Abstract
The cell/environment interface is composed of the proteins of plasma membrane which face the extracellular space and by the proteins secreted directly by the cell of origin or by neighboring cells. The secreted proteins can act as extracellular matrix proteins and/or autocrine/paracrine proteins. This report discusses the technical aspects involved in the identification and characterization of the secreted proteins of specific cell types that comprise the heart. These aspects include the culturing of the cells, cell co-culturing and quantitative labeling, conditioned media collection and dealing with high abundant serum proteins, post-translational modification enrichment, the use of protein separation methods and mass spectrometry, protein identification and validation and the incorporation of pathway analysis to better understand the novel discovery on the background of already known experimental biological systems. The proteomic methods have the solid emplacement in cardiovascular research and the identification of proteins secreted by cardiac cells has been used in various applications such as determination the specificity between secretomes of different cell types, e.g. cardiac stem cells and cardiac myocytes, for the global secretome screening of e.g. human arterial smooth muscle cells, for the mapping of the beneficial effect of conditioned medium of one cell type on the other cell type, e.g. conditioned medium of human mesenchymal stem cells on cardiac myocytes, and for the searching the candidate paracrine factors and potential biomarkers.
Keywords: secretomes, proteomics, technology, cardiovascular diseases
1. Introduction
Concept of the cell/environment interface
Interaction among different types of cells is a common response of the heart to the injury. The interplay between various cell types comprising the heart or the vasculature occurs either through direct cell contact (e.g. gap junctions or adhesion molecules) or via paracrine and autocrine action. The cardiac myocytes and fibroblasts, the primary cells comprising the heart, as well as resident cardiac stem cells respond to the changes in their local environment, while it is the cell surface which translates the extracellular signal into a cellular response through direct or indirect interactions. The main site of this response is the cell/environment interface and it includes both the cell surface proteins and the proteins which are secreted by the cells as a reaction to these changes. Various methods have been developed to identify these subproteomes and clarify the protein alteration in vivo. To target and define the proteins responsible for the repair process could lead to improved therapy and to the development of potential biomarkers for disease diagnosis and prognosis. In this article, we will discuss the technical issues related to identification of one of these subproteomes, i.e. the proteins secreted by cardiac cell as a response to the alteration in their environment due to cardiovascular diseases.
2. Secretome proteomics
The proteins secreted by particular type of cells (the secretome) play important roles in regulation of many physiological processes via paracrine/autocrine mechanisms and they are of increasing interest as potential biomarkers and therapeutic targets in diseases. The proteins released by cells into conditioned media in vitro have been studied to better understand the pathological conditions and mechanisms in vivo. For damaged myocardium, current therapeutic approaches are limited since postnatal cardiac myocytes have minimum or no regenerative capacity. Thus, being able to regenerate the myocardium using the cell therapy has a potential and several experimental and clinical studies have demonstrated an improvement in cardiac function following myocardial infarction (MI) and heart failure (HF) [1–9]. In addition, soluble proteins secreted by cardiac myocytes, fibroblast and/or (cardiac) stem cells into the damaged myocardium microenvironment have the functional benefit seen in studies of cell therapy [10–16], and identifying the secretomes that have salutary effects on injured cardiac myocytes and cardiac stem cells introduces the possibility of administering “off the shelf” protein therapy. The proteins secreted by various cardiac cells have been identified and characterized over years and used e.g. for determination the specificity between secretomes of different cell types such as cardiac stem cells and cardiomyocytes [17], peripheral blood-derived smooth muscle progenitors and aortic smooth muscle cells [18]; for the global secretome screening of arterial smooth muscle cells [19], endothelial cells [20], atheroma plaque [21] and early pro-angiogenic cells [22]; for the mapping of the beneficial effect of conditioned medium of one cell type on the other cell type such as conditioned medium of mesenchymal stem cells on cardiac myocytes [13]; and for searching the potential paracrine/autocrine factors [12,14,23–24]. With technology development, proteomics has become a powerful identification tool, and mass spectrometry is the major and commonly applied technique for detection of proteins in cell secretomes. Even though each cell type has its own challenges, many of the issues and considerations described herein for secretome proteomics apply regardless of differences in cell type under investigation.
2.1 Technology
To identify cell specific secreted proteins, the investigators often start their experiments in cell culture. Yet, the goal is more complex in vivo setting which consists of multiple cell types existing in a 3D structure and includes the local environment changes. For example, in vivo setting, co-cultures can mimic co-existence of multiple cell types, and the cells grown in the culture on the scaffold can mimic the existence of 3D structure. In following sections, we discuss the issues and challenges leading to ultimate goal of identification and characterization of secreted proteins in cell conditioned media.
Cell culture
To ensure the optimal growth and viability of the cells, the cell cultured in vitro are generally grown in media containing bovine serum. Although high abundant serum proteins may mask the presence of lower abundance secreted proteins, making their detection and identification by mass spectrometry (MS) difficult to achieve, there are a number of strategies by which to handle this issue. The first is to reduce the amount of serum in the medium by moving towards serum free conditions. However, there is a need to minimize the cell death and this requirement often results in a reduction but not complete elimination of the serum. Thus, to have the strategies for depletion of the serum are important, and they include the affinity techniques to deplete or remove at least the high abundant serum proteins or the protein fractionation methods such as SDS-PAGE, two dimensional gel electrophoresis (2-DE) or liquid chromatography (LC) in which the serum albumin is physically separated from the remaining proteins. Most common is to replace the medium rich of serum by conditioned medium with lower serum content or by serum-free medium [25–26]. Although many cells tolerate starvation conditions for short period of time (usually 12 to 48 hours), the monitoring and optimization of the starvation condition for each particular type of cells is essential. The important step between changing the medium rich of serum to conditioned medium with lower or no serum concentration is a rinsing step in order to ensure that any contamination from high serum content is eliminated. On the other hand, extensive rinsing or the choice of improper washing buffer can cause the cell death as well. Generally, the cells are washed several times with PBS buffer or with reduced or serum-free culture medium. Recently, the comparison of the efficiency of BSA removal from medium of rat vascular endothelial cells by three rinsing techniques was published [27]. The study has shown that the most effective technique to change from medium with 20% fetal bovine serum (FBS) to serum-free medium appeared to be the combination of two rinses with Dubelcco’s phosphate buffered saline containing calcium and magnesium and one rinse with serum-free medium. Even though high precautions are kept and protocol optimized, handling the cells in vitro results in cell necrosis, and percentage of dead cells as well as cell viability in reduced serum medium should be measured and evaluated, as the intracellular proteins released by necrotic and apoptotic cells distort the results obtained for secreted proteins. In addition, the medium itself should be analyzed the same way as conditioned medium and used as a control to eliminate the proteins delivered by medium. Lately, the technique has been published in which secreted proteins can be distinguished from proteins derived from both media residual fetal calf serum (FCS) and proteins released by dead cells [28]. The method is based on combination of in vitro metabolic labeling of cells ([35S]-labeled methionine and cysteine) and subsequent detection of proteins by fluorescence analysis and autoradiography. Whilst fluorescence analysis detects all proteins present in medium, autoradiography detects only proteins synthesized by living cells during the metabolic labeling period (secreted proteins).
Co-culture and quantitative labeling
Numerous studies have been published on identification of proteins in cardiac myocyte, non-myocyte and stem cell secretomes and on contribution of secreted proteins to functional improvement during various cardiac pathologies. Secretomes obtained from either single cultures [29–30] or from co-cultures of various cells [31] have been studied in vitro as well as changes induced in one cell type conditioned by secretome of the other cell types [13,23,32–35]. Although the latter case is technically easier and most often used, the cell co-culture more closely reflects the situation in vivo and enables to evaluate the possible cross-talk between both cell types. In co-culture studies, especially when secreted proteins should be assigned to cells from which they originated, experimental design is more challenging. First, the labeling methods are mostly used where one of the cell types is labeled to distinguish between the same proteins delivered from two different cell types mixed together. Second, the labeling should not alter the cells and secretome and third, heterogeneity of both cell systems complicates the co-culturing conditions in terms of the medium type selection, serum concentration, culture dish coating, etc.
