Abstract
Cancer-related fatigue (CRF) is influenced and modulated by a number of critical factors, and the mechanism that is both necessary and sufficient to induce development of severe fatigue in patients with cancer has not yet been identified. Specific research efforts on understanding the factors that may contribute to development of CRF have been made, such as studies of the direct effects of tumor burden, the effects of cancer treatment, and other pathophysiological and psychosocial conditions. Compelling new hypotheses regarding the pathophysiology of CRF have been proposed, such as the pro-inflammatory hypothesis, the serotonin hypothesis, the vagal-afferent-activation hypothesis, the anemia hypothesis, and the adenosine triphosphate hypothesis; some of these have been tested in both animal models and humans and some in animals only.
Gaining an understanding of the specific mechanisms related to the development of fatigue in patients with cancer and survivors of cancer requires further investigation. Pathophysiological research in CRF could be applied in the clinic to improve diagnosis of CRF and to enable administration of mechanism-driven interventions. A targeted intervention study with CRF as a primary end point would also be useful.
Introduction
Cancer-related fatigue (CRF) is one of the most common and complex symptoms experienced by patients with cancer, occurring across the spectrum of malignant disease diagnoses and major therapies. Gaining an understanding of the mechanisms underlying this highly prevalent and burdensome symptom is of great interest to researchers and clinicians alike, yet relatively few studies have evaluated the etiology of CRF or the factors that mediate multiple, related physiologic effects.1,2 The multifactorial and multidimensional nature of CRF has hindered the development of methodologies for evaluating its underlying CRF; consequently, a lack of mechanism-driven clinical trials exploring effective pharmacologic therapies has hampered the effective management of CRF.3 In sum, CRF is a challenging and controversial subject for both researchers and clinical caregivers, and it is also a significant issue for the many patients with cancer who are unable to get out of bed and function normally.
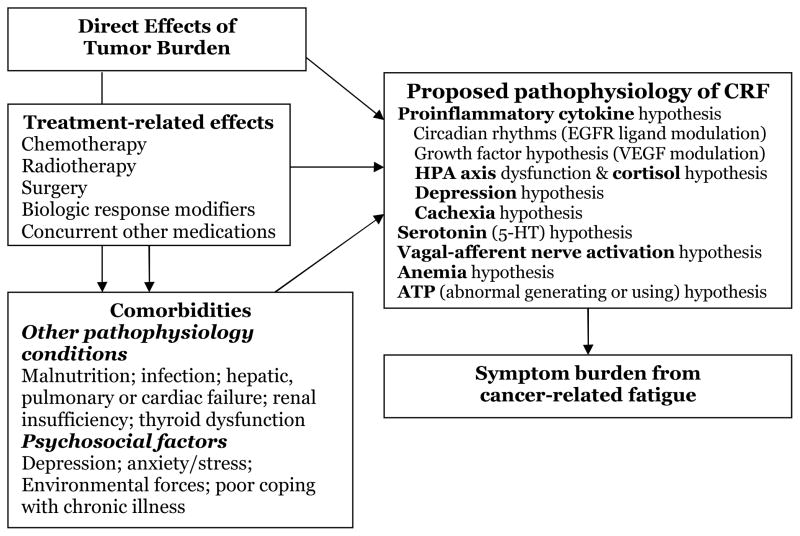
The pathophysiology of CRF has not been adequately elucidated. Clinical studies have focused on understanding factors that contribute to CRF, including the disease itself, treatments received, and a variety of chronic physical or psychological comorbid conditions, such as anemia, pain, depression, anxiety, cachexia, sleep disturbance, and immobility (Figure 1). Although several mechanisms for the pathophysiology of CRF have been proposed, little progress has been made toward identifying reliable physiological marker(s) as objective measures of fatigue.
Figure 1. Proposed potential causes of CRF.
ATP, adenosine triphosphate; EGFR, epidermal growth factor receptor; HPA, hypothalamic-pituitary-adrenal axis; VEGF, vascular endothelial growth factor.
CRF has been analyzed from physiological, anatomical, and psychological perspectives.4 The central governor model posits that fatigue develops in the brain and spinal cord (“central fatigue,” as opposed to “peripheral fatigue,” which occurs in the neuromuscular junctions and muscle tissues).5,6 Central fatigue, defined as difficulty in the initiation or maintenance of voluntary activities,7 manifests as “a failure to complete physical and mental tasks that require self-motivation and internal cues, in the absence of demonstrable cognitive failure or motor weakness”.8,9 In this model, to which CRF seems well fitted, fatigue is a complex emotion affected by motivation and drive, fear and anger, and memory of prior activity. It has been proposed that a centrally mediated disorder of perception may underlie many syndromes with symptoms that lack clear pathophysiologic explanations.4,10 However, although a failure of nonmotor function of basal ganglia has been proposed as one of the potential pathogenic mechanisms of central fatigue,8 there is little research on human brain imaging of fatigue, and it is unknown if the conscious sensation of fatigue is associated with particular brain locations or related to whole-brain activity. The inherent subjectivity of CRF has limited development of preclinical models.11
Establishing the causality of CRF presents numerous difficulties and challenges.12 First, not all at-risk patients will experience this symptom. According to National Comprehensive Cancer Network (NCCN) guidelines, causes of CRF include the cancer itself, chemotherapy, bone marrow transplants, immunotherapy and radiation therapy, and anemia; factors identified as frequently contributing to CRF include pain, emotional distress, sleep disturbance, anemia, nutritional deficiencies, cardiac deconditioning, and comorbidities.13 Even so, not all patients with these serious conditions will develop fatigue.14,15 Variability in disease prognosis and response to cancer or symptom treatment (including placebo effects) may further affect development of this symptom. In addition, CRF is more likely to be caused by a constellation of risk factors (sometimes referred to as a “web of causation”) than by a single factor. Complex interplay may be seen between the etiologic agent (eg, cancer treatment, infections, use of central-acting drugs), and host susceptibility. Clinical observations indicate that multiple physical and psychosocial factors are involved for each individual patient.
This paper will review the clinical correlates of CRF development and propose potential mechanisms underlying the pathophysiology of CRF, supported by data related to both single and multiple mechanisms.
