Abstract
Survival following myocardial infarction (MI) has improved substantially over the last 40 years; however, the incidence of subsequent congestive heart failure has dramatically increased as a consequence. Discovering plasma markers that signify adverse cardiac remodeling may allow high-risk patients to be recognized earlier and may provide an improved way to assess treatment efficacy. Alterations in extracellular matrix (ECM) regulate cardiac remodeling following MI and potentially provide a large array of candidate indicators.
The field of cardiac proteomics has progressed rapidly over the past 20 years, since publication of the first two-dimensional electrophoretic gels of left ventricle proteins. Proteomic approaches are now routinely utilized to better understand how the left ventricle responds to injury.
In this review, we will discuss how methods have developed to allow comprehensive evaluation of the ECM proteome. We will explain how ECM proteomic data can be used to predict adverse remodeling for an individual patient and highlight future directions. Although this review will focus on the use of ECM proteomics to better understand post-MI remodeling responses, these approaches have applicability to a wide-range of cardiac pathologies, including pressure overload hypertrophy, viral myocarditis, and non-ischemic heart failure.
Keywords: proteomics, remodeling, myocardial infarction, biomarker, MMP
Introduction
Short term (i.e., 30 day) survival rates for the post-myocardial infarction (MI) patient have improved from 60% in the 1970’s to >90% currently, primarily as a result reperfusion therapy.1–3 However, up to 45% of MI survivors subsequently develop congestive heart failure (CHF).4–6 Even with currently available therapies (reperfusion, angiotensin converting enzyme inhibitors and β adrenergic receptor inhibitors),2, 7, 8 novel strategies are needed to identify and treat patients who are at-risk for CHF.9 Examining changes at the extracellular matrix (ECM) level is a promising avenue to find new mechanisms to limit adverse remodeling.10
One clinical trial that highlights the importance of ECM in post-MI remodeling is the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival (EPHESUS) Study. In this study, the investigators evaluated the effects of treatment with an aldosterone receptor antagonist compared to placebo on post-MI outcomes. In a sub-study of 476 post-MI patients that developed CHF, serum analytes of collagen turnover were measured.11 The authors combined baseline serum levels of type I collagen telopeptide and brain natriuretic peptide into one composite index of collagen turnover. All-cause mortality and the composite end-point of cardiovascular death or CHF hospitalization were both associated with the collagen turnover composite index, with hazard ratios of 2.49 (p=0.039) and 3.03 (p=0.002), respectively. Furthermore, patients treated with eplerenone showed reduced levels of collagen turnover markers compared to those who did not receive eplerenone, indicating that collagen synthesis and degradation are active processes in post-MI patients. These results are consistent with the numerous animal studies that have shown a relationship between ECM remodeling and the development of CHF.12–14 Therefore, a better understanding of how the cardiac ECM proteome changes during disease progression may provide additional targets for therapeutic intervention.
This review will concentrate on the strengths and limitations of different proteomic approaches to glean information that is specific to ECM turnover in the post-MI setting. Proteomic strategies provide us with a means to index the proteins present, quantify levels, determine function, and explore interactions.15 This review will not concentrate on mass spectrometry technological advances that are well described in other reviews.15 Rather, we will focus on how sample preparation and labeling protocols have changed to increase the likelihood of cataloguing the cardiac ECM proteome. From our own perspective, we will describe how proteomic approaches focusing on the ECM compartment have progressed over time to current gel-free approaches using decellularized fractions. Strengths and limitations of the various proteomic approaches are summarized in Table 1.
Table 1.
Strengths And Limitations Of Proteomic Approaches for Evaluation of ECM
|
Abbreviations: 2-DE, two dimensional electrophoresis; ECM, extraceullar matrix; MW, molecular weight; pI, isoelectric point
Resolving whole left ventricular (LV) tissue extracts by 2-dimensional gel electrophoresis (2-DE)
In 1992, Dunn and colleagues were the first to establish a human myocardial two-dimensional electrophoresis database, which was updated in 1994.16, 17 The database contained 1,388 protein spots characterized by molecular weight (MW) and isoelectric point (pI). From a total of 103 protein spots analyzed, 49 were assigned by immunoblotting and 32 by N-terminal sequencing, for a 79% identification success rate. An additional six proteins were tentatively assigned by comparison with the human heart 2-DE protein database of Jungblut and colleagues.18 The databases from these two groups are the standards by which cardiac extract 2-DE gels are still compared. Annotated web-based 2-DE maps for healthy and diseased cardiac tissue, such as failing myocardium, have also been developed from these databases.19 These maps, which contain a preponderance of calcium signaling proteins and mitochondrial enzymes, highlight impairments in sarcoplasmic reticulum calcium cycling and mitochondrial signaling as markers of cardiac injury.
A major advantage of the 2-DE gel approach is that it provides apparent MW and pI information. Additionally, gel analysis is broad-based and effectively unbiased from the investigator point of view (i.e., you do not need to have a target already selected to examine changes). Because gel approaches are non-targeted, they can, in theory, reveal novel discoveries. Detailed image analysis of gel spots, using software such as SameSpots (Nonlinear Dynamic) affords the advantage that subsequent mass spectrometry analyses only need to be conducted on spots that exhibit significant differences among comparison groups.