There are a number of available labeling strategies by which to address these challenges. In addition to chemical derivatization which introduces a mass tag into proteins/peptides (e.g. tandem mass tag (TMT) and isobaric tag for relative and absolute quantification (iTRAQ)) [36–37], the other stable isotope labeling methods are applied based on metabolic labeling of living cells [38]. In 2002, stable isotope labeling by amino acids in cell culture (SILAC) method was introduced [39] for mass spectrometry – based quantitative proteomics. SILAC method is based on a metabolic labeling approach and can be used in any cell culture system for incorporation of specific amino acid tag into all mammalian proteins. By using this method, the cells are grown in media lacking a standard essential amino acid but supplemented with a non-radioactive, isotopically labeled form of that amino acid (“heavy” amino acid, e.g. 13C- and 15N-Arg). Complete incorporation of “heavy” amino acid occurs after several doublings in cell lines, and does not affect the growth and morphology of cells compared to cells in normal medium. Since free amino acids present in serum-containing medium can interfere with results, dialyzed serum should be used. When “heavy” and “light” amino acid (e.g. arginine or lysine) cell populations are mixed, they are distinguishable by MS (e.g. 6 Da for 13C6-Arg), and protein abundances are determined from the relative MS signal intensities [40]. Compared to other metabolic or chemical labeling methods (18O, 15N, iTRAQ and TMT), SILAC has several advantages, among them, no differences in labeling efficiency between samples (100% tag incorporation), no chemical labeling or affinity purification steps and thus no sample loss during handling, and since proteins are uniformly labeled, several peptides from the same protein can be compare to ensure that change magnitude is the same. Despite the advantages, there are some issues with SILAC labeling, e.g. the method does not work for every cell type and can alter the proliferation of some cell types, and the labeling requires sufficient cell doubling to incorporate the tag and obtain 100% labeling efficiency which can be problematic, especially for ventricular cardiac myocytes for which no dividing cell culture system exists. The method was shown to be compatible with gel electrophoresis [39] and LC methods [41] followed by MS, and it was used as a tool for many applications [42–44]. Since designed for comparative proteomic experiments, the benefit of SILAC application in co-culture secretome identification studies is apparent and the method is tested in several laboratories for this purpose.
The use of an insert well co-culture system is another possible approach by which to evaluate cross-talk between different cell types [45–46]. Although the purity of each tested cells is preserved in this system, the cells can be easily separated and the components of this system can be easily changed to achieve desired experimental conditions, there are the limitations, e.g. the effect of direct cell-to-cell contact between different types of cells cannot be evaluated and it is difficult to identify which cell type is responsible for the secretion of a particular protein. Thus, the co-culturing setting depends on the goal of each particular study. In case that the origin of the proteins is not the issue and/or a direct cell–to-cell contact is desirable, various cell types can be co-cultured without protein labeling either altogether or in various insert arrangements. But to be able to distinguish among the proteins originated in different cell types, the protein labeling is required (SILAC) in the co-culture. Two approaches are mostly used at present, either one cell type is labeled in the cell co-culture or the secretome of one cell type is labeled and used as a conditioned medium for the other cell type (the labeling can be vice versa).
Conditioned medium collection
After conditioning, cell supernatants are collected, centrifuged or filtered to remove non-adherent cells. Conditioned media are then stored at −80°C before analysis. Prior to fractionation by separation methods, conditioned medium can be further processed, e.g. high abundant serum proteins can be depleted and medium concentrated. As secreted protein concentration in the conditioned medium is usually low and the proteins are in a soluble form, it can be desirable to retain the solubility during concentration and use the methods that do not involve precipitation, e.g. centrifuge filtration, dialysis, evaporation, etc. The centrifuge filtration using microconcentrators can result in protein binding to the membranes (even though low protein binding membrane devices are used) and the proteins of interest can be lost. As well, the membrane cut-off must be chosen to be well below the size of proteins of interest. During dialysis, the protein can precipitate due to low salt concentration in the dialysis buffer. In our group, we mostly use SpeedVac concentrator to concentrate conditioned medium approximately six to tenfold before analysis by reversed phase liquid chromatography (RPLC) and we do not experience any protein precipitation.
It should be pointed out that in addition to secreted proteins, conditioned medium may also contain shed membrane proteins and secretory vesicles (e.g. microvesicles, exosomes, membrane particles) after its collection. As the size of the secretory vesicles is mostly in the range of tens to hundred nanometers (except for microvesicles: 100 – 1000 nm), the filters used for removal of non-adherent cells and debris (typically 40 µm in size) will not remove the secretory vesicles from the medium. As well, the centrifugation of the medium after collection (typically 450–600 g) will leave the secretory vesicles in medium since they constitute (together with Golgi membranes) the slowest sedimenting membranes. They are retained in the supernatant fraction after centrifugation at 17,000–25,000 g for 15 minutes and collected in pellet fraction after sedimentation at 100,000 g for 60 minutes [47]. Thus, secretory vesicle proteins can be released into conditioned medium during processing and they constitute the fraction of the secretome [48–51].
It is also possible to consider analysis of in vivo secretome. Certainly, there are instances where proteomics has successfully identified proteins in fluid obtained from proximal tissue/cells of interest (such as with cancer where the tumor drainage can be obtained) [52–53] and the pericardial fluid could be source for enrichment of cardiac specific secreted proteins. Furthermore, one can envision that with injury there would be an increase in secreted proteins. However, the proteins secreted could originate from one or more of the cell types that comprise the heart and coronary vasculature. The blood sample will also contain proteins that originate from other organs in the body as they can respond in a systematic way. The other challenges which were outlined for cell culture in vitro apply as well: ensuring that the proteins being detected in vivo do not arise from the cell death, the need to maximize proteome coverage and quantification of the proteins.
Dealing with high abundant serum proteins
Since media containing serum (e.g. FBS) in concentration ranging from 10% to 20% are generally used in vitro cell cultures, the identification of low abundant secreted proteins in presence of serum proteins (albumin and immunoglobulins) means the challenge. The relatively easy solution is to decrease the serum concentration in conditioned media [17] or use serum-free media during the cell conditioning [12,19,54] as mentioned above. The other option could be to keep the serum in conditioned media at the same concentration level as was used for the cells in the culture with albumin and/or other high abundance serum protein removal prior to 2-DE, LC and subsequent MS. There are numerous methods developed and commercially available for high abundant protein removal from the serum that can be applied for FBS in secretomes as well. Most often, the antibody affinity depletion systems for the removal of various number of top serum proteins are applied [55–57], as well as fractionation of the samples by chromatography methods without the necessity of albumin or IgG depletion [17,58]. Recently, a new ligand base (not antibody) affinity columns were developed to remove albumin from serum (www.proteabio.com). These columns are highly efficient (99% human albumin and 80% bovine albumin depleted) and they are not based on the antibody but rather on a unique motif found in other species. Figure 1 shows the results of albumin depletion in the smooth muscle cell medium containing 1% FBS using SDS-PAGE and RPLC (Stastna M, PhD, unpublished data, 2011). In our opinion, the high abundant serum proteins should be removed from medium before protein identification to increase the possibility of low abundant secreted proteins detection. But it is not quite straightforward which depletion method to recommend. It depends e.g. on the quality and suitability of particular depletion methods for given experiment, on the type of medium and the medium components as they can interfere with depletion assay. As well, it should be remarked that most of the depletion methods will result in sample losses and in removal of the proteins bound to albumin as well. This can be partly overcome by using the agents that will break off the bounds between albumin and its bound proteins prior to depletion provided that it will not interfere with purpose of the protein study. As very important step in secretome analysis, we recommend to optimize the depletion step for each particular case. This is also true if other samples such as coronary sinus serum or plasma are analyzed instead of cell conditioned media.
