Abstract
The general principles of retinal organization are now well known. It may seem surprising that retinal organization in the primate, which has a complex visual behavioral repertoire, appears relatively simple. In this review, we primarily consider retinal structure and function in primate species. Photoreceptor distribution and connectivity are considered as are connectivity in the outer and inner retina. One key issue is the specificity of retinal connections; we suggest that the retina shows connectional specificity but this is seldom complete, and we consider here the functional consequences of imprecise wiring. Finally, we consider how retinal systems can be linked to psychophysical descriptions of different channels, chromatic and luminance, which are proposed to exist in the primate visual system.
1. Introduction
The primate retina is an interesting locus to assess how neuronal connectivity defines function. Responses of retinal elements in primates can be related to their synaptic relationships with other retinal neurons, and to visual performance. The retina is an accessible part of the brain (Dowling, 1987) and each of its neural elements is conveniently lain out in a two-dimensional array, in demarcated layers. This propitious arrangement has allowed great progress in understanding relations between retinal structure and function. However, many aspects of retinal connectivity remain unexplored, and many described connections are controversial.
To relate retinal connectivity to vision, there must be behavioral data available to act as a yardstick against which to measure retinal responses. Old World primates such as the various macaque monkey species are the model of choice for human vision; available evidence suggests that psychophysical performance on simple visual detection tasks is similar in macaque and human (Crawford et al., 1990; Merigan and Maunsell, 1993). Direct comparison of retinal physiology from the macaque and human psychophysics has proved possible (Kallomiatis and Harwerth, 1991; Lee et al., 1988).
Humans and other Old World primates show routine trichromatic color vision, based on three photoreceptor classes sensitive to short (S), medium (M) or long wavelengths (L) in the visible spectrum. A comparative aspect relevant to understanding color vision has been provided by New-World primates. In most New-World monkey species, the males are “red–green color blind” dichromats whereas most of the females show trichromatic color vision similar to that of most humans or to human anomalous trichromats (Jacobs, 2008; Jacobs et al., 1993b). New-World monkeys are thus an interesting model for testing the functional consequences of changes in the input stage of the visual process, that is, changes in the M and L cone photoreceptor populations.
One central and unresolved issue is how far retinal elements are specific in their connectivity as opposed to indiscriminately contacting their neighbors. For example, there appear to be gap junctional connections between neighboring cone photoreceptors (Massey, 2008; O'Brian et al., 2004). These are indiscriminate between the M and L cones, although S cones participate in such junctions only rarely. However, physiological (Lee et al., 1999) and psychophysical evidence (Stiles, 1959) for the functional independence of the M- and L-cone mechanisms is not compatible with strong gap junction coupling. The viewpoint stressed here is that retinal connectivity is as specific as it needs to be for functional purposes, but absolute specificity may be a chimera.
This review concentrates on those cells and circuits that can be related to specific visual functions. Emphasis is given to three best-understood pathways in the primate retina: the parasol, midget and small bistratified pathways shown schematically in Fig. 1. We address specifically the question of selectivity, that is, what is the wiring precision of these parallel neural circuits? A key question in considering primate retina is how far acquisition of receptors serving red–green color vision has prompted remodeling of retinal circuitry. We also discuss the way retinal receptive fields are dependent on retinal connectivity. We first consider the cone photoreceptors and their distributions, then the S-cone pathway and then the M,L-cone systems of primates.
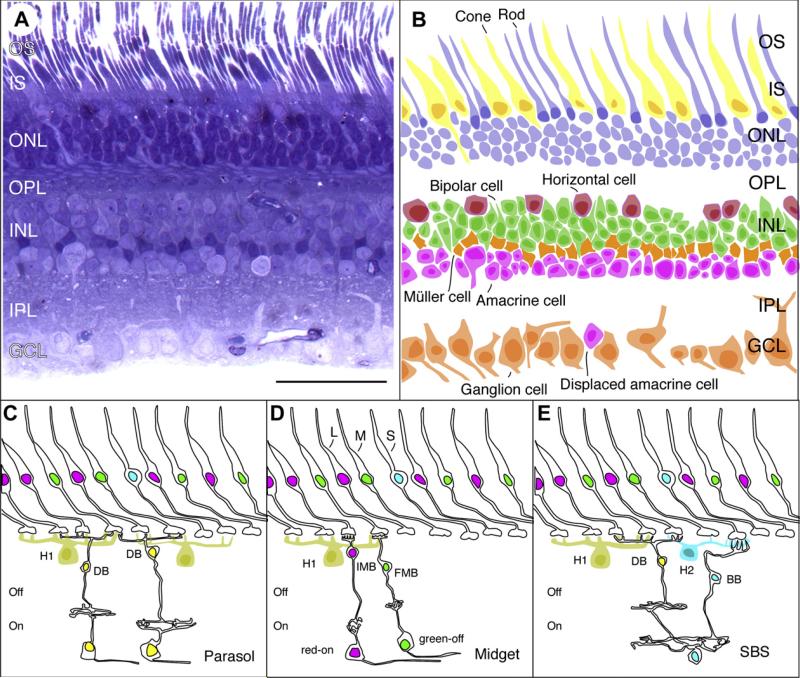
Fig. 1.
Moving from histology to functional circuitry in primate retina. Scale bar (50 μm) in A applies to all panels allowing relative size and disposition of neuron populations to be compared. A, semithin radial section through macaque monkey retina. Toluidine blue (Nissl) stain near 3 μm eccentricity. OS, outer segments; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. B, disposition of neuron populations in the same area. Silhouettes show cell somata and nuclei visible in the section from panel A; inner and outer segments of some rod and cone receptors are also drawn. C, parasol pathway. Excitation to off-parasol cells is through several flat diffuse bipolar cells (only one is shown); excitation to on-parasol cells is through invaginating diffuse bipolar cells. Surround inhibition to diffuse bipolar cells derives from H1 class of horizontal cells in OPL; additional inhibition may be present in IPL (not drawn). H1, horizontal cell; fdb, flat diffuse bipolar cell; idb, invaginating diffuse bipolar cell. D, midget pathway. Excitation to midget ganglion cells is through single-cone contacting midget bipolar cells; Surround inhibition to midget bipolar cells derives from H1 class of horizontal cells in OPL; additional inhibition may be present in IPL (not drawn). imb, invaginating midget bipolar cell; fmb, flat midget bipolar cell. L, long wavelength-sensitive cone; M, medium wavelength sensitive cone. S, short-wavelength sensitive cone. E, small bistratified (“blue-on”) pathway. On-sign excitation to blue-on cells from S cones is through blue cone bipolar cells. Off excitation from ML cones is through diffuse bipolar cells. Inhibition is from H2 class of horizontal cells in the OPL to S cones and from H1 horizontal cells to ML cones.
In addition to the parasol, midget and small bistratified pathways that are the main topic of this review there are many other ganglion cell types. Among those that have received recent attention are the intrinsically photosensitive (“melanopsin”) ganglion cell, which helps control the circadian rhythm and pupillomotor responses (Dacey et al., 2005) and direction selective ganglion cells (Taylor and Vaney, 2002). Reviews of these (and other classes) can be found elsewhere (Vaney and Taylor, 2002; Wässle, 2004).
2. Basic principles of retinal circuitry
Fig. 1 illustrates the major neuron populations in primate retina, together with three well-established functional circuits feeding distinct visual parallel pathways. The retina is a multilayered structure in all mammals, as seen in the toluidine blue stained section of primate retina in Fig. 1A. The neuron populations contained in this section are sketched in Fig. 1B. Photosensitive segments of rod and cone photoreceptors occupy the most scleral layer; the receptor cell bodies constitute the Outer Nuclear Layer (ONL). The Outer Plexiform Layer (OPL) contains a synaptic plexus consisting of the synaptic terminals of the receptors and dendritic processes of bipolar and horizontal cells. The Inner Nuclear Layer (INL) contains cell bodies of the horizontal and bipolar cells, Müller cells, and most amacrine cells. The Inner Plexiform Layer (IPL) contains axon terminals of the bipolar cells, and a rich connective mesh of amacrine cell processes and ganglion cell dendrites. Each population of ganglion cells makes synaptic connections at a different sub-level within the IPL. As a broad rule, on-center ganglion cells stratify in the vitreal half of the IPL and off-center ganglion cells stratify in the scleral half of the IPL. The Ganglion Cell Layer (GCL) contains the cell bodies of the ganglion cells and (especially in peripheral retina) the cell bodies of displaced amacrine cells. The proportion of displaced amacrine cells increases with retinal eccentricity, although the absolute density is highest near the fovea; it then declines at a shallower rate with eccentricity than the density of ganglion cells (Wässle et al., 1990; Lima et al., 1996).
Fig. 1C–E shows simplified views of the circuitry of the three main ganglion cell types which project to the thalamus. On- and off-center parasol cells (Fig. 1C) receive input predominantly from M and L cones via one or more classes of diffuse bipolar cell (Boycott and Wässle, 1991; Jacoby et al., 1996, 2000), and they project to the magnocellular layers of the lateral geniculate nucleus (LGN); they are thus frequently termed MC (or M) cells. A second pathway begins in the midget ganglion cells (Fig. 1D), which in the fovea receive dominant anatomical input from a single cone via a single midget bipolar cell (Calkins et al., 1994; Kolb and Dekorver, 1991). Trichromatic primates such as most humans and macaque species express both M- and L-type cones. Each foveal M or L cone thus provides input to one on-type and one off-type midget ganglion cell, yielding four distinct receptive field profiles: red (L cone) on-center, red (L cone) off-center, green (M cone) on-center and green (M cone) off-center (Derrington et al., 1984; DeValois and DeValois, 1975; Dreher et al., 1976; Lee et al., 1987; Wiesel and Hubel, 1966). There is only one anatomical array of on-center and one array of off-center midget ganglion cells. Thus, one midget on-center ganglion cell with an L-cone center can have a midget ganglion cell with an M-cone center as its neighbor and so have distinct spectral characteristics, i.e., a mixture of functional types within the same ganglion cell array. As discussed below, this multiplexing of chromatic and spatial channels in a single nerve pathway is an apparent violation of Müller's principle of specific nerve energies (Müller, 1838 p. 250ff; Helmholtz, 1962; Kremer, 1993), with still poorly-understood consequences for visual processing. The midget ganglion cells project to the parvocellular layers of the LGN, and are termed PC (or P) cells.
A third group of cells receives S-cone input. One of these is the small bistratified cell (Fig. 1E), first identified anatomically (Dacey, 1993a; Rodieck, 1991) and then physiologically (Dacey and Lee, 1994). As detailed below, small bistratified cells receive on-excitation from the S cones via blue cone on-bipolar cells, and inhibitory (off) input from the other cone types via diffuse off- bipolar cells. Fewer details are known of other ganglion cell types receiving S-cone input, but at least one receives an off, inhibitory S-cone signal (Dacey et al., 2003); in common with small bistratified cells they are thought to project to the koniocellular layers of the LGN, and are termed KC- (or K) cells (Martin et al., 1997; Szmajda et al., 2006).
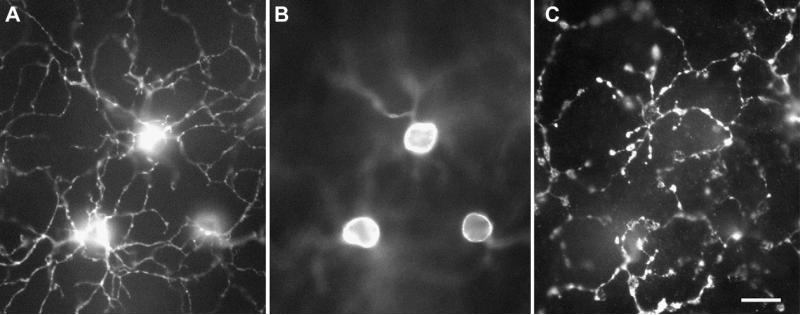
Early descriptions of retinal circuitry were derived from trans-verse sections through the retina in the plane shown in Fig. 1 (Cajal, 1893; Polyak, 1941). This approach makes certain principles of retinal connectivity obvious, for example the relation of bipolar cells to the photoreceptors. However, visual space and receptive fields of retinal neurons are mapped onto the retina in a plane orthogonal to the transverse section. It proved much easier to identify relationships between, say, ganglion cells identified anatomically and functional types identified physiologically when ganglion cell morphology was viewed in the wholemount, from the direction of the pupil (e.g., Boycott and Wässle, 1974). This is illustrated in Fig. 2, which shows wholemount views of a bipolar cell mosaic in macaque retina. Changes in retinal morphology with eccentricity are obvious in the wholemount preparation, and this permitted a more unified view of retinal structure. It is now recognized that almost all retinal neurons are laid out in semi-regular arrays across the retinal surface. However, it remains true that connectivity is best studied from a transverse perspective, which is orthogonal to the dimensions of visual space in which receptive fields are constructed. This has hampered elucidation of the retinal connectivity underlying receptive field structure.
Fig. 2.
Mosaic of bipolar cells. Whole mount view of immunolabelled DB6 cone bipolar cells in macaque retina. A, The focus is on the dendritic trees; B, the focus is on the somata; C, the focus is on the axon terminals. Scale bar 10 μm. For further details see Chan et al. (2001).
As discussed above, a main theme of this review is how wiring in the retina produces functional specificity. Long-known examples of functional specificity in the retina include the separation of on- and off pathways, distinct mechanisms to generate sustained and transient responses in different cell types, and the functional segregation of rod and cone input pathways to ganglion cells. However, detailed organization even in these well-established instances is seldom straightforward. The schemata in Fig. 1 imply specific retinal connectivity in cone pathways reaching ganglion cells. As described in following sections, where studied in detail retinal connections do not show completely specific wiring. But on the other hand, psychophysical and physiological evidence suggests functional specificity in the cone inputs to the MC, PC and KC pathways, and evidence discussed below suggests that the retina does select the connections it makes. We will argue that absolute specificity is not necessary to provide adequate visual function: the retina is not like a computer chip. A moderate degree of connectional specificity may be adequate for functional purposes, but complete specificity might yield little further biological advantage. This makes the task of the anatomist more of a challenge; in addition to demonstrating connectivity, a quantitative approach is required to estimate synaptic weighting. In addition, the anatomical substrates of some physiological properties are challenging to define; for example, the basis of connections supporting the receptive field center is well studied, but the wiring responsible for surround mechanisms is much less certain. In summary, the schemata in Fig. 1 are idealized and neglect a complex and messy biological reality.
3. The photoreceptors
The distribution of cone receptors across the retina has important implications for models of retinal connectivity. In addition, there is evidence for connectivity between cones, between cone and rods, and between rods. We summarize current views of these issues in the next sections.
