Abstract
Gene therapy in the craniofacial region provides a unique tool for delivery of DNA to coordinate protein production in both time and space. The drive to bring this technology to the clinic is derived from the fact that over 85% of the global population may at one time require repair or replacement of a craniofacial structure. This need ranges from mild tooth decay and tooth loss to temporomandibular joint disorders and large-scale reconstructive surgery. Our ability to insert foreign DNA into a host cell has been developing since early uses of gene therapy to alter bacterial properties for waste cleanup in the 1980s followed by successful human clinical trials in the 1990s to treat severe combined immunodeficiency. In the past twenty years the emerging field of craniofacial tissue engineering has adopted these techniques to enhance regeneration of mineralized tissues, salivary gland, periodontium, and to reduce tumor burden of head and neck squamous cell carcinoma. Studies are currently pursuing research on both biomaterial-mediated gene delivery as well as more clinically efficacious, though potentially more hazardous, viral methods. Though hundreds of gene therapy clinical trials have taken place in the past twenty years, we must still work to ensure an ideal safety profile for each gene and delivery method combination. With adequate genotoxicity testing, we can expect gene therapy to augment protein delivery strategies and potentially allow for tissue-specific targeting, delivery of multiple signals, and increased spatial and temporal control with the goal of natural tissue replacement in the craniofacial complex.
Keywords: tissue engineering, gene therapy, bone, salivary gland, craniofacial regeneration
INTRODUCTION
The basic concept of gene therapy is derived from our ability to insert new genetic material into a cell to manipulate the proteins that are produced by endogenous cell machinery. This concept was first successfully applied in humans in 1990 when W. French Anderson and his team at the National Institutes of Health used gene therapy to treat a four-year old girl born with severe combined immunodeficiency (SCID) 1. Ten years later in 2000, two infant boys with x-linked SCID were also successfully treated when the γc cytokine receptor was reintroduced to their blood stem cells using a retrovirus 2. The direct treatment of stem cells increased the likelihood that the gene would remain permanently expressed and result in a true cure for the disease. In a very different scenario, genetic engineering was used in 1988 to generate phenol-degrading strains of pseudomonas bacterium to aid in environmental bioremediation after a fire in an Estonian oil shale mine 3,4. We now use this technology to engineer protein producing bacteria for treatment of diseases such as leukemia and hepatitis-C, alter plants to resist disease and destructive insects, and perhaps in the future to create new forms of energy producing organisms. In basic research, gene transfer is a technique that is used daily around the world to enhance our understanding of cellular signaling pathways and their effects on development and disease. Most recently it has been used to reprogram the fate of cells to generate induced-pluripotent stem (iPS) cells. IPS cells return to a state of pluripotency and can differentiate to multiple different cell types. Clinical application of these findings is limited by safety and efficacy of gene transfer into human patients. Despite these limitations there were over 300 gene therapy clinical trials to treat over 3,000 patients from 1990–2000 5 and many more in the past decade.
The field of craniofacial regeneration is driven by the reality that more than 85% of the global population requires repair or replacement of a craniofacial structure 6. However, even something as seemingly innocuous as mild tooth decay presents a significant challenge when replacement with natural tissue is desired. This complex regenerative goal requires synthesis of clinical science, basic science and engineering. Identification of the appropriate spatial and temporal signals, scaffolds, and cell sources will allow us to biologically address tissue loss in the craniofacial region and ideally improve long-term patient outcomes. Gene therapy provides a unique tool for delivery of previously identified signaling molecules in both time and space that may significantly augment our progress toward clinical craniofacial regeneration.
