Abstract
Objective:
Adolescent problem behaviors such as conduct disorder and attention-deficit/hyperactivity disorder (ADHD) are often associated with alcohol problems in adulthood, particularly alcohol dependence. This association is partly a result of shared genetic liability. However, it is unclear whether ADHD, or an ADHD subtype, shares genetic influences with alcohol dependence beyond those also shared by conduct disorder.
Method:
We evaluated phenotypic associations between adolescent conduct disorder and ADHD phenotypes with adult alcohol dependence in a population-based sample of adult male twins (N = 1,774). We then assessed genetic and environmental relationships among phenotypes using structural equation modeling.
Results:
Individually, conduct disorder and each ADHD factor were associated with adult alcohol dependence. Results from twin modeling indicate that a genetic factor common to conduct disorder and ADHD also loads strongly onto alcohol dependence. Even after controlling for genetic factors shared with conduct disorder and other ADHD factors, the hyperactivity component of ADHD shared significant residual genetic influences with alcohol dependence.
Conclusions:
Most of the genetically mediated association between adolescent ADHD and adult alcohol dependence is shared with conduct disorder, reflecting a generalized risk to externalizing behaviors. The significant residual genetic covariance between the ADHD factor hyperactivity/impulsivity and alcohol dependence implies that impulsive behaviors less destructive/harmful than those manifested by conduct disorder can be indicative of genetic risk for adult alcohol dependence. However, the ADHD factors inattention and forgetfulness are not uniquely predictive of genetic/environmental risk for alcohol dependence.
Epidemiological studies have consistently identified a phenotypic association between adolescent problem behaviors and adult alcohol use. In particular, conduct disorder (CD) and adolescent attention-deficit hyperactivity disorder (ADHD) are often correlated with later alcohol use disorders, especially alcohol dependence (AD) (Arias et al., 2008; Friedrichs et al., 2010; Knop et al., 2009; Langley et al., 2010; Weiss et al., 1985; Whalen et al., 2002; White et al., 2001). Each of these disorders is characterized to some extent by externalizing behavior, raising the possibility that a general liability to such behavior contributes to the association among phenotypes.
Evidence from twin studies and molecular genetic studies indicates that some portion of the observed association is due to a shared genetic liability among disorders. Multiple reports support the existence of a substantial genetic correlation (rG) between CD or adult antisocial behavior and AD (Haber et al., 2010; Kendler et al., 2003; Slutske et al., 1998; Young et al., 2000), with estimates ranging from rG ≈ 4 to .8. Studies examining shared genetic liability between ADHD and AD are less common. A genetic correlation between these phenotypes has been reported in adults (Knopik et al., 2006; Young et al., 2000), although such a relationship was not detected in a sample of adolescents (Knopik et al., 2009). That a genetic correlation between ADHD and AD is detected in adults but not in adolescents parallels findings for CD and AD; although the genetic correlation between CD and adult AD is robust (see above), the relationship between CD and adolescent AD symptoms is largely environmentally mediated (Rose et al., 2004). Thus, these childhood or adolescent behavioral disorders are potentially useful indicators of genetic risk of adult AD but might be less informative of genetic risk of earlier alcohol problems.
In addition to shared latent genetic influences, molecular and statistical genetic studies have identified specific genes in which allelic variation has been associated with both CD and AD, or with ADHD and AD. These include DAT1 (Sharp et al., 2009; van der Zwaluw et al., 2009), GABRA2 (Dick et al., 2006; Edenberg et al., 2004), CHRM2 (Dick et al., 2008), and CDH13 (Franke et al., 2009; Treutlein et al., 2009). Furthermore, Dick and colleagues (2010a) identified 23 genes on chromosome 2 that harbor markers associated with a “behavioral disinhibition” phenotype in which individuals were considered “affected” if they were alcohol dependent and had either met criteria for CD or had attempted suicide. Although different variants within each gene might be associated with these outcomes, such findings reinforce the notion that common genetic factors or networks underlie the phenotypic associations among CD, ADHD, and AD. These shared genetic influences could generally influence externalizing, even if those tendencies manifest in different ways (e.g., CD in one individual, ADHD in another, and a comorbid phenotype in a third).
Despite much progress in this area, unanswered questions remain with regard to the relationship among CD, ADHD, and AD. For example, because CD and ADHD are genetically correlated (Dick et al., 2005; Knopik et al., 2009; Silberg et al., 1996; Young et al., 2000), it is not clear whether ADHD is independently genetically correlated with AD or whether most (or all) of the genetic variation shared between ADHD and AD is also common to CD. This is an important distinction. Is shared liability limited to a genetic factor that broadly influences multiple externalizing pheno-types (including adult AD), or are the various manifestations of externalizing behavior (CD, ADHD, etc.) differentially genetically informative? This point is applicable within the range of ADHD phenotypes as well. The multidimensional nature of ADHD raises the question of whether any genetic correlation between ADHD and AD is specific to a particular component of the disorder. For example, we might hypothesize that the association is primarily related to hyperactive/ impulsive manifestations of ADHD and less relevant to the inattentive subtype. This distinction has potential clinical relevance; if ADHD subtypes are differentially indicative of genetic risk to adult alcohol problems, treatment and prevention efforts could be tailored accordingly.
