Abstract
A major goal in the application of hydrogels for tissue engineering scaffolds, especially for load-bearing tissues such as cartilage, is to develop hydrogels with high mechanical strength. In this study, a double-network (DN) strategy was used to engineer strong hydrogels that can encapsulate cells. We improved upon previously studied double-network (DN) hydrogels by using a processing condition compatible with cell survival. The DN hydrogels were created by a two-step photocrosslinking using gellan gum methacrylate (GGMA) for the rigid and brittle first network, and gelatin methacrylamide (GelMA) for the soft and ductile second network. We controlled the degree of methacrylation of each polymer so that they obtain relevant mechanical properties as each network. The DN was formed by photocrosslinking the GGMA, diffusing GelMA into the first network, and photocrosslinking the GelMA to form the second network. The formation of the DN was examined by diffusion tests of the large GelMA molecules into the GGMA network, the resulting enhancement in the mechanical properties, and the difference in mechanical properties between GGMA/GelMA single networks (SN) and DNs. The resulting DN hydrogels exhibited the compressive failure stress of up to 6.9 MPa, which approaches the strength of cartilage. It was found that there is an optimal range of the crosslink density of the second network for high strength of DN hydrogels. DN hydrogels with a higher mass ratio of GelMA to GGMA exhibited higher strength, which shows promise in developing even stronger DN hydrogels in the future. Three dimensional (3D) encapsulation of NIH-3T3 fibroblasts and the following viability test showed the cell-compatibility of the DN formation process. Given the high strength and the ability to encapsulate cells, the DN hydrogels made from photocrosslinkable macromolecules could be useful for the regeneration of load-bearing tissues.
Keywords: hydrogel, IPN, photopolymerization, cell encapsulation, mechanical properties
1. Introduction
Load-bearing tissues, such as cartilage, tendon, and muscle exhibit high strength and toughness, despite their softness [1, 2]. For example, cartilage frequently takes compressive stress of several MPa, and withstands up to 14–59 MPa without failure [2–4]. To approximate these mechanical properties, various biomaterials have been developed and studied as artificial soft tissues; however, the large difference between natural tissues and artificial biomaterials still presents a challenge [5–7].
Hydrogels are promising candidates for tissue engineering scaffolds due to their high water content, high permeability to small molecules, biocompatibility, and mechanical properties which resemble natural tissues [2, 8, 9]. Cells can be encapsulated within hydrogels for cell delivery and three dimensional (3D) cell culture. Such cultures better mimic the natural cellular environment to understand the role of the native microenvironment on cellular functions and tissue formation [10, 11]. However, hydrogels are often too soft and weak to be applied for a number of tissue engineering applications that require extensive load bearing behavior. Therefore, developing hydrogels with high mechanical strength is a critical challenge for expanding the range of applications of hydrogels for tissue engineering scaffolds.
Double-network (DN) hydrogels have attracted a great deal of attention for their high fracture toughness and high fracture stress [12, 13]. They are specialized interpenetrating networks (IPNs) in that they are composed of networks with opposite mechanical properties, which results in high strength. In the case of DN hydrogels, the first network is stiff and brittle whereas the second is soft and ductile. In this scheme, the rigid network sustains the stress throughout the material, and the ductile network dissipates energy near the crack tip, preventing the fracture of gels [14]. Various DN hydrogels from different materials have been reported [12, 13, 15, 16], however, these previous studies did not attempt to encapsulate cells to make cell-laden 3D tissue constructs due to synthetic conditions for DN formation that were incompatible with cell encapsulation. These include the use of toxic crosslinkers or the long crosslinking time needed for the network formation from small molecules. Normal IPNs prepared from biomacromolecules that can encapsulate cells were developed; however, the fracture stress did not reach to the order of MPa [17].
Photocrosslinking of macromolecules provides a method for hydrogel fabrication that has been demonstrated to be compatible with cell encapsulation [18, 19]. The photocrosslinking method has been used to make hydrogels for many tissue engineering studies by modifying various polymers with photoreactive groups. This approach does not need toxic crosslinkers, allows for injection of polymers into the body without large incisions, and enables temporal and spatial control in the fabrication of complicated structures [8, 11, 20, 21]. Since chemical crosslinks are irreversible, photocrosslinked hydrogels are relatively stiff and stable. However, the heterogeneous distribution of crosslinks in photocrosslinked hydrogels results in concentrated stress around the dense crosslinks, causing the hydrogels to fail at low stress [2]. Typically, the compressive moduli of photocrosslinked hydrogels range up to a few hundreds of kPa, and the failure stresses are on the order of tens to hundreds of kPa at the polymer concentrations of 0.5–20% [22–25].
In this study, by a two-step photocrosslinking of two modified biomacromolecules, gellan gum methacrylate (GGMA) and gelatin methacrylamide (GelMA), we developed DN hydrogels with high strength that can encapsulate cells. Gellan gum (GG) is a bacterial polysaccharide consisting of a tetrasaccharide repeating unit. GG has been approved by FDA as a food additive and has been recently receiving attention for tissue engineering applications [26–28]. Metharylated GG was created to make stiff hydrogels, and highly methacrylated GG hydrogels were reported to have a modulus of more than 100 kPa at only 1% polymer concentration[23]. Gelatin is denatured collagen, which is a major constituent of the extracellular matrix (ECM). Due to the natural cell binding motifs, such as RGD, gelatin exhibits great biological properties such as cell adhesion and cell elongation, which makes it an attractive material for tissue engineering applications. These two polymers were modified into photocrosslinkable GGMA and GelMA and fabricated into DN hydrogels. The formation of the DN was examined by diffusion tests of the large GelMA molecules into the GGMA network, measuring the resultant enhancement in the mechanical properties, and comparing the mechanical properties between GGMA/GelMA single networks (SN) and DNs. The mechanical properties of DN hydrogels were also compared with those of each GGMA and GelMA SN hydrogels. The effect of the crosslink density of the second network and the concentration of each component in preparation of DN hydrogels on the mechanical properties of DN hydrogels was studied. Lastly, NIH-3T3 fibroblasts were encapsulated in DN gels and the viability was assessed to demonstrate the cell-compatibility of the whole process of DN network formation.
