Abstract
Inhibitors of PI3K/Akt signaling are being actively developed for tumor therapy owing to the frequent mutational activation of the PI3K-Akt-mTORC1 pathway in many cancers, including glioblastomas (GBMs). NVP-BEZ235 is a novel and potent dual PI3K/mTOR inhibitor that is currently in phase 1/2 clinical trials for advanced solid tumors. Here, we show that NVP-BEZ235 also potently inhibits ATM and DNA-PKcs, the two major kinases responding to ionizing radiation (IR)-induced DNA double-strand breaks (DSBs). Consequently, NVP-BEZ235 blocks both nonhomologous end joining and homologous recombination DNA repair pathways resulting in significant attenuation of DSB repair. In addition, phosphorylation of ATMtargets and implementation of the G2/M cell cycle checkpoint are also attenuated by this drug. As a result, NVP-BEZ235 confers an extreme degree of radiosensitization and impairs DSB repair in a panel of GBM cell lines irrespective of their Akt activation status. NVP-BEZ235 also significantly impairs DSB repair in a mouse tumor model thereby validating the efficacy of this drug as a DNA repair inhibitor in vivo. Our results, showing that NVP-BEZ235 is a potent and novel inhibitor of ATM and DNA-PKcs, have important implications for the informed and rational design of clinical trials involving this drug and also reveal the potential utility of NVP-BEZ235 as an effective radiosensitizer for GBMs in the clinic.
Introduction
The phosphatidylinositol 3-kinase (PI3K)-Akt-mTORC1 pathway is frequently activated in a variety of human cancers, including glioblastomas (GBMs). Therefore, inhibitors of PI3K/Akt signaling are being actively developed for tumor therapy (reviewed in Liu et al. [1] and Garcia-Echeverria and Sellers [2]). Dual PI3K-mTOR inhibitors are particularly effective in blocking Akt activation because they prevent the feedback activation of PI3K signaling normally observed with mTORC1 inhibitors, such as rapamycin. NVP-BEZ235 is a potent dual PI3K-mTOR inhibitor [3] that has shown great efficacy in inhibiting tumor growth in preclinical mouse models [4–11] and is currently being evaluated in phase 1/2 clinical trials for advanced solid tumors (colorectal, breast, non-small cell lung carcinoma, renal, and sarcoma; reviewed in Garcia-Echeverria and Sellers [2]). The design of these trials is based on the premise that this compound specifically inhibits PI3Ks [half-maximal inhibitory concentration (IC50) = 4–75 nM] and mTOR (IC50 = 20 nM) with little cross-reactivity toward other kinases, as previously reported [3]. However, mTOR belongs to the PI3K-like kinase (PI3KK) family, which includes the two major kinases that respond directly to DNA double-strand breaks (DSBs), ATM and DNA-PKcs, both of which have catalytic domains highly homologous to that of the PI3Ks [1,12]. Because DSBs are induced during radiotherapy and commonly generated during chemotherapy, it is particularly important to understand the effects of NVP-BEZ235 on these two kinases.
A number of recent reports identify an important role for the PI3K-Akt pathway in radioresistance (reviewed in Mukherjee et al. [13] and Begg et al. [14]). Our laboratory and those of others have shown that GBM-relevant cells and tumors with epidermal growth factor receptor vIII (EGFRvIII) amplification or PTEN loss exhibit proficient DSB repair, which confers radioresistance [15–19]. Because radiotherapy plays a key role in the treatment of GBM, we investigated the potential utility of NVP-BEZ235 as a radiosensitizing agent against human GBM lines. Surprisingly, we found that very low concentrations of this drug conferred a high degree of radiosensitization that was significantly greater than that previously reported with PI3K/Akt inhibition [15–19], and this correlated with attenuation of DSB repair. Detailed experimentation revealed that NVP-BEZ235 potently inhibits ATM and DNA-PKcs, thereby blocking both nonhomologous end joining (NHEJ) and homologous recombination (HR), the two major pathways of DSB repair. In addition, phosphorylation of ATM targets and implementation of the G2/M cell cycle checkpoint are also attenuated by this drug. The consequence is profound radiosensitization at very low concentrations of NVP-BEZ235 (100 nM). This radiosensitizing effect is significantly more potent than that seen with much higher concentrations (10 µm) of current inhibitors of DNA-PKcs [20] or ATM [21] that are being optimized for clinical testing (reviewed in Ding et al. [22]). These results have significant translational importance, both for the design of current clinical trials involving this drug and for the potential use of this drug as a powerful radiosensitizer in the clinic.
Materials and Methods
Cell Culture and Drug Treatment
The cell lines used in this study are U251, U118, LN18, T98G, LN229, SF188, 1BR3, AT5, M059K, and M059J. The U87 glioma line expressing EGFRvIII has been described before [19]. All cell lines were maintained in Dulbecco modified Eagle medium containing 10% fetal bovine serum in a humidified 37°C incubator with 5% CO2. All cells were Mycoplasma free. NVP-BEZ235 (Selleck Chemicals, Houston, TX), NU7026 (Sigma, St Louis, MO), and KU55933 (EMD Chemicals, Darmstadt, Germany) were dissolved in dimethyl sulfoxide (DMSO), and 10-mM stocks were stored at -20°C. Cells were treated with drugs for 1 hour before irradiation.
Irradiation of Cells
Cells were irradiated with gamma rays from a 137Cs source (JL Shepherd and Associates, CA) at the indicated doses. Subcutaneous tumors in mice were irradiated with an x-ray device (X-RAD 320, Precision X-ray, North Branford, CT; 300 kV, 12 mA, 1.65 mm Al) fitted with a specifically designed collimator providing a 1-cm-diameter field size iso-dose exposure. For laser irradiation, cells were microirradiated with a pulsed nitrogen laser (Spectra-Physics, Newport Corporation, Santa Clara, CA; 365 nm, 10 Hz) with output set at 75% of the maximum, as described [23].