Here, we report findings from a population-based, genetically informative sample of adult men regarding the genetic relationship between AD and adolescent ADHD and CD. The primary goal of the study was to determine whether AD shares genetic and/or environmental influences with ADHD in addition to those that are accounted for by CD. Do we gain information about liability to AD by considering ADHD in addition to CD? In particular, we examined whether different phenotypic factors of ADHD are differentially related, genetically or environmentally, to AD. By separating ADHD into phenotypic dimensions (e.g., hyperactivity and inattention) and including these components alongside CD in a single model, we can determine the specificity of genetic factors shared between adult AD and each manifestation of adolescent externalizing behavior.
Method
Sample
Participants were part of the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders, which has been previously described (Kendler and Prescott, 2006). The current sample consisted of male pairs who participated in Waves 2 and 3 of the study (comparable externalizing data were not collected on female pairs). This use of data across different assessments reduces the possibility of occasion-specific reporter bias. The sample consisted of 1,774 individuals, all with complete data and of known zygosity, comprising 1,073 monozygotic and 701 dizygotic twins. There were 458 complete monozygotic pairs and 282 complete dizygotic pairs; 294 individuals were members of incomplete pairs. Zygosity was determined using a combination of self-report measures, photographs, and genotyping (Kendler and Prescott, 2006). Mean age in Wave 2 was 36.1 years (range: 20–57 years); in Wave 3, mean age was 40.3 years (range: 24–62 years). The project received human subjects approval from Virginia Commonwealth University, and participants provided informed consent before all interviews.
Measures
Conduct disorder.
Data for the current analyses were obtained in Wave 2 (AD) and Wave 3 (ADHD and CD). Participants were asked 11 items corresponding to CD symptoms and were further asked to report on these symptoms during three age ranges: 8–11 years, 12–14 years, and 15–17 years. A mean symptom count was computed across these age ranges for preliminary analyses; this mean was converted into an ordinal symptom score ranging from 0 to 4 for twin modeling (see below). For simplicity, we will refer to this phenotype as “CD”; however, the items administered did not include all of the possible CD diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR; American Psychiatric Association, 2000), and this phenotype does not represent a formal diagnosis.
Alcohol dependence.
AD was assessed according to lifetime DSM-IV diagnostic criteria, requiring that symptoms are temporally clustered. Individuals meeting diagnostic criteria received a score of 1; those who did not received a score of 0. Individuals who had never used alcohol were scored as missing the AD diagnosis.
Attention-deficit/hyperactivity disorder.
Participants were administered a scale to assess childhood/adolescent (before age 18) ADHD symptoms; these items were worded differently than were those aimed at assessing CD in that respondents were asked about symptoms they experienced before age 18, rather than being asked about three different age ranges. Each item corresponded approximately to a DSM symptom; however, as with the CD phenotype, the items administered did not include all possible ADHD diagnostic criteria and do not represent a formal diagnosis. Each item had four responses: never, rarely, sometimes, and often (scored 0, 1, 2, and 3, respectively). These scores were used to conduct a factor analysis (see below) in Mplus (Muthén and Muthén, 1998–2007), which indicated that the ADHD construct comprised three factors: inattention (INATT), hyperactivity/impulsivity (HYPER), and forgetfulness (FORGET). Three items loaded preferentially onto the INATT factor, five items loaded onto HYPER, and three items loaded onto FORGET. Broadly speaking, DSM symptoms of inattention were split across the INATT and FORGET factors, whereas DSM symptoms of hyperactivity/impulsivity were grouped together. Once the factor structure was determined, the original 11 items were recoded such that participants who reported that the item “sometimes” or “often” applied to them were considered to have endorsed that symptom and were given a score of 1 on that item; otherwise, they were given a score of 0. Each individual received a separate symptom count for each of the three factors identified previously; thus, a participant could have a symptom count of 0–3 for INATT, 0–5 for HYPER, and 0–3 for FORGET. These were treated as ordinal variables in twin analyses.
Statistical analyses
Descriptive statistics and preliminary analyses.
Descriptive statistics and preliminary analyses were obtained/ conducted using SAS 9.2 and JMP 8 (SAS Institute Inc., Cary, NC). When appropriate, significance levels were adjusted for the twin structure of the data using generalized estimating equations. Regressions (which included age at Wave 2 interview as a covariate) were conducted in which adolescent externalizing phenotypes were included as predictive variables, with adult AD as the outcome variable.
Factor analysis.
Factor analysis was conducted in MPlus 5.2. A quartimin rotation was used to accommodate correlations among factors. Based on results from a separate, unpublished analysis, and in agreement with model fit statistics, we selected the three-factor model. Results were similar regardless of whether items were coded as continuous or ordinal variables. As described above, we named the three factors INATT, HYPER, and FORGET, with three, five, and three items preferentially loading onto these factors, respectively. Additional information on the factor structure of ADHD in this sample is the topic of a forthcoming report and is thus not included here. Details are also available by request.