Clinical Correlates of Cancer-Related Fatigue
Potential Tumor-Related Causes of CRF
Unusual tiredness often is the first signal that causes people to seek medical care. Significant fatigue often is observed in patients with newly diagnosed cancer, especially patients with renal or small cell lung cancer who develop paraneoplastic syndrome. Patients with advanced-stage cancer may suffer from even more distressing CRF. Progressive cancer directly affects several organ systems and causes neurophysiologic changes in skeletal muscles. Abnormal production of certain substances (eg, inflammatory cytokines)13,16 may inhibit metabolism or normal muscle function. Decreased availability of metabolic substrates in patients with cancer also be involved.17 As an example, CRF is one of the main symptoms of cachexia, which presents in approximately 50% of patients with cancer and is characterized by loss of body mass and skeletal muscle that cannot be explained solely by decreased food intake. Cachexia has been associated with increased levels of certain inflammatory cytokines, including interleukins and tumor necrosis factor (TNF-α) and may also be related to abnormalities in energy metabolism.1,11,18
Potential Treatment-Related Causes of CRF
Surgery
Fatigue is common after major surgery and delays recovery.19,20 It is usually attributed to the physiological response to surgery. Postoperative fatigue has been reported immediately after curative surgery21–23 and may be related to such factors as having received anesthesia, type of analgesia, decreased ventilatory capacity, immobilization, infection, or anxiety.24 The mechanisms of postoperative fatigue have been examined only during early times after surgery. Salmon and Hall, in a study of patients who underwent hip arthroplasty, found that the severity of postoperative fatigue was predicted not by physiological changes but by the preoperative level of fatigue.21 In contrast, Rubin and colleagues have suggested that psychological processes are relevant in the etiology of postoperative fatigue.19 In particular, their results relating to mood and expectations suggest that somatization may be particularly important in the first few weeks after surgery, whereas cognitive-behavioral factors and cardiovascular deconditioning may be more important in determining later-stage recovery.19
Chemotherapy
The nausea, diarrhea, and vomiting induced by chemotherapy can influence CRF symptoms.1 Chemotherapy-related fatigue may also be associated with anemia or with accumulation of end products from cell destruction.11 Available evidence supports the correlation between anemia and fatigue.25,26 Chemotherapy drugs that cross the blood-brain barrier may induce neurotoxicities that produce fatigue.24 Most patients experience fluctuations in fatigue during high-dose chemotherapy paired with stem cell transplantation, with fatigue increasing as the patients’ blood counts approach white blood cell nadir but improving as counts recover.27 The association of fatigue and chemotherapy has been extensively studied in patients with breast cancer.28–33 Even existing anemia and fatigue can be exacerbated by chemotherapy and radiotherapy.34
Radiotherapy
Fatigue may be the most severe symptom experienced by patients during radiation therapy.35 Treatment with radiation can lead to anemia, diarrhea, weight loss, anorexia, and chronic pain, any of which can influence fatigue severity.1 For example, in a longitudinal study of patients with colorectal cancer receiving chemoradiation, the severity of pain before treatment and the severity of diarrhea during treatment predicted the development of severe fatigue.36 Patients who receive radiotherapy may experience a gradual deepening of fatigue with ongoing treatment.36,37 Combined modality therapy (eg, concurrent chemotherapy and radiation) is a known risk factor for persistent fatigue.38
Biologic-response modification
Patients treated with biologic-response modifiers, such as proinflammatory cytokines, may experience such intense and intolerable fatigue that it limits their ability to continue with these agents.39–41 Administration of cytokines often results in a “flu-like syndrome” with a set of symptoms that includes fatigue, fever, chills, headache, myalgias, and malaise.42 The most-studied of these biologic agents is interferon (IFN)-α, which may cause fatigue in approximately 70% of patients and may induce hypothyroidism, which also may cause fatigue, in up to 20% of patients.43
Hormone therapy
Side effects of hormone therapy have not been well assessed and are frequently underestimated.44 Lethargy and lack of energy related to hormone treatment have been reported in patients with breast cancer.45 Hormone ablation may double the incidence of reported fatigue in patients with prostate cancer, which supports the correlation between gonadotrophin function and fatigue.46
CRF in Cancer Survivors
Not only have patients repeatedly identified CRF as the most distressing symptom during the acute phase of cancer treatment but also published literature shows that CRF may continue for years after treatment is completed and even when the cancer is cured. CRF may so affect a cancer survivor’s physical and cognitive abilities that he or she is not able to function normally either at work or at home, which can cause significant difficulties.47,48
Relationship of Fatigue to Other Symptoms
Although fatigue is often singled out as the most common symptom across many different diseases, it almost always clusters with other significant symptoms. The greater the number of symptoms and perceived disabilities, the more likely clinicians are to identify psychological, behavioral, or social contributors to illness.10 Cluster analyses of multiple cancer symptoms, such as fatigue, pain, and sleep disturbance, have confirmed this phenomenon.49,50 This is consistent with observations from cancer patients, who rarely have solitary symptoms during active therapy or with advanced disease and who often experience accompanying medical comorbidities and psychological disorders.51,52
Pain and sleep disturbance
The interaction of fatigue with pain and sleep disturbance has been well addressed in the symptom research literature.47,53–58 Parameter estimates in patients with newly diagnosed lung cancer indicated that the three-way interaction of pain, fatigue, and insomnia was statistically significant.52 Higher total and subscale fatigue scores were correlated with most components of poorer subjective sleep quality (r = 0.25–0.42, P ≤ 0.005).58
Distress and depression
Chronic emotional distress can contribute to development of CRF.28,29 Proposed mechanisms in this regard include dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis.59 In an international study of chronic fatigue syndrome in the primary care setting, a temporal relationship between fatigue and depression, when adjusted for demographics, physical morbidity, and intercenter variability, supported the concept that unexplained fatigue and depression might act as independent risk factors for each other.60 It has also been hypothesized that activation of inflammatory pathways in otherwise healthy individuals may influence individual depression-related symptoms, such as fatigue, insomnia, and anger/hostility.61,62
Other pathophysiological conditions