Western Analyses and Immunofluorescence Staining
For Western blot analysis of ATM and DNA-PKcs, nuclear extracts were prepared as described [24]. For Western blot analysis of all other proteins, whole-cell extracts were prepared as described [25]. Western blot analysis was carried out as described [24]. Immunofluorescence (IF) staining of cells and tumor sections was performed as described [19]. Antibodies used were as follows: anti-phospho-Akt(S473), anti-Akt, anti-phospho-Chk2(T68), anti-phospho-p53(S15), and anti-phospho-S6(S235/236) (Cell Signaling, Danvers, MA); anti-actin and anti-ATM (Sigma); anti-Rad51, anti-cyclin A, anti-p53, and anti-53BP1 (Santa Cruz, Biotechnology, Santa Cruz, CA); anti-phospho-SMC1(S966), anti-SMC1, anti-phospho-KAP-1(S824), anti-KAP-1, anti-Chk2, and anti-H2AX (Bethyl Laboratories, Montgomery, TX); anti-γH2AX and pHistone-H3(S10) (Upstate, Billerica, MA); anti-phospho-ATM(S1981) (GenScript, Piscataway, NJ); anti-phospho-DNA-PKcs (S2056) (Abcam, Cambridge, MA); anti-DNA-PKcs (Thermo Fisher, Waltham, MA); anti-Ku80 (kind gift from Dr. B. Chen); HRP-conjugated secondary antibodies (Biorad, Hercules, CA); and Alexa488/568-conjugated secondary antibodies (Molecular Probes, Grand Island, NY). Primary antibodies were incubated overnight at 4°C, and secondary antibodies were incubated for 1 hour at room temperature for both IF and Western blot analysis. Antibody dilutions are provided in Table W1.
Colony Formation Assays
Cells were plated in triplicate onto 60-mm dishes (1000 cells per dish), treated with the indicated drugs, and irradiated 1 hour later with graded doses of radiation. At 16 hours after irradiation, drug-containing medium was replaced with drug-free medium. Surviving colonies were stained with crystal violet approximately 10 to 14 days later as described [19].
DSB Repair Assays
DSB repair rates were assessed by quantifying the rates of dissolution of 53BP1 foci after irradiation of cells with 1 Gy of gamma rays as described [19]. For quantifying Rad51 foci, cells were irradiated with 6 Gy of gamma rays and coimmunostained with Rad51 and cyclin A antibodies 3 hours later as described [23]. The average numbers of Rad51 foci for cyclin A-positive (S/G2) nuclei were determined after scoring at least 50 nuclei.
HR and NHEJ Assays
In the HR assay, GFP expression was quantified (by flow cytometry) in MCF7-DRGFP cells transfected with an I-SceI plasmid as described [26]. In the NHEJ assay, repair of site-specific I-SceI-induced breaks in engineered 293T cells by NHEJ results in the conversion of a GFP signal to RFP (quantified by flow cytometry) as described [27].
G2/M Checkpoint Assay
The G2/M checkpoint was evaluated by quantifying histone H3 phosphorylation by flow cytometry as described [23].
DNA-PKcs Kinase Assays
In vitro kinase assays were carried out with purified DNA-PKcs (Invitrogen, Grand Island, NY) using GST-p53 (Santa Cruz) as substrate as described [28]. Sheared herring testis DNA (Clontech, Mountain View, CA) was added to the DNA-PKcs kinase assays to stimulate kinase activity.
Mouse Tumor Studies
Tumors were generated by subcutaneous injection of U87 cells overexpressing EGFRvIII [19] into 6-week-old female Nu/Nu mice. Once these tumors reached an average size of 150 mm3, mice were treated with a single dose of 45 mg/kg NVP-BEZ235 by oral gavage or with vehicle (NMP/polyethylene glycol 300; 10:9 vol/vol) as control. Two hours later, tumors were irradiated with 2 Gy of x-rays, and tumors were excised at the indicated time points. All animal studies were performed under protocols approved by the Institutional Animal Care and Use Committee of UT Southwestern Medical Center.
Statistical Analyses
Statistical significance was determined by a 2-tailed t test using GraphPad Prism software (San Diego, CA; **P < .01, ***P < .001). Error bars represent the SEM for all plots.
Results
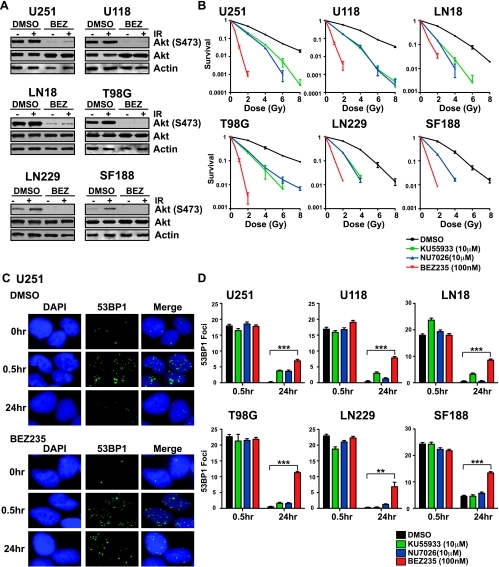
Because PI3K-Akt signaling has been shown to promote DSB repair in GBM cells and tumors [15–19], we assessed whether NVP-BEZ235 could radiosensitize human GBM cells by inhibiting DNA repair. We chose a panel of six GBM lines, of which four (U251, U118, LN18 and T98G) exhibit high levels of activation of the PI3K-Akt pathway as evidenced by high levels of Akt phosphorylation (Ser473), whereas two (SF188 and LN229) exhibit lower levels of Akt activation (Figure 1A). Radiation survival was measured by the colony formation assay. We chose a concentration of 100 nM NVP-BEZ235 for colony survival assays because this was the highest dose at which plating efficiency was largely unaffected for most cell lines (Table W2). We observed significant attenuation of Akt signaling with NVP-BEZ235 treatment at 100 nM, in accord with previous reports [3,5,6,8,11] (Figure 1A). We tested the radiosensitizing potential of NVP-BEZ235 (100 nM) compared with the established radiosensitizers and DNA repair inhibitors KU55933 (ATM inhibitor, 10 µM) [21] and NU7026 (DNA-PKcs inhibitor, 10 µM) [20]. We found that NVP-BEZ235 elicited a significantly greater degree of radiosensitization compared with KU55933 or NU7026, and this was consistent among all cell lines irrespective of their Akt activation status (Figure 1B). Radiosensitization was drug dose dependent with a lesser degree of sensitization seen with lower concentrations of NVP-BEZ235 (Figure W1). As indicated by IF staining for 53BP1 foci [19] (Figure 1C), all glioma lines could complete DSB repair by 24 hours after irradiation. However, NVP-BEZ235-treated cells showed higher numbers of unresolved foci at 24 hours (Figure 1D), which correlates with the high degree of radiosensitization observed.
