Background: The mechanism by which anemia results in lowered hepcidin levels is not clear.
Results: Bone morphogenetic protein (BMP)-binding endothelial cell precursor-derived regulator (BMPER), a known BMP antagonist, was found to be up-regulated in anemic Trfhpx/hpx mice and to suppress hepcidin transcription both in vivo and in vitro.
Conclusion: BMPER is involved in suppressing hepcidin levels in Trfhpx/hpx mice.
Significance: BMPER is a novel regulator of hepcidin and iron metabolism.
Keywords: Angiogenesis, Hepatocyte, Iron, Iron metabolism, Liver, BMPER, CV-2, Anemia, Crossveinless, Hepcidin
Abstract
The BMP/SMAD4 pathway has major effects on liver hepcidin levels. Bone morphogenetic protein-binding endothelial cell precursor-derived regulator (Bmper), a known regulator of BMP signaling, was found to be overexpressed at the mRNA and protein levels in liver of genetically hypotransferrinemic mice (Trfhpx/hpx). Soluble BMPER peptide inhibited BMP2- and BMP6-dependent hepcidin promoter activity in both HepG2 and HuH7 cells. These effects correlated with reduced cellular levels of pSMAD1/5/8. Addition of BMPER peptide to primary human hepatocytes abolished the BMP2-dependent increase in hepcidin mRNA, whereas injection of Bmper peptide into mice resulted in reduced liver hepcidin and increased serum iron levels. Thus Bmper may play an important role in suppressing hepcidin production in hypotransferrinemic mice.
Introduction
Hepcidin is secreted into the circulation by hepatocytes and plays a central role in the regulation of iron metabolism (1–3). Hepatic hepcidin levels are regulated by iron stores (1), hypoxia, erythropoietic rate, and inflammatory status (4, 5). Hepcidin works by blocking the entry of iron into the circulation from the gut and the macrophages by binding to the iron transporter Ferroportin resulting in its ubiquitylation and degradation (6, 7). Low levels of circulating hepcidin are associated with many forms of genetic iron overload (8, 9). Various signaling pathways have been shown to regulate hepatic hepcidin levels with bone morphogenetic proteins, Bmps2 (especially Bmp2, Bmp4, Bmp6, and Bmp9), which signal via SMADs, emerging as key players (10–13). Bmp signaling is extraordinarily complex with a number of extracellular Bmp ligands, including crossveinless-2 (CV-2), Twisted gastrulation (Twsg1), chordin, noggin, and hemojuvelin (hjv), which can act as either pro- and anti-Bmp effectors depending on the context and concentration (14). TWSG1 was recently shown to be elevated in patients with thalassemia and to negatively regulate hepcidin by inhibiting the effects of BMP2 and 4 (15).
Crossveinless-2 (Cv-2) was identified in Drosophila where it was implicated in Bmp signaling and shown to be essential for the formation of small blood vessels or “crossveins” in the wing (16). The orthologous mouse protein named bone morphogenetic protein (Bmp)-binding endothelial cell precursor-derived regulator (Bmper) is highly expressed in developing endothelial cells and binds to Bmp2, -4, and -6 inhibiting BMP signaling (17). Further evidence in zebrafish Bmper mutants supports a role in Bmp signaling during vascular development (18). Bmper has been shown to have both pro- and anti-Bmp activity (16, 17, 19, 20). The protein exists in two forms, a full-length membrane-associated form and a soluble form consisting of a heterodimer of C- and N-terminal cleavage fragments connected via disulfide bonding (19). The cleavage is autocatalytic via a conserved acid-sensitive cleavage site (FGDPH) and is suggested to account for the ability of the protein to act as a pro- or anti-Bmp in vivo (19). The N terminus of Bmper contains five cysteine-rich von Willebrand type C-like domains (named CR1–5) responsible for ligand binding that are conserved in other BMP-binding proteins such as chordin. Bmper binds Bmps with high affinity similar to that of Bmp type I and type II receptors themselves (19). The C-terminal of Bmper contains a furin-like, a von Willebrand type D, and a trypsin inhibitor domain (16).
Hypotransferrinemic mice (Trfhpx/hpx) have defective transferrin gene expression leading to low levels of plasma transferrin (<1% of wild type) and, despite massive iron loading, have very low levels of hepcidin in hepatocytes (21). The precise mechanism of hepcidin suppression in Trfhpx/hpx mice, as in other conditions of enhanced erythropoiesis, anemia, and hypoxia, remains unclear and may involve a number of signaling pathways. We sought to identify potential hepcidin regulators produced locally in liver of Trfhpx/hpx mice. We identified a known Bmp regulator Bmper as being highly up-regulated in liver of Trfhpx/hpx mice. In this report we show that Bmper can suppress hepcidin promoter activity and reduce hepcidin levels in liver cells both in vitro and in vivo via effects on the BMP pathway.
EXPERIMENTAL PROCEDURES
Animals
HPX mice (Trfhpx/hpx) were bred and maintained as described previously (22). Homozygous Trfhpx/hpx mice were identified at birth. Normal littermates (mixture of Trf+/+ and Trfhpx/+) were used as controls. To study the effect of Bmper injections, male 6-week-old CD-1 mice (4 mice per group) were injected intraperitoneally with 50 μg of mouse Bmper peptide dissolved in PBS (R&D Systems) or PBS alone. After 18 h mice were sacrificed and liver RNA extracted. Hepcidin mRNA was determined by real-time PCR (described below). Serum iron was determined using a commercial kit (Quanti chrom, Bioassays). All animal experiments were performed under a UK Home Office License.
