Abstract
Higher-order executive tasks such as learning, working memory, and behavioral flexibility depend on the prefrontal cortex (PFC), the brain region most elaborated in primates. The prominent innervation by serotonin neurons and the dense expression of serotonergic receptors in the PFC suggest that serotonin is a major modulator of its function. The most abundant serotonin receptors in the PFC, 5-HT1A, 5-HT2A and 5-HT3A receptors, are selectively expressed in distinct populations of pyramidal neurons and inhibitory interneurons, and play a critical role in modulating cortical activity and neural oscillations (brain waves). Serotonergic signaling is altered in many psychiatric disorders such as schizophrenia and depression, where parallel changes in receptor expression and brain waves have been observed. Furthermore, many psychiatric drug treatments target serotonergic receptors in the PFC. Thus, understanding the role of serotonergic neurotransmission in PFC function is of major clinical importance. Here we review recent findings concerning the powerful influences of serotonin on single neurons, neural networks, and cortical circuits in the PFC of the rat, where the effects of serotonin have been most thoroughly studied.
Keywords: Prefrontal Cortex, Serotonin Receptors, Neural Oscillations, Pyramidal neuron, Fast-spiking interneuron, Electrophysiology
Introduction
The PFC is an associational cortical area that comprises the most anterior structures of the frontal lobes. It has been defined across species according to its reciprocal anatomical connections with the mediodorsal nucleus of the thalamus, although its exact anatomical boundaries are variable across mammalian species [1–3]. The PFC plays a central role in top-down control of many higher-order executive tasks. It is involved in learning [4,5], memory [6], categorization [5,7], inhibitory control [8,9] and cognitive flexibility [10–12], among others. In order to accomplish these tasks, the PFC is densely interconnected with numerous cortical and subcortical structures, such as the thalamus and the brainstem [1,3]. Importantly, it receives remarkably dense inputs from the neuromodulatory centers of the brainstem and forebrain, including the serotonergic nuclei in the raphe nuclei. Specifically, serotonin (5-hydroxytryptamine, 5-HT) neurons in the dorsal and median raphe nuclei send axons to the several subregions of the rodent PFC: the cingulate, prelimbic, and infralimbic cortices [13]. Early anatomical studies utilizing retrograde and anterograde tracing methods revealed that all of these cortices send projections back to the raphe nuclei, providing the substrate for feedback control of cortical 5-HT release [14–16]. Electrophysiological studies have confirmed the functionality of these bidirectional projections in vivo, yielding insights into the relative conduction velocities of descending glutamatergic axons originating in the PFC and ascending serotonergic axons from the raphe nuclei [17–21]. The robust anatomical and functional interconnections between the PFC and the raphe nuclei further suggest that 5-HT plays an important role in regulating executive function. Yet, despite many years of research, the mechanisms by which 5-HT facilitates higher-order cognition remains poorly understood.
Two observations help explain why defining the role of 5-HT in PFC function has been so challenging: 1) the PFC is an extraordinarily complex cellular microcircuit comprising numerous specialized neuron subtypes, and 2) the expression of serotonergic receptors among these cellular components is highly selective and sophisticated. Excitatory neurons in the PFC are primarily glutamatergic pyramidal neurons. Although similar in many regards, cortical pyramidal neurons are not homogenous, but have been subdivided into discrete subtypes based on morphological, physiological, and axonal target projections [22–30]. More importantly, subtypes of pyramidal neurons are very selective in their connectivity and form multiple parallel excitatory pathways within the cortical circuit that target specific cortical and subcortical structures via long-distance axonal projections [23,27–29,31]. In addition to excitatory pyramidal neurons, the PFC also contains a diverse complement of inhibitory GABAergic interneurons that are also selective in forming connections with each other and with networks of pyramidal neurons. Cortical interneurons are classified into subtypes based on their morphology, chemical neuroanatomy, and electrophysiological properties [32–36]. While a detailed description of cortical neuron diversity is beyond the scope of this review, we will discuss several pyramidal and interneuron subtypes modulated by 5-HT.
The rat medial PFC consists of 5 layers (a discrete layer 4 is lacking), in which populations of excitatory and inhibitory neurons are organized in precise arrangements. Layer 1 is the most superficial and is dominated by neuropil composed of the apical dendritic tufts of cortical pyramidal neurons, and the axons from local and long-distance cortical and subcortical inputs. Layer 1 also contains the somata of several types of slow-spiking GABAergic interneurons that provide feed-forward inhibition onto pyramidal neuron dendrites. Layers 2 and 3 contain the somata of small pyramidal neurons and several types of interneurons, including both slow- and fast-spiking interneurons. Layers 5 and 6, on the other hand, contain the somata of large pyramidal neurons and several interneuron subtypes, of which fast-spiking interneurons are the most abundant. 5-HT influences cortical information processing by activating multiple receptor subtypes selectively expressed by specific subclasses of pyramidal neurons and interneurons. Because the cellular organization of the PFC is complex and the expression patterns of 5-HT receptors not fully characterized, elucidating the functional impact of serotonergic modulation of cortical neurons and networks has been difficult. In the following sections we explore how selective expression of single or multiple 5-HT receptor subtypes differentially modulates the activity of cortical neuron subpopulations. We also tackle the crucial question of how these receptors regulate network activity, as reflected in changes in the amplitude and frequency of neural oscillations generated when populations of neurons fire in a synchronous manner.
Expression of Serotonin Receptors in the Prefrontal Cortex
5-HT is an evolutionarily ancient neurotransmitter, and is implicated in a large variety of behavioral processes. Based on pharmacological, structural, and transductional characteristics, 5-HT receptors are classified into seven subfamilies, 5-HT1 to 5-HT7, which comprise 14 receptor subtypes associated with unique genes. With the exception of 5-HT3 receptors, which are ligand-gated cation channels, 5-HT receptors couple to G-proteins to exert their effects on neuron activity [37]. The PFC is highly enriched with 5-HT1A and 5-HT2A receptors [38,39]. 5-HT1A receptors are generally considered inhibitory, whereas 5-HT2A receptors are excitatory (see below). In the rat, 60% of prefrontal pyramidal neurons express 5-HT1A or 5-HT2A receptors, particularly in layer 5 [38–47]. Interestingly, around 80% of these neurons co-express both receptors [17,46,48], despite the opposing influences they exert on neuronal excitability (Table 1). While the functional interaction of 5-HT1A and 5-HT2A receptors within single neurons is not yet fully understood, differential distribution of these receptors in cellular compartments is thought to play a critical role. 5-HT1A receptors are densely expressed on the axon initial segment of pyramidal neurons where they act to suppress action potential generation [44,49–52]. On the other hand, 5-HT2A receptors are abundant in apical dendrites [44,53], where they may amplify the impact of excitatory synaptic currents [54] (Figure 1). Recently, it was revealed that prefrontal pyramidal neurons also express 5-HT2C receptors, but the degree of co-expression with 5-HT1A and 5-HT2A receptors is still unknown [48].
Table 1.
Serotonergic regulation of neuron excitability in the neocortex
| Cell type | Pharmacology | 5-HT effects in vitro* | 5-HT effects in vivo* |
|---|---|---|---|
| Pyramidal | 5-HT1A | Inhibition87,88,89 | Inhibition17,20,123 |
| 5-HT2A | Excitation89,90,91 | Excitation17,19 | |
| 5-HT1A + 5-HT2A | Biphasic responses89 | Biphasic responses17 | |
|
| |||
| FS Interneuron | 5-HT1A | Inhibition106,107 | Inhibition48 |
| 5-HT2A | Excitation40,106 | Excitation48 | |
|
| |||
| SS Interneuron | 5-HT1A | Inhibition106 | N/A |
| 5-HT2A | Excitation60,85,106 | N/A | |
| 5-HT3A | Excitation60,63,85 | Excitation59 | |
FS, fast-spiking; SS, slow-spiking.
Note that the effects of 5-HT in these preparations have been examined in distinct stages of development: immature cortex in in vitro experiments and adult cortex in in vivo experiments. References indicated.
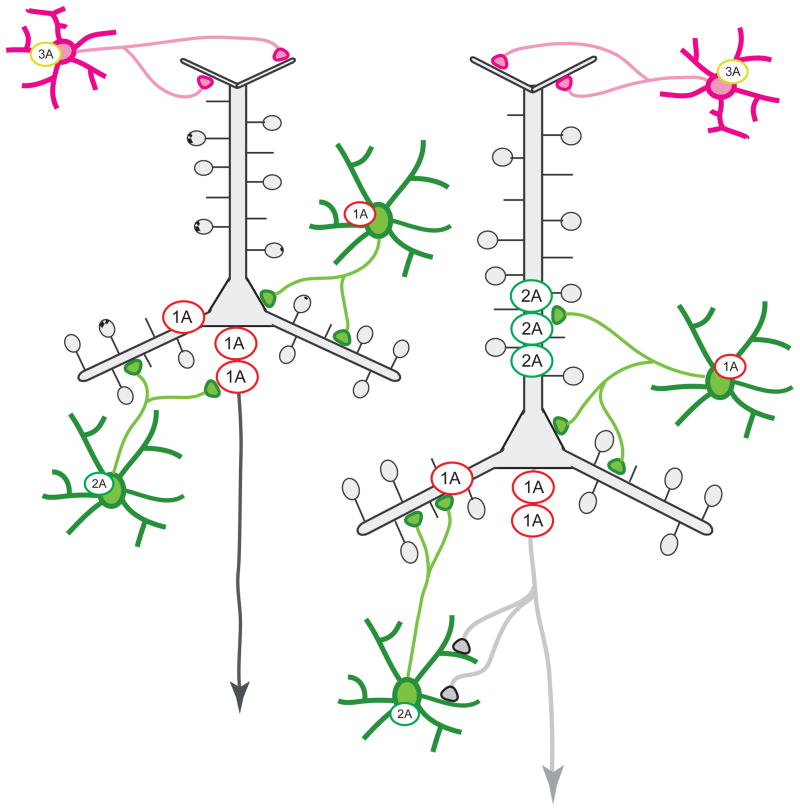
Figure 1. Localization of 5-HT receptors within the prefrontal cortex microcircuit.
