Abstract
Dynamic body acceleration (DBA) has been used as a proxy for energy expenditure in logger-equipped animals, with researchers summing the acceleration (overall dynamic body acceleration - ODBA) from the three orthogonal axes of devices. The vector of the dynamic body acceleration (VeDBA) may be a better proxy so this study compared ODBA and VeDBA as proxies for rate of oxygen consumption using humans and 6 other species. Twenty-one humans on a treadmill ran at different speeds while equipped with two loggers, one in a straight orientation and the other skewed, while rate of oxygen consumption (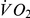 ) was recorded. Similar data were obtained from animals but using only one (straight) logger. In humans, both ODBA and VeDBA were good proxies for
) was recorded. Similar data were obtained from animals but using only one (straight) logger. In humans, both ODBA and VeDBA were good proxies for 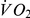 with all r2 values exceeding 0.88, although ODBA accounted for slightly but significantly more of the variation in
with all r2 values exceeding 0.88, although ODBA accounted for slightly but significantly more of the variation in 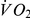 than did VeDBA (P<0.03). There were no significant differences between ODBA and VeDBA in terms of the change in
than did VeDBA (P<0.03). There were no significant differences between ODBA and VeDBA in terms of the change in 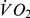 estimated by the acceleration data in a simulated situation of the logger being mounted straight but then becoming skewed (P = 0.744). In the animal study, ODBA and VeDBA were again good proxies for
estimated by the acceleration data in a simulated situation of the logger being mounted straight but then becoming skewed (P = 0.744). In the animal study, ODBA and VeDBA were again good proxies for 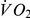 with all r2 values exceeding 0.70 although, again, ODBA accounted for slightly, but significantly, more of the variation in
with all r2 values exceeding 0.70 although, again, ODBA accounted for slightly, but significantly, more of the variation in 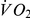 than did VeDBA (P<0.03). The simultaneous contraction of muscles, inserted variously for limb stability, may produce muscle oxygen use that at least partially equates with summing components to derive DBA. Thus, a vectorial summation to derive DBA cannot be assumed to be the more ‘correct’ calculation. However, although within the limitations of our simple study, ODBA appears a marginally better proxy for
than did VeDBA (P<0.03). The simultaneous contraction of muscles, inserted variously for limb stability, may produce muscle oxygen use that at least partially equates with summing components to derive DBA. Thus, a vectorial summation to derive DBA cannot be assumed to be the more ‘correct’ calculation. However, although within the limitations of our simple study, ODBA appears a marginally better proxy for 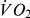 . In the unusual situation where researchers are unable to guarantee at least reasonably consistent device orientation, they should use VeDBA as a proxy for
. In the unusual situation where researchers are unable to guarantee at least reasonably consistent device orientation, they should use VeDBA as a proxy for 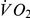 .
.
Introduction
The broad interest in animal optimal foraging [1] underpins the central concept that creatures should behave in such a way as to maximize their inclusive fitness by maximizing their net rate of energy intake [see 2,3]. This includes optimized harvesting solutions [e.g. 4], [5], [6] but also minimizing locomotion costs in animals that have to move to acquire food [7]. Thus, information on the rate at which organisms expend energy during movement is fundamental to informing models of optimal foraging and indeed, ultimately, the efficiency of movement affects the survival of wild animals [8]. Understanding optimality in foraging is only one example that demonstrates the importance of being able to determine energy expenditure but it, like many other biological processes, is best informed by energy expenditure at a fine-scale temporal resolution, something that is notably rare in published studies with some exceptions [9], [10], [11], [12]. This situation stems from a paucity of appropriate methods for determining the power use of wild animals.
The most common methods of measuring animal energy expenditure have used doubly labelled water (DLW) [13], [14], [15], [16], [17], direct and indirect calorimetry [e.g. 18], or some proxy for energy expenditure such as heart beat rate (fH) [19], [20], [21], [22], [23]. All the above systems have disadvantages [reviewed by 20], [24] which distill out into giving poor temporal resolution (doubly labelled water), being confined to a laboratory situation (calorimetry) or generally involving invasive methods of instrumentation (heart rate). See Halsey [25] and references therein for further details.
Beyond these approaches, however, some researchers have examined the use of mechanical motion sensors [26], [27] in studies of animal power use. In fact, as early as 1963, researchers proposed that the extent of body movement should act as a proxy for energy expenditure [28] because in order to elicit movement, animals need to expend energy, with more pronounced and vigorous movements presumed to arise as a result of more energy expended [e.g. 29], [30], [31]. Thus, specifically, energy expenditure should correlate with the extent of movement in some manner [32], [33]. In 2006, an acceleration-based proxy for energy expenditure focusing on dynamic body acceleration (DBA) was proposed, using tri-axial acceleration data derived from a logger recording at high rates (>10 Hz) and placed close to the participant's centre of gravity [34]. The specific proxy for metabolic rate was overall dynamic body acceleration (ODBA), determined by adding the dynamic acceleration from three orthogonally-placed accelerometers orientated so as to represent the main axes of the animal's body; in the surge, heave and sway dimensions [34]. Although this original work was conducted on birds, subsequent studies confirmed linear, strong correlations between the rate of oxygen consumption (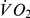 ) and ODBA in fish [35], amphibians [36], birds [34], [37], [38] and mammals [12], [39], including man [39].
) and ODBA in fish [35], amphibians [36], birds [34], [37], [38] and mammals [12], [39], including man [39].
Despite the promise of the newly proposed ODBA method, however, Gleiss, Wilson et. al. [24] point to an uncertainty in its formulation. Actually, acceleration is a vectorial quantity so it might seem incorrect that ODBA should treat each axis as independent because the implication is that the ODBA metric represents work done by three distinct straight-line paths and thus overestimates the work done for any specific movement. Furthermore, ODBA values are expected to differ according to the alignment of the axes of the logger with respect to the equipped participant, something that should not affect a vectorial solution [24]. The suggestion, therefore, is that properly calculated vectorial dynamic body acceleration (VeDBA) may, in fact, prove a better, and a more appropriate proxy for metabolic rate than does ODBA. Indeed, a recent study by McGregor, Busa et al. [40] uses VeDBA (although not referred to as such) rather than ODBA as a proxy of 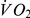 . On the other hand, from a mathematical perspective both ODBA and VeDBA are norms and so are equally valid ways to measure the length of a vector. To ascertain which derivative is a better proxy of
. On the other hand, from a mathematical perspective both ODBA and VeDBA are norms and so are equally valid ways to measure the length of a vector. To ascertain which derivative is a better proxy of 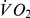 is difficult because there is no expected mathematical relationship that can be examined to calculate the impact of using different norms. Furthermore, the empirical relationship will depend on the species, the data logger location on the animal, and the behaviour and locomotion gait(s) of the animal. Thus a direct test of the predictive power of ODBA and VeDBA is required, yet Gleiss, Wilson et al. [24] note that no studies have explicitly sought to determine whether ODBA or VeDBA is a better predictor of metabolic rate and whether the outcome of such a test might be influenced by logger orientation on the animal.
is difficult because there is no expected mathematical relationship that can be examined to calculate the impact of using different norms. Furthermore, the empirical relationship will depend on the species, the data logger location on the animal, and the behaviour and locomotion gait(s) of the animal. Thus a direct test of the predictive power of ODBA and VeDBA is required, yet Gleiss, Wilson et al. [24] note that no studies have explicitly sought to determine whether ODBA or VeDBA is a better predictor of metabolic rate and whether the outcome of such a test might be influenced by logger orientation on the animal.