2. Materials and methods
2.1. Synthesis of GGMA and GelMA polymers
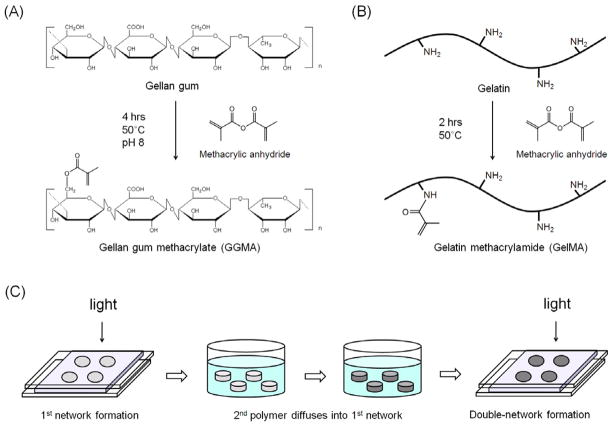
GG (Gelzan™, Molecular weight: 1,000,000), gelatin (from procine skin, Type A), and methacrylic anhydride were purchased from Sigma-Aldrich. GGMA and GelMA were synthesized by reacting GG and gelatin with methacrylic anhydride (Fig. 1A–B) [23, 29]. Briefly, 1g of GG was dissolved in 100ml of distilled water at 90°C for 30min, and 8ml of methacrylic anhydride was added at 50°C. The reaction was continued for 4hrs at 50°C while the pH of the solution was maintained at 8 by adding 5N NaOH solution. Then the solution was dialyzed in distilled water using dialysis tubing (molecular weight cutoff: 12–14 kDa, Spectrum Labs, Inc.) at 4°C for 4 days. The solution was lyophilized to obtain pure GGMA, and it was stored at −40°C until further use. Similarly, 20g of gelatin was dissolved in 200ml of phosphate buffered saline (PBS, 1X, Invitrogen) at 50°C, and varying amounts of methacrylic anhydride was added to vary the degree of methacrylation. The reaction was continued for 2hrs at 50°C, and then the solution was dialyzed against distilled water at 40°C for at least 3 days. The solution was lyophilized to obtain pure GelMA, and it was stored at −40°C until further use.
Fig. 1.
Synthesis scheme of (A) gellan gum methacrylate (GGMA) (pictured as above for simplicity, although methacrylic anhydride can react with any hydroxyl group in gellan gum) and (B) gelatin methacrylamide (GelMA). (C) Fabrication of DN hydrogels through a two-step photocrosslinking process.
2.2. 1H NMR
The degree of methacrylation (DM) of GGMA and GelMA was measured by 1H NMR (Varian Inova 500). GGMA was dissolved in D2O at 10 mg/ml at 50°C and the spectrum was obtained at 50°C. The DM of GGMA, defined as the number of methacrylate groups attached to GG divided by the number of hydroxyl groups of unreacted GG, was calculated by integrating the peak at 1.5 ppm from the methyl group of the rhamnose unit, and the peak at 2.0 ppm from the methyl group of the methacrylate group [30]. GelMA polymers were dissolved in D2O at 30 mg/ml at 40°C and the spectra were obtained at 40°C. The DM of GelMA, defined as the number of methacrylate groups attached to gelatin divided by the number of amine groups of unreacted gelatin, was calculated by integrating the peaks at 7.4 ppm from the aromatic residues of gelatin, and the peaks at 5.5 ppm and 5.7 ppm from the double bond hydrogens of methacrylate groups [31, 32].
2.3. Fabrication of SN and DN hydrogels
GGMA polymer solutions were prepared by dissolving GGMA polymer at different concentrations (0.5, 1.0, 1.5% (w/v)) at 50°C for 1 day in distilled water containing 0.1% (w/v) photoinitiator, 2-hydroxy-1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-1-propanone (Irgacure 2959, Ciba Specialty Chemicals). The solutions were molded into disks with ~8mm diameter and ~1mm thickness, and exposed to light (320–500 nm, ~7 mW/cm2, EXFO OmniCure S2000) for crosslinking for varying times. Likewise, GelMA SN hydrogels were fabricated by preparing GelMA polymer solutions at different concentrations at 40°C in distilled water containing 0.1% (w/v) photoinitiator, and crosslinking the solutions in the same manner at 37°C for 120 s. GGMA/GelMA SN hydrogels were fabricated by preparing GGMA/GelMA mixed solutions at different concentrations in distilled water containing 0.1% (w/v) photoinitiator at 50°C for 1 day, and crosslinking the solutions in the same manner at 37°C for 120 s. To fabricate GGMA/GelMA DN hydrogels (Fig. 1C), GGMA hydrogels were immersed in GelMA solutions (5, 10, 15, 20% (w/v) in distilled water) containing 0.1% (w/v) photoinitiator at 37°C for 2 days on a shaker to allow GelMA molecules to diffuse into GGMA hydrogels. Subsequently, the hydrogels were taken out of the GelMA solutions, and after removing the excess GelMA solutions from the surface of the hydrogels, they were exposed to light again for varying times. All the resulting SN and DN hydrogels were immersed in distilled water at 37°C for 12 hrs and used for further experiments.
2.4. Diffusion test
To make fluorescent GelMA polymers, fluorescein isothiocyanate (FITC, Sigma-Aldrich) was conjugated to GelMA polymer based on the manufacturer’s instruction. Briefly, 1g of GelMA (DM: 14.7%) was dissolved in 50ml of sodium bicarbonate (Sigma-Aldrich) aqueous solution (100 mM) at 40°C, and 20mg of FITC was dissolved in 10ml dimethyl formamide (DMF, EMD chemicals). The two solutions were mixed and reacted for 6 hrs at 40°C. The resulting solution was dialyzed against distilled water at 40°C for 7 days, and lyophilized to obtain solid FITC-GelMA. The reaction and purification were performed in the dark to minimize fluorescein photobleaching. To examine the diffusion of GelMA molecules into GGMA hydrogels, FITC-GelMA and GelMA (DM: 14.7%) were dissolved together (mass ratio of 1:23) at the concentration of 20% in distilled water containing 0.1% (w/v) photoinitiator. Previously prepared cylindrical (~8mm diameter, ~1mm thickness) 0.5% GGMA hydrogels were immersed in the solution on a shaker at 37°C. At each time point, the hydrogels were taken out to be exposed to light for 120 s, immersed in distilled water for ~10 mins at 37°C, and cross-sectioned in the middle to be observed under a fluorescence microscope (Nikon TE 2000-U). Relative fluorescence intensity profiles over the thickness of the cross section were plotted by using ImageJ software. The diffusion coefficient of GelMA molecules in GGMA hydrogel was estimated by fitting the fluorescence intensity profiles in the solution to the diffusion equation by using Origin 6.0 software.
