Abstract
Background
Primary monosymptomatic nocturnal enuresis (PMNE) is a common disorder in school-aged children. Previous studies have suggested that a developmental delay might play a role in the pathology of children with PMNE. However, microstructural abnormalities in the brains of these children have not been thoroughly investigated.
Methodology/Principal Findings
In this work, we evaluated structural changes in the brains of children with PMNE using diffusion tensor imaging (DTI). Two groups consisting of 26 children with PMNE and 26 healthy controls were scanned using magnetic resonance DTI. The diffusion parameters of fractional anisotropy (FA) and mean diffusivity (MD) were subjected to whole-brain, voxel-wise group comparisons using statistical parametric mapping (SPM). When compared to healthy subjects, children with PMNE showed both a decrease in FA and an increase in MD in the thalamus. MD also increased in the frontal lobe, the anterior cingulate cortex and the insula; these areas are all involved in controlling micturition. The significant changes seen in the thalamus could affect both urine storage and arousal from sleep.
Conclusions/Significance
The microstructure abnormalities were observed in the thalamus, the medial frontal gyrus, the anterior cingulate cortex and the insula, which are involved in micturition control network. This indicates developmental delay in these areas may be the cause of PMNE.
Introduction
Nocturnal enuresis is a common developmental disorder that affects 15–20% of 5-year-old children [1] , and it has important negative effects on the self-image and performance of these children [2]. Enuresis in children without additional lower urinary tract (LUT) symptoms (excluding nocturia) or a history of bladder dysfunction is defined as monosymptomatic nocturnal enuresis [3], while children with both enuresis and any other LUT symptoms are classified as having non-monosymptomatic enuresis [3]. When a child with monosymptomatic enuresis has never had a period of established urinary continence for more than six months, this is considered primary monosymptomatic nocturnal enuresis (PMNE).
Several factors are associated with and contribute to nocturnal enuresis, including heredity, polyuria, detrusor overactivity, sleep and central nervous system mechanisms [4]. In the past, electroencephalograph (EEG) [5], event-related brain potential (ERP) [6], and the startle blink [7] have all been used to study enuresis. It has been observed that the maturational delay of the central nervous system is an important factor in the pathogenesis of nocturnal enuresis [5], [6], [7], because it can induce functional and structural abnormalities in some brain areas. Using functional magnetic resonance imaging (fMRI), a number of studies have reported alterations in several brain functions in patients with urgency and urge incontinence [8], [9]. One study utilized event-related fMRI in PMNE subjects to reveal that children with PMNE had deficits in working memory [10]. We have performed a series of fMRI experiments to investigate the functional abnormalities that are associated with PMNE. In our previous studies, we reported that forebrain activation was altered during a response inhibition task [11] and that spontaneous brain activity changed during the resting state in children with PMNE [12]. These functional changes may originate from a structural abnormality. This idea is supported by a study from Kuchel et al., which has suggested that the presence of white matter hyperintensities seen by magnetic resonance imaging (MRI) both in the right inferior frontal region and in selected white matter tracts predicts the presence and severity of incontinence [13]. We therefore speculate that the structure of the brain is likely abnormal in PMNE patients. However, the exact cerebral areas involved in the pathogenesis of PMNE remain unclear and the brain structure of these patients is poorly understood. Therefore, we evaluated changes the brain structure of children with PMNE using diffusion tensor imaging (DTI).
DTI is an MRI method which provides information about tissue microstructure and its physiologic state [14]. Changes in these parameters can be determined by two important measurements: fractional anisotropy (FA) and mean diffusivity (MD). FA values are most often used to characterize the integrity of white matter tracts, while MD reflects the overall magnitude of diffusional motion within a given voxel. Recently, researchers observed an increase of FA in 11 white matter tracts from childhood to adolescence [15]. Previous research has reported that linear increases in FA and age-related MD decreases are observed in white matter during adolescence [16], [17], [18]. The values of FA and MD would change if the child was developmentally delayed.
In this study, we used statistical parametric mapping (SPM) to perform a whole-brain analysis of the data and to explore structural differences in the brains of children with PMNE and healthy controls. In addition, we also performed a region of interest (ROI)-based analysis of FA and MD to investigate potential changes in the thalamus of children with PMNE.