3.1. The short-wavelength-sensitive (S) cones
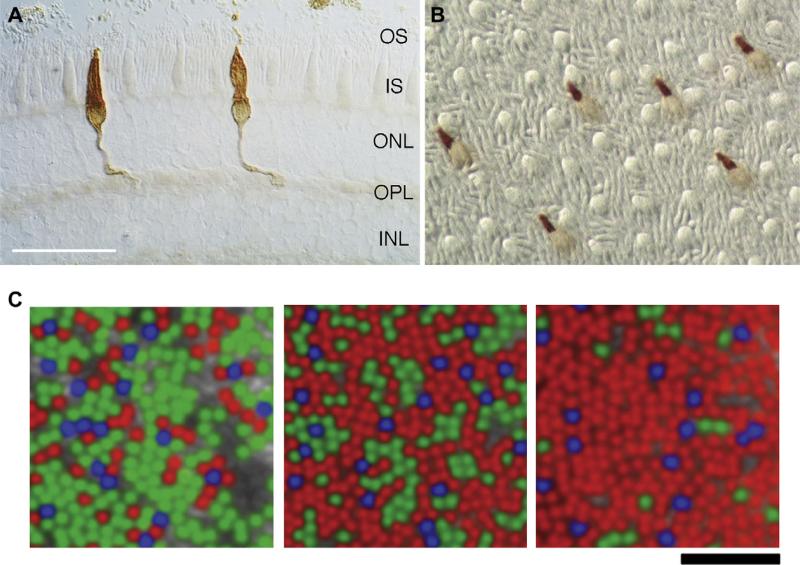
The visual pigments of vertebrates evolved about 500 million years ago and derive from five classes of opsin genes: four spectrally distinct classes of cone opsin gene families and one class of rod opsin (Bowmaker, 2008). The four cone opsin families consist of two short-wavelength sensitive classes (SWS1, SWS2), one middle-wavelength sensitive (Rh2), and one long-wavelength sensitive (LWS) class. The cone opsin classes evolved through a series of gene duplications, and the rod opsin class (Rh1) likely evolved by duplication of the Rh2 cone opsin gene. Nearly all mammals possess two types of cone opsin (SWS1 and LWS) in addition to rod opsin. The distribution of S cones has been studied in a large number of mammalian species using immunohistochemistry and in situ hybridization (Ahnelt and Kolb, 2000; Martin et al., 2000; Peichl, 2005; Wikler and Rakic, 1990). Fig. 3A and B shows preparations of macaque retina processed with antibodies against S cone opsin. As in most primate retinae, S cones form a minority (between 5 and 15%) of the cone population. Short wavelength sensitive cones are usually distributed across the retina with a higher density in central compared to peripheral retina.
Fig. 3.
A. Comparison of vertical (radial) and wholemount (tangential) visualization of retinal nerve populations. Short-wavelength sensitive (S) cones are labelled with an antibody (JH445) against human S-cone opsin pigment. In these differential interference contrast images, inner and outer segments of unlabelled cones and rods are visible interspersed between the labelled S cones. A, Vertical section. OS, outer segments; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer. B, wholemount view. Scale bar in A (50 μm) applies to A and B. C Pseudo color images illustrating organization of the cone mosaic predicted by adaptive optics imaging and reflection densitometry in living human observers (Carroll et al., 2009). Cones were classified and pseudo-colored (red, L; green, M; blue, S) according to relative absorptance after 650 nm and 470 nm bleaching lights. Uncolored cones were not classifiable. Note inter-subject variance of M/L ratio among classified cones.
The following exceptions to the rules of S-cone expression and distribution outlined above have been reported. Firstly, a lack of S cones was found in a number of nocturnal mammals including two species of primates (owl monkey and bushbaby) and in some marine species (whales, seals). Secondly, in some mammalian species, an uneven distribution of S cones was found, such that S cones are concentrated in ventral (mouse, guinea pig) or dorsal (ground squirrel, some marsupials) retina. In two species of nocturnal primates an increased S-cone density has been found in peripheral retina with a low density in central retina (Hendrickson et al., 2000) Finally, in some species co-expression of S and L opsins has been reported (mouse, guinea pig) but “true blue” cones expressing only S opsin have also been found (Haverkamp et al., 2005). In primates, co-expression of different cone opsins has not been found (Bumsted et al., 1997).
The proportion of S cones is relatively low in the central-most fovea of all diurnal primates described so far (de Monasterio et al., 1981; Shapiro et al., 1985; Curcio et al., 1991; Marc and Sperling, 1977; Martin et al., 2000; Wikler and Rakic, 1990) and S cones are absent from the central quarter degree of the human retina (Curcio et al., 1991; Williams et al., 1981a,b). The S-cone mosaic is customarily described as organized in quasi-hexagonal array (Marc and Sperling, 1977; de Monasterio et al., 1981; Shapiro et al., 1985; Wikler and Rakic,1990) but the exact pattern of the array is unlikely to be important for retinal wiring. In many New-World monkeys, and in human perifoveal retina, the S-cone mosaic is randomly organized, but the pattern of connections to the post-receptoral bipolar array and the properties of S cone-recipient neurons (see below) are preserved across all primates studied so far.
3.2. The M and L cones
The expression of M and L cones is governed by genetic machinery on the X-chromosome (Sharpe et al., 1999; Wang et al., 1999) that selects either the M- or L-cone opsin gene. Development of adaptive optics imaging techniques has permitted visualization and identification of the cones and any deviations from a random distribution appear to be minor in the majority of individuals (Hofer et al., 2005; Roorda et al., 2001). There is however substantial variability between individuals in the relative number of M and L cones (Carroll et al., 2009). There is a good correlation between relative numerosity determined by adaptive optics and that predicted on the basis of electrotretinographic measurements, which in turn is correlated with psychophysical estimates (Kremers et al., 2000). Fig. 3C shows an example of such distributions (Carroll et al., 2009). Hofer et al. (2005) specifically addressed the question of inter-individual variation in cone proportion. They show that individuals with “abnormally” high proportion of M or L cones contain clumps of the more commonly expressed cone type, but for the majority of individuals the distribution of M and L cones shows only minor deviations from randomness. Implications of these facts for receptive field organization are taken up in a later section.
3.3. Cone connectivity
Gap junctions between neighboring cones were first demonstrated anatomically (Raviola and Gilula, 1973) and later functional connectivity was demonstrated (Hornstein et al., 2004, 2005). Anatomically, it appears that S cones make few contacts with other cones in the ground squirrel (Li and DeVries, 2004). The situation appears to be similar in the primate (O'Brian et al., 2004; Massey, 2008). Hornstein et al. (2004) calculated that crosstalk between M and L cones would cause a moderate decrease in chromatic sensitivity with a small increase in luminance sensitivity due to an improvement in signal-to-noise ratio. On the other hand, there is evidence that individual M and L cones can adapt independently of their neighbors (Lee et al., 1999; MacLeod et al., 1992), which is not consistent with substantial crosstalk. This conundrum has led to the suggestion that resistance of cone-to-cone gap junctions is low at low light levels (to permit a better signal-to-noise ratio), but high at higher light levels (to improve chromatic selectivity) (Tsukamoto et al., 1992). This is an attractive hypothesis but direct tests have not been made.
3.4. Rod pathways
In the specialized scotopic (night vision) pathway, rod bipolar cells receive input from rod photoreceptors and provide output to GABAergic amacrine cell types and the glycinergic AII amacrine cell. The AII amacrine cell in turn contacts off bipolar and off ganglion cells via inhibitory synapses and on bipolar cells via sign-conserving gap junctions (Kolb and Famiglietti, 1974). In this way, signals from rod photoreceptors can feed into on and off cone pathways. Gap junction coupling between rods and cones provides a second pathway, likely active at mesopic light levels (Schneeweiss and Schnapf, 1995; Sharpe et al., 1989). These rod pathways are well conserved across mammalian retinas studied so far (Wässle, 2004). A third rod pathway involving contacts between rods and off cone bipolar cells has been found in a number of mammals (Hack et al., 1999; Li et al., 2010, 2004; Pang et al., 2010; Tsukamoto et al., 2001; Wässle et al., 2009) but has not yet been detected in primates.
Physiological evidence shows strong rod input to the MC pathway, but weak contribution of rod signals to the PC pathway (Lee et al., 1997; Purpura et al., 1988; Wiesel and Hubel, 1966). Data from in vitro recordings also indicate rod input to peripheral small bistratified cells, at light levels predicted to produce photoisomerisation rates equivalent to scotopic conditions for the intact eye (Crook et al., 2009; Field et al., 2009). An earlier in vivo study had found less rod input to more centrally located cells (Lee et al., 1997).
Anatomical studies in macaque retina show the postsynaptic targets of AII cells involve different types of off bipolar cells; namely off midget bipolar (PC pathway) and DB3 cells (MC pathway) (Grünert, 1997; Grünert and Wässle, 1996). The AII array likely is the sampling matrix which sets the limit for scotopic acuity (Mills and Massey, 1999; Wässle et al., 1995). However, the connectivity between AII cells and off bipolar cells has not been evaluated quantitatively, and thus the question of whether the differences seen in contribution of rod signals to PC, MC, and blue-on pathways are correlated to anatomical differences in the synaptic connectivity remains open.
Recent data obtained from in vitro recordings in mice and guinea pig retinas show that AII cells are active at high light levels, and may amplify the response range of off-type ganglion cells by “pushepull” disinhibition (Manookin et al., 2008; Pang et al., 2002). This raises the intriguing possibility that synapses from AII cells to PC pathway bipolar cells could contribute to chromatic opponency by a “pushepull” mechanism but a direct experimental test of this possibility in primate retina is still lacking. Interestingly, in the owl monkey, rod input usually dominates the visual response in all cell types, even at photopic (2000 td) light levels (Silveira et al., 2000). As the owl monkey has a single cone type, study of rodecone interactions through AII amacrine cells and off bipolar cells would be feasible and of interest in this species.
4. Horizontal cells as an example of connectional specificity
Horizontal cells in most mammals are generally considered to fall into two types. They may be designated in different ways, for example type A and B (in the cat) and Type H1 and H2 (in primates). Cross-order homology between these types is not clear, although often just one supports an axonal arbor making connections to rods (Class B in cat retina; Type I in primate retina). There appears to be a qualitative difference in horizontal cell function between mammalian and other vertebrates. In the latter, the first stages of chromatic processing occur in outer retina, so that some cell types are color opponent (excited by some wavelengths and inhibited by others), but in the former (at least in primates) horizontal cells are cone specific but not spectrally opponent (Dacey et al., 1996; Kolb and Nelson, 1995).
Horizontal cells in monkey retina show connectional and functional specificity. H1 cells are strongly hyperpolarized by luminance increments, and also by an increase in excitation of the M and L cones. The underlying anatomy reconstructed after neurobiotin injection indicates that H1 cells contact certain cones densely, but others are avoided; it has been shown through immunocytochemical staining that the non-contacted cones are the S cones (Chan and Grünert, 1998; Goodchild et al., 1996). The selectivity is not complete; occasional contacts are made to the S cones, but S-cone responses are not detected in intracellular recordings from H1 cells (Dacey et al., 1996). The sparse S-cone connections therefore are either functionally insignificant or have their effect only locally within the dendritic tree of the H1 cell, and are not detected in recordings from cell somata.
In contrast to the connections of H1 cells, the H2 horizontal cells make strong contacts with S cones as well as sparser contacts with M and L cones. Consistent with the anatomical wiring, H2 cells show vigorous responses to S-cone modulation and also respond to M- and L-cone modulation. All responses are hyperpolarizing; there is cone selectivity but no opponency.
The physiology of H1 horizontal cells has been subject to extensive study (Dacey et al., 2000; Dacheux and Raviola, 1990; Lee et al., 1999, 2003; Smith et al., 2001; Verweij et al., 1999). Recording from H2 cells is difficult and fewer data are available. Nevertheless, the specificity with which S cones are avoided by H1 cells and targeted by H2 cells indicates a role in chromatic processing.
5. The S cone pathways
5.1. S-Cone bipolar cells
The sparseness of the S-cone array was exploited in an early study of primate retinal connectivity. Mariani (1984) identified in Golgi preparations a bipolar cell “selective for the cones likely to be blue-sensitive”, because dendrites of these bipolar cells course horizontally through the outer plexiform layer to reach the position of putative S-cone pedicles. The ‘blue’ cone bipolar cell array is the dominant or exclusive source of on-type (invaginating, metabotrobic glutamate receptor type mGluR6) bipolar contacts with S cones (Herr et al., 2003; Kouyama and Marshak, 1992; Luo et al., 1999; Wässle et al., 1994a). Although not directly demonstrated in primates, the conclusion that S-cone bipolar cells transmit on-type signals to the inner plexiform layer is logically compelling. Existence of a cone-opponent surround mechanism in S-cone bipolar cells is likely (Packer et al., 2010), and implied by recent recordings from ganglion cells (Crook et al., 2009). This surround itself may be inherited from the S cones, which show ML inhibitory surrounds (Packer et al., 2010).
5.2. Other bipolar cell classes
Diffuse cone bipolar cells contact multiple cone photoreceptors. Analysis of Golgi preparations (Boycott and Dowling, 1969; Boycott and Wässle, 1991; Hopkins and Boycott, 1997) suggested indiscriminate contacts with all cones. On the assumption that each cone-to-bipolar synapse carries the same functional weight, this anatomical result would predict 5e10% functional input from S cones to diffuse bipolar cells. But more recent results reveal a subtle bias in connections, whereby diffuse bipolar cells studied so far make fewer contacts with S cones than with M and L cones (Lee and Grünert, 2007; Lee et al., 2004). Thus, the functional strength of S-cone inputs to downstream visual pathways should be very low: the S cones form only a small proportion of cones, and diffuse bipolar connections with S cones are weaker than connections with M and L cones.
The question whether S cones provide strong off-type signals to any bipolar class has not been resolved conclusively. Ultrastructural analysis of a patch of foveal macaque retina showed five presumed S cones which made contact with off-type (flat contacting (Kolb et al., 1969)) midget bipolar cells (Klug et al., 2003). A study of marmoset retina however failed to reveal contacts between immunolabelled off-midget bipolar cells and immunolabelled S cones (Lee et al., 2005), and an ultrastructural study of a single S cone in peripheral human retina likewise failed to find contact with off-midget bipolar cells (Kolb et al., 1997).
Haverkamp et al. (2001) compared S with M/L cones in macaque retina and found no difference between S and M/L cones with respect to the expression of (off-type) AMPA receptor subunit GluR1 on-bipolar cell membranes facing cones. Because midget bipolar cells express this subunit, the result implies that off-midget bipolar cells contact S cones in macaque. By contrast, Puller et al. (2007) studied marmoset retina and found that M/L cones, but not S cones, were associated with the AMPA receptor subunit GluR1. This implies that midget bipolar cells do not contact S cones in marmoset.
The foregoing results could mean that New World and Old World monkeys have different retinal circuitry serving color vision. But they must be interpreted with caution: the GluR1 label at S cones of macaques could arise from non-midget bipolar cells. In all other respects measured so far the retinal circuitry in marmosets and macaques is functionally identical, with any differences attributable to higher cone photoreceptor density in marmoset.
5.3. Horizontal cells and the S-cone pathway
As discussed above each H2 cell makes substantial connections with each S cone in its dendritic field and much sparser connections with M and L cones (Ahnelt and Kolb, 1994; Chan and Grünert, 1998; Dacey et al., 1996; Goodchild et al., 1996). This strong connectional bias is largely counteracted by the numerical dominance of M and L cones. The upshot of this is that the H2 cells are to date the only retinal element showing additive summation of (L + M + S) spectral inputs. By analogy with the demonstrated contribution of horizontal cells to the inhibitory surround of bipolar cells (Perlman et al., 2003; Werblin and Dowling, 1969) the inhibitory action of H2 cells would be expected to influence S cones to a greater extent than M and L cones (because the S cones make much stronger connections to H2 cells). This is the most likely explanation for cone-opponent spatial receptive field characteristic in S cones and blue cone bipolar cells (Packer et al., 2010; Verweij et al., 2003). As discussed above (Section 4), the connectivity of H2 cells with all cone types stands in contrast to the connectivity of H1 cells, which contact almost exclusively M and L cones and show no functional sign of S-cone input (Dacey and Lee, 1994). This is one piece of evidence (more is summarized below) that the S-cone pathway constitutes a primordial color pathway in the retina.