Microarrays
Liver RNA was extracted using TRIzol reagent (Invitrogen) from two male HPX mice and two male WT mice. Labeled cDNA was synthesized according to the manufacturer's instructions (Affymetrix, Santa Clara, CA) and hybridized to Mouse Genome 430 2.0 Arrays (Affymetrix). Gene expression analysis was performed using Genespring software (Agilent Technologies, Santa Clara, CA).
Western Blotting and Immunocytochemistry
Whole cell lysates were extracted from mouse liver tissue samples by homogenization in 500 μl of RIPA buffer (10 mm Tris, 150 mm Nacl, 1 mm EDTA, 1% Nonidet P-40, 0.1% SDS) and protease inhibitor mixture (1:200 dilution, Sigma-Aldrich). The homogenates were centrifuged at 3000 rpm at 4 °C for 5 min. The resulting supernatant was quantified using a BSA assay (Bio-Rad), resolved by SDS-PAGE, and transferred onto a PVDF membrane. Membranes were incubated sequentially with primary and secondary antibodies. Primary antibodies, Crossveinless-2 (R&D Systems), pSMAD 1/5/8, and SMAD1 (Cell Signaling Technology), or actin (Sigma-Aldrich) were used for Western blots and/or immunocytochemistry. Following incubation with the appropriate secondary antibodies, blots were visualized by chemiluminescence (Pierce, Thermo Scientific). For immunocytochemistry, liver samples from mice were frozen in freezing medium (Bright Instruments) using liquid nitrogen. Sections (5-μm thickness) were cut using a cryostat microtome (Bright Instruments) and placed on polylysine-coated slides (VWR International, Leuven, Belgium). Sections were fixed in 100% acetone for 10 min at 4 °C before being incubated for 20 min in blocking buffer (1% BSA in PBS) followed by incubation in primary antibody for crossveinless-2 (1:100) for 1 h. Thereafter, sections were washed in PBS and incubated with FITC-conjugated anti-goat secondary (Dako), 1:50 for 20 min, followed by washing in PBS and addition of mounting medium containing propidium iodide (Vector Laboratories). Images were captured using an LS-2 confocal microscope (Leica).
Real-time PCR
One microgram of total RNA was reverse-transcribed, and cDNA was synthesized using a Transcriptor High Fidelity cDNA kit (Roche Diagnostics). All primers were designed using Universal Probe Library system (Roche Diagnostics) software, and qPCR was performed using an ABI Prism 3700 PCR machine (ABI). See supplemental materials for all primer sequences. Results were normalized to the housekeeping gene RPL-19 and expressed as -fold change using the method of Livak and Schmittgen (23).
Cell Culture and Hepcidin Promoter Assays
Hepatic cells HuH7 and HepG2 were obtained from the ATCC and cultured in Dulbecco's modified Eagle's medium (DMEM, Sigma) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Sigma), penicillin-streptomycin (Sigma), and glutamine (Sigma). Cell cultures were maintained at 37 °C under 95% air/5% CO2. Cells (2.5 × 105 cells/ml) were seeded onto 24-well plates for transfections. Recombinant human peptides for BMP2 and BMP6, BMPER, and TWSG1 were obtained from R&D Systems. Approximately 2.7 kb of the human hepcidin promoter relative to the transcriptional start site containing the three known BMP response elements (BMP-RE1–3) (24) was subcloned into the pGL3-basic luciferase reporter vector (Promega) utilizing MluI and HindIII restriction sites. In addition, a mutant hepcidin promoter was generated by substituting two nucleotides in the BMP RE2 from GGCGCC to GGAACC (position −2250 to −2246 relative to the transcriptional start site) using a QuikChange site-directed mutagenesis kit (Stratagene). All constructs were sequenced. The pGL3-hepcidin reporter constructs were co-transfected into cells with a TK-Renilla control reporter at a 3:1 ratio using Fugene-6 (Roche) according to the manufacturer's instructions. After 6 h, the cells were treated with human BMP-2 (25 ng/ml, 16–18 h) or human BMP-6 (25 ng/ml, 16–18 h), in the presence or absence of human BMPER (1–2500 ng/ml, 16–18 h) or human TWSG1 (1–1000 ng/ml, 16–18 h). Luminescence was detected using the Dual-Luciferase Reporter Assay system (Promega) and measured by using a luminometer (Promega). Relative luciferase activity was calculated and expressed as the ratio of the signal of firefly luciferase to TK-Renilla.
Primary Hepatocytes
Human hepatocytes were isolated from donor liver segments/lobes. All tissues were consented for research in accordance with the Research Ethics Committee of King's College Hospital. Cell isolation was carried out using a modified two-step collagenase perfusion technique, and hepatocytes were purified by low speed centrifugation at 50 × g for 5 min at 4 °C (25). Cells were cryopreserved in University of Wisconsin solution containing 10% DMSO using a controlled-rate freezer and stored at −140 °C before rapid thawing for use.