2.5. Mechanical test
The mechanical properties of hydrogels were measured by unconfined, uniaxial compression tests by using an Instron 5542 mechanical tester. Cylindrical hydrogels were prepared as described above and immersed in distilled water until they reached a swelling equilibrium. They were compressed at a rate of 0.3 mm/min−1 until failure. The compressive modulus was determined as the slope of the linear region in the 0–10% strain range of the stress-strain curve. The failure strain and the failure stress were taken from the point where a crack starts to be observed. This happened when the stress-strain curve dropped suddenly or the slope of the curve started to decrease, according to the brittleness of the hydrogels. As it was difficult to identify the failure point in case of GelMA SN hydrogels, the hydrogels were compressed to an ending stress, removed from the tester, and manually checked for cracks while varying the ending stress by 0.5 MPa.
2.6. Hydrogel characterization
To determine the fraction of unreacted double bonds in GGMA hydrogels, Fourier transform infrared spectroscopy (FTIR) analysis was performed. To prepare samples for FTIR, 0.5% GGMA hydrogels were fabricated as described above varying crosslinking time from 45s to 360s. The resulting hydrogels were immersed in distilled water and then lyophilized, and the spectra were taken by a FTIR spectrometer (Bruker Alpha) with an attenuated total reflection (ATR) module.
Water content of hydrogels was determined by measuring the mass of hydrogels in the swollen state and in the dried state. It was calculated as the difference between the mass in the swollen state and that in the dried state divided by the mass in the swollen sate. The mass ratio of each network in DN hydrogels was calculated by using the water content of GGMA SN hydrogels and DN hydrogels.
2.7. Cell culture and encapsulation
NIH-3T3 fibroblasts were cultured using Dulbecco’s Modified Eagle Medium (DMEM, high glucose, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin-streptomycin (Invitrogen) in an atmosphere with 5% of CO2 at 37°C. Cells were trypsinized and replated every 3–4 days and media was replaced every 2–3 days.
A cell suspension of NIH-3T3 fibroblasts (5 × 106 cells/ml) was prepared by trypsinization and resuspension into the media described above. 1% GGMA solution in distilled water containing 0.2% photoinitiator was also prepared and mixed with the cell suspension at 1:1 volume ratio. The resulting mixture was pipetted on a Petri dish and the mixture drop was covered by a 3-(trimethoxysilyl)propyl methacrylate (TMSPMA)-coated glass slide with two 300 μm spacers between the Petri dish and the glass slide. Subsequently, a photomask with square patterns (900 × 900 μm) was placed on the glass slide and the mixture was exposed to light (320–500 nm, ~7 mW/cm2) for 120s. The resulting microgels attached to the glass slide were then immersed in previously prepared 20% GelMA (DN:14.7%) solution in media containing 0.1% photoinitiator, and placed on a shaker in an incubator. After 24 hrs, the samples were taken out and exposed to light again for 120s. A calcein AM/ethidium homodimer-1 live/dead assay (Invitrogen) was performed according to the manufacturer’s instructions to assess the cell viability in the resulting DN gels following 1 hr (Day 0) and 3 days of culture in media. Similarly, NIH-3T3 fibroblasts were encapsulated in each 0.5% GGMA and 20% GelMA SN hydrogels, and the live/dead assay was performed at day 0 and day 3.
2.8. Statistics
All data were expressed as mean ± standard deviation. T-test, one-way, or two-way ANOVA followed by Tukey’s test or Bonferroni test was performed where appropriate to measure statistical significance (GraphPad Prism 5.02, GraphPad Software). Differences were taken to be significant for p < 0.05.
3. Results and discussion
3.1. GGMA and GelMA synthesis
The mechanical properties of hydrogels mainly depend on the original rigidity of polymer chains and the crosslinking density [8]. GG, a polysaccharide, is composed of rigid repeat units that have six-membered ring structure, and have many hydroxyl groups that can be functionalized with the photoreactive methacrylate groups of which the amount determines the crosslink density of the resulting network. Thus, if GG is highly methacrylated, it can be a suitable candidate for the stiff first network of a DN hydrogel. In contrast, gelatin has a more flexible chain, and a relatively small amount of the amine groups of lysine or hydroxylysine residues are spread throughout the polymer. Consequently, methacrylated gelatin can be adequate for the soft and ductile second network. Molecular weight was also an important factor to be considered. As it was reported that the concentration of the second component must be much higher than the first component for the DN to effectively improve the mechanical properties [2], the first polymer had to form rigid hydrogels at low polymer concentration, which meant that the molecular weight of the first polymer had to be very high. Furthermore, the stiffness of a hydrogel increases as the molecular weight of the polymer increases, because the effective number of crosslinked chains increases [33]. Considering all these factors, GG with the molecular weight of ~1 MDa and gelatin were chosen as each the first and the second polymer for this study.
Both polymers were methacrylated by reacting them with methacrylic anhydride [23, 29] (Fig. 1A–B). The DM of GGMA analyzed by using 1H NMR spectroscopy was 24.5%. Since one repeating unit has 10 hydroxyl groups, the DM of 24.5% means that there were 2.45 methacrylate groups per repeating unit, so the molecular weight between two methacrylate groups is about 300 g/mol on average. In case of gelatin, the DM was calculated to range from 5.7% to 76.0%, which was created by varying the amount of methacrylic anhydride added to the synthesis reaction to examine the effect of the crosslink density of the second network to the mechanical properties of DN hydrogels. Since the molecular weight of a gelatin molecule is around 100 kDa [32], the molecular weight between two metharylate groups in GelMA was calculated to range from 4,000 to 50,000 g/mol on average. Consequently, highly methacrylated gellan gum and methacrylated gelatin were successfully prepared as the first and the second component of DN hydrogels.