Results
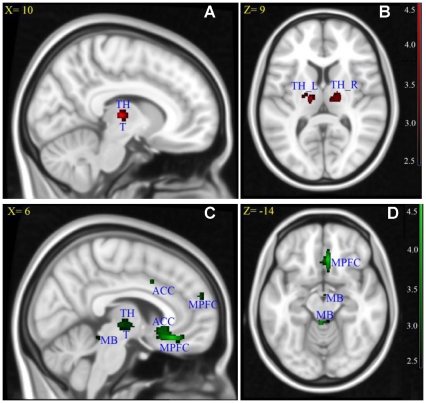
When compared to healthy children, children with PMNE demonstrated a significant decrease in FA in the bilateral thalamus, the left frontal lobe (sub-gyral) and the left limbic lobe (uncus).
Children with PMNE also showed an increase in MD in the bilateral anterior cingulate (ACC), the bilateral insula, the right medial frontal gyrus, the right thalamus, the right midbrain, the left anterior lobe of the cerebellum, the right lentiform nucleus and the right sub-lobar (extra-nuclear white matter) when compared to healthy children.
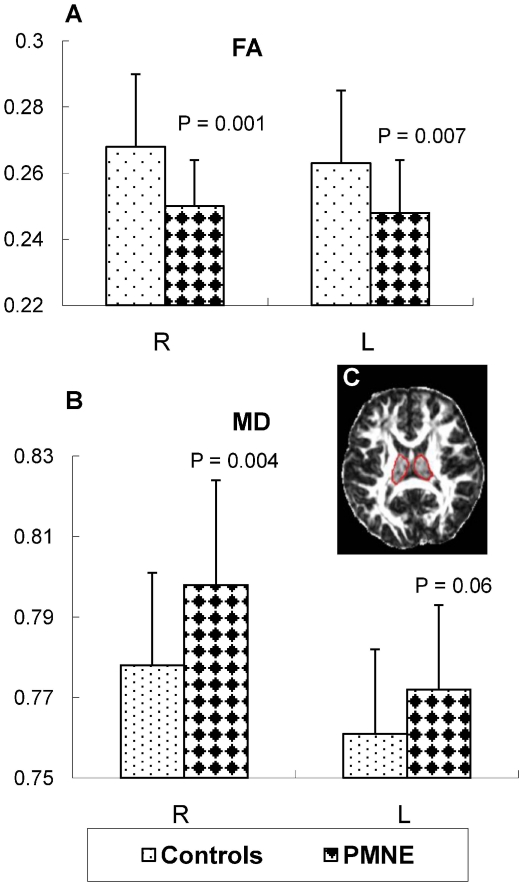
A significant decrease in FA and increase in MD were revealed in the thalamus by both an SPM- and an ROI-based analysis. Detailed results are shown in Fig. 1, Fig. 2 and Table 1.
Figure 1. Results of fractional anisotropy (FA) and mean diffusivity (MD) for group analysis between children with PMNE and healthy children.
(A) and (B) FA decreases in when compared to healthy children. (C) and (D) MD increases in children with PMNE when compared to TH: thalamus, T: hypothalamus, TH_L: left side of the thalamus, TH_R: right side of the thalamus, MPFC: medial prefrontal cortex, ACC: anterior cingulate cortex, MB: midbrain. Also, X = : X coordinate in MNI space, Z = : Z coordinate in MNI space. (A) and (C) are located in the right brain.
Figure 2. The results of the region of interest (ROI) analysis of fractional anisotropy (FA) and mean diffusivity (MD).
P values were analyzed by a two-sample t-test between the two groups. L: left side of the thalamus, R: right side of the thalamus.
