Abstract
In nonmammalian vertebrates, photic cues that regulate the timing of seasonal reproductive cyclicity are detected by nonretinal, nonpineal deep brain photoreceptors. It has long been assumed that the underlying mechanism involves the transmission of photic information from the photoreceptor to a circadian system, and thence to the reproductive axis. An alternative hypothesis is that there is direct communication between the brain photoreceptor and the reproductive axis. In the present study, light and confocal microscopy reveal that gonadotropin releasing hormone (GnRH) neurons and processes are scattered among photoreceptor cells (identified by their opsin-immunoreactivity) in the lateral septum (SL). In the median eminence (ME), opsin and GnRH immunoreactive fibers overlap extensively. Single and double label ultrastructural immunocytochemistry indicate that in the SL and preoptic area (POA), opsin positive terminals form axo-dendritic synapses onto GnRH dendrites. In the ME, opsin and GnRH terminals lie adjacent to each other, make contact with tanycytes, or terminate on the hypophyseal portal capillaries. These results reveal that brain photoreceptors communicate directly with GnRH-neurons; this represents a means by which photoperiodic information reaches the reproductive axis.
Keywords: circadian, avian, brain photoreceptor, GnRH, median eminence, opsin, VIP
It is well established that photic stimuli associated with changing day length regulate seasonal cycles of reproduction. In mammals, light cues are transmitted from the retina to the circadian system of the suprachiasmatic nuclei (SCN), ultimately reaching the pineal gland where the duration of melatonin secretion determines the timing of seasonal endocrine secretions (Bartness et al., 1993). In birds, there is no evidence that melatonin secretion controls reproductive cycles, though there is a reduction in the amplitude of melatonin in midsummer and midwinter in some high-latitude birds (reviewed in Gwinner et al., 1997).
In comparing mammals and birds, there are differences not only in the function(s) of the pineal gland but also in the significance of photic input from the eyes. In small mammals, ocular enucleation results in complete loss of seasonal cyclicity. It has been assumed that a similar pathway, from photic receptors to circadian pacemakers to GnRH effectors, occurs in nonmammalian vertebrates. In these species, however, the sensory element entails a nonretinal, nonpineal photoreceptor residing deep within the brain. In evidence, there is no change in the pattern of seasonal changes in gonadal growth and secretion of LH (Luteinizing hormone) following enucleation and/or pinealectomy (reviewed in Oliver and Baylé, 1982;Wilson, 1991). In contrast, if the head is covered so that light cannot penetrate the skull, photoperiodic responses are eliminated (Menaker et al., 1970), providing compelling evidence for the importance of brain photoreceptors.
To understand how photic information reaches the gonadal system, it was necessary to locate and characterize brain photoreceptors. The existence of brain photoreceptors in nonmammalian vertebrates has been known for more than 8 decades (reviewed in Oliver and Baylé, 1982); however, they have been difficult to find anatomically. Initial attempts to localize brain photoreceptors involved lesion studies and directed illumination using fiber optics. Lesion studies brought the problems of transecting fibers of passage, among other issues. Fiber optic implants resulted in the spread of the light signal from the point of illumination within the brain. Not surprisingly, these studies implicated many brain regions as potential loci for encephalic photoreceptors.
More recently, there has been success in finding brain photoreceptors with the use of anti-opsin antibodies. Rod and cone photopigments are well conserved. All photopigments consist of an opsin protein (part of a family of transmembrane proteins), which binds to a chromophore (Kandel et al., 2000). Initial studies using a variety of anti-opsin antibodies over a period of 15 years failed to reveal these neural elements. These failures may have resulted from the fact that the immunopositive neurons are highly localized to very small brain regions. In 1988, however, we (Silver et al., 1988) used an antibody directed against rod opsin and successfully identified cerebrospinal fluid–(CSF-) contacting neurons in the septum lateralis (SL) and hypothalamus. This was subsequently confirmed and extended using other anti-opsin antibodies (reviewed in Foster et al., 1994; Foster and Soni, 1998). The immunocytochemical results have been extended and confirmed in recent years using molecular biological techniques. In situ hybridization using rod opsin–specific probes substantiated the immunocytochemical studies (Wada et al., 1998). Furthermore, the occurrence of novel opsins in extraocular sites has been reported by several laboratories (reviewed in Foster and Soni, 1998). The next step in understanding the interface between the photic and reproductive system requires exploration of the route where by photic input from brain photoreceptors reaches GnRH neurons. To this end, we examined opsin- and GnRH-positive cells using light, confocal, and electron microscopic immunocytochemistry.
MATERIALS AND METHODS
Animals
Subjects were adult ring doves (Streptopelia roseogrisea), housed in visual isolation in suspended metal cages (10 × 8 × 14 inches), and maintained at 20 to 22 °C under a 14:10 light-dark schedule with lights on at 0600 h. Animals were sacrificed between 1000 and 1400 h. Food, water, and grit were available ad libitum. All animal care was carried out according to the National Institutes of Health guidelines, approved by the IACUC (Institutional animal care and use committee) of Columbia University.
Perfusion
For light microscopy, animals were perfused using 4% paraformaldehyde and 0.1% glutaraldehyde, with the pH shift postfix (from pH 7.4 to pH 10.4) described below. For electron microscopy, the perfusate (pH 7.4) was 4% paraformaldehyde and 0.2% glutaraldehyde. Brains were postfixed in the perfusate at 20 °C for 2 h and overnight in 4% paraformaldehyde at pH 10.4 (Eldred et al., 1983). This fixation was critical to the identification of both opsin and GnRH in the same section. Finally, brains were embedded in 12% gelatin and cut on a vibratome.
