Summary
Intracellular recording and intracellular dye injection of single cells in the dentate region of rat hippocampal slices have been used to understand the different types of cells in the dentate and their possible functional organization. On the basis of combined electrophysiological and morphological data, the cells that have been sampled fall into three distinct groups: the granule cells, the spiny cells located in the hilus (the ‘mossy’ cell being the prototype), and the aspiny, ‘fast-spiking’ cells located throughout the region (many of which are likely to be GABAergic interneurons). Although there is some variability within each group, this variability is minor compared to the large differences between groups. To clarify these groups, each one is described first morphologically, at the level of the light microscope and histochemically, and then the three groups are described electrophysiologically, in terms of intrinsic electrophysiological characteristics, synaptic responses to perforant path stimulation, and possible roles in dentate circuitry. It is proposed that this apparent organization of neurons into three major classes be used as a starting point in our evolving understanding of the functional organization of the dentate region, and, in particular, the hilus. In addition, the possibility is raised that area CA3c cells of the hippocampus could be included in the dentate region as a fourth group. Together with the hilar cells, area CA3c could have the obviously important role of integrating the dentate circuitry with that of the hippocampus proper.
Keywords: Dentate gyrus, Hippocampus, Granule cell, Spiny hilar cell, Aspiny cell, Fast-spiking, Rat
Introduction
The rat fascia dentata is a complex area containing numerous neurons in addition to the granule cells. For example, the hilus contains many non-granule cells of diverse morphologies1. The granule cell layer itself contains numerous non-granule cells, such as the GABAergic basket cells42,44.
In order to understand the physiology as well as the anatomy of different cell types, we examined cells throughout the fascia dentata by intracellular recording in hippocampal slices. Individual dentate neurons were recorded intracellularly, stimulated synaptically, and identified morphologically following intracellular dye injection. Surprisingly, our data did not suggest that there were innumerable cell types in the fascia dentata. Rather, our data supported a division of all cells into three main groups: granule cells, spiny hilar cells (the prototype being the mossy cell), and aspiny ‘fast-spiking’ cells (the prototype being the local circuit cell or interneuron). Each group is relatively homogeneous morphologically and electrophysiologically, although it is certainly possible to make subdivisions within each category.
The purpose of this chapter is to describe the distinguishing morphological and electrophysiological characteristics of these three cell types, using both our own data as well as the data already available in the literature. The granule cells are described cursorily, since so much material is already available concerning this cell type. In addition, area CA3c pyramidal cells are described, since they: (1) are an additional, major class of cells that interdigitate with the processes of dentate neurons; and (2) are somewhat different from other pyramidal cells of area CA3a and CA3b.
Methods
The methods used in these studies have been described previously48,52,53. Transverse or longitudinal hippocampal slices from young (100–150 g) adult male or female Sprague–Dawley rats were cut on a vibratome. Only slices with strong paired-pulse inhibition of the granule cell population spike were used for experiments (see ‘pre stim’ conditions in Figs. 3B, 5B and 7A, B). Cells were impaled with microelectrodes containing 1 M potassium acetate, 1–2% Lucifer yellow dissolved in 1 M LiCl, or 2% Neurobiotin (similar to biocytin) in 1 M potassium acetate. A twisted metal bipolar electrode (100 μm between the center of the poles) placed in the outer molecular layer, at least 500 μm from all recording sites, was used to stimulate the perforant path. Prolonged ‘intermittent’ stimulation entailed repeating the following 30 s period of stimuli: 25 s of paired-pulse stimuli (interstimulus interval = 30 ms) at 2 Hz, followed by 5 s of single stimuli at 20 Hz. The buffer that perfused slices contained (in mM): NaCl, 126; KCl, 5; CaCl2, 2.0; MgCl2, 1.0, 1.5 or 2.0; NaHCO3, 26; NaH2PO4, 1.25; and D-glucose, 10.
Fig. 3.

Synaptic responses of granule cells. (A) The typical response of a granule cell to stimulation of the perforant path, at a stimulus strength below threshold and at a membrane potential of −55 mV, is an EPSP followed by an IPSP. As the cell is hyperpolarized the IPSP reverses polarity so that only a depolarization is recorded. Membrane potentials are at the left of each response. Each stimulus (0.1 mA, 50 μs) is indicated by the filled circle underneath the stimulus artifact. Calibration (in B) = 10 mV, 5 ms. (B) Simultaneous extracellular recording from a site in the granule cell layer (top), and intracellular recording from a cell located near the extracellular recording electrode (bottom). Before repetitive stimulation, paired-pulse inhibition was present in the population as well as the individual granule cell (left, ‘PRE’). After several minutes of repetitive ‘intermittent’ stimulation (see Methods) granule cells lost paired-pulse inhibition (right, ‘POST’). (C) Granule cells are hyperpolarized by fast-spiking cells. A recording is shown from a fast-spiking cell located in the granule cell layer (top, ‘GCL INT’) and a granule cell (bottom, ‘GC’). When intracellular current injection was used so that the fast-spiking cell fired a train of action potentials, hyperpolarizations occurred in the granule cell when the granule cell was depolarized to −52 mV. The hyperpolarizations reversed polarity between −65 and −75 mV. Interestingly, the hyperpolarizations evoked by the fast-spiking cell were very similar to spontaneous hyperpolarizations (arrows). Calibration = 10 mV (GCL INT) and 4 mV (GC). Reprinted with permission from Scharfman and Schwartzkroin52 and Scharfman et al.53.
Fig. 5.

Responses of spiny hilar cells to synaptic stimulation. (A) Most spiny hilar cells have lower thresholds than granule cells in response to stimulation of the perforant path. Simultaneous recordings are shown of granule cell population spikes recorded extracellularly from the granule cell layer (top, ‘GCL’) and intracellular recordings from a spiny hilar cell (bottom, ‘HIL’). At a low stimulus strength, below threshold for a population spike, the spiny hilar cells burst (left). A higher stimulus intensity was able to elicit a population spike (right, arrow). Arrowheads point to capacitative artifacts of the hilar cell action potentials. Small circles mark stimulus artifacts. (B) Prior to prolonged intermittent stimulation (see Methods) the granule cell population spike shows strong paired-pulse inhibition (top, left, ‘PRE STIM’) and spiny hilar cells have high RMPs and large, overshooting action potentials (bottom, left). Following prolonged intermittent stimulation, granule cells lose paired-pulse inhibition (top, right, ‘POST STIM’) and spiny hilar cells depolarize, lose input resistance, and lose the capacity to fire action potentials (bottom, right). An arrow marks the capacitative artifact of the hilar cell action potential. (C) Individual granule cells excite spiny hilar cells strongly. Simultaneous intracellular recordings are shown from a granule cell (top) and a spiny hilar cell (bottom). Intracellular injection of depolarizing current (0.3 nA, 150 ms) in the granule cell evoked three action potentials. Immediately following each granule cell action potential, the spiny hilar cell depolarized. The start and finish of the current pulse are marked by small, filled circles. The granule cell was depolarized with DC current. Capacitative artifacts of the granule cell action potentials are marked by asterisks. The arrowhead points to a spontaneous EPSP that did not follow activity of the impaled granule cell. Reprinted with permission from Scharfman and Schwartzkroin52 and Scharfman et al.53.
Fig. 7.

