Background: Toll-like Receptor 4 activation multiple signaling cascades with unique requirements.
Results: PLCγ2-IP3-Ca2+ mediate Toll-like Receptor 4 translocation to the endosome.
Conclusion: PLCγ2-IP3-Ca2+ are selectively required for Toll-like Receptor 4 signaling.
Significance: Novel role of the PLCγ2-IP3-Ca2+ cascade in LPS signaling.
Keywords: Calcium Signaling; Inositol 1,4,5-trisphosphate; Lipopolysaccharide (LPS); Phospholipase C; Toll-like Receptors (TLR)
Abstract
Toll-like receptor 4 (TLR4) is unique among the TLRs in its use of multiple adaptor proteins leading to activation of both the interferon regulatory factor 3 (IRF3) and nuclear factor κB (NF-κB) pathways. Previous work has demonstrated that TLR4 initiates NF-κB activation from the plasma membrane, but that subsequent TLR4 translocation to the endosomes is required for IRF3 activation. Here we have characterized several components of the signaling pathway that governs TLR4 translocation and subsequent IRF3 activation. We find that phospholipase C γ2 (PLCγ2) accounts for LPS-induced inositol 1,4,5-trisphosphate (IP3) production and subsequent calcium (Ca2+) release. Blockage of PLCγ2 function by inhibitors or knockdown of PLCγ2 expression by siRNAs in RAW 264.7 macrophages lead to reduced IRF3, but enhanced NF-κB activation. In addition, bone marrow-derived macrophages from PLCγ2-deficient mice showed impaired IRF3 phosphorylation and expression of IRF3-regulated genes after LPS stimulation. Using cell fractionation, we show that PLCγ2-IP3-Ca2+ signaling cascade is required for TLR4 endocytosis following LPS stimulation. In conclusion, our results describe a novel role of the PLCγ2-IP3-Ca2+ cascade in the LPS-induced innate immune response pathway where release of intracellular Ca2+ mediates TLR4 trafficking and subsequent activation of IRF3.
Introduction
Mammalian Toll-like receptors (TLRs)3 are germ line-encoded pattern-recognizing receptors (PPRs) that recognize variety of conserved microbial components known as pathogen-associated molecular patterns (PAMPs). PAMPs are essential for the survival of the microorganism and are therefore difficult for the microorganism to alter (1). Different TLRs react with specific PAMPs, show distinct expression patterns, and activate specific signaling pathways, which leads to initiation of anti-microbial responses (2).
Toll-like receptor 4 (TLR4) recognizes lipopolysaccharide (LPS), which is the main cell wall component of Gram-negative bacteria (3, 4). TLR4 is unique among TLRs in utilizing both myeloid differentiation primary response gene 88 (MyD88) and TIR domain-containing adaptor protein-inducing interferon-β (TRIF) adaptor proteins. The MyD88-dependent pathway leads to activation of nuclear factor κB (NF-κB) while TRIF pathway mediates activation of interferon regulatory factor 3 (IRF3) (5–7). Recent reports have shown that TLR4 localization has a key role in determining, which of these signal transduction pathways are activated. TLR4 engagement on the plasma membrane leads to MyD88-dependent NF-κB activation, which is followed by translocation of TLR4 into endosomes where TRIF-dependent IRF3 activation takes place (8). Although TLR4 endocytosis is dependent on Dynamin and Rab11a GTPase (8–10) the exact molecular mechanisms controlling TLR4 internalization have not been fully elucidated.
Calcium (Ca2+) signals are required to initiate several types of transcriptional events and growth responses such as proliferation and apoptosis (11). In most eukaryotic cells, Ca2+ signals are triggered by the secondary messenger inositol 1,4,5-trisphosphate (IP3), the cleavage product of phosphatidylinositol 4,5-bisphosphate (PIP2) by the enzymatic activity of phospholipase C (PLC). Binding of IP3 to IP3 receptors (IP3Rs) in the endoplasmic reticulum results in the release of Ca2+ from internal stores (12). We have previously shown that LPS-induced IRF3 activation and subsequent expression of interferon-stimulated genes (ISGs) requires the generation of reactive oxygen species (ROS) by the NADPH-dependent oxidase NOX4 (13). As the production of ROS occurs frequently concomitant with an increase in cytosolic Ca2+ (14, 15), and several TLR ligands including LPS have been shown to induce transient Ca2+ flux in myeloid cells (16–19), we decided to elucidate the role of intracellular Ca2+ in LPS-induced activation of inflammatory response.