Table 1. Results from the SPM analysis of fractional anisotropy (FA) and mean diffusivity (MD).
| Predominant regions in the cluster | T valuea | P valuea | Number of voxels | Peak location (X Y Z) | ||
| FA decrease in PMNE | ||||||
| Right thalamus | 4.33 | <0.001 | 168 | 10 | −18 | 6 |
| Left thalamus | 3.81 | <0.001 | 92 | −12 | −14 | 10 |
| Left frontal lobe/sub-gyral | 4.04 | <0.001 | 23 | −24 | 12 | 40 |
| Left limbic lobe/uncus | 3.74 | <0.001 | 10 | −18 | 6 | −28 |
| MD increase in PMNE | ||||||
| Right medial frontal gyrus | 4.50 | <0.001 | 124 | 4 | 28 | −16 |
| Right anterior cingulate | 3.40 | <0.001 | 55 | 6 | 18 | −8 |
| Right anterior cingulate | 3.29 | = 0.001 | 12 | 8 | 10 | 38 |
| Left anterior cingulate | 3.63 | <0.001 | 21 | −4 | 26 | 22 |
| Right thalamus | 3.52 | <0.001 | 48 | 8 | −18 | 0 |
| Right insula | 3.49 | <0.001 | 31 | 34 | 16 | −2 |
| Left insula | 3.35 | = 0.001 | 20 | −36 | 20 | 0 |
| Right medial frontal gyrus | 3.33 | <0.001 | 15 | 8 | 54 | 24 |
| Right brainstem/midbrain | 3.42 | <0.001 | 29 | 2 | −14 | −18 |
| Left cerebellum anterior lobe/midbrain | 3.75 | <0.001 | 29 | −2 | −40 | −14 |
| Right lentiform nucleus/lateral globus pallidus | 3.52 | <0.001 | 71 | 16 | 0 | 4 |
| Right sub-lobar/extra-nuclear white matter | 2.94 | <0.001 | 17 | 10 | 6 | −4 |
X, Y, Z = MNI coordinates.
For peak areas of activation.
Discussion
When compared to healthy subjects, children with PMNE showed both a decrease in FA and an increase in MD in the thalamus; MD also increased in the medial frontal gyrus, the ACC and the insula, which are all involved in the control of micturition [8]. Previous studies have reported that FA increases and MD decreases with age throughout childhood and adolescence [15], [16], [17], [18]. These changes in FA and MD were observed in children with PMNE but not in healthy children, which suggests a delay in central nervous system maturation in children with PMNE.
3.1 Thalamus
All information that reaches the cerebral cortex is relayed by the thalamus including sensation, spatial sense, perceptions and voluntary movements; the thalamus also regulates consciousness, sleep, and alertness [19], [20], [21]. We observed a significant decrease in FA and an increase in MD in the thalamus of children with PMNE, which suggests that the microstructure of the thalamus is abnormal in these patients. The previous research showed that anisotropic increase with age in the anterior thalamic radiations during adolescence, suggesting that the relay between the cortex and thalamus may be undergoing a refinement of connections during adolescence [16].
In the currently accepted model of bladder control, the thalamus plays an important role in relaying the signal coming from the midbrain periaqueductal gray (PAG) by transmitting it to the ACC, the insula and the lateral prefrontal cortex (LPFC) [8], [9]. Previous research has shown that the thalamus is activated during urine storage or bladder infusion [22], [23]. In fact, the thalamus is involved in relaying sensory afferent information from the bladder, which is important during the urine storage phase. The significant reduction in FA and the increase of MD (Fig. 1B) in the thalamus, which may affect thalamic neuronal signal transmission, could influence signal transmission from the PAG to the ACC, the insula and the prefrontal cortex (PFC). If this occurs, urine cannot be properly stored in children with PMNE.
The thalamus also plays an important role in regulating states of sleep and wakefulness and is affected in some sleep disorders [24]. Because children with PMNE do not have involuntary urination during the daytime, a sleep disorder is one of the pathogenic factors of PMNE [25]. Previous research has suggested that an immaturity in the function of the thalamus might be a cause of the arousal dysfunction in patients with Type I enuresis (Type I enuresis was defined as: the cystometrogram is stable and when the bladder is filled, and the EEG pattern changes to that for arousal; and enuresis occurs without the patients awakening.) [26]. A high arousal threshold and an inability to wake during sleep in response to the need to void may directly cause bed-wetting. Therefore, a dysfunctional thalamus may affect the ability of children of PMNE to wake up when they need to void.