(A) Aspiny cells fall into two patterns of responsiveness to perforant path stimulation. (A) Simultaneous extracellular recordings from the granule cell layer (top, ‘GCL’) and intracellular recordings from an aspiny cell (bottom, ‘INT’). This fast-spiking cell was very sensitive to stimulation of the perforant path compared to granule cells, since it burst at a stimulus intensity that produced only a small population spike (left, ‘PRE STIM’). After several minutes of intermittent stimulation (right, ‘POST STIM’), the fast-spiking cell was depolarized and unable to fire action potentials. This brief period of intermittent stimulation was not sufficient to decrease paired-pulse inhibition of granule cells, as does happen after prolonged intermittent stimulation (see B). Calibration = 5 mV (GCL), 10 mV (INT), 10 ms. (B) Similar experimental arrangement as in A, except recordings were from a different slice. This fast-spiking cell was not more sensitive to perforant path stimuli than granule cells, since it fired one action potential at a stimulus strength which produced a large population spike. After prolonged intermittent stimulation blocked paired-pulse inhibition (right, ‘POST STIM’), the responses of the fast-spiking cell were similar to the ‘PRE STIM’ condition. Calibration (in A) = 12 mV, 20 ms. (C) Individual granule cells excite individual fast-spiking cells. Simultaneous intracellular recordings are shown of a granule cell (top) and a fast-spiking cell (bottom). Stimulation of the granule cell by current injection evoked a series of action potentials. Following each granule cell action potential the fast-spiking cell depolarized or fired an action potential. A spontaneous action potential is marked by the arrow. Small filled circles mark the start and end of the current pulse used to cause granule cell discharge. Calibration (in A) = 12 mV, 30 ms. Reprinted with permission from Scharfman and Schwartzkroin52 and Scharfman et al.53.
Before describing the cell types of the fascia dentata, the use of certain terms that signify different areas of the fascia dentata should be clarified, since several of these terms have different interpretations. In the following discussion, the term fascia dentata (or ‘dentate’) will refer to the entire area between the hippocampal fissure and the border of the hilus with area CA3c (Fig. 1). The hilus will refer to the area that extends from the base of the granule cell layer to within 100 μm of the border of the CA3c pyramidal cell layer (Fig. 1). Area CA3c will refer to the part of the hippocampal pyramidal cell layer that extends between the blades of the granule cell layer (Fig. 1). Since the borders of the area CA3c cell body layer are indistinct in slices, cells in the studies described below have not been examined in this indistinct zone. All cells were impaled either well within the hilus (i.e. within 200 μm of the granule cell layer) or well within the cell body layer of area CA3c (i.e. in the middle of the pyramidal cell layer).
Fig. 1.

A schematic of the fascia dentata is shown, including the area referred to as the ‘hilus’ (shaded) and the area CA3c pyramidal cell layer. Reprinted with permission from Scharfman48.
Granule cells
ANATOMY
General morphology
Granule cells can be discriminated easily from other dentate cell types by their stereotypical morphology and location, which has been described by others and will not be repeated here32,40 (Fig. 2A).
Fig. 2.
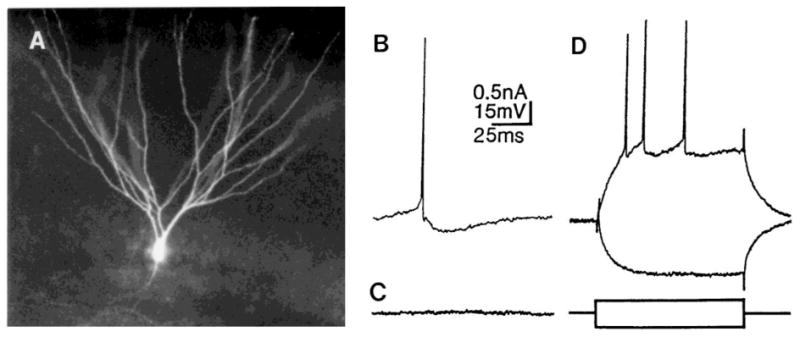
General morphology and membrane properties of granule cells. (A) A typical granule cell that was filled by intracellular dye injection of Lucifer yellow. (B) Recordings from a typical granule cell demonstrate a triphasic afterhyperpolarization following a single action potential if the cell is depolarized with DC current (membrane potential = −55 mV). (C) At the resting membrane potential (RMP), there is little spontaneous activity recorded from a typical granule cell. A recording from a typical granule cell is shown, at its RMP (−81 mV). (D) Granule cells have strong spike frequency adaptation, demonstrated by the response to intracellular injection of depolarizing current for 150 ms. The response to intracellular injection of hyperpolarizing current is superimposed.
Axonal projection
The projection of the axons of granule cells (the ‘mossy fibers’) to hilar neurons and area CA3 pyramidal cells, as well as the impressive large mossy fiber boutons, have been described in detail8,11.
Neurotransmitter/Immunocytochemistry
There is strong evidence that the granule cells are excitatory (see below), and use an excitatory amino acid as their neurotransmitter65. Granule cells are immunoreactive for the calcium-binding protein calbindinD28K (CaBP), but not parvalbumin3,59. The presence of an intracellular calcium-binding protein may be one of the reasons for the resistance of granule cells to prolonged intermittent stimulation of the perforant path (see below), which appears to be excitotoxic to many hilar cells (see below).
ELECTROPHYSIOLOGICAL PROPERTIES
Like the morphological characteristics of granule cells, the electrophysiology of granule cells is also quite stereotypical12,15. Granule cells have an unusually high resting membrane potential (RMP), approximately −75 to −80 mV when the perfusing buffer contains 5 mM K+. When a more physiological concentration (3 mM K+) is used, RMPs may be even more hyperpolarized (−90 mV (ref. 30)). The question arises how these extremely negative RMPs relate to the RMPs of granule cells in vivo; in anesthetized preparations granule cell RMPs that are recorded intracellularly are more depolarized (−50 to −60 mV (ref. 39)). However, cells may be more depolarized in vivo than in vitro because impalement injury is more likely to occur in vivo.
Granule cells are also distinguished from other dentate cell types by their afterhyperpolarizations (AHPs), which are similar to pyramidal cells in many ways63 (Fig. 2B). The different components of the AHP are easiest to observe when the cell is depolarized from its RMP with DC current. At membrane potentials near −55 mV the AHP following a single action potential is triphasic (Fig. 2B); at RMP the afterpotential following a single action potential is purely depolarizing. Granule cells also possess little spontaneous activity at their resting potential (Fig. 2C), which is quite different from the high level of spontaneous activity of the other cells in the fascia dentata. However, some granule cells do have extremely small (1–3 mV), brief (3–6 ms) spontaneous depolarizations (frequency ~0.2 Hz). When the cell is depolarized to approximately −50 mV, the depolarizations reverse polarity and may become quite large, so they are likely to reflect spontaneous inhibitory postsynaptic potentials (IPSPs).
A final differentiating characteristic of granule cells is their strong spike frequency adaptation and the absence of burst firing (Fig. 2D). This characteristic, perhaps more than any other, distinguishes granule cells from other dentate cell types. However, granule cells which have been depolarized due to impalement damage may lose their adaptive properties and certain components of their AHPs; in these cases the injured granule cell may appear similar electrophysiologically to an aspiny, ‘fast spiking’ cell (see below), which normally lacks strong adaptation and has a brief, monophasic AHP (see below). This possible confusion is exacerbated by the fact that numerous aspiny, fast-spiking cells exist in the granule cell layer, and there is some overlap in action potential duration between aspiny, fast-spiking cells and granule cells: the briefest granule cell action potential approaches the longest action potential of fast spiking cells (approximately 1.5 ms total duration). In summary, it is easy to confuse a damaged granule cell with an aspiny, fast-spiking cell that is located in the granule cell layer. This potential problem may be circumvented by using intracellular dye injection to morphologically identify the impaled neuron.
SYNAPTIC RESPONSES
Synaptic responses to stimulation of perforant path axons
The response of granule cells to single or repetitive stimuli of the perforant path is different from almost all other dentate cell types. A single stimulus to the outer molecular layer leads to a stereotypical sequence of postsynaptic potentials while recording intracellularly from a granule cell: an excitatory postsynaptic potential (EPSP) is followed by a mono- or biphasic IPSP (Fig. 3A). The EPSP is thought to be a result of glutamate release from perforant path terminals, mainly onto non-N-methyl-D-aspartate (NMDA) receptors, but there is some NMDA receptor activation13,30. The IPSP is thought to result from granule cell activation of GABAergic inhibitory neurons that make recurrent synapses on granule cell somata2,7.
