Abstract
It is has been shown that the major afferent input to the dentate gyrus, the perforant path, excites dentate hilar neurons. However, little is known about the other inputs to hilar cells. Therefore, we examined the responses of hilar neurons to stimulation of the fimbria. We positioned our stimulating electrodes so that granule cells were not excited antidromically by fimbria stimulation, although action potentials were easily triggered in area CA3b and CA3c pyramidal cells by such stimulation. In these experiments, fimbria stimulation evoked responses from every hilar cell tested, including examples of both of the major cell types, the spiny hilar ‘mossy’ cells (n=15) and the relatively aspiny. ‘fast-spiking’ cells (putative interneurons, n=5). Hilar cell responses consisted primarily of EPSPs that could trigger action potentials, but small IPSPs were also evoked in some cases, particularly in the fast-spiking cells. Excitation was blocked by an antagonist of the AMPA/kainate receptor subtype of excitatory amino acid receptors, 6-cyano-7-nitroquinoxaline-2,3-dione(CNQX, 5μM, n=5), whereas the cholinergic antagonist atropine (10μM) had no effect (n=4). When sequential intracellular recordings were made from hilar cells and area CA3 pyramidal cells in the same slice, hilar cell EPSPs began after action potentials of CA3b pyramidal cells, and stimulus strengths required to evoke hilar cell EPSPs were above threshold for area CA3b pyramidal cells. Taken together with the evidence that area CA3 pyramidal cells use an excitatory amino acid as a neurotransmitter [7, 21], and the demonstrations of area CA3 axon collaterals in the hilus [11, 16], the results raise the possibility that some area CA3 pyramidal cells excite dentate hilar neurons.
Keywords: Interneuron, Pyramidal cell, Mossy cell, Fast-spiking, Area CA3, α-Amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA)/kainate receptor, 6-Cyano-7-nitroquinoxaline-2, 3-dione (CNQX), Atropine
Although the trisynaptic circuit is without a doubt a fundamental aspect of hippocampal circuitry [3], there are also indications that the trisynaptic circuit is not the only sequence of activation of hippocampal neurons [2]. One potential example, which is suggested by recent anatomical and physiological data, is the possible projection of area CA3 pyramidal cells to the dentate gyrus. The anatomical data come from studies of intracellularly labelled CA3 pyramidal cells, which show that the axons of area CA3 pyramidal cells can project deep into the hilar region, in addition to their well-documented projections elsewhere in the hippocampus [11, 16]. The physiological data are from a study using hippocampal slices, where stimulation of the area CA3 pyramidal cell layer evoked EPSPs in hilar ‘mossy’ cells [27]. However, it was not necessarily clear from the slice study [27] that pyramidal cells mediated the excitation of hilar neurons, because stimulation of the pyramidal cell layer and its vicinity could have activated granule cells antidromically. Since granule cells innervate hilar neurons [6], hilar neurons could have been activated irrespective of pyramidal cell activity. Therefore, one aim of the present study was to determine if area CA3 pyramidal cells could excite hilar neurons in the absence of granule cell activation.
It was also of interest to determine whether excitation was restricted to the spiny hilar cells, since the previous slice work had only sampled spiny hilar cells [27], Although the hilus is a region of morphologically diverse neurons [1], based on combined electrophysiology and morphology, two broad categories of cells can be appreciated [25]. One group is composed of spiny hilar cells, which are similar to area CA3 pyramidal cells electro-physiologically, and are densely covered by spines and/or thorny excrescences. The prototype of this group is the ‘mossy’ cell [1], which is thought to be glutamatergic [33] and projects to the inner molecular layer [9, 22, 34, 36]. The other group is composed of relatively aspiny hilar cells, which are similar to ‘fast-spiking’ inhibitory interneurons electrophysiologically and morphologically [25]. However, some of the aspiny hilar cells have commissural projections [4] and may not use GABA as a neurotransmitter [14, 15, 32], so there is some heterogeneity within this group.
