Background: Expression of activating immune ligands promotes inflammation.
Results: A pro-inflammatory immune ligand (MICA) is expressed in atherosclerotic lesions and is regulated by different transcription factors (NF-κB and HSF1) that compete for binding to overlapping promoter sites.
Conclusion: Competition for overlapping promoter sites integrates input from multiple transcriptional pathways.
Significance: Inflammatory immune responses may be influenced by genetic targeting of overlapping promoter sites.
Keywords: Atherosclerosis, Endothelial Cell, Innate Immunity, Natural Killer Cell, NF-kappa B, Tumor Necrosis Factor (TNF), MICA, NKG2D
Abstract
Endothelial cells form a barrier between blood and the underlying vessel wall, which characteristically demonstrates inflammatory damage in atherosclerotic disease. MICA is a highly polymorphic ligand for the activating immune receptor NKG2D and can be expressed on endothelial cells. We hypothesized that damaged vessel walls, such as those involved in atherosclerosis, might express MICA, which could contribute to the vascular immunopathology. Immune activation resulting from MICA expression could play a significant role in the development of vascular damage. We have demonstrated that TNFα up-regulates MICA on human endothelial cells. The up-regulation is mediated by NF-κB, and we have defined the regulatory control site responsible for this at −130 bp upstream of the MICA transcription start site. This site overlaps with a heat shock response element and integrates input from the two pathways. We have shown that in atherosclerotic lesions there is expression of MICA on endothelial cells. Using lentivirus-mediated gene delivery in primary human endothelial cells, we were able to inhibit the MICA response to TNFα with a truncated HSF1 that lacked a transactivation domain. This highlights the potential for transcription-based therapeutic approaches in atherosclerotic vascular disease to reduce immune-mediated endothelial and vessel wall damage.
Introduction
Endothelial cells on the luminal surface of blood vessels provide an active interface between blood and underlying tissues, including those of the vessel wall itself (1). Damage to endothelial cells occurs in a wide range of pathological situations, including atherosclerotic disease, infections, and autoimmune diseases (1). Endothelial damage can lead to exposure of the vessel wall and underlying tissues to blood cells and other blood components. Potential consequences of these interactions include luminal thrombosis or further vessel wall damage with the influx of immune cells and consequent vascular inflammation (1, 2). Endothelial cell damage could arise if endothelial cells up-regulate ligands for activating innate immune receptors. We hypothesized that pro-inflammatory stimuli might promote the up-regulation of such ligands, which would promote innate immune attack and so contribute to vessel wall inflammation and damage.
Human MICA is a highly polymorphic molecule and is a ligand for the activating receptor NKG2D, which is expressed on human natural killer cells and cytotoxic T lymphocytes. The engagement of NKG2D on a human natural killer cell by MICA on a potential target cell can result in cytotoxic killing of the target cell and inflammatory cytokine release (3). The interaction of MICA with NKG2D on T cells also exerts an activating influence on the T cells (4–6). MICA is encoded in the major histocompatibility complex (MHC) and is the most highly polymorphic human gene after the canonical MHC molecules, with over 70 alleles identified to date (7). MICA has structural similarities to the canonical MHC class I molecules but does not bind peptides (8). MICA is absent from the surface of most normal cells but is expressed on stressed cells, tumor cells, and pathogen-infected cells; the molecular mechanisms regulating MICA expression are poorly understood (3, 8, 9).
Endothelial cells can be activated by a range of stimuli, including bacteria or viruses and certain cytokines such as TNFα and IL1β (10, 11). Activation is associated with the release of cytokines, which can include TNFα itself and with changes at the endothelial cell surface, including expression of MHC class II molecules and enhanced capacity for leukocyte adhesion (12–15). These factors contribute to local inflammation. Endothelial dysfunction arises at an early stage in the atherosclerotic process, and there is much interest in factors that might contribute to this, especially inflammation (14, 16). There is an established correlation between inflammation and cardiovascular disease, and TNFα levels are elevated in patients with atherosclerotic vascular disease (17, 18).
The role of MICA in atherosclerosis has not been studied, but there is increasing evidence for the expression of MICA on endothelial cells in several other disease contexts. Cytomegalovirus infection of endothelial cells has been shown to up-regulate MICA (4), and MICA is expressed on circulating inflammatory endothelial cells and kidney endothelium in patients with systemic vasculitis, but it is absent from normal kidney (19). Antibodies against MICA can develop following organ transplantation and are associated with reduced transplant organ survival (20). MICA is certainly expressed on the endothelium of the transplanted organ, in the context of an allo-immune attack and likely cellular stress from ischemia and other acute changes associated with transplantation (21).
MICA could play an important role in the interaction between the endothelium and the immune system by acting as a marker in the vascular system for areas of endothelium overlying abnormal regions of vessel wall, such as those affected by atherosclerosis, or overlying abnormal tissues, such as infected or tumor tissues. The mechanisms responsible for triggering MICA expression on endothelial cells have not been identified. Up-regulation of MICA will promote an immune attack from cells expressing NKG2D, in particular from natural killer cells that constitute 5–10% of peripheral blood cells (22). This attack could lead to endothelial damage and contribute to inflammatory changes in the vessel wall.
We hypothesized that inflammatory stimuli might up-regulate MICA on human endothelial cells, thus increasing the probability of an immune attack against these endothelial cells. Such immune-mediated endothelial cell damage could play a role in the development of vascular damage such as atherosclerosis. We show that TNFα up-regulates MICA surface expression through the NF-κB pathway. This transcriptional activation is mediated by a master regulatory DNA element in the MICA promoter. MICA is up-regulated on endothelium overlying atherosclerotic lesions, and up-regulation of MICA on endothelial cells can be inhibited by genetically targeting the master regulatory DNA element.
EXPERIMENTAL PROCEDURES
Plasmid Construction
The −3.8-kb MICA promoter reporter plasmid pOC347 MICA-3756-WT was constructed by PCR amplification of a 3.8-kb MICA promoter fragment from a genomic DNA template. This was cloned into the HindIII/NcoI sites of the pGL3-Basic plasmid (pGL3B, Promega, Madison, WI). The −230-bp reporter plasmid pOC149 MICA-233-WT was constructed in a similar way. Site-directed mutagenesis was carried out by PCR with reverse complementary primers containing the mutation followed by DpnI digestion to remove template plasmid DNA. The details of mutations for luciferase plasmids are specified in Fig. 5A. The lentiviral plasmid pHR-SIN-BX-IRES-Emerald and accessory plasmids for lentiviral production pMD.G (VSV-G expression plasmid) and p8.91 (Gag-Pol, Rev, and Tat expression plasmid) are as described (23). The truncated HSF1 expression plasmid pOC352 HSF1T and lentiviral plasmid pOC1129 LV-HSF1T were constructed by PCR amplification of the region of the HSF1 cDNA corresponding to amino acids 1–379 and cloning of this fragment into pcDNA3 and pHR-SIN-BX-IRES-Emerald, respectively. The dominant negative IκBα lentiviral expression plasmid pOC1130 LV-IKBA-DN expressing IκBα S32A/S36A was constructed by site-directed mutagenesis of a pHR-SIN-IRES-Emerald-based wild-type IκBα lentiviral expression plasmid. The empty lentiviral expression plasmid pOC845 LV-control was constructed by removal of the BamHI/XhoI insert of pHR-SIN-BX-IRES-Emerald followed by blunt-ending with T4 DNA polymerase and re-ligation. All PCRs for cloning were carried out using Pfu polymerase (Stratagene, La Jolla, CA), and all constructs were verified by sequencing. All coordinates are relative to the transcriptional start site that was determined experimentally as described in the supplemental material.
