Abstract
Background
Chronic disease is a risk factor for frailty. Previous studies typically consider individual diagnosed diseases, but disease builds over time, possibly in several organs simultaneously.
Objective
We hypothesize that disease burden is associated with frailty independent of diagnosed chronic disease and that physiologic measurements provide greater understanding of the etiology of frailty.
Design
Cross-sectional study.
Setting
Community.
Participants
Cardiovascular Health Study, 1992–93 examination (N=2437, mean (SD) age 74.8 (4.8) years, 43.4% male, 95.8% white).
Measurements
Disease burden and frailty were tabulated using 10-point scales (0=healthy, 10=unhealthy). Disease burden was the sum of measurements characterizing the vasculature, brain, kidneys, lungs, and glucose metabolism. Frailty was assessed with the frailty index reported by Fried. Multivariate linear models were used to determine the association of disease burden (predictor) to frailty (outcome).
Results
Unadjusted, 1 point higher disease burden was associated with a 0.28 point higher frailty score (p<0.0001). White matter grade, forced vital capacity, and cystatin-C were particularly strongly and significantly associated with frailty. Disease burden attenuated the association of frailty with age by 29%, and disease burden and age had similar associations with frailty. Disease burden attenuated the association of frailty with fibrinogen, Factor VIII, and CRP by 32%, 56%, and 83%. Frailty was associated with diagnosed depression, stroke, cognitive impairment, arthritis, and pulmonary disease but not coronary heart disease, diabetes, or kidney disease in the presence of a summary of disease burden. In the adjusted model disease burden remained significantly associated with frailty (β=0.11, p<0.0001).
Conclusion
Disease burden was independently and significantly associated with frailty. These results emphasize that typically unrecognized physiologic changes may importantly contribute to frailty.
Keywords: Frailty, etiology, disease burden
Introduction
Frailty commonly occurs in older adults, is associated with poor wellbeing, and increases mortality. (1–5) Previous studies show that chronic disease is a major risk factor for frailty. Using this etiologic model, preventing frailty may focus on preventing or ameliorating diagnosed chronic disease. But, in cohort studies of older individuals who do not report diagnosed disease, the prevalence and severity range of disease can in fact be substantial when assessed using several noninvasive methods. (6–9) Undiagnosed disease can also powerfully predict incident adverse events independent of diagnosed disease. (6–12) Determining the risk of frailty from chronic disease measured without regard to clinical diagnosis could reveal an earlier point of intervention to prevent frailty.
Recent evidence also supports the hypothesis that frailty is a complex syndrome which appears in the presence of a sufficient number of risk factors – in essence, that the association of the sum is more than the association of the individual parts. This is illustrated by recent findings in which dysregulation in markers of anemia, endocrine function, metabolism, inflammation, micronutrient status, adiposity, and fine motor status increased the risk of frailty when considered together, but their individual dysregulation was not or was modestly associated with frailty. (13) Subsequently, studying the etiology of frailty using tools which aggregate several measurements simultaneously, rather than focusing on individual chronic diseases, might suggest that interventions which effect several organs would be most advantageous for preventing frailty.
Recently, Newman et al. introduced the physiologic index of comorbidity (physiologic index), a 10-point scale that tabulates the severity of chronic disease using tests across the vasculature, lungs, kidneys, brain, and glucose metabolism. (14) It is not specific to “subclinical disease,” which can be “asymptomatic, presymptomatic, atypically symptomatic, or simply undiagnosed.” (14) Rather, the physiologic index is a more encompassing measure of “disease burden,” which we define as the sum of markers of structure or function representing different organ systems. This physiologic index powerfully predicts mortality and disability independent of age and diagnosed disease, and does so better than a count of diagnosed diseases. Thus, the physiologic index could be a useful means to study the association of chronic disease to frailty in an attempt to find an earlier point of intervention.
Using data from the Cardiovascular Health Study (CHS), we tested three hypotheses in this analysis: 1) Frailty is associated with disease burden independent of diagnosed chronic conditions; 2) Frailty is less strongly associated with disease markers in individual systems (carotid thickness, pulmonary vital capacity, serum cystatin-C, white matter grade, and serum fasting glucose) than with disease burden; 3) Disease burden alone attenuates the association of frailty to markers of inflammation and coagulation, previously identified risk factors for frailty. (15) We chose these hypotheses to determine the independent contribution of disease burden to frailty, which, if significant, could identify an earlier point for clinicians to intervene to prevent frailty. Examining the strength of the association of frailty with disease in individual systems might suggest which systems contribute most to frailty, and thus which may be most worthwhile to target for interventions. Finally, the hierarchical approach considering molecular, tissue, and organ level risk factors helps refine the etiologic model of frailty.
Methods
Design Overview, Setting, and Participants
The CHS is an ongoing community-based study of cardiovascular risk in 5888 men and women over the age of 65 years, from four regions of the United States. (16) The cohort was enrolled in 1989–1990 and was supplemented with added minority recruitment in 1992–1993. Participants and eligible household members were identified from a random sample of Medicare enrollees at each field center. To be eligible, participants were ≥65 years old, did not have cancer under active treatment, could not be wheelchair- or bed-bound in the home, and did not plan to move out of the area within 3 years. We used data from the 1992–1993 examination to include all of the minority participants and to include the brain magnetic resonance imaging (MRI) scan conducted at that time. Of 3660 individuals with a brain MRI scan, 2928 had a clinical examination with complete data for the other major components used to calculate the physiologic index score. Of those 2928, 483 did not have sufficient data to calculate frailty, and we excluded 8 participants undergoing medical treatment for Parkinson’s disease or Alzheimer’s disease, leaving 2437 participants for this analysis (mean (SD) age 74.8 (4.8) years, range 67–96; 43.4% male; 95.8% white). The subset analyzed here had a better health profile regarding some covariates (younger age, lower BMI, etc.) than the sample without available measurements, though most differences were not statistically significant, and significant differences were minor in absolute terms. The CHS is approved by the Institutional Review Boards of all participating institutions.