Physical conditions, such as various infections, malnutrition, thyroid dysfunction, and other organ failure could all either cause or contribute to CRF (Figure 1). Multiple physical symptoms interact with affective symptoms, and the patient’s perception of illness, coping skills, and mood may have important and long-lasting effects on eventual adaptation to chronic fatigue and should be considered for effective intervention. This is one area where cancer researchers might make good use of the psychology literature, such as the study by Edwards and colleagues on illness representations by patients with chronic fatigue.63
Proposed Mechanisms of CRF Pathophysiology
While the underlying etiology of and risk factors for CRF are not fully resolved, the following hypotheses provide independent and overlapping potential mechanisms for the pathophysiology of this complex phenomenon (Figure 1). These proposed mechanisms include proinflammatory cytokines, growth factors, circadian rhythm modulation, HPA-axis disruption, serotonin dysregulation, vagal-afferent activation, anemia, and abnormalities of generation or use of adenosine triphosphate (ATP).5,16,34,64,65
Proinflammatory Cytokine Hypothesis
Symptoms reported by patients with cancer undergoing treatment are strikingly similar to characteristics of the evolving animal models of cytokine-induced sickness behavior.65–67 Sickness behavior refers to clusters of behavioral and physiological responses (eg, hyperalgesia, sleep disturbance, and reduced activity and food intake) observed in animals after physical insult or administration of inflammatory agents or specific proinflammatory cytokines.68–70 There is growing awareness that cytokines may play a mechanistic role in CRF as common biologic mechanisms.35,60,71–73 The theoretical underpinning for the proinflammatory cytokine hypothesis, based on the animal model of inflammation-induced sickness behavior, is that dysregulated inflammation and its downstream toxic effects represent a significant biologic basis for subjectively reported CRF and a cluster of other symptoms.65,66,74
Cytokine dysregulation appears to play a part in cancer-related–symptom production. Elevated inflammatory biomarkers (eg, interleukin [IL]-6 and TNF-α) have been shown in studies of persistent fatigue in survivors of breast cancer.75 These cytokines might be associated with a chronic inflammatory process involving the T-cell compartment.76 In patients with advanced cancer, cachexia-related tissue catabolism is reported to be mediated by IL-6 and TNF-α.77
Increased inflammation is also a prime candidate for the mechanism behind increases in treatment-related symptoms. The insult of cancer treatment, including radiotherapy and chemotherapy, increases production of inflammatory cytokines, especially IL-6 and TNF variants.78,79 Increases in IL-6 in response to paclitaxel therapy for breast cancer have been associated with reported symptoms.80 Patients with CRF exhibit elevations in IL-6, IL-1 receptor antagonist (IL-1RA), IL-1, and TNF, along with decreased albumin.81
It has been suggested that reduction of this treatment-induced inflammatory response might significantly reduce the morbidity associated with radiotherapy.82 The use of a TNF inhibitor (etanercept) was associated with significantly less fatigue in patients with cancer, along with a trend for lower mean nuclear factor-kappa B (NF-κB, a cytokine precursor molecule) activity.83 Prechemotherapy and chemotherapy-induced changes in inflammation in response to chemotherapy have been related to changes in fatigue and quality of life.84
Molecules downregulated by cytokines, such as albumin and C-reactive protein (CRP), also show a high correlation with the presence of CRF. For example, fatigue was associated with low albumin in patients with hematologic malignancies and with greater CRP in patients with advanced lung cancer.85,86
A recent article reviewed 18 well-designed published studies (N = 1037) to evaluate the strength of evidence supporting the relationship between inflammation and fatigue.87 Analyses based on weighting according to sample size showed a significant positive correlation between fatigue and levels of circulating inflammatory markers (r = 0.11, P < 0.0001). Analyses of individual inflammatory markers revealed significantly positive correlations between fatigue and IL-6 (r = 0.12, P = 0.004), IL-1RA (r = 0.24, P= 0.0005), and neopterin (r = 0.22, P = 0.0001). The author proposed that the reason for insignificant correlation with IL-1β or TNF-α was because serum TNF-α was not detectable, possibly due to the sampling process or to storage conditions or duration.87,88
Growth Factor Hypothesis
The growth factor hypothesis posits that vascular endothelial growth factor (VEGF) level is associated with treatment-induced fatigue. VEGF is an angiogenic cytokine with high relevance to cancer, stimulating the formation of new blood vessels necessary for tumor growth and metastasis89 and an independent predictor of poorer survival.90 Patients with breast cancer undergoing chemotherapy were found to have significantly increased fatigue and reduced quality of life correlated with elevated VEGF and soluble intracellular adhesion molecule-1 levels.84 Sunitinib is a VEGF receptor inhibitor that is hypothesized to decrease thyroid function by preventing binding of VEGF to normal thyroid cells or impairing thyroid blood flow, which results in thyroiditis. Sunitinib-induced hypothyroidism (without autoantibodies) has been observed in patients with advanced renal cell cancer91 or gastrointestinal stromal cancer.92 Thyroid hormone replacement improved fatigue and other symptoms in 9 of 17 patients with renal cancer.91
Circadian Rhythm Modulation Hypothesis
Research examining possible links between circadian rhythms and CRF has focused on secretion rhythms of the stress hormone cortisol and on rest-activity patterns. Preclinical studies have shown that epidermal growth factor receptor (EGFR) ligands, such as transforming growth factor-alpha (TGF-α), inhibit hypothalamic signaling of rhythmic behavior. Clinical observations indicate that elevated levels of TGF-α are associated with fatigue, flattened circadian rhythms, and loss of appetite in patients with metastatic colorectal cancer.93 A slower decline in salivary cortisol levels correlated with increased fatigue severity was observed in fatigued survivors of cancer over the course of the day.92 These data support the hypothesis that a symptom cluster of fatigue, appetite loss, and sleep disruption commonly seen in patients with cancer may be related to EGFR ligands, released either by the cancer itself or by the host in response to the stress associated with cancer, and suggest that further examination of their role in the production of symptom clustering is warranted.95
Sleep disorders are commonly observed in patients with cancer and may result from altered circadian rest-activity rhythms. An inverse correlation between fatigue and daily activity levels and a positive correlation between fatigue and restless sleep at night have been reported.31,96 Changes in fatigue between chemotherapy cycles have also been correlated with changes in the rest-activity rhythm.97 The association between fatigue and circadian disruption was reported as being independent of the presence of depression, although depression did correlate with altered circadian rhythm.