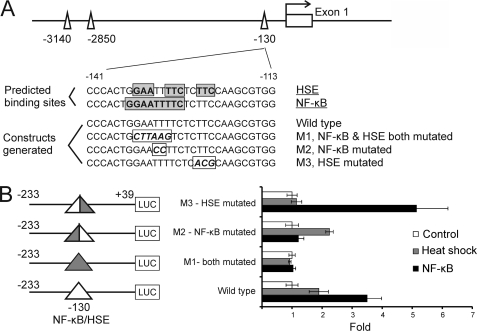
FIGURE 5.
MICA regulatory control site integrates input from both heat shock and NF-κB pathways. A, locations and sequences of the mutations introduced to the reporter constructs. Bases corresponding to the predicted binding sites for NF-κB or heat shock factor (HSE) sites are boxed and highlighted. Mutations designed to specifically disrupt either the NF-κB or HSE site in the corresponding reporter constructs are shown in boxed italics. B, different sets of mutations at the −130-bp NF-κB/HSE site selectively abolish the induction of the MICA promoter by NF-κB or heat shock. HeLa cells were transfected with the indicated reporter constructs bearing specific mutations to the −130-bp site together with either an NF-κB p65 expression vector (NF-κB) or empty control vector (Control and Heat Shock), and cells were harvested 48 h post-transfection for reporter assay (NF-κB and Control) or subjected to heat shock (Heat Shock) for 1 h at 42 °C followed by 5-h recovery at 37 °C before cells were harvested. The error bars represent standard deviations of three replicates. LUC, luciferase.
Cell Culture and Lentivirus Infection
Human umbilical vein endothelial cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Sigma) with 10% fetal calf serum, 4 mm l-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin (Sigma), supplemented with 30 μg/ml endothelial cell growth supplement (Sigma) and 5 units/ml heparin (Sigma). Human aortic arterial endothelial cells (Invitrogen) were maintained in Medium 200 supplemented with Low Serum Growth Supplement (Invitrogen), 50 units/ml penicillin, and 50 μg/ml streptomycin. Primary arterial and venous endothelial cells before passage 6 were used for experiments. HeLa and 293T cells were maintained in DMEM supplemented with 10% fetal calf serum, 4 mm l-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. Where appropriate, TNFα (R&D Systems, Minneapolis, MN) was added at 10 ng/ml. Lentivirus was generated by co-transfection of the lentiviral expression plasmid, pMD.G and p8.91, into 293T cells.
Flow Cytometry
Cells were detached from tissue culture plates by incubation with enzyme-free cell dissociation buffer (Invitrogen) and stained with anti-MICA monoclonal antibody (clone 2C10, Santa Cruz Biotechnology) or isotype control followed by FITC-conjugated goat anti-mouse IgG (Serotec, Oxford, UK) or Dylight649-conjugated goat anti-mouse IgG (Serotec). Lentivirally transduced cells were identified by their GFP positivity. Flow cytometry was performed using a FACSCanto machine (BD Biosciences), and data were analyzed using Flowjo software (Tree Star, Ashland, OR).
Quantitative RT-PCR
Total RNA was extracted using Tri-Reagent (Sigma), further purified using the RNeasy mini kit (Qiagen, Hilden, Germany), and reverse-transcribed into cDNA using oligo(dT) and avian myeloblastosis virus reverse transcriptase (Invitrogen) or random hexamers and Bioscript Moloney murine leukemia virus reverse transcriptase (Bioline, London, UK). Real time PCR was carried out with SYBR Green supermix (Bio-Rad) using an IQ real time PCR machine (Bio-Rad). The data were normalized to GAPDH expression using the ΔΔCt method, and all the results represent the mean of at least two replicates. Real time PCR primers are listed in supplemental Table 1.
Reporter Assays
For reporter assays with NF-κB transfection, HeLa cells were cultured in 24-well plates and co-transfected with 150 ng of each reporter construct and pCMVβ (Clontech) using FuGENE 6 (Roche Applied Science). When appropriate, cells were also co-transfected with 150 ng of p65 expression plasmid or pcDNA3 empty vector control at this stage. Cells were lysed 48 h post-transfection for luciferase assay using the luciferase assay system (Promega) and a TD-2020 luminometer (Turner Designs, Sunnyvale, CA), as well as β-galactosidase assay using ortho-nitrophenyl-β-galactoside (Sigma) as substrate. The luciferase activity of each sample was normalized to β-galactosidase activity and shown as relative luminescence units. For reporter assay with TNFα treatment, human arterial endothelial cells were transfected with 1 μg of each reporter construct and 50 ng of pRL-SV40 (Promega) by nucleofection using human coronary artery endothelial cell nucleofector kit and a Nucleofector II device (Lonza BioWhittaker, Portsmouth, NH), plated to recover for 24 h, and treated with 10 ng/ml TNFα for 24 h. Cells were lysed for luciferase assay using the Dual-Luciferase Reporter Assay System (Promega). The luciferase activity of each sample was normalized to Renilla luciferase activity and shown as relative luminescence units.
EMSA
EMSA was performed using nuclear extracts from endothelial cells treated with TNFα and [γ-32P]ATP end-labeled double-stranded DNA probes. The forward strand probe sequences are CAGCCCACTGGAATTTTCTCTTCCA (wild type), CAGCCCACTGCTTAAGTCTCTTCCA (mutant), and AGTTGAGGGGACTTTCCCAGGC (NF-κB consensus). The mutations introduced to disrupt the NF-κB site are underlined. The mutations are identical to those introduced into the luciferase reporter plasmids pOC234 MICA-233P-M1 and pOC348 MICA-3756P-M1. For standard EMSA, 5 μg of nuclear extract was incubated with 100 fmol of labeled probes in a 10-μl binding reaction containing 1 μg of poly(dI-dC) and 100 ng of denatured sonicated salmon sperm DNA. For EMSA with limiting probe condition, 30 μg of nuclear extract was incubated with 2.5 fmol of labeled probes in a 20-μl binding reaction. For supershift assay, the nuclear extract was preincubated with 1 μg of antibody for 30 min on ice before the probe was added. The following antibodies were used for supershift assay: anti-p65 (clone F-6, Santa Cruz Biotechnology), anti-p50 (clone 4D, Biolegend, San Diego), anti-c-Rel (Calbiochem), and anti-HSF1 (clone 10H8, StressGen, Victoria, Canada). For competition assays, unlabeled probe at 100-fold excess was added to the binding mixture before the addition of labeled probes.
ChIP Assay
Sonicated chromatin prepared from endothelial cells treated with TNFα was immunoprecipitated with anti-p65 antibody or mouse IgG1 isotype control using protein G-agarose beads (Millipore, Bedford, MA). ChIP samples were analyzed by PCR amplification of the MICA proximal promoter region containing the putative NF-κB site, as well as a control region at the end of MICA intron1 6 kb downstream. ChIP assay primers are listed in supplemental Table 2.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue samples of nonatheromatous aorta (n = 5), atheromatous aorta (n = 4), and aorta with giant cell arteritis (n = 3), obtained with full ethical approval from the National Research and Ethics Service (Oxfordshire Research and Ethics Committee A, reference 04/Q1604/21), were immunostained for MICA with anti-MICA antibody (ab62540, Abcam, Cambridge, UK) and detected with the NovolinkTM max polymer detection system (Leica Microsystems, Wetzlar, Germany), as per the manufacturer's instructions, or with detection reagents only as a negative control. Slides were mounted in Aquatex mounting medium (Merck). Stained sections were photographed with a Nikon DS-FI1 camera with a Nikon DS-L2 control unit (Nikon UK Ltd., Kingston-upon-Thames, UK) and an Olympus BX40 microscope (Olympus UK Ltd., Watford, UK).