Twin modeling.
Twin modeling was conducted in Mx (Neale et al., 2006) using the raw ordinal data option. All twins were included in these analyses, including those who were members of incomplete pairs. In Mx, ordinal and continuous phenotypes cannot be included in the same model; because AD is necessarily an ordinal model, the CD and ADHD phenotypes had to be coded as ordinal variables as well. The use of ordinal data assumes that the categories are representative of an underlying normal distribution of liability, with thresholds in liability discriminating between categories.
In twin modeling, liability to phenotypes such as depression or alcohol use can be attributed to several latent sources of variance: additive genetic factors (A), shared environment (C), and unique environment (E). The C variance component represents environmental exposures and experiences that are shared by both members of a twin pair and contribute to twins’ increased similarity, irrespective of zygosity, in a given phenotype. Environmental factors that are unique to one twin are accounted for by the E component; these factors reduce twin similarity for a given phenotype. The E component also includes measurement error. Estimates of each of these variance components are calculated by comparing the phenotypic correlation between monozygotic twins (who share all their genes), with dizygotic twins (who share half of their genes, on average, identical by descent). In the current sample, phenotypic correlations were higher for monozygotic twins than for dizygotic twins for each phenotype, suggesting that genetic variance contributes to manifestation of these phenotypes.
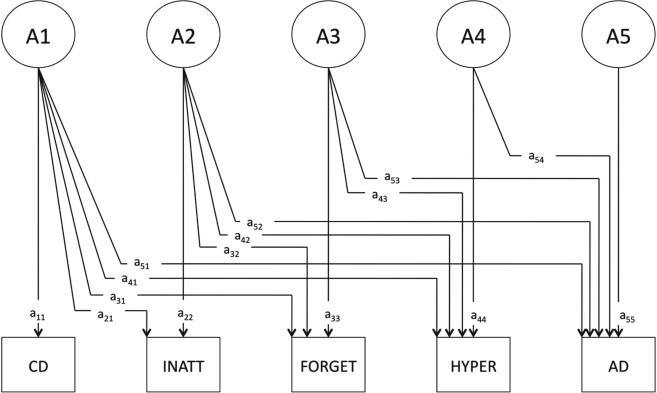
We used three Cholesky decomposition models, each with five observed variables: CD, INATT, FORGET, HYPER, and AD (see Figure 1 for an example). The models were constructed such that CD was the first (leftmost) variable and AD was the last (right-most) variable; the order of the ADHD factors varied across models such that a different ADHD phenotypic factor was immediately adjacent to AD in each of the three models. The ordering of the variables in the model made it possible to determine the degree to which each ADHD factor shared genetic influences with AD after genetic influences shared with CD and the other ADHD factors had been accounted for (i.e., allowed testing for residual genetic covariance). This structure is analogous to a regression model in which CD and other ADHD factors are included as covariates. An identical structure existed for shared and unique environmental factors.
Figure 1.
Genetic factor structure of Cholesky decomposition. The model is constructed such that A1 represents a genetic factor that can load onto all five observed variables, A2 loads onto all three ADHD phenotypic factors (inattention [INATT], forgetfulness [FORGET], and hyperactivity/impulsivity [HYPER]) as well as alcohol dependence (AD), etc. Path a54 represents genetic covariance specific to the HYPER factor and AD. A model that is identical except for the juxtaposition of HYPER and FORGET was used to assess genetic covariance specific to FORGET and AD; likewise for INATT. CD = conduct disorder.
Based on prior reports of positive genetic correlations among phenotypes (Slutske et al., 1998; Young et al., 2000), we restricted genetic covariance among all phenotypes to be greater than or equal to zero. This constraint decreases the risk of statistical artifact, and when compared with the corresponding model without such a restriction, model fit statistics did not differ substantially (difference in -2 log likelihood = 0.163). The full model included A, C, and E influences. Sampling only twins, we cannot simultaneously model C and dominance (D) genetic effects; furthermore, our sample size does not provide sufficient power for the robust detection of D variance (Martin et al., 1978). Models included age at Wave 2 interview as a covariate. Submodels were compared with the full model (the genetic component of which is depicted in Figure 1) using the p value of the chi-square and Akaike’s Information Criterion (AIC; Akaike, 1987) as an indicator of model fit and parsimony.
Results
Descriptive statistics
The mean (SE) CD score was 0.76 (0.02); these scores ranged from 0 to 7.33 out of a possible 11. Nearly one quarter (n = 419, 23.6%) of the sample met lifetime criteria for AD. As expected, all adolescent externalizing phenotypes were phenotypically correlated (r = .30–.54). In unadjusted regressions, mean CD and all three ADHD factors were each significantly (p<.01) associated with AD (Table 1). We then conducted regressions in which mean CD, INATT, HYPER, and FORGET scores were all included in the model to predict AD, to determine whether any ADHD factor had predictive value above and beyond that of CD. Mean CD score and HYPER score, but not INATT and FORGET scores, remained significantly associated with AD in this adjusted model.
Table 1.