3.2. Fabrication of DN hydrogels
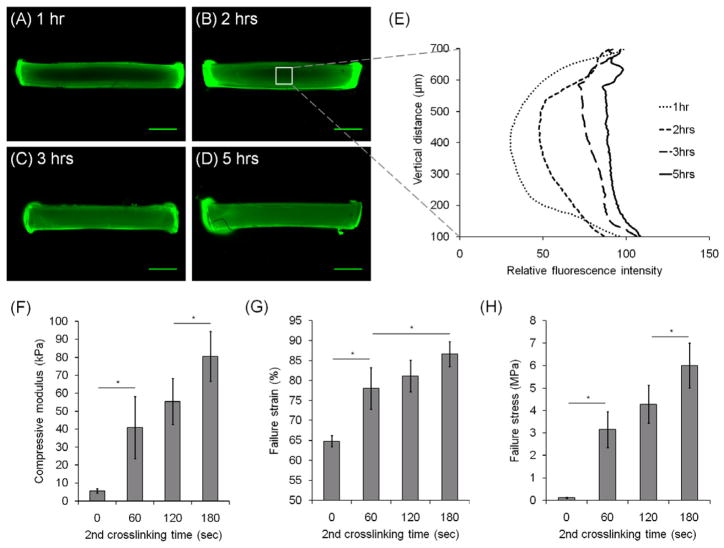
DN hydrogels were fabricated by a two-step crosslinking (Fig. 1C). First, the GGMA solutions (0.5%, 1.0%, 1.5% (w/v)) were photocrosslinked to form the first network, GGMA hydrogels. The GGMA hydrogels then were immersed in GelMA solution (5%, 10%, 15%, 20% (w/v)) so that the GelMA molecules can diffuse into the GGMA hydrogels. Since the initial polymer concentration of the GGMA hydrogels was much lower than the concentration of GelMA solution, the hydrogels deswelled in GelMA solution likely due to the osmotic pressure. Subsequently, the gels were taken out and exposed to light for the second crosslinking. To confirm that the GelMA molecules diffused into the GGMA gels, 0.5% GGMA hydrogels were immersed in 20% GelMA (DM:14.7%) solution containing FITC-GelMA, and the fluorescence image of the gel cross-section was taken at each time point (Fig. 2A–E). The relative fluorescence intensity profile in the middle of the cross-section at each time point was plotted to show that GelMA molecules diffused into the GGMA gel, and the concentration of GelMA molecules became almost uniform in the GGMA gel in several hours. We estimated the diffusion coefficient (D) of GelMA molecules in the GGMA hydrogel by using these plots. Assuming that the diffusivity is constant and the diffusion in this case can be seen as a one-dimensional diffusion, the diffusion equation has the solution [34, 35]
Fig. 2.
Formation of double-network (DN) hydrogels. (A–D) Diffusion of FITC-GelMA molecules into GGMA hydrogels over time. (A) 1hr, (B) 2hrs, (C) 3hrs, (D) 5hrs. Scale bars represent 1 mm. (E) Vertical fluorescence profile of the cross-section of hydrogels over time. (F) Compressive modulus, (G) failure strain, and (H) failure stress of DN hydrogels with varying 2nd crosslinking time. 0.5% GGMA hydrogels crosslinked for 120 seconds and 20% GelMA(DM: 14.7%) solutions were used for (A)–(H). (*) indicates significant difference (P < 0.05).
where c0 is the initial concentration at boundaries, d is the thickness of the hydrogel, and erf is the error function. Although the thickness of the GGMA hydrogels decreased due to the deswelling in the GelMA solution, we used the final thickness of DN hydrogels for d, because most of the deswelling occurred within an hour, and the expected error was not significant in further estimation and comparison. By fitting the fluorescence profiles in the solution above retaining the terms with n = 0, ±1, and ±2, D was estimated as (8 ± 3) × 10−8 cm2/s. In the literature, the diffusion coefficient of gelatin in water was reported to be on the order of 10−7 cm2/s [36, 37]. The hydrodynamic radius of gelatin molecules can be estimated to be ~10 nm; the ratio of diffusion coefficients in water and in hydrogels of macromolecules of this size was reported to be around 0.5 for several studies [38, 39]. Therefore, the estimated value of D above is feasible. Using the calculated value of diffusivity, it was estimated that it would take ~6 hrs for the concentration of GelMA at the center of GGMA hydrogel with thickness of 800 μm to be 90% of that at the boundaries, ~7 hrs for 95%, and ~11 hrs for 99%. Using the equation above retaining more terms with higher n does not make significant difference. The effect of shaking is presumably to reduce the resistance to mass transfer in the interfacial boundary layer outside the gel.
Diffusion of GelMA molecules into GGMA hydrogels was also confirmed by the enhancement of the mechanical properties of the resulting gels. The immersed gels were taken out, exposed to light for the second crosslinking for varying times, and tested by compressions on a mechanical tester. Compared to the gels that were not exposed to light (0 s), the gels that were exposed to light presented enhanced mechanical properties, which confirms the formation of the second network (Fig. 2F–H). The compressive modulus, failure stress, and failure strain of the gels all continued to increase with increasing crosslinking time up to 180 s, the maximum time that was tested. The formation of the second network might not be complete in 180 s, so further crosslinking may further enhance the mechanical properties. However, longer crosslinking times are potentially detrimental for cell encapsulation, so for further experiments the second crosslinking time was set at 120 s.