Here we report that release of Ca2+ from intracellular stores is required for efficient IRF3 activation in LPS-stimulated macrophages. In contrast, LPS-induced activation of NF-κB pathway did not require Ca2+. Mechanistically, PLCγ2 activity and subsequent release of intracellular Ca2+ was required for translocation of TLR4 from plasma membrane to endosomes, where TRIF-dependent IRF3 activation takes place. Our results describe novel regulatory role for Ca2+ in the activation of immune signaling.
RESULTS AND DISCUSSION
Intracellular Calcium Regulates LPS-induced ISG Expression
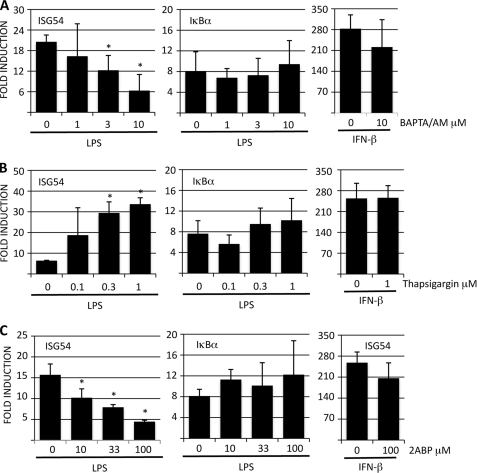
To address the role of Ca2+ in LPS-induced signaling, RAW 264.7 cells were pretreated with cell permeable intracellular Ca2+ chelator BAPTA/AM and then stimulated with LPS. Expression level of IRF3-regulated ISG54 and NF-κB-dependent IκBα genes was determined by RT-QPCR. BAPTA/AM treatment reduced LPS-induced ISG54 expression dose-dependently and ∼70% reduction in ISG54 levels was observed with the highest inhibitor dose (Fig. 1A). A similar response profile was observed when additional IRF3-induced genes such as IFIT1 and IFIT3 were analyzed (supplemental Fig. S1). In contrast to these ISGs, LPS-induced expression of IκBα was not affected by BAPTA/AM treatment suggesting that intracellular Ca2+ flux is required for IRF3 but not NF-κB activation. To exclude the possibility that BAPTA/AM was regulating ISG54 expression in a more general way, RAW 264.7 cells were pretreated BAPTA/AM and then stimulated with IFN-β, which activates ISGs independently of IRF3 via Jak/Stat pathway. IFN-β-induced ISG expression was not significantly altered in BAPTA/AM-treated cells compared with those treated with DMSO vehicle (Fig. 1A, and supplemental Fig. S1A) demonstrating that inhibition of intracellular Ca2+ flux only affects LPS-induced IRF3 activation and ISG54 expression. Elevation of intracellular Ca2+ levels can also originate from extracellular sources. However, chelating extracellular Ca2+ with EGTA prior to LPS stimulation did not have an effect on LPS-induced expression of ISG54 (data not shown).
FIGURE 1.
Calcium signaling modulates LPS-induced expression of IRF3-regulated genes. RAW264.7 cells were pretreated with A, BAPTA/AM; B, thapsigargin; or C, 2-ABP for 30 min and then stimulated with LPS (100 ng/ml) or IFN-β (100 IU/ml) for 4 h. ISG54 and IκBα expressions were analyzed by RT-QPCR and normalized to GAPDH. Results are presented as fold induction compared with unstimulated controls and are the means of three independent experiments. Error bars indicate S.D. and statistical significance was determined by paired Student's t test.