Phenotypic associations between adolescent externalizing phenotypes and the outcome variable, adult AD diagnosis. See the Method section for details.
| Predictive variable | Unadjusted regressionsa β estimate (SE) | Adjusted regressionsb β estimate (SE) |
| Mean CD | .37 (.06), p < .01 | .31 (.06), p < .01 |
| FORGET | .15 (.06), p < .01 | -.05 (.06), p = .40 |
| INATT | .23(.06), p < .01 | .10 (.07), p = .14 |
| HYPER | .25(.04), p < .01 | .17 (.05), p < .01 |
Notes: AD = alcohol dependence; CD = conduct disorder; FORGET = forgetfulness; INATT = inattention; HYPER = hyperactivity/impulsivity.
In unadjusted regressions, only age at Wave 2 interview is included as a covariate with each externalizing phenotype;
adjusted regressions include all externalizing phenotypes as predictive variables and age as a covariate.
Twin modeling
Establishment of the full model.
We next fit twin models as described in the Method section. The fit of the full models differed slightly (by <1.3 AIC units) depending on the order of the ADHD factors. Based on prior evidence of potential shared environmental influences on CD (Jacobson et al., 2000), we first tested whether the C path specific to CD (y11) could be set to 0. This resulted in a deterioration in model fit (Table 2; Model 2 in A, B, and C). However, shared environmental influences could be dropped from the other phenotypes (Table 2; Model 3 in A, B, and C). Submodels were fit within this reduced model with the specific aim of testing whether each ADHD factor shared genetic (A) or environmental (E) influences with AD beyond those that were shared with CD and the other ADHD phenotypic factors. This was accomplished by testing the significance of paths a54 and e54.
Table 2.
Tests of genetic and environmental covariance between ADHD factors and AD
| A. Variable order: CD–INATT–HYPER–FORGET–AD; tests relationship between FORGET and AD | ||||||
| Model no. | Model description | Model comparison | Δχ2 | Δdf | p | ΔAIC |
| 1 | Full model | N.A. | (20,250.29) | (8,707) | N.A. | (2,836.29) |
| 2 | Drop C influences on CD | 2 vs. 1 | 7.06 | 1 | <.01 | 5.06 |
| 3 | Drop C influences on other phenotypes | 3 vs. 1 | 6.00 | 14 | .97 | −22.00 |
| 4 | Set genetic covariance between FORGET and AD to 0 | 4 vs. 3 | 1.49 | 1 | .22 | −0.51 |
| 5 | Set environmental covariance between FORGET and AD to 0 | 5 vs. 4 | 3.46 | 1 | .06 | 1.46 |
| B. Variable order: CD–HYPER–FORGET–INATT–AD; tests relationship between | ||||||
| Model no. | Model description | Model comparison | Δχ2 | Δdf | p | ΔAIC |
| 1 | Full model | N.A. | (20,251.44) | (8,707) | N.A. | (2,837.44) |
| 2 | Drop C influences on CD | 2 vs. 1 | 7.13 | 1 | .01 | 5.13 |
| 3 | Drop C influences on other phenotypes | 3 vs. 1 | 8.38 | 14 | .87 | −19.62 |
| 4 | Set genetic covariance between INATT and AD to 0 | 4 vs. 3 | 0.05 | 1 | .83 | −1.95 |
| 5 | Set environmental covariance between INATT and AD to 0 | 5 vs. 4 | 0.65 | 1 | .42 | −1.35 |
| C. Variable order: CD–INATT–FORGET–HYPER–AD; tests relationship between HYPER and AD | ||||||
| Model no. | Model description | Model comparison | Δχ2 | Δdf | p | ΔAIC |
| 1 | Full model | N.A. | (20,250.34) | (8,707) | N.A. | (2,836.34) |
| 2 | Drop C influences on CD | 2 vs. 1 | 7.01 | 1 | <.01 | 5.01 |
| 3 | Drop C influences on other phenotypes | 3 vs. 1 | 6.03 | 14 | .99 | −21.97 |
| 4 | Set genetic covariance between HYPER and AD to 0 | 4 vs. 3 | 8.21 | 1 | <.01 | 6.21 |
| 5 | Set environmental covariance between HYPER and AD to 0 | 5 vs. 4 | 0.02 | 1 | .88 | −1.98 |
Notes: In each case, the full model is as described in the Method section; the order of the variables is different in A, B, and C, but the model structure is identical. ADHD = attention-deficit/hyperactivity disorder; AD = alcohol dependence; CD = conduct disorder; INATT = inattention; HYPER = hyperactivity/impulsivity; FORGET = forgetfulness; AIC = Akaike’s Information Criterion; N.A. = not applicable.
Genetic influences.
