Abstract
A hallmark of tumor cell survival is the maintenance of elongated telomeres. It is known that antiviral reverse transcriptase inhibitors (RTIs) such as azidothymidine (AZT) and didanosine (ddI) lead to telomere shortening at high, potentially toxic concentrations. We hypothesized that those drugs might have synergistic effects enabling successful therapy with low, nontoxic concentrations. Biologic effects of AZT and ddI were analyzed at concentrations that correspond to minimal plasma levels achieved during human immunodeficiency virus therapy. Long-term coapplication of low-dose AZT and ddI induced a significant shortening of telomeres in the tumor cell lines HCT-116, SkMel-28, MelJuso, and Jurkat. Treatment of cells with both RTI, but not with single RTI, led to a significant accumulation of γH2AX, to p53 phosphorylation, and to cell apoptosis in all cell lines. Oral low-dose dual RTI application but not low-dose single RTI application was associated with a significantly reduced tumor growth of HCT-116 cells in mice. This antiproliferative activity of the combined use of AZT and ddI at low, clinically applicable concentrations warrants clinical testing in human solid cancer.
Introduction
Telomeres at the end of linear chromosomes contain tandem arrays of the GT-rich nucleotide sequence 5′-TTAGGG-3′ [1]. In normal somatic cells, telomeres generally shorten with every cell division [2]. Physiological erosion of telomeres induces cell senescence until critically shortened telomeres result in natural cell death [3,4]. On a molecular level, telomere shortening is associated with binding and phosphorylation of DNA damage recognition proteins such as the histone H2AX [5]. Cancer cells develop mechanisms that inhibit the physiological shortening of telomeres leading to the unlimited capacity to proliferate that inhibits not only cancer cell death but also cancer cell senescence. Thus, drugs leading to telomere shortening should allow selective purging of tumor cells [6–9].
Most approaches targeting telomeres in cancer cells have focused on the inhibition of telomerase [6], a ribonucleoenzyme promoting telomere elongation. Because telomerase is a reverse transcriptase, research initially focused on known nucleoside reverse transcriptase inhibitors (RTIs) such as azidothymidine (AZT) [6]. High concentrations of RTIs effectively inhibited telomerase activity [7–9]. The advantage of these drugs was their established pharmacodynamic and toxicity profile because RTIs were used for a long time as a cornerstone in human immunodeficiency virus (HIV) therapy. It was therefore reasonable to initially assess AZT in HIV-associated lymphomas [10]. Today, AZT is established as first-line therapy in human T-cell lymphatic virus type I-associated adult T-cell leukemia/lymphoma [11,12].
This prompted the investigation of the antiproliferative effect of AZT in other tumor entities. It had been established that short-term (2–3 days) AZT treatment induced telomere shortening [13–15] and cell cycle arrest [16] at high concentrations (>100 µM) [9,14,15]. Long-term treatment required the reduction of the biologically effective dose to concentrations below 100 µM [17,18]. However, animal data revealed only marginal effects of low-dose AZT monotherapy in solid cancers [19].
In HIV therapy, RTIs are usually given as a combination therapy. We have therefore hypothesized that synergistic effects of RTIs might also enhance their antiproliferative activity in a cancer setting. We reasoned that synergism might allow for a reduction in the required effective therapeutic dose of the drugs to concentrations that are well tolerated in humans. In support of our hypothesis, we found that prolonged treatment of different types of cancer cells with AZT in combination with didanosine (ddI) induced telomere attrition at concentrations applicable to humans. The RTI combination also significantly increased apoptosis in vitro and led to distinct therapeutic benefits in vivo.
Materials and Methods
Cell Cultures
Human colorectal carcinoma cells HCT-116, SkMel-28, and Jurkat cell lines were purchased from ATCC (Wesel, Germany). Melanoma cell line MelJuso was a gift from J. Lehman from the Institute of Immunology, University of Munich. HCT-116 and melanoma cell lines SkMel-28 and MelJuso were maintained in Dulbecco modified Eagle medium/F12 (GibcoLife Technologies, Grand Island, NY). Acute T-cell leukemia (Jurkat) cells were maintained in RPMI-1640 (GibcoLife). Media were supplemented with 10% fetal bovine serum (GibcoLife) and 1 mM L-glutamine (Sigma-Aldrich, Vienna, Austria). Cells were maintained at 37°C/5% CO2 and propagated twice weekly with 0.5% trypsin (GibcoLife). 3′-Azido-3′-deoxythymidine (AZT) and 2′,3′-dideoxyinosin (ddI) (Sigma-Aldrich) were dissolved in Dulbecco modified Eagle medium, and the inhibitor-containing medium was changed every 48 hours. Cells were harvested every 72 to 96 hours, counted in a Burker chamber (two counting sessions per sample), and replated at the same density.