Serotonin Dysregulation Hypothesis
The theory behind dysregulation of serotonin as an explanation of CRF is that cancer and/or treatment cause an increase in brain serotonin (5-hydroxytryptemine [5-HT]) levels in localized regions of the brain and an upregulation of certain 5-HT receptors. This can lead to decreases in somatomotor drive, modified HPA-axis function, and a sensation of decreased capacity to perform physical work.98–100
Taken primarily from studies of exercise-induced fatigue or chronic fatigue syndrome, increased evidence supports a role for 5-HT metabolism and neurotransmission in the genesis of central fatigue. Animal studies have shown that 5-HT concentrations increase in the hypothalamus and brain stem with sustained exercise, reaching a maximum at the point of fatigue.101,102 Similarly, administration of 5-HT to rats produced a dose-related decrease in running endurance,103 whereas administration of a 5-HT antagonist improved performance.102 Studies in patients with chronic fatigue syndrome have demonstrated raised plasma levels of free tryptophan, which could potentially lead to high levels of central 5-HT.104,105 In several human studies, administration of selective serotonin reuptake inhibitors has been shown to reduce the capacity to perform exercise. However, other investigators have shown that central 5-HT concentrations do not influenceCRF.106,107
Hypothalamic-Pituitary-Adrenal–Axis Disruption Hypothesis
The HPA axis is the central system regulating release of the stress hormone cortisol. The HPA-dysfunction hypothesis proposes that cancer or its treatment either directly or indirectly cause alterations in HPA function, leading to endocrine changes that either cause or contribute to fatigue.108,109 Fatigue has been associated with reduced HPA-axis function, such as defective central corticotropin-releasing hormone (CRH) release and downregulation of CRH receptors in response to chronic stress, and with hypocortisolemia in patients with cancer, chronic fatigue syndrome, and rheumatoid arthritis.5 Alterations in HPA-axis function may be caused by various factors in patients with cancer. For example, proinflammatory cytokines (e.g., IL-1, IL-6, and TNF-α) and some comorbidities (eg, sleep disturbance) can stimulate the HPA axis.110,111 Certain cancer treatments (eg, glucocorticoids, radiotherapy, and some chemotherapeutic regimens) may lead to direct suppression of the HPA axis.112–114 Cortisol has an inhibitory effect on cytokine production; thus, cytokine levels may rise in the presence of reduced cortisol concentrations.115
Vagal-Afferent–Activation Hypothesis
Based on animal studies, the vagal-afferent–activation hypothesis suggests that cancer and its treatment cause peripheral release of a spectrum of neuroactive molecules (such as serotonin, cytokines, and prostaglandins) that may activate vagal-afferent nerves.116–118 The overall effects may manifest as decreased somatic motor output and sustained changes in particular regions of the brain associated with fatigue by induction of IL-1β sickness behavior.119,120 In response to injection of IL-1β, the vagal-afferent nerves of rats mediate IL-1β production at multiple sites in the central nervous system.121 Considering the effect of the HPA axis on fatigue, cytokine production in the hypothalamus is especially significant.
Anemia Hypothesis
Cancer-related anemia has a profound impact on patients experiencing the associated complications of fatigue, dyspnea, palpitations, dizziness, and decreased cognitive function.122 Anemia results in decreased oxygen delivery to tissue, despite the body’s attempts to compensate for the effects of a decrease in red blood cells.123 Hypoxia-related compromise in organ functioning is one of the suspects related to anemia or hemoglobin dysfunction that might cause fatigue.5 A direct relationship between increases in hemoglobin (within the recommended range) and improvements in fatigue and quality of life has been documented in randomized trials in elderly patients with chronic anemia and in patients with cancer.124–128
Adenosine Triphosphate Hypothesis
Feelings of “weakness” and “lack of energy” are reported by patients with cancer, who often have decreased ability to perform mechanical work.129,130 Cancer or its treatments may lead to a defect in ATP regeneration and the buildup of metabolic byproducts in the neuromuscular junctions and skeletal muscle. ATP is a major source of energy for skeletal-muscle contraction, and a disruption of its metabolism in patients with cancer could decrease their physical abilities. This mechanism of producing fatigue symptoms has been referred to as “peripheral fatigue”.5 Results from pilot studies of agents thought to affect metabolic response or short-term endurance performance in muscle are inconclusive. Whether the “peripheral fatigue” may be a part of CRF is unknown.
Conclusions
Research on understanding the mechanisms underlying both the development and management of CRF has attracted multidisciplinary attention. Specific efforts have been made to understand factors, such as anemia, pain, depression, anxiety, sedating medications (opioids), sleep disturbance, and immobility, that may contribute to fatigue. Compelling new hypotheses regarding the pathophysiology of CRF have been proposed, some with evidence in both animal models and humans and some in animals only. Investigation of these potential mechanisms will contribute to improved understanding of the nature of fatigue, one of the most complicated symptoms experienced by patients with cancer.
Continued research will no doubt improve our understanding of the etiology of CRF. Clearly, CRF is influenced and modulated by a number of critical factors, and the mechanism that is both necessary and sufficient to induce development of severe fatigue in patients with cancer has not yet been identified. Although some effort is being expended to develop objective measures of the physical and cognitive changes caused by fatigue, no well-accepted physiologically or behaviorally objective measures are currently available for diagnostic use. Additionally, whereas fatigue is often objectively described by clinicians as a decrease in performance,131 it may also result in behavioral manifestations that are more likely to be noted by concerned family members and partners.
Pathophysiological research of CRF could be applied in the clinic to improve diagnosis of CRF and to administration of mechanism-driven interventions. Gaining an understanding of the specific mechanisms related to the development of fatigue in patients with cancer and survivors of cancer requires further investigation. A targeted intervention study with CRF as a primary end point would also be useful.
At a Glance.
The pathophysiology of cancer-related fatigue (CRF) has not yet been adequately elucidated.
No physiological markers of CRF have been established from ongoing research with hypotheses proposing underlying mechanisms.
A web of causation may be reflected in an interaction of etiology and host susceptibility.
References
- 1.Gutstein HB. The biologic basis of fatigue. Cancer. 2001;92(6 Suppl):1678–1683. doi: 10.1002/1097-0142(20010915)92:6+<1678::aid-cncr1496>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. British Journal of Cancer. 2004;91:822–828. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. Journal of the National Cancer Institute Monographs. 2004:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 4.St Clair Gibson A, Baden DA, Lambert MI, Lambert EV, Harley YX, Hampson D, et al. The conscious perception of the sensation of fatigue. Sports Medicine. 2003;33:167–176. doi: 10.2165/00007256-200333030-00001. [DOI] [PubMed] [Google Scholar]
- 5.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. The Oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 6.Weir JP, Beck TW, Cramer JT, Housh TJ. Is fatigue all in your head? A critical review of the central governor model. British Journal of Sports Medicine. 2006;40:573–586. doi: 10.1136/bjsm.2005.023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri A, Behan PO. Fatigue and basal ganglia. Journal of the Neurological Sciences. 2000;179(S 1–2):34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 9.Okada T, Tanaka M, Kuratsune H, Sadato N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurology. 2004;4:14. doi: 10.1186/1471-2377-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wessely S. Chronic fatigue: symptom and syndrome. Annals of Internal Medicine. 2001;134(9 Pt 2):838–843. doi: 10.7326/0003-4819-134-9_part_2-200105011-00007. [DOI] [PubMed] [Google Scholar]
- 11.Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98:1786–1801. doi: 10.1002/cncr.11742. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K. Studying symptoms: sampling and measurement issues. Annals of Internal Medicine. 2001;134(9 Pt 2):844–853. doi: 10.7326/0003-4819-134-9_part_2-200105011-00008. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. NCCN Cancer-Related Fatigue Panel. NCCN Practice Guidelines in Oncology. Cancer-related fatigue. 2007 Retrieved December 14, 2007, from http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf.