When stimuli are repeated in the form of brief (0.3–10s) high-frequency (20–300-Hz) trains, long-term potentiation (LTP) results in granule cells9. When similar high frequency trains are triggered in 5s periods every 25 s (i.e. ‘intermittent’ stimulation, see Methods), LTP may also occur (see Fig. 8 of ref. 52). When intermittent stimulation is prolonged for several minutes, other changes also occur in granule cell physiology. As previously shown by extracellular recordings in vivo, granule cells lose paired-pulse inhibition after many hours of such intermittent stimulation58. In slices, although a reversible loss of inhibition can occur quite quickly (after several minutes) an irreversible loss of paired-pulse inhibition requires up to 2 h of stimulation (using 2 mM Mg2+ in the perfusate; Fig. 3B). When 1 mM [Mg2+]O is used, it usually requires less than 15 min to block paired-pulse inhibition irreversibly.
Several other interesting changes in granule cell physiology also occur when intermittent stimulation is prolonged and there is a loss of paired-pulse inhibition. Granule cells increase their input resistance, decrease spike frequency adaptation and they often hyperpolarize (3–10 mV; see Fig. 5 of ref. 52). In addition, the response to a single stimulus changes from an EPSP–IPSP sequence to a prolonged EPSP52. It has not yet been proved that the prolonged EPSP following intermittent stimulation is a result of NMDA receptor activation, but this is likely given the sensitivity of this prolonged PSP to [Mg2+]O (ref. 50), and the findings that an NMDA-receptor mediated component of granule cell EPSPs occurs after repetitive stimulation used to elicit LTP14 or the repeated stimulation used for kindling37.
Synaptic responses to stimulation of individual dentate neurons
By recording from two synaptically connected neurons simultaneously, effects of a single presynaptic cell on a single granule cell have been recorded. Such simultaneous intracellular recordings have demonstrated the effects of individual aspiny, ‘fast-spiking’ cells (possible inhibitory interneurons) on individual granule cells. Regardless of the location of the fast-spiking cell (i.e. in the granule cell layer or in the hilus), the effect on the granule cell has always been a brief hyperpolarization53 (Fig. 3C). The hyperpolarizations reversed polarity near the chloride equilibrium potential. Therefore, as has been assumed by many, it appears that some of the aspiny, fast-spiking cells that are present in the granule cell layer and hilus may be inhibitory interneurons, and mediate chloride-dependent, GABAA receptor-mediated, fast IPSPs in granule cells. However, the number of synaptically-connected fast-spiking cells and granule cells that have been recorded is low, so there may be several other types of connections present that remain to be identified.
Interestingly, there were numerous ‘failures’ of transmission from individual fast-spiking cells to individual granule cells (Fig. 3C). Such failures did not occur in the other synaptic connections observed in simultaneous recordings of dentate cells (such as granule cells and spiny hilar cells), so it is unlikely that the ‘failures’ were a result of the dual recording method or the use of slices to examine synaptic connections. Such failures do not appear to be common in area CA3 and area CA121,28,29,35,36,42. Whether failures are a characteristic feature of inhibitory input to granule cells, and what this might imply about inhibitory transmission in the dentate, remains to be clarified.
Spiny hilar cells
Although there are many ways to subdivide spiny hilar cells on the basis of detailed anatomy1, spiny hilar cells are quite homogeneous electrophysiologically, and these electrophysiological characteristics are very different from all other dentate cells (i.e. granule cells and aspiny cells). Therefore, spiny hilar cells have been grouped together as one separate category.
ANATOMY
General morphology
It has already been shown that there are numerous subtypes of spiny cells in the hilus on the basis of Golgi material1. However, on the basis of light microscopy of intracellularly filled cells in slices, spiny hilar cells appear to belong to two main groups. One group has a cell body that is multipolar (Fig. 4A, C), and a large dendritic tree that extends in numerous directions, covering a large part of the hilus and often reaching into the area CA3c pyramidal cell layer (see also Fig. 2 of ref. 49). The other major type of spiny hilar cell has a fusiform cell body, and its dendritic tree is bipolar (Fig. 4B, D). These cells are similar to some of the fusiform neurons described from Golgi material by Ribak and Seress43. Although thorny excrescences can occur on both types of spiny hilar cell, the more heavily encrusted cells with the largest dendritic trees are usually multipolar, and these multipolar cells correspond best to the ‘mossy’ cells described by Amaral1 (i.e. Fig. 4A, C). Most of the dendrites of both types of spiny hilar cell are restricted to the hilus, as previously described1. However, use of confocal microscopy on thick tissue sections has demonstrated that spiny hilar cells usually possess at least one dendrite that enters the granule cell layer and passes into the molecular layer48 (Fig. 4B, D). This may be one of the reasons for the great sensitivity of spiny hilar cells to perforant path stimulation (see below).
Fig. 4.

Morphology of spiny hilar cells. (A) A typical multipolar spiny hilar ‘mossy’ cell is shown. The soma and proximal dendrites are encrusted with thorny excrescences (arrowheads) and smaller spines are present on the rest of the dendritic tree. This photomicrograph, and others in other figures, were made using a confocal microscope (Biorad MRC 600). Calibration (in B) = 30 μm. (B) Another spiny hilar cell with a fusiform soma and a bipolar dendritic tree. The arrow points to a dendrite that curved and entered the granule cell layer (see drawing in D). Calibration = 30 μm. (C) A drawing of the cell in A. MOL, molecular layer; GCL, granule cell layer; HIL, hilus. (D) A drawing of the cell in B. This cell was located near the upper blade. Area CA3 is to the left. (E) The response of the cell shown in B to a 0.2-nA, 150-ms period of depolarizing and hyperpolarizing current injection are superimposed. Recordings from spiny hilar cells show that they usually have depolarizing afterpotentials following action potentials, large and frequent spontaneous EPSPs (arrows), a long time constant, and high input resistance50. Calibration = 15 mV, 25 ms. B, D, and E are reprinted with permission from Scharfman48.
Axonal projection
The axons of spiny hilar cells clearly differentiate them from other dentate cells. Most, if not all, spiny hilar neurons (both ‘mossy’ and ‘non-mossy’ cells) possess a thick axon that projects ipsilaterally and contralaterally, and terminates in the inner molecular layer5,6,17,31,66–69, where it makes asymmetric synapses45. Spiny hilar cell axons also have local collaterals that synapse with aspinous processes in the hilus (probably the dendrites of aspiny cells)53. Thus the axonal projection and relatively conventional bouton size of spiny hilar cells contrast with the axonal projection and terminals of granule cells.
Neurotransmitter/immunocytochemistry
The neurotransmitter of spiny hilar cells is likely to be glutamate, based on immunocytochemical evidence64. However, other immunocytochemical studies have shown that many hilar neurons are immunoreactive for other possible neurotransmitters, such as GABA24,44, various peptides4,24,61, and possibly acetylcholine34. Although in most cases these substances appear to label aspiny hilar cells, it is possible that some spiny hilar cells might also have been labeled; unfortunately immunocytochemistry lacks the resolution to definitively differentiate aspiny cells from spiny cells.
Very few, if any, spiny hilar cells contain either the calcium-binding protein CaBP or parvalbumin59. The lack of immunoreactivity of spiny hilar cells for calcium-binding proteins contrasts with the granule cells, and may be one reason for the sensitivity of spiny hilar cells to perforant path stimulation59,60. In support of this hypothesis, it has been shown that intracellular calcium chelation blocks the damage to spiny hilar cells which follows prolonged intermittent stimulation of the perforant path50.
ELECTROPHYSIOLOGICAL PROPERTIES
Electrophysiologically, spiny hilar cells are striking in their differences from granule cells. Large, spontaneous depolarizations occur almost continuously, unlike the quiescent granule cells49 (compare Figs. 2C and 4E). Spiny hilar cells are prone to fire in bursts (either spontaneously or in response to intracellular current injection; Fig. 4E), unlike granule cells49 (Fig. 2D). Spiny hilar cells also have longer time constants and higher input resistances than granule cells49 and are distinguished from granule cells by their afterpotential, which is usually large and depolarizing at −50 to −60 mV (Fig. 4E), unlike that of granule cells (where AHPs have a mixture of voltage-dependent depolarizing and hyperpolarizing components (Fig. 2B), or the AHPs of aspiny cells (where AHPs are brief and hyperpolarizing; Fig. 6C). The only cell type similar to spiny hilar cells is the area CA3c pyramidal cell. Spiny hilar cells are similar to area CA3c pyramidal cells in the frequent spontaneous EPSPs. Both spiny hilar cells and area CA3c pyramidal cells are able to fire in bursts or trains, and have long time constants. Spiny hilar cells and area CA3c pyramidal cells are also similar morphologically, since both are endowed with numerous spines and excrescences. However, spiny hilar cells generally have more spontaneous activity, longer time constants, larger input resistances, and often have more excrescences than area CA3c pyramidal cells. Therefore, taking both morphological and electrophysiological characteristics into consideration, spiny hilar cells appear to be an exaggerated or extreme form of area CA3c pyramidal cells.