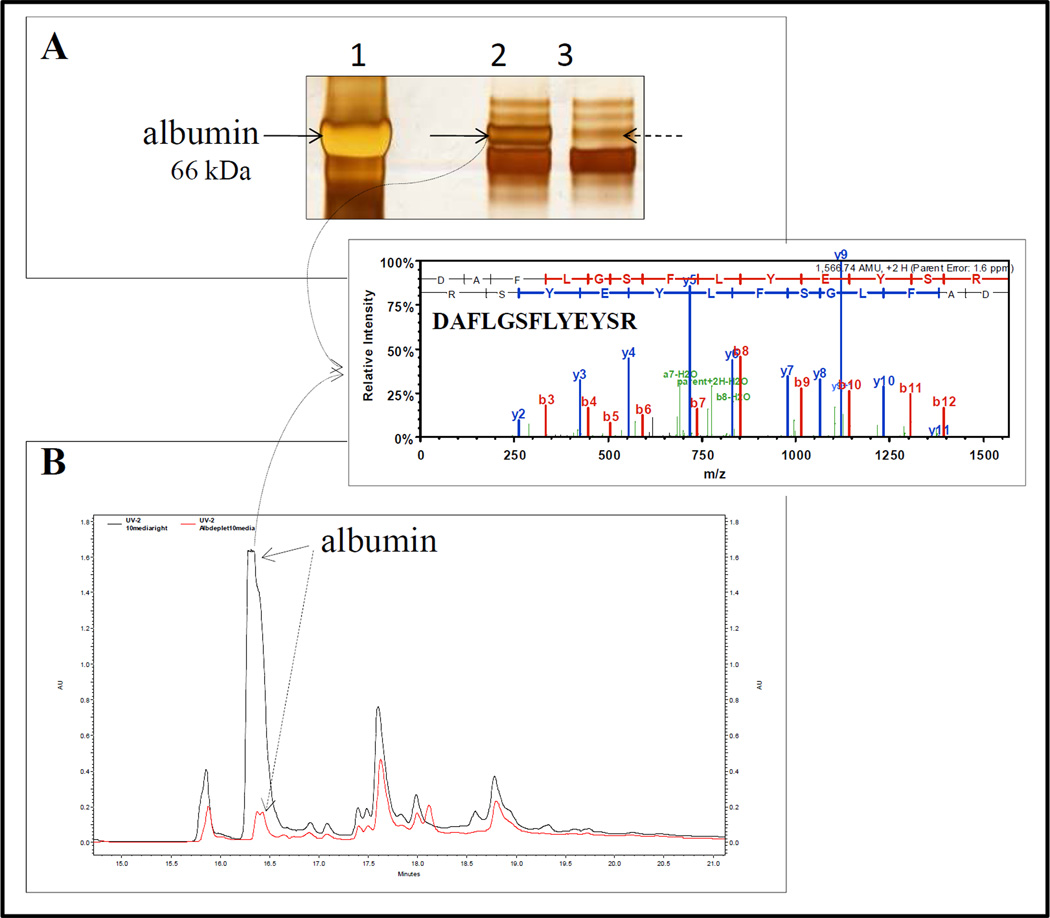
Figure 1.
Depletion of albumin in culture medium containing FBS. A - image of SDS-PAGE gel after silver staining; lane 1 - bovine serum albumin (6 µg loaded), lane 2 - medium containing 2 % FBS before albumin depletion (5 µl loaded is equal to 1.25 µg of total protein), lane 3 - medium containing 2% FBS after albumin depletion (5 µl of flow through loaded). B - chromatograms of RPLC of the medium containing 10 % FBS before albumin depletion (solid arrow; 100 µl loaded is equal to 56 µg of total protein) and after albumin depletion (dashed arrow; 100 µl of flow through loaded). Inset – example of spectrum of one peptide DAFLGSFLYEYSR identifying bovine albumin (accession # P02769; ALBU_BOVIN) present in the FBS culture medium. ProteaPrep Albumin Depletion Sample Prep Kit was from Protea Biosciences (WV, USA; cat. # SP-200-12), albumin was from Sigma (MO, USA; cat. # A7517) and media was Clonetics smooth muscle cell basal medium (Lonza, MD, USA; cat. # CC-3181).
Separation methods
Since proteomes of biological samples consist of high number of different proteins in broad dynamic ranges, separation methods are used in order to fractionate and reduce the complexity of the samples, enhance the proteome coverage and improve the protein identification by subsequent MS. As well, they can be used to physically separate the high abundant serum proteins, e.g. albumin, from the remaining proteins. Most frequent proteomic methods used for fractionation of complex protein samples are 2-DE [19,21] and LC methods [30,59] which can be used for fractionation of proteins in secretome samples as well [17,27]. Despite disadvantages such as poor separation of membrane and hydrophobic proteins or inability to analyze high/low molecular mass proteins, 2-DE is the method of choice in many laboratories since relatively inexpensive equipment, its versatile applicability to wide range of samples, and it is one of the few methods which allows to determine post-translational modification (PTM) and specific protein isoforms. The method is based on the separation of proteins by isoelectric points (pIs) in the first dimension and by molecular masses in the second dimension [60]. Protein identification (or confirmation) can be done by using Western blot or MS analysis. Prior to MS, the protein spots are excised from gel, destained if the gel was Coomassie blue or silver stained, reduced, alkylated and subjected to enzymatic digestion.
The modification of 2-DE called two-dimensional difference gel electrophoresis (2-D DIGE) is method designed for reproducible detection and quantification of differences in protein levels/expression between two proteomes [61] and it should be considered as a one of the methods for evaluation of protein changes between conditioned media of different types of cells as well. In this method, two protein samples are fluorescently tagged with two different cyanine dyes, samples are mixed and run on the same 2-DE gel, post-run imaged into two images which are then superimposed to compare the differences in protein composition. Since the samples are run on the same gel in both dimensions and thus subjected to the same procedure and environment, DIGE is highly reproducible without interference due to gel-to-gel variation. The combination of DIGE and shotgun proteomics has been used recently for characterization and mapping the proteome and secretome of human early pro-angiogenic cells [22].
The other methods used abundantly in proteomics are LC methods which can be used either as a single or can be combined and used sequentially in various multidimensional arrangements either off-line or on-line. Most often RPLC is the final separation method in multidimensional arrangement as it is compatible with downstream mass spectrometry. In RPLC, proteins/peptides are separated in the chromatographic column based on differences in retention magnitude of the protein/peptide to column stationary phase by using of relatively non-polar stationary phase and polar solvent. The proteins/peptides are eluted from column in order of increasing degree of hydrophobicity and elution times depend on the percentage of organic solvent and the type of stationary phase. The use of gradient elution (linear or discontinuous; mostly with increasing concentration of organic solvent) is preferred for complex samples in order to speed the elution of strongly retained proteins/peptides and to obtain sufficient resolution. Recently, we used RPLC for fractionation of intact proteins in conditioned media of rat cardiac stem cells and neonatal cardiac myocytes (containing 1% of FBS) prior to MS/MS protein identification [17]. Since low amount of secreted protein is usually presented, the cell conditioned media are concentrated after collection and mixed with solvent compatible with RPLC (e.g. 20% ACN, 1% trifluoroacetic acid (TFA) in final concentration, pH 2.3), centrifuged at 18000 × g for 30 min at 4°C and injected into RPLC column. For secretome protein separation with reduced FBS (1%) in media, we used a linear AB gradient from 0 to 100 % B, where solvent A was water/0.1% TFA (v/v) and B was ACN/0.08% TFA (v/v) with C18 column. The eluted fractions were collected (the albumin rich fraction was excluded), concentrated, neutralized by ammonium bicarbonate and digested (250 ng of enzyme/fraction) prior to MS analysis.
The other potential methods for secretome fractionation are non-gel based electrophoretic methods, such as free-flow electrophoresis (FFE) [62]. In FFE based on isoelectric focusing (IEF), the proteins are continuously injected into a thin liquid film flowing between two parallel glass plates, and IEF conditions are established by using carrier ampholytes by introducing an electric field perpendicular to the flow direction. Proteins are separated based on their different pIs and are collected into fractions for further processing [63]. For example, the combination of FFE to reduce sample complexity and nanoflow LC-MS/MS has been used for the analysis of secretome of human umbilical vein endothelial cells [20].
PTM protein enrichment
Since proteins may be secreted by cells in very low concentrations, as mentioned previously, the various enrichment methods can be applied. The enrichment method can be based on PTM that occurs to secreted proteins, e.g. glycosylation. The examples of secreted proteins which are known to be glycosylated and play important roles in cardiovascular diseases are e.g. VCAM1, BNP, IL6, ST2, TNFα, MMP-9 and proteins of ICAM superfamily. Glyco-forms of some of these proteins e.g. could affect proBNP-108 processing by furin [64] and affect proBNP stability [65] and thus, they could have the effect on cardiovascular and cellular functions. Recently, the method termed cell surface capturing (CSC) was published [66] which is based on the conjugation of glycoproteins to a solid support using hydrazide chemistry and stable isotope labeling of N- and O-linked glycopeptides. The method has been optimized to date for N-linked glycosylation based on the specific release of formerly N-linked glycosylated peptides via peptide- N-glycosidase F (PNGase F). The recovered peptides are then identified and quantified by MS. Although the method was used for the selective isolation of N-linked glycosylation sites from glycoproteins in blood serum [66], for selective enrichment and detection of the cell surface N-glycoproteomes of T and B cells [67] and for the identification of the cell surface N-linked glycoprotein subproteome of myoblasts [68], the use of this method for enrichment of secreted proteins in conditioned medium could be a good approach as well. O-linked glycoprotein and peptide analysis (just like N-linked) can be carried out based on lectin affinity and other click chemistry reagents [69]. Nevertheless, the methods for O-linked glycoproteins are still challenging.