Light Microscopy and Use of Fluorophore
Two protocols were used to screen for regions of overlapping opsin- and GnRH-positive neurons. First, alternate sections (50 µm) from 4 birds were reacted immunocytochemically with antibodies against opsin (RET-P1, gift of C. Barnstable) or GnRH (LR-1, gift of R. Benoit; see Silver et al., 1988; Saldanha et al., 1994 for details of methods used). Next, for a more detailed examination of overlapping distributions of the two antigens, 20 µm sections were cut in two brains through the SL, preoptic area (POA), and median eminence (ME) and were double labeled for opsin and GnRH. On half the sections, opsin antibody (1:20 K, 48 h) was reacted first and visualized with Vector SG chromogen (stains blue-black), followed by incubation with GnRH antiserum (1:5 K, 96 h), visualized with 0.03% diaminobenzidene (DAB; stains brown). The alternate set was treated for detection of GnRH (1:40 K, 48 h) and visualized with the blue-black color (SG) followed by incubation with anti–opsin antibody (1:10 K, 48 h), which was visualized as brown (DAB). The distribution of opsin and GnRH-immunoreactive elements was traced from the lobus parolfactorius to the level of the oculomotor nerve, using a camera lucida.
Confocal Microscopy
Animals (N = 6) were perfused as above, and 50 µm vibratome sections were cut. For GnRH, sections were incubated in LR-1 (LR-1 1:5 K) followed by goat anti-rabbit FITC (Fluorescein isothiocyanate). For opsin, the same sections were incubated in RET-P1 (1:15 K) followed by biotinylated horse anti-mouse secondary and avid in Texas Red. Sections through SL, POA, and ME were examined.
Electron Microscopy
Three approaches were used in the ultrastructural analysis.
Single Label, Single Antibody
To determine the relative synaptic density onto opsin and GnRH neurons (N = 3 doves), sections were cut at three levels separated by 350 to 450 nm. Montages were made of the cells and synapses counted. Synapses on dendrites present in these same thin sections were also tallied.
The single label, single antibody protocol for GnRH and for opsin was also used to establish the general staining pattern for each cell type. This material was used to measure granule size within opsin and GnRH neurons. For this analysis, 8 cells of each type (cut through the plane of the nucleus) were photographed at 3 levels separated by ~450 nm. Cells were harvested from the brain region in which they are most numerous: The opsin positive cells were from the SL and the GnRH cells from the POA. Granules in opsin (N = 121) and GnRH (N = 88) cells were examined by scanning the photomicrographs and measuring granule sizes (surface area) using the NIH Image (v1.61) program.
Double Label, Two Antibodies
A double label procedure was used to mark simultaneously, in a single section, GnRH and opsin-positive neurons (N = 5 doves). The chromogens used were TMB and DAB (described in Chen et al., 1989), with the following minor modifications. Sections from SL, POA, or ME were exposed to1% NaBH2 for 30 min, 10% normal goat serum 0.05% saponin overnight, and 1:5 K LR-1 in saponin for 7 days at 4 °C. Immunoproduct was visualized by treating the sections with 0.03% DAB in glucose-glucose oxidase reaction mixture to generate hydrogen peroxide. Sections were subsequently washed in 20% normal horse serum in saponin overnight and then incubated in 1:15 K RET-P1 in saponin for 8 days at 4 °C. Sections were washed for 3 days in 0.1MPB at 4 °C, exposed to 1:250 biotinylated horse anti-mouse IgG (Vector) in saponin overnight at 4 °C, washed 3 times for 15 min each, exposed to 1:100 avidin-biotin-HRP (Vector) for 2 h, and washed again 3 times for 15 min. Opsin was visualized using TMB. Following staining, tissue was microdissected, postfixed in 2% osmium tetroxide in 0.9% NaCl for 1 h, and embedded in Epon for ultrathin sectioning.
Single Label, Two Antibodies
The foregoing protocol did not reveal any synapses of opsin terminals onto GnRH cell bodies or vice versa. To verify these results and to optimally reveal all synapses of GnRH and opsin neurons, we used antibodies to opsin and to GnRH on the same section and localized both using DAB (N = 5 doves). This procedure was used to determine whether any labeled synapses occurred at all (either GnRH or opsin) onto labeled cell bodies or their processes.
An extensive survey of both SL and POA tissue was performed. From epon-embedded blocks (approximately 1 mm2), semithin sections were examined in the light microscope to determine if any immunoreactive cell bodies and/or processes were present. Serial sections (5–7 sections, each 70 nm thick) were collected. In this fashion, we surveyed a total of 16 mm2 of SL and 21 mm2 of POA from 5 birds. These represent the regions of highest density of opsin and GnRH, respectively (see Fig. 1). Photomicrographs for more detailed analysis were taken of all sections where interactions between the two systems were seen.
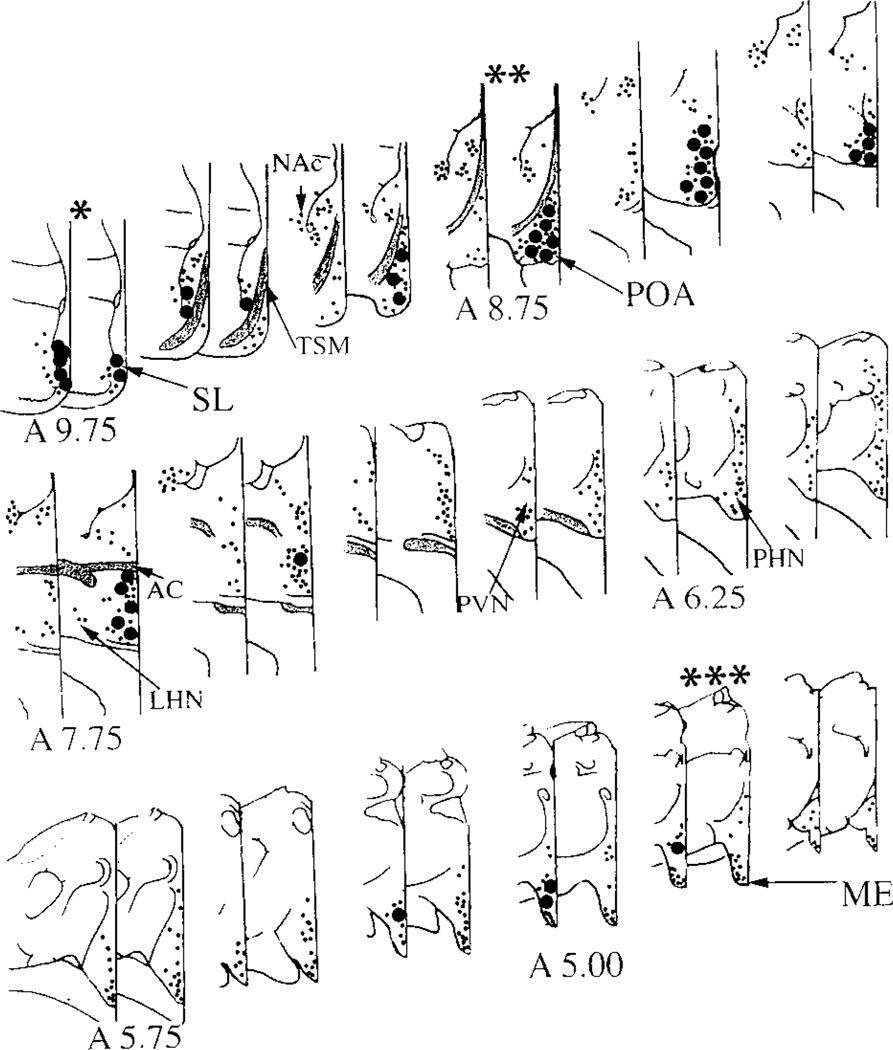
Figure 1.