- 14.Mock V. Evidence-based treatment for cancer-related fatigue. Journal of the National Cancer Institute Monographs. 2004:112–118. doi: 10.1093/jncimonographs/lgh025. [DOI] [PubMed] [Google Scholar]
- 15.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer. 2001;92(6 Suppl):1684–1688. doi: 10.1002/1097-0142(20010915)92:6+<1684::aid-cncr1497>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Shaw JH, Wolfe RR. Glucose and urea kinetics in patients with early and advanced gastrointestinal cancer: the response to glucose infusion, parenteral feeding, and surgical resection. Surgery. 1987;101:181–191. [PubMed] [Google Scholar]
- 18.Tisdale MJ. Pathogenesis of cancer cachexia. The Journal of Supportive Oncology. 2003;1:159–168. [PubMed] [Google Scholar]
- 19.Rubin GJ, Cleare A, Hotopf M. Psychological factors in postoperative fatigue. Psychosomatic Medicine. 2004;66:959–964. doi: 10.1097/01.psy.0000143636.09159.f1. [DOI] [PubMed] [Google Scholar]
- 20.Rubin GJ, Hardy R, Hotopf M. A systematic review and meta-analysis of the incidence and severity of postoperative fatigue. Journal of Psychosomatic Research. 2004;57:317–326. doi: 10.1016/S0022-3999(03)00615-9. Erratum in: Journal of Psychosomatic Research, 58, 113 (2005) [DOI] [PubMed] [Google Scholar]
- 21.Salmon P, Hall GM. A theory of postoperative fatigue: an interaction of biological, psychological, and social processes. Pharmacology, Biochemistry, and Behavior. 1997;56:623–628. doi: 10.1016/s0091-3057(96)00429-7. [DOI] [PubMed] [Google Scholar]
- 22.Forsberg C, Björvell H, Cedermark B. Well-being and its relation to coping ability in patients with colo-rectal and gastric cancer before and after surgery. Scandinavian Journal of Caring Sciences. 1996;10:35–44. doi: 10.1111/j.1471-6712.1996.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 23.Galloway SC, Graydon JE. Uncertainty, symptom distress, and information needs after surgery for cancer of the colon. Cancer Nursing. 1996;19:112–117. doi: 10.1097/00002820-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Smets EM, Garssen B, Schuster-Uitterhoeve AL, de Haes JC. Fatigue in cancer patients. British Journal of Cancer. 1993;68:220–224. doi: 10.1038/bjc.1993.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner R, Anglin P, Burkes R, Couture F, Evans W, Goss G, et al. Epoetin alfa in cancer patients: evidence-based guidelines. Journal of Pain and Symptom Management. 2001;22:954–965. doi: 10.1016/s0885-3924(01)00357-8. [DOI] [PubMed] [Google Scholar]
- 26.Demetri GD, Kris M, Wade J, Degos L, Cella D. Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a perspective community oncology study; Procrit Study Group. Journal of Clinical Oncology. 1998;16:3412–3425. doi: 10.1200/JCO.1998.16.10.3412. [DOI] [PubMed] [Google Scholar]
- 27.Anderson KO, Giralt SA, Mendoza TR, Brown JO, Neumann JL, Mobley GM, et al. Symptom burden in patients undergoing autologous stem-cell transplantation. Bone Marrow Transplantation. 2007;39:759–766. doi: 10.1038/sj.bmt.1705664. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course and correlates. Journal of Pain and Symptom Management. 1999;1:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 29.Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy: A comparison with the fatigue experienced by healthy individuals. Cancer Nursing. 1994;17:367–378. [PubMed] [Google Scholar]
- 30.Pickard-Holley S. Fatigue in cancer patients: A descriptive study. Cancer Nursing. 1991;14:13–19. [PubMed] [Google Scholar]
- 31.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncology Nursing Forum. 1998;25:51–62. [PubMed] [Google Scholar]
- 32.Richardson A, Ream E, Wilson-Barnett J. Fatigue in patients receiving chemotherapy: patterns of change. Cancer Nursing. 1998;21:17–30. doi: 10.1097/00002820-199802000-00003. Erratum in: Cancer Nursing, 21, 195 (1998) [DOI] [PubMed] [Google Scholar]
- 33.Greene D, Nail LM, Fieler VK, Dudgeon D, Jones LS. A comparison of patient-reported side effects among three chemotherapy regimens for breast cancer. Cancer Practice. 1994;2:57–62. [PubMed] [Google Scholar]
- 34.Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Supportive Care in Cancer. 2002;10:389–398. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- 35.Wang XS, Fairclough DL, Liao Z, Komaki R, Chang JY, Mobley GM, et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. Journal of Clinical Oncology. 2006;24:4485–4491. doi: 10.1200/JCO.2006.07.1126. [DOI] [PubMed] [Google Scholar]
- 36.Wang XS, Janjan NA, Guo H, Johnson BA, Engstrom MC, Crane CH, et al. Fatigue during preoperative chemoradiation for resectable rectal cancer. Cancer. 2001;92(6 Suppl):1725–1732. doi: 10.1002/1097-0142(20010915)92:6+<1725::aid-cncr1504>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 37.Curt GA. The impact of fatigue on patients with cancer: overview of FATIGUE 1 and 2. The Oncologist. 2000;5(Suppl 2):9–12. doi: 10.1634/theoncologist.5-suppl_2-9. [DOI] [PubMed] [Google Scholar]
- 38.Jereczek-Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy-related fatigue. Critical Reviews in Oncology/Hematology. 2002;41:317–325. doi: 10.1016/s1040-8428(01)00143-3. [DOI] [PubMed] [Google Scholar]
- 39.Kirkwood JM, Ernstoff MS, Davis CA, Reiss M, Ferraresi R, Rudnick SA. Comparison of intramuscular and intravenous recombinant alpha-2 interferon in melanoma and other cancers. Annals of Internal Medicine. 1985;103:32–36. doi: 10.7326/0003-4819-103-1-32. [DOI] [PubMed] [Google Scholar]
- 40.Robinson KD, Posner JD. Patterns of self-care needs and interventions related to biologic response modifier therapy: fatigue as a model. Seminars in Oncology Nursing. 1992;8(4 Suppl 1):17–22. doi: 10.1016/0749-2081(92)90050-d. [DOI] [PubMed] [Google Scholar]
- 41.Dean GE, Spears L, Ferrell BR, Quan WD, Groshon S, Mitchell MS. Fatigue in patients with cancer receiving interferon alpha. Cancer Practice. 1995;3:164–172. [PubMed] [Google Scholar]
- 42.Piper BF, Rieger PT, Brophy L, Haeuber D, Hood LE, Lyver A, et al. Recent advances in the management of biotherapy-related side effects: fatigue. Oncology Nursing Forum. 1989;16(6 Suppl):27–34. [PubMed] [Google Scholar]
