Abstract
Resting and actively degranulating mast cells are found on the brain side of the blood–brain barrier. In the periphery, exocytosis of mast cell granules results in the release of soluble mediators and insoluble granule remnants. These mast cell constituents are found in a variety of nearby cell types, acquired by fusion of granule and cellular membranes or by cellular capture of mast cell granule remnants. These phenomena have not been studied in the brain. In the current work, light and electron microscopic studies of the medial habenula of the dove brain revealed that mast cell-derived material can enter neurons in three ways: by direct fusion of the granule and plasma membranes (mast cell and neuron); by capture of insoluble granule remnants and, potentially, via receptor-mediated endocytosis of gonadotropin-releasing hormone, a soluble mediator derived from the mast cell. These processes result in differential subcellular localization of mast cell material in neurons, including free in the neuronal cytoplasm, membrane-bound in granule-like compartments or in association with small vesicles and the trans-Golgi network. Capture of granule remnants is the most frequently observed form of neuronal acquisition of mast cell products and correlates quantitatively with mast cells undergoing piecemeal degranulation. The present study indicates that mast cell-derived products can enter neurons, a process termed transgranulation, indicating a novel form of brain–immune system communication.
Keywords: dove, gonadal steroids, gonadotropin-releasing hormone, mast cell activation, medial habenula, neuro–immune interactions
Introduction
It is well established that the central nervous system (CNS) contains mast cells that are located on the brain side of the blood–brain barrier (Ibrahim, 1974; Dropp, 1976; Dimitriadou et al., 1990; Theoharides, 1990; Manning et al., 1994; Silverman et al., 1994; Florenzano & Bentivoglio, 2000). Mast cells are members of the immune system with properties of both the innate and acquired immune response (Galli et al., 2005). They are characterized by a large complement of secretory granules which store a wide variety of mediators, including biogenic amines, neuropeptides, cytokines, sulfated proteoglycans and neutral proteases (Johnson & Krenger, 1992; Silver et al., 1996; Galli et al., 2005). When released in the CNS, mast cell secretory products can alter the function of both neural (Khalil et al., 2004) and vascular (Esposito et al., 2001) elements.
In addition to the secretory granules that fill the cytoplasm, mast cells are distinguished from neurons, astroglia and microglia by other morphological criteria. The nucleus of mast cells has a distinctive pattern of heterochromatin associated with the nuclear envelope, not seen in other neural cells. Subcellular organelles of mast cells, such as the rough endoplasmic reticulum and Golgi apparatus, are much reduced in comparison to neurons and glia (see Silverman et al., 1994; Wilhelm et al., 2000). Mast cells lack the bundles of intermediate filaments and irregular lipofuscin inclusions that typify astroglia and microglia, respectively (see Peters et al., 1976 for ultrastructural details on CNS cell types).
In the periphery, mast cells interact with other cell types by transgranulation, a term first used by Greenberg & Burnstock (1983). Using time-lapse photomicrography and electron microscopy, they observed a multistep process that began with the elaboration of a mast cell pseudopodium containing granules followed by the attachment of the pseudopodium to the plasma membrane of an adjacent cell. Thereafter, the pseudopodium with its mast cell granules was transferred to the cell to which it was attached.
Mast cells degranulate by compound exocytosis (Lagunoff, 1973; Burwen & Satir, 1977; Guo et al., 1998). During exocytosis the electron-dense core of the granule is hydrated, acquires a particulate morphology and the contents are released into the extracellular space. In addition to the soluble elements of the granule, the insoluble core of proteoglycans, referred to as a granule remnant, is also released (Wang et al., 1995). In vitro capture of mast cell granule remnants has been demonstrated by macrophages (Lindahl et al., 1979; Kovanen, 1991), neutrophils and eosinophils (Baggiolini et al., 1982). In neutrophils, the captured material has been identified in ‘azurophilic’ granules (endosomes/lysosomes) and both condensed and disaggregating granules occur within the host cell (Baggiolini et al., 1982). In vivo mast cell granule remnants are present in human macrophages isolated from the peritoneal cavity during the early phase of allergic inflammatory events (Oliani et al., 2001), suggesting that granule capture is a common consequence of mast cell granule release.
Using light and electron microscopy, the present study documents, for the first time, transfer of mast cell granular material to neurons in the CNS. Doves have a high density of mast cells in the medial habenula (Zhuang et al., 1993), enabling quantitative electron microscopic studies. The medial habenula, a relay center between limbic forebrain and midbrain, has a role in motivation and arousal (Sutherland, 1982). Mast cells in the habenular neural parenchyma are in close contact with neuronal and glial elements (Wilhelm et al., 2000). In addition, mast cell numbers increase dramatically in this nucleus within hours of the initiation of courtship behavior (Zhuang et al., 1993) or following gonadal steroid treatment (Wilhelm et al., 2000) so that there is a larger population to survey.
Materials and methods
Subjects were ring doves (Streptopelia roseogrisea), aged between 1 month and 2 years, housed in visual isolation from conspecifics in cages (24 × 42 × 18 cm). Animals were provided with food, water and grit ad libitum. Rooms were maintained at a temperature of 22–24 °C with a 14/10-h light/dark cycle. The methods employed were described in detail in Silver et al. (1992). All procedures, including housing and surgery, were approved by the Columbia University Institutional Animal Care and Use Committee and meet the NIH guidelines.
The tissue studied here had previously been examined for a quantitative study of mast cell activational state and reproductive status. Animals (n = 20) were treated with sex-specific gonadal steroid hormones or control cholesterol (Wilhelm et al., 2000). Steroid hormone treatment did not change the frequency of transgranulation compared with controls (data not shown).
Tissue preparation for light and transmission electron microscopy
A lethal dose of chloropent (1.0 mL) was administered (i.m.), followed 5–10 min later by 0.33 mL of heparin i.v. (300 units, Sigma). Birds were perfused via the carotid artery using a peristaltic pump (Harvard Apparatus, Holliston, MA, USA) at 8 mL/min with 2% paraformaldehyde and 2.5% glutaraldehyde. Blocks containing the medial habenula were embedded in 12% gelatin and cut in the coronal plane on a vibratome (Microslicer 1500E, Ted Pella Inc., Redding, CA, USA) at 50 μm. Areas of interest were microdissected and post-fixed with 2% osmium (Ted Pella Inc.) in saline containing 1.5% potassium ferricyanide (Sigma), the latter to maximize the preservation of membranes (Langford & Coggeshall, 1980). All osmicated tissue was flat embedded in EPON (Ted Pella Inc.). Sections (1 μm) were cut using a diamond knife and stained with basic toluidine blue (borate buffer, Sigma, pH 11; this basic pH is necessary for the dye to penetrate the epoxy). Ultra-thin sections were collected on formvar-coated slot-hole grids (Ted Pella Inc.) and photographed using a 1200EX microscope (JEOL USA, Peabody, MA, USA).
For the quantitative electron microscopy, 20–51 cells per animal were examined for a total of 564 mast cells (see Table 1). Ten serial ultra-thin sections were collected per cell and the first section in each series was photographed. If areas of interest were found, the remaining serial sections were also photographed. Serial sections confirmed that the granules observed within neurons (see Results) were not an artifact of the plane of section. Furthermore, this ‘rule’ meant that all mast cell–neuropil interactions were identified in a consistent manner across experimental groups and states of mast cell activation.