Many pyramidal neurons in deep layers co-express 5-HT1A and 5-HT2A receptors. In addition, distinct populations of local inhibitory interneurons that express 5-HT receptors innervate different compartments of the pyramidal tree: 5-HT1A- and 5-HT2A-expressing fast-spiking interneurons are preferentially located in deep layers where they contact pyramidal neurons at the soma and proximal dendrites; slow-spiking interneurons that express 5-HT3A receptors are located in superficial layers where they innervate pyramidal neurons at the distal dendrites. Modified from references 21,178.
Inhibitory interneurons in the cortex express 5-HT1A, 5-HT2A, or 5-HT3A receptors [40,41,46–48,55–60] (Figure 1 and Table 1). Double and triple in situ hybridization histochemistry have revealed that, unlike pyramidal neurons, two separate populations of parvalbumin-expressing fast-spiking interneurons express either 5-HT1A or 5-HT2A receptors, but not both together, and not 5-HT3 receptors [58]. These interneurons are present in layers 2, 3 and 5, but are particularly abundant in layer 5, where each receptor subtype is expressed by ~50% of interneurons [48]. Thus, fast-spiking interneurons expressing either 5-HT1A or 5-HT2A mRNAs are enriched in layer 5, just as are pyramidal neurons expressing these receptors. In addition, several populations of slow-spiking interneurons express 5-HT3A receptors [58,59,61–63]. These interneurons are particularly abundant in layer 1, where they are expressed by 40% of inhibitory cells [59,64], and in layers 2 and 3 [55,61]. In the rat, these neurons also express cholecystokinin (CCK), vasoactive intestinal peptide (VIP), or neuropeptide Y [55,61]. A similar segregation of 5-HT2A- and 5-HT3-expressing interneurons is present in the non-human primate cortex [56]. It is noteworthy that 5-HT3A-expressing interneurons are located primarily in superficial cortical layers, whereas 5-HT1A- and 5-HT2A-expressing interneurons dominate deeper layers (Figure 1). This complementary distribution of receptors may allow 5-HT to independently regulate the excitability of different pyramidal neuron compartments to fine-tune synaptic integration and action potential generation.
5-HT receptors are also expressed at axon terminals in the cortex, where they can regulate impulse-dependent release of glutamate and GABA [60,65–75]. Although presynaptic regulation of transmitter release in the central nervous system is generally thought to involve 5-HT1B receptors [76,77], the receptor subtype(s) responsible for presynaptic serotonergic regulation of transmitter release in the PFC is not well established. Pharmacological data exist indicating the involvement of 5-HT1A, 5-HT1B, and/or 5-HT2 receptors, yet conclusive data regarding the presynaptic localization of 5-HT receptors in cortex is lacking. To date, the only ultrastructural data available from the rat PFC indicate that a minority of 5-HT2A receptors are localized to axon terminals, and those presynaptic receptors appear to regulate the release of monoamines, rather than glutamate or GABA [78]. Interestingly, serotonergic fibers are present in all cortical layers of the rat frontal cortex, but synaptic specializations are associated with only about 25% of 5-HT release sites, suggesting that the vast majority of 5-HT release is non-synaptic [79]. Similar results have been found in the cat [80] and primate [81] cortex. Those 5-HT release sites that are associated with synaptic specializations occur predominantly onto GABAergic interneurons in superficial cortical layers [82–84]. This pattern of serotonergic innervation suggests that the bulk of 5-HT signaling to pyramidal neurons and deep-layer interneurons (mostly parvalbumin-positive and somatostatin-positive interneurons) is via volume transmission from non-synaptic 5-HT release sites, while serotonergic input to certain interneuron subtypes in superficial layers [83] is synapse-specific. This selectivity in synapse formation makes conceptual sense because the ionotropic 5-HT3 receptors expressed by some superficial interneurons are capable of responding rapidly to synaptic release of transmitter [60,85]. Thus, it appears that serotonergic input to the medial PFC may tonically regulate the excitability of pyramidal neurons and interneurons in deep cortical layers, while generating phasic excitation in interneurons in superficial layers.
Serotonin Modulates Neuronal Activity in the Prefrontal Cortex
Effects of 5-HT on cortical neurons in vitro
As noted above, most adult pyramidal neurons in the rat PFC co-express both 5-HT1A and 5-HT2A receptors. Together, these receptors are responsible for the range of physiological responses to 5-HT observed in pyramidal neurons in vitro (Figure 2 and Table 1). Activation of 5-HT1A receptors, which are linked to Gi/o-type G-protein alpha subunits, generate inhibitory responses in pyramidal neurons via the activation of GIRK channels [86–88]. On the other hand, 5-HT2A receptors couple to Gq-type alpha subunits to promote neuronal excitation by mechanisms that are not fully characterized. Activation of 5-HT2A receptors reduce resting potassium conductances [89–91], which may contribute to depolarization of layer 5 pyramidal neurons. In addition, remarkable similarities between 5-HT2A-mediated afterdepolarizations (ADPs) that follows spike trains [92], and the ADPs mediated by metabotropic muscarinic [93,94] and glutamate receptors [95,96], suggest these excitatory responses may share a common mechanism that enhances neuron excitability. Indeed, a similar convergence of these transmitter systems generates excitation in human cortical pyramidal neurons [97]. While not well studied in regard to 5-HT, ADPs generated by Gq-linked acetylcholine and glutamate receptors are mediated by a calcium-sensitive nonspecific cation conductance [93,98–100]. The resulting calcium-dependent inward current, coupled with Gq-mediated inhibition of calcium-dependent potassium conductances [89–91], may therefore be responsible for increased action potential output in the presence of 5-HT. The calcium dependency of these mechanisms may explain why direct serotonergic excitation of layer 5 pyramidal neurons requires increases in intracellular calcium [91].
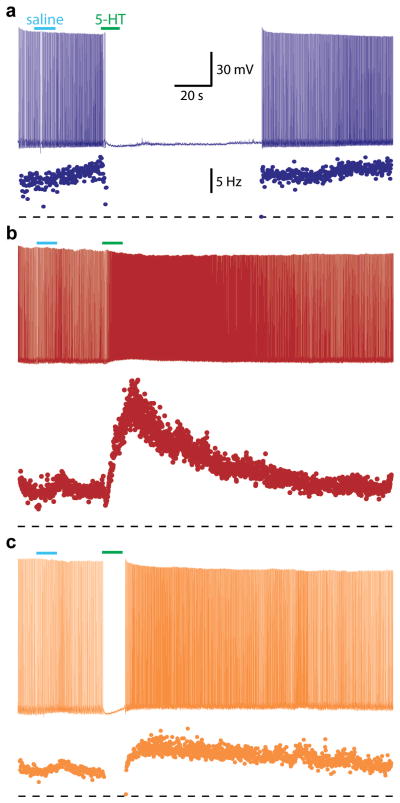
Figure 2. Three 5-HT response types are observed in layer 5 pyramidal neurons from the mouse prefrontal cortex slice.
(A) In most layer 5 pyramidal neurons, focal application of 5-HT (100 μM, green), but not drug-free saline (blue), generates a long-lasting inhibition of action potential firing (top). A plot of instantaneous spike frequency for each action potential is shown below. (B) In a minority of layer 5 pyramidal neurons, focal 5-HT application increases action potential generation. (C) Some layer 5 pyramidal neurons display biphasic responses to 5-HT. In all examples shown, DC somatic current injection was used to depolarize neurons and induce action potential generation. Dashed lines show the level of zero Hz. Scale bars in A apply to data in B and C. Unpublished data courtesy of Daniel Avesar and Allan T. Gulledge.
Because the endogenous transmitter, 5-HT, activates both 5-HT1A and 5-HT2A receptors, it is difficult to predict the net effect of 5-HT on individual pyramidal neuron activity. However it is clear that the majority of adult pyramidal neurons are functionally inhibited by 5-HT in a 5-HT1A-dependent manner [87–89,91,101,102], suggesting that 5-HT1A receptors play a dominant role in regulating pyramidal neuron activity. However, a minority of adult pyramidal neurons is excited by 5-HT in a 5-HT2A-dependent manner [89,102], and 5-HT2A receptor activation leads to an increase in spontaneous excitatory synaptic transmission in pyramidal neurons [102,103]. The ability of 5-HT2A receptor activation to depolarize layer 5 pyramidal neurons from their resting membrane potential is limited, and typically only a few millivolts of depolarization is observed [68,89,91,92,104]. However, 5-HT2A activation can enhance action potential generation in response to other sources of excitatory input [89,91]. This increase in the “gain” of pyramidal neurons likely acts synergistically with 5-HT2A receptor-dependent enhancement of excitatory synaptic transmission [54,60,88,105] to enhance synaptically driven action potential generation in layer 5 pyramidal neurons.