GENE THERAPY METHODS
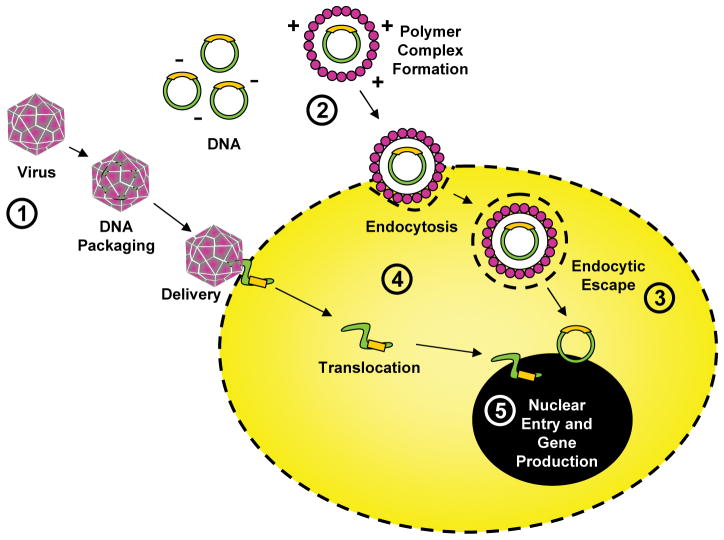
There is often a misconception that gene therapy requires viral transduction of genetic material. This view neglects to consider gene transfer using DNA either in solution or conjugated to a biomaterial. In both applications genes are most commonly delivered within a plasmid that contains the genetic information necessary for the cell to begin making the protein product of that gene once the plasmid is taken into the nucleus (Fig.1). Delivery of the plasmid to the nucleus and its subsequent retention are two challenges that limit the effectiveness of gene therapy (Fig.1). Though viruses can enhance this process, delivery of plasmid DNA alone from a biomaterial is desired due to low immunogenicity and toxicity, relative safety, ease of manufacture, and lack of DNA insert size limitation 7.
Figure 1.
Diagram depicting regulatory points for optimization of gene delivery to the cell including: (1) virus type selection, (2) cationic polymer optimization, (3) regulation of endocytic escape, (4) control of cytoplasmic trafficking, and (5) regulation of nuclear DNA uptake and transcription.
To use a biomaterial to introduce plasmid DNA to the cell, the polymer must first be able to neutralize the negative charge of the DNA through electrostatic interactions (Fig.1) 7. The resulting positively charged complex can then interact with the cell surface and be internalized by endocytosis. Once inside the cell the DNA must escape its endosome, move into the cytosol, and finally enter the nucleus (Fig.1). If it manages this without damage, the cell can then begin producing the protein product of the introduced gene. Though there have been several strategies employed to enhance endosomal escape of the DNA 8,9, standards for overall clinical efficiency have not yet been met. However, optimization of both natural and synthetic polymeric delivery systems may provide benefit in the future. For example, addition of calcium phosphate precipitates to gene activated matrix has improved transfection of a bone morphogenetic protein-2 plasmid and resulted in enhanced repair of critical sized tibial defects in rats 10.
In contrast to biomaterial-mediated DNA delivery techniques, viruses have evolved for millions of years to optimize their ability to introduce foreign DNA to host cells 11. Though there is not a universal vector or viral system for all applications, their innate ability to infect both dividing and non-dividing cells drives tailoring of viral gene therapy to meet specific needs such as tissue regeneration, correction of genetic defects, and cure of disease. As mentioned previously, retroviral transduction has been used successfully in humans to cure x-linked SCID. In one clinical trial, however, this success was tempered when two of the nine cured SCID patients later developed leukemia due to activation of an oncogene after retroviral DNA integration 12. Incidents such as this highlight both the major promises and the potential dangers inherent in viral gene therapy. In addition to insertional oncogenesis, use of viral vectors holds the risk of viral replication and dissemination as well as the potential for toxic immune responses (Table 1)13. Despite this, viral transduction is by far the most efficacious method for gene transfer and researchers are rapidly moving to overturn the safety concerns (Table 1) 14,15.
Table 1.