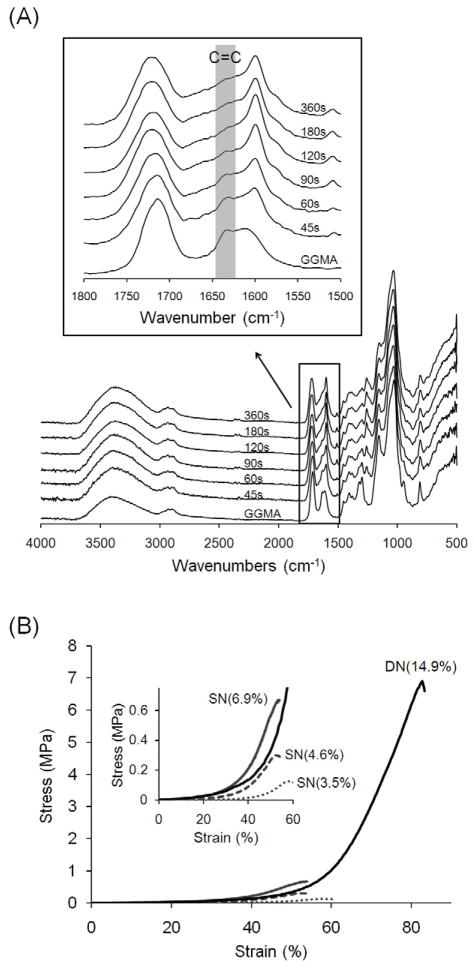
The interconnection between the two networks is an important factor that affects the mechanical properties of DN hydrogels. It was reported that DN hydrogels with no interconnection between the two networks can be stronger than those with the interconnection because the interconnection sites serve as the crosslinking sites of the second network, so if there are too many interconnections, the second network would not be crosslinked loosely enough [40]. Since both GGMA and GelMA networks are formed via the same mechanism, the unreacted C=C double bonds of the metheacrylate groups in the GGMA network can react during the GelMA network formation. To examine the amount of the unreacted double bonds in GGMA hydrogels, FTIR spectra were taken for GGMA hydrogels with varying crosslinking time (Fig. 3A). It was found that the shoulder peak appearing around 1640cm−1 corresponding to the unreacted C=C double bonds decreased over crosslinking time and almost disappeared in 120s, which meant most of the C=C double bonds reacted. Further crosslinking up to 360s did not significantly change the spectrum. Although this is a qualitative result and there may be a small fraction of unreacted double bonds, the crosslinking time was kept at 120s for further experiments since shorter crosslinking time is better for cell viability. Even though there may be a small amount of interconnection, the resulting DN hydrogels (prepared from 0.5% GGMA hydrogels immersed in 20% GelMA solution) were significantly stronger than the GGMA/GelMA SN hydrogels with the same mass ratio (GelMA/GGMA = 8.2, this will be discussed later in Fig. 6) prepared by a single crosslinking of GGMA/GelMA mixed solutions (Fig. 3B). It was found that GGMA/GelMA SN hydrogels with the polymer content as high as that of DN hydrogels (14.9%) could not be prepared since it was not possible to dissolve both polymers together at such a high concentration. The GGMA/GelMA SN hydrogels with lower polymer content failed at much lower strain and stress, which indicates that they are more brittle and weaker than DN hydrogels. Based on Fig. 3B, it is expected that even if we made the SN hydrogels with the same polymer content, they would fail at lower strain and stress than DN hydrogels.
Fig. 3.
(A) FTIR spectra of GGMA and dried GGMA hydrogels crosslinked for varying time. The shoulder peak appearing around 1640cm−1 corresponds to the unreacted C=C bonds. (B) Stress-strain curves of GGMA/GelMA SN and DN hydrogels with the same mass ratio (GelMA/GGMA = 8.2). Every crosslinking time was 120 seconds, and GelMA (DM: 32.3%) was used. The number in parenthesis refers to the polymer content of the hydrogels.
Fig. 6.
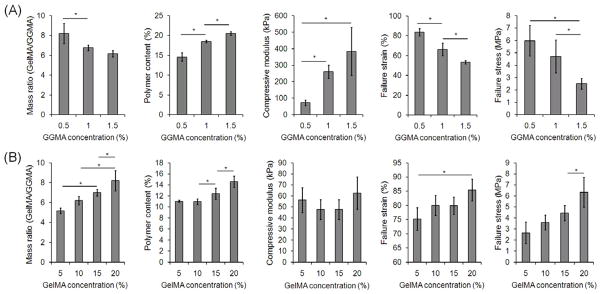
Mass ratio (GelMA/GGMA), polymer content, compressive modulus, failure strain, and failure stress of DN hydrogels with varying concentration of either component: (A) varying concentration of GGMA hydrogels + 20% GelMA solution, and (B) 0.5% GGMA hydrogels + varying concentration of GelMA solution. GelMA (DM: 32.3%) was used for (A)–(B). (*) indicates significant difference (P < 0.05).
