Abstract
The Golgi apparatus undergoes extensive disassembly during mitosis and reassembly in post-mitotic daughter cells. This process has been mimicked in vitro by treating Golgi membranes with mitotic and interphase cytosol. To determine the minimal machinery that controls the morphological change, we have developed a defined Golgi disassembly and reassembly assay that reconstitutes this process using purified proteins instead of cytosol. Treatment of Golgi membranes with mitotic kinases and COPI coat proteins efficiently disassembles the membranes into mitotic Golgi fragments, whereas further incubation with p97 or N-ethylmaleimide-sensitive factor (two AAA ATPases involved in membrane fusion) and their cofactors, in combination with protein phosphatase PP2A, leads to reassembly of the membranes into new Golgi stacks. The whole process takes 3–4 d and is applicable for identification and determination of novel cytosolic and membrane proteins that regulate Golgi membrane dynamics in the cell cycle.
INTRODUCTION
The Golgi apparatus, which consists of discrete Golgi stacks laterally linked together by tubules, undergoes extensive fragmentation during mitosis. The generated fragments are equally distributed in the daughter cells in which new Golgi stacks are formed after mitosis. To determine the mechanism that controls the Golgi disassembly and reassembly processes, biochemical reconstitution assays have been performed. One approach involves semi-permeabilized cells, in which the cells are first semi-permeabilized either by freeze-thaw1, using a mild detergent digitonin2 or using a bacterial toxin streptolysin O (ref. 3). Cells are then washed with 1 M KCl to remove the endogenous cytosol, incubated with mitotic cytosol or other proteins and then analyzed by immunofluorescence or electron microscopy (EM) to detect Golgi morphological changes. This approach has been used to determine the roles of a few known proteins (e.g., MEK and Cdc2 kinases) in the cell cycle-regulated Golgi morphological change4. Another approach, which we refer to as ‘standard Golgi disassembly and reassembly assay’, involves treatment of purified Golgi membranes with mitotic or interphase cytosol followed by EM morphological analysis5,6. This cell-free system is particularly powerful in the identification of proteins involved in the cell cycle-regulated Golgi disassembly and reassembly process7,8. This system, or its modified forms, has been used to identify or analyze the roles of GRASP65 (refs. 9,10), GRASP55 (ref. 11), GM130 (ref. 12), Giantin13, Golgin 84 (ref. 14), p115 (ref. 15), the membrane fusion machineries SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptor), NSF (N-ethylmaleimide-sensitive factor), p97 and their cofactors16–18, as well as ubiquitination19,20 in the Golgi disassembly and reassembly process. However, as the cytosol used in both systems contains many proteins, it is difficult to identify the key regulators responsible for the morphological change of the Golgi.
In this study, we detail a ‘defined Golgi disassembly and reassembly assay’ in which purified proteins, instead of cytosol, are used to treat Golgi membranes. This improved assay allowed us to reveal the minimal machinery involved in the regulation of the Golgi disassembly and reassembly in the cell cycle21. For Golgi disassembly, two sets of proteins are used to treat Golgi membranes. The first set includes mitotic kinases, cdc2 and polo-like kinase 1 (plk-1), which phosphorylate the Golgi stacking proteins (e.g., GRASP65)10,22,23. Treatment of Golgi membranes with these mitotic kinases leads to cisternal unstacking, but the length of the cisternae does not change21. The second set of proteins is the COPI vesicle budding machinery, ARF1 (ADP ribosylation factor 1) and coatomer, which sequesters the cisternal membranes into vesicles during mitosis5,21,24. Incubation of Golgi membranes with this COPI budding machinery leads to partial vesiculation of the Golgi membranes with the core of the stack remaining as mini stacks21. When both sets of proteins (kinases and COPI budding machinery) are included, disassembly of the Golgi membranes is nearly complete. Up to 80% of the Golgi membranes are disassembled into mitotic Golgi fragments (MGFs), which is more efficient than what was observed using mitotic cytosol21. After the disassembly reaction, separation of the vesicles from the Golgi remnants is necessary to identify and quantify proteins associated with the COPI vesicles or the Golgi remnants. This can be achieved using equilibrium sucrose gradient, as the coated vesicles have a higher density than the Golgi remnants. Fractions of the gradient can be analyzed by western blotting or by EM21. The resulting information may be valuable for better understanding of Golgi structure formation and Golgi function (e.g., intra-Golgi trafficking)21.
For Golgi reassembly, there are also two sets of proteins involved. The first includes two AAA ATPases, NSF with their adaptor proteins α-SNAP (soluble NSF attachment protein) and γ-SNAP16,25–27, and p97 (ref. 17) with its adaptor protein p47 (refs. 1,17–20,28). It has been shown that these proteins are capable of fusing MGFs into cisternal membranes7,8. Treatment of MGFs with these proteins generates single Golgi cisternae, which normally do not form stacks. The second set of proteins includes protein phosphatases, such as PP2A, which dephosphorylates GRASP65 and other Golgi structural proteins and thus leads to Golgi membrane restacking21. In addition, the Golgi tethering protein, p115, is also required for Golgi cisterna formation15. Therefore, we treat MGFs generated in the Golgi disassembly reaction with a combination of the membrane fusion proteins, phosphatases and p115; this leads to reassembly of the membranes into new stacks21.
In this improved reconstitution assay, Golgi disassembly by purified kinases and ARF1/coatomer is more efficient than by mitotic cytosol, and the generated MGFs are capable of reassembly into new Golgi stacks with the treatment of purified membrane fusion proteins and phosphatase PP2A. The result of the experiment reveals the minimal machinery sufficient to regulate Golgi morphological change during the cell division, i.e., disassembly by unstacking and vesiculation and reassembly by membrane fusion and cisternal restacking. Compared with the standard Golgi disassembly and reassembly assay, which is also included in this protocol, the defined Golgi disassembly and reassembly assay may be technically more challenging, but it allows us to explore detailed molecular mechanisms that mediate Golgi structure formation. For example, the defined Golgi disassembly allowed us to dissect this process into two different but related processes, namely, cisternal unstacking and membrane vesiculation. Furthermore, this protocol is suitable for the identification and characterization of novel cytosolic or membrane proteins that have a role in the Golgi disassembly and reassembly process. It is necessary to point out that the disassembly and reassembly reactions can be uncoupled, or combined with the standard Golgi disassembly or reassembly reactions. For example, disassembly using purified proteins can be performed without a subsequent reassembly reaction; a defined reassembly reaction can be performed using MGFs raised by incubation of Golgi membranes with mitotic HeLa cell cytosol or vice versa. In addition, the methods described in this protocol for purification and handling of Golgi membranes, preparation of HeLa interphase and mitotic cytosol, separation of COPI vesicles from mitotic Golgi remnants, as well as purification of Golgi-related proteins could be used in other studies. For example, the high efficiency of the Golgi disassembly in this protocol and the separation of Golgi-derived vesicles by sucrose gradient will allow the preparation of mitotic Golgi vesicles, which could be fluorescently labeled and micro-injected into cells and followed by time-lapse imaging to monitor their incorporation into the existing Golgi stacks.
Experimental design
Preparation of rat liver Golgi
The Golgi membranes are purified from rat liver homogenate by two sequential sucrose gradients, a method first described by Hui et al.29 and modified by Wang et al.30. This method uses rat liver as the source because liver cells have abundant Golgi membranes owing to the high activity in protein and lipid secretion. In addition, liver tissue is soft and easy to homogenize. The tissue is homogenized by passing through a 150-μm mesh sieve; this mild homogenization method is essential for the preservation of the stacked structure of the Golgi membranes. The first gradient separates the Golgi membranes from unbroken cells, other cellular organelles (e.g., nuclei and mitochondria) and the majority of cytosol and free lipid. The second sucrose gradient further separates the Golgi membranes from cytosolic contaminants and concentrates the Golgi membranes. The relative purity of the Golgi stacks is assessed by measuring the increase in activity of a Golgi enzyme, β-1,4-galactosyltransferase (GalT), over that of the total liver homogenate. The yields of Golgi membranes can be calculated from the ratio between the total GalT activity in the Golgi fractions and that of the rat liver homogenate. A typical preparation can yield Golgi membranes that are purified 80- to 100-fold over the homogenate and 60–70% in stacks, which can be confirmed by examination by EM. The protein content in the purified Golgi membranes can be determined by western blot analysis. The addition of protease inhibitors, although not essential, is recommended in all of the experimental procedures including the Golgi preparation, especially when the Golgi protein of interest is sensitive to proteases. All membranes and proteins are aliquoted, snap frozen in liquid nitrogen and stored in − 80 °C.
Preparation of interphase and mitotic HeLa cell cytosol
Inter-phase or mitotic cytosol is prepared from HeLa S3 cells (ATCC CCL-2.2)5,6. These cells have been adapted to grow in suspension (spinner) culture. More importantly, these cells can be efficiently blocked to prometaphase by nocodazole treatment, with over 95% of the cells in mitosis and a relatively low number of apoptotic cells. The cells can be cultured on Petri dishes and later transferred into spinner flasks. For mitotic cytosol, the cells are synchronized to mitosis by nocodazole treatment; the mitotic index is examined by fluorescence microscopy with chromosomes stained by a fluorescence DNA dye before cytosol preparation. After homogenization using a ball-bearing homogenizer, the cytosol is obtained by pelleting the large cellular components such as membrane organelles by ultracentrifugation.
Preparation of purified protein
The proteins used in this assay are either purified from animal liver homogenate or as recombinant proteins expressed and purified using baculoviral and bacterial expression systems. Cdc2 kinase (also called cdk1, complexed with cyclin B1) and plk-1 are expressed in insect Hi-5 cells by baculoviral infection and purified through the His- or GST-tagging as described10. Coatomer complex is purified from rat or rabbit liver cytosol by gel filtration31. Myristoylated ARF1 is coexpressed with a second plasmid encoding the yeast N-myristoyltransferase in BL21 bacterial cells and purified by gel filtration, as previously described24,32,33. His-tagged NSF, α-SNAP, γ-SNAP and p47 are expressed in bacteria and purified with nickel beads17,19,34. p97 is prepared using a bacterial expression system or purified from rat liver cytosol by gel filtration18,19. p115 is purified from rat liver cytosol by fast protein liquid chromatography as previously described15,35–37. Protein phosphatase PP2A is expressed and purified using a baculoviral expression system38 or purchased from Millipore.