The present study attempts to determine whether ODBA or VeDBA is a better proxy for rate of oxygen consumption. Humans are used as a model species [cf. 39] and primary data collected while people move at different speeds on a treadmill are analysed in detail. This is supplemented by reanalysis of published data [12] for six other animal species. The implications of the findings are discussed in terms of the most appropriate way to derive dynamic body acceleration in the future.
Materials and Methods
The human study
Twenty-one healthy adults (mean age ± SD: 20.44±3.28 years) were involved in the study. Before the start of the experiment, the height (1.75±0.07 m) and weight (70.66±9.78 kg) of the participants were measured according to the International Standards for Anthropometric Assessment (2001). The experimental protocol was approved by the Swansea University Ethics Committee. All participants were asked to give informed consent before the trials began.
Broadly, the investigations compared the rate of oxygen consumption during locomotion by humans on a treadmill while back-mounted loggers recorded tri-axial acceleration.
Specifically, all participants performed a VO2 max test [40] on a treadmill (Woodway Ergo ELG 55; Woodway GmbH, Germany) that started at 3 km/h and increased in speed every 3 min by 1 km/h until participant volitional exhaustion. During this process the participants breathed into a mask, and expired air was analyzed for oxygen and carbon dioxide content using an Oxycon Pro (Jaeger Oxycon Manual (Version 4.5), VIASYS Healthcare GmbH, Hoechberg, Germany) on a breath-by-breath basis. Acceleration was measured using two tri-axial accelerometers (X6-1A USB; Gulf Coast Data Concepts, LLC, Waveland, USA; 16 bit resolution, recording range ±6 g), each set to record at 80 Hz on each of the three orthogonal axes. The loggers were placed within holding moulds cut into a single polystyrene saddle to ensure correct orientation; one unit was mounted in accordance with the main body axes of surge, heave and sway while the other was set to be 30° displaced from this on all axes. This skew-mounted accelerometer was rotated by 30° about the roll, pitch and yaw axes respectively, where the roll axis was taken as the long axis of the accelerometer. The saddle was optimised by trial and error during pilot studies to move properly with the participant's body. It was placed in the centre of the participant's back between the shoulder blades and held in place using a specially made Silastic® (Silastic® P1 Base and Curing Agent, Thomson Bros Newcastle Ltd) harness which kept the system in a stable position even during the most vigorous of movement.
The animal study
Data previously gathered comparing 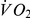 with acceleration data for animals during activity on a treadmill at Buenos Aires Zoo [12] were reanalyzed to supplement the work on humans. Species used were; coypu (Myocastor coypus) (4 individuals), larger hairy armadillo (Chaetophractus villosus) (1 individual), Muscovy duck (Cairina moschata) (1 individual), greylag goose (Anser anser) (2 individuals), Magellanic penguin (Spheniscus magellanicus) (2 individuals) and rockhopper penguin (Eudyptes chrysocome) (1 individual). Briefly, animals were equipped with acceleration data loggers, attached variously, before being exposed to a treadmill with the tread moving at a range of speeds between 0 and 2.52 km/h, the upper limit dependent on their capacities. The animals were given rests between the higher speeds where the predominant behavior was locomotion however at the lower speeds the animal typically exhibited a range of behaviors including searching, scratching and lying. An open circuitry respirometry system was used to measure
with acceleration data for animals during activity on a treadmill at Buenos Aires Zoo [12] were reanalyzed to supplement the work on humans. Species used were; coypu (Myocastor coypus) (4 individuals), larger hairy armadillo (Chaetophractus villosus) (1 individual), Muscovy duck (Cairina moschata) (1 individual), greylag goose (Anser anser) (2 individuals), Magellanic penguin (Spheniscus magellanicus) (2 individuals) and rockhopper penguin (Eudyptes chrysocome) (1 individual). Briefly, animals were equipped with acceleration data loggers, attached variously, before being exposed to a treadmill with the tread moving at a range of speeds between 0 and 2.52 km/h, the upper limit dependent on their capacities. The animals were given rests between the higher speeds where the predominant behavior was locomotion however at the lower speeds the animal typically exhibited a range of behaviors including searching, scratching and lying. An open circuitry respirometry system was used to measure 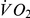 . Full details of the protocol are given in Halsey et al. [12].
. Full details of the protocol are given in Halsey et al. [12].
Data analysis
The raw accelerometer data were converted to DBA by first smoothing each channel to derive the static acceleration using a running mean over 2 s [7] and then subtracting this static acceleration from the raw data [24]. The resulting values for dynamic acceleration were all then converted to positive values. These values for DBA were then either summed to provide ODBA [34];
| (1) |
where Ax, Ay and Az are the derived dynamic accelerations at any point in time corresponding to the three orthogonal axes of the accelerometer, or their vectorial sum (VeDBA) using;
| (2) |
Means for ODBA and VeDBA were derived for all data corresponding to particular running speeds (for each individual used in the experiments) and plotted against speed and 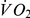 . Because measurements of
. Because measurements of 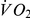 are most indicative of rate of energy expenditure when metabolism is mainly aerobic,
are most indicative of rate of energy expenditure when metabolism is mainly aerobic, 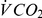 and
and 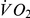 were also plotted against one another and the gas exchange threshold determined for each human participant using the v-slope method [41]: The plot of
were also plotted against one another and the gas exchange threshold determined for each human participant using the v-slope method [41]: The plot of 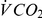 and
and 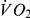 typically shows two slopes corresponding to the way
typically shows two slopes corresponding to the way 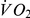 changes with respect to
changes with respect to 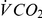 and the point at which these slopes intersect is considered to be the gas exchange threshold, which closely corresponds to the ventilatory threshold [41], [cf. 42], the point which approximately indicates when the participant changes from aerobic to anaerobic respiration as a main source of energy production. All data where participants were running at speeds which suggested that there was considerable anaerobic metabolism were excluded from the analysis.
and the point at which these slopes intersect is considered to be the gas exchange threshold, which closely corresponds to the ventilatory threshold [41], [cf. 42], the point which approximately indicates when the participant changes from aerobic to anaerobic respiration as a main source of energy production. All data where participants were running at speeds which suggested that there was considerable anaerobic metabolism were excluded from the analysis.
Simple linear regressions were used to test the strength of relationships between ODBA and VeDBA for both humans and animals. Mixed linear models tested the relationships between data recorded from the straight mounting and data recorded from the skewed mounting in the human trials. The coefficient of variation for ODBA and VeDBA was calculated for each human participant for the two logger data sets combined. Mixed linear models were used to generate equations for 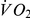 against the two acceleration metrics for all participants together, including participant as a random factor, separately for the straight- and skew-mounted logger data. To compare the error on estimates of
against the two acceleration metrics for all participants together, including participant as a random factor, separately for the straight- and skew-mounted logger data. To compare the error on estimates of 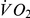 using ODBA or VeDBA caused by an acceleration logger becoming skewed, the difference between measured
using ODBA or VeDBA caused by an acceleration logger becoming skewed, the difference between measured 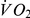 and
and 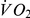 estimated by a skew-mounted logger using calibrations for a straight-mounted logger at speed 5 (an average walking speed) was calculated for both these derived metrics. Paired tests were used to test for differences between ODBA and VeDBA. Mean values are provided ±1 S.E. (standard error).
estimated by a skew-mounted logger using calibrations for a straight-mounted logger at speed 5 (an average walking speed) was calculated for both these derived metrics. Paired tests were used to test for differences between ODBA and VeDBA. Mean values are provided ±1 S.E. (standard error).
