Abstract
The identification and clinical use of more sensitive and specific biomarkers in the field of solid organ transplantation is an urgent need in medicine. Solid organ transplantation has seen improvements in the short-term survival of transplanted organs due to recent advancements in immunosuppressive therapy. However, the currently available methods of allograft monitoring are not optimal. Recent advancements in assaying methods for biomolecules such as genes, mRNA and proteins have helped to identify surrogate biomarkers that can be used to monitor the transplanted organ. These high-throughput ‘omic’ methods can help researchers to significantly speed up the identification and the validation steps, which are crucial factors for biomarker discovery efforts. Still, the progress towards identifying more sensitive and specific biomarkers remains a great deal slower than expected. In this article, we have evaluated the current status of biomarker discovery using proteomics tools in different solid organ transplants in recent years. This article summarizes recent reports and current status, along with the hurdles in efficient biomarker discovery of protein biomarkers using proteomics approaches. Finally, we will touch upon personalized medicine as a future direction for better management of transplanted organs, and provide what we think could be a recipe for success in this field.
Keywords: acute rejection, biomarker, biomarker discovery, noninvasive biomarkers, organ transplantation, peptidomics, proteomics, urine
Risk of rejection of transplanted organ is a major concern for transplant recipients and transplant physicians [1,2]. Currently available strategies to monitor transplanted organs are not efficient and accurate enough to assess the risk of drug toxicity and predict acute or chronic rejection. Therefore, there is an urgent need for noninvasive or minimally invasive biomarkers that could help in monitoring the health status of the transplanted organ. Such clinically applicable biomarkers would enable clinicians to utilize broad-spectrum clinical screening and diagnosis and also assess each individual patient’s risk for acute and chronic allograft injury and tolerance for the transplanted organ, and to monitor efficacy of immunosuppressive therapy. Accurate assessment of the status of these organs achieved through such biomarkers would help either increase or decrease immunosuppressive drugs in time so that further injury or damage to the organ could be prevented [3].
Rapid advancement in the field of genomics and transcriptomics assays to accurately measure changes in the gene level or mRNA level has helped our understanding of the role of gene polymorphisms and changes in the transcription level of genes and its regulation [4,5]. The impact of translational regulation, post-translational modifications, protein kinetics, protein–protein interactions and turnovers of proteins makes study of proteins distinctly advantageous over profiling of genes or mRNAs. The emergence of and developments in mass spectrometry (MS)-based proteomics has aided in the high-throughput profiling of proteins and peptides present in all the tissue types and fluids in an organism. Because of the dire need of more sensitive and specific clinical biomarkers, it is high time for researchers to come together and apply genomics, proteomics, metabolomics and bioinformatics to identify and validate such biomarkers. In this article, we focus on recent advancements in proteomics methods and critically review the current status of application of proteomics in the field of solid organ transplantation.
Recent biomarker discovery efforts in solid organ transplantation using proteomics
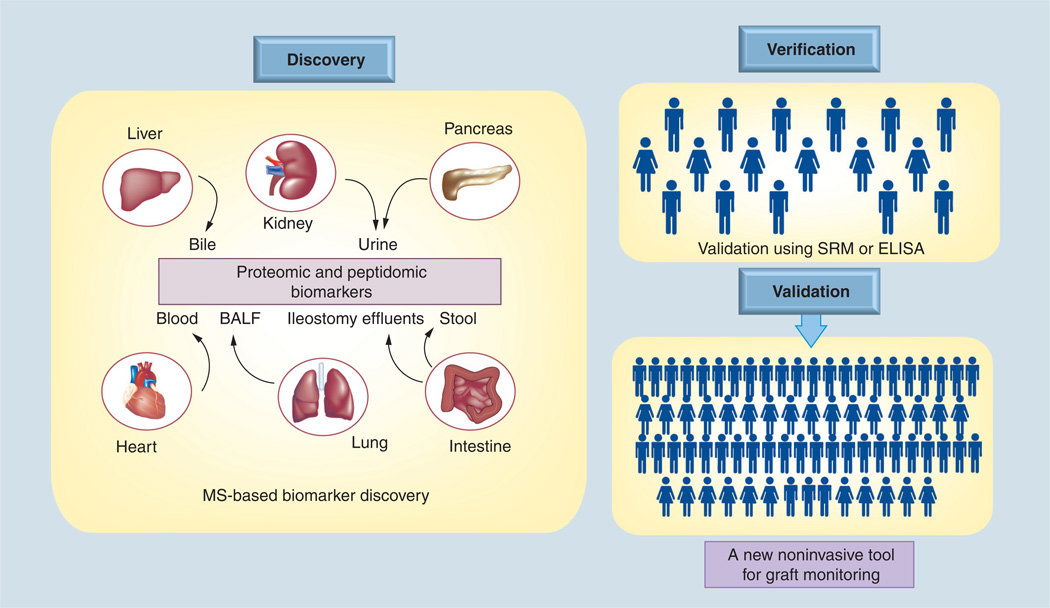
Development and advancement in MS-based proteomic analysis, along with the publication of human genome data, generated a lot of enthusiasm among researchers to use these newly available tools in analyzing the proteome of different organisms [6,7]. It has been realized that proteins are much more complicated entities to profile than their nucleotide counterparts because of the post-translational modifications and dynamic nature of protein turnover. However, there is a continuous thrust towards identifying new proteins and peptides and the analysis of their role in different disease conditions that were previously not identified. The Human Protein Organization launched the Human Protein Project at its 9th Annual Congress 2010 in Sydney, Australia. The transplant community has started to utilize advantages and utility of proteomic methods not only in the field of discovery and validation of biomarkers but also in understanding the molecular mechanisms of graft dysfunction and tolerance. Progress is still very slow and it is expected that in the near future the efforts will escalate and we will be able to see an increased number of reports published in the field of transplantation proteomics [8,9]. Below is a brief summary in the field of application of proteomics in the field of solid organ transplantation. A schematic of use of proteomics in analyzing different biospecimens for different organs towards a clinically useful biomarker discovery is shown in Figure 1.
Figure 1. A schematic of how mass spectrometry-based proteomics could be used in analyzing different biospecimens for different organs in the discovery and validation of clinically useful biomarkers.
Once the potential biomarker molecules are identified, they could be validated through a larger patient cohort and subsequently by a clinical trial.
BALF: Bronchoalveolar lavage fluid; MS: Mass spectrometry; SRM: Selected reaction monitoring.
Kidney
Kidneys are the most transplanted organs. Improvements in immunosuppressive drug regimens have facilitated the short-term health of the transplanted kidney; however, long-term maintenance of the kidney is still a challenge, partly due to the lack of effective methods for diagnosis and prognosis [10]. The need for a noninvasive and specific biomarker has prompted new efforts in this regard. Several immune-monitoring assays have been tested that focused on adaptive T-cell activity and innate immunity, such as ELISPOT [11] and the ImmuKnow® assay [12]; however, none of these tools have yet been considered in clinical practice. Thus far, major attention has been focused on identifying gene markers using microarrays and quantitative (Q)-PCR [13–15] and a few proteomic studies [16–21].
Prediction and early detection of alloimmune specific and nonspecific injury processes prior to the change of clinically observable parameters is very important as it allows for timely clinical intervention. Identifying such parameters (genes, mRNA transcripts, peptides and proteins) has been investigated by monitoring these parameters in allograft biopsy, blood and urine [10,22,23]. According to Vidal et al., an ideal biomarker of for renal tubular injury would be easy to collect (e.g., urine or blood), sensitive to be detected early in the course of acute injury, specific to be able to distinguish tubular from perenal and glomerular injury, and ideally expressed in the kidney [24].
