in a time-dependent manner was evident in the chemosensitive cells treated with CDDP (i.e. cytoplasmic → mitochondrial → nuclear). However, chemoresistant cells displayed only cytoplasmic p53 at all time points (Fig. 1A). One-way ANOVA indicates that there was a significant time effect in all subcellular fractions examined in OV2008 cells (p < 0.01) but not C13* cells. PARC overexpression in OV2008 cells attenuated CDDP-induced mitochondrial and nuclear p53 accumulation and increased cytoplasmic p53 retention at 6 h, suggesting that PARC is involved in the regulation of p53 subcellular localization (Fig. 1B; two-way ANOVA, PEF6-PARC (p < 0.001), fraction (p < 0.001), and interaction between the two factors (p < 0.001)).
FIGURE 1.
CDDP induces p53 mitochondrial and nuclear accumulation in chemosensitive but not chemoresistant OVCA cells, regulation by PARC. A, chemosensitive (OV2008) and resistant (C13*) OVCA cells were cultured with CDDP (10 μm; DMSO as vehicle control). Arrows indicate exact locations across fields and serve to highlight the presence or absence of p53 co-localization with TOM20 and DAPI, mitochondrial and nuclear markers, respectively, by immunofluorescence/confocal microscopy. Quantification of the confocal images shows the population of cells exhibiting p53 accumulation in the cytoplasm (Cyto), mitochondria (Mito), both mitochondria and nucleus (Nuc), and only the nucleus. At least 200 cells were counted for each time point in each replicate. B, PARC was overexpressed in OV2008 cells (PEF6-PARC; PEF6-LacZ as control; 0.5 μg, 24 h) and treated with CDDP (10 μm, 6 h). Subcellular p53 accumulation was assessed as above. Results are expressed as mean ± S.E. (n = 3 replicate experiments); ***, p < 0.001.
Inhibition of Akt Is Required to Sensitize Chemoresistant OVCA Cells to CDDP in a p53-dependent Manner, an Effect Enhanced by PARC Down-regulation, and PARC Overexpression Attenuates CDDP-induced Apoptosis in Chemosensitive OVCA Cells
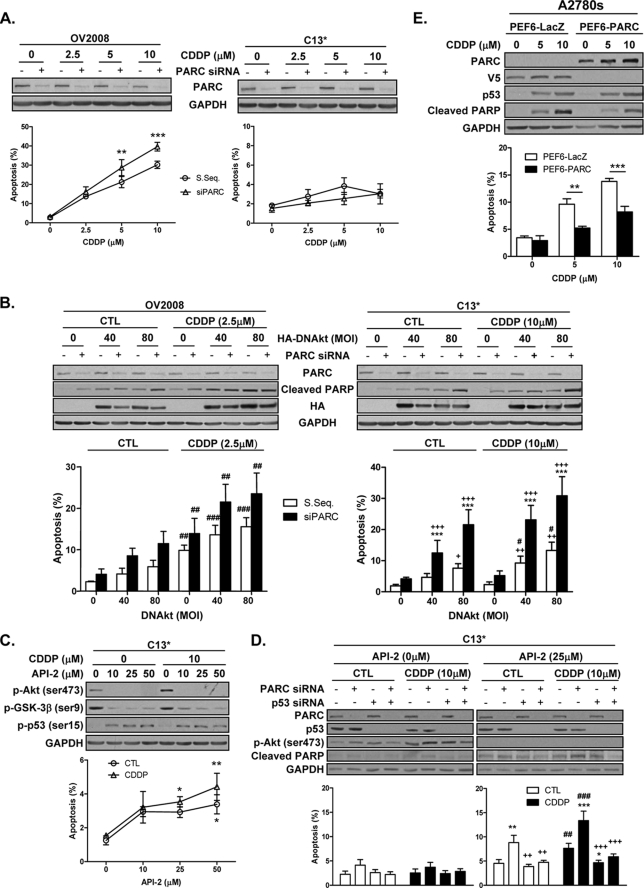
To assess the regulatory role of Akt in the anti-apoptotic role of PARC, we first determine the role of PARC in the regulation of CDDP sensitivity following PARC down-regulation in chemosensitive (OV2008) and chemoresistant (C13*) OVCA cells prior to CDDP treatment. As expected, CDDP alone induced apoptosis in OV2008 but not C13* cells. PARC down-regulation enhanced CDDP-induced apoptosis in the chemosensitive but not the resistant OVCA cells (Fig. 2A; two-way ANOVA, OV2008, PARC siRNA (p < 0.001), CDDP (p < 0.001), and PARC siRNA × CDDP (p < 0.01); C13*, PARC siRNA CDDP effect, and PARC siRNA × CDDP (p > 0.05 for all)).
FIGURE 2.
Inhibition of Akt is required to sensitize chemoresistant OVCA cells to CDDP in a p53-dependent manner, an effect enhanced by PARC down-regulation. PARC overexpression attenuates CDDP-induced apoptosis in chemosensitive OVCA cells. Chemosensitive (OV2008) and/or chemoresistant (C13*) OVCA cells were subjected to the following manipulations in the presence or absence of CDDP (10 μm; 24 h). A, PARC siRNA (scrambled sequence (S. Seq.) as control; 100 nm, 48 h; **, p < 0.01; ***, p < 0.001 (versus scrambled sequence)). B, DN-Akt (LacZ as control (CTL); 48 h) with and without PARC siRNA (scrambled sequence control, 100 nm, 48 h; ***, p < 0.001 (versus scrambled sequence); ++, p < 0.01; +++, p < 0.001 (versus respective DN-Akt (multiplicity of infection (MOI), 0)); #, p < 0.05; ##, p < 0.01; ###, p < 0.001 (versus respective CDDP CTL)). C, API-2 (1 h of pretreatment) (*, p < 0.05; **, p < 0.01 (versus no API-2)). D, PARC siRNA (scrambled sequence as control; 100 nm, 48 h) in combination with p53 siRNA (100 nm, 48 h) followed by API-2 (25 μm; 1 h pretreatment prior to CDDP) (**, p < 0.01; ***, p < 0.001 (versus no PARC siRNA and no p53 siRNA); ++, p < 0.01; +++, p < 0.001 (versus PARC siRNA); ##, p < 0.01; ###, p < 0.001 (versus respective CDDP control)). E, PARC was overexpressed (PEF6-PARC; PEF6-LacZ as control; 1 μg, 24 h) in PARC-null chemosensitive (A2780s) OVCA cells and treated with CDDP (10 μm; 24 h) (**, p < 0.01; ***, p < 0.001). Results are expressed as mean ± S.E. (n = 3–5 replicate experiments).