5.4. S-Cone ganglion cells
5.4.1. Small bistratified cells: morphology
Polyak (1941) and Boycott and Dowling (1969) described shrub ganglion cells with a dendritic extent close to that of parasol cells. The dendrites of shrub cells are “sprinkled with a few thornlike and hook-shaped shoots and buds” and resemble “the runners of a climbing plant” (Polyak, 1941: p. 314). Polyak's shrub cells are almost certainly the cells later classified as small-field bistratified cells (Dacey, 1993a; Rodieck, 1991) and shown to display blue-on/ yellow-off response characteristics in macaque retina (Dacey and Lee, 1994). Homologous cells have been described in marmoset and capuchin monkeys (Ghosh et al., 1997; Silveira et al., 1999; Szmajda et al., 2008) consistent with the idea that this pathway is preserved across diurnal simian primates. It is noteworthy that in the nocturnal simian owl monkey Aotus, which lacks S cones (Jacobs, 1993; Levenson et al., 2007), the small bistratified cell may also be absent (Yamada et al., 1996a, 2001).
5.4.2. Small bistratified cells: function and circuitry
When measured through the natural optics, blue-on/yellow-off receptive fields show approximately matched spatial regions yielding on and off response sign (Crook et al., 1987; Derrington and Lennie, 1984; DeValois et al., 1966; Dreher et al., 1976; Tailby et al., 2008a,b; Wiesel and Hubel, 1966); this arrangement traditionally has been explained as optimizing the receptive field for transmitting chromatic contrast (Ingling and Martinez-Uriegas, 1985; Solomon and Lennie, 2007; Wiesel and Hubel, 1966). Discrete spatial analyses in isolated retina however revealed “hot spots” of excitatory input likely attributable to individual S cones in the afferent array (Chichilnisky and Baylor, 1999). Likewise, responses to achromatic gratings of S-cone receptive fields in the LGN are consistent with non-concentric on- and off-regions (Tailby et al., 2008a,b). This suggests the canonical “Type II” organization (Wiesel and Hubel, 1966) may be accidental, that is, the cell draws input from roughly overlapping regions of the afferent array, and the otherwise punctate input from S cones is “smoothed” in the eye by axial chromatic aberration (Chichilnisky and Baylor, 1999; McLellan et al., 2002; Wyszecki and Stiles, 1967).
Anatomical evidence that blue cone bipolar cells provide the depolarizing (on) signal to blue-on cells is strong: the blue cone bipolar cells co-stratify with inner dendrites of the small bistratified cells in the b-sublamina (the “on” sublamina) of the inner plexiform layer, and bipolar synapses are present on these dendrites (Calkins et al., 1998; Ghosh and Grünert, 1999; Ghosh et al., 1997; Percival et al., 2009).
The presence of dendrites with bipolar input synapses in the outer (off) half of the inner plexiform layer is an obvious channel for yellow-off signals; diffuse-off bipolar cell classes might provide off-excitation. As outlined above, diffuse bipolar cells show bias against input from S cones, predicting a yellow-off response. Current data from in vitro recordings confirmed by pharmacological isolation off-excitation to small bistratified cells (Crook et al., 2009), are consistent with diffuse-off bipolar cell input. On the other hand there is also evidence that the yellow-off subfield of small bistratified cells is larger than the blue-on subfield (Field et al., 2007). The receptive field structure of small bistratified cells may be the deceptively simple result of complex inputs, if both blue cone and diffuse bipolar cells show M + L surrounds (Crook et al., 2009; Field et al., 2007). These surrounds have opposite sign and should largely show mutual annihilation under most stimulus conditions.
In summary, all data suggest that blue-on cells are the major afferent channel for excitatory S cone signals. There is good evidence that off-excitatory inputs from M L cones is derived from diffuse bipolar cells, but surround components + deriving from horizontal cells may well also contribute.
5.4.3. Other S cone ganglion cells
At least two additional ganglion cell populations may receive substantial functional input from S cones, but in comparison to blue-on/small bistratified cells, little is known about these populations.
Dacey et al. (2003) labelled multiple populations of ganglion cells by retrograde tracing from the pretectal nucleus and lateral geniculate complex. They identified pretectal-projecting cells which were later revealed to show melanopsin-based intrinsic photosensitivity (Gamlin et al., 2007). A recorded sample of seven melanopsin cells all showed S-cone off/M,L-cone on responses (Dacey et al., 2005). One published recording from a large sparse monostratified ganglion cell shows an S-cone off/yellow on characteristic (Dacey and Packer, 2003) but the relation of this cell type to melanopsin cells and small bistratified cells is not clearly established. The melanopsin ganglion cells are unusual in that both inner- and outer-stratifying subtypes show on-type response to 550 nm lights (Dacey et al., 2005), yet whether both subtypes show S-off chromatic response property has not been established. Dacey et al. (2003) showed one further example of S-cone input cells: a large bistratified cell showing a blue-on/yellow-off response characteristic. The population properties of these cells have not been established with certainty; large blue-on/yellow-off fields were not reported in a multi-electrode array study suggesting that factors such as electrode bias may impede study (Petruska et al., 2007).
One electron microscopic study (Klug et al., 2003) reports that S cone-contacting midget bipolar cells (see above) make contact with midget ganglion cells. This predicts the existence of small-field, S-off ganglion cells, but as noted above no physiological study has so far reported small-field S-off receptive fields near the fovea. Physiological studies in retina (Dacey and Packer, 2003) and LGN (Szmajda et al., 2006; Tailby et al., 2008b) describe only large-field S-off receptive fields so the functional correlate of this anatomical circuit in the fovea is unclear.
Recordings made in vitro from ganglion cells in peripheral macaque retina (Field et al., in press) showed S-cone input to off-midget ganglion cells, but at a functional strength (on a cone-by-cone basis) below the strength of M-cone and L-cone inputs. In peripheral macaque retina the midget ganglion cells receive input from multiple bipolar cells, and midget bipolar cells contact multiple cones. This means the dominant input to off-midget ganglion cells is from M cones and L cones; a situation quite different to the anatomical prediction of a “private line” for S-off signals. The occasional connections from S cones to midget bipolar cells described by Lee et al. (2005) in marmosets may account for the S cone input seen by Field et al. (in press). In summary, the key question whether S cones contact “private line” midget ganglion cells in central retina has not been addressed physiologically, and remains an outstanding question for understanding color vision circuitry in primate retina.
5.5. S-Cone circuits in other mammals
Because most mammals show dichromatic color vision (Jacobs, 1993; Nathans, 1999) the blue/yellow opponent pathway has been described as the basic or primordial pathway for color vision (Mollon, 1991). On-type inputs to homologous blue cone bipolar classes in mouse and ground squirrel retina derive exclusively from S cones (Haverkamp et al., 2005; Li and DeVries, 2006). An offstratifying type bipolar cell selective for S cones was reported in rabbit retina (Liu and Chiao, 2007) but no homologous cell type has been reported for any other species so this intriguing finding remains isolated.
On-sign S-cone responses have been recorded in ground squirrel optic nerve (Jacobs et al., 1981; Jacobs and Tootell, 1981) and cat and rabbit retina (Cleland and Levick, 1974; Vaney et al., 1981); off-sign S-cone responses have been recorded in ground squirrel optic nerve (Jacobs et al., 1981; Jacobs and Tootell, 1981) and tammar wallaby Macropus eugenii retina (Hemmi et al., 2002). The question whether a ganglion cell homologous to the small bistratified cell is present in other diurnal mammals remains however unresolved. Recent recordings made in vitro from guinea pigs show rarely-encountered blue-on/yellow-off opponent cells with monostratified fields (Yin et al., 2009). However as outlined above the functional and anatomical specificity of blue cone bipolar cell appears to be preserved between mouse, ground squirrel and primate retina, so how S-cone signals could reach this cell type remains unknown.
6. Parasol cells
6.1. Cone connectivity to the receptive field
The connectivity of parasol ganglion cells to cones through diffuse bipolar cells provides an instructive example of retinal connectional specificity; connectivity is not random, but is not completely specific. There are six types of diffuse bipolar in the primate retina (Boycott and Wässle, 1991), of which three (DB1-3) are likely to be off-type bipolar cells, and the other three (DB4-6) are likely to be on-type bipolar cells. Which of these bipolar types provide input to the parasol cells is not fully established. Boycott and Wässle (1991) noted that on- and off-center parasol cells stratify near the boundary between the inner and outer sublaminae of the IPL, which corresponds to the axonal ramifications of DB4 and DB3 bipolar cells respectively. Definitive evidence of connectivity to on-parasol cells is still wanting but connections of off-parasol cells to DB3 cells has been confirmed by electron microscopy (Jacoby et al., 2000).
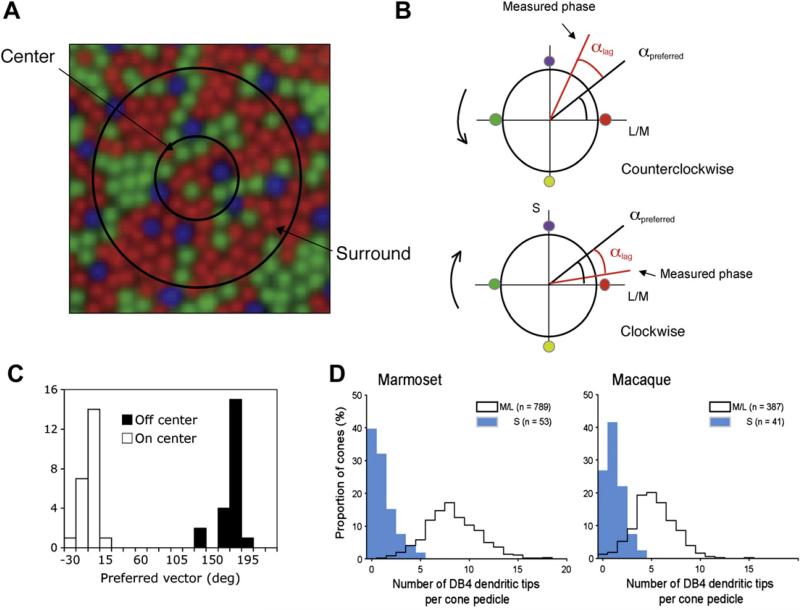
Ganglion cells’ center size is usually well-correlated to the diameter of the dendritic tree (Wässle and Boycott, 1991). Under this assumption, the receptive field center size of parasol cells near the fovea would appear to be ~6–8 cones in diameter. If the cone inputs derive randomly from the underlying M- and L-cone mosaic, then some variability in L/M cone weighting would be expected (possible S-cone input is addressed below). This can be visualized in Fig. 4A, where the size of a parasol (MC) cell dendritic tree has been superimposed on a cone mosaic, with circles representing putative center diameter; assuming a Gaussian profile of input strength, the central-most cones should have greatest weight. Consistent with this prediction, variability of spectral sensitivity of parasol ganglion cells is observed (de Monasterio and Schein, 1980; Valberg et al., 1992).
Fig. 4.
A. Sketch of hypothetical parasol cell receptive field on the foveal cone array. Center (and surround) would be expected to receive a mixed M, L cone input, with some variability in proportion depending on the precise location of in relation to the underlying cone mosaic. It would also be expected that some S cone input should be present, since S cones would sometimes be within the dendritic tree/receptive field. B. A method of determination of cone weighting by rotating the chromaticity of a stimulus field around the circumference of a cone space; the radii relative to the origin represent cone excitations. Rotation in clockwise and counter-clockwise directions makes it possible to compensate for response phase delays (Sun et al., 2006b). C. Distribution of parasol cell preferred vectors; on- and off-cells cluster around 0 and 180 . This is not consistent with a simulation of the weightings expected with random input from S cones (Sun et al., 2006a). D. Quantification of diffuse bipolar cell bias against S-cone connections. The histograms show for (left) marmoset and (right) macaque monkeys the number of putative flat synaptic contact points between diffuse bipolar cell type 4 (DB4) with short-wavelength sensitive (S, blue shading) cones and medium/long wavelength sensitive (M/L, solid line) cones. Data obtained from horizontal sections where DB4 cells were identified by protein kinase C immunoreactivity, cones were labelled with peanut agglutinin. For details see Lee and Grünert (2007).
The possibility of S-cone input to parasol cells has recently been the focus of attention. Physiological evidence had suggested that activity in the parasol-magnocellular pathway underlies the photopic luminosity function (Kaiser et al., 1990; Lee et al., 1988), which does not include an S-cone contribution (Smith and Pokorny, 1975). However, it was recently proposed that magnocellular cells receive S-cone input, consistent with random sampling from the cone mosaic (Chatterjee and Callaway, 2002). To re-examine the possibility of S-cone input to parasol cells, a novel method was developed to estimate cone weights (Sun et al., 2006b) and sketched in Fig. 4B. The result showed no evidence for S-cone input to parasol cells; on and off-center cells cluster around +/-180° (Fig. 4C; see Sun et al., 2006a). Thus, under normal conditions, S-cone input to the parasol-magnocellular pathway cells is functionally insignificant. The anatomical evidence however shows limited S-cone input to diffuse bipolar cells. Fig. 4D shows histograms of DB4 contacts to S and M,L cones in macaque and marmoset. Although many S cones are avoided, some get a limited number of contacts. Thus the retina is not concerned with absolute, but only functional specificity. With a similar approach, it was shown that midget, PC ganglion cells also lack functional S-cone input (Sun et al., 2006b). Midget PC cells may avoid S-cone input to preserve the M,L-cone opponent signal, but why parasol cells should avoid S-cone input is less certain. Such bias of midget and parasol cells against S-cone input might be connected to spatial processing.
6.2. Connectivity and parasol cell surrounds
Centers of ganglion cell receptive fields have a ready anatomical substrate; connectivity responsible for receptive field surrounds is less obvious. A major role for surround antagonism inherited from outer retina for primate parasol cells was proposed by McMahon et al. (2004), who used carbanoxolone and other blocking agents in order to dissect outer and inner retina contributions. However, there is also evidence from other mammals that surrounds derive from both inner and outer retinal components (Flores-Herr et al., 2001; see Lukasiewicz, 2005 for review). In addition, parasol cells show evidence of non-classical surround effects, as well as contrast gain control mechanisms (Solomon et al., 2006), and a chromatic input to the surround mechanism explained in detail below.