Urine remains a popular biospecimen because of the noninvasive nature of its collection and its proximity to the injury site [25]. Proteome analysis of urine not only helps to identify a potential biomarker for kidney transplant dysfunction (such as acute and chronic rejection) but also to understand the mechanism of graft dysfunction, as urine proteins could be originated from multiple sources such as filtration of plasma proteins, secretion of nephrons, proteolytic degradation products, secretion by the lower urinary tract, and physiological and/or pathological cell death. Vasconcellos et al. reported an increased expression of T-cell transcripts such as granzyme B, perforin and FasL at the time of acute rejection in peripheral blood mononuclear cells and validated their increase at the time of acute rejection [26]. The increase of expression was also found to be associated in the case of cytomegalovirus infection, urinary tract infection and delayed graft function by subsequent studies performed in the blood and urine [27–29]. Schaub et al. have reported an increase of CXCL9 and CXCL10 protein in the urine of patients with acute interstitial inflammation and tubulitis in both subclinical and clinical acute rejection [30], and the Schaub and colleagues also identified β2-microglobulin (β2-m) for acute rejection in a separate study [17]. In addition to β2-m, retinol-binding protein [31] and α1-microglobulin (α1-m) [32] are elevated in the urine of patients with acute rejection. Neutrophil-gelatinase-associated lipocalin has been reported as a marker of graft recovery after kidney transplantation [33]. In a recent study, we have reported an elevation of UMOD, SERPINF1 and CD44 in the urine of patients with active acute rejection [20]. With a novel integrative strategy that utilized both gene expression and peptidomics data, a 40-peptide panel including UMOD and collagen peptides has been reported to be acute rejection-specific in the urine of kidney transplant patients [19]. In a recent report, Nakorchevsky et al. took a proteogenomic approach to analyze kidney transplant biopsies using transcriptomics and proteomics in parallel to demonstrate how this novel approach could help not only in identifying injury-specific genes and proteins but also in providing a mechanistic insight into the graft injury [34]. In yet another novel effort, using a bioinformatics approach across different solid organ transplants (kidney and heart) Chen et al. identified a number of proteins as potential biomarkers for acute rejection that were successfully validated by ELISA [35]. A detailed summary of recent proteomics approach in the field of kidney transplantation is provided in Table 1.
Table 1.
A shortlist of recent publications in kidney transplantation that used proteomics.
| Study (year) | Organ | Phenotype | Study size | Discovery technology | Validation technology |
Biosample used |
Molecules identified (n) |
Interesting molecules | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Freue et al. (2010) | Kidney | Acute rejection | AR = 11, nAR = 21 |
iTRAQ-MALDI-TOF/TOF | ELISA | Plasma | n = 18 | TTN, KNG1, LBP, VASN, AFM | [82] |
| Kurian et al. (2009) | Kidney | Chronic allograft nephropathy | noCAN = 18, mild CAN = 15, severe CAN = 9 |
Shotgun LC/MS/MS | No validation | Peripheral blood lymphocytes | Mild CAN = 302, severe CAN = 509 |
No specific candidate proteins reported; however, 50 protein/mRNA matches were used in a discrimination analysis | [83] |
| Narkorchevsky et al. (2010) | Kidney | Chronic allograft nephropathy | noCAN = 8, mild CAN = 9, severe CAN = 15 |
Shotgun LC/MS/MS | SRM | Tissue biopsy | Unique = 492, differential expression = 904 |
No specific core molecules, but lists pathways/biological functions of interest: complement, actin cytoskeleton and lymphocyte/macrophage cell signaling | [34] |
| Sigdel et al. (2010) | Kidney | Acute rejection | 40 (92 samples) | Shotgun LC/MS/MS | ELISA | Urine | 1446 | UMOD, SERPINF1, CD44 | [20] |
| Dai et al. (2008) | Kidney (rat) | Acute rejection | 18 | MALDI-TOF-TOF-MS | No validation | Tissue | 11 | Chain D, rat transthyretin, apolipoprotein A-IV and Rho GDP | [84] |
| Jahnukainen et al. (2006) | Kidney | BK | 78 | SELDI-TOF-MS | No validation | Urine | A number of peptide peaks | [85] | |
| Quintana et al. (2009) | Kidney | CAN | 71 | LC-ESI-MS | MRM | Urine | A number of peptides | UMOD, kininogen | [86] |
| Ling et al. (2010) | Kidney | Acute rejection | 70 | LC-MS-MS | MRM | Urine | 40 peptides for AR | COL1A2, COL3A1, UMOD, MMP7, SERPING1 and TIMP1 | [19] |
| Metzger et al. (2011) | Kidney | Acute rejection | AR = 44 nAR = 59 |
CE-MS | CE-MS on blinded samples | Urine | A panel of peptides | COL α(I), α(III) chain fragments, MMP8 | [87] |
AFM: Afamin precursor; AR: Acute rejection; BK: BK virus nephropathy; CAN: Chronic allograft nephropathy; CE: Capillary electrophoresis; COL: Collagen; iTRAQ: Isobaric tags for relative and absolute quantitation; KNG1: Kininogen-1; LBP: Lipopolysaccharide-binding protein precursor; LC: Liquid chromatography; MMP: Matrix metalloproteinase; MRM: Multi-reaction monitoring; MS: Mass spectrometry; nAR: Non-acute rejection; noCAN: No chronic allograft nephropathy; SELDI: Surface-enhanced laser desorption ionization; SERPIN: Serine protease inhibitor; SRM: Selected reaction monitoring; TIMP1: Tissue inhibitor of metalloproteinase 1; TTN: Titin; UMOD: Uromodulin; VASN: Vasorin precursor.
Liver
The liver is the second most transplanted organ after the kidney. Liver transplantation is the only treatment option for patients with end-stage liver disease. Survival rates after liver transplantation have steadily improved over the past decade, with 1-year survival now exceeding 85%. This improvement has been attributed to new and better use of immunosuppressive drugs, improved diagnostic methods for identifying and preventing infections, and better surgical techniques. Despite these improvements, morbidity and mortality due to infectious complications in liver transplantation remain a major challenge. In many centers, the most common cause of death following liver transplantation remains infection. In addition, the molecular mechanisms of rejection are poorly understood [36]. Identification and use of reliable biomarkers to timely diagnose viral infection and acute rejection, which usually happens within weeks after transplantation remains a critical need. Chronic rejection, which occurs from the early months to years after post-transplantation, is associated with bile duct loss. These issues, along with recurrent infection and cirrhosis, are still important factors for the long-term survival of the transplanted liver. The liver proteome has been published, with a total 6788 proteins with at least two peptides matches [37]. By analyzing plasma samples collected from hepatitis C virus-hepatocellular carcinoma (HCV-HCC) and HCV cirrhotic patients, Mas et al. identified 2320 proteins [38]. Seven proteins, including properdin, apolipoprotein D and transferrin, showed significant changes between cirrhosis and tumor groups [38]. The identified proteins are suggested to be useful in screening HCC high-risk patients. Emadali et al. reported the results of a study that analyzed liver biopsies from early phases of ischemia and reperfusion [39]. Global expression and phosphorylation patterns of cytoskeleton-related proteins suggested that IQGAP1, a Cdc42/Rac1 effector, regulates actin cytoskeleton remodeling and maintenance of bile canaliculi (BC) integrity [39]. Using a differential proteomics approach, Avellini et al. identified early targets of oxidative injury due to ischemia-reperfusion (I/R) during liver transplantation [40]. The experiment revealed a specific modification of the peroxiredoxins active site thiol into sulfinic and/or sulfonic acid and immunophenotypic expression of APE1/Ref-1. Hyperoxidation of the peroxiredoxin family of hydroperoxide scavengers, PrxI, PrxII and Prx VI, was observed during I/R upon liver transplantation, which showed a dependence on the time of warm ischemia. This could be helpful in enabling a better understanding of graft preservation and evaluation for a successful outcome of liver transplant [40]. Using MALDITOF MS, Vascotto et al. identified 36 altered proteins during I/R injury that were significantly involved in lipid and energy metabolism [41].