5-HT also modulates the excitability of GABAegic interneurons in the cortex, primarily through activation of 5HT1A, 5-HT2A, and 5HT3 receptors expressed on different interneuron subpopulations (see above and Table 1). However, there are only limited data regarding physiological responses of interneurons to 5-HT in the slice, with much of it restricted to neurons from immature (< 3-week-old) animals and non-prefrontal cortical areas [40,60,63,85,106]. In immature somatosensory cortex, Foehring et al. (2002) [85] described 5-HT2A/C-mediated excitatory responses occurring in most layer 2/3 interneurons, and in a population of layer 1 interneurons having descending axonal projections. Similarly, Weber et al. (2010) [40] reported 5-HT2A-dependent excitation in a subpopulation of fast-spiking interneurons in the medial PFC. On the other hand, most layer 1 interneurons with horizontal axonal projections were inhibited via 5-HT1A receptors. However a more recent study by Lee et al. (2010) [63] found in the juvenile somatosensory cortex that many slow-spiking interneurons in superficial cortical layers are excited by 5-HT3A receptors. In layer 5 of immature visual cortex, Xiang and Prince (2003) [106] found that 81% of low-threshold-spiking (likely somatostatin-expressing) and about 32% of fast-spiking (likely parvalbumin-expressing) interneurons were inhibited by 5-HT1A receptors, while another 40% of fast-spiking interneurons were excited via ionotropic 5-HT3 receptors (but see [107]). In the immature PFC, Zhou and Hablitz (1999) [60] found evidence for 5-HT2A and 5-HT3 receptor dependent increases in interneuron activity, largely as reflected in increased spontaneous, but not miniature, inhibitory synaptic transmission onto interneurons and pyramidal neurons. However, few direct effects of 5-HT on interneuron excitability were reported. Thus, our current knowledge of serotonergic regulation of specific interneuron classes in the adult neocortex is incomplete, and additional studies looking at serotonergic actions in identified interneuron subtypes will be necessary to characterize serotonergic regulation of inhibition in the adult PFC.
As mentioned above, 5-HT receptors are also expressed at presynaptic locations where they can modulate the impulse-dependent release of neurotransmitters, including glutamate and GABA. 5-HT1B receptors suppress glutamate release from corticostriatal terminals [74], suggesting that 5-HT may also regulate glutamate release from these same neurons at cortico-cortical synapses. Indeed, Troca-Marin and Geijo-Barrientos (2010) [65] found that 5-HT can suppress glutamate release from callosal projection axons. However involvement of 5-HT1B receptors was not specifically tested and clear identification of the receptor subtypes involved was lacking. Perhaps of more significance, serotonergic suppression of callosal fiber transmission was not universal, with synapses onto some neurons left unaffected by 5-HT. This suggests that serotonergic modulation of synaptic transmission may be highly selective to combinations of specific pre- and postsynaptic elements. It is also possible that serotonergic suppression of transmitter release in the cortex may be developmentally regulated. 5HT1B receptors are transiently expressed at thalamocortical terminals in the somatosensory cortex during the first two weeks of life [108,109], and the resulting serotonergic suppression of glutamate release at these synapses is critical for barrel field formation [71,110]. Whether thalamocortical transmission in the PFC is also developmentally regulated by 5-HT is not known. Finally, 5-HT can also suppress GABA release at central synapses [111–119], including in the cerebral cortex [60,73], yet little is known about whether serotonergic suppression of GABA release is selective to specific synaptic connection types.
Effects of 5-HT on cortical neurons in vivo
One way to investigate the actions of endogenous 5-HT in vivo is to stimulate electrically the raphe nuclei – a procedure that induces measurable increases of prefrontal 5-HT release [120,121] – while simultaneously recording the activity of individual neurons of the PFC. We have conducted such experiments in chloral hydrate anesthetized rats, where 5-HT evokes in pyramidal neurons a variety of responses similar to those observed in vitro: inhibitions (66%), excitations (13%), and biphasic responses (20%) comprising an excitation preceded by a short-latency inhibition [20,122] (Figure 3). Taking into account the overwhelming proportion of inhibitory responses found in the slice and the intact brain, it is clear that the preferential actions of 5-HT on prefrontal pyramidal activity are inhibitory. Pharmacological blockade of serotonergic responses with the 5-HT1A receptor antagonist WAY100635 or the 5-HT2A receptor antagonist MDL100907 confirms that the decreases and increases of activity are mediated by 5-HT1A and 5-HT2A receptors, respectively, and that biphasic responses likely correspond to pyramidal neurons co-expressing both receptors [17,19,20,123]. It is noteworthy that the amount of biphasic responses (20%) recorded in vivo is smaller than the reported proportion of pyramidal neurons co-expressing 5-HT1A and 5-HT2A receptors (45–50%). It may be that 5-HT1A receptor-mediated inhibition can dominate weaker 5-HT2A receptor-mediated excitation when both receptors are co-expressed. Three different features of serotonergic receptor expression may account for this. First, as described earlier, 5-HT1A receptors are localized on the soma and axon initial segment of pyramidal neurons, locations that maximize their ability to limit action potential generation, while 5-HT2A receptors are located more distal from the axon, in the apical dendrite. A second possibility is that stimulation in the dorsal raphe may activate non-serotonergic inhibitory afferents to the PFC. Indeed, GABA projection neurons have been found in the dorsal raphe nucleus [124], and some inhibitory responses recorded in PFC neurons have a fast component blocked by the GABAA receptor antagonist picrotoxinin [20]. The involvement of direct GABAergic projections to the PFC is also suggested by the short latency (≤ 9 ms) of some inhibitions recorded in prefrontal pyramidal neurons, because signal propagation in unmyelinated serotonergic fibers is much slower (>15 ms; [20]). A third possibility is that 5-HT may activate prefrontal interneurons via 5-HT3 receptors to initiate short-latency feed-forward inhibition [58].
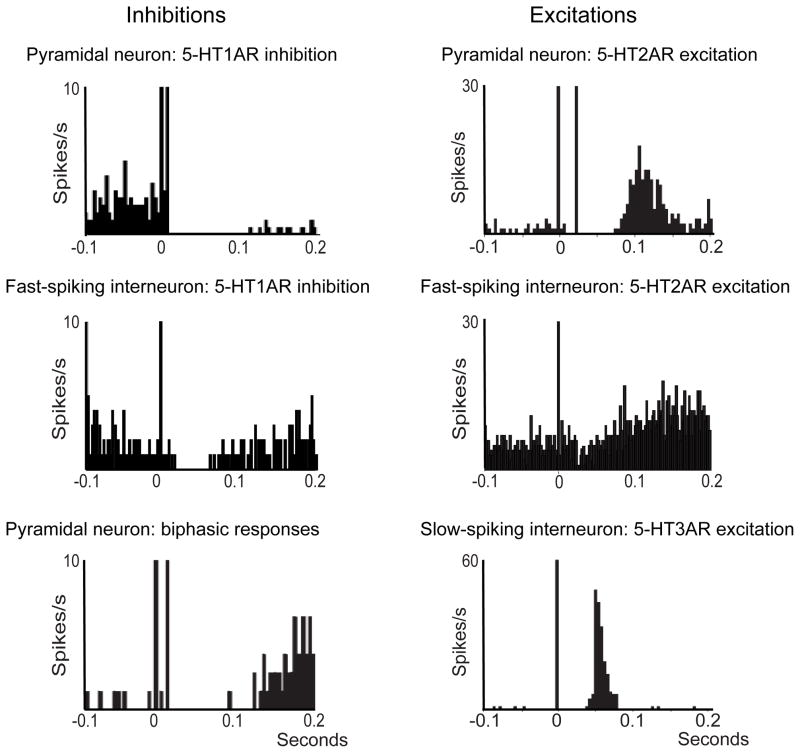
Figure 3. 5-HT inhibits and activates distinct populations of prefrontal neurons in vivo.
Peri-stimulus histograms depicting the firing rate of pyramidal neurons, fast-spiking and slow-spiking interneurons recorded in the prefrontal cortex of anesthetized rats during electrical stimulation of the dorsal raphe nucleus (time 0), which induces release of 5-HT in the prefrontal cortex. Note that 5-HT1A-mediated inhibitions are shorter in fast-spiking interneurons compared to pyramidal neurons and that 5-HT3A-mediated excitations have a shorter delay and duration than 5-HT2A-mediated excitations. Modified from references 17,48,59,178.
We recently found that subgroups of prefrontal fast-spiking interneurons are modulated by 5-HT1A and 5-HT2A receptors in vivo [48]. Akin to previous studies, we stimulated electrically the dorsal raphe nucleus and recorded the responses of parvalbumin-expressing fast-spiking interneurons of the PFC. We observed 5-HT1A-mediated decreases and 5-HT2A-mediated increases of activity in 61% and 10% of the recorded cells, respectively (Figure 3). However, unlike pyramidal neurons, we found very few biphasic responses (6.5%). This may be due to the fact that separate populations of fast-spiking interneurons express 5-HT1A and 5-HT2A receptors [48]. Again, a predominance of 5-HT1A-mediated inhibitions indicates that 5-HT exerts a potent suppression of the activity of cortical fast-spiking interneurons, similar to that observed in pyramidal neurons. The impact of inhibition of fast-spiking interneurons on network activity would be further enhanced by 5-HT-dependent suppression of GABA release at their synapses with pyramidal neurons [73]. Slow-spiking interneurons were also identified in superficial layers of the PFC that are excited by 5-HT through 5-HT3A receptors [59]. As expected for a ionotropic receptor, the latency and duration of the 5-HT3A-mediated excitations were shorter than the excitations elicited by G-protein-coupled 5-HT2A receptors in pyramidal and fast-spiking neurons of the same area (Figure 3). Thus, not only is the expression pattern of 5-HT2A- and 5-HT3A-positive interneurons complementary, but the timing of their activation by 5-HT is finely tuned as well.