Results
The human study
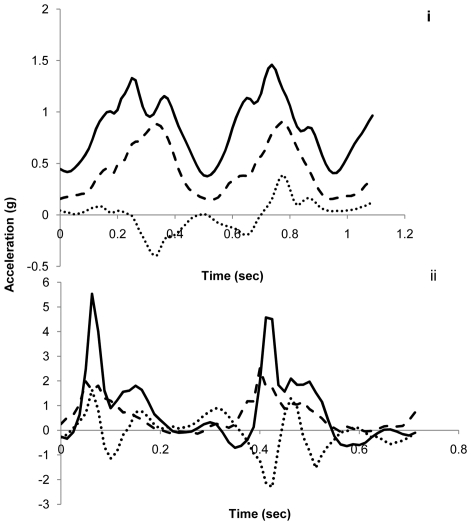
The accelerometers recorded a very precise profile of tri-axial acceleration from each participant during walking and running (Fig. 1) with clear peaks in heave and surge in particular, denoting each stride, although peaks in sway were also apparent.
Figure 1. Heave (continuous line), sway (dotted line) and surge (dashed line) acceleration axes displayed graphically over one stride (from each leg) during walking (i) and running (ii).
Gas analytical data and data from the straight-mounted logger were obtained for all participants, while data from the skew-mounted logger were obtained for 18 of the participants (on three occasions the logger failed). Q-Q plots indicated that the distribution for all the 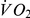 s data together was reasonably normal. ODBA and VeDBA appeared non-normally distributed as did the values representing the percentage change in estimated
s data together was reasonably normal. ODBA and VeDBA appeared non-normally distributed as did the values representing the percentage change in estimated 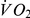 due to the logger becoming skewed. However, for the majority of analyses, ODBA and VeDBA represented the independent variable. The r2 values for
due to the logger becoming skewed. However, for the majority of analyses, ODBA and VeDBA represented the independent variable. The r2 values for 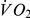 against ODBA and VeDBA for both straight and skewed logger orientations were reasonably normal.
against ODBA and VeDBA for both straight and skewed logger orientations were reasonably normal.
For all participants and both logger mountings, ODBA and VeDBA from trials were highly correlated with each other (Figs. 2 and 3), with r2 values on means derived from all participants typically being ca. 0.999. Mixed linear models (straight ODBA∼skewed ODBA+participant(random), or, straight VeDBA∼skewed VeDBA+participant(random)) indicated that during the trials, ODBA values from the straight-mounted devices were highly correlated with the ODBA values from the skew-mounted devices (r2 = 0.99), as were VeDBA values from the straight- and skew-mounted devices (r2 = 0.99) (Fig. 4). Both ODBA and VeDBA were highly correlated with 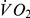 (Fig. 5). Mixed linear models (
(Fig. 5). Mixed linear models (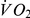 ∼ODBA+participant(random), or,
∼ODBA+participant(random), or, 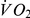 ∼VeDBA+participant(random)) returned significant relationships for both the straight and the skewed mountings (Table 1).
∼VeDBA+participant(random)) returned significant relationships for both the straight and the skewed mountings (Table 1).
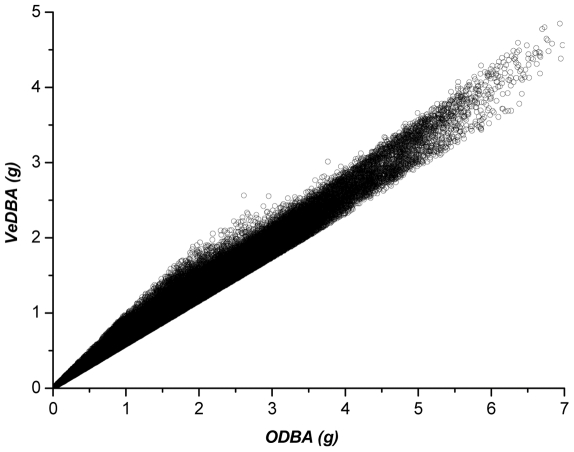
Figure 2. Instantaneous ODBA plotted against VeDBA using all data from a participant recorded during a full 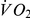 max test.
max test.
In this example, as with all other participants, the relationship between ODBA and VeDBA was highly significant (VeDBA = 0.014+0.6418 ODBA, r2 = 0.987, P<0.001).
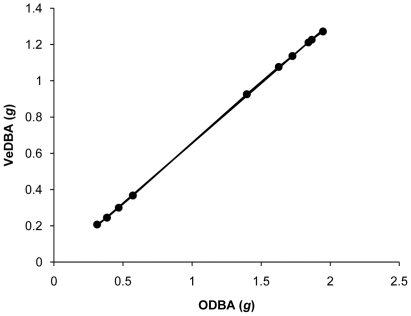
Figure 3. Relationship between mean ODBA and mean VeDBA (means taken for each running speed) for a test participant during a 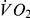 max test.
max test.
Only data during the period when the participant did not exceed the ventilatory threshold (for definition see text) are included. as with all other participants, the relationship between ODBA and VeDBA was highly significant (VeDBA = 0.014+0.6418 ODBA, r2 = 0.987, P<0.001).
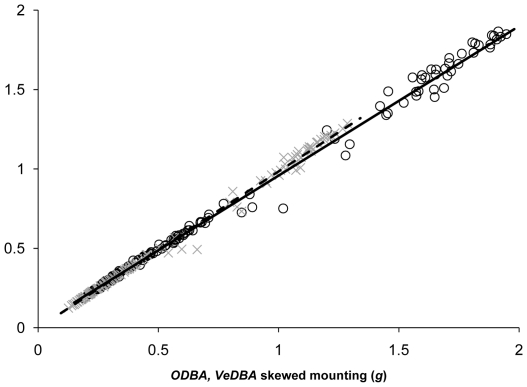
Figure 4. Dynamic body accelerations (ODBA – circles, and VeDBA –crosses) from straight- versus skew-mounted accelerometers (for details see text).
Each point denotes a mean value derived from a three-minute trial of a participant moving at one particular speed below the lactate threshold. Data from all participants are included.
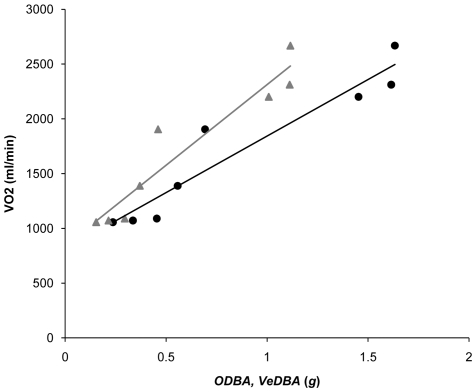
Figure 5. An example plot of 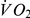 uptake against ODBA (black circles) and VeDBA (grey triangles) over the duration of the trial following removal of the points above the participant's anaerobic threshold.
uptake against ODBA (black circles) and VeDBA (grey triangles) over the duration of the trial following removal of the points above the participant's anaerobic threshold.