Other evidence for excitatory connectivity between area CA3 and the dentate gyrus comes from another slice study where area CA3 pyramidal cells were examined under epileptiform conditions that produced synchronous bursts of area CA3 pyramidal cells [28, 29]. In such slices, many hilar cells burst simultaneous to the area CA3 pyramidal cell population burst [28], suggesting connectivity between area CA3 and the hilus. However, it was not determined whether hilar neurons excited CA3 pyramidal cells or vice versa. Thus, another reason for the present study was to examine excitation of hilar cells and area CA3 pyramidal cells in the absence of epileptiform conditions.
Slice preparation was similar to methods described elsewhere [24]. Adult male Sprague–Dawley rats (100–200 g) were anesthetized with ether and decapitated. Animals were treated in accordance with guidelines for the humane treatment of animals set by the National Institutes of Health and the New York State Department of Health. The brain was quickly removed and placed in 4°C buffer (in mM: NaCl 126.0, KCl 5.0. NaH2PO4 1.25, CaCl2 2.0, MgSO4 2.0, NaHCO3 26.0, and D-glucose 10.0). One hemisphere was trimmed to a rectangular block containing one hippocampus, and transverse hippocampal slices (400 μm thick) were cut with a Vibroslice (Campden Instruments). Slices were immediately transferred to a modified interface chamber (Fine Science Tools) where they lay at an interface of warmed (34–35°C), humidified oxygen (95% O2 5% CO2).
Recording electrodes were pulled horizontally (Model P-87 PC, Sutter Instruments) from borosilicate glass containing a capillary fiber (A and M Systems). Extracellular electrodes were filled with 1 M NaCl (2–15 MΩ) and intracellular electrodes were filled with 1 M potassium acetate (80–175 MΩ). A high input impedance amplifier with a bridge circuit was used for recording (Axoclamp 2A, Axon Instruments) and the bridge was balanced whenever current was passed. Data were collected on a digitizing oscilloscope (Model 410. Nicolet Instruments) and stored on tape (Model IR-284, Neurodata Instruments) for analysis offline. Stimulating electrodes were made from two Teflon-coated stainless steel wires (50 μm diameter) twisted tightly together so that the dimensions of the electrode surface contacting the slice were approximately 50 μm × 100 μm. For ‘fimbria stimulation’, electrode tips were oriented parallel to the area CA3 pyramidal cell layer and positioned at the border of the fimbria and stratum oriens/alveus of area CA3b. For molecular layer stimulation, electrode tips were oriented parallel to and adjacent to the hippocampal fissure in the upper blade. Stimuli consisted of 50–500 μA, 20–200 μs unipolar current pulses triggered at 0.1 Hz. For a given site of stimulation, current amplitude was fixed (i.e. 100 μA) and stimulus duration was varied to produce different intensities of stimulation (i.e. 20, 30, 40 μs). When responses to stimulation of cells from the same slice were compared, impalements were made sequentially rather than simultaneously.
The selective antagonist of the AMPA/kainate receptor subtype of excitatory amino acid receptors, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), or the muscarinic cholinergic antagonist atropine, was applied by adding an appropriate volume of stock solution (1 mM CNQX in 0.1 N NaOH, Tocris Neuramin; 1 mM atropine sulfate in 0.9% NaCl, Sigma) to the buffer that bathed the slices so that the final concentration was 5 μM CNQX or 10 μM atropine. Aliquots were kept frozen at −20°C until the day of the experiment. Reversal of the effect of drug was tested by replacing buffer that contained drug with drug-free buffer. There were no effects of drug application on resting membrane potential or input resistance.
As would be expected from stimulating area CA3 pyramidal cell axons as well as other fibers in the fimbria, stimulation of the fimbria strongly excited area CA3 pyramidal cells. When assessed by intracellular and extracellular recording, area CA3 cells were activated antidromically and/or orthodromically. In these slices, no antidromic population spikes were ever recorded from the granule cell layer following fimbria stimulation, and no antidromic responses were recorded from granule cells that were examined intracellularly (Fig. 1C). In each slice (n=20), 3–6 sites in the granule cell layer were sampled extracellularly (at least 2 in the upper blade and 1 in the lower blade), and 2–4 granule cells were sampled intracellularly.