Schematic of the distribution of opsin and gonadotropin releasing hormone (GnRH) cells and fibers from the septum lateralis (SL) to the median eminence (ME) in the dove brain. The coordinates from Karten and Hodos’s (1967) atlas of the pigeon brain are indicated for reference. Immunoreactive cell bodies (large dots) and fibers (small dots) are depicted on paired sections with opsin on the left and GnRH on the right. Opsin neurons and fibers are concentrated in the ventromedial aspect of the SL. GnRH cells and fibers are also seen in this region. Opsin fibers are present in the preoptic area (POA), an area rich in GnRH neurons and fibers. Throughout the diencephalon, opsin cells and fibers are seen along the medial and dorsal borders of the 3rd ventricle. The ME contains a dense plexus of opsin and GnRH fibers. The SL (*), POA(**), and the ME (***) were studied at the ultrastructural level. AC = anterior commissure; TSM = tractus septomesancephalicus; LHN = lateral hypothalamic nucleus; NAc = Nucleus Accumbens; PVN = paraventricular nucleus; PHN = periventricular nucleus.
RESULTS
Light Microscopy
As previously reported, opsin and GnRH neurons and/or their axons were abundant in 3 areas: SL, POA, and ME (Silver et al., 1988, 1992). The distribution of both opsin and GnRH elements is shown diagrammatically in Figure 1. In SL, opsin and GnRH perikarya and fibers lie in close proximity, with most opsin perikarya lining the lateral ventricles and most GnRH perikarya lying more laterally. In the POA, scattered opsin fibers lie amid abundant GnRH neurons. Opsin-containing fibers course from the SL to the ME close to the midline and do not reach the lateral hypothalamic retinorecipient area. In ME, opsin and GnRH fibers overlap extensively.
Confocal Microscopy
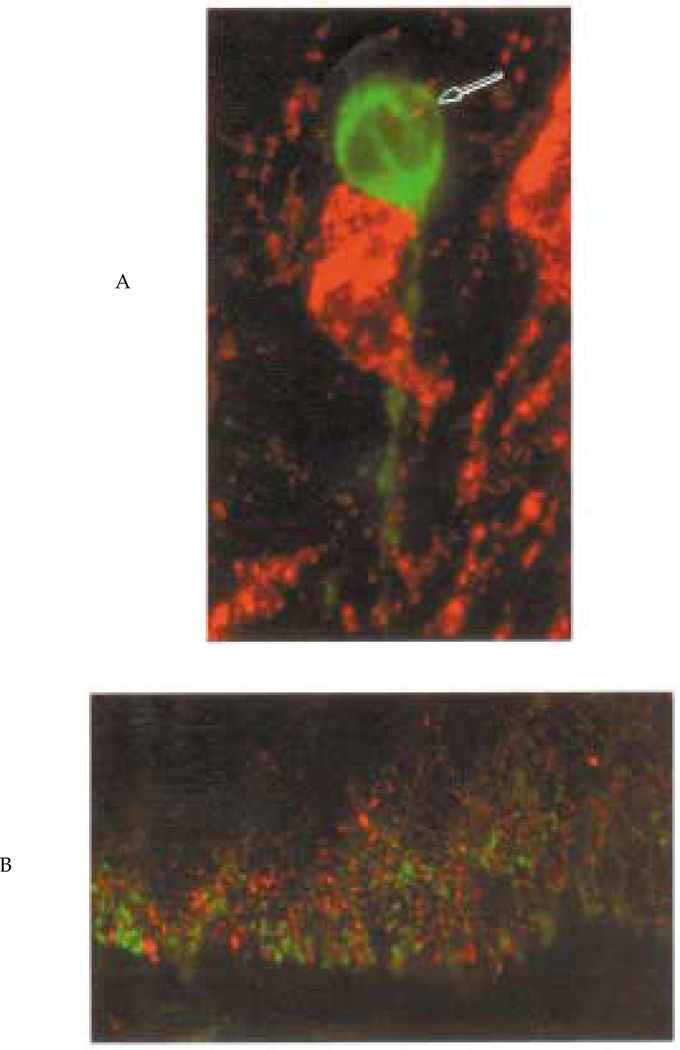
The foregoing observations were extended using confocal microscopy (Fig. 2) to determine whether there were appositions between opsin and GnRH in regions where the two systems overlap. In SL and POA, opsin-GnRH interactions include apparent contacts between soma and fibers (Fig. 2A). In the ME, GnRH and opsin fibers are very abundant and their distribution largely overlaps through most of this region (Fig. 2B).
Figure 2.
Confocal microscopy. Figure panels are scanning confocal projections of 18 serial 1 µm images of tissue double stained for opsin (red) and gonadotropin releasing hormone (GnRH) (green). The upper panel depicts two opsin neurons in the lateral septum, one of which is in apparent contact with a GnRH cell. The arrow indicates a presumptive opsin terminal near theGnRH neuron. The lower panel shows the median eminence; GnRH and opsin fibers are abundant and their distribution overlaps extensively.