Because Akt attenuates CDDP-induced mitochondrial p53 accumulation and apoptosis and confers resistance in chemosensitive OVCA cells (5), we then examined if p53 function and CDDP sensitivity in OVCA cells is dependent on both PARC and Akt-mediated regulation. Akt down-regulation by DN-Akt expression had no effect on CDDP-induced apoptosis in OV2008 cells, irrespective of PARC silencing. In contrast, down-regulation of Akt activity significantly induced apoptosis in C13* cells, an effect enhanced by PARC down-regulation (Fig. 2B; three-way ANOVA-OV2008, PARC siRNA (p < 0.01), DN-Akt (p < 0.01), CDDP (p < 0.001), with no interaction between these factors; C13*, PARC siRNA (p < 0.001), DN-Akt (p < 0.001), CDDP (p < 0.01), with interaction between PARC siRNA and DN-Akt (p < 0.05)). To further investigate the role of Akt, a second inhibitor was used. Pretreatment of C13* cells with API-2, an Akt inhibitor (34), attenuated the activation and phosphorylation of Akt and its downstream target GSK-3β and induced p53 phosphorylation (Ser-15) and apoptosis irrespective of the presence of CDDP (Fig. 2C; two-way ANOVA-API-2 dose response, API-2 (p < 0.01)).
We then examined the role of PARC in Akt-mediated CDDP resistance in OVCA cells and examined whether the enhancement of CDDP sensitivity as a result of PARC down-regulation was p53-dependent. Down-regulation of Akt activity with API-2 facilitated CDDP-induced apoptosis in chemoresistant cells (C13*), a response further enhanced by PARC down-regulation (Fig. 2D). These responses were p53-dependent and were attenuated by p53 silencing (Fig. 2D; three-way ANOVA-API-2 (25 μm), CDDP (p < 0.01), PARC siRNA (p < 0.001), p53 siRNA (p < 0.001), PARC siRNA × p53 siRNA interaction (p < 0.05)). Taken together, these findings suggest that PARC down-regulation can enhance p53-dependent CDDP sensitivity once Akt activity is inhibited.
PARC overexpression in PARC-null A2780s cells (chemosensitive) decreased CDDP-induced apoptosis without affecting p53 content (Fig. 2E). Two-way ANOVA indicates a significant effect of PARC expression (p < 0.001) and CDDP (p < 0.001) and interaction between these two factors (p < 0.01). The fusion PARC protein produced from this expression vector interacted with p53 (data not shown).
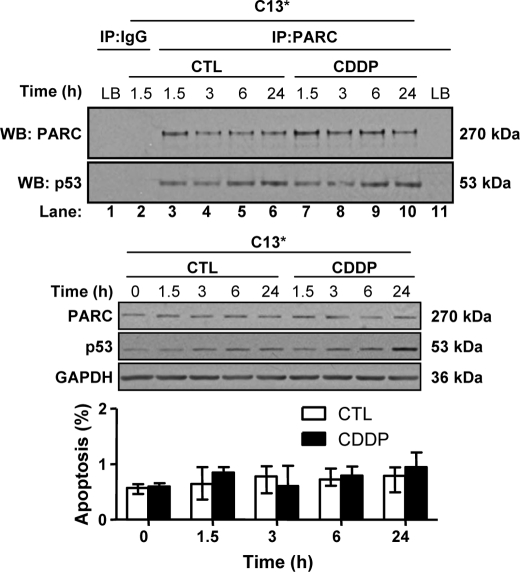
CDDP Has No Effect on PARC and p53 Interaction in Chemoresistant OVCA Cells
Basal PARC and p53 content are abundant and stably expressed in C13* cells and could be co-immunoprecipitated, a phenomenon unaffected by CDDP treatment. CDDP had no effect on PARC and p53 content and did not induce apoptosis, as expected (Fig. 3).
FIGURE 3.
CDDP has no effect on PARC and p53 interaction in chemoresistant OVCA cells. Protein A Dynabeads were incubated with PARC antibody (Bethyl, A300-096A; 2 μg/mg lysate; 1 h) and subsequently incubated for 1 h with C13* whole cell lysate (1,000 μg; lysis buffer control (LB)) obtained from CDDP treated (0–10 μm) cells for various durations. PARC pulldown and p53 content resulting from co-immunoprecipitation is unaffected by time or CDDP treatment (lanes 3–10). Also shown are controls (CTL) for potential nonspecific bands involving antibody IgG (lanes 1 and 2) and from the lysis buffer (lanes 1 and 11). To confirm the effectiveness of the CDDP treatment, PARC, p53 and GAPDH content and apoptosis counts of the above samples were examined. Results are expressed as mean ± S.E. (n = 3 replicate experiments). IP, immunoprecipitation; WB, Western blot.