RESULTS
TNFα Induces MICA Expression on Human Endothelial Cells via NF-κB
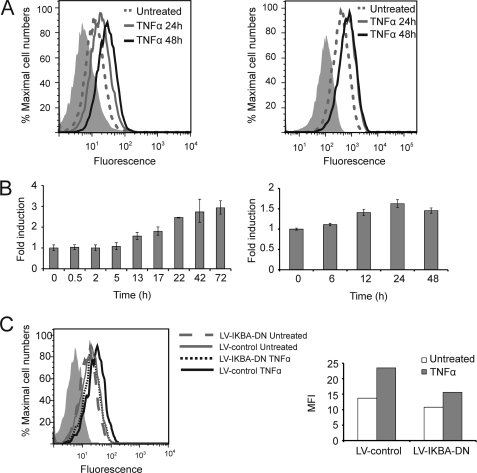
We hypothesized that MICA expression might be regulated by TNFα in endothelial cells. To test this hypothesis, primary human arterial and venous cells were treated with TNFα, and the cell surface expression of MICA was assessed by flow cytometry. Surface expression of MICA was significantly up-regulated at 24 and 48 h following TNFα treatment in both arterial and venous cells (Fig. 1A). Quantitative real time RT-PCR analysis demonstrated marked MICA mRNA up-regulation in response to TNFα, with a time course that is consistent with regulation of MICA by TNFα at the transcription level (Fig. 1B).
FIGURE 1.
TNFα up-regulates MICA expression on human endothelial cells via the NF-κB pathway. A, up-regulation of MICA surface expression on endothelial cells by TNFα. Human primary endothelial cells were treated with TNFα for 24 or 48 h and analyzed for MICA surface expression by flow cytometry. The left panel shows venous cells, and the right panel shows arterial cells, and in both cases MICA expression is up-regulated by TNFα. Shaded histograms represent isotype controls. B, up-regulation of MICA mRNA expression by TNFα. Human primary endothelial cells were treated with TNFα for the indicated times. Cells were then harvested, and MICA mRNA expression was quantified by real time RT-PCR. The left panel shows venous cells, and the right panel arterial endothelial cells. The error bars represent standard deviations of two (venous) or three (arterial) replicates. C, dominant negative IκBα blocks the induction of MICA by TNFα in endothelial cells. Primary human arterial endothelial cells were infected with a lentivirus which expressed a dominant negative IκBα (LV-IKBA-DN) or empty vector control (LV-control). 24 h later, the cells were treated with TNFα for 24 h and then analyzed for surface expression of MICA by flow cytometry. The shaded histogram represents an isotype control. The right panel illustrates the median fluorescence intensity (MFI) of MICA staining after background isotype control subtraction.
TNFα activates multiple pathways in endothelial cells, including the MAPK, JNK, and NF-κB pathways (24). The NF-κB pathway is activated by TNFα through phosphorylation-dependent degradation of IκB, which under normal circumstances retains NF-κB transcription factors in the cytoplasm (25). IκB degradation results in translocation of NF-κB transcription factors into the nucleus. Using bioinformatic analysis, we identified putative NF-κB response elements in the MICA promoter. The role of NF-κB in TNFα-induced MICA expression in endothelial cells was studied using lentivirus-mediated expression of a dominant negative IκBα. The dominant negative IκBα is mutated to prevent serine phosphorylation by the activating kinases and so specifically sequesters NF-κB components in the cytoplasm, thus blocking NF-κB-mediated transcriptional activation (26). Expression of the dominant negative IκBα inhibited the induction of MICA expression at the cell surface by TNFα (Fig. 1C) in primary endothelial cells. This confirms that the NF-κB pathway is the dominant pathway in the regulation of MICA by TNFα in endothelial cells.
NF-κB Regulates MICA through a Master Control Site at −130 bp
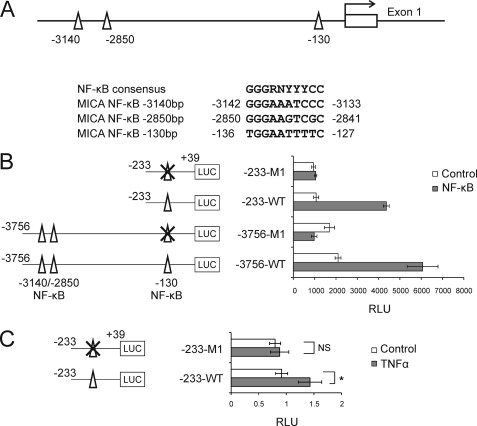
A detailed bioinformatic analysis identified three putative NF-κB-binding sites in the MICA promoter at −3140, −2850, and −130 bp upstream of the experimentally determined transcriptional start site (Fig. 2A). To determine whether any of these sites contribute to the transcriptional regulation of MICA by NF-κB, a series of reporter constructs were created in which MICA promoter regions containing the putative NF-κB sites drive the expression of luciferase. As Fig. 2B indicates, constructs containing the −3.8-kb and −230-bp regions displayed similar levels of NF-κB-mediated transactivation of the MICA promoter. These data are consistent with NF-κB acting at the predicted −130-bp site and demonstrate that there is no requirement for the predicted −3140- or −2850 bp sites. To confirm this, the constructs covering the −3.8-kb and −230-bp region were both similarly mutated within the −130-bp site to abolish NF-κB binding. This mutation abolished the inducibility of both the −3.8-kb and −230-bp constructs by NF-κB, confirming that the −130-bp site is necessary and sufficient for transcriptional transactivation of MICA by NF-κB (Fig. 2B). The role of the −130-bp regulatory element in the response to TNFα in primary endothelial cells was also tested by reporter assay. As shown in Fig. 2C, TNFα significantly induces the wild-type MICA promoter in primary endothelial cells and mutation of the −130-bp site completely abolishes this induction. A previously suggested NF-κB site in intron 1 of MICA was not responsive to NF-κB activation (supplemental Fig. 1) (27).
FIGURE 2.
NF-κB regulates MICA expression through a control site at −130 bp. A, locations and sequences of the three predicted NF-κB sites. Numbers indicate locations in base pairs with respect to the experimentally determined MICA transcriptional start site. B, mutation of the −130-bp NF-κB abolishes the induction of MICA promoter by NF-κB. HeLa cells were co-transfected with the indicated luciferase (LUC) reporter constructs plus either an NF-κB p65 expression vector (NF-κB) or control vector (Control). Data were normalized against β-galactosidase activity. C, treatment of primary arterial endothelial cells with TNFα regulates transcriptional activity of the MICA promoter through the −130-bp control site. Cells were transfected with the reporter constructs shown, treated with TNFα for 24 h, and harvested for luciferase activity. Data were normalized against Renilla luciferase activity. The error bars represent standard deviations of three replicates. Statistical significance was assessed using Student's t test: NS, not significant; *, p < 0.05; RLU, relative luminescence units.