A major disadvantage of the 2-DE gel approach is that even though the approach is unbiased, it is limited by issues of experimental design. Another limitation is the inability to fully resolve the entire complement of LV proteins on a single 2-DE gel. While using multiple IPG strips with overlapping pI ranges has increased the number of proteins resolved, for each gel only approximately 10% of the total number of proteins can be visualized. Furthermore, LV proteomic profiles represent mixed cell populations that are dominated by the most abundant cell type. Therefore, comparing a normal ventricle to an infarcted ventricle will merely reflect the change from a myocyte-dominated to an inflammatory cell and fibroblast-dominated tissue. Additionally, approaches using unfractionated LV extracts will yield a predominance of cytoplasmic and mitochondrial proteins, since the myocyte is the major cell type in the LV and mitochondria comprise 30% of the myocyte volume.20, 21 The inability to add ECM proteins to the LV proteome catalog using this global strategy has been disappointing. In addition to being present in relatively low abundance, ECM proteins are also hydrophobic, which requires that solubility issues be addressed.
Protein labeling strategies
From 2-DE gels, methods progressed to stable isotopic labeling strategies that directly couple quantification and identification. A major advantage of multiplexed labeling strategies is that the experimental groups are mixed prior to mass spectrometry analysis, thereby minimizing issues related to analytical variability such as differences in retention time or differential suppression of ionization.22, 23 Strategies in use over recent years have been based on a variety of approaches,24 including stable isotope incorporation (16O/18O labeling), derivatization for MS [e.g., isotope-coded affinity tags (ICAT), dimethyl labeling], derivatives that yield reporter ions in MS/MS [e.g., isobaric tag for relative and absolute quantitation (iTRAQ) and tandem mass tags (TMT)], and metabolic labeling [e.g., stable isotope labeling with amino acids in cell culture (SILAC)].25–31
A major strength of these strategies is that proteins are not first separated on 2-DE gels, which avoids issues of solubility and extremes of pI associated with the isoelectric focusing step. However, there are advantages to separating proteins by 1-DE prior to mass spectrometry analysis in that MW information is obtained and minimal sample cleanup is required. Labeling strategies can also be effectively coupled with multi-dimensional chromatography [combinations of ion exchange/reversed-phase (e.g., MudPIT) or high-pH/low-pH reversed phase (termed RP-RP) in conjunction with tandem mass spectrometry to allow quantification and identification in one step.32–34
With iTRAQ and TMT, all tryptic peptides are labeled, which increases confidence in the relative quantification. A major advantage of both iTRAQ and TMT is that both approaches can be highly multiplexed. Incorporation of 16O/18O during tryptic digestion and dimethyl labeling are straightforward methods that are relatively inexpensive but do not afford a high degree of multiplexing.