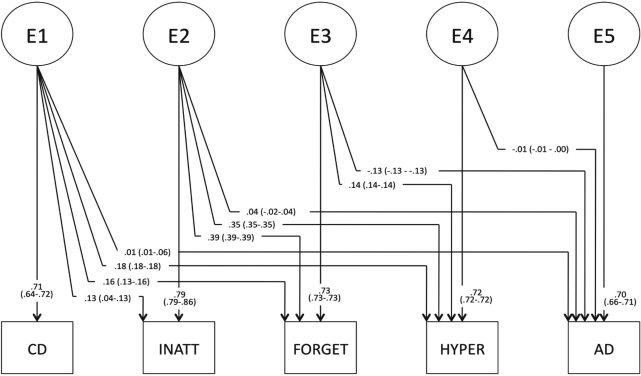
Genetic path estimates, before testing whether path a54 could be fixed to 0, are provided in Figure 2A. Table 3 provides genetic and environmental variance and covariance estimates from the full model, in which the ADHD factors were ordered INATT-FORGET-HYPER. The total genetic covariance between ADHD factors and AD ranged from .19 to .25, with the highest covariation between AD and HYPER. The corresponding genetic correlations are moderate (rG = .44–.65). Genetic covariance specific to each factor and AD was low (.00–.13). Total genetic covariance between CD and AD was similar to that between AD and each of the ADHD factors at .28, corresponding to rG = .76. Genetic covariance specific to CD and AD was low at .08 (29% of the total genetic covariance). The heritabilities of each phenotype are presented in Table 3.
Figure 2A.
Genetic (Figure 2A) and unique environmental (Figure 2B) path estimates (95% confidence intervals) for the full model testing the relationship between hyperactivity/impulsivity (HYPER) and alcohol dependence (AD), after setting shared environmental factor loadings to 0 (except that loading onto conduct disorder [CD]), but before testing the significance of genetic and unique environmental covariances specific to each attention-deficit/hyperactivity (ADHD) factor and AD. Although path estimates differed slightly depending on the order of ADHD factors, these estimates are generally representative. The shared environmental path estimate loading onto CD was .33 (.33–.43) (not pictured). INATT = inattention; FORGET = forgetfulness.
Table 3.
Genetic and unique environmental variances and covariances
| Genetic influences |
Environmental influences |
|||||
| Phenotype | Total variance | Total covariance with AD | Covariance shared only with AD | Total variance | Total covariance with AD | Covariance shared only with AD |
| CDa | .28 | .28 | .08 | .51 | <.01 | <.01 |
| FORGET | .29 | .22 | .05 | .71 | -.08 | -.09 |
| INATT | .34 | .19 | .00 | .66 | <.01 | .03 |
| HYPER | .31 | .25 | .13 | .69 | <-.01 | <-.01 |
| ADb | .50 | N.A. | N.A. | .50 | N.A. | N.A. |
Notes: The total variance, total covariance with AD, and covariance specific to each phenotype with alcohol dependence (AD) are presented; both are derived from models in which the phenotype is adjacent to AD in the twin model, which is required for the calculation of the residual variance. Estimates are based on full models, before testing the significance of genetic and environmental covariance specific to AD and the other phenotypes.
Shared environmental influences account for the remaining variance (21 %);
derived from a model structured as in Figure 1. CD = conduct disorder; FORGET = forgetfulness; INATT = inattention; HYPER = hyperactivity/impulsivity; N.A. = not applicable.
To test whether any of the ADHD factors shared genetic influences with AD beyond those shared with CD, we tested the significance of the genetic covariance path specific to AD and each ADHD factor in the model. By alternating the order of the ADHD factors in the model, we tested this relationship (represented by path a54 in Figure 1) for each factor (HYPER, FORGET, and INATT). Results from these tests are provided in Table 2. The genetic covariance specific to FORGET and AD, and that specific to INATT and AD, could be dropped from the model without significant deterioration of fit (Tables 2A and 2B, respectively). However, setting the genetic covariance specific to HYPER and AD to 0 resulted in a significant deterioration in fit (Table 2C).
Environmental influences.
Environmental path estimates, before model fitting, are provided in Figure 2B. Total environmental covariances between ADHD phenotypes and AD were very low, ranging from -.08 to <.01. Covariances specific to AD and each ADHD factor were similarly small. The total environmental variance of each ADHD factor was substantial, ranging from .65 to .71. Environmental influences accounted for less of the total variance of CD (.52) and AD (.50). Shared environmental factors accounted for 21% of the total variance of CD.
Figure 2B.
We tested the significance of unique environmental covariance specific to each ADHD factor and AD in the same manner that we tested the corresponding genetic covariance. Results are presented in Table 2. Setting the covariance between FORGET and AD to 0 resulted in a deterioration in fit (Table 2A), although the p value of the chi-square test was greater than .05; the environmental covariance between these two variables was negative. Unique environmental covariance between INATT and AD, and that between HYPER and AD, could be dropped without a significant deterioration in fit (Tables 2B and 2C).
Discussion
The primary goal of these analyses was to determine whether AD shares genetic and/or environmental influences with ADHD above and beyond those that are accounted for by CD. In other words, do we gain additional information about risk of adult AD by considering multiple manifestations of adolescent externalizing problems? Our results indicate that the majority of genetic liability shared by adult AD and adolescent externalizing is accounted for by a genetic factor shared across all phenotypes. However—and critical to our research question—the ADHD factor HYPER provides additional information about genetic risk to AD above and beyond this general externalizing factor. Therefore, consideration of multiple adolescent externalizing phenotypes can improve prediction of genetic risk to adult AD.
