Summary
A three-dimensional 1H chemical shift/1H-15N dipolar coupling/15N chemical shift correlation spectrum was obtained on a sample of specifically 15N-labeled magainin peptides oriented in lipid bilayers between glass plates in a flat-coil probe. The spectrum showed complete resolution of the resonances from two labeled amide sites in all three dimensions. The three orientationally dependent frequencies associated with each resonance enabled the orientation of the peptide planes to be determined relative to the direction of the applied magnetic field. These results demonstrate the feasibility of multiple-pulse spectroscopy in a flat-coil probe, the ability to measure three spectral parameters from each site in a single experiment, and the potential for resolving among many labeled sites in oriented membrane proteins.
Keywords: Solid-state NMR, Magainin, Membranes, Oriented samples, Structure determination
Solid-state NMR spectroscopy of macroscopically oriented systems is a particularly appealing approach to the structural characterization of peptides and proteins associated with bilayer membranes (Cross and Opella, 1994), since their three-dimensional structures are intimately related to the highly ordered and anisotropic environment of phospholipid bilayers, and suitable well-oriented samples can be prepared for these compounds. Orientationally dependent spectral parameters are measured and then interpreted in terms of the angles between the individual bonds and the direction of the applied magnetic field. Generally, this has been accomplished using specifically labeled samples, where the spectral parameters from one or, at most, a few sites are measured in each experiment (Bechinger et al., 1991). The implemenuation of three- and four-dimensional solid-state NMR experiments (Ramamoorthy et al., 1995a,b) in Structural studies of oriented membrane samples is attractive, because it gives opportunities for resolving among multiple overlapping resonances and for measuring multiple spectral parameters from experiments on uniformly labeled peptides and proteins.
In this communication we describe the application of a recently developed three-dimensional solid-state NMR experiment (Ramamoorthy et al., 1995a) that correlates 1H chemical shift, 1H-15N heteronuclear dipolar coupling, and 15N chemical shift frequencies of a sample of specifically 15N-labeled magainin peptides in lipid bilayers oriented between glass plates in a flat-coil probe (Bechinger and Opella, 1991). Each resonance in the resulting 3D spectrum is characterized by three frequencies that can be analyzed by the graphical restriction plot method (Stewart et al., 1987) to yield the orientations of the individual peptide planes, which can then be assembled to form the polypeptide structure (Opella et al., 1987). The spectrum also demonstrates the potential for resolution among resonances from multiple sites in uniformly labeled proteins. An important technical finding is that a flat-coil probe has adequate rf homogeneity for multiple-pulse line narrowing of 1H resonances.
The samples used for these studies consisted of specifically 15N-labeled magainin2 peptides in hydrated phospholipid bilayers oriented between glass plates. The magainins are a family of 21–26-residue amphipathic helical peptides, found originally in frog skins, which have potent antibiotic activities (Zasloff, 1987). There is strong evidence that their biological activities are due to interactions with membranes rather than protein receptors. These peptides have been shown to be helical in several membrane environments by multidimensional solution NMR spectroscopy (Marion et al., 1988; J. Gesell, M. Zasloff and S.J. Opella, unpublished results). Our earlier 1D and 2D solid-state NMR studies of magainins showed that residues throughout the peptide form an α-helix with its axis aligned parallel to the plane of the bilayers (Bechinger et al., 1991,1992,1993; Opella et al., 1993).
The sequence of magainin2 is n-GIGKFLHSAKKFGKAFVGEIMNS-amide. Two peptides were synthesized separately with 15N-valine at position 17 or with 15N-phenylalanine at position 16 (Bechinger et al., 1993). The sample with two different labeled magainins was prepared by mixing 7 mg of each of the labeled peptides with sufficient palmitoyl-oleoyl-phosphatidylcholine (POPC) (80%) and palmitoyl-oleoyl-phosphatidylglycerol (POPG) (20%) to obtain a final molar ratio of 4% peptide in the lipids. The mixtures of peptides and lipids were co-solubilized in chloroform with a trace amount of trifluoroethanol and then spread on the surface of 20 18×18 mm glass plates, the thickness of which was reduced from 0.1 to 0.06 mm by etching in a solution of 8% HF in ethanol (v/v) as described by Prosser et al. (1995), to give the highest possible filling factor within the basic geometry of a fiat-coil probe. After air drying of the sample, residual solvent was removed under vacuum for 2 h. The plates were then stacked and the sample was hydrated at 45 °C for more than 12 h. After hydration, the sample was equilibrated at 93% relative humidity for an extended period of time in a closed chamber. The sample was wrapped with a plastic film prior to insertion in the rf coil of the probe.