Transplant tolerance is very interesting as it is the ultimate goal of all transplant communities. However, unraveling the mechanism of tolerance of transplanted organ is still a matter of active research. Proteomics/peptidomics may enable us to study these tolerance-specific changes in a number of proteins and identify an appropriate time to wean down the immunosuppressive drugs to avoid the possible immunosuppressive drug-related complications and injuries. Hsu et al. studied one orthotopic liver transplant patient who was out of immunosuppression for last 5 years, following post-transplant lymphoproliferative disease [42]. Their study demonstrated that 12 protein spots were specific to the patient with the tolerance.
Heart
The heart is the third most transplanted organ after the kidney and liver. In case of heart transplantation, acute rejection is the cause of 12% of deaths occurring between 1 month and 1 year after transplantation and it is critical to avoid acute rejection episodes [43,44]. Owing to the lack of an established biomarker, heart transplant patients have to go through serial endomyocardial biopsies with histologic evaluation of myocardial tissue. The biopsy is the gold standard to allow assessment of the status of the graft and provides some prognosis. However, its invasive nature means that it is associated with stress, pain and complications. The heart transplantation field has seen a number of high-throughput studies aimed towards biomarker discovery [45]. Meirovich et al. used plasma collected from 16 transplant patients and applied a cytokine profiling array to observe a significant correlation of regulated-on-activation, normal T-expressed and secreted, neutrophil-activating protein-2 (NAP-2) and IGF-binding protein-1 (IGFBP-1) with brain natriuretic peptide (BNP) plasma levels. The correlation was measured during grade 3A (grade 2 revised [2R]) or above rejection as diagnosed by endomyocardial biopsy score, according to the International Society for Heart and Lung Transplantation grading system [46]. The authors demonstrated a good correlation between BNP plasma levels observed during acute rejection episodes of heart transplant and cytokines other than proinflammatory cytokines. In a recent report, Kienzl et al. used a 2DE followed by MS approach to identify 17 proteins in mouse model of heart transplantation that were increased by at least 1.5-fold or more in acute rejection [47]. The list of proteins included peroxideroxin 6, pyruvate kinase isozyme M2 and coronin 1A, among others [47]. Cardiac allograft vasculopathy (CAV) is one of the major risk factors influencing heart dysfunction and eventual loss and patient survival [48]. Several studies have indicated that protection against CAV may result because of a number of protective genes. De Souza et al. applied 2DE followed by MS to identify heat-shock protein (HSP)127 as a marker. This protein was identified in four out of six heart biopsies from patients without CAV but was present in one out of six biopsies from patients with CAV [49]. The study validated its results using immunohistochemistry and concluded that the expression of the diphosphorylated form of HSP27 could be a marker of healthy blood vessels, which slowly gets depleted from vessels of patients with CAV. Corbett et al. evaluated changes in the protein level in heart tissue from patients with dilated cardiomyopathy (DCM) to ischemic heart disease (IHD) [50]. The study applied the 2DE method to 28 DCM heart biopsies, 21 explanted hearts from IHD patients and nine donor heart biopsies. A total of 88 proteins differed in their level in tissues of DCM and IHD patients, including myosin light chain 2 and a group of proteins identified as desmins.
Lung
Lung transplantation is the only therapy option for patients with end-stage lung disease. However, chronic rejection of the transplanted lung remains a major hurdle in long-term lung survival [51]. Current methods for lung transplant dysfunction diagnosis are based on lung biopsies or the presence of bronchiolitis obliterans syndrome (BOS). Bronchoalveolar lavage fluid (BALF) remains the biospecimen of choice for its noninvasive nature and its proximity to the transplanted lung. Nelsestuen et al. performed proteomic profiling using 126 BALF samples from 57 individuals on a MALDI-TOF MS platform [52]. The study reported increased levels of three human neutrophil peptides (HNP) 1–3 in individuals who experienced BOS. The study verified its discovery by ELISA and correlated an elevated level of HNP peptides with onset of BOS. In a separate study, Zhang et al. analyzed 431 BALF samples collected from 101 lung transplant patients and identified clara cell protein (CCP) to lysozyme ratio as a good predictor of BOS [53]. Another concerning issue in lung transplantation is idiopathic pulmonary fibrosis (IPF), which is a progressive fatal disease for which there is no effective therapy. Korfei et al. analyzed 24 lung tissues from 14 patients with sporadic IPF and ten control human donor lungs using 2DE and MALDI-TOF MS. The study reported 51 upregulated and 38 downregulated proteins in IPF. A detailed analysis revealed that IPF is characterized by epithelial cell injury, apoptosis and aberrant epithelial proliferation [54].
Pancreas
Pancreas transplantation is considered preferred method of treatment for diabetes mellitus and Type 1 diabetes (T1D) that can revert long-term insulin dependence and hyperglycemia [55,56]. The transplanted organ still faces a slow progression to impaired pancreatic function, which poses a risk for graft loss. As in case of other organ organs, the field of pancreas or kidney–pancreas transplant urgently needs biomarkers that help monitor the status of the transplant organ(s). There is almost no report in the literature database that involves proteomics and pancreas transplantation. In a recent report, Folli et al. utilized proteomics and ultrastructural approaches to examine pathways that may be normalized by restoration of normoglycemia with kidney–pancreas transplantation [57]. The study utilized 2DE followed by MS/MS to find alterations in the levels of a number of proteins involved in oxidative stress, such as catalase, superoxide dismutase 1, Hsp27, Hsp60, ATP synthase δ chain and flavin reductase, which were all altered in patients with T1D and T1D with end stage renal disease (ESRD). Also, the proteins involved in aerobic and anaerobic glycolysis (ACBP, pyruvate kinase muscle isozyme and phosphoglycerate kinase 1) and intracellular signaling (stratifin-14-3-3, S100-calcyclin, cathepsin and PPI rotamase), as well as endothelial vascular abnormalities, were altered in their level in patients with T1D and T1D and ESRD. These alterations due to T1D and T1D and ESRD were found to be normalized after kidney–pancreas transplantation [57]. It is therefore now time for researchers in the field of pancreas or kidney–pancreas transplantation to utilize proteomics to identify potential biomarkers for graft monitoring and to discover the underlying mechanisms of graft rejection and dysfunction.
Roadblocks in identifying clinically useful biomarkers
Current monitoring of all the transplants is achieved with the help of the biopsy, which is the gold standard not only to assess underlying pathological processes but also to determine the causes of graft dysfunction. Even with this diagnostic and some prognostic capability of the biopsy, a need for more sensitive and specific biomarkers has been realized. A lack of standardized scoring of biopsies and sampling error contributes to the subjectivity of pathology read. The advancements in molecular assay methods, including MS-based proteomics and peptidomics, have provided hope in finding more sensitive and specific biomarkers in the field of solid organ transplantation. Apart from the difficulty in comparing results from two different laboratories because of the instrumental settings for retention and identification of proteins/peptides, there are several hurdles in protein peptide biomarker discovery.