Physiologic Index of Comorbidity
The instruments and methods used to construct the physiologic index have been described previously. (14) Briefly, the clinical examination conducted in 1992–1993 included cardiovascular and pulmonary function tests, blood tests for glucose tolerance and kidney function, and a brain MRI. The choice of tests to include in the physiologic index was based on previous reports that each is individually an important predictor of mortality, and that each represents a major, common age-related chronic disease. (16,17) Carotid ultrasound was obtained in the left and right internal and common carotid arteries to assess near and far wall thicknesses and Doppler flow. The mean of the maximum wall thickness of the internal carotid artery was used to represent the extent of vascular disease. (18) Spirometry was conducted according to the standards of the American Thoracic Society. (16) Fasting glucose was assessed as described previously. (19) Cystatin-C, a serum marker of glomerular filtration rate, was assessed using a BNII nephelometer that used a particle-enhanced immunonephelometric assay. (20) Brain MRI was obtained according to a standard scanning protocol and data was interpreted at a central MRI Reading Center by a neurologist trained in a standardized protocol. (21) The white matter grade score was used to indicate small-vessel vascular disease in the brain. (17)
To construct the physiologic index, each of the five measures was divided into three groups based on tertiles with the best values classified as 0 and the worst as 2. (14) Although the choice of cut points was arbitrary, the best score of “0” was generally found to represent a healthy, young normal value, and values of “2” were in the range of individuals with diagnosed chronic disease. Individual scores were summed for a total score ranging from 0 to 10. For the carotid wall thickness, tertile cut points were scored as 0: 0.60 to 1.06 mm, 1: >1.06 to 1.53 mm, 2: >1.53 to 3.94 mm. Tertile cut points for forced vital capacity (FVC) were sex-specific (Women: 0: >2.6 to 3.8 L, 1: >2.2 to 2.6 L, 2: 0.6 to 2.2 L; Men: 0: >3.9 to 6.5 L, 1: >3.2 to 3.9 L, 2: 0.3 to 3.2 L). Tertile cut points for cystatin-C were scored as 0: 0.6 to 1.0 mg/L, 1: >1.0 to 1.1 mg/L, 2: >1.1 to 3.5 mg/L. For white matter grade, tertile cut points were scored as 0: 0 to 1 units, 1: 2 units, 2: 3 to 9 units on the 0–9 ordinal scale. Fasting glucose was the only measure not classified by tertile. Although results were similar, for clinical interpretation, this presentation uses clinical cut points defined by the American Diabetes Association (0: <100 mg/dL, 1: 100 to 126 mg/dL, 2: >126 mg/dL) (Table 1). (22)
Table 1.
Characteristics of study participants by physiologic index score: the Cardiovascular Health Study, 1992–1993 examination
| Physiologic Index Score | |||||
|---|---|---|---|---|---|
| Characteristics | 0–2 (N=457) | 3–4 (N=794) | 5–6 (N=719) | 7–10 (N=467) | P* |
| Demographics | |||||
| Age, y, mean (SD) | 72.5 (3.7) | 73.9 (4.0) | 75.6 (4.9) | 77.2 (5.3) | <0.0001 |
| Male gender, n (%) | 145 (31.7) | 313 (39.4) | 350 (48.7) | 250 (53.3) | <0.0001 |
| Non-white race, n (%) | 13 (2.8) | 25 (3.2) | 41 (5.7) | 24 (5.1) | 0.03 |
| Behavioral risk factors | |||||
| Education, y, mean (SD) | 15.2 (4.5) | 14.8 (4.5) | 14.2 (4.6) | 13.8 (4.6) | <0.0001 |
| Body mass index, kg/m2, mean (SD) | 24.9 (3.7) | 26.0 (4.0) | 26.3 (4.2) | 27.4 (4.3) | <0.0001 |
| Smoking status | |||||
| Current, n (%) | 24 (5.3) | 56 (7.1) | 77 (10.7) | 53 (11.4) | <0.0001 |
| Past, n (%) | 182 (39.8) | 331 (41.7) | 339 (47.2) | 245 (52.5) | |
| Never, n (%) | 251 (54.9) | 407 (51.3) | 303 (42.1) | 169 (36.2) | |
| Diagnosed chronic conditions | |||||
| Coronary heart disease, n (%) | 54 (11.8) | 129 (16.3) | 187 (26.0) | 168 (36.0) | <0.0001 |
| Cerebrovascular disease, n (%) | 17 (3.7) | 26 (3.3) | 47 (6.5) | 57 (12.2) | <0.0001 |
| Diabetes, n (%) | 4 (0.9) | 48 (6.1) | 97 (13.5) | 151 (32.3) | <0.0001 |
| Chronic obstructive lung disease, n (%) | 104 (22.8) | 235 (29.6) | 188 (26.2) | 135 (28.9) | 0.05 |
| Kidney disease, n (%) | 1 (0.2) | 4 (0.5) | 6 (0.8) | 11 (2.4) | 0.005 |
| Arthritis, n (%) | 196 (42.9) | 347 (43.7) | 323 (44.9) | 227 (48.6) | 0.28 |
| Depression (CES-D >10), n (%) | 49 (10.7) | 90 (11.3) | 87 (12.1) | 92 (19.7) | <0.0001 |
| Cognitive impairment (MMSE < 80), n (%) | 8 (1.8) | 24 (3.0) | 44 (6.1) | 43 (9.2) | <0.0001 |
| No. of conditions, 0–8, mean (SD) | 0.9 (0.9) | 1.1 (1.0) | 1.4 (1.0) | 1.9 (1.3) | <0.0001 |
| Inflammation and coagulation markers | |||||
| lnCRP, mean (SD) | 0.435 (0.888) | 0.741 (0.984) | 0.880 (0.914) | 1.13 (1.00) | <0.0001 |
| Fibrinogen, mg/dl, mean (SD) | 298 (53.9) | 309 (59.0) | 319 (60.0) | 329 (66.0) | <0.0001 |
| Factor VIII, (%), mean (SD) | 111 (30.4) | 118 (35.0) | 122 (35.6) | 128 (39.7) | <0.0001 |
Overall p-value from test for trend, chi-square test, or Fisher's exact test.