Mass spectrometry and protein identification
For protein identification, two basic MS approaches are use in order to analyze the peptides resulting from enzymatic digestion of protein samples, the analysis assisted by matrix (matrix-assisted laser desorption ionization - time of flight mass spectrometry (MALDI-TOF-TOF MS)) and the electrospray ionization - mass spectrometry (ESI-MS/MS). Today, most scientists use tandem MS instruments which allow determination of amino acid sequence of the observed peptides. Since MALDI-TOF-TOF MS suppresses the low abundant proteins in the favor of most abundant proteins and it is often successfully used for analysis of simple protein mixtures (unless the peptides are pre-separated by LC), the second approach is generally of choice for identification of the proteins in cell secretomes where RPLC is on-line coupled directly by an electrospray interface to MS/MS instrument. Using RPLC-ESI-MS/MS or MALDI-TOF-TOF MS, the extensive datasets are generated and various search engines are used to match the MS/MS fragmentation spectrum with peptides from appropriate databases and thus, the mass accuracy of MS data is of high importance. In order to get as high confidence as possible for final protein identifications, various criteria and limits are selected for data filtering. As well, all identified proteins/peptides must be examined for redundancy. In some cases, it is advantageous to use a combination of more different enzymes simultaneously, as it can lead to higher proteome coverage [70] since they cleave the protein at different amino acid sites into more variety of peptides.
In addition to MS-based proteomic methods that compare peptide ratios of two proteomes labeled separately with a tag containing heavy and light isotopes, the other methods with potential utility to secretome analysis are label-free quantification methods [71–72] including spectral counting (based on the frequency of identifications) [73–74] and selected/multiple reaction monitoring (SRM, MRM, respectively) [75–77]. SRM (MRM) is a technology for the consistent detection and absolute quantification of specific, predetermined sets of peptides that are representative of their parent proteins in a complex background and in multiple samples [78]. Usually, triple quadrupole MS instrument is used to monitor parent peptide-fragment ions (transitions) in combination with peptide retention time during MRM assay development. Although high amount of transitions with high reproducibility can be performed in a single experiment run, development of the assay for each protein is still time-consuming process despite the improvements published recently [78–79]. Nonetheless, MRM technique has a great potential, and as a validation method might replace the classical immunoassay approaches still depending on antibody specificity and availability.
To identify or predict the subcellular location of proteins and to assess which proteins identified from a secretome study are truly secreted, the computational tools to assess the proteins which are undergoing the classical secretory pathways as well non-classical protein secretion have been published [80–81]. Many proteins are secreted by a classical secretory mechanism, i.e. with signal peptide (an N-terminal peptide, typically 15–30 amino acids long) which is cleaved off during translocation of the protein across the membrane, and can be predicted from e.g. the amino-acid sequence of the protein as the input by using bioinformatic tools, e.g. TargetP or SignalP [80]. For secretory proteins not having N-terminal signal peptides (e.g. fibroblast grow factors, interleukins and galectins found in the extracellular matrix), alternative pathways called non-classical secretory pathways exist and the sequence-based method SecretomeP for the prediction of mammalian secretory proteins targeted to the nonclassical secretory pathway can be implemented [81]. The method is also capable of predicting secretory proteins signal peptide-containing where only the mature part of the protein has been annotated or in the cases, where the signal peptide remains uncleaved.
2.2 Validation and biological assays
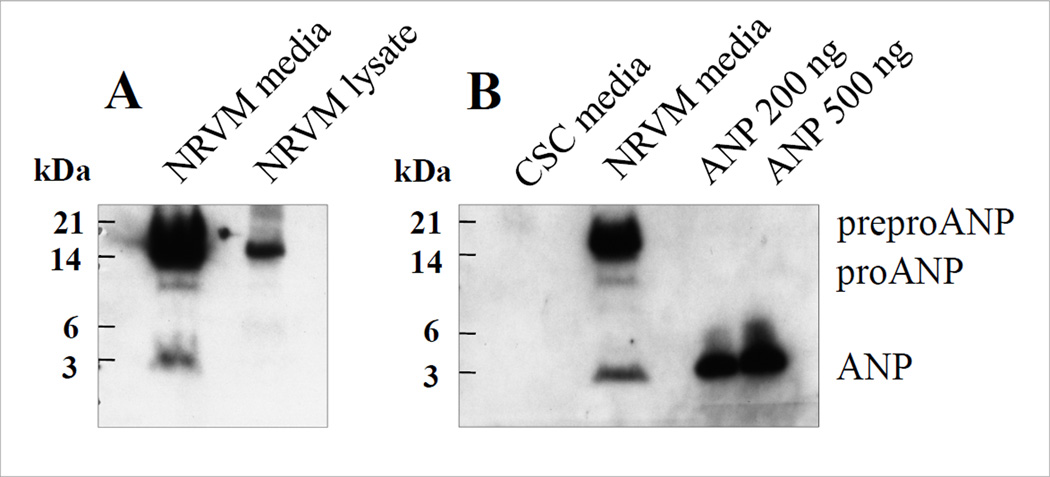
After identification by MS, presence of proteins should be validated qualitatively and/or quantitatively by other methods. One of the methods applied can be newly developed MRM technique [82–83], which is antibody independent and it possesses a great potential, especially when the antibody for a particular protein is unavailable, and since it can be run in the multiplexed format for many proteins simultaneously [84]. Our laboratory recently built a MRM assay for interleukin-1 receptor-like 1 (ST2) protein (Fu Q, PhD, unpublished data, 2011) which is schematically shown in Figure 2. Unfortunately, the MRM method has not been yet broadly utilized by proteomic community and thus, mostly various immunoassays, e.g. ELISA and Western blotting, are the methods of the choice, at least for proteins to which antibodies are available. For example, the absence of atrial natriuretic peptide (ANP) in conditioned medium of cardiac stem cells (CSCs) but presence in conditioned medium of neonatal rat ventricular myocytes (NRVMs) as found by LC-MS/MS has been confirmed by Western blotting [17] and in [30], heat shock protein 90-α (HSP90-α) but not HSP90-β was found in conditioned medium of vascular smooth muscle cells by LS-MS/MS and the results confirmed by Western blotting as well. Figure 3 shows Western blot validation of ANP as identified by LC-MS/MS [17]. ANP is synthesized as a pre-prohormone (152 aa; 16556 Da) and within the endoplasmic reticulum the signal peptide is cleaved to produce the prohormone (128 aa) with ANP hormone consisting of 28 amino acid residues (from C terminus) with molecular weight of 3063 Da. Western blots of ANP carried out on medium or lysate of NRVMs and medium of CSCs with recombinant protein as control (200 and 500 ng loaded) are shown in panels A and B of Figure 3. Three bands corresponding to preproANP, proANP and ANP peptide were detected in medium of NRVMs, but none of ANP bands were found in conditioned medium from CSCs. These findings confirmed the MS results in which 3 peptides were exclusively identified only in medium of NRVMs: 2 peptides covering a part of ANP prohormone sequence and 1 peptide matching the partial sequence of ANP hormone [17]. ANP is considered to be a cardiac lineage marker as it is expressed early during the differentiation of myocytes [85] and early during heart development [86]. This suggests that since CSCs are like embryonic and other progenitor cells [87], they do not secrete ANP but they would during differentiation into cardiac myocytes. Similarly to ANP, brain natriuretic peptide (BNP), another early marker of cardiac differentiation, exists as prepropeptide that is further processed to a prohormone (proBNP; 108 aa). The proBNP is enzymatically cleaved to form a biologically active C-terminal peptide (BNP-32; 32 aa) and biologically inactive N-terminal peptide (NT-proBNP; 76 aa) [88]. It is generally believed that both peptides (BNP-32 and NT-proBNP) are up-regulated and released into circulation in patients with HF and although both peptides are used to monitor the existence and severity of HF, very little information is known about BNP molecular forms in relation to HF. Since BNP is present in circulation in very low concentration (9.29 pg/mL to 1119 pg/mL) [89], both high sensitive and specific methods are essential for the identification. Recently, the studies have been published which demonstrated the improved separation and detection of BNP and its proteolytic peptides by experimental optimization and using MS instruments [88,90].