Because depletion of intracellular Ca2+ reduced LPS-induced ISG54 expression, we hypothesized that increasing intracellular Ca2+ levels might potentiate LPS-induced responses. To test this, RAW 264.7 cells were pretreated with thapsigargin (Tg), which has been shown to trigger Ca2+ release from internal stores in macrophages (17). Thapsigargin-treated cells showed ∼4-fold higher ISG54 expression after LPS stimulation compared with DMSO-treated cells (Fig. 1B). Thapsigargin alone did not, however, induce ISG54 expression in RAW 264.7 cells (data not shown). In line with the BAPTA/AM data, thapsigargin treatment did not have an effect on LPS-induced IκBα expression or on IFN-β-induced ISG54 or IFIT1/3 expression (Fig. 1B and supplemental Fig. S1B). Similar results were also observed when RAW 264.7 cells were pretreated with another Ca2+ ionophore A23187 (data not shown).
The endoplasmic reticulum (ER) and its muscle equivalent, sarcoplasmic reticulum (SR), are the major sites for intracellular Ca2+ storage. Release of Ca2+ into cytoplasm from intracellular stores is mediated via IP3 receptors and ryanodine receptors (RyRs) on the ER and SR, respectively. Because IP3Rs are virtually universal, while RyRs are most evident in excitable cells such as skeletal and cardiac muscles, we hypothesized that IP3R might be the most likely pathway modulating LPS-induced increase in intracellular Ca2+. To address this, RAW 264.7 cells were pretreated with IP3R antagonist 2-ABP followed by LPS stimulation. LPS-induced ISG54 expression was reduced by ∼70% in RAW cells treated with highest dose of 2-ABP when compared with cells pretreated with DMSO vehicle (Fig. 1C). Moreover, the effect of 2-ABP was IRF3-specific, since LPS-induced IκBα expression or IFN-β induced ISG54 expression was not markedly altered in 2-ABP-treated cells (Fig. 1C). RyR antagonist Dantrolene did not have an effect on LPS-induced ISG54 expression (data not shown), which suggests that in macrophages IP3Rs mediate the release of intracellular Ca2+ following LPS stimulation.
Collectively these data demonstrate that LPS-induced ISG54 expression requires the release of intracellular Ca2+ from ER, and that the release of Ca2+ is mediated via IP3 receptors. Moreover, the data suggests that intracellular Ca2+ is specifically required for efficient LPS-induced expression of IRF3- but not NF-κB-regulated genes, and that this effect is specific, since modulating intracellular Ca2+ levels did not have an effect on IFN-β-induced ISG54 expression. In addition, intracellular Ca2+ most likely works upstream of IRF3, because 2-ABP did not have an effect on ISG54 expression induced by constitutively active IRF3–5D expression construct (data not shown).
Phospholipase Cγ Mediates LPS-induced ISG Expression
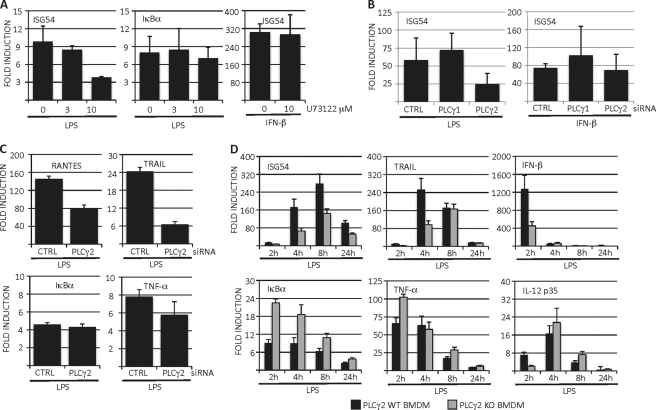
Phospholipase C activity is the major cellular biochemical pathway generating IP3. These enzymes cleave PIP2 into IP3 and diacyl glycerol. Of the thirteen mammalian PLC isoforms described, PLCγ1 and PLCγ2 are structurally unique in a way that they can be activated via both receptor and non-receptor tyrosine kinases. Moreover, both PLCγ1 and PLCγ2 have been shown to be phosphorylated following LPS stimulation in myeloid cells (18, 20). Although a previous report showed that PLCγ2 is required for LPS-induced Ca2+ flux in macrophages (18) the contribution of Ca2+ in LPS-induced intracellular