Golgi disassembly assay
In the standard Golgi disassembly assay5,6, Golgi membranes are treated with mitotic cytosol followed by analysis of the membranes by EM. In total, 20 μg of membranes per reaction mixture will give an appropriate size of pellet for EM processing. The final reaction mixture should contain 0.1 mg ml−1 of Golgi membranes in a 200 μl solution. The ratio of Golgi membranes to mitotic cytosol is maintained as 1:100 in terms of the protein amount, which is an estimate of the ratio in rat liver cells based on the activity of GalT. This ratio can be reduced to 1:50 if the cytosol is limited or if the protein concentration in cytosol is not high. An ATP regeneration system is included in the reaction mixture to provide energy and to allow protein phosphorylation. The rest of the volume of the reaction mixture is filled by Magnesium, EGTA and β-glycerophosphate (MEB) buffer, which contains ATP, GTP and β-glycerophosphate that inhibit phosphatase activity. In addition to the cytosol, antibodies to Golgi structural proteins, recombinant Golgi proteins, ubiquitin and its mutants19,20 or chemicals (e.g., kinase inhibitors and GTPγS), can be included in the reaction mixture to test their effects on the Golgi disassembly or reassembly. The nonhydrolyzable GTP analog GTPγS enhances the Golgi disassembly, but the resulting Golgi fragments cannot be reassembled into new Golgi stacks6. Some proteins can be immunodepleted from the cytosol before the reaction to test the role of these proteins in Golgi disassembly or reassembly12. The reaction mixture should be incubated at 37 °C for 20 min, which is about the time necessary for mitotic Golgi disassembly in living cells5,39. Extension of the time improves the efficiency of the disassembly, but the subsequent reassembly using the resulted MGFs will become less efficient. In addition, kinase inhibitors must be avoided in the disassembly reaction.
In the defined disassembly assay, Golgi membranes are incubated with purified proteins instead of mitotic cytosol. Purified kinases (e.g., cdc2 and plk) or the COPI budding machinery (e.g., ARF1 and coatomer) or both are added in the reaction mixture to treat Golgi membranes. The same amount or activity of the purified proteins, equivalent to that in the mitotic cytosol used in the standard disassembly reaction, should be used. The activity of cdc2 kinases is measured using histone H1 as the substrate12 and plk activity on His-GRASP65 phosphorylation10. For reactions mixtures with 20 μg Golgi membranes, 5 μg recombinant myristoylated ARF1 and 10 μg coatomer purified from rat liver cytosol are used on the basis of quantification of western blotting results of ARF1 and β-COP in mitotic HeLa cell cytosol. The reaction mixture also includes the ATP regeneration system and MEB buffer as in the standard assay. The current protocol has combined, modified and improved similar protocols used in a number of studies10,14,19–21,24,40,41.
Isolation of MGFs from the mitotic environment
After the Golgi disassembly reaction, the membranes need to be reisolated to remove the mitotic cytosol or kinases before they can be used in the reassembly assay; otherwise, the remaining mitotic cytosol or kinases will inhibit Golgi reassembly. This purpose is achieved by centrifugation. The disassembly reaction mixture is loaded on top of a 0.4 M sucrose cushion in KCl, HEPES and magnesium (KHM) buffer in a 1.5 ml centrifuge tube, with a 6 μl 2 M sucrose cushion added to the bottom of the tube. After centrifugation at 136,000g (55,000 r.p.m.) in a TLA55 rotor at 4 °C, the membranes would run through the 0.4 M sucrose cushion and accumulate at the top of the 2 M sucrose, whereas the cytosolic proteins would remain on top of the 0.4 M sucrose cushion. In addition, the centrifugation also allows the change of the membranes from MEB buffer, which contains β-glycerophosphate (a general phosphatase inhibitor), into KHM buffer, which is used in the reassembly reaction. The addition of a small volume of high-concentration sucrose at the bottom of the centrifuge tube would maintain the membranes in a suspension so that they could be uniformly distributed, which is critical for the subsequent Golgi reassembly.
Golgi reassembly assay
In a standard Golgi reassembly reaction, MGFs derived from the Golgi disassembly assay and reisolated by centrifugation are treated with interphase HeLa cell cytosol. A similar assay using rat liver cytosol has been previously reported6, but we found that the efficiency is relatively higher with HeLa cell cytosol. Different from the disassembly reaction, the membranes were kept at a higher concentration to facilitate membrane fusion and tethering. For a reaction with 20 μg MGF, the reaction mixture contains 400 μg interphase cytosol and 1× ATP regeneration system, with a final volume of 30 μl adjusted by KHM buffer. Incubation is carried out for 60 min at 37 °C, with gentle mixing every 20 min. It has been shown that Golgi reassembly takes longer than disassembly in the cell42,43.
In the defined Golgi reassembly reaction, purified proteins instead of interphase cytosol are used to treat the MGFs. These proteins include the membrane fusion proteins, NSF with its adaptor proteins, α-SNAP and γ-SNAP, or p97 with its adaptor protein p47, protein phosphatase PP2A, as well as the Golgi tethering protein p115. Before the addition of MGFs, the purified protein are mixed and left on ice for 5–10 min to allow to form the functional complexes, which could be carried out during the centrifugation for MGF reisolation.
Considering the fact that activity of the purified proteins in the Golgi reassembly may be lower compared with those in the inter-phase cytosol, two to three types of the same proteins were used for the 20 μg MGF in comparison with that in 400 μg interphase cytosol, based on quantification of the western blotting results of these proteins. Similar amounts have been used in previously published studies20,21,44. The following reactions have been performed to test the two membrane fusion pathways: (1) the NSF pathway: 3 μg of NSF (final concentration 100 ng μl−1 in a 30 μl reaction mixture), 0.75 μg of α-SNAP (25 ng μl−1), 0.75 μg of γ-SNAP (25 ng μl−1), 0.9 μg of p115 (30 ng μl−1) and 2 U of PP2A (0.07 U μl−1) and (2) the p97 pathway: 3 μg p97 (100 ng μl−1), 0.75 μg p47 (25 ng μl−1), 0.9 μg p115 (30 ng μl−1) and 2 U PP2A (0.07 U μl−1). p97 and p47 should be mixed and kept on ice for 15 min before membranes are added into the reaction. In addition, kinase inhibitors can be included, but phosphatase inhibitors should be avoided in the reassembly reaction.
Separation of Golgi-derived vesicles from Golgi remnants using sucrose gradients
A 0.5–2.0 M sucrose gradient is used to separate the vesicles from larger Golgi membranes such as the mini stacks and long Golgi cisternae. After centrifugation, the coated vesicles are enriched in a fraction equivalent to the density of 1.2–1.3 M sucrose45, whereas the Golgi remnants remain in the top fractions of the gradient with the density of about 0.8 M sucrose46, which is consistent with the density of the Golgi membranes. Fractions of the gradient can be analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting to determine the proteins associated with either the vesicles or the Golgi remnants or by EM to analyze the morphology of the membranes in the fractions.
Detailed procedures for the standard and defined Golgi disassembly and reassembly reconstitution assays, the analysis of the membranes by sucrose gradients and EM, as well as the stereological quantification criteria are described below. It is necessary to note that the disassembly and reassembly reactions can be uncoupled, and the improved defined protocol can be combined with the standard assay, depending on whether the purpose of the experiment is to search for factors involved in either disassembly or reassembly. For example, disassembled Golgi membranes by mitotic cytosol treatment can be used for reassembly with purified components or vice versa. This has allowed us to uncover the role of ubiquitination in cell cycle regulation of Golgi membrane dynamics19,20.
MATERIALS
REAGENTS
Preparation of rat liver Golgi membranes
Rat: Sprague–Dawley rats (body weight 150–200 g) ! CAUTION Experiments using live rodents must conform to the National and Institutional regulations.
CO2 (Cryogenic gases, cat. no. 51676)
K2HPO4 (American Bioanalytical, cat. no. AB01650-01000)
KH2PO4 (American Bioanalytical, cat. no. AB01660-01000)
Sucrose (American Bioanalytical, cat. no. AB01900-01000)
MgCl2·6H2O (Fisher Scientific, cat. no. M33-500)
EDTA-free protease inhibitors (Roche, cat. no. 14512300)
Pepstatin A (American Bioanalytical, cat. no. AB01555-00010)
DMSO (dimethyl sulfoxide; American Bioanalytical, cat. no. AB00435-00500)
Bio-Rad Protein Assay (Bio-Rad, cat. no. 500-0006)
Determination of GalT activity
Sodium cacodylate (Sigma, cat. no. C4945)
HCl (10 N, American Bioanalytical, cat. no. AB00831-01000)
Ovomucoid (Sigma, cat. no. T9253)
-
[3H]UDP-galactose (American Radiolabeled Chemicals, cat. no. ART 0131)
! CAUTION This is a radioactive compound and needs permission and caution for handling radioactive chemicals.
Triton X-100 (American Bioanalytical, cat. no. AB02025-00500)
ATP (Roche, cat. no. 10127531001)
MnCl2·4H2O (Fisher, cat. no. AC20589-5000)
Phosphotungstic acid (PTA, Sigma, cat. no. 455970)
Sodium dodecyl sulfate (SDS, American Bioanalytical, cat. no. AB01920-00500) ! CAUTION This compound is toxic when inhaled; handle the powder with a mask.
Tris base (American Bioanalytical, cat. no. AB02000-02000)
Preparation of cytosol
Sucrose (American Bioanalytical, cat. no. AB01900-01000)
β-glycerophosphate (Sigma-Aldrich, cat. no. 50020)
EGTA (ethylene glycol tetraacetic acid, American Bioanalytical, cat. no. AB00505-00100)
MgCl2·6H2O (Fisher Scientific, cat. no. M33-500)
ATP (Roche, cat. no. 10127531001)
Glutathione (Sigma-Aldrich, cat. no. G4251)
EDTA-free protease inhibitor cocktail (Roche, cat. no. 14512300),
Pepstatin A (American Bioanalytical, cat. no. AB01555-00010)
KOH (American Bioanalytical, cat. no. AB01485-00500)
Liquid nitrogen ! CAUTION As this involves very low temperature, handle with cryo gloves.