Statistical Analysis
Data are presented as means ± S.D. Data analysis was performed using SPSS (IBM), and statistical differences were determined where appropriate using either ANOVA followed by Tukey's post hoc test or two-tailed Student's t test.
RESULTS
BMPER Levels in Trfhpx/hpx Mice
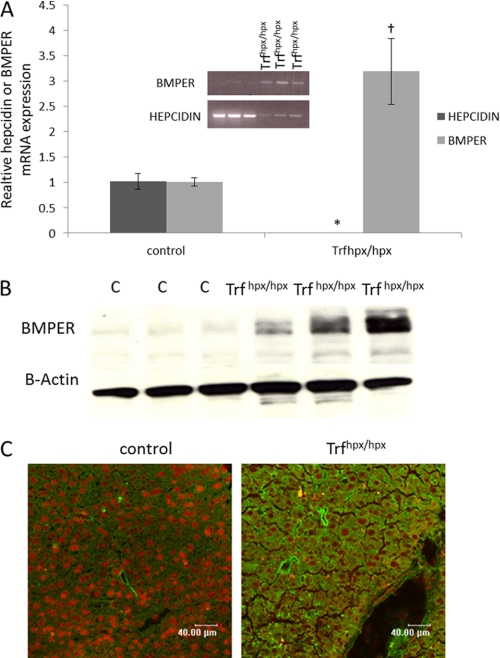
As previously shown by others (21, 26) hepcidin levels are lowered in Trfhpx/hpx relative to control mice (Fig. 1A). In contrast Bmper protein and mRNA levels were both markedly increased in liver of Trfhpx/hpx mice compared with control mice (Fig. 1, A–C). In liver sections from control mice BMPER protein expression appeared to be restricted to cells lining blood vessels, whereas in Trfhpx/hpx mice, BMPER expression was observed in other cell types, including hepatocytes (Fig. 1C).
FIGURE 1.
BMPER expression in Trfhpx/hpx mice. A, qPCR shows relative Bmper and hepcidin mRNA levels (the results were normalized to the housekeeping gene RPL-19) in 10- and 11-week-old male Trfhpx/hpx mice (n = 3). Trfhpx/+ were used as control (C) mice (n = 3). The inset gel picture shows a semiquantitative RT-PCR assay for Bmper and hepcidin in the same samples. B, Western blots showing BMPER expression in the same mice as in A. C, BMPER staining (green) in liver sections from male, 7-week-old Trfhpx/hpx compared with age- and sex-matched control (Trfhpx/?), counterstain is propidium iodide (*, p < 0.003; †, p < 0.03, compared with control mice, Student's t test).
Effect of BMPER Peptide on Hepcidin Transcription
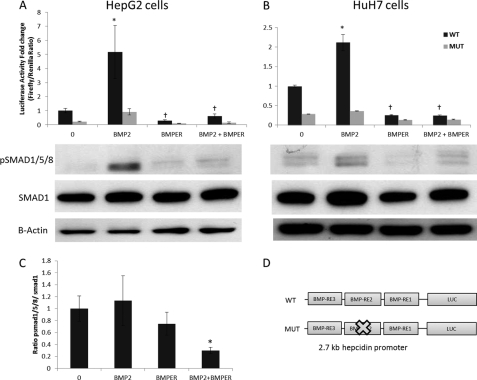
We investigated the effect of recombinant soluble human BMPER peptide on hepcidin transcription using hepcidin promoter reporter (luciferase) assays in two liver cell lines, HepG2 and HuH7. BMPER treatment strongly inhibited BMP2-dependent hepcidin promoter activity at a molar ratio of 1:15 (BMP2:BMPER) in both cell lines (Fig. 2, A and B). The hepcidin promoter contains three known BMP response elements (BMP-RE1–3, Fig. 2D). BMP-RE1 and -2 are essential for BMP-dependent regulation of hepcidin transcription (24). In agreement with previous studies of Casanovas and colleagues (24), we found that mutation of the BMP-RE2 greatly reduced the responsiveness of the hepcidin promoter to BMP2 (Fig. 2, A and B). In agreement with the previous results we found the residual promoter activity of the mutated construct showed a similar pattern to the WT construct in the presence of BMP2 and BMPER albeit at a lower level (Fig. 2, A and B). To confirm that the effects of BMPER were via SMAD signaling, pSMAD1/5/8 levels were measured by Western blotting (Fig. 2, A and B, lower panels). As expected pSMAD 1/5/8 levels were increased by BMP2 treatment, and this induction was suppressed by the presence BMPER in both cell lines (Fig. 2, A and B, lower panels). The ratio of pSMAD1/5/8:SMAD1 was significantly reduced in HepG2 cells treated with BMP2 and BMPER compared with BMP2 treatment alone (Fig. 2C).
FIGURE 2.