TNFα Induces NF-κB Binding to the Regulatory Control Site in Endothelial Cells
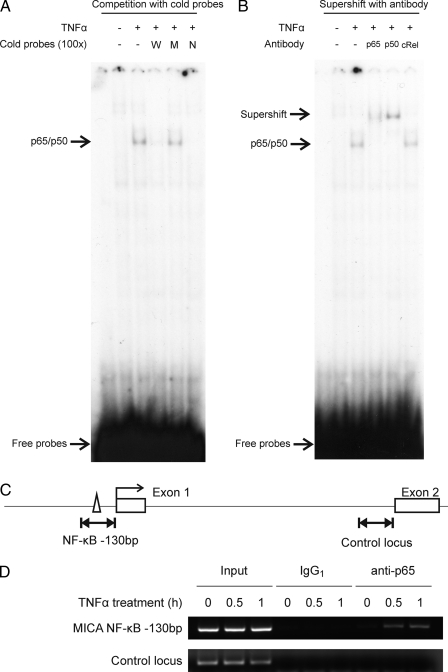
To establish whether the −130-bp NF-κB site is involved in TNFα-mediated up-regulation of MICA in endothelial cells, EMSAs were performed (Fig. 3A). Following TNFα treatment of human arterial endothelial cells, a nuclear species was induced that bound specifically to the −130-bp site. Binding of the induced species persists for at least 24 h after the initiation of TNFα treatment (supplemental Fig. 2). This species was competed off by an excess of an unlabeled oligonucleotide corresponding to the wild-type −130-bp site but not by an equivalent excess of an unlabeled oligonucleotide corresponding to the mutated form of the −130-bp site, which was inactive in the luciferase reporter assay. It was also competed off by an excess of an oligonucleotide corresponding to a consensus NF-κB site. Therefore, the specific DNA-binding species has the same sequence specificity for binding as that identified for regulatory activity at the −130-bp site using reporter constructs. To identify the bound species, supershift assays were undertaken using antibodies against specific NF-κB subunits. The bound species was supershifted by either anti-p65 or anti-p50 antibody but not by anti-c-Rel antibody (Fig. 3B). These results demonstrate that TNFα acts on human endothelial cells to induce binding of NF-κB p65/p50 heterodimers to a probe corresponding in sequence to the −130-bp site of the MICA gene. Similar experiments using nuclear extracts form venous endothelial cells confirmed that TNFα induces binding of p65/p50 heterodimers and also some p65/p65 homodimers to the MICA −130-bp site in these cells (supplemental Fig. 3).
FIGURE 3.
TNFα induces NF-κB transcription factor binding to the −130-bp control site. A and B, in vitro binding of NF-κB to the −130-bp MICA NF-κB site demonstrated by EMSA in primary human arterial endothelial cells. 32P-Labeled DNA probes containing the −130-bp site were incubated with nuclear extract from primary endothelial cells treated with TNFα for 1 h or from untreated cells. In the competition assay (A), nuclear extracts were preincubated with 100-fold excess of unlabeled probe containing the intact wild-type −130-bp site (W), a mutated site (M), or a consensus NF-κB site (N); in the supershift assay (B), nuclear extracts were preincubated with antibodies against NF-κB p65, p50 or c-Rel before the addition of 32P-labeled probe. Protein-probe complexes were resolved by native PAGE. Results are representative of four experiments using different batches of independently purified nuclear extracts. C, locations of the PCR amplicons used in the ChIP assay at the −130-bp site or at a control locus in the first intron of the MICA gene. D, in vivo binding of p65 to the MICA locus demonstrated by ChIP assay. Primary endothelial cells were treated with TNFα for the times shown or untreated. Chromatin was immunoprecipitated with anti-NF-κB p65 antibody (anti-p65) or IgG1 isotype control (IgG1) and analyzed by PCR in parallel with 50-fold diluted pre-immunoprecipitated chromatin (input).
To confirm that NF-κB binds to the −130-bp site in primary endothelial cells, ChIP assays were undertaken. Following treatment with TNFα or otherwise, sheared chromatin was immunoprecipitated with an anti-p65 antibody and analyzed by PCR for the presence of the −130-bp site (Fig. 3, C and D). As shown in Fig. 3D, TNFα treatment of primary endothelial cells rapidly induced binding of NF-κB to the −130-bp promoter element but not to a control region in intron 1 of the MICA gene.
MICA Is Up-regulated on Endothelial Cells in Atherosclerotic and Inflamed Vessels
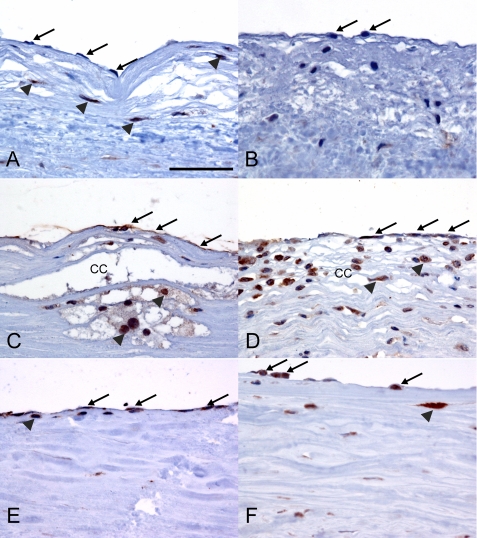
Atherosclerotic lesions are characterized by the presence of pro-inflammatory lipid material and inflammatory cells, including macrophages that can produce TNFα (28). In this environment, our data suggest that local TNFα production could potentially influence endothelial cell MICA expression. Therefore, MICA expression was analyzed in normal and diseased human vessels using immunohistochemistry. In nondiseased vessels very occasional endothelial cells showed low levels of MICA expression, but the vast majority were negative on MICA immunostaining (Fig. 4, A and B). In contrast, in atheromatous vessels, endothelial cells showed strong immunostaining for MICA, indicating high levels of expression (Fig. 4, C and D). In giant cell arteritis, a form of large vessel vasculitis, the majority of endothelial cells showed MICA expression (Fig. 4, E and F), but at a lower level than that in the endothelial cells in atheromatous vessels. Significant MICA expression was also seen on foamy macrophages associated with atherosclerotic lesions.
FIGURE 4.
MICA is up-regulated in endothelial cells in atherosclerotic and inflamed human arteries. Sections of human aorta were immunostained for MICA (brown) using the peroxidase method. A and B, normal aorta showing minimal staining of endothelial cells (indicated with arrows). Some positive immunostaining of medial smooth muscle cells (arrowheads) is also seen in A. The scale bar in A (50 μm) refers to all panels. C and D, atheromatous aorta showing strong staining of endothelial cells for MICA (arrows). Staining is also seen in foamy macrophages (arrowheads) associated with cholesterol clefts (CC) in plaques. E and F, in giant cell arteritis, there is less intense staining of endothelial cells (arrows) with some scattered MICA-positive macrophages seen (arrowheads).
Regulatory Control Site Integrates Regulatory Input from the NF-κB and Heat Shock Pathways
Given the potential importance of TNFα-mediated up-regulation of MICA in endothelial cells in vascular inflammation and in the pathogenesis of atherosclerotic vascular disease, we sought to identify ways to target this up-regulating activity on the MICA gene. The NF-κB site at −130 bp of the MICA promoter overlaps with a heat shock element (HSE)2 previously shown to mediate the induction of MICA by heat shock (8). We hypothesized that this overlap creates a master regulatory control site that integrates input from these two important pathways of transcriptional regulation. To test this, we designed and constructed detailed sets of mutations to this site to differentially disrupt either the NF-κB-binding elements or the heat shock factor-binding elements alone. The design was based on the different sequence specificities for NF-κB binding and heat shock factor binding (Fig. 5A) (29, 30). Mutation of the NF-κB elements alone abolished induction through this site by NF-κB but not by heat shock (Fig. 5B). In contrast, mutation of the heat shock element alone abolished the induction through the site by heat shock but not by NF-κB. This demonstrates that NF-κB and heat shock regulate MICA transcription through overlapping, but independent, elements at the −130-bp NF-κB/HSE regulatory control site.