Frailty
The 5 point frailty scale developed by Fried et al. is based on dichotomous components of unintentional weight loss, low physical activity, low strength, slow motor performance, and low energy. (1,15) The 5-point frailty scale was scored as follows: weight loss (1: unintentional weight loss of ≥10 pounds in prior year or, at follow-up, of ≥5% of body weight in prior year; 0: criteria not fulfilled); physical activity (1: lowest 20%, men: <383 Kcal/week, women: <270 Kcal/week; 0: highest 80%, men ≥383 Kcal/week, women: ≥270 Kcal/week); strength (1: lowest 20% grip strength adjusted for gender and body mass index; 0: highest 80% grip strength adjusted for gender and body mass index); motor performance (1: slowest 20% time to walk 15 feet adjusted for gender and height; 0: fastest 80% time to walk 15 feet adjusted for gender and height); energy (1: self reported exhaustion from 2 questions on CES-D; 0: no self reported exhaustion from 2 questions on CES-D). The 5-point scale is typically compressed into 3 broader categories: “frail” (subjects with 3, 4, or 5 components present), “intermediate frail” (subjects with 1 or 2 components present), and “not frail” (subjects with 0 components present). This scaling method well differentiates individuals who are very frail but not individuals who are exceptionally robust, i.e. there is a ceiling effect (Figure 1). To remove the ceiling effect and achieve greater differentiation of healthy aging on par with the physiologic index we created a more normalized, distributed version of the 5-point scale which was 10 points.
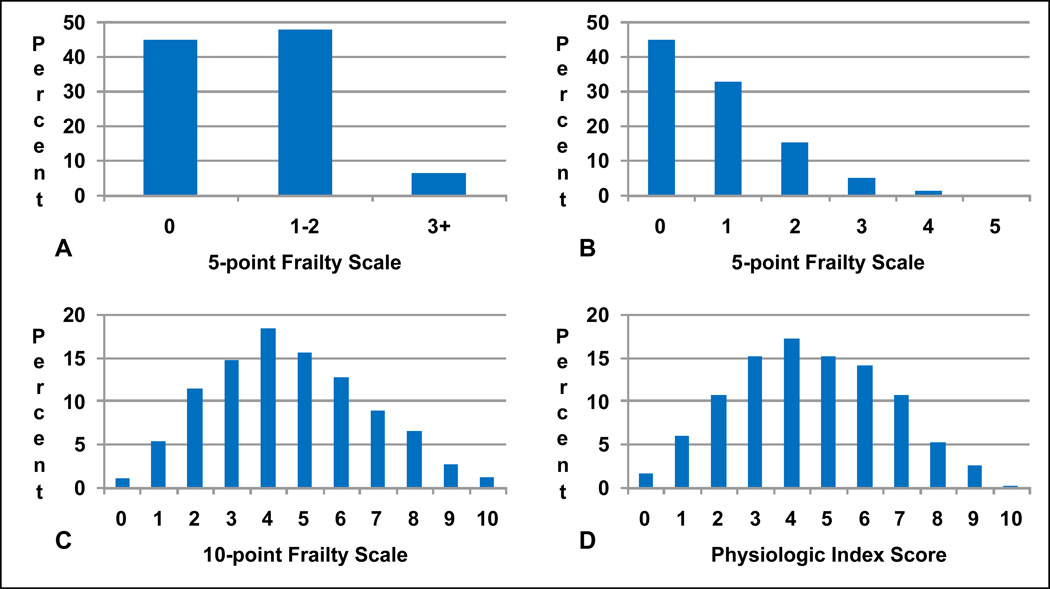
Figure 1.
Comparison of the distribution of the frailty scales and the physiologic index. Percentages are the proportion of the study population in each score group. (A) The 5-point scale categorizes participants as 0 (not frail), 1–2 (intermediate frail), and 3+ (frail) based on the presence of low scores on five domains. (B) The ceiling effect is apparent even when each domain is summed. (C) The new 10-point frailty scale is constructed using tertiles and scores are summed as a continuous variable. (D) The physiologic index is constructed using tertiles and scores are also summed as a continuous variable.