Effect of soluble BMPER on hepcidin promoter activity and pSMAD levels in HepG2 and HuH7 cells. A, luciferase assays showing effect of BMPER on BMP2-dependent activation on the WT hepcidin promoter and mutant (BMP-RE2) constructs in HepG2 cells. B, HuH7 cells treated as above. Lower panels, Western blots showing pSMAD1/5/8 levels in the respective cells under the same conditions. Cells were exposed to 25 ng/ml BMP2 and 2500 ng/ml BMPER or a combination of these. When given together, the molar ratio was 1:15 (BMP2:BMPER). C, densitometry from Western blots performed in triplicate showing the ratio of pSMAD1/5/8:SMAD1 in HepG2 cells (*, p < 0.05 BMP2 alone compared with BMP2+BMPER, Student's t test). D, diagrammatic representation of WT hepcidin promoter luciferase construct used with three intact BMP response elements (BMP-RE1–3) and mutated construct in which BMP-RE2 was mutated. Luciferase assays shown are means ± S.D. derived from a single experiment with three biological replicates, and the experiment shown is representative of three similar experiments (*, p < 0.001 control versus BMP2; †, p < 0.001 BMP2 versus BMPER and BMP2 versus BMPER+BMP2, multiple ANOVA with Tukey's post hoc analysis). Densitometry was performed on Western blots using three biological replicates for each condition.
Effect of BMPER Peptide on HAMP Levels in Human Primary Hepatocytes
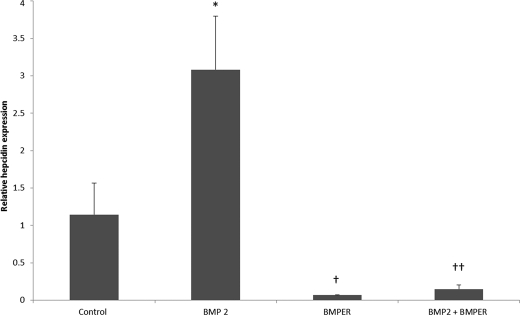
To confirm the effects of BMPER on endogenous Hamp levels we used isolated primary human hepatocytes treated with and without BMP2 and measured Hamp mRNA levels by qPCR. Exposure of primary hepatocytes to BMP2 alone produced a 3-fold induction in Hamp mRNA levels, however in the presence of BMPER this effect was abolished (Fig. 3). Thus, consistent with the effect of BMPER on the hepcidin promoter assays, BMPER can robustly modulate endogenous Hamp mRNA levels in primary hepatocytes.
FIGURE 3.
Effect of BMPER on hepcidin mRNA levels in primary human hepatocytes. Effect of BMPER (2500 ng/ml) added to medium for 16 h on hepcidin levels in primary hepatocytes in the presence and absence of BMP2 (25 ng/ml). Experiment shows mean ± S.D. of three biological replicates for each condition. *, p < 0.05 control versus BMP2; †, p < 0.01 BMP2 versus BMPER; ††, p < 0.01 BMP2 versus BMP2+BMPER (one-way ANOVA with Tukey's post hoc analysis).
BMPER Inhibits BMP6-mediated Induction of Hepcidin Transcription
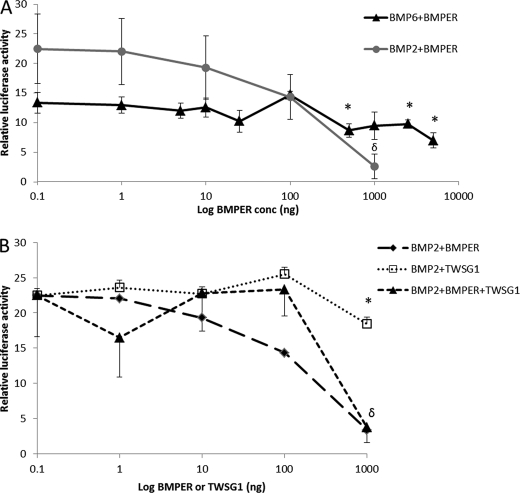
Because BMP6 is thought to be the iron stores regulator and has been shown to be important in modulating iron metabolism (13), we tested the effect of BMPER against BMP6 using luciferase hepcidin promoter assays. Addition of increasing amounts of BMPER resulted in a significant inhibition of BMP6-induced hepcidin promoter activity (Fig. 4A), although the effect was less marked than for BMP2 (35% reduction of Bmp6-induced activity compared with a 90% reduction of BMP2-mediated activity).
FIGURE 4.
Effect of BMPER and TWSG1 on BMP2- and BMP6-dependent hepcidin promoter activity in HepG2 cells. A, luciferase reporter assays showing the effect of increasing doses of BMPER (1–5000 ng/ml) on BMP6 (25 ng/ml)- and BMP2 (25 ng/ml)-dependent hepcidin promoter activity (*, p < 0.03; δ, p < 0.01 compared with control levels, multiple ANOVA with Tukey's post hoc analysis). B, effect of BMPER (1–1000 ng/ml) and TWSG1 (1–1000 ng/ml) alone or in combination on BMP2 (25 ng/ml)-dependent hepcidin promoter activity (*, p < 0.05; δ, p < 0.01; multiple ANOVA with Tukey's post hoc analysis). Luciferase assays shown are means ± S.D. derived from a single experiment with three biological replicates, and the experiment shown is representative of three similar experiments.