The MC pathway delivers a small response to red–green sinusoidal alternation at twice the stimulus frequency (2F response) (Lee et al., 1989), and an excitatory response to both directions of movement of an equiluminant red–green border (Kaiser et al., 1990; Schiller and Colby, 1983). In addition, when changing the relative phase of slowly (1e5 Hz) modulated red and green lights, a response minimum of MC cells occurs not to chromatic modulation (with the lights out of phase) but at an intermediate value. This latter property can be modeled by assuming a chromatic input to the cells (Smith et al., 1992). Recent evidence (Lee and Sun, 2009) suggests that both effects derive from a rectified chromatic signal. These receptive field features have plausible functional consequences for vision. Cells in the middle temporal area of visual cortex receive an ‘unsigned’ motion signal to isoluminant chromatic targets (Dobkins and Albright, 1994), as might be provided by the MC-pathway 2F response. A similar motion signal can also be demonstrated psychophysically (Dobkins and Albright, 1993). In addition, there is much evidence that moving chromatic patterns affect luminance motion mechanisms (Cavanagh and Favreau, 1985; Cropper and Wuerger, 2005; Derrington and Badcock, 1985; Mullen and Baker, 1985). It is thus possible that the MC 2F response is a means of extracting motion signals from red–green patterns close to equiluminance.
6.3. Cross-order homology for parasol cells
The transient responses of parasol-MC cells led to the suggestion that they were homologous to the Y cells of the cat (Dreher et al., 1976) and this suggestion received support from other sources (de Monasterio, 1978). However, most MC cells show linear spatial summation: this finding and other functional considerations (Shapley and Perry, 1986) led the idea of homology between primate MC and cat Y cells into disfavor. Recently this hypothesis has however been revived (Crook et al., 2008b), based on measurements from the in vitro primate retina and the anatomical demonstration of a parasol cell projection to the superior colliculus. The in vitro measurements showed non-linearity of spatial summation for high spatial frequency gratings. On the other hand a smooth monostratified ganglion cell showing non-linear spatial summation in the in vitro preparation has also been described (Crook et al., 2008a) with a receptive field center 2e3 times the diameter of the parasol cell. This cell may correspond to the population previously identified by parallel electrode arrays in vitro as showing large fields with non-linear spatial summation (Petruska et al., 2007). In summary, drawing trans-order homologies remains a difficult task. Discussion of this issue often fails to distinguish functional homology (which was certainly implied in early reports, but now appears unlikely) and anatomical and evolutionary homology (which is possible but difficult to establish). The authors of this review are agnostic on this latter issue.
7. Midget ganglion cells
In trichromatic primates such as humans and Old World monkeys, the midget-parvocellular system is considered to transmit signals that support the red–green axis of color vision. Fig. 5A shows a sketch of the standard textbook models of midget-parvocellular receptive field structure in the foveal visual field. As explained below, the center is thought to derive dominant excitatory input from a single cone. The surround may either be cone specific, or receive a mixed cone input; both schemes would generate |M–L| described opponency. Both center and surround are with a Gaussian profile. Fig. 5B shows such a field structure superimposed upon a random cone mosaic; the dimensions of receptive field center and surrounds have been based on literature estimates (Derrington and Lennie, 1984). This receptive field can, in principle, transmit high-acuity signals from a single receptor and also transmit a chromatic |M–L| opponent signal.
Fig. 5.
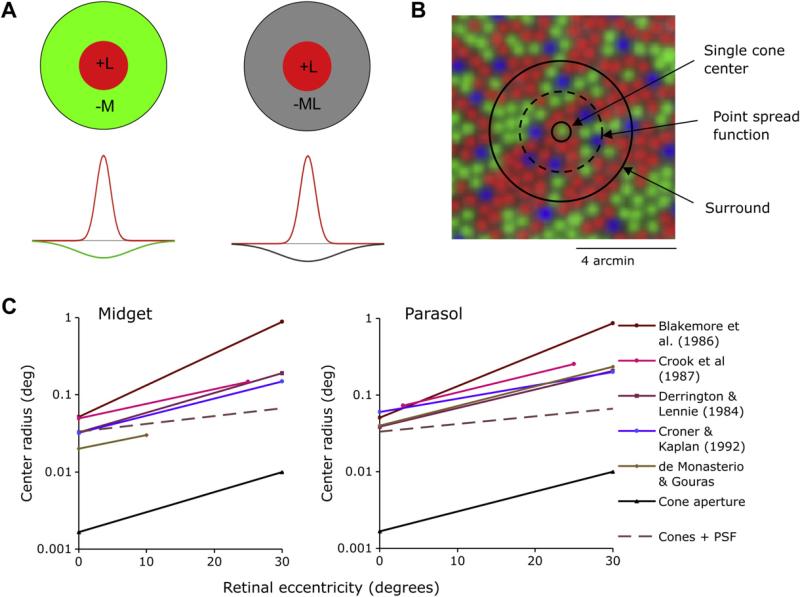
A. Possible receptive field structures of midget ganglion cells. The center, if derived from a single cone (L cone in the example shown), provides chromatic specificity, independent of the cone input to the surround, which may be cone specific or mixed. B. Projection of hypothetical midget ganglion cell receptive field structure upon the cone array near the fovea. A single cone center would be expected to yield a small receptive field center consistent with the cone aperture. Optical blur is expected to expand the center beyond the size of a single cone. C. Estimates of center size derived from the literature.
Textbook descriptions of the roles of PC and MC pathways rarely go beyond the description of the MC pathway as specialized for motion signaling and the PC pathway as serving high resolution spatial vision. It is clear that the PC pathway cells must play major role in spatial vision in the natural world, to code the complex spatio-chromatic-surface structure of natural scenes. However, PC pathway activity cannot support an achromatic spatial channel with spectral sensitivity corresponding to the luminosity function; its low achromatic contrast sensitivity argues against such a function. There have been attempts to show that an achromatic, luminance channel could be built out of PC cell activity (Ingling and Martinez-Uriegas, 1983) but it is not possible to construct an achromatic signal in, for example, the minimally distinct border paradigm by combining PC cell signals (Kaiser et al., 1990; Valberg et al., 1992). Also, hyperacuity tasks appear to rely on the MC pathway (e.g., Lee et al., 1995; Sun et al., 2004). We discuss this issue further in a later section, but it is largely beyond the scope of this review.
7.1. Connectivity and center structure near the fovea
The foveal midget system was first described by Polyak (1941) and further elaborated by Boycott and Dowling (1969) and Kolb (1970). Each cone in central retina contacts a single on and a single off-midget bipolar cell. Each cone makes about 20 triad invaginating synapses near the fovea, of which the majority probably derive from the associated on-midget bipolar cell (Calkins et al., 1996; Chun et al., 1996). Each on-bipolar and each off-bipolar makes dominant contact with one midget ganglion cell (Calkins et al., 1994; Jusuf et al., 2006b; Kolb and Dekorver, 1991). A chromatically specific signal is thus provided to the midget ganglion cell, in that either an M or an L cone is thought to provide dominant functional input to the receptive field centre.
This apparently simple picture has interesting functional consequences. Firstly, each on-center midget ganglion cell receives major input from a single cone (e.g., M), and its neighbors, depending on the cone distributions in the matrix, could receive input from the other cone type (i.e., L) and thus have different spectral characteristics. This type of heterogeneous functionality within a single ganglion cell array is not present, for example, in X and Y cells of the cat or primate parasol cells. Secondly, Peichl and Wässle (1981), and Wässle et al. (1981) showed that dendritic tree and receptive field center diameters roughly correspond in size for X and Y cells of the cat, i.e., the dendritic tree is the locus of spatial summation for the center. This relationship does not strictly apply to midget cells in and near the fovea, because center diameter should be limited by the sampling aperture of a single cone, which is small compared to dendritic tree diameter. Thirdly, ganglion cell classes usually show dendritic tree/receptive field center overlap, so that each point in visual space is covered by 3 or more receptive fields, a factor known as the coverage factor (Peichl and Wässle, 1979) Midget ganglion cells that receive input from a single cone, must have an anatomical coverage factor of one.
Physiologically, functional center size of midget cells must be increased by optical factors. Fig. 5B shows a cone mosaic, with cone density as present in the fovea; the midget center as derived from a single cone and the dotted circle represents the point spread function (Navarro et al., 1993), though it should be stressed that the point spread function is poorly represented by a Gaussian function (see, for example, Williams and Hofer, 2003). Consistently, functional estimates of midget center diameter have been larger than those expected of single cones. Fig. 5C (see Lee, 2004 for details) collates center Gaussian radius estimates for PC and MC cells from a number of studies of primate retina and LGN. All studies report the expected increase in center size with eccentricity. The PC cell centers are on average smaller than diameter of MC cell centers, by a factor of 0.7. The dashed curve indicates the expected relationship if sampling aperture of the cone as determined by MacLeod et al. (1992) is related to cone diameters derived from the literature (Packer et al., 1989). If optical blur is taken into account (Navarro et al., 1993) midget center size comes closer to the experimental estimates.
7.2. Retinal eccentricity and the midget system
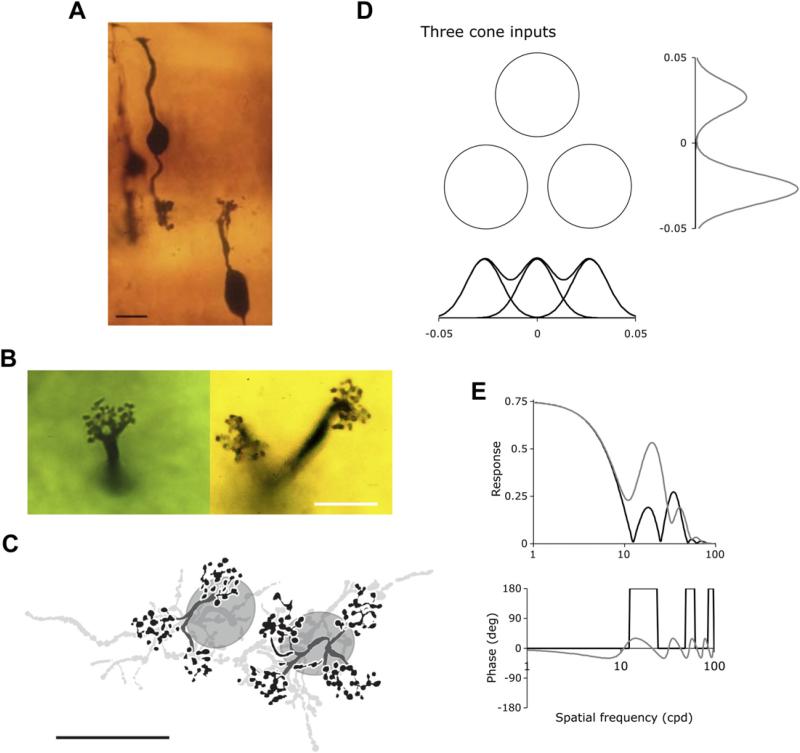
Fig. 6A shows a pair of midget bipolar and ganglion cells in close proximity (Fig. 6A and B from Boycott and Dowling, 1969; re-photographed and kindly provided by H. Wässle), characteristic of eccentricities up to ~10°. The one-to-one relationship between cones to midget bipolar cells persists to ~40° eccentricity after which some (but not all) on-midget bipolar cells may contact two or three cones (Fig. 6B); off-midget bipolars show convergence to a greater degree (Wässle et al., 1994b). Convergence from midget bipolar to ganglion cell begins at ~10°, and by 30–40° eccentricity a typical midget ganglion cell gets convergent input from over 30 cone photoreceptors. In the best-studied New-World primate (marmosets) the private line connectivity from cones to bipolar cells persists only to ~10° (Telkes et al., 2008). In the example from mid-peripheral (10–40°) marmoset retina shown in Fig. 6C an average of 8 midget bipolar cells converge onto a midget ganglion cell (Jusuf et al., 2006a). This ratio increases to an average of 13 midget bipolar cells per midget ganglion cell in far peripheral retina.
Fig. 6.
Cone convergence in the midget system. A, Vertical view of an OFF midget bipolar and an OFF midget ganglion cell near the fovea in a Golgi preparation of macaque retina. B, Horizontal view of a single-cone-contacting and a two-cone-contacting midget bipolar cell in peripheral macaque retina. Images shown in A and B kindly provided by Heinz Wässle. C, Drawings of OFF midget bipolar cells in peripheral marmoset retina. The cell on the left contacts three cones, the one on the right contacts four cones. Somata and axon terminal are drawn in light grey. Modified from Telkes et al. (2008, Fig. 2). Scale bar = 10 μm in A, B and C. D, With just a few cone inputs (3 in this example, as in C) potentially anisotropic receptive fields may occur, with more than one peak constituting the center. In the example shown, cone diameters and spacing have been derived from the literature (Packer et al., 1989) The horizontal and vertical profiles indicate expected receptive field center characteristics. E. Spatial frequency tuning curves for amplitude and phase would be expected to show irregularities with such a receptive field structure. Black and grey curves refer to horizontal and vertical gratings respectively.
Convergence from bipolar cells onto midget ganglion cells was invoked to account for the rapid decrease in red–green chromatic sensitivity with eccentricity in human observers (Mullen and Kingdom, 1996). Although broadly consistent with physiological measurements which show an increased proportion of non-opponent PC cells with increasing eccentricity (de Monasterio and Gouras, 1975; Diller et al., 2004; Solomon et al., 2005) detailed results are not consistent with this “random wiring” hypothesis. Firstly, red–green psychophysical sensitivity has decreased substantially by 10° eccentricity, where one-to-one connectivity is still intact. Secondly, some strongly opponent midget ganglion cells are found up to 30–40° eccentricity (Martin et al., 2001; Solomon et al., 2005). The details of midget ganglion cell properties at different eccentricities remain a puzzling and unresolved issue. Alreadyat 20–40° eccentricity about 20–30% of putative midget ganglion cells show little or no M,L opponency and in others the M,L cone balance showed more variability than in central retina (Solomon et al., 2005). Recordings in vitro, mainly at higher eccentricities, have failed to find much opponency (Diller et al., 2004), and it was suggested that the often anisotropic shapes of midget ganglion cell dendritic trees at this eccentricity represented connectional specificity to the underlying cone mosaic (Dacey,1993b; Martin et al., 2001), but dendritic trees of midget ganglion cells in dichromatic New-World primates are also anisotropic (Yamada et al., 1996b), and a recent study has shown no evidence of specific connectivity of midget ganglion cells in trichromatic or dichromatic marmosets (Jusuf et al., 2006a). In summary, chromatic responses of peripheral midget ganglion cells may be maintained by subtle changes in synaptic connections rather than on overall changes in cell morphology. Finally, it is noteworthy that dendritic trees of peripheral midget ganglion cells do not overlap (Dacey, 1993b), leading to an anatomical coverage factor around one as in central retina, rather than the higher coverage factors of parasol cells.
Anisotropic receptive field structure might be expected from cells in the 10–20° eccentricity range that receive input from just a few cones. Fig. 6C shows morphology of a midget bipolar cell that receives input from 3 cones, and Fig. 6 D and E shows analysis of the behavior expected. Three cones are drawn with the separation and size expected at 15° eccentricity (Packer et al., 1989). The receptive field profiles expected in the horizontal and vertical directions are drawn next to the cones, with cone sampling apertures calculated based on the analysis of MacLeod et al. (1992). The spatial frequency amplitude and phase plots expected for such a receptive field show complex orientation dependency due to aliasing of the grating with the individual cone inputs within the putative receptive field center. With increasing numbers of cones providing input, distortions continue to occur until the center derives from a patch ~5–6 cones across, with a Gaussian weighting across the center profile. With such a diameter, the center profile can be well approximated by a continuous Gaussian. However, midget ganglion cells at these intermediate eccentricities have not received detailed study, although some cells of this sort were described by de Monasterio and Gouras (1975; their Figure 15).