Table 1.
Association of transgranulation events with activity state of the adjacent mast cells
| Activity state |
||||||
|---|---|---|---|---|---|---|
| Resting | Partial degranulation | Full degranulation | Piecemeal degranulation | Resynthesizing degranulation | Totals | |
| Number of cells analysed | 158 | 150 | 89 | 121 | 46 | 564 |
| Number of transgranulation events | 5 | 6 | 2 | 28* | 4 | 45 |
| Transgranulation events (% of total)† | (11.1%) | (13.3%) | (4.4%) | (62.2%) | (8.9%) | (100%) |
Activity states: resting, partial, full and piecemeal degranulation, and resynthesizing (see Wilhelm et al., 2000).
Percentage of hits that occur next to a mast cell in a specified activity category.
Significantly different from all other groups (see text).
To ascertain that transgranulation was not a fixation artifact, three animals were perfused with a second fixative. This involved perfusion for 20 min at 37 °C with oxygenated avian Ringer's solution (Sigma) containing 0.5% tannic acid (Sigma) (pH 6.8) to preserve membranes (Buma & Roubos, 1986) followed by perfusion with 1% paraformaldehyde and 2.5% glutaraldehyde (pH 7.4). In all cases, fixation continued overnight at 4 °C. For these electron microscopic studies, the medial habenula was dissected and osmicated as above.
Tissue preparation for ultrastructrual immunocytochemistry
For electron microscopic immunocytochemistry, three birds were perfused as above using 4% paraformaldehyde and 0.5% glutaraldehyde. The immunocytochemical procedures were performed with free-floating sections and the LR-1 antiserum (gift of Robert Benoit, McGill University, Montreal, Quebec, Canada) directed against mammalian gonadotropin-releasing hormone (GnRH) at a dilution of 1 : 5000 for 48–72 h. Absorption controls using this antiserum have been reported previously (Silver et al., 1993). Primary and secondary antisera were diluted with phosphate-buffered saline containing 0.05% saponin (Sigma). Two procedures were used to detect bound antibody. Both employed a biotinylated secondary antibody (Vector, Burlingame, CA, USA) and avidin-biotin-horseradish peroxidase (Vector). The first made use of conventional staining with 3,3′ diaminobenzidine (DAB) (Immunopure DAB, Pierce) and the second, a silver intensification followed by gold toning of the DAB product (not shown). Both procedures have been described previously for this antibody (Silverman et al., 1990; Zhuang et al., 1999).
Relationship of mast cell activation state to transgranulation
A total of 542 micrographs containing 564 mast cells (occasionally more than 1 cell/micrograph; see Table 1) ranging in magnification from 5000 to 10 000× were used in the quantitative analysis. This represents an area of ~55 000 μm2. Each of the 564 cells was initially assigned by two independent raters to one of the following categories: resting, partially degranulated, fully degranulated, piecemeal degranulation and resynthesizing and are described in detail in Wilhelm et al. (2000).
Results
Semi-thin sections
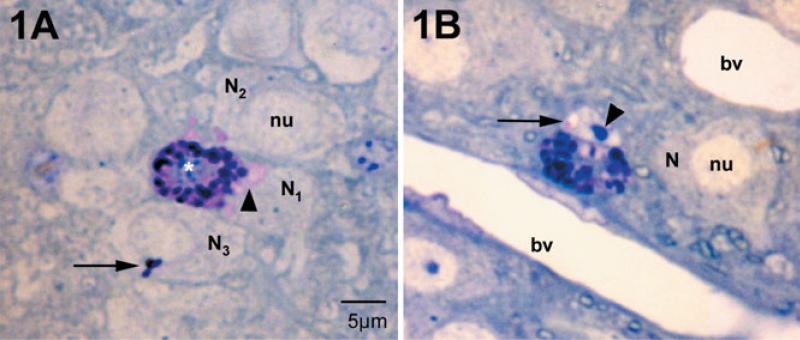
In the dove, medial habenular mast cells were located deep within the brain parenchyma surrounded by neurons, glia and their processes (Fig. 1A and B; also see Zhuang et al., 1999). Basic toluidine blue deeply stained mast cell granules (Fig. 1A and B). It is known that when the mast cell is stimulated and exocytosis initiated, the interior of the mast cell granule is exposed to extracellular fluids, causing the granule to swell as the sulfated proteoglycan core becomes hydrated (Lagunoff, 1973; Dvorak et al., 1992). Such swollen granules appeared as paler (pink) patches in the mast cell cytoplasm (Fig. 1A). Adjacent neurons (N1, N2 and N3) had both pink and deep blue material within their cytoplasm. Mast cell granular material was also found in neuronal nuclei, in this case as a sac-like compartment (Fig. 1B, arrow; an area of pale pink, also see Fig. 4). These intraneuronal staining patterns represented transgranulation events and were observed (to date) only in neuronal elements adjacent to mast cells.
Fig. 1.
Mast cells and neurons in the medial habenula as visualized in 1-μm plastic sections stained with toluidine blue, pH 11. Mast cells are distinguished from neurons by their large secretory granules and heterochromatic nucleus (white asterisk). Nuclei (nu) of neighboring neurons (N) are euchromatic. (A) The mast cell cytoplasm is filled with intensely stained granules. Those that are intact are blue to purple. Small regions of the cytoplasm are pink and are sites of granules undergoing degranulation. In the neighboring neurons, N1 and N2, patches of pink are present in the cytoplasm (arrowhead). In N3 a small cluster of stained granules is present (arrow). N1 and N2 correspond to the electron micrograph in Fig. 3. (B) In this instance the neuronal nucleus contains pink material apparently in a small sac (arrow); this cell is cut through the nucleolus (arrowhead). This cell is also shown in the electron micrograph in Fig. 4. bv, blood vessel.
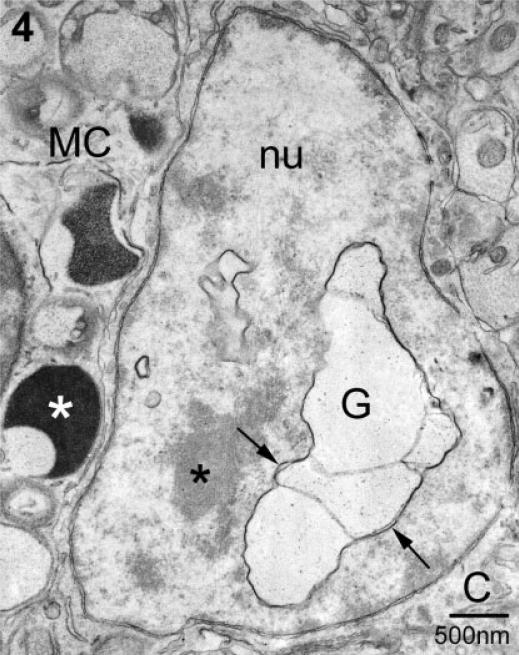
Fig. 4.