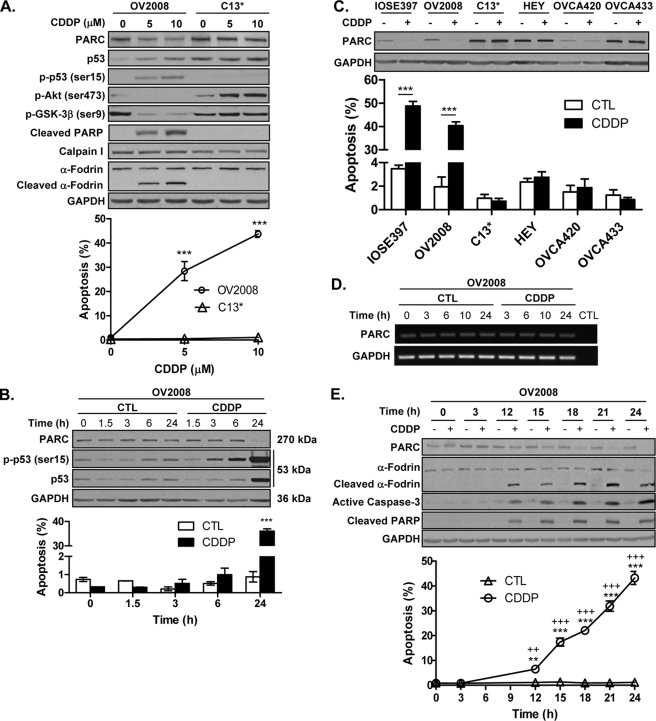
CDDP Effect on PARC, p53, p-p53 (Ser-15), and Akt in Chemosensitive and Resistant OVCA Cells
Chemoresistance in OVCA is associated with high Akt expression and activity and decreased p53 phosphorylation and function (5, 9). Because PARC regulates p53 localization and nuclear function (11, 35), we compared the influence of CDDP on PARC, p53, and Akt in chemosensitive and chemoresistant OVCA cells. CDDP decreased PARC content and Akt activity, increased caspase and calpain activity and p53 and p-p53 (Ser-15) content, and induced apoptosis in OV2008 but not C13* cells. Basal PARC and p53 content was higher in C13* cells compared with OV2008 cells (Fig. 4A). Two-way ANOVA indicates that there was a significant effect of CDDP treatment (p < 0.001) and cell line (p < 0.001) and interaction between both factors (p < 0.001). CDDP-induced phosphorylation of p53 (Ser-15) in OV2008 cells was an early event, occurring before an increase in total p53 content was detectable (Fig. 4B; two-way ANOVA, CDDP (p < 0.001), time (p < 0.001), and CDDP × time interaction (p < 0.001)). CDDP decreased PARC content and induced apoptosis in the chemosensitive (IOSE397 and OV2008) but not the chemoresistant (C13*, HEY, OV420, and OV433) cells, and basal PARC content was generally higher in the chemoresistant compared with the chemosensitive cells (Fig. 4C; two-way ANOVA, cell line (p < 0.001), CDDP (p < 0.001), and cell line × CDDP interaction (p < 0.001)). CDDP-induced PARC down-regulation in chemosensitive cells was not associated with changes in PARC mRNA content (Fig. 4D) but rather with caspase and calpain activation and apoptosis (Fig. 4E; two-way ANOVA, time (p < 0.001), CDDP (p < 0.001), and time × CDDP interaction (p < 0.001)).
FIGURE 4.
CDDP down-regulates PARC and Akt activity and increases p-p53 (Ser-15) content and apoptosis in chemosensitive but not chemoresistant OVCA cells. A, CDDP treatment (24 h) increased p53 content in both chemosensitive (OV2008) and resistant (C13*) cells, with increased p-p53 (Ser-15) content, caspase, and calpain activation (represented by PARP and α-fodrin cleavage, respectively) in the sensitive cells only. CDDP decreased PARC content and Akt activity (represented by p-Akt (Ser-473) and p-GSK-3β (Ser-9)) in the sensitive cells only. A dose-dependent increase in apoptosis was seen only with CDDP-treated OV2008 cells (***, p < 0.001 (versus no CDDP)). B, CDDP decreased PARC content and increased p-p53 (Ser-15) and total p53 content and apoptosis in a time-dependent manner in OV2008 cells (***, p < 0.001 (versus control (CTL))). C, CDDP induced PARC down-regulation and apoptosis in chemosensitive (IOSE397 and OV2008) but not chemoresistant (C13*, HEY, OVCA420, and OVCA433) OVCA cells. Cells were treated with CDDP (10 μm, 24 h; DMSO control), and PARC content and apoptosis were assessed. Basal PARC content was lower in the chemosensitive cells compared with the resistant cells, except in the OVCA420 cell, where both PARC and GAPDH (loading control) content were consistently low (***, p < 0.001). D, CDDP (10 μm; DMSO control) does not affect PARC mRNA content in chemosensitive (OV2008) OVCA cells. E, CDDP (10 μm) decreased PARC content and increased calpain and caspase-3 activity (represented by α-fodrin and PARP cleavage) and apoptosis in a time-dependent manner. (**, p < 0.01; ***, p < 0.001 (versus no CDDP); ++, p < 0.01; +++, p < 0.001 (versus time = 0)). Results are expressed as mean ± S.E. (n = 3–4 replicate experiments).
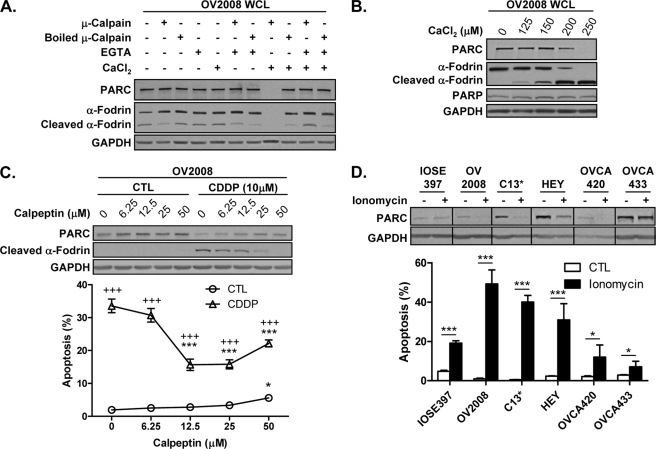
CDDP-induced, Calpain-mediated PARC Down-regulation
Based on reports that calpain is involved in CDDP-induced apoptosis (15, 36), we then examined whether PARC is a substrate for calpain. In vitro experiments confirm that PARC is a calpain substrate (Fig. 5A) and that calpain-mediated PARC down-regulation is Ca2+-dependent (Fig. 5B). The simultaneous incubation of OV2008 whole cell lysate with calpain and the calcium chelator EGTA prevented the degradation of PARC and α-fodrin, a known calpain substrate (37–39). Calpain inactivation (by boiling) also prevented the calpain-induced loss of PARC and α-fodrin (Fig. 5A). Furthermore, the concurrent degradation of both PARC and α-fodrin by calpain in a Ca2+-dependent manner was independent of caspase activity as PARP content was unchanged (Fig. 5B). These results suggest that calpain is the primary mediator of PARC processing.
FIGURE 5.