In summary, the spatial characteristics of responses of a cell with just a few cone inputs do not conform to the standard difference-of-Gaussians model. Under a linear model of neural information processing (Marr, 1982) this presents a problem for the midget system as the exclusive mediator of fine spatial signals. The possibility remains that IPL interactions and optical imperfections extend center size beyond that expected on an anatomical basis, but the question how well primate receptive fields really do conform to standard models derived from study of the cat visual system is worth renewed examination.
7.3. Surrounds – selective, random or partial selectivity?
Early studies of midget ganglion cell receptive fields used differential adaptation to isolate cone inputs (de Monasterio, 1978), and this gave rise to the hypothesis that the center (putatively derived from a single cone) and the surround were both cone selective. However, it was soon pointed out that a mixed surround would also generate opponency (Paulus and Kröger-Paulus, 1983) and this model later received a quantitative validation (Lennie et al., 1991). In outer retina, H1 horizontal cells receive mixed input from both L and M cones (Dacey et al., 1996), so any surround derived from the horizontal cell network is likely to be non-selective. There might be an inner retinal origin for surrounds, but there is no evidence for any cone-selective connectivity in inner retina (Calkins and Sterling, 1996; Jusuf et al., 2006a). But all this evidence is indirect. Direct physiological measurements in macaque have been consistent with cone specific or partially selective surrounds (Lee et al., 1998; Reid and Shapley, 1992, 2002), and as detailed below, measurements from trichromatic marmosets likewise showed partial selectivity in both center and surround mechanism (Buzás et al., 2006).
In summary, the attraction of the idea of mixed surrounds is that no wiring specificity is required; it would be an economical way of generating M,L-cone opponency. When two opsins developed during primate evolution, an opponent signal was presumably derived without specific wiring. Selective and mixed surrounds are often considered distinct alternatives, but partial selectivity is a possibility. Random, mixed surrounds degrade the opponent signal by decreasing signal-to-noise ratio in the presence of receptor noise (Lee, 2008). Partial selectivity significantly improves signal-to-noise ratio, but complete selectivity yields little further advantage.
7.4. Recent electrophysiological findings
Experiments that mitigate optical imperfections of the eye have also indicated that the standard concentric receptive field models are incomplete. McMahon et al. (2000) measured responses of foveal PC cells to achromatic gratings produced by interference fringes up to high spatial frequencies normally attenuated by the optics of the eye. The resultant spatial frequency tuning curves were complex and showed amplitude plots similar to those in Fig. 6, with one or more extra peaks at the high spatial frequencies. It is not possible to measure response phase reliably using this technique, and so this remains an intriguing result without a clear explanation.
More recently, adaptive optic techniques have been used to improve the eye's optics such that it is possible to target individual cones with a probe spot (Sincich et al., 2009). Recordings were obtained from parafovea, where a one-to-one relation between cone and ganglion cell center would be expected to hold. Their data show (their Fig. 1; ~4° eccentricity) that excitatory responses can be evoked from a patch 5–6 cones across. These data indicate that neural convergence enlarges center diameter beyond the size of a single cone, but the cell sample provided is limited.
A striking feature of M,L-cone opponency in PC cells is that the opponent inputs have similar weighting (Derrington et al., 1984; Lee et al., 1987), which may be optimal for visual coding (Buchsbaum and Gottschalk, 1983; MacLeod and von der Twer, 2003). The M- and L-cone distributions, although random in assignment, shows considerable local patchiness (Fig. 3). The relation of cone opponency to the underlying cone distributions remains unexplored, but might throw light on some of the unexpected receptive field features noted in the previous paragraph.
7.5. New-World primates; a test of retinal malleability
Old World primates possess a tandem gene array on the X-chromosome coding for the L- and M-cone opsins, whereas most New-World primates have only one opsin gene, but with several opsin alleles present in the population; males are thus obligatory dichromats whereas females with two different opsin genes on their two X chromosomes achieve trichromacy (Mollon, 1991). There are two main New-World primate groups, the callitrichids (marmosets and tamarins) and cebids (the squirrel and capuchin monkeys, amongst others). Most species show three alleles, but these differ between the callitrichids and cebids and there are some exceptions to this rule (Jacobs, 2008). The presence of two different opsin genes in female squirrel monkeys lead to their expression in different cones and behavioral trichromacy (Bowmaker et al., 1985; Jacobs, 1983b; Mollon et al., 1984).
The midget-parvocellular and parasol-magnocellular pathways are readily identified in both New and Old World primates studied so far, including monochromatic nocturnal New-World monkeys, and the monochromatic nocturnal prosimian bushbaby (Silveira et al., 1994, 2004a; Yamada et al., 1996a,b, 1998, 2001; Lima et al., 1996; Ghosh et al., 1996). Electrophysiological data showed that M,L-cone opponent neurons could readily be found in the LGN or retinae of trichromatic females, whereas in dichromatic animals cells PC or midget cells were ‘color-blind’ versions of such cells in trichromats (Blessing et al., 2004; Jacobs, 1983a; Lee et al., 2000; Yeh et al., 1995). Analysis of parvocellular cell responses in trichromatic marmosets (Buzás et al., 2006) showed wide variation in M,L cone weighting in center and surround, but consistent bias of opposing cone types to center and surround. Thus, in this species as in macaque (see above) a partially cone selective receptive field appears adequate to transmit signals supporting red–green color vision.
As outlined above, the organization of all retinal pathways studied so far shows great similarity between New World and Old World primates. Differences in detail can be primarily attributed to differences in the density and distribution of the cone and rod receptor arrays, which in turn can be related to the habitats of different species. Where examined, no anatomical differences between dichromatic and trichromatic members of the same species have been detected, consistent with the idea that the fundamental wiring of the retina is not altered by the pattern of expression of M:L-cone opsins (Ghosh et al., 1996; Jusuf et al., 2006a,b; Wilder et al., 1996).
There are two exceptions to the standard New-World primate pattern. The owl monkey (Aotus) is the only nocturnal simian primate. It appears to have separated from the cebid branch about two million years ago (Martin, 1990). It is a cone monochromat (Jacobs et al., 1993a), possessing only one middle-wavelength pigment and having lost the short-wavelength opsin (Jacobs, 1993; Levenson et al., 2007). The foveal depression is usually lost or minimal, and rods have invaded the central fovea (Silveira et al., 1993; Finlay et al., 2008; Yamada et al., 1996c). Nevertheless, the midget morphology appears to be maintained. Electrophysiological studies have shown that PC and MC cell responses are similar to those in other New-World primates (O'Keefe et al., 1998; Usrey and Reid, 2000). The howler monkey is the only New-World monkey described so far in which males show full trichromacy (Jacobs et al., 1996), a gene duplication having occurred to provide two opsins on the X-chromosome. In howler monkeys, opsins are still selectively expressed (Saito et al., 2004) despite the duplication of the locus control regions customarily considered to control M,L gene expression. How opsin expression is controlled in howler monkeys remains unresolved. Electrophysiologically, howler monkey retinal ganglion cells show standard M,L opponency (Saito et al., 2004). Anatomically, the howler monkey retina conforms to the standard pattern, except for a high cone density in the fovea (Silveira et al., 2004b).
In summary two main results have emerged from study of New-World monkeys. Firstly, distinct mechanisms of M,L cone expression yield consistent red–green opponent properties in parvocellular cells, without obvious changes in retinal morphology. Second, in trichromatic New-World monkeys, opponent responses appear as an extra dimension on the small, low contrast gain receptive fields of parvocellular cells.
7.6. Concluding remarks
Polarized debate as to the origin of the midget-parvocellular system was initiated over 20 years ago. Shapley and Perry (1986) proposed that the midget, PC system arose through a “hyper-plasic enlargement” of a color-selective ganglion cell class separate from the X- and Y-like groups present in most mammals. Alternatively, Wässle and Boycott (1991) hypothesized the midget system derived from a high-resolution system for purposes of spatial vision. The strong form of the latter hypothesis would be that the one-to-one midget connectivity was already present in the primordial system (and automatically provided cone selectivity as a substrate for an M,L opponent signal when dual opsins evolved). The weak form would be that the original small-field system input from just a few cones, and after evolution of two opsins, random distribution of cones in the PC array meant that some cells had some degree of chromatic selectivity; the selective advantage so conferred would lead to reduction of cone convergence, leading to midget morphology. The authors of this review are divided as to the relative merits of these scenarios. Both account for the fact that two physiological classes of ganglion cell (L and M on- or off-center) are mixed in the same ganglion cell array. Cortical mechanisms must disentangle these signals; how this is achieved is unknown.
When comparing PC pathways and behavior, some discrepancies are apparent. For example, PC cells respond to chromatic modulation at high temporal frequencies (30–40 Hz), whereas psychophysical sensitivity decreases steeply above ~4 Hz, so that PC pathway signals do not reach conscious perception. These examples illustrate limitations in trying to correlate retinal anatomy and physiology with function; properties of central mechanisms, such as dorsal and ventral pathways, become entangled with properties of ganglion cells and linking physiology and psychophysics becomes more challenging.
If the midget system derived from a high resolution spatial system, was it then taken over for chromatic processing or does it maintain a critical role for precision spatial vision? From a functional viewpoint, as summarized in the foregoing sections, an intermediate scenario may be more likely than either of these extreme views. Rather than providing an exclusive subcortical pathway for either color or spatial signals, the PC pathway provides spatial signals in dichromatic primates, with color signals appearing as an extra response dimension in trichromatic primates. Alternatively, the different temporal properties of PC- and MC-pathways may indicate a concentration on different aspects of spatial structure, those based on surface characteristics or contours respectively.
8. Conclusions and future directions
Recent insights into the workings of the retina have received a major stimulus from an explosion of new techniques for investigating connectivity and function, for example transgenic and related molecular approaches. Ironically the great majority of such studies are of mouse retina: arguably a poor mammalian model for understanding human vision and visual dysfunction. However, recent application of adenovirus transfection techniques to primate retina (Ivanova et al., 2010) holds great promise for applying advances made in mouse retina to primates. Research on non-human primates remains however difficult (and expensive), and supply of tissue is appropriately and stringently limited. In another direction, recent techniques for recording from multiple ganglion cells with multi-electrode arrays using in vitro methods (e.g., Petruska et al., 2007; Shlens et al., 2006) have produced important basic results and new insights, but there remain important differences to the in vivo recording situation (most importantly, lack of natural optics and possible compromise of the retinal tissue) which make these data difficult to relate to visual performance in the same way as traditional recording methods. On the other hand, the spectacular success of adaptive optics in application to longstanding problems such as the organization of the cone mosaic and more recently to receptive field organization (Roorda et al., 2001; Roorda and Williams, 1999; Sincich et al., 2009) mean that in vivo and in vitro approaches are finding common ground at least in regard to spatial resolution.
From a functional perspective, the primate retina is better understood than that of many other species, but this has made lacunae in our understanding more striking. One issue of significance is how synchronization between neighboring ganglion cells (predominantly MC cells (Shlens et al., 2006)) affects the sampling of the retinal image by the ganglion cell array. Another is the receptive field structure of cells of the midget/PC pathway, and its relation to eccentricity, which seems to be more complex than originally thought.
In conclusion, this review spans a wide range of topics, from receptor connectivity to the behavioral significance of retinal signals. The retina may adhere to common principles of organization, with common mechanisms of connectivity and physiology, in many different species, but the relative weighting of, for example, different mechanisms determining ganglion cell receptive field structure, may vary widely from species to species depending on environmental requirements. We have tried to stress in this review that nuanced, quantitative approaches are required to resolve such questions, seldom with black-and-white solutions. This is perhaps a cautionary message for the retinal neurobiologist.
Acknowledgements
Preparation of this review was partially supported by NEI EY13112 (BBL), NHMRC 56558 (PRM) and NHMRC 632640 (UG).