Confounder-related issues
High-abundance proteins
The presence of high-abundant proteins poses a major problem in studies that involve analysis of blood and urine samples. In the serum, ten high-abundant proteins, such as albumin and immunoglobulin, account for more than 95% of the total protein [58]. Therefore, removal of these major abundant proteins is mandatory to allow detection of the remaining lower abundant proteins. In urine proteomics, major abundant urinary proteins obscure the identification of low-abundant proteins. However, eliminating high-abundant proteins could itself cause another confounder in ‘quantitative’ data analysis and can cause the loss of other biologically relevant proteins. Known fragmentation patterns of abundant proteins and proteolytic activity in nephritic syndrome and oxidation properties of plasma albumin in focal segmental glomerulosclerosis suggest that these abundant proteins also could be informative in biomarker discovery [59–61]. Therefore, it is important that removal of major abundant proteins should be decided based on the study objectives and goals.
Sample-related confounders
For successful proteomic analysis of a sample, all the sample-related variables, such as identification and appropriate selection, processing, handling and storage should be defined. For example, in the case of urine, urine has a low and highly heterogeneous protein milieu, high levels of salts and a number of interfering compounds. The high degree of variation in its concentration, combined with hematuria, bacterial contamination and the presence of different proteases, requires careful consideration of the protein extraction method. In addition, different origins of urine proteins add to its complexity. These include the fact that the origin of protein in proteinuric patients could be severe, such as filtrate of plasma through intact (overflow proteinuria) or damaged filtration barriers (glomerular proteinuria), impaired reabsorption of the filtered protein caused by the tubular injury, secreted protein from the renal tubule, proteins originating from injured kidney tissue (nephrinuria in diabetic nephropathy and tubular enzymuria in acute kidney injury), and excretory vesicles (exosomes) or membrane-shed vesicles (microparticles, also referred to as ectosomes) from kidney and uroepithelial cells. A number of reports have been published with a number of different appropriate handling methods of urine samples [62–64]. In order to create a so-called ‘universal standard’, a group of researchers have worked to develop a well-characterized ‘real-life’ sample that can be used as reference standard in urine clinical proteomics studies [65]. The control urine standards are available to other researchers, and are believed to provide a standard for the comprehensive characterization of the urinary proteome. Still, several issues exist such as sample collection, removal method of high-abundant proteins and the use of protease inhibitor for proteinuric urine, among others. A similar approach is important while using blood, BALF, bile and other bodily fluids for proteomic studies.
Proteolytic activity in the bladder
The pH has a significant influence on the activity of proteolytic processes, not only in the blood and the kidney but also in the bladder. Therefore, proteomics and peptidomics analyses to evaluate proteins and native peptides could be impacted by the duration that the urine was in the bladder. For example, first morning void and second morning void urine may have differences in protein and peptide composition because of this potential confounder. This confounding issue needs to be evaluated with the use of appropriate controlled experiments.
Bioinformatics hurdles
With the explosion of data generated by high-throughput genomics and proteomics methods, and their availability through public repositories such as the Gene Expression Omnibus, a tremendous amount of molecular data is being cataloged. Several reports have been published utilizing such publicly available data. However, these works have been limited to the researchers who have well-trained bioinformaticists. In the absence of such a tool, the massive amount of data with the potential to be utilized to screen for clinically useful biomarkers is mostly unexploited. Tools and algorithms that enable researchers to query these repositories based on gene expression and protein information content would enable good use of the data towards discovery of novel biomarkers [66].
Recipes of success
Fractionation
One of the strategies for successful proteomics analysis is to fractionate the samples. Blood samples can be fractionated and analyzed to reach deeper into the blood proteome for discovery of clinically meaningful biomarkers. Urine contains a mixture of analytes and they can be split into different fractions, such as high-molecular-weight proteins, low-molecular-weight peptides, metabolites, exosomeal vesicles and cells present in the urine, among others. These compartments can be analyzed separately by different high-throughput platforms.
Collaboration among individual researchers
Limited availability of clinical samples for translational research often hinders biomarker discovery efforts. It is very difficult for a research project with a small sample size to produce results with enough statistical significance. Collaboration among different research groups working in the same field could provide a remedy for this. For such collaborative multicenter studies, it is very critical that sample collections, sample processing and sample storage methods are optimized and distributed to all the centers participating in the study.
Consortiums through government & private organizations
As a result of preliminary proteomic analyses, we now have a list of potential gene and protein biomarker molecules in the field of different solid organ transplantation. Owing to the fact that individual laboratories and clinical centers can only have access to a relatively small number of patients, the importance of a broader collaboration has been realized. Such a realization has been materialized in the field of transplantation under the National Institute of Allergy and Infectious Diseases and is called The Clinical Trials in Organ Transplantation [101]. This is a cooperative research program sponsored by the National Institute of Allergy and Infectious Diseases, with cofunding from the National Heart, Lung and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases. This consortium helps in conducting clinical and associated mechanistic studies that are believed to contribute to sophisticated methods in helping transplant patients and improving long-term organ and patient survival.
Collaboration through organizations such as, the NIH, American Society of Transplantation, European Society for Organ Transplantation, Reprogramming the Immune System for Establishment of Tolerance, Canadian Society of Transplantation and Roche Organ Transplant Research Foundation can help establish and support such collaborations. Samples collected through such large collaborations can be used towards validation of clinically useful biomarkers. In the absence of interventional trials, it would be difficult to test the utility of these biomarkers.
Data repository
The importance of publicly available data has been realized [67]. With the help of public repositories and encouraging researchers to deposit the raw data in a standardized format is a thoughtful way to utilize the results of hard work and invest it for future purposes. In transcriptomics, this practice already exists in the form of the Minimum Information about Microarray Experiment (MIAME) guidelines. The MIAME guidelines outline the minimum information that should be included in describing a microarray experiment, and several repositories such as the Gene Expression Omnibus and the Stanford Microarray Database require researchers to deposit data in compliance with MIAME. These public repositories, with their collected MIAME compliant microarray data, have encouraged several integrated approaches to mining the deposited data, with early signs of success. Such an approach has already been successfully applied with microarray data to gain new insights into human diseases [68,69]. Integrating the high volume of proteomic and peptidomic data with genomic and transcriptomic data would further the effectiveness of the biomarker discovery process.