In this example of transgranulation, a multicompartment, particle-containing granule remnant (G) is present within the neuronal nucleus (nu) (neuron in Fig. 1B). The arrows indicate the membrane associated with this granule remnant. A small portion of a mast cell (MC), as identified by its granules (white asterisk), borders the neuron. *, nucleolus; C, neuronal cytoplasm.
Ultrastructure of mast cell granules in neurons
The light microscopic observation of transgranulation was extended to the ultrastructural level. We characterized the phenomenon of transfer of material from mast cell to neurons (transgranulation) and verified the phenomenon using a second fixation protocol. We then determined the frequency of transgranulation relative to the activity state of the mast cell. Finally, using immunocytochemistry, we identified a mast cell product in the cytoplasm of adjacent neurons and processes.
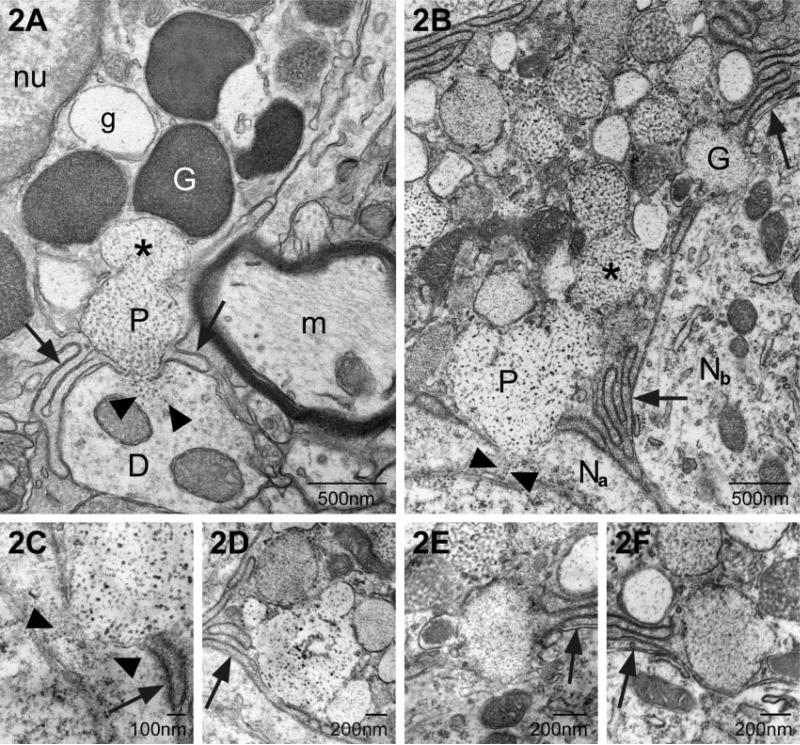
Three forms of transgranulation were observed: (1) membrane fusion between mast cell and neuron; (2) capture of mast cell granule remnants by neurons and (3) acquisition of a soluble mast cell mediator (GnRH). The first involved fusion of the mast cell's granular and plasma membranes with the neuronal plasma membrane. As seen in Fig. 2A and B, the mast cell extended filopodia or multilayered finger-like processes, excluding glial elements at the site of contact between the mast cell and the dendrite (Fig. 2A) or the neuronal soma (Fig. 2B, shown at higher magnification in Fig. 2C). When the membranes fused, there was a transcellular, exocytotic release of particles derived from the mast cell granule into the neural cytoplasm. These particles were morphologically similar to those in the parent granule (Fig. 2A–C). The site of fusion was confined to a single 70-nm section. For example, Fig. 2C illustrates a point of particle transfer. In the next ultra-thin section, shown in Fig. 2D, the membranes of the mast cell and neuron were intact and filopodia formed a barrier between the mast cell and neural compartments (Fig. 2D). Additional sites of granule–neuron fusion were identified but without any apparent accumulation of mast cell particles in the neuron; an example is shown in Fig. 2E. As in the previous examples, mast cell filopodia were present lateral to the fusion site (arrow). In the next section (Fig. 2F) the two cell types were separated once again. Also note in Fig. 2B that a second granule within the mast cell had fused with the granule making contact with the neuron (asterisk) forming a fusion chamber.
Fig. 2.
Two examples of transcytosis via transcellular exocytosis. (A) In this electron micrograph a portion of a mast cell is shown with its electron-dense granules (white G) and a particulate granule (P). The arrows indicate fine mast cell filopodia in contact with a small dendrite (D). The membrane of the particulate granule (P) and plasma membranes of both the mast cell and dendrite have fused. Small particles, morphologically similar to those in the particulate granule, are in the dendritic cytoplasm at the site of fusion (arrowheads). m, myelinated axon; g, electron-lucent granule; nu, nucleus. (B) As in A mast cell filopodia (arrow) come in contact with a neuron (Na) and small particles of the same size and morphology as those in the granule (P) are present in the neuronal cytoplasm (arrowheads). Another granule (G) makes close contact with an adjacent neuron (Nb). In both A and B the granule fusing with the neuron is part of a fusion chamber in which one or more granules have fused with the granule which is at the plasma membrane (* in both). (C) A higher magnification of the contact zone in B. The mast cell particles appear as two separate streams (arrowheads). Arrow indicates a mast cell filopodium. (D) This is the serial section to C. Within the intervening ~70 nm the fusion point has closed and mast cell filopodia (arrow) are inserted between the two cell types. (E) A higher magnification of the granule marked G in B. Note that it is apposed to the neuronal plasma membrane but its own membrane is intact. There is no evidence of mast cell material in the neuron in this or the next serial section. (F) Arrow points to mast cell filopodia.