- 43.Kirkwood JM, Bender C, Agarwala S, Tarhini A, Shipe-Spotloe J, Smelko B, et al. Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. Journal of Clinical Oncology. 2002;20:3703–3718. doi: 10.1200/JCO.2002.03.052. [DOI] [PubMed] [Google Scholar]
- 44.Obe SD. Exploring the impact of treatment: communications, perceptions, reality! European Journal of Cancer Care. 1996;5(3 Suppl):3–4. doi: 10.1111/j.1365-2354.1996.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 45.Leonard RC, Lee L, Harrison ME. Impact of side effects associated with endocrine treatments for advanced breast cancer: clinicians’ and patients’ perception. Breast. 1996;5:259–264. [Google Scholar]
- 46.Stone P, Hardy J, Huddart R, A’Hern R, Richards M. Fatigue in patients with prostate cancer receiving hormone therapy. European Journal of Cancer. 2000;36:1134–1141. doi: 10.1016/s0959-8049(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 47.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. Journal of Clinical Oncology. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 48.Servaes P, Verhagen S, Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: a cross-sectional study. Annals of Oncology. 2002;13:589–598. doi: 10.1093/annonc/mdf082. [DOI] [PubMed] [Google Scholar]
- 49.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. Journal of the National Cancer Institute Monographs. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 50.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncology Nursing Forum. 2001;28:465–470. [PubMed] [Google Scholar]
- 51.Hickok JT, Morrow GR, Roscoe JA, Mustian K, Okunieff P. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. Journal of Pain Symptom Management. 2005;30:433–442. doi: 10.1016/j.jpainsymman.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman AJ, Given BA, von Eye A, Gift AG, Given CW. Relationships among pain, fatigue, insomnia, and gender in persons with lung cancer. Oncology Nursing Forum. 2007;34:785–792. doi: 10.1188/07.ONF.785-792. [DOI] [PubMed] [Google Scholar]
- 53.Gaston-Johansson F, Fall-Dickson JM, Bakos AB, Kennedy MJ. Fatigue, pain, and depression in pre-autotransplant breast cancer patients. Cancer Practice. 1999;7:240–247. doi: 10.1046/j.1523-5394.1999.75008.x. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz AL, Mori M, Gao R, Nail LM, King ME. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Medicine and Science in Sports and Exercise. 2001;33:718–723. doi: 10.1097/00005768-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Mock V, Pickett M, Ropka ME, Muscari Lin E, Stewart KJ, Rhodes VA, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Practice. 2001;9:119–127. doi: 10.1046/j.1523-5394.2001.009003119.x. [DOI] [PubMed] [Google Scholar]
- 56.Berger AM, Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncology Nursing Forum. 1999;26:1663–1671. [PubMed] [Google Scholar]
- 57.Lee KA. Sleep and fatigue. Annual Review of Nursing Research. 2001;19:249–273. [PubMed] [Google Scholar]
- 58.Berger AM, Farr LA, Kuhn BR, Fischer P, Agarwal S. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. Journal of Pain and Symptom Management. 2007;33:398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott LV, Dinan TG. The neuroendocrinology of chronic fatigue syndrome: focus on the hypothalamic-pituitary-adrenal axis. Functional Neurology. 1999;14:3–11. [PubMed] [Google Scholar]
- 60.Skapinakis P, Lewis G, Mavreas V. Temporal relations between unexplained fatigue and depression: longitudinal data from an international study in primary care. Psychosomatic Medicine. 2004;66:330–335. doi: 10.1097/01.psy.0000124757.10167.b1. [DOI] [PubMed] [Google Scholar]
- 61.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119–1128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Edwards R, Suresh R, Lynch S, Clarkson P, Stanley P. Illness perceptions and mood in chronic fatigue syndrome. Journal of Psychosomatic Research. 2001;50:65–68. doi: 10.1016/s0022-3999(00)00204-x. [DOI] [PubMed] [Google Scholar]
- 64.Cleeland CS, Wang XS. Measuring and understanding fatigue. Oncology. 1999;13:91–97. [Google Scholar]
- 65.Lee BN, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279–292. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- 66.Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 67.Miller AH. Cytokines and sickness behavior: implications for cancer care and control. Brain, Behavior, and Immunity. 2003;17(Suppl 1):S132–S134. doi: 10.1016/s0889-1591(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 68.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain, Behavior, and Immunity. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 69.Hart BL. Biological basis of the behavior of sick animals. Neuroscience and Biobehavioral Reviews. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 70.Kent S, Bluthé RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends in Pharmacological Sciences. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- 71.Dunlop RJ, Campbell CW. Cytokines and advanced cancer. Journal of Pain and Symptom Management. 2000;20:214–232. doi: 10.1016/s0885-3924(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 72.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: conceptual, design, measurement, and analysis issues. Journal of Pain and Symptom Management. 2006;31:85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 73.Dodd MJ, Cho MH, Cooper B, Miaskowski C, Lee KA, Bank K. Advancing our knowledge of symptom clusters. The Journal of Supportive Oncology. 2005;3(6 Suppl 4):30–31. [PubMed] [Google Scholar]
- 74.Payne JK. The trajectory of biomarkers in symptom management for older adults with cancer. Seminars in Oncology Nursing. 2006;22:31–35. doi: 10.1016/j.soncn.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clinical Cancer Research. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 76.Bower JE, Ganz PA, Aziz N, Fahey JL, Cole SW. T-cell homeostasis in breast cancer survivors with persistent fatigue. Journal of the National Cancer Institute. 2003;95:1165–1168. doi: 10.1093/jnci/djg0019. [DOI] [PubMed] [Google Scholar]
- 77.Argilés JM, Busquets S, López-Soriano FJ. Cytokines as mediators and targets for cancer cachexia. Cancer Treatment Research. 2006;130:199–217. doi: 10.1007/0-387-26283-0_9. [DOI] [PubMed] [Google Scholar]