HeLa S3 cells (ATCC, cat. no. CCL-2.2)
RPMI medium (Invitrogen, cat. no. 23400062)
Donor bovine serum with iron (Invitrogen, cat. no. 10371029)
Penicillin/streptomycin/glutamine (100×, Invitrogen, cat. no. 10738-016)
Trypsin (2.5%, wt/vol, 10×, Invitrogen, cat. no. 15090-046)
EDTA (0.5 M, Invitrogen, cat. no. 15575-020)
Nocodazole (98% pure, Acros Organics, cat. no. AC35824-0100)
Formaldehyde solution (37%, wt/wt, Fisher, cat. no. BP531-500) ! CAUTION This chemical is toxic. Wear goggles and gloves and use an exhaust hood.
Triton X-100 (American Bioanalytical, cat. no. AB02025-00500)
Hoechst 33258 (Sigma, cat. no. B-2883)
Golgi disassembly and reassembly assay
HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; American Bioanalytical, cat. no. AB00892-00500)
Sucrose (American Bioanalytical, cat. no. AB01900-01000)
KCl (American Bioanalytical, cat. no. AB01652-01000)
Mg(OAc)2·4H2O (Sigma-Aldrich, cat. no. M5661)
ATP (Roche, cat. no. 10127531001)
GTP (Roche, cat. no. 10106399001)
Glutathione (Sigma-Aldrich, cat. no. G4251)
EDTA-free protease inhibitor cocktail (Roche, cat. no. 14512300)
Tris base (American Bioanalytical, cat. no. AB02000-02000)
β-glycerolphosphate (Sigma-Aldrich, cat. no. 50020)
EGTA (American Bioanalytical, cat. no. AB00505-00100)
Creatine phosphate (Sigma-Aldrich, cat. no. 27920)
Cytochalasin B (Sigma-Aldrich, cat. no. C6762)
Creatine phosphokinase (Sigma-Aldrich, cat. no. C3755)
Bio-spin 6-Tris column (Bio-Rad, cat. no. 732-6227)
Electron microscopy
Glutaraldehyde (50% solution, wt/wt, Electron Microscopy Sciences, cat. no. 16320) ! CAUTION This is a toxic chemical. Wear goggles and gloves and use an exhaust hood.
-
OsO4 (4% aqueous solution, wt/wt, Electron Microscopy Sciences, cat. no. 19190)
! CAUTION This is a toxic compound. Wear gloves and use an exhaust hood.
Potassium ferricyanide (Electron Microscopy Sciences, cat. no. 20150)
Sodium cacodylate (Sigma Ultra, cat. no. C4945)
Propylene oxide (Electron Microscopy Sciences, cat. no. 20401)
Embed 812 (Electron Microscopy Sciences, cat. no. 14900)
NMA (methyl-5-norbornene-2,3-dicarboxylic anhydride; Electron Microscopy Sciences, cat. no. 19000)
DDSA (dodecenyl succinic anhydride; Electron Microscopy Sciences, cat. no. 13710)
DMP-30 (2,4,6-tri(dimethylaminomethyl) phenol; Electron Microscopy Sciences, cat. no. 13600)
Uranyl acetate (Electron Microscopy Sciences, cat. no. 22400) ! CAUTION It is a heavy metal. Wear gloves and use an exhaust hood.
Lead nitrate (Electron Microscopy Sciences, cat. no. 17900) ! CAUTION It is a heavy metal. Wear gloves and use an exhaust hood.
Sodium citrate (Fisher Scientific, cat. no. S93364)
EQUIPMENT
150-mm mesh stainless-steel sieve
Pasteur pipette
Refractometer
Spectrophotometer
Standard tissue culture equipments and Petri dishes
Spinner flask
Fluorescence microscope
Ball-bearing homogenizer (Chamber size, 8.020 mm; ball size, 8.010 mm, HGM Lab Equipment)
0.22 μm syringe filter
SW-41 rotor and Ultraclear tubes (size 14 × 89 mm; Beckman, cat. no. 344059)
Beckman L8-70M ultracentrifuge, SW-41 rotor and centrifuge tubes (Beckman, cat. no. 344059) or equivalents
Beckman Max-XP ultracentrifuge, TLA55 rotors and centrifuge tubes (Beckman, cat. no. 357448) and TLA 100.3 rotor and centrifuge tubes (Beckman, cat. no. 349622) or equivalents
Eppendorf 5417R microcentrifuge with swing bucket rotor
Standard equipment for epon embedding of EM samples, microtome and electron microscope
REAGENT SETUP
For rat liver Golgi preparation
Potassium phosphate buffer (0.5 M; pH 6.7)
Make up 500 ml solutions of 0.5 M anhydrous K2HPO4 (43.55 g) and 0.5 M anhydrous KH2PO4 (34.02 g). To 400 ml of the latter, gradually add the former until the pH reaches 6.7. This solution is stable for at least 2 months at 4 °C.
Sucrose (2 M)
Dissolve 342.3 g sucrose in water pre-warmed to 50 °C. Make up to a final volume of 500 ml. This solution is stable at 4 °C for months.
MgCl2 (2 M)
Dissolve 40.7 g of MgCl2 6H2O in water to a final volume of 100 ml. This solution is stable at room temperature (18–22 °C) for years.
Protease inhibitors
For each 50 ml solution (e.g., gradient buffers), directly dissolve one EDTA-free tablet and then add 50 μl pepstatin A (5 mM stock). To prepare the stock solution of pepstatin A, dissolve 3.43 mg of the powder in 1 ml DMSO and store the aliquots at − 20 °C. This solution is stable for at least 6 months at − 20 °C.
Gradient buffers
Make up buffers A–E from the preceding three stock solutions and ice-cold water as shown in Table 1. In total, 50 ml of each buffer A, B and E and 100 ml of buffers C and D are needed. EDTA-free protease inhibitor tablet and pepstatin A are added to buffer C and D. Prepare 500 ml Buffer C without protease inhibitors for washing the liver. Water should be pre-cooled to 4 °C overnight to ensure that all the buffers are ice cold.
TABLE 1.
Components of sucrose gradient buffers for rat liver Golgi preparation.
| Buffer | A | B | C | D | E |
|---|---|---|---|---|---|
| Sucrose concentration (M) | 0 | 0.25 | 0.5 | 0.86 | 1.3 |
| phosphate buffer (0.5 M, pH 6.7) (ml) | 10 | 10 | 20 | 20 | 10 |
| Sucrose (2 M, ml) | 0 | 6.25 | 25 | 43 | 32.5 |
| MgCl2(2 M, ml) | 0.125 | 0.125 | 0.25 | 0.25 | 0.125 |
| Water (ml) | 39.9 | 33.6 | 54.8 | 36.8 | 7.4 |
| Total volume (ml) | 50 | 50 | 100 | 100 | 50 |
| Sucrose % (wt/wt) | 0 | 8.6 | 16.0 | 26.4 | 38.6 |
| Refractive index | 1.3330 | 1.3456 | 1.3574 | 1.3747 | 1.3973 |
▴ CRITICAL It is important to be as accurate as possible when mixing various components and to check the refractive index of each buffer using a refractometer. The final refractive index should be adjusted to within ± 0.5% sucrose (about 0.001 in refractive index) for buffer C and D in particular.
For Golgi purity determination and GalT activity measurement
0.4 M sodium cacodylate (pH 6.6)
Dissolve 17.1 g in 150 ml of water and adjust the pH to 6.6 with HCl. Make up to 200 ml. This solution is stable for 6 months at 4 °C.
Ovomucoid (175 mg ml−1)
Dissolve 1 g in water and make up to a final volume of 5.7 ml. Filter through a 0.45 μm nitrocellulose filter and aliquot. This solution is stable for 6 months at − 20 °C.
UDP-galactose (10 mM)
Dissolve 25 mg in a final volume of 4.4 ml of water. Aliquot and store at − 20 °C. This solution is stable for 6 months at the storage temperature.
Triton X-100 (10% (wt/vol))
Dissolve 10 g in 80 ml of water and make up to 100 ml. This solution is stable for 6 months at 4 °C.
ATP (0.2 M)
Dissolve 242 mg in 1.5 ml of 250 mM HEPES-KOH (pH 7.2). Adjust the pH to 6.5–7.0 with 1 M NaOH and make up to a final volume of 2 ml with water. Aliquot and store at − 20 °C. This solution is stable for 2 months at the storage temperature.
MnCl2 (2 M)
Dissolve 9.9 g of MnCl2·4H2O in 15 ml of water and make up to 25 ml. This solution is stable for 6 months at 4 °C.
Phosphotungstic acid (1%)/0.5 M HCl
Dissolve 5 g of phosphotungstic acid (PTA) in 400 ml of water. Add 22 ml of concentrated HCl and make up to 500 ml with water. This solution is stable for 2 months at 4 °C.
SDS (5% (wt/vol))
Dissolve 5 g of SDS in 80 ml of water and make up to 100 ml. This solution is stable for 6 months at room temperature.
Tris (2 M)
Dissolve 24.2 g of Tris in 70 ml of water and make up to 100 ml. This solution is stable at room temperature for years.
Assay mixture
Make a fresh batch of the assay mixture from the above stocks as follows: 200 μl sodium cacodylate, 6 μl β-mercaptoethanol, 200 μl ovomucoid, 40 μl UDP-galactose, 40 μl Triton X-100, 20 μl ATP, 40 ml MnCl2, 10 μl [3H]UDP-galactose and 1,040 μl of water.
For cytosol preparation
EGTA, β-glycerophosphate and sucrose (EBS) buffer
Mix Sucrose (0.1 M), 80 mM β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 2 mM ATP, 1 mM glutathione, EDTA-free protease inhibitor cocktail (Roche, 1 in 50 ml) and 5 mM pepstatin A. Adjust to pH 7.2 with KOH. Freshly made before the experiment.