Telomere Length Determination by Southern Blot
Genomic DNA was extracted from 1 x 107 cultured tumor cells using Maxwell 16 Cell and Tissue DNA Purification Kit (Promega, Madison, WI). Mean telomere length was measured using the Telo-TAGGG telomere length assay (Roche Diagnostics GmbH, Mannheim, Germany). Briefly, 1 µg of genomic DNA was digested with HinfI/RsaI mixture (5 U per restriction enzyme) and separated on 1% agarose gels. Fractionated DNA fragments were transferred onto nylon membrane (Roche Diagnostics GmbH) and hybridized with a digoxygenin-labeled telomere oligo-probe (TTAGGG)3. The hybridized probe was incubated with a digoxygenin-specific antibody covalently coupled to alkaline phosphatase. The immobilized telomere probe was visualized by a highly sensitive chemiluminescent substrate for alkaline phosphatase, and telomere DNA detection was finally achieved by the ChemiSmart 2000 chemiluminescence imaging system (Vilber Lourmat, Eberhardzell, Germany). The signal intensity was evaluated by densitometric analysis with ImageQuant software 5.0, Molecular Dynamics, Sunnyvale, CA, and the telomeric repeat fragments (TRFs), an indicator for mean telomere length of a sample, were calculated according to ∑(ODi)/∑(ODi/Li), where OD is the signal intensity and L is the length (kb) at the gel point i. Li represents the mean molecular size of 40 equal intervals of the telomeric smears in the range of 0.5 to 23 kb.
Real-time Polymerase Chain Assay
Mean telomere length was further measured from total genomic DNAbyusing areal-timequantitativepolymerasechain reaction (PCR) method previously described [20]. The premise of this assay is to measure an average telomere length ratio by quantifying telomeric DNA with specifically designed primer sequences and normalizing to the quantity of a single-copy gene.
Briefly, the telomere repeat copy number to single gene copy number (T/S) ratio was determined using an Applied Biosystems (Foster City, CA) 7500 ThermoCycler in a 96-well format. Twenty nanograms of genomic DNA or water as nontemplate control was distributed in a 96-well plate. The same volume of 2x Power SYBR Green MasterMix (Applied Biosystems, Life Technologies, Inchinnan, Scotland) was added with 400 nM each of forward and reverse primers to amplify the telomere repeats (T) or the 36B4 single-copy gene (S) in single-plex PCR assays. The telomere assay consisted of Tel-1 primer (CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT) and of Tel-2 primer (GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT). The 36B4 assay consisted of the 36B4u primer (CAGCAAGTGGGAAGGTGTAATCC) and the 36B4d primer (CCCATTCTATCATCAACGGGTACAA). The 36B4 gene, which encodes acidic ribosomal phosphoprotein PO, is located in chromosome 12 [21].
The reaction proceeded for 1 cycle at 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and at 60°C for 1 minute. All samples of telomere reactions were determined in triplicate, whereas single-copy gene (36B4) reactions were performed in duplicates, and the SD threshold for both reactions was set to 0.5. Results exceeding this deviation limit were excluded and repeated. The collected data of telomere repeats were evaluated by the ΔΔCt method [22], normalized to the single-copy gene 36B4 and set in relation to the untreated cells for reference resulting in relative quantity values for telomere DNA repeat content. PCR products of T and S reactions were validated by gel electrophoresis (data not shown).
Fluorescence In Situ Hybridization Analysis
For quantitative fluorescence in situ hybridization of interphase nuclei (Q-FISH), 1 x 106 cancer cells were seeded at a low density and cultured in growth medium with 10% fetal calf serum (FCS) for 48 hours. Colcemid (10 ng/ml) was given for 120 minutes, and nuclei were prepared according to standard methods. Telomere length based on telomere signals of individual chromosomes was detected using the Telomere PNA FISH kit/FITC (Dako Denmark A/S, Glostrup, Denmark) by essentially following the reported protocol [23]. Interphase nuclei were captured by confocal microscopy (63x 1.4 oil magnification) and were quantitatively evaluated with Telomere Measurement Software (obtained from British Columbia Cancer Research Centre).
Immunofluorescence Staining
Adherent cells were cultured on four-chamber coverslips (NUNC, lab-tek chamber slide with cover; Dako) for 48 hours to minimize stress that may arise during passage. Nonadherent cells (Jurkat) were seeded on coverslips by using a cytospin device. Cell density was 1 x 105 per slide. After preparing slides, all cells were washed and fixed in 4% formaldehyde for 10 minutes at 37°C. The coverslips were subsequently washed three times with PBS, and nonspecific binding sites were blocked in blocking solution (PBS containing 10% FCS, 0.3% Triton X-100, and 3% bovine serum albumin) for 30 minutes. Cells were then incubated with purified mouse anti-human p53 (Ser-15) (1:400 dilution 554294; BD Biosciences, Erembodegem, Belgium) or rabbit anti-γH2AX (Ser-139) (1:1000 dilution; Bethyl A300-031, Bethyl Laboratories, Inc, Montgomery, TX) in blocking solution for 60 minutes. Subsequently, cells were washed three times with PBS and incubated with goat anti-rabbit Alexa Fluor 568 (1:1000 dilution; Molecular Probes, Life Technologies, Inchinnan, Scotland) or rabbit antimouse Alexa Fluor 488 (1:1000 dilution; Molecular Probes) in blocking solution for 60 minutes at 37°C. The nuclei were stained with Draq5 (1:750 dilution; Sigma), washed with PBS, and mounted in a mounting medium for fluorescence (Vectashield; VectorLabs, Burlingame, CA). Images were captured with a confocal microscope at 40x 1.3 oil or 63x 1.4 oil magnification.