Effect of BMPER and TWSG1, Alone or in Combination, on Hepcidin Promoter Activity
TWSG1 has also been shown to inhibit BMP-mediated effects in hepcidin promoter studies, whereas other studies have suggested Bmper and Twsg1 interact to form a ternary complex together with that of Bmp (27). When given alone, BMPER exhibited a dose response inhibition against BMP2 (25 ng/ml)-mediated activity resulting in an 83% reduction in activity at 1000 ng/ml (equivalent to 1:6 molar ratio BMP2:BMPER) (Fig. 4B). TWSG1 also exhibited a dose response but was less potent than BMPER, inhibiting BMP2 effects by only 46% at 1000 ng/ml (equivalent to a 1:23 molar ratio BMP2:TWSG1). When both TWSG1 and BMPER were added jointly BMP2-induced hepcidin transcriptional activity was decreased to similar levels to that observed with BMPER alone indicating there was no additive effect.
Effect of Bmper Injection in Normal Mice
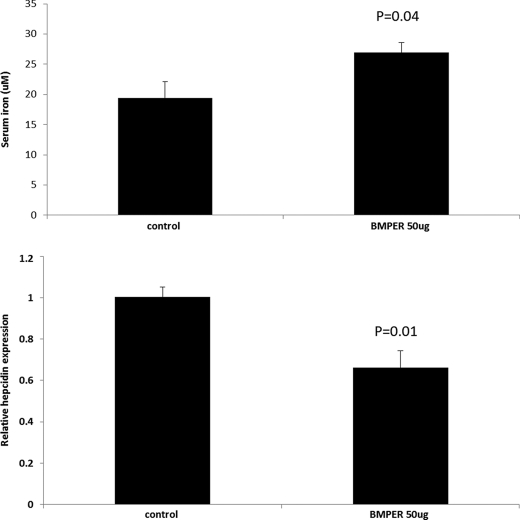
To investigate the effect of Bmper in vivo CD1 mice were injected with a single dose of 50 μg of mouse Bmper peptide. After 18 h mice were sacrificed, and serum iron and liver hepcidin mRNA levels were measured. Compared with control saline-injected mice, mice injected with BMPER peptide showed a significant increase in serum iron together with a significant decrease in liver hepcidin mRNA levels (Fig. 5).
FIGURE 5.
Effect of BMPER peptide on serum iron and liver hepcidin in CD1 mice. 7-week-old male mice received an intraperitoneal injection of either 50 μg of BMPER in PBS or PBS alone. Mice were sacrificed 18 h after injection, and serum iron and liver hepcidin levels were measured (n = 4 mice per group; p values are shown from Student's t test).
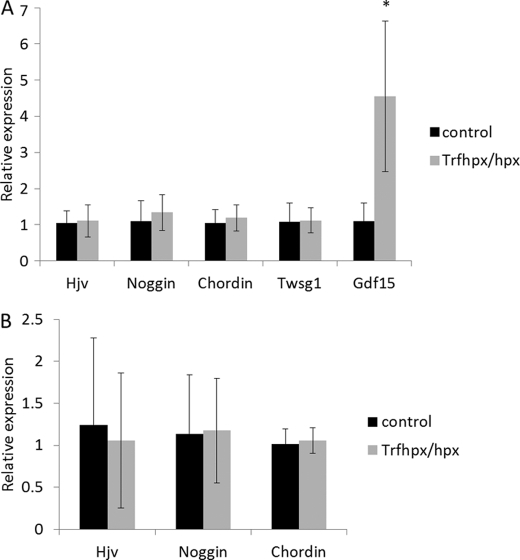
We also measured the mRNA levels of other known BMP antagonists (Hjv, Noggin, Chordin, and Twsg1) as well as the hepcidin inhibitor Gdf15 by qPCR in liver of Trfhpx/hpx mice and controls. We found no significant changes in any of the BMP antagonists listed, however levels of Gdf15 were significantly raised in liver of Trfhpx/hpx mice (Fig. 6A). Because some BMP antagonists such as Hjv are highly expressed in other tissues such as skeletal muscle we also measured levels of Hjv, Noggin, and Chordin in skeletal muscle of Trfhpx/hpx mice and controls (Fig. 6B). No significant changes were found.
FIGURE 6.
Expression levels of other potential hepcidin modifiers in liver and muscle of Trfhpx/hpxversus control mice. A, qPCR showing relative expression of BMP antagonists, Hjv, Noggin, Chordin, Twsg1, and hepcidin modulator Gdf15 expression in liver of male Trfhpx/hpx mice versus age-matched Trfhpx/+ male mice (four mice per group). B, qPCR showing relative expression of Hjv, Noggin, and Chordin mRNA levels in muscle in the same mice as above.
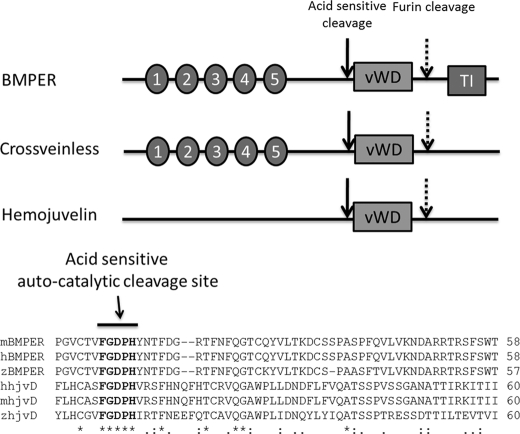
Multiple sequence alignment of BMPER and HJV protein sequences from several species (human, mouse, and zebrafish) reveal that both proteins share a von Willebrand type D domain and a fully conserved acid-sensitive autocatalytic domain (Fig. 7). BMPER contains five well conserved cysteine repeat regions (CR1–5), which are involved in BMP binding. Interestingly, these domains are absent in HJV, suggesting HJV must bind BMPs by an alternative mechanism.