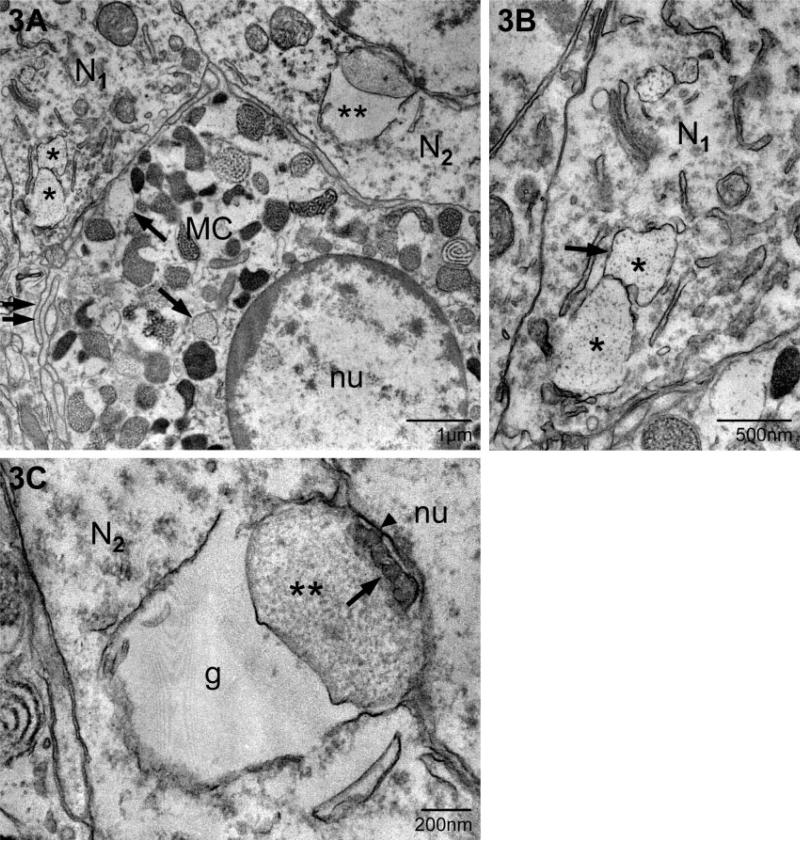
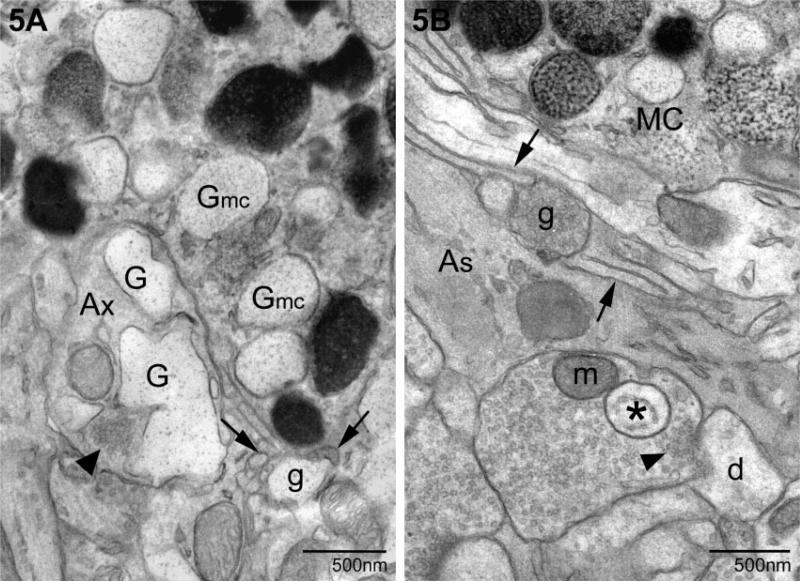
The second form of transgranulation, acquisition of granule remnants, differed from the first in that mast cell material was found in membrane-bound, particle-filled structures within several compartments of neurons (Figs 3–5). Figure 3 illustrates the apposition of a mast cell and two adjacent neurons, each of the latter containing mast cell granules (N1 and N2 previously photographed at the light microscopic level; see Fig. 1A). Figure 3A is a low magnification photomicrograph demonstrating the close relationship between neurons and the mast cell. Single and/or multiple layers of mast cell filopodia (arrows) were in direct contact with the neuronal plasma membrane. Within the mast cell there were granules of varying density and subgranular architecture, including those with finely particulate interiors. These particulate granules were similar to those present in N1 (asterisks) and shown at higher magnification in Fig. 3B. In these instances of transgranulation the internalized granule remnants formed a closely associated chain as in Fig. 3A and B (marked by asterisks). In serial sections of N1 four separate ‘granules’ were observed in the cytoplasm (not shown) and had no obvious association with other neuronal organelles. In another instance, N2 (Fig. 3A and C), two granules (double asterisk) abutted the outer nuclear envelope. As seen in Fig. 3C, the granule closest to the nucleus retained both a particulate and vesicular substructure (asterisks). The more swollen element (g) lacked any apparent internal structure.
Fig. 3.
Localization of mast cell granule remnants in neighboring neurons. (A) Low magnification of a mast cell (MC) and adjacent neurons. Note the heterogeneity in size, shape and electron density of the granules that fill the mast cell cytoplasm. The arrows indicate electron-lucent, particle-filled granules characteristic of ongoing or recovery from exocytosis. Two neurons (N1 and N2; as shown in Fig. 1) contain mast cell granules (* and **, respectively). (B) A higher magnification of the granule remnants in A designated with *. In this section, two of the four contiguous remnants (*) (as identified in a set of serial sections, not shown) are seen. The granular material is enclosed by membrane (arrow). (C) A higher magnification of the granule remnant (**) in N2. This section is separated from that shown in A by ~200 nm. The remnant is composed of two chambers, one swollen and devoid of particles (g) and a compartment which retains particulate (**) and vesicular substructure (arrow). The second compartment is in contact with the outer nuclear envelope (arrowhead). nu, nucleus.
Fig. 5.
Two examples of mast cell granules in pre-synaptic elements (as defined by the clustering of synaptic vesicles, arrowheads). (A) The inclusion is an expanded remnant (G) similar to the particulate granules in the neighboring mast cell (Gmc). Also note a granule remnant (g) external to either cell and surrounded by filopodia (arrows). In B a pre-synaptic terminal contains a granule (*) with a well-defined membrane and vesicular interior (*). An externalized granule (g) is present, surrounded by mast cell filopodia (arrows). Ax, axon; As, astrocyte; d, dendrite; MC, mast cell; m, mitochondrion.
A second site for localization of mast cell granule chains was the neuronal nucleus (Fig. 4; see light microscopic equivalent in Fig. 1B). In this case the neuron was cut through the plane of the nucleus/nucleolus and scant cytoplasm was present in this section. The neuronal plasma membrane was immediately adjacent to that of the mast cell (MC) whose granules, in turn, were at the cell boundary. Serial section analysis of this neuron indicated that several contiguous granule compartments were present in both the nucleus (nu) and cytoplasm (not shown) of this neuron. There was no anatomical evidence for apoptosis or necrosis of this or any other neuron with or without mast cell granules.
Most surprising was the discovery of mast cell granules in pre-synaptic elements as illustrated in Fig. 5. In Fig. 5A, a portion of a mast cell with its characteristic collection of electron-dense and particulate (G) granules was apposed to a pre-synaptic terminal with its clustering of synaptic vesicles (arrowhead). Numerous mast cell filopodia, cut in both longitudinal and cross section, were present at one pole of the mast cell (arrows). These cellular extensions surrounded an extruded granule remnant (g). In the nerve terminal there were several membrane-bound particulate structures (G) similar to those in the mast cell (Gmc) but more pleiomorphic. In Fig. 5B another extruded granule (g) was observed surrounded by mast cell filopodia. The granule remnant (asterisk) had a vesicular substructure and was present in the next three serial sections (data not shown).
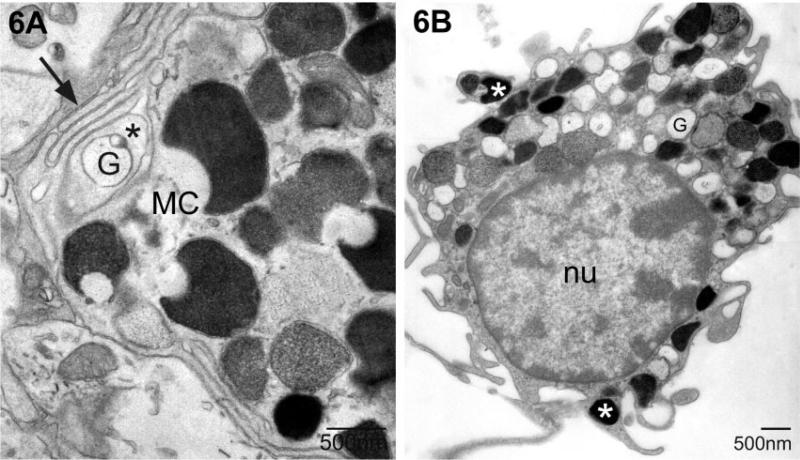
Mast cell granules were found outside the main body of the mast cell, enfolded in mast cell filopodial processes (Wilhelm et al., 2000). Examples of this phenomenon are shown in Figs 5 and 6A. When mast cells were on the pia mater adherent to the medial habenula, granules were found in fine cytoplasmic extensions (Fig. 6B).