For Golgi disassembly and reassembly assay
KHM buffer
Mix HEPES (20 mM; pH 7.4), 0.2 M sucrose, 60 mM KCl, 5 mM Mg(OAc)2, 2 mM ATP, 1 mM GTP, 1 mM glutathione and protease inhibitors (1 EDTA-free tablet for 50 ml buffer). Freshly made before the experiment.
MEB buffer
Mix Tris-HCl (50 mM; pH 7.4), 0.2 M sucrose, 50 mM KCl, 20 mM β-glycerophosphate, 15 mM EGTA, 10 mM MgCl2, 2 mM ATP, 1 mM GTP, 1 mM glutathione and protease inhibitors (1 Roche EDTA-free tablet for 50 ml buffer). Freshly made before the experiment.
ATP regeneration system (10×)
Creatine phosphate (100 mM from 200 mM stock, in distilled water), 1 mM ATP (10 mM stock, in 250 mM HEPES-KOH (pH 7.3)), 0.2 mg ml−1 cytochalasin B (2 mg ml−1 stock, in DMSO), 0.2 mg ml−1 creatine kinase (2 mg ml−1 stock, in 50% glycerol). Freshly mix the stock solutions with distilled water before use. Dilute them in the ratio of 1:10 into the reaction mixtures.
For sucrose gradient
Sucrose gradient
Make sucrose solutions by mixing the proper amount of 2.4 M (wt/vol) sucrose solution, 5 × MEB or KHM buffer (containing no sucrose) to reach the required final sucrose concentrations (0.5 M, 0.8 M, 1.2 M, 1.4 M and 1.6 M), adjust the final volume by H2O. Justify the concentration of each solution using a refractometer. The gradients are always freshly made.
For EM
EM fixation buffer
Glutaraldehyde (2%) in KHM buffer. Dilute glutaraldehyde from a 25% or 50% stock into KHM buffer freshly, warm to room temperature before use.
Post fixative
OsO4 (1% (wt/vol)), 1.5% K3(Fe(CN)6), 0.1 M sodium cacodylate (pH 7.4). Mix freshly before use.
Epon-mix
Before use, mix 14.5 g Embed 812, 10.5 g NMA and 5.0 g DDSA. Mix well until no streaks are left; add 0.54 g DMP-30 and mix. Prepare freshly before use.
Uranyl acetate solution
Dissolve 2% uranyl acetate in H2O. This solution is stable for 2 weeks at 4 °C. Pass the solution through 0.22 μm syringe filter before use.
Lead citrate solution
Weigh 1.33 g lead nitrate and 1.76 g sodium citrate, add 30 ml H2O and shake for 2 min at room temperature. Add 8 ml 1 M NaOH, adjust the volume to 50 ml with H2O. This solution is stable for months at 4 °C. Pass through 0.22 μm syringe filter before use.
PROCEDURE
Purification of Golgi membranes from rat liver ● TIMING 2 d
-
1
Starve six female Sprague–Dawley rats (body weight 150–200 g) for 24 h. Provide water during this time period.
-
2
Kill the rats using high doses of CO2 followed by cervical dislocation. Rapidly remove the livers into a large volume of ice-cold buffer C (without protease inhibitors) to wash off the blood. Transfer the livers into ice-cold buffer C with EDTA-free protease inhibitors and pepstatin A. Mince the liver into small pieces with a pair of scissors.
-
3
Weigh livers, which is normally 40–45 g, from six rats. Do not use more than 50 g liver for 12 gradients.
-
4
Homogenize the tissue by gently pressing through a 150-mm mesh stainless-steel sieve, with the bottom of a 200 ml conical flask in a rolling action. Addition of a small amount of buffer C (with protease inhibitor) to the sieve will help the liver homogenate press through more easily. The final volume of the homogenate should be about 50 ml. Keep 200 μl on ice for enzyme assay.
-
5
To prepare the gradients, place 6 ml of buffer D (buffered 0.86 M sucrose) in each of the 12 SW-41 Ultraclear tubes, overlay 4.5 ml of homogenates and 1.5 ml of buffer B (buffered 0.25 M sucrose) on the top of the gradient and balance the tubes.
-
6
Centrifuge at 103,800g (29,000 r.p.m.) in a SW-41 rotor for 60 min at 4 °C.
-
7
Aspirate the lipid at the top and the cytosol indicated by red color of hemoglobin (the cytosol can be kept and accumulated for preparation of p115 and coatomer, if necessary); collect Golgi fractions that accumulate at the 0.5/0.86 M interfaces with a plastic Pasteur pipette.
-
8
Pool the collected fractions. Measure the sucrose concentration of the sample by a refractometer, and adjust the sucrose concentration to 0.25 M (refractive index, 1.3456) using buffer A. Keep 200 μl on ice for enzyme measurement.
-
9
Pool the second gradient with 1 ml 1.3 M sucrose (buffer E) and 2 ml 0.5 M sucrose (buffer C) in each tube; overlay 9 ml diluted Golgi sample.
-
10
Centrifuge at 7,900g (8,000 r.p.m.) in a SW-41 rotor for 30 min at 4 °C.
-
11
Discard the supernatant and collect the membranes at the 0.5 M/1.3 M sucrose interface. Adjust the sucrose concentration to 0.5 M (refractive index, 1.3574) using buffer A.
-
12
Check the protein concentration using Bio-Rad Bradford assay, following the manufacturer’s instructions. Check morphology and purity of the purified Golgi membranes by EM (Steps 60–73).
? TROUBLESHOOTING
-
13
Aliquot and freeze samples in liquid nitrogen and store at − 80 °C.
Determination of GalT activity of purified Golgi membrane ● TIMING 3 h
-
14
Once the Golgi preparation has been completed, make 1:20 dilutions of the homogenate and Golgi fractions with water.
-
15
Add 80 μl of assay mixture to screw-capped Eppendorf tubes containing 20 μl of the diluted samples or of water (blanks). Make duplicates. Vortex and incubate at 37 °C for 30 min.
-
16
Stop the reaction by adding 1 ml of ice-cold PTA/HCl and spin at 18,000g (13,000 r.p.m.) on a bench-top centrifuge for 10 s.
-
17
Aspirate and discard the supernatants, and add 1 ml of PTA/HCl. Resuspend the pellets by vortexing and spin at 18,000g (13,000 r.p.m.) on a bench-top centrifuge for 10 s.
-
18
Aspirate and discard the supernatants, add 1 ml of ice-cold 95% ethanol and resuspend the pellets by vortexing.
-
19
Spin at 18,000g (13,000 r.p.m.) on a bench-top centrifuge for 10 s and remove the supernatant. Add 50 μl of 2 M Tris followed by 200 μl of 5% SDS. Shake or vortex until dissolved. Add 10 μl of assay mixture, 40 μl of water and 200 μl of 5% SDS to a fresh tube to allow determination of the specific activity of [3H]UDP-galactose in the mixture.
-
20
Add 1 ml of scintillation fluid to each sample. Vortex and count in a scintillation counter using the tritium channel.
-
21
Calculate the yield of Golgi membranes from the ratio of the total GalT activity in the Golgi fractions to that of the homogenate. The average purification fold in 10 of our preparations was 97.4 ± 4.2 (mean ± s.e.m.).
Preparation of interphase and mitotic HeLa cell cytosol ● TIMING 12 d
-
22
Grow HeLa S3 cells (with a passage below 25) in RPMI1640 medium containing 10% bovine serum, nonessential amino acids, 1% penicillin/streptomycin and glutamine until 90% confluent.
-
23
Collect cells from 15 Petri dishes (15 cm) by trypsinization and resuspend the cells in 1.5 liter medium. Divide the cells into three 1 liter spinner flasks; the final cell density should be around 3–4 × 105 cells per ml. Incubate the cells with constant rotation at 90 r.p.m. in a 37 °C incubator with 5% CO2.
-
24
On the second day, dilute cells into 3 × 1.0 liter (3.0 liter total) spinner flasks, with a final cell density of around 4 × 105 cells per ml. On the third day, dilute the cells into 5 × 1 liter medium to reach a final cell density of about 5 × 105 cells per ml. For interphase cytosol preparation, incubate the cells overnight in growth medium as above. For mitotic cell cytosol, incubate the cells with 100 ng ml−1 nocodazole in growth medium for 20–22 h before harvesting.
-
25
To determine whether the cells are synchronized to mitosis, take 1 ml of the cell culture, spin at maximum speed in an Eppendorf bench-top centrifuge for 30 s and fix in 50 μl of 4% formaldehyde in PBS with 0.5% Triton X-100; stain the chromosome with 5 μl of 0.5 mg ml−1 Hoechst 33258 as a indicator of the cell phase. Check the mitotic index under a fluorescence microscope by counting cells with condensed chromosomes, which should be ≥95% after nocodazole block. If the mitotic index is below 95%, continue the incubation for another 1–2 h. Do not incubate the cells over 24 h, as apoptosis may occur afterward. The mitotic index of nonsynchronized cells is 3–4%.
-
26
For both interphase and mitotic cells, spin down the cells for 5 min at 1,000g (4,000 r.p.m.) using 250 ml centrifuge tubes in a Sorvall GSA rotor and a RC-5B centrifuge (or equivalent).
-
27
Resuspend and combine the pellets in a total of 100 ml of ice-cold 33% EBS (1:3 dilution of EBS buffer into ice-cold water) in a 250 ml tube. Leave the cells for 10 min on ice.
-
28
Spin for 10 min at 650g (2,500 r.p.m.) at 4 °C in an Eppendorf 5810R centrifuge. Discard the supernatant and resuspend the pellet in 30 ml of 100% EBS buffer, move to a 50 ml Falcon tube. Spin again for 5 min at 650g (2,500 r.p.m.) at 4 °C and discard the supernatant.