Fig. 6.
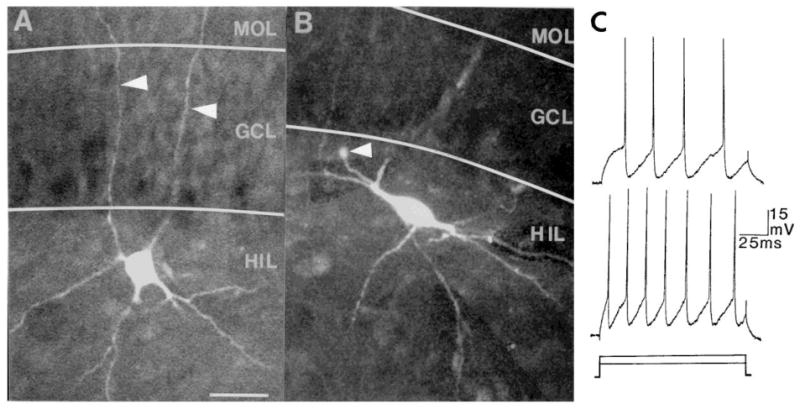
Morphology of aspiny cells. Aspiny cells of the fascia dentata have varied morphologies, but all share a lack of spines. Many are round or multipolar (A) whereas others are fusiform (B). The arrowheads in A point to dendrites that crossed the granule cell layer and entered the molecular layer. The arrowhead in B points to the cut end of the axon, which appears to collects dye and swell. Calibration (in A) = 30 μm for both A and B. (C) All aspiny cells have electrophysiological properties of ‘fast-spiking’ cells. Action potentials are followed by large, brief afterhyperpolarizations and spike frequency adaptation is weak. The responses of the cell in A to intracellular current injection (150 ms rectangular current pulses: top, +0.2 nA; bottom, +0.3 nA) are shown. A and C are reprinted with permission from Scharfman48.
SYNAPTIC RESPONSES
Synaptic responses to stimulation of perforant path axons
The responses of spiny hilar cells even to single stimuli of the perforant path set them apart from granule cells. At very low stimulus strengths a spiny hilar cell may burst in response to a single stimulus52 (Fig. 5A); bursts do not follow single stimuli in the case of granule cells. In fact, these low stimulus strengths may be below those necessary to evoke a granule cell population spike (Fig. 5A). That is, at a stimulus strength that evokes a burst in a spiny hilar cell, granule cells have not even reached firing threshold. Even when the recording site for the granule cell population spike is closer to the stimulating electrode than the hilar cell, the stimulus threshold for a spiny hilar cell is still below that for the granule cell population spike48.
When hilar cells are examined for their subthreshold responses to stimulation, they differ from granule cells in their lack of large IPSPs49,52 (Fig. 5). It is rare to find large IPSPs in spiny hilar cells, even when granule cells in the same area of the slice exhibit robust IPSPs. It is unlikely that the small IPSPs in spiny hilar cells are due to the presence of chloride in the Lucifer yellow-filled recording electrodes (chloride is present since Lucifer yellow is dissolved in LiCl), since similar responses are recorded with other electrodes that contain potassium acetate. These data imply a preferential innervation of granule cells by GABAergic inhibitory interneurons relative to spiny hilar cells. This preferential innervation could be a factor in the relative resistance of granule cells to excitotoxic injury and the relative vulnerability of hilar neurons.
Given the sensitivity of spiny hilar cells to single stimuli, it is perhaps not surprising that spiny hilar cells depolarize and fire multiple action potentials following intermittent stimulation of the perforant path (Fig. 5B). It has been known for some time that hilar cells degenerate histologically after prolonged intermittent stimulation in vivo61; studies in vitro have shown that deterioration also occurs electrophysiologically51,52. Specifically, the changes in hilar cell physiology during prolonged intermittent stimulation are: (1) depolarization, often over 50 mV; (2) loss of input resistance; (3) decrease in time constant; and (4) inability to fire fast (Na+-dependent) action potentials, either by current injection or synaptic stimuli52. Interestingly, a hilar cell may recover if intermittent stimulation is brief (1–2 min) or [Mg2+]O is high (2 mM), but after a certain critical period spiny hilar cells apparently lose their capacity for recovery. The fact that this critical period for irreversible hilar cell damage is so similar to the period required for loss of paired-pulse inhibition of granule cells, suggests a functional relationship between healthy spiny hilar cells and inhibition of granule cells. This is consistent with the observation that slices without strong paired-pulse inhibition also are slices where few cells are encountered in the hilus with intracellular recording techniques, or where all of the hilar cells that are found are unhealthy (i.e. hilar cells are depolarized, have low input resistance, and APs are short and broad; unpublished data). However, a direct role of spiny hilar cells in the inhibition of granule cells has not yet been demonstrated.
Synaptic responses determined by simultaneous intracellular recordings
Many simultaneous recordings have been obtained between synaptically connected granule cells and spiny hilar neurons53. The dramatic excitatory effects of granule cell on spiny hilar cells was evident in every pair of synaptically-connected cells53 (Fig. 5C). Granule cells produced large unitary EPSPs in spiny hilar cells and could drive spiny hilar cells to fire action potentials, or even bursts of action potentials53 (Fig. 5C). The excitatory drive of a single granule cell on an individual spiny hilar cell is quite different from the limited excitatory effects of principal cell pairs in the hippocampus, such as the effect of a single area CA3c pyramidal cell on an area CA1 pyramidal cell47. This possibly reflects the requirements of the circuitry in the dentate as compared to area CA1.
Aspiny, ‘fast-spiking’ cells
The aspiny hilar cells are very similar to the ‘fast-spiking’ cells reported in hippocampus, which are thought to be GABAergic inhibitory interneurons20,21,27,29,48,53,55. However, regardless of the similarities in morphology and electrophysiology, the aspiny, fast-spiking cells of the fascia dentata may not all be GABAergic, nor are they all necessarily local circuit interneurons. The aspiny, fast-spiking cells of the fascia dentata that have been recorded intracellularly and filled with dye in slices have not been assessed immunocytochemically, so it is not clear whether they were GABAergic. In addition, it is unclear whether the axons of the aspiny cells that have been labeled in slices were entirely restricted to a local area. Since there is a great deal of immunocytochemical evidence that aspiny hilar cells are immunoreactive for neurotransmitters besides GABA4,16,22,24,34,61,64, and other studies indicate that some aspiny cells project to the contralateral dentate4,46,56,57, some of the aspiny cells of the dentate may not be GABAergic, may not be inhibitory, and may not be local circuit interneurons.
ANATOMY
General morphology and electron microscopy
Aspiny cells are easily differentiated from other dentate cells by their morphology. Their lack of spines is the most obvious differentiating morphological characteristic. However, among aspiny cells there is considerable morphological variability. For example, aspiny cell body shapes can be round, multipolar or fusiform (Fig. 6A, B). Some dendrites are extremely thin, whereas other cells have dendrites as thick as granule cell dendrites. Although the fast-spiking cells filled by intracellular dye injections have not borne spines, sparsely spiny hilar neurons have been reported1,43.
Several ultrastructural features of aspiny cells also distinguish this cell type from granule cells or spiny hilar cells. For example, infolded nuclei and intracellular rods and sheets are found in aspiny cells but not in granule cells or spiny hilar cells26,33,41,45.
Axonal projection
Many aspiny cell axons arborize around the initial axon segments or somata of granule cells62; these aspiny cells are thought to correspond to the GABAergic inhibitory interneurons that control recurrent inhibition7,23,44. Other aspiny cells, however, have very different axonal arbors. Aspiny cells of the hilus that are immunoreactive for somatostatin or neuropeptide Y have an axonal plexus in the outer molecular layer4,22,61. Other aspiny cells project to the contralateral hippocampus4,46,56,57.