SILAC involves the metabolic incorporation of stable isotope analogs of amino acids into cellular proteins. SILAC has been used to quantify protein complexes, enzyme substrates, membrane proteins, and temporal dynamics.35–37 Since up to five labels can be used, multiple comparisons can be made simultaneously. This technique is especially useful for monitoring cell secretomes since newly synthesized proteins are evaluated. Pinto and colleagues demonstrated the utility of SILAC for exploring sheddase activity in cultured cells to identify two novel substrates for snake venom metalloproteinases; a similar approach in cardiac fibroblasts may identify novel ECM substrates of matrix metalloproteinases (MMPs).38, 39 A limitation of using SILAC to analyze a cell secretome is that the cells must be cultured in protein-free medium to allow analysis of secreted proteins in the supernatant; here, the assumption is that the proteins secreted under serum starvation conditions reflect in vivo secretion patterns.
Label-free quantification, including spectral counting (based on the number of spectra assigned to a given protein) and comparison of precursor ion intensities, can be used to determine differential protein expression levels in two or more experimental groups.40, 41 The enhanced mass accuracy and resolution of high-performance mass spectrometers are particularly important for relative quantification. The availability of software for relative quantification, [e.g., Progenesis LC-MS (Nonlinear), SIEVE (Thermo Fisher) based on precursor ion intensities, and Scaffold (Proteome Software) and ProteoIQ (NuSep) for spectrum counting] have advanced the label-free approach by providing the computational framework necessary to analyze the data.42–44
Xu and colleagues used the label-free approach to identify MMP-9 substrates in cancer cells.45 Others are working on label-free analysis in studies of other cardiovascular diseases, such as hypertension and atherosclerotic plaque formation.46–48
Focusing on natural tissue enrichment strategies
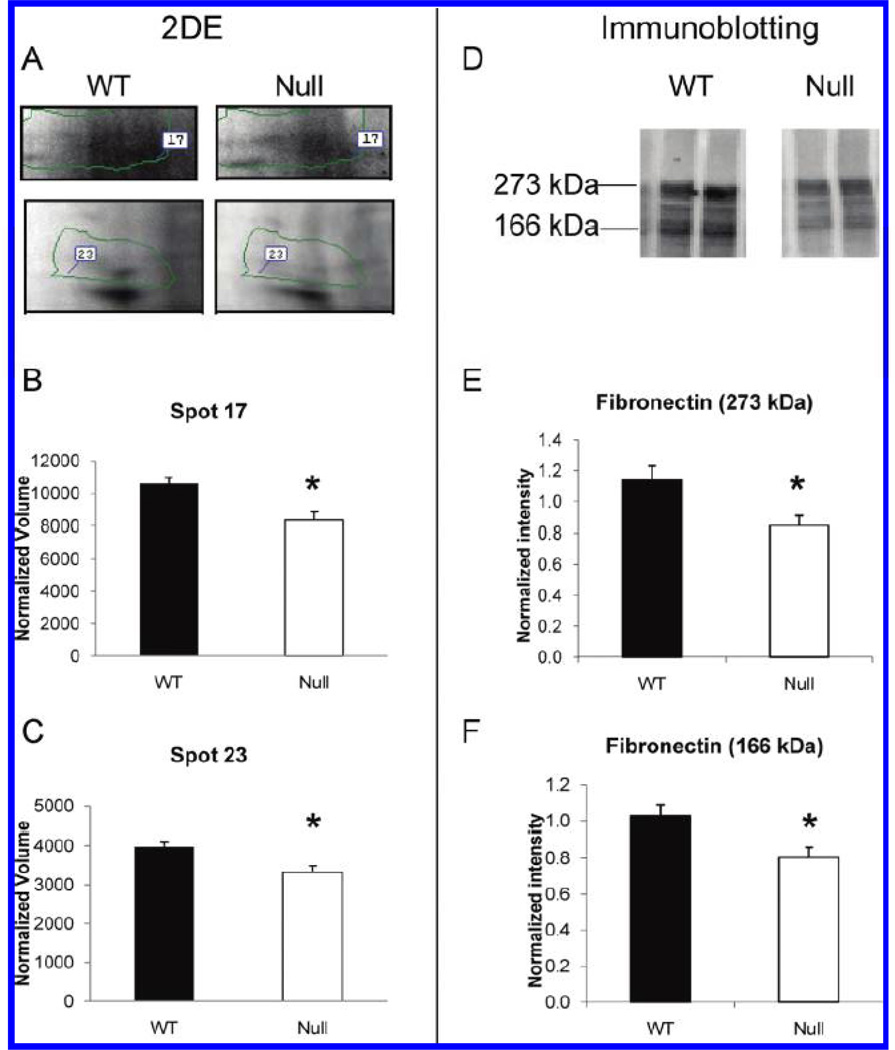
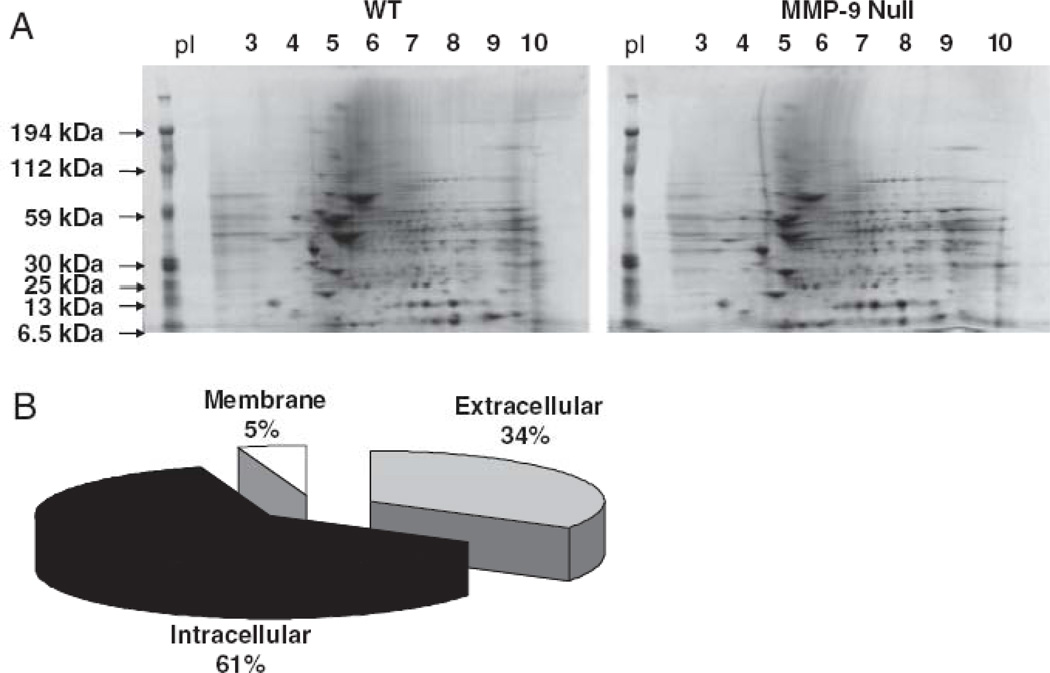
Our team used a 2-DE gel approach to identify MMP-7 and MMP-9 related changes in the left ventricle post-MI.49, 50 Using infarct tissue only, which provided a natural enrichment for ECM, we evaluated wild-type and MMP-7 or MMP-9 null left ventricular infarcts at day 7 post-MI. In both MMP-7 and MMP-9 null mice, we identified left ventricular ECM proteins with lower expression compared to wild-type mice. Included in the list was fibronectin, a known in vitro substrate for MMP-7 and MMP-9 (Figure 1). In the wild type and MMP-7 null post-MI extract comparison, we further showed that adding exogenous recombinant MMP-7 resulted in the generation of the fibronectin fragments seen in the wild type LV samples. We also demonstrated a rescue in phenotype for tenascin C, indicating that both fibronectin and tenascin C are in vivo substrates of MMP-7. Importantly, these were the first reports of multiple ECM proteins being identified in cardiac extracts using a 2-DE gel approach, indicating success in enriching for ECM by limiting analysis to the infarct region. In the wild type and MMP-9 null samples, 34% of the proteins present in differentially expressed spots were identified as extracellular proteins (Figure 2). A further 5% were identified as membrane proteins. However, multiple cytoplasmic and mitochondrial proteins were also present, indicating that this enrichment strategy was not fully optimized.