3.3. Mechanical properties of DN hydrogels
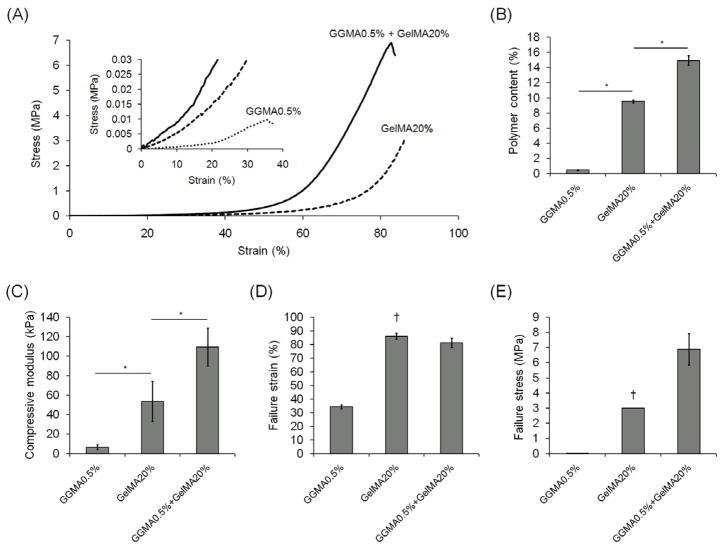
The mechanical properties of DN hydrogels were measured by unconfined, uniaxial compression tests on a mechanical tester, and compared with those of GGMA and GelMA SN hydrogels (Fig. 4). The modulus of DN hydrogels made from 0.5% GGMA hydrogels immersed in 20% GelMA (DM: 32.3%) solution was significantly higher than that of SN hydrogels. It may seem strange that the modulus of GGMA hydrogel is lower than that of GelMA hydrogel as the first network must be stiffer than the second network, and that the modulus of DN hydrogel became very high by combining two networks with relatively low modulus. In order to explain this, it should be considered that these mechanical data were obtained from swollen hydrogels, and the modulus of a hydrogel greatly depends on the polymer content in the swollen state. Even though the GGMA hydrogel barely swelled in water, since it was formed at very low concentration (0.5%), the polymer content of the swollen GGMA hydrogel was only 0.46 ± 0.05 %, while the 20% GelMA hydrogel swelled to a much higher degree, but still had a high polymer content, 9.5 ± 0.2 %. Considering that the modulus of 1.0% GGMA hydrogel with the polymer content of 1.1 ± 0.1% had a similar modulus to that of GelMA 20% (data not shown), it is true that GGMA network is stiffer than GelMA network. Also, GGMA hydrogels were brittle, breaking at less than 40% of strain, while GelMA hydrogels did not break up to 80% of strain. The polymer content of the resulting DN hydrogel was even higher, 14.9 ± 0.6 %. This is likely because the swelling of the GelMA network was restricted since it was formed in the GGMA network. In addition, as mentioned earlier, the GGMA hydrogels deswelled in GelMA solution likely due to the osmotic pressure, and the final volume of the DN gels in the swollen state was smaller than that of the initial GGMA hydrogels. Thus, the increased concentration of GGMA in DN hydrogels was also the reason for the high polymer content. Along with the effect of DN, this higher polymer content also caused the DN hydrogels to have much higher modulus.
Fig. 4.
(A) Stress-strain curves for SN and DN hydrogels under uniaxial compression. (B) Polymer content, (C) compressive modulus, (D) failure strain, and (E) failure stress of SN and DN hydrogels. (†) indicates the stress under which the majority of GelMA SN gels started to break, and the strain at that stress. Every crosslinking time was 120 seconds, and GelMA (DM: 32.3%) was used for (A)–(E). (*) indicates significant difference (P < 0.05).
We found that GelMA (DM: 32.3%) SN hydrogels were quite strong by themselves, such that the failure stress reached up to a few MPa (80% of hydrogels tested broke under 3 MPa, while 20% broke under 2.5 MPa), which is not usually observed for SN hydrogels. The DN hydrogels exhibited even higher failure stress than SN hydrogels, 6.9 ± 1.0 MPa. This higher strength of the DN hydrogels results from a combination of an increase in the equilibrium polymer concentration and the DN structure. DN formation enabled higher polymer content in the swollen state without increasing the crosslink density as is required to increase concentration for SN hydrogels, which leads to brittle gels. Thus, along with the crack energy dissipation to the second network, the higher polymer content of these DN hydrogels without the increase of crosslink density is a great advantage that enables them to resist higher stress. To confirm that DN hydrogels are stronger than SN hydrogels even when their polymer contents are similar, GelMA SN hydrogels with polymer content of 15.3 ± 0.3 % were made from a 15% solution of GelMA with higher DM (65.2%). It was found that 80% of these DN hydrogels tested broke under 2 MPa, which shows that they were weaker than DN hydrogels with similar polymer content, and even weaker than GelMA (DM: 32.3%) SN hydrogels with a lower polymer content.
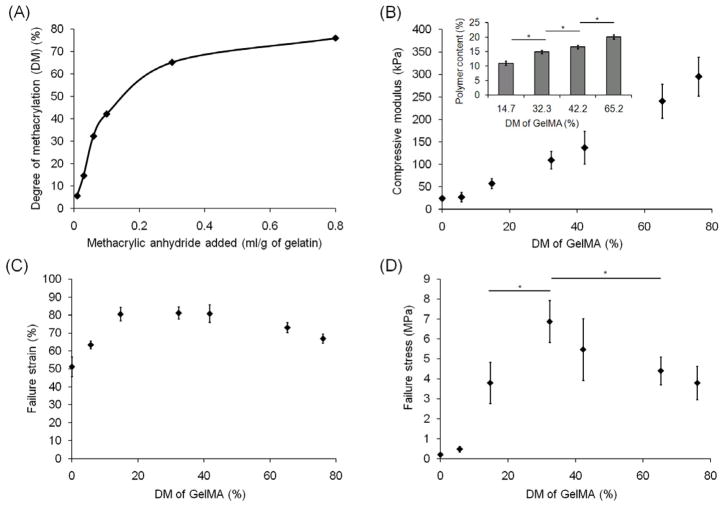
To examine the effect of the crosslink density of the GelMA network on the mechanical properties of DN hydrogels, GelMA polymers with varying DM were synthesized and used to make DN hydrogels (Fig. 5). The DM of GelMA increased up to ~30% almost linearly with the amount of metharcrylic anhydride added to the reaction, but over ~30%, the conversion increased more slowly with increasing methacrylic anhydride concentration. The compressive modulus of DN hydrogels increased as the DM of GelMA increased due to the increase in crosslink density of the second network and the polymer content of DN hydrogels. Since the GelMA polymer with higher DM is more hydrophobic, the DN hydrogels resulting from it swelled less in water and presented higher polymer content in swollen state. However, the failure strain and the failure stress had a maximal value, 81 ± 3 % and 6.9 ± 1.0 MPa at the DM of 32.3%. This demonstrates that a certain amount of crosslink is needed to make substantial gel that can work effectively as the second network, but if the second network gets crosslinked too much, it becomes brittle and its capacity of energy dissipation decreases. The intermediate crosslink density that shows maximal failure stress optimizes the trade-off between stiffness and the strength of the DN hydrogels.
Fig. 5.
(A) Degree of methacrylation (DM) of GelMA with varying amount of methacrylic anhydride added to the reaction. (Inset in B) Polymer content of DN hydrogels with varying DM of GelMA. Effect of DM of GelMA on (B) compressive modulus, (C) failure strain, and (D) failure stress of DN hydrogels. Every crosslinking time was 120 seconds. 0.5% GGMA hydrogels and 20% GelMA(each DM) solutions were used for (A)–(E). (*) indicates significant difference (P < 0.05).
