Abstract
Lifelong antibody responses to vaccination require reorganization of lymphoid tissue and dynamic inter-cellular communication called the germinal center reaction. B lymphocytes undergo cellular polarization during antigen stimulation, acquisition, and presentation, which are critical steps for initiating humoral immunity. Here we show that germinal center B lymphocytes asymmetrically segregate the transcriptional regulator Bcl6, the receptor for interleukin-21, and the ancestral polarity protein atypical PKC to one side of the plane of division, generating unequal inheritance of fate-altering molecules by daughter cells. Germinal center B lymphocytes from mice with a defect in leukocyte adhesion fail to divide asymmetrically. These results suggest that motile cells lacking constitutive attachment can receive provisional polarity cues from the micro-environment to generate daughter cell diversity and self-renewal.
Humoral immunity requires an antigen-specific B cell to proliferate, differentiate, and self-renew. Refinement of antibody responses occurs in the germinal center (GC), a dynamic lymphoid structure where B cells with high-affinity receptors are selected to proliferate and give rise to antibody-secreting plasma cells and memory B cells (1, 2). Intravital imaging has suggested that B cells engage in prolonged, motile contacts with follicular helper T (TFH) cells prior to dividing and entering the GC (3, 4). In the GC, B cells acquire antigen from synapsis with follicular dendritic cells (FDCs) (5, 6), and clones with higher affinity receptors are thought to compete for subsequent contact with TFH cells by presenting greater abundance of antigen (3, 7). What signals promote disparate fate choices and regeneration of selected B cells are incompletely understood.
T lymphocytes engaged in immune responses appear to obtain polarity cues from antigen-presenting cells, yielding asymmetric division and diversification of daughter cell fates (8–10). Selection of B cells before and within the GC is linked to acquisition and presentation of antigen, processes characterized by provisional polarity of B cells (11). We, therefore, asked whether selected B cells might receive external polarity cues that could foster asymmetric division. To test this, we examined the morphology of splenic GC B cells from immunized mice to ascertain localization of key proteins (12). We initially examined the receptor for interleukin 21 (IL-21R) and the transcriptional regulator Bcl6, which function to initiate and maintain GC B cell identity, respectively (13–16).
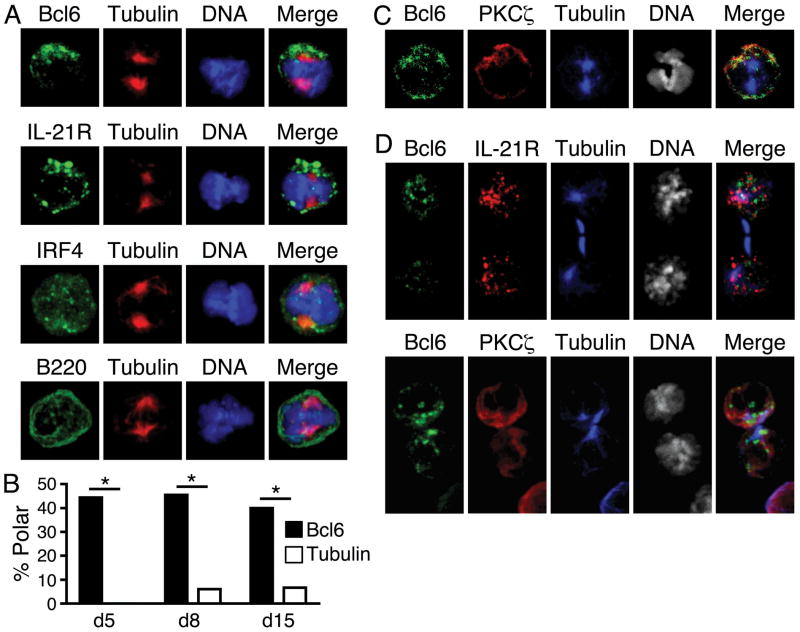
In GC B cells undergoing mitosis 8d following immunization, Bcl6 and IL-21R asymmetrically segregated to one side of the plane of division in 44% and 43% of cells (Fig. 1A). By contrast, the transcription factor IRF4 (17) and the transmembrane receptor B220 were symmetrically distributed (11% asymmetric). Asymmetry of Bcl6 in mitotic cells was also observed earlier (d5) and later (d15) after immunization (Fig. 1B). In pre-mitotic GC B cells, Bcl6 was localized within the nucleus, while IL-21R and the cell polarity regulator atypical PKCζ co-localized with the microtubule organizing center (MTOC) in a polarized manner (Fig. S1). PKCζ remained polarized in mitotic cells (Fig. 1C), suggesting that B cell asymmetric division has evolutionarily conserved features (8–11, 18).
Fig. 1.