Cell Cycle and Cell Death Analysis Using Annexin V/Propidium Iodide Double Staining Assay
After treatment, 106 cancer cells were harvested, washed twice with PBS, and resuspended in 500 µl of PBS. Then cells were slowly immersed in 5 ml of 70% ethanol and frozen at -20°C for 2 hours. Cells were then stained with 200 µl of propidium iodide (PI; Sigma) dissolved in 10 ml of PBS and 2 mg of DNase-free RNase (Sigma) and 0.1% Triton-X for 15 minutes. Subsequently, cells were measured by FC500 flow cytometer (Becton Dickinson, Franklin Lakes, NJ). For annexin V staining, the harvested cells were adjusted to a concentration of 1 x 106/ml. One hundred microliters was stained with 5 µl of PI solution and 5 µl of FITC-conjugated annexin V (BD Pharmingen, Erembodegem, Belgium). Measurements were performed by flow cytometer and followed the protocol and the calculations of Multi-Cycle AV for Windows, Version 6.0 (Phoenix Flow Systems, Inc, San Diego, CA).
Tumor Xenografts and Treatment of Animals
Athymic nude mice (5 weeks old; Charles River, Sulzfeld, Germany), kept in accordance with the European Union guidelines, were inoculated subcutaneously with 2 x 106 HCT-116 colon carcinoma cells in 100 µl of PBS. AZT and ddI were dissolved in ddH2O and added to the drinking water. Animals received AZT at a concentration of 10 mg/kg and ddI at 20 mg/kg, respectively, with daily changes of the drinking water. Mice were randomized to treatment groups at the day of tumor inoculation. Treatment started 14 days after tumor implantation when tumor size was ∼35 mm3. The total number of animals in each treatment group was 8. Tumor growth was monitored every third day by caliper measurements; volumes were calculated using the formula: length x length x width x 0.52 [24]. After reaching a tumor volume of 600 mm3, the mice were killed. Tumors were excised and fixed in formaldehyde.
Immunohistochemistry
To detect the Ki-67 antigen in formalin-fixed/paraffin-embedded tissue sections, slides were deparaffinized in xylene and rehydrated in graded ethanol. Ten-micrometer-thick sections were immersed in a trypsin buffer solution (0.05% trypsin, 1 mM CaCl2, PBS) for 15 minutes at 37°C. Slides were incubated with an anti-Ki-67 antibody (1:250 dilution; Abcam, Cambridge, UK). The nonspecific staining was blocked by incubation for 30 minutes with 10% FCS. The sections were incubated with Alexa Fluor 568 as a secondary antibody (1:1000 dilution; Molecular Probes). Nuclei staining positive for Ki-67 were defined by visual determination in three or more randomly selected fields per cross section at 40x magnification. Cross sections were done at the biggest diameter of the tumor. Ki-67 “positive” staining was defined as at least three positive areas per cross section.
Microscopy
Deconvolution microscopy was carried out using a Zeiss Axioplan 2 microscope, with LSM 510 Meta microscopy laser system, and a ZEN 2008 restoration microscopy system (Zeiss, Inc, Jena, Germany). In each case, five independent fields were captured, and representative images were selected. All images were digitally processed for presentation with Adobe Photoshop CS4.
Statistical Analysis
All data are represented by the mean and SEM of least two or three independent experiments. Statistical analysis was performed using the statistical software SPSS Statistics 17.0.0 (SPSS Software, Armonk, NY) and with GraphPad Prism Version 5 (GraphPad Software, La Jolla, CA). P < .05 was considered statistically significant.
Results
Effect of AZT and ddI Double Treatment on Telomere Length
First, we investigated a possible additive effect of AZT and ddI with respect to telomere shortening. We used AZT at a concentration of 0.4 µM and ddI at a concentration of 6.0 µM. Those concentrations correspond to the lowest serum concentrations measured in HIV patients treated orally with those drugs.
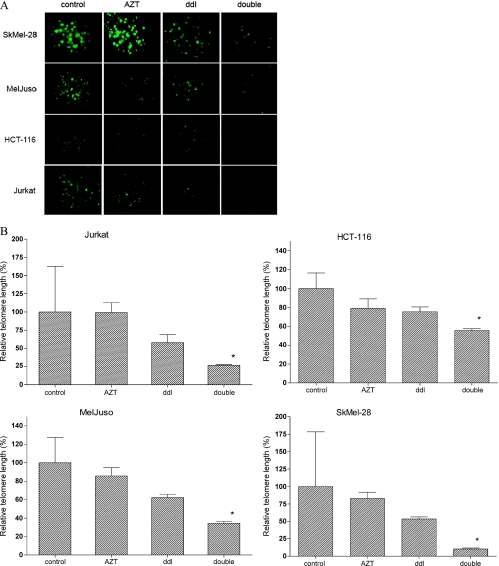
We treated the following tumor cell lines with AZT and ddI for 32 days: the colorectal carcinoma cell line HCT-116, the T-lymphocyte cell line Jurkat and two human skin melanoma cell lines, MelJuso and SkMel-28. The size of telomeres was first assessed by Q-FISH analysis of interphase nuclei. The combined treatment of cells with AZT and ddI induced a substantial and significant decline of telomere length in all four cell lines (Figure 1, A and B). As expected, there was no major change of telomeric length in the presence of AZT alone.
Figure 1.