FIGURE 7.
Sequence comparison of BMPER, Cv-2, and Hjv. Upper panel, BMPER, Hjv, and Cv-2 share a common von Willebrand type D domain (vWD), a fully conserved acid-sensitive autocatalytic cleavage site, and a C-terminal furin cleavage site. BMPER and Cv-2 contain five N-terminal cysteine-rich regions (CR1–5), which are involved in binding BMPs; these domains are absent in Hjv. Lower panel, multiple alignment (Clustal) of mouse, human, and zebrafish BMPER and Hjv sequences showing a fully conserved acid-sensitive autocatalytic cleavage site. TI = trypsin inhibitor domain.
DISCUSSION
Anemia has been shown to strongly down-regulate liver hepcidin expression, even when iron stores are greatly increased as found in Trfhpx/hpx mice (4, 21). This has led to the notion that one or more secreted humoral factors are released from the marrow under increased erythropoietic drive that can influence hepatic hepcidin levels. Levels of GDF15 and TWSG1 in the marrow are increased in patients with β-thalassemia, and both have been shown to inhibit hepcidin production in vitro, the latter by inhibiting the action of BMP4 and BMP2 (15). Bartnikas et al. found that both Gdf15 and Twsg1 levels were raised in spleen of Trfhpx/hpx mice; however, when erythropoiesis was inhibited with doxorubicin, levels of both decreased without significant change in liver hepcidin levels. This suggests Gdf15 and Twsg1 may be markers for increased erythropoiesis but do not directly regulate hepcidin in vivo (26). We found that Gdf15 levels were increased in liver of Trfhpx/hpx mice, which may be reflective of some extra medullary or stress erythropoiesis occurring in liver of Trfhpx/hpx mice. However, no changes in Twsg1, Hjv, or other known BMP antagonists Noggin and Chordin were observed. We also found no changes in Hjv, Noggin, or Chordin in skeletal muscle of Trfhpx/hpx. Hence, of the BMP antagonists tested, only Bmper was highly up-regulated in Trfhpx/hpx mice.
Our results revealed that excess soluble BMPER peptide inhibited BMP-dependent hepcidin transcription as well as endogenous hepcidin production in hepatocytes by acting as an anti-Bmp and inhibiting the SMAD pathway. The mechanism is likely based on the ability of BMPER to bind and sequester BMP molecules with high affinity and thereby prevent BMPs from binding to BMP type I and II receptors (28, 29). It is noteworthy that in some situations Bmper has been shown to elicit pro-BMP responses, a function that may be dependent on whether the protein is membrane-associated or soluble (28). In this respect, BMPER may function in a similar manner to HJV where the soluble form is anti-BMP and membrane form is pro-BMP. Both HJV and BMPER share several structural features such as a conserved autocatalytic cleavage site and C-terminal furin cleavage sites. In our study we did not test the effect of full-length membrane-associated BMPER; however, we did not observe pro-BMP effects using the soluble BMPER peptide.
BMPER has been shown to bind to BMP2, -4, and -6 (17). In agreement our results showed that BMPER inhibited the effects of both BMP2 and BMP6 on hepcidin production, although the effect on BMP6 was less pronounced than that for BMP2. The reasons for this are not clear as yet; however, BMPER is likely to bind to the various BMPs with different affinities.
TWSG1 has been shown to inhibit hepcidin (15) production induced by BMP2 and -4 in hepatocytes, whereas other reports suggest TWSG1 forms a ternary complex with Bmper and Bmp4 (27) thereby increasing the binding of Bmp4 to Bmper. In our experiment comparing relative effects of BMPER and TWSG1 we used 25 ng of BMP2 and 1000 ng of TWSG1 and BMPER. This is equivalent to a 1:23 molar ratio of BMP2 to TWSG1 and a 1:6 molar ratio of BMP2 to BMPER. Hence using four times less BMPER than TWSG1 resulted in an 80% inhibition of BMP2-dependent activity, compared with ∼40% for TWSG1. We therefore conclude that BMPER is more potent than TWSG1 at inhibiting BMP-induced promoter activity. This is consistent with data of others showing that the zebrafish orthologue of BMPER, Cv-2, had a binding affinity for BMP2 some 20-fold higher than TWSG1 and was considerably more potent at inhibiting the binding of BMP2 to both BMP type I and type II receptors (28). In summary we found that BMPER was more potent at inhibiting BMP effects compared with TWSG1, and there was no evidence of a significant additive anti-BMP effect of BMPER and TWSG1 together.