signaling and gene expression was not characterized in detail. With this in mind, we evaluated the possible role of PLCγ in LPS-induced ISG activation. When RAW 264.7 cells were pretreated with PLCγ inhibitor U73122 we observed a clear reduction in LPS-induced ISG54 expression, while the IκBα expression remained intact (Fig. 2A). Moreover, U73122 treatment did not have any effect on IFN-β-induced ISG54 expression. To further characterize which PLCγ isoform mediates LPS-induced ISG54 expression RAW 264.7 cells were transfected with siRNAs targeting PLCγ1 or PLCγ2. Quantitation of Western blots revealed that the protein expression of PLCγ1 and PLCγ2 after siRNA transfection was reduced by 90 and 60%, respectively (Fig. 2B). Interestingly, we observed that knockdown of only PLCγ2, but not PLCγ1, reduced LPS-induced ISG54 expression (Fig. 2B). In line with the previous data, knockdown of PLCγ1 or PLCγ2 did not have an effect on IFN-β induced ISG54 expression (Fig. 2B). To confirm that our observations were not limited to ISG54 but reflect a general requirement for PLCγ2 in LPS-induced expression of IRF3-dependent genes, we analyzed the effect of PLCγ2 knockdown on expression of RANTES/CCL5 and TRAIL. Similar to ISG54, knockdown of PLCγ2 reduced LPS-induced expression of these IRF3-dependent genes, while expression of NF-κB-dependent IκBα and TNF-α was unaffected by PLCγ2 knockdown (Fig. 2C). We also generated bone marrow-derived macrophages (BMDM) from wild-type and PLCγ2 knock-out mice. When these cells were stimulated with LPS, we observed that the expression of ISG54, TRAIL and IFN-β was markedly delayed and reduced in PLCγ2 knock-out macrophages compared with wild type macrophages (Fig. 2D). Importantly, the expression of NF-κB-regulated IκBα, TNF-α and IL-12 p35 genes was similar or even slightly higher in PLCγ2 knock-out macrophages compared with wild type cells (Fig. 2D). PLCγ2 knock-out macrophages also produced more TNF-α and less IRF3-regulated RANTES/CCL5 chemokine in response to LPS stimulation as determined by ELISA analysis (data not shown). Thus, by using chemical PLCγ inhibition, siRNA knockdown and bone marrow-derived macrophages from PLCγ2-deficient mice we have shown that PLCγ2 is required for efficient LPS-induced expression of IRF3-dependent genes while the NF-κB response remains intact.
FIGURE 2.
PLCγ is required for expression of IRF3-regulated genes in LPS-stimulated macrophages. A, RAW 264.7 cells were pretreated for 30 min with PLCγ inhibitor U73122 followed by LPS or IFN-β stimulation. ISG54 and IκBα expression was analyzed by RT-QPCR. B, RAW 264.7 cells were transiently transfected with control siRNAs or siRNAs targeting PLCγ1 or PLCγ2. After 48 h, cells were stimulated with LPS or IFN-β and ISG54 expression was analyzed by RT-QPCR. An aliquot of the cell lysates was analyzed for knock-down efficiency. C, RAW 264.7 cells were transfected with control or PLCγ2 siRNAs and stimulated with LPS. Expression of RANTES, TRAIL, IκBα, and TNF-α was analyzed by RT-QPCR. D, bone marrow-derived macrophages from wild type or PLCγ2 knock-out mice were stimulated with LPS (100 ng/ml) for the times indicated and expression of inflammatory cytokines was analyzed by RT-QPCR. Results are mean of three independent experiments and error bars represent standard deviations of means, and statistical significance was determined by paired Student's t test.