-
29
Estimate the size of the pellet and resuspend it in 0.8 volume of 100% EBS buffer and homogenize using a ball-bearing homogenizer (chamber size, 8.020 mm; ball size, 8.010 mm) on ice until 70–80% cells are broken, estimated by trypan blue exclusion.
-
30
Spin the homogenates in 3 ml thick-wall polycarbonate tubes in a TLA 100.3 rotor (Beckman) for 30 min at 386,000g (100,000 r.p.m.) at 4 °C.
-
31
Remove supernatant (cytosol) using a syringe needle. Avoid the lipids (white thin layer) on the surface and the pellet. For mitotic cytosol, respin as above for 10 min.
-
32
Measure protein concentration of the cytosol with the Bio-Rad protein assay kit. The protein concentration should be 18–22 mg ml−1. Aliquot and snap-freeze in liquid nitrogen; store at − 80 °C.
Golgi disassembly using mitotic HeLa cell cytosol ● TIMING 2 h
-
33
Calculate the amounts of membranes and mitotic cytosol needed. The final concentrations in each reaction mixture should be 0.1 mg ml−1 Golgi membranes, 10 mg ml−1 mitotic cytosol and 1 × ATP regeneration system. The additional volume should be filled by MEB buffer. For each reaction, 20 μg membranes are used, as it will give a good size of pellet for EM processing. For more than one subsequent reassembly reactions, the reaction mixture can be scaled up, e.g., for five subsequent reassembly reactions, use 100 μg membranes and 10 mg mitotic cytosol in 1 ml reaction mixture, and later divide the membranes for five subsequent reassembly reactions.
-
34
Buffer exchange for mitotic cytosol. Before use, centrifuge the mitotic cytosol in a bench-top microcentrifuge for 20 min at 18,000g (14,000 r.p.m.) at 4 °C to remove protein precipitates. Desalt the mitotic cytosol into MEB buffer using Bio-spin 6-Tris column according to the manufacturer’s instruction.
-
35
In a 1.5 ml Eppendorf tube, mix the reagents in the order of MEB buffer, ATP regeneration system, mitotic cytosol and membranes.
▴ CRITICAL STEP The Golgi membranes should be thawed on ice right before the reaction. Unused membranes or cytosol can be snap frozen and kept in − 80 °C.
-
36
Incubate the reactions at 37 °C in a water bath for 20 min. Then, either directly process the generated MGFs for EM analysis or reisolate from mitotic cytosol and use for subsequent reassembly reactions (Steps 37–41) or analyze by sucrose gradients (described in Steps 54–59).
? TROUBLESHOOTING
Isolation of MGFs from the mitotic cytosol after disassembly ● TIMING 1 h
-
37
Add 400 μl of 0.4 M sucrose in KHM buffer in a 1.5 ml ultracentrifuge tube for a Beckman TLA55 rotor.
-
38
Carefully load the disassembly reaction (e.g., 1 ml) above the 0.4 M sucrose cushion.
▴ CRITICAL STEP If the reaction is in a smaller volume, increase the volume of the 0.4 M sucrose cushion so that the total volume in the tube is between 1.0 and 1.4 ml. The centrifuge tube may collapse after the high-speed spin if the volume is too small.
-
39
Add 6 μl of 2 M sucrose to the bottom of the centrifuge tube, which will keep the membranes in suspension after the centrifugation.
-
40
Spin the tube in a TLA55 rotor at 136,000g (55,000 r.p.m.) for 20 min at 4 °C. Remove the supernatant carefully. Remove the 2 M sucrose underneath the membranes using a 10 μl pipette (this may not need to be complete).
-
41
Take out the Golgi membrane suspension. Use the pipette to measure the volume of the MGFs and adjust the volume by adding KHM buffer to 4 mg ml−1 membranes (if the disassembly assay uses 100 μg Golgi membranes, adjust the MGF volume to 25 μl). Then either use the membrane for Golgi reassembly using interphase HeLa cell cytosol (Steps 42–44) or purified components (Steps 49–53), or analyze by EM (Steps 60–73) or western blotting.
Golgi reassembly using interphase HeLa cell cytosol ● TIMING 2 h
-
42
Buffer exchange. Before use, centrifuge the cytosol in a microcentrifuge for 20 min at 18,000g (14,000 r.p.m.) at 4 °C to remove protein precipitates. Desalt the interphase cytosol into KHM buffer using Bio-spin 6-Tris column according to the manufacturer’s instruction.
-
43
In 500 μl Eppendorf tubes, mix 5 μl (20 μg) of the reisolated MGFs with 1× ATP regeneration system and 400 μg (about 20 μl) interphase cytosol. Adjust the volume with KHM buffer to 30 μl per reaction.
▴ CRITICAL STEP The use of 500 μl Eppendorf tube allows the samples to concentrate in a relatively sharp tip of the tube, which makes the following EM processing and sectioning easier. Label the tubes properly and cover the label with a transparency tape, otherwise the label may be washed off by the dehydration solutions in the EM process.
-
44
Incubate the reaction in a 37 °C water bath for 60 min. Gently mix the samples every 20 min by tapping the Eppendorf tube. Then directly fix the reaction and analyze by EM (Steps 60–73). Alternatively, analyze disassembly and reassembly reactions by western blotting for phosphorylation of Golgi structural proteins10,21,23,24,41,47.
? TROUBLESHOOTING
Golgi disassembly assay using purified proteins ● TIMING 2 h
-
45
Calculate the amounts of membranes and purified proteins needed. Use 20 μg purified Golgi membranes per reaction, which gives an appropriate pellet size for EM processing. The total reaction volume should be 200 μl, which gives a final concentration of 0.1 mg ml−1 Golgi membranes. Calculate the amount of kinases, i.e., recombinant cdc2 and plk purified from Hi-5 cells10, based on their activity, which should be equivalent to 2 mg mitotic cytosol. In addition, calculate the amount of COPI budding machinery, i.e., 5 μg recombinant myristoylated ARF124,32,33 and 10 μg coatomer purified from rat liver cytosol31. Add 1× ATP regeneration system and use MEB buffer to adjust the volume to 200 μl. Use mitotic HeLa cell cytosol as a control for disassembly (Steps 33–36).
▴ CRITICAL STEP Kinases and COPI budding machinery can be used separately or in combination in the disassembly assay.
-
46
Before use, spin the proteins in a microcentrifuge for 20 min at 18,000g (14,000 r.p.m.) and 4 °C to remove protein precipitates. Thaw the Golgi membranes on ice; do not spin.
-
47
Add the reagents into 1.5 ml Eppendorf tube in the order of MEB buffer, ATP regeneration system, kinases, coatomer, Golgi membranes and myristoylated ARF1. Mix with a 200 μl pipette. Incubate the mixture at 37 °C for 20 min in a water bath.
▴ CRITICAL STEP ARF1 has to be added after the membranes. ARF1 is prepared in the GDP form. When added into the reaction mixture that contains GTP, it undergoes GDP–GTP exchange and becomes membrane bound. Without membranes, ARF1 will precipitate and become nonfunctional. To test the function of each group of proteins, kinases or COPI vesicle machinery can be added into the reaction separately. In addition to the listed components, additional proteins or antibodies can also be tested using this method. Protein phosphatase inhibitors such as 5 μM microcystin LR can also be included into the reaction. The reaction can be scaled up if the membranes will be used for several subsequent reassembly reactions.
-
48
Isolate the MGFs from the soluble proteins following the procedures described in Steps 37–41. Resuspend the pellet into KHM buffer and then use the aliquots for the reassembly assay or fix for EM analysis. Alternatively, analyze the samples by sucrose gradients, as described in Steps 54–59.
? TROUBLESHOOTING
Golgi reassembly assay using purified proteins ● TIMING 1 h
-
49
Calculate the amount of each protein that needs to be added. Add 1 × ATP regeneration system and use KHM buffer to adjust the volume to 30 μl when 20 μg MGFs are used in each reaction. As described in the Experimental design, calculate the amount of proteins on the basis of the amount of the same protein in 400 μg interphase cytosol, based on quantitation of western blot results. The amounts of the purified proteins can be increased to two to threefolds of that contained in 400 μg cytosol owing to the reduced protein activity or optimized based on published literatures and in our experiments. For the two membrane fusion pathways, use the following amount of proteins in a 30 μl reaction system: (1) the NSF pathway17,34: 3 μg of NSF (recombinant protein, final concentration: 100 ng μl−1), 0.75 μg of α-SNAP (25 ng μl−1), 0.75 μg of γ-SNAP (25 ng μl−1), 0.9 μg of p115 (purified from rat liver cytosol)15,35,36,48 (30 ng μl−1) and 2 U of PP2A (purchased from Millipore) (0.07 U μl−1) or (2) the p97 pathway17,18,49,50: 3 μg p97 (recombinant protein or purified from rat liver cytosol; 100 ng μl−1), 0.75 μg p47 (recombinant protein, 25 ng μl−1), 0.9 μg p115 (30 ng μl−1) and 2 U PP2A (0.07 U μl−1). p97 and p47 should be mixed and kept on ice for 10 min before membranes are added into the reaction17,20. Use interphase HeLa cytosol (Steps 42–44) as a control for the reassembly assay.
-
50
Before use, centrifuge the proteins in a tabletop microcentrifuge for 20 min at 18,000g (14,000 r.p.m.) at 4 °C to remove protein precipitates.
-
51
In 500 μl Eppendorf tubes, mix KHM buffer, an ATP regeneration system, purified proteins and 20 μg MGFs. Mix well.
▴ CRITICAL STEP When purified kinases are used in the disassembly assay, adding a general kinase inhibitor (e.g., 5 μM staurosporine) to inhibit the kinases associated with the membranes may help the reassembly.
-
52
Incubate the reaction in a water bath at 37 °C for 60 min; gently mix the reaction every 20 min by tapping the tube.
-
53
Process the samples directly for EM analysis (Steps 60–73) or pellet the membranes by centrifugation at 136,000g (55,000 r.p.m.) for 30 min in a TLA55 rotor, and then analyze by western blotting. Alternatively, analyze the samples by sucrose gradients as described in Steps 54–59.