Fig. 6.
Examples of granules in mast cell cytoplasmic extensions. (A) The granule (G) is isolated from the mast cell cytoplasm by several layers of filopodia (*, first layer; arrow, multiple layers). The cell in B is in the pia and electron-dense granules (white asterisk) are frequently present in the small cellular extensions. This mast cell is primarily filled with electron-lucent granules associated with piecemeal degranulation (G). MC, mast cell; nu, nucleus.
Immunoelectron microscopy
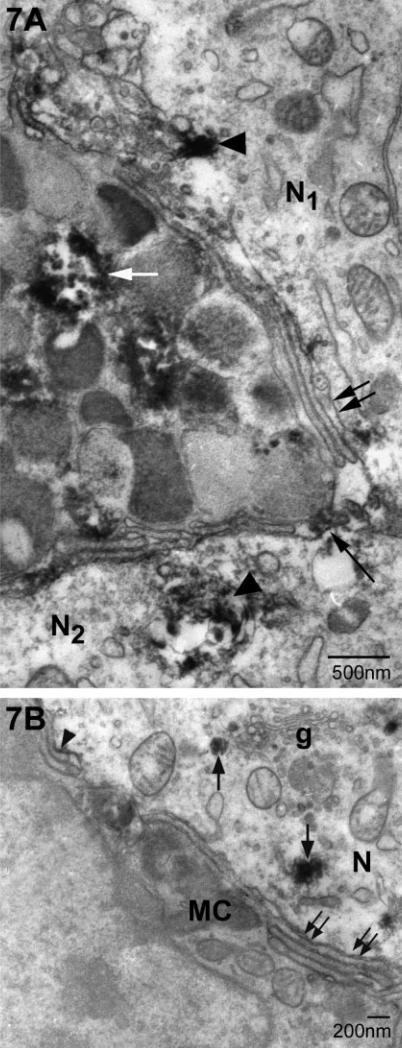
Gonadotropin-releasing hormone immunoreactivity was present in some but not all mast cell granules (Fig. 7A, white arrow) (Zhuang et al., 1993; Silverman et al., 1994). We used this antigen to mark the presence of a mast cell constituent within medial habenular neurons (Fig. 7A and B). Immunoreactive sites were found within neurons adjacent to mast cells (Fig. 7A) but not in neurons that did not border a mast cell (not shown). Immunoreactive material was identified as accumulations of flocculent DAB precipitate on mast cell filopodia abutting the neuron and in vesicular structures within the neuron (Fig. 7B). The spread of the DAB reaction product made it difficult to discern membranes. In Fig. 7B, these deposits adhered to the side of the filopodia facing the neuron (arrowhead) or in small vesicles in the cytoplasm which are occasionally near the trans-Golgi network (arrows).
Fig. 7.
Ultrastructural immunocytochemical evidence for mast cell-derived gonadotropin-releasing hormone (GnRH) in neurons. (A) 3,3′ diaminobenzidine (DAB), used to detect sites of antibody binding, forms a flocculent deposit (which can obscure finer ultrastructural detail) in some (white arrow) but not all granules within the mast cell. DAB deposits are also present in two adjacent neurons (N1 and N2; arrowheads). There are multiple layers of mast cell filopodia cut in both longitudinal and cross section (double arrows). Some of these are associated with DAB deposits (long arrows). (B) In this plane of section the mast cell (MC) has few granules. Within the neuron (N), small deposits of immunoreactive material are found associated with vesicles (arrows). Some of these vesicles are close to the Golgi apparatus (g). DAB is also associated with the surface of filopodia (arrowhead). Double arrows indicate the characteristic multiple filopodial layers of the MC.
Is transgranulation associated with a mast cell activational state?
A total of 45 transgranulation events were identified in ultra-thin sections through 564 mast cell profiles representing ~55 mm2 or a volume of ~3.85 mm3. Each incidence of mast cell granule remnant(s) in neurons was scored as was the neuronal compartment within which the phenomenon was noted. The most frequent anatomical site for granule remnant capture was within the neuronal cell body as illustrated in Fig. 3A–C. When granules were observed in neurons, a mast cell or a portion thereof was present in the same ultra-thin section. Similar results were obtained with both fixation protocols used.
The relationship of transgranulation to mast cell activity state is shown in Table 1. Transfer of granules to neurons occurred regardless of the activity state of the mast cell but was observed with a significantly greater frequency in neurons adjacent to mast cells undergoing piecemeal degranulation. The majority of transgranulation events (28 events or 62.2% of the total) were associated with mast cells undergoing piecemeal degranulation even though this state represented only ~21% of the total number of cells examined (chi square = 302.8, d.f. = 4, P < 0.0001).
Discussion
Documentation of transgranulation
This is the first documentation of transgranulation of mast-derived material in neurons of the CNS and the phenomenon is demonstrated in vivo. Mast cell products are either released directly into neuronal cytoplasm (Fig. 2) or present in membrane-bound organelles (Fig. 3). This conclusion is based on the morphological identification of mast cell material in neurons and the demonstration of a peptide of mast cell origin in neurons adjacent to mast cells (Fig. 7). Furthermore, granule capture is independent of the fixative used, as well as age and reproductive status of the animal.
Importance of this phenomenon in the central nervous system
In the present series of micrographs, 45 instances of neuronal acquisition of granule remnants are recorded in a very small tissue volume (~3.85 mm3), representing a fraction of the total volume of the medial habenula. The estimation of frequency is based on examination of a 70-nm section through each of 564 mast cells and not on the number of neurons photographed. These data support the hypothesis that brain transgranulation events are frequent and represent a new mechanism of neuron–immune communication in the CNS.
Association of transgranulation with activational state
Mast cell activation occurs in normal animals under basal conditions (Wilhelm et al., 2000; Florenzano & Bentivoglio, 2000) and following stress (Theoharides et al., 1995; Esposito et al., 2001). The present results indicate a significant relationship between the activity status of the mast cell and the frequency of transgranulation events. Mast cell granule remnants are most commonly observed in neural elements adjacent to mast cells which are undergoing piecemeal degranulation. Although this might seem counter-intuitive, in that piecemeal release is associated with preferential secretion of small molecules (Kops et al., 1990), these mast cells also contain granules that could undergo conventional exocytosis and be the source of the granule remnants.