Intracellular Calcium Mediates IRF3 Phosphorylation and Nuclear Localization
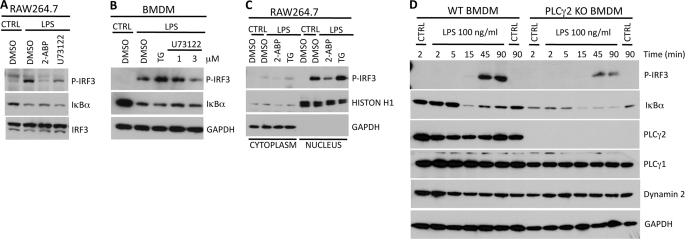
LPS stimulation triggers phosphorylation of IRF3. This leads to translocation of IRF3 into nucleus where it binds to promoter elements of IRF3-regulated genes. Western blot analysis of RAW 264.7 whole cell lysates revealed that LPS-induced IRF3 phosphorylation was significantly reduced in cells pretreated with IP3R antagonist 2-ABP or PLCγ inhibitor U73122 (Fig. 3A). Inhibition of intracellular Ca2+ release did not however affect LPS-induced degradation of IκBα, a hallmark of NF-κB activation (Fig. 3A). The requirement for intracellular Ca2+ for IRF3 activation was also confirmed in bone marrow-derived macrophages, where enforced increase of intracellular Ca2+ concentration by thapsigargin augmented LPS-induced IRF3 phosphorylation and inhibition of PLCγ activity by U73122 reduced IRF3 phosphorylation (Fig. 3B). Again, the activation of NF-κB pathway remained intact as determined by the level of IκBα degradation after LPS stimulation. To evaluate the effect of intracellular Ca2+ on nuclear translocation of IRF3 RAW 264.7 cells were treated with 2-ABP or thapsigargin. After LPS stimulation, cells were fractionated into cytosolic and nuclear fractions, and IRF3 levels were analyzed by Western blotting. Similar to experiments done with whole cell extracts, we noted that 2-ABP inhibited nuclear import of phosphorylated IRF3, whereas thapsigargin pretreatment enhanced LPS-induced translocation of IRF3 into nucleus (Fig. 3C).
FIGURE 3.
Calcium is required for efficient phosphorylation and nuclear import of IRF3. A, RAW 264.7 cells or B, bone marrow-derived macrophages were pretreated with the indicated inhibitors for 30 min and subsequently stimulated with LPS (100 ng/ml) for 45 min. Whole cell lysates were analyzed for phosphorylated IRF3 and IκBα. C, RAW 264.7 cells were pretreated with inhibitors for 30 min and stimulated with LPS for 45 min. Cells were lysed and cytoplasmic and nuclear fractions were analyzed for phosphorylated IRF3. D, bone marrow-derived macrophages from wild type or PLCγ2 knock-out mice were stimulated with LPS and levels of phosphorylated IRF3 and IκBα were analyzed by Western blotting from whole cell lysates.
To obtain definitive proof for involvement of PLCγ2 in LPS-induced IRF3 activation, bone marrow-derived macrophages from wild type or PLCγ2 knock-out mice were stimulated with LPS for various times. Analysis of whole cell lysates revealed that IRF3 phosphorylation was markedly impaired in macrophages derived from PLCγ2 knock-out mice (Fig. 3D). In contrast, the activation kinetics of IκBα degradation was similar in both wild type and PLCγ2 knock-out macrophages but the NF-κB signaling was sustained longer in knock-out cells. In addition, we noticed that the IκBα expression levels were somewhat lower in unstimulated PLCγ2 knock-out macrophages suggesting that also the steady-state activation level of NF-κB pathway might be higher in PLCγ2 knock-out macrophages compared with wild type cells.