In addition, the administration of the 5-HT2A/C receptor agonist DOI (1-[2,5-dimethoxy-4-iodophenyl-2-aminopropane]) in vivo increases and decreases the firing rate of distinct subpopulations of pyramidal neurons [19,125,126]. Increases of activity might follow direct stimulation of 5-HT2A/C receptors, whereas the decreases likely involve activation of nearby interneurons expressing 5-HT2A receptors (previous studies have failed to show expression of 5-HT2C receptors by cortical interneurons [48]). The increases in activity of pyramidal cells were reversed in most neurons by the 5-HT2A receptor antagonist MDL100907 [19,44]. However, in a small population of neurons MDL100907 failed to reverse DOI’s induced excitation, suggesting that 5-HT2C receptors may excite a subpopulation of pyramidal neurons, in accordance with their pattern of expression in the PFC [48].
In summary, 5-HT acting at 5-HT1A receptors is a potent inhibitor of both pyramidal neuron and fast-spiking interneuron activity in the PFC. This is evident when the dorsal raphe is electrically stimulated with paired pulses that induce enormous 5-HT release in the cortex [120]. In this condition, 5-HT significantly increases the duration of 5-HT1A-mediated inhibitions and can even convert some excitations into inhibitions [20]. Hence, greater 5-HT release in the PFC preferentially induces inhibition of activity. However, the overall effects of 5-HT release on the cortical microcircuit will be complex and difficult to predict. Direct inhibition of pyramidal neuron excitability coexists with enhanced activity of 5-HT2A- and 5-HT3A-expressing interneurons targeting apical dendritic compartments, and decreased excitability and GABA release from 5-HT1A-expressing fast-spiking interneurons targeting pyramidal neuron somata. Clearly, more work is needed to elucidate how 5-HT shapes information flow through neural circuits within the PFC. One approach to this challenging problem is to examine how 5-HT alters the activity of neural networks. In the following section we describe our recent advances in this field.
Serotonin Modulates Network Activity in the Prefrontal Cortex
Neural networks can be defined as populations of neurons that fire synchronously, independent of their anatomical inter-connectivity. As such, they oscillate, generating small electrical waves that can be detected outside the skull through EEGs or intracerebrally through local field potentials (LFPs). Neural network activity can generate oscillations of different frequencies, typically from 0.1 Hz up to 100 Hz. Some of these oscillations have been recorded in the PFC during diverse behavioral tasks. For instance, slow waves (< 2 Hz) are thought to be critical for memory consolidation [127–131], whereas alpha (10–14 Hz) and gamma waves (30–80 Hz) are involved in attention [132–136] and memory [137–139]. In recent years, an enormous literature on neural oscillations has been published. However virtually nothing is known about the involvement of 5-HT in their generation or modulation.
To address this question we examined the effects of endogenous 5-HT (released after electrical stimulation of the dorsal raphe) on neural oscillations recorded in the PFC of anesthetized rats. Under chloral hydrate anesthesia, the predominant oscillatory activities recorded through intracortical field potentials are slow waves (< 2 Hz), that resemble the slow rhythms of natural slow-wave sleep. Slow waves are generated by synchronized neuronal ensembles that oscillate between periods of activity (UP states) and silence (DOWN states). UP and DOWN states reflect alternating periods of membrane depolarization and hyperpolarization synchronized within large neuronal networks [140–142]. Stimulation of the dorsal raphe nucleus at a frequency similar to the awake discharge rate of serotonergic neurons (1 spike/s) increased the frequency of slow waves. UP and DOWN states appeared more irregular and of shorter duration and the peak of the power spectra (that marks the predominant frequency) increased significantly from 0.74 to 0.94 Hz (Figure 4A and 4B). This suggests that 1 Hz stimulations in the dorsal raphe impose their frequency onto the cortical network. Increasing the release of 5-HT into the cortex by augmenting the intensity of stimulations (but not changing the frequency) reliably increased the frequency of slow oscillations to 1 Hz [48]. This indicates that the amount of 5-HT released, and not merely the frequency of stimulation, is enough to modulate the frequency of slow waves. 5-HT induced this increase in frequency by promoting rapid initiation of UP states. Thus, the activity of serotonergic neurons in the raphe nuclei may directly regulate the frequency of cortical slow oscillations by promoting UP states. Moreover, during raphe stimulations, both the amplitude of slow waves and the duration of DOWN states were reduced compared to pre- and post-stimulation epochs, and thus the duration of UP-state potentials was greater. Since UP states are generated by the synchronous depolarization of large ensembles of cortical neurons, these results suggest 5-HT may have a net excitatory effect on cortical networks in vivo. Indeed, high frequency stimulation of the dorsal raphe nucleus (100 Hz), which induces a massive release of 5-HT in the cortex, completely suppressed cortical slow waves by eliminating DOWN states altogether [48]. These results support the proposed role of 5-HT in modulating the transition between sleep and wakefulness [143,144]. Furthermore, the administration of the 5-HT2A/C receptor antagonist ritanserin reduced slow waves, while the 5-HT1A or 5-HT2C receptor antagonists WAY100635 and SB242084 did not (Figure 4C). This implicates 5-HT2A receptors in the regulation of slow waves. Blockade of 5-HT2A receptors desynchronized slow oscillations by reducing the number, duration, and amplitude of DOWN states, which resulted in a significant increase in UP state potentials similar to that observed after raphe stimulation [48]. In sum, 5-HT modulates the frequency and amplitude of slow waves in the PFC via activation of 5-HT2A receptors, and its overall effects are excitatory.
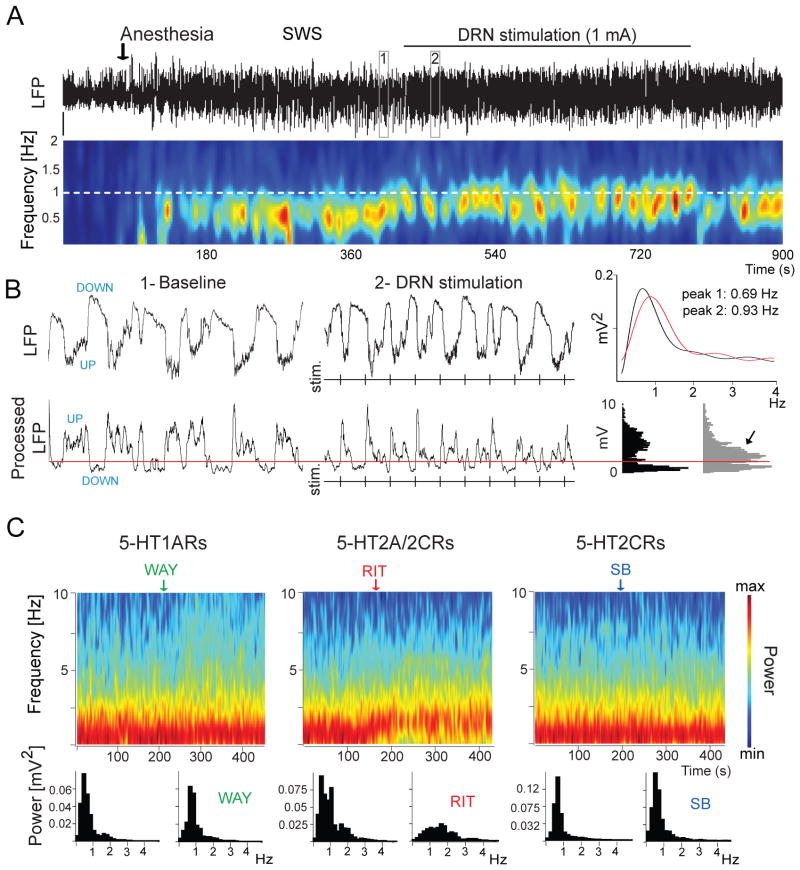
Figure 4. 5-HT modulates slow waves in prefrontal cortex.
(A) Stimulation of the dorsal raphe nucleus (DRN) increases the frequency of cortical slow waves (< 1 Hz). Top, Local field potential (LFP) signal depicting an epoch of desynchronization (absence of slow waves) preceding anesthesia-induced slow-wave sleep (SWS), during which the DRN was stimulated electrically at 1 Hz. Boxes 1 and 2 are expanded in (B). Bottom, Change in power (root mean square of the amplitude) of slow waves over time (red indicates high power, blue low power). White dashed line marks the frequency of stimulation. Note that the predominant band (~ 0.7 Hz) increases in frequency towards the frequency of stimulation during DRN stimulation. (B) Top, expanded 10-second traces from (A). Vertical lines correspond to times of DRN stimulation. Power spectra for 1 min segments that contain the 10 s traces in boxes 1 and 2 are shown on the far right. Bottom, LFPs were processed off-line for an accurate measure of UP-state duration. A threshold was set (red line) to discriminate UP states [21,141]. Note the increase in UP-state potentials during the stimulations (arrow). (C) Effect of WAY (WAY100635, 5-HT1A receptor antagonist), RIT (ritanserin, 5-HT2A/2C receptor antagonist) and SB (SB242084, 5-HT2C receptor antagonist) on the power of slow waves in the rat prefrontal cortex. The power of slow waves decreases after injection of RIT but not SB or WAY, indicating a modulation by 5-HT2A receptors. Modified from reference 48.