Fig. 1.
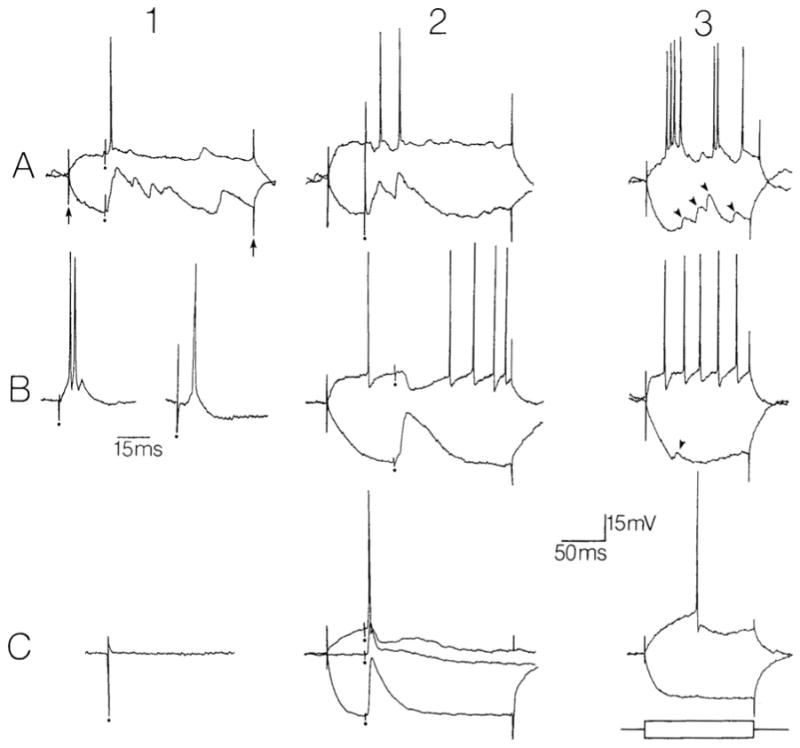
Representative responses of hilar cells and granule cells to fimbria stimulation. A: responses of spiny hilar ‘mossy’ cells. 1,2: responses to fimbria stimulation (at the dots) are superimposed on hyperpolarizing and depolarizing intracellular current steps (arrows point to the start and end of the current injection). Current amplitudes were +0.3 nA, −0.3 nA in part 1 and +0.4 nA, −0.3 nA for part 2. Membrane potentials, −65 mV (1), −67 mV (2). 3: responses to depolarizing and hyperpolarizing current injection (± 0.3 nA) are shown in the absence of stimulation to illustrate the electrophysiological characteristics of the spiny hilar cell type (for review, see ref. 25). Note the irregular firing behavior and large spontaneous potentials (arrowheads). Membrane potential, −62 mV. B: responses of ‘fast-spiking’ hilar cells (possible interneurons). 1: excitatory responses of two different cells to fimbria stimulation. Membrane potentials, −60 mV (left) and −58 mV (right). Calibration for 1 and 2, 15 ms; for all other parts of this figure, calibration, 50 ms. 2: inhibitory response of a different cell to fimbria stimulation, superimposed on responses to ± 0.3 n A current steps. Membrane potential, −68 mV. 3: responses to current injection (± 0.3 nA) are shown in the absence of stimulation. Same cell as in B2. Note the lack of spike frequency adaptation and the large afterhyperpolarization after each action potential. Membrane potential, −64 mV. C: responses of a granule cell. 1,2: the responses of the same granule cell are shown following fimbria stimulation (1) and molecular layer stimulation (2). In part 2, the response is shown in the absence of current injection as well as in the presence of +0.2 nA and −0.5 nA current steps. Membrane potential for both 1 and 2, −70 mV. 3: the responses of the same granule cell as in parts 1 and 2 are shown to current injection (±0.3 nA) without synaptic stimulation.