References
- Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in human retina: a Golgi-electron microscopic study of spectral connectivity. J. Comp. Neurol. 1994;343:406–427. doi: 10.1002/cne.903430306. [DOI] [PubMed] [Google Scholar]
- Ahnelt PK, Kolb H. The mammalian photoreceptor mosaic-adaptive design. Prog. Retin. Eye Res. 2000;19:711–777. doi: 10.1016/s1350-9462(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Blessing EM, Solomon SG, Hashemi-Nezhad M, Morris BJ, Martin PR. Chromatic and spatial properties of parvocellular cells in the lateral geniculate nucleus of the marmoset (Callithrix jacchus). J. Physiol. 2004;557:229–245. doi: 10.1113/jphysiol.2003.058065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker JK. Evolution of vertebrate visual pigments. Vision Res. 2008;48:2022–2041. doi: 10.1016/j.visres.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Bowmaker JK, Jacobs GH, Spiegelhalter DJ, Mollon JD. Two types of trichromatic squirrel monkey share a pigment in the redegreen spectral range. Vision Res. 1985;25:1937–1946. doi: 10.1016/0042-6989(85)90018-5. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Dowling JE. Organization of the primate retina: light microscopy. Phil. Trans. Roy. Soc. Lond. B. Biol. Sci. 1969;255:109–184. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J. Physiol. 1974;240:397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. Morphological classification of bipolar cells of the primate retina. Eur. J. Neurosci. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Buchsbaum G, Gottschalk A. Trichromacy, opponent colours coding and optimum colour information transmission in the retina. Proc. Roy. Soc. Lond. B. 1983;220:89–113. doi: 10.1098/rspb.1983.0090. [DOI] [PubMed] [Google Scholar]
- Bumsted K, Jasoni C, Szel A, Hendrickson A. Spatial and temporal expression of cone opsins during monkey retinal development. J. Comp. Neurol. 1997;378:117–134. [PubMed] [Google Scholar]
- Buzás P, Blessing EM, Szmadja BA, Martin PR. Specificity of M and L cone inputs to receptive fields in the parvocellular pathway: random wiring with functional bias. J. Neurosci. 2006;26:11148–11161. doi: 10.1523/JNEUROSCI.3237-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR. La retine des vertebres. La Cellule. 1893;9:17–257. [Google Scholar]
- Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones in macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses. Nature. 1994;371:70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Sterling P. Absence of spectrally specific lateral inputs to midget ganglion cells in primate retina. Nature. 1996;381:613–615. doi: 10.1038/381613a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Tsukamato Y, Sterling P. Foveal cones form basal as well as invaginating junctions with diffuse ON bipolar cells. Vision Res. 1996;36:3373–3381. doi: 10.1016/0042-6989(95)00333-9. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Tsukamoto Y, Sterling P. Microcircuitry and mosaic of a blue–yellow ganglion cell in the primate retina. J. Neurosci. 1998;18:3373–3385. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J, Yoon GY, Williams DR. The photoreceptor mosaic in normal and defective color vision. In: Gazzinaga MS, editor. The Cognitive Neurosciences. fourth MIT Press; Boston: 2009. pp. 383–394. [Google Scholar]
- Cavanagh P, Favreau O. Color and luminance share a common motion pathway. Vision Res. 1985;25:1595–1601. doi: 10.1016/0042-6989(85)90129-4. [DOI] [PubMed] [Google Scholar]
- Chan TL, Grünert U. Horizontal cell connections with short wavelength-sensitive cones in the retina: a comparison between New World and Old World primates. J. Comp. Neurol. 1998;393:196–209. [PubMed] [Google Scholar]
- Chan TL, Martin PR, Clunas N, Grünert U. Bipolar cell diversity in the primate retina: morphologic and immunocytochemical analysis of a new world monkey, the marmoset Callithrix jacchus. J. Comp. Neurol. 2001;437:219–239. doi: 10.1002/cne.1280. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Callaway EM. S cone contributions to the magnocellular visual pathway in macaque monkey. Neuron. 2002;35:1135–1146. doi: 10.1016/s0896-6273(02)00874-7. [DOI] [PubMed] [Google Scholar]
- Chichilnisky EJ, Baylor DA. Receptive-field microstructure of blue–yellow ganglion cells in primate retina. Nat. Neurosci. 1999;2:889–893. doi: 10.1038/13189. [DOI] [PubMed] [Google Scholar]
- Chun MH, Grünert U, Martin PR, Wässle H. The synaptic complex of cones in the fovea and in the periphery of the macaque monkey retina. Vision Res. 1996;36:3383–3395. doi: 10.1016/0042-6989(95)00334-7. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Levick WR. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J. Physiol. 1974;240:457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MLJ, Anderson RA, Blake R, Jacobs GH, Neumeyer C. Inter-species comparisons in the understanding of human visual perception. In: Spillmann L, Werner JS, editors. Visual Perception: The Neurological Foundations. Academic Press; San Diego: 1990. pp. 23–52. [Google Scholar]
- Crook JD, Davenport CM, Peterson BB, Packer OS, Detwiler PB, et al. Parallel ON and OFF cone bipolar inputs establish spatially coextensive receptive field structure of blue–yellow ganglion cells in primate retina. J. Neurosci. 2009;29:8372–8387. doi: 10.1523/JNEUROSCI.1218-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Peterson BB, Packer OS, Robinson FR, Gamlin PD, et al. The smooth monostratified ganglion cell: evidence for spatial diversity in the Y-cell pathway to the lateral geniculate nucleus and superior colliculus in the macaque monkey. J. Neurosci. 2008a;28:12654–12671. doi: 10.1523/JNEUROSCI.2986-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Peterson BB, Packer OS, Robinson FR, Troy JB, et al. Y-cell receptive field and collicular projection of parasol ganglion cells in macaque monkey retina. J. Neurosci. 2008b;28:11277–11291. doi: 10.1523/JNEUROSCI.2982-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JM, Lee BB, Tigwell DA, Valberg A. Thresholds to chromatic spots of cells in the macaque geniculate nucleus as compared to detection sensitivity in man. J. Physiol. 1987;392:193–211. doi: 10.1113/jphysiol.1987.sp016776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper S, Wuerger S. The perception of motion in chromatic stimuli. Behav. Cogn. Neurosci. Rev. 2005;4:192–217. doi: 10.1177/1534582305285120. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Kimberly AA, Sloan KR, Lerea CL, Hurley JB, et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J. Comp. Neurol. 1991;312:610–624. doi: 10.1002/cne.903120411. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Vis. Neurosci. 1993a;10:1081–1098. doi: 10.1017/s0952523800010191. [DOI] [PubMed] [Google Scholar]
- Dacey DM. The mosaic of midget ganglion cells in the human retina. J. Neurosci. 1993b;13:5334–5355. doi: 10.1523/JNEUROSCI.13-12-05334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Diller LC, Verweij J, Williams DR. Physiology of L- and M-cone inputs to H1 horizontal cells in the primate retina. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2000;17:589–596. doi: 10.1364/josaa.17.000589. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The blue-ON opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB, Stafford DK, Pokorny J, Smith VC. Horizontal cells of the primate retina: cone specificity without spectral opponency. Science. 1996;271:656–659. doi: 10.1126/science.271.5249.656. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Packer OS. Colour coding in the primate retina: diverse cell types and cone-specific circuitry. Curr. Opin. Neurobiol. 2003;13:421–427. doi: 10.1016/s0959-4388(03)00103-x. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. Physiology of H1 horizontal cells in the primate retina. Proc. Roy. Soc. Lond. B. 1990;239:213–230. doi: 10.1098/rspb.1990.0014. [DOI] [PubMed] [Google Scholar]
- de Monasterio FM. Properties of concentrically organised X and Y ganglion cells of macaque retina. J. Neurophysiol. 1978;41:1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]
- de Monasterio FM, Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J. Physiol. 1975;251:167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Monasterio FM, Schein SJ. Protan-like spectral sensitivity of foveal Y ganglion cells of macaque monkeys. J. Physiol. 1980;299:385–396. doi: 10.1113/jphysiol.1980.sp013131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Monasterio FM, Schein SJ, McCrane EP. Staining of blue-sensitive cones of the macaque retina by a fluorescent dye. Science. 1981;213:1278–1281. doi: 10.1126/science.7268439. [DOI] [PubMed] [Google Scholar]
- Derrington A, Badcock D. The low level motion system has both chromatic and luminance inputs. Vision Res. 1985;25:1879–1884. doi: 10.1016/0042-6989(85)90011-2. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J. Physiol. 1984;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J. Physiol. 1984;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeValois RL, Abramov I, Jacobs GH. Analysis of response patterns of LGN cells. J. Opt. Soc. Am. 1966;56:966–977. doi: 10.1364/josa.56.000966. [DOI] [PubMed] [Google Scholar]
- DeValois RL, DeValois KK. Neural coding of color. In: Carterette ED, Friedman MP, editors. Handbook of Perception. V. Academic Press; New York: 1975. [Google Scholar]
- Diller L, Packer OS, Verweij J, McMahon MJ, Williams DR, et al. L and M cone contributions to the midget and parasol ganglion cell receptive fields of macaque monkey retina. J. Neurosci. 2004;24:1079–1088. doi: 10.1523/JNEUROSCI.3828-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkins KR, Albright TD. What happens if it changes color when it moves?: psychophysical experiments on the nature of chromatic input to motion detectors. Vision Res. 1993;33:1019–1036. doi: 10.1016/0042-6989(93)90238-r. [DOI] [PubMed] [Google Scholar]
- Dobkins KR, Albright TD. What happens if it changes color when it moves?: the nature of chromatic input to visual area MT. J. Neurosci. 1994;14:4854–4870. doi: 10.1523/JNEUROSCI.14-08-04854.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. The Retina, an Approachable Part of the Brain. Harvard University Press; Cambridge: 1987. [Google Scholar]
- Dreher B, Fukuda Y, Rodieck RW. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J. Physiol. 1976;258:433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Gauthier JL, Sher A, Greschner M, Machado T, Jepson LH, Shlens J, Gunning DE, Mathieson K, Dabrowski W, Paninski L, Litke AM, Chichilnisky EJ. Functional connectivity in the retina at the resolution of photo-receptors. Nature. doi: 10.1038/nature09424. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Grescher M, Gauthier JL, Rangel C, Shlens J, Sher A, Marshak DW, Litke AM, Chichilnisky EJ. High-sensitivity rod input to the blue–yellow color opponent pathway in macaque retina. Nat. Neurosci. 2009;12:1159–1164. doi: 10.1038/nn.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Sher A, Gauthier JL, Greschner M, Shlens J, et al. Spatial properties and functional organization of small bistratified ganglion cells in primate retina. J. Neurosci. 2007;27:13261–13272. doi: 10.1523/JNEUROSCI.3437-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, Franco EC, Yamada ES, Crowley JC, Parsons M, et al. Number and topography of cones, rods and optic nerve axons in New and Old World primates. Vis. Neurosci. 2008;25:289–299. doi: 10.1017/S0952523808080371. [DOI] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA, Wässle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J. Neurosci. 2001;21:4852–4863. doi: 10.1523/JNEUROSCI.21-13-04852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, et al. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Goodchild AK, Sefton AE, Martin P. The morphology of retinal ganglion cells in the New World marmoset monkey Callithrix jacchus. J. Comp. Neurol. 1996;366:76–92. doi: 10.1002/(SICI)1096-9861(19960226)366:1<76::AID-CNE6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Grünert U. Synaptic input to small bistratified (blue-ON) ganglion cells in the retina of a new world monkey, the marmoset Callithrix jacchus. J. Comp. Neurol. 1999;413:417–428. doi: 10.1002/(sici)1096-9861(19991025)413:3<417::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Martin PR, Grünert U. Morphological analysis of the blue cone pathway in the retina of a New World monkey, the marmoset Callithrix jacchus. J. Comp. Neurol. 1997;379:211–225. [PubMed] [Google Scholar]
- Goodchild AK, Chan TL, Grünert U. Horizontal cell connections with short-wavelength-sensitive cones in macaque monkey retina. Vis. Neurosci. 1996;13:833–845. doi: 10.1017/s0952523800009093. [DOI] [PubMed] [Google Scholar]
- Grünert U. Anatomical evidence for rod input to the parvocellular pathway in primate. Eur. J. Neurosci. 1997;9:617–621. doi: 10.1111/j.1460-9568.1997.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Grünert U, Wässle H. Glycine receptors in the rod pathway of the macaque monkey retina. Vis. Neurosci. 1996;13:101–115. doi: 10.1017/s0952523800007161. [DOI] [PubMed] [Google Scholar]
- Hack I, Peichl L, Brandstatter JH. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells, and the localization of glutamate receptors. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14130–14135. doi: 10.1073/pnas.96.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. The synaptic architecture of AMPA receptors at the cone pedicle of the primate retina. J. Neurosci. 2001;21:2488–2500. doi: 10.1523/JNEUROSCI.21-07-02488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, et al. The primordial, blue-cone color system of the mouse retina. J. Neurosci. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmholtz H.v. Treatise on Physiological Optics. third ed. Dover; New York: 1962. [Google Scholar]
- Hemmi JM, James A, Taylor WR. Color opponent retinal ganglion cells in the tammar wallaby retina. J. Vis. 2002;2:608–617. doi: 10.1167/2.9.3. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Djajadi HR, Nakamura L, Possin DE, Sajuthi D. Nocturnal tarsier retina has both short and long/medium-wavelength cones in an unusual topography. J. Comp. Neurol. 2000;424:718–730. doi: 10.1002/1096-9861(20000904)424:4<718::aid-cne12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Herr S, Klug K, Sterling P, Schein S. Inner S-cone bipolar cells provide all of the central elements for S cones in macaque retina. J. Comp. Neurol. 2003;457:185–201. doi: 10.1002/cne.10553. [DOI] [PubMed] [Google Scholar]
- Hofer H, Carroll J, Neitz J, Neitz M, Williams DR. Organization of the human trichromatic cone mosaic. J. Neurosci. 2005;25:9669–9679. doi: 10.1523/JNEUROSCI.2414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins JM, Boycott BB. The cone synapses of cone bipolar cells of primate retina. J. Neurocytol. 1997;26:313–325. doi: 10.1023/a:1018504718282. [DOI] [PubMed] [Google Scholar]
- Hornstein EP, Verweij J, Li PH, Schnapf JL. Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. J. Neurosci. 2005;25:11201–11209. doi: 10.1523/JNEUROSCI.3416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein EP, Verweij J, Schnapf JL. Electrical coupling between red and green cones in primate retina. Nat. Neurosci. 2004;7:745–750. doi: 10.1038/nn1274. [DOI] [PubMed] [Google Scholar]