During natural sleep and anesthesia, gamma oscillations are present along with slow waves [145]. During alertness, gamma rhythms play a role in numerous cognitive tasks such as attention, sensory processing, and memory [133,134,137,146–148]. By contrast, the functions of these oscillations during sleep are unknown. It has been recently demonstrated that gamma oscillations are generated by the synchronous discharge of fast-spiking interneuron networks [149,150], which exerts potent inhibition onto pyramidal neurons and other interneurons, including fast-spiking neurons [33,34,151,152]. In fact, gamma oscillations generated in hippocampal slices in vitro are mainly derived from synchronized rhythmic IPSCs in pyramidal neurons produced by perisomatic fast-spiking interneurons [153,154]. Therefore, we propose that 5-HT may regulate gamma rhythms through fast-spiking interneurons expressing 5-HT1A and 5-HT2A receptors, provided that these two cell subpopulations are predominantly localized in layer 5, close to the soma of pyramidal neurons. There are a number of observations that support this. First, we recently discovered that 5-HT exerts a strong modulation of gamma oscillations in the PFC of anesthetized rats via 5-HT1A and 5-HT2A receptors [48]. Specifically, we found that blockade of 5-HT1A receptors increases, whereas blockade of 5-H2A receptors decreases, the amplitude of gamma waves. Second, blockade of 5-HT1A receptors increases the spiking of 5-HT1A-expressing fast-spiking interneurons, while sharpening the synchronization of these neurons to gamma cycles. Moreover, blockade of 5-HT2A receptors desynchronizes 5-HT2A-expressing fast-spiking interneurons from gamma waves. Thus, stimulation of cortical 5-HT1A receptors by endogenous 5-HT may desynchronize gamma oscillations by reducing the activity and synchronization of 5-HT1A-expressing fast-spiking interneurons, whereas stimulation of cortical 5-HT2A receptors may potentiate gamma oscillations by enhancing the synchronization of 5-HT2A-expressing fast-spiking interneurons. Third, high-frequency stimulation of the dorsal raphe nucleus reduces the amplitude of gamma waves in the PFC and, as mentioned earlier, 5-HT predominantly inhibits cortical fast-spiking interneuron activity. And fourth, fast-spiking interneurons in the PFC do not express 5-HT2C receptors and, consequently, blockade of these receptors does not alter the amplitude of gamma oscillations. Finally, the interplay between pyramidal neurons and fast-spiking interneurons further enhances gamma oscillations. Thus, during anesthesia-induced sleep-like states, 5-HT may down regulate gamma oscillations simply by inhibiting the overall activity of most pyramidal and fast-spiking neurons. Altogether, 5-HT is a strong modulator of prefrontal gamma oscillations, with its major effect being reduction of their amplitude, likely through inhibition of 5-HT1A-expressing fast-spiking interneurons.
The Paradox: Serotonin Opposing Actions on Prefrontal Pyramidal Networks
In previous sections we have described in some detail the impact of 5-HT on individual neurons and on neural oscillations in the rodent PFC. Converging data suggest that 5-HT predominantly reduces activity of pyramidal neurons via stimulation of 5-HT1A receptors. By contrast, 5-HT simultaneously excites the membranes of large ensembles of these neurons through 5-HT2A receptors so that there is a switch from DOWN-hyperpolarizing to UP-depolarizing states. The paradox emerges when we consider that 5-HT is inhibiting the activity of individual neurons but exciting the network as a whole. Key to understanding this complex phenomenon is that 5-HT may be acting upon 5-HT1A receptors on the soma and axon initial segment of pyramidal neurons to prevent generation of action potentials while promoting the increase of excitatory postsynaptic potentials (EPSCs) on their apical dendrites via 5-HT2A -and perhaps 5-HT2C- receptors. Importantly, recent studies suggest that oscillations recorded through local field signals are independent of action potential generation, and rather indicate synchronous postsynaptic potentials in soma [153,154] or dendrites [155,156] of a large number of neurons. We propose that 5-HT plays a dual action on cortical pyramidal networks by enhancing synaptic inputs onto the dendrites while down-regulating action potential output at the axon.
It is well known that interneurons are potent modulators of pyramidal neuron output, and that single interneurons can innervate many pyramidal neurons simultaneously. Thus, it is fair to assume that interneurons may participate in the generation of UP and DOWN states by inhibiting large populations of pyramidal cells synchronously. Nevertheless, a role for inhibitory interneurons in the proposed dual model of 5-HT actions is unclear. On the one hand, superficial layers of the cortex rich in pyramidal neuron dendrites contain 5-HT3A-expressing interneurons, whose activation by 5-HT should indeed decrease excitability of distal pyramidal dendrites. On the other hand, it is less clear to what pyramidal neuron compartments 5-HT1A- and 5-HT2A-expressing fast-spiking interneurons interact with. In a configuration consonant with our model, 5-HT2A-containing interneurons would preferentially innervate the soma and axon of pyramidal neurons, whereas 5-HT1A-containing interneurons would innervate pyramidal neuron dendrites (Figure 1). Clearly, additional work is needed to elucidate the exact role of 5-HT-modulated interneurons on the cortical microcircuit.
Future Directions
Serotonin Actions on Prefrontal Cortex Activity During Different Behavioral States
We have reported the influences of 5-HT on neurons in PFC slices and in the PFC of rats anesthetized with choral hydrate. Although the anesthetized preparation shares many electrophysiological properties with natural slow-wave sleep, it is important to note that chloral hydrate acts as a GABA agonist and reduces glutamatergic neurotransmission [157,158], putatively inducing confounding effects on the results. Thus, to better determine the role of 5-HT in PFC function it is imperative that the actions of 5-HT are examined during natural sleep and alertness. It has been known for decades that the serotonergic system is implicated in the regulation of the sleep-wake cycle [144,159,160], but it is currently unknown how 5-HT differentially modulates prefrontal microcircuits during distinct arousal states. One reason for this is the challenges of performing stable electrophysiological recordings in non-anesthetized animals during cognitive tasks. To overcome this problem we have taken an indirect approach that involves adjusting the level of anesthesia in our experiments. We allowed short-lasting epochs of light anesthesia that promoted cortical desynchronization, periods of time with absence of slow waves that resemble awake states, yet occur while rats are still unconscious. This manipulation let us examine the firing patterns of cortical neurons during sleep-like states (deep anesthesia) and wake-like states (light anesthesia), where neuron activity may resemble more to that occurring during cognitive tasks. We identified two populations of fast-spiking interneurons based on their activity during cortical UP states, and on differences in their spiking during sleep-like and wake-like states. One population preferentially discharged action potentials during the first half of UP states (‘early’ cells) and decreased spiking during wake-like states. The second population behaved in the opposite manner: it fired action potentials during the second half of UP states (‘late’ cells) and increased dramatically the activity during wake-like states [21]. So, it is likely that this latter population is responsible for generating the gamma oscillations associated with cognitive processing during wakefulness. ‘Early’ and ‘late’ pyramidal neurons were also found, although their low firing rate made it difficult to quantify changes in activity between sleep-like and alert-like states (Puig and Kawaguchi, unpublished results). Collectively, these data suggest that distinct populations of prefrontal neurons display opposing but complementary discharge patterns during sleep and alertness. Whether these neuron populations play different roles in sleep processes and cognitive tasks will need to be tested in non-anesthetized animals during natural sleep-wake cycles.
We assessed the effects of 5-HT on the ‘early’ and ‘late’ fast-spiking interneuron populations during sleep-like and wake-like states [48]. Although 5-HT inhibited most fast-spiking neurons during both states, there was a remarkable increase in the proportion of excited cells during wake-like states. Consistently, the ‘late’ population (which is more active during alertness) showed greater serotonergic excitation. Thus, 5-HT may activate a larger population of fast-spiking interneurons during alertness, perhaps to facilitate a balanced ratio of inhibition to excitation to fine tune gamma oscillations during cognitive tasks. Again, future research could test this directly by comparing the proportion of excitatory and inhibitory responses elicited in fast-spiking interneurons by endogenous 5-HT in awake animals. If the hypothesis holds, it would suggest 5-HT exerts a potent inhibition on prefrontal circuits during sleep but a more balanced excitation/inhibition during alertness. This could have profound implications for our understanding of how 5-HT modulates cognitive tasks encoded in the PFC.
Serotonin Modulation of Prefrontal Cortex Function
Previous studies have shown that prefrontal 5-HT may play a role in short-term memory [161,162], attention [163,164], and cognitive flexibility [10,165]. In addition, optimized 5-HT levels appear to be critical for behavioral inhibition, as elevated or reduced 5-HT in the PFC is followed by increased impulsivity [166–169]. However, although these studies point to a prominent role of 5-HT in cognition, the cellular basis for this modulation has remained largely undetermined.
A seminal work by Williams et al. (2002) [161] revealed that 5-HT2A receptors modulate delay activity or ‘memory fields’ of neurons in the dorsolateral PFC of monkeys performing a spatial working memory task. Interestingly, in this study the authors recorded regular-spiking pyramidal neurons and putative fast-spiking interneurons and reported that both cell types displayed 5-HT2A-modulated tuning. Two distinct, and not mutually exclusive, neural mechanisms could account for this: 1) 5-HT2A-expressing pyramidal neurons and fast-spiking interneurons directly modulate short-term memory processes, and 2) network activity in the PFC exerts this control. The participation of 5-HT1A receptors in short-term memory processes, however, has been more controversial. Separate studies have reported both memory improvements and impairments following 5-HT1A receptor blockade [170,171]. In these studies, no electrophysiological recordings were implemented and different SSRIs were either pre- or co-administered together with the 5-HT1A blocker, making it difficult to interpret the results at the cellular level.