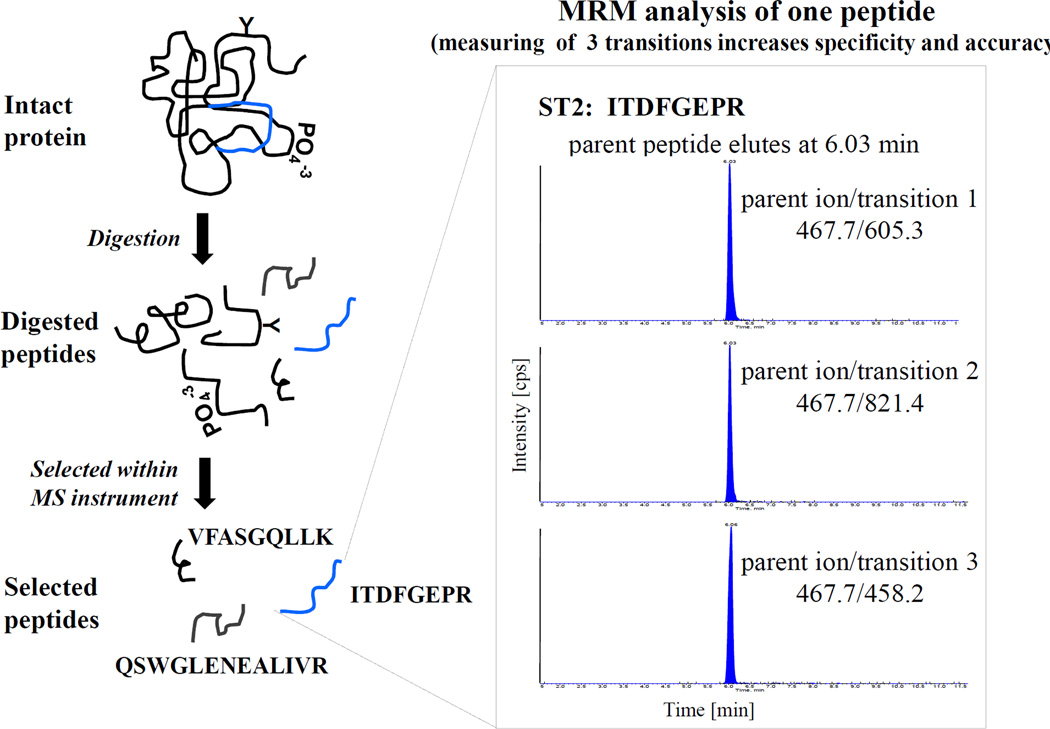
Figure 2.
Schematic workflow and MRM analysis of one peptide unique to the target protein ST2. The ST2 protein was digested with trypsin and the resulting peptides were analyzed using a triple quadrupole MS instrument. Peptides were targeted within the MS instrument and only those peptides selected based on their mass (parent ion) were analyzed. The specificity was obtained based on the peptides elution times from the online RPLC and the corresponding MS/MS data (transitions). Three transitions are shown for one selective parent peptide (ITDFGEPR) that is unique for the protein ST2 (accession # Q01638; ILRL1_HUMAN). The MS/MS data provides amino acid sequence information and ensures a high level of specificity. Three peptides and two transitions per peptide are commonly used for quantification.
Figure 3.
Validation of ANP by Western blotting as identified by LC-MS/MS. A - ANP identified in NRVM medium and NRVM lysate; amounts of total protein loaded into SDS-PAGE gel were 75 µg and 180 µg, respectively; B - ANP in CSC medium and NRVM medium; amounts of total protein loaded into SDS-PAGE gel were 130 µg and 75 µg, respectively. SDS-PAGE conditions: NuPAGE 4–12% Bis-Tris 1.0 mm thick gel (cat. # NP0321; Invitrogen, CA, USA); MES running buffer; 200 V for 35 minutes [17]. The primary antibody (rabbit anti-rat ANP; Pab A4152-35; US Biological, MA, USA) was used in 1:1,000 dilution and secondary antibody-enzyme conjugate (alkaline phosphatase) was used in 1:10,000 dilution. Recombinant rat ANP (1–28 aa from C-terminus, 3063 Da) was from US Biological (MA, USA; cat. # A4152-05). Detailed explanation is given in the text.
The relatively high number of studies has been aimed to evaluate and compare the secretomes of various cells under both normal and pathological conditions. Different assays in vitro have been developed to monitor the influence of various secreted factors and to simulate the pathological conditions in vivo, such as effect of oxidative stress, hypoxia, hyperoxia, cyclical stretch, proliferation, differentiation, etc. It was demonstrated that cardiotrophin 1 (CT-1) expression is augmented after hypoxic stimulation, that hypoxic conditioned medium presented enhanced ability to activate the signal transducer and activator of transcription (STAT3) in cardiac myocytes and CT-1 might play an important role in the pathogenesis of ischemic heart disease [91]. Assaying the conditioned medium of cardiac myocytes subjected to influence of different concentration of oxygen has shown self-protection ability of cardiac myocytes against hypoxia and hyperoxia by producing increasing amount of angiotensin I and adenosine with oxygen concentration above 6% and below 6%, respectively [92]. Placenta growth factor (PGF) expression was increased 3.88 fold in cultured neonatal rat cardiac myocytes after 12 hours of hypoxia compared to normoxia, and shorter periods of hypoxia, conditioned medium from hypoxic cells and cyclical stretch conditions did not significantly alter PGF or its receptor VEGFR1 expression [93]. CTGF and ANP proteins identified in secretome of NRVMs and ST2 protein identified in secretome of cardiac stem cells (CSCs) have been shown to affect cardiac stem cell proliferation. Whilst NRVM-specific CTGF and ANP altered cardiac stem cell proliferation rates in a paracrine manner (but in opposite direction), CSC-specific ST2 (inhibits cardiac stem cell proliferation) acted as an autocrine factor [17].
In addition, one can move to analysis of the secretome in vivo. As discussed earlier, there are body fluids that are in close proximity to the cells that comprise the heart and they could capture the changes in their secretomes. These proteins, if quantified, could give rise to circulation biomarkers for the diagnostic and prognostic indications.
2.3 Pathway analysis
To manage and interpret the large-scale proteomic data sets and to model, analyze and understand the complex biological and chemical systems, various pathway analysis software have been adopted by the research community [94]. One of the proof-of knowledge based software often used is the Ingenuity Pathways Analysis (IPA) which helps to understand biology at multiple levels by integrating data from a variety of experimental platforms and providing insight into the molecular and chemical interactions, cellular phenotypes, and disease processes of examined system. IPA contains extensive library of well-characterized signaling and metabolic pathways which helps to find a connection between novel discovery and known in vivo and in vitro experimental biological systems. It provides information on known protein-protein relationships between members of large datasets through graphical representation. As numerous papers incorporating the pathway analysis have been published in cardiovascular research in recent years [20,95–105], this approach has an increasing use and it seems to be a valuable tool in many research fields and especially in the profiling the secretomes and potential biomarkers.
3. Conclusions
An increasing number of studies showing the affirmative effect of conditioned media with secreted proteins derived from various stem cells in cardiac regeneration confirm the hypothesis for using paracrine/autocrine factors secreted by cells rather than the cells themselves to affect myocardium tissue repair. This has several advantages, e.g. the potential to generate a therapeutic cocktail that effects in vivo cardiac regeneration and can be administered immediately following injury, thus limiting scar formation and preventing HF and arrhythmias, and the avoidance of the issues such as tumorigenicity and immune compatibility associated with ex vivo cell culture. And thus, identifying those secreted modulators and cardioprotective proteins is of a great interest. In addition, secreted proteins can serve as an essential source for biomarker discovery. Despite all this knowledge, only a few proteomic studies have identified actual protein candidates, presumably due to various technical issues that render analysis of secreted proteins challenging, and some of them have been mentioned in previous sections. Proteomics is a field which developed rapidly within several past years. It represents a broad group of technologies and methods comprising analytical protein biochemistry, analytical separation, mass spectrometry and bioinformatics. Many technologies are synergistic and complement to each other providing different data for each proteome. Although there are still many aspects of proteomics which need to be further developed, up to date implemented sensitive MS instruments, robust methods (e.g. enhanced protein and peptide separations and use of multiple enzymes) and expanded databases are valuable tools to better understand the reasons of proteome alteration in the context to health and disease. Proteomics is incorporated rapidly into cardiovascular research [106], and as the broader scientific community will adopt and apply proteomic methods into common practice, the novel findings expect to be discovered. This includes further studies on secreted proteins to find new biomarkers and enhance the understanding of the cell/environment interface. The ability to manipulate the cell/environment interface for therapeutic intervention starts with knowing the proteins involved. Thus, the use of proteomics enables better secretome analysis profiling in the context of the broader perspectives.