? TROUBLESHOOTING
Separation of Golgi-derived vesicles from Golgi remnants using sucrose gradients ● TIMING 5–6 h
-
54
Prepare a step gradient: from the bottom to the top of a centrifuge tube (Beckman, cat. no. 362181) for a Beckman VTi65.1 rotor, carefully overlay each of the following sucrose solutions: 2.0 ml of 1.6 M, 2.0 ml of 1.4 M, 2.0 ml of 1.2 M, 1.5 ml of 0.8 M and 1.0 ml of 0.5 M sucrose. For disassembly reactions, the solutions should be prepared in MEB buffer and sucrose concentration verified using a refractometer.
-
55
Add ice-cold 3 M KCl solution to the disassembly reactions to a final concentration of 250 mM, leave the tube on ice for 2 min. The addition of high salt (KCl) will stop the reaction and facilitate the dissociation of the vesicles from the Golgi remnants.
-
56
Overlay the membrane sample on top of the gradient.
-
57
Centrifuge the gradient to equilibrium for 2.5 h at 372,000g (65,000 r.p.m.) in a Beckman VTi65 rotor at 4 °C.
▴ CRITICAL STEP Coated vesicles have a higher density (equivalent to 1.2–1.3 M sucrose) than Golgi remnants (similar to 0.8 M sucrose). A vertical or near vertical rotor generates a higher g-force (over 200,000g) than a swing out rotor and thus allows a quick separation of vesicles from the Golgi remnants. It will also avoid the effect caused by uncoating of the vesicles over time.
-
58
Fractionate the gradient from top to bottom using a pipette. The fractions can be directly analyzed by SDS-PAGE and western blotting. To exclude soluble proteins from membranes, proceed to the next step.
-
59
Dilute each fraction by threefold with MEB buffer containing no sucrose and pellet the membrane by centrifugation in a Beckman TLA55 rotor at the maximal speed for 60 min at 4 °C. The membranes can be analyzed by western blotting or processed for EM.
▴ CRITICAL STEP The samples need to be diluted by MEB buffer containing no sucrose, otherwise the high sucrose solution will not allow sedimentation of the membranes. The soluble proteins, most of which remain in the top two fractions in the gradient, are not pelleted after this centrifugation. Therefore, only the proteins that are associated with Golgi remnants and vesicles will be tested. Ensure that the pellets are completely dissolved in SDS buffer before loading onto a SDS-PAGE gel. If the isolated membranes are used for further reactions, add 6 μl 2 M sucrose to the bottom of the 1.5 centrifuge tube before centrifugation. This will maintain the membranes in solution; otherwise, they will be difficult to resuspend once they form a tight pellet at the bottom of the tube.
? TROUBLESHOOTING
Electron microscopy ● TIMING 3 d
-
60
Fixation: Resuspend the membrane pellet into 400 μl of 2% glutaraldehyde solution (in MEB buffer for disassembly reactions or in KHM buffer for reassembly reactions), mix well and leave for 30 min at room temperature. For membrane solutions from disassembly reactions (200 μl for each reaction), add 200 μl of 4% glutaraldehyde in KHM buffer and mix. Ensure that the fixative is always freshly prepared.
-
61
Spin down the membrane for 20 min at maximal speed in a swing out rotor in an Eppendorf tabletop 5417R microcentrifuge, and then spin for 15 min in a fixed angle rotor in an equivalent centrifuge.
▴ CRITICAL STEP The first spin will accumulate the membranes right at the bottom of the tube; the second spin generates higher g-force to make the pellet more compact. Fixation of the membranes allows uniform distribution of the membranes into the pellet, as they are cross-linked by the fixative before the centrifugation. We use 0.5 ml Eppendorf tubes, as the pellet is sharper at the tip of the tube, which is easier for sectioning without much trimming. The membrane pellet should not be too big (more than 3 mm in diameter), as a large size of the pellet prevents penetration of the dehydration and infiltration buffer in the later steps. There is no need to dissociate the pellets from the bottom of the tube during epon embedding.
-
62
Wash the pellet with 0.5 ml 0.1 M sodium cacodylate (pH 7.4) three times.
▪ PAUSE POINT Membrane pellets after fixation can be maintained in cacodylate buffer overnight at 4 °C.
-
63
Next day, wash the pellet with 0.5 ml of 0.1 M sodium cacodylate (pH 7.4) three times.
-
64
Post fix the pellet for 60 min with 200 μl of 1% (wt/vol) OsO4, 1.5% K3(Fe(CN)6) and 0.1 M sodium cacodylate (pH 7.4). Membranes should become dark after post-fixation step.
-
65
Wash the pellet with water three times.
-
66
Dehydrate the membrane by sequentially treating the pellet with 500 μl of 50% (2 × 5 min), 70% (2 × 5 min), 90% (2 × 5 min) and 100% (3 × 15 min) ethanol.
▴ CRITICAL STEP From this step on, the samples should be kept on a rotator at room temperature for better dehydration and infiltration results. Keep the lid closed when 100% ethanol is used. Embedding should be performed in an exhaust hood.
-
67
Remove 100% ethanol; treat the pellet with 0.5 ml propylene oxide for 15 min with closed lid.
-
68
Infiltrate with epon-mix/propylene oxide (1:1) for 1 h with the lid closed, then repeat the infiltration for another 1 h with the lid open.
-
69
Infiltrate with epon-mix (pure) with open lid for 3 × 1 h.
▪ PAUSE POINT Sample in pure epon-mix can be left rotating at room temperature overnight.
-
70
Add 500 μl of epon-mix and polymerize at 60 °C overnight.
-
71
Cut the sample into ultrathin (50–70 nm) sections using a diamond knife.
-
72
Collect the sections onto carbon-coated grids, stain the sample with 2% uranyl acetate at room temperature for 5 min, wash extensively with water, contrast with lead citrate solution at room temperature for 2 min, wash with water and dry on filter paper.
▴ CRITICAL STEP Omit uranyl acetate stain if cytosolic proteins precipitate in the samples.
-
73
Observe the membranes with transmission electron microscope at 80 kV.
? TROUBLESHOOTING
Stereology and quantitation ● TIMING 1 d
-
74
To analyze the efficiency of Golgi disassembly and reassembly, the percentage of membrane in either Golgi stacks, single cisternae, vesicles or tubular structures can be quantified. The criteria of each membrane structure are listed below5,6,10,21,24.
Cisternae: long membrane profiles with a length greater than four times their width, and the width no more than 60 nm. Normal cisterna ranges from 20 to 30 nm in width and longer than 200 nm.
Stacks: two or more cisternae that were separated by no more than 15 nm and overlapped in parallel by more than 50% of their length.
Vesicles: spherical or nearly spherical membrane profiles with 50–200 nm diameter, often can be seen with the protein coat. The vesicles in our study are ~70 nm in diameter.
Tubular network: single tubules with a length more than 1.5 times but less than four times their maximal width. The width is often uneven, sometimes more than 60 nm and sometimes with branches.
-
75
To quantify the percentage of membrane amount in each category, take photographs of the membranes in a random manner at a magnification of ×14,000. Enlarge and print the images onto 11 × 8.5 inch (US letter) papers, overlay them by a transparency paper with line grids of 4 mm width, and count the number of intersections of each membrane structure. Set the number of intersections of all membranes in the sample to 100% and calculate the percentage of membrane in each category. Following this, statistical analysis can be performed.
● TIMING
Steps 1–13, Purification of rat liver Golgi: 2 d. Starvation of the rats takes 24 h; preparation of the solutions takes 2 h; and the actual purification procedure takes up to 4 h
Steps 14–21, Determination of Golgi purity: 3 h. As the purification results were highly reproducible, we only do the GalT assay once every two to three preparations
Steps 22–32, Preparation of cytosol: 12 d. Most of the time is spent waiting for the cell to grow in large numbers. The actual purification procedure takes up to 4 h
Steps 33–36 or Steps 45–48, Golgi disassembly assay: 2 h. Buffer preparation takes about 1 h, incubation of the reaction takes 20 min
Steps 37–41, Isolation of MGFs: 1 h
Steps 42–44 or Steps 49–53, Golgi reassembly assay: 2 h. Preparation of the solutions takes 1 h, incubation of the reaction takes 1 h
Steps 54–59, Separation of Golgi-derived vesicles from Golgi remnants: 5–6 h. Solution and gradient preparation takes 1 h, centrifugation takes 3 h and fractionation takes 1 h. Reisolation of membranes in each fraction take another 1 h
Steps 60–73: Electron microscopy, 3 d. Embedding, 1 d; sectioning and observation, 1 d; and further analysis and image processing, 1 d
Steps 74–75: Quantitation of Golgi disassembly or reassembly efficiency, 1 d
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
TABLE 2.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 5–12 | Low yield with Golgi preparations |
|
|
| 5–12 and 60–73 | Golgi stacked structure not preserved when examined by EM |
|
|
| 33–36 | Golgi disassembly by mitotic cell cytosol does not work |
|
|
| 42–44 | Golgi reassembly by interphase cell cytosol does not work |
|
|
| 45–48 | Golgi disassembly by purified proteins does not work, with a large number of long cisternae remaining | ARF1 or coatomer preparations have low activity |
|
| Golgi disassembly by purified proteins does not work, with the core of the Golgi remaining as mini-stacks | Kinase activity is low |
|
|
| 49–53 | Golgi reassembly by purified proteins does not work, with new mini-stacks generated but the cisternae are short and a large number of vesicles are remaining |
|
|
| Golgi reassembly by purified proteins does not work, new cisternae are formed, but they do not form stacks | Phosphatase (PP2A) activity too low |
|
|
| 54–59 | No membranes in the vesicle fractions |
|
|
| 60–73 | Under the EM, samples showed dark precipitates with less membranes after the standard disassembly and reassembly (this never happens with the defined assay) | Proteins in the cytosol precipitated |
|
ANTICIPATED RESULTS
One successful reconstitution of Golgi disassembly with purified rat liver Golgi and mitotic cytosol will lead to fragmentation of a large fraction (> 60%) of the Golgi membranes from stacked cisternae into vesicles, when analyzed by electron microscope (Fig. 1). When the purified proteins, including both kinases and COPI budding machinery, are used in the disassembly reaction, the efficiency reaches 80% (ref. 21).