Membrane potential, −77 mV.
Both of the major cell types of the hilus responded to fimbria stimulation in every case where it was tested (n=20). Hilar cells were identified on the basis of their physiological properties either as spiny hilar ‘mossy’ cells (n=15, [27]), or relatively aspiny ‘fast-spiking’ cells (n=5; Fig. 1; [13, 24]), and were sampled in many different parts of the hilus (i.e. close to the upper blade, close to the lower blade, etc.). Spiny hilar cells responded homogeneously to fimbria stimulation. These responses were composed of EPSPs that were able to trigger one or more action potentials (Fig. 1A). All EPSPs increased in amplitude with hyperpolarization (Fig. 1A). In three cells, small hyperpolarizations were also evoked (Fig. 1A). The hyperpolarizations occurred prior to the EPSP in one cell (Fig. 1A), and in the other 2 cells hyperpolarizations occurred after the onset of the EPSPs. In contrast to spiny hilar cells, responses of fast-spiking cells to fimbria stimulation were relatively heterogeneous. The most common response was an EPSP followed by an IPSP (n=3). EPSPs were able to trigger 1–2 action potentials (Fig. 1B). In addition, EPSPs were observed in the absence of IPSPs (n=1), and an IPSP without an EPSP was also evoked (n= 1, Fig. 1B). In contrast, fast-spiking cells that were located in the granule cell layer did not respond to fimbria stimulation at all (n=4).
When the responses of hilar cells and CA3 pyramidal cells to fimbria stimulation were compared, area CA3b pyramidal cell action potentials occurred prior to hilar cell responses. In these experiments, area CA3b pyramidal cells were impaled approximately 500 μm from the stimulating electrode. The mean time to onset of area CA3b action potentials was 4.8 ± 0.4 ms (mean ± S.E.M., n=25), which is significantly different from the mean time to onset of hilar cell EPSPs (6.7 ± 0.3 ms, n= 18; Student’s t-test, P < 0.05). These data are consistent with the possibility that area CA3b pyramidal cells excite hilar cells. In contrast, the action potentials of area CA3c pyramidal cells did not necessarily precede hilar cell responses; the mean time to peak of area CA3c pyramidal cell action potentials (7.1 ± 0.5 ms, n=19) was not significantly different from the mean time to onset of hilar cell EPSPs (Student’s t-test, P > 0.05).
The stimulus strengths required to produce EPSPs in hilar cells were consistently greater than the stimulus strengths required to activate CA3 pyramidal cells. In no case did the stimulus strength required to evoke a hilar cell EPSP fall below the stimulus strength required to cause discharge of an area CA3b pyramidal cell in the same slice. Comparisons of stimulus strength were difficult to make across all slices because different stimulating electrodes as well as different levels of stimulus current and stimulus durations were used. However, in 8 experiments where both area CA3b pyramidal cells (n=25) and hilar cells (n=8) were impaled within the same slice (so that the same stimulating electrode, electrode position, and same level of current were identical), the stimulus duration required to produce an EPSP in the hilar cell always was above threshold for the CA3b pyramidal cells sampled in that slice.
To determine the mechanism(s) of excitation evoked by fimbria stimulation, a pharmacological approach was taken. When the glutamate receptor antagonist CNQX (selective for the AMPA/kainate receptor, 5 μM) was bath-applied to 5 cells, all hilar cell excitation was blocked reversibly (Fig. 2). The 5 cells included 4 spiny hilar cells and one fast-spiking cell. In contrast, the muscarinic cholinergic antagonist atropine (10 μM) had no effect on 4 cells (3 spiny hilar cells and one fast-spiking cell; Fig. 2), arguing against a role of the large septohippocampal projection (which appears to exert its effects mainly by muscarinic receptors; [20, 23, 30, 35]) in generating hilar cell responses.
Fig. 2.