Acknowledgments
We would like to thank Qin Fu, PhD (Johns Hopkins University, Baltimore, MD) for the use of her MRM data of ST2 and James V. Snider, PhD (Critical Diagnostics, San Diego, CA) for a gift of ST2 recombinant protein.
Funding Sources: Supported by the National Heart, Lung, and Blood Institute Proteomic Initiative - contract NHLBI-HV-10-05 (2), by the AHA grant in-aid and by the Institutional Research Plan AV0Z40310501 of the Academy of Sciences of the Czech Republic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: JEV has a sponsored research program with Protea Biosciences (Morgantown, WV), and ST2 recombinant protein was a gift from Critical Diagnostics (San Diego, CA).
References
- 1.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 2.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh ETH, Zhang S, Wu HD, Körbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 5.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimen. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 6.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, et al. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathur A, Martin JF. Stem cells and repair of the heart. Lancet. 2004;364:183–192. doi: 10.1016/S0140-6736(04)16632-4. [DOI] [PubMed] [Google Scholar]
- 8.Lipinski MJ, Biondi-Zoccai GG, Abbate A. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction:a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle B, Sorajja P, Hynes B, Kumar AH, Araoz PA, Stalboerger PG, et al. Progenitor cell therapy in a porcine acute myocardial infarction model induces cardiac hypertrophy, mediated by paracrine secretion of cardiotrophic factors including TGFbeta1. Stem Cells Dev. 2008;17:941–951. doi: 10.1089/scd.2007.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cells-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timmers L, Lim SK, Arslan F, Armtrong JS, Hoefer IE, Doevendans PA, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Rossini A, Zacheo A, Mocini D, Totta P, Facchiano A, Castoldi R, et al. HMGB1-stimulated human primary cardiac fibroblasts exert a paracrine action on human and murine cardiac stem cells. J Mol Cell Cardiol. 2008;44:683–693. doi: 10.1016/j.yjmcc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signalling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis and tissue repair. 2008;1:4. doi: 10.1186/1755-1536-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stastna M, Chimenti I, Marban E, Van Eyk JE. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics. 2010;10:245–253. doi: 10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simper D, Mayr U, Urbich C, Zampetaki A, Prokopi M, Didangelos A, et al. Comparative proteomics profiling reveals role of smooth muscle progenitors in extracellular matrix production. Arterioscler Thromb Vasc Biol. 2010;30:1325–1332. doi: 10.1161/ATVBAHA.110.204651. [DOI] [PubMed] [Google Scholar]
- 19.Dupont A, Corseaux D, Dekeyzer O, Drobecq H, Guihot AL, Susen S, et al. The proteome and secretome of human arterial smooth muscle cells. Proteomics. 2005;5:585–596. doi: 10.1002/pmic.200400965. [DOI] [PubMed] [Google Scholar]
- 20.Tunica DG, Yin X, Sidibe A, Stegemann C, Nissum M, Zeng L, et al. Proteomic analysis of the secretome of human umbilical vein endothelial cells using a combination of free-flow electrophoresis and nanoflow LC-MS/MS. Proteomics. 2009;9:4991–4996. doi: 10.1002/pmic.200900065. [DOI] [PubMed] [Google Scholar]
- 21.Durán MC, Martín-Ventura JL, Mas S, Barderas MG, Dardé VM, Jensen ON, et al. Characterization of the human atheroma plaque secretome by proteomic analysis. Methods Mol Biol. 2007;357:141–150. doi: 10.1385/1-59745-214-9:141. [DOI] [PubMed] [Google Scholar]
- 22.Urbich C, De Souza AI, Rossig L, Yin X, Xing Q, Prokopi M, et al. Proteomic characterization of human early pro-angiogenic cells. J Mol Cell Cardiol. 2011;50:333–336. doi: 10.1016/j.yjmcc.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Pedrotty DM, Klinger RY, Kirkton RD, Bursac N. Cardiac fibroblasts paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc Res. 2009;83:688–697. doi: 10.1093/cvr/cvp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu BS, Landeen LK, Aroonsakool N, Giles WR. An analysis of the effects of stretch on IGF-I secretion from rat ventricular fibroblasts. Am J Physiol Heart Circ Physiol. 2007;293:H677–H683. doi: 10.1152/ajpheart.01413.2006. [DOI] [PubMed] [Google Scholar]
- 25.Gissel C, Doss MX, Hippler-Altenburg R, Hescheler J, Sachinidis A. Generation and characterization of cardiomyocytes under serum-free conditions. Methods Mol Biol. 2006;330:191–219. doi: 10.1385/1-59745-036-7:191. [DOI] [PubMed] [Google Scholar]
- 26.Xu C, He JQ, Kamp TJ, Police S, Hao X, O’Sullivan C, et al. Human embryonic stem cell-derived cardiomyocytes can be maintained indefined medium without serum. Stem Cells Dev. 2006;15:931–941. doi: 10.1089/scd.2006.15.931. [DOI] [PubMed] [Google Scholar]
- 27.Pellitteri-Hahn MC, Warren MC, Didier DN, Winkler EL, Mirza SP, Greene AS, et al. Improved mass spectrometric proteomic profiling of the secretome of rat vascular endothelial cells. J Proteome Res. 2006;5:2861–2864. doi: 10.1021/pr060287k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwickl H, Traxler E, Staettner S, Parzefall W, Grasl-Kraupp B, Karner J, et al. A novel technique to specially analyze the secretome of cells and tissues. Electrophoresis. 2005;26:2779–2785. doi: 10.1002/elps.200410387. [DOI] [PubMed] [Google Scholar]
- 29.Dostal DE, Rothblum KN, Conrad KM, Cooper GR, Baker KM. Detection of angiotensin I and II in cultured rat cardiac myocytes and fibroblasts. Am J Physiol. 1992;263:C851–C863. doi: 10.1152/ajpcell.1992.263.4.C851. [DOI] [PubMed] [Google Scholar]
- 30.Liao DF, Jin ZG, Baas AS, Daum G, Gygi SP, Aebersold R, et al. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–196. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- 31.Harada M, Itoh H, Nakagawa O, Ogawa Y, Miyamoto Y, Kuwahara K, et al. Significance of ventricular myocytes and nonmyocytes interaction during cardiocyte hypertrophy: evidence for endothelin-1 as a paracrine hypertrophic factor from cardiac nonmyocytes. Circulation. 1997;96:3737–3744. doi: 10.1161/01.cir.96.10.3737. [DOI] [PubMed] [Google Scholar]
- 32.Kado M, Lee JK, Hidaka K, Miwa K, Murohara T, Kasai K, et al. Paracrine factors of vascular endothelial cells facilitate cardiomyocyte differentiation of mouse embryonic stem cells. Biochem Biophys Res Commun. 2008;377:413–418. doi: 10.1016/j.bbrc.2008.09.160. [DOI] [PubMed] [Google Scholar]
- 33.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007;581:3961–3966. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 34.Burstein B, Qi XY, Yeh YH, Calderone A, Nattel S. Atrial cardiomyocyte tachycardia alters cardiac fibroblast function: a novel consideration in atrial remodeling. Cardiovasc Res. 2007;76:442–452. doi: 10.1016/j.cardiores.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 35.LaFramboise WA, Scalise D, Stoodley P, Graner SR, Guthrie RD, Magovern JA, et al. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am J Physiol Cell Physiol. 2007;292:C1799–C1808. doi: 10.1152/ajpcell.00166.2006. [DOI] [PubMed] [Google Scholar]
- 36.Arntzen MO, Koehler CJ, Barsnes H, Berven FS, Treumann A, Thiede B. IsobariQ: software for isobaric quantitative proteomics using IPTL, iTRAQ, and TMT. J Proteome Res. 2011;10:913–920. doi: 10.1021/pr1009977. [DOI] [PubMed] [Google Scholar]
- 37.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Julka S, Regnier F. Quantification in proteomics through stable isotope coding: a review. J Proteome Res. 2004;3:350–363. doi: 10.1021/pr0340734. [DOI] [PubMed] [Google Scholar]
- 39.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 40.Ong SE, Mann M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC) Nature Protocols. 2006;1:2650–2620. doi: 10.1038/nprot.2006.427. [DOI] [PubMed] [Google Scholar]