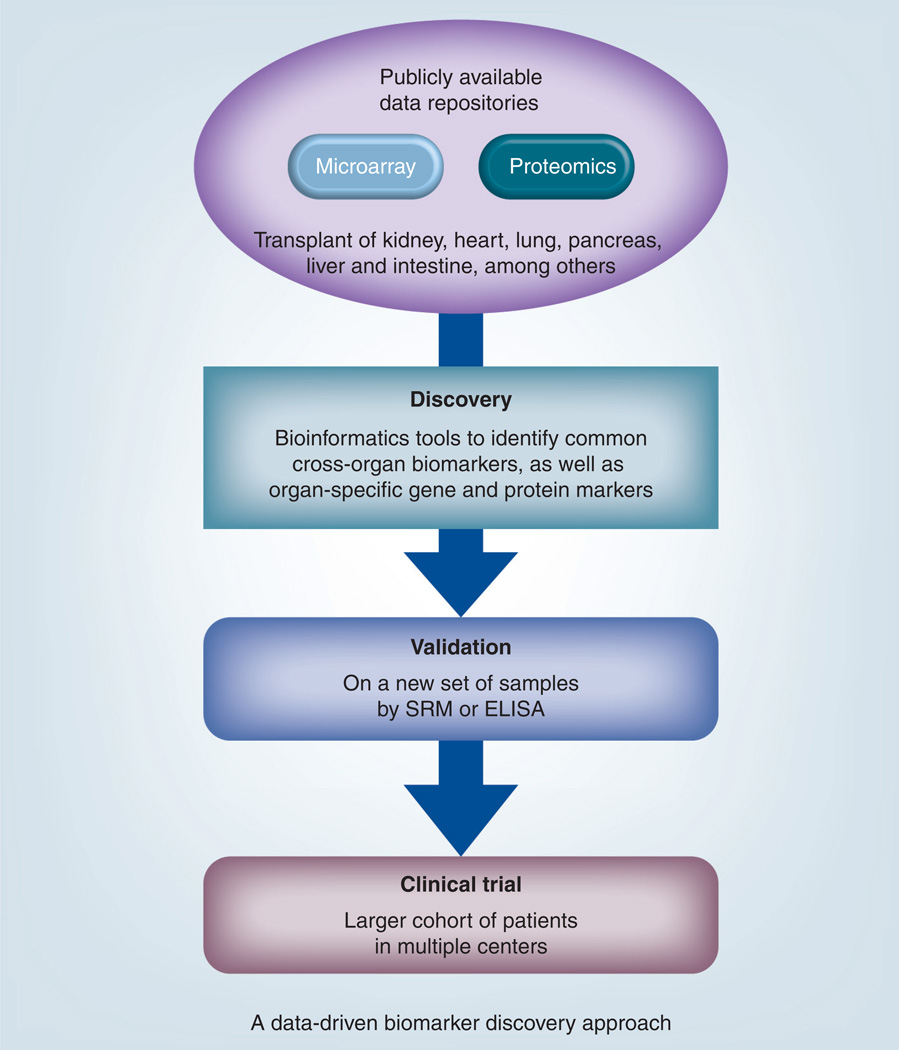
A recently published work by Li et al. used publicly available microarray data and protein array data to identify non-HLA antigen targets after renal transplantation [70]. In a recent report published by Chen et al., the authors used novel bioinformatics strategy to integrate microarray data to screen for potential biomarker proteins, which could be validated in the serum by ELISA. The beauty of these novel strategies is that they could even identify markers that are a part of the common rejection mechanism across different solid organ transplant fields [35]. The benefit of such an integrated approach is that is not only helps to analyze data collected from a number of laboratories but also in identifying the biomarkers that could be common link to different solid organ transplantation. The schematic of utility of such an approach is presented in Figure 2.
Figure 2. A new data-driven approach of biomarker discovery that can utilize publicly available data and use mass spectrometry-based selected reaction monitoring or ELISA for validation of potential biomarkers.
SRM: Selected reaction monitoring.
Personalized medicine in organ transplantation
Improvements and advancements in immunosuppressive regimens have contributed to short-term organ survival. However, the effects of immunosuppressive agents are not specific and eventually they contribute to organ failure. A concentration-based dose strategy undermines patient-to-patient variation in drug susceptibility, which has been ignored thus far [71]. Cellular and humoral immunities are known to be common pathways of immune response towards the transplanted organ [72]. It has also been appreciated that organ injury is a complicated process and depends on donor and recipient pairing. Customizing treatment strategies has remained a major challenge for the scientific community, which has contributed in continuation of the current ‘one size fits all’ treatment approach [73,74]. In this direction, associations of several polymorphisms in CYP3A, ABCB1, TPMT and ABCC2 genes that affect the requirements of common immunosuppressive agents in solid organ transplantation have been studied [75–78]. Still, it remains unclear how a drug-related polymorphism can impact whole-drug metabolism and the pathogenesis of organ dysfunction. Next-generation DNA sequencing has provided a powerful tool to perform genomic, epigenomic and transcriptomic studies and has allowed the determination of large numbers of short sequences (30–40 million reads 36–100 bp in length for an Illumina® GAIIx) from one or both ends of DNA fragments in a high-throughput manner [79]. RNA-Seq enables precise monitoring of distinct splicing isoforms to acquire singlebase resolution in a high-throughput manner that can be used to screen for individual gene variants between the donor–recipient pair [80]. Based on the information obtained from these sequencing methods, donor-specific antigenic targets can be identified, which can be used to monitor the alloresponse of the recipient by assaying antibodies targeted to donor-specific antigens. This might open up a new avenue in the field of personalized medicine, which is a major demand in current medicine.
Focus on markers for positive outcomes
The studies that have been presented in this article are more focused on the markers that are associated with negative outcomes, such as acute and chronic rejection. Instead of focusing on the markers that are specific to different transplant injury, researchers may focus on identifying protein or peptide markers whose presence or absence is a marker of good health. This will help physicians to identify the patient as at risk or nonrisk. All the non-risk patients can be sent home without further biopsy evaluation. For the risk patients, more tests such as biopsy can be scheduled to further specify the type of risk and the severity of the injury.
Conclusion
Reliable and effective methods to diagnose rejection and other forms of transplant injury to the organs are currently unavailable. Identification and validation of clinically useful protein and peptide biomarkers requires a concerted effort from surgeons and physicians in the field of transplantation and researchers in basic science and bioinformatics. The lack of sufficient patients in one laboratory or center requires researchers from different states and countries to collaborate in this effort to validate clinically useful biomarkers that will eventually be transferred to the clinical laboratories as a clinical testing tool to provide help to the patients being treated in the hospital beds.
Expert commentary
The proteome is metastable, cell specific and has a wide range of concentration. The proteome is dependent on several physiological and environmental factors. As a result, despite numerous studies of human tissues, no comprehensive proteomic map has been published, except for biofluids such as human urine, serum and plasma. Still, proteome analysis is a very important in discovering clinically relevant biomarkers for existing health problems, including dysfunction of transplanted organs. Several strategies, including fractionation of protein samples, study of different compartments of the proteome, (e.g., the glycoproteome, phosphoproteome, membrane proteome and exosomes) is a preferred way to identify low-abundant protein markers. Collaboration among different researchers for a larger cohort of patients will help to obtain large enough sample sizes to validate clinically useful biomarkers [81]. Once these noninvasive biomarker/s are validated, these could be used to stratify biopsy-requiring patients and those that are safe without a biopsy evaluation. Once the use of these noninvasive biomarkers has been established and their utility is tested, biopsy can be replaced, and these biomarkers can be used to wean immunosuppression based on the health status of the transplanted organ. With this effort, we can ensure substantial help is provided to the transplant patients by turning the table to a new age of patient care by replacing nonspecific, invasive diagnostic tests with noninvasive, effective and specific tests for solid organ transplant monitoring.
Five-year view
With rapid progress in high-throughput assay technology, along with advanced bioinformatics tools to integrate ‘omics’ data, it is expected that a set of clinically useful biomarkers will be identified. Since, the first ‘dirty’ step of discovering potential markers is almost completed, the next couple of years will see testing of these markers in clinical setting. The first step towards such testing would perhaps be to use these molecules in stratifying ‘risk’ groups of patients from ‘no-risk’ group of patients who do not need to go through invasive procedures such as biopsy. A simple blood or urine test is expected to be a reality within the next 5-year period.
Key issues.
The current ‘not-so-gold’ gold standard for diagnostic tools for transplant dysfunction is not very specific or sensitive and there is a dire need for better and noninvasive diagnostic methods.
Recent developments in profiling of biomolecules using ‘omic’ methods have provided new platforms for biomarker discovery.
Recent biomarker discovery efforts in organ transplantation have shown some promise.
There are a number of roadblocks in identifying the best biomarkers.
There are a number of important factors that are critical for successful biomarker discovery.
Personalized medicine in transplantation is the next step in transplantation that has the potential to revolutionize how the transplanted organs are managed and monitored.
Acknowledgements
The authors would like to thank Matthew Vitalone and Marianne Delville for their help during manuscript preparation.
