Abstract
With the rapidly expanding field of tissue engineering, surgeons have been eager to apply these principles to craniofacial surgery. Tissue engineering strategies combine the use of a cell type placed on a scaffold and subsequently implanted in vivo to address a tissue defect or tissue dysfunction. In this review we will discuss the current clinical need for skeletal and soft tissue engineering faced by craniofacial surgeons and subsequently we will explore cell types and scaffold designs being employed for tissue engineering treatment options. We will conclude by discussing ways to enhance the vascularity of tissue engineered constructs as this will ultimately allow for a definitive repair. Current “stem cell” options include pluripotent stem cells as well as multipotent mesenchymal cells. Biomimetic scaffolds can function to protect and enhance differentiation of stem cells by providing inductive cues. Larger scale studies including prospective, randomized control trials must be performed to determine the optimum cell delivery method and cytokine stimuli for tissue engineering driven strategies to address the clinical needs in skeletal and soft tissue reconstruction.
Keywords: Stem Cells, Craniofacial Surgery, adipose derived mesenchymal cells, bone marrow derived mesenchymal cells, scaffolds, vascularization
Introduction
Craniofacial malformations can have devastating psychosocial implications for many children and represents a significant socioeconomic burden. The mean total hospital cost per child with a craniofacial malformation has been shown to be over $20,000 during the first two years of life, compared to $2500 for children without craniofacial malformations.(1) Additionally, trauma and tumor resections create a large biomedical burden in the craniofacial skeleton of adults. Such oncogenic reconstructions pose difficult reconstructive challenges due to concurrent use of chemotherapy and radiation. Addressing these craniofacial defects often require soft tissue transfer, bone grafting techniques and other technically challenging operations such as microvascular free flap procedures that create large secondary donor site defects. These autogenous techniques are limited in quantity, often lack strength and long term viability and fail to restore pre-operative form and function. Alternatively, alloplastic materials allow for the creation of a more precise form but often lack in function and can be complicated by infection, mechanical failure and extrusion.
To address these current shortcomings in craniofacial surgery, physicians as well as biotechnology companies have sought to develop alternate less invasive approaches. With the rapidly expanding field of tissue engineering, surgeons have been eager to apply these principles to craniofacial surgery. Tissue engineering strategies combine the use of a cell type placed on a scaffold and subsequently implanted in vivo to address a tissue defect or tissue dysfunction. More recently, excitement has surrounded the use of pluripotent and multipotent cells types when utilizing tissue engineering technologies. Though these pluiripotent or “stem” cells are attractive, it is crucial that physicians and surgeons understand the cell type they are using, assess whether it is the optimal cell type to address the clinical need, and most importantly, understand the risks of such therapies before using them clinically. Furthermore, craniofacial surgeons must understand methods of cell delivery using different scaffold designs. In this review we will discuss the current clinical need for skeletal and soft tissue engineering faced by craniofacial surgeons and subsequently we will explore current cell types and scaffold designs being employed for tissue engineering treatment options. We will conclude by discussing ways to enhance the vascularity of tissue engineered constructs as this will ultimately allow for a definitive repair.
Clinical need for stem cell therapy in Craniofacial Surgery
Craniofacial surgery is particularly suited to tissue engineering as significant progress has been made in adipose and osseous tissue induction. With regards to soft tissue needs, craniofacial surgeons are faced with the aesthetic challenges of facial rejuvenation such as malar and lip augmentation which currently rely on the use of synthetic fillers such as hyaluronic acid and fat transfer. Similarly, reconstructive needs for soft tissue contour such as in patients suffering from Parry-Romberg syndrome and HIV related lipoatrophy are addressed with a number of treatment options. Autologous lipoinjection has become recently popularized and been shown to provide excellent outcomes, however, even in the best hands, lipotransfer does not provide a self-renewing population of cells.(2, 3) Thus, recent efforts by Yoshimura et al to augment transferred adipose tissue with adipose derived stromal cells (ASCs) offer a promising application of stem cell therapy.(4–7)
A second medical bioburden faced by craniofacial surgeons is the need for bone replacement. The craniofacial skeleton provides structural stability and mastication puts load bearing stress on several craniofacial bones. There are over 500,000 bone grafts performed annually, of which 6% are for craniofacial indications.(8, 9) Bone is a responsive, highly vascularized tissue that responds to local stress and strain. Bone consists of three different cell types (osteocytes, osteoblasts, and osteoclasts) which are surrounded by a matrix composed of hydroxyapatite, collagens, glycoproteins, proteoglycans and sialoproteins.(10, 11) With regards to the pediatric population, cleft palate represents an area where bony tissue regeneration to address the alveolar ridge and enable subsequent tooth eruption and midface development would greatly benefit from tissue engineered bone. Autogenous bone grafts, often harvested from the iliac crest have become the current gold standard to treat the alveolar cleft, however, such tissues are painful to harvest, and can be complicated by hematoma, infection and resorption.