- 78.Linard C, Marquette C, Clarencon D, Galonnier M, Mathieu J, Pennequin A, et al. Acute ileal inflammatory cytokine response induced by irradiation is modulated by subdiaphragmatic vagotomy. Journal of Neuroimmunology. 2005;168:83–95. doi: 10.1016/j.jneuroim.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 79.Linard C, Marquette C, Mathieu J, Pennequin A, Clarencon D, Mathé D. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-κ B inhibitor. International Journal of Radiation Oncology, Biology, Physics. 2004;58:427–434. doi: 10.1016/j.ijrobp.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 80.Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 82.Garden AS. Mucositis: current management and investigations. Seminars in Radiation Oncology. 2003;13:267–273. doi: 10.1016/S1053-4296(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 83.Madhusudan S, Muthuramalingam SR, Braybrooke JP, Wilner S, Kaur K, Han C, et al. Study of etanercept, a tumor necrosis factor-alpha inhibitor, in recurrent ovarian cancer. Journal of Clinical Oncolology. 2005;23:5950–5959. doi: 10.1200/JCO.2005.04.127. [DOI] [PubMed] [Google Scholar]
- 84.Mills PJ, Parker B, Dimsdale JE, Sadler GR, Ancoli-Israel S. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biological Psychology. 2005;69:85–96. doi: 10.1016/j.biopsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 85.Wang XS, Giralt SA, Mendoza TR, Engstrom MC, Johnson BA, Peterson N, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin’s lymphoma. Journal of Clinical Oncology. 2002;20:1319–1328. doi: 10.1200/JCO.2002.20.5.1319. [DOI] [PubMed] [Google Scholar]
- 86.Brown DJ, McMillan DC, Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer. 2005;103:377–382. doi: 10.1002/cncr.20777. [DOI] [PubMed] [Google Scholar]
- 87.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain, Behavior, and Immunity. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 88.Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR. Measuring cytokine levels in blood. Importance of anticoagulants, processing, and storage conditions. Journal of Immunological Methods. 1992;153:115–124. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- 89.Boudreau N, Myers C. Breast cancer-induced angiogenesis: multiple mechanisms and the role of the microenvironment. Breast Cancer Research. 2002;5:140–146. doi: 10.1186/bcr589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishimura R, Nagao K, Miyayama H, Matsuda M, Baba K, Yamashita H, et al. Higher plasma vascular endothelial growth factor levels correlate with menopause, overexpression of p53, and recurrence of breast cancer. Breast Cancer. 2000;10:120–128. doi: 10.1007/BF02967636. [DOI] [PubMed] [Google Scholar]
- 91.Rini BI, Tamaskar I, Shaheen P, Salas R, Garcia J, Wood L, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. Journal of the National Cancer Institute. 2007;99:81–83. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- 92.Desai J, Yassa L, Marqusee E, George S, Frates MC, Chen MH, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Annals of Internal Medicine. 2006;145:660–664. doi: 10.7326/0003-4819-145-9-200611070-00008. [DOI] [PubMed] [Google Scholar]
- 93.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clinical Cancer Research. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 94.Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 95.Rich TA. Symptom clusters in cancer patients and their relation to EGFR ligand modulation of the circadian axis. The Journal of Supportive Oncology. 2007;5:167–174. discussion 176–177. [PubMed] [Google Scholar]
- 96.Mormont MC, Hecquet B, Bogdan A, Benavides M, Touitou Y, Levi F. Non-invasive estimation of the circadian rhythm in serum cortisol in patients with ovarian or colorectal cancer. International Journal of Cancer. 1998;78:421–424. doi: 10.1002/(sici)1097-0215(19981109)78:4<421::aid-ijc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 97.Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Supportive Care in Cancer. 2002;10:329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 98.Andrews PLR, Morrow GR, Hickok JT, et al. Mechanisms and models of fatigue associated with cancer and its treatment: evidence of pre-clinical and clinical studies. In: Armes J, Krishnasamy M, Higginson I, editors. Fatigue in cancer. Oxford, England: Oxford University Press; 2004. pp. 51–87. [Google Scholar]
- 99.Newsholme EA, Blomstrand E. Tryptophan, 5-hydroxytryptamine and a possible explanation for central fatigue. Advances in Experimental Medicine and Biology. 1995;384:315–320. doi: 10.1007/978-1-4899-1016-5_25. [DOI] [PubMed] [Google Scholar]
- 100.Gandevia SC, Allen GM, McKenzie DK. Central fatigue. Critical issues, quantification and practical implications. Advances in Experimental Medicine and Biology. 1995;384:281–294. [PubMed] [Google Scholar]
- 101.Blomstrand E, Perrett D, Parry-Billings M, Newsholme EA. Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions in the rat. Acta Physiologica Scandinavica. 1989;136:473–481. doi: 10.1111/j.1748-1716.1989.tb08689.x. [DOI] [PubMed] [Google Scholar]
- 102.Bailey SP, Davis JM, Ahlborn EN. Neuroendocrine and substrate responses to altered brain 5-HT activity during prolonged exercise to fatigue. Journal of Applied Physiology. 1993;74:3006–3012. doi: 10.1152/jappl.1993.74.6.3006. [DOI] [PubMed] [Google Scholar]
- 103.Bailey SP, Davis JM, Ahlborn EN. Effect of increased brain serotonergic activity on endurance performance in the rat. Acta Physiologica Scandinavica. 1992;145:75–76. doi: 10.1111/j.1748-1716.1992.tb09338.x. [DOI] [PubMed] [Google Scholar]
- 104.Badawy AA, Morgan CJ, Llewelyn MB, Albuquerque SR, Farmer A. Heterogeneity of serum tryptophan concentration and availability to the brain in patients with the chronic fatigue syndrome. Journal of Psychopharmacology. 2005;19:385–391. doi: 10.1177/0269881105053293. [DOI] [PubMed] [Google Scholar]
- 105.Castell LM, Yamamoto T, Phoenix J, Newsholme EA. The role of tryptophan in fatigue in different conditions of stress. Advances in Experimental Medicine and Biology. 1999;467:697–704. doi: 10.1007/978-1-4615-4709-9_90. [DOI] [PubMed] [Google Scholar]
- 106.Morrow GR, Hickok JT, Roscoe JA, Raubertas RF, Andrews PL, Flynn PJ, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Journal of Clinical Oncology. 2003;21:4635–4641. doi: 10.1200/JCO.2003.04.070. [DOI] [PubMed] [Google Scholar]