Neurotransmitter/immunocytochemistry
Immunocytochemical studies have shown that aspiny cells may contain a wide variety of possible neurotransmitters, such as GABA44, several peptides16,22,24,61, and possibly acetylcholine34. Many of the GABAergic neurons contain both GABA as well as a peptide22,24,61. Regarding their immunoreactivity for calcium-binding proteins, aspiny hilar cells also vary. Many aspiny hilar cells are clearly immunoreactive for parvalbumin or CaBP, while others are apparently devoid of immunoreactivity for either or these compounds25,59.
ELECTROPHYSIOLOGICAL PROPERTIES
Although aspiny cells vary anatomically, they share a very specific type of electrophysiological ‘signature’, and this signature is completely different from that of granule cells and spiny hilar cells. This is the main reason for placing the aspiny cells into a single, separate category.
The large, brief AHP following each action potential, and weak spike frequency adaptation, are the two electrophysiological characteristics that most easily distinguish aspiny hilar cells from granule cells or spiny hilar cells (Fig. 6C). Although aspiny cell electrophysiology is very different from granule cell and spiny hilar cell electrophysiology, there are slight variations among aspiny cells. For example, although most aspiny cell action potentials are extremely brief (hence the name ‘fast-spiking’), some aspiny cells have action potentials that are shorter than others (range of total duration of action potentials: 0.5–1.5 ms). This is similar to the differences in action potential duration between different fast-spiking interneurons of area CA119,20 such as the oriens/alveus interneurons, stratum lacunosum-moleculare neurons and the area CA1 basket cells located in the pyramidal cell layer27,29,55. Although some aspiny cells of the dentate possess time constants that are very short, others are quite long (range: 10–20 ms). At this time no relationship has been found between these slight variations in electrophysiology and the variations in morphology, histochemistry or possible function.
SYNAPTIC RESPONSES
Synaptic responses to stimulation of perforant path axons
Although the membrane properties are relatively homogeneous, the responses of aspiny cells to stimulation of the perforant path are divided: either cells are very sensitive to single stimuli, and depolarize and deteriorate during intermittent stimulation (like spiny hilar cells; Fig. 7A), or aspiny cells have sequential EPSP–IPSP responses to single stimuli (like granule cells), and maintain their resting potential and other electrophysiological properties during intermittent stimulation (Fig. 7B). Since calcium-binding protein immunoreactivity is similarly divided (see above), it is tempting to speculate that the aspiny cells which possess calcium-binding proteins are those that are relatively resistant to excitotoxic stimulation, while the cells without calcium-binding proteins are vulnerable to similar stimulation59. However, it has not yet been established that those cells with intracellular responses similar to the resistant granule cells are immunoreactive for calcium-binding proteins, nor have the cells that have responses like vulnerable spiny hilar cells been shown to lack immunoreactivity for calcium-binding proteins.
Synaptic responses to stimulation of individual dentate neurons
Paired recordings between granule cells and fast-spiking cells have shown that granule cells have strong excitatory effects on fast-spiking cells53 (Fig. 7C). It is interesting that granule cell excitation of fast-spiking cells is somewhat different from granule cell excitation of spiny hilar cells. This difference is apparent in the different waveform and smaller unitary EPSPs recorded from fast-spiking cells, and that a single granule cell never was able to evoke bursts of action potentials in a fast spiking cell, although bursts were often produced in spiny hilar cells. However, one cannot be sure these differences can be generalized to all fast-spiking cells.
Paired recordings have also documented hyperpolarizing actions of fast spiking cells on other fast-spiking cells53. In all cases these hyperpolarizations were similar to the hyperpolarizations recorded from granule cells in response to single fast-spiking cells, in their unitary size, reversal potential, and high rate of failure. One is struck by the similarity of effects between different putative inhibitory neurons on granule cells and the effects of putative inhibitory cells on each other. This may reflect a uniformity of inhibitory action in dentate, with small differences between cell types. But such uniformity is surprising in light of the complexity of dentate inhibition that has been described in other studies10,38.
Area CA3c pyramidal cells
Although area CA3c pyramidal cells are not generally considered to be part of the dentate32, there are sufficient similarities between area CA3c pyramidal cells and spiny hilar cells to indicate that it may not be so obvious where the hippocampus ends and the dentate begins. It is of specific interest to compare the characteristics of CA3c pyramidal cells with spiny hilar cells to address why area CA3c pyramidal cells are so much less vulnerable to prolonged intermittent perforant path stimulation. Both spiny hilar cells and area CA3c pyramidal cells lack calcium-binding protein immunoreactivity, so this factor may not be the critical difference. One emerges with the sense that it is the differential innervation by granule cells, perforant path, and possibly GABAergic interneurons, that underlies the difference in vulnerability.
ANATOMY
General morphology and axonal projection
The cells of area CA3c can be differentiated morphologically into two types: the cells with classical pyramidal cell body shape and dendritic tree (Fig. 8A), and non-classical pyramidal cells (Fig. 8B). Both cell types possess thorny excrescences on their proximal dendrites, and, although far less common, on the proximal portions of their basal dendrites. The classical pyramidal cells are similar to those in area CA3a or CA3b, with the exception that area CA3c pyramidal cells often have an apical dendrite that bends away from the granule cell layer, instead of being straight (Fig. 8A). The non-classical cells, which are a substantial population in area CA3c but not in areas CA3a and b, have many different types of cell body shape and usually have more than one ‘apical’ dendrite (Fig. 8B). The basal dendritic tree usually consists of one large tuft of fine processes, instead of a dendritic tree arising from two large primary dendrites that originate at the base of the pyramid (Fig. 8B). These non-classical pyramidal cells are referred to as pyramidal since they possess a dendritic tree that is oriented the same way as classical pyramidal cells (i.e., with ‘apical’ and ‘basal’ sections, with the major dendritic axis perpendicular to the cell layer), and the major branch of the axon follows the identical course as area CA3a and CA3b pyramidal cell axons (i.e., the axon projects into stratum oriens and the alveus). However, some axon collaterals of area CA3c pyramidal cells have been traced into the hilar region18, travelling in the opposite direction from the major branch of the axon. We have also seen this projection upon examination of Lucifer yellow or Neurobiotin-filled cells (Scharfman et al., unpublished). However, although intriguing, the functional significance of these collaterals remains to be elucidated.
Fig. 8.
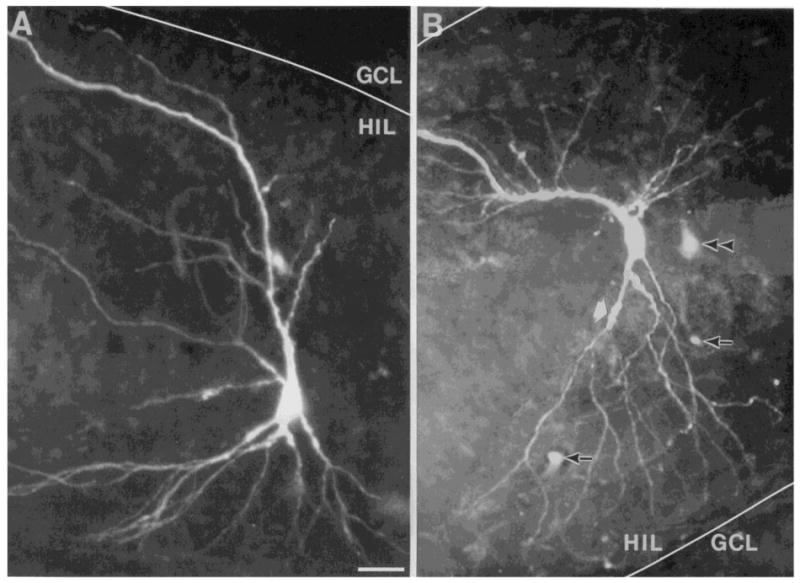
Morphology of area CA3c pyramidal cells. Area CA3c cells have variable morphologies. Cells may be ‘classical’ in the pyramidal shape of their cell body and dendritic tree (A), or they may be non-pyramidal in cell body shape (B) and have dendrites which, though orientated with an ‘apical’ and ‘basal’ dendritic tree, are not classically pyramidal. The cell in A was located at the end of the area CA3c cell layer and its apical dendrite was bent towards area CA3 as it approached the upper blade. The border of the upper blade (GCL) and the hilus (HIL) is marked by a white line. The cell in B was located in the center of the area CA3c pyramidal cell layer. The lower blade is to the lower right. Arrows mark autofluorescence of blood vessels; the double arrowhead points to a lightly filled cell; the thick white arrow points to a thorny excrescence. Calibration = 25 μm for A and 20 μm for B. A is reprinted with permission from Scharfman48.