In patients of all ages above 2 years old, a time when the calvarium can no longer regenerate on its own, calvarial defects also represent a significant reconstructive challenge. Whether from congenital malformations, trauma or cancer extirpation, the loss of bone in the craniofacial skeleton has significant structural and functional consequences. Current treatments for such skeletal defects include nonvascularized bone grafting, which are at risk of resorption, and the use of alloplastic materials which are wrought with infection, extrusion and mechanical failure. Thus, the development and clinical introduction of a biomimetic, osteoconductive scaffold would greatly benefit surgeons treating patients with osseous defects of the craniofacial skeleton.
Cancer resections and trauma can also often lead to maxillary and mandibular bony defects. Current approaches of bone grafting using nonvascularized as well as vascularized bone grafts such as those harvested from the fibula and scapula are often employed. Though vascularized grafts undergo less resorption, they often create large secondary defects and are available in a limited supply. When autologous transplantation is not possible, allogenic and xenogenic bone grafts have been employed, however, these substitutes are constrained in their osteogenicity, stability and strength.(10)
Current State of Stem Cell Therapy in Craniofacial Surgery
Cell Sources
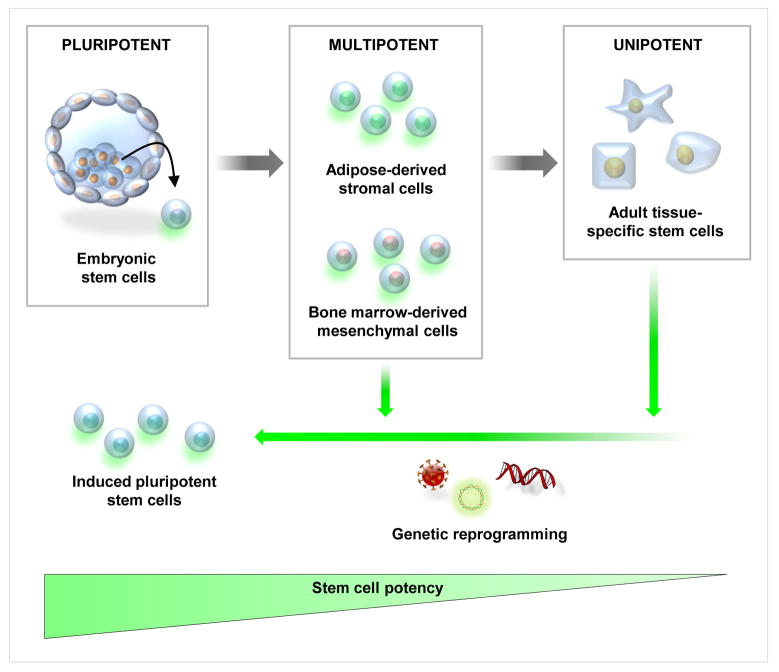
Current concepts of what is referred to as a “stem cell” have only been validated in the last 40 years (Fig. 1). Prior to the 1960s all dividing cells were thought to contribute to tissue growth and turnover and were considered resident stem cells. (12) Early studies demonstrated that by infusing a lethally irradiated mouse with bone marrow cells would migrate to the spleen and give rise to hematopoetic cell nodules that were proven to derive from the same single cell or clone. These clones were named colony forming units that eventually were shown to be hematopoietic stem cells. These early studies provided the groundwork for the understanding of the existence of an undifferentiated self-renewing cell population. However, when referring to a “stem cell” surgeons and scientists need to be specific as to the type of cell they are referring to as a pluripotent stem cell can differentiate into any of the 3 germ layers: endoderm, ectoderm or mesoderm, whereas cells such as a mesenchymal stem cell are multipotent and can only differentiate into cells of mesodermal lineage (i.e bone, fat, cartilage and muscle).