A Dominant Negative Truncated HSF1 Blocks NF-κB Binding to the −130-bp Regulatory Control Site
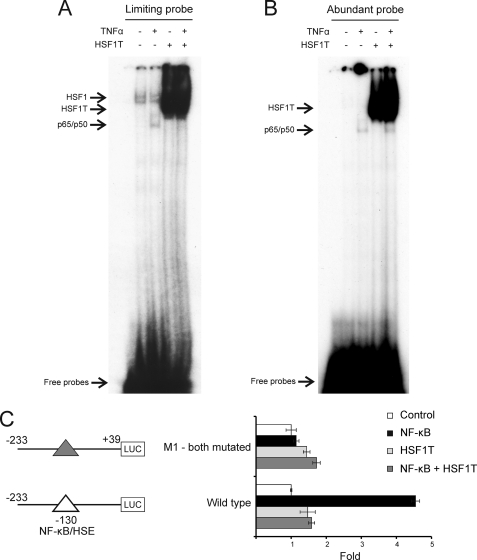
The existence of a single overlapping regulatory control site suggests a potentially powerful approach to inhibit the TNFα-mediated up-regulation of MICA on endothelial cells. We hypothesized that a dominant negative HSF1, which lacks a transactivation domain, could compete with NF-κB for binding to this master regulatory control site and so block the NF-κB-mediated induction of MICA by TNFα. To establish whether there was direct competition between truncated HSF1 and NF-κB for binding to the −130-bp MICA promoter site, EMSAs were undertaken using nuclear extracts from TNFα-treated endothelial cells transduced with lentivirus expressing truncated HSF1 (HSF1T). The TNFα-induced binding of NF-κB to the −130-bp regulatory element was inhibited by truncated HSF1 overexpression, as seen when the probe concentration was limiting (Fig. 6A). When abundant probe is available in the binding reaction, TNFα-induced binding of NF-κB to the probe is similar in the presence or absence of truncated HSF1 (Fig. 6B), confirming that NF-κB activation by TNFα is not affected by truncated HSF1 expression. These observations demonstrate that truncated HSF1 competes directly with NF-κB for binding to the −130-bp regulatory site of the MICA promoter.
FIGURE 6.
Truncated HSF1 blocks transactivation of MICA promoter by NF-κB through direct competition with NF-κB for binding to the −130-bp site. A and B, direct competition between NF-κB and truncated HSF1 for the −130-bp NF-κB site is demonstrated by EMSA under limiting probe condition. 32P-Labeled DNA probes containing the −130-bp site were incubated with nuclear extract from primary arterial endothelial cells transduced with lentivirus expressing a dominant negative truncated HSF1 that lacks a transactivation domain (HSF1T) or with empty vector control and treated with TNFα for 1 h. The binding reaction was carried out under limiting probe conditions (A) or standard abundant probe conditions (B) as described under “Experimental Procedures.” The protein-probe complex was resolved by native PAGE, and the gel was subjected to autoradiography for 72 h (A) or 9 h (B). The identities of the complexes indicated by the arrows were confirmed by supershift assay (supplemental Fig. 4). A, it can be seen that TNFα induces an NF-κB band, but the truncated HSF1 successfully competes for probe binding resulting in loss of this band. C, dominant negative HSF1 blocks the induction of MICA promoter by NF-κB through the −130-bp NF-κB site. HeLa cells were transfected with the indicated luciferase (LUC) reporters (as illustrated in Fig. 5A) together with either an NF-κB p65 expression vector (NF-κB) or a truncated HSF1 expression vector (HSF1T) or an NF-κB p65 and a truncated HSF1T expression vector together (NF-κB + HSF1T) or an empty control vector (Control). Cells were harvested 48 h post-transfection for reporter assay. The error bars represent standard deviations of three replicates.
In addition to the NF-κB p65/p50 heterodimer induced by TNFα, there is also constitutive basal level HSF1 binding to the −130-bp site (Fig. 6A), as confirmed by supershift assay (supplemental Fig. 4). With abundant probe, the TNFα-induced band seen for NF-κB bound to the −130-bp site is much stronger than that for HSF1, which is not seen with a short exposure time (Fig. 6B), although with limiting probe the bands are comparable (Fig. 6A). This demonstrates that the level of NF-κB-probe complex falls more than that of the HSF1-probe complex when the probe concentrations are reduced. Therefore, the affinity of NF-κB for the −130-bp regulatory site is weaker than that of HSF1 for the site. For this reason, HSF1 can compete successfully with NF-κB for binding to the −130-bp overlapping site.
The ability of truncated HSF1 to block NF-κB-mediated transactivation of the MICA promoter was tested by reporter assay. To explore this, cells were co-transfected with MICA promoter reporter constructs and expression plasmids for NF-κB and for a truncated HSF1 lacking a transactivation domain. As shown in Fig. 6C, co-transfection of the truncated HSF1 completely blocks the induction of the MICA promoter by NF-κB, confirming that a functional interaction occurs at this site and that transcriptional regulation by NF-κB at this site can be inhibited by the truncated HSF1.
Gene-based Functional Antagonism of MICA Up-regulation at the Master Regulatory Control Site
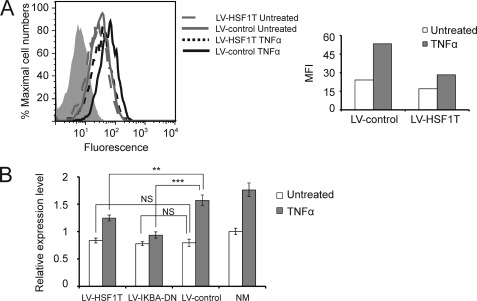
To formally test the hypothesis that truncated HSF1 can block NF-κB-mediated MICA up-regulation by TNFα in endothelial cells, truncated HSF1 was overexpressed in primary human arterial endothelial cells using lentiviral gene delivery, and the cells were treated with TNFα and analyzed for MICA expression. Expression of truncated HSF1 in endothelial cells had an inhibitory effect on TNFα-induced up-regulation of cell surface MICA (Fig. 7A). Quantitative real time RT-PCR demonstrated that lentiviral infection of endothelial cells with the truncated HSF1 significantly inhibited the transcriptional up-regulation of MICA by TNFα, as did a dominant negative IκBα (Fig. 7B). This result confirms the importance of the −130-bp control site in the up-regulation of MICA by TNFα in endothelial cells and suggests a potential gene-based therapeutic approach to influence immune activation and inflammation in vascular disease utilizing overlapping transcription factor-binding sites.
FIGURE 7.
Gene-based functional antagonism of MICA up-regulation at the master control site. A, truncated HSF1 inhibits induction of MICA surface expression by TNFα in primary arterial endothelial cells. Human primary arterial endothelial cells were infected with lentivirus expressing a dominant negative truncated HSF1 that lacks a transactivation domain (LV-HSF1T) or empty vector control (LV-control). 48 h later, cells were treated with TNFα for 24 h and analyzed for MICA surface expression by flow cytometry. The shaded histogram represents an isotype control. The right panel illustrates the median fluorescence intensity (MFI) of MICA staining after background isotype control subtraction. B, quantitative real time RT-PCR analysis demonstrates that a truncated HSF1 inhibits induction of MICA transcription by TNFα in primary arterial endothelial cells. Endothelial cells were infected with the lentivirus expressing either a truncated HSF1 lacking a transactivation domain (LV-HSFIT) or a dominant negative IκBα (LV-IKBA-DN) or an empty vector control (LV-HSFIT) and treated with TNFα. The transcript expression level relative to the uninfected untreated sample is shown. Statistical significance was assessed using Student's t test: NS, not significant; **, p < 0.01; ***, p < 0.001.