A dysfunction in the thalamus could affect both the relay of sensory afferent information that originates in the bladder and the ability to wake during sleep in response to a need to void. Although each of these thalamic dysfunction mechanisms could affect micturition control separately, it is more likely that both of them occur in PMNE. Taken together, these data suggest that a developmental delay or an abnormality of the thalamus could be an important factor behind the bed-wetting seen in children with PMNE.
3.2 Medial frontal gyrus
The most significant cluster revealed by SPM was the increase in MD that was observed in the medial frontal gyrus, which suggested a dysfunction of this brain region in children with PMNE. In our previous studies, abnormal activation of the PFC was seen both during a Go/NoGo task and during the resting state in children with PMNE. The PFC has been implicated in planning complex cognitive behaviors, decision making and moderating proper social behavior [27]. A This brain area is connected to the PAG, the ACC, the insula, the hypothalamus and the thalamus; these brain areas are all involved in the control of micturition [8]. Previous brain imaging research has shown that the PFC is activated during both the voiding and filling phases of micturition [8], [9], [28], [29]. In Fowler's preliminary working model of lower urinary tract control by higher brain centers, the medial prefrontal cortex (MPFC) is involved in the storage and voiding phases [8]. Generally, the PFC is associated with decision making in voiding [8], [9]. Patients with bilateral MPFC lesions and multiple sclerosis experience a disturbance in micturition [30], implying that the dysfunction of the MPFC may reduce the ability to withhold urine and cause inappropriate micturition.
3.3 Anterior cingulate cortex
The ACC has a wide range of cerebral functions that involve both cognitive function and emotion [31]. This structure may also play a role in mediating bodily arousal states and may take part in interoceptive awareness [32]. Beckel and Holstege have suggested that the ACC is one of most important brain areas that is involved in voluntary voiding and that the ACC may be involved in attention and introspection (i.e., being aware that the bladder is full and that it is time to void) in addition to executive control (i.e., voiding in an “appropriate” place) [33]. Previous research has suggested that ACC responses seem to be associated with pontine micturition center inhibition because reduced ACC activity accompanies the failure of inhibition [34].
In our study, SPM revealed increases in MD in the ACC, suggesting that this abnormality in the ACC might induce micturition at an inappropriate time and place.
3.4 Insula
The insula is widely considered to be involved in bladder control. When the bladder is full, the insular cortex is activated in healthy people [22]. Insular and visceral sensations are related; in the insula, the visceral sensation signals are changed into comprehensible consciousness [32], [35]. For example, the insula translates the signal from a fully filled bladder to a conscious internal signal; therefore, the insula aids in interoceptive awareness of bladder states. Our observation that MD increases on both sides of insula may suggest that there are problems in the translation of this signal in the insula. While asleep, the signal from a full bladder might not be transmitted to the brain in time, which would prevent the brain from being aware of the state of the bladder and result in a delay or a missed warning signal indicating that the bladder is full.
3.5 Other brain regions
Our results also showed an increase in MD in the right lateral globus pallidus in children with PMNE when compared to healthy children. The globus pallidus has been shown to be activated in normal men during micturition but not at rest [28]. Tai et al. also detected the activation of the globus pallidus during a micturition contraction in rats [36]. Although the activation of this region has been reported in several studies, its exact function in micturition control is still unclear.
Finally, we found two peak areas with increased MD. One was in the midbrain, while the other was near the PAG (x = 2, y = −14, z = −18). It has been established that the PAG plays an important role in bladder control [8]. Moreover, an SPM-based analysis also revealed differences in the uncus, the sub-gyral portion of the left frontal lobe and the midbrain. However, the role of these areas in the pathogenesis of PMNE remains unclear.