Scoring the 10-point frailty scale was done similarly to the physiologic index for interpretability and to directly compare agreement between scores: for each component, the best tertile received a score of 0, the middle tertile a score of 1, and the worst tertile a score of 2, and adding the five component scores created the new frailty scale from 0 (healthiest) to 10 (frailest) (Figure 1). Weight change in the past year (measured at the current and previous year’s clinical examination) was scored as: 0: >1.5 lbs, 1: −2 lbs to 1.5 lbs, 2: ≤−2 lbs for women; 0: >0 lbs, 1: −4 lbs to 0 lbs, 2: ≤−4 lbs for men. Physical activity was based on the Modified Minnesota Leisure Time Activities questionnaire and involved self-report of performing any of 18 activities in the prior week, along with the frequency and duration of these activities. Kilocalories of energy expended in a week on leisure time activity were calculated. Physical activity was scored as: 0: >1430 kcal, 1: 472.5 to 1430 kcal, 2: ≤472.5 kcal for women; 0: >2025 kcal, 1: 740 to 2025 kcal, 2: ≤740 kcal for men. Walk time for crossing a 15 ft (4.5 m) length at usual pace (to assess slow motor performance) was determined by a trained examiner according to a standardized protocol. Walk time tertiles were height specific (above or below mean height of 159 cm) for women. Because walk times were recorded as integers and tertile cut points were close, tertiles were not different for men above or below their mean height of 173 cm. For women ≤159 cm: 0: ≤5 sec, 1: 5 to 6 sec, 2: >6 sec. For women >159 cm: 0: ≤4 sec, 1: 4 to 5 sec, 2: >5 sec. For men: 0: ≤4 sec, 1: 4 to 5 sec, 2: >5 sec. Grip strength (to assess low strength) tertiles were gender and body mass index (BMI)-quartile specific. For women BMI ≤22.75: 0: >23.33 kg, 1: 18.67 to 23.33 kg, 2: ≤18.67 kg; BMI 22.75 to 25.5: 0: >23.67 kg, 1: 19.33 to 23.67 kg, 2: ≤19.33 kg; BMI 25.5 to 28.35: 0: >24 kg, 1: 19.33 to 24 kg, 2: ≤19.33 kg; BMI >28.35: 0: >25.33 kg, 1: 20.67 to 25.33 kg, 2: ≤20.67 kg. For men BMI ≤23.94: 0: >38.67 kg, 1: 32.33 to 38.67 kg, 2: ≤32.33 kg; BMI 23.94 to 25.93: 0: >40.33 kg, 1: 34 to 40.33 kg, 2: ≤34 kg; BMI 25.93 to 28.28: 0: >41.33 kg, 1: 34.67 to 41.33 kg, 2: ≤34.67 kg; BMI >28.28: 0: >41.33 kg, 1: 34.44 to 41.33 kg, 2: ≤34.33 kg. Two items from the Center for Epidemiologic Studies Short Depression Scale (CES-D) were used to characterize exhaustion. These were (1) “I felt that everything I do is an effort” and (2) “I cannot get going.” Individuals were asked to indicate if they felt that way 0 (none of the time), 1 (some of the time [1–2 days a week]), 2 (a moderate amount of time [3–4 days]), or 3 (most of the time). The sum of possible responses ranged from 0 to 6, and the best effort to tertile exhaustion was grouping as 0, 1, and 2–6.
Inflammation and Coagulation Markers
At each CHS field center, blood samples were drawn after an overnight fast, processed for storage, and shipped to a central laboratory at the University of Vermont (Colchester) following standardized protocols. Methods of phlebotomy sample handling and quality assurance have been described previously. (23) C-reactive protein (CRP) was assessed with a high-sensitivity enzyme-linked immunosorbent assay using purified protein and polyclonal anticlonal anti-CRP antibodies. (24) The interassay coefficient of variation was 5.50%. Plasma fibrinogen was measured using a semiautomated modified clot-rate method with a BBL Fibrometer (Becton Dickinson and Company, Bedford, MA). The mean monthly coefficient of variation for the fibrinogen assay was 3.09%. Factor VIII was measured with the Coag-A-Mate X2 instrument (Organon Teknika Corp, Durham, NC) using a partial thromboplastin reagent (Organon Teknika Corp). Values were reported as a percentage of normal plasma pool, and standardization was performed by assaying reference plasma from the World Health Organization. The mean monthly coefficient of variation for the Factor VIII assay was 9.67%.
Demographic, Behavioral Health, and Clinical Disease Variables
Other variables included age, sex, race (black, white, or other), and years of education, which were ascertained by self-report. Smoking was assessed by a standardized interview. (25) Blood pressure, height, and weight were assessed by standardized protocols. BMI was calculated as kilograms per meter squared. For consistency, we tabulated clinically diagnosed chronic conditions using the same methods as in the original report of the physiologic index. (14) Pulmonary disease, diabetes, kidney disease, and arthritis were assessed by self-report of physician diagnosis to depict what would be diagnosed disease. Depression was defined on the basis of a score >10 on a modified 10-item CES-D score. (26, 27) Reports of cardiovascular disease and stroke were confirmed by review of medications and medical records. Additionally, we defined cognitive impairment as a score <80 on a 100-point Mini Mental Status Exam. Using this information, a count of diagnosed chronic conditions was constructed for each person with a maximum of 8 for these conditions: cardiovascular disease, stroke, pulmonary disease, diabetes, kidney disease, arthritis, depression, and cognitive impairment. (14)
Statistical Analysis
We calculated descriptive statistics for all covariates by previously used categories of the physiologic index (scores: 0–2, 3–4, 5–6, 7–10) and 10-point frailty scale (scores: 0–2, 3–4, 5–6, 7–10) with a test for trend, the Χ2-test, or Fisher’s exact test. We constructed histograms to visually compare the distribution of the new 10-point frailty scale to the previous 5-point frailty scale. Gender-stratified age-adjusted Spearman correlations were calculated between components of the frailty scale to assess their independence.