Effect of combined AZT/ddI treatment on telomere length in tumor cell lines as assessed by Q-FISH analysis of interphase nuclei. Treatment was applied for 4 weeks. (A) Representative FISH microscopy images of interphase nuclei stained for telomeres with FITC-labeled PNA oligonucleotides (green pixels; magnification, x40). (B) Relative telomere length values as determined by Q-FISH in relation to the untreated control cells set to 100%. Columns represent mean values of at least two independent experiments; the error bar indicates the standard deviation. *P ≤ .05.
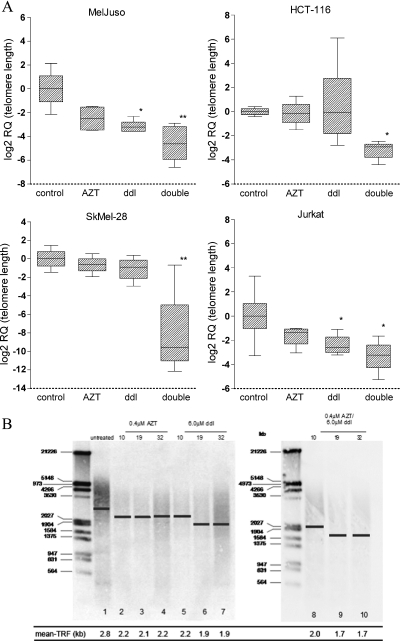
We further verified the observed effect of RTI on the length of telomeres by real-time PCR (Figure 2A). Corresponding to the data obtained by Q-FISH analysis, real-time PCR results revealed that double RTI treatment induced a significant shortening of telomeres in all four cell lines analyzed. Single-agent treatment with ddI only induced significant telomere shortening in MelJuso and Jurkat cells.
Figure 2.
Effect of combined AZT/ddI treatment on telomere length in tumor cell lines as determined by real-time PCR and Southern blot analysis. (A) Telomeric length as assessed by real-time PCR analysis in different tumor cell lines after 4 weeks of treatment. The y axis of box plots indicates the relative quantity (RQ). RQs are normalized to a single-copy gene (36B4) and a reference sample and are log-transformed (see Materials and Methods). The box plot summarizes the mean, maximum, and minimum; error bars indicate the SEM from at least two independent experiments performed in triplicates. *P ≤ .05. **P ≤ .001. (B) Telomeric length as assessed by Southern blot hybridization in HCT-116 cells after 4 weeks of treatment. The DNA molecular weight (kb) of a standard sample is given on the left. The calculated mean TRF is indicated by a bar in each sample lane, and the calculated values are given below.
To further illustrate the reduction of telomeric DNA during RTI treatment, we determined the TRFs by Southern blot analysis in one of the cell lines (Figure 2B). We used the HCT-116 cell line in this experiment because we have also chosen this cell line for in vivo experiments. The calculated mean TRF in the untreated control was 2.8 kb. Double treatment induced a slow shortening to 2.0, 1.68, and 1.66 kb after 11, 19, and 32 days, respectively. This attrition became significant at day 19. Single treatment with ddI was associated with a moderate and nonsignificant shortening of mean telomere length. AZT treatment did not affect the length of telomeres in this assay.
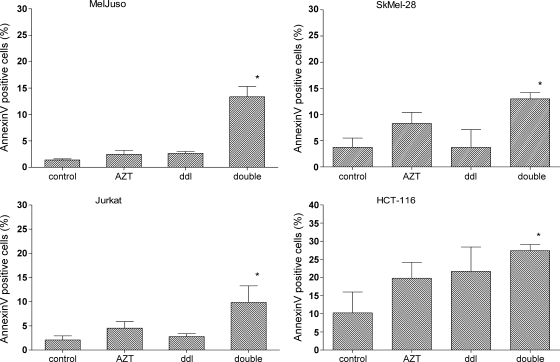
Combined Treatment of Cells with AZT and ddI Increases Apoptosis
Telomere shortening can promote cell death [2–4]. We therefore determined the level of apoptosis in treated and untreated tumor cell lines by annexin V staining (Figure 3). The double RTI treatment induced substantially higher levels of apoptosis in all four cancer cell lines compared with untreated cells. In contrast, single-agent treatment did not lead to significantly higher levels of annexin V-positive cells. Lowering the concentrations of RTI (to 0.04 µM AZT and 0.6 µM ddI) in double treatment abrogated induction of apoptosis (data not shown).
Figure 3.
Effect of combined AZT/ddI treatment on apoptosis in tumor cell lines. Treatment was carried out for 3 weeks. Apoptosis was determined by annexin V/PI double staining and flow cytometry. *P ≤ .05.