The NMR experiments were performed on a Chemagnetics CMX-400 spectrometer equipped with a wide-bore Oxford 400/89 magnet. The home-built double-resonance probe, tuned for 1H at 400.6 MHz and 15N at 40.6 MHz, had a rectangular four-turn coil with inner dimensions of 18×18×5 mm. During the experiments, the sample temperature and hydration were maintained by flowing large volumes of prehumidified air over the coil and sample. For the one-dimensional experiments, an rf field strength corresponding to 61 kHz was employed with MOIST phase-alternated single-contact cross-polarization (Levitt, 1986) to generate magnetization prior to data acquisition. The three-dimensional pulse sequence has been recently described (Ramamoorthy et al., 1995a). It utilizes flip-flop Lee–Goldburg homonuclear decoupling (Lee and Goldburg, 1965; Mehring and Waugh, 1972; Bielecki et al., 1990) to achieve line narrowing of the 1H resonances during t1 as well as during t2, when spin-exchange at the magic angle (SEMA) (Wu et al., 1994) is effected for measurement of the 1H-15N heteronuclear dipolar couplings. Continuous 1H irradiation is applied during data acquisition in t3 to provide 1H decoupling of the observed 15N resonances.
Multiple-pulse line-narrowing experiments are critically dependent on rf homogeneity, and this was of considerable concern with regard to the potential applicability of these pulse sequences in flat-coil probes. However, we found that the line narrowing achievable in a flat-coil probe is similar to that for a solenoidal-coil probe, demonstrating that the rf homogeneity is adequate, at least with flip-flop Lee–Goldburg sequences, for experimental studies of large samples oriented between glass plates. The line widths observed for the oriented bilayer samples in a flat-coil probe were about 1.3 ppm for the 1H resonances and about 400 Hz for the 1H-15N dipolar couplings, which, although somewhat broader than those observed (0.8 ppm and 180 Hz) for a single crystal sample in a solenoidal-coil probe, are quite favorable. Since the 15N resonance line width is 10 ppm in the oriented bilayer sample compared to 1.5–4 ppm in single crystal samples, at least some of the residual line widths are likely to be due to imperfections in sample orientation rather than limitations of the pulse sequences or coil geometry. The 15N resonance line widths observed in these spectra are somewhat narrower than those in our previous studies. This reflects better sample orientation resulting from the optimization of conditions and preparative procedures; however, considerable room for improvement remains. The experiments started with an rf field strength of 42 kHz on both the 1H and 15N frequencies, resulting from rf irradiations of 0.2 and 1.1 kW, respectively; during t2, the 15N rf field strength was increased to 51 kHz (1.5 kW), and the 1H frequency was shifted by 29.5 kHz to satisfy the Lee–Goldburg off-resonance condition for magic angle irradiation. The 1H rf field strength was increased to 61 kHz (0.4 kW) to provide adequate decoupling during data acquisition. The drift of the magnet was compensated by shifting the carrier frequencies every 6 h during the course of the experiment. The 1H and 15N chemical shifts are referenced to 0 ppm for external liquid samples of TMS and ammonia, respectively. Several 1D and 2D tune-up experiments on test samples were used to optimize the spectrometer conditions. To minimize the effects of offset in the 1H frequency dimension, the central rf carrier frequency was placed near the 1H resonance frequencies of the peptides.
Figure 1 compares the 1D 15N NMR spectra obtained from two different samples of specifically 15N-labeled magainin peptides in oriented bilayers. Two resonances are present in the spectrum in Fig. 1A, because it was obtained on a sample containing a mixture of magainin peptides labeled in two different residues. In contrast, the spectrum in Fig. 1B, which was obtained from a sample containing only 15N-Phe16 labeled magainin, has one single-line resonance at 73.8 ppm, in good agreement with previous measurements (Bechinger et al., 1993). The resonance from Phe16 in the spectrum in Fig. 1A can be assigned by direct comparison with the spectrum in Fig. 1B; thus, the resonance at 83.8 ppm can be assigned by difference to Val17, also in agreement with previous results (Bechinger et al., 1993).
Fig. 1.
15N solid-state NMR spectra of specifically 15N-labeled magainin peptides in oriented lipid bilayers. (A) Mixture of 15N-Phe16 and 15N-Val17 labeled peptides. (B) 15N-Phe16 labeled peptide. The spectra were obtained by cross-polarization with 1H decoupling. The mixing time was 1 ms and the recycle delay was 2.5 s. For each spectrum, obtained at 25 °C, 6000 acquisitions were signal averaged.