- Ingling CR, Jr., Martinez-Uriegas E. The spatiotemporal properties of the r-g X-cell channel. Vision Res. 1985;25:33–38. doi: 10.1016/0042-6989(85)90077-x. [DOI] [PubMed] [Google Scholar]
- Ingling CR, Martinez-Uriegas E. The relationship between spectral sensitivity and spatial sensitivity for the primate r-g X channel. Vision Res. 1983;23:1495–1500. doi: 10.1016/0042-6989(83)90161-x. [DOI] [PubMed] [Google Scholar]
- Ivanova E, Hwang GS, Pan ZH, Troilo D. Evaluation of AAV-mediated expression of Chop2-GFP in the Marmoset retina. Invest. Ophthalmol. Vis. Sci. 2010 doi: 10.1167/iovs.10-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH. Differences in spectral response properties of LGN cells in male and female squirrel monkeys. Vision Res. 1983a;23:461–468. doi: 10.1016/0042-6989(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Within-species variations in visual capacity among squirrel monkeys (Saimiri sciureus): sensitivity differences. Vision Res. 1983b;23:239–248. doi: 10.1016/0042-6989(83)90112-8. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. The distribution and nature of color vision among the mammals. Biol. Rev. Camb. Philos. Soc. 1993;68:413–471. doi: 10.1111/j.1469-185x.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Primate color vision: a comparative perspective. Vis. Neurosci. 2008;25:619–633. doi: 10.1017/S0952523808080760. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Blakeslee B, Tootell RB. Color-discrimination tests on fibers in ground squirrel optic nerve. J. Neurophysiol. 1981;45:903–914. doi: 10.1152/jn.1981.45.5.903. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Deegan JF, Neitz J, Crognale MA, Neitz M. Photopigments and color vision in the nocturnal monkey, Aotus. Vision Res. 1993a;33:1773–1783. doi: 10.1016/0042-6989(93)90168-v. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J, Neitz M. Genetic basis of polymorphism in the color vision of platyrrhine monkeys. Vision Res. 1993b;33:269–274. doi: 10.1016/0042-6989(93)90083-9. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz M, Deegan JF, Neitz J. Trichromatic color vision in New-World monkeys. Nature. 1996;382:156–158. doi: 10.1038/382156a0. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Tootell RB. Spectral-response properties of optic-nerve fibers in the ground squirrel. J. Neurophysiol. 1981;45:891–902. doi: 10.1152/jn.1981.45.5.891. [DOI] [PubMed] [Google Scholar]
- Jacoby RA, Stafford D, Kouyama N, Marshak D. Synaptic inputs to ON parasol ganglion cells in the primate retina. J. Neurosci. 1996;16:8041–8056. doi: 10.1523/JNEUROSCI.16-24-08041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RA, Wiechmann AF, Amara SG, Leighton BH, Marshak DQ. Diffuse bipolar cells provide input to OFF parasol ganglion cells in the macaque retina. J. Comp. Neurol. 2000;416:6–18. doi: 10.1002/(sici)1096-9861(20000103)416:1<6::aid-cne2>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Martin PR, Grünert U. Random wiring in the midget pathway of primate retina. J. Neurosci. 2006a;26:3908–3917. doi: 10.1523/JNEUROSCI.4891-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Martin PR, Grünert U. Synaptic connectivity in the midget-parvocellular pathway of primate central retina. J. Comp. Neurol. 2006b;494:260–274. doi: 10.1002/cne.20804. [DOI] [PubMed] [Google Scholar]
- Kaiser PK, Lee BB, Martin PR, Valberg A. The physiological basis of the minimally distinct border demonstrated in the ganglion cells of the macaque retina. J. Physiol. 1990;422:153–183. doi: 10.1113/jphysiol.1990.sp017978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalloniatis M, Harwerth RS. Effects of chromatic adaptation on opponent interactions in monkey increment-threshold spectral-sensitivity functions. J. Opt. Soc. Am. A. 1991;8:1818–1831. doi: 10.1364/josaa.8.001818. [DOI] [PubMed] [Google Scholar]
- Klug K, Herr S, Ngo IT, Sterling P, Schein S. Macaque retina contains an S-cone OFF midget pathway. J. Neurosci. 2003;23:9881–9887. doi: 10.1523/JNEUROSCI.23-30-09881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. Organization of the outer plexiform layer of the primate retina: electron microscopy of Golgi-impregnated cells. Phil. Trans. Roy. Soc. Lond. B, Biol. Sci. 1970;258:261–283. doi: 10.1098/rstb.1970.0036. [DOI] [PubMed] [Google Scholar]
- Kolb H, Boycott BB, Dowling JE. A second type of midget bipolar cell in the primate retina. Phil. Trans. Roy. Soc. Lond. B, Biol. Sci. 1969;255:177–184. [Google Scholar]
- Kolb H, Dekorver L. Midget ganglion cells of the parafovea of the human retina: a study by electron microscopy and serial section reconstructions. J. Comp. Neurol. 1991;303:617–636. doi: 10.1002/cne.903030408. [DOI] [PubMed] [Google Scholar]
- Kolb H, Famiglietti EV. Rod and cone pathways in the inner plexiform layer of cat retina. Science. 1974;186:47–49. doi: 10.1126/science.186.4158.47. [DOI] [PubMed] [Google Scholar]
- Kolb H, Goede P, Roberts S, McDermott R, Gouras P. Uniqueness of the S-cone pedicle in the human retina and consequences for color processing. J. Comp. Neurol. 1997;386:443–460. doi: 10.1002/(sici)1096-9861(19970929)386:3<443::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Kolb H, Nelson R. The organization of photoreceptor to bipolar synapses in the outer plexiform layer. In: Djamgoz MBA, Archer SN, Vallerga S, editors. Neurobiology and Clinical Aspects of the Outer Retina. Chapman and Hall; London: 1995. pp. 273–296. [Google Scholar]
- Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. J. Neurosci. 1992;12:1233–1252. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer RW. Innovation through synthesis: Helmholtz and color research. In: Cahan D, editor. Hermann von Helmholtz and the Foundations of Nineteenth Century Science. University of California Press; Berkeley, CA: 1993. pp. 205–258. [Google Scholar]
- Kremers J, Scholl HP, Knau H, Berendschot TT, Usui T, et al. L/M cone ratios in human trichromats assessed by psychophysics, electroretinography, and retinal densitometry. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2000;17:517–526. doi: 10.1364/josaa.17.000517. [DOI] [PubMed] [Google Scholar]
- Lee BB. Paths to color in the retina. Aust. J. Optom. 2004;87:239–248. doi: 10.1111/j.1444-0938.2004.tb05054.x. [DOI] [PubMed] [Google Scholar]
- Lee BB. Neural models and physiological reality. Vis. Neurosci. 2008;251:231–241. doi: 10.1017/S0952523808080140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Dacey DM, Smith VC, Pokorny J. Horizontal cells reveal cone type-specific adaptation in primate retina. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14611–14616. doi: 10.1073/pnas.96.25.14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Dacey DM, Smith VC, Pokorny J. Dynamics of sensitivity regulation in primate outer retina: the horizontal cell network. J. Vis. 2003;3:513–526. doi: 10.1167/3.7.5. [DOI] [PubMed] [Google Scholar]
- Lee BB, Kremers J, Yeh T. Receptive fields of primate ganglion cells studied with a novel technique. Vis. Neurosci. 1998;15:161–175. doi: 10.1017/s095252389815112x. [DOI] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. The physiological basis of heterochromatic flicker photometry demonstrated in the ganglion cells of the macaque retina. J. Physiol. 1988;404:323–347. doi: 10.1113/jphysiol.1988.sp017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. Nonlinear summation of M- and L-cone inputs to phasic retinal ganglion cells of the macaque. J. Neurosci. 1989;9:1433–1442. doi: 10.1523/JNEUROSCI.09-04-01433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Pokorny J, Smith VC, Kremers J. Rod inputs to macaque ganglion cells. Vision Res. 1997;37:2813–2828. doi: 10.1016/s0042-6989(97)00108-9. [DOI] [PubMed] [Google Scholar]
- Lee BB, Silveira LCL, Yamada ES, Hunt DM, Kremers J, et al. Visual responses of ganglion cells of a New-World primate, the capuchin monkey, Cebus apella. J. Physiol. 2000;528:573–590. doi: 10.1111/j.1469-7793.2000.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Sun H. The chromatic input to cells of the magnocellular pathway of primates. J. Vis. 2009;9:151–158. doi: 10.1167/9.2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Valberg A, Tigwell DA, Tryti J. An account of responses of spectrally opponent neurons in macaque lateral geniculate nucleus to successive contrast. Proc. Roy. Soc. Lond. B. 1987;230:293–314. doi: 10.1098/rspb.1987.0021. [DOI] [PubMed] [Google Scholar]
- Lee BB, Wehrhahn C, Westheimer G, Kremers J. The spatial precision of macaque ganglion cell responses in relation to Vernier acuity of human observers. Vision Res. 1995;35:2743–2758. doi: 10.1016/0042-6989(95)00015-r. [DOI] [PubMed] [Google Scholar]
- Lee SC, Grünert U. Connections of diffuse bipolar cells in primate retina are biased against S-cones. J. Comp. Neurol. 2007;502:126–140. doi: 10.1002/cne.21284. [DOI] [PubMed] [Google Scholar]
- Lee SC, Jusuf PR, Grünert U. S-cone connections of the diffuse bipolar cell type DB6 in macaque monkey retina. J. Comp. Neurol. 2004;474:353–363. doi: 10.1002/cne.20139. [DOI] [PubMed] [Google Scholar]
- Lee SC, Telkes I, Grünert U. S-cones do not contribute to the OFF-midget pathway in the retina of the marmoset, Callithrix jacchus. Eur. J. Neurosci. 2005;22:437–447. doi: 10.1111/j.1460-9568.2005.04231.x. [DOI] [PubMed] [Google Scholar]
- Lennie P, Haake PW, Williams DR. The design of chromatically opponent receptive fields. In: Landy MS, Movshon JA, editors. Computational Models of Visual Processing. MIT Press; Cambridge, MA, USA: 1991. pp. 71–82. [Google Scholar]
- Levenson DH, Fernandez-Duque E, Evans S, Jacobs GH. Mutational changes in S-cone opsin genes common to both nocturnal and cathemeral Aotus monkeys. Am. J. Primatol. 2007;69:757–765. doi: 10.1002/ajp.20402. [DOI] [PubMed] [Google Scholar]
- Li W, Chen S, DeVries SH. A fast rod photoreceptor signaling pathway in the mammalian retina. Nat. Neurosci. 2010;13:414–416. doi: 10.1038/nn.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, DeVries SH. Separate blue and green cone networks in the mammalian retina. Nat. Neurosci. 2004;7:751–756. doi: 10.1038/nn1275. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Bipolar cell pathways for color and luminance vision in a dichromatic mammalian retina. Nat. Neurosci. 2006;9:669–675. doi: 10.1038/nn1686. [DOI] [PubMed] [Google Scholar]
- Li W, Keung JW, Massey SC. Direct synaptic connections between rods and OFF cone bipolar cells in the rabbit retina. J. Comp. Neurol. 2004;474:1–12. doi: 10.1002/cne.20075. [DOI] [PubMed] [Google Scholar]
- Lima SMA, Silveira LCL, Perry VH. Distribution of M retinal ganglion cells in diurnal and nocturnal New-World monkeys. J. Comp. Neurol. 1996;368:538–552. doi: 10.1002/(SICI)1096-9861(19960513)368:4<538::AID-CNE6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Liu PC, Chiao CC. Morphologic identification of the OFF-type blue cone bipolar cell in the rabbit retina. Invest. Ophthalmol. Vis. Sci. 2007;48:3388–3395. doi: 10.1167/iovs.06-1531. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD. Synaptic mechanisms that shape visual signaling at the inner retina. Prog. Brain Res. 2005;147:205–218. doi: 10.1016/S0079-6123(04)47016-2. [DOI] [PubMed] [Google Scholar]
- Luo X, Ghosh KK, Martin PR, Grünert U. Analysis of two types of cone bipolar cells in the retina of a New World monkey, the marmoset, Callithrix jacchus. Vis. Neurosci. 1999;16:707–719. doi: 10.1017/s0952523899164101. [DOI] [PubMed] [Google Scholar]
- MacLeod DIA, von der Twer T. The pleistochrome: optimal opponent codes for natural colours. In: Mausfeld R, Heyer D, editors. Color Perception: Mind and the Physical World. Oxford University Press; Oxford: 2003. [Google Scholar]
- MacLeod DIA, Williams DR, Makous W. A visual non-linearity fed by single cones. Vision Res. 1992;32:347–363. doi: 10.1016/0042-6989(92)90144-8. [DOI] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J. Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Sperling HG. Chromatic organization of primate cones. Science. 1977;143:454–456. doi: 10.1126/science.403607. [DOI] [PubMed] [Google Scholar]
- Mariani AP. Bipolar cells in monkey retina selective for the cone likely to be blue-sensitive. Nature. 1984;308:184–186. doi: 10.1038/308184a0. [DOI] [PubMed] [Google Scholar]
- Marr D. Vision: a Computational Investigation into the Human Representation and Processing of Visual Information. Freeman; San Francisco: 1982. [Google Scholar]
- Martin PR, Grünert U, Chan TL, Bumsted K. Spatial order in short-wavelength-sensitive cone photoreceptors: a comparative study of the primate retina. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2000;17:557–567. doi: 10.1364/josaa.17.000557. [DOI] [PubMed] [Google Scholar]
- Martin PR, Lee BB, White AJ, Solomon SG, Rüttiger L. Chromatic sensitivity of ganglion cells in peripheral primate retina. Nature. 2001;410:933–936. doi: 10.1038/35073587. [DOI] [PubMed] [Google Scholar]
- Martin PR, White AJR, Goodchild AK, Wilder HD, Sefton AE. Evidence that blue-on cells are part of the third geniculocortical pathway in primates. Eur. J. Neurosci. 1997;9:1536–1541. doi: 10.1111/j.1460-9568.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Martin RD. Primate Origins and Evolution. Princeton University Press; Princeton: 1990. [Google Scholar]
- Massey SC. Circuit functions of gap junctions in mammalian retina. In: Masland R, Albright TD, editors. The Senses: a Comprehensive Reference. Vision 1. Vol. 1. Academic Press; San Diego: 2008. pp. 457–472. [Google Scholar]
- McLellan JS, Marcos S, Prieto PM, Burns SA. Imperfect optics may be the eye's defence against chromatic blur. Nature. 2002;417:174–176. doi: 10.1038/417174a. [DOI] [PubMed] [Google Scholar]
- McMahon MJ, Lankheet MJ, Lennie P, Williams DR. Fine structure of parvocellular receptive fields in the primate fovea revealed by laser interferometry. J. Neurosci. 2000;20:2043–2053. doi: 10.1523/JNEUROSCI.20-05-02043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon MJ, Packer OS, Dacey DM. The classical receptive field surround of primate parasol ganglion cells is mediated primarily by a non-GABAergic pathway. J. Neurosci. 2004;24:3736–3745. doi: 10.1523/JNEUROSCI.5252-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? Annu. Rev. Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. AII amacrine cells limit scotopic acuity in central macaque retina: a confocal analysis of calretinin labeling. J. Comp. Neurol. 1999;411:19–34. [PubMed] [Google Scholar]
- Mollon JD. Uses and evolutionary origins of primate color vision. In: Cronly-Dillon JR, Gregory RL, editors. Evolution of the Eye and Visual System. Vol. 2. MacMillan; London: 1991. pp. 306–319. [Google Scholar]
- Mollon JD, Bowmaker JK, Jacobs GH. Variations in colour vision in a new-world monkey can be explained by a polymorphism of retinal photopigments. Proc. Roy. Soc. Lond. B. 1984;222:373–389. doi: 10.1098/rspb.1984.0071. [DOI] [PubMed] [Google Scholar]
- Mullen K, Baker CL., Jr. A motion aftereffect from an isoluminant stimulus. Vision Res. 1985;25:685–688. doi: 10.1016/0042-6989(85)90174-9. [DOI] [PubMed] [Google Scholar]
- Mullen KT, Kingdom FA. Losses in peripheral colour sensitivity predicted from “hit or miss” post-receptoral cone connections. Vision Res. 1996;36:1995–2000. doi: 10.1016/0042-6989(95)00261-8. [DOI] [PubMed] [Google Scholar]
- Müller J. Handbuch der Physiology des Menschen Coblenz. 1838.
- Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- Navarro R, Artal P, Williams DR. Modulation transfer of the human eye as a function of retinal eccentricity. J. Opt. Soc. Am. A. 1993;10:201–212. doi: 10.1364/josaa.10.000201. [DOI] [PubMed] [Google Scholar]
- O'Brian JJ, Chen X, MacLeish PR, Massey SC. Connexin 36 forms gap junctions between telodendria of primate cones. Invest. Ophthalmol. Vis. Sci. 2004 45 E-Abstract 1146. [Google Scholar]
- O'Keefe LP, Levitt JB, Kiper DC, Shapley RM, Movshon JA. Functional organization of owl monkey lateral geniculate nucleus and visual cortex. J. Neurophysiol. 1998;80:594–609. doi: 10.1152/jn.1998.80.2.594. [DOI] [PubMed] [Google Scholar]
- Packer O, Hendrickson AE, Curcio CA. Photoreceptor topography of the retina in the adult pigtail macaque (Macaca nemestrina). J. Comp. Neurol. 1989;288:165–183. doi: 10.1002/cne.902880113. [DOI] [PubMed] [Google Scholar]
- Packer OS, Verweij J, Li PH, Schnapf JL, Dacey DM. Blue–yellow opponency in primate S cone photoreceptors. J. Neurosci. 2010;30:568–572. doi: 10.1523/JNEUROSCI.4738-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J-J, Gao F, Wu SM. Relative contributions of bipolar cell and amacrine cell inputs to light responses of ON, OFF and ON–OFF retinal ganglion cells. Vision Res. 2002;42:19–27. doi: 10.1016/s0042-6989(01)00258-9. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Lem L, Bramblett DE, Paul DL, et al. Direct rod input to cone BCs and direct cone input to rod BCs challenge the traditional view of mammalian BC circuitry. Proc. Natl. Acad. Sci. U.S.A. 2010;107:395–400. doi: 10.1073/pnas.0907178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus W, Kröger-Paulus A. A new concept of retinal colour coding. Vision Res. 1983;23:529–540. doi: 10.1016/0042-6989(83)90128-1. [DOI] [PubMed] [Google Scholar]
- Peichl L. Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle? Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005;287:1001–1012. doi: 10.1002/ar.a.20262. [DOI] [PubMed] [Google Scholar]
- Peichl L, Wässle H. Size, scatter and coverage of ganglion cell receptive field centres in the cat retina. J. Physiol. 1979;291:117–141. doi: 10.1113/jphysiol.1979.sp012803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L, Wässle H. Morphological identification of on- and off-centre brisk transient (Y) cells in the cat retina. Proc. Roy. Soc. Lond. B. 1981;212:139–156. doi: 10.1098/rspb.1981.0030. [DOI] [PubMed] [Google Scholar]
- Percival KA, Jusuf PR, Martin PR, Grünert U. Synaptic inputs onto small bistratified (blue-ON/yellow-OFF) ganglion cells in marmoset retina. J. Comp. Neurol. 2009;517:655–669. doi: 10.1002/cne.22183. [DOI] [PubMed] [Google Scholar]
- Perlman I, Kolb H, Nelson R. Anatomy, circuitry and physiology of vertebrate horizontal cells. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. MIT Press; Cambridge, MA: 2003. pp. 410–421. [Google Scholar]
- Petruska D, Grivich MI, Sher A, Field GD, Gauthier GL, et al. Identification and characterization of a Y-like primate retinal ganglion cell type. J. Neurosci. 2007;27:8254–8266. doi: 10.1523/JNEUROSCI.2836-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak SL. The Retina. University of Chicago Press; Chicago: 1941. [Google Scholar]
- Puller C, Haverkamp S, Grünert U. OFF midget bipolar cells in the retina of the marmoset, Callithrix jacchus, express AMPA receptors. J. Comp. Neurol. 2007;502:442–454. doi: 10.1002/cne.21315. [DOI] [PubMed] [Google Scholar]
- Purpura K, Kaplan E, Shapley RM. Background light and the contrast gain of primate P and M retinal ganglion cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:4534–4537. doi: 10.1073/pnas.85.12.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Gap junctions between photoreceptor cells in the vertebrate retina. Proc. Natl. Acad. Sci. U.S.A. 1973;70:1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RC, Shapley RM. Spatial structure of cone inputs to receptive fields in primate lateral geniculate nucleus. Nature. 1992;356:716–718. doi: 10.1038/356716a0. [DOI] [PubMed] [Google Scholar]
- Reid RC, Shapley RM. Space and time maps of cone photoreceptor signals in macaque lateral geniculate nucleus. J. Neurosci. 2002;22:6158–6175. doi: 10.1523/JNEUROSCI.22-14-06158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck RW. Which cells code for color? In: Valberg A, Lee BB, editors. From Pigments to Perception. Plenum; New York and London: 1991. [Google Scholar]
- Roorda A, Metha AB, Lennie P, Williams DR. Packing arrangement of the three cone classes in primate retina. Vision Res. 2001;41:1291–1306. doi: 10.1016/s0042-6989(01)00043-8. [DOI] [PubMed] [Google Scholar]
- Roorda A, Williams DR. The arrangement of the three cone classes in the living human eye. Nature. 1999;397:520–522. doi: 10.1038/17383. [DOI] [PubMed] [Google Scholar]
- Saito CA, de Silvo MF, Lee BB, Bowmaker JK, Kremers J, et al. Alouatta trichromatic color vision – single-unit recordings from retinal ganglion cells and microspectrophotometry. Invest. Ophthalmol. Vis. Sci. 2004 45 E-Abstract 4276. [Google Scholar]
- Schiller PH, Colby CL. The responses of single cells in the lateral geniculate nucleus of the rhesus monkey to color and luminance contrast. Vision Res. 1983;23:1631–1641. doi: 10.1016/0042-6989(83)90177-3. [DOI] [PubMed] [Google Scholar]
- Schneeweiss DM, Schnapf JL. Photovoltages of rods and cones in the macaque retina. Science. 1995;268:1053–1056. doi: 10.1126/science.7754386. [DOI] [PubMed] [Google Scholar]
- Shapiro MB, Schein SJ, de Monasterio FM. Regularity and structure of the spatial pattern of blue cones of macaque retina. J. Am. Stat. Assoc. 1985;80:803–812. [Google Scholar]
- Shapley R, Perry VH. Cat and monkey retinal ganglion cells and their visual functional roles. Trends Neurosci. 1986;9:229–235. [Google Scholar]
- Sharpe LT, Stockman A, Jägle H, Nathans J. Opsin genes, cone photopigments, color vision and color blindness. In: Gegenfurtner KR, Sharpe LT, editors. Color Vision: From Genes to Perception. Cambridge University Press; Cambridge: 1999. pp. 3–51. [Google Scholar]
- Sharpe LT, Stockman A, MacLeod DIA. Scotopic flicker perception: scotopic, duality, phase lags and destructive interference. Vision Res. 1989;29:1539–1559. doi: 10.1016/0042-6989(89)90137-5. [DOI] [PubMed] [Google Scholar]
- Shlens J, Field GD, Gauthier JL, Grivich MI, Petrusca D, et al. The structure of multi-neuron firing patterns in primate retina. J. Neurosci. 2006;26:8254–8266. doi: 10.1523/JNEUROSCI.1282-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira LC, Saito CA, Lee BB, Kremers J, da Silva Filho M, et al. Morphology and physiology of primate M- and P-cells. Prog. Brain Res. 2004a;144:21–46. doi: 10.1016/S0079-6123(03)14402-0. [DOI] [PubMed] [Google Scholar]
- Silveira LCL, Lee BB, Kremers J, da Silva Filho M, Saito CA, Kilavek BE. Receptor inputs and temporal dynamics of owl monkey ganglion cells. Invest. Ophthalmol. Vis. Sci. 2000;41:S937. [Google Scholar]
- Silveira LCL, Lee BB, Yamada ES, Kremers J, Hunt DM, et al. Ganglion cells of a short wavelength sensitive cone pathway in New-World monkeys: morphology and physiology. Vis. Neurosci. 1999;16:333–343. doi: 10.1017/s0952523899162138. [DOI] [PubMed] [Google Scholar]
- Silveira LCL, Zurawski JD, Parsons MP, Finlay BL. Unusual retinal organization in the howler monkey, Alouatta caraya. Invest. Ophthalmol. Vis. Sci. 2004b 45 E-abstract 44. [Google Scholar]
- Silveira LCL, Perry VH, Yamada ES. The retinal ganglion cell distribution and the representation of the visual field in area 17 of the owl-monkey Aotus trivirgatus. Vis. Neurosci. 1993;10:887–897. doi: 10.1017/s095252380000609x. [DOI] [PubMed] [Google Scholar]
- Silveira LCL, Yamada ES, Perry VH, Picanço-Diniz CW. M and P retinal ganglion cells of diurnal and nocturnal New-World monkeys. Neuroreport. 1994;5:2077–2081. doi: 10.1097/00001756-199410270-00022. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Zhang Y, Tiruveedhula P, Horton JC, Roorda A. Resolving single cone inputs to visual receptive fields. Nat. Neurosci. 2009;12:967–969. doi: 10.1038/nn.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VC, Lee BB, Pokorny J, Martin PR, Valberg A. Responses of macaque ganglion cells to the relative phase of heterochromatically modulated lights. J. Physiol. 1992;458:191–221. doi: 10.1113/jphysiol.1992.sp019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VC, Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 1975;15:161–171. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- Smith VC, Pokorny J, Lee BB, Dacey DM. Primate horizontal cell dynamics: an analysis of sensitivity regulation in outer retina. J. Neurophysiol. 2001;85:545–558. doi: 10.1152/jn.2001.85.2.545. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Lee BB, Sun H. Suppressive surrounds and contrast gain in magnocellular-pathway retinal ganglion cells of macaque. J. Neurosci. 2006;26:8715–8726. doi: 10.1523/JNEUROSCI.0821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SG, Lee BB, White AJ, Ruttiger L, Martin PR. Chromatic organization of ganglion cell receptive fields in the peripheral retina. J. Neurosci. 2005;25:4527–4539. doi: 10.1523/JNEUROSCI.3921-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SG, Lennie P. The machinery of colour vision. Nat. Rev. Neurosci. 2007;8:276–286. doi: 10.1038/nrn2094. [DOI] [PubMed] [Google Scholar]
- Stiles WS. Color vision: the approach through increment threshold sensitivity. Proc. Natl. Acad. Sci. U.S.A. 1959;45:100–114. [Google Scholar]
- Sun H, Ruttiger L, Lee BB. The spatiotemporal precision of ganglion cell signals: a comparison of physiological and psychophysical performance with moving gratings. Vision Res. 2004;44:19–33. doi: 10.1016/j.visres.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Sun H, Smithson H, Zaidi Q, Lee BB. Do magnocellular and parvocellular ganglion cells avoid short-wavelength cone input. Vis. Neurosci. 2006a;23:323–330. doi: 10.1017/S0952523806233042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Smithson H, Zaidi Q, Lee BB. Specificity of cone inputs to macaque ganglion cells. J. Neurophysiol. 2006b;95:837–849. doi: 10.1152/jn.00714.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmajda BA, Buzás P, Fitzgibbon T, Martin PR. Geniculocortical relay of blue-off signals in the primate visual system. Proc. Natl. Acad. Sci. U.S.A. 2006;103:19512–19517. doi: 10.1073/pnas.0606970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmajda BA, Grünert U, Martin PR. Retinal ganglion cell inputs to the koniocellular pathway. J. Comp. Neurol. 2008;510:251–268. doi: 10.1002/cne.21783. [DOI] [PubMed] [Google Scholar]
- Tailby C, Solomon SG, Lennie P. Functional asymmetries in visual pathways carrying S-cone signals in macaque. J. Neurosci. 2008a;28:4078–4087. doi: 10.1523/JNEUROSCI.5338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailby C, Szmajda BA, Buzás P, Lee BB, Martin PR. Transmission of blue (S) cone signals through the primate lateral geniculate nucleus. J. Physiol. 2008b;586:5947–5967. doi: 10.1113/jphysiol.2008.161893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. J. Neurosci. 2002;22:7712–7720. doi: 10.1523/JNEUROSCI.22-17-07712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telkes I, Lee SC, Jusuf PR, Grünert U. The midget-parvocellular pathway of marmoset retina: a quantitative light microscopic study. J. Comp. Neurol. 2008;510:539–549. doi: 10.1002/cne.21813. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Masarachia P, Schein SJ, Sterling P. Gap junctions between the pedicles of macaque foveal cones. Vision Res. 1992;32:1809–1815. doi: 10.1016/0042-6989(92)90042-h. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J. Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reid RC. Visual physiology of the lateral geniculate nucleus in two species of New World monkey: Saimiri sciureus and Aotus trivirgatis. J. Physiol. 2000;523:755–769. doi: 10.1111/j.1469-7793.2000.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valberg A, Lee BB, Kaiser PK, Kremers J. Responses of macaque ganglion cells to movement of chromatic borders. J. Physiol. 1992;458:579–602. doi: 10.1113/jphysiol.1992.sp019435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Levick WR, Thibos LN. Rabbit retinal ganglion cells. Receptive field classification and axonal conduction properties. Exp. Brain Res. 1981;44:27–33. doi: 10.1007/BF00238746. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Taylor WR. Direction selectivity in the retina. Curr. Opin. Neurobiol. 2002;12:7712–7720. doi: 10.1016/s0959-4388(02)00337-9. [DOI] [PubMed] [Google Scholar]
- Verweij J, Dacey DM, Peterson BB, Buck SL. Sensitivity and dynamics of rod signals in H1 horizontal cells of the macaque monkey retina. Vision Res. 1999;39:3662–3672. doi: 10.1016/s0042-6989(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J. Neurosci. 2003;23:10249–10257. doi: 10.1523/JNEUROSCI.23-32-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Smallwood PM, Cowan M, Blesh D, Lawler A, et al. Mutually exclusive expression of human red and green visual pigment-reporter transgenes occurs at high frequency in murine cone photoreceptors. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5251–5256. doi: 10.1073/pnas.96.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol. Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB, Illing R-B. Morphology and mosaic of on- and off-beta-cells of the cat retina and some functional considerations. Proc. Roy. Soc. Lond. B. 1981;212:177–195. doi: 10.1098/rspb.1981.0033. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Chun MH, Boycott BB. The rod pathway of the macaque monkey retina: identification of AII-amacrine cells with antibodies against calretinin. J. Comp. Neurol. 1995;361:537–551. doi: 10.1002/cne.903610315. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Martin PR, Boycott BB. Color coding in the primate retina: predictions and constraints from anatomy. In: Albowitz B, Albus K, Kuhnt U, Nothdurft HC, Wahle P, editors. Structural and Functional Organization of the Neocortex. A Symposium in the Memory of Otto D. Creutzfeldt. New York. Springer; Berlin, Heidelberg: 1994a. pp. 94–104. [Google Scholar]
- Wässle H, Grünert U, Martin PR, Boycott BB. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vision Res. 1994b;34:561–579. doi: 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Röhrenbeck J, Boycott BB. Cortical magnification factor and the ganglion cell density of the primate retina. Vision Res. 1990;30:1897–1911. doi: 10.1016/0042-6989(90)90166-i. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J. Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J. Neurophysiol. 1969;32:339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wiesel T, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J. Neurophysiol. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Wikler KC, Rakic P. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J. Neurosci. 1990;10:3390–3401. doi: 10.1523/JNEUROSCI.10-10-03390.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder HD, Grünert U, Lee BB, Martin PR. Topography of ganglion cells and photoreceptors in the retina of the new-world marmoset monkey. Vis. Neurosci. 1996;13:335–352. doi: 10.1017/s0952523800007586. [DOI] [PubMed] [Google Scholar]
- Williams DR, Hofer H. Formation and acquisition of the retinal image. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. MIT Press; Cambridge, MA: 2003. [Google Scholar]
- Williams DR, MacLeod DIA, Hayhoe MM. Foveal tritanopia. Vision Res. 1981a;21:1341–1356. doi: 10.1016/0042-6989(81)90241-8. [DOI] [PubMed] [Google Scholar]
- Williams DR, MacLeod DIA, Hayhoe MM. Punctate sensitivity of the blue-sensitive mechanism. Vision Res. 1981b;21:1357–1375. doi: 10.1016/0042-6989(81)90242-x. [DOI] [PubMed] [Google Scholar]
- Wyszecki G, Stiles W. Color Science – Concepts and Methods, Quantitative Data and Formulas. John Wiley & Sons; New York: 1967. [Google Scholar]
- Yamada E, Silveira LCL, Gomes FL, Lee BB. The retinal ganglion cell classes of new-world primates. Revista Brasileira de Biologia. 1996a;56:381–396. [PubMed] [Google Scholar]
- Yamada ES, Marshak DW, Silveira LCL, Casagrande VA. Morphology of P and M retinal ganglion cells of the bush baby. Vision Res. 1998;38:3345–3352. doi: 10.1016/s0042-6989(97)00412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada ES, Silveira LCL, Perry VH. Morphology, dendritic field size, somal size, density and coverage of M and P retinal ganglion cells of dichromatic Cebus monkeys. Vis. Neurosci. 1996b;13:1011–1029. doi: 10.1017/s0952523800007677. [DOI] [PubMed] [Google Scholar]
- Yamada ES, Silveira LCL, Perry VH. The morphology and photoreceptor convergence of Aotus M and P retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 1996c;37:S631. [Google Scholar]
- Yamada ES, Silveira LCL, Perry VH, Franco ECS. M and P retinal ganglion cells of the owl monkey: morphology, size and photoreceptor convergence. Vision Res. 2001;41:119–132. doi: 10.1016/s0042-6989(00)00244-3. [DOI] [PubMed] [Google Scholar]
- Yeh T, Lee BB, Kremers J, Cowing JA, Hunt DM, et al. Visual responses in the lateral geniculate of dichromatic and trichromatic marmosets (Callithrix jacchus). J. Neurosci. 1995;15:7892–7904. doi: 10.1523/JNEUROSCI.15-12-07892.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P, Brainard DH. Physiology and morphology of color-opponent ganglion cells in a retina expressing a dual gradient of S and M opsins. J. Neurosci. 2009;29:2706–2724. doi: 10.1523/JNEUROSCI.5471-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]