Results from twin modeling were largely consistent with those from intra-individual regression analyses, with twin modeling clarifying the etiology of phenotypic associations. Unadjusted regressions support previous reports of a phenotypic association between each manifestation of adolescent externalizing behavior and adult AD. Among the ADHD factors, this relationship with AD is strongest for HYPER, which remains significantly associated with outcome in adjusted regressions. Twin modeling revealed that genetic covariance is critical to the association between HYPER and AD. This relationship holds true even when influences shared with CD (i.e., factor loadings from A1) and with other ADHD factors (i.e., factor loadings from A2 and A3) are accounted for. The association between FORGET and AD—which is negative and is due to environmental factors—is more tenuous than that between HYPER and AD, as evidenced by the nonsignificant chi-square test of the environmental covariance between the former (Tables 2A and 2C). Therefore, we will focus our discussion on the apparently more robust, and genetically mediated, relationship between HYPER and AD.
Items loading onto the HYPER factor included, “How often did you have difficulty staying seated?” “How often did you find it hard to wait in line or wait your turn to do something?” and “How often did you interrupt or butt into the activities of others?” These items are designed to measure impulsivity; they are derived from the “hyperactivity–impulsivity” symptoms described in DSM-IV. Importantly, CD symptoms are largely related to harmful or destructive behavior and are not limited to impulsive behavior. Because HYPER shares genetic influences with AD beyond those accounted for by CD, we conclude that the genetic association between impulsivity and AD is not limited to influences specific to aggressive or delinquent behavior; rather, seemingly innocuous measures of impulsivity, such as those captured by HYPER, are uniquely predictive of genetic risk to AD.
Environmental correlations between AD and each measure of adolescent behavior were low, indicating that the phenotypic associations observed are primarily because of shared genetic liability. Another possible explanation for the observed phenotypic associations is that a causal relationship exists between adolescent problem behavior and later alcohol use problems. A path from genetic factors to ADHD and one from ADHD to AD is distinct from the current model, in which paths from the same genetic factors load onto both phenotypes. This could be the topic of future analyses, although it is typically difficult to determine whether a causal model or a shared liability model provides superior fit when the sources of variance do not differ between traits (Heath et al., 1993).
The genetic correlation between adolescent externalizing problems and adult AD is consistent with prior evidence linking a variety of impulsive behaviors—both disruptive/ destructive and more “harmless”—with alcohol misuse (Dick et al., 2010b). Our findings are also broadly consistent with findings from a prospective study of adolescents (Elkins et al., 2007), in which symptoms of the HYPER ADHD subtype were uniquely associated with alcohol initiation, even after controlling for the explanatory power of CD symptoms; symptoms of the inattentive subtype of ADHD did not uniquely predict initiation. However, important differences exist between that study and the current report: Elkins and colleagues did not incorporate a genetic component; there are potentially relevant differences in the ages at which the externalizing and alcohol-related phenotypes were assessed; and symptoms of the HYPER subtype did not uniquely predict age 18 alcohol abuse/ dependence (a slightly different phenotype than AD alone, used in the current report) in adjusted analyses. Furthermore, another recent phenotypic analysis of an adult sample found that the INATT ADHD subtype, but not the HYPER subtype, was significantly associated with AD (Friedrichs et al., 2010). These inconsistencies could reflect genuine population differences, changes in the relationships among phenotypes across the life span, or some other unknown factor; clearly, additional research is warranted.
We note that, despite the moderate genetic correlations among CD, ADHD factors, and AD, ∼41% of the total genetic variance of AD is AD-specific. Without incorporating measures of adult ADHD or antisocial personality disorder, or measures of adolescent alcohol problems into the model, we cannot determine whether this large proportion of unique genetic variance is a function of genetic influences related to development (that is, adolescence vs. adulthood) or whether it is capturing influences that are truly specific to the alcohol phenotype, beyond factors shared with hyperactivity/ impulsivity. Examples of AD-specific genes would be those involved in alcohol metabolism, such as alcohol dehydrogenase and aldehyde dehydrogenase (Kuo et al., 2008). A previous analysis, which included adult antisocial behavior but not adult ADHD in the model, suggests that AD-specific genetic variance is substantial (Kendler et al., 2003). Future analyses should attempt to replicate this finding when developmentally informative data are available.