Table 1. Overall relationships between 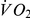 and ODBA or VeDBA recorded for humans locomoting on a treadmill using an acceleration logger in a straight orientation or a skewed orientation.
and ODBA or VeDBA recorded for humans locomoting on a treadmill using an acceleration logger in a straight orientation or a skewed orientation.
| Straight | Skewed | |
| ODBA | VO2 = 1132.ODBA+615 r2 = 0.915 | VO2 = 1466.ODBA+776 r2 = 0.94 |
| VeDBA | VO2 = 1664.VeDBA+636 r2 = 0.914 | VO2 = 1659.VeDBA+629 r2 = 0.91 |
ODBA accounted for significantly more of the variation in 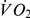 than did VeDBA for the straight-mounted loggers (mean r2, ODBA: 0.95±0.01; VeDBA: 0.94±0.01; t20 = 2.29, P = 0.03) and for the skew-mounted logger (t17 = 2.44, p = 0.03) (mean r2, ODBA: 0.94±0.01; VeDBA: 0.91±0.01). The difference in r2 values for single linear regressions of
than did VeDBA for the straight-mounted loggers (mean r2, ODBA: 0.95±0.01; VeDBA: 0.94±0.01; t20 = 2.29, P = 0.03) and for the skew-mounted logger (t17 = 2.44, p = 0.03) (mean r2, ODBA: 0.94±0.01; VeDBA: 0.91±0.01). The difference in r2 values for single linear regressions of 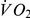 against ODBA versus
against ODBA versus 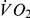 against VeDBA for each participant, for both the straight-mounted logger and the skew-mounted logger, are very small. 95% confidence intervals derived from paired t tests indicate that the true difference in r2 for the straight-mounted logger is in the range of 0.0004 to 0.0078, while for the skew-mounted logger is in the range of 0.0003 to 0.0040. These ranges represent less than 0.1% of mean r2 values.
against VeDBA for each participant, for both the straight-mounted logger and the skew-mounted logger, are very small. 95% confidence intervals derived from paired t tests indicate that the true difference in r2 for the straight-mounted logger is in the range of 0.0004 to 0.0078, while for the skew-mounted logger is in the range of 0.0003 to 0.0040. These ranges represent less than 0.1% of mean r2 values.
To test for differences between ODBA and VeDBA in the effect on estimates of 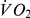 in the case of an initially straight-mounted logger subsequently becoming skewed
in the case of an initially straight-mounted logger subsequently becoming skewed 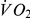 measured during speed 5 on the treadmill was compared to
measured during speed 5 on the treadmill was compared to 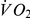 estimated from acceleration measurements recorded by the skew-mounted logger using the straight-mounted logger calibrations. For both ODBA and VeDBA the percentage difference between
estimated from acceleration measurements recorded by the skew-mounted logger using the straight-mounted logger calibrations. For both ODBA and VeDBA the percentage difference between 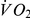 measured and
measured and 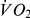 estimated was small (median, ODBA: 0.93; VeDBA: 0.81), and the size of this difference was similar for the two metrics (Wilcoxon signed ranks test: Z = −0.327, N = 18, P = 0.744).
estimated was small (median, ODBA: 0.93; VeDBA: 0.81), and the size of this difference was similar for the two metrics (Wilcoxon signed ranks test: Z = −0.327, N = 18, P = 0.744).
Table 1
Overall relationships between 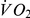 and ODBA or VeDBA recorded for humans locomoting on a treadmill using an acceleration logger in a straight orientation or a skewed orientation.
and ODBA or VeDBA recorded for humans locomoting on a treadmill using an acceleration logger in a straight orientation or a skewed orientation.
The animal study
In a manner similar to the human study, the coefficients of determination for the relationships between ODBA or VeDBA and 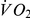 were all high, ranging between r2 = 0.70 for the VeDBA versus
were all high, ranging between r2 = 0.70 for the VeDBA versus
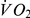 relationship for coypu 4 and r2 = 0.99 for the VeDBA versus
relationship for coypu 4 and r2 = 0.99 for the VeDBA versus
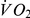 relationship for the rockhopper penguin (Table 2). The ODBA values had significantly higher coefficients of determination than the VeDBA values (t = 2.54, P<0.03).
relationship for the rockhopper penguin (Table 2). The ODBA values had significantly higher coefficients of determination than the VeDBA values (t = 2.54, P<0.03).
Table 2. r2-values for relationships between ODBA and VeDBA and 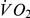 recorded using an acceleration data logger on a range of animals during activity at Buenos Aires Zoo.
recorded using an acceleration data logger on a range of animals during activity at Buenos Aires Zoo.
| Species | ODBA | VeDBA |
| Chaetophractus villosus | 0.9775 | 0.942 |
| Myocastor coypus 1 | 0.9594 | 0.94 |
| Myocastor coypus 2 | 0.7449 | 0.7019 |
| Myocastor coypus 3 | 0.9473 | 0.9486 |
| Myocastor coypus 4 | 0.8617 | 0.8568 |
| Cairina moschata | 0.9853 | 0.9841 |
| Anser anser 1 | 0.9022 | 0.8904 |
| Anser anser 2 | 0.9427 | 0.9242 |
| Spheniscus magellanicus 1 | 0.975 | 0.9662 |
| Spheniscus magellanicus 2 | 0.8979 | 0.811 |
| Eudyptes chrysocome | 0.9914 | 0.9957 |
Table 2
r2-values for relationships between ODBA or VeDBA and 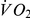 recorded using an acceleration data logger on a range of animals during activity at Buenos Aires Zoo.
recorded using an acceleration data logger on a range of animals during activity at Buenos Aires Zoo.
Discussion
In the purely physical sense, ODBA and VeDBA are derived using the same terms and, although the relative importance of the terms differs, the precise formulation of them means that larger VeDBA values will generally also accompany larger ODBA values, although VeDBA values will almost invariably be lower and never higher than ODBA. How much higher ODBA is than VeDBA will depend, inter alia, on the type of motion recorded which, in turn depends on animal type, gait and tag location. Our study was limited in scope, incorporating data from only 7 species, all of which were travelling in a straight line on a treadmill (although some species exhibited a range of behaviors at slower speeds), so it is unwise to over-interpret. Nonetheless, the treadmill approach has been used as a general method to simulate increased activity of all types by researchers examining the relationship between heart rate and 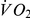 for many years [e.g. 20], [21] and two studies have explicitly sought to incorporate behaviours other than straight-line treadmill locomotion within the treadmill context with success [38], [43]. With these provisos in mind, generally, it is to be expected that an important finding of this study is the close correlation between ODBA and VeDBA where no human participant had an r2 of less than 0.998 for mean values derived from either skew or straight loggers with, unsurprisingly, the slope of the relationship always being less than 1. In addition, simple comparisons of the correlations between ODBA and VeDBA with
for many years [e.g. 20], [21] and two studies have explicitly sought to incorporate behaviours other than straight-line treadmill locomotion within the treadmill context with success [38], [43]. With these provisos in mind, generally, it is to be expected that an important finding of this study is the close correlation between ODBA and VeDBA where no human participant had an r2 of less than 0.998 for mean values derived from either skew or straight loggers with, unsurprisingly, the slope of the relationship always being less than 1. In addition, simple comparisons of the correlations between ODBA and VeDBA with 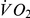 in animals show that they differ minimally (Table 2). This makes the discussion of whether ODBA or VeDBA is a better predictor of metabolic rate [24] rather academic. Nonetheless, given concerns about potential differences in the utility of ODBA with respect to VeDBA
[24], the present study was conducted to carry out tests into the matter.
in animals show that they differ minimally (Table 2). This makes the discussion of whether ODBA or VeDBA is a better predictor of metabolic rate [24] rather academic. Nonetheless, given concerns about potential differences in the utility of ODBA with respect to VeDBA
[24], the present study was conducted to carry out tests into the matter.