Figure 1.
MMP-7 null infarcted LV showed lower levels of fibronectin by 2-DE and immunoblotting. (A) Fibronectin was identified in spot 17 and spot 23 of the 2-DE gels. Both spot 17 (B) and spot 23 (C) showed significant lower intensity in infarct LV of MMP-7 null mice compared to WT. (D) Immunoblotting of fibronectin in WT and MMP-7 null LV extracts were performed. The densitometry of the 273 kDa full-length (E) and the 166 kDa fragments (F) of fibronectin indicated both bands showed significantly lower intensity in MMP-7 null LV infarct when compared with the wt (p<0.05). Reproduced from reference 49 with permission from the American Chemical Society.
Figure 2.
Representative 2-DE gels of the wt and MMP-9 null post-MI groups (A), and of the candidate MMP-9 substrates identified (B), showing that 61% are classified as intracellular, 5% as membrane, and 34% as extracellular proteins. Reproduced from reference 50 with permission from Wiley-VCH Verlag GmbH & Co.
Proteome simplification by suborganelle fractionation
Fractionation techniques include separation by organelle, which is based, to a large extent, on solubility or physiochemical properties such as size and pI. In the heart, mitochondria, caveolae, proteasome, and myofilament organelles have been examined using sub-fractionation coupled with proteomic approaches.25, 51–55 Taylor and colleagues have comprehensively characterized the human heart mitochondrial proteome,56 and several groups continue with this endeavor.57 An excellent review on the cardiac mitochondrial proteome is provided by Gucek and colleagues.25
With the sub-fractionation approach, the accuracy of the cardiac organelle proteome catalog generated is dependent on the purity of the isolation. Protein correlation profiling can be used to assign a protein as either a true component of an organelle or a contaminating component, thereby monitoring isolation purity and reducing false positive identifications.51 However, the profiling strategy is complicated by the fact that proteins often shuffle among compartments and, therefore, may not be assignable to only one organelle. Additionally, while profiling provides information on whether the protein is present in a particular compartment, it does not elucidate function.
We have recently developed a sub-fractionation approach whereby the left ventricle is decellularized to remove cells while leaving the ECM intact (Figure 3).58, 59 Our protocol was modified from work done by the Christman lab, which uses a decellularization protocol to prepare tissue specific ECM coatings for cell culture and other bioengineering applications.58 They found that human embryonic derived stem cells grown in cardiac derived ECM matured more fully into a cardiomyocyte (e.g., formed desmosomes) compared to cells grown on the standard gelatin substrate. This technique uses sodium dodecyl sulfate (SDS) to break up cell and organelle membranes. A premise to this approach is that the cytosolic proteins and intracellular organelles will be washed away in the SDS buffer. In order for this technique to be successful, the intracellular protein content must be washed away. Additionally, residual SDS needs to be washed away to avoid interference with downstream mass spectrometry analyses. Similar techniques have been developed by the Mayr laboratory to examine vascular ECM.60 An advantage of this strategy is that it can be coupled with protein separation on a 1-DE gel to provide MW information which can reflect ECM proteolysis that may occur during disease progression. This approach is currently being used to examine ECM and matrix metalloproteinase-dependent ECM peptide formation during left ventricular remodeling, with a representative experimental design shown in Table 2.
Figure 3.
Decellularization strategy to enrich for relatively low abundance ECM proteins. The image shows a mouse untreated left ventricle (left) and a decellularized left ventricle (right).
Table 2.
Strategies To Evaluate MMP-Mediated ECM Peptide Generation and Determine Effects of the Peptides on Post-MI Remodeling
|
Incorporating functional proteomics into systems biology approaches to assign cause and effect relationships between proteases and specific ECM targets
Proteomics can be used to better understand protein, cell, and tissue function, for the purpose of identifying mechanistic indicators, prognostic predictors, therapeutic assessors of cardiovascular disease and biosignatures that inform us on individual responses61, 62. Functional proteomic strategies can also be applied to isolated cell proteomes to give us information on cell function during disease development and progression. For example, isolating cardiac fibroblasts during different stages of post-MI remodeling can provide functional clues about how the fibroblast regulates cardiac remodeling. However, an approach that involves examination of cell-specific proteomes has the potential to overlook secreted proteins. Evaluating both the isolated cell proteome and the corresponding cell secretome is necessary to fully understand the contribution of that particular cell.63 In left ventricular samples, relevant cells include the cardiac myocyte, fibroblasts, endothelial cells, smooth muscle cells, and (during injury responses) infiltrating inflammatory cells. Of these cell types, the fibroblast is the major source of cardiac ECM and the most prominent target of ECM proteomic approaches.
Future Directions and Conclusions
Several areas actively under development that have relevance to cardiac ECM proteomics include:
1. Target ECM using unbiased, informed approaches
Protein microarrays, including currently available antibody arrays, typically ignore ECM proteins in their arrays. Protein array data can be coupled with mRNA expression array analysis of the same samples to increase the confidence that any observed changes are accurate and true. While this approach minimizes false positive results, it does not provide information about protein post-translational modifications, which are an abundant component of ECM complexity. This approach also assumes equal protein breakdown and synthesis rates, which may be true at equilibrium but not in the post-MI setting.