Figure 1. Schematic of available stem cells for tissue engineering.
Embryonic stem cells (left) are pluirpotent and can be differentiated into multipotent mesenchymal stem cells. Similarly, mesenchymal multipotent cells can be harvested from adipose tissue and bone marrow and can differentiate to adult tissue–specific stem cells. Recent studies have demonstrated that mulipotent cells and adult tissue–specific stem cells can be reprogrammed to induced pluirpotent stem cells (iPS) which, similar to embryonic stem cells, can differentiate to any of the three main germ layers (endoderm, ectoderm and mesoderm).
Before the recent discovery and characterization of induced Pluripotent Stem Cells (iPS), human embryonic stem cells (hESCs) represented the only truly pluripotent cell type. Research on or potential clinical uses of any tissue of embryonic origin, however, have been surrounded by contentious political and ethical debate surrounding creation, usage, and destruction of human embryos. The creation of hESCs necessitates destruction of a human embryo, which naturally raised various value of life objections. Despite improved federal funding by President Obama for the use of existing and newly created hESC cell lines and regional funding such as the California Institute of Regenerative Medicine, the use of hESCs remains controversial. For example, promising data in the field of neuroregeneration exists, however, there are only limited animal studies employing hESCs for craniofacial diseases. (13) Obstacles which must be overcome include the risk of tumorigenicity, immunocompatibility, and isolation of desired differentiated cell types for therapeutic use.
In 2006, scientists first described the process of reprogramming or “inducing” pluripotency in an adult lineage committed cell by transfecting a few key transcription factors in the cell for a period of weeks.(14) These cells have been named “induced” pluripotent stem (iPS) cells as they have ESC-like properties. Despite the excitement generated behind the use of iPS cells, preclinical studies are still not safe enough for transition into human trials. Recently, a human sickle cell anemia mouse model was successfully treated with iPS cells that were genetically corrected. (15) Although this was a human sickle cell model, significant safety studies are required to avoid teratoma formation. The reason for teratoma formation after injection is not completely understood, though it is currently believed that undifferentiated hESCs or hiPSCs residing in the differentiated cell populations survive and go on to create three germ layer tumors.(16) Teratoma formation, however, is also affected by cell number as the cut off of >50hESCs has been shown to induce teratomas in SCID mice.(16) Similarly, the site of implantation has been shown to affect tumor formation as injection of hESCs into the liver will form tumors as early as 3–4 weeks, whereas subcutaneous implantation takes up to 8 weeks to form tumors.(16) Other hurdles for the use of pluripotent cells include but are not limited to cell number and immunorejection. Lastly, from a commercialization standpoint, it has yet to be determined if a viable business model for patient specific iPS treatments is possible given the challenge of their reprogramming and the oncogenic concerns. (17)