The concentration of each component in the preparation of DN hydrogels also had great influence on the mechanical properties (Fig. 6). When the concentration of the first GGMA hydrogels varied from 0.5% to 1.5%, while that of GelMA (DM: 32.3%) solution was kept at 20%, the mass ratio of GelMA to GGMA in the resulting DN hydrogels decreased from 8.2 to 6.2. The reason for that the mass ratio decreased by only 24% of the initial value while the concentration of GGMA was tripled is that the higher the GGMA concentration, the less the GGMA hydrogels deswelled in GelMA solution. Due to the stiffness of the GGMA network, the modulus of the DN hydrogels significantly increased as the GGMA concentration increased. However, the failure strain and stress dropped greatly. This indicates that the mass ratio of the second to the first network must be very high to get strong DN hydrogels, consistent with previous results [2, 12]. When the concentration of GelMA solution was varied from 5% to 20% while the GGMA concentration was kept at 0.5%, the compressive modulus did not vary significantly. However, increasing the concentration of GelMA increased the mass ratio from 5.2 to 8.2, resulting in significant increases in the failure strain and stress. Achieving a high mass ratio of the second to the first network was an important reason for choosing the GGMA with a high molecular weight as the first component since polymers with high molecular weights can form hydrogels at very low concentrations. It was difficult to prepare DN hydrogels with even higher mass ratios because GGMA polymer could not form hydrogels at lower concentrations than 0.5%, and GelMA solutions with higher concentration than 20% were too viscous to process. However, increasing the molecular weight of GG may allow gelation at a lower concentration, enabling stronger DN hydrogels by further increasing the mass ratio of the second network to the first network.
3.4. Encapsulation of cells in DN hydrogels
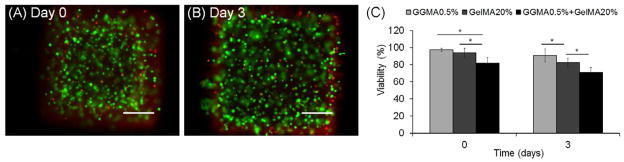
To fabricate cell-laden tissue constructs for tissue engineering applications, cells encapsulated in the DN hydrogels must survive the entire DN synthesis process. To make cell-laden DN hydrogels, we encapsulated cells in the first network, GGMA hydrogels, by crosslinking the mixture of cell suspension and GGMA solution. Subsequently, the cell-laden GGMA hydrogels were immersed in GelMA solution containing the photoinitiator, followed by the second crosslinking. The cell viability of encapsulated NIH-3T3 fibroblasts in the DN hydrogels was measured at two time points, day 0 and day 3 (Fig. 7). The cell viability tested by using live/dead staining 1 hour after the DN formation (Day 0) was 82%, and after 3 days of culture, the cell viability was measured to be 71%. Although the cell viability in DN hydrogels was lower than that in each GGMA and GelMA SN hydrogels, it is as good as in some previous reports on the cell viability in photocrosslinked hyrogels [19, 22, 29]. This result demonstrates that the majority of cells survived the whole DN formation process, that is, the two crosslinking steps under light and the immersion step between them in a viscous solution containing the photoinitiator. As shown in Fig. 2, longer crosslinking time may enhance the mechanical properties of the DN hydrogels; however, it is likely to result in a lower cell viability. Immersion in the viscous solution with the photoinitiator may also harm the cells, thus making the immersion time shorter would increase the cell viability. However, shortening the immersion time will limit the size of the DN constructs since time is required to allow the second polymer to diffuse in the first network. Thus, encapsulating cells in this DN system can be further optimized by controlling the crosslinking conditions and immersion time that closely relates to the cell viability, the size of the constructs, and the mechanical properties. Further cell experiments such as a tissue formation in DN hydrogels and the resulting change in mechanical properties over time will be performed in the future.
Fig. 7.
Fluorescence images of live/dead stained NIH-3T3 fibroblasts encapsulated in DN hydrogels: (A) day 0 and (B) day 3 of culture after DN hydrogel formation. Scale bars represent 200μm. (C) Viability of 3T3 fibroblasts encapsulated in SN and DN hydrogels. (*) indicates significant difference (P < 0.05). 0.5% GGMA hydrogels and 20% GelMA (DM: 14.7%) solutions were used for (A)–(C).
4. Conclusions
In this study we developed mechanically strong DN hydrogels that can encapsulate cells for applications as scaffolds for load-bearing tissues. Cell-laden DN formation was made possible by using a two-step photocrosslinking of two modified biomacromolecules, GGMA and GelMA, which are photoreactive versions of gellan gum and gelatin. As compared to SN hydrogels, DN hydrogels exhibited higher strength, which approaches closer to the strength of cartilage. It was found that a certain range of DM of the second network is optimal to achieve highest strength of the DN hydrogels, and a large mass ratio of the second to the first network is needed to obtain strong DN hydrogels. The encapsulation of NIH-3T3 fibroblasts and the following cell viability assay presented that the whole DN formation process was cell-compatible. Given the high mechanical strength and the cell-compatibility, our DN hydrogels made from photocrosslinkable macromolecules have great potential in applications as scaffolds for the load-bearing tissues.