In summary, these findings indicate that, although adolescent ADHD is phenotypically associated with adult AD, much of the shared genetic liability between these two disorders is shared broadly across multiple measures of adolescent externalizing behavior. Only one phenotypic ADHD factor, hyperactivity, is significantly genetically correlated with AD after factoring out influences shared with CD and the other ADHD factors. Somewhat unexpectedly, the environmental correlation between forgetfulness and AD approaches significance (and is negative), although overall environmental covariances among CD, ADHD, and AD were quite low. These findings are generally in agreement with research positing that externalizing behavior is the key commonality between adolescent behavior problems and adult alcohol problems. However, they also indicate that individual measures of adolescent externalizing share genetic influences with AD beyond those accounted for by this broad genetic factor. Specifically, the HYPER ADHD factor indexes risky impulsive behavior that is not captured by the harmful/destructive behaviors typical of CD; both of these manifestations of externalizing can be independently predictive of genetic risk of AD. Future molecular genetic studies seeking to identify genetic risk variants underlying ADHD and AD might benefit from focusing on the hyperactive/impulsive subtype. Likewise, clinicians concerned with adolescent behaviors indicative of adult alcohol problems should be aware of potential differences in predictive value across ADHD subtypes and should note that externalizing behaviors beyond those encompassed by CD are potentially relevant to risk.
Limitations
These results should be considered in the context of several limitations. First, reports of childhood/adolescent behavior were retrospective and might have been subject to recall bias; this could account for the fact that our heritability estimates of ADHD factors were lower than previous studies (Faraone et al., 2005), most of which reported estimates greater than or equal to 0.6. However, our estimate is in agreement with others from retrospective reports (Haberstick et al., 2008) and with an estimate of the heritability of adult ADHD (Boomsma et al., 2010). In addition, assessments from other reporters (parents, teachers) could reduce error in the measure of adolescent behavior; such reports are unavailable for this sample.
Other studies have modeled, and found support for, genetic dominance (D) effects underlying ADHD. However, we did not incorporate these effects into our models, in part because the sample size is likely insufficient for robust detection and interpretation of those effects (Martin et al., 1978). In addition, their inclusion would lead to unnecessarily complicated models. Twin correlations did not suggest D loadings onto CD or AD. C and D cannot be modeled simultaneously on a given phenotype using only twin data, and we prioritized the inclusion of C effects based on prior evidence in this sample of C influences on CD. Further, interpretation of D effects would have been peripheral to our primary research question, namely shared genetic influences between ADHD factors and AD, which would likely be quite minimal given the absence of evidence for D effects on AD. However, we do note that twin correlations were consistent with modest D influences on ADHD, and such influences will be explored in future work.
Because the range of the sum-score phenotype is higher for HYPER (which ranged from 0 to 5) than for the other ADHD factors (which ranged from 0 to 3), the HYPER phenotype might be a more powerful variable than FORGET and INATT. It is possible that differential sensitivity in assessment exists across the ADHD factors, which could influence the phenotypic associations and other results presented here. In addition, data were available only for men, and results might not be generalizable to women; not only do the phenotypes of interest differ by sex, but there could be significant qualitative differences in genetic and environmental influences on all phenotypes (Prescott et al., 1999). Last, the population used for these analyses is Caucasian, and the results might not be applicable to other ethnicities.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grants F32AA19849 (to Alexis C. Edwards), R37AA011408, and P20AA017828 (to Kenneth S. Kendler).
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision. 4th ed. Washington, DC: Author; 2000. [Google Scholar]
- Arias AJ, Gelernter J, Chan G, Weiss RD, Brady KT, Farrer L, Kranzler HR. Correlates of co-occurring ADHD in drug-dependent subjects: Prevalence and features of substance dependence and psychiatric disorders. Addictive Behaviors. 2008;33:1199–1207. doi: 10.1016/j.addbeh.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma DI, Saviouk V, Hottenga J-J, Distel MA, de Moor MHM, Vink JM, Willemsen G. Genetic epidemiology of attention deficit hyperactivity disorder (ADHD index) in adults. PLoS ONE. 2010;5(5):e10621. doi: 10.1371/journal.pone.0010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Wang JC, Grucza RA, Schuckit M, Kuperman S, Goate A. Using dimensional models of externalizing psychopathology to aid in gene identification. Archives of General Psychiatry. 2008;65:310–318. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behavior Genetics. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, Meyers J, Aliev F, Nurnberger J, Jr, Kramer J, Kuperman S, Bierut L. Evidence for genes on chromosome 2 contributing to alcohol dependence with conduct disorder and suicide attempts. American Journal of Medical Genetics, Part B: Neuropsychi-atric Genetics. 2010a;153B:1179–1188. doi: 10.1002/ajmg.b.31089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010b;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Understanding the covariation among childhood externalizing symptoms: Genetic and environmental influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. Journal of Abnormal Child Psychology. 2005;33:219–229. doi: 10.1007/s10802-005-1829-8. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer H, Begleiter H. Variations in GABRA2, encoding the α2 subunit of the GABAA receptor, are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Franke B, Neale BM, Faraone SV. Genome-wide association studies in ADHD. Human Genetics. 2009;126:13–50. doi: 10.1007/s00439-009-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs B, Igl W, Larsson H, Larsson J-O. Coexisting psychiatric problems and stressful life events in adults with symptoms of ADHD—A large Swedish population-based study of twins. Journal of Attention Disorders. 2010 doi: 10.1177/1087054710376909. Advance online publication. Retrieved from http://jad.sagepub.com/content/early/2010/08/02/1087054710376909. [DOI] [PubMed] [Google Scholar]