Neurotransmitter/immunocytochemistry
The area CA3c cells are quite similar to the spiny hilar cells histochemically. It is generally accepted that area CA3c pyramidal cells use an excitatory amino acid as their neurotransmitter, as is probably the case for spiny hilar cells64,65. In addition, area CA3c pyramidal cells lack immunoreactivity for both CaBP and parvalbumin, as do the spiny hilar cells59.
ELECTROPHYSIOLOGICAL PROPERTIES
There are few clear electrophysiological differences among area CA3c pyramidal cells. Both the classical and non-classical pyramidal cells have electrophysiological characteristics intermediate between the spiny hilar neurons and the pyramidal cells of area CA3a/b. There appears to be a continuum, starting in area CA1, continuing into CA3a/b, then CA3c, and finally the hilus. Starting at area CA1, pyramidal cells are relatively quiescent (low spontaneous firing rate, few spontaneous EPSPs), they have short time constants and low input resistances, and usually fire in trains as opposed to bursts. Their spines are relatively small. Area CA3a/b pyramidal cells have slightly higher input resistances and longer time constants, may burst as well as fire in trains, and may have thicker dendrites. The spines often form large clusters or excrescences. In CA3c and the hilus, spiny neurons have numerous spontaneous EPSPs, the longest time constants and largest input resistances, and are quite prone to bursting. They are densely covered with spines and excrescences, especially the spiny hilar cells.
The similar morphology and electrophysiology of spiny hilar cells and hippocampal pyramidal cells, and the dissimilarity between spiny hilar cells and other dentate neurons, suggests that spiny hilar cells are related more to the hippocampus than to the fascia dentata. One might consider the spiny hilar cells as displaced area CA3c pyramidal cells, as did Lorente de Nó32. However, there certainly are differences between spiny hilar cells and pyramidal cells, such as their birth dates54, and indeed the origins of spiny hilar cells are more similar to dentate neurons than hippocampal pyramidal cells1.
SYNAPTIC RESPONSES
The responses of area CA3c pyramidal cells to stimulation of the perforant path distinguish them from cells of the fascia dentata more than any other characteristic. The response to a single stimulus is much different from that of spiny hilar cells, in that: (1) the thresholds of area CA3c pyramidal cells to single perforant path stimuli are not lower than those of granule cells (almost all spiny hilar cells have lower thresholds than granule cells); and (2) far more area CA3c cells display IPSPs in response to perforant path stimuli than spiny hilar cells. Since the general morphology and electrophysiology of area CA3c cells and spiny hilar cells may be somewhat similar, it is useful to use the cell’s response to perforant path stimuli to differentiate an area CA3c pyramidal cell from a spiny hilar cell (i.e., if the location of the intracellular electrode is unclear and the cell is not morphologically identified following dye injection).
The responses to prolonged intermittent perforant path stimulation of area CA3c pyramidal cells are also different from spiny hilar cells. Area CA3c cells located close to the granule cell layer (as opposed to those located near area CA3b) respond similarly to the spiny hilar cells, but the responses are not as dramatic and the cells usually can recover after stimulation ends51. Area CA3c cells located closer to area CA3b are even more resistant to the deleterious effects of prolonged intermittent stimulation.51. Another interesting difference between area CA3c cells and other dentate cells regarding prolonged intermittent stimulation is that area CA3c cells may begin to burst spontaneously following the termination of prolonged intermittent stimulation51. These bursts occur in approximately 46% of slices where the perforant path was stimulated in a prolonged intermittent manner, and where granule cell paired-pulse inhibition was irreversibly lost. The bursts start at least 15 min after cessation of prolonged intermittent stimulation, and are spontaneous, synchronized events that last 100–500 ms and occur between 0.15 and 0.05 Hz. Subsequent experiments have shown that granule cells synchronously hyperpolarize during the bursts of area CA3 cells, and that the aspiny, fast spiking cells in the dentate may underlie these hyperpolarizations. Spontaneous events continue for several hours after prolonged intermittent stimulation has stopped.
These comparisons, both of the morphology/immunocytochemistry and the electrophysiology, suggests that it is the circuitry, as opposed to the calcium-binding protein content, that is the critical difference between the extremely vulnerable spiny hilar cells, such as the mossy cell, and the area CA3c pyramidal cells.
Conclusions
The results of numerous intracellular recordings from dye-filled neurons in rat hippocampal slices have made three types of cells obvious in the fascia dentata (Table I). First are the granule cells, which are the principal cells of the dentate and strongly excitatory to other dentate cells types. For reasons that are unclear, granule cells are endowed with membrane properties, inhibitory innervation, and other characteristics, that enable them to be stable, resistant cells in the face of potentially excitotoxic afferent excitation. Second are the aspiny cells, which are located throughout the dentate gyrus, contain numerous combinations of neuroactive substances, and share very specific morphological and electrophysiological features that set them apart from other dentate cells. Although some of the aspiny cells correspond to GABAergic inhibitory interneurons, there is still a great deal to be determined about the functions of the other aspiny cells. Third are the spiny hilar cells, which appear to be an electrophysiological and morphological extreme of area CA3 pyramidal cells. The spiny hilar cells are extremely vulnerable to excitotoxicity, and when these cells are damaged the fascia dentata and area CA3 become hyperexcitable. Spiny hilar cells clearly must be important to the maintenance of healthy dentate and hippocampal function, yet their exact role in dentate circuitry remains to be elucidated.
TABLE I.
COMPARISON OF THREE CELL TYPES OF THE FASCIA DENTATA AND AREA CA3C PYRAMIDAL CELLS
| Granule cells | Spiny hilar cells (i.e. mossy cells) | Aspiny fast-spiking cells (i.e. interneurons) | Area CA3c pyramidal cells | |
|---|---|---|---|---|
| Anatomy | ||||
| Cell body | round oval |
multipolar fusiform |
variable other |
pyramidal and non-pyramidal |
| Dendrites | ||||
| Location | MOL | mostly HIL | all areas | HIL |
| Spines | spiny | extremely spiny with excrescences | aspiny | spiny some excrescences |
| Axon | ||||
| Projection | ipsi HIL and str. lucidum | ipsi HIL, IML contra IML |
ipsi FD sometimes contra FD |
ipsi CA3, CA1 contra CA3, CA1 |
| Targets | FD: BC, HC | ? | FD: GC, INT | CA1: PC, BC |
| CA3: PC, BC | CA3:? | CA3 BC | ||
| Terminal size | often large |
small | small | small |
| Neurotransmitter | Glu/Asp | Glu? | GABA, peptides, perhaps others | Glu/Asp |
| Electrophysiological properties | ||||
| RMP (mV) | high | intermediate | variable | intermediate |
| −75 to −80 | −60 to −70 | −50 to −80 | −60 to −70 | |
| Input resistance (MΩ) | intermediate | high | very high | high |
| 50 to 80 | 70 to 100 | 100 to 200 | 50 to 90 | |
| Time constant (ms) | intermediate | very long | variable | long |
| 15 | >20 | 10 to 20 | >20 | |
| Spike frequency adaptation | very strong | variable | weak | intermediate |
| After potential after a single action potential at −55 mV | triphasic | depolarizing | hyperpolarizing | triphasic |
| Synaptic responses | ||||
| Spontaneous PSPs | ||||
| Type | IPSPs | EPSPs | EPSPs and IPSPs | EPSPs |
| Frequency | rare | very frequent | variable | intermediate |
| Single perforant path stimuli | EPSP–IPSP | EPSP | EPSP sometimes IPSP | EPSP sometimes IPSP |
| Prolonged intermittent perforant path stimulation | ||||
| Vulnerability | resistant | vulnerable | divided | less vulnerable than SHC |
| Specific changes | small hyperpol. | large depolarization | either like SHC or like GC | like SHC but more resistant than SHC |
| ↑ Rin | ↓ Rin | |||
| CaBP immuno. | yes | no | divided | some |
| Synaptic effects | + | ? | − | + (*) |
MOL, molecular layer; HIL, hilus; ipsi, ipsilateral; contra, contralateral; IML, inner molecular layer; str., stratum; FD, fascia dentata; BC, basket cell; HC, hilar cell; GC, granule cell; INT, interneuron; PC, pyramidal cell; Glu/Asp, glutamate or aspartate; SHC, spiny hilar cell; Rin, input resistance; adapt., adaptation; APs, action potentials; CaBP immuno., immunoreactivity for calbindin or parvalbumin; +, excitatory; −, inhibitory.