Gene therapy methods, summary of advantages and risks.
| Method | Advantages | Disadvantages/Risks |
|---|---|---|
| Polymeric | Low toxicity Relative safety Ease of manufacture Lack of DNA insert size limitation |
Insufficient clinical efficacy Endosomal escape required |
| Viral | High efficency gene transfer | Replication and dissemination of virus Induction of toxic immune response Insertional oncogenesis |
| Retroviral | Infection of non-dividing cells (Lentivirus only) Long-term gene expression (years+) |
Infection of dividing cells only (Non-lentivirus) Smaller DNA insert allowed (<10kbp) |
| Adenoviral | “common cold” virus, self-limiting non-fatal infection Large DNA insert possible (30kbp) Infection of dividing and non-dividing cells |
Short-term gene expression (days) |
| AAV | Superior safety - can not replicate without helper virus Infection of dividing and non-dividing cells Potential for tissue-specific targeting Moderate to long-term expression (months to years) |
Very small DNA insert allowed (<5kbp) |
The three main classes of viruses used for gene therapy are retroviruses, adenoviruses, and adeno-associated viruses (Table 1) (for review see 14). Retroviruses are ideal for long-term gene therapy since once introduced, their DNA integrates and becomes part of the genome of the host cell. Indeed, the current human genome contains up to 5–8% of endogenous retroviral sequences that have been acquired over the course of evolution 16. Adenoviruses are more suited for short term gene delivery and are highly targeted for tissue engineering strategies that desire protein production over the course of several weeks. In addition, since the adenovirus is well known as the “virus of the common cold,” infection is generally non-toxic and self-limiting. Though, determination of genotoxicity for each specific application is necessary to keep the safety profile within acceptable parameters. Adeno-associated viruses (AAV) have become the focus of much research in recent years due to their complete inability to replicate without a helper virus, potential for tissue-specific targeting, and gene expression on the order of months to years. The ability to specifically target one tissue type without untoward effects on neighboring tissues is highly desired in fields such as craniofacial regeneration.
GENETIC ENGINEERING OF iPS CELLS
The genetic manipulation of cells has expanded the possible use of stem cells in regenerative medicine with great promise for craniofacial reconstruction. Through the insertion of specific genes involved in pluripotency of embryonic stem (ES) cells, somatic cells have been reprogrammed into cells that have unlimited self-renewal and differentiation capabilities. Due to their similarities to ES cells, these cells are named induced-pluripotent stem (iPS) cells. Pluripotent stem cells are distinct from stem cells present in adult tissues and can generate cellular derivatives from all three germ layers. Unlimited self-renewal of pluripotent iPS cells can overcome issues of cellular senescence encountered during proliferation of adult stem cells. In addition, the derivation of patient specific human iPS cells can circumvent ethical issues related to use of embryos to generate human ES cells and can also bypass concerns of immunorejection of allotransplanted cells.
The first reprogramming efforts of mouse and human fibroblasts into iPS cells were accomplished using lentivirus, a retroviral family member, to overexpress Oct3/4, Sox2, c-Myc and Klf4 genes 17,18. Soon after, a different combination of genes, Oct3/4, Sox2, Nanog, and Lin28 also expressed on a lentiviral vector, was used to successfully reprogram adult cells into iPS cells 19. Since then, the potential for iPS cells in regenerative medicine has been recognized, but concerns regarding the safety of integrating lentiviral vectors remain. In response, several non-integrating viral and non-viral methods have been used including adenovirus 20, episomal plasmids 21, and proteins 22. There is also significant concern about the use of oncogenes c-Myc and Klf4 as reprogramming factors, due to increased risk of tumorogenesis. It has been shown that these oncogenes are not required for iPS cell reprogramming 19, though c-Myc improves reprogramming efficiency 23. To reduce the tumoregenic propensity of iPS cells and to maintain or increase the reprogramming efficiency, replacement of c-Myc with another member of the Myc proto-oncogene family such as l-Myc 24 or with the maternal transcription factor Glis1 has been proposed 25. Suppression or stimulation of specific molecular signaling pathways such as p53 26,27 and chromatin remodeling factors by small molecule inhibitors 28 could also be used to enhance iPS cell reprogramming. Alternatively, overexpression of microRNA miR302/367 can efficiently reprogram somatic cells without exogenous transcription factors 29. Future efforts will continue to focus on increasing safety and reprogramming efficency of iPS cells.