Aspects of transgranulation: particles, granule remnant, and neuromodulator
We report that fusion events between mast cells and neurons result in intragranular particles entering neurons. A similar phenomenon has been reported for bladder nerve terminals (Keith & Saban, 1995). The particles are probably large complexes of stored heparin sulfated proteoglycans with attached neutral proteases (Serafin et al., 1986; Serafin & Austen, 1987; see below). The apparent low frequency of this form of transgranulation may be due to the swift dispersion of particles, rapidity of the fusion events or restriction of the fusion site to a single ultra-thin section.
The mechanism for the second form of transgranulation is either engulfment of mast cell processes containing a granule(s) or capture of released granule remnants. In the brain we cannot as yet differentiate between these possibilities although our data support capture of granule remnants. Granule capture is a multistep process (Greenberg & Burnstock, 1983). Mast cells, cocultured with fibroblasts or endothelial cells, shed processes containing granules (Fig. 6) which then attach to the neighboring cell's plasma membrane followed by internalization. Enclosure of mast cell processes may be analogous to the transfer of granular inclusions in other cell–cell interactions. For example, melanosomes are transferred from the melanocyte to the keratinocyte via the engulfment by the latter of melanosome-filled dendritic process (Klaus, 1969; Okazaki et al., 1976; Yammoto, 1994) by a protease-activated receptor 2 mechanism (Seiberg et al., 2000).
The capture of granule remnants is described in several studies using immune (see Introduction) and non-immune system cells. Fibroblasts and endothelial cells extend processes to capture intact (released) mast cell granules or granule remnants (Subba et al., 1983; Atkins et al., 1985). Using mast cell granule remnants tagged with gentamicin (which has an intrinsic fluorescence), Decorti et al. (2000) demonstrated that remnants are rapidly internalized by macrophages. The internalization of mast cell granule remnants also occurs in vivo in humans. DeSchryver-Kecskemeti et al. (1992) reported that mast cell granules were present in the endothelia of colonic capillaries in a patient with active colitis. As in the periphery, mast cell degranulation and the presence of extracellular granule remnants occur in the CNS of normal animals (see Fig. 5; Florenzano & Bentivoglio, 2000; Wilhelm et al., 2000) and are available for capture. Therefore, the phenomenon of transgranulation is found under both normal and disease conditions. Recent experiments in vitro using rat thalamic neurons and purified mast cells or mast cell granules suggest that capture of granule remnants is not limited to any specific granule subtype (Silverman & Silver, 2005).
Gonadotropin-releasing hormone, a neuromodulator, serves as an excellent marker for the transfer of a peptide of mast cell origin (Silver et al., 1993; Silverman et al., 2002; Khalil et al., 2003) to adjacent neurons. Mast cells are the only source of GnRH in the medial habenula as GnRH axons do not cross the nuclear boundaries (Silver et al., 1996). The LR-1 antibody recognizes amino acids 6–10 of the GnRH decapeptide within the prohormone and as the cleaved and amidated active molecule but it does not recognize degradation products (Silverman et al., 1990). These data on the specificity of the antibody suggest that the epitope present within habenular neurons persists intact (perhaps as the prohormone).
The intraneuronal localization of GnRH immunoreactivity in discrete vesicles is different from both the particle streams following membrane fusion and the capture of mast cell granule remnants. GnRH is a small peptide, unlike the attached proteoglycans and molecules (the particles), and is likely to be soluble in extracellular space upon mast cell degranulation. It is possible that released GnRH becomes bound to its receptors on the mast cell filopodial plasma membrane (see Fig. 7; Rivier et al., 1986). Filopodia are frequently pinched off from the parent mast cell (Fig. 7A) and these cellular ‘bits’ may be incorporated into the neighboring neuron. Alternatively, habenular neurons in the dove may have GnRH receptors and the peptide could enter the neurons by receptor-mediated endocytosis. The mechanism triggering such incorporation is unknown. Although this material may eventually be targeted to lysosomes, we speculate that GnRH might be available for re-release as demonstrated for captured mast cell-derived β-hexosaminidase and collagenase by fibroblasts (Subba et al., 1983).
Role of the captured mast cell material
It is well documented that mast cell mediators alter neuronal membrane properties (Undem et al., 1993, 1995; Weinreich et al., 1997; Moore et al., 2002; Khalil et al., 2004). Histamine, which is present in dove medial habenular mast cells (Silverman et al., 1994), would fall into this category. A role for the internalization of mast cell components into neurons is unknown but could represent an efficient mechanism for termination of the stimulus of mast cell secretory products as suggested by Metcalfe and others (Subba et al., 1983; Atkins et al., 1985) and/or provide a limit on the potential destructiveness of their serine proteases. In vitro, peritoneal macrophage ingestion of mast cell granules alters cellular physiology resulting in degradation of nuclear factor-kappa B, the loss of which inhibits the up-regulation of inducible nitric oxide synthetase and tumor necrosis factor-α mRNA expression (Ito et al., 1998).
A potentially powerful effect of internalized mast cell particles (Fig. 2) could be mediated by heparin, a substance synthesized only by mast cells (Zehnder & Galli, 1999 and references therein). Intracellular heparin is a well-known pharmacological tool which blocks release of intracellular calcium via inhibition of the inositol triphosphate (IP3) pathway (Nilsson et al., 1988). The hypothesis that heparin, delivered to neurons, can alter their responsiveness is substantiated by the following examples. Activation of metabotropic glutamate receptors on mid-brain dopaminergic neurons results in hyperpolarization. This membrane response is due to the activation of Ca2+-sensitive K+ channels. Neurotransmitter-induced Ca2+ mobilization is due, in part, to the second messenger, IP3. The IP3 receptor is antagonized by intracellular perfusion of the neuron with heparin, blocking the neurotransmitter's effect (Morikawa et al., 2003). Similarly, cytoplasmic heparin inhibits the mobilization of internal Ca2+ stores via IP3 in layers 2/3 and 5 of rat neocortical somatosensory neurons (Larkum et al., 2003). When mast cell granules are captured by fibroblasts, the heparin sulfated proteoglycan is metabolized within the ‘host’ cell (Atkins & Metcalfe, 1983). Similarly, when macrophages take up granule remnants containing 35S-labelled heparin sulfate proteoglycans, the radioactivity appears rapidly in the medium. It is not clear how neurons metabolize captured mast cell material; Cy-3 avidin-labeled mast cell granules (avidin binds to heparin) can be captured and remain in the neuronal cytoplasm as fluorescent puncta for up to 7 days (Silverman & Silver, 2005). It is not known whether heparin is ever free in the cytoplasm of any cell type, including neurons, although the appearance of the particle streams (Fig. 2) strongly suggests that this could happen. It should be emphasized that mast cell degranulation within the normal CNS is not accompanied by an inflammatory response (no invasion of immune system cells) nor by induction of apoptosis/necrosis.
In summary, we have described a novel form of intercellular communication between mast cells of CNS and neighboring neurons. In addition to altering the neuronal microenvironment via actions on blood vessel permeability (Zhuang et al., 1996; Esposito et al., 2001), mast cells can alter the internal environment/constituents of neurons that acquire mast cell products with the potential to alter the responsiveness of a neuron (i.e. via the presence of intracellular heparin) or by supplying products that the neuron can re-release.