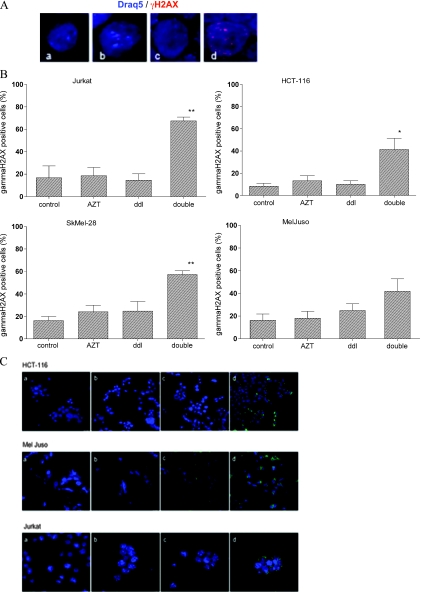
Combined Treatment of Cells with AZT and ddI Enhances the Number of γH2AX Foci and the Phosphorylation of p53
We next analyzed the effect of RTI treatment on the phosphorylation of histone H2AX (Figure 4, A and B). The phosphorylated γH2AX serves as a marker for DNA damage, senescence, and cell death. It is known that tumor cells have higher spontaneous phosphorylation of H2AX, when compared to nonmalignant cells. In accordance with the literature [25], γH2AX-specific signals were found in 8.3%, 13.4%, 16.3%, and 16.2% of the nuclei in untreated HCT-116, Jurkat, MelJuso, and SkMel-28 cells (mean values). Double treatment of tumor cell lines for 4 weeks induced a significant rise of γH2AX-positive signals to 41.4%, 54.0%, 41.9%, and 57.3%, respectively. In contrast, single-drug treatment never led to a significant increase in γH2AX-specific signals.
Figure 4.
Effect of combined AZT/ddI treatment on the nuclear accumulation of γH2AX and phosphorylated p53 in tumor cell lines. Tumor cells were treated for 4 weeks: AZT, 0.4 µM azidothymidine; ddI, 6.0 µM didanosine; double, 0.4 µM AZT and 6.0 µM ddI. (A) Representative immunofluorescence images of γH2AX (Alexa Fluor 568, red) and stained nuclei (Draq5, blue) (magnification, x60) of Jurkat cells. Treatment of cells as indicated (a) control, (b) AZT, (c) ddI, and (d) double. (B) For quantitative information, the percentage of positive cells is indicated by graphs. Results represent the mean of two independent experiments. Error bar, SD. (C) Representative images of fluorescence microscopy of p53 staining (FITC, green) and DNA counterstaining (Draq5, blue) for Jurkat cells (magnification, x60) and MelJuso and HCT-116 cells (magnification, x40). Treatment as indicated (a) control, (b) AZT, (c) ddI, and (d) double. *P ≤ .05. **P ≤ .001.
Previously, it has been shown that γH2AX is associated with activation (phosphorylation) of p53 [5]. We therefore determined serine-15 phosphorylation of p53 in response to RTI therapy by immunocytochemistry. A functional p53 pathway has been reported for HCT-116, MelJuso, and Jurkat cells. Thus, we focused on these three cell lines for analysis. RTI treatment induced a strong (>70% positive cells) signal specific for serine-15 phosphorylation of p53 in all three cell lines, whereas the effect of single agents was comparably modest (Figure 4C).
Effect of RTIs on Cell Cycle and Cell Growth of HCT-116 Cells
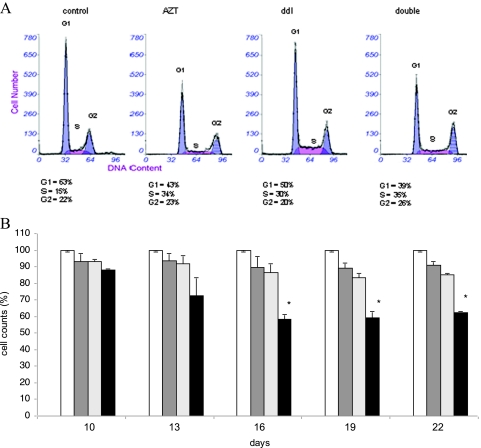
DNA damage, telomere length attrition, and induction of γH2AX usually lead to cell cycle arrest. We determined the effect of RTI on the cell cycle status of HCT-116 cells. Double and single RTI treatment led to an accumulation of cells in the S phase and a corresponding decrease in the G0/G1 peak: a representative picture is shown in Figure 5A (flow cytometry histogram of HCT-116 cells). We next determined the effect of RTI therapy on cell proliferation and survival by establishing the cell count. Double low-dose RTI therapy significantly reduced the cell number by approximately 40% after 16 to 22 days of treatment, whereas single agents had no substantial effect on cell count (Figure 5B).
Figure 5.
Effect of combined AZT/ddI treatment on cell cycle and proliferation of HCT-116 cells. Cells were exposed to RTI up to 22 days. (A) Effect of combined AZT/ddI treatment on cell cycle progression as determined by PI staining. The percentage of cells in distinct cycle phases is specified. The experiment was repeated once with similar results. (B) Effect of AZT/ddI treatment on cell proliferation and death. Columns represent the mean of triplicate cell counts ± SD. The cell number is expressed in percent to control cultures receiving no treatment. Empty bar indicates untreated control; dark gray bar, AZT; light gray bar, ddI; black bar, double treatment. *P ≤ .05.