Initially, it was proposed that any somatic cell could be reprogrammed into an iPS cell as a stochastic event, however recent evidence opposes this hypothesis, suggesting that only certain cells can give rise to iPS cells 30. Cells endogenously expressing one or more of the reprogramming factors are more susceptible to iPS induction, even with smaller combinations of reprogramming factors 31. Adult stem cells are a good example of this and are excellent candidates for reprogramming due to their relative ease of isolation from different tissues, including many in the craniofacial region. Human gingival fibroblasts 32 and dental pulp stem cells 33,34 isolated from molar teeth are examples of donor cells of craniofacial origin successfully reprogrammed into iPS cells. These cells are also ideal donors because their precursor tissue can be aseptically obtained from the mandible, it is protected from UV and other damage by surrounding hard tissue, and in the case of 3rd molar extraction, it is considered medical waste. Epigenetic memory of craniofacial-derived iPS cells may additionally enhance their ability to differentiate towards their tissue of origin 35,36 and enhance their use for craniofacial reconstruction.
CLINICAL GENE THERAPY
Though the theoretical advantages of gene therapy have so far outweighed practical application, the potential of this field promotes continued research and discovery. There are several significant hurdles that must be overcome for theoretical promise to match clinical reality. This includes optimizing the mechanics of gene delivery to the nucleus as discussed above, safety challenges, timing of gene expression, control of regional versus systemic effects, and the identification of appropriate signals for regeneration. The craniofacial region contains tissue targets that are common to other body regions such as bone, cartilage, muscle, nerve, ligament, skin, and mucosa. However, it also contains unique structures such as teeth and salivary glands and is under constant challenge due to the presence of bacteria and inflammation in the oral cavity. In addition, many of these tissues exist at an interface with another. For example, bone, cartilage, and ligament in the joint space or cementum, periodontal ligament, and bone around the tooth root. The question then evolves, even if identified, will we be able to deliver multiple signals in the correct order to achieve regeneration of these complex tissues? To address this we must thoroughly master the regeneration of each individual structure. The following sections will summarize our current progress.
Cancer Therapy
Though not a direct target of craniofacial regeneration, removal of oral tumors often generates defects that require significant reconstruction. In addition to using gene therapy to repair or regenerate the complex tissues at these sites, treatment of the tumor itself with genetic transfer has made significant progress in the last decade. This includes approval of the ‘H101’ oncolytic adenovirus for treatment of head and neck cancer in China in November of 2005 37. In the United States an oncolytic herpes simplex virus encoding granulocyte macrophage colony stimulating factor (GM-CSF, OncoVEXR) is in active phase III clinical trials for treatment of both stage III/IV melanoma and locally advanced head and neck squamous cell carcinoma 38,39. Oncolytic viruses work by specifically targeting and replicating in tumor cells to result in cell death and decrease in overall tumor size. In addition, OncoVEXR works to regulate the immune response to the tumor by induction of antigen-specific T cell responses 40. Genetic treatments of the tumor itself may limit the subsequent amount of reconstruction required.
Mineralized Tissues
In the craniofacial region, gene transfer studies are more often directed at regenerating tissues than correcting genetic defects. Gene therapy for tissue regeneration can transform cells at the site of injury into sustainable protein producing reactors and help to overcome some of the issues associated with use of recombinant proteins such as low half-life, poor site retention, and cost 41. Adenoviral, retroviral, and AAV-mediated delivery of osteogenic genes have enhanced fracture repair of intramembranous and endochondral bone formation in vivo in animal models (for review see 42). Though many retroviral therapies utilize ex vivo gene therapy of cultured cells, to mitigate costs associated with gene therapy procedures, direct delivery of the virus to the site is desired. This can be accomplished with an “expedited ex vivo” strategy in which host tissue is transduced during the course of an operation and then implanted at the site requiring regeneration to serve as a biocompatible protein producing reactor 43. This technique has been successfully used to enhance regeneration of rat femoral defects with adenovirus transduced adipose tissue 43. Like plasmid DNA, viruses can also be delivered on biocompatible scaffolds that not only generate the desired protein production but can also guide tissue growth via spatial coordination of cells (for review see 6).