Agreement between the physiologic index and the 10-point frailty scale was assessed using the kappa statistic. A lowess smoothed curve modeling the unadjusted association of the physiologic index to the frailty scale suggested a linear trend. Subsequently, we built a series of general linear models to determine the association of disease burden as a predictor with the 10-point frailty scale as an outcome adjusting for covariates as follows: model 1 (unadjusted); model 2 (adjusted for age, gender, and race); model 3 (additionally adjusted for years of education, BMI, and smoking); model 4 (additionally adjusted for natural logarithm of CRP, fibrinogen, and Factor VIII); model 5 (additionally adjusted for the number of diagnosed chronic conditions). We also replaced the number of diagnosed chronic conditions with the conditions themselves to test if disease burden was associated with frailty independent of specific conditions. In the full model, we tested for interaction between the physiologic index and all covariates.
Lowess smoothers suggested a linear trend was most likely between frailty and components of the physiologic index, so to test if disease in individual physiologic systems was associated with frailty we used separate linear models of each index component predicting frailty. We constructed a larger linear model including all physiologic index components simultaneously to assess if the components were significant predictors of frailty independent of each other. We calculated standardized regression coefficients to compare the strength of the components to the strength of the physiologic index for predicting frailty.
To see if disease burden alone attenuates the association between frailty and inflammation or coagulation, we constructed an additional linear model using only CRP, fibrinogen, and Factor VIII as predictors of frailty, then adjusted for the physiologic index, and examined the change in magnitude and significance of the parameters. For all analyses we used a two-sided alpha of 0.05 to determine significance and SAS 9.2 (Cary, NC).
Results
Those with higher disease burden were older, less educated, more likely to be non-white or have some smoking history, had a higher BMI, a greater number of diagnosed chronic conditions, and higher CRP, fibrinogen, and Factor VIII (Table 1). Population trends by frailty were nearly identical (data not shown). Notably, male gender and kidney disease were associated with higher disease burden but not frailty, and arthritis was associated with frailty (p<0.0001) but not disease burden.
Re-distributing the frailty scale resulted in a much rarer “not frail” phenotype (Figure 1). While 45% of participants were categorized as “not frail” using the previous 5-point frailty scale, only 1.2% of participants had a score of 0 on the new 10-point frailty scale. The distribution of the 10-point frailty scale also matched the distribution of the physiologic index almost exactly and both appeared relatively normal (Figure 1). It is possible that the distributions take this form because the data are derived from large general population samples and the components are weakly though significantly correlated (reference 14). Indeed, after adjustment for age, the largest correlations between frailty scale components were between walk time and physical activity (Men: r = −0.170, p<0.0001; Women: r = −0.184, p<0.0001) and walk time and exhaustion (Men: r = 0.168, p<0.0001; Women: r = 0.204, p<0.0001), and though many other correlations were significant (p<0.05) they were generally weak (r<0.13). The mean (SD) of the 10-point frailty scale and physiologic index was 4.6 (2.2) and 4.5 (2.1), respectively.
The linear weighted kappa statistic between the physiologic index and 10-point frailty scale was 0.17 (95% CI 0.14–0.19), indicating significant agreement though a modest chance that individuals had the same score on both scales. Using the 5-point frailty scale, the mean physiologic index score was 4.0 for those who were not frail, 4.8 for those who were intermediate frail, and 5.9 for those who were frail. Only 0.8% of participants had a physiologic index score ≤3 and were frail. Using the 10-point frailty scale, the mean physiologic index score was 3.6 for those with a frailty score of 0, 4.6 for those with a frailty score of 5, and 5.9 for those with a frailty score of 10. In an unadjusted model, each 1 point higher physiologic index score was associated with a 0.28 point higher 10-point frailty scale (p<0.0001, model r2 = 0.08) (Table 2). For comparison, each 1 point higher physiologic index score was associated with a 0.12 point higher 5-point frailty scale (p<0.0001). The crude association between age and frailty was attenuated by 29% after inclusion of the physiologic index score in the model (β(SE) = 0.14(0.01) per year of age, p<0.0001, model r2 = 0.09 without the physiologic index; β(SE) = 0.10(0.01) per year of age, p<0.0001, model r2 = 0.12 with the physiologic index), indicating the physiologic index accounted for a substantial proportion of the age effect on frailty.
Table 2.