DISCUSSION
The role of innate immunity in human disease is increasingly recognized, as is the role of inflammation in vascular disease. We have demonstrated that the activating immune ligand MICA is up-regulated on human endothelial cells by the pro-inflammatory cytokine TNFα. This up-regulation is controlled at the transcriptional level by a master regulatory control element positioned −130-bp upstream of the MICA transcription start site. This element integrates input from the NF-κB and heat shock pathways and in the presence of NF-κB activation exerts a strong influence on MICA expression at the cell surface. The overlapping nature of the control element for these two pathways allows targeted transcriptional blockade that inhibits TNFα-induced up-regulation of MICA. These results define a precise molecular mechanism of MICA up-regulation, of particular relevance in the pro-inflammatory state. The persistent binding of NF-κB to the regulatory site and the long half-life of the MICA transcript (supplemental Fig. 5) indicate that TNFα stimulation will cause prolonged activation at the −130-bp site, and transcript levels will continue to rise for a considerable time while the stimulus persists. Slow prolonged NF-κB-mediated transcriptional up-regulation has been described for genes with long transcript half-lives (31). In arterial endothelial cells, the main NF-κB species bound is p65/p50 heterodimer, whereas in venous endothelial cells there is significant p65/p65 homodimer binding. This may be a factor in the slightly stronger induction of MICA in venous cells as p65/p65 homodimers contain two transactivating domains, whereas the p65/p50 heterodimers contain only one. We found MICA expression on the endothelium overlying atherosclerotic lesions and in autoimmune vascular disease. The display of MICA on the surface of endothelial cells will promote immune attack of the endothelial cells (3, 4), resulting in damage to the endothelium and contributing to ongoing vessel inflammation. Our data define a new mechanism whereby MICA expression could play a role in the development of vascular damage.
MICA may contribute to the pathogenesis of vascular disease at one or more stages of its development. Risk factors for atherosclerosis are well appreciated and include elevated lipid levels, diabetes, hypertension, age, and genetic risk. These factors apply systemically, but the disease is patchy and favors certain regions of the vascular tree. The cause for this distribution is unclear, but in some anatomical locations physical stressors, such as pressure or flow characteristics, may play a role. Flow-related stresses can have substantial effects on endothelial cells function and could be significant factors in the development of vascular disease and its anatomical distribution (32, 33). MICA is known to respond to a wide range of cellular stresses (9, 34), and it is possible that it may be up-regulated by flow-related stresses at particular locations in the vascular tree.
Inflammatory cytokines such as TNFα are implicated in the pathogenesis of vascular disease. Patients with atherosclerosis have elevated levels of TNFα in their blood compared with matched controls (18, 35). Traditional risk factors correlate with markers of inflammation in patients with atherosclerosis (17, 35). This association may indicate that traditional risk factors, such as raised oxidized lipid levels, act by triggering inflammation. TNFα levels increase with age in humans, as does atherosclerosis (36). Polymorphisms in TNFα receptors are associated with risk for age-related atherosclerosis in humans (37). Our data raise the possibility that TNFα acts via NF-κB on the MICA gene, by the mechanism that we have defined, to lower the threshold required for local anatomically variable stimuli, such as abnormal flow or pressure to up-regulate MICA and so promote endothelial cell attack by the immune system. There are precedents for this type of synergism between TNFα and a physical stressor, and the effect of TNFα on endothelial cell recruitment of monocytes has been shown to increase by an order of magnitude in the presence of nonuniform shear stress (38). In addition, there is evidence that TNFα can increase the susceptibility of endothelial cells to cytotoxic destruction by natural killer cells (39).
Up-regulation of MICA expression on endothelial cells by other stimuli could also contribute to vascular damage. Cytomegalovirus can infect endothelial cells and cause MICA expression (4), which will promote immune attack with the potential for endothelial damage and consequent influx of blood components, including immune cells and lipid species into the vessel wall. There is evidence that cytomegalovirus infection is associated with increased cardiovascular mortality long after primary infection (40–42).
The mechanism of MICA up-regulation that we have defined could clearly play a role in the propagation of an established atherosclerotic lesion. The lesions are rich in inflammatory cells, especially macrophages and lymphocytes (including T cells and natural killer cells) (2, 43). Macrophages release TNFα in response to lipid species, especially oxidized low density lipoprotein (44). Atherosclerotic lesions contain a range of pro-inflammatory lipid species, and the macrophages within the lesions have been shown to produce TNFα (45, 46). Within lesions, endothelial cells are often physically close to macrophages or foam cells, and production of TNFα by these cells will expose overlying endothelial cells to high local levels of TNFα. Thus, in the development of inflammatory atherosclerotic lesions, macrophages that have migrated beneath the endothelium release TNFα that can activate NF-κB in the overlying endothelial cells. Binding of activated NF-κB components to the regulatory control site in the MICA gene would trigger expression of MICA on the surface of the endothelial cells; this would promote an immune attack resulting in further vascular damage with loss of endothelial integrity and influx of blood cells and components to the subendothelial space. The consequence of this is likely to be further macrophage influx, further local TNFα release and further MICA expression on neighboring endothelial cells, thus propagating and exacerbating an inflammatory immune attack on the vessel wall.
Even chronic low grade immune attack of endothelial cells may damage endothelial integrity and reduce the capacity of the vessel wall to generate NO. Such endothelial dysfunction has been implicated in the pathogenesis of vascular disease and has been shown to arise in the context of inflammation and improve with resolution of the inflammation (47). MICA-mediated immune damage to the endothelium could contribute to the development of endothelial dysfunction. Endothelial damage could also expose subendothelial structures to blood, potentially triggering thrombosis and vessel occlusion. MICA could play a role in the pathology of unstable plaques or plaque rupture if MICA expression results in endothelial destruction and local immune activity sufficient to cause plaque herniation into the vessel lumen and consequent thrombosis.
Genome-wide analyses have not identified a strong association between MICA and atherosclerotic vascular disease. This indicates either that MICA does not play a role in the disease or that its effect does not differ between individuals with different MICA alleles. Although MICA is highly polymorphic, the regulatory control site is conserved unchanged in over 70 alleles that have been sequenced to date. This is consistent with an important function for this site and selection for an effective pathway for NF-κB and heat shock to drive MICA expression in endothelial cells and likely other cells too. Furthermore, the site is completely conserved across those species that have been sequenced (chimpanzee, rhesus, and orangutan). In the marmoset sequence, there is only a single base difference, and this is in a part of the site not involved in NF-κB or heat shock factor binding (29, 30). This pathway is likely to be particularly important in endothelial cells, which are a key interface between the circulating immune system and infected or diseased tissue. Our data indicate that the use of the regulatory site by one of NF-κB or heat shock factor is exclusive of the other. Therefore, there will be no additive effect of heat shock and NF-κB on MICA expression. This could prevent low level activation of the two pathways combining to trigger MICA expression but allow high level stimulation of just one pathway to do so.