Currently, one of the most well defined and clinically utilized multipotent cell types are bone marrow derived mesenchymal cells (MSCs). Much of our understanding of MSCs comes from work by Pittenger and colleagues.(18) Similar to bone marrow, scientists in the early 1960s also described a stromal-vascular fraction (SVF) that contained fibroblastic-like cells. In 2001, however, the understanding of the potential of these SVF cells greatly changed as Zuk et al. demonstrated the ability of the SVF to undergo adipogenic, chondrogenic, myogenic, and osteogenic differentiation. (19) These cells were eventually renamed adipose-derived stromal cells (ASCs). The ease of harvest of a large quantity of ASCs derived from a simple liposuction procedure, has led to a rapidly gaining popularity in their use in tissue engineering research and clinical trials. With regards to basic science research, numerous studies have demonstrated the potential of ASCs seeded onto a polyglycolic scaffold to engineer calvarial bone.(20, 21)
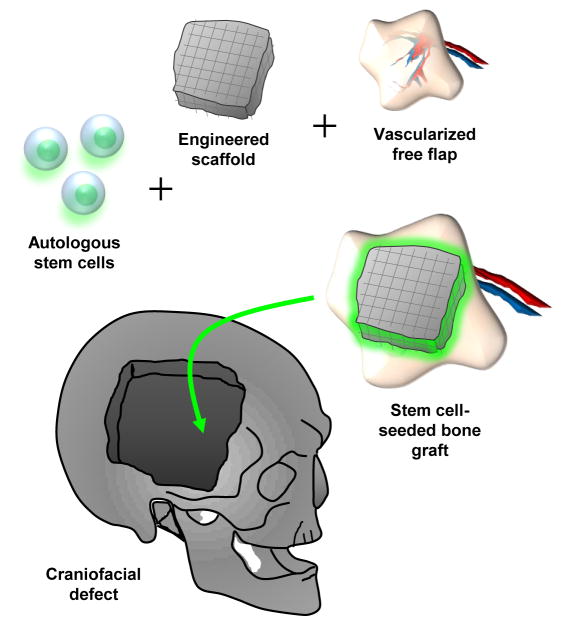
From a clinical standpoint, limited case reports describe using ASCs to treat defects of the maxilla, (22) mandible (23) and calvaria.(24) Such reconstructions lead to significant hope as they eliminate the need for alloplastic materials, and thus reduce the risk of infection, breakdown or rejection. Lendeckel et al treated a 7 year old patient with widespread calvarial defects after calvarial trauma by placing autogenous ASCs on nonvascularized bone grafts using fibrin glue.(24) Mesimaki et al. delivered ASCs on a vascularized bed by harvesting a microvascular flap. They describe seeding this flap with ASCs, beta-tricalcium phosphate and BMP-2 to treat a large defect in the maxilla, reporting promising outcomes up to 8 months post-operatively. (22) For mandibular reconstruction, Warnke et al implanted a prefabricated titanium mesh loaded with HA and rhBMP-2 and seeded this with bone marrow MSCs which was then implanted into the latissiumus dorsi muscle.(25) After 7 weeks, this mandible was transplanted using the thorocodorsal vessel and placed into a 4cm mandibular defect.(25) These findings are encouraging as they begin to use multipotent cells and combine them with proven surgical procedures such as bone grafts and microvascular free flaps to augment osseous healing. One could perhaps envision future applications where a scaffold is constructed in the shape of a calvarial bone, seeded with osteocapable cells such as hASCs, and transferred along with a microvascular tissue flap providing vascularized autogenous de novo bone (Fig. 2).
Figure 2. Tissue Engineering for Craniofacial Bone Tissue Reconstruction.
Future options for calvarial defect repair involve seeding a multipotent stem cell on an engineered scaffold, placing this construct on a vascularized microvascular free flap and using this vascularized stem cell–seeded scaffold to address a tissue defect such as a calvarial defect.