- 41.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 42.Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010;9:2482–2496. doi: 10.1074/mcp.M110.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng W, de Hoog CL, Yu HB, Li Y, Croxen MA, Thomas NA, et al. A comprehensive proteomic analysis of the type III secretome of Citrobacter rodentium. J Biol Chem. 2010;285:6790–6800. doi: 10.1074/jbc.M109.086603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rigbolt KT, Blagoev B. Proteome-wide quantitation by SILAC. Methods Mol Biol. 2010;658:187–204. doi: 10.1007/978-1-60761-780-8_11. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda K, Tojo K, Udagawa T, Otsubo C, Ishikawa M, Tokudome G, et al. Cellular physiology of rat cardiac myocytes in cardia fibrosis: In vitro simulation using the cardiac myocyte/cardiac non-myocyte co-culture system. Hypertens Res. 2008;31:693–706. doi: 10.1291/hypres.31.693. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda K, Tojo K, Otsubo C, Udagawa T, Hosoya T, Tajima N, et al. Effects of urocortin II on neonatal rat cardiac myocytes and non-myocytes. Peptides. 2005;26:2473–2481. doi: 10.1016/j.peptides.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 47.Frauzusoff A, Rothblatt J, Schekman R. Analysis of polypeptide transit through yeast secretory pathway. Chapter 45. In: Guthrie C, Fink GR, editors. Methods in Enzymology. Guide to yeast genetics and molecular and cell biology. San Diego, CA, USA: Elsevier Academic Press; 2004. [DOI] [PubMed] [Google Scholar]
- 48.Klein-Scory S, Kübler S, Diehl H, Eilert-Micus C, Reinacher-Schick A, Stühler K, et al. Immunoscreening of the extracellular proteome of colorectal cancer cells. BMC Cancer. 2010;10:70. doi: 10.1186/1471-2407-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raimondo F, Morosi L, Chinello C, Magni F, Pitto M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics. 2011;11:709–720. doi: 10.1002/pmic.201000422. [DOI] [PubMed] [Google Scholar]
- 50.Brunner Y, Schvartz D, Coute Y, Sanchez JC. Proteomics of regulated secretory organelles. Mass Spectrom Rev. 2009;28:844–867. doi: 10.1002/mas.20211. [DOI] [PubMed] [Google Scholar]
- 51.Xiao Z, Conrads TP, Beck GR, Veenstra TD. Analysis of the extracellular matrix and secreted vesicle proteomes by mass spectrometry. Methods Mol Biol. 2008;428:231–244. doi: 10.1007/978-1-59745-117-8_13. [DOI] [PubMed] [Google Scholar]
- 52.Xue H, Lu B, Lai M. The cancer secretome: a reservoir of biomarkers. J Transl Med. 2008;6:52. doi: 10.1186/1479-5876-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang CM. In vivo secretome sampling technology for proteomics. Proteomics Clin Appl. 2007;1:953–962. doi: 10.1002/prca.200700031. [DOI] [PubMed] [Google Scholar]
- 54.Duran MC, Mas S, Martin-Ventura JL, Meilhac O, Michel JB, Gallego-Delgado J, et al. Proteomic analysis of human vessels: Application to atherosclerotic plagues. Proteomics. 2003;3:973–978. doi: 10.1002/pmic.200300389. [DOI] [PubMed] [Google Scholar]
- 55.Polaskova V, Kapur A, Khan A, Molloy MP, Baker MS. High-abundance protein depletion: Comparison of methods for human plasma biomarker discovery. Electrophoresis. 2010;31:471–482. doi: 10.1002/elps.200900286. [DOI] [PubMed] [Google Scholar]
- 56.Pernemalm M, Lewensohn R, Lehtio J. Affinity prefractionation for MS-based plasma proteomics. Proteomics. 2009;9:1420–1427. doi: 10.1002/pmic.200800377. [DOI] [PubMed] [Google Scholar]
- 57.Liu T, Qian W, Mottaz HM, Gritsenko MA, Norbeck AD, Moore RJ, et al. Evaluation of multiprotein immunoaffinity subtraction for plasma proteomics and candidate biomarker discovery using mass spectrometry. Mol Cell Proteomics. 2006;5:2167–2174. doi: 10.1074/mcp.T600039-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tucholska M, Bowden P, Jacks K, Zhu P, Furesz S, Dumbrovsky M, et al. Human serum proteins fractionated by preparative partition chromatography prior to LC-ESI-MS/MS. J Proteome Res. 2009;8:1143–1155. doi: 10.1021/pr8005217. [DOI] [PubMed] [Google Scholar]
- 59.Stastna M, Van Eyk J. Protein separation: Liquid chromatography. Chapter 3. In: Van Eyk JE, Dunn MJ, editors. Clinical proteomics: From diagnosis to therapy. Weinheim: Wiley-VCH Verlag GmbH & Co.; 2008. [Google Scholar]
- 60.O’Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 61.Unlu M, Morgan ME, Minden JS. Difference gel electrophoresis: A single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18:2071–2077. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- 62.Hannig K. New aspects in preparative and analytical continuous free-flow cell electrophoresis. Electrophoresis. 1982;3:235–243. [Google Scholar]
- 63.Moritz RL, Ji H, Schütz F, Connolly LM, Kapp EA, Speed TP, et al. A proteome strategy for fractionating proteins and peptides using continuous free-flow electrophoresis coupled off-line to reversed-phase high-performance liquid chromatography. Anal Chem. 2004;76:4811–4824. doi: 10.1021/ac049717l. [DOI] [PubMed] [Google Scholar]
- 64.Nishikimi T, Minamino N, Nakao K. Diverse molecular forms of plasma B-type natriuretic peptide in heart failure. Curr Heart Fail Rep. 2011;8:140–146. doi: 10.1007/s11897-011-0051-y. [DOI] [PubMed] [Google Scholar]
- 65.Jiang J, Pristera N, Wang W, Zhang X, Wu Q. Effect of sialylated O-glycans in pro-brain natriuretic peptide stability. Clin Chem. 2010;56:959–966. doi: 10.1373/clinchem.2009.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, Li XJ, Martin DB, Aebersold R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat Biotechnol. 2003;21:660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 67.Wollscheid B, Bausch-Fluck D, Henderson C, O'Brien R, Bibel M, Schiess R, et al. Mass-spectrometric identification and relative quantification of N-linked cell surface glycoproteins. Nat Biotechnol. 2009;27:378–386. doi: 10.1038/nbt.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gundry RL, Raginski K, Tarasova Y, Tchernyshyov I, Bausch-Fluck D, Elliott ST, et al. The mouse C2C12 myoblast cell surface N-linked glycoproteome: identification, glycosite occupancy, and membrane orientation. Mol Cell Proteomics. 2009;8:2555–2569. doi: 10.1074/mcp.M900195-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suksrichavalit T, Yoshimatsu K, Prachayasittikul V, Bülow L, Ye L. “Clickable" affinity ligands for effective separation of glycoproteins. J Chromatogr A. 2010;1217:3635–3641. doi: 10.1016/j.chroma.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 70.Temporini C, Calleri E, Cabrera K, Felix G, Massolini G. On-line multi-enzymatic approach for improved sequence coverage in protein analysis. J Sep Sci. 2009;32:1120–1128. doi: 10.1002/jssc.200800596. [DOI] [PubMed] [Google Scholar]
- 71.Walther TC, Mann M. Mass-spectrometry-based proteomics in cell biology. J Cell Biol. 2010;190:491–500. doi: 10.1083/jcb.201004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Becker CH, Bern M. Recent developments in quantitative proteomics. Mutat Res. 2010;722:171–182. doi: 10.1016/j.mrgentox.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collier TS, Sarkar P, Franck WL, Rao BM, Dean RA, Muddiman DC. Direct comparison of stable isotope labeling by amino acids in cell culture and spectral counting for quantitative proteomics. Anal Chem. 2010;82:8696–8702. doi: 10.1021/ac101978b. [DOI] [PubMed] [Google Scholar]
- 74.Li M, Gray W, Zhang H, Chung CH, Billheimer D, Yarbrough WG, et al. Comparative shotgun proteomics using spectral count data and quasi-likelihood modeling. J Proteome Res. 2010;9:4295–4305. doi: 10.1021/pr100527g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf-Yadlin A, Hautaniemi S, Lauffenburger DA, White FM. Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. Proc Natl Acad Sci USA. 2007;104:5860–5865. doi: 10.1073/pnas.0608638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baek JH, Kim H, Shin B, Yu MH. Multiple products monitoring as a robust approach for peptide quantification. J Proteome Res. 2009;8:3625–3632. doi: 10.1021/pr800853k. [DOI] [PubMed] [Google Scholar]