Vectorial versus summed tri-axial acceleration as a proxy for 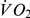
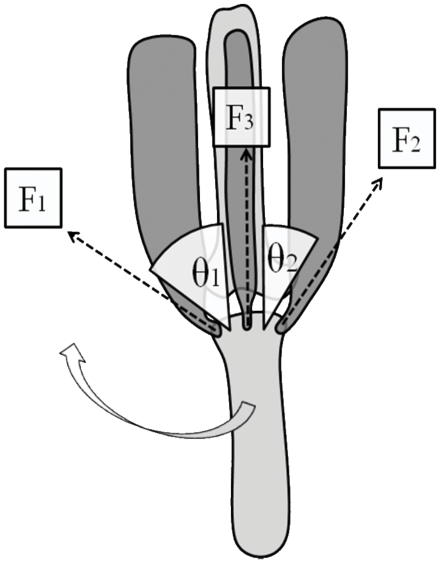
From a theoretical standpoint it may seem perplexing that VeDBA does not outperform ODBA as a proxy for 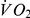 and a specific explanation is warranted. If the simple scenario of one limb articulating on another is considered (Fig. 6) where muscles emanating from the upper limb are inserted at various angles (θ) on the lower limb [e.g. 44], each exerting a force (F), then the overall force along the longitudinal y-axis (Fytot) is given by the vectorial solution;
and a specific explanation is warranted. If the simple scenario of one limb articulating on another is considered (Fig. 6) where muscles emanating from the upper limb are inserted at various angles (θ) on the lower limb [e.g. 44], each exerting a force (F), then the overall force along the longitudinal y-axis (Fytot) is given by the vectorial solution;
| (3) |
where the subscripts refer to each of the specific muscles with their defined forces and angles of insertion relative to the y-axis of the lower limb. In a similar manner, the total force along axis x is;
| (4) |
The torque (τ) along the y-axis produced by the contraction of these muscles depends on the overall force generated along that axis by each muscle (Eq 3) and the moment arm (d), defined as the perpendicular distance between the line of action of the muscle force and the pivot point of the articulation so that;
The torque is related to the angular acceleration (α) via;
| (6) |
where I is the moment of inertia. The linear acceleration ( ) perceived by an accelerometer placed on the moving lower limb is dependent on the distance between pivot and transducer (r) by;
) perceived by an accelerometer placed on the moving lower limb is dependent on the distance between pivot and transducer (r) by;
| (7) |
Thus, the (linear) acceleration perceived by an accelerometer mounted in the y-axis and measuring in the plane of movement can be determined by substituting Eq (5) into equation (7) and is approximated by;
| (8) |
which is clearly a vectorial solution. However, the work done (W) during muscular contraction to produce the forces necessary for the movement is given by;
| (9) |
for each muscle involved, where ΔD is the distance contracted. The total amount of energy used during contraction by all the muscles involved in moving the limb (Wto t) is;
| (10) |
a necessarily non-vectorial derivation, where the energy used equates directly with the oxygen consumed [45]. Thus, seen from a pure physics perspective, VeDBA is the proper way to derive the total magnitude of the acceleration vector at any one moment in time. However, the issue of interest to biologists studying energetics is not the total acceleration but how the DBA signal relates to rate of energy expenditure, and specifically the rate of energy expenditure used by the muscles involved. The rate of energy expenditure is not just dependent on the movement arc, which is described by VeDBA [see 24 for treatment of this], but also dependent, among other things, on the force exerted by the contracting muscles at the points of their insertion. A single muscle can contract to produce a movement arc of one limb by exerting an appropriate force (Fig. 6) while exactly the same movement arc can result from the contraction of two or more differently inserted muscles (Fig. 6), each of which exerts a force that leads to a vectorial solution that accords with that exhibited by the single muscle. In both cases the overall result for movement and physical work done is the same but in the latter case the oxygen consumed by the multiple muscles will exceed that of the single muscle because forces are developed that are not equally manifest in the movement. Fundamental to the amount of oxygen used by a body is the amount of muscle tissue that is active [46] and the precise orientation of various muscle groups involved in limb movement is critical in this regard. Human walking and running is brought about by a complex interplay of interacting, and variously inserted, muscles [44] which, nonetheless, produces a relatively simple movement arc which equates to the vectorial component of the variously contracting muscles even though the muscular work produced may more appropriately be represented by a sum value of muscular contraction. The ‘inefficiencies’ that result from partially opposing contracting muscles are, in fact, necessary for increasing limb stability [cf. 8]. For example, animals moving over rough terrain could not afford to have limb movement that is overly sensitive to lateral forces.
Figure 6. Schematic representation of a movement arc (curved arrow) elicited by one bone (light grey) with respect to another and brought about by contraction of multiple muscles (dark grey) with varying forces (F) with differing angles of insertion (θ).
Skew versus straight-mounted logger orientation
A specific concern, and one that perhaps might lead to the greatest discrepancy between ODBA and VeDBA, is what happens when device orientation is not standardized. Importantly, in our study on humans, the difference in recorded 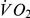 at a speed of 5 km/h compared to
at a speed of 5 km/h compared to 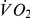 estimated for the same speed from the data recorded by the skew-mounted logger using the calibrations obtained from the straight-mounted logger was small. Further, it did not differ between ODBA and VeDBA. Thus, apparently, even if a logger is deployed in the straight position but then subsequently slips out of true, perhaps, for example, due to the intensity of the exercise, both ODBA and VeDBA would appear similarly powerful proxies for
estimated for the same speed from the data recorded by the skew-mounted logger using the calibrations obtained from the straight-mounted logger was small. Further, it did not differ between ODBA and VeDBA. Thus, apparently, even if a logger is deployed in the straight position but then subsequently slips out of true, perhaps, for example, due to the intensity of the exercise, both ODBA and VeDBA would appear similarly powerful proxies for 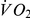 . In fact, contrary to what might be expected from a purely physical treatise, VeDBA did not outperform ODBA based on any of statistical analyses conducted.
. In fact, contrary to what might be expected from a purely physical treatise, VeDBA did not outperform ODBA based on any of statistical analyses conducted.
The actual acceleration values recorded by a straight- with respect to skew-mounted tri-axial accelerometer can be derived for any scenario by considering the relative rotations for each of the axes. Here, the matrix representation for the acceleration vector transformation is
| (11) |
where 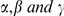 are the angles of roll, pitch and yaw angles of the skew-mounted accelerometer relative to the straight-mounted one, where the rotations are carried out in that order.
are the angles of roll, pitch and yaw angles of the skew-mounted accelerometer relative to the straight-mounted one, where the rotations are carried out in that order.