Along these same lines, profiling ECM pathways that are perturbed early in the initiation of congestive heart failure, before symptoms become irreversible, is needed. Utilizing multiple techniques that overlap in diagnostic or prognostic capabilities and combining datasets by using pathway analysis software can increase the effect size by several orders of magnitude and allow more accurate detection of protein changes. These proteins can then be confirmed to occur in human patients using case-controlled studies.
2. Include post-translational modifications specific to ECM
In the mitochondrial proteome, post-translational modifications notably include phosphorylation, nitrosylation, and O-GlcNAcylation, which are transient and, therefore, difficult to detect.25, 64 In the ECM, in contrast, post-translational modifications tend to be more permanent, with the most common modifications being hydroxylation, glycosylation, and cross-linking.65, 66 Additionally, global and site-specific glycosylation can be visualized using stains such as the Pro-Q Emerald stain.67 Therefore, adding the posttranslational dimension to the ECM proteome catalog is quite possible.
3. Incorporate ECM into systems biology approaches
One limitation of existing pathway analysis software programs is that the ECM is largely ignored or is listed as an intracellular component. While cluster analysis is a useful method to analyze proteomic datasets, clusters are often grouped by cytosolic, microsomal, mitochondrial, and nuclear fractions, totally excluding the extracellular compartment. The same is true for computational tools, including protein correlation profiling software.26 Another limitation is that currently available programs do not take into consideration the fact that left ventricular tissue is heterogeneous in terms of cell composition. Normal functioning myocytes, hypoxic myocytes, necrotic myocytes, and apoptotic myocytes all co-exist in the post-MI LV but vary in number temporally and spatially. Likewise, included in the general designation of fibroblasts are resident fibroblast cells, infiltrating fibrocytes, myofibroblasts, and protomyofibroblasts, all of which alter ECM composition in different ways.68–70 Therefore, consideration of the cell complexity needs to be incorporated into systems biology software tools.
4. Extend tissue proteomics to the plasma
Plasma can be a valuable source of accessible protein markers, providing distinct barcodes to reflect cardiac disease status.61, 71, 72 As recently as 35 years ago, only 40 proteins could be routinely measured in plasma.73 Currently, thousands of proteins can be analyzed, after depletion of albumin and other highly abundant proteins.63, 74, 75 Analyses based on the Luminex technology (also available commercially, for example, from Rules-Based-Medicine, Austin, TX), permit multi-analyte profiling of up to 100 cytokines, growth factors, and other analytes in a 100-µl plasma sample. While plasma analysis serves as a promising avenue for clinical applications, there is a need for uniform blood collection and sample processing protocols to minimize handling artifacts. Consistent sampling procedures are necessary to reproducibly evaluate plasma for clinical diagnostic potential.26
As in other areas of scientific investigation, the validity of the results obtained with proteomic analysis is dependent upon proper study design, controls, statistical analysis, and validation. In terms of predicting which post-MI patients are likely to progress to congestive heart failure, techniques to monitor a panel of complementary markers, rather than a single marker, will likely need to be developed in order to examine ECM patterns and obtain a more complete prognostic picture. Additionally, the most informative markers will likely be found in multiple screens and demonstrate a functional role in the initiation or progression of CHF. In conclusion, the progress in ECM proteomics seen in the last 10 years will undoubtedly continue into the future as we develop this list of markers.
Acknowledgments
Funding Sources: The authors acknowledge grant and contract support from the National Institutes of Health (HL75360 and HHSN268201000036C), the Max and Minnie Tomerlin Voelcker Foundation, and the Veterans Administration. We also acknowledge support from UTHSCSA for the Institutional Mass Spectrometry Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Takemura G, Nakagawa M, Kanamori H, Minatoguchi S, Fujiwara H. Benefits of reperfusion beyond infarct size limitation. Cardio Res. 2009;83:269–276. doi: 10.1093/cvr/cvp032. [DOI] [PubMed] [Google Scholar]
- 2.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa K, Kimura A, Taniwa T, Takenaka T, Hayashi T, Kanamasa K. Modification of treatment strategies over a period of 14 years has markedly reduced cardiac events among post-myocardial infarction patients. Circ J. 2002;66:881–885. doi: 10.1253/circj.66.881. [DOI] [PubMed] [Google Scholar]
- 4.Lewis EF, Velazquez EJ, Solomon SD, Hellkamp AS, McMurray JJ, Mathias J, et al. Predictors of the first heart failure hospitalization in patients who are stable survivors of myocardial infarction complicated by pulmonary congestion and/or left ventricular dysfunction: A valiant study. Eur Heart J. 2008;29:748–756. doi: 10.1093/eurheartj/ehn062. [DOI] [PubMed] [Google Scholar]