RTIs Reduce the Growth of Human Tumor Xenografts in Athymic Nude Mice
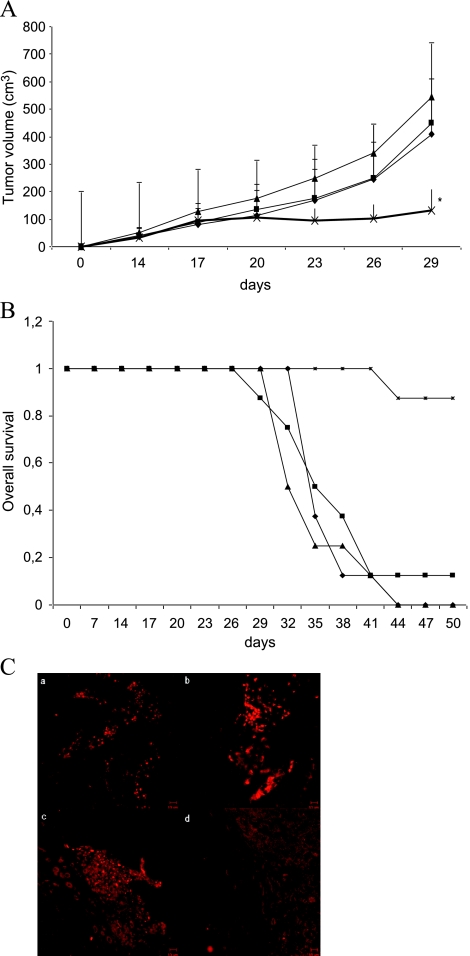
We then investigated whether low concentrations of AZT in combination with ddI would affect tumor growth in vivo. HCT-116 cells were injected in the right flank of nude mice. Treatment of animals with AZT and/or ddI was started when the tumors were grown to a volume of 35 mm3. The drug concentration applied in drinking water to the mice corresponded to the therapeutic dose recommended for HIV patients. Tumor growth was significantly reduced in animals treated with both drugs compared to untreated mice (Figure 6A). After 29 days, the mean tumor volume was 163 ± 84 mm3 in the combined treatment group compared with a mean volume of 593 ± 99 mm3 in the control group (P ≤ .05). In contrast, single treatment was not associated with significant therapeutic effects. The corresponding Kaplan-Meier graph illustrates the increased survival of animals treated with the RTI combination (Figure 6B).
Figure 6.
Therapeutic effect of combined AZT/ddI treatment on HCT-116 derived tumors in nude mice. Treatment was started when tumors reached the size of ∼35 mm3. Groups (n = 8) are indicated as follows: -◆- Bidest.water; -■- 10 mg/kg per day AZT; -▲- 20 mg/kg per day ddI; -x- combination of AZT and ddI. (A) Mean values of tumor volumes of different groups are given. Error bars, SD. *P ≤ .05. (B) Kaplan-Meier plot illustrating survival of the different groups. (C) Immunohistochemical staining of the proliferation marker Ki-67 in excised murine tumors. A representative picture (magnification, x40) for each group is shown. Treatment of mice as indicated on the top: (a) control, (b) AZT, (c) ddI, and (d) double.
To further investigate the effect of RTI treatment on the growth of tumor tissue in vivo, tumor samples of killed mice were analyzed for the cellular proliferation marker Ki-67 (Figure 6C) as detected by immunohistochemical staining. In untreated animals, each tumor showed more than five Ki-67-positive spots per cross section. In single agent-treated mice, each tumor revealed at least three Ki-67-positive spots per slide. In contrast, histologic slides of xenografts derived from mice receiving double RTI treatment only revealed a single positive spot in two of eight samples. Thus, the staining pattern of Ki-67 correlated with the rate of tumor growth.
Discussion
This is the first demonstration that the combined use of low-dose AZT and ddI reduces tumor cell growth in vivo. Most importantly, anticancer effects were observed at drug concentrations applied during oral treatment of HIV patients. Thus, an important aspect of our preclinical finding is the fact that drug dosing acceptable to human patients was effective.
With regard to the molecular mechanism, we could demonstrate that low-dose dual RTI treatment leads to a significant shortening of telomeres and induces a DNA damage response and apoptosis of tumor cells. In line with previous observations [26,27], we propose that it is essential to apply RTIs for several weeks to achieve biologic effects at low drug concentrations. Furthermore, our assays clearly demonstrated that cotreatment with AZT and ddI, but not single treatment, was able to induce telomere shortening in all tested human cancer cell lines. This indicates that the combination of these drugs may be widely applicable to various tumor entities.
It is well known that RTIs efficiently inhibit telomerase activity when applied at high concentrations. However, studies conducted by others [6,7,13] and us (TRAP assay; data not shown) indicate that, at low levels, RTIs do not interact with the known telomere maintenance proteins such as telomerase, indicating another mechanism of action. Alternative mechanisms of action can be envisioned by an inhibition of other cellular reverse transcriptases such as encoded in LINE-1 elements or human endogenous retroviral sequences. This notion is also supported by the facts that 1) LINE-1 and human endogenous retroviral elements are known to be activated in cancer cells and 2) the finding that AZT and ddI combination treatment does not lead to telomere attrition in nonmalignant cells. Ongoing experiments are performed to elucidate the interaction of RTI with those tumor-associated reverse transcriptases.
We have demonstrated an accumulation of γH2AX during the dual RTI treatment. H2AX is activated through double-strand breaks and cell stress. Thus, H2AX activation can occur as a consequence of telomere attrition, but it can also accumulate by telomere-unrelated mechanisms. On the basis of the present data, we cannot conclusively determine the mechanism of γH2AX activation in our setting: Telomere-independent mechanisms can be envisioned because AZT is incorporated into the DNA. Alternatively, ddI may enhance H2AX incorporation [16]. However, a telomere-dependent activation of H2AX is supported by the fact that H2AX was not detected before 4 weeks of treatment (data not shown). Four weeks seem to be necessary for progressive degradation of telomere ends and for triggering the DNA damage signal by uncapped telomeres. Direct damage of DNA by the drugs would be expected to occur more rapidly. Irrespective of the mechanism of activation, we conclude that the activation of H2AX by dual RTI treatment but not by single-agent therapy greatly supports this drug combination as an anticancer regimen.