Thus, if the acceleration vector measured by the device in the straight-mounted position is
| (12) |
then in the skew-mounted position, that same acceleration is measured as a vector;
| (13) |
where
| (14) |
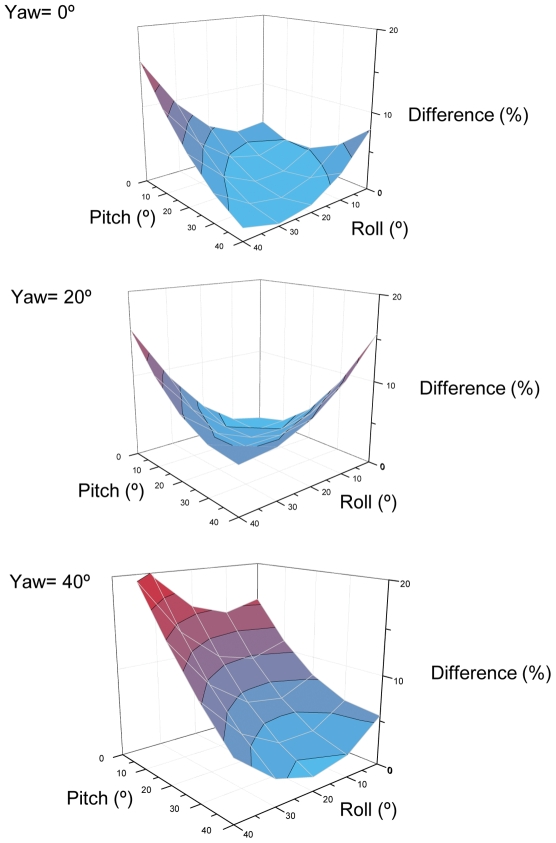
Derived values for these vector components can be used to produce VeDBA, (using Eq 2) which does not change with orientation and, more particularly, to produce ODBA (using Eq 1) which does change with orientation (Fig. 7). This approach shows that under given conditions of triaxial acceleration (the case shown in Fig. 7 shows equal amounts of dynamic acceleration in the heave surge and sway axes), deviations of up to 10° in any one axis produce only up to a 1% change in ODBA (although a 10% change in two or three axes simultaneously produces about a 1% and 0.03% change in ODBA, respectively). In fact, to produce a 5% change in ODBA requires skew placement of >20° in one or more axes, something which would be immediately obvious during many device attachment protocols. Thus, although many situations where loggers containing tri-axial accelerometers are attached to animals for derivation of energy expenditure will not have to worry overly about orientation [e.g. 47], there are a number of obvious situations, such as having round, unmarked devices in which the orientation of the transducers is unclear and placing devices via suction cups onto whales [48], [49] that should only use VeDBA. Importantly though, the latter situation is also likely to incur substantial additional errors in any derivation of DBA and its relation to 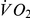 resulting from the non-standardized positioning of the device on the body relative to the animal's centre of gravity, which would markedly affect both ODBA and VeDBA signals [24]. This effect is apparent even in our results on humans where, despite placing the straight and skew tag as closely together as we could, the correlation coefficient between
resulting from the non-standardized positioning of the device on the body relative to the animal's centre of gravity, which would markedly affect both ODBA and VeDBA signals [24]. This effect is apparent even in our results on humans where, despite placing the straight and skew tag as closely together as we could, the correlation coefficient between 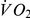 and VeDBA for straight and skewed tag orientations was (marginally) different. Allusion to this phenomenon, in an albeit simplistic form, can be accessed via equation (7) which shows the extent to which distance from the pivot point (or distance from the moving body part such as a fish's tail [24]) affects DBA.
and VeDBA for straight and skewed tag orientations was (marginally) different. Allusion to this phenomenon, in an albeit simplistic form, can be accessed via equation (7) which shows the extent to which distance from the pivot point (or distance from the moving body part such as a fish's tail [24]) affects DBA.
Figure 7. Predicted difference between straight- and skew-mounted ODBA derived from recordings on a tri-axial accelerometer subjected to equal acceleration in the heave, surge and sway axes as a function of pitch, roll and yaw differences between straight and skew.
Contour lines show 2.5% intervals.
Overall, our results showed that ODBA was insensitive to the accelerometer when its axes were skewed off the major axes of the body (by 30° in roll, pitch and yaw), which we attribute to the general variance between 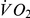 and DBA and the fact that the degree of skew tested was insufficient to elicit a marked difference in the way ODBA reacts to changes in orientation. Thus, against predictions based on physical theory, our study indicates that ODBA is, in fact, not worse than VeDBA at predicting
and DBA and the fact that the degree of skew tested was insufficient to elicit a marked difference in the way ODBA reacts to changes in orientation. Thus, against predictions based on physical theory, our study indicates that ODBA is, in fact, not worse than VeDBA at predicting 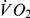 ; if anything it is better (though the difference is minimal) as long as devices can be attached close to the major axes of the body. This indicates that whether researchers use ODBA or VeDBA may not be the most critical issue in treatment of DBA signals and
; if anything it is better (though the difference is minimal) as long as devices can be attached close to the major axes of the body. This indicates that whether researchers use ODBA or VeDBA may not be the most critical issue in treatment of DBA signals and 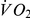 because variation in device positioning is likely to introduce much more variability [39]. Future work will have to address this aspect more carefully. For the moment, researchers should certainly be working towards positioning their devices in the same anatomical location as far as possible and, with the exception of a few species such as cetaceans [47] and animals that have tags implanted [50], this will tend to lead to device orientation being correct anyway. There are other particularly germane reasons for researchers to orientate devices on their study species in a comparable manner. In particular, it underpins powerful behaviour identification protocols based on posture and dynamic acceleration [51], a process which, itself, requires device orientation to be controlled rigorously. Subsequently, both the known behaviour and the ODBA-derived estimate of metabolic rate can be used to determine activity-specific metabolic rate. VeDBA would, however, clearly be a better proxy of
because variation in device positioning is likely to introduce much more variability [39]. Future work will have to address this aspect more carefully. For the moment, researchers should certainly be working towards positioning their devices in the same anatomical location as far as possible and, with the exception of a few species such as cetaceans [47] and animals that have tags implanted [50], this will tend to lead to device orientation being correct anyway. There are other particularly germane reasons for researchers to orientate devices on their study species in a comparable manner. In particular, it underpins powerful behaviour identification protocols based on posture and dynamic acceleration [51], a process which, itself, requires device orientation to be controlled rigorously. Subsequently, both the known behaviour and the ODBA-derived estimate of metabolic rate can be used to determine activity-specific metabolic rate. VeDBA would, however, clearly be a better proxy of 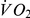 than ODBA where device orientation cannot be maintained within a 30° arc in any of the angular dimensions and so should be used when loggers cannot be implanted [52] reasonably precisely, or attached reasonably precisely [50], or are ingested [e.g. 53]. Importantly though, determination of behaviour using inconsistently placed accelerometers is more problematic.
than ODBA where device orientation cannot be maintained within a 30° arc in any of the angular dimensions and so should be used when loggers cannot be implanted [52] reasonably precisely, or attached reasonably precisely [50], or are ingested [e.g. 53]. Importantly though, determination of behaviour using inconsistently placed accelerometers is more problematic.