- 5.Hellermann JP, Goraya TY, Jacobsen SJ, Weston SA, Reeder GS, Gersh BJ, et al. Incidence of heart failure after myocardial infarction: Is it changing over time? Am J Epidemiol. 2003;157:1101–1107. doi: 10.1093/aje/kwg078. [DOI] [PubMed] [Google Scholar]
- 6.Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, et al. Predictors of late development of heart failure in stable survivors of myocardial infarction: The care study. J American College of Card. 2003;42:1446–1453. doi: 10.1016/s0735-1097(03)01057-x. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA. Ace inhibitors in acute myocardial infarction. Circulation. 1998;97:2192–2194. doi: 10.1161/01.cir.97.22.2192. [DOI] [PubMed] [Google Scholar]
- 8.Hu K, Gaudron P, Ertl G. Long-term effects of beta-adrenergic blocking agent treatment on hemodynamic function and left ventricular remodeling in rats with experimental myocardial infarction: Importance of timing of treatment and infarct size. J of American College of Cardiology. 1998;31:692–700. doi: 10.1016/s0735-1097(97)00527-5. [DOI] [PubMed] [Google Scholar]
- 9.Anavekar NS, McMurray JJV, Velazquez EJ, Solomon SD, Kober L, Rouleau J-L, White HD, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. New Eng J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 10.Tsuruda T, Costello-Boerrigter LC, Burnett JC., Jr Matrix metalloproteinases: Pathways of induction by bioactive molecules. Heart Fail Rev. 2004;9:53–61. doi: 10.1023/B:HREV.0000011394.34355.bb. [DOI] [PubMed] [Google Scholar]
- 11.Iraqi W, Rossignol P, Angioi M, Fay R, Nuee J, Ketelslegers JM, et al. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: Insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (ephesus) study. 2009;119:2471–2479. doi: 10.1161/CIRCULATIONAHA.108.809194. [DOI] [PubMed] [Google Scholar]
- 12.Zamilpa R, Lindsey ML. Extracellular matrix turnover and signaling during cardiac remodeling following mi: Causes and consequences. Journal of molecular and cellular cardiology. 2010;48:558–563. doi: 10.1016/j.yjmcc.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh M, Foster CR, Dalal S, Singh K. Osteopontin: Role in extracellular matrix deposition and myocardial remodeling post-mi. Journal of molecular and cellular cardiology. 2010;48:538–543. doi: 10.1016/j.yjmcc.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fedak PWM, Verma S, Weisel RD, Li R-K. Cardiac remodeling and failure: From molecules to man (part ii) Cardiovascular Pathology. 2005;14:49–60. doi: 10.1016/j.carpath.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Drake TA, Ping P. Thematic review series: Systems biology approaches to metabolic and cardiovascular disorders. Proteomics approaches to the systems biology of cardiovascular diseases. Journal of lipid research. 2007;48:1–8. doi: 10.1194/jlr.R600027-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Baker CS, Corbett JM, May AJ, Yacoub MH, Dunn MJ. A human myocardial two-dimensional electrophoresis database: Protein characterisation by microsequencing and immunoblotting. Electrophoresis. 1992;13:723–726. doi: 10.1002/elps.11501301154. [DOI] [PubMed] [Google Scholar]
- 17.Corbett JM, Wheeler CH, Baker CS, Yacoub MH, Dunn MJ. The human myocardial two-dimensional gel protein database: Update 1994. Electrophoresis. 1994;15:1459–1465. doi: 10.1002/elps.11501501209. [DOI] [PubMed] [Google Scholar]
- 18.Jungblut P, Otto A, Zeindl-Eberhart E, Plessner KP, Knecht M, et al. Protein composition of the human heart: The construction of a myocardial two-dimensional electrophoresis database. Electrophoresis. 1994;15:685–707. doi: 10.1002/elps.1150150197. [DOI] [PubMed] [Google Scholar]
- 19.Mayr M, Zhang J, Greene AS, Gutterman D, Perloff J, Ping P. Proteomics-based development of biomarkers in cardiovascular disease: Mechanistic, clinical, and therapeutic insights. Mol Cell Proteomics. 2006;5:1853–1864. doi: 10.1074/mcp.R600007-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. American journal of physiology. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 21.Vo TD, Greenberg HJ, Palsson BO. Reconstruction and functional characterization of the human mitochondrial metabolic network based on proteomic and biochemical data. J. Biol. Chem. 2004;279:39532–39540. doi: 10.1074/jbc.M403782200. [DOI] [PubMed] [Google Scholar]
- 22.Kline KG, Sussman MR. Protein quantitation using isotope-assisted mass spectrometry. Annu Rev Biophys. 2010;39:291–308. doi: 10.1146/annurev.biophys.093008.131339. [DOI] [PubMed] [Google Scholar]
- 23.Iliuk A, Galan J, Tao WA. Playing tag with quantitative proteomics. Anal Bioanal Chem. 2009;393:503–513. doi: 10.1007/s00216-008-2386-0. [DOI] [PubMed] [Google Scholar]
- 24.Fenselau C, Yao X. 18o2-labeling in quantitative proteomic strategies: A status report. Journal of proteome research. 2009;8:2140–2143. doi: 10.1021/pr8009879. [DOI] [PubMed] [Google Scholar]