Electron Microscopy
To determine whether the appositions between the opsin and GnRH systems observed with confocal microscopy represented synaptic contacts, ultrastructural immunocytochemistry was used. Several different EM protocols were used, each with a different rationale. Single-label DAB provides maximal ultrastructural preservation and was used to characterize the cellular morphology and the distribution of immunoreactivity in cells and processes.
Single Label, Single Antibody-Opsin
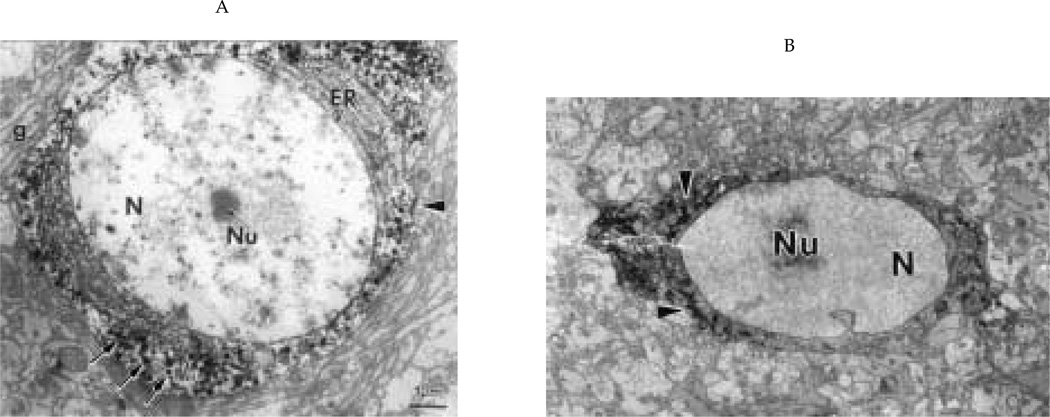
Opsin neurons (Fig. 3A) have abundant rough endoplasmic reticulum (RER), a large Golgi apparatus, and a cilium that projects into the neuropil (Silver et al., 1988). Reaction product for opsin is restricted within secretory granules within the cell body, adventricular knobs (terminal structure penetrating between ependymal cell and contacting the cerebrospinal fluid), axons, and terminals and is absent from the RER and Golgi.
Figure 3.
Single-label electron micrographs illustrating general cytological characteristics of opsin (A) and gonadotropin releasing hormone (GnRH) (B) neurons. (A) An opsin-positive cell body in the septum with its large, euchromatic nucleus (N) and central nucleolus (Nu). The cytoplasm contains numerous immunoreactive granules (arrows). There is abundant endoplasmic reticulum (ER), which is not immunoreactive. Asynapse (arrowhead) innervates this cell. g = glia; X = 6000. (B) A GnRH-positive cell body with a large nucleus (N) and nucleolus (Nu), abundant immunoreactive material in the ER (arrowheads), and few secretory granules. X = 4000.
As summarized in Table 1, opsin cells receive axosomatic (2.3 ± 0.4 synapses/soma; N = 18 cells) and axodendritic afferents (3.2 ± 0.8 synapses/dendritic profile). The surface area of the opsin granule is 22,798 ± 581 nm2 (X̅ ± SEM), measured using the NIH Image program (see Methods). No synapses were seen on the CSF-contacting terminal. Opsin-containing axons form synapses in the SL and the POA with unidentified dendrites and cell bodies (Table 2). No opsin-opsin synapses were found.
Table 1.
Quantitative data on the occurrence of unidentified synapses onto opsin and GnRH immunoreactive elements in the SL and POA. Each cell was sampled in 3 planes separated by 650 nm. The dendritic elements were present in the same micrographs. The data are expressed as mean ± SEM.
| Antibody | Brain Region | Soma (N =) | Dendrite (N =) |
|---|---|---|---|
| Opsin | SL | 2.3 ± 0.4 (N = 18) | 3.3 ± 0.8 (N = 5) |
| GnRH | SL | 2.6 ± 0.9 (N = 17) | 2.9 ± 1.1 (N = 5) |
| GnRH | POA | 2.1 ± 1.1 (N = 15) | 2.4 ± 1.4 (N = 5) |
NOTE: GnRH = gonadotropin releasing hormone; POA = preoptic area; SL = lateral septum.
Table 2.
Summary of the number of opsin and GnRH immunoreactive synapses onto unidentified elements in the SL and POA.
| Antibody | Region | Soma | Dendrite |
|---|---|---|---|
| Opsin | SL | 3 | 17 |
| Opsin | POA | 2 | 12 |
| GnRH | SL | 5 | 9 |
| GnRH | POA | 4 | 14 |
NOTE: GnRH = gonadotropin releasing hormone; POA = preoptic area; SL = lateral septum.
Single Label, Single Antibody-GnRH
GnRH immunoreactivity, in contrast to opsin, is present within the RER of the soma and dendrites as well as in secretory granules in each neuronal compartment (Fig. 3B). In GnRH processes and synaptic terminals, the reaction product covers not only the synaptic granule but also the adjacent cytoplasm.
As summarized in Table 1, GnRH cells are innervated at a density of 2.6 ± 0.9 synapses/soma; 2.9 ± 1.1 synapses/dendritic profiles in the SL (n = 17), and at a density of 2.1 ± 1.1 synapses/soma; 2.4 ± 1.4 synapses/dendritic profile in thePOA(n = 15). The area of GnRH granules (14,134 ± 641 nm2; X̅ ± SEM) in GnRH cells was not significantly different from that of opsin neurons (t test, p > 0.05). In the SL and POA, GnRH axons also form synapses on unidentified cell bodies and dendrites (Table 2). No GnRH-GnRH synapses were identified.