Additionally, our results shows difference mainly in gray matter, which is further discussed as following: firstly, DTI measures the restricted diffusion of water through brain tissue, which is sensitive to local microstructure. White matter and gray matter consist of many neuronal processes, which both contribute to water diffusion. It is generally accepted that FA is more sensitive in white matter because of the special structure of nerve fiber. However, FA and MD can also be used to measure microstructure connections of dendrites and soma in gray matter. DTI has been used to detect microstructure changes of gray matter in many researches [37], [38], [39], [40]. Secondly, both gray matter and white matter are involved in structure changes in the maturing brain [40]. However, our results that microstructure changes were primarily in gray matter, may suggest that the gray matter of the PMNE children have more significant abnormality than their white matter. In other words, it suggested that the dysfunction of gray matter, rather than white matter, may play a key role in PMNE. Finally, previous studies showed that thalamus microstructure is involved in developmental changes from children to adults. For example, Snook et al. observed that FA increased and MD decreased in the thalamus of young adults compared to children [40]. Literatures also reported that FA value of thalamus was observed to be abnormal in some disease, such as neurofibromatosis type 1 [41] and autism spectrum disorders [42]. So the significant changes in thalamus that is observed in our experiment suggest that a possible developmental delay or abnormality in the thalamus of PMNE children.
In summary, our results revealed microstructural abnormalities in the thalamus, the medial frontal gyrus, the ACC and the insula of children with PMNE. These areas are all involved in the control of micturition; therefore, a developmental delay in any of these regions may be an important cause of PMNE. In particular, thalamic dysfunction may play an important role in PMNE.
Materials and Methods
4.1 Ethics statement
This study was approved by the Institutional Review Board of Shanghai Children's Medical Center, which is affiliated with Shanghai Jiao Tong University School of Medicine (No: SCMC-201014). All participants involved in our study have known the Informed Consent before the experiments. This consent was written, which was signed by each participant and their guardians with their consent. And the ethics committees approved this consent procedure.
4.2 Subjects
We studied 30 children with PMNE and 48 healthy children with the consent of children and their guardians. After exclusion of children with excessive head motion as well as match of age, IQ and sex, 26 children with primary nocturnal enuresis (mean age 10.5; range 7–15; 8 female) and 26 healthy controls (mean age 10.7; range 7–15; 9 female) were available for further analysis. All of the children were right-handed with an IQ>75, and there was no significant difference between the IQs of the two groups (p = 0.218) as assessed by the Wechsler Intelligence Scale for Children revised (WISC-R). The children with PMNE have been excluded suffered from spina bifida occulta by lumbosacral X-ray imaging. And their bladder volumes were measured by b-ultrasound examination. Additionally, all neurological and psychiatric diseases were excluded both by a clinical examination and a structured interview based on the criteria listed in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). All of the 26 children with PMNE were outpatients at Shanghai Children's Medical Center. Additional clinical data about the patient group are listed in Table 2.
Table 2. Clinical data collected from the patient group.
| Age (years) | Gender | Weight (kg) | Bed-wetting frequency (per day) | Bed-wetting frequency (per week) | Bladder volumeb(ml) | Frequency of waking up for voluntary voiding | |
| 1 | 10 | M | 40 | 1 | 6 | 173 | Sometimes |
| 2a | 10 | M | 30 | 1 | 1–2 | 303 | Sometimes |
| 3a | 10 | M | 30 | 1 | 2 | 130 | Sometimes |
| 4 | 8 | M | 35 | 1 | 1 | 100 | Often |
| 5 | 14 | M | 75 | 1 | 2 | 114 | Never |
| 6a | 11 | F | 35 | 1 | 4 | 132 | Sometimes |
| 7 | 8 | F | 25 | 2 | Everyday | 140 | Sometimes |
| 8 | 9 | M | 48 | 0–1 | 1 | 130 | Sometimes |
| 9 | 10 | M | 29 | 2 | Everyday | 100 | Sometimes |
| 10 | 10 | M | 49 | 1 | 2–3 | 155 | Sometimes |
| 11 | 15 | F | 50 | 2–3 | Everyday | 510 | Never |
| 12a | 15 | F | 45 | 1 | 1 | 450 | Often |
| 13a | 8 | M | 22 | 1 | 1 | 235 | Sometimes |
| 14a | 11 | F | 25 | 1–2 | 1–2 | 251 | Often |
| 15 | 15 | M | 54 | 1–2 | Everyday | 192 | Never |
| 16a | 8 | M | 34 | 2 | 5–6 | 260 | Sometimes |
| 17 | 8 | M | 26 | 2 | Everyday | 190 | Sometimes |
| 18a | 12 | M | 32 | 2 | 5 | 350 | Sometimes |
| 19a | 11 | F | 34 | 1 | Everyday | 165 | Sometimes |
| 20a | 8 | M | 40 | 1 | 1 | 90 | Often |
| 21a | 13 | M | 41 | 1 | 2–3 | 245 | Often |
| 22a | 10 | F | 31 | 1 | 3–4 | 310 | Sometimes |
| 23a | 7 | F | 23 | 1–2 | 6 | 115 | Sometimes |
| 24a | 11 | M | 37 | 1 | 6 | 120 | Sometimes |
| 25a | 10 | M | 41 | 1–2 | 1–2 | 153 | Often |
| 26a | 10 | M | 50 | 1 | 3–4 | 304 | Never |
All patients wet the bed only at night. The age is given in years; M = male; F = female.