Recent developments in genetic regeneration of osseous tissues include inducible vector systems, use of rAAV, and generation of tissue interfaces. Inducible vectors allow for gene expression only after stimulation of the transformed cells with a drug such as dexamethasone or doxycycline and provide an additional level of temporal control of protein expression 44,45. Use of recombinant AAV is desired for bone repair due to promises of superior safety, tissue targeting, and in vivo transduction of non-dividing cells. For example, in vivo AAV-mediated expression of constitutively active activin receptor-like kinase-2 (caAlk2), VEGF/RANKL, and BMP-7 has successfully enhanced healing of osseous defects in rodent models 46,47. Craniofacial interfaces such as the temporomandibular joint (TMJ) have been targeted for regeneration through focused production of an osteo-chondral graft using a combination of differentiated chondrocytes and BMP-7 transduced gingival fibroblasts on pre-fabricated scaffolds 48. TMJ repair has also been attempted in a rodent model with localized injection of AAV expressing VEGF to condylar tissue 49. Further exploration may make both TMJ replacement and repair of developmental deformities within the limits of gene therapy.
Arguably one of the most complex mineralized interfaces in the body, the tooth is comprised of three unique mineral layers (enamel, dentin, and cementum), encased in a proprioceptive periodontal ligament, and housed in alveolar bone. To date, all strategies for whole tooth bioengineering have relied on the use of stem cells derived from the dental pulp, periodontal ligament, and/or developing tooth germ with very little emphasis on gene delivery (for review see 6). Gene transfer, however, has been extensively studied in animal models for regeneration of the periodontium and for augmenting alveolar bone prior to placement of dental implants 50,51.
Salivary Gland
Need for augmentation of salivary gland function may occur due to side effects of medication, radiation therapy, or autoimmune disorders. The cells of the salivary gland duct are not capable of fluid secretion. To accomplish this, acinar cells require four membrane proteins to generate an osmotic gradient and mediate fluid movement: (1) the N+K+-ATPase used to maintain membrane potential, (2) a Ca2+ activated K+ channel, (3) the secretory isoform of the Na+/K+/2Cl− cotransporter, and (4) the apical membrane bound Ca2+ activated Cl− channel 52,53. The osmotic gradient then directs fluid movement through water channels in the apical membrane known as aquaporins (AQP) 52. Normal ductal epithelial cells lack AQP expression54, however, introduction of AQP using adenoviral transduction has helped to increase salivary secretion in rat and mini-pig salivary gland tissue in vivo 55. A human clinical trial is currently underway to treat patients with impaired salivary flow post-radiation therapy with injection of AQP1 encoding adenovirus to the parotid gland 56.
FUTURE DIRECTIONS
Though the potential for success drives research activity, coordination of gene transfer and craniofacial regeneration will require cautious, regulated progress to minimize safety concerns while maximizing patient outcomes. One of the major challenges facing tissue engineers is the ability to scale up a technology that has proven successful in rodent models. Regeneration of tissues such as bone in rodents is more easily accomplished due to controlled geometry of the defect, smaller size, and higher remodeling rate. As the size of a defect gets larger, the ability to engineer a vascular supply becomes more difficult as cells must be within 100μm of an oxygen source to survive 6,57. In addition to vascular supply, accurate craniofacial reconstruction demands production of tissue interfaces to repair structures such as joint, tooth, and muscle attachments. This will require identification and controlled delivery of complex signals in both time and space. Despite these challenges, use of gene transfer to engineer a cell to produce proteins of interest and drive coordination of tissue repair will help to overcome limitations of traditional recombinant protein therapy and advance craniofacial regeneration efforts.