Scaffolds and Delivery systems for craniofacial tissue engineering
When using stem cells for tissue engineering applications, one must take into account the environmental niche or microenvironment in which these cells are placed. This microenvironment can significantly modify stem cell behavior and ability to undergo differentiation towards a specific lineage. Part of this microenvironment depends on the region of implantation; however, the niche can also be manipulated by placing stem cells on unique biomimetic scaffolds. Biomaterial scaffolds can function to protect and enhance differentiation of stem cells by providing inductive cues. Ideally a scaffold would be constructed from inert materials that can withstand any immune response, protect the stem cells that are seeded onto the scaffold and eventually undergo resorption by the body. Other important scaffold considerations for craniofacial reconstruction include porosity, permeability and pore architecture which have been shown to affect cell viability, differentiation and vascularization.(26–28)
Natural scaffolds include collagen and hyaluronic acid which have been shown to be osteoconductive, however, they fail to offer significant mechanical strength.(26) Synthetic polymers, commonly used materials for bone tissue engineering, include demineralized bone matrix as well as poly-l-lactic acid (PLGA) which provide greater structural strength. Though PLGA lacks a significant osteogenic capability on its own, when combined with hydroxyapatite, it has proven to create a significantly osteogenic environment.(20, 21) Such scaffolds can perhaps be further augmented through the use of osteogenic cytokines such as Bone Morphogenic Protein 2 (BMP-2). In a laboratory setting, several investigators have used BMP-2 delivery methods on a scaffold to enhance the osteogenic capability of ASCs. (29) BMP-2 is osteoinductive and likely enhances the osteogenesis of surrounding osteoblasts, periosteal cells and underlying dura mater in calvarial defects. Clinically, BMP-2 has been successfully employed to enhance spinal fusion treatments opening the window for craniofacial applications to follow suit. (30)
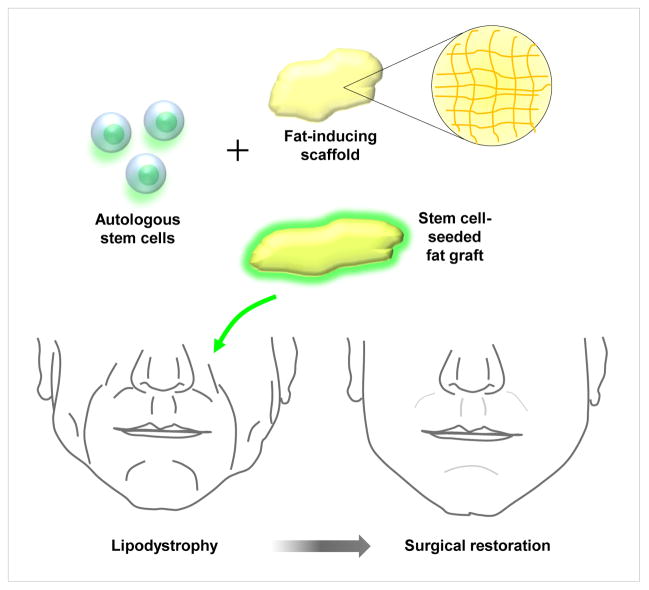
With regards to soft tissue augmentation of the face, rigid scaffolds such as demineralized bone matrix are ill suited. Instead, stem cells are better placed in a soft matrigel-type environment or seeded on autogenous adipose tissue. Yoshimura et al have demonstrated impressive outcomes by combining ASCs with adipose tissue for breast augmentation and recently for facial lipoatrophy. In both uses, Yoshimura et al employ ‘cell assisted lipotransfer” where a fat graft is transferred along with freshly isolated ASCs.(4–7) Despite promising results, it remains to be seen if transferred “stem cells” remain viable and undergo directed differentiation into adipocytes or vascular structures in vivo or whether using adipose tissue with a nutrient rich cell population augments survival of the implanted adipocytes. Future efforts to treat patients with significant craniofacial soft tissue deficits will likely involve combining an autogenous, multipotent stem cell with the capability of undergoing adipogenic differentiation, and seeding these cells on a tissue derived scaffold such as adipose tissue (Fig. 3). This graft could subsequently be injected or implanted into regions of lipodystrophy or soft tissue asymmetries.
Figure 3. Tissue Engineering for Craniofacial Soft Tissue Reconstruction.
Treatment of patients with lipodystrophy employs the combination of autogenous stem cells seeded on an adipose tissue scaffold and placed in areas of soft tissue deficits.
Enhancing Vascularization of Tissue Engineered Constructs
Tissue engineered constructs utilizing stem cells seeded or incorporated into a biomimetic matrix have tremendous potential for use in craniofacial and other reconstructive surgical therapies, but those examples described above depend mainly on extrinsic neovascularization.(31) One challenge to craniofacial tissue engineering is creating an intrinsic or de novo vascularization of the engineered graft. Once a two- or three-dimensional construct becomes more than 5–8mm in thickness, vascularization must occur within the graft in order to promote survival of implanted stem cells and stem cell-derived regenerative tissues (32). Several groups have attempted to address this concern by enhancing the vascularization potential of tissue engineered scaffold/stem cell constructs before implantation.(33–35)
One approach is to incorporate vasculogenic growth factors and stimulatory ligands to a synthetic biomimetic scaffold, which would then encourage neovascularization within the engineered graft after implantation (36). By providing stimulatory cues for stem cell differentiation into endothelium and smooth muscles cells in addition to encouraging paracrine function of stem cell populations within the engineered construct, a pro-angiogenic environment can be created, thereby facilitating vascularization of the graft (37). Several groups have reported incorporation of stem cell-seeded engineered grafts into the recipient vasculature after implantation, thus demonstrating the ability of stem cells within tissue engineered constructs to form vascular tissues (38–41).