- 25.Gucek M, Murphy E. What can we learn about cardioprotection from the cardiac mitochondrial proteome? Cardiovascular research. 2010;88:211–218. doi: 10.1093/cvr/cvq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arab S, Gramolini AO, Ping P, Kislinger T, Stanley B, van Eyk J, et al. Cardiovascular proteomics: Tools to develop novel biomarkers and potential applications. Journal of the American College of Cardiology. 2006;48:1733–1741. doi: 10.1016/j.jacc.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 27.Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJ. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nature protocols. 2009;4:484–494. doi: 10.1038/nprot.2009.21. [DOI] [PubMed] [Google Scholar]
- 28.Hsu JL, Huang SY, Chow NH, Chen SH. Stable-isotope dimethyl labeling for quantitative proteomics. Analytical chemistry. 2003;75:6843–6852. doi: 10.1021/ac0348625. [DOI] [PubMed] [Google Scholar]
- 29.Yao X, Freas A, Ramirez J, Demirev PA, Fenselau C. Proteolytic 18o labeling for comparative proteomics: Model studies with two serotypes of adenovirus. Analytical chemistry. 2001;73:2836–2842. doi: 10.1021/ac001404c. [DOI] [PubMed] [Google Scholar]
- 30.Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 31.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, et al. Multiplexed protein quantitation in saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Yates JR, Ruse CI, Nakorchevsky A. Proteomics by mass spectrometry: Approaches, advances, and applications. Annual review of biomedical engineering. 2009;11:49–79. doi: 10.1146/annurev-bioeng-061008-124934. [DOI] [PubMed] [Google Scholar]
- 33.Gilar M, Olivova P, Daly AE, Gebler JC. Two-dimensional separation of peptides using rp-rp-hplc system with different ph in first and second separation dimensions. J Sep Sci. 2005;28:1694–1703. doi: 10.1002/jssc.200500116. [DOI] [PubMed] [Google Scholar]
- 34.Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 35.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, et al. Stable isotope labeling by amino acids in cell culture, silac, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 36.Amanchy R, Kalume DE, Pandey A. Stable isotope labeling with amino acids in cell culture (silac) for studying dynamics of protein abundance and posttranslational modifications. Sci STKE. 2005;2005:l2. doi: 10.1126/stke.2672005pl2. [DOI] [PubMed] [Google Scholar]
- 37.Harsha HC, Molina H, Pandey A. Quantitative proteomics using stable isotope labeling with amino acids in cell culture. Nature protocols. 2008;3:505–516. doi: 10.1038/nprot.2008.2. [DOI] [PubMed] [Google Scholar]
- 38.Pinto AF, Ma L, Dragulev B, Guimaraes JA, Fox JW. Use of silac for exploring sheddase and matrix degradation of fibroblasts in culture by the piii svmp atrolysin a: Identification of two novel substrates with functional relevance. Archives of biochemistry and biophysics. 2007;465:11–15. doi: 10.1016/j.abb.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 39.Gioia M, Foster LJ, Overall CM. Cell-based identification of natural substrates and cleavage sites for extracellular proteases by silac proteomics. Methods in molecular biology (Clifton, N.J. 2009;539:131–153. doi: 10.1007/978-1-60327-003-8_8. [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, Smith JW, Huang CM. Mass spectrometry-based label-free quantitative proteomics. J Biomed Biotechnol. 2010;2010:840518. doi: 10.1155/2010/840518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Analytical chemistry. 2005;77:6218–6224. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- 42.Lundgren DH, Hwang SI, Wu L, Han DK. Role of spectral counting in quantitative proteomics. Expert review of proteomics. 2010;7:39–53. doi: 10.1586/epr.09.69. [DOI] [PubMed] [Google Scholar]
- 43.Becker CH, Bern M. Recent developments in quantitative proteomics. Mutat Res. 2010 doi: 10.1016/j.mrgentox.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christin C, Bischoff R, Horvatovich P. Data processing pipelines for comprehensive profiling of proteomics samples by label-free lc-ms for biomarker discovery. Talanta. 2011;83:1209–1224. doi: 10.1016/j.talanta.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 45.Xu D, Suenaga N, Edelmann MJ, Fridman R, Muschel RJ, et al. Novel mmp-9 substrates in cancer cells revealed by a label-free quantitative proteomics approach. Mol Cell Proteomics. 2008;7:2215–2228. doi: 10.1074/mcp.M800095-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, et al. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovascular research. 2010;85:376–384. doi: 10.1093/cvr/cvp344. [DOI] [PubMed] [Google Scholar]
- 47.Cutillas PR, Vanhaesebroeck B. Quantitative profile of five murine core proteomes using label-free functional proteomics. Mol Cell Proteomics. 2007;6:1560–1573. doi: 10.1074/mcp.M700037-MCP200. [DOI] [PubMed] [Google Scholar]
- 48.Mayr M, Grainger D, Mayr U, Leroyer AS, Leseche G, Sidibe A, et al. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ Cardiovasc Genet. 2009;2:379–388. doi: 10.1161/CIRCGENETICS.108.842849. [DOI] [PubMed] [Google Scholar]
- 49.Chiao YA, Zamilpa R, Lopez EF, Dai Q, Escobar GP, Hakala KW, et al. In vivo matrix metalloproteinase-7 substrates identified in the left ventricle post-myocardial infarction using proteomics. Journal of proteome research. 2010;9:2649–2657. doi: 10.1021/pr100147r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zamilpa R, Lopez EF, Chiao YA, Dai Q, Escobar GP, Hakala K, et al. Proteomic analysis identifies in vivo candidate matrix metalloproteinase-9 substrates in the left ventricle post-myocardial infarction. Proteomics. 2010;10:2214–2223. doi: 10.1002/pmic.200900587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dengjel J, Jakobsen L, Andersen JS. Organelle proteomics by label-free and silac-based protein correlation profiling. Methods in molecular biology (Clifton, N.J. 2010;658:255–265. doi: 10.1007/978-1-60761-780-8_15. [DOI] [PubMed] [Google Scholar]