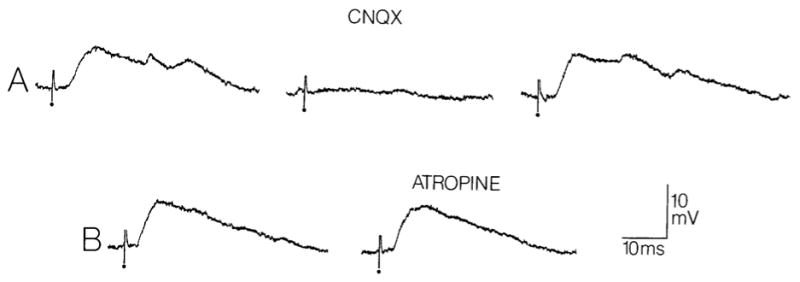
Blockade of hilar cell responses to fimbria stimulation by the AMPA/kainate receptor antagonist CNQX but not by the muscarinic antagonist atropine. A: the response of a spiny hilar cell to fimbria stimulation is shown before (left) and 15 min following (center) perfusion of the slice with 5 μM CNQX. The trace at right shows a response to the same stimulus 75 min after perfusion with drug-free buffer was resumed. Dots indicate the stimulus artifact. Membrane potential, −65 mV. B: two responses to the same fimbria stimulus are shown for a different spiny hilar cell. At left is a response elicited before 10 μM atropine was added to the bathing medium, and at right is a response elicited 30 min after addition of atropine.
Membrane potential, −64 mV.
In summary, dentate hilar cells, CA3 pyramidal cells, and granule cells were sampled consecutively in the same slice and assessed for their response to fimbria stimulation. Dentate hilar cells and pyramidal cells were excited but granule cells were not. Excitation was blocked by CNQX but not atropine. The latencies of hilar cell responses were greater than the latency of action potentials of area CA3b pyramidal cells, and hilar cell responses required stimuli stronger than those necessary to discharge the same area CA3b pyramidal cells. These data support the hypothesis that some area CA3 pyramidal cells may excite hilar neurons.
However, other pathways besides area CA3 pyramidal cells could have mediated the excitatory responses of hilar cells. For example, fimbria stimulation could have activated extrahippocampal glutamatergic fibers that travel in the fimbria and innervate hilar cells. However, such a pathway has not been documented. Another potential source of excitatory afferents are commissurally projecting area CA3 pyramidal cells, or commissurally projecting glutamatergic hilar cells. The cut axons of these cells could have been stimulated by fimbria stimulation. However, a specific projection of area CA3 pyramidal cells or hilar cells to contralateral hilar neurons has not been demonstrated. Blackstad showed that the commissural input to the hilus, regardless of the cells that contributed to that pathway, was very weak [5]. A different possibility is that a glutamatergic interneuron that is activated by stimulation of the fimbria innervates hilar neurons. One candidate for such a cell is the recently described calretinin-immunoreactive cell located in stratum lucidum of area CA3 [10, 18]. However, it is not known whether these cells receive input from the fimbria or area CA3 pyramidal cells, the transmitter of these cells could be GABA and not glutamate, and it is not clear that they project to the hilus [10, 18]. Therefore, although other possibilities may unfold in the future, given the present anatomical and physiological understanding of the hippocampus it reasonable to suggest that area CA3 pyramidal cells innervate hilar neurons.
If area CA3 pyramidal cells innervate hilar neurons, even if they do so indirectly, the consequences will still be important. First, such a pathway could lead to effects on large populations of granule cells, since spiny hilar ‘mossy’ cells project heavily to the inner molecular layer of both the ipsilateral and contralateral dentate gyrus [9, 22, 34, 36], and fast-spiking hilar cells can innervate granule cells [19, 26]. Second, such a pathway could help explain why repetitive excitatory events are triggered in hilar cells following stimulation of a single granule cell [26]; secondary excitation may have been due to area CA3 pyramidal cells that were excited by the granule cell. Such repetitive excitation is important to understand because it may be one of the reasons hilar cells are relatively vulnerable to prolonged perforant path excitation [28, 29, 31], as well as other insults [8, 12, 17].