Linear regression models of the association of the physiologic index score to 10-point frailty scale
| Model 1* | Model 2† | Model 3‡ | Model 4§ | Model 5□ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P |
| Physiologic index score, 1 point |
0.28 (0.02) |
<0.0001 | 0.21 (0.02) |
<0.0001 | 0.17 (0.02) |
<0.0001 | 0.16 (0.02) |
<0.0001 | 0.11 (0.02) |
<0.0001 |
| Demographics | ||||||||||
| Age, 1 year | 0.11 (0.01) |
<0.0001 | 0.12 (0.01) |
<0.0001 | 0.12 (0.01) |
<0.0001 | 0.11 (0.01) |
<0.0001 | ||
| Male gender | −0.27 (0.08) |
<0.0001 | −0.22 (0.09) |
0.02 | −0.20 (0.09) |
0.03 | −0.10 (0.09) |
0.24 | ||
| Non-white race | 0.47 (0.20) |
0.03 | 0.37 (0.20) |
0.07 | 0.39 (0.20) |
0.06 | 0.34 (0.20) |
0.09 | ||
| Behavioral risk factors | ||||||||||
| Education, 1 year | −0.03 (0.01) |
0.0003 | −0.03 (0.01) |
0.002 | −0.02 (0.01) |
0.01 | ||||
| Body mass index, 1 kg/m2 | 0.03 (0.01) |
0.002 | 0.03 (0.01) |
0.009 | 0.02 (0.01) |
0.02 | ||||
| Current smoking | 0.67 (0.16) |
<0.0001 | 0.64 (0.16) |
<0.0001 | 0.64 (0.16) |
<0.0001 | ||||
| Past smoking | −0.05 (0.09) |
0.61 | −0.06 (0.09) |
0.53 | −0.13 (0.09) |
0.14 | ||||
| Inflammation and coagulation markers | ||||||||||
| lnCRP, 1 unit | 0.05 (0.05) |
0.34 | 0.01 (0.05) |
0.90 | ||||||
| Fibrinogen, 10 mg/dl | 0.01 (0.01) |
0.27 | 0.01 (0.01) |
0.09 | ||||||
| Factor VIII, 10 % | 0.00 (0.01) |
0.99 | −0.00 (0.01) |
0.83 | ||||||
| No. of chronic health conditions, 1 point | 0.43 (0.04) |
<0.0001 | ||||||||
Unadjusted. Model r2 = 0.08.
Adjusted for age, gender, and race. Model r2 = 0.13.
Additionally adjusted for education, body mass index, and smoking status. Model r2 = 0.14.
Additionally adjusted for lnCRP, fibrinogen, and Factor VIII. Model r2 = 0.14.
Additionally adjusted for number of diagnosed chronic conditions. Model r2 = 0.18.
Adjustment for covariates sequentially attenuated the effect of the physiologic index but it remained a significant independent predictor of frailty (Table 2). In the fully adjusted model, each 1 point higher physiologic index score was associated with a 0.11 point higher 10-point frailty scale (p<0.0001, model r2 = 0.18). For comparison, each 1 point higher physiologic index score was associated with a 0.04 point higher 5-point frailty scale (p<0.0001). Current smoking was strongly associated with frailty while education was weakly, though significantly, protective. Replacement of the count of diagnosed chronic conditions with the conditions themselves revealed that many were significantly and independently associated with frailty (depression β(SE) = 1.49(0.12), p<0.0001; cerebrovascular disease β(SE) = 0.64(0.17), p=0.0001; cognitive impairment β(SE) = 0.44(0.19), p=0.02; arthritis β(SE) = 0.39(0.08), p<0.0001; chronic lung disease β(SE) = 0.23(0.09), p=0.01), though some were not (coronary heart disease β(SE) = 0.03(0.10), p=0.80; diabetes β(SE) = 0.12(0.13), p=0.35; kidney disease β(SE) = 0.22(0.43), p=0.62) with disease burden in the model.
The physiologic index components were also significantly associated with frailty (Table 3). Adjustment attenuated the association for all components, but white matter grade, cystatin-C, and FVC remained significantly associated with frailty. Adjustment for diagnosed diabetes alone attenuated serum glucose by ~50% and to non-significance. Adjustment for age attenuated carotid thickness by ~50%, though additional adjustment for several combinations of covariates was necessary to attenuate the association to non-significance. White matter grade interacted with current smoking (interaction β(SE) = 0.23(0.10), p=0.03), indicating that its effect was augmented in current smokers. There was no interaction with cystatin-C or FVC. Inclusion of all physiologic index components in the same crude model illustrated that the components attenuate each other slightly though their independent contribution to frailty remained (Table 3). The association of frailty to cystatin-C and FVC was greatest and on par with the association of frailty to disease burden (Table 3).
Table 3.
Association of the physiologic index or components of the physiologic index to the 10-point frailty scale
| Physiologic Index or Component | Mean (SD) | Crude β* | P | Adjusted β*,† | P† |
|---|---|---|---|---|---|
| Physiologic Index (point) | 4.5 (2.1) | 0.60 | <0.0001 | 0.23 | <0.0001 |
| Separate model for each component | |||||
| Carotid artery thickness (mm) | 1.42 (0.56) | 0.23 | <0.0001 | 0.06 | 0.14 |
| White matter grade (unit) | 2.19 (1.38) | 0.36 | <0.0001 | 0.15 | 0.0004 |
| Serum fasting glucose (mg/dL) | 106 (30) | 0.19 | <0.0001 | −0.00 | 0.99 |
| Serum cystatin-C (mg/L) | 1.10 (0.28) | 0.44 | <0.0001 | 0.21 | <0.0001 |
| Forced vital capacity (L) | 2.95 (0.88) | −0.41 | <0.0001 | −0.26 | <0.0001 |
| All components in the same model | |||||
| Carotid artery thickness (mm) | 0.14 | 0.001 | 0.02 | 0.60 | |
| White matter grade (unit) | 0.25 | <0.0001 | 0.14 | 0.0007 | |
| Serum fasting glucose (mg/dL) | 0.18 | <0.0001 | 0.01 | 0.87 | |
| Serum cystatin-C (mg/L) | 0.38 | <0.0001 | 0.19 | <0.0001 | |
| Forced vital capacity (L) | −0.38 | <0.0001 | −0.25 | <0.0001 |
The increase in the 10-point frailty scale per standard deviation increase in the physiologic index or component of the physiologic index.