Prefrontal 5-HT is also involved in the regulation of attention. Specifically, acute serotonin depletion in humans improves attentional control [164]. The systemic administration of the 5-HT1A/2A receptor agonist psilocybin to healthy volunteers impaired attentional tracking abilities during a multiple-object tracking task [163], an effect that was independent of 5-HT2A receptors. This implicates 5-HT1A receptors in the control of attention in humans. In rats, where more invasive experiments can be implemented, both stimulation of 5-HT1A receptors or blockade of 5-HT2A receptors in the PFC abolished attentional impairments induced by the NMDA receptor antagonist CPP [172,173]. So, an overall reduction in prefrontal activity dependent on 5-HT neurotransmission is beneficial for attention. This suggests that an inadequate serotonergic inhibitory tone of prefrontal areas may underlie some attentional deficits.
Previous studies have also implicated 5-HT in the regulation of both cognitive flexibility and impulsivity (see ref. [174] for a review). Clarke et al. (2004 and 2005) [10,165] have shown that local depletions of 5-HT in the PFC increase perseverative (or compulsive) behavior in monkeys. It seems like prefrontal 5-HT2A, but not 5-HT2C, receptors may be involved in the control of cognitive flexibility, as measured by increased perseverance [173] and impairments in reversal learning [175,174]. 5-HT exerts a strong modulation of impulsivity as well. Global 5-HT depletion increases impulsivity, as reflected by premature responding [167,169]. Recent work has reported that impulsivity is modulated by 5-HT1A, 5-HT2A and 5-HT2C receptors. Stimulation of 5-HT1A or blockade of 5-HT2A receptors in the PFC decrease impulsivity [173,176,177], suggesting that a downregulation of cortical activity may effectively promote behavioral control. Intriguingly, blockade of 5-HT2C receptors, which presumably reduces cortical activity as well, increases impulsivity to levels similar to 5-HT depletion [167]. In this study though, the administration of the antagonist was systemic, so subcortical effects cannot be ruled out.
Altogether these studies reveal that prefrontal 5-HT exerts a complex modulation of cognitive functions. First, a decreased 5-HT tone in the PFC is beneficial for attention, but detrimental for cognitive flexibility and impulsivity. Moreover, drug treatments that enhance/restore attention and decrease impulsivity involve a reduction of prefrontal activity via stimulation of 5-HT1A receptors or blockade of 5-HT2A receptors. This indicates a deficient 5-HT inhibition of prefrontal activity during impairment of these tasks. A deeper knowledge of the cortical microcircuits underlying executive functions will be necessary to understand how alterations in prefrontal 5-HT neurotransmission lead to cognitive impairments and perhaps psychiatric disorders.
Conclusions
Despite decades of intense research, the role of 5-HT in PFC function is still largely unresolved. This may be due to the complex microcircuit of the PFC and sophisticated patterns of expression for 5-HT receptor subtypes among distinct populations of prefrontal neurons. From previous investigations we can conclude that 5-HT primarily inhibits most pyramidal and fast-spiking neurons via 5-HT1A receptors, while exciting smaller populations of these neurons via 5-HT2A receptors. Intriguingly, although 5-HT actions on neuronal activity are overwhelmingly inhibitory, many pyramidal neurons co-express 5-HT1A and 5-HT2A receptors, and about half of fast-spiking interneurons express 5-HT2A receptors. Determining the role of 5-HT2A receptors in modulating the activity of these neurons, and hence the cortical circuit in general, remains an important priority. It is likely that selective expression of 5-HT2A receptors in functionally distinct neuron subgroups may help explain their ultimate role in cortical function. Similarly, the exquisite expression pattern of 5-HT1A, 5-HT2A and 5-HT3A receptors in separate populations of fast-spiking and slow-spiking interneurons located in distinct cortical layers points to selective and independent serotonergic control of the excitability of specific neuronal compartments within pyramidal neurons. These sophisticated mechanisms of control likely facilitate the precise activity patterns required during cognitive tasks, as reflected in serotonergic modulation of neural oscillations in the PFC. Recent studies have revealed that both neural oscillations and GABAergic neurotransmission may be altered in the PFC of patients with severe psychiatric illnesses. Comprehensive studies on the brain mechanisms underlying serotonergic modulation of neural oscillations and GABA neurotransmission will provide valuable information regarding why many psychiatric treatments are largely ineffective at improving cognition.
Acknowledgments
We are grateful to F. Artigas, M. Bosch, P. Celada, and Y. Kawaguchi for their guidance and support. We also thank D. Avesar for contributing the data for Figure 2. This work has been supported by a number of grants and fellowships from the Spanish, Japanese and U.S. governments. These include a fellowship from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS; M.V.P), a fellowship from the Japan Society for the Promotion of Science (JSPS, M.V.P.), a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation (A.T.G.) and PHS grant R01 MH83806 (A.T.G.).
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 2.Fuster J. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 3.Fuster JM. The Prefrontal Cortex- Anatomy, physiology and neuropsychology of the frontal lobe. Linpicott-Raven; Philadelphia-New York: 1997. [Google Scholar]
- 4.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 5.Antzoulatos EG, Miller EK. Differences between neural activity in prefrontal cortex and striatum during learning of novel abstract categories. Neuron. 2011;71:243–249. doi: 10.1016/j.neuron.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warden MR, Miller EK. Task-dependent changes in short-term memory in the prefrontal cortex. J Neurosci. 2010;30:15801–15810. doi: 10.1523/JNEUROSCI.1569-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman DJ, et al. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 8.Chudasama Y, et al. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Dalley J, Everitt B, Robbins T. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Clarke HF, et al. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 11.Gruber AJ, et al. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rygula R, et al. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci. 2010;30:14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- 14.Hajos M, et al. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- 15.Peyron C, et al. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 16.Sesack SR, et al. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 17.Amargós-Bosch M, et al. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- 18.Celada P, et al. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABAA, and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puig MV, et al. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- 20.Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- 21.Puig MV, Ushimaru M, Kawaguchi Y. Two distinct activity patterns of fast-spiking interneurons during neocortical UP states. Proc Nat Acad Sci. 2008;105:8428–8433. doi: 10.1073/pnas.0712219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell BD, Macklis JD. Large-scale maintenance of dual projections by callosal and frontal cortical projection neurons in adult mice. J Comp Neurol. 2005;482:17–32. doi: 10.1002/cne.20428. [DOI] [PubMed] [Google Scholar]
- 23.Otsuka T, Kawaguchi Y. Cell diversity and connection specificity between callosal projection neurons in the frontal cortex. J Neurosci. 2011;31:3862–3870. doi: 10.1523/JNEUROSCI.5795-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson CJ. Morphology and synaptic connections of crossed corticostriatal neuron in the rat. J Comp Neurol. 1987;263:567–580. doi: 10.1002/cne.902630408. [DOI] [PubMed] [Google Scholar]
- 25.Rumberger A, et al. Correlation of electrophysiology, morphology, and functions in corticotectal and corticopretectal projection neurons in rat visual cortex. Exp Brain Res. 1998;119:375–390. doi: 10.1007/s002210050353. [DOI] [PubMed] [Google Scholar]
- 26.Kasper EM, et al. Pyramidal neurons in layer 5 of the rat visual cortex. I. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets. J Comp Neurol. 1994;339:459–474. doi: 10.1002/cne.903390402. [DOI] [PubMed] [Google Scholar]
- 27.Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci. 2006;26:4394–4405. doi: 10.1523/JNEUROSCI.0252-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morishima M, et al. Highly differentiated projection-specific cortical subnetworks. J Neurosci. 2011;31:10380–10391. doi: 10.1523/JNEUROSCI.0772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457:1133–1136. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- 31.Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci. 2010;30:16922–16937. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- 35.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 36.Uematsu M, et al. Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex. 2008;18:315–330. doi: 10.1093/cercor/bhm056. [DOI] [PubMed] [Google Scholar]
- 37.Hoyer D, et al. International union of pharmacology classification of receptors for 5-Hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 38.Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 40.Weber E, Andrade R. Htr2a gene and 5-HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Frontiers Neurosci. 2010;4:1–12. doi: 10.3389/fnins.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Almeida J, Mengod G. Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT2A receptors in human and monkey prefrontal cortex. J Neurochem. 2007;103:475–486. doi: 10.1111/j.1471-4159.2007.04768.x. [DOI] [PubMed] [Google Scholar]
- 42.Kia HK, et al. Ultrastructural localization of 5-hydroxytryptamine(1A) receptors in the rat brain. J Neurosci Res. 1996;46:697–708. doi: 10.1002/(SICI)1097-4547(19961215)46:6<697::AID-JNR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Gimenez JF, et al. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- 44.Martín-Ruiz R, et al. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- 46.Santana N, et al. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- 47.Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 48.Puig MV, et al. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci. 2010;30:2211–2222. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azmitia EC, et al. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacol. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 50.Cruz DA, et al. Serotonin1A receptors at the axon initial segment of prefrontal pyramidal neurons in schizophrenia. Am J Psychiatry. 2004;161:739–742. doi: 10.1176/appi.ajp.161.4.739. [DOI] [PubMed] [Google Scholar]