Figure 1.
The dual PI3K/mTOR inhibitor NVP-BEZ235 potently impairs DSB repair and confers significant radiosensitization in a panel of human GBM lines. (A) Inhibition of PI3K/Akt signaling in mock-irradiated or irradiated human GBM lines after treatment with 100 nM NVP-BEZ235 was analyzed by Western blot analysis with α-phospho-Akt (Ser473) antibody. Cells were irradiated (10 Gy) after 1 hour of drug treatment and harvested at 30 minutes after IR. (B) Radiation survival of GBM lines after treatment with the indicated concentrations of NVP-BEZ235 (PI3K/mTOR inhibitor), KU55933 (ATM inhibitor), NU7026 (DNA-PKcs inhibitor), or DMSO (solvent) was quantified by colony formation assays. The fraction of surviving colonies (y axis) was plotted against corresponding radiation dose (x axis). (C) GBM cells were irradiated with 1 Gy of gamma rays after a 1-hour period of drug treatment. Cells were immunostained for 53BP1 foci (green) at 0.5 and 24 hours after IR. Nuclei were stained with DAPI (blue). Representative pictures are shown for DMSO- or NVP-BEZ235-treated U251 cells. (D) 53BP1 foci were scored at 0.5 and 24 hours after IR (average of 50 nuclei), and after subtracting background (number of foci in mock-irradiated nuclei), the average foci per nucleus was plotted against the indicated times.
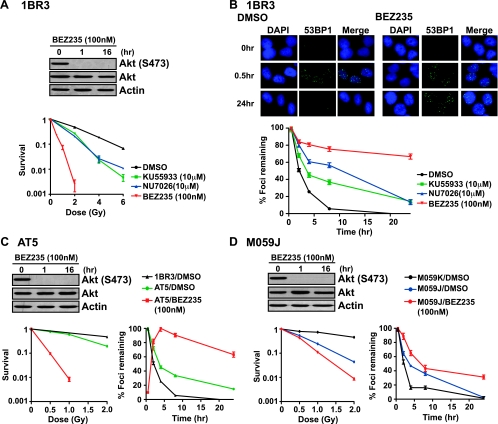
Because the effect of NVP-BEZ235 on DSB repair and radioresistance was significantly greater than what has been previously observed due to inactivation of PI3K-Akt signaling [15–19], we hypothesized that this compound might be inhibiting other kinases in addition to PI3K and mTOR. The most likely candidates, given the striking inhibition of DSB repair, are the DSB-responsive kinases, ATM and DNA-PKcs, whose catalytic domains are highly homologous to that of PI3K and mTOR [1,12]. We therefore examined the effects of NVP-BEZ235 in wild-type (1BR3) and ATM-deficient (AT5) human fibroblasts [23] as well as in DNA-PKcs-proficient (MO59K) and -deficient (MO59J) human glioma lines [29]. We found that NVP-BEZ235 could sensitize 1BR3 cells to IR, and the degree of sensitization was significantly greater than that seen with KU55933 or NU7026 (Figure 2A). As expected, NU7026 and KU55933 treatments resulted in attenuated DSB repair, consistent with the role of DNA-PKcs in NHEJ [30] and the role of ATM in promoting HR [31] and heterochromatic DSB repair [32] (Figure 2B). Strikingly, NVP-BEZ235 treatment resulted in a repair defect that was much more severe than that seen with either NU7026 or KU55933 and affected both “early” and “late” phases of DSB repair [32], with almost 70% of breaks remaining unrepaired at 24 hours after irradiation.
Figure 2.
NVP-BEZ235 impairs both “early” and “late” phases of DSB repair and can exacerbate the repair defects of ATM- and DNA-PKcs-null cells. (A) Radiation survival of wild-type 1BR3 fibroblasts on treatment with the indicated concentrations of NVP-BEZ235, KU55933, NU7026, or DMSO was quantified by colony formation assays. The fraction of surviving colonies (y axis) was plotted against the corresponding radiation dose (x axis). Inhibition of PI3K/Akt signaling after NVP-BEZ235 treatment for 1 hour or 16 hours was analyzed by Western blot analysis with α-phospho-Akt (Ser473) antibody. (B) DSB repair kinetics of 1BR3 cells irradiated with 1 Gy of gamma rays after a 1-hour period of drug treatment. Cells were immunostained for 53BP1 foci (green), and nuclei were stained with DAPI (blue). Representative pictures are shown for DMSO- or NVP-BEZ235-treated 1BR3 cells. 53BP1 foci were scored at the indicated times after IR, and percentage foci remaining was plotted against time. (C) Radiation sensitivity and DSB repair kinetics of ATM-deficient AT5 cells on treatment with NVP-BEZ235. (D) Radiation sensitivity and DSB repair kinetics of DNA-PKcs-proficient (M059K) and -deficient (M059J) cells on treatment with NVP-BEZ235.
These data suggest that the profound radiosensitization conferred by NVP-BEZ235 is due to the inhibition of more than one DNA repair pathway. Indeed, we found that NVP-BEZ235 could further radiosensitize ATM-null [23] and DNA-PKcs-null [29] cell lines, indicating that the radiosensitizing effect of the drug was not due to its effect on just one PI3KK i.e., either ATM or DNA-PKcs (Figure 2, C and D). Similarly, the DSB repair defects of both lines could be further exacerbated after NVP-BEZ235 treatment (Figure 2, C and D), clearly indicating that this compound can block multiple PI3KK family members.
