Abstract
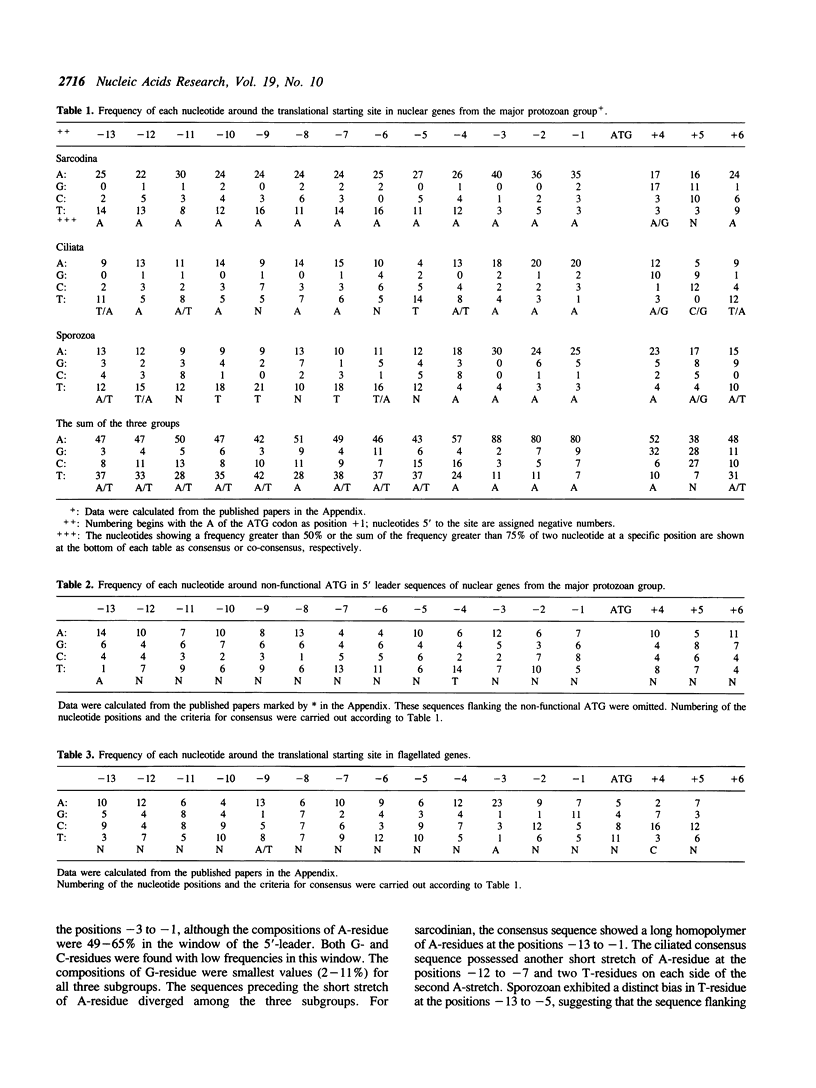
Nucleotide sequences flanking the translational initiation site were compiled from protozoan nuclear genes and were compared in every protozoan group. The entire 5'-untranslated sequences were very rich in A- and T-residues, but poor in G- and C-residues in most protozoan genes except for the flagellated ones. The sequence AAAAATTTTTAAAATTTAAAATGANAT emerged as a consensus sequence flanking the initiation site in the major protozoan group, although the sequences upstream from -4 (four nucleotides upstream from the ATG codon) were divergent among ciliates, sarcodinians, and sporozoans. On the other hand, the consensus sequence for flagellates was revealed to be a simple feature. Only the nucleotide position -3 was occupied with a high frequency of A-residue, in other positions it appeared randomly. These facts suggest that the strong preference for A-residue at the position -3 is a universal feature in nuclear genes for all eukaryotes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroin A., Perasso R., Qu L. H., Brugerolle G., Bachellerie J. P., Adoutte A. Partial phylogeny of the unicellular eukaryotes based on rapid sequencing of a portion of 28S ribosomal RNA. Proc Natl Acad Sci U S A. 1988 May;85(10):3474–3478. doi: 10.1073/pnas.85.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garreau H., Williams J. G. Two nuclear DNA binding proteins of Dictyostelium discoideum with a high affinity for poly(dA)-poly(dT). Nucleic Acids Res. 1983 Dec 10;11(23):8473–8484. doi: 10.1093/nar/11.23.8473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R., Watanabe C. K., de Boer H. A. Compilation and comparison of the sequence context around the AUG startcodons in Saccharomyces cerevisiae mRNAs. Nucleic Acids Res. 1987 Apr 24;15(8):3581–3593. doi: 10.1093/nar/15.8.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutzel R., Lacombe M. L., Simon M. N., de Gunzburg J., Veron M. Cloning and cDNA sequence of the regulatory subunit of cAMP-dependent protein kinase from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1987 Jan;84(1):6–10. doi: 10.1073/pnas.84.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]