- 51.Czyrak A, et al. Serotonin 5-HT1A receptors might control the output of cortical glutamatergic neurons in rat cingulate cortex. Brain Res. 2003;989:42–51. doi: 10.1016/s0006-8993(03)03352-3. [DOI] [PubMed] [Google Scholar]
- 52.De Felipe J, et al. Pyramidal cell axons show a local specialization for GABA and 5-HT inputs in monkey and human cerebral cortex. J Comp Neurol. 2001;433:148–155. doi: 10.1002/cne.1132. [DOI] [PubMed] [Google Scholar]
- 53.Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Nat Acad Sci. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marek GJ, Aghajanian GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- 55.Vucurovic K, et al. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex. 2010;20:2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 57.Jansson A, et al. Relationships of 5-hydroxytryptamine immunoreactive terminal-like varicosities to 5-hydroxytryptamine-2A receptor-immunoreactive neuronal processes in the rat forebrain. J Chem Neuroanat. 2001;22:185–203. doi: 10.1016/s0891-0618(01)00133-8. [DOI] [PubMed] [Google Scholar]
- 58.Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puig MV, et al. In vivo excitation of GABA interneurons in the medial prefrontal cortex through 5-HT3 receptors. Cereb Cortex. 2004;14:1365–1375. doi: 10.1093/cercor/bhh097. [DOI] [PubMed] [Google Scholar]
- 60.Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]
- 61.Ferezou I, et al. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morales M, et al. Cellular and subcellular immunolocalization of the type 3 serotonin receptor in the rat central nervous system. Mol Brain Res. 1996;36:251–260. doi: 10.1016/0169-328x(96)88406-3. [DOI] [PubMed] [Google Scholar]
- 63.Lee S, et al. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morales M, et al. The type 3 serotonin receptor is expressed in a subpopulation of GABAergic neurons in the rat neocortex and hippocampus. Brain Res. 1996;731:199–202. doi: 10.1016/0006-8993(96)00557-4. [DOI] [PubMed] [Google Scholar]
- 65.Troca-Marin J, Geijo-Barrientos E. Inhibition by 5-HT of the synaptic responses evoked by callosal fibers on cortical neurons in the mouse. Eur J Physiol. 2010;460:1073–1085. doi: 10.1007/s00424-010-0875-4. [DOI] [PubMed] [Google Scholar]
- 66.Crino PB, et al. Cellular localization of serotonin 1A, 1B and uptake sites in cingulate cortex of the rat. J Pharmacol Exp Ther. 1990;252:651–656. [PubMed] [Google Scholar]
- 67.Maroteaux L, et al. Mouse 5HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proc Nat Acad Sci. 1992;89:3020–3024. doi: 10.1073/pnas.89.7.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanaka E, North RA. Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol. 1993;69:1749–1757. doi: 10.1152/jn.1993.69.5.1749. [DOI] [PubMed] [Google Scholar]
- 69.Compan V, et al. Differential effects of serotonin (5-HT) lesions and synthesis blockade on neuropeptide-Y immunoreactivity and 5-HT1A, 5-HT1B/1D and 5-HT2A/2C receptor binding sites in the rat cerebral cortex. Brain Res. 1998;795:264–276. doi: 10.1016/s0006-8993(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 70.Murakoshi T, et al. Multiple G-protein-coupled receptors mediate presynaptic inhibition at single excitatory synapses in the rat visual cortex. Neurosci Lett. 2001;309:117–120. doi: 10.1016/s0304-3940(01)02051-1. [DOI] [PubMed] [Google Scholar]
- 71.Laurent A, et al. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J Neurosci. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Torres-Escalante JL, et al. 5-HT1A, 5-HT2, and GABAB receptors interact to modulate neurotransmitter release probability in layer 2/3 somatosensory rat cortex as evaluated by the paired pulse protocol. J Neurosci Res. 2004;78:268–278. doi: 10.1002/jnr.20247. [DOI] [PubMed] [Google Scholar]
- 73.Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathur BN, et al. Serotonin induces long-term depression at corticostriatal synapses. J Neurosci. 2011;31:7402–7411. doi: 10.1523/JNEUROSCI.6250-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bianchi C, et al. Serotonin modulation of cell excitability and of [3H]GABA and [3H]d-aspartate efflux in primary cultures of rat cortical neurons. Neuropharmacol. 2007;52:995–1002. doi: 10.1016/j.neuropharm.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 76.Boschert U, et al. The mouse 5-Hydroxytryptamine(1B) receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 77.Sari Y, et al. Cellular and subcellular localization of 5- hydroxytryptamine(1b) receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- 78.Miner LA, et al. Ultrastructural localization of serotonin(2A) receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–117. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- 79.Seguela P, Watkins KC, Descarries L. Ultrastructural relationships of axon terminals in the cerebral cortex of the adult rat. J Comp Neurol. 1989;289:129–142. doi: 10.1002/cne.902890111. [DOI] [PubMed] [Google Scholar]
- 80.Mulligan KA, Törk I. Serotonergic innervation of the cat cerebral cortex. J Comp Neurol. 1988;270:86–110. doi: 10.1002/cne.902700108. [DOI] [PubMed] [Google Scholar]
- 81.DeFelipe J, Jones EG. A light and electron microscopic study of serotonin-immunoreactive fibers and terminals in the monkey sensory-motor cortex. Exp Brain Res. 1988;71:171–182. doi: 10.1007/BF00247532. [DOI] [PubMed] [Google Scholar]
- 82.DeFelipe J, et al. Synaptic relationships of serotonin-immunoreactive terminal baskets on GABA neurons in the cat auditory cortex. Cereb Cortex. 1991;1:117–133. doi: 10.1093/cercor/1.2.117. [DOI] [PubMed] [Google Scholar]
- 83.Hornung JP, Celio MR. The selective innervation by serotonergic axons of calbindin-containing interneurons in the neocortex and hippocampus of the marmoset. J Comp Neurol. 1992;320:457–467. doi: 10.1002/cne.903200404. [DOI] [PubMed] [Google Scholar]
- 84.Smiley JF, Goldman-Rakic PS. Serotonergic axons in monkey prefrontal cerebral cortex synapse predominantly on interneurons as demonstrated by serial section electron microscopy. J Comp Neurol. 1996;367:431–43. doi: 10.1002/(SICI)1096-9861(19960408)367:3<431::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 85.Foehring RC, et al. Serotonergic modulation of supragranular neurons in rat sensorimotor cortex. J Neurosci. 2002;22:8238–8250. doi: 10.1523/JNEUROSCI.22-18-08238.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrade R, Malenka RC, Nicoll RA. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- 87.Goodfellow NM, et al. Layer II/III of the prefrontal cortex: Inhibition by the serotonin 5-HT1A receptor in development and stress. J Neurosci. 2009;29:10094–10103. doi: 10.1523/JNEUROSCI.1960-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beique JC, et al. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- 90.Villalobos C, et al. Serotonergic regulation of calcium-activated potassium currents in rodent prefrontal cortex. Eur J Neurosci. 2005;22:1120–1126. doi: 10.1111/j.1460-9568.2005.04307.x. [DOI] [PubMed] [Google Scholar]
- 91.Zhang ZW, Arsenault D. Gain modulation by serotonin in pyramidal neurones of the rat prefrontal cortex. J Physiol. 2005;566:379–394. doi: 10.1113/jphysiol.2005.086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spain WJ. Serotonin has different effects on two classes of Betz cells from the cat. J Neurophysiol. 1994;72:1925–1937. doi: 10.1152/jn.1994.72.4.1925. [DOI] [PubMed] [Google Scholar]
- 93.Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex. J Neurosci. 1996;16:3848–3861. doi: 10.1523/JNEUROSCI.16-12-03848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gulledge AT, et al. M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J Neurosci. 2009;29:9888–9902. doi: 10.1523/JNEUROSCI.1366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greene CC, Schwindt PC, Crill WE. Properties and ionic mechanisms of a metabotropic glutamate receptor-mediated slow afterdepolarization in neocortical neurons. J Neurophysiol. 1994;72:693–704. doi: 10.1152/jn.1994.72.2.693. [DOI] [PubMed] [Google Scholar]
- 96.Libri V, et al. Metabotropic glutamate receptor subtypes mediating slow inward tail current (IADP) induction and inhibition of synaptic transmission in olfactory cortical neurones. Br J Pharmacol. 1997;120:1083–1095. doi: 10.1038/sj.bjp.0701021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McCormick DA, Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Nat Acad Sci. 1989;86:8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haj-Dahmane S, Andrade R. Ionic mchanism of the slow afterdepolarization induced by muscarinic receptor activation in rat prefrontal cortex. J Neurophysiol. 1998;80:1197–1210. doi: 10.1152/jn.1998.80.3.1197. [DOI] [PubMed] [Google Scholar]
- 99.Fowler MA, et al. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS ONE. 2007;2:e573. doi: 10.1371/journal.pone.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan HD, Villalobos C, Andrade R. TRPC channels mediate a muscarinic receptor-induced afterdepolarization in cerebral cortex. J Neurosci. 2009;29:10038–10046. doi: 10.1523/JNEUROSCI.1042-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhong P, Yan Z. Differential regulation of the excitability of prefrontal cortical fast-spiking interneurons and pyramidal neurons by serotonin and fluoxetine. PLoS ONE. 2011;6:e16970. doi: 10.1371/journal.pone.0016970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beique JC, et al. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Nat Acad Sci. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lambe EK, Goldman-Rakic PS, Aghajanian GK. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb Cortex. 2000;10:974–980. doi: 10.1093/cercor/10.10.974. [DOI] [PubMed] [Google Scholar]
- 104.Davies MF, et al. Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res. 1987;423:347–352. doi: 10.1016/0006-8993(87)90861-4. [DOI] [PubMed] [Google Scholar]
- 105.Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacol. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 106.Xiang Z, Prince DA. Heterogeneous actions of serotonin on interneurons in rat visual cortex. J Neurophysiol. 2003;89:1278–1287. doi: 10.1152/jn.00533.2002. [DOI] [PubMed] [Google Scholar]
- 107.Gulledge AT, et al. Heterogeneity of phasic cholinergic signaling in neocortical neurons. J Neurophysiol. 2007;97:2215–2229. doi: 10.1152/jn.00493.2006. [DOI] [PubMed] [Google Scholar]
- 108.Leslie MJ, Bennett-Clarke CA, Rhoades RW. Serotonin 1B receptors form a transient vibrissa-related pattern in the primary somatosensory cortex of the developing rat. Brain Res Dev. 1992;69:143–148. doi: 10.1016/0165-3806(92)90132-g. [DOI] [PubMed] [Google Scholar]