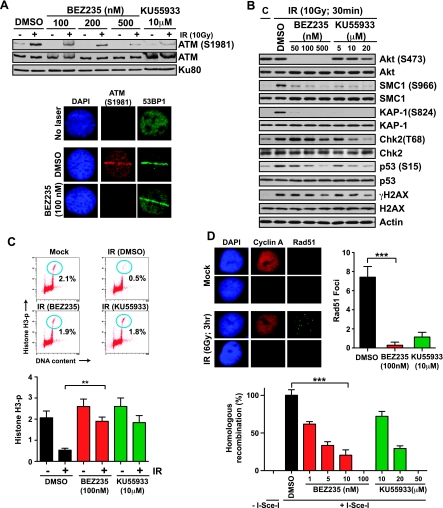
Among the PI3KK family members, ATM plays a central role in the mammalian DNA damage response (DDR), triggering cell cycle arrest and promoting DSB repair [33]. To examine if NVP-BEZ235 attenuates the activation of ATM, we irradiated 1BR3 cells and analyzed the autophosphorylation of ATM at Ser1981 by Western blot analysis [35]. We found that NVP-BEZ235 attenuated IR-induced activation of ATM, similar to the specific ATM inhibitor KU55933 (Figure 3A, top panel). Moreover, autophosphorylation of ATM at the sites of micro-laser-induced DSBs [23] was reduced by NVP-BEZ235 pretreatment (Figure 3A, bottom panel). We irradiated 1BR3 cells after pretreatment with NVP-BEZ235 and, by Western blot analysis with phospho-specific antibodies as described [23], assessed the phosphorylation status of the following key ATM substrates: Chk2 (Thr68), SMC1 (Ser966), p53 (Ser15), KAP-1 (Ser824), and H2AX (Ser139). Phosphorylation of all of these ATM substrates was attenuated (to varying extents) by pretreatment with NVP-BEZ235, similar to that seen with KU55933 (Figure 3B). We validated the biologic significance of this inhibition by examining the G2/M cell cycle checkpoint in 1BR3 cells as described [23]. The G2/M block manifests as a decrease in M-phase cells at 2 hours after irradiation, and this was attenuated by NVP-BEZ235 to the same extent seen with KU55933 (Figure 3C). In addition to checkpoint signaling, ATMplays an important role in DSB repair by HR in S/G2 phases [34]. Consistent with inhibition of HR, we found that IR-induced Rad51 foci formation was attenuated in 1BR3 cells pretreated with NVP-BEZ235 similar to that seen with KU555933 treatment (Figure 3D, top panel). Abrogation of HR by NVP-BEZ235 was also seen in a GFP-based assay, which measures reconstitution of a GFP gene by HR after the induction of DSBs by I-SceI [26] (Figure 3D, bottom panel). These results indicate that NVP-BEZ235 inhibits ATM activation, ATM-mediated phosphorylation events, cell cycle checkpoints, and HR.
Figure 3.
NVP-BEZ235 impairs ATM activation, phosphorylation of ATM substrates, G2/M cell cycle checkpoint, and HR repair. (A) Top panel. 1BR3 cells were pretreated with the indicated concentrations of NVP-BEZ235 or KU55933 and irradiated with 10 Gy of gamma rays. Autophosphorylation of ATM was assayed after 30 minutes by Western blot analysis with a α-phospho-ATM (Ser1981) antibody. Bottom panel. 1BR3 cells, pretreated with DMSO or NVP-BEZ235, were laser microirradiated and co-IF stained with α-phospho-ATM (Ser1981) antibody (red) and α-53BP1 antibody (green) after 30 minutes. (B) 1BR3 cells were pretreated for 1 hour with the indicated concentrations of NVP-BEZ235 or KU55933 and irradiated with 10 Gy of gamma rays. Phosphorylation of key DDR proteins was assayed after 30 minutes by Western blot analysis with phospho-specific antibodies as indicated. (C) IR-induced G2/M checkpoint in 1BR3 cells treated with NVP-BEZ235 or KU55933 was analyzed by dual-parameter flow cytometry. Representative distributions show staining for DNA content (x axis) and for histone H3 phosphorylation (y axis); cells in M phase are demarcated with blue circles. Percent cells in M-phase in mock-irradiated and irradiated (4 Gy) cells are plotted. The G2/M checkpoint manifests as a decrease in mitotic cells at 2 hours after irradiation in DMSO-treated cells; this decrease is not seen in NVP-BEZ235- or KU55933-treated cells. (D) Top panel. Representative image of gamma-irradiated 1BR3 cells costained with α-cyclin A antibody (red) and α-Rad51 antibody (green) after 3 hours. Nuclei are stained with DAPI (blue). Average numbers of Rad51 foci for cyclin A-positive (S/G2) nuclei at 3 hours after irradiation are plotted for 1BR cells pretreated with NVP-BEZ235 or KU55933. Bottom panel. HR was measured by quantifying GFP expression (by flow cytometry) in MCF7-DRGFP cells transfected with an I-SceI plasmid in the presence of NVP-BEZ235 or KU55933 as indicated. Plots show percentages of drug-treated cells expressing GFP relative to DMSO-treated cells. GFP expression after transfection with a control plasmid with full-length GFP was quantified to ensure that transfection efficiencies were comparable between the different drug treatments.