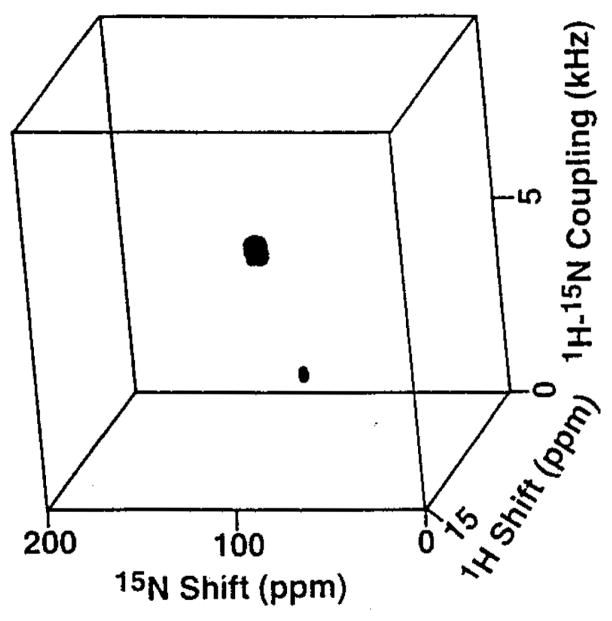
The 3D spectrum in Fig. 2 was obtained on the same sample used for the spectrum in Fig. 1A, which contains magainin peptides 15N-labeled at Phe16 and Val17. Each of the resonances is characterized by the three frequencies that represent the orientational dependence of the operative 1H chemical shift, the 1H-15N dipolar coupling, and the 15N chemical shift interactions. This is illustrated in Fig. 3, which contains slices through the resonance of Val17 along all three frequency axes. These frequencies provide the basis for spectral resolution and structure determination.
Fig. 2.
Three-dimensional correlation spectrum obtained on the sample used in Fig. 1A. The spectrum was obtained from 6 t1 and 6 t2 experiments with dwell times of 38 and 47.5 μs, respectively. In each experiment, 3000 acquisitions were collected. Linear prediction was applied on the time-domain signals. The data were processed with the program FELIX (Biosym Technologies, San Diego, CA).
Fig. 3.

One-dimensional spectral slices taken from the three-dimensional correlation spectrum in Fig. 2 for the resonance from Val17. (A) 15N chemical shift; (B) 1H-15N dipolar coupling; and (C) 1H chemical shift.
The anisotropies of the chemical shift and dipolar interactions are the sources of orientational information in this approach to structure determination. The measured frequencies are a function of the magnitudes and orientations of the principal elements of the spin-interaction tensors in the molecular frame and the orientation of the molecular site with respect to the applied magnetic field. It is possible to calculate unique values for the expected frequencies when the spin-interaction tensors have been determined and the orientation of the site is known. The converse is not true, since there are generally two angles associated with each frequency of an axially symmetric interaction, such as the heteronuclear dipolar coupling, and there may be many angles that correspond to a frequency from a non-axially symmetric interaction, including most chemical shift tensors. The measurement of frequencies from multiple interactions enables these ambiguities to be reduced to a manageable level. In order to evaluate the restrictions in possible molecular orientations associated with each measured frequency, we utilize a graphical restriction plot method (Stewart et al., 1987).