Acknowledgements
This work was supported by NIMH 54088 (A.J.S.) and NIMH 067782 (R.S.). We acknowledge the technical help of Honor Kirwan, Heather McKellar and Katalin Pula. We thank our colleagues Drs Joan W. Witkin and Guilia Baldini for critique of previous versions of the manuscript. The LR-1 antiserum was a gift of Robert Benoit, McGill University, Montreal, Quebec, Canada.
Abbreviations
- CNS
central nervous system
- DAB
3,3′ diaminobenzidine
- GnRH
gonadotropin-releasing hormone
- IP3
inositol triphosphate
References
- Atkins F, Metcalfe DD. Degradation of the heparin matrix of mast cell granules by cultured fibroblasts. J. Immunol. 1983;131:1420–1425. [PubMed] [Google Scholar]
- Atkins F, Friedman MM, Metcalfe DD. Biochemical and microscopic evidence for the internalization and degradation of heparin-containing mast cell granules by bovine endothelial cells. Lab. Invest. 1985;52:278–286. [PubMed] [Google Scholar]
- Baggiolini M, Horisberger U, Martin U. Phagocytosis of mast cell granules by mononuclear phagocytes, neutrophils and eosinophils during anaphylaxis. Int. Arch. Allergy Appl. Immunol. 1982;67:219–226. doi: 10.1159/000233022. [DOI] [PubMed] [Google Scholar]
- Buma P, Roubos EW. Ultrastructural demonstration of non-synaptic release sites in the CNS of the snail, Lymnaea stagnalis, the insect, Periplaneta americana, and the rat. Neuroscience. 1986;17:867–879. doi: 10.1016/0306-4522(86)90051-5. [DOI] [PubMed] [Google Scholar]
- Burwen S, Satir B. A freeze-fracture study of early membrane events during mast cell secretion. J. Cell Biol. 1977;73:660–671. doi: 10.1083/jcb.73.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decorti G, Klugman FB, Crivellato E, Malusa N, Furlan G, Candussio L, Giraldi T. Biochemical and microscopic evidence for the internalization of drug-containing mast cell granules by macrophages and smooth muscle cells. Toxicol. Appl. Pharm. 2000;169:269–275. doi: 10.1006/taap.2000.9072. [DOI] [PubMed] [Google Scholar]
- DeSchryver-Kecskemeti K, Williamson JR, Jakschik BA, Clouse RE, Alpers DH. Mast cell granules within endothelial cells: a possible signal in the inflammatory process? Mod. Path. 1992;5:343–347. [PubMed] [Google Scholar]
- Dimitriadou V, Lambracht-Hall M, Reichler J, Theoharides TC. Histochemical and ultrastructural characteristics of rat brain perivascular mast cells stimulated with compound 48/80 and carbachol. Neuroscience. 1990;39:209–224. doi: 10.1016/0306-4522(90)90234-u. [DOI] [PubMed] [Google Scholar]
- Dropp JJ. Mast cells in the mammalian brain. Acta Anat. 1976;94:1–21. doi: 10.1159/000144540. [DOI] [PubMed] [Google Scholar]
- Dvorak A, McLeod RS, Onderdonk A, Monahan-Earley RA, Cullen JB, Antonioli DA, Morgan E, Blair JE, Estrella P, Cisneros RL, Silen W, Cohen Z. Ultrastructural evidence for piecemeal and anaphylactic degranulation of human gut mucosal mast cells in vivo. Intern. Arch. Allergy Immunol. 1992;99:74–83. doi: 10.1159/000236338. [DOI] [PubMed] [Google Scholar]
- Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, Jacobson S, Theoharides TC. Acute stress increases permeability of the blood–brain barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Florenzano F, Bentivoglio M. Degranulation, density and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J. Comp. Neurol. 2000;424:651–669. [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Greenberg G, Burnstock G. A novel cell-to-cell interaction between mast cells and other cell types. Exp. Cell Res. 1983;147:1–13. doi: 10.1016/0014-4827(83)90265-3. [DOI] [PubMed] [Google Scholar]
- Guo Z, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellopodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- Ibrahim MZM. The mast cells of the mammalian central nervous system. Part I. Morphology, distribution, and histochemistry. J. Neurol. Sci. 1974;21:431–478. doi: 10.1016/0022-510x(74)90044-6. [DOI] [PubMed] [Google Scholar]
- Ito N, Li Y, Suzuki T, Stechschulte DJ, Dileepan KN. Transient degradation of NF-kappaB proteins in macrophages after interaction with mast cell granules. Med. Inflamm. 1998;7:397–407. doi: 10.1080/09629359890776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Krenger W. Interactions of mast cells with the nervous system – recent advances. Neurochem. Res. 1992;17:939–951. doi: 10.1007/BF00993271. [DOI] [PubMed] [Google Scholar]
- Keith IM, Saban R. Nerve–mast cell interaction in normal guinea pig bladder. J. Comp. Neurol. 1995;363:28–36. doi: 10.1002/cne.903630104. [DOI] [PubMed] [Google Scholar]
- Khalil MH, Silverman AJ, Silver R. Mast cells in the rat brain synthesize gonadotropin-releasing hormone. J. Neurobiol. 2003;56:113–124. doi: 10.1002/neu.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil MH, Silverman AJ, Silver R. Keystone Symposium on Mast Cells in Physiology, Host Defense and Disease: Beyond IgE. Taos; New Mexico: 2004. Mast cell mediators alter electrical activity of rat thalamic neurons. p. 57. [Google Scholar]
- Klaus S. Post-transfer digestion of melanosome complexes and saltatory movement of melanin granules within mammalian epidermal cells. J. Invest. Dermatol. 1969;53:440–444. doi: 10.1038/jid.1969.172. [DOI] [PubMed] [Google Scholar]
- Kops SK, Theoharides TC, Cronin CT, Kashgarian MG, Askenase PW. Ultrastructural characteristics of rat peritoneal mast cells undergoing differential release of serotonin without histamine and without degranulation. Cell Tiss. Res. 1990;262:415–424. doi: 10.1007/BF00305238. [DOI] [PubMed] [Google Scholar]
- Kovanen P. Mast cell granule mediated uptake of low density lipoproteins by macrophages: a novel carrier mechanism leading to the formation of foam cells. Ann. Med. 1991;23:551–119. doi: 10.3109/07853899109150517. [DOI] [PubMed] [Google Scholar]
- Lagunoff D. Membrane fusion during mast cell secretion. J. Cell Biol. 1973;57:252–259. doi: 10.1083/jcb.57.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford LA, Coggeshall RE. The use of potassium ferricyanide in neural fixation. Anat. Rec. 1980;197:297–303. doi: 10.1002/ar.1091970304. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Watanabe S, Nakamura T, Lasser-Ross N, Ross WN. Synaptically activated Ca2+ waves in layer 2/3 and layer 5 rat neocortical pyramidal neurons. J. Physiol. 2003;549:471–488. doi: 10.1113/jphysiol.2002.037614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U, Pertoft H, Seljelik R. Uptake and degradation of mast cell granules by mouse peritoneal macrophages. Biochem. J. 1979;182:189–193. doi: 10.1042/bj1820189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning KA, Pienkowski TP, Uhlrich DJ. Histaminergic and non-histamine-immunoreactive mast cells within the cat lateral geniculate complex examined with light and electron microscopy. Neuroscience. 1994;63:191–206. doi: 10.1016/0306-4522(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Moore KA, Oh EJ, Weinreich D. 5-HT(3) receptors mediate inflammation-induced unmasking of functional tachykinin responses in vitro. J. Appl. Physiol. 2002;92:2529–2534. doi: 10.1152/japplphysiol.00974.2001. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J. Neurosci. 2003;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Zwiller J, Boynton AL, Berggren PO. Heparin inhibits IP3-induced Ca2+ release in permeabilized pancreatic beta-cells. FEBS Lett. 1988;229:211–214. doi: 10.1016/0014-5793(88)80829-9. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Uzuka M, Morikawa F, Toda K, Seiji M. Transfer mechanism of melanosomes in epidermal cell culture. J. Invest. Dermatol. 1976;67:541–547. doi: 10.1111/1523-1747.ep12664554. [DOI] [PubMed] [Google Scholar]