Acknowledgments
I would like to thank Dr. Robert Sloviter, Dr. Daniel H. Lowenstein, and Dr. Peter Somogyi for their comments on the manuscript. This study was supported by NIH Grant NS 30831.
References
- 1.Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- 2.Amaral DG, Witter M. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1990;31:571–791. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Bliss TVP, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Exp Brain Res. 1971;13:222–238. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- 4.Bakst I, Avendano C, Morrison JH, Amaral DG. An experimental analysis of origins of somatostatin-like immunoreactivity in dentate gyrus of rat. J Neurosci. 1986;6:1452–1462. doi: 10.1523/JNEUROSCI.06-05-01452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackstad T. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol. 1956;105:417–537. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- 6.Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;146:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- 7.Cotman CW, Nadler JV. Glutamate and aspartate as hippocampal transmitters: histochemical and pharmacological evidence. In: Roberts PJ, Storm-Mathisen J, Johnston GAR, editors. Glutamate: Transmitter in the Central Nervous System. Wiley; New York: 1981. pp. 117–154. [Google Scholar]
- 8.Crain BJ, Westerkam WD, Harrison AH, Nadler JV. Selective neuronal death after transient forebrain ischemia in the Mongolian gerbil: a silver impregnation study. Neuroscience. 1987;27:387–402. doi: 10.1016/0306-4522(88)90276-x. [DOI] [PubMed] [Google Scholar]
- 9.Frotscher M, Seress L, Schwerdtfeger WK, Buhl E. The mossy cells of the fascia dentala: a comparative study of their fine structure and synaptic connections in rodents and primates. J Comp Neurol. 1991;312:145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- 10.Gulyás AI, Miettinen R, Jacobowitz DM, Freund TF. Calretinin is present in non-pyramidal cells of the rat hippocampus. I. A new type of neuron specifically associated with the mossy fibre system. Neuroscience. 1992;48:1–27. doi: 10.1016/0306-4522(92)90334-x. [DOI] [PubMed] [Google Scholar]
- 11.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 12.Johansen FF, Zimmer J, Diemer NH. Early loss of somatostatin neurons in the dentate hilus after cerebral ischemia in the rat precedes CA-1 pyramidal cell loss. Acta Neuropathol. 1987;73:110–114. doi: 10.1007/BF00693775. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi Y, Hama K. Fast-spiking non-pyramidal cells in the hippocampal CA3 region, dentate gyrus and subiculum of rats. Brain Res. 1987;425:351–355. doi: 10.1016/0006-8993(87)90518-x. [DOI] [PubMed] [Google Scholar]
- 14.Köhler C, Eriksson S, Davies S, Chan-Palay V. Co-localization of neuropeptide tyrosine and somatostatin immunoreactivity in neurons of individual subfields of the rat hippocampal region. Neurosci Lett. 1987;78:1–6. doi: 10.1016/0304-3940(87)90551-9. [DOI] [PubMed] [Google Scholar]
- 15.Kosaka T, Hama K, Wu J-Y. GABAergic synaptic boutons in the granule cell layer of rat dentate gyrus. Brain Res. 1984;293:353–359. doi: 10.1016/0006-8993(84)91242-3. [DOI] [PubMed] [Google Scholar]
- 16.Li X-G, Tepper JM, Jandó G, Buzsáki G. Axon arborization of CA3 pyramidal cells in vivo: an intracellular labelling study. Soc Neurosci Abstr. 1992;18:320. [Google Scholar]
- 17.Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miettinen R, Gulyás AI, Baimbridge KG, Jacobowitz DM, Freund TF. Calretinin is present in non-pyramidal cells of the rat hippocampus. II. Co-existence with other calcium binding proteins and GABA. Neuroscience. 1992;48:29–43. doi: 10.1016/0306-4522(92)90335-y. [DOI] [PubMed] [Google Scholar]