Intracellular Calcium Regulates TLR4 Trafficking
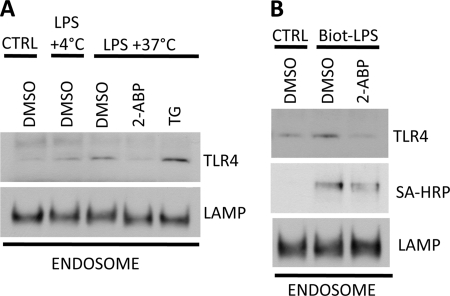
Recent reports have shown that LPS-induced signal transduction is a multistep process. Binding of LPS to TLR4 on plasma membrane activates NF-κB signaling and after this initial phase TLR4 is translocated into endosomes (9). Endosomal localization results in, and is required for efficient IRF3 activation (8, 10). Because our data showed that inhibiting the PLCγ2-IP3-Ca2+ cascade only affected LPS-induced IRF3 activation, we hypothesized that Ca2+ might have a role in mediating TLR4 internalization. Moreover, PLCγ2 activity has been previously linked into activation and mobilization of actin network in myeloid cells (22, 23). To test this, RAW 264.7 cells were pretreated with 2-ABP or thapsigargin followed by LPS stimulation. Endosomes were enriched by ultracentrifugation (supplemental Fig. S2) and TLR4 levels in endosomal fraction were analyzed by Western blotting. LPS stimulation resulted in translocation of TLR4 into endosomal compartment and this process was inhibited when cells where incubated at +4 °C instead of +37 °C (Fig. 4A). More importantly, the recruitment of TLR4 into endosomes was completely abrogated when IP3R activation and subsequent Ca2+ release was blocked by 2-ABP treatment prior to LPS stimulation. When intracellular Ca2+ levels were elevated by thapsigargin pretreatment LPS-induced TLR4 endocytosis was significantly higher compared with cells treated with DMSO vehicle (Fig. 4A). In another set of experiments RAW 264.7 cells were pretreated with 2-ABP and stimulated with biotinylated LPS to evaluate if both TLR4 and LPS are recruited to endosomes in Ca2+-dependent manner. Again, 2-ABP treatment resulted in nearly complete inhibition TLR4 endocytosis after LPS stimulation (Fig. 4B). 2-ABP treatment also inhibited the recruitment of biotinylated LPS into endosomal compartment revealed by staining the Western blot membranes with streptavidin-HRP (Fig. 4B). Unfortunately the limiting number of PLCγ2 mice available did not enable us to perform endosomal fractionation experiments with bone marrow-derived macrophages.
FIGURE 4.
Calcium signaling regulates TLR4 endocytosis. A, RAW 264.7 cells were pretreated with inhibitors for 30 min prior to stimulation with LPS. Cells were lysed and endosomes were enriched by sucrose gradient centrifugation. TLR4 and LAMP-1 levels were analyzed by Western blotting. B, RAW 264.7 cells were treated similarly to A but stimulated with biotin-conjugated LPS (Biot-LPS). Levels of TLR4 and biotin-LPS in the endosome enriched fraction were determined by using TLR4-specific antibody and streptavidin-HRP (SA-HRP), respectively. Results from one out of three experiments are shown.
Our results show that PLCγ2-IP3-Ca2+ cascade is essential for LPS-induced expression of IRF3-regulated genes in macrophages. Mechanistically, the release of intracellular Ca2+ was required for recruitment of TLR4 into endosomes, where TRIF-dependent IRF3 signaling platform is assembled (8, 10). What remains to be addressed is the pathway(s) leading from TLR4 into activation of PLCγ2. In B cells PLCγ2 is activated by Bruton's tyrosine kinase (Btk) and spleen tyrosine kinase (Syk) (12). When RAW 264.7 cells were treated with Btk inhibitor prior to LPS stimulation we did not observe any inhibition of IRF3-regulated genes (data not shown). Thus, Btk is not the PLCγ2 activating kinase in macrophages. There is some controversy on the role of Syk in PLCγ2 activation. We have previously observed that piceatannol, which inhibits Syk, specifically inhibits IRF3-dependent gene activation after LPS challenge (24). Syk has also been shown to be associated with TLR4 in LPS-stimulated macrophages (25). A report published during the revision of this report demonstrated the regulation of TLR4 internalization by CD14, and implicated both Syk and PLCγ2 in this process (26). In conclusion we have described here a novel regulatory component controlling LPS-induced IRF3 activation in macrophages. Our results reveal a previously unrecognized role for intracellular Ca2+ as an important regulator of TLR4 signaling and trafficking.
MATERIALS AND METHODS
Cells Lines, Mice, and Bone Marrow-derived Macrophages
All mice used in these experiments were housed in a pathogen-free environment and were bred and cared for in accordance with University of California, San Diego Animal Care Facility regulations. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the UCSD Animal Subjects Committee.
RAW 264.7 cells were obtained from American Type Culture Collection (ATCC) and cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum and antibiotics. PLCγ2-deficient mice have been described elsewhere (27). Bone marrow-derived macrophages were differentiated for 7 days in complete RPMI supplemented with 50 ng/ml M-CSF (Peprotech Inc.).
Reagents
All inhibitors and Ca2+ ionophores were obtained from Calbiochem. Ultrapure and biotin-conjugated Escherichia coli LPS were purchased from Invivogen and murine IFN-β was a generous gift from Biogen. siRNAs against PLCγ1 and PLCγ2 were purchased from Dharmacon with the catalogue numbers 040978 and 040979, respectively. RAW 264.7 cells were transfected with Lipofectamine 2000 (Invitrogen).