CDDP-induced PARC processing and chemosensitivity is mediated in part by the Ca2+-dependent activation of calpains. A, OV2008 whole cell lysates (WCL) (30 μg) were incubated with purified calpain I from human plasma (1 h; 30 °C). Calpain activity was inhibited by boiling, EGTA treatment, and absence of CaCl2 during the incubation process. PARC cleavage occurs when native calpain is activated by CaCl2 (8th lane). α-Fodrin cleavage is indicative of calpain activity. B, PARC processing as a result of calpain activity is Ca2+-dependent. OV2008 cell lysates were incubated as above with increasing CaCl2 concentrations. Calpain-mediated PARC processing was complete at 250 μm CaCl2. α-Fodrin cleavage occurred at lower CaCl2 concentrations and was used as an indicator of calpain activity. C, chemosensitive OVCA cells (OV2008) pretreated with the cell-permeable calpain inhibitor calpeptin (1 h; DMSO control) attenuated PARC down-regulation, α-fodrin cleavage, and apoptosis induced by CDDP (24 h) (*, p < 0.05; ***, p < 0.001 (versus no calpeptin); +++, p < 0.001 (versus no CDDP)). CTL, control. D, ionomycin induced PARC down-regulation in both chemosensitive (OV2008) and chemoresistant (C13*, HEY and OVCA420) OVCA cells, with increases in apoptosis in all cell types. Ionomycin did not induce PARC down-regulation in the chemosensitive IOSE397 and chemoresistant OVCA433 cells (*, p < 0.05; ***, p < 0.001). Results are expressed as mean ± S.E. (n = 3 replicate experiments).
To determine the effects of the calpain system on CDDP-induced PARC processing, OV2008 cells were pretreated with the cell-permeable calpain inhibitor calpeptin followed by CDDP. Pretreatment with calpeptin prevented CDDP-induced PARC down-regulation, α-fodrin cleavage, and apoptosis. Maximal effects were seen at 25 μm calpeptin, with cytotoxic effects becoming apparent at higher concentrations (Fig. 5C). Two-way ANOVA indicates that there was a significant effect of calpeptin (p < 0.001) and CDDP (p < 0.001) and the calpeptin × CDDP interaction (p < 0.001).
Calpain activation is Ca2+-dependent, and CDDP increases intracellular Ca2+ levels in chemosensitive OV2008 cells but not in its resistant variant (C13*) (40). Because calpain content is the same in both chemosensitive and resistant OVCA cells (Fig. 4A), we examined if ionomycin-induced Ca2+ influx in chemosensitive and resistant OVCA cells would result in PARC down-regulation and apoptosis. As demonstrated in Fig. 5D, ionomycin decreased PARC content in both chemosensitive (OV2008) and chemoresistant (C13*, HEY, and OVCA420) OVCA cells and induced apoptosis in all cell types examined. Two-way ANOVA indicates that there was a significant effect of ionomycin (p < 0.001) and cell line (p < 0.001) and interaction between both factors (p < 0.001). Ionomycin did not induce PARC down-regulation in the chemosensitive IOSE397 and chemoresistant OVCA433 OVCA cells, which may reflect alternative mechanisms of Ca2+ regulation in these cells. These data implicate Ca2+/calpain in CDDP-induced PARC processing and chemosensitivity.
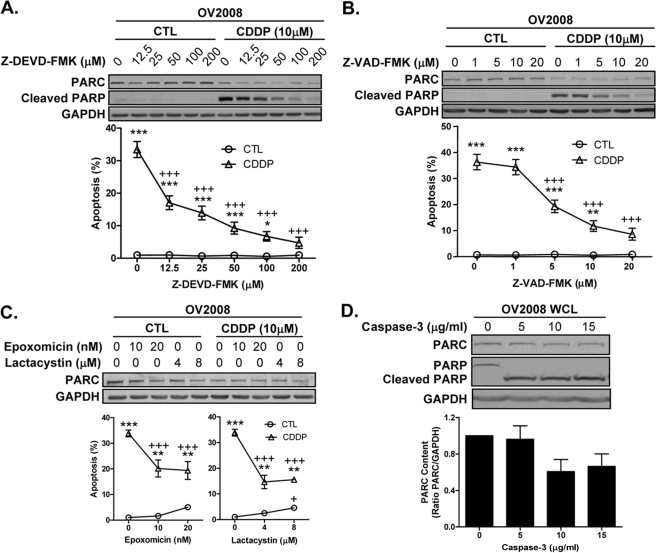
In addition, CDDP-induced PARC down-regulation was not prevented by the caspase-3-specific and the pan-caspase inhibitors Z-DEVD-FMK and Z-VAD-FMK, respectively (Fig. 6, A and B; two-way ANOVA, Z-DEVD-FMK (p < 0.001), Z-VAD-FMK (p < 0.001), CDDP (p < 0.001), and interaction between each caspase inhibitors with CDDP (p < 0.001)), nor by lactacystin and epoxomicin, two specific inhibitors of the 26 S proteasome (Fig. 6C; two-way ANOVA; epoxomicin (p < 0.01), CDDP (p < 0.01), interaction (p < 0.001); lactacystin (p < 0.001), CDDP (p < 0.001), interaction (p < 0.001)). Furthermore, in vitro experiments indicate that caspase-3 is not the primary mediator of PARC processing (Fig. 6D).
FIGURE 6.
Caspases and ubiquitin proteasome pathway are not the primary mediators of CDDP-induced PARC processing. CDDP-induced PARC down-regulation is caspase-independent. OV2008 cells were pretreated for 2 h with the caspase-3 specific inhibitor Z-DEVD-FMK (A) and the pan-caspase inhibitor Z-VAD-FMK (B), followed by CDDP (24 h; DMSO as control). Pretreatment with both drugs prevented CDDP-induced caspase activity (indicated by PARP cleavage) and apoptosis in a concentration-dependent manner (*, p < 0.05; **, p < 0.01; ***, p < 0.001 (versus no CDDP); +++, p < 0.001 (versus no caspases inhibitor); A and B), but failed to inhibit CDDP-induced PARC down-regulation compared with control (CTL). Results are expressed as mean ± S.E. (n = 6 replicate experiments). C, CDDP-induced PARC down-regulation is not mediated by the ubiquitin proteasome pathway. OV2008 cells pretreated with the proteasome inhibitors epoxomicin (30 min; DMSO as control) and lactacystin (30 min; DMSO as control) did not prevent CDDP-induced (24 h) PARC down-regulation but attenuated apoptosis (**, p < 0.01; ***, p < 0.001 (versus no CDDP); +++, p < 0.001 (versus no proteasome inhibitor)). D, purified active caspase-3 incubated with OV2008 whole cell lysate (WCL; 2 h, 30 °C) resulted in complete PARP cleavage at 5 μg/ml with no significant effect on PARC content even at the highest caspase-3 concentration tested (15 μg/ml); p > 0.05. Results are expressed as mean ± S.E. (n = 3 replicate experiments; C and D).