Double Label, Two Antibodies
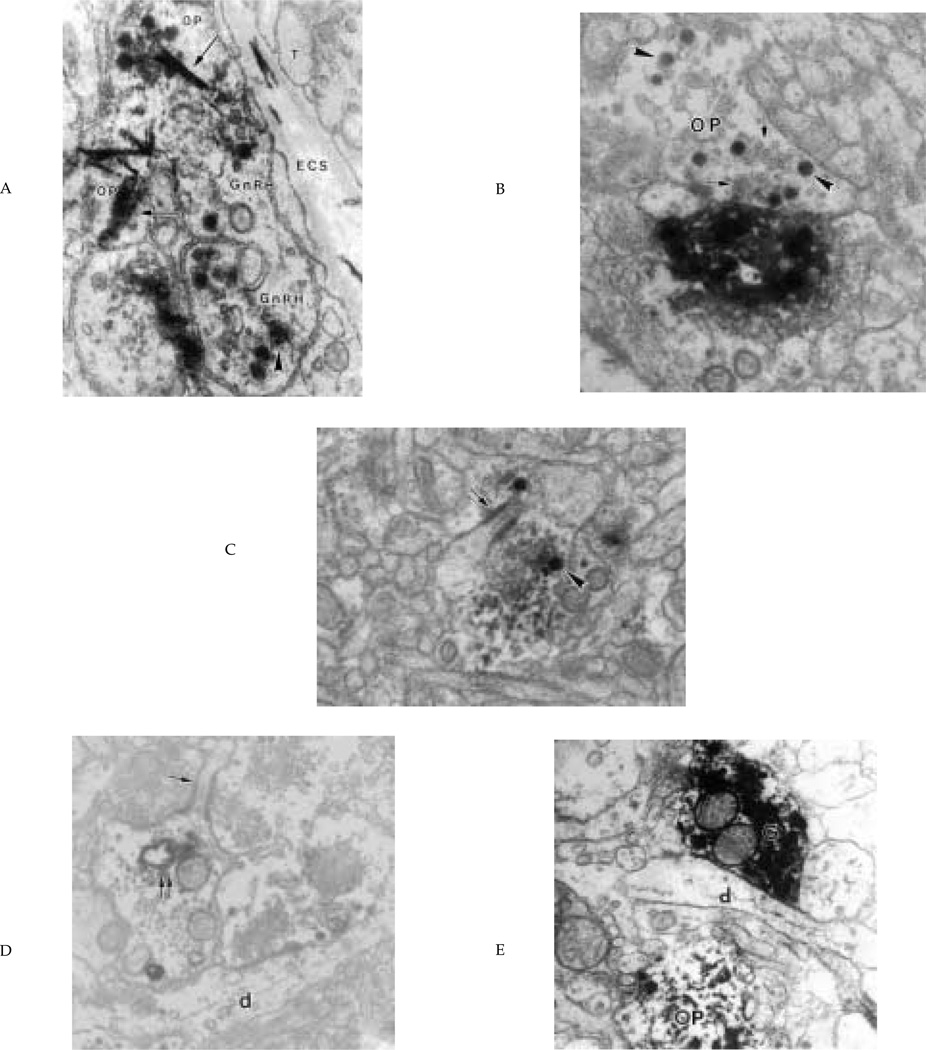
As revealed by the conventional and confocal light microscopic work, opsin and GnRH fibers are in contact through much of the zona externa of the ME. These opsin- and GnRH-expressing axons touch each other with no intervening glial elements (Fig. 4A). These points of contact occur close to the hypophyseal portal capillaries, particularly at the lateral aspects of the zona externa. Opsin- and GnRH-positive terminals end on either tanycyte foot processes or the basal lamina of the hypophyseal portal capillaries. No synapses were apparent on or between opsin- or GnRH-expressing terminals.
Figure 4.
Interactions between opsin and GnRH elements. (A) Opsin axons (OP), identified by the TMB crystal (arrows), and gonadotropin releasing hormone (GnRH) axons (GnRH, identified by DAB reaction product, arrowhead) are adjacent to each other in the median eminence and make contact with the extracellular space (ECS) of the hypophyseal portal capillaries. T = tanycytic end foot; X = 30,000. (B) Example of an opsin fiber (OP) terminating on a GnRH dendrite in preoptic area (POA). Characteristically, opsin immunoreactivity is confined to dense granules (arrowheads). Electron lucent synaptic vesicles are also present (arrows). X = 30,000. (C, D) Two sections from the septum lateralis (SL) of the same GnRH dendrite and opsin terminal separated by 700 nm. In C, the opsin axon terminates on a dendritic spine (arrow). The opsin immunoreactivity is in secretory granules (arrowhead). In (D) the spine (arrow) is identified as part of a GnRH dendrite by the presence of immunoreactive material in the RER (double arrow). The opsin terminal also forms a synapse on an unidentified dendrite (d). X = 24,000. (E) GnRH (G) and opsin (OP) processes in the lateral septum. They are separated by a small dendritic (d) process. X = 24,000.
Of the 18 opsin and 22 GnRH cells examined, neither opsin synapses on GnRH cell bodies nor GnRH terminals on opsin cell bodies were found, though the confocal micrographs suggested appositions. Instead, we found that immunoreactive axons/terminals were separated from the immunoreactive cell bodies by intervening elements of neuropil. This is addressed further in the next study.
The acid pH and the size of the TMB crystals can result in suboptimal preservation of the finest structural elements within the opsin cells and their processes. For this reason, a single-label DAB/DAB strategy was used as described below.
Single Label, Two Antibodies
In the first EM protocol (single label, single antibody), which produced excellent ultrastructure, no GnRH terminals onto GnRH cells and no opsin terminals onto opsin cell bodies were observed. In addition, there were differences between GnRH and opsin cells dendrites and axons in the distribution and appearance of the DAB reaction product (see above). We reasoned that the use of DAB as the sole chromogen would maximize the possibility of finding synaptic interactions between the two systems in the neuropil (i.e., axodendritic).
Nine examples of opsin innervation of GnRH dendrites (Fig. 4B) or dendritic spines (Fig. 4 C,D) were found in the SL. Note that opsin terminals contain immunoreactive secretory granules and small lucent synaptic vesicles; a synaptic cleft and symmetrical pre- and postsynaptic densities were present (Fig. 4 B–D). In some instances where structures containing both proteins were seen, GnRH- and opsin-positive processes were often separated from each other by unidentified dendrites (Fig. 4E) or astrocytes. Such small distances cannot be resolved by confocal microscopy, hence the necessity for ultrastructural studies. GnRH synapses onto opsin dendrites were not found.