Asymmetric division of germinal center (GC) B cells. (A) Mitotic GC B cells from pooled spleens of 3 mice at d8 post-immunization stained for Bcl6, IL-21R, IRF4, or B220 (green), β-tubulin (red), and DNA (blue). Compared to tubulin, Bcl6 asymmetry 44%, n=16 mitoses, p<0.05; IL-21R asymmetry 43%, n=14, p<0.05; IRF4 asymmetry 11%, n=18, p>0.05; B220 asymmetry 11%, n=18, p>0.05. (B) Asymmetric mitoses of Bcl6 (filled) and tubulin (open) at d5, d8, d15 post-immunization, n=9, 16, 15, respectively, * = p<0.05. p values calculated with chi-squared tests. (C) Bcl6 and PKCζ co-segregate asymmetrically during mitosis at d8 post-immunization. Bcl6 and PKCζ were asymmetric in 44% and 38% of mitoses, respectively, and were 100% co-segregated when both were asymmetric, n=16 mitoses. (D) Isolated GC B cells undergoing cytokinesis maintain asymmetry. Bcl6, IL-21R, and PKCζ were asymmetric in 45%, 53%, and 61% of divisions, respectively, and had 100% co-segregation when both were asymmetric. Analysis of Bcl6 and IL-21R, n=13 cytokinetic cells; analysis of Bcl6 and PKCζ, n=13. All data are representative of 2 or more experiments.
GC B cell division is thought to occur in a zone relatively free of FDCs and TFH cells (7). We, therefore, tested whether B cells could break free of polarity cues and retain an imprint of asymmetry. We mechanically disrupted splenic inter-cellular contacts into single-cell suspensions and cultured unattached GC B cells for 3h in the presence of cytochalasin B to arrest cells undergoing cytokinesis. Cells cleaving in isolation retained the property of asymmetric distribution of Bcl6, IL-21R, and PKCζ (Fig. 1D). In mitotic and cytokinetic cells, PKCζ co-segregated with Bcl6 and IL-21R in all instances where both were asymmetric (Fig. 1, C and D). PKCζ also co-localized with IL-21R at the single MTOC of interphase blasts in vivo (Fig. S1), as has been observed in vitro for PKCζ and antigen-presenting components of the B cell synapse (11). Thus, the daughter cell with preferential inheritance of Bcl6, IL-21R, and PKCζ may have arisen from the side of the B cell that was once proximal, and presenting antigen, to a TFH cell.
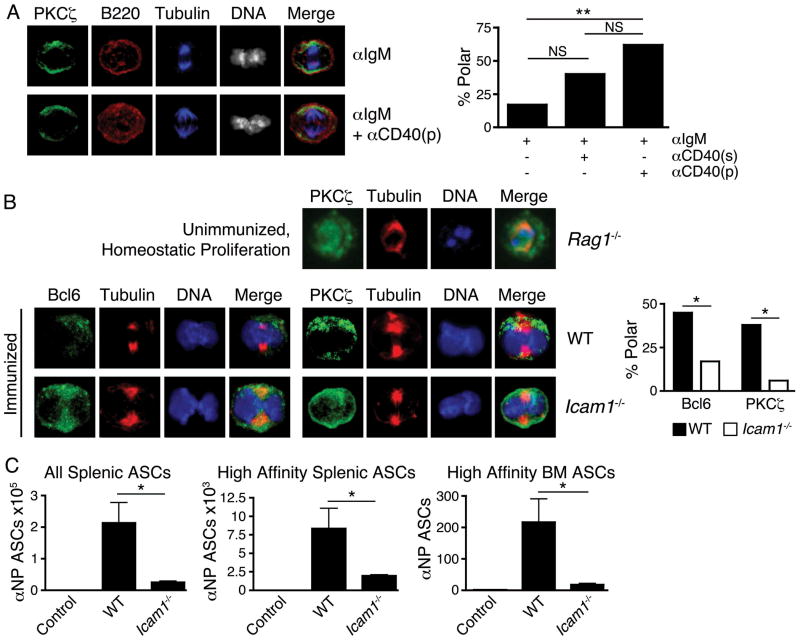
To determine whether TFH cell-associated signals promote mitotic B cell polarity, we stimulated naïve, CD23+ B cells in vitro with or without addition of anti-CD40 antibody to mimic a critical signal from TFH cells. Anti-CD40 in the culture media increased mitotic PKCζ polarity compared to baseline stimulation, while provision of anti-CD40 by adherence to the culture-dish bottom further increased the frequency of mitotic PKCζ asymmetry (Fig. 2A). CD40 signaling, as occurs when B cells present antigen to and receive cognate help from TFH cells, thus appears to facilitate asymmetry by providing and/or focusing a polarity cue to the B cell.
Fig. 2.