- 19.Müller W, Misgeld U. Inhibitory role of dentate hilus neurons in guinea pig hippocampal slice. J Neurophysiol. 1990;64:46–56. doi: 10.1152/jn.1990.64.1.46. [DOI] [PubMed] [Google Scholar]
- 20.Nicoll RA. The septo-hippocampal projection: a model cholinergic pathway. Trends Neurosci. 1985;8:533–536. [Google Scholar]
- 21.Ottersen OP, Storm-Mathisen J. Excitatory and inhibitory amino acids in the hippocampus. In: Chan-Palay V, Köhler C, editors. The Hippocampus. New Vistas, Liss; New York: 1989. pp. 97–117. [Google Scholar]
- 22.Ribak CE, Seress L, Amaral DC. The development, ultra-structure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- 23.Ropert N. Modulation of inhibition in the hippocampus in vivo. Can J Physiol Pharmacol. 1984;63:838–842. doi: 10.1139/y85-138. [DOI] [PubMed] [Google Scholar]
- 24.Scharfman HE. Dentate hilar cells with dendrites in the molecular layer have lower thresholds for synaptic activation by perforant path than granule cells. J Neurosci. 1991;11:1660–1673. doi: 10.1523/JNEUROSCI.11-06-01660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scharfman HE. Differentiation of rat dentate neurons by morphology and electrophysiology in hippocampal slices: granule cells, spiny hilar cells and aspiny ‘fast-spiking’ cells. In: Ribak CE, Gall C, Mody I, editors. The Dentate Gyrus And Its Role In Seizures. Elsevier; Amsterdam: 1992. pp. 93–109. [PMC free article] [PubMed] [Google Scholar]
- 26.Scharfman HE, Kunkel DD, Schwartzkroin PA. Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recording and intracellular HRP injections. Neuroscience. 1990;37:693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- 27.Scharfman HE, Schwartzkroin PA. Electrophysiology of morphologically identified mossy cells of the rat dentate hilus. J Neurosci. 1988;8:3412–3421. doi: 10.1523/JNEUROSCI.08-10-03812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharfman HE, Schwartzkroin PA. Consequences of prolonged afferent stimulation of the rat fascia dentata: epileptiform activity in area CA3 of hippocampus. Neuroscience. 1990;35:505–517. doi: 10.1016/0306-4522(90)90325-x. [DOI] [PubMed] [Google Scholar]
- 29.Scharfman HE, Schwartzkroin PA. Responses of cells of the rat fascia dentata to prolonged stimulation of the perforant path: sensitivity of hilar cells and changes in granule cell excitability. Neuroscience. 1990;35:491–504. doi: 10.1016/0306-4522(90)90324-w. [DOI] [PubMed] [Google Scholar]
- 30.Segal M. Multiple actions of acetylcholine at a muscarinic receptor studied in the rat hippocampal slice. Brain Res. 1982;246:77–87. doi: 10.1016/0006-8993(82)90144-5. [DOI] [PubMed] [Google Scholar]
- 31.Sloviter RS. Decreased inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- 32.Sloviter RS, Nilaver G. Immunocytochemical localization of GABA-, cholecystokinin-, vasoactive intestinal polypeptide- and somatostatin-like immunoreactivity in the area dentata and hippocampus of the rat. J Comp Neurol. 1987;256:42–60. doi: 10.1002/cne.902560105. [DOI] [PubMed] [Google Scholar]
- 33.Storm-Mathisen J, Wold JE. In vivo high-affinity uptake and axonal transport of D-[2,33H]aspartate in excitatory neurons. Brain Res. 1980;230:427–435. doi: 10.1016/0006-8993(81)90428-5. [DOI] [PubMed] [Google Scholar]
- 34.Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–716. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- 35.Wheal HV, Miller JJ. Pharmacological identification of acetylcholine and glutamate excitatory systems in the dentate gyrus of the rat. Brain Res. 1980;182:145–155. doi: 10.1016/0006-8993(80)90837-9. [DOI] [PubMed] [Google Scholar]
- 36.Zimmer J. Ipsilateral afferents to the commissural zone of the fascia dentata, demonstrated in decommissurated rats by silver impregnation. J Comp Neurol. 1971;142:93–416. doi: 10.1002/cne.901420402. [DOI] [PubMed] [Google Scholar]