Acknowledgments
Hyeongho Shin acknowledges financial support from the Samsung Scholarship. This paper was supported by the National Institutes of Health (HL092836, EB02597, AR057837), the National Science Foundation CAREER award (DMR0847287) and the Office of Naval Research Young Investigator award. The authors would like to thank Daniela F. Coutinho for discussions and aid of GGMA synthesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fung Y. Biomechanics: mechanical properties of living tissues. Springer; 1993. [Google Scholar]
- 2.Gong J. Why are double network hydrogels so tough? Soft Matter. 2010;6(12):2583–2590. [Google Scholar]
- 3.Kerin AJ, Wisnom MR, Adams MA. The compressive strength of articular cartilage. Proc Inst Mech Eng Part H-J Eng Med. 1998;212(H4):273–280. doi: 10.1243/0954411981534051. [DOI] [PubMed] [Google Scholar]
- 4.McCutchen C. Lubrication of joints, the joints and synovial fluid. Academic Press: New York. 1978;10:437. [Google Scholar]
- 5.Amsden B. Curable, biodegradable elastomers: emerging biomaterials for drug delivery and tissue engineering. Soft Matter. 2007;3:1335–1348. doi: 10.1039/b707472g. [DOI] [PubMed] [Google Scholar]
- 6.Calvert P. Hydrogels for soft machines. Adv Mater. 2009;21(7):743–756. [Google Scholar]
- 7.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 8.Lee K, Mooney D. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 9.Peppas N, Hilt J, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345. [Google Scholar]
- 10.Nuttelman CR, Rice MA, Rydholm AE, Salinas CN, Shah DN, Anseth KS. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Prog Polym Sci. 2008;33(2):167–179. doi: 10.1016/j.progpolymsci.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ifkovits J, Burdick J. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13(10):2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 12.Gong J, Katsuyama Y, Kurokawa T, Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv Mater. 2003;15(14):1155–1158. [Google Scholar]
- 13.Nakayama A, Kakugo A, Gong JP, Osada Y, Takai M, Erata T, et al. High mechanical strength double network hydrogel with bacterial cellulose. Adv Funct Mater. 2004;14(11):1124–1128. [Google Scholar]
- 14.Na YH, Kurokawa T, Katsuyama Y, Tsukeshiba H, Gong JP, Osada Y, et al. Structural characteristics of double network gels with extremely high mechanical strength. Macromolecules. 2004;37(14):5370–5374. [Google Scholar]
- 15.Weng L, Gouldstone A, Wu Y, Chen W. Mechanically strong double network photocrosslinked hydrogels from N,N-dimethylacrylamide and glycidyl methacrylated hyaluronan. Biomaterials. 2008;29(14):2153–2163. doi: 10.1016/j.biomaterials.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myung D, Koh W, Ko J, Noolandi J, Carrasco M, Smithc A, et al. Characterization of poly(ethylene glycol)-poly(acrylic acid) (PEG-PAA) double networks designed for corneal implant applications. Invest Ophthalmol Vis Sci. 2005;46:1. [Google Scholar]
- 17.Brigham MD, Bick A, Lo E, Bendali A, Burdick JA, Khademhosseini A. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng Part A. 2009;15(7):1645–1653. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryant S, Nuttelman C, Anseth K. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11(5):439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 19.Fedorovich NE, Oudshoorn MH, van Geemen D, Hennink WE, Alblas J, Dhert WJA. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30(3):344–353. doi: 10.1016/j.biomaterials.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 20.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103(8):2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28(34):5087–5092. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Burdick J, Chung C, Jia X, Randolph M, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6(1):386. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coutinho DF, Sant SV, Shin H, Oliveira JT, Gomes ME, Neves NM, et al. Modified gellan gum hydrogels with tunable physical and mechanical properties. Biomaterials. 2010;31(29):7494–7502. doi: 10.1016/j.biomaterials.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae H, Ahari AF, Shin H, Nichol JW, Hutson CB, Masaeli M, et al. Cell-laden microengineered pullulan methacrylate hydrogels promote cell proliferation and 3D cluster formation. Soft Matter. 2011;7(5):1903–1911. doi: 10.1039/C0SM00697A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryant SJ, Bender RJ, Durand KL, Anseth KS. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol Bioeng. 2004;86(7):747–755. doi: 10.1002/bit.20160. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira J, Martins L, Picciochi R, Malafaya P, Sousa R, Neves N, et al. Gellan gum: a new biomaterial for cartilage tissue engineering applications. J Biomed Mater Res A. 2010;93(3):852–863. doi: 10.1002/jbm.a.32574. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira JT, Santos TC, Martins L, Picciochi R, Marques AP, Castro AG, et al. Gellan gum injectable hydrogels for cartilage tissue engineering applications: in vitro studies and preliminary in vivo evaluation. Tissue Eng Part A. 2009;16(1):343–353. doi: 10.1089/ten.TEA.2009.0117. [DOI] [PubMed] [Google Scholar]
- 28.Gong Y, Wang C, Lai RC, Su K, Zhang F, Wang D. An improved injectable polysaccharide hydrogel: modified gellan gum for long-term cartilage regeneration in vitro. J Mater Chem. 2009;19(14):1968–1977. [Google Scholar]
- 29.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamcerencu M, Desbrieres J, Khoukh A, Popa M, Riess G. Synthesis and characterization of new unsaturated esters of gellan gum. Carbohydr Polym. 2008;71(1):92–100. [Google Scholar]
- 31.Brinkman WT, Nagapudi K, Thomas BS, Chaikof EL. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: mechanical properties, cell viability, and function. Biomacromolecules. 2003;4(4):890–895. doi: 10.1021/bm0257412. [DOI] [PubMed] [Google Scholar]
- 32.Podczeck F. Pharmaceutical capsules. Pharmaceutical Pr; 2004. [Google Scholar]
- 33.Flory P. Principles of polymer chemistry. Cornell Univ Pr; 1953. [Google Scholar]
- 34.Jost W. Diffusion in solids, liquids, gases. Academic Press; New York: 1960. [Google Scholar]
- 35.Pawel R. Diffusion in a finite system with a moving boundary. J Nucl Mater. 1974;49(3):281–290. [Google Scholar]
- 36.Griffith P, Stilbs P, Howe A, Cosgrove T. A self-diffusion study of the complex formed by sodium dodecyl sulfate and gelatin in aqueous solutions. Langmuir. 1996;12(12):2884–2893. [Google Scholar]
- 37.Chang T, Yu H. Self-diffusion of gelatin by forced Rayleigh scattering. Macromolecules. 1984;17(1):115–117. [Google Scholar]
- 38.Amsden B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules. 1998;31(23):8382–8395. [Google Scholar]
- 39.Pluen A, Netti PA, Jain RK, Berk DA. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophys J. 1999;77(1):542–552. doi: 10.1016/S0006-3495(99)76911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakajima T, Furukawa H, Tanaka Y, Kurokawa T, Osada Y, Gong JP. True chemical structure of double network hydrogels. Macromolecules. 2009;42(6):2184–2189. [Google Scholar]