Activation of H2AX can lead to cell death, which has been described to be mediated by serine-15 phosphorylation of p53 [5]. Along this line, we could demonstrate a concurrent phosphorylation of γH2AX and increased p53 phosphorylation in HCT-116, MelJuso, and Jurkat cells. However, RTI treatment also induced cell death in SkMel-28, which lacks a functional p53 pathway. Thus, RTI-induced cell death is not necessarily linked to a functional p53 pathway. This correlates with the fact that p53-independent γH2AX-mediated cell death has been described in other studies [25].
With respect to cell cycle arrest, we could observe a shift from the G0/G1 phase to the S phase in HCT-116 cells by double treatment. In line with previous publications, single RTI treatment also induced comparable changes [28]. This observation suggests that effects of RTI on cell cycle might be uncoupled to—and do not explain—drug effects on telomere lengthening, apoptosis, and growth inhibition in vitro. It should be noted that there is also only limited amount of consistent data of RTI effects on cell cycle progression in the literature supporting a loose interaction.
Lately, a number of clinical trials have been initiated with new telomere-interacting agents and telomerase inhibitors as well as with antitelomerase vaccines [29]. These agents have been developed owing to the apparent therapeutic potential resulting from the pathologic telomere regulation in cancer cells. They include drug candidates inhibiting telomerase by nonnucleoside small molecules such as BiBR1532 [30] or oligonucleotide inhibitors of telomerase activity such as GRN163L [26,27,31], the latter being applied in phase 2 trials for breast cancer, lung cancer, and myeloma. A major concern of the clinical application of AZT as a telomerase inhibitor is its toxic side effects on healthy tissue observed at high drug concentrations. Specifically, it was shown that RTI can enhance mutagenesis in vitro and can lead to transplacental genotoxicity [32]. The herein used therapeutic concentrations of the AZT/ddI combination had no effect on telomeres in human fibroblasts (T.A. and M.B., unpublished observations) indicating a differential susceptibility of malignant and nonmalignant cells to these drugs at low concentrations. Similarly, a differential reactivity toward malignant versus nonmalignant tissue has been described for other telomerase interactive agents [32,33]. In conclusion, the successful use of the combination of two known RTI such as AZT and ddI at low concentrations in a murine cancer model might reintroduce these drugs in the oncological therapy for solid cancers. The important aspect of using the drugs in combination mirrors their application and effectiveness in HIV therapy.
Acknowledgments
The authors thank the careful reading of Christine Brostjan and the technical help of Monika Sachet and the members of the Surgical Research Laboratories.
Abbreviations
- AZT
azidothymidine
- ddI
didanosine
- RTI
reverse transcriptase inhibitor
References
- 1.Kim NW. Specific association of human telomerase activity with immortal calls and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 2.Harley CB, Futcher AB, Greider CW. Telomere shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 3.Lundblad V, Szostak JW. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 4.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 5.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 6.Mergny JL, Riou JF, Mailliet P, Teulade-Fichou MP, Gilson E. Natural and pharmacological regulation of telomerase. Nucleic Acids Res. 2002;30:839–865. doi: 10.1093/nar/30.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 8.Brower V. Telomerase-based therapies emerging slowly. J Natl Cancer Inst. 2010;102:520–521. doi: 10.1093/jnci/djq145. [DOI] [PubMed] [Google Scholar]
- 9.Strahl C, Blackburn EH. The effects of nucleoside analogs on telomerase and telomeres in Tetrahymena. Nucleic Acids Res. 1994;22:893–900. doi: 10.1093/nar/22.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill PS, Harrington W, Jr, Kaplan MH, Ribeiro RC, Bennett JM, Liebman HA, Bernstein-Singer M, Espina BM, Cabral L, Allen S. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon α and zidovudine. N Engl J Med. 1995;332:1744–1748. doi: 10.1056/NEJM199506293322603. [DOI] [PubMed] [Google Scholar]
- 11.Bazarbachi A, Plumelle Y, Carlos Ramos J, Tortevoye P, Otrock Z, Taylor G, Gessain A, Harrington W, Panelatti G, Hermine O. Meta-analysis on the use of zidovudine and interferon-α in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28:4177–4183. doi: 10.1200/JCO.2010.28.0669. [DOI] [PubMed] [Google Scholar]
- 12.Datta A, Bellon M, Sinha-Datta U, Bazarbachi A, Lepelletier Y, Canioni D, Waldmann TA, Hermine O, Nicot C. Persistent inhibition of telomerase reprograms adult T-cell leukemia to p53-dependent senescence. Blood. 2006;108:1021–1029. doi: 10.1182/blood-2006-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez DE, Tejera AM, Olivero OA. Irreversible telomere shortening by3′-azido-2′,3′-dideoxythymidine (AZT) treatment. Biochem Biophys Res Commun. 1998;246:107–110. doi: 10.1006/bbrc.1998.8555. [DOI] [PubMed] [Google Scholar]