Figure 1.
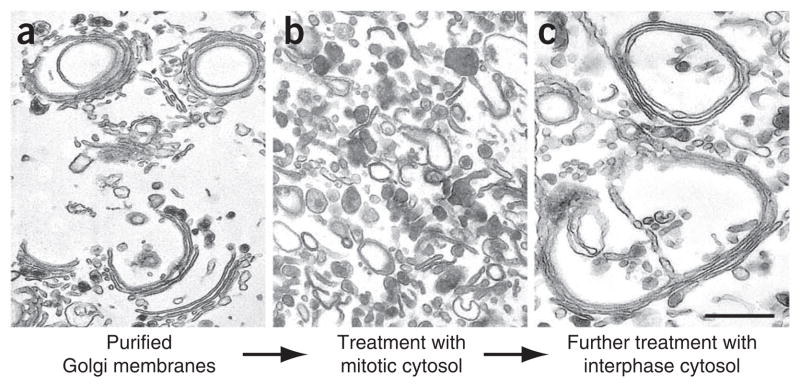
Standard Golgi disassembly and reassembly assay in which purified Golgi membranes were treated with mitotic and interphase HeLa cell cytosol (a,b). (a) Purified rat liver Golgi membranes were (b) first treated with mitotic HeLa cell cytosol at 37 °C for 20 min. (c) Membranes were reisolated by centrifugation and subsequently treated by interphase cytosol at 37 °C for 60 min. Membranes were fixed and processed for EM. Scale bar, 0.5 μm. Note that treatment with mitotic cytosol led to fragmentation of the Golgi membranes, whereas further treatment of the Golgi fragments with interphase cytosol allowed the reassembly of new Golgi stacks. Reprinted and modified from Tang et al.21 with permission from the journal. Our plan for animal use is approved by the University of Michigan Committee on Use and Care of Animals (UCUCA, approval number 09146).
There are two major types of morphological changes in Golgi disassembly. One is cisternal unstacking; a large number of single cisternae will be observed after treatment with purified cdc2 and plk kinases. Previous studies showed that Golgi sacking is mediated by the oligomerization of the Golgi stacking proteins GRASP65 and GRASP55; phosphorylation of these proteins by mitotic kinases leads to their dephosphorylation, oligomerization and thus to Golgi cisternal unstacking10,21,41. Treatment of Golgi membrane with mitotic kinases leads to cisternal unstacking, but the cisternae remain to be of the same length. A second morphological change involves vesiculation of the Golgi membranes, which is mediated by the COPI budding machinery ARF1 and coatomer. Treatment of Golgi membranes with purified ARF1 and coatomer generates a large number of vesicles; however, the core of the Golgi stack often remains as mini stacks after this treatment. Only when both treatments are combined, the disassembly becomes extensive and complete, with about 80% of the membranes in vesicles and the rest in short tubules, and no stacks or long cisternae remained (Fig. 2)21.
Figure 2.
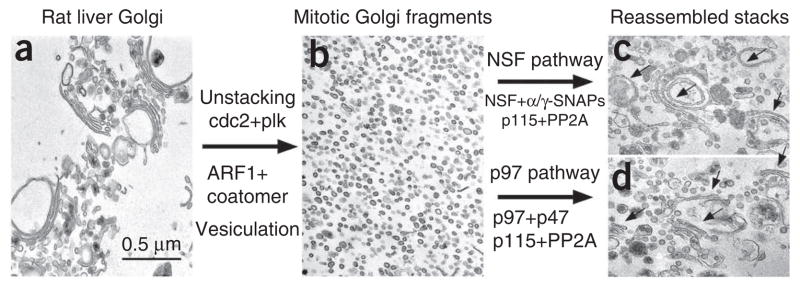
Defined Golgi disassembly and reassembly assay using purified proteins (a,b). (a) Purified Golgi membranes were first treated with (b) purified ARF1, coatomer and mitotic kinases at 37 °C for 20 min (c,d). Membrane fragments were reisolated and further treated by purified proteins (c) in the p97 pathway (p97 and p47) or (d) in the NSF pathway (NSF and α/γ-SNAP), at 37 °C for 60 min. Each reassembly reaction also included the Golgi tethering protein p115 and the protein phosphatase PP2A. Membranes were fixed and processed for EM. Scale bar, 0.5 μm. Note the newly formed Golgi stacks and single cisternae (arrows) after reassembly. Reprinted and modified from Tang et al.21 with permission from the journal.
The Golgi membrane disassembled by either mitotic cytosol or purified proteins described above can be reassembled into new Golgi stacks when the Golgi fragments are further incubated with interphase cytosol (Fig. 1c) or purified proteins (Fig. 2c). Purified proteins include two AAA ATPases, NSF and p97 (ref. 17), which function together with their cofactors in post-mitotic Golgi membrane fusion and thus generate single Golgi cisternae and the phosphatase PP2A, which dephosphorylates the Golgi stacking protein GRASP65 (ref. 21). Reassembly using NSF (and its cofactors) is normally more efficient that using p97 and p47 (ref. 21), we reason this to be because of the lack of ubiquitination machineries required for the reassembly reaction19,20. In general, the efficiency of the disassembly using purified components is higher than with mitotic HeLa cell cytosol, possibly because of the loss of kinase or COPI budding activities during the preparation of the cytosol or because of the existence of membrane fusion proteins and phosphatases in the mitotic cytosol. The defined reassembly using purified proteins is normally less efficient than the standard reassembly using interphase cytosol, possibly because of the high efficiency in the defined disassembly or because of the lacking of regulatory proteins in the reassembly reaction. In this sense, this assay could be used to screen for additional proteins or chemicals that can enhance the reaction, which may lead to the discovery of novel proteins that regulate Golgi biogenesis during the cell cycle.
Vesicles derived from the Golgi membranes can be isolated from the Golgi remnants using equilibrium sucrose gradient. Western blotting results have shown that they contain different protein components (Fig. 3a). For example, Golgi matrix proteins such as GRASP65 and GM130 are concentrated in the Golgi remnants, whereas some of the Golgi enzymes such as α-mannosidase II are more enriched in vesicles. Further analysis of the distributions of these proteins may provide information on the mechanism of protein trafficking across the Golgi stack and Golgi division into the daughter cells. In addition, this method can be used to prepare Golgi-derived vesicles (Fig. 3c) and Golgi core machineries (or Golgi remnants, Fig. 3b), which could be useful for analysis of the mechanism of Golgi structure formation. For example, these membranes can be labeled by a fluorescence dye and microinjected into cells followed by time-lapse microscopy to monitor its integration into the Golgi in living cells.
Figure 3.
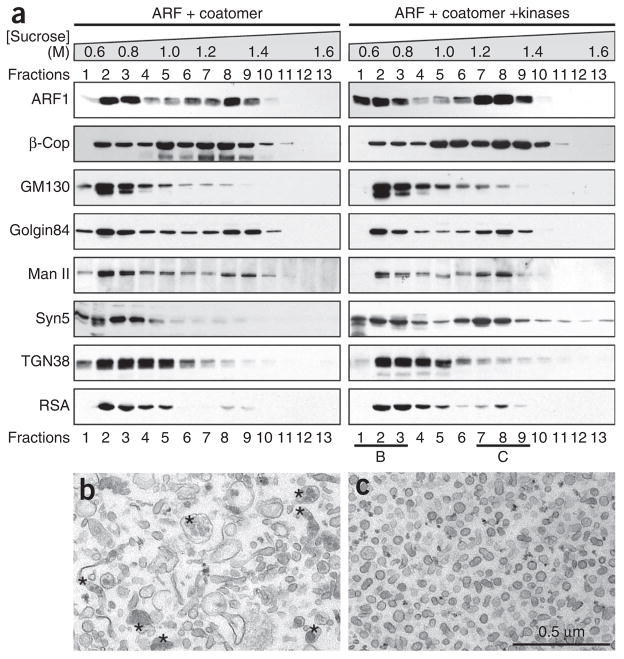
Distribution of Golgi proteins in vesicles and Golgi remnants after mitotic Golgi disassembly. Purified rat liver Golgi membranes were treated with purified ARF1 and coatomer in the absence or presence of mitotic kinases at 37 °C for 20 min. Reactions were fractionated by equilibrium centrifugation on sucrose gradients (0.5–1.6 M, indicated on the top). (a) Membranes in each fraction were pelleted and equal fractions (by volume) were analyzed by western blotting for indicated proteins. Man II, α-mannosidase II; syn5, syntaxin 5; RSA, rat serum albumin. Membranes in fractions 1–3, which contained 0.6–0.8 M sucrose and Golgi remnants, and in fractions 7–9, which contained 1.3–1.4 M sucrose and COPI vesicles, were collected and processed for EM. (b,c) The EM images are shown in (b) and (c), respectively. Asterisks in b indicate lipoprotein-enriched trans-Golgi network elements. The average diameter of the vesicles in c is 70 ± 6 nm (mean ± s.d.). Scale bar, 0.5 μm. Reprinted and modified from Tang et al.21 with permission from the journal.
Acknowledgments
The current Golgi disassembly and reassembly assay was modified from a protocol developed by T. Misteli and C. Rabouille who used purified Golgi membranes, mitotic HeLa cell cytosol and rat liver cytosol5,6. We thank J. Shorter and G. Warren for help and suggestions during our work, M. Jackman, K. Mar, J. Malsam, H. Meyer and T. Taguchi for protein preparations and J. Rothman and G. Thomas for reagents. This work was supported by the Pardee Cancer Research Foundation, the National Institute of Health (GM087364), the American Cancer Society (RGS-09-278-01-CSM), a University of Michigan Rackham Faculty Research Grant, the NIH-funded Michigan Alzheimer’s Disease Research Center (P50 AG08761) and an anonymous donation to Y.W.