The authors acknowledge funding support from the NIH (grant numbers 5R21AI075256-02 and 5R01DK083447-02) to MM Sarwal, the Transplant and Tissue Engineering Center of Excellence Award to MM Sarwal and TK Sigdel, and CHRP fellowship to TK Sigdel.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N. Engl. J. Med. 2010;363(15):1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 2.de Fijter JW. Rejection and function and chronic allograft dysfunction. Kidney Int. 2010;78 Suppl. 119:S38–S41. doi: 10.1038/ki.2010.421. [DOI] [PubMed] [Google Scholar]
- 3.Turka LA, Lechler RI. Towards the identification of biomarkers of transplantation tolerance. Nat. Rev. Immunol. 2009;9(7):521–526. doi: 10.1038/nri2568. [DOI] [PubMed] [Google Scholar]
- 4.Green ED, Guyer MS. Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470(7333):204–213. doi: 10.1038/nature09764. [DOI] [PubMed] [Google Scholar]
- 5.Ozsolak F, Platt AR, Jones DR, et al. Direct RNA sequencing. Nature. 2009;461(7265):814–818. doi: 10.1038/nature08390. [DOI] [PubMed] [Google Scholar]
- 6.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422(6928):198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 7.Cox J, Mann M. Is proteomics the new genomics? Cell. 2007;130(3):395–398. doi: 10.1016/j.cell.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Norden AG, Rodriguez-Cutillas P, Unwin RJ. Clinical urinary peptidomics: learning to walk before we can run. Clin. Chem. 2007;53(3):375–376. doi: 10.1373/clinchem.2006.084038. [DOI] [PubMed] [Google Scholar]
- 9.Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat. Biotechnol. 2006;24(8):971–983. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- 10.Brouard S, Giral M, Soulillou JP, Ashton-Chess J. Elaboration of gene expression-based clinical decision AIDS for kidney transplantation: where do we stand? Transplantation. 2011;91(7):691–696. doi: 10.1097/TP.0b013e31820c4559. [DOI] [PubMed] [Google Scholar]
- 11.Hricik DE, Rodriguez V, Riley J, et al. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am. J. Transplant. 2003;3(7):878–884. doi: 10.1034/j.1600-6143.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 12.Kowalski RJ, Post DR, Mannon RB, et al. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation. 2006;82(5):663–668. doi: 10.1097/01.tp.0000234837.02126.70. [DOI] [PubMed] [Google Scholar]
- 13. Sarwal M, Chua MS, Kambham N, et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N. Engl. J. Med. 2003;349(2):125–138. doi: 10.1056/NEJMoa035588. •• This work was first of its kind to use microarray method to identify genes that are actively involved in different acute rejection (AR) grades and other injuries.
- 14.Li L, Ying L, Naesens M, et al. Interference of globin genes with biomarker discovery for allograft rejection in peripheral blood samples. Physiol. Genomics. 2008;32(2):190–197. doi: 10.1152/physiolgenomics.00216.2007. [DOI] [PubMed] [Google Scholar]
- 15.Morgun A, Shulzhenko N, Perez-Diez A, et al. Molecular profiling improves diagnoses of rejection and infection in transplanted organs. Circ. Res. 2006;98(12):e74–e83. doi: 10.1161/01.res.0000228714.15691.8a. [DOI] [PubMed] [Google Scholar]
- 16.Schaub S, Rush D, Wilkins J, et al. Proteomic-based detection of urine proteins associated with acute renal allograft rejection. J. Am. Soc. Nephrol. 2004;15(1):219–227. doi: 10.1097/01.asn.0000101031.52826.be. [DOI] [PubMed] [Google Scholar]
- 17. Schaub S, Wilkins JA, Antonovici M, et al. Proteomic-based identification of cleaved urinary beta2-microglobulin as a potential marker for acute tubular injury in renal allografts. Am. J. Transplant. 2005;5(4 Pt 1):729–738. doi: 10.1111/j.1600-6143.2005.00766.x. • This work was the first of its kind to demonstrate usefulness of a proteomics method in identifying a peptide signature specific to AR.
- 18.O’Riordan E, Orlova TN, Mei JJ, et al. Bioinformatic analysis of the urine proteome of acute allograft rejection. J. Am. Soc. Nephrol. 2004;15(12):3240–3248. doi: 10.1097/01.ASN.0000145241.83482.68. [DOI] [PubMed] [Google Scholar]
- 19. Ling XB, Sigdel TK, Lau K, et al. Integrative urinary peptidomics in renal transplantation identifies biomarkers for acute rejection. J. Am. Soc. Nephrol. 2010;21(4):646–653. doi: 10.1681/ASN.2009080876. •• In this carefully designed work, the authors implemented an integrative approach to analyze native urinary peptides and also gene-expression data to identify a panel of peptides that are AR specific and a set of genes from kidney biopsy that are AR specific.
- 20. Sigdel TK, Kaushal A, Gritsenko M, et al. Shotgun proteomics identifies proteins specific for acute renal transplant rejection. Proteomics Clin. Appl. 2010;4(1):32–47. doi: 10.1002/prca.200900124. • The authors implemented a shotgun proteomics approach to analyze urine proteins specific to AR and validated by ELISA in an independent set of samples.
- 21.Sigdel TK, Ling XB, Lau K, et al. Urinary peptidomic analysis identifies potential biomarkers for acute rejection of renal. Clin. Proteom. 2009;5(2):103–113. [Google Scholar]
- 22.Sigdel TK, Klassen RB, Sarwal MM. Interpreting the proteome and peptidome in transplantation. Adv. Clin. Chem. 2009;47:139–169. doi: 10.1016/s0065-2423(09)47006-9. [DOI] [PubMed] [Google Scholar]
- 23.Sigdel TK, Sarwal MM. The proteogenomic path towards biomarker discovery. Pediatr. Transplant. 2008;12(7):737–747. doi: 10.1111/j.1399-3046.2008.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidal BC, Bonventre JV, I-Hong Hsu S. Towards the application of proteomics in renal disease diagnosis. Clin. Sci. (Lond.) 2005;109(5):421–430. doi: 10.1042/CS20050085. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Buitrago JM, Ferreira L, Lorenzo I. Urinary proteomics. Clin. Chim. Acta. 2007;375(1–2):49–56. doi: 10.1016/j.cca.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Vasconcellos LM, Schachter AD, Zheng XX, et al. Cytotoxic lymphocyte gene expression in peripheral blood leukocytes correlates with rejecting renal allografts. Transplantation. 1998;66(5):562–566. doi: 10.1097/00007890-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 27.Simon T, Opelz G, Wiesel M, Ott RC, Susal C. Serial peripheral blood perforin and granzyme B gene expression measurements for prediction of acute rejection in kidney graft recipients. Am. J. Transplant. 2003;3(9):1121–1127. doi: 10.1034/j.1600-6143.2003.00187.x. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Hartono C, Ding R, et al. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N. Engl. J. Med. 2001;344(13):947–954. doi: 10.1056/NEJM200103293441301. [DOI] [PubMed] [Google Scholar]
- 29.Yannaraki M, Rebibou JM, Ducloux D, et al. Urinary cytotoxic molecular markers for a noninvasive diagnosis in acute renal transplant rejection. Transpl. Int. 2006;19(9):759–768. doi: 10.1111/j.1432-2277.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- 30.Schaub S, Nickerson P, Rush D, et al. Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am. J. Transplant. 2009;9(6):1347–1353. doi: 10.1111/j.1600-6143.2009.02645.x. [DOI] [PubMed] [Google Scholar]
- 31.Camara NO, Silva MS, Nishida S, Pereira AB, Pacheco-Silva A. Proximal tubular dysfunction is associated with chronic allograft nephropathy and decreased long-term renal-graft survival. Transplantation. 2004;78(2):269–275. doi: 10.1097/01.tp.0000128333.46949.a4. [DOI] [PubMed] [Google Scholar]
- 32.Teppo AM, Honkanen E, Finne P, Tornroth T, Gronhagen-Riska C. Increased urinary excretion of alpha1-microglobulin at 6 months after transplantation is associated with urinary excretion of transforming growth factor-beta1 and indicates poor long-term renal outcome. Transplantation. 2004;78(5):719–724. doi: 10.1097/01.tp.0000131816.51366.6b. [DOI] [PubMed] [Google Scholar]
- 33.Hollmen ME, Kyllonen LE, Inkinen KA, Lalla ML, Salmela KT. Urine neutrophil gelatinase-associated lipocalin is a marker of graft recovery after kidney transplantation. Kidney Int. 2011;79(1):89–98. doi: 10.1038/ki.2010.351. [DOI] [PubMed] [Google Scholar]
- 34. Nakorchevsky A, Hewel JA, Kurian SM, et al. Molecular mechanisms of chronic kidney transplant rejection via large-scale proteogenomic analysis of tissue biopsies. J. Am. Soc. Nephrol. 2010;21(2):362–373. doi: 10.1681/ASN.2009060628. •• In this thorough study report, the authors implemented an integrative proteogenomics method to look into the molecular mechanisms of chronic rejection of kidney transplantation.