Atherosclerotic vascular disease is the major cause of death globally (48). Despite improvements in both treatment and prevention of vascular disease, there is a pressing need for a detailed understanding of all aspects of the biology of atherosclerotic vascular disease. Statin drugs, which have had a major beneficial effect on cardiovascular disease, lower lipid levels by inhibiting HMG-CoA reductase, but they also have some inhibitory action on NF-κB (49). Different statins differ in their ability to block NF-κB in monocytes (50), so developing statins with a more pronounced NF-κB inhibitory effect in specific cellular contexts might improve their efficacy.
Future studies will be required to investigate the effects of modulating MICA expression on the development of atherosclerotic disease in vivo, but as rodents do not have a polymorphic NKG2D ligand like MICA, such studies will require careful design. Direct studies of activation of primary immune cells by MICA on endothelial cells are also limited by the need to avoid allo-responses, ideally by using autologous endothelial cells, which is not generally possible. Our findings establish a new mechanism whereby endothelial cells can flag themselves as potential targets for cytotoxic destruction by the immune system in atherosclerotic vascular disease. Other factors may contribute to the regulation of MICA on endothelial cells, and it will be important to define these factors. We have shown that targeted lentivirus-mediated genetic intervention can down-regulate the MICA response to TNFα in primary human endothelial cells. The identification of the overlapping NF-κB-HSE site in the MICA gene allows specific genetic targeting of this NF-κB site while leaving other NF-κB sites unaffected. In the longer term, it may prove possible and beneficial to engineer specific blood-borne therapeutics to control MICA expression in endothelial cells. These could be in the form of genetic therapy, recombinant protein, or small molecules. Such approaches may be of benefit in a wide range of circumstances including atherosclerosis, other inflammatory vascular conditions, and organ transplantation. Furthermore, our results indicate that therapeutic regulation of other genes with overlapping transcription factor-binding sites may be possible using gene therapy with an altered form of one of the transcription factors lacking an activation domain. This approach will be relatively gene-specific in its blockade of the target transcription factor.
Supplementary Material
Acknowledgments
We are grateful to Margaret Jones and Jackie Cordell for advice about immunohistochemical staining, to Dr. Xiaoning Xu for the lentiviral constructs, and Dr Matthew Cockman for the NF-κB p65 expression plasmid and the NF-κB-Luc reporter plasmid.
This work was supported by the Medical Research Council and the National Institute for Health Research Oxford Comprehensive Biomedical Research Centre Programme.

This article contains supplemental Tables 1 and 2, Figs. 1–5, Materials, and an additional reference.
- HSE
- heat shock response element.
REFERENCES
- 1. Cines D. B., Pollak E. S., Buck C. A., Loscalzo J., Zimmerman G. A., McEver R. P., Pober J. S., Wick T. M., Konkle B. A., Schwartz B. S., Barnathan E. S., McCrae K. R., Hug B. A., Schmidt A. M., Stern D. M. (1998) Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91, 3527–3561 [PubMed] [Google Scholar]
- 2. Galkina E., Ley K. (2009) Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 27, 165–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bauer S., Groh V., Wu J., Steinle A., Phillips J. H., Lanier L. L., Spies T. (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 [DOI] [PubMed] [Google Scholar]
- 4. Groh V., Rhinehart R., Randolph-Habecker J., Topp M. S., Riddell S. R., Spies T. (2001) Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2, 255–260 [DOI] [PubMed] [Google Scholar]
- 5. Jamieson A. M., Diefenbach A., McMahon C. W., Xiong N., Carlyle J. R., Raulet D. H. (2002) The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 17, 19–29 [DOI] [PubMed] [Google Scholar]
- 6. Ehrlich L. I., Ogasawara K., Hamerman J. A., Takaki R., Zingoni A., Allison J. P., Lanier L. L. (2005) Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J. Immunol. 174, 1922–1931 [DOI] [PubMed] [Google Scholar]
- 7. Robinson J., Mistry K., McWilliam H., Lopez R., Parham P., Marsh S. G. (2011) The IMGT/HLA database. Nucleic Acids Res. 39, D1171–D1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Groh V., Bahram S., Bauer S., Herman A., Beauchamp M., Spies T. (1996) Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc. Natl. Acad. Sci. U.S.A. 93, 12445–12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mistry A. R., O'Callaghan C. A. (2007) Regulation of ligands for the activating receptor NKG2D. Immunology 121, 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryan U. S. (1987) Activation of endothelial cells. Ann. N.Y. Acad. Sci. 516, 22–38 [DOI] [PubMed] [Google Scholar]
- 11. Mantovani A., Sozzani S., Introna M. (1997) Endothelial activation by cytokines. Ann. N.Y. Acad. Sci. 832, 93–116 [DOI] [PubMed] [Google Scholar]
- 12. McGuire T. R., Trickler W. J., Smith L., Hoie E. B., Miller D. W. (2005) Release of TNF-α and IL-1β from porcine brain endothelium corresponds to the pyrogenic potential of three marketed formulations of amphotericin. Inflamm. Res. 54, 375–379 [DOI] [PubMed] [Google Scholar]
- 13. Gourin C. G., Shackford S. R. (1997) Production of tumor necrosis factor-α and interleukin-1β by human cerebral microvascular endothelium after percussive trauma. J. Trauma 42, 1101–1107 [DOI] [PubMed] [Google Scholar]
- 14. Csiszar A., Wang M., Lakatta E. G., Ungvari Z. (2008) Inflammation and endothelial dysfunction during aging. Role of NF-κB. J. Appl. Physiol. 105, 1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gerritsen M. E., Bloor C. M. (1993) Endothelial cell gene expression in response to injury. FASEB J. 7, 523–532 [DOI] [PubMed] [Google Scholar]
- 16. Félétou M., Vanhoutte P. M. (2006) Endothelial dysfunction. A multifaceted disorder (The Wiggers Award Lecture). Am. J. Physiol. Heart Circ. Physiol. 291, H985–H1002 [DOI] [PubMed] [Google Scholar]
- 17. Haddy N., Sass C., Droesch S., Zaiou M., Siest G., Ponthieux A., Lambert D., Visvikis S. (2003) IL-6, TNF-α, and atherosclerosis risk indicators in a healthy family population. The STANISLAS cohort. Atherosclerosis 170, 277–283 [DOI] [PubMed] [Google Scholar]
- 18. Ridker P. M., Rifai N., Pfeffer M., Sacks F., Lepage S., Braunwald E. (2000) Elevation of tumor necrosis factor-α and increased risk of recurrent coronary events after myocardial infarction. Circulation 101, 2149–2153 [DOI] [PubMed] [Google Scholar]
- 19. Holmén C., Elsheikh E., Stenvinkel P., Qureshi A. R., Pettersson E., Jalkanen S., Sumitran-Holgersson S. (2005) Circulating inflammatory endothelial cells contribute to endothelial progenitor cell dysfunction in patients with vasculitis and kidney involvement. J. Am. Soc. Nephrol. 16, 3110–3120 [DOI] [PubMed] [Google Scholar]
- 20. Zou Y., Stastny P., Süsal C., Döhler B., Opelz G. (2007) Antibodies against MICA antigens and kidney transplant rejection. N. Engl. J. Med. 357, 1293–1300 [DOI] [PubMed] [Google Scholar]