Polarity cues are required for asymmetric division of B cells. (A) Naive B cells simulated 36h with anti-IgM, with or without anti-CD40 to mimic a key contact-mediated signal from TFH cells. Anti-CD40 was withheld, added solubly to media (“s”), or coated on cell culture plates (“p”). Left, representative microscopy of no added anti-CD40 (upper) and plate-bound anti-CD40 (lower). Right, quantification of mitoses exhibiting asymmetry of PKCζ in indicated conditions. Left-to-right, n=18, 20, 21 mitoses, respectively. (B) Upper row, B cells undergoing homeostatic proliferation in Rag1−/− mice exhibit low incidence of asymmetric mitoses (11%, n=19 mitoses from pooled spleens of 3 recipients) compared to GC B cells from immunized mice (38%, n=16 mitoses); p<0.05. Lower rows, defective asymmetric division in adhesion-deficient mice. Left, GC B cells from 3 pooled spleens of d8 immunized wild-type (“WT”) and Icam1−/− mice stained for indicated components. Right, quantification of GC B cell mitoses exhibiting asymmetry of Bcl6 and PKCζ from indicated genotypes. Left-to-right, n=33, 18, 16, 18 mitoses, respectively. NS = p>0.05, * = p<0.05, ** = p<0.01 by chi-squared test. Similar results obtained at d5. (C) Icam1−/− mice have impaired antibody responses to immunization. Quantification of antibody secreting cells (ASCs) from immunized WT and Icam1−/− mice d14 post-immunization, compared to adjuvant-only injected WT mice (“Control”). n=6 mice/group, NS = p>0.05; * = p<0.05 by unpaired student’s t-test. All data are representative of 2 or more experiments.
Further evidence that extrinsic contact may be critical for B cell asymmetric division was obtained from two separate in vivo approaches. Firstly, naïve B cells were transferred into lymphocyte-deficient mice (Rag1−/−) wherein excess growth factors support brisk lymphocyte division without need of antigen stimulation or cell-cell contact. Unimmunized B cells undergoing homeostatic division exhibited infrequent polarity of PKCζ (11%) compared to B cells from immunized mice (38%) (Fig. 2B).
It has been suggested that optimal B cell-T cell adhesion requires LFA-1-ICAM-1 interactions (19) and humans with leukocyte adhesion defects may exhibit impaired antibody production (20). Secondly, we tested whether ICAM-1 is required for B cells to divide asymmetrically following immunization. The frequencies of mitotic GC B cells that asymmetrically segregated Bcl6 and PKCζ were substantially reduced in the absence of ICAM-1 (Fig. 2B), suggesting cell-cell adhesion may be critical to the polarity cue received by asymmetrically dividing B cells. Absence of optimal cell adhesion had functional correlates beyond defective asymmetric B cell division insofar as immunized Icam1−/− mice exhibited substantial defects in generating antibody-secreting cells (Fig. 2C), despite relative preservation of germinal center composition (Fig. S2).
GC selection appears to license a B cell with a high-affinity receptor to proliferate (7). The present findings suggest that coupling receptor affinity to proliferation may do more than amplify the fittest cells. Coupling affinity to proliferation and asymmetric division may also provide the fittest cell with a robust mechanism, like that of a stem cell, to ensure its lifelong renewal (8, 18). Further investigation will be required to identify the fate of daughter B cells arising from asymmetric divisions before and during the GC reaction, and the requirements for cell diversification and regeneration in the immune response to pathogens and vaccines. Finally, it will be important to determine whether asymmetric division following facultative polarity is a widely conserved feature of mammalian differentiation and regeneration in normal and neoplastic cells.
Supplementary Material
Acknowledgments
We thank J. Chaix, S. Gordon, M. Paley, and L. Rupp for assistance. We are grateful for funding from NIH (AI042370 and AI076458 to S.L.R., T32GM07229 to B.E.B., T32HD007516 and 3T32GM007170-36S1 to M.L.C., T32AI055428 to R.G., AI065644 to J.K.B.), DOD (W81WWXWH1010185 to M.P.C.), VA (BX000435 to T.M.L.), and the Abramson family (S.L.R.). The data reported in this paper are tabulated in the main paper and in the Supporting Online Material.
Footnotes
The authors declare no conflicts of interest.
References and Notes
- 1.MacLennan IC. Annu Rev Immunol. 1994;12:117. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 2.Nutt SL, Tarlinton DM. Nat Immunol. 2011;12:472. doi: 10.1038/ni.2019. [DOI] [PubMed] [Google Scholar]
- 3.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. Nature. 2008;455:764. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada T, et al. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki K, Grigorova I, Phan TG, Kelly LM, Cyster JG. J Exp Med. 2009;206:1485. doi: 10.1084/jem.20090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwickert TA, et al. Nature. 2007;446:83. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 7.Victora GD, et al. Cell. 2010;143:592. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JT, et al. Science. 2007;315:1687. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 9.Chang JT, et al. Immunity. 2011;34:492. doi: 10.1016/j.immuni.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliaro J, et al. J Immunol. 2010;185:367. doi: 10.4049/jimmunol.0903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuseff MI, et al. Immunity. 2011;35:361. doi: 10.1016/j.immuni.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Materials and methods are available as supporting material on Science Online.
- 13.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Science. 1997;276:589. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 14.Ye BH, et al. Nat Genet. 1997;16:161. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- 15.Linterman MA, et al. J Exp Med. 2010;207:353. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tunyaplin C, et al. J Immunol. 2004;173:1158. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- 17.Klein U, et al. Nat Immunol. 2006;7:773. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 18.Knoblich JA. Nat Rev Mol Cell Biol. 2010;11:849. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannons JL, et al. Immunity. 2010;32:253. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer A, Durandy A, Sterkers G, Griscelli C. J Immunol. 1986;136:3198. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.