Adjusted for age, gender, race, education, BMI, smoking status, lnCRP, fibrinogen, Factor VIII, and number of diagnosed chronic conditions.
Finally, in a model predicting frailty using only CRP, fibrinogen, and Factor VIII, these markers were independently and significantly associated with frailty. But, their effect was substantially attenuated with the addition of the physiologic index. The CRP coefficient decreased 83% from 0.123 per unit (p=0.02) to 0.021 (p=0.67). The fibrinogen coefficient decreased 32% from 0.022 per 10 mg/dl fibrinogen (p=0.01) to 0.015 (p=0.06). The Factor VIII coefficient decreased 56% from 0.034 per 10% Factor VIII (p=0.007) to 0.015 (p=0.22). In this model, the physiologic index was significant (β=0.27 per 1 point higher physiologic index score, p<0.0001).
Discussion
These data strongly suggest that disease burden is directly and independently associated with frailty. In comparison, frailty was weakly associated with markers of inflammation and coagulation and more strongly with several diagnosed chronic conditions. These observations help refine the current etiologic model of frailty. They support the hypothesis that inflammation and coagulation are risk factors for frailty. (15) Furthermore, they suggest that the effects of inflammation and coagulation may be mediated through structural and functional changes in specific organs. In turn, these changes may build upon each other to engender a cycle of inflammation, disease, and frailty that is reinforced by worsening disease. Although age remained a significant independent predictor of frailty, illustrating there are age-associated factors that contribute to frailty that were not included in our models, disease burden accounted for a substantial portion of the age effect. This implies aging is partly the aggregation of structural and functional changes in organs, which is captured by the physiologic index. In future frailty research, because subclinical disease may be present before clinically-recognized disease, physiologic measurements may be used to identify older adults at increased risk of frailty earlier than is often done, which would allow the development of preventive interventions targeting early risk factors to maximally mitigate their effects. (28)
Recalibrating the frailty index to remove the ceiling effect and better differentiate healthier individuals resulted in two notable achievements. First, the reference category of “not frail” became much rarer (1.2% rather than 45%). Second, the strength of the association between disease burden and frailty doubled with recalibration. These observations might be due to removing the ceiling effect of the old frailty scale, doubling the range of the scale, and/or reclassifying individuals more finely. These results suggest that the recalibrated 10-point frailty scale may be more useful in the search for longevity-associated factors because it can identify a more exceptional and homogenous phenotype that can be used as a reference category. It should be validated further in prospective analyses.
Cystatin-C, FVC, and white matter grade were the only physiologic index components independently associated with frailty in this analysis. Cystatin-C is a marker of glomerular filtration rate and has been associated with greater overall and cardiovascular-specific mortality, incident cardiovascular disease, and incident non-cardiovascular outcomes. (20,29) FVC is a marker of tidal volume and pulmonary reserve and is intimately tied to cardiorespiratory fitness. White matter grade is a marker of brain lesions which interfere with neural tract communication. (30) These lesions occur in normal aging and many age-associated conditions and predict mortality. (17,31) Previous reports do not explore continuous measurement of these factors in association with frailty, so more research is needed to corroborate these findings and clarify their meaning. Although continuous carotid thickness was not associated with frailty, several categorical classifications of subclinical cardiovascular disease markers have been associated with frailty. (32) More extreme separation of carotid thickness groups may be required to achieve significance. These findings suggest which markers may be most worthwhile for monitoring or developing targeted preventive interventions.
We also found that non-white race, higher BMI, and current smoking were risk factors for frailty while education was protective. Previous reports agree with these racial findings but it is difficult to know whether race is a surrogate for other biologic or environmental factors, such as access, quality, and adherence to care. (33–35) Data from the Women’s Health and Aging Studies suggests that lower education is a significant risk factor for frailty and accounts for the effect of race, and that race is confounded by socioeconomic position. (33) A recent report from the Canadian Study of Health and Aging implies that the association between BMI and frailty may be U-shaped and that older adults with a BMI 25–29.9 have the lowest prevalence of frailty. (36) The Canadian study, along with the Women’s Health Initiative Observational Study, also show smoking likely contributes to frailty. (37,38) Education, BMI, and smoking are modifiable factors so they may be useful targets for decreasing frailty risk. In particular, the large effect of current smoking warrants continued attention to smoking cessation.
This study has several strengths and weaknesses. The size and community-based sampling of the CHS suggest results may be generalizable, though selection from the complete CHS cohort may limit generalizability somewhat. Measurement of many covariates allowed us to adjust for potential confounders, strengthening internal validity. The main weakness of this study is that the observational and cross-sectional data do not permit definitive causal inference. Because the CHS was developed to examine mainly cardiovascular disease, our analysis might not include factors unrelated to cardiovascular disease which nonetheless impact frailty. Finally, we used a phenotype of frailty with mainly physical components vs. cognitive ones. Subsequently, our analysis depends in part on this classification of health status.