- 21. Sumitran-Holgersson S., Wilczek H. E., Holgersson J., Söderström K. (2002) Identification of the nonclassical HLA molecules, mica, as targets for humoral immunity associated with irreversible rejection of kidney allografts. Transplantation 74, 268–277 [DOI] [PubMed] [Google Scholar]
- 22. Whiteside T. L., Herberman R. B. (1994) Role of human natural killer cells in health and disease. Clin. Diagn. Lab. Immunol. 1, 125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li D., Chen N., McMichael A. J., Screaton G. R., Xu X. N. (2008) Generation and characterization of CD1d tetramer produced by a lentiviral expression system. J. Immunol. Methods 330, 57–63 [DOI] [PubMed] [Google Scholar]
- 24. Madge L. A., Pober J. S. (2001) TNF signaling in vascular endothelial cells. Exp. Mol. Pathol. 70, 317–325 [DOI] [PubMed] [Google Scholar]
- 25. Perkins N. D. (2007) Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 8, 49–62 [DOI] [PubMed] [Google Scholar]
- 26. Beauparlant P., Kwon H., Clarke M., Lin R., Sonenberg N., Wainberg M., Hiscott J. (1996) Transdominant mutants of IκBα block Tat-tumor necrosis factor synergistic activation of human immunodeficiency virus type 1 gene expression and virus multiplication. J. Virol. 70, 5777–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molinero L. L., Fuertes M. B., Girart M. V., Fainboim L., Rabinovich G. A., Costas M. A., Zwirner N. W. (2004) NF-κB regulates expression of the MHC class I-related chain A gene in activated T lymphocytes. J. Immunol. 173, 5583–5590 [DOI] [PubMed] [Google Scholar]
- 28. Rayment N. B., Moss E., Faulkner L., Brickell P. M., Davies M. J., Woolf N., Katz D. R. (1996) Synthesis of TNFα and TGFβ mRNA in the different micro-environments within atheromatous plaques. Cardiovasc. Res. 32, 1123–1130 [DOI] [PubMed] [Google Scholar]
- 29. Abravaya K., Phillips B., Morimoto R. I. (1991) Heat shock-induced interactions of heat shock transcription factor and the human hsp70 promoter examined by in vivo footprinting. Mol. Cell. Biol. 11, 586–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verma I. M., Stevenson J. K., Schwarz E. M., Van Antwerp D., Miyamoto S. (1995) Rel/NF-κB/IκB family. Intimate tales of association and dissociation. Genes Dev. 9, 2723–2735 [DOI] [PubMed] [Google Scholar]
- 31. Hao S., Baltimore D. (2009) The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat. Immunol. 10, 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frangos J. A., Eskin S. G., McIntire L. V., Ives C. L. (1985) Flow effects on prostacyclin production by cultured human endothelial cells. Science 227, 1477–1479 [DOI] [PubMed] [Google Scholar]
- 33. Chiu J. J., Lee P. L., Chen C. N., Lee C. I., Chang S. F., Chen L. J., Lien S. C., Ko Y. C., Usami S., Chien S. (2004) Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-α in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24, 73–79 [DOI] [PubMed] [Google Scholar]
- 34. Champsaur M., Lanier L. L. (2010) Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 235, 267–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jovinge S., Hamsten A., Tornvall P., Proudler A., Båvenholm P., Ericsson C. G., Godsland I., de Faire U., Nilsson J. (1998) Evidence for a role of tumor necrosis factor α in disturbances of triglyceride and glucose metabolism predisposing to coronary heart disease. Metabolism 47, 113–118 [DOI] [PubMed] [Google Scholar]
- 36. Bruunsgaard H., Andersen-Ranberg K., Hjelmborg J. B., Pedersen B. K., Jeune B. (2003) Elevated levels of tumor necrosis factor α and mortality in centenarians. Am. J. Med. 115, 278–283 [DOI] [PubMed] [Google Scholar]
- 37. Zhang L., Connelly J. J., Peppel K., Brian L., Shah S. H., Nelson S., Crosslin D. R., Wang T., Allen A., Kraus W. E., Gregory S. G., Hauser E. R., Freedman N. J. (2010) Aging-related atherosclerosis is exacerbated by arterial expression of tumor necrosis factor receptor-1. Evidence from mouse models and human association studies. Hum. Mol. Genet. 19, 2754–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cicha I., Beronov K., Ramirez E. L., Osterode K., Goppelt-Struebe M., Raaz D., Yilmaz A., Daniel W. G., Garlichs C. D. (2009) Shear stress preconditioning modulates endothelial susceptibility to circulating TNF-α and monocytic cell recruitment in a simplified model of arterial bifurcations. Atherosclerosis 207, 93–102 [DOI] [PubMed] [Google Scholar]
- 39. Maurus C. F., Schneider M. K., Schmidt D., Zünd G., Seebach J. D. (2006) Activation of human microvascular endothelial cells with TNF-α and hypoxia/reoxygenation enhances NK-cell adhesion, but not NK-cytotoxicity. Transplantation 81, 1204–1211 [DOI] [PubMed] [Google Scholar]
- 40. Sorlie P. D., Nieto F. J., Adam E., Folsom A. R., Shahar E., Massing M. (2000) A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease. The atherosclerosis risk in communities (ARIC) study. Arch. Intern. Med. 160, 2027–2032 [DOI] [PubMed] [Google Scholar]
- 41. Espinola-Klein C., Rupprecht H. J., Blankenberg S., Bickel C., Kopp H., Rippin G., Victor A., Hafner G., Schlumberger W., Meyer J. (2002) Impact of infectious burden on extent and long term prognosis of atherosclerosis. Circulation 105, 15–21 [DOI] [PubMed] [Google Scholar]
- 42. Yamashiroya H. M., Ghosh L., Yang R., Robertson A. L., Jr. (1988) Herpesviridae in the coronary arteries and aorta of young trauma victims. Am. J. Pathol. 130, 71–79 [PMC free article] [PubMed] [Google Scholar]
- 43. Bobryshev Y. V., Lord R. S. (2005) Identification of natural killer cells in human atherosclerotic plaque. Atherosclerosis 180, 423–427 [DOI] [PubMed] [Google Scholar]
- 44. Jovinge S., Ares M. P., Kallin B., Nilsson J. (1996) Human monocytes/macrophages release TNF-α in response to Ox-LDL. Arterioscler. Thromb. Vasc. Biol. 16, 1573–1579 [DOI] [PubMed] [Google Scholar]
- 45. Woollard K. J., Geissmann F. (2010) Monocytes in atherosclerosis. Subsets and functions. Nat. Rev. Cardiol. 7, 77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tipping P. G., Hancock W. W. (1993) Production of tumor necrosis factor and interleukin-1 by macrophages from human atheromatous plaques. Am. J. Pathol. 142, 1721–1728 [PMC free article] [PubMed] [Google Scholar]
- 47. Tonetti M. S., D'Aiuto F., Nibali L., Donald A., Storry C., Parkar M., Suvan J., Hingorani A. D., Vallance P., Deanfield J. (2007) Treatment of periodontitis and endothelial function. N. Engl. J. Med. 356, 911–920 [DOI] [PubMed] [Google Scholar]
- 48. Jamison D. T., Breman J. G., Measham A. R., Alleyne G., Claeson M., Evans D. B., Jha P., Mills A., Musgrove P. (2006) Priorities in Health, The World Bank, Washington, D. C: [PubMed] [Google Scholar]
- 49. Hölschermann H., Schuster D., Parviz B., Haberbosch W., Tillmanns H., Muth H. (2006) Statins prevent NF-κB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis 185, 240–245 [DOI] [PubMed] [Google Scholar]
- 50. Hilgendorff A., Muth H., Parviz B., Staubitz A., Haberbosch W., Tillmanns H., Hölschermann H. (2003) Statins differ in their ability to block NF-κB activation in human blood monocytes. Int. J. Clin. Pharmacol. Ther. 41, 397–401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.