The successful use of iPS cells in regenerative medicine will require efficient methods to differentiate them into functional cells. Although this represents a major challenge, significant progress has been made in controlling differentiation of human ES cells that is theoretically transferable to iPS cell populations. For example, multiple groups including our own have differentiated iPS cells into functional mesenchymal stem cells (MSC) with osteogenic, chondrogenic and adipogenic differentiation capabilities 58. IPS cell technology is also useful in the derivation of cell lines from patients affected by congenital disorders to establish disease models that recapitulate the pathophysiology of the disease and can be used as a cellular tool for drug screening, diagnosis and treatment. Over 700 genetic syndromes are known to have craniofacial defects and more than 250 have been associated with clefting of the lip and/or palate 59,60. Currently, iPS cell lines have been generated from patients with disorders including Lou Gehrig’s disease, spinal muscular atrophy, familial dysautonomia, diskerastosis congenita, Hutchinson Gilford Progeria, Rett syndrome, and Friedreich’s ataxia (for review see 61). Gene therapy has been used to rescue function of hepatocyte-like cells derived from iPS cells of a patient with Wilson’s disease 62, and to restore β-globin production by erythroid cells derived from patients with β-thalassemia 63. As a novel strategy, somatic cells from patients with Fanconi anaemia were genetically corrected and then reprogrammed into iPS cells than in turn were differentiated into disease-free haematopoietic progenitors of the myeloid and erythroid lineages 64. Similarly, although not in cells derived from iPS cells, gene therapy was used to partially correct a mineralization defect of MSCs from a patient with osteogenesis imperfecta 65. In combination with gene and cell therapy, iPS cell technology has great potential to enhance our understanding of and to treat congenital disorders.
Acknowledgments
Supported by R01 DE13835 (PHK), F30 DE019577 (ELS), and the NIDCR T32 Tissue Engineering and Regeneration Training Program (LGV)
References
- 1.Anderson WF. September 14, 1990: the beginning. Hum Gene Ther. 1990;1(4):371–2. doi: 10.1089/hum.1990.1.4-371. [DOI] [PubMed] [Google Scholar]
- 2.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–72. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 3.Peters M, Heinaru E, Talpsep E, et al. Acquisition of a deliberately introduced phenol degradation operon, pheBA, by different indigenous Pseudomonas species. Appl Environ Microbiol. 1997;63(12):4899–906. doi: 10.1128/aem.63.12.4899-4906.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kivisaar MA, Habicht JK, Heinaru AL. Degradation of phenol and m-toluate in Pseudomonas sp. strain EST1001 and its Pseudomonas putida transconjugants is determined by a multiplasmid system. J Bacteriol. 1989;171(9):5111–6. doi: 10.1128/jb.171.9.5111-5116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKie R. The Observer, January. 2. London: 2000. Jan 2, Gene researchers face crisis as man's saviour turns killer. [Google Scholar]
- 6.Scheller EL, Krebsbach PH, Kohn DH. Tissue engineering: state of the art in oral rehabilitation. J Oral Rehabil. 2009;36(5):368–89. doi: 10.1111/j.1365-2842.2009.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58(4):467–86. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Cho YW, Kim JD, Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J Pharm Pharmacol. 2003;55(6):721–34. doi: 10.1211/002235703765951311. [DOI] [PubMed] [Google Scholar]
- 9.Mahato RI, Lee M, Han S, et al. Intratumoral delivery of p2CMVmIL-12 using water-soluble lipopolymers. Mol Ther. 2001;4(2):130–8. doi: 10.1006/mthe.2001.0425. [DOI] [PubMed] [Google Scholar]
- 10.Endo M, Kuroda S, Kondo H, et al. Bone regeneration by modified gene-activated matrix: effectiveness in segmental tibial defects in rats. Tissue Eng. 2006;12(3):489–97. doi: 10.1089/ten.2006.12.489. [DOI] [PubMed] [Google Scholar]