Conclusions
The assumption that DBA derived by a vectorial rather than an absolute summation is more appropriate as a proxy for 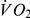 is not founded for devices mounted in a standardized manner and issues of force generation by muscles likely account for this rather than just the physics associated with measures of acceleration. ODBA and VeDBA are very closely correlated with each other and both can be excellent proxies for movement-based metabolic rate. Proponents of DBA as a proxy of metabolic rate must choose which derivation to use based on (a) the value they place on the derivation representing the biology of muscle metabolism (b) whether they are concerned that logger orientation could vary markedly (c) whether they wish to compare their DBA values with values in the literature. Critically, neither ODBA nor VeDBA deals with the problem of variation in device positioning.
is not founded for devices mounted in a standardized manner and issues of force generation by muscles likely account for this rather than just the physics associated with measures of acceleration. ODBA and VeDBA are very closely correlated with each other and both can be excellent proxies for movement-based metabolic rate. Proponents of DBA as a proxy of metabolic rate must choose which derivation to use based on (a) the value they place on the derivation representing the biology of muscle metabolism (b) whether they are concerned that logger orientation could vary markedly (c) whether they wish to compare their DBA values with values in the literature. Critically, neither ODBA nor VeDBA deals with the problem of variation in device positioning.
Acknowledgments
We are grateful to Mr. Lindsay A. D'Silva and Dr. Michael J. Lewis for helpful discussions and to Oliver Stroud for doing his stuff.
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflicts: They declare that part of the funding for this work was obtained through a grant to Rory Wilson from the Rolex Awards for Enterprise. Note that Lewis Halsey is a co-author and is an editor for PLoS ONE. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This study was supported by the Rolex Awards for Enterprise and Damascus University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bartumeus F, Catalan J. Optimal search behavior and classic foraging theory. Journal of Physics a-Mathematical and Theoretical. 2009;42 [Google Scholar]
- 2.Pyke GH. Optimal Foraging Theory: A Critical Review. Annual Review of Ecology and Systematics. 1984;15:523–575. [Google Scholar]
- 3.Murray MG. Maximizing Energy Retention in Grazing Ruminants. Journal of Animal Ecology. 1991;60:1029–1045. [Google Scholar]
- 4.Bergman CM, Fryxell JM, Gates CC, Fortin D. Ungulate foraging strategies: energy maximizing or time minimizing? Journal of Animal Ecology. 2001;70:289–300. [Google Scholar]
- 5.Shipley LA. The influence of bite size on foraging at larger spatial and temporal scales by mammalian herbivores. Oikos. 2007;116:1964–1974. [Google Scholar]
- 6.May R, van Dijk J, Landa A, Andersen R. Spatio-temporal ranging behaviour and its relevance to foraging strategies in wide-ranging wolverines. Ecological Modelling. 2010;221:936–943. [Google Scholar]
- 7.Shepard ELC, Wilson RP, Liebsch N, Quintana F, Laich AG, et al. Flexible paddle sheds new light on speed: a novel method for the remote measurement of swim speed in aquatic animals. Endangered Species Research. 2008;4:157–164. [Google Scholar]
- 8.Alexander RMN. Principles of animal locomotion. Princeton: Princeton University Press; 2003. [Google Scholar]
- 9.Wilson RP, Grémillet D, Syder J, Kierspel MAM, Garthe S, et al. Remote-sensing systems and seabirds: their use, abuse and potential for measuring marine environmental variables. Marine Ecology Progress Series. 2002;228:241–261. [Google Scholar]
- 10.Halsey LG, Fahlman A, Handrich Y, Schmidt A, Woakes AJ, et al. How accurately can we estimate energetic costs in a marine top predator, the king penguin? Zoology. 2007;110:81–92. doi: 10.1016/j.zool.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RP, Shepard ELC, Liebsch N. Prying into the intimate details of animal lives: use of a daily diary on animals. Endangered Species Research. 2007;4:123–137. [Google Scholar]
- 12.Halsey LG, Shepard ELC, Quintana F, Gomez Laich A, Green JA, et al. The relationship between oxygen consumption and body acceleration in a range of species. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology. 2009;152:197–202. doi: 10.1016/j.cbpa.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Nagy KA, Siegfried WR, Wilson RP. Energy utilization by free-ranging jackass penguins, Spheniscus demersus. Washington, DC,, ETATS-UNIS: Ecological Society of America; 1984. [Google Scholar]
- 14.Nagy KA. Field Metabolic Rate and Food Requirement Scaling in Mammals and Birds. Ecological Monographs. 1987;57:112–128. [Google Scholar]
- 15.Speakman JR, Racey PA. The doubly-labelled water technique for measurement of energy expenditure in free-living animals. Science Progress Oxford. 1988;72:227–237. [PubMed] [Google Scholar]
- 16.Nagy KA. Food requirements of wild animals: predictive equations for freeliving mammals, reptiles, and birds. Nutrition Abstracts and Reviews Series B: Livestock Feeds and Feeding. 2001;71:21–31. [Google Scholar]
- 17.Shaffer SA, Costa DP, Weimerskirch H. Field metabolic rates of black-browed albatrosses Thalassarche melanophrys during the incubation stage. Journal of Avian Biology. 2004;35:551–558. [Google Scholar]
- 18.Frappell PB, Blevin HA, Baudinette RV. Understanding respirometry chambers: What goes in must come out. Journal of Theoretical Biology. 1989;138:479–494. doi: 10.1016/s0022-5193(89)80046-3. [DOI] [PubMed] [Google Scholar]
- 19.Owen RB. Heart rate, a measure of metabolism in blue-winged teal. Comparative Biochemistry and Physiology. 1969;31:431–436. doi: 10.1016/0010-406x(69)90024-3. [DOI] [PubMed] [Google Scholar]
- 20.Butler PJ, Woakes AJ, Boyd IL, Kanatous S. Relationship between heart rate and oxygen consumption during steady-state swimming in California sea lions. Journal of Experimental Biology. 1992;170:35–42. doi: 10.1242/jeb.170.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Bevan RM, Butler PJ, Woakes AJ, Boyd IL. The energetics of Gentoo Penguins, Pygoscelis papua, during the breeding season. Functional Ecology. 2002;16:175–190. [Google Scholar]
- 22.Green JA, Butler PJ, Woakes AJ, Boyd IL. Energy requirements of female Macaroni Penguins breeding at South Georgia. Functional Ecology. 2002;16:671–681. [Google Scholar]
- 23.Froget G, Butler PJ, Woakes AJ, Fahlman A, Kuntz G, et al. Heart rate and energetics of free-ranging king penguins (Aptenodytes patagonicus). Journal of Experimental Biology. 2004;207:3917–3926. doi: 10.1242/jeb.01232. [DOI] [PubMed] [Google Scholar]