- 107.Roscoe JA, Morrow GR, Hickok JT, Mustian KM, Griggs JJ, Matteson SE, et al. Effect of paroxetine hydrochloride (Paxil) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Research and Treatment. 2005;89:243–249. doi: 10.1007/s10549-004-2175-1. [DOI] [PubMed] [Google Scholar]
- 108.Swain MG, Maric M. Defective corticotropin-releasing hormone mediated neuroendocrine and behavioral responses in cholestatic rats: implications for cholestatic liver disease-related sickness behaviors. Hepatology. 1995;22:1560–1564. [PubMed] [Google Scholar]
- 109.Bakheit AM, Behan PO, Dinan TG, Gray CE, O’Keane V. Possible upregulation of hypothalamic 5-hydroxytryptamine receptors in patients with postviral fatigue syndrome. BMJ (Clinical Research Edition) 1992;304:1010–1012. doi: 10.1136/bmj.304.6833.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. International Journal of Neuropsychopharmacology. 2002;5:375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- 111.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic–pituitary–adrenal axis, and cytokines: Multiple interactions and disturbances in sleep disorders. Endocrinology and Metabolism Clinics of North America. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 112.Del Priore G, Gurski KJ, Warshal DP, Angel C, Dubeshter B. Adrenal function following high-dose steroids in ovarian cancer patients. Gynecologic Oncology. 1995;59:102–104. doi: 10.1006/gyno.1995.1274. [DOI] [PubMed] [Google Scholar]
- 113.Morrow GR, Hickok JT, Andrews PL, Stern RM. Reduction in serum cortisol after platinum based chemotherapy for cancer: A role for the HPA axis in treatment-related nausea? Psychophysiology. 2002;39:491–495. doi: 10.1017.S0048577202991195. [DOI] [PubMed] [Google Scholar]
- 114.Schmiegelow M, Feldt-Rasmussen U, Rasmussen AK, Lange M, Poulsen HS, Müller J. Assessment of the hypothalamo-pituitary-adrenal axis in patients treated with radiotherapy and chemotherapy for childhood brain tumor. Journal of Clinical Endocrinology and Metabolism. 2003;88:3149–3154. doi: 10.1210/jc.2002-021994. [DOI] [PubMed] [Google Scholar]
- 115.Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: Regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–312. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- 116.Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. Journal of the Autonomic Nervous System. 1993;45:41–50. doi: 10.1016/0165-1838(93)90360-7. [DOI] [PubMed] [Google Scholar]
- 117.Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1β: Role of endogenous prostaglandins. Journal of Neuroscience. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Niijima A. The afferent discharges from sensors for interleukin-1β in the hepatoportal system in the anaesthetized rat. Journal of the Autonomic Nervous System. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- 119.Opp MR, Toth LA. Somnogenic and pyrogenic effects of interleukin-1β and lipopolysaccharide in intact and vagotomized rats. Life Sciences. 1998;62:923–936. doi: 10.1016/s0024-3205(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 120.Hansen MK, Krueger JM. Subdiaphragmatic vagotomy blocks the sleep-and fever-promoting effects of interleukin-1β. American Journal of Physiology. 1997;273(4 Pt 2):R1246–R1253. doi: 10.1152/ajpregu.1997.273.4.R1246. [DOI] [PubMed] [Google Scholar]
- 121.Hansen MK, Taishi P, Chen Z, Krueger JM. Vagotomy blocks the induction of interleukin-1β (IL-1β) mRNA in the brain of rats in response to systemic IL-1β. Journal of Neuroscience. 1998;18:2247–2253. doi: 10.1523/JNEUROSCI.18-06-02247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cella D. Quality of life and clinical decisions in chemotherapy-induced anemia. Oncology (Williston Park) 2006;20(8 Suppl 6):25–28. [PubMed] [Google Scholar]
- 123.Hurter B, Bush NJ. Cancer-related anemia: Clinical review and management update. Clinical Journal of Oncology Nursing. 2007;11:349–359. doi: 10.1188/07.CJON.349-359. [DOI] [PubMed] [Google Scholar]
- 124.Agnihotri P, Telfer M, Butt Z, Jella A, Cella D, Kozma CM, et al. Chronic anemia and fatigue in elderly patients: results of a randomized, double-blind, placebo-controlled, crossover exploratory study with epoetin alfa. Journal of the American Geriatrics Society. 2007;55:1557–1565. doi: 10.1111/j.1532-5415.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- 125.Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn LH. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: Improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. Journal of Clinical Oncology. 2001;19:2875–2882. doi: 10.1200/JCO.2001.19.11.2875. [DOI] [PubMed] [Google Scholar]
- 126.Vadhan-Raj S, Mirtsching B, Charu V, Terry D, Rossi G, Tomita D, et al. Assessment of hematologic effects and fatigue in cancer patients with chemotherapy-induced anemia given darbepoetin alfa every two weeks. The Journal of Supportive Oncology. 2003;1:131–138. [PubMed] [Google Scholar]
- 127.Vansteenkiste J, Pirker R, Massuti B, Barata F, Font A, Fiegl M, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. Journal of the National Cancer Institute. 2002;94:1211–1220. doi: 10.1093/jnci/94.16.1211. [DOI] [PubMed] [Google Scholar]
- 128.Cella D, Kallich J, McDermott A, Xu X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: Results from five randomized clinical trials. Annals of Oncology. 2004;15:979–986. doi: 10.1093/annonc/mdh235. [DOI] [PubMed] [Google Scholar]
- 129.Dimeo F, Stieglitz RD, Novelli-Fischer U, Fetscher S, Mertelsmann R, Keul J. Correlation between physical performance and fatigue in cancer patients. Annals of Oncology. 1997;8:1251–1255. doi: 10.1023/a:1008234310474. [DOI] [PubMed] [Google Scholar]
- 130.Akechi T, Kugaya A, Okamura H, Yamawaki S, Uchitomi Y. Fatigue and its associated factors in ambulatory cancer patients: A preliminary study. Journal of Pain and Symptom Management. 1999;17:42–48. doi: 10.1016/s0885-3924(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 131.Stone P. The measurement, causes and effective management of cancer-related fatigue. International Journal of Palliative Nursing. 2002;8:120–128. doi: 10.12968/ijpn.2002.8.3.10248. [DOI] [PubMed] [Google Scholar]