- 11.Dewannieux M, Harper F, Richaud A, et al. Identification of an infectious progenitor for the multiple-copy HERV-K human endogenous retroelements. Genome Res. 2006;16(12):1548–56. doi: 10.1101/gr.5565706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser J. Gene therapy. Seeking the cause of induced leukemias in X-SCID trial. Science. 2003;299(5606):495. doi: 10.1126/science.299.5606.495. [DOI] [PubMed] [Google Scholar]
- 13.Yi Y, Hahm SH, Lee KH. Retroviral gene therapy: safety issues and possible solutions. Curr Gene Ther. 2005;5(1):25–35. doi: 10.2174/1566523052997514. [DOI] [PubMed] [Google Scholar]
- 14.Scheller EL, Krebsbach PH. Gene therapy: design and prospects for craniofacial regeneration. J Dent Res. 2009;88(7):585–96. doi: 10.1177/0022034509337480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NIH . Assessment of adenoviral vector safety and toxicity: report of the National Institutes of Health Recombinant DNA Advisory Committee. Hum Gene Ther. 2002;13(1):3–13. doi: 10.1089/10430340152712629. [DOI] [PubMed] [Google Scholar]
- 16.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 20.Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–9. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–4. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa M, Takizawa N, Narita M, et al. Promotion of direct reprogramming by transformation-deficient Myc. Proc Natl Acad Sci U S A. 2010;107(32):14152–7. doi: 10.1073/pnas.1009374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maekawa M, Yamaguchi K, Nakamura T, et al. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature. 2011;474(7350):225–9. doi: 10.1038/nature10106. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Yin X, Qin H, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3(5):475–9. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–5. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huangfu D, Maehr R, Guo W, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26(7):795–7. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8 (4):376–88. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460(7251):49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- 31.Kim JB, Greber B, Arauzo-Bravo MJ, et al. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461(7264):649–3. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 32.Egusa H, Okita K, Kayashima H, et al. Gingival fibroblasts as a promising source of induced pluripotent stem cells. PLoS One. 2010;5(9):e12743. doi: 10.1371/journal.pone.0012743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamaoki N, Takahashi K, Tanaka T, et al. Dental pulp cells for induced pluripotent stem cell banking. J Dent Res. 2010;89(8):773–8. doi: 10.1177/0022034510366846. [DOI] [PubMed] [Google Scholar]
- 34.Oda Y, Yoshimura Y, Ohnishi H, et al. Induction of pluripotent stem cells from human third molar mesenchymal stromal cells. J Biol Chem. 2010;285(38):29270–8. doi: 10.1074/jbc.M109.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–90. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polo JM, Liu S, Figueroa ME, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28(8):848–55. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7(2):141–8. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 2010;6(6):941–9. doi: 10.2217/fon.10.66. [DOI] [PubMed] [Google Scholar]
- 39.NIH. ClinicalTrials.gov [database online] Bethesda, MD: National Institutes of Health; 2011. [Google Scholar]
- 40.Kaufman HL, Kim DW, DeRaffele G, et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17(3):718–30. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 41.Krebsbach PH, Gu K, Franceschi RT, et al. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11(8):1201–10. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 42.Franceschi RT. Biological approaches to bone regeneration by gene therapy. J Dent Res. 2005;84(12):1093–103. doi: 10.1177/154405910508401204. [DOI] [PubMed] [Google Scholar]
- 43.Betz VM, Betz OB, Harris MB, et al. Bone tissue engineering and repair by gene therapy. Front Biosci. 2008;13:833–41. doi: 10.2741/2724. [DOI] [PubMed] [Google Scholar]