Western Blotting and Antibodies
For Western blotting, cells were lysed into Cell Lysis Buffer (Cell Signaling Technology) supplemented with protease and phosphatase inhibitor cocktails (Calbiochem). Antibodies for IκBα, IRF3, P-IRF3, PLCγ1, PLCγ2, and GAPDH were from Cell Signaling Technology. TLR4 antibody was from Invivogen and Histon H1 and LAMP1 antibodies from Santa Cruz Biotechnology.
RNA Isolation and Real Time PCR
Total cellular RNA was isolated with TRIzol reagent (Invitrogen). For cDNA synthesis, High Capacity cDNA synthesis kit from Applied Biosystems was used. RT-QPCR was performed with Fast SYBR Green master mix from Applied Biosystems (primer sequences are available upon request).
Subcellular Fractionation
Endosomes were enriched by sucrose gradient centrifugation (21). Briefly, after LPS stimulation, RAW 264.7 cells were lysed into homogenization buffer (0.25% sucrose, 3 mm imidazole, 0.5 mm EDTA) and homogenized with Dounce homogenizer (20 strokes). Nuclei were removed by centrifugation, and lysate was loaded on top of 64% sucrose and 17% Percoll gradient followed by centrifugation at 56,000 × g for 1 h. Endosome-enriched fraction was collected and washed with PBS by centrifugation at 100,000 × g for 30 min. Remaining pellet was suspended into homogenization buffer and expression of TLR4 or biotinylated-LPS was analyzed by Western blotting.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AI 047182 (to M. D.) and grants from the Academy of Finland, Sigrid Juselius Foundation, and Finnish Cultural Foundation (to V. V.).

This article contains supplemental Figs. S1 and S2.
- TLR
- Toll-like receptor
- IRF
- interferon regulatory factor
- PLC
- phospholipase C
- PPR
- pattern-recognizing receptor
- PAMP
- pathogen-associated molecular patterns
- IP3
- inositol 1,4,5-trisphosphate
- ISG
- interferon-stimulated gene
- ROS
- reactive oxygen species
- NOX
- NADPH-dependent oxidase
- Tg
- thapsigargin
- RyR
- ryanodine receptor
- Btk
- Bruton's tyrosine kinase
- Syk
- spleen tyrosine kinase.
REFERENCES
- 1. Akira S., Takeda K., Kaisho T. (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675–680 [DOI] [PubMed] [Google Scholar]
- 2. Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 3. Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 4. Qureshi S. T., Larivière L., Leveque G., Clermont S., Moore K. J., Gros P., Malo D. (1999) Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (TLR4) J. Exp. Med. 189, 615–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. (1999) Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11, 115–122 [DOI] [PubMed] [Google Scholar]
- 6. Fitzgerald K. A., Palsson-McDermott E. M., Bowie A. G., Jefferies C. A., Mansell A. S., Brady G., Brint E., Dunne A., Gray P., Harte M. T., McMurray D., Smith D. E., Sims J. E., Bird T. A., O'Neill L. A. (2001) Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413, 78–83 [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., Akira S. (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 [DOI] [PubMed] [Google Scholar]
- 8. Kagan J. C., Su T., Horng T., Chow A., Akira S., Medzhitov R. (2008) TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon β. Nat. Immunol. 9, 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Husebye H., Halaas Ø., Stenmark H., Tunheim G., Sandanger Ø., Bogen B., Brech A., Latz E., Espevik T. (2006) Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 25, 683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Husebye H., Aune M. H., Stenvik J., Samstad E., Skjeldal F., Halaas O., Nilsen N. J., Stenmark H., Latz E., Lien E., Mollnes T. E., Bakke O., Espevik T. (2010) The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity 33, 583–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signaling: dynamics, homeostasis and remodeling. Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 12. Kurosaki T., Tsukada S. (2000) BLNK: connecting Syk and Btk to calcium signals. Immunity 12, 1–5 [DOI] [PubMed] [Google Scholar]