Trfhpx/hpx mice have very low circulating levels of diferric transferrin, and in common with human patients with β- thalassemia, very low hepatic hepcidin levels associated with increased erythropoiesis. However, unlike β-thalassemic patients, Trfhpx/hpx mice do not have ineffective erythropoiesis. Instead they have expanded marrow due to iron-restricted erythropoiesis and exhibit life-long anemia and tissue hypoxia due to the lack of transferrin. Reduced levels of Tfr2 have also been reported in Trfhpx/hpx mouse liver, which may also contribute to reduced hepcidin transcription (30). Although it is not known whether one or a combination of these factors contributes to the lowered hepcidin in Trfhpx/hpx mice, evidence suggests that both diferric transferrin levels and the anemia are important (26, 31). Iron absorption is much higher and hepcidin levels lower in Trfhpx/hpx mice compared with b-thalassemic mice, and as a result iron accumulation in the liver is some 6-fold higher as well (32). This implies other factors are more important than the anemia alone for hepcidin down-regulation in Trfhpx/hpx mice. Bmp6 levels are increased in Trfhpx/hpx mouse liver indicating that iron sensing is intact (26). Our finding that the BMP ligand BMPER is significantly increased in Trfhpx/hpx liver could explain why Trfhpx/hpx mice fail to elicit a hepcidin response despite increased hepatic iron and BMP6 levels.
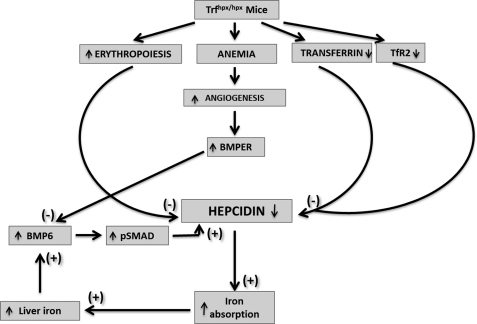
It has been suggested that BMPER plays a role in blood vessel sprouting and remodeling, because mutations in the Bmper gene (Cv-2) adversely affect the formation of small blood vessels in Drosophila wing (16), whereas in zebrafish mutations affect vasculogenesis and blood vessel remodeling (18). We hypothesize that, under severe chronic anemia as found in Trfhpx/hpx mice, BMPER levels are raised to support blood vessel development and remodeling, to counteract the anemia, leading to increased levels of soluble BMPER, sequestration of BMPs, and the down-regulation of hepatic hepcidin levels through inhibition of the SMAD signaling pathway (Fig. 8).
FIGURE 8.
Model showing mechanisms of hepcidin down-regulation in Trfhpx/hpx mice. Hepcidin levels in Trfhpx/hpx mice are suppressed by several mechanisms: increased erythropoietic drive, decreased plasma Trf, decreased TfR2, and increased BMPER levels. The resulting increase in iron absorption leads to liver iron loading, which increases liver BMP6 levels. Increased BMPER levels prevent positive regulation of hepcidin via BMP6.
In summary, hepcidin down-regulation in Trfhpx/hpx mice is likely to be a complex interplay of a number of factors exerting downward repression on hepcidin. BMPER adds to the list of factors that can suppress hepcidin levels and therefore affect iron metabolism. Increased BMPER levels in Trfhpx/hpx mice would also explain why increased BMP6 fails to induce an increase in hepcidin levels. BMPER may play a role in suppressing hepcidin in other forms of severe chronic anemia with iron loading or in diseases where there is a significant amount of angiogenesis.
Supplementary Material
Acknowledgment
We acknowledge the help of Estibaliz Aldecoa-Otalora Astarloa.
This work was supported by the Kings College Graduate School Bursary (to N. P.) and by the Anandamahidol Foundation (to P. M.).

This article contains supplemental list of primers.
- Bmp
- bone morphogenetic protein
- Bmper
- Bmp-binding endothelial cell precursor-derived regulator
- CV-2
- crossveinless-2
- Twsg1
- Twisted gastrulation
- hjv
- hemojuvelin
- ANOVA
- analysis of variance.
REFERENCES
- 1. Pigeon C., Ilyin G., Courselaud B., Leroyer P., Turlin B., Brissot P., Loréal O. (2001) A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 276, 7811–7819 [DOI] [PubMed] [Google Scholar]
- 2. Park C. H., Valore E. V., Waring A. J., Ganz T. (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 276, 7806–7810 [DOI] [PubMed] [Google Scholar]
- 3. Nicolas G., Bennoun M., Devaux I., Beaumont C., Grandchamp B., Kahn A., Vaulont S. (2001) Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc. Natl. Acad. Sci. U.S.A. 98, 8780–8785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicolas G., Chauvet C., Viatte L., Danan J. L., Bigard X., Devaux I., Beaumont C., Kahn A., Vaulont S. (2002) The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Invest. 110, 1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nemeth E., Valore E. V., Territo M., Schiller G., Lichtenstein A., Ganz T. (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101, 2461–2463 [DOI] [PubMed] [Google Scholar]
- 6. Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., Kaplan J. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 [DOI] [PubMed] [Google Scholar]
- 7. De Domenico I., Ward D. M., Langelier C., Vaughn M. B., Nemeth E., Sundquist W. I., Ganz T., Musci G., Kaplan J. (2007) The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol. Biol. Cell 18, 2569–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nemeth E., Ganz T. (2006) Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 26, 323–342 [DOI] [PubMed] [Google Scholar]