These children can wake up after bed-wetting.
The bladder volume was defined by b-ultrasound results: children with PMNE were asked to try their best to hold back urine for three times while their bladder volumes were measured by b-ultrasound. The largest measured value was defined as the bladder volume.
4.3 Data acquisition
MRI data were acquired on a Siemens 3 T Trio MR scanner with a 12-channel phased array coil. The DTI acquisition used a single-shot spin-echo planar imaging sequence in contiguous axial planes covering the whole brain. The diffusion sensitizing gradients were applied along 12 non-collinear directions together with an acquisition without diffusion weighting (b = 0). Imaging parameters were set to the following values: TR = 6600 ms, TE = 89 ms, average = 4, b-value = 1000 s/mm2, slice thickness = 2.5 mm, and 50 slices. Matrix size was acquired with a 128×128 and reconstructed to 256×256. The resolution was 2×2×2.5 mm3. The subjects were told not to move during the scans, and the acquisition time was 6 minutes and 5 seconds.
4.4 Data processing and statistical analysis
Preprocessing
SPM 8 (http://www.fil.ion.ucl.ac.uk/spm/), Matlab 2009 (The MathWorks, Natick, MA) and FSL4.1 (http://www.fmrib.ox.ac.uk/fsl/) were used to analyze the data. First, the DICOM files of each DWI acquisition were converted into a single multivolume NIFTI file. Then, FSL's “eddy current correction” was used to correct the distortions induced by the eddy current and head motion in the dataset. The brain was extracted using BET (Brain Extraction Tool, http://fsl.fmrib.ox.ac.uk/fsl/bet2/) for further processing steps. Finally, the FA and MD maps were calculated with FSL DTIFit (http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_dtifit.html).
SPM analysis
A whole brain voxel-wise analysis was performed using SPM 8. First, to reduce the potential errors caused by the adult template, we used the FA maps from our control group to generate our FA-specific pediatric template, which included following steps:
We normalized the FA maps of the control group based on the deformation information that was generated from the corresponding unweighted images (first b = 0 image) and the echo-planar imaging (EPI) template (in MNI152 space) in SPM; we called these the wFA maps.
These wFA maps were averaged to generate a mean map.
The mean map was smoothed (using a [6,6,6] FWHM Gaussian kernel) to obtain our FA-specific pediatric template for further analysis [43]. Subsequently, we normalized all of the original FA maps (from both patient and control groups) based on the deformation field produced from both the original FA maps and our FA-specific pediatric template. Then, the normalized FA maps were smoothed (using a [6,6,6] filter) for the statistical analysis. Finally, a fractional design specification was set up to compare the PMNE group against the controls using a two-sample t-test. The same processes were applied to the MD maps. For all analyses, the statistical maps were thresholded at p<0.005 (uncorrected). Moreover, an extent threshold of 10 contiguous voxels was applied to exclude small clusters that emerged by chance [44].
ROI analysis
To confirm the significant differences in the thalamus seen in PMNE children and unaffected controls, we also performed a ROI analysis. Each child's thalamus mask was manually drawn on one of the FA slices according to the cerebral surgery expert's opinion; the thalamus was visualized to be the biggest and the most distinct (Fig. 2C). Because the FA and the related MD maps are generated from the same individual DTI dataset, the ROIs were translated onto the corresponding MD maps. Finally, the mean values of FA and MD in all ROIs were calculated using Matlab, and a two-sample t-test was carried out between the two groups.