- 24.Gleiss AC, Wilson RP, Shepard ELC. Making overall dynamic body acceleration work: on the theory of acceleration as a proxy for energy expenditure. Methods in Ecology and Evolution. 2011;2:23–33. [Google Scholar]
- 25.Halsey LG. The challenge of measuring energy expenditure: Current field and laboratory methods. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology. 2011;158:247–251. doi: 10.1016/j.cbpa.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Terrier P, Ladetto Q, MermInod B, Schutz Y. Measurement of the mechanical power of walking by satellite positioning system (GPS). Medicine & Science in Sports & Exercise. 2001;33:1912–1918. doi: 10.1097/00005768-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Pfau T, Witte TH, Wilson AM. Centre of mass movement and mechanical energy fluctuation during gallop locomotion in the Thoroughbred racehorse. Journal of Experimental Biology. 2006;209:3742–3757. doi: 10.1242/jeb.02439. [DOI] [PubMed] [Google Scholar]
- 28.Cavagna GA, Kaneko M. Mechanical work and efiiciency in level walking and running. Journal of Physiology-London. 1963;268:467–481. doi: 10.1113/jphysiol.1977.sp011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatner P, Bryant DM. Flight Cost of a Small Passerine Measured Using Doubly Labeled Water: Implications for Energetics Studies. The Auk. 1986;103:169–180. [Google Scholar]
- 30.Boisclair D, Siros P. The importance of activity in bioenergetics modelsapplied to actively foraging fishes. Canadian Journal of Fisheries and Aquatic Sciences. 1989;46:1856–1876. [Google Scholar]
- 31.Karasov WH. Daily energy expenditure and the cost of activity in mammals 1. Integrative and Comparative Biology. 1992;32:238–248. [Google Scholar]
- 32.Meijer GA, Westerterp KR, Koper H, Ten Hoor F. Assessment of energy expenditure by recording heart rate and body acceleration. Medicine& Science in Sports & Exercise. 1989;21:343. [PubMed] [Google Scholar]
- 33.Bouten CV, Westerterp KR, Verduin M, Janssen JD. Assessment of energy expenditure for physical activity using a triaxial accelerometer. Medicine & Science in Sports & Exercise. 1994;26:1516–1523. [PubMed] [Google Scholar]
- 34.Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, et al. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. Journal of Animal Ecology. 2006;75:1081–1090. doi: 10.1111/j.1365-2656.2006.01127.x. [DOI] [PubMed] [Google Scholar]
- 35.Gleiss AC, Dale JJ, Holland KN, Wilson RP. Accelerating estimates of activity-specific metabolic rate in fishes: Testing the applicability of acceleration data-loggers. Journal of Experimental Marine Biology and Ecology. 2010;385:85–91. [Google Scholar]
- 36.Halsey LG, White CR. Measuring Energetics and Behaviour Using Accelerometry in Cane Toads Bufo marinus. PLoS ONE. 2010;5:e10170. doi: 10.1371/journal.pone.0010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halsey LG, Portugal SJ, Smith JA, Murn CP, Wilson RP. Recording raptor behavior on the wing via accelerometry Grabando la conducta de rapaces a vuelo vía acelerometría. Journal of Field Ornithology. 2009;80:171–177. [Google Scholar]
- 38.Green JA, Halsey LG, Wilson RP, Frappell PB. Estimating energy expenditure of animals using the accelerometry technique: activity, inactivity and comparison with the heart-rate technique. Journal of Experimental Biology. 2009;212:471–482. doi: 10.1242/jeb.026377. [DOI] [PubMed] [Google Scholar]
- 39.Halsey LG, Shepard ELC, Hulston CJ, Venables MC, White CR, et al. Acceleration versus heart rate for estimating energy expenditure and speed during locomotion in animals: Tests with an easy model species, Homo sapiens. Zoology. 2008;111:231–241. doi: 10.1016/j.zool.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 40.McGregor SJ, Busa MA, Yaggie JA, Bollt EM. High Resolution MEMS Accelerometers to Estimate VO2 and Compare Running Mechanics between Highly Trained Inter-Collegiate and Untrained Runners. PLoS ONE. 2009;4:e7355. doi: 10.1371/journal.pone.0007355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. Journal of Applied Physiology. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto Y, Miyashita M, Hughson R, Tamura S-i, Shinohara M, et al. The ventilatory threshold gives maximal lactate steady state. European Journal of Applied Physiology and Occupational Physiology. 1991;63:55–59. doi: 10.1007/BF00760802. [DOI] [PubMed] [Google Scholar]
- 43.Gómez Laich A, Wilson RP, Gleiss AC, Shepard ELC, Quintana F. Use of overall dynamic body acceleration for estimating energy expenditure in cormorants: Does locomotion in different media affect relationships? Journal of Experimental Marine Biology and Ecology. 2011;399:151–155. [Google Scholar]
- 44.Zajac FE, Neptune RR, Kautz SA. Biomechanics and muscle coordination of human walking: Part I: Introduction to concepts, power transfer, dynamics and simulations. Gait & Posture. 2002;16:215–232. doi: 10.1016/s0966-6362(02)00068-1. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt-Nielsen K. Locomotion: Energy Cost of Swimming, Flying, and Running. Science. 1972;177:222–228. doi: 10.1126/science.177.4045.222. [DOI] [PubMed] [Google Scholar]
- 46.Zhang K, PI-Sunyer FX, Boozer CN. Improving Energy Expenditure Estimation for Physical Activity. Medicine & Science in Sports & Exercise. 2004;36:883–889. doi: 10.1249/01.mss.0000126585.40962.22. [DOI] [PubMed] [Google Scholar]
- 47.Shepard ELC, Wilson RP, Quintana F, Gómez Laich A, Forman DW. Pushed for time or saving on fuel: fine-scale energy budgets shed light on currencies in a diving bird. Proceedings of the Royal Society B: Biological Sciences. 2009;276:3149–3155. doi: 10.1098/rspb.2009.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson MP, Tyack PL. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. Oceanic Engineering, IEEE Journal of. 2003;28:3–12. [Google Scholar]
- 49.Hooker SK, Baird RW. Deep–diving behaviour of the northern bottlenose whale, Hyperoodon ampullatus (Cetacea: Ziphiidae). Proceedings of the Royal Society of London Series B: Biological Sciences. 1999;266:671–676. [Google Scholar]
- 50.Sakai M, Aoki K, Sato K, Amano M, Baird RW, et al. Swim speed and acceleration measurements of short-finned pilot whales (Globicephala macrorhynchus) in Hawai'i. Mammal Study. 2011;36:55–59. [Google Scholar]
- 51.Shepard ELC, Wilson RP, Albareda D, Glesis A, Laich AG, et al. Identification of animal movement patterns using tri-axial accelerometry. Endang Species Res. 2008;10:47–60. [Google Scholar]
- 52.Clark T, Sandblom E, Hinch S, Patterson D, Frappell P, et al. Simultaneous biologging of heart rate and acceleration, and their relationships with energy expenditure in free-swimming sockeye salmon Oncorhynchus nerka. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2010;180:673–684. doi: 10.1007/s00360-009-0442-5. [DOI] [PubMed] [Google Scholar]
- 53.Gleiss AC, Jorgensen SJ, Liebsch N, Sala JE, Norman B, et al. evolution in locomotory patterns of flying and swimming animals. Nature Communications. in press doi: 10.1038/ncomms1350. [DOI] [PubMed] [Google Scholar]