DISCUSSION
Confinement of p53 to the cytoplasm decreases responsiveness of cancer cells to genotoxic stress induced by radiotherapy and chemotherapy (9, 11, 41). Therefore, it is important to understand the molecular mechanisms by which p53 subcellular localization is regulated. In this study, we have shown for the first time that PARC regulates p53 subcellular localization and CDDP sensitivity, two responses dependent on Akt status of the cells (5, 9). We have also demonstrated for the first time the post-translational processing of PARC by the Ca2+-dependent cysteine protease calpain.
Translocation of p53 to the mitochondria is an early event in p53-dependent apoptosis (8, 9, 42) and is thought to initiate and amplify the slower transcription-based response. Due to the pleiotropic role of p53 in apoptosis, it is possible that cytoplasmic sequestration of p53 by PARC represents the most basic form of regulation. Indeed, PARC content is present in both chemosensitive and resistant OVCA cells, and with higher levels present in resistant ones. CDDP decreased PARC content in the sensitive but not resistant cells, suggesting that higher levels of PARC may be important in conferring CDDP resistance. This idea is further supported by the identification of a PARC-null, p53-WT, chemosensitive OVCA cell line (A2780s), whereas PARC is highly expressed in the chemoresistant A2780cp counterpart (data not shown). The PARC-null phenotype in the A2780s cells is dependent on DNA methylation, as 5-aza-2′-deoxycytidine induced PARC expression (data not shown). This represents, to our knowledge, the first demonstration of a physiological condition where PARC content is altered.
A major determinant of chemoresistance in OVCA cells is the activation of the PI3K/Akt pathway. Although no modified or mutated Akt genes have been reported in humans, Akt gene amplification is common in high grade aggressive ovarian tumors, corresponding with elevated protein content and high kinase activity in various OVCA cell lines. We have previously demonstrated that CDDP promotes Akt cleavage in a caspase-3-dependent fashion in chemosensitive but not resistant OVCA cells (1).
Akt activation attenuates CDDP-induced p53 phosphorylation, localization, and apoptosis (5, 9). Here, we demonstrated for the first time that CDDP decreased Akt activity which corresponded with an increase in p53 phosphorylation (Ser-15) and apoptosis in OV2008 cells but not in the resistant C13* counterparts. Inhibition of Akt activity with the Akt-specific inhibitor API-2 increased p-p53 (Ser-15) content and apoptosis in chemoresistant C13* cells. These results are consistent with reports implicating Akt activation as an important determinant in chemoresistance in OVCA (1, 9, 34, 43).
In chemoresistant OVCA cells, CDDP failed to induce p53-mediated apoptosis, and this is partially attributed to the inability of p53 to accumulate to the mitochondria and/or nucleus as a result of high Akt activity (5, 9). We demonstrate that PARC and p53 interact in OVCA cells and that this interaction is unaffected by CDDP in chemoresistant OVCA cells. The use of chemoresistant C13* cells for future studies involving PARC/p53 interaction is attractive as it would not require ectopic expression of mutated p53 to avoid WT-p53-dependent apoptosis, as C13* cells express high basal WT-p53 and PARC levels and are resistant to CDDP. In contrast, investigation of PARC/p53 interaction in chemosensitive OV2008 cells revealed various complications as basal p53 content is maintained at a low level, and CDDP is required to induce p53 up-regulation and would subsequently result in PARC down-regulation, p53 translocation, and apoptosis. The cellular mechanisms regulating PARC/p53 interaction is unclear and may involve modifications to PARC, p53, or both. Possible modifications would include site-specific phosphorylation and monoubiquitination as both have been reported to influence p53 translocation (9, 44). The role of Akt in these processes also requires further investigation.
PARC down-regulation enhanced CDDP-induced apoptosis in chemosensitive but not resistant OVCA cells. Interestingly, inhibition of Akt is required to sensitize chemoresistant cells to CDDP in a p53-dependent manner, an effect enhanced by PARC down-regulation. Although p53 is free to move to the various subcellular compartments, we propose that elevated Akt activity in the chemoresistant cells inhibits p53 phosphorylation and function (5). Phosphorylation of p53 on Ser-15 and/or Ser-20 is required for p53-induced apoptosis (45). CDDP-induced phosphorylation of Ser-15 in OVCA cells appears to be a critical residue for apoptosis as mutations at this site attenuate CDDP-induced apoptosis (5). The mechanisms by which Ser-15 phosphorylation affects the apoptotic capacity of p53 is unclear; however, evidence suggest it is not mediated by p53-dependent transactivation (5). The possibility that phosphorylation of p53 may affect PARC/p53 interaction requires further investigation.
It has recently been reported that PARC functions as a tumor suppressor and promotes p53-dependent apoptosis in mice (46). These results are contrary to our data and those of others (11, 13, 35). Pei et al. (46) examined the effect of PARC knock-out in γ-irradiated murine thymus and thymocytes, although PARC expression is very low in human thymus (11). Furthermore, although it is interesting that their PARC knock-out mice exhibited decreased survival times and developed spontaneous tumors, previous PARC-null mice demonstrated no physical deformities, growth defects, or health problems (47). Although the reasons for these discrepancies are not immediately apparent, whether it is related to the stress signal and/or species/cell type used remains to be determined.