The Gurtner laboratory has developed another approach utilizing decellularized microvascular free flaps as expandable microvascular beds (EMBs) to form the basis of a vascularized tissue engineered construct (42). This approach utilizes the native capillary structure as the basis for a tissue engineering construct by digesting the surrounding perivascular tissue from a soft tissue bed, which leaves the large vessels and capillary bed intact. Stem cells can then be seeded onto the EMB and the engineered construct will already have a developed vasculature before implantation. While there are still unresolved technical issues associated with this approach, we are actively working to scale up this technique to provide complex soft tissue constructs for use in complex reconstructive applications (43).
Conclusion
Though encouraging, these early studies offer only level 4 and 5 evidence data and lack significant power to help tailor clinical practice. Furthermore, significant hurdles remain to bring large scale tissue engineering into craniofacial surgery. Large studies including prospective, randomized control trials, must verify the preclinical and early clinical findings, and to determine the optimum cell delivery method and cytokine stimuli for tissue engineering driven strategies for craniofacial reconstruction.
Acknowledgments
Sources of Support:
This study was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research grant 1 R21 DE019274, 1 RC2 DE020771, the Oak Foundation and Hagey Laboratory for Pediatric Regenerative Medicine to M.T.L. B.L was supported by the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1F32AR057302. National Endowment of Plastic Surgery
Footnotes
The authors have no potential conflicts of interest.
References
- 1.Weiss J, Kotelchuck M, Grosse SD, et al. Hospital use and associated costs of children aged zero-to-two years with craniofacial malformations in Massachusetts. Birth defects research. 2009;85:925–934. doi: 10.1002/bdra.20635. [DOI] [PubMed] [Google Scholar]
- 2.Coleman SR. Facial augmentation with structural fat grafting. Clin Plast Surg. 2006;33:567–577. doi: 10.1016/j.cps.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118:108S–120S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178–1185. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 6.Kurita M, Matsumoto D, Shigeura T, et al. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg. 2008;121:1033–1041. doi: 10.1097/01.prs.0000299384.53131.87. discussion 1042-1033. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic plastic surgery. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. discussion 56-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenwald AS, Boden SD, Goldberg VM, et al. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am. 2001;83-A(Suppl 2 Pt 2):98–103. doi: 10.2106/00004623-200100022-00007. [DOI] [PubMed] [Google Scholar]
- 9.Zuk PA. Tissue engineering craniofacial defects with adult stem cells? Are we ready yet? Pediatr Res. 2008 doi: 10.1203/PDR.0b013e31816bdf36. [DOI] [PubMed] [Google Scholar]
- 10.Kneser U, Schaefer DJ, Polykandriotis E, et al. Tissue engineering of bone: the reconstructive surgeon’s point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robey PG, Fedarko NS, Hefferan TE, et al. Structure and molecular regulation of bone matrix proteins. J Bone Miner Res. 1993;8 (Suppl 2):S483–487. doi: 10.1002/jbmr.5650081310. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman R. Hematology: basic principles and practice. 5. Philadelphia, PA: Churchill Livingstone/Elsevier; 2009. [Google Scholar]
- 13.Kim BG, Hwang DH, Lee SI, et al. Stem cell-based cell therapy for spinal cord injury. Cell transplantation. 2007;16:355–364. doi: 10.3727/000000007783464885. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 16.Fong CY, Gauthaman K, Bongso A. Teratomas from pluripotent stem cells: A clinical hurdle. J Cell Biochem. 111:769–781. doi: 10.1002/jcb.22775. [DOI] [PubMed] [Google Scholar]