Footnotes
AUTHOR CONTRIBUTIONS Y.W. and Y.X. prepared Golgi membranes, Y.W. performed the Golgi disassembly and reassembly assay and Y.W. and D.T. analyzed the results. Y.W., D.T. and Y.X. wrote the paper.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Acharya U, et al. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. doi: 10.1016/0092-8674(95)90269-4. [DOI] [PubMed] [Google Scholar]
- 2.Jamora C, et al. Regulation of Golgi structure through heterotrimeric G proteins. Cell. 1997;91:617–626. doi: 10.1016/s0092-8674(00)80449-3. [DOI] [PubMed] [Google Scholar]
- 3.Kano F, Nagayama K, Murata M. Reconstitution of the Golgi reassembly process in semi-intact MDCK cells. Biophys Chem. 2000;84:261–268. doi: 10.1016/s0301-4622(00)00133-2. [DOI] [PubMed] [Google Scholar]
- 4.Kano F, et al. MEK and Cdc2 kinase are sequentially required for Golgi disassembly in MDCK cells by the mitotic Xenopus extracts. J Cell Biol. 2000;149:357–368. doi: 10.1083/jcb.149.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misteli T, Warren G. COP-coated vesicles are involved in the mitotic fragmentation of Golgi stacks in a cell-free system. J Cell Biol. 1994;125:269–282. doi: 10.1083/jcb.125.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabouille C, Misteli T, Watson R, Warren G. Reassembly of Golgi stacks from mitotic Golgi fragments in a cell-free system. J Cell Biol. 1995;129:605–618. doi: 10.1083/jcb.129.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y. Golgi apparatus inheritance. In: Mironov A, Pavelka M, Luini A, editors. The Golgi Apparatus. State of the Art 110 Years After Camillo Golgi’s Discovery. Chapter 4.3. Springer-Verlag Gmbh; Wien-New York: 2008. pp. 580–607. [Google Scholar]
- 9.Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. doi: 10.1016/s0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Seemann J, Pypaert M, Shorter J, Warren G. A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 2003;22:3279–3290. doi: 10.1093/emboj/cdg317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shorter J, et al. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 1999;18:4949–4960. doi: 10.1093/emboj/18.18.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowe M, et al. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 1998;94:783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- 13.Sonnichsen B, et al. A role for giantin in docking COPI vesicles to Golgi membranes. J Cell Biol. 1998;140:1013–1021. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh A, Wang Y, Malsam J, Beard MB, Warren G. Golgin-84 is a rab1 binding partner involved in Golgi structure. Traffic. 2003;4:153–161. doi: 10.1034/j.1600-0854.2003.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shorter J, Warren G. A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling golgi cisternae in a cell-free system. J Cell Biol. 1999;146:57–70. doi: 10.1083/jcb.146.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabouille C, et al. Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell. 1998;92:603–610. doi: 10.1016/s0092-8674(00)81128-9. [DOI] [PubMed] [Google Scholar]
- 17.Rabouille C, Levine TP, Peters JM, Warren G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell. 1995;82:905–914. doi: 10.1016/0092-8674(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 18.Kondo H, et al. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–78. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- 19.Meyer HH, Wang Y, Warren G. Direct binding of ubiquitin conjugates by the mammalian p97 adaptor complexes, p47 and Ufd1-Npl4. EMBO J. 2002;21:5645–5652. doi: 10.1093/emboj/cdf579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Satoh A, Warren G, Meyer HH. VCIP135 acts as a deubiquitinating enzyme during p97-p47-mediated reassembly of mitotic Golgi fragments. J Cell Biol. 2004;164:973–978. doi: 10.1083/jcb.200401010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang D, Mar K, Warren G, Wang Y. Molecular mechanism of mitotic Golgi disassembly and reassembly revealed by a defined reconstitution assay. J Biol Chem. 2008;283:6085–6094. doi: 10.1074/jbc.M707715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CY, et al. Peripheral Golgi protein GRASP65 is a target of mitotic polo-like kinase (Plk) and Cdc2. Proc Natl Acad Sci USA. 2000;97:12589–12594. doi: 10.1073/pnas.220423497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Satoh A, Warren G. Mapping the functional domains of the Golgi stacking factor GRASP65. J Biol Chem. 2005;280:4921–4928. doi: 10.1074/jbc.M412407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang Y, Seemann J, Bisel B, Punthambaker S, Wang Y. Active ADP-ribosylation factor-1 (ARF1) is required for mitotic Golgi fragmentation. J Biol Chem. 2007;282:21829–21837. doi: 10.1074/jbc.M611716200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otter-Nilsson M, Hendriks R, Pecheur-Huet EI, Hoekstra D, Nilsson T. Cytosolic ATPases, p97 and NSF, are sufficient to mediate rapid membrane fusion. EMBO J. 1999;18:2074–2083. doi: 10.1093/emboj/18.8.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller JM, et al. An NSF function distinct from ATPase-dependent SNARE disassembly is essential for Golgi membrane fusion. Nat Cell Biol. 1999;1:335–340. doi: 10.1038/14025. [DOI] [PubMed] [Google Scholar]
- 27.Muller JM, et al. Sequential SNARE disassembly and GATE-16-GOS-28 complex assembly mediated by distinct NSF activities drives Golgi membrane fusion. J Cell Biol. 2002;157:1161–1173. doi: 10.1083/jcb.200202082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchiyama K, et al. p37 is a p97 adaptor required for Golgi and ER biogenesis in interphase and at the end of mitosis. Dev Cell. 2006;11:803–816. doi: 10.1016/j.devcel.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Hui N, Nakamura N, Slusarewicz P, Warren G. Purification of rat liver Golgi stacks. In: Celis J, editor. Cell Biology: A Laboratory Handbook. Vol. 2. Academic Press; San Diego, California: 1998. pp. 46–55. [Google Scholar]
- 30.Wang Y, Taguchi T, Warren G. Purification of rat liver Golgi stacks. In: Celis J, editor. Cell Biology: A Laboratory Handbook. 3. Elsevier Science; San Diego, California: 2006. pp. 33–39. [Google Scholar]
- 31.Pavel J, Harter C, Wieland FT. Reversible dissociation of coatomer: functional characterization of a beta/delta-coat protein subcomplex. Proc Natl Acad Sci USA. 1998;95:2140–2145. doi: 10.1073/pnas.95.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randazzo PA, Weiss O, Kahn RA. Preparation of recombinant ADP-ribosylation factor. Methods Enzymol. 1995;257:128–135. doi: 10.1016/s0076-6879(95)57018-7. [DOI] [PubMed] [Google Scholar]
- 33.Ha VL, Thomas GM, Stauffer S, Randazzo PA. Preparation of myristoylated Arf1 and Arf6. Methods Enzymol. 2005;404:164–174. doi: 10.1016/S0076-6879(05)04016-4. [DOI] [PubMed] [Google Scholar]
- 34.Whiteheart SW, et al. N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J Cell Biol. 1994;126:945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine TP, Rabouille C, Kieckbusch RH, Warren G. Binding of the vesicle docking protein p115 to Golgi membranes is inhibited under mitotic conditions. J Biol Chem. 1996;271:17304–17311. doi: 10.1074/jbc.271.29.17304. [DOI] [PubMed] [Google Scholar]
- 36.Satoh A, Beard M, Warren G. Preparation and characterization of recombinant golgin tethers. Methods Enzymol. 2005;404:279–296. doi: 10.1016/S0076-6879(05)04026-7. [DOI] [PubMed] [Google Scholar]
- 37.Beard M, Satoh A, Shorter J, Warren G. A cryptic Rab1-binding site in the p115 tethering protein. J Biol Chem. 2005;280:25840–25848. doi: 10.1074/jbc.M503925200. [DOI] [PubMed] [Google Scholar]
- 38.Molloy SS, Thomas L, Kamibayashi C, Mumby MC, Thomas G. Regulation of endosome sorting by a specific PP2A isoform. J Cell Biol. 1998;142:1399–1411. doi: 10.1083/jcb.142.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucocq JM, Berger EG, Warren G. Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J Cell Biol. 1989;109:463–474. doi: 10.1083/jcb.109.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bisel B, et al. ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65. J Cell Biol. 2008;182:837–843. doi: 10.1083/jcb.200805045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiang Y, Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol. 2010;188:237–251. doi: 10.1083/jcb.200907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shima DT, Haldar K, Pepperkok R, Watson R, Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altan-Bonnet N, et al. Golgi inheritance in mammalian cells is mediated through endoplasmic reticulum export activities. Mol Biol Cell. 2006;17:990–1005. doi: 10.1091/mbc.E05-02-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malsam J, Satoh A, Pelletier L, Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307:1095–1098. doi: 10.1126/science.1108061. [DOI] [PubMed] [Google Scholar]
- 45.Nickel W, et al. Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTPgammaS in vitro. J Cell Sci. 1998;111 (Part 20):3081–3090. doi: 10.1242/jcs.111.20.3081. [DOI] [PubMed] [Google Scholar]
- 46.Serafini T, Rothman JE. Purification of Golgi cisternae-derived non-clathrin-coated vesicles. Methods Enzymol. 1992;219:286–299. doi: 10.1016/0076-6879(92)19029-6. [DOI] [PubMed] [Google Scholar]
- 47.Vielemeyer O, et al. Direct selection of monoclonal phosphospecific antibodies without prior phosphoamino acid mapping. J Biol Chem. 2009;284:20791–20795. doi: 10.1074/jbc.M109.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satoh A, Malsam J, Warren G. Tethering assays for COPI vesicles mediated by golgins. Methods Enzymol. 2005;404:125–134. doi: 10.1016/S0076-6879(05)04013-9. [DOI] [PubMed] [Google Scholar]
- 49.Meyer HH, Kondo H, Warren G. The p47 co-factor regulates the ATPase activity of the membrane fusion protein, p97. FEBS Lett. 1998;437:255–257. doi: 10.1016/s0014-5793(98)01232-0. [DOI] [PubMed] [Google Scholar]
- 50.Meyer HH, Shorter JG, Seemann J, Pappin D, Warren G. A complex of mammalian ufd1 and npl4 links the AAA-ATPase, p97, to ubiquitin and nuclear transport pathways. EMBO J. 2000;19:2181–2192. doi: 10.1093/emboj/19.10.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]