- Haber JR, Bucholz KK, Jacob T, Grant JD, Scherrer JF, Sartor CE, Heath A. Effect of paternal alcohol and drug dependence on offspring conduct disorder: Gene-environment interplay. Journal of Studies on Alcohol and Drugs. 2010;71:652–663. doi: 10.15288/jsad.2010.71.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Timberlake D, Hopfer CJ, Lessem JM, Ehringer MA, Hewitt JK. Genetic and environmental contributions to retrospectively reported DSM-IV childhood attention deficit hyperactivity disorder. Psychological Medicine. 2008;38:1057–1066. doi: 10.1017/S0033291707001584. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS. Testing hypotheses about direction of causation using cross-sectional family data. Behavior Genetics. 1993;23:29–50. doi: 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- Jacobson KC, Prescott CA, Neale MC, Kendler KS. Cohort differences in genetic and environmental influences on retrospective reports of conduct disorder among adult male twins. Psychological Medicine. 2000;30:775–787. doi: 10.1017/s0033291799002561. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, environment, and psycho-pathology: Understanding the causes of psychiatric and substance use disorders. New York, NY: Guilford Press; 2006. [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Knop J, Penick EC, Nickel EJ, Mortensen EL, Sullivan MA, Murtaza S, Gabrielli WF. Childhood ADHD and conduct disorder as independent predictors of male alcohol dependence at age 40. Journal of Studies on Alcohol and Drugs. 2009;70:169–177. doi: 10.15288/jsad.2009.70.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Bucholz KK, Madden PA, Waldron M. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: A female twin study. Pharmacology, Biochemistry, and Behavior. 2009;93:313–321. doi: 10.1016/j.pbb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, Martin G. Maternal alcohol use disorder and offspring ADHD: Disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine. 2006;36:1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- Kuo P-H, Kalsi G, Prescott CA, Hodgkinson CA, Goldman D, van den Oord EJ, Riley BP. Association of ADH and ALDH genes with alcohol dependence in the Irish Affected Sib Pair Study of alcohol dependence (IASPSAD) sample. Alcoholism: Clinical and Experimental Research. 2008;32:785–795. doi: 10.1111/j.1530-0277.2008.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K, Fowler T, Ford T, Thapar AK, van den Bree M, Harold G, Thapar A. Adolescent clinical outcomes for young people with attention-deficit hyperactivity disorder. The British Journal of Psychiatry. 2010;196:235–240. doi: 10.1192/bjp.bp.109.066274. [DOI] [PubMed] [Google Scholar]
- Martin NG, Eaves LJ, Kearsey MJ, Davies P. The power of the classical twin study. Heredity. 1978;40:97–116. doi: 10.1038/hdy.1978.10. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 5th ed. Los Angeles, CA: Authors; 1998–2007. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 7th ed. Richmond, VA: Dept. of Psychiatry, Virginia Commonwealth University; 2006. [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcoholism: Clinical and Experimental Research. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcoholism: Clinical and Experimental Research. 2004;28:1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- Sharp SI, McQuillin A, Gurling HM. Genetics of attention-deficit hyperactivity disorder (ADHD) Neuropharmacology. 2009;57:590–600. doi: 10.1016/j.neuropharm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Silberg J, Rutter M, Meyer J, Maes H, Hewitt J, Simonoff E, Eaves L. Genetic and environmental influences on the covariation between hyperactivity and conduct disturbance in juvenile twins. Journal of Child Psychology and Psychiatry. 1996;37:803–816. doi: 10.1111/j.1469-7610.1996.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PAF, Bucholz KK, Dunne MP, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. Journal of Abnormal Psychology. 1998;107:363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, Rietschel M. Genome-wide association study of alcohol dependence. Archives of General Psychiatry. 2009;66:773–784. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaluw CS, Engels RCME, Buitelaar J, Verkes RJ, Franke B, Scholte RHJ. Polymorphisms in the dopamine transporter gene (SLC6A3/DAT 1) and alcohol dependence in humans: A systematic review. Pharmacogenomics. 2009;10:853–866. doi: 10.2217/pgs.09.24. [DOI] [PubMed] [Google Scholar]
- Weiss G, Hechtman L, Milroy T, Perlman T. Psychiatric status of hyperactives as adults: A controlled prospective 15-year follow-up of 63 hyperactive children. Journal of the American Academy of Child Psychiatry. 1985;24:211–220. doi: 10.1016/s0002-7138(09)60450-7. [DOI] [PubMed] [Google Scholar]
- Whalen CK, Jamner LD, Henker B, Delfino RJ, Lozano JM. The ADHD spectrum and everyday life: Experience sampling of adolescent moods, activities, smoking, and drinking. Child Development. 2002;73:209–227. doi: 10.1111/1467-8624.00401. [DOI] [PubMed] [Google Scholar]
- White HR, Xie M, Thompson W, Loeber R, Stouthamer-Loeber M. Psychopathology as a predictor of adolescent drug use trajectories. Psychology of Addictive Behaviors. 2001;15:210–218. [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibi-tion. American Journal of Medical Genetics. 2000;96:684–695. [PubMed] [Google Scholar]