- 35.Chen R, Sigdel TK, Li L, et al. Differentially expressed RNA from public microarray data identifies serum protein biomarkers for cross-organ transplant rejection and other conditions. PLoS Comput. Biol. 2010;6(9) doi: 10.1371/journal.pcbi.1000940. e1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasui H, Yoshimura N, Kobayashi Y, et al. Microstructural changes of bile canaliculi in canine liver: the effect of cold ischemia-reperfusion in orthotopic liver transplantation. Transplant. Proc. 1998;30(7):3754–3757. doi: 10.1016/s0041-1345(98)01222-6. [DOI] [PubMed] [Google Scholar]
- 37.Chinese Human Liver Proteome Profiling Consortium. First insight into the human liver proteome from PROTEOME(SKY)-LIVERHu) 1.0, a publicly available database. J. Proteome Res. 2010;9(1):79–94. doi: 10.1021/pr900532r. [DOI] [PubMed] [Google Scholar]
- 38.Mas VR, Maluf DG, Archer KJ, Yanek K, Bornstein K, Fisher RA. Proteomic analysis of HCV cirrhosis and HCV-induced HCC: identifying biomarkers for monitoring HCV-cirrhotic patients awaiting liver transplantation. Transplantation. 2009;87(1):143–152. doi: 10.1097/TP.0b013e318191c68d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emadali A, Muscatelli-Groux B, Delom F, et al. Proteomic analysis of ischemia-reperfusion injury upon human liver transplantation reveals the protective role of IQGAP1. Mol. Cell. Proteomics. 2006;5(7):1300–1313. doi: 10.1074/mcp.M500393-MCP200. [DOI] [PubMed] [Google Scholar]
- 40.Avellini C, Baccarani U, Trevisan G, et al. Redox proteomics and immunohistology to study molecular events during ischemia-reperfusion in human liver. Transplant. Proc. 2007;39(6):1755–1760. doi: 10.1016/j.transproceed.2007.05.082. [DOI] [PubMed] [Google Scholar]
- 41.Vascotto C, Cesaratto L, D’Ambrosio C, et al. Proteomic analysis of liver tissues subjected to early ischemia/reperfusion injury during human orthotopic liver transplantation. Proteomics. 2006;6(11):3455–3465. doi: 10.1002/pmic.200500770. [DOI] [PubMed] [Google Scholar]
- 42.Hsu LW, Goto S, Nakano T, et al. Immunosuppressive activity of serum taken from a liver transplant recipient after withdrawal of immunosuppressants. Transpl. Immunol. 2007;17(2):137–146. doi: 10.1016/j.trim.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Taylor DO, Stehlik J, Edwards LB, et al. Registry of the international society for heart and lung transplantation: twenty-sixth official adult heart transplant report-2009. J. Heart Lung Transplant. 2009;28(10):1007–1022. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Mehra MR, Uber PA, Uber WE, Park MH, Scott RL. Anything but a biopsy: noninvasive monitoring for cardiac allograft rejection. Curr. Opin. Cardiol. 2002;17(2):131–136. doi: 10.1097/00001573-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Dos Remedios CG, Liew CC, Allen PD, Winslow RL, Van Eyk JE, Dunn MJ. Genomics, proteomics and bioinformatics of human heart failure. J. Muscle Res. Cell. Motil. 2003;24(4–6):251–260. doi: 10.1023/a:1025433721505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meirovich YF, Veinot JP, de Bold ML, et al. Relationship between natriuretic peptides and inflammation: proteomic evidence obtained during acute cellular cardiac allograft rejection in humans. J. Heart Lung Transplant. 2008;27(1):31–37. doi: 10.1016/j.healun.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 47.Kienzl K, Sarg B, Golderer G, et al. Proteomic profiling of acute cardiac allograft rejection. Transplantation. 2009;88(4):553–560. doi: 10.1097/TP.0b013e3181b119b1. [DOI] [PubMed] [Google Scholar]
- 48.Valantine H. Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J. Heart Lung Transplant. 2004;23(5 Suppl.):S187–S193. doi: 10.1016/j.healun.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 49.De Souza AI, Wait R, Mitchell AG, Banner NR, Dunn MJ, Rose ML. Heat shock protein 27 is associated with freedom from graft vasculopathy after human cardiac transplantation. Circ. Res. 2005;97(2):192–198. doi: 10.1161/01.RES.0000174815.10996.08. [DOI] [PubMed] [Google Scholar]
- 50.Corbett JM, Why HJ, Wheeler CH, et al. Cardiac protein abnormalities in dilated cardiomyopathy detected by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1998;19(11):2031–2042. doi: 10.1002/elps.1150191123. [DOI] [PubMed] [Google Scholar]
- 51.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am. J. Respir. Crit. Care Med. 2002;166(4):440–444. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 52.Nelsestuen GL, Martinez MB, Hertz MI, Savik K, Wendt CH. Proteomic identification of human neutrophil alpha-defensins in chronic lung allograft rejection. Proteomics. 2005;5(6):1705–1713. doi: 10.1002/pmic.200401036. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Wroblewski M, Hertz MI, Wendt CH, Cervenka TM, Nelsestuen GL. Analysis of chronic lung transplant rejection by MALDI-TOF profiles of bronchoalveolar lavage fluid. Proteomics. 2006;6(3):1001–1010. doi: 10.1002/pmic.200500105. [DOI] [PubMed] [Google Scholar]
- 54.Korfei M, Schmitt S, Ruppert C, et al. Comparative proteomic analysis of lung tissue from patients with idiopathic pulmonary fibrosis (IPF) and lung transplant donor lungs. J. Proteome Res. 2011;10(5):2185–2205. doi: 10.1021/pr1009355. [DOI] [PubMed] [Google Scholar]
- 55.Egidi FM. Management of hyperglycaemia after pancreas transplantation: are new immunosuppressants the answer? Drugs. 2005;65(2):153–166. doi: 10.2165/00003495-200565020-00001. [DOI] [PubMed] [Google Scholar]
- 56.Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann. Surg. 2001;233(4):463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Folli F, Guzzi V, Perego L, et al. Proteomics reveals novel oxidative and glycolytic mechanisms in type 1 diabetic patients’ skin which are normalized by kidney-pancreas transplantation. PLoS ONE. 2010;5(3):e9923. doi: 10.1371/journal.pone.0009923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tirumalai RS, Chan KC, Prieto DA, Issaq HJ, Conrads TP, Veenstra TD. Characterization of the low molecular weight human serum proteome. Mol. Cell Proteomics. 2003;2(10):1096–1103. doi: 10.1074/mcp.M300031-MCP200. [DOI] [PubMed] [Google Scholar]
- 59.Candiano G, Musante L, Bruschi M, et al. Repetitive fragmentation products of albumin and alpha1-antitrypsin in glomerular diseases associated with nephrotic syndrome. J. Am. Soc. Nephrol. 2006;17(11):3139–3148. doi: 10.1681/ASN.2006050486. [DOI] [PubMed] [Google Scholar]