Acknowledgments
The authors would like to thank Dr. Min-Lu Tian of the Shanghai Institute of Physical Education and Dr. Jiaxin Du of the Queensland Brain Institute at the University of Queensland for their English writing assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was funded by the Science and Technology Commission of Shanghai (08DZ1900702), the Shanghai Children's Medical Center Fund, the Shanghai Key Laboratory of Children's Environmental Health (10DZ2272200, 09DZ2200900), and the Development of Science and Technology Fund of Pudong New Area, Shanghai (PKJ2009-Y03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Riccabona M. [Evaluation and management of enuresis. An update]. Urologe A. 2010;49:861–869; quiz 870. doi: 10.1007/s00120-010-2328-2. [DOI] [PubMed] [Google Scholar]
- 2.Theunis M, Van Hoecke E, Paesbrugge S, Hoebeke P, Vande Walle J. Self-image and performance in children with nocturnal enuresis. Eur Urol. 2002;41:660–667; discussion 667. doi: 10.1016/s0302-2838(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 3.Neveus T, von Gontard A, Hoebeke P, Hjalmas K, Bauer S, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children's Continence Society. J Urol. 2006;176:314–324. doi: 10.1016/S0022-5347(06)00305-3. [DOI] [PubMed] [Google Scholar]
- 4.Neveus T. Diagnosis and management of nocturnal enuresis. Curr Opin Pediatr. 2009;21:199–202. doi: 10.1097/MOP.0b013e3283229b12. [DOI] [PubMed] [Google Scholar]
- 5.Hallioglu O, Ozge A, Comelekoglu U, Topaloglu AK, Kanik A, et al. Evaluation of cerebral maturation by visual and quantitative analysis of resting electroencephalography in children with primary nocturnal enuresis. J Child Neurol. 2001;16:714–718. doi: 10.1177/088307380101601002. [DOI] [PubMed] [Google Scholar]
- 6.Karlidag R, Ozisik HI, Soylu A, Kizkin S, Sipahi B, et al. Topographic abnormalities in event-related potentials in children with monosymptomatic nocturnal enuresis. Neurourol Urodyn. 2004;23:237–240. doi: 10.1002/nau.20031. [DOI] [PubMed] [Google Scholar]
- 7.Freitag CM, Rohling D, Seifen S, Pukrop R, von Gontard A. Neurophysiology of nocturnal enuresis: evoked potentials and prepulse inhibition of the startle reflex. Dev Med Child Neurol. 2006;48:278–284. doi: 10.1017/S0012162206000600. [DOI] [PubMed] [Google Scholar]
- 8.Fowler CJ, Griffiths DJ. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn. 2010;29:49–55. doi: 10.1002/nau.20740. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn. 2008;27:466–474. doi: 10.1002/nau.20549. [DOI] [PubMed] [Google Scholar]
- 10.Yu B, Guo Q, Fan G, Ma H, Wang L, et al. Evaluation of working memory impairment in children with primary nocturnal enuresis: Evidence from event-related functional magnetic resonance imaging. J Paediatr Child Health. 2011 doi: 10.1111/j.1440-1754.2010.02000.x. [DOI] [PubMed] [Google Scholar]
- 11.Lei D, Ma J, Du X, Shen G, Tian M, et al. Altered brain activation during response inhibition in children with primary nocturnal enuresis: An fMRI study. Hum Brain Mapp: 2011 doi: 10.1002/hbm.21411. doi: 10.1002/hbm.21411. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei D, Ma J, Du X, Shen G, Tian M, et al. Spontaneous brain activity changes in children with primary monosymptomatic nocturnal enuresis: A resting-state fMRI study. Neurourol Urodyn: 2011 doi: 10.1002/nau.21205. doi: 10.1002/nau.21205. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Kuchel GA, Moscufo N, Guttmann CR, Zeevi N, Wakefield D, et al. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64:902–909. doi: 10.1093/gerona/glp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 15.Verhoeven JS, Sage CA, Leemans A, Van Hecke W, Callaert D, et al. Construction of a stereotaxic DTI atlas with full diffusion tensor information for studying white matter maturation from childhood to adolescence using tractography-based segmentations. Hum Brain Mapp. 31:470–486. doi: 10.1002/hbm.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, et al. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 18.Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, et al. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 20.Sherman SM. Thalamic relays and cortical functioning. Prog Brain Res. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- 21.Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17:417–422. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, et al. Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J Urol. 2002;168:2035–2039. doi: 10.1016/S0022-5347(05)64290-5. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urol. 2005;174:1862–1867. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- 24.Jan JE, Reiter RJ, Wasdell MB, Bax M. The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders. J Pineal Res. 2009;46:1–7. doi: 10.1111/j.1600-079X.2008.00628.x. [DOI] [PubMed] [Google Scholar]
- 25.Neveus T. The role of sleep and arousal in nocturnal enuresis. Acta Paediatr. 2003;92:1118–1123. doi: 10.1080/08035250310005837. [DOI] [PubMed] [Google Scholar]
- 26.Kawauchi A, Imada N, Tanaka Y, Minami M, Watanabe H, et al. Changes in the structure of sleep spindles and delta waves on electroencephalography in patients with nocturnal enuresis. Br J Urol. 1998;81(Suppl 3):72–75. doi: 10.1046/j.1464-410x.1998.00012.x. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- 28.Nour S, Svarer C, Kristensen JK, Paulson OB, Law I. Cerebral activation during micturition in normal men. Brain. 2000;123(Pt 4):781–789. doi: 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- 29.Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997;120(Pt 1):111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- 30.Namatame S, Mochizuki H, Ebitani M, Matsuda N, Ugawa Y. Micturitional disturbance due to bilateral medial frontal lobe lesions in a patient with multiple sclerosis. Neurol Sci. 2010;31:205–207. doi: 10.1007/s10072-009-0189-5. [DOI] [PubMed] [Google Scholar]
- 31.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 32.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 33.Beckel JM, Holstege G. Neurophysiology of the lower urinary tract. Handb Exp Pharmacol. 2011:149–169. doi: 10.1007/978-3-642-16499-6_8. [DOI] [PubMed] [Google Scholar]
- 34.Griffiths D, Tadic SD, Schaefer W, Resnick NM. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage. 2007;37:1–7. doi: 10.1016/j.neuroimage.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 36.Tai C, Wang J, Jin T, Wang P, Kim SG, et al. Brain switch for reflex micturition control detected by FMRI in rats. J Neurophysiol. 2009;102:2719–2730. doi: 10.1152/jn.00700.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 38.Alkonyi B, Juhasz C, Muzik O, Behen ME, Jeong JW, et al. Thalamocortical connectivity in healthy children: asymmetries and robust developmental changes between ages 8 and 17 years. AJNR Am J Neuroradiol. 2011;32:962–969. doi: 10.3174/ajnr.A2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckert U, Metzger CD, Buchmann JE, Kaufmann J, Osoba A, et al. Preferential networks of the mediodorsal nucleus and centromedian-parafascicular complex of the thalamus-A DTI tractography study. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snook L, Plewes C, Beaulieu C. Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage. 2007;34:243–252. doi: 10.1016/j.neuroimage.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 41.Ferraz-Filho JR, da Rocha AJ, Muniz MP, Souza AS, Goloni-Bertollo EM, et al. Diffusion tensor MR imaging in neurofibromatosis type 1: expanding the knowledge of microstructural brain abnormalities. Pediatr Radiol. 2011 doi: 10.1007/s00247-011-2274-1. [DOI] [PubMed] [Google Scholar]
- 42.Cheon KA, Kim YS, Oh SH, Park SY, Yoon HW, et al. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: a Diffusion Tensor Imaging study. Brain Res. 2011;1417:77–86. doi: 10.1016/j.brainres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 44.Wilke M, Kaufmann C, Grabner A, Putz B, Wetter TC, et al. Gray matter-changes and correlates of disease severity in schizophrenia: a statistical parametric mapping study. Neuroimage. 2001;13:814–824. doi: 10.1006/nimg.2001.0751. [DOI] [PubMed] [Google Scholar]