In conclusion, we found that a particular characterization of disease burden was significantly associated with frailty independent of inflammation, coagulation, and diagnosed conditions. This underscores the importance of possibly unrecognized physiologic changes as potential causes of frailty. Depicting simultaneous change in these measurements over time would provide meaningful data on aging and, most importantly, how the body adapts to these changes. Further definition of the frailty risk factor network, particularly through longitudinal analysis juxtaposing individuals who age well vs. individuals who age poorly, will enable development of interventions which target specific biologic pathways to promote healthy aging and longevity rather than forestalling decline.
Acknowledgments
The research reported in this article was supported by grants R01-AG-023629 and 5-P30-AG-024827 and by contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contributions from the National Institute of Neurological Disorders and Stroke. A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Footnotes
Meetings submitted to: Jason L. Sanders was awarded the 2011 Scientist-In-Training Award from the American Geriatrics Society for this abstract submission to the 2011 AGS Annual Meeting.
Author Contributions, Role of Sponsor, and Conflict of Interest
Study concept and design: LPF, TBH, ABN. Acquisition of subjects and/or data: RMB, LPF, TBH, ABN. Analysis and interpretation of data: JLS, RMB, LPF, JDW, TBH, ABN. Preparation of manuscript: JLS, RMB, LPF, JDW, TBH, ABN. Sponsor’s Role: The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication. Conflict of Interest: The authors declare no conflicts of interest.
Contributor Information
Robert M. Boudreau, Email: BoudreauR@edc.pitt.edu.
Linda P. Fried, Email: lpfried@columbia.edu.
Jeremy D. Walston, Email: jwalston@jhmi.edu.
Tamara B. Harris, Email: harrist@gw.nia.nih.gov.
Anne B. Newman, Email: NewmanA@edc.pitt.edu.
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Walston J. Frailty and failure to thrive. In: Hazzard WR, Blass JP, Ettinger WH Jr, Halter JB, Ouslander J, editors. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill; 2003. pp. 1487–1502. [Google Scholar]
- 3.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 4.Rockwood K, Mitnitski A, Song X, et al. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 5.Studenski S, Hayes RP, Leibowitz RQ, et al. Clinical global impression of change in physical frailty: development of a measure based on clinical judgement. J Am Geriatr Soc. 2004;52:1560–1566. doi: 10.1111/j.1532-5415.2004.52423.x. [DOI] [PubMed] [Google Scholar]
- 6.Waterer GW, Wan JY, Kritchevsky SB, et al. Airflow limitation is underrecognized in well-functioning older people. J Am Geriatr Soc. 2001;49:1032–1038. doi: 10.1046/j.1532-5415.2001.49205.x. [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 8.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Haggerty CL, Kritchevsky SB, et al. Walking performance and cardiovascular response: associations with age and morbidity – The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2003;58:715–720. doi: 10.1093/gerona/58.8.m715. [DOI] [PubMed] [Google Scholar]
- 10.Kuller LH, Shemanski L, Psaty BM, et al. Subclinical disease as an independent risk factor for cardiovascular disease. Circulation. 1995;92:720–726. doi: 10.1161/01.cir.92.4.720. [DOI] [PubMed] [Google Scholar]
- 11.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 12.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Xue Q-L, Cappola AR, et al. Nonlinear multisystem physiologic dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2010;64A:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman AB, Boudreau RM, Naydeck BL, et al. A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63A:603–609. doi: 10.1093/gerona/63.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 17.Kuller LH, Arnold AM, Longstreth WT, Jr., et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 18.O’Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 19.Barzilay JI, Kronmal RA, Gottdiener JS, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults ≥65 years. J Am Coll Cardiol. 2004;43:2236–2241. doi: 10.1016/j.jacc.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 20.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 21.Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 22.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 23.Cushman M, Meilahn EN, Psaty BM, et al. Hormone replacement therapy, inflammation, and hemostasis in elderly women. Arterioscler Thromb Vasc Biol. 1999;19:893–899. doi: 10.1161/01.atv.19.4.893. [DOI] [PubMed] [Google Scholar]
- 24.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 25.Higgins MW, Enright PL, Kronmal RA, et al. Smoking and lung function in elderly men and women: the Cardiovascular Health Study. JAMA. 1993;269:2741–2748. [PubMed] [Google Scholar]
- 26.Orme J, Reis J, Herz E. Factorial and discriminate validity of the center for epidemiological studies depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 27.Schulz R, Beach SR, Ives DG, et al. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 28.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 29.Madero M, Sarnak MJ. Association of cystatin C with adverse outcomes. Curr Opin Nephrol Hypertens. 2009;18:258–263. doi: 10.1097/mnh.0b013e328326f3dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmahmann JD, Smith EE, Eichler FS, et al. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry. 2008;64:273–280. doi: 10.1016/j.biopsych.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 33.Szanton SL, Seplaki CL, Thorpe RJ, Jr, et al. Socioeconomic status is associated with frailty: the Women’s Health and Aging Studies. J Epidemiol Community Health. 2010;64:63–67. doi: 10.1136/jech.2008.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging: evidence from the U.S. older adult population. J Gerontol B Psychol Sci Soc Sci. 2010;65B:246–255. doi: 10.1093/geronb/gbp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirsch C, Anderson ML, Newman A, et al. The association of race with frailty: the cardiovascular health study. Ann Epidemiol. 2006;16:545–553. doi: 10.1016/j.annepidem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Hubbard RE, Lang IA, Llewellyn DJ, et al. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65:377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 37.Hubbard RE, Searle SD, Mitnitski A, et al. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging. 2009;13:468–472. doi: 10.1007/s12603-009-0085-y. [DOI] [PubMed] [Google Scholar]
- 38.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]