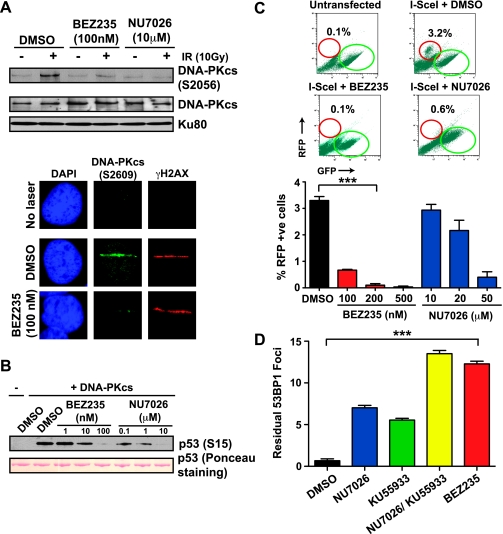
Apart from ATM, the other PI3KK family member that responds directly to DSBs is DNA-PKs, a key enzyme in the NHEJ pathway of DSB repair [30]. To investigate whether NVP-BEZ235 also inhibits the activation of DNA-PKcs, we irradiated 1BR3 cells and examined DNA-PKcs autophosphorylation at Ser2056 by Western blot analysis [35]. We found that NVP-BEZ235 attenuated IR-induced activation of DNA-PKcs, similar to the specific DNA-PKcs inhibitor NU7026 (Figure 4A, top panel). Similarly, autophosphorylation of DNA-PKcs at the sites of micro-laser-induced DSBs [23] was impaired on NVP-BEZ235 pretreatment (Figure 4A, bottom panel). To determine whether NVP-BEZ235 can directly block DNA-PKcs kinase activity, we carried out in vitro kinase assays with purified DNA-PKcs using GST-p53 (1–393) as a substrate [28]. DNA-PKcs efficiently phosphorylated p53 at Ser15 in vitro, and this was attenuated by NVP-BEZ235, indicating that NVP-BEZ235 can directly block DNA-PKcs, similar to NU7026 (Figure 4B). Because DNA-PKcs is a key enzyme in the NHEJ pathway of DSB repair [30], we used a GFP to RFP-conversion assay to investigate if NVP-BEZ235 might block NHEJ in addition to attenuating HR [27]. We found that NVP-BEZ235 could potently block NHEJ (no RFP signal after transfection with an I-SceI-expressing plasmid) (Figure 4C). These results clearly indicate that NVP-BEZ235 potently blocks both ATM and DNA-PKcs, resulting in a DSB repair defect that is more striking than that seen on the inhibition of ATM or DNA-PKcs alone. Indeed, blocking both ATM and DNA-PKcs by combining KU55933 and NU7026 resulted in greater numbers of unrepaired DSBs, similar to that seen with NVP-BEZ235 alone, both in 1BR3 cells (Figure 4D) as well as in the panel of GBM cell lines (Figure W2). Given the potential cross-talk between ATM and DNA-PKcs [36], we investigated whether NVP-BEZ235 could attenuate IR-induced ATM activation in DNA-PKcs-null M059J cells and DNA-PKcs activation in ATM-null AT5 cells. We found inhibition of kinase activation in both cell lines, demonstrating that this drug can independently block either kinase (Figure W3). We also examined ATM and DNA-PKcs activation in the panel of glioma lines that were radiosensitized by NVP-BEZ235 (Figure 1B) and observed inhibition of both ATM (Figure W4) and DNA-PKcs (Figure W5) to varying extents. Taken together, these data implicate the impairment of both HR and NHEJ repair pathways, due to inhibition of both ATM and DNA-PKcs, as the underlying mechanism behind the profound radiosensitization conferred by NVP-BEZ235.
Figure 4.
NVP-BEZ235 impairs DNA-PKcs activation and NHEJ. (A) Top panel. 1BR3 cells were pretreated with NVP-BEZ235 or NU7026 and irradiated with 10 Gy of gamma rays. Autophosphorylation of DNA-PKcs was assayed after 30 minutes by Western blot analysis with a α-phospho-DNA-PKcs (Ser2056) antibody. Bottom panel. 1BR3 cells, pretreated with DMSO or NVP-BEZ235, were laser microirradiated and co-IF stained with α-phospho-DNA-PKcs (Ser2056) antibody (green) and α-γH2AX antibody (red) after 30 minutes. (B) In vitro kinase assays were carried out with purified DNA-PKcs incubated with recombinant GST-p53 (1–393) in the presence of sheared herring testis DNA and ATP. Increasing concentrations of NVP-BEZ235 or NU7026 were added to the reaction mixture as indicated. Phosphorylation of p53 by DNA-PKcs was assessed by Western blot analysis with α-phospho-p53 (Ser15) antibody. (C) The NHEJ assay was initiated by the transfection of an I-SceI plasmid into engineered 293T cells harboring a GFP gene (flanked by I-SceI sites) followed by a RFP gene. A switch from GFP to RFP expression indicates ligation of I-SceI-generated breaks by NHEJ. Representative distributions show RFP +ve cells (red circles) and GFP +ve cells (green circles) for different drug treatments; percentages of RFP +ve cells are indicated and are plotted for cells treated with increasing concentrations of NVP-BEZ235 or NU7026. (D) 1BR3 cells were irradiated with 1 Gy of gamma rays after a 1-hour period of treatment with NVP-BEZ235, KU55933, or NU7026 alone or with a combination of KU55933 and NU7026, as indicated. To quantify residual (unrepaired) DSBs, cells were immunostained for 53BP1 foci at 24 hours after IR. Residual 53BP1 foci in irradiated cells were scored (average of 50 nuclei), and after subtracting background (number of foci in mock-irradiated nuclei), average foci per nucleus were plotted against the indicated treatment conditions.