- 9. Nemeth E., Ganz T. (2006) Hepcidin and iron-loading anemias. Haematologica 91, 727–732 [PubMed] [Google Scholar]
- 10. Babitt J. L., Huang F. W., Wrighting D. M., Xia Y., Sidis Y., Samad T. A., Campagna J. A., Chung R. T., Schneyer A. L., Woolf C. J., Andrews N. C., Lin H. Y. (2006) Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat. Genet. 38, 531–539 [DOI] [PubMed] [Google Scholar]
- 11. Babitt J. L., Huang F. W., Xia Y., Sidis Y., Andrews N. C., Lin H. Y. (2007) Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J. Clin. Invest. 117, 1933–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andriopoulos B., Jr., Corradini E., Xia Y., Faasse S. A., Chen S., Grgurevic L., Knutson M. D., Pietrangelo A., Vukicevic S., Lin H. Y., Babitt J. L. (2009) BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Genet. 41, 482–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meynard D., Kautz L., Darnaud V., Canonne-Hergaux F., Coppin H., Roth M. P. (2009) Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat. Genet. 41, 478–481 [DOI] [PubMed] [Google Scholar]
- 14. Bier E. (2008) Intriguing extracellular regulation of BMP signaling. Dev. Cell 15, 176–177 [DOI] [PubMed] [Google Scholar]
- 15. Tanno T., Porayette P., Sripichai O., Noh S. J., Byrnes C., Bhupatiraju A., Lee Y. T., Goodnough J. B., Harandi O., Ganz T., Paulson R. F., Miller J. L. (2009) Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood 114, 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conley C. A., Silburn R., Singer M. A., Ralston A., Rohwer-Nutter D., Olson D. J., Gelbart W., Blair S. S. (2000) Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127, 3947–3959 [DOI] [PubMed] [Google Scholar]
- 17. Moser M., Binder O., Wu Y., Aitsebaomo J., Ren R., Bode C., Bautch V. L., Conlon F. L., Patterson C. (2003) BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol. Cell. Biol. 23, 5664–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moser M., Yu Q., Bode C., Xiong J. W., Patterson C. (2007) BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish. J. Mol. Cell. Cardiol. 43, 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rentzsch F., Zhang J., Kramer C., Sebald W., Hammerschmidt M. (2006) Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development 133, 801–811 [DOI] [PubMed] [Google Scholar]
- 20. Binnerts M. E., Wen X., Canté-Barrett K., Bright J., Chen H. T., Asundi V., Sattari P., Tang T., Boyle B., Funk W., Rupp F. (2004) Human Crossveinless-2 is a novel inhibitor of bone morphogenetic proteins. Biochem. Biophys. Res. Commun. 315, 272–280 [DOI] [PubMed] [Google Scholar]
- 21. Weinstein D. A., Roy C. N., Fleming M. D., Loda M. F., Wolfsdorf J. I., Andrews N. C. (2002) Inappropriate expression of hepcidin is associated with iron refractory anemia. Implications for the anemia of chronic disease. Blood 100, 3776–3781 [DOI] [PubMed] [Google Scholar]
- 22. McKie A. T., Marciani P., Rolfs A., Brennan K., Wehr K., Barrow D., Miret S., Bomford A., Peters T. J., Farzaneh F., Hediger M. A., Hentze M. W., Simpson R. J. (2000) A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 5, 299–309 [DOI] [PubMed] [Google Scholar]
- 23. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 24. Casanovas G., Mleczko-Sanecka K., Altamura S., Hentze M. W., Muckenthaler M. U. (2009) Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J. Mol. Med. 87, 471–480 [DOI] [PubMed] [Google Scholar]
- 25. Mitry R. R., Hughes R. D., Aw M. M., Terry C., Mieli-Vergani G., Girlanda R., Muiesan P., Rela M., Heaton N. D., Dhawan A. (2003) Human hepatocyte isolation and relationship of cell viability to early graft function. Cell Transplant. 12, 69–74 [DOI] [PubMed] [Google Scholar]
- 26. Bartnikas T. B., Andrews N. C., Fleming M. D. (2011) Transferrin is a major determinant of hepcidin expression in hypotransferrinemic mice. Blood 117, 630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ambrosio A. L., Taelman V. F., Lee H. X., Metzinger C. A., Coffinier C., De Robertis E. M. (2008) Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev. Cell 15, 248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J. L., Huang Y., Qiu L. Y., Nickel J., Sebald W. (2007) von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J. Biol. Chem. 282, 20002–20014 [DOI] [PubMed] [Google Scholar]
- 29. Zhang J. L., Qiu L. Y., Kotzsch A., Weidauer S., Patterson L., Hammerschmidt M., Sebald W., Mueller T. D. (2008) Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev. Cell 14, 739–750 [DOI] [PubMed] [Google Scholar]
- 30. Robb A., Wessling-Resnick M. (2004) Regulation of transferrin receptor 2 protein levels by transferrin. Blood 104, 4294–4299 [DOI] [PubMed] [Google Scholar]
- 31. Raja K. B., Pountney D. J., Simpson R. J., Peters T. J. (1999) Importance of anemia and transferrin levels in the regulation of intestinal iron absorption in hypotransferrinemic mice. Blood 94, 3185–3192 [PubMed] [Google Scholar]
- 32. Latunde-Dada G. O., McKie A. T., Simpson R. J. (2006) Animal models with enhanced erythropoiesis and iron absorption. Biochim. Biophys. Acta 1762, 414–423 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.