- 44.Gafni Y, Pelled G, Zilberman Y, et al. Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther. 2004;9 (4):587–95. doi: 10.1016/j.ymthe.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Peng H, Usas A, Gearhart B, et al. Development of a self-inactivating tet-on retroviral vector expressing bone morphogenetic protein 4 to achieve regulated bone formation. Mol Ther. 2004;9(6):885–94. doi: 10.1016/j.ymthe.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 46.Ulrich-Vinther M. Gene therapy methods in bone and joint disorders. Evaluation of the adeno-associated virus vector in experimental models of articular cartilage disorders, periprosthetic osteolysis and bone healing. Acta Orthop Suppl. 2007;78(325):1–64. [PubMed] [Google Scholar]
- 47.Kang Y, Liao WM, Yuan ZH, et al. In vitro and in vivo induction of bone formation based on adeno-associated virus-mediated BMP-7 gene therapy using human adipose-derived mesenchymal stem cells. Acta Pharmacol Sin. 2007;28(6):839–49. doi: 10.1111/j.1745-7254.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 48.Schek RM, Taboas JM, Hollister SJ, et al. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthod Craniofac Res. 2005;8(4):313–9. doi: 10.1111/j.1601-6343.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 49.Rabie AB, Dai J, Xu R. Recombinant AAV-mediated VEGF gene therapy induces mandibular condylar growth. Gene Ther. 2007;14(12):972–80. doi: 10.1038/sj.gt.3302943. [DOI] [PubMed] [Google Scholar]
- 50.Jin Q, Anusaksathien O, Webb SA, et al. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9(4):519–26. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang PC, Seol YJ, Cirelli JA, et al. PDGF-B gene therapy accelerates bone engineering and oral implant osseointegration. Gene Ther. 2010;17(1):95–104. doi: 10.1038/gt.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melvin JE, Yule D, Shuttleworth T, et al. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–69. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 53.Aframian DJ, Palmon A. Current Status of the Development of an Artificial Salivary Gland. Tissue Eng Part B Rev. 2008 doi: 10.1089/ten.teb.2008.0044. [DOI] [PubMed] [Google Scholar]
- 54.Tran SD, Wang J, Bandyopadhyay BC, et al. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng. 2005;11 (1–2):172–81. doi: 10.1089/ten.2005.11.172. [DOI] [PubMed] [Google Scholar]
- 55.Baum BJ, Zheng C, Cotrim AP, et al. Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim Biophys Acta. 2006;1758(8):1071–7. doi: 10.1016/j.bbamem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Baum BJ, Zheng C, Cotrim AP, et al. Aquaporin-1 gene transfer to correct radiation-induced salivary hypofunction. Handb Exp Pharmacol. 2009;(190):403–18. doi: 10.1007/978-3-540-79885-9_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lightfoot E. The roles of mass transport in tissue function. In: Bronzino JD, editor. CRC Handbook of Biomedical Engineering. CRC Press; Boca Raton FL: 1995. pp. 1656–1670. [Google Scholar]
- 58.Lian Q, Zhang Y, Zhang J, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113–23. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 59.Gorlin RJ, Cohen MM, Jr, Levin LS. Press OU. Syndromes of the Head and Neck. 3. Oxford University Press; New York: 1990. [Google Scholar]
- 60.Tewfik TL, der Kaloustian VM. Press OU. Congenital Abnormalities of the Ear, Nose, and Throat. Oxford University Press; New York, NY: 1997. [Google Scholar]
- 61.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13(6):734. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S, Chen S, Li W, et al. Rescue of ATP7B function in hepatocyte-like cells from Wilson's disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr223. [DOI] [PubMed] [Google Scholar]
- 63.Papapetrou EP, Lee G, Malani N, et al. Genomic safe harbors permit high beta- globin transgene expression in thalassemia induced pluripotent stem cells. Nat Biotechnol. 2011;29 (1):73–8. doi: 10.1038/nbt.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raya A, Rodriguez-Piza I, Guenechea G, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460(7251):53–9. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pochampally RR, Horwitz EM, DiGirolamo CM, et al. Correction of a mineralization defect by overexpression of a wild-type cDNA for COL1A1 in marrow stromal cells (MSCs) from a patient with osteogenesis imperfecta: a strategy for rescuing mutations that produce dominant-negative protein defects. Gene Ther. 2005;12(14):1119–25. doi: 10.1038/sj.gt.3302514. [DOI] [PubMed] [Google Scholar]