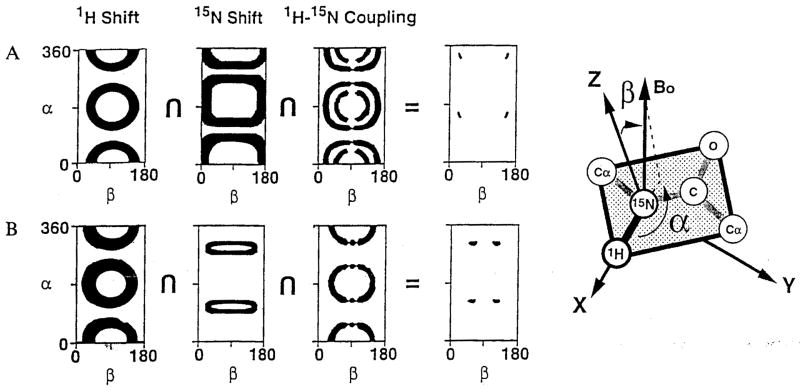
The graphical method for determining the orientations of peptide planes from the spectral parameters measured in solid-state NMR experiments on oriented samples is illustrated in Fig. 4 for the peptide planes from Phe16 and Val17 in magainin, using the data obtained from the single 3D spectrum in Fig. 2. The dark areas correspond to those pairs of α and β angles, as defined in the right part of Fig. 4, that are consistent with the experimental measurements. The full ranges of angles reflect experimental errors, uncertainty in the magnitudes and principal values of the spin-interaction tensors, as well as the fundamental geometrical dependencies of the various spin interactions (Stewart et al., 1987; Chirlian and Opella, 1990). Each experimental measurement restricts the number of possible orientations that can be present for an individual peptide plane in the sample. Generally, the intersection of the restriction plots from three measurements is needed to determine the orientation of a peptide plane. In most cases, four symmetry-related orientations are the successful outcome of the experimental determination. This reflects the symmetries inherent in the spin interactions of nuclei with spin S=1/2. Further reductions are possible with the interactions of quadrupolar nuclei with S=1 (McNamara et al., 1995). In practice, the finding of four symmetry-related orientations for a peptide plane is rarely a limitation, because of the additional geometrical and chemical requirements for assembling the peptide planes into a satisfactory covalent structure. The orientations for Phe16 and Val17 of magainin derived from the restriction plots in Fig. 4 are shown in Fig. 5. These restriction plots were generated using orientations of the 1H chemical shift, the 1H-15N heteronuclear dipolar coupling, and the 15N chemical shift tensors determined for the model peptide AIa-15N-Leu (Wu et al., 1995). The 1H-15N dipolar coupling and σ11 of the 1H chemical shift tensor are collinear with the N-H bond. σ33 of the 15N chemical shift tensor is tilted 17° with respect to the N-H bond. The magnitude of the dipolar coupling corresponded to an N-H bond length of 1.08 Å, a typical value for peptide bonds measured by solid-state NMR methods. The magnitudes of the principal elements of the 1H and 15N chemical shift tensors were measured directly from powder patterns of unoriented samples of magainin in lipid bilayers.
Fig. 4.
Restriction plots derived from the data in Fig. 2 listed in Table 1. The measured values reflect uncertainties of ± 1.2 ppm for both chemical shift measurements and ±0.2 kHz for the dipolar coupling. The orientations of the principal elements of the tensors were derived from the Ala-Leu dipeptide. The magnitudes were measured from unoriented magainin samples. The pertinent angles are defined in the axis system depicted on the right.
Fig. 5.
The four possible peptide plane orientations that are consistent with the experimental data for Phe16 and Val17 of magainin2 in oriented bilayers. In this representation, the magnetic field direction is out of the page toward the viewer.
The peptide plane orientations in Fig. 5 can be assembled into the two low-energy helical structures shown in Fig. 6 using the computer program TOTLINK (Opella and Stewart, 1989). They are consistent with our previous structural interpretation based on two measurements for each of four contiguous peptide planes (Bechinger et al., 1993). The results summarized in the intersection plots in Fig. 4 are substantially improved compared to those presented previously. The 3D spectrum gives the additional spectral parameter of 1H chemical shift. Since the pulse sequence incorporates spin exchange at the magic angle to measure the dipolar couplings, these values have greatly enhanced precision compared to those obtained from conventional separated local field experiments. An additional, smaller improvement is obtained due to the narrower 15N resonance line widths that result from improved sample orientation. The improvement in the characterization of the magnitudes and orientations of the principal values of the spin-interaction tensors at the amide nitrogen site in a peptide bond, resulting from the application of 3D solid-state NMR experiments to powder samples, also helps to reduce the number of possible orientations that are consistent with the experimental data (Wu et al., 1995).
Fig. 6.
The two helical arrangements of the peptide planes obtained from the 16 possible combinations of the Phe16 and Val17 peptide plane orientations shown in Fig. 5.
The 3D solid-state NMR spectrum of an oriented sample of a membrane-associated peptide demonstrates, by itself, several important aspects of the feasibility of applying NMR spectroscopy to membrane proteins (Opella et al., 1994). First of all, it shows that higher dimensional experiments utilizing multiple-pulse procedures can be performed in a flat-coil probe. Second, it demonstrates that the three spectral parameters required for determination of peptide plane orientations can be measured in a single spectrum. And, third, it shows the potential for resolution among resonances from multiple-labeled sites. These findings set the stage for studies of uniformly labeled proteins obtained by expression in bacteria rather than chemical synthesis. This will have an important influence on the generality of the application of solid-state NMR spectroscopy for protein structure determination.
TABLE 1.