We have shown that the CDDP-induced PARC down-regulation was not a result of decreased mRNA content. Although PARC contains three Asp-Xaa-Xaa-Asp (DXXD) motifs, indicative of potential caspase-3 cleavage sites, PARC processing was caspase-independent. Importantly, our results demonstrated that CDDP-induced PARC down-regulation is not merely a consequence of apoptosis, as caspase inhibition significantly attenuated cell death but was unable to reverse PARC down-regulation. In addition, PARC has been shown to self-ubiquitylate in vitro, and highly ubiquitylated species of PARC have been detected in human cells with overexpression of PARC and ubiquitin (11, 12). In this study we show that CDDP-induced PARC down-regulation is not mediated by the ubiquitin proteasome pathway. Substrates modified by Lys-48-linked polyubiquitin chains are best characterized as being targeted to the proteasome (48), whereas monoubiquitylation or chains conjugated to other lysine residues, such as Lys-63 and Lys-29, provide nonproteolytic signals (48–50). It is possible that the previously reported ubiquitylated PARC species were not of the Lys-48-type but were modifications controlling PARC function or localization. Those studies did not examine the ubiquitin chain configurations modifying PARC, as well as the corresponding functional outcomes. PARC regulation by post-translational modification has been proposed and may involve the covalent modification by NEDD8 (12, 49) (related to ubiquitin; also known as Rub1), which is a regulatory modification controlling ligase activity (51). Interestingly, although PARC fails to ubiquitylate p53, p53 activity can be inhibited by neddylation (52). If and how PARC is modified by ubiquitylation or neddylation in OVCA and its significance in CDDP sensitivity remain to be determined.
In this study, we provided evidence that implicate calpain as the primary mediator of CDDP-induced PARC down-regulation. This represents, to our knowledge, the first demonstration of a signaling pathway that regulates PARC processing and function. The role of calpains in apoptosis is reflected in a growing list of substrates, including p53, Bcl-2, Bax, apoptosis-inducing factor, caspases, and cytoskeletal proteins (14, 36, 53–55). Calpain activity is Ca2+-dependent, and calpain activation in response to Ca2+ signaling is an early event in CDDP-induced apoptosis (56), mediating Bid cleavage (15) and preceding apoptosis-inducing factor release and caspase-9/-3 activation (36). Furthermore, a decrease in Ca2+ signaling could prevent PARC processing and promote chemoresistance. This agrees with recent reports that CDDP induces an increase in intracellular calcium concentration in chemosensitive but not resistant OVCA cells (40).
In addition to regulating calpain activation, Ca2+ is shown to inhibit Akt activity (57) and therefore presents itself as an important regulator of apoptosis and CDDP sensitivity. Due to the fact that calpain is expressed at the same levels in both chemosensitive and resistant cells, our results suggest that this difference to CDDP sensitivity involves the Ca2+ response (40). By inducing a Ca2+ influx in chemosensitive and chemoresistant OVCA cells with ionomycin, PARC cleavage and apoptosis were induced in both cell types. Ionomycin treatment did not induce PARC processing in the IOSE397-sensitive immortalized ovarian surface epithelial cells nor in the chemoresistant OVCA433 cells, possibly because of different Ca2+ regulatory mechanisms (56, 58).
In summary, we have demonstrated that CDDP-induced PARC processing and apoptosis is regulated differently in chemosensitive and chemoresistant human OVCA cells. Processing of PARC is mediated by the Ca2+-dependent cysteine protease calpain, although CDDP-induced apoptosis may involve the interaction of different proteolytic pathways. Determining the molecular mechanisms controlling PARC processing may contribute to the current understanding of p53 function and apoptosis and ultimately of the underlying mechanisms of chemoresistance in human ovarian cancer.
Acknowledgments
We thank Dr. Kenneth Walsh (Whitaker Cardiovascular Institute, Boston University School of Medicine, Boston) for the generous supply of DN-Akt and David G. Dagenais for technical advice on the presentation of the confocal microscopy data.
This work was supported in part by Canadian Institute of Health Research Grant MOP-15691 and World Class University Program, National Research Foundation of Korea, supported by Ministry of Education, Science and Technology Grant R31-10056.
- OVCA
- ovarian cancer
- CDDP
- cisplatin
- PARC
- p53-associated, Parkin-like cytoplasmic protein
- PARP
- poly(ADP-ribose) polymerase
- DN-Akt
- dominant-negative Akt
- UPP
- ubiquitin proteasome pathway
- ANOVA
- analysis of variance
- Z
- benzyloxycarbonyl
- FMK
- fluoromethyl ketone.
REFERENCES
- 1. Asselin E., Mills G. B., Tsang B. K. (2001) XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 61, 1862–1868 [PubMed] [Google Scholar]
- 2. Dan H. C., Sun M., Kaneko S., Feldman R. I., Nicosia S. V., Wang H. G., Tsang B. K., Cheng J. Q. (2004) Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J. Biol. Chem. 279, 5405–5412 [DOI] [PubMed] [Google Scholar]
- 3. Yuan Z. Q., Feldman R. I., Sussman G. E., Coppola D., Nicosia S. V., Cheng J. Q. (2003) AKT2 inhibition of cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK1. Implication of AKT2 in chemoresistance. J. Biol. Chem. 278, 23432–23440 [DOI] [PubMed] [Google Scholar]
- 4. Hu L., Hofmann J., Lu Y., Mills G. B., Jaffe R. B. (2002) Inhibition of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 62, 1087–1092 [PubMed] [Google Scholar]