DISCUSSION
The question of how photic information reaches the brain to alter reproductive responses has gained renewed interest with the demonstration of photoreceptors and photoreceptor proteins in extraocular neural sites of nonmammalian species (reviewed in Foster and Soni, 1998). In birds, photic stimuli modulating seasonal reproduction are not detected by the retina (see Introduction) and there is compelling evidence of encephalic photoreceptors. Optical enucleation and/or pinealectomy does not alter seasonal cycles of gonadal changes or LH secretion (Wilson, 1991). In contrast, in mammals, photic stimuli are detected by the retina, and there is no evidence of the existence of any encephalic photoreceptor (Nelson and Zucker, 1981; Yamazuki et al., 1999; Lockley et al., 1998).
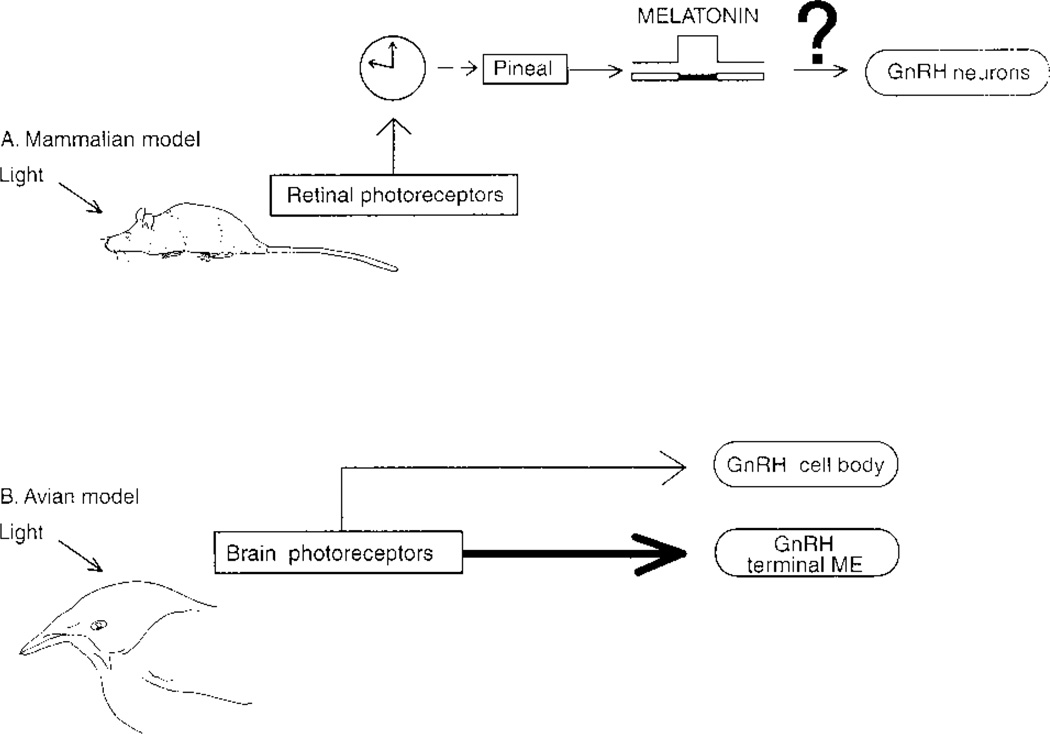
In mammals, photic information from the retina reaches the circadian system in the SCN, and from there information is relayed to the pineal gland (schematized in Fig. 5). Pineal melatonin modulates GnRH secretion by pathways yet to be determined. Based on a mammalian model (Van der Beek et al., 1997), it had been assumed that in nonmammalian vertebrates, photic information would reach circadian pacemaker(s) in the SCN and that output from the circadian system would act on GnRH neurons to regulate the reproductive axis.
Figure 5.
Schematic comparing mammalian and avian systems whereby photic information reaches the reproductive axis. In mammals, photic input reaches the suprachiasmatic nucleus directly, via the retinohypothalamic tract. Efferents from the SCN regulate pineal melatonin secretion via a multisynaptic pathway (modified from Goldman, 1999). The duration of melatonin secretion determines gonadotropin releasing hormone (GnRH) secretion and time of seasonal breeding. In contrast, in birds, the eyes are not necessary for photic determination of seasonal cyclicity (see Introduction). The present results indicate that photic input reaching brain photoreceptors communicates directly with GnRH cell bodies and their terminals in the median eminence. ME = median eminence.
Contrary to expectation, the present results indicate the occurrence of direct interactions between brain photoreceptors and the reproductive axis. Opsin-positive synapses are seen on GnRH dendrites in the septum. The present finding constitutes the first description of the pathway for direct photic input to the neuroendocrine system. Although few, the synapses seen on dendritic shafts and spines are noteworthy. It is well established that innervation of GnRH cells in all species is quite sparse (reviewed in Silverman et al., 1994; present results); therefore, those synapses that do occur can play a large role in regulation of GnRH release. This is particularly true in light of the fact that GnRH neurons are connected by intercellular bridges (Witkin et al., 1995), serving to amplify the effectiveness of individual synapses.
The present results indicate that the opsin-containing photoreceptors of the lateral ventricle send terminals to the vascular supply of the median eminence, supporting the notion that they are important in regulating pituitary function (directly or indirectly). At the level of the median eminence, there is very substantial direct contact among terminals. Opsin and GnRH axons course together across the zona interna of the median eminence, and within the zona externa their terminals end side by side on the perivascular space of the hypophysial portal capillaries. At the perivascular space, GnRH and opsin terminals can be separated from the fenestrated capillary by tanycytic end feet. Based on the structural evidence, we propose that secretions from the opsin terminals modulate the release of GnRH into the portal system. This observation is consistent with the emerging view that neurotransmitters and neuropeptides act in the median eminence in a nonclassical, terminal-to-terminal mode (Crowley, 1999).