- 52.Huber LA, Pfaller K, Vietor I. Organelle proteomics: Implications for subcellular fractionation in proteomics. Circ Res. 2003;92:962–968. doi: 10.1161/01.RES.0000071748.48338.25. [DOI] [PubMed] [Google Scholar]
- 53.Taylor RS, Wu CC, Hays LG, Eng JK, Yates JR, 3rd, Howell KE. Proteomics of rat liver golgi complex: Minor proteins are identified through sequential fractionation. Electrophoresis. 2000;21:3441–3459. doi: 10.1002/1522-2683(20001001)21:16<3441::AID-ELPS3441>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 54.Taylor SW, Fahy E, Ghosh SS. Global organellar proteomics. Trends Biotechnol. 2003;21:82–88. doi: 10.1016/S0167-7799(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 55.Wu CC, Yates JR. The application of mass spectrometry to membrane proteomics. Nat Biotechnol. 2003;21:262–267. doi: 10.1038/nbt0303-262. [DOI] [PubMed] [Google Scholar]
- 56.Taylor CF, Paton NW, Garwood KL, Kirby PD, Stead DA, Yin Z, et al. A systematic approach to modeling, capturing, and disseminating proteomics experimental data. Nat Biotechnol. 2003;21:247–254. doi: 10.1038/nbt0303-247. [DOI] [PubMed] [Google Scholar]
- 57.Arrell DK, Elliott ST, Kane LA, Guo Y, Ko YH, Pedersen PL, et al. Proteomic analysis of pharmacological preconditioning: Novel protein targets converge to mitochondrial metabolism pathways. Circ Res. 2006;99:706–714. doi: 10.1161/01.RES.0000243995.74395.f8. [DOI] [PubMed] [Google Scholar]
- 58.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PloS one. 2010;5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Didangelos A, Yin X, Mandal K, Baumert M, Jahangiri M, Mayr M. Proteomics characterization of extracellular space components in the human aorta. Mol Cell Proteomics. 2010;9:2048–2062. doi: 10.1074/mcp.M110.001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berhane BT, Zong C, Liem DA, Huang A, Le S, Edmondson RD, et al. Cardiovascular-related proteins identified in human plasma by the hupo plasma proteome project pilot phase. Proteomics. 2005;5:3520–3530. doi: 10.1002/pmic.200401308. [DOI] [PubMed] [Google Scholar]
- 62.MacLellan WR, Ping P, Vondriska T. Heart disease leaves its mark: Proteomics-based biosignatures in acute coronary syndromes. Journal of the American College of Cardiology. 2004;44:1584–1586. doi: 10.1016/j.jacc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Mayr M, Van Eyk JE. Cardiovascular proteomics. Proteomics Clin Appl. 2008;2:785–786. doi: 10.1002/prca.200890020. [DOI] [PubMed] [Google Scholar]
- 64.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-glcnac signaling in the cardiovascular system. Circ Res. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krane SM. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino acids. 2008;35:703–710. doi: 10.1007/s00726-008-0073-2. [DOI] [PubMed] [Google Scholar]
- 66.Robins SP. Biochemistry and functional significance of collagen cross-linking. Biochem Soc Trans. 2007;035:849–852. doi: 10.1042/BST0350849. [DOI] [PubMed] [Google Scholar]
- 67.Roeser J, Bischoff R, Bruins AP, Permentier HP. Oxidative protein labeling in mass-spectrometry-based proteomics. Anal Bioanal Chem. 2010;397:3441–3455. doi: 10.1007/s00216-010-3471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Espira L, Michael PC. Emerging concepts in cardiac matrix biology. Canadian journal of physiology and pharmacology. 2009;87:996–1008. doi: 10.1139/Y09-105. [DOI] [PubMed] [Google Scholar]
- 69.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-ii-induced cardiac hypertrophy. Journal of molecular and cellular cardiology. 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson L. Candidate-based proteomics in the search for biomarkers of cardiovascular disease. J Physiol (Lond) 2005;563:23–60. doi: 10.1113/jphysiol.2004.080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mateos-Caceres PJ, Garcia-Mendez A, Lopez Farre A, Macaya C, Nunez A, Gomez J, et al. Proteomic analysis of plasma from patients during an acute coronary syndrome. Journal of the American College of Cardiology. 2004;44:1578–1583. doi: 10.1016/j.jacc.2004.06.073. [DOI] [PubMed] [Google Scholar]
- 73.Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, Conrads TP, et al. The human plasma proteome: A nonredundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004;3:311–326. doi: 10.1074/mcp.M300127-MCP200. [DOI] [PubMed] [Google Scholar]
- 74.Rothemund DL, Locke VL, Liew A, Thomas TM, Wasinger V, Rylatt DB. Depletion of the highly abundant protein albumin from human plasma using the gradiflow. Proteomics. 2003;3:279–287. doi: 10.1002/pmic.200390041. [DOI] [PubMed] [Google Scholar]
- 75.Tam SW, Pirro J, Hinerfeld D. Depletion and fractionation technologies in plasma proteomic analysis. Expert review of proteomics. 2004;1:411–420. doi: 10.1586/14789450.1.4.411. [DOI] [PubMed] [Google Scholar]