- 109.Bennett-Clarke CA, et al. Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Proc Nat Acad Sci. 1993;90:153–157. doi: 10.1073/pnas.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salichon N, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase A and 5-HT transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oleskevich S, Lacalle JC. Reduction of GABAb inhibitory postsynaptic potentials by serotonin via pre- and postsynaptic mechanisms in CA3 pyramidal cells of rat hippocampus in vitro. Synapse. 1992;12:173–188. doi: 10.1002/syn.890120302. [DOI] [PubMed] [Google Scholar]
- 112.Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kishimoto K, Koyama S, AKAIKE N. Synergistic mu-opioid and 5-HT1A presynaptic inhibition of GABA release in rat periaqueductal gray neurons. Neuropharmacol. 2001;41:529–538. doi: 10.1016/s0028-3908(01)00100-9. [DOI] [PubMed] [Google Scholar]
- 114.Yan QS, Yan SE. Serotonin-1B receptor-mediated inhibition of [3H]GABA release from rat ventral tegmental area slices. J Neurochem. 2001;79:914–922. doi: 10.1046/j.1471-4159.2001.00643.x. [DOI] [PubMed] [Google Scholar]
- 115.Koyama S, et al. Role of presynaptic 5-HT1A and 5-HT3 receptors in modulation of synaptic GABA transmission in dissociated rat basolateral amygdala neurons. Life Sci. 2002;72:375–387. doi: 10.1016/s0024-3205(02)02280-4. [DOI] [PubMed] [Google Scholar]
- 116.Katsurabayashi S, et al. A distinct distribution of functional presynaptic 5-HT receptor subtypes on GABAergic nerve terminals projecting to single hippocampal CA1 pyramidal neurons. Neuropharmacol. 2003;44:1022–1030. doi: 10.1016/s0028-3908(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 117.Mlinar B, Falsini C, Corradetti R. Pharmacological characterization of 5-HT1B receptor-mediated inhibition of local excitatory synaptic transmission in the CA1 region of rat hippocampus. Br J Pharmacol. 2003;138:71–80. doi: 10.1038/sj.bjp.0705026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matsuoka T, Hasuo H, Akasu T. 5-Hydroxytryptamine 1B receptors mediate presynaptic inhibition of monosynaptic IPSC in the rat dorsolateral septal nucleus. Neurosci Res. 2004;48:229–238. doi: 10.1016/j.neures.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 119.Hashimoto K, Kita H. Serotonin activates presynaptic and postsynaptic receptors in rat globus pallidus. J Neurophysiol. 2008;99:1723–1732. doi: 10.1152/jn.01143.2007. [DOI] [PubMed] [Google Scholar]
- 120.Gartside SE, et al. Neurochemical and electrophysiological studies on the functional significance of burst firing in serotonergic neurons. Neuroscience. 2000;98:295–300. doi: 10.1016/s0306-4522(00)00060-9. [DOI] [PubMed] [Google Scholar]
- 121.McQuade R, Sharp T. Release of cerebral 5-hydroxytryptamine evoked by electrical stimulation of the dorsal and median raphe nuclei: Effect of a neurotoxic amphetamine. Neuroscience. 1995;68:1079–1088. doi: 10.1016/0306-4522(95)00214-4. [DOI] [PubMed] [Google Scholar]
- 122.Puig MV, Celada P, Artigas F. Serotonergic control of prefrontal cortex. Rev Neurol. 2004;39:539–547. [PubMed] [Google Scholar]
- 123.Hajós M, et al. In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacol. 2003;45:72–81. doi: 10.1016/s0028-3908(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 124.Li YQ, et al. Morphological features and electrophysiological properties of serotonergic and non-serotonergic projection neurons in the dorsal raphe nucleus. An intracellular recording and labeling study in rat brain slices. Brain Res. 2001;900:110–118. doi: 10.1016/s0006-8993(01)02272-7. [DOI] [PubMed] [Google Scholar]
- 125.Wang S, et al. In vivo effects of activation and blockade of 5-HT2A/2C receptors in the firing activity of pyramidal neurons of medial prefrontal cortex in a rodent model of Parkinson’s disease. Exp Neurol. 2009;219:239–248. doi: 10.1016/j.expneurol.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 126.Celada P, et al. The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biol Psychiatry. 2008;64:392–400. doi: 10.1016/j.biopsych.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 127.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 128.Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 129.Landsness EC, et al. Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32:1273–1284. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 131.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 132.Fries P, et al. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 133.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 134.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 135.Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 136.Bollimunta A, et al. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J Neurosci. 2011;31:4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Siegel M, Warden MR, Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc Nat Acad Sci. 2009;106:21341–21346. doi: 10.1073/pnas.0908193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jensen O, et al. Oscillations in the alpha band (9-2 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- 139.Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol. 2011;21:475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 140.Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mukovski M, et al. Detection of active and silent states in neocortical neurons from the field potential signal during slow-wave sleep. Cereb Cortex. 2007;17:400–414. doi: 10.1093/cercor/bhj157. [DOI] [PubMed] [Google Scholar]
- 142.Steriade M, Nuñez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dringenberg HC, Vanderwolf CH. Neocortical activation: modulation by multiple pathways acting on central cholinergic and serotonergic systems. Exp Brain Res. 1997;116:160–174. doi: 10.1007/pl00005736. [DOI] [PubMed] [Google Scholar]
- 144.Portas CM, Bjorvatn B, Ursin R. Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies. Prog Neurobiol. 2000;60:13–35. doi: 10.1016/s0301-0082(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 145.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 146.Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cog Sci. 2003;7:553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 147.Howard MW, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 148.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 149.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sohal VS, et al. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 152.Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 153.Oren I, Hájos N, Paulsen O. Identification of the current generator underlying cholinergically induced gamma frequency field potential oscillations in the hippocampal CA3 region. J Physiol. 2010;588:785–797. doi: 10.1113/jphysiol.2009.180851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gulyás AI, et al. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Monosov IE, Trageser JC, Thompson KG. Measurements of simultaneously recorded spiking activity and local field potentials suggest that spatial selection emerges in the frontal eye field. Neuron. 2008;57:614–625. doi: 10.1016/j.neuron.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nielsen KJ, Logothetis NK, Rainer G. Dissociation between local field potentials and spiking activity in macaque inferior temporal cortex reveals diagnosticity-based encoding of complex objects. J Neurosci. 2006;26:9639–9645. doi: 10.1523/JNEUROSCI.2273-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kreuter JD, et al. Cocaine-induced Fos expression in rat striatum is blocked by chloral hydrate or urethane. Neuroscience. 2004;127:233–242. doi: 10.1016/j.neuroscience.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 158.Lu J, Greco MA. Sleep circuitry and the hypnotic mechanism of GABA-A drugs. J Clin Sleep Med. 2006;2:S19–26. [PubMed] [Google Scholar]
- 159.Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev. 2011;15:269–281. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 160.Monti JM, Jantos H. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog Brain Res. 2008;172:625–646. doi: 10.1016/S0079-6123(08)00929-1. [DOI] [PubMed] [Google Scholar]
- 161.Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liy-Salmeron G, Meneses A. Effects of 5-HT drugs in prefrontal cortex during memory formation and the ketamine amnesia-model. Hippocampus. 2008;18:965–974. doi: 10.1002/hipo.20459. [DOI] [PubMed] [Google Scholar]
- 163.Carter OL, et al. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cog Neurosci. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- 164.Scholes KE, et al. Acute serotonin and dopamine depletion improves attentional control: findings from the Stroop task. Neuropsychopharmacol. 2006;32:1600–1610. doi: 10.1038/sj.npp.1301262. [DOI] [PubMed] [Google Scholar]
- 165.Clarke HF, et al. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dalley JW, et al. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacol. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- 167.Winstanley CA, et al. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacol. 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- 168.Dalley JW, et al. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacol. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- 169.Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: Interactions with dopaminergic mechanisms. Psychopharmacol. 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- 170.Wingen M, Kuypers KPC, Ramaekers JG. Selective verbal and spatial memory impairment after 5-HT1A and 5-HT2A receptor blockade in healthy volunteers pre-treated with an SSRI. J Psychopharmacol. 2007;21:477–485. doi: 10.1177/0269881106072506. [DOI] [PubMed] [Google Scholar]
- 171.Fernández-Pérez S, Pache DM, Sewell RDE. Co-administration of fluoxetine and WAY100635 improves short-term memory function. Eur J Pharmacol. 2005;522:78–83. doi: 10.1016/j.ejphar.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 172.Carli M, et al. The serotonin 5-HT2A receptors antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacol. 2004;29:1637–1647. doi: 10.1038/sj.npp.1300479. [DOI] [PubMed] [Google Scholar]
- 173.Carli M, et al. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacol. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- 174.Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:517–542. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- 175.Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacol. 2007;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- 176.Winstanley CA, et al. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacol. 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- 177.Talpos JC, Wilkinson LS, Robbins TW. A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J Psychopharmacol. 2006;20:47–58. doi: 10.1177/0269881105056639. [DOI] [PubMed] [Google Scholar]
- 178.Puig MV. Serotonergic modulation of the prefrontal cortex: from neurons to brain waves. In: Uehara T, editor. Psychiatric Disorders - Worldwide Advances. InTech; 2011. [Google Scholar]