- 5. Fraser M., Bai T., Tsang B. K. (2008) Akt promotes cisplatin resistance in human ovarian cancer cells through inhibition of p53 phosphorylation and nuclear function. Int. J. Cancer 122, 534–546 [DOI] [PubMed] [Google Scholar]
- 6. Miyashita T., Reed J. C. (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293–299 [DOI] [PubMed] [Google Scholar]
- 7. Mihara M., Erster S., Zaika A., Petrenko O., Chittenden T., Pancoska P., Moll U. M. (2003) p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11, 577–590 [DOI] [PubMed] [Google Scholar]
- 8. Marchenko N. D., Zaika A., Moll U. M. (2000) Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J. Biol. Chem. 275, 16202–16212 [DOI] [PubMed] [Google Scholar]
- 9. Yang X., Fraser M., Moll U. M., Basak A., Tsang B. K. (2006) Akt-mediated cisplatin resistance in ovarian cancer. Modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 66, 3126–3136 [DOI] [PubMed] [Google Scholar]
- 10. Kmet L. M., Cook L. S., Magliocco A. M. (2003) A review of p53 expression and mutation in human benign, low malignant potential, and invasive epithelial ovarian tumors. Cancer 97, 389–404 [DOI] [PubMed] [Google Scholar]
- 11. Nikolaev A. Y., Li M., Puskas N., Qin J., Gu W. (2003) Parc. A cytoplasmic anchor for p53. Cell 112, 29–40 [DOI] [PubMed] [Google Scholar]
- 12. Skaar J. R., Florens L., Tsutsumi T., Arai T., Tron A., Swanson S. K., Washburn M. P., DeCaprio J. A. (2007) PARC and CUL7 form atypical cullin RING ligase complexes. Cancer Res. 67, 2006–2014 [DOI] [PubMed] [Google Scholar]
- 13. Mulhall J. P., Barnas J., Kobylarz K., Mueller A. (2010) p53-associated Parkin-like cytoplasmic protein (Parc) short interfering RNA (siRNA) alters p53 location and biology of Peyronie disease fibroblasts. BJU Int. 106, 1706–1713 [DOI] [PubMed] [Google Scholar]
- 14. Kubbutat M. H., Vousden K. H. (1997) Proteolytic cleavage of human p53 by calpain. A potential regulator of protein stability. Mol. Cell. Biol. 17, 460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu L., Xing D., Chen W. R., Chen T., Pei Y., Gao X. (2008) Calpain-mediated pathway dominates cisplatin-induced apoptosis in human lung adenocarcinoma cells as determined by real time single cell analysis. Int. J. Cancer 122, 2210–2222 [DOI] [PubMed] [Google Scholar]
- 16. Schafer D. P., Jha S., Liu F., Akella T., McCullough L. D., Rasband M. N. (2009) Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 29, 13242–13254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DiSaia P. J., Sinkovics J. G., Rutledge F. N., Smith J. P. (1972) Cell-mediated immunity to human malignant cells. A brief review and further studies with two gynecologic tumors. Am. J. Obstet. Gynecol. 114, 979–989 [DOI] [PubMed] [Google Scholar]
- 18. Molthoff C. F., Calame J. J., Pinedo H. M., Boven E. (1991) Human ovarian cancer xenografts in nude mice. Characterization and analysis of antigen expression. Int. J. Cancer 47, 72–79 [DOI] [PubMed] [Google Scholar]
- 19. Shaw T. J., Senterman M. K., Dawson K., Crane C. A., Vanderhyden B. C. (2004) Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol. Ther. 10, 1032–1042 [DOI] [PubMed] [Google Scholar]
- 20. Andrews P. A., Albright K. D. (1992) Mitochondrial defects in cis-diamminedichloroplatinum(II)-resistant human ovarian carcinoma cells. Cancer Res. 52, 1895–1901 [PubMed] [Google Scholar]
- 21. Havrilesky L. J., Elbendary A., Hurteau J. A., Whitaker R. S., Rodriguez G. C., Berchuck A. (1995) Chemotherapy-induced apoptosis in epithelial ovarian cancers. Obstet. Gynecol. 85, 1007–1010 [DOI] [PubMed] [Google Scholar]
- 22. Bast R. C., Jr., Zbar B., Borsos T., Rapp H. J. (1974) BCG and cancer (first of two parts). N. Engl. J. Med. 290, 1413–1420 [DOI] [PubMed] [Google Scholar]
- 23. Bast R. C., Jr., Zbar B., Borsos T., Rapp H. J. (1974) BCG and cancer. N. Engl. J. Med. 290, 1458–1469 [DOI] [PubMed] [Google Scholar]
- 24. Buick R. N., Pullano R., Trent J. M. (1985) Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 45, 3668–3676 [PubMed] [Google Scholar]
- 25. Xu G., Zhou H., Wang Q., Auersperg N., Peng C. (2006) Activin receptor-like kinase 7 induces apoptosis through up-regulation of Bax and down-regulation of Xiap in normal and malignant ovarian epithelial cell lines. Mol. Cancer Res. 4, 235–246 [DOI] [PubMed] [Google Scholar]
- 26. Wolf J. K., Mills G. B., Bazzet L., Bast R. C., Jr., Roth J. A., Gershenson D. M. (1999) Adenovirus-mediated p53 growth inhibition of ovarian cancer cells is independent of endogenous p53 status. Gynecol. Oncol. 75, 261–266 [DOI] [PubMed] [Google Scholar]
- 27. Yan X., Fraser M., Qiu Q., Tsang B. K. (2006) Overexpression of PTEN sensitizes human ovarian cancer cells to cisplatin-induced apoptosis in a p53-dependent manner. Gynecol. Oncol. 102, 348–355 [DOI] [PubMed] [Google Scholar]
- 28. Astanehe A., Arenillas D., Wasserman W. W., Leung P. C., Dunn S. E., Davies B. R., Mills G. B., Auersperg N. (2008) Mechanisms underlying p53 regulation of PIK3CA transcription in ovarian surface epithelium and in ovarian cancer. J. Cell Sci. 121, 664–674 [DOI] [PubMed] [Google Scholar]
- 29. Fraser M., Leung B. M., Yan X., Dan H. C., Cheng J. Q., Tsang B. K. (2003) p53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Res. 63, 7081–7088 [PubMed] [Google Scholar]
- 30. Sasaki H., Sheng Y., Kotsuji F., Tsang B. K. (2000) Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 60, 5659–5666 [PubMed] [Google Scholar]
- 31. Grusch M., Polgar D., Gfatter S., Leuhuber K., Huettenbrenner S., Leisser C., Fuhrmann G., Kassie F., Steinkellner H., Smid K., Peters G. J., Jayaram H. N., Klepal W., Szekeres T., Knasmüller S., Krupitza G. (2002) Maintenance of ATP favors apoptosis over necrosis triggered by benzamide riboside. Cell Death Differ. 9, 169–178 [DOI] [PubMed] [Google Scholar]
- 32. Mullen P. (2004) PARP cleavage as a means of assessing apoptosis. Methods Mol. Med. 88, 171–181 [DOI] [PubMed] [Google Scholar]