- 13. Chiang E., Dang O., Anderson K., Matsuzawa A., Ichijo H., David M. (2006) Cutting edge: apoptosis-regulating signal kinase 1 is required for reactive oxygen species-mediated activation of IFN regulatory factor 3 by lipopolysaccharide. J. Immunol. 176, 5720–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higo T., Hattori M., Nakamura T., Natsume T., Michikawa T., Mikoshiba K. (2005) Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120, 85–98 [DOI] [PubMed] [Google Scholar]
- 15. Singh D. K., Kumar D., Siddiqui Z., Basu S. K., Kumar V., Rao K. V. (2005) The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell 121, 281–293 [DOI] [PubMed] [Google Scholar]
- 16. Watanabe N., Suzuki J., Kobayashi Y. (1996) Role of calcium in tumor necrosis factor-α production by activated macrophages. J. Biochem. 120, 1190–1195 [DOI] [PubMed] [Google Scholar]
- 17. Jin S. W., Zhang L., Lian Q. Q., Yao S. L., Wu P., Zhou X. Y., Xiong W., Ye D. Y. (2006) Close functional coupling between Ca2+ release-activated Ca2+ channels and reactive oxygen species production in murine macrophages. Mediators Inflamm. 2006, 36192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aki D., Minoda Y., Yoshida H., Watanabe S., Yoshida R., Takaesu G., Chinen T., Inaba T., Hikida M., Kurosaki T., Saeki K., Yoshimura A. (2008) Peptidoglycan and lipopolysaccharide activate PLCγ2, leading to enhanced cytokine production in macrophages and dendritic cells. Genes Cells 13, 199–208 [DOI] [PubMed] [Google Scholar]
- 19. Zanoni I., Ostuni R., Capuano G., Collini M., Caccia M., Ronchi A. E., Rocchetti M., Mingozzi F., Foti M., Chirico G., Costa B., Zaza A., Ricciardi-Castagnoli P., Granucci F. (2009) CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 460, 264–268 [DOI] [PubMed] [Google Scholar]
- 20. Zhou X., Yang W., Li J. (2006) Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-κB activation, inducible nitric-oxide synthase expression, and tumor necrosis factor α production in lipopolysaccharide-stimulated rat peritoneal macrophages. J. Biol. Chem. 281, 31337–31347 [DOI] [PubMed] [Google Scholar]
- 21. Hagen E., Myhre A. M., Tjelle T. E., Berg T., Norum K. R. (1999) Retinyl esters are hydrolyzed in early endosomes of J774 macrophages. J. Lipid Res. 40, 309–317 [PubMed] [Google Scholar]
- 22. Dearden-Badet M. T., Mouchiroud G. (2005) Re-distribution of phospholipase C γ2 in macrophage precursors is mediated by the actin cytoskeleton under the control of the Src kinases. Cell Signal. 17, 1560–1571 [DOI] [PubMed] [Google Scholar]
- 23. Cremasco V., Benasciutti E., Cella M., Kisseleva M., Croke M., Faccio R. (2010) Phospholipase C γ2 is critical for development of a murine model of inflammatory arthritis by affecting actin dynamics in dendritic cells. PLoS. One 5, e8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dang O., Navarro L., David M. (2004) Inhibition of lipopolysaccharide-induced interferon regulatory factor 3 activation and protection from septic shock by hydroxystilbenes. Shock 21, 470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaudhary A., Fresquez T. M., Naranjo M. J. (2007) Tyrosine kinase Syk associates with Toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol. Cell Biol. 85, 249–256 [DOI] [PubMed] [Google Scholar]
- 26. Zanoni I., Ostuni R., Marek L. R., Barresi S., Barbalat R., Barton G. M., Granucci F., Kagan J. C. (2011) CD14 Controls the LPS-Induced Endocytosis of Toll-like Receptor 4. Cell 147, 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang D., Feng J., Wen R., Marine J. C., Sangster M. Y., Parganas E., Hoffmeyer A., Jackson C. W., Cleveland J. L., Murray P. J., Ihle J. N. (2000) Phospholipase C γ2 is essential in the functions of B cell and several Fc receptors. Immunity 13, 25–35 [DOI] [PubMed] [Google Scholar]
- 28.Deleted in proof
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.