Previous work demonstrated that all opsin-expressing cells co-localize vasoactive intestinal peptide (VIP) in birds (Silver et al., 1988; Saldanha et al., 1994), and this has been confirmed in other species (Foster and Soni, 1998). The VIP within the opsin system has the potential to regulate reproductive function via synaptic interactions all along the trajectory of its axons through the SL, POA, and hypothalamus. Similar results were obtained in the pigeon with VIP terminals from CSF contacting-cells making synapses onto GnRH neurons in the SL (Kiyoshi et al., 1998). Furthermore, in the present work, opsin synapses on nonidentified neurons were abundant, indicating the occurrence of additional routes of communication.
The ME is another possible site of interaction. It is known that VIP receptors are present in the ME (Hof et al., 1991). There is substantial evidence that VIP can modulate GnRH activity, with high VIP at times of low GnRH expression, both during the annual reproductive cycle (Saldanha et al., 1994) and within a breeding cycle (Cloues et al., 1990; Mauro et al., 1992). VIP administration lowers plasma LH in ovariectomized rats, an effect not mediated by altered pituitary responsiveness to GnRH (Alexander et al., 1985; Stobie and Weick, 1989). The data suggest that opsin/VIP-containing photoreceptor cells can relay photic information to GnRH terminals in the ME.
The involvement of tanycytes in the regulation of neurohormone release at the ME is well documented in mammals (reviewed in Wittkowski, 1998). The degree of ensheathment of GnRH neurosecretory terminals is regulated by the reproductive status of the animal. In rats, GnRH terminals are ensheathed by tanycytes at all times of the ovulatory cycle except the morning of proestrous (King and Rubin, 1994). Furthermore, the proximity of GnRH terminals to the portal vasculature increases following gonadectomy (King and Letourneau, 1994). These dynamic changes in the median eminence are thought to be vital for the regulation of gonadotropin secretion within the ovulatory cycle as well as at the onset of puberty (Ojeda et al., 1990; Terasawa, 1998). Given the close apposition of the opsin and GnRH terminals in the median eminence, similar mechanisms may mediate seasonally regulated events such as the onset of puberty (McNaughton et al., 1995) and steroid feedback (Wilson, 1985).
Opsins constitute a large family of proteins, some of which have recently been found in mammalian brain (e.g., Blackshaw and Snyder, 1999). The precise functions of these mammalian proteins remain to be elucidated. In nonmammalian vertebrates, many aspects of brain photoreception is understood. Encephalic photoreceptors are necessary and sufficient (vide supra) for the detection of changes in day length that determine seasonal reproductive readiness. The present results indicate how the link between the brain sensory cells and the reproductive axis is achieved and unexpectedly reveal that the circadian system is not a necessary intermediary between the sensory and reproductive components.
ACKNOWLEDGMENTS
We thank Drs. C. Barnstable and R. Benoit for the gifts of RET-P1 and LR-1, respectively. Kate Rosa and Honor Kirwan provided invaluable help in electron microscopic work. The research was supported by NSF grant IBN-9511300. The research described herein was submitted in partial fulfillment of the Ph.D. thesis at Columbia University by Colin Saldanha. The confocal facility was established by NIH Shared Instrumentation Grant No. 1S10 RR10506 and is supported by NIH Grant No. P30CA13696 as part of the Herbert Irving Cancer Center at Columbia University.
REFERENCES
- Alexander MJ, Clifton DK, Steiner RA. Vasoactive intestinal polypeptide effects a central inhibition of pulsatile luteinizing hormone secretion in ovariectomized rats. Endocrinology. 1985;117:2134–2139. doi: 10.1210/endo-117-5-2134. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Snyder SH. Encephalopsin: A novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci. 1999;19:3681–3690. doi: 10.1523/JNEUROSCI.19-10-03681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Witkin JW, Silverman AJ. beta-endorphin and gonadotropin-releasing hormone synaptic input to gonadotropin-releasing hormone neurosecretory cells in the male rat. J Comp Neurol. 1989;286:85–95. doi: 10.1002/cne.902860106. [DOI] [PubMed] [Google Scholar]
- Cloues R, Ramos C, Silver R. Vasoactive intestinal polypeptide-like immunoreactivity during reproduction in doves: Influence of experience and number of offspring. Horm Behav. 1990;24:215–231. doi: 10.1016/0018-506x(90)90006-j. [DOI] [PubMed] [Google Scholar]
- Crowley WR. Toward multifactorial hypothalamic regulation of anterior pituitary hormone secretion. News Physiol Sci. 1999;14:54–58. doi: 10.1152/physiologyonline.1999.14.2.54. [DOI] [PubMed] [Google Scholar]
- Eldred WD, Zucker C, Karten HJ, Yazulla S. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J Histochem Cytochem. 1983;31:285–292. doi: 10.1177/31.2.6339606. [DOI] [PubMed] [Google Scholar]
- Foster RG, Grace MS, Provencio I, DeGrip WJ, Garcia-Fernandez JM. Identification of vertebrate deep brain photoreceptors. Neurosci Biobehav Rev. 1994;18:541–546. doi: 10.1016/0149-7634(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Foster RG, Soni BG. Extraretinal photoreceptors and their regulation of temporal physiology. Rev Reprod. 1998;3:145–150. doi: 10.1530/ror.0.0030145. [DOI] [PubMed] [Google Scholar]
- Goldman BD. The circadian timing system and reproduction in mammals. Steroids. 1999;64:679–685. doi: 10.1016/s0039-128x(99)00052-5. [DOI] [PubMed] [Google Scholar]
- Gwinner E, Hau M, Heigl S. Melatonin: Generation and modulation of avian circadian rhythms. Brain Res Bull. 1997;44:439–444. doi: 10.1016/s0361-9230(97)00224-4. [DOI] [PubMed] [Google Scholar]