- 33. Jang J. S., Lee S. J., Choi Y. H., Nguyen P. M., Lee J., Hwang S. G., Wu M. L., Takano E., Maki M., Henkart P. A., Trepel J. B. (1999) Post-translational regulation of the retinoblastoma gene family member p107 by calpain protease. Oncogene 18, 1789–1796 [DOI] [PubMed] [Google Scholar]
- 34. Yang L., Dan H. C., Sun M., Liu Q., Sun X. M., Feldman R. I., Hamilton A. D., Polokoff M., Nicosia S. V., Herlyn M., Sebti S. M., Cheng J. Q. (2004) Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 64, 4394–4399 [DOI] [PubMed] [Google Scholar]
- 35. Vitali R., Cesi V., Tanno B., Ferrari-Amorotti G., Dominici C., Calabretta B., Raschellà G. (2008) Activation of p53-dependent responses in tumor cells treated with a PARC-interacting peptide. Biochem. Biophys. Res. Commun. 368, 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu L., Xing D., Chen W. R. (2009) Micro-calpain regulates caspase-dependent and apoptosis-inducing factor-mediated caspase-independent apoptotic pathways in cisplatin-induced apoptosis. Int. J. Cancer 125, 2757–2766 [DOI] [PubMed] [Google Scholar]
- 37. Siman R., Baudry M., Lynch G. (1984) Brain fodrin. Substrate for calpain I, an endogenous calcium-activated protease. Proc. Natl. Acad. Sci. U.S.A. 81, 3572–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takamure M., Murata K. Y., Tamada Y., Azuma M., Ueno S. (2005) Calpain-dependent α-fodrin cleavage at the sarcolemma in muscle diseases. Muscle Nerve 32, 303–309 [DOI] [PubMed] [Google Scholar]
- 39. Sato K., Hattori S., Irie S., Sorimachi H., Inomata M., Kawashima S. (2004) Degradation of fodrin by m-calpain in fibroblasts adhering to fibrillar collagen I gel. J. Biochem. 136, 777–785 [DOI] [PubMed] [Google Scholar]
- 40. Al-Bahlani S., Fraser M., Wong A. Y., Sayan B. S., Bergeron R., Melino G., Tsang B. K. (2011) P73 regulates cisplatin-induced apoptosis in ovarian cancer cells via a calcium/calpain-dependent mechanism. Oncogene 30, 4219–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moll U. M., LaQuaglia M., Bénard J., Riou G. (1995) Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors. Proc. Natl. Acad. Sci. U.S.A. 92, 4407–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Erster S., Mihara M., Kim R. H., Petrenko O., Moll U. M. (2004) In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol. Cell. Biol. 24, 6728–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shayesteh L., Lu Y., Kuo W. L., Baldocchi R., Godfrey T., Collins C., Pinkel D., Powell B., Mills G. B., Gray J. W. (1999) PIK3CA is implicated as an oncogene in ovarian cancer. Nat. Genet. 21, 99–102 [DOI] [PubMed] [Google Scholar]
- 44. Marchenko N. D., Wolff S., Erster S., Becker K., Moll U. M. (2007) Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J. 26, 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Unger T., Sionov R. V., Moallem E., Yee C. L., Howley P. M., Oren M., Haupt Y. (1999) Mutations in serines 15 and 20 of human p53 impair its apoptotic activity. Oncogene 18, 3205–3212 [DOI] [PubMed] [Google Scholar]
- 46. Pei X. H., Bai F., Li Z., Smith M. D., Whitewolf G., Jin R., Xiong Y. (2011) Cytoplasmic CUL9/PARC ubiquitin ligase is a tumor suppressor and promotes p53-dependent apoptosis. Cancer Res. 71, 2969–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Skaar J. R., Arai T., DeCaprio J. A. (2005) Dimerization of CUL7 and PARC is not required for all CUL7 functions and mouse development. Mol. Cell. Biol. 25, 5579–5589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boname J. M., Thomas M., Stagg H. R., Xu P., Peng J., Lehner P. J. (2010) Efficient internalization of MHC I requires lysine 11 and lysine 63 mixed linkage polyubiquitin chains. Traffic 11, 210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hicke L. (2001) Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2, 195–201 [DOI] [PubMed] [Google Scholar]
- 50. Hoeller D., Hecker C. M., Dikic I. (2006) Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat. Rev. Cancer 6, 776–788 [DOI] [PubMed] [Google Scholar]
- 51. Hochstrasser M. (2000) Evolution and function of ubiquitin-like protein-conjugation systems. Nat. Cell Biol. 2, E153–E157 [DOI] [PubMed] [Google Scholar]
- 52. Xirodimas D. P., Saville M. K., Bourdon J. C., Hay R. T., Lane D. P. (2004) Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell 118, 83–97 [DOI] [PubMed] [Google Scholar]
- 53. Mandic A., Viktorsson K., Strandberg L., Heiden T., Hansson J., Linder S., Shoshan M. C. (2002) Calpain-mediated Bid cleavage and calpain-independent Bak modulation. Two separate pathways in cisplatin-induced apoptosis. Mol. Cell. Biol. 22, 3003–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wood D. E., Thomas A., Devi L. A., Berman Y., Beavis R. C., Reed J. C., Newcomb E. W. (1998) Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17, 1069–1078 [DOI] [PubMed] [Google Scholar]
- 55. Tan Y., Dourdin N., Wu C., De Veyra T., Elce J. S., Greer P. A. (2006) Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 281, 16016–16024 [DOI] [PubMed] [Google Scholar]
- 56. Splettstoesser F., Florea A. M., Büsselberg D. (2007) IP(3) receptor antagonist, 2-APB, attenuates cisplatin-induced Ca2+ influx in HeLa-S3 cells and prevents activation of calpain and induction of apoptosis. Br. J. Pharmacol. 151, 1176–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith I. J., Dodd S. L. (2007) Calpain activation causes a proteasome-dependent increase in protein degradation and inhibits the Akt signaling pathway in rat diaphragm muscle. Exp. Physiol. 92, 561–573 [DOI] [PubMed] [Google Scholar]
- 58. Aagaard-Tillery K. M., Jelinek D. F. (1995) Differential activation of a calcium-dependent endonuclease in human B lymphocytes. Role in ionomycin-induced apoptosis. J. Immunol. 155, 3297–3307 [PubMed] [Google Scholar]