Acknowledgments
I would like to thank Drs. P.A. Schwartzkroin and D.D. Kunkel for their contributions to many of the studies described above. I also thank Dr. P.R. Adams for his support, and Drs. R.S. Sloviter and J.H. Goodman for helpful comments on the manuscript.
References
- 1.Amaral DG. A Golgi study of the cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Eccles JC, Løyning Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature (Lond) 1963;198:540–542. doi: 10.1038/198540a0. [DOI] [PubMed] [Google Scholar]
- 3.Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–229. doi: 10.1016/0006-8993(82)90804-6. [DOI] [PubMed] [Google Scholar]
- 4.Bakst I, Avendano C, Morrison JH, Amaral DG. An experimental analysis of origins of somatostatin-like immunoreactivity in dentate gyrus of rat. J Neurosci. 1986;6:1452–1462. doi: 10.1523/JNEUROSCI.06-05-01452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger TW, Semple-Rowland S, Basset JL. Hippocampal polymorph neurons are the cells of origin for ipsilateral, association, and commissural afferents to the dentate gyrus. Brain Res. 1980;215:329–336. doi: 10.1016/0006-8993(81)90512-6. [DOI] [PubMed] [Google Scholar]
- 6.Blackstad TW. Commissural connections of the hippocampal region in the rat. J Comp Neurol. 1956;105:417–538. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- 7.Blackstad TW, Flood PR. Ultrastructure of hippocampal axosomatic synapses. Nature (Lond) 1963;198:542–543. doi: 10.1038/198542a0. [DOI] [PubMed] [Google Scholar]
- 8.Blackstad TW, Kjaerheim A. Special axo-dendritic synapses in the hippocampal cortex. Electron and light microscopic studies on the layer of mossy fibers. J Comp Neurol. 1961;117:133–159. doi: 10.1002/cne.901170202. [DOI] [PubMed] [Google Scholar]
- 9.Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;231:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgard EC, Sarvey JM. Long-lasting potentiation and epileptiform activity produced by GABAB receptor activation in the dentate gyrus of rat hippocampal slice. J Neurosci. 1991;11:1198–1209. doi: 10.1523/JNEUROSCI.11-05-01198.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- 12.Crunelli V, Assaf SY, Kelly JS. Intracellular recordings from granule cells of the dentate gyrus in vitro. In: Seifert W, editor. The Neurobiology of the Hippocampus. Academic Press; London: 1983. pp. 197–214. [Google Scholar]
- 13.Dahl D, Burgard EC, Sarvey JM. NMDA antagonists reduce medial, but not lateral, perforant pathway-evoked EPSPs in dentate gyrus of rat hippocampal slices. Exp Brain Res. 1990;83:172–177. doi: 10.1007/BF00232206. [DOI] [PubMed] [Google Scholar]
- 14.Errington ML, Lynch MA, Bliss TVP. Long-term potentiation in the dentate gyrus: induction and increased glutamate release are blocked by D(−) aminovale-rate. Neuroscience. 1987;20:279–284. doi: 10.1016/0306-4522(87)90019-4. [DOI] [PubMed] [Google Scholar]
- 15.Fricke RA, Prince DA. Electrophysiology of dentate gyrus granule cells. J Neurophysiol. 1984;51:195–209. doi: 10.1152/jn.1984.51.2.195. [DOI] [PubMed] [Google Scholar]
- 16.Gall C, Brecha N, Karten HJ, Chang K-J. Localization of enkephalin-like immunoreactivity to identified axonal and neuronal populations of the rat hippocampus. J Comp Neurol. 1981;198:335–350. doi: 10.1002/cne.901980211. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb DI, Cowan WM. Autoradiographic studies of the commissural and associational connections of the hippocampus and dentate gyrus of the rat. I. The commissural connections. J Comp Neurol. 1973;149:383–422. doi: 10.1002/cne.901490402. [DOI] [PubMed] [Google Scholar]
- 18.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi Y, Hama K. Two subtypes of non-pyramidal cells in rat hippocampal formation identified by intracellular recording and HRP injection. Brain Res. 1987;411:190–195. doi: 10.1016/0006-8993(87)90700-1. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi Y, Hama K. Physiological heterogeneity of nonpyramidal cells in rat hippocampal CA1 region. Exp Brain Res. 1988;72:494–502. doi: 10.1007/BF00250594. [DOI] [PubMed] [Google Scholar]
- 21.Knowles WD, Schwartzkroin PA. Local circuit synaptic interactions in hippocampal brain slices. J Neurosci. 1981;1:318–322. doi: 10.1523/JNEUROSCI.01-03-00318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Köhler C, Eriksson S, Davies S, Chan-Palay V. Co-localization of neuropeptide tyrosine and somatostatin immunoreactivity in neurons of individual subfields of the rat hippocampal region. Neurosci Lett. 1987;78:1–6. doi: 10.1016/0304-3940(87)90551-9. [DOI] [PubMed] [Google Scholar]
- 23.Kosaka T, Hama K, Wu J-Y. GABAergic synaptic boutons in the granule cell layer of rat dentate gyrus. Brain Res. 1984;293:353–359. doi: 10.1016/0006-8993(84)91242-3. [DOI] [PubMed] [Google Scholar]
- 24.Kosaka T, Kosaka K, Tateishi K, Hamaoka Y, Yanaihara N, Wu J-Y, Hama K. GABAergic neurons containing CCK-8-like and/or VIP-like immunoreactivities in the rat hippocampus and dentate gyrus. J Comp Neurol. 1985;239:420–430. doi: 10.1002/cne.902390408. [DOI] [PubMed] [Google Scholar]
- 25.Kosaka T, Katsumaru H, Hama K, Wu J-Y, Heizmann CW. GABAergic neurons containing the Ca2+ binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res. 1987;419:119–130. doi: 10.1016/0006-8993(87)90575-0. [DOI] [PubMed] [Google Scholar]
- 26.Laatsch RH, Cowan WM. Electron microscopic studies of the dentate gyrus of the rat. I. Normal structure with special reference to synaptic organization. J Comp Neurol. 1966;128:359–396. doi: 10.1002/cne.901280305. [DOI] [PubMed] [Google Scholar]
- 27.Lacaille J-C, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. J Neurosci. 1988;8:1400–1410. doi: 10.1523/JNEUROSCI.08-04-01400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacaille J-C, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci. 1988;8:1411–1424. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacaille JC, Mueller AM, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert JDC, Jones RSG. A reevaluation of excitatory amino acid-mediated synaptic transmission in rat dentate gyrus. J Neurophysiol. 1990;64:19–132. doi: 10.1152/jn.1990.64.1.119. [DOI] [PubMed] [Google Scholar]
- 31.Laurberg S, Sorensen KE. Associational and commissural collaterals of neurons in the hippocampal formation (hilus fasciae dentatae and subfield CA3) Brain Res. 1981;212:287–300. doi: 10.1016/0006-8993(81)90463-7. [DOI] [PubMed] [Google Scholar]
- 32.Lorente de Nó R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol (Leipzig) 1934;46:113–142. [Google Scholar]
- 33.Lübbers K, Frotscher M. Fine structure and synaptic connections of identified neurons in the rat fascia dentata. Anat Embryol. 1987;177:1–14. doi: 10.1007/BF00325285. [DOI] [PubMed] [Google Scholar]
- 34.Matthews DA, Salvaterra PM, Crawford GD, Houser CR, Vaughn JE. An immunocytochemical study of choline acetyltransferase-containing neurons and axon terminals in normal and partially deafferented hippocampal formation. Brain Res. 1987;402:30–43. doi: 10.1016/0006-8993(87)91044-4. [DOI] [PubMed] [Google Scholar]