EXPERIMENTAL VALUES FOR THE MEASURED SPECTRAL PARAMETERS OF TWO 15N-LABELED RESIDUES IN AN ORIENTED BILAYER SAMPLE OF MAGAININ
| Residue | 1H shift (ppm) | 1H-15N coupling (kHz) | 15N shift (ppm) |
|---|---|---|---|
| Phe16 | 9.1 | 0.6 | 73.8 |
| Val17 | 9.9 | 6.6 | 83.8 |
The values have an estimated precision of ± 1.2 ppm for the chemical shift measurements and ±0.2 kHz for the dipolar coupling measurements.
Acknowledgments
We thank R.S. Prosser and R.R. Vold for helpful discussions and sending a preprint on the use of ultrathin glass plates for oriented NMR samples. This research was supported by Grants RO1GM29754 and RO1A120770 from the General Medical Sciences and Allergy and Infectious Disease Institutes, National Institutes of Health, and utilized the Resource for Solid-State NMR of Proteins at the University of Pennsylvania, supported by Grant P41RR09731 from the Biomedical Research Technology Program, Division of Research Resources, National Institutes of Health. F.M.M. was supported by postdoctoral fellowship 9304FEN-1004-43344 from the Medical Research Council of Canada.
References
- Bechinger B, Opella SJ. J Magn Reson. 1991;95:585–588. [Google Scholar]
- Bechinger B, Kim Y, Chirlian LE, Gesell J, Neumann JM, Montal M, Tomich J, Zasloff M, Opella SJ. J Biomol NMR. 1991;1:167–173. doi: 10.1007/BF01877228. [DOI] [PubMed] [Google Scholar]
- Bechinger B, Zasloff M, Opella SJ. Biophys J. 1992;62:12–14. doi: 10.1016/S0006-3495(92)81763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechinger B, Zasloff M, Opella SJ. Protein Sci. 1993;2:2077–2084. doi: 10.1002/pro.5560021208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki A, Kolbert AC, De Groot HJM, Griffin RG, Levitt MH. Adv Magn Reson. 1990;14:111–124. [Google Scholar]
- Chirlian LE, Opella SJ. New Polymeric Mater. 1990;2:279–290. [Google Scholar]
- Cross TA, Opella SJ. Curr Opin Struct Biol. 1994;4:574–581. [Google Scholar]
- Lee M, Goldburg WI. Phys Rev. 1965;A140:1261–1271.
- Levitt MH. J Chem Phys. 1986;94:30–38. [Google Scholar]
- Marion D, Zasloff M, Bax A. FEBS Lett. 1988;227:21–26. doi: 10.1016/0014-5793(88)81405-4. [DOI] [PubMed] [Google Scholar]
- McNamara R, Wu CH, Chirlian LE, Opella SJ. J Am Chem Soc. 1995;117:7805–7811. [Google Scholar]
- Mehring M, Waugh JS. Phys Rev. 1972;B5:3459–3471. [Google Scholar]
- Opella SJ, Stewart PL, Valentine KG. Q Rev Biophys. 1987;19:7–49. doi: 10.1017/s0033583500004017. [DOI] [PubMed] [Google Scholar]
- Opella SJ, Stewart PL. Methods Enzymol. 1989;176:242–275. doi: 10.1016/0076-6879(89)76015-8. [DOI] [PubMed] [Google Scholar]
- Opella SJ, Gesell J, Bechinger B. In: The Amplupathic Helix. Epand R, editor. CRC Press; Boca Raton, FL: 1993. pp. 87–106. [Google Scholar]
- Opella SJ, Kim Y, McDonnell P. Methods Enzymool. 1994;239:536–560. doi: 10.1016/s0076-6879(94)39021-5. [DOI] [PubMed] [Google Scholar]
- Prosser RS, Hunt SA, Void RR. J Magn Reson Ser B. 1995;109:109–111. [Google Scholar]
- Ramamoorthy A, Wu CH, Opella SJ. J Magn Reson Ser B. 1995a;107:88–90. doi: 10.1006/jmrb.1995.1063. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy A, Gierasch LM, Opella SJ. J Magn Reson Ser B. 1995b;109:112–116. doi: 10.1006/jmrb.1995.1157. [DOI] [PubMed] [Google Scholar]
- Stewart PL, Valentine KG, Opella SJ. J Magn Reson. 1987;71:45–61. [Google Scholar]
- Wu CH, Ramamoorthy A, Opella SJ. J Magn Reson Ser A. 1994;109:270–272. [Google Scholar]
- Wu CH, Ramamoorthy A, Gierasch LM, Opella SJ. J Am Chem Soc. 1995;117:6148–6149. [Google Scholar]
- Zasloff M. Proc Natl Acad Sci USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]