- 77.Lange V, Picotti P, Domon B, Aebersold R. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol. 2008;4:222. doi: 10.1038/msb.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Picotti P, Rinner O, Stallmach R, Dautel F, Farrah T, Domon B, et al. High-throughput generation of selected reaction-monitoring assays for proteins and proteomes. Nature Methods. 2010;7:43–46. doi: 10.1038/nmeth.1408. [DOI] [PubMed] [Google Scholar]
- 79.Bertsch A, Jung S, Zerck A, Pfeifer N, Nahnsen S, Henneges C, et al. Optimal de novo design of MRM experiments for rapid assay development in targeted proteomics. J Proteome Res. 2010;9:2696–2704. doi: 10.1021/pr1001803. [DOI] [PubMed] [Google Scholar]
- 80.Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nature Protocols. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- 81.Bendtsen JD, Jensen LJ, Blom N, von Heijne, Brunak S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Engineering, Design & Selection. 2004;17:349–356. doi: 10.1093/protein/gzh037. [DOI] [PubMed] [Google Scholar]
- 82.Kitteringham NR, Jenkins RE, Lane CS, Elliott VL, Park BK. Multiple reaction monitoring for quantitative biomarker analysis in proteomics and metabolomics. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1229–1239. doi: 10.1016/j.jchromb.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 83.Turtoi A, Mazzucchelli GD, De Pauw E. Isotope coded protein label quantification of serum proteins-comparison with the label-free LC-MS and validation using the MRM approach. Talanta. 2010;80:1487–1495. doi: 10.1016/j.talanta.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 84.Kuzyk MA, Smith D, Yang J, Cross TJ, Jackson AM, Hardie DB, et al. Multiple reaction monitoring-based, multiplexed, absolute quantitation of 45 proteins in human plasma. Mol Cell Proteomics. 2009;8:1860–1877. doi: 10.1074/mcp.M800540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zang MC, Li Y, Xue LX, Jia HT, Jing H. Cooperative activation of atrial naturetic peptide promoter by dHAND and MEF2C. J Cell Biochem. 2004;93:1255–1266. doi: 10.1002/jcb.20225. [DOI] [PubMed] [Google Scholar]
- 86.McMullen NM, Zhang F, Hotchkiss A, Bretzner F, Wilson JM, Ma H, et al. Functional characterization of cardiac progenitor cells and their derivatives in the embryonic heart post-chamber formation. Dev Dyn. 2009;238:2787–2799. doi: 10.1002/dvdy.22112. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka T, Tohyama S, Murata M, Nomura F, Kaneko T, Chen H, et al. In vitro pharmacologic testing using human induced pluripotent stem cell-derived cardiomyocytes. Biochem Biophys Res Commun. 2009;385:497–502. doi: 10.1016/j.bbrc.2009.05.073. [DOI] [PubMed] [Google Scholar]
- 88.Andrews GL, Shuford CM, Burnett JC, Jr, Hawkridge AM, Muddiman DC. Coupling of a vented column with splitless nanoRPLC-ESI-MS for the improved separation and detection of brain natriuretic peptide-32 and its proteolytic peptides. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:948–954. doi: 10.1016/j.jchromb.2009.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wieczorek SJ, Wu AH, Christenson R, Krishnaswamy P, Gottlieb S, Rosano T, Hager D, et al. A rapid B-type natriuretic peptide assay accurately diagnoses left ventricular dysfunction and heart failure: a multicenter evaluation. Am Heart J. 2002;144:834–839. doi: 10.1067/mhj.2002.125623. [DOI] [PubMed] [Google Scholar]
- 90.Miller WL, Phelps MA, Wood CM, Schellenberger U, Van Le A, Perichon R, et al. Comparison of Mass Spectrometry and Clinical Assay Measurements of Circulating Fragments of B-Type Natriuretic Peptide in Patients with Chronic Heart Failure. Circ Heart Fail. 2011;4:355–360. doi: 10.1161/CIRCHEARTFAILURE.110.960260. [DOI] [PubMed] [Google Scholar]
- 91.Hishinuma S, Funamoto M, Fujio Y, Kunisada K, Yamauchi-Takihara K. Hypoxic stress induces cardiotrophin-1 expression in cardiac myocytes. Biochem Biophys Res Commun. 1999;264:436–440. doi: 10.1006/bbrc.1999.1535. [DOI] [PubMed] [Google Scholar]
- 92.Winegrad S, Henrion D, Rappaport L, Samuel JL. Self-protection by cardiac myocytes against hypoxia and hyperoxia. Circ Res. 1999;85:690–698. doi: 10.1161/01.res.85.8.690. [DOI] [PubMed] [Google Scholar]
- 93.Torry RJ, Tomanek RJ, Zheng W, Miller SJ, Labarrere CA, Torry DS. Hypoxia increases placenta growth factor expression in human myocardium and cultured neonatal rat cardiomyocytes. J Heart Lung Transplant. 2009;28:183–190. doi: 10.1016/j.healun.2008.11.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Henderson-MacLennan NK, Papp JC, Talbot CC, Jr, McCabe ERB, Presson AP. Pathway analysis software: Annotation errors and solutions. Molecular Genetics and Metabolism. 2010;101:134–140. doi: 10.1016/j.ymgme.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Z, Liu N, Zhang L, Gong K, Cai Y, Gao W, et al. Proteomic profiling reveals comprehensive insights into adrenergic receptor-mediated hypertrophy in neonatal rat cardiomyocytes. Proteomics Clin Appl. 2009;3:1407–1421. doi: 10.1002/prca.200900029. [DOI] [PubMed] [Google Scholar]
- 96.Lawlor K, Nazarian A, Lacomic L, Tempst P, Villanueva J. Pathway-based biomarker search by high-throughput proteomics profiling of secretomes. J Proteome Res. 2009;8:1489–1503. doi: 10.1021/pr8008572. [DOI] [PubMed] [Google Scholar]
- 97.Arrell DK, Niederländer NJ, Faustino RS, Behfar A, Terzic A. Cardioinductive network guiding stem cell differentiation revealed by proteomic cartography of tumor necrosis factor alpha-primed endodermal secretome. Stem Cells. 2008;26:387–400. doi: 10.1634/stemcells.2007-0599. [DOI] [PubMed] [Google Scholar]
- 98.Chung S, Arrell DK, Faustino RS, Terzic A, Dzeja PP. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J Mol Cell Cardiol. 2010;48:725–734. doi: 10.1016/j.yjmcc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agnetti G, Kaludercic N, Kane LA, Elliott ST, Guo Y, Chakir K, et al. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ Cardiovasc Genet. 2010;3:78–87. doi: 10.1161/CIRCGENETICS.109.871236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zlatkovic-Lindor J, Arrell DK, Yamada S, Nelson TJ, Terzic A. ATP-sensitive K(+) channel-deficient dilated cardiomyopathy proteome remodeled by embryonic stem cell therapy. Stem Cells. 2010;28:1355–1367. doi: 10.1002/stem.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arrell DK, Zlatkovic J, Kane GC, Yamada S, Terzic A. ATP-sensitive K+ channel knockout induces cardiac proteome remodeling predictive of heart disease susceptibility. J Proteome Res. 2009;8:4823–4834. doi: 10.1021/pr900561g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zlatkovic J, Arrell DK, Kane GC, Miki T, Seino S, Terzic A. Proteomic profiling of KATP channel-deficient hypertensive heart maps risk for maladaptive cardiomyopathic outcome. Proteomics. 2009;9:1314–1325. doi: 10.1002/pmic.200800718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Behfar A, Perez-Terzic C, Faustino RS, Arrell DK, Hodgson DM, Yamada S, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010;5:e13984. doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Isserlin R, Merico D, Alikhani-Koupaei R, Gramolini A, Bader GD, Emili A. Pathway analysis of dilated cardiomyopathy using global proteomic profiling and enrichment maps. Proteomics. 2010;10:1316–1327. doi: 10.1002/pmic.200900412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stastna M, Abraham MR, Van Eyk JE. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett. 2009;583:1800–1807. doi: 10.1016/j.febslet.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]