- Oliani SM, Lim LH, Christian HC, Pell K, Das AM, Perretti M. Morphological alteration of peritoneal mast cells and macrophages in the mouse peritoneal cavity during the early phases of an allergic inflammatory reaction. Cell Biol. Int. 2001;25:795–803. doi: 10.1006/cbir.2001.0770. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay S, Webster H. de F. The Fine Structure of the Nervous System. W.B. Saunders Co.; Philadelphia: 1976. [Google Scholar]
- Rivier JE, Porter J, Rivier CL, Perrin M, Corrigan A, Hook WA, Siraganian RP, Vale WW. New effective gonadotropin releasing hormone antagonists with minimal potency for histamine release in vitro. J. Med. Chem. 1986;29:1846–1851. doi: 10.1021/jm00160a008. [DOI] [PubMed] [Google Scholar]
- Seiberg M, Paine C, Sharlow E, Andrade-Gordon P, Costanzo M, Eisinger M, Shapiro S. The protease-activated receptor 2 regulates pigmentation via keratinocyte–melanocyte interactions. Exp. Cell Res. 2000;254:25–32. doi: 10.1006/excr.1999.4692. [DOI] [PubMed] [Google Scholar]
- Serafin WE, Austen KF. Mediators of immediate hypersensitivity reactions. NEJM. 1987;317:30–34. doi: 10.1056/NEJM198707023170106. [DOI] [PubMed] [Google Scholar]
- Serafin WE, Katz HR, Austen KF, Stevens RL. Complexes of heparin proteoglycans, chondroitin sulfate E proteoglycans and [3H]diisopropyl fluorophosphate-binding proteins are exocytosed from activated mouse bone marrow-derived mast cells. J. Biol. Chem. 1986;261:15 017–15 021. [PubMed] [Google Scholar]
- Silver R, Ramos CL, Silverman AJ. Sex behavior triggers appearance of non-neural cells containing gonadotropin-releasing hormone in doves. J. Neuroendo. 1992;4:207–210. doi: 10.1111/j.1365-2826.1992.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Silver R, Zhuang X, Millar RP, Silverman AJ. Mast cells containing GnRH-like immunoreactivity in the CNS of doves. In: Sharp PJ, editor. Avian Endocrinology. J. Endocrinology Ltd; Bristol: 1993. pp. 87–89. [Google Scholar]
- Silver R, Silverman AJ, Vitkovic L, Lederhendler I. Mast cells in the brain: evidence and functional significance. TINS. 1996;19:25–31. doi: 10.1016/0166-2236(96)81863-7. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Silver R. Neurons acquire mast cell granule remnants. Soc. Neurosci. 2005 doi: 10.1111/j.1460-9568.2005.04429.x. Abstr., no. 6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Witkin JW, Millar RP. Light and electron microscopic immunocytochemical analysis of antibodies directed against GnRH and its precursor in hypothalamic neurons. J. Histochem. Cytchem. 1990;38:803–813. doi: 10.1177/38.6.2186087. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Millar RP, King JA, Zhuang X, Silver R. Mast cells with gonadotropin releasing hormone-like immunoreactivity in the brain of doves. Proc. Natl Acad. Sci. (U.S.A.) 1994;91:3695–3699. doi: 10.1073/pnas.91.9.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman A, Asarian L, Khalil M, Silver R. GnRH, brain mast cells and behavior. Prog. Brain Res. 2002;141:315–325. doi: 10.1016/S0079-6123(02)41102-8. [DOI] [PubMed] [Google Scholar]
- Subba P, Friedman M, Atkins FM, Metcalfe DD. Phagocytosis of mast cell granules by cultured fibroblasts. J. Immunol. 1983;183:341–349. [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. Mast cells: The immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-f. [DOI] [PubMed] [Google Scholar]
- Theoharides T, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, Rozniecki JJ, Webster E, Chrousos GP. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- Undem B, Hubbard W, Weinreich D. Immunologically induced neuromodulation of guinea pig nodose ganglion neurons. J. Autonom. Nerv. Syst. 1993;44:35–44. doi: 10.1016/0165-1838(93)90376-6. [DOI] [PubMed] [Google Scholar]
- Undem B, Riccio M, Weinreich D, Ellis J, Myers A. Neurophysiology of mast cell–nerve interactions in the airways. Int. Arch. Allergy Immunol. 1995;107:199–201. doi: 10.1159/000236976. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lindstedt K, Kovanen P. Mast cell granule remnants carry LDL into smooth muscle cells of the synthetic phenotype and induce their conversion into foam cells. Arterioscler. Thromb. Vasc. Biol. 1995;15:801–810. doi: 10.1161/01.atv.15.6.801. [DOI] [PubMed] [Google Scholar]
- Weinreich D, Moore KA, Taylor GE. Allergic inflammation in isolated vagal sensory ganglia unmasks silent NK-2 tachykinin receptors. J. Neurosci. 1997;17:7683–7693. doi: 10.1523/JNEUROSCI.17-20-07683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, King B, Silverman AJ, Silver R. Gonadal steroids regulate the number and activational state of mast cells in the medial habenula. Endocrinology. 2000;141:1178–1186. doi: 10.1210/endo.141.3.7352. [DOI] [PubMed] [Google Scholar]
- Yammoto O. Three modes of melanosome transfer in caucasian facial skin: hypothesis based on an ultrastructural study. Pigment Cell Res. 1994;7:158–169. doi: 10.1111/j.1600-0749.1994.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Zehnder JL, Galli SJ. Mast-cell heparin demystified. Nature. 1999;400:714–715. doi: 10.1038/23360. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Reproductive behavior, endocrine state, and the distribution of GnRH-like immunoreactive mast cells in dove brain. Horm. Behav. 1993;27:283–295. doi: 10.1006/hbeh.1993.1021. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Brain mast cell degranulation regulates the blood brain barrier. J. Neurobiol. 1996;31:393–403. doi: 10.1002/(SICI)1097-4695(199612)31:4<393::AID-NEU1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Distribution and local differentiation of mast cells in the parenchyma of the forebrain. J. Comp. Neurol. 1999;408:477–488. doi: 10.1002/(sici)1096-9861(19990614)408:4<477::aid-cne3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]