- 35.Miles R, Wong RKS. Unitary inhibitory synaptic potentials in the guinea pig hippocampus in vitro. J Physiol (Lond) 1984;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miles R, Wong RKS. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol (Lond) 1986;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mody I, Heinemann U. NMDA receptors of dentate gyrus granule cells participate in synaptic transmission following kindling. Nature (Lond) 1987;326:702–704. doi: 10.1038/326701a0. [DOI] [PubMed] [Google Scholar]
- 38.Mott DD, Bragdon AC, Lewis DV, Wilson WA. Baclofen has a pro-epileptic effect in the rat dentate gyrus. J Pharmacol Ther. 1989;249:721–725. [PubMed] [Google Scholar]
- 39.Muñoz MD, Nuñez A, Garcia-Austi E. In vivo intracellular analysis of rat dentate granule cells. Brain Res. 1990;509:91–98. doi: 10.1016/0006-8993(90)90313-z. [DOI] [PubMed] [Google Scholar]
- 40.Ramón y Cajal S. The Structure of Ammon’s Horn. Charles Thomas; Illinois: 1968. p. 78. [Google Scholar]
- 41.Ribak CE, Anderson J. Ultrastructure of the pyramidal basket cells in the dentate gyrus of the rat. J Comp Neurol. 1980;192:903–916. doi: 10.1002/cne.901920416. [DOI] [PubMed] [Google Scholar]
- 42.Ribak CE, Seress L. Five types of basket cell in the hippocampal dentate gyrus: a combined Golgi and electron microscopic study. J Neurocytol. 1983;12:577–597. doi: 10.1007/BF01181525. [DOI] [PubMed] [Google Scholar]
- 43.Ribak CE, Seress L. A Golgi-electron microscopic study of fusiform neurons in the hilar region of the dentate gyrus. J Comp Neurol. 1988;271:67–78. doi: 10.1002/cne.902710108. [DOI] [PubMed] [Google Scholar]
- 44.Ribak CE, Vaughn J, Saito K. Immunocytochemical localization of glutamic acid decarboxylase in neuronal somata following colchicine inhibition of axonal transport. Brain Res. 1978;140:315–332. doi: 10.1016/0006-8993(78)90463-8. [DOI] [PubMed] [Google Scholar]
- 45.Ribak CE, Seress L, Amaral DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- 46.Ribak CE, Seress L, Peterson GM, Seroogy KB, Fallon JH, Schmued L. A GABAergic inhibitory component within the hippocampal commissural pathway. J Neurosci. 1986;6:3492–3498. doi: 10.1523/JNEUROSCI.06-12-03492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayer RJ, Redman SJ, Andersen P. Amplitude fluctuations in small EPSPs recorded from CA1 pyramidal cells in the guinea pig hippocampal slice. J Neurosci. 1990;9:840–850. doi: 10.1523/JNEUROSCI.09-03-00840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scharfman HE. Dentate hilar cells with dendrites in the molecular layer have lower thresholds for synaptic activation by perforant path than granule cells. J Neurosci. 1991;11:1660–1673. doi: 10.1523/JNEUROSCI.11-06-01660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scharfman HE, Schwartzkroin PA. Electrophysiology of morphologically identified mossy cells of the rat dentate hilus. J Neurosci. 1988;8:3412–3421. doi: 10.1523/JNEUROSCI.08-10-03812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scharfman HE, Schwartzkroin PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science. 1989;246:257–260. doi: 10.1126/science.2508225. [DOI] [PubMed] [Google Scholar]
- 51.Scharfman HE, Schwartzkroin PA. Consequences of prolonged afferent stimulation of the rat fascia dentata: epileptiform activity in area CA3 of hippocampus. Neuroscience. 1990;35:505–517. doi: 10.1016/0306-4522(90)90325-x. [DOI] [PubMed] [Google Scholar]
- 52.Scharfman HE, Schwartzkroin PA. Responses of cells of the rat fascia dentata to prolonged stimulation of the perforant path: sensitivity of hilar cells and changes in granule cell excitability. Neuroscience. 1990;35:491–504. doi: 10.1016/0306-4522(90)90324-w. [DOI] [PubMed] [Google Scholar]
- 53.Scharfman HE, Kunkel DD, Schwartzkroin PA. Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience. 1990;37:693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- 54.Schlessinger A, Cowan RWM, Swanson LW. The time of origin of neurons in ammon’s horn and the associated retrohippocampal fields. Anat Embryol. 1978;154:153–173. doi: 10.1007/BF00304660. [DOI] [PubMed] [Google Scholar]
- 55.Schwartzkroin PA, Mathers L. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978;157:1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- 56.Schwerdtfeger WK, Buhl E. Various types of non-pyramidal hippocampal neurons project to the septum and contralateral hippocampus. Brain Res. 1986;386:146–154. doi: 10.1016/0006-8993(86)90151-4. [DOI] [PubMed] [Google Scholar]
- 57.Seress L, Ribak CE. GABAergic cells in the dentate gyrus appear to be local circuit and projection neurons. Exp Brain Res. 1983;50:173–182. doi: 10.1007/BF00239181. [DOI] [PubMed] [Google Scholar]
- 58.Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- 59.Sloviter RS. Calcium binding protein (calbindin D28K) and parvalbumin immunocytochemistry: location in the rat hippocampus with specific reference to selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- 60.Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the ‘dormant basket cell’ hypothesis and its possible relevance to Temporal Lobe Epilepsy. Hippocampus. 1991;1:31–40. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- 61.Sloviter RS, Nilaver G. Immunocytochemical localization of GABA-, cholecystokinin-, vasoactive intestinal polypeptide-, and somatostatin-like immunoreactivity in the area dentata and hippocampus of the rat. J Comp Neurol. 1987;256:42–60. doi: 10.1002/cne.902560105. [DOI] [PubMed] [Google Scholar]
- 62.Soriano E, Frotscher M. A GABAergic axo-axonic cell in the fascia dentata controls the main excitatory hippocampal pathway. Brain Res. 1990;503:170–174. doi: 10.1016/0006-8993(89)91722-8. [DOI] [PubMed] [Google Scholar]
- 63.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–188. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- 64.Storm-Mathisen J, Wold JE. In vivo high-affinity uptake and axonal transport of D-[2,33H] aspartate in excitatory neurons. Brain Res. 1981;230:427–435. doi: 10.1016/0006-8993(81)90428-5. [DOI] [PubMed] [Google Scholar]
- 65.Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug F-MS, Ottersen OP. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature (Lond) 1983;301:518–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- 66.Swanson LW, Wyss JM, Cowan WM. An auto-radiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:621–716. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- 67.Swanson LW, Sawchenko PE, Cowan WM. Evidence for collateral projections by neurons in Ammon’s horn, the dentate gyrus, and the subiculum: a multiple retrograde labeling study in the rat. J Neurosci. 1981;1:548–559. doi: 10.1523/JNEUROSCI.01-05-00548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.West JR, Nornes HO, Barnes CL, Bronfenbrenner M. The cells of origin of the commissural afferents to the area dentata of the mouse. Brain Res. 1979;160:203–215. doi: 10.1016/0006-8993(79)90419-0. [DOI] [PubMed] [Google Scholar]
- 69.Zimmer J. Ipsilateral afferents to the commissural zone of the fascia dentata, demonstrated in decommissurated rats by silver impregnation. J Comp Neurol. 1971;142:93–416. doi: 10.1002/cne.901420402. [DOI] [PubMed] [Google Scholar]