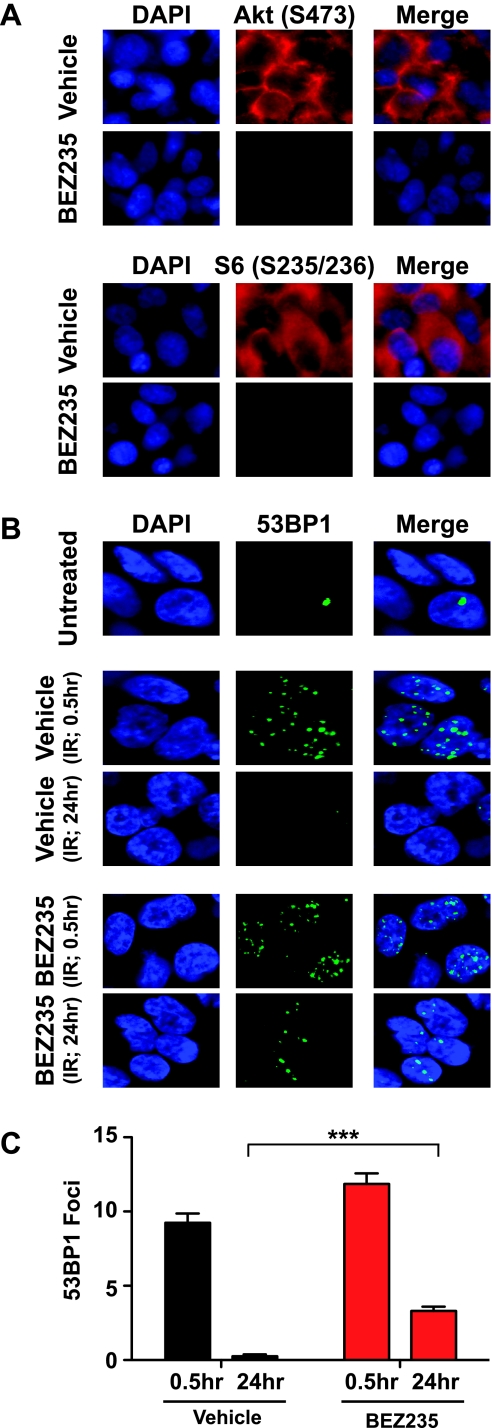
Finally, to examine the effect of NVP-BEZ235 on DSB repair in tumors, we generated subcutaneous tumors in Nu/Nu mice using U87 cells overexpressing EGFRvIII [19]. We first confirmed that NVP-BEZ235 could inhibit Akt activation and block DSB repair in U87-EGFRvIII cells in culture (Figure W6). Next, tumor-bearing mice were treated with a single dose of 45 mg/kg NVP-BEZ235 or with vehicle as control. Tumors were mock irradiated or irradiated (2 Gy of x-rays) 2 hours later, collected at 0.5 and 24 hours after IR, and sectioned for IF. Tumors from NVP-BEZ235-treated mice exhibited a marked reduction in the phosphorylation of Akt (Ser473) and abrogation of phosphorylation of an mTOR substrate, the ribosomal protein S6 (Ser235/236), thereby confirming intratumoral delivery of the drug and consequent inhibition of the PI3K-Akt-mTOR pathway [7] (Figure 5A). Irradiated NVP-BEZ235- or vehicle-treated tumor sections were IF stained for 53BP1 foci as described [19]. Vehicle-treated tumors were able to completely repair radiation-induced DSBs by 24 hours after IR. Interestingly, NVP-BEZ235-treated tumors exhibited higher levels of unresolved 53BP1 foci at 24 hours after IR, indicating attenuation of DSB repair (Figure 5, B and C). These results unequivocally demonstrate that the striking inhibition of DSB repair by NVP-BEZ235 is also valid in a tumor setting.
Figure 5.
NVP-BEZ235 impairs the repair of IR-induced DNA damage in tumors. (A) Nu/Nu mice bearing subcutaneous tumors (U87-EGFRvIII) were treated with NVP-BEZ235 (45 mg/kg) or vehicle alone and tumors were irradiated (2 Gy) 2 hours after drug treatment. Tumors were collected at 0.5 or 24 hours after IR and sectioned for IF staining. Tumor sections were stained for phosphorylation of Akt (Ser473) and ribosomal protein S6 (Ser235/236) to confirm intratumoral drug delivery and consequent inhibition of the PI3K-Akt-mTOR pathway. (B) Tumor sections were IF stained for 53BP1 foci (green) to visualize radiation-induced DSBs (at 0.5 hours) and residual breaks (at 24 hours). Representative pictures are shown for NVP-BEZ235- or vehicle-treated tumors. (C) 53BP1 foci were scored at 0.5 and 24 hours after IR (average of 50 nuclei), and after subtracting background (average number of foci in mock-irradiated tumors), average foci per nucleus were plotted against the indicated times. Please note residual 53BP1 foci in NVP-BEZ235-treated tumors, confirming that the impairment of DSB repair by NVP-BEZ235 is also valid in a tumor setting.
Discussion
Using multiple approaches, we find that NVP-BEZ235, a drug already in clinical trials, can inhibit IR-induced activation of ATM and DNA-PKcs, the two major kinases responding to DSBs. This results in inhibition of DSB repair, attenuation of cell cycle arrest, and profound radio sensitization. In the original report describing the compound, the authors examined the effects of NVP-BEZ235 only on doxorubicin-induced phosphorylation of ATM(Ser1981) and DNA-PKcs (Thr2609) and found attenuation only at high concentrations of the drug (1250 nm); no other end points were analyzed, and it was inferred that the compound has no cross-reactivity toward these two kinases [3]. However, we observe attenuation of ATM (Ser1981) and DNA-PKcs (Ser2056) autophosphorylations at low concentrations (100 nM) of NVP-BEZ235. Differences in cell lines, phospho-specific antibodies, DNA-damaging agents, and duration of incubation with drugs probably underlie these discrepancies. It is also important to point out that, as DNA-PKcs is phosphorylated at Thr2609 by ATM, Ser2056 autophosphorylation might be a better indicator of DNA-PKcs activity in vivo [36]. Regardless of the extent of inhibition of ATM and DNA-PKcs autophosphorylation, our results clearly show that a low concentration of NVP-BEZ235 is sufficient to block key DDR events triggered by ATM and DNA-PKcs — phosphorylation of DDR proteins, implementation of cell cycle checkpoints, and repair of IR-induced DSBs by HR and NHEJ. Moreover, we show that NVP-BEZ235 inhibits purified DNA-PKcs in vitro indicating that the observed effects of NVP-BEZ235 are likely due to a direct blockage of the kinase activity of this protein.
Cross-inhibition of ATM and DNA-PKcs by a dual PI3K-mTOR inhibitor is not surprising because these kinases have homologous catalytic domains [1,12]. In accord with our results, a previous report demonstrated that NVP-BEZ235 could radiosensitize non-small cell lung carcinoma cells expressing oncogenic K-RAS and this correlated with higher levels of IR-induced DNA breaks, although the underlying mechanism(s) were not investigated [8]. Also, an earlier report demonstrated inhibition of DNA-PKcs kinase activity by NVP-BEZ235 in vitro, although the radiosensitizing effect of this drug was not tested in this study [37]. At the time this article was in preparation, NVP-BEZ235 was shown to induce senescence in irradiated cancer cells by inhibiting DNA-PKcs thereby supporting our data showing radiosensitization by this drug due to inhibition of both ATM and DNA-PKcs [38]. Moreover, NVP-BEZ235 was recently identified in a cell-based screen as a potent ATR inhibitor [39]. Like ATM and DNA-PKcs, ATR also belongs to the PI3KK family and responds to ssDNA generated by the stalling of replication forks or by the resection of DSBs [12]. Our findings, in combination with these reports, unequivocally demonstrate that NVP-BEZ235 is a potent inhibitor of all members of the PI3KK family that respond to IR-induced DNA breaks, either directly (ATM and DNA-PKcs) or indirectly (ATR), resulting in profound radiosensitization at low concentrations of the drug.
Our study not only shows that NVP-BEZ235 is a novel inhibitor of both ATM and DNA-PKcs but also establishes that this drug is significantly more potent than specific inhibitors of ATM and DNA-PKcs that are being optimized for use as radiosensitizers in the clinic [22]. A very high degree of radiosensitization was seen with 100-fold lower concentrations of NVP-BEZ235 (100 nM) relative to KU55933 (10 µM) or NU7026 (10 µM). Indeed, radiosensitization by NVP-BEZ235 was greater than the higher degree of sensitization achieved by combining both ATM and DNA-PKcs inhibitors (data not shown), indicating that radiosensitization by NVP-BEZ235 is not just due to combined inhibition of both DSB-responsive kinases. Thus, the extreme radiosensitivity conferred by NVP-BEZ235 is clearly a combinatorial effect of disabling multiple pathways impinging on 1) DSB repair (due to DNA-PKcs and ATM inhibition), 2) cell cycle checkpoints (due to ATM and ATR inhibition), and 3) cell survival (due to PI3K and mTOR inhibition). These results have two important clinical implications. On one hand, considering the significant impairment of DDRs resulting from NVP-BEZ235 treatment, combining the drug with genotoxic chemotherapy could result in significant systemic toxicity and limit therapeutic gain. On the other hand, radiotherapy, like surgery, is a local treatment and its efficacy could be significantly enhanced by the use of potent radiosensitizers like NVP-BEZ235, which confers radiosensitization even at radiation doses used in conventional fractionation schemes (1.8–2 Gy per fraction). Thus, highly conformal techniques, such as intensity-modulated radiotherapy, might be able to exploit the radiosensitizing potential of the drug while minimizing normal tissue toxicity. Current inhibitors of DNA-PKcs or ATM have not yet reached clinical trials; however, NVP-BEZ235 is approved for phase 1/2 trials, and some preliminary clinical data already exist showing that the drug is well tolerated [40]. Thus, it can be more readily tested as a radiosensitizer for GBMs and other solid tumors in the clinic. Further preclinical evaluation is warranted regarding the effects of NVP-BEZ235 in combination with chemotherapy or radiation in mouse tumor models of GBMs and other cancers.
Supplementary Material
Acknowledgments
The authors thank Matthew Porteus for providing cells for the NHEJ assay and Kum Kum Khanna for cells for the HR assay. The authors also thank David Chen for facilitating laser microirradiation experiments and Donglai Qi for preliminary work on the DNA-PKcs kinase assay.
Footnotes
S.B. is supported by grants from the National Institutes of Health (RO1 CA149461), National Aeronautics and Space Administration (NNX10AE08G), and the Cancer Prevention and Research Institute of Texas (RP100644).
This article refers to supplementary materials, which are designated by Tables W1 and W2 and Figures W1 to W6 and are available online at www.neoplasia.com.
References
- 1.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 3.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo anti-tumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 4.Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, Wang S, Garcia-Echeverria C, Maira SM. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci USA. 2009;106:22299–22304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiarini F, Grimaldi C, Ricci F, Tazzari PL, Evangelisti C, Ognibene A, Battistelli M, Falcieri E, Melchionda F, Pession A, et al. Activity of the novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 against T-cell acute lymphoblastic leukemia. Cancer Res. 2010;70:8097–8107. doi: 10.1158/0008-5472.CAN-10-1814. [DOI] [PubMed] [Google Scholar]
- 6.Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, Beijersbergen RL, Valero V, Seoane J, Bernards R, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konstantinidou G, Bey EA, Rabellino A, Schuster K, Maira MS, Gazdar AF, Amici A, Boothman DA, Scaglioni PP. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69:7644–7652. doi: 10.1158/0008-5472.CAN-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu TJ, Koul D, LaFortune T, Tiao N, Shen RJ, Maira SM, Garcia-Echevrria C, Yung WK. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–2210. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnell CR, Stauffer F, Allegrini PR, O'Reilly T, McSheehy PM, Dartois C, Stumm M, Cozens R, Littlewood-Evans A, Garcia-Echeverria C, et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Res. 2008;68:6598–6607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- 11.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 12.Abraham RT. PI 3-kinase related kinases: “big” players in stress-induced signaling pathways. DNA Repair (Amst) 2004;3:883–887. doi: 10.1016/j.dnarep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee B, Choy H, Nirodi C, Burma S. Targeting nonhomologous end-joining through epidermal growth factor receptor inhibition: rationale and strategies for radiosensitization. Semin Radiat Oncol. 2010;20:250–257. doi: 10.1016/j.semradonc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 15.Golding SE, Morgan RN, Adams BR, Hawkins AJ, Povirk LF, Valerie K. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8:730–738. doi: 10.4161/cbt.8.8.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Z, Pore N, Cerniglia GJ, Mick R, Georgescu MM, Bernhard EJ, Hahn SM, Gupta AK, Maity A. Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer Res. 2007;67:4467–4473. doi: 10.1158/0008-5472.CAN-06-3398. [DOI] [PubMed] [Google Scholar]
- 17.Kao GD, Jiang Z, Fernandes AM, Gupta AK, Maity A. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem. 2007;282:21206–21212. doi: 10.1074/jbc.M703042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEllin B, Camacho CV, Mukherjee B, Hahm B, Tomimatsu N, Bachoo RM, Burma S. PTEN loss compromises homologous recombination repair in astrocytes: implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res. 2010;70:5457–5464. doi: 10.1158/0008-5472.CAN-09-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee B, McEllin B, Camacho CV, Tomimatsu N, Sirasanagandala S, Nannepaga S, Hatanpaa KJ, Mickey B, Madden C, Maher E, et al. EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009;69:4252–4259. doi: 10.1158/0008-5472.CAN-08-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veuger SJ, Curtin NJ, Richardson CJ, Smith GC, Durkacz BW. Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 2003;63:6008–6015. [PubMed] [Google Scholar]
- 21.Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 22.Ding J, Miao ZH, Meng LH, Geng MY. Emerging cancer therapeutic opportunities target DNA-repair systems. Trends Pharmacol Sci. 2006;27:338–344. doi: 10.1016/j.tips.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Tomimatsu N, Mukherjee B, Burma S. Distinct roles of ATR and DNA-PKcs in triggering DNA damage responses in ATM-deficient cells. EMBO Rep. 2009;10(6):629–635. doi: 10.1038/embor.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson SR, Kurimasa A, Oshimura M, Dynan WS, Bradbury EM, Chen DJ. Loss of the catalytic subunit of the DNA-dependent protein kinase in DNA double-strand-break-repair mutant mammalian cells. Proc Natl Acad Sci USA. 1995;92:3171–3174. doi: 10.1073/pnas.92.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Anna JA, Valdez JG, Habbersett RC, Crissman HA. Association of G1/S-phase and late S-phase checkpoints with regulation of cyclin-dependent kinases in Chinese hamster ovary cells. Radiat Res. 1997;148:260–271. [PubMed] [Google Scholar]
- 26.Bolderson E, Tomimatsu N, Richard DJ, Boucher D, Kumar R, Pandita TK, Burma S, Khanna KK. Phosphorylation of Exo1 modulates homologous recombination repair of DNA double-strand breaks. Nucleic Acids Res. 2010;38(6):1821–1831. doi: 10.1093/nar/gkp1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma GG, So S, Gupta A, Kumar R, Cayrou C, Avvakumov N, Bhadra U, Pandita RK, Porteus MH, Chen DJ, et al. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol Cell Biol. 2010;30:3582–3595. doi: 10.1128/MCB.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurimasa A, Kumano S, Boubnov NV, Story MD, Tung CS, Peterson SR, Chen DJ. Requirement for the kinase activity of human DNA-dependent protein kinase catalytic subunit in DNA strand break rejoining. Mol Cell Biol. 1999;19:3877–3884. doi: 10.1128/mcb.19.5.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee B, Kessinger C, Kobayashi J, Chen BP, Chen DJ, Chatterjee A, Burma S. DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair (Amst) 2006;5:575–590. doi: 10.1016/j.dnarep.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Burma S, Chen DJ. Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA Repair (Amst) 2004;3:909–918. doi: 10.1016/j.dnarep.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Morrison C, Sonoda E, Takao N, Shinohara A, Yamamoto K, Takeda S. The controlling role of ATM in homologous recombinational repair of DNA damage. EMBO J. 2000;19:463–471. doi: 10.1093/emboj/19.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodarzi AA, Jeggo P, Lobrich M. The influence of heterochromatin on DNA double strand break repair: getting the strong, silent type to relax. DNA Repair (Amst) 2010;9:1273–1282. doi: 10.1016/j.dnarep.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 34.Beucher A, Birraux J, Tchouandong L, Barton O, Shibata A, Conrad S, Goodarzi AA, Krempler A, Jeggo PA, Lobrich M. ATM and Artemis promote homologous recombination of radiation-induced DNA double-strand breaks in G2. EMBO J. 2009;28:3413–3427. doi: 10.1038/emboj.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee B, Camacho CV, Tomimatsu N, Miller J, Burma S. Modulation of the DNA-damage response to HZE particles by shielding. DNA Repair (Amst) 2008;7:1717–1730. doi: 10.1016/j.dnarep.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Chen BP, Uematsu N, Kobayashi J, Lerenthal Y, Krempler A, Yajima H, Lobrich M, Shiloh Y, Chen DJ. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J Biol Chem. 2007;282:6582–6587. doi: 10.1074/jbc.M611605200. [DOI] [PubMed] [Google Scholar]
- 37.Kong D, Yaguchi S, Yamori T. Effect of ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor, on DNA-dependent protein kinase. Biol Pharm Bull. 2009;32:297–300. doi: 10.1248/bpb.32.297. [DOI] [PubMed] [Google Scholar]
- 38.Azad A, Jackson S, Cullinane C, Natoli A, Neilsen PM, Callen DF, Maira SM, Hackl W, McArthur GA, Solomon B. Inhibition of DNA-dependent protein kinase induces accelerated senescence in irradiated human cancer cells. Mol Cancer Res. 2011;9(12):1696–1707. doi: 10.1158/1541-7786.MCR-11-0312. [DOI] [PubMed] [Google Scholar]
- 39.Toledo LI, Murga M, Zur R, Soria R, Rodriguez A, Martinez S, Oyarzabal J, Pastor J, Bischoff JR, Fernandez-Capetillo O. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat Struct Mol Biol. 2011;18(6):721–727. doi: 10.1038/nsmb.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peyton JD, Rodon Ahnert J, Burris H, Britten C, Chen LC, Tabernero J, Duval V, Rouyrre N, Silva AP, Quadt C, et al. A dose-escalation study with the novel formulation of the oral pan-class I PI3K inhibitor BEZ235, solid dispersion system (SDS) sachet, in patients with advanced solid tumors. J Clin Oncol. 2011;29 ASCO Annual Meeting Proceedings, Abstract 3066. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.