- Hof PRM, Dietl M, Charnay Y, Martin JL, Bouras C, Palaios MM, Magistretti PJ. Vasoactive intestinal peptide binding sites and fibers in the brain of the pigeon Columba livia: An autoradiographic and immunohistochemical study. J Comp Neurol. 1991;305:393–411. doi: 10.1002/cne.903050304. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessel TM. Principles of Neuroscience. 4th ed. New York: McGraw-Hill; 2000. [Google Scholar]
- Karten H, Hodos W. A Stereotaxic Atlas of the Brain of the Pigeon (Columba livia) Baltimore: Johns Hopkins University Press; 1967. [Google Scholar]
- King JC, Letourneau RJ. Luteinizing hormone-releasing hormone terminals in the median eminence of rats undergo dramatic changes after gonadectomy, as revealed by electron microscopic image analysis. Endocrinology. 1994;134:1340–1351. doi: 10.1210/endo.134.3.8119174. [DOI] [PubMed] [Google Scholar]
- King JC, Rubin BS. Dynamic changes in LHRH neurovascular terminals with various endocrine conditions in adults. Horm Behav. 1994;28:349–356. doi: 10.1006/hbeh.1994.1031. [DOI] [PubMed] [Google Scholar]
- Kiyoshi K, Kondoh M, Hirunagi K, Korf H. Confocal laser scanning and electron-microscopic analyses of the relationship between VIP-like and GnRH-like-immunoreactive neurons in the lateral septal-preoptic area of the pigeon. Cell Tissue Res. 1998;293:39–46. doi: 10.1007/s004410051096. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Thapan K, English J, Ribeiro D, Haimov I, Hampton S, Middletown B, von Shantz M, Arendt J. Extraocular light exposure does not suppress plasma melatonin in humans. J Clin Endocrinol Metab. 1998;83:3369–3372. doi: 10.1210/jcem.83.9.5244. [DOI] [PubMed] [Google Scholar]
- Mauro LJ, Youngren OM, Proudman JA, Phillips RE, el Halawani ME. Effects of reproductive status, ovariectomy, and photoperiod on vasoactive intestinal peptide in the female turkey hypothalamus. Gen Comp Endocrinol. 1992;87:481–493. doi: 10.1016/0016-6480(92)90056-p. [DOI] [PubMed] [Google Scholar]
- McNaughton FJ, Dawson A, Goldsmith AR. A comparison of the responses to gonadotrophin-releasing hormone of adult and juvenile, and photosensitive and photorefractory European starlings, Sturnus vulgaris. Gen Comp Endocrinol. 1995;97:135–144. doi: 10.1006/gcen.1995.1013. [DOI] [PubMed] [Google Scholar]
- Menaker M, Roberts R, Elliott J, Underwood H. Extraretinal light perception in the sparrow: 3. The eyes do not participate in photoperiodic photoreception. Proc Natl Acad Sci U S A. 1970;67:320–325. doi: 10.1073/pnas.67.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Zucker I. Photoperiodic control of reproduction in olfactory-bulbectomized rats. Neuroendocrinol. 1981;32:266–271. doi: 10.1159/000123171. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholt-Siebert M. Involvement of transforming growth factor alpha in the release of luteinizing hormone-releasing hormone from the developing female hypothalamus. Proc Natl Acad Sci U S A. 1990;87:9698–9702. doi: 10.1073/pnas.87.24.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J, Baylé JD. Brain photoreceptors for the photo-induced testicular response in birds. Experientia. 1982;38:1021–1029. doi: 10.1007/BF01955346. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Leak RK, Silver R. Detection and transduction of daylength in birds. Psychoneuroendocrinology. 1994;19:641–656. doi: 10.1016/0306-4530(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Silver R, Ramos C, Machuca H, Silverin B. Immunocytochemical distribution of GnRH in the brain of adult and posthatching Great Tit (Parus major) and Ring Dove (Streptopelia roseogrisea) Ornis Scand. 1992;23:222–232. [Google Scholar]
- Silver R, Witkovsky P, Horvath P, Alones V, Barnstable CJ, Lehman MN. Co-expression of opsin- and VIP-like immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res. 1988;253:189–198. doi: 10.1007/BF00221754. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Livne I, Witkin J. The gonadotropin-releasing hormone (GnRH), neuronal systems: Immunocytochemistry and in situ hybridization. In: Knobil E, Neill J, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 1683–1710. [Google Scholar]
- Stobie KM, Weick RF. Vasoactive intestinal peptide inhibits luteinizing hormone secretion: The inhibition is not mediated by dopamine. Neuroendocrinology. 1989;49:597–603. doi: 10.1159/000125175. [DOI] [PubMed] [Google Scholar]
- Terasawa E. Cellular mechanism of pulsatile LHRH release. Gen Comp Endocrinol. 1998;112:283–295. doi: 10.1006/gcen.1998.7155. [DOI] [PubMed] [Google Scholar]
- Van der Beek EM, Horvath TL, Weigant VM, Van der Hurk R, Buijs RM. Evidence for a direct neuronal pathway fromthe suprachiasmatic nucleus to the gonadotropin-releasing hormone system: Combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 1997;384:569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Wada Y, Okano T, Adachi A, Ebihara S, Fukada Y. Identification of rhodopsin in the pigeon deep brain. FEBS Lett. 1998;425:53–56. doi: 10.1016/s0014-5793(98)00138-0. [DOI] [PubMed] [Google Scholar]
- Wilson FE. Androgen feedback-dependent and -independent control of photoinduced LH secretion in male tree sparrows (Spizella arborea) J Endocrinol. 1985;105:141–152. doi: 10.1677/joe.0.1050141. [DOI] [PubMed] [Google Scholar]
- Wilson FE. Neither retinal nor pineal photoreceptors mediate photoperiodic control of seasonal reproduction in American tree sparrows (Spizella arborea) J Exp Zool. 1991;259:117–127. [Google Scholar]
- Witkin JW, O’Sullivan H, Silverman AJ. Novel associations among gonadotropin-releasing hormone neurons. Endocrinology. 1995;136:4323–2330. doi: 10.1210/endo.136.10.7664651. [DOI] [PubMed] [Google Scholar]
- Wittkowski W. Tanycytes and pituicytes: Morphological and functional aspects of neuroglial interaction. Microsc Res Tech. 1998;41:29–42. doi: 10.1002/(SICI)1097-0029(19980401)41:1<29::AID-JEMT4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Goto M, Menaker M. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J Biol Rhythms. 1999;14:197–201. doi: 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]