- 17.Sun N, Longaker MT, Wu JC. Human iPS cell-based therapy: Considerations before clinical applications. Cell Cycle. :9. doi: 10.4161/cc.9.5.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 20.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 21.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesimaki K, Lindroos B, Tornwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Kulakov AA, Goldshtein DV, Grigoryan AS, et al. Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bulletin of experimental biology and medicine. 2008;146:522–525. doi: 10.1007/s10517-009-0322-8. [DOI] [PubMed] [Google Scholar]
- 24.Lendeckel S, Jodicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Warnke PH, Wiltfang J, Springer I, et al. Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials. 2006;27:3163–3167. doi: 10.1016/j.biomaterials.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 26.Ward BB, Brown SE, Krebsbach PH. Bioengineering strategies for regeneration of craniofacial bone: a review of emerging technologies. Oral Dis. 16:709–716. doi: 10.1111/j.1601-0825.2010.01682.x. [DOI] [PubMed] [Google Scholar]
- 27.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 28.Hutmacher DW, Schantz JT, Lam CX, et al. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. Journal of tissue engineering and regenerative medicine. 2007;1:245–260. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 29.Peterson B, Zhang J, Iglesias R, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–129. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 30.Glassman SD, Carreon LY, Djurasovic M, et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: a randomized, controlled trial in patients over sixty years of age. Spine (Phila Pa 1976) 2008;33:2843–2849. doi: 10.1097/BRS.0b013e318190705d. [DOI] [PubMed] [Google Scholar]
- 31.Cassell OC, Hofer SO, Morrison WA, et al. Vascularisation of tissue-engineered grafts: the regulation of angiogenesis in reconstructive surgery and in disease states. Br J Plast Surg. 2002;55:603–610. doi: 10.1054/bjps.2002.3950. [DOI] [PubMed] [Google Scholar]
- 32.Uygun BE, Soto-Gutierrez A, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh S, Wu B, Dunn J. Accelerating Vascularization in Polycaprolactone Scaffolds by Endothelial Progenitor Cells. Tissue Eng Part A. doi: 10.1089/ten.tea.2010.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao M, Zhou J, Li X, et al. Repair of bone defect with vascularized tissue engineered bone graft seeded with mesenchymal stem cells in rabbits. Microsurgery. 31:130–137. doi: 10.1002/micr.20854. [DOI] [PubMed] [Google Scholar]
- 35.Hegen A, Blois A, Tiron CE, et al. Efficient in vivo vascularization of tissue-engineering scaffolds. Journal of tissue engineering andregenerative medicine. 5:e52–62. doi: 10.1002/term.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453:338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- 37.Kawamoto A, Asahara T. Role of progenitor endothelial cells in cardiovascular disease and upcoming therapies. Catheter Cardiovasc Interv. 2007;70:477–484. doi: 10.1002/ccd.21292. [DOI] [PubMed] [Google Scholar]
- 38.Matsumura G, Miyagawa-Tomita S, Shin’oka T, et al. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 39.Murayama T, Tepper O, Silver M, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002 Aug;30:967–972. doi: 10.1016/s0301-472x(02)00867-6. [DOI] [PubMed] [Google Scholar]
- 40.Brennan MP, Dardik A, Hibino N, et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg. 2008;248:370–377. doi: 10.1097/SLA.0b013e318184dcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roh JD, Brennan MP, Lopez-Soler RI, et al. Construction of an autologous tissue-engineered venous conduit from bone marrow-derived vascular cells: optimization of cell harvest and seeding techniques. J Pediatr Surg. 2007;42:198–202. doi: 10.1016/j.jpedsurg.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 42.Chang EI, Bonillas RG, El-ftesi S, et al. Tissueengineering using autologous microcirculatory beds as vascularized bioscaffolds. FASEB J. 2009;23:906–915. doi: 10.1096/fj.08-114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rustad KC, Sorkin M, Levi B, et al. Strategies for organ level tissue engineering. Organogenesis. 2010 March 25;6:151–157. doi: 10.4161/org.6.3.12139. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]