- 60.Magistroni R, Ligabue G, Lupo V, et al. Proteomic analysis of urine from proteinuric patients shows a proteolitic activity directed against albumin. Nephrol. Dial. Transplant. 2009;24(5):1672–1681. doi: 10.1093/ndt/gfp020. [DOI] [PubMed] [Google Scholar]
- 61.Musante L, Candiano G, Petretto A, et al. Active focal segmental glomerulosclerosis is associated with massive oxidation of plasma albumin. J. Am. Soc. Nephrol. 2007;18(3):799–810. doi: 10.1681/ASN.2006090965. [DOI] [PubMed] [Google Scholar]
- 62.Thongboonkerd V, Chutipongtanate S, Kanlaya R. Systematic evaluation of sample preparation methods for gel-based human urinary proteomics: quantity, quality, and variability. J. Proteome Res. 2006;5(1):183–191. doi: 10.1021/pr0502525. [DOI] [PubMed] [Google Scholar]
- 63.Sigdel TK, Lau K, Schilling J, Sarwal MM. Optimizing protein recovery for urinary proteomics, a tool to monitor renal transplantation. Clin. Transplant. 2008;22(5):617–623. doi: 10.1111/j.1399-0012.2008.00833.x. [DOI] [PubMed] [Google Scholar]
- 64.Khan A, Packer NH. Simple urinary sample preparation for proteomic analysis. J. Proteome Res. 2006;5(10):2824–2838. doi: 10.1021/pr060305y. [DOI] [PubMed] [Google Scholar]
- 65.Mischak H, Kolch W, Aivaliotis M, et al. Comprehensive human urine standards for comparability and standardization in clinical proteome analysis. Proteomics Clin. Appl. 2010;4(4):464–478. doi: 10.1002/prca.200900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engreitz JM, Morgan AA, Dudley JT, et al. Content-based microarray search using differential expression profiles. BMC Bioinformatics. 2010;11:603. doi: 10.1186/1471-2105-11-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mons B, van Haagen H, Chichester C, et al. The value of data. Nat. Genet. 2011;43(4):281–283. doi: 10.1038/ng0411-281. [DOI] [PubMed] [Google Scholar]
- 68.Dudley J, Butte AJ. Enabling integrative genomic analysis of high-impact human diseases through text mining. Pac. Symp. Biocomput. 2008;2008:580–591. [PMC free article] [PubMed] [Google Scholar]
- 69.Basic-Jukic N, Juric I, Kes P, Bubic-Filipi L, Brunetta B, Pasini J. Arterial hypertension in renal transplant recipients. Acta Med. Croatica. 2007;61(2):171–176. [PubMed] [Google Scholar]
- 70.Li L, Wadia P, Chen R, et al. Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and ‘antibodyome’ measures. Proc. Natl Acad. Sci. USA. 2009;106(11):4148–4153. doi: 10.1073/pnas.0900563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Millan O, Benitez C, Guillen D, et al. Biomarkers of immunoregulatory status in stable liver transplant recipients undergoing weaning of immunosuppressive therapy. Clin. Immunol. 2010;137(3):337–346. doi: 10.1016/j.clim.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Kirk AD. Location, location, location: regional immune mechanisms critically influence rejection. Nat. Med. 2010;8(6):553–555. doi: 10.1038/nm0602-553. [DOI] [PubMed] [Google Scholar]
- 73.Ginsburg GS, McCarthy JJ. Personalized medicine: revolutionizing drug discovery and patient care. Trends Biotechnol. 2001;19(12):491–496. doi: 10.1016/s0167-7799(01)01814-5. [DOI] [PubMed] [Google Scholar]
- 74.Goldfarb-Rumyantzev AS. Personalized medicine and prediction of outcome in kidney transplant. Am. J. Kidney Dis. 2010;56(5):817–819. doi: 10.1053/j.ajkd.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Lampreabe I, Gainza de los Rios FJ, Arrieta Gutierrez A, et al. Toward personalized medicine in renal transplantation. Transplant. Proc. 2010;42(8):2864–2867. doi: 10.1016/j.transproceed.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Coto E, Tavira B. Pharmacogenetics of calcineurin inhibitors in renal transplantation. Transplantation. 2009;88(3 Suppl.):S62–S67. doi: 10.1097/TP.0b013e3181afe9e7. [DOI] [PubMed] [Google Scholar]
- 77.Wavamunno MD, Chapman JR. Individualization of immunosuppression: concepts and rationale. Curr. Opin. Organ Transplant. 2008;13(6):604–608. doi: 10.1097/MOT.0b013e3283193bc5. [DOI] [PubMed] [Google Scholar]
- 78.Hesselink DA, van Gelder T, van Schaik RH. The pharmacogenetics of calcineurin inhibitors: one step closer toward individualized immunosuppression? Pharmacogenomics. 2005;6(4):323–337. doi: 10.1517/14622416.6.4.323. [DOI] [PubMed] [Google Scholar]
- 79.Holt KE, Parkhill J, Mazzoni CJ, et al. High-throughput sequencing provides insights into genome variation and evolution in Salmonella typhi. Nat. Genet. 2008;40(8):987–993. doi: 10.1038/ng.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butte AJ. Medicine. The ultimate model organism. Science. 2008;320(5874):325–327. doi: 10.1126/science.1158343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freue GV, Sasaki M, Meredith A, et al. Proteomic signatures in plasma during early acute renal allograft rejection. Mol. Cell Proteomics. 2010;9(9):1954–1967. doi: 10.1074/mcp.M110.000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurian SM, Heilman R, Mondala TS, et al. Biomarkers for early and late stage chronic allograft nephropathy by proteogenomic profiling of peripheral blood. PLoS ONE. 2009;4(7):e6212. doi: 10.1371/journal.pone.0006212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dai Y, Lv T, Wang K, Huang Y, Li D, Liu J. Detection of acute renal allograft rejection by analysis of renal tissue proteomics in rat models of renal transplantation. Saudi J. Kidney Dis. Transpl. 2008;19(6):952–959. [PubMed] [Google Scholar]
- 85.Jahnukainen T, Malehorn D, Sun M, et al. Proteomic analysis of urine in kidney transplant patients with BK virus nephropathy. J. Am. Soc. Nephrol. 2006;17(11):3248–3256. doi: 10.1681/ASN.2006050437. [DOI] [PubMed] [Google Scholar]
- 86.Quintana LF, Sole-Gonzalez A, Kalko SG, et al. Urine proteomics to detect biomarkers for chronic allograft dysfunction. J. Am. Soc. Nephrol. 2009;20(2):428–435. doi: 10.1681/ASN.2007101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Metzger J, Chatzikyrkou C, Broecker V, et al. Diagnosis of subclinical and clinical acute T-cell-mediated rejection in renal transplant patients by urinary proteome analysis. Proteomics Clin. Appl. 2011;5(5–6):322–333. doi: 10.1002/prca.201000153. [DOI] [PubMed] [Google Scholar]
Website
- 101.Clinical Trials in Organ Transplantation. www.ctotstudies.org/index.htm.