- 14.Strahl C, Blackburn EH. Effects of reverse transcriptase inhibitors on telomere length and telomerase activity in two immortalized human cell lines. Mol Cell Biol. 1996;16:53–65. doi: 10.1128/mcb.16.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Takahashi H, Harada Y, Ogawara T, Ogimura Y, Mizushina Y, Saneyoshi M, Yamaguchi T. 3′-Azido-2′,3′-dideoxynucleoside 5′-triphosphates inhibit telomerase activity in vitro, and the corresponding nucleosides cause telomere shortening in human HL60 cells. Nucleic Acids Res. 2007;35:7140–7149. doi: 10.1093/nar/gkm859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng Q, Walker DM, Olivero OA, Shi X, Antiochos BB, Poirier MC, Walker VE. Zidovudine-didanosine coexposure potentiates DNA incorporation of zidovudine and mutagenesis in human cells. Proc Natl Acad Sci USA. 2000;97:12667–12671. doi: 10.1073/pnas.220203197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji HJ, Rha SY, Jeung HC, Yang SH, An SW, Chung HC. Cyclic induction of senescence with intermittent AZT treatment accelerates both apoptosis and telomere loss. Breast Cancer Res Treat. 2005;93:227–236. doi: 10.1007/s10549-005-5156-0. [DOI] [PubMed] [Google Scholar]
- 18.Fang JL, Beland FA. Long-term exposure to zidovudine delays cell cycle progression, induces apoptosis, and decreases telomerase activity in human hepatocytes. Toxicol Sci. 2009;111:120–130. doi: 10.1093/toxsci/kfp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humer J, Ferko B, Waltenberger A, Rapberger R, Pehamberger H, Muster T. Azidothymidine inhibits melanoma cell growth in vitro and in vivo. Melanoma Res. 2008;18:314–321. doi: 10.1097/CMR.0b013e32830aaaa6. [DOI] [PubMed] [Google Scholar]
- 20.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boulay JL, Reuter J, Ritschard R, Terracciano L, Herrmann R, Rochlitz C. Gene dosage by quantitative real-time PCR. Biotechniques. 1999;27:228–230. doi: 10.2144/99272bm03. 232. [DOI] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, Dirks RW, Raap AK, Tanke HJ. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- 24.Airoldi I, Di Carlo E, Banelli B, Moserle L, Cocco C, Pezzolo A, Sorrentino C, Rossi E, Romani M, Amadori A, et al. The IL-12Rβ2 gene functions as a tumor suppressor in human B cell malignancies. J Clin Invest. 2004;113:1651–1659. doi: 10.1172/JCI20303. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Nakamura AJ, Redon CE, Bonner WM, Sedelnikova OA. Telomere-dependent and telomere-independent origins of endogenous DNA damage in tumor cells. Aging (Albany NY) 2009;1:212–218. doi: 10.18632/aging.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochreiter AE, Xiao H, Goldblatt EM, Gryaznov SM, Miller KD, Badve S, Sledge GW, Herbert BS. Telomerase template antagonist GRN163L disrupts telomere maintenance, tumor growth, and metastasis of breast cancer. Clin Cancer Res. 2006;12:3184–3192. doi: 10.1158/1078-0432.CCR-05-2760. [DOI] [PubMed] [Google Scholar]
- 27.Marian CO, Cho SK, McEllin BM, Maher EA, Hatanpaa KJ, Madden CJ, Mickey BE, Wright WE, Shay JW, Bachoo RM. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and tumor growth. Clin Cancer Res. 2010;16:154–163. doi: 10.1158/1078-0432.CCR-09-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivero OA, Tejera AM, Fernandez JJ, Taylor BJ, Das S, Divi RL, Poirier MC. Zidovudine induces S-phase arrest and cell cycle gene expression changes in human cells. Mutagenesis. 2005;20:139–146. doi: 10.1093/mutage/gei019. [DOI] [PubMed] [Google Scholar]
- 29.Nava-Parada P, Emens LA. GV-1001, an injectable telomerase pep-tide vaccine for the treatment of solid cancers. Curr Opin Mol Ther. 2007;9:490–497. [PubMed] [Google Scholar]
- 30.Damm K, Hemmann U, Garin-Chesa P, Hauel N, Kauffmann I, Priepke H, Niestroj C, Daiber C, Enenkel B, Guilliard B, et al. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J. 2001;20:6958–6968. doi: 10.1093/emboj/20.24.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uziel O, Beery E, Dronichev V, Samocha K, Gryaznov S, Weiss L, Slavin S, Kushnir M, Nordenberg Y, Rabinowitz C, et al. Telomere shortening sensitizes cancer cells to selected cytotoxic agents: in vitro and in vivo studies and putative mechanisms. PLoS One. 2010;5:e9132. doi: 10.1371/journal.pone.0009132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rha SY, Izbicka E, Lawrence R, Davidson K, Sun D, Moyer MP, Roodman GD, Hurley L, Von Hoff D. Effect of telomere and telomerase interactive agents on human tumor and normal cell lines. Clin Cancer Res. 2000;6:987–993. [PubMed] [Google Scholar]
- 33.Castelo-Branco P, Zhang C, Lipman T, Fujitani M, Hansford L, Clarke I, Harley CB, Tressler R, Malkin D, Walker E, et al. Neural tumor-initiating cells have distinct telomere maintenance and can be safely targeted for telomerase inhibition. Clin Cancer Res. 2011;17:3–5. doi: 10.1158/1078-0432.CCR-